Abstract
Glucagon is classically described as a counterregulatory hormone that plays an essential role in the protection against hypoglycemia. In addition to its role in the regulation of glucose metabolism, glucagon has been described to promote ketosis in the fasted state. Sodium–glucose cotransporter 2 inhibitors (SGLT2i) are a new class of glucose-lowering drugs that act primarily in the kidney, but some reports have described direct effects of SGLT2i on α-cells to stimulate glucagon secretion. Interestingly, SGLT2 inhibition also results in increased endogenous glucose production and ketone production, features common to glucagon action. Here, we directly test the ketogenic role of glucagon in mice, demonstrating that neither fasting- nor SGLT2i-induced ketosis is altered by interruption of glucagon signaling. Moreover, any effect of glucagon to stimulate ketogenesis is severely limited by its insulinotropic actions. Collectively, our data suggest that fasting-associated ketosis and the ketogenic effects of SGLT2 inhibitors occur almost entirely independent of glucagon.
Introduction
The current view of glucagon physiology is that it acts as the catabolic counterbalance to insulin, maintaining adequate amounts of blood glucose in states where it is threatened, such as starvation, exercise, and hypoglycemia (1,2). Glucagon is secreted into the hepato-portal circulation and has well-described effects to promote hepatic glucose production by increasing glycogenolysis and gluconeogenesis (1). Another important effect of glucagon in the liver is to reduce glucose consumption by increasing the oxidation of fatty acids, a shift in fuel utilization that coordinates energy needs and glucose production (2). The actions of glucagon to increase lipid oxidation, including the production of ketone bodies that is a downstream end point of this process, have been defined by numerous experiments with cultured hepatocytes (3–5). Moreover, the classic studies of Gerich et al. (6), using somatostatin to reduce circulating glucagon and mitigate diabetic ketoacidosis (DKA), add to the now ingrained belief that glucagon has both glucogenic and ketogenic activities.
Sodium–glucose cotransporter 2 inhibitors (SGLT2i) are a new class of medications used in type 2 diabetes (T2D) that have been implicated in causing ketosis. These drugs act by blocking reabsorption of glucose in the proximal tubules of the kidneys, leading to substantial glucosuria (7). In addition to improved glycemia and modest weight loss, reductions of adverse cardiovascular and renal complications of T2D have been demonstrated with use of SGLT2i in recent clinical trials (8). However, it is now clear that SGLT2i have consistent effects to raise circulating ketone bodies and, in uncommon instances, can precipitate DKA, particularly among insulinopenic patients (9–16). While the mechanism by which SGLT2i causes ketosis has not been established, patients treated with these agents have elevations in circulating glucagon and increased hepatic glucose production. Remarkably, there is some evidence that SGLT2 is expressed by the islet α-cells that secrete glucagon, leading to the hypothesis that increased glucagon mediates SGLT2i effects in the liver (17–19).
Ketogenesis is considered to be controlled by the islet hormones, insulin and glucagon (20). Insulin strongly inhibits ketosis, predominantly by reducing lipolysis in adipocytes and reducing the supply of free fatty acids, the substrate for ketone body production. In addition, insulin may have direct effects at the level of the hepatocyte by lowering intracellular cAMP (21). In contrast, glucagon potently increases cAMP in hepatocytes, a signal that has been tied to both lipid and glucose metabolism (22). Consequently, the plasma insulin-to-glucagon ratio has long been purported to dictate the rates of ketogenesis (23). For example, it has been proposed that DKA secondary to SGLT2 inhibition occurs through a drug-induced increase in glucagon in an insulinopenic patient (10). However, this explanation was recently put in doubt in a study that implicated dehydration and insulinopenia as necessary components of dapagliflozin-induced ketosis in a rat model (24). In this study, the authors demonstrated that glucagon was elevated in response to SGLT2i independent of hydration state, while ketosis in this study required dehydration in the setting of insulinopenia, raising some doubt as to the contribution of glucagon to SGLT2i-induced ketosis.
We recently challenged the primacy of a catabolic, counterregulatory role for glucagon by demonstrating that glucagon has a significant impact on glucose metabolism as an insulin secretagogue (25,26), an observation also noted by other investigators (27,28). In the current study, we sought to directly investigate the importance of glucagon in the regulation of physiologically (fasting) or pharmacologically (SGLT2i) induced ketosis. Herein, we demonstrate that loss of glucagon signaling does not modulate ketone production in response to fasting or SGLT2i. Importantly, we demonstrate that glucagon is only capable of increasing ketone production in the context of complete loss of insulin signaling. Moreover, we reveal that SGLT2i-induced ketosis occurs independently of the actions of insulin and glucagon.
Research Design and Methods
Reagents
Dapagliflozin was purchased from Advanced ChemBlocks Inc. and prepared fresh in PBS. Glucagon receptor–blocking antibody (Ab-4) was prepared in PBS and kindly provided by Eli Lilly and Company. Glucagon (Gcg) was purchased from Sigma-Aldrich, and stocks were prepared in 0.3% acetic acid. Epinephrine was purchased from Sigma-Aldrich and prepared fresh in PBS for each experiment. Streptozotocin [STZ] was purchased from Sigma-Aldrich and prepared in sodium citrate buffer. S661, an insulin receptor antagonist (29), was prepared in PBS and was kindly provided by B.F. from Novo Nordisk.
Animals
All mouse procedures were approved and performed in accordance with the Duke University Institutional Animal Care and Use Committee. Experiments were performed in 8- to 20-week-old mice of the C57Bl6 background. Mice were housed under a 12-h light/dark cycle and provided free access to a normal chow diet. WT mice were either bred in-house or purchased from The Jackson Laboratory. Gcg−/− mice, described previously (30), were generated by breeding Gcg+/− mice. Cage-matched Gcg+/+ mice served as controls.
Dapagliflozin Treatment
Mice were fasted for 3 h in the morning before receiving orally administered dapagliflozin (10 mg/kg) in PBS. Plasma was collected 3 or 6 h post–dapagliflozin treatment. Mice had ad libitum access to water throughout the experiments. For glucagon studies, glucagon (20 µg/kg) was injected 3 h post–dapagliflozin treatment. For studies using Ab-4, Ab-4 (10 mg/kg) was administered 24 h prior to dapagliflozin treatment.
Fast and Refeed
Food was removed from mice at the end of the light cycle. Plasma was collected at the beginning of the following light cycle after ∼12–14 h of fasting. Food was then provided and plasma collected after 30–60 min of refeeding. For studies using Ab-4, 10 mg/kg i.p. was administered 24 h prior to fasting plasma collection.
S661 and STZ Treatment
S661 was injected (20 nmol/mouse i.p.). For STZ treatment, mice were injected for two consecutive days with 120 mg/kg i.p. STZ in sodium citrate buffer. Control mice were injected with sodium citrate buffer. Mice received subsequent doses of STZ as needed until fed glycemia was ≥350 mg/dL. For experiments using STZ/S661, only mice with postfeeding blood glucose >400 mg/dL were used. For STZ/S661 plus dapagliflozin experiments, mice were fasted for 3 h prior to dapagliflozin administration at t = 0. S661 was administered 1 h before blood glucose and ketone measurement at t = 3 h. For STZ/S661 plus glucagon experiments, mice were fasted for 5 h and then injected at t = −60 min with S661, and glucagon was injected 1 h later at t = 0.
Metabolic and Hormone Measurements
Blood glucose was measured from tail blood using a glucometer (Contour). β-Hydroxybutyrate (βOHB) was measured using a ketometer (Precision Xtra), which we validated by the measurement of known concentrations of βOHB spiked into either plasma from a fed mouse to achieve low βOHB levels. Values from the ketometer averaged ±14.25% of the expected concentrations across a range of βOHB from 0 to 2 mmol/L. Assay variability was 12.9% when the same sample was read three times. Plasma nonesterified fatty acid (NEFA) concentrations were measured by enzymatic assay (Wako Diagnostics). Plasma insulin and glucagon were measured by ELISA (Mercodia). Insulin and glucagon levels that were undetectable were assigned the lowest value on the standard curve.
Statistical Analysis
All data are presented as mean ± SEM. Statistical analyses were performed using GraphPad Prism 7. t tests or one-way or two-way ANOVA were performed, depending on the experiment, with a Bonferroni post hoc analysis. P < 0.05 was determined to identify statistically significant differences.
Data and Resource Availability
All original data and key resources supporting this work will be made available upon reasonable request.
Results
Physiologic and Pharmacologic Ketosis Is Not Temporally Associated With Peripheral Glucagon Levels
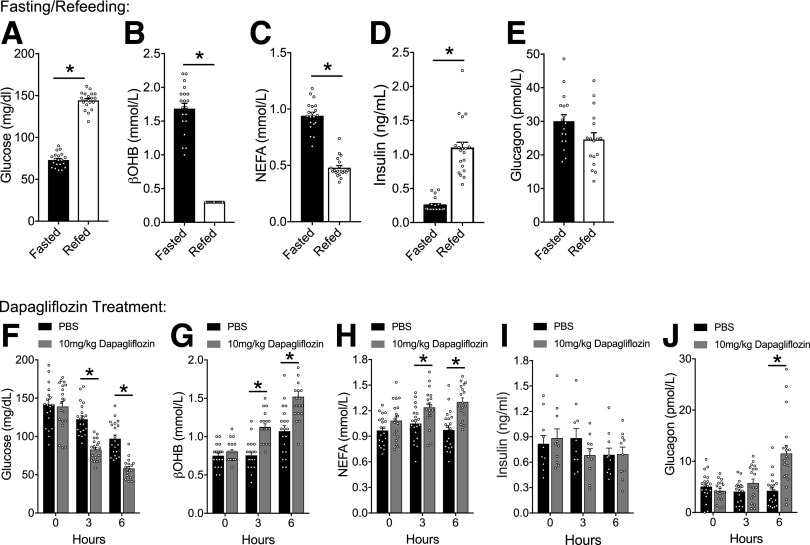
To establish a dynamic range for the circulating concentration of ketones and hormones, we fasted wild-type (WT) animals overnight (∼16 h), followed by a 1-h refeeding period. We measured βOHB to assess ketosis, as it is the predominant circulating ketone and shows the greatest dynamic range in humans (31). As expected, prolonged fasting was characterized by low glycemia, which was quickly reversed by refeeding (Fig. 1A). The fasted state was also characterized by elevated circulating βOHB and NEFA concentrations, which were substantially reduced by refeeding (Fig. 1B and C). Plasma insulin was low following the fast, with many of the samples below the level of detection, and increased robustly following refeeding (Fig. 1D). Plasma glucagon levels were elevated by fasting (ambient concentrations are typically <10 pmol/L [see Fig. 1J]), and refeeding led to a modest, but nonsignificant, reduction in glucagon levels (P = 0.06) (Fig. 1E). This paradigm of fasting/refeeding produces clear and easily measurable changes in ketosis, reflected in βOHB levels, accompanied by the expected effects on glycemia, NEFA, insulin, and glucagon concentrations. Consequently, we used fasting as a physiological stimulus for ketosis.
Figure 1.
Physiologic and pharmacologic ketosis is not temporally associated with peripheral glucagon levels. Mice were fasted overnight and refed at 8:00 a.m. for 30 min, and glycemia (A), βOHB (B), NEFA (C), insulin (D), and glucagon (E) were measured. Mice were fasted for 3 h and then administered PBS or dapagliflozin (10 mg/kg), and glycemia (F), βOHB (G), NEFA (H), insulin (I), and glucagon (J) were measured over the following 6 h. Data are shown as mean ± SEM. *P < 0.05. Data were analyzed by a two-tailed Student t test (A–E) or two-way ANOVA (F–J).
We next established a pharmacological ketogenic stimulus with the SGLT2i dapagliflozin. Dapagliflozin-induced ketosis has been demonstrated in both rodent and human studies (17,18,24,32–34). We determined the kinetic profile of dapagliflozin by fasting WT mice for 3 h at the beginning of the light cycle (7:00 a.m.) to establish a starting baseline, followed by gavage of dapagliflozin and monitoring for an additional 6 h. This protocol involves a total of 9 h of fasting during the lights-on period in both control and treatment groups (7:00 a.m.–4:00 p.m.). Dapagliflozin treatment induced a greater drop in glycemia compared with control groups (Fig. 1F). Analysis of urine from mice collected at the end of the 6-h treatment confirmed substantial glucosuria (Supplementary Fig. 1) (mean ± SEM for control: 17.1 ± 1.9 mg/dL and dapagliflozin: 8,767.2 ± 578.7 mg/dL). We also observed significantly elevated βOHB levels in dapagliflozin-treated mice at 3 h and 6 h post-treatment (Fig. 1G), concurrent with elevated concentrations of plasma NEFA (Fig. 1H). Insulin levels were not significantly altered by dapagliflozin (Fig. 1I), but treatment did increase plasma glucagon at the 6-h time point (Fig. 1J). Thus, dapagliflozin-stimulated plasma glucagon occurred after detectable changes in glycemia, ketones, and NEFAs.
We next tested direct effects of SGLT2i on islet cell secretion in perifusion experiments. Mouse islets treated with 200 ng/mL dapagliflozin at various glucose concentrations failed to increase glucagon secretion at either low- or high-glucose concentrations (Supplementary Fig. 2). We have previously shown that stimulating glucagon secretion at high glucose concentrations produces a concurrent increase in insulin secretion (25). Dapagliflozin did not stimulate insulin secretion at low glucose or high glucose concentrations. Together, these findings demonstrate that there is little to no direct effect of SGLT2 inhibition on islet hormone secretion in mice.
Recently, SGLT2i has been postulated to increase ketones through a mechanism linked to dehydration and insulinopenia but independent of glucagon secretion (24). Our initial observations were made in mice with ad libitum access to water, but we hypothesized that our results could be amplified in dehydrated mice. To test this, we administered dapagliflozin and measured circulating ketones in mice with restricted or ad libitum access to water. Dapagliflozin treatment reduced body weight (a marker of dehydration [24]) and increased βOHB to a similar extent in both conditions (Supplementary Fig. 3A and B). Interestingly, restricting water intake independent of dapagliflozin treatment doubled the amount of weight loss (not significant) and limited the rise of βOHB concentrations over the treatment period (Supplementary Fig. 3). Consequently, while we did see a modest interaction between hydration status and ketone levels, we were unable to demonstrate that this interaction influenced dapagliflozin-induced ketogenesis in the 3-h protocol used. Hereafter, all experiments were performed in mice provided water ad libitum.
Loss of Glucagon Signaling Does Not Impair Physiologic or Pharmacologic Ketosis
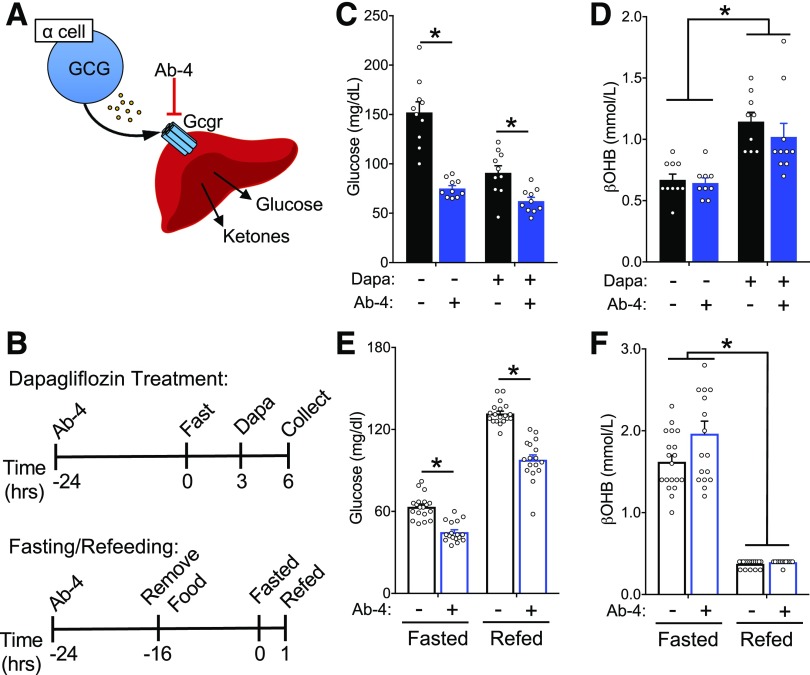
Our observation that dapagliflozin elevates ketone levels prior to a rise in glucagon levels supports a mechanism of lipolysis and ketogenesis independent of glucagon. However, we cannot completely rule out the contribution of glucagon based on our measurement of temporal changes in tail vein plasma concentrations. Indeed, measuring glucagon levels in the tail vein is not always reflective of the dynamic changes occurring in the portal circulation (26,35), which is proximal to hepatocytes and more relevant for ketone metabolism. To directly assess the contribution of glucagon to ketogenesis, we interrupted glucagon signaling through two independent strategies: 1) blocking glucagon action by treatment with Ab-4 (25,36,37), an anti-GCGR human IgG4 antibody (GRA) (Fig. 2) and 2) studying mice with deletion of the preproglucagon gene (Gcg) and complete loss of proglucagon-derived peptides (Gcg−/−) (30) (Fig. 3). For the GRA experiments, mice were injected 24 h prior to the initiation of a 3-h fast, after which dapagliflozin or control gavage was performed, and the mice were monitored for an additional 3 h (Fig. 2A and B). As expected, both GRA and dapagliflozin independently lowered glycemia (Fig. 2C). However, dapagliflozin increased βOHB levels comparably in both control and GRA-treated mice (Fig. 2D), demonstrating that glucagon receptor activity is dispensable for SGLT2i-induced ketosis. To test this in the context of physiological ketosis, we administered the GRA prior to the fasting and refeeding protocol (Fig. 2B). The GRA significantly lowered both fasting and fed glycemic levels, a biomarker of its pharmacological activity (Fig. 2E), but did not alter βOHB levels in the fasting or fed state (Fig. 2F). Together, these data demonstrate that antagonism of the glucagon receptor does not interfere with physiological or pharmacological ketosis, suggesting that glucagon receptor signaling is not essential for ketogenesis.
Figure 2.
Glucagon receptor blockade with a blocking antibody does not impair physiologic or pharmacologic ketosis. A: Schematic of strategy to block glucagon receptor with blocking antibody Ab-4 (10 mg/kg). B: Timing of treatment with glucagon blockade with respect to intervention with dapagliflozin (Dapa) or fasting and refeeding. Glycemia (C) and βOHB (D) in response to dapagliflozin following Ab-4 treatment. Glycemia (E) and βOHB (F) in response to fasting and refeeding following Ab-4 treatment. Data are shown as mean ± SEM. *P < 0.05. Data were analyzed by two-way ANOVA (C–F). hrs, hours.
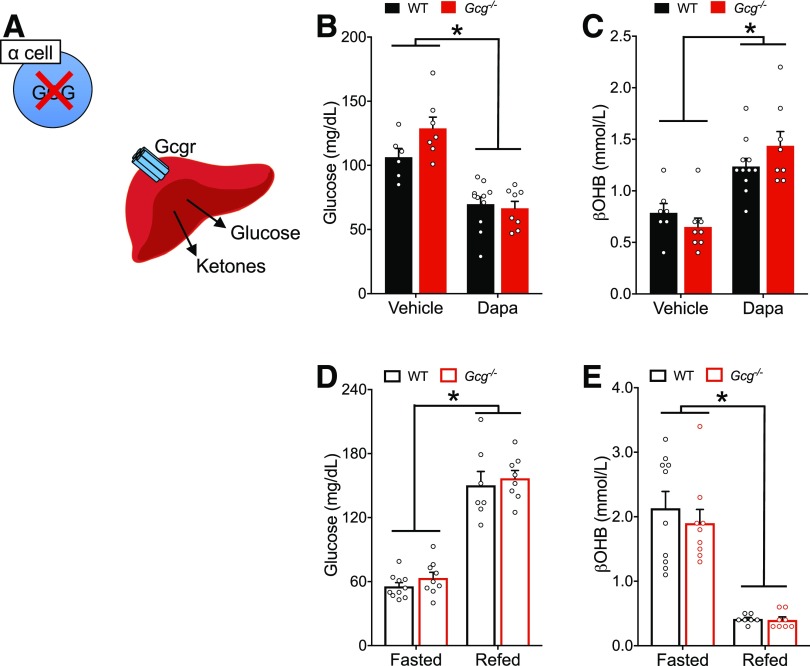
Figure 3.
Loss of proglucagon products does not impair physiologic or pharmacologic ketosis. A: Schematic of Gcg−/− loss of function, which is characterized by loss of glucagon from the α-cell but intact Gcgr signaling in the liver. Glycemia (B) and βOHB (C) in response to dapagliflozin (Dapa) in Gcg−/− mice or cage-matched controls. Glycemia (D) and βOHB (E) in response to fasting and refeeding in Gcg−/− mice or cage-matched controls. Data are shown as mean ± SEM. *P < 0.05. Data were analyzed by two-way ANOVA (B–E).
Glucagon receptor antagonism leads to substantial increases in both glucagon and glucagon-like peptide 1 (GLP-1) levels (38,39). We, and others, have shown that proglucagon products, including glucagon and GLP-1, strongly increase β-cell tone and insulin secretion through the GLP-1 receptor (GLP-1R) (25,26,40). Elevated insulin secretion in response to the GRA could inhibit ketogenesis and confound our interpretation of the direct involvement of glucagon. To avoid this possibility, we complemented our GRA experiments with an alternative approach using Gcg−/− mice (30) (Fig. 3A). Following the same experimental protocol used in the GRA experiments (Fig. 2B), we found that dapagliflozin reduced glycemia and increased βOHB levels to the same extent in both WT and Gcg−/− mice (Fig. 3B and C). Moreover, Gcg−/− mice displayed similar glycemia and βOHB compared with WT mice in both the fasted and refed states (Fig. 3D and E). These data provide additional evidence that loss of glucagon signaling does not impair ketosis in mice in response to either physiological or pharmacological stimuli.
Glucagon and Epinephrine Have Opposing Effects on Ketogenesis, Lipolysis, and Insulin Levels
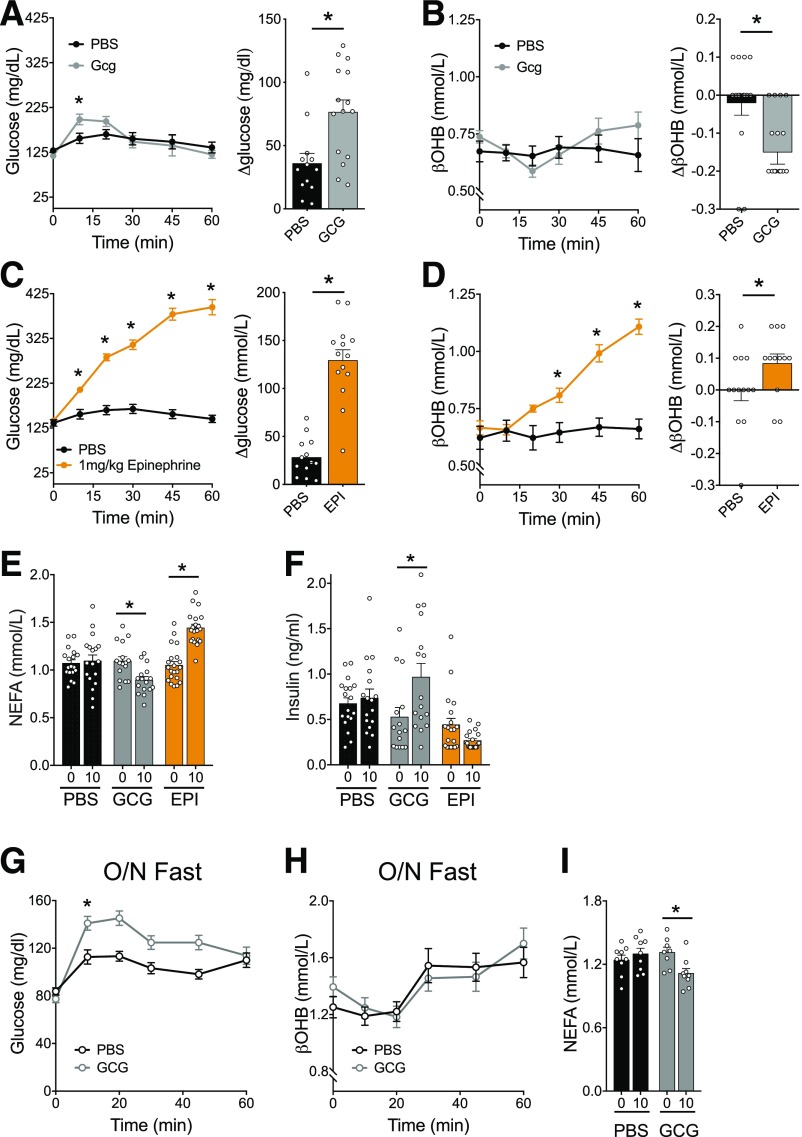
Although we demonstrate that endogenous glucagon signaling is dispensable for ketosis in response to either fasting or SGLT2 inhibition, we cannot rule out that supraphysiologic glucagon concentrations, e.g., associated with disease states such as decompensated diabetes or exogenous hormone administration, can induce ketosis. Indeed, previous studies using a perfused rodent liver technique suggest that infusion of glucagon should amplify ketogenesis (4). To test the hypothesis that exogenous glucagon administration can enhance ketosis, we administered glucagon (20 µg/kg i.p.) to WT mice fasted for 5 h. We previously reported that this dose is sufficient to activate glucagon receptors on hepatocytes, with minimal activity on β-cells (26). Exogenous glucagon transiently elevated glycemia at 10 min post–glucagon injection (Fig. 4A) but decreased βOHB levels 20 min post-injection (Fig. 4B). The reduction of ketone levels was exaggerated at a higher dose of glucagon (1 mg/kg)—a dose that does activate β-cells and potently enhances insulin secretion while lowering glycemia (26) (Supplementary Fig. 4). Together these findings are consistent with insulinotropic effects of glucagon apparent in the level of ketosis, which is very sensitive to suppression by insulin. In contrast, epinephrine given under the same experimental conditions robustly increased both glycemia and βOHB (Fig. 4C and D). Further analysis revealed that glucagon stimulated insulin secretion and reduced NEFA concentrations, while epinephrine reduced insulin levels and enhanced NEFA concentrations (Fig. 4E and F). These data align with our recent reports demonstrating the potent insulinotropic properties of glucagon (26), while epinephrine inhibits the β-cell as previously described (41,42). These findings suggest that insulinotropic responses to glucagon severely limit any direct hepatic ketogenic effects in vivo.
Figure 4.
Glucagon and epinephrine have opposing effects on ketogenesis, lipolysis, and insulin levels. A and B: Mice were injected with 20 μg/kg glucagon, and glycemia (A) and βOHB (B) were measured. The change from baseline to 20 min was calculate for both glucose and βOHB. C and D: Mice were injected with 1 mg/kg epinephrine (EPI), and glycemia (C) and βOHB (D) were measured. The change from baseline to 20 min was calculated for both glucose and βOHB. E and F: Baseline (0) and 10 min (10)-stimulated NEFA (E) and insulin (F) were measured in response to 20 μg/kg glucagon or 1 mg/kg epinephrine. G–I: Mice were fasted overnight (O/N), followed by an injection of glucagon (20 µg/kg). Glycemia (G), βOHB (H), and NEFA (I) were measured. Data are shown as mean ± SEM. *P < 0.05. Data were analyzed by two-way ANOVA (A–I) or two-tailed Student t test (Δ values for A–D).
To more directly test the hypothesis that glucagon-stimulated insulin secretion accounts for the apparent lack of ketogenic activity, we used a model to limit the insulinotropic potential of glucagon while also increasing NEFA levels to ensure sufficient substrate for ketogenesis. Glucagon stimulates insulin through the GLP-1R and GCGR on β-cells, which are glucose-dependent GPCRs that have minimal activity at low glucose concentrations (26). Based on this, we administered glucagon to mice fasted overnight to reduce glycemia and plasma insulin levels and limit the impact of glucagon on β-cell activity (Fig. 1D). In this setting, 20 µg/kg glucagon produced a more robust increase in glycemia compared with a 5-h fast, suggesting that the lower ambient glycemia after overnight fasting blunted glucagon action on β-cells (Fig. 4G). Even under these conditions, though, glucagon did not produce any effect on βOHB levels (Fig. 4H), but it reduced NEFA levels (Fig. 4I), suggesting that an overnight fast is not sufficient to prevent glucagon-stimulated insulin secretion in response to glucagon treatment. However, both unstimulated and stimulated insulin concentrations measured from the tail vein remained below the detection level of the assay (data not shown). Thus, we are only able to infer that the decrease in NEFA levels in response to glucagon is due to small increases in insulin secretion undetected in our sampling. Treatment with dapagliflozin, which significantly increased NEFA levels (Fig. 1H), limited the glycemic excursion in response to glucagon. However, glucagon did not further increase βOHB levels (Supplementary Fig. 5B), or lower circulating NEFA (Supplementary Fig. 5C), in response to dapagliflozin. Thus, exogenous glucagon is unable to induce ketosis in healthy mice even in the context of high circulating NEFA and low glycemia.
Blockade of Insulin Receptor Signaling Is Not Sufficient to Permit Glucagon-Stimulated Ketogenesis
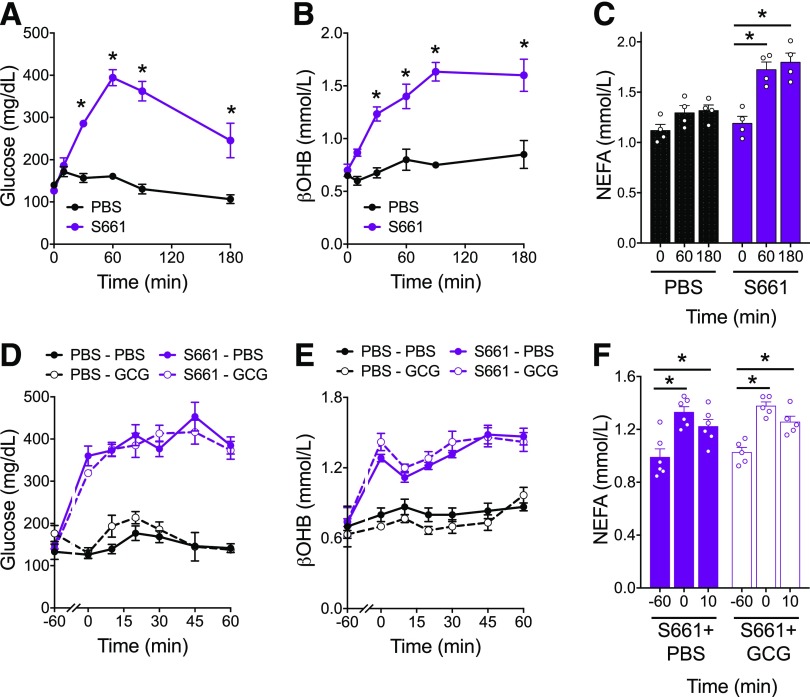
Although the insulinotropic potential of glucagon is substantially decreased following the reduction in glycemia with fasting, we cannot rule out small changes in insulin secretion induced by exogenous glucagon in fasted mice. This could be attributed either to modest effects of glucagon directly on the β-cell or to a secondary effect due to the rise in glycemia. Experimental ketosis stimulated by glucagon has been demonstrated in isolated hepatocytes, in perfused pancreas, or following somatostatin infusion to suppress insulin secretion (3–6)—all scenarios that eliminate the potential contribution of glucose- or glucagon-stimulated insulin secretion. Based on our findings, we hypothesized that any glucagon activity on β-cells could be sufficient to prevent ketosis. To test this, we sought to eliminate any contribution of insulin action. First, we used the insulin receptor antagonist S661 (29) to block the effects of any glucagon-stimulated insulin secretion. Treatment of healthy WT mice with i.p. injection of S661 potently elevated glycemia (Fig. 5A), βOHB levels (Fig. 5B), and plasma NEFA (Fig. 5C), confirming the strong inhibition of ketogenesis by insulin. To determine whether exogenous glucagon enhanced ketone production in the setting of insulin receptor blockade, we coadministered glucagon and S661. However, in mice treated acutely with S661, glucagon did not have an additive effect to increase glycemia, ketone levels, or NEFA concentrations (Fig. 5D–F). We conclude from these data that even with insulin receptor blockade, glucagon does not stimulate ketosis.
Figure 5.
Blockade of insulin receptor signaling is not permissive of glucagon-stimulated ketogenesis. Mice were injected with S661 (20 nmol/mouse), and glycemia (A), βOHB (B), and NEFA (C) were assessed over a 3-h period. Mice were injected with S661 (20 nmol/mouse) 1 h before injection with glucagon (20 µg/kg). Glycemia (D), βOHB (E), and NEFA (F) were assessed. Data are shown as mean ± SEM. *P < 0.05. Data were analyzed by two-way ANOVA.
Glucagon Stimulates Ketosis in the Context of Complete Lack of Insulin Signaling
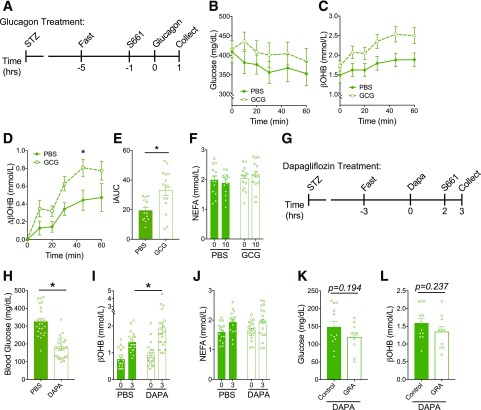
Previous work demonstrated that blockade of insulin receptors by a structurally similar insulin receptor antagonist, S961, was unable to completely eliminate insulin signaling and mimic the situation of C-peptide–negative diabetes (43,44). However, lack of insulin signaling can be reasonably obtained by the combination of insulin receptor antagonism in mice with near complete β-cell destruction with STZ (43). To test the effects of glucagon in a model of complete loss of insulin signaling, we first treated mice with STZ to induce hyperglycemia and then acutely added S661 (Fig. 6A). In this setting, glucagon enhanced the level of hyperglycemia (Fig. 6B) and potentiated an increase in βOHB (Fig. 6C–E). NEFA levels were comparable between control and glucagon-treated groups (Fig. 6F), indicating that the ketogenic effect of glucagon is likely not mediated peripherally through effects on lipolysis. These data demonstrate that glucagon is capable of increasing ketone levels in the setting of complete lack of insulin signaling, which is in agreement with previous reports documenting the ketogenic potential of glucagon in isolated hepatocytes (3,5) or experimental models that prevent insulin signaling (4,6).
Figure 6.
Glucagon stimulates ketosis in the absence of insulin signaling but is not necessary for dapagliflozin (Dapa)-induced ketosis. A: Mice were made hyperglycemic with STZ treatment and then injected with S661 (20 nmol/mouse) 1 h before injection of glucagon (20 µg/kg), and glycemia (B) and βOHB (C) were measured. Data are depicted as the change in βOHB from t = 0 (D), and incremental area under the curve (iAUC) of the βOHB curve was assessed (E). F: NEFA at baseline (0) and 10 min post–S661 injection (10). G: STZ-treated hyperglycemic mice were then given dapagliflozin (10 mg/kg) for 3 h and were injected 1 h before assessment with S661 (20 nmol/mouse). Glycemia (H), βOHB (I), and NEFA (J) were measured from dapagliflozin-treated mice vs. controls. Hyperglycemic mice were then given a glucagon-blocking antibody (Ab-4) (10 mg/kg) 24 h before dapagliflozin/S661 treatment, and glycemia (K) and βOHB (L) were measured. Data are shown as mean ± SEM. *P < 0.05. Data were analyzed by two-way ANOVA (B–D, F, I, and J) or Student t test (E, H, K, and L). hrs, hours.
Next, we tested the ketogenic potential of dapagliflozin in the experimental setting of absent insulin signaling (Fig. 6G). Following treatment with the combination of STZ and S661, the addition of dapagliflozin decreased glycemia and further increased blood βOHB (Fig. 6H and I), similar to the effects noted in healthy, untreated WT mice (Fig. 1F and G). Interestingly, there were no apparent changes in NEFA levels induced by dapagliflozin treatment in the STZ+S661 mice (Fig. 6J), whereas dapagliflozin robustly increased NEFA levels in untreated WT mice (Fig. 1H). This suggests that lipolysis was already maximally stimulated by STZ/S661 treatment. Dapagliflozin also failed to alter NEFA levels in untreated, healthy WT mice fasted overnight where insulin concentrations are considerably decreased (Supplementary Fig. 6). Together, these data suggest that in the setting of extremely low to absent insulin signaling, lipolysis and NEFA levels are elevated and unable to be further enhanced by SGLT2i. Thus, the increase in ketone levels induced by dapagliflozin in these settings is independent of changes in insulin or NEFA levels (Fig. 6 and Supplementary Fig. 6).
Finally, based on our observation that glucagon can stimulate ketosis with absent insulin signaling, we sought to test the potential role of glucagon for dapagliflozin-induced ketosis in the setting of absent insulin signaling. To do so, we administered the GRA to mice made hyperglycemic with STZ and acutely treated with S661. STZ-treated mice received an IgG control antibody or GRA 24 h before dapagliflozin treatment. S661 was then administered 2 h post–dapagliflozin administration, and glycemia and ketones were assessed 1 h after S661. Blocking glucagon action in mice treated with dapagliflozin/STZ/S661 did not impact glycemia (Fig. 6K) or circulating βOHB (Fig. 6L), indicating that glucagon is not necessary for dapagliflozin-induced ketosis even in the context of absent insulin signaling.
Discussion
Our findings directly challenge the dogma that glucagon is a primary factor in the regulation of ketone production. Here we demonstrate that glucagon is neither necessary nor sufficient to facilitate the increase in ketone production in response to either physiological (fasting) or pharmacological (SGLT2i) stimuli. A key feature of our experiments is that they were performed in vivo, while previous work supporting a central role for glucagon in ketone production used isolated cells or study conditions that limited the input of other regulatory factors on the liver (3–6). A crucial aspect of this study is our focus on the effects of glucagon action in the context of normal physiology, which, importantly, incorporates the actions of glucagon on β-cells. Our findings clearly demonstrate that the insulinotropic actions of glucagon in β-cells severely limit any ketogenic activity at the liver. Even reducing the ability for glucagon to enhance insulin signaling by either lowering glycemia with prolonged fasting (Fig. 5 and Supplementary Fig. 6) or using an insulin receptor antagonist (Fig. 6) did not permit a ketogenic role for glucagon. It is only when insulin signaling is completely abolished that glucagon is able to modestly stimulate ketone production, aligning with the previously reported direct actions of glucagon on hepatocytes (3–6). However, even in the setting of complete lack of insulin signaling, glucagon is not necessary for the enhanced ketone production in response to SGLT2i. Consequently, our studies in mice indicate that the rise in ketones induced by SGLT2i is independent of islet hormones.
To extrapolate our findings to human physiology, there are important differences to consider about glucagon secretion and action between rodents in humans. There is no evidence to support a direct lipolytic effect of glucagon at physiologic ranges in humans (45–48), while glucagon-stimulated lipolysis has been observed in isolated rat adipocytes (48,49). We were unable to detect a significant effect of glucagon to increase NEFA levels in mice, regardless of the level of glycemia or insulin action. On the other hand, there was a modest increase in NEFA concentrations accompanying each of our ketogenic interventions, including fasting (Fig. 1C), dapagliflozin (Fig. 1H), epinephrine administration (Fig. 4C), or insulin receptor blockade (Fig. 5C). These findings support the hypothesis that availability of substrate (NEFAs) is a key driver of ketone body production. It is interesting to note that elevations in β-adrenergic signaling robustly increased NEFA concentrations, while activation of GCGR did not (Fig. 4). Insulin is a potent inhibitor of lipolysis (50), and the elevations in plasma insulin in our refeeding paradigm lowered both NEFA and ketone concentrations significantly. We recently demonstrated that glucagon is a potent stimulus of insulin secretion and lowers glycemia (26). The results of the current study are consistent with this effect in that exogenous glucagon lowered NEFA levels in any of our experiments that permitted insulinotropic actions on β-cells (Figs. 4C and 5C). The only situation where glucagon did not lower NEFA levels was in mice treated with STZ/S661 (Fig. 6E). These findings are consistent with the hypothesis that even small increases of insulin secretion in response to glucagon administration obscure any effect on ketone production. This hypothesis will require direct testing but aligns with our previous observations of the importance of insulinotropic actions in the systemic actions of glucagon (25,26).
The direct effect of dapagliflozin on α-cells has been debated (17–19,24), and our data indicate that SGLT2i in mouse islets does not directly enhance glucagon secretion. Yet, dapagliflozin significantly elevates circulating glucagon in mice (Fig. 1J), indicating there are indirect effects and the potential for multiple mechanisms by which SGLT2i could increase glucagon secretion. It is possible that human islets would respond differently to SGLT2i by increasing glucagon secretion. However, the data presented here (Supplementary Fig. 2), and previously (25, 26), show that stimulating glucagon secretion when glucose levels are elevated subsequently stimulates an increase in insulin secretion. These observations strongly argue against SGLT2i actions in α-cells as the mechanism for increased ketone production, as glucagon-stimulated insulin secretion would provide a strong inhibitory tone on ketosis. This would not be applicable in the setting of C-peptide–negative type 1 diabetes (T1D), where glucagon cannot stimulate insulin secretion. It appears that SGLT2i-induced DKA is more prominent in T1D than T2D, but this comparison is difficult, since the level of β-cell function varies in patients with T2D (51). However, our preclinical model of T1D (STZ+S661) revealed a significant increase in ketone production in response to SGLT2i that was independent of glucagon signaling (Fig. 6I and J).
While using dapagliflozin as a tool to stimulate ketogenesis, we made the interesting observation that insulin and glucagon do not appear to be the primary mediators of dapagliflozin-stimulated ketogenesis. This is in contrast to a prevailing explanation in the clinical literature that SGLT2i results in a lower need for insulin, and this decrease in circulating insulin or insulin action in patients with diabetes contributes to DKA (10,14,24,33,52). We clearly demonstrate that glucagon does not play a role in dapagliflozin-induced ketogenesis either in the presence (Figs. 2D and 3C) or absence (Fig. 6J) of insulin signaling. Surprisingly, we also found that dapagliflozin was an effective ketogenic stimulus in conditions of low (Supplementary Fig. 6) or absent (Fig. 6G) insulin action. These findings demonstrate that a component of ketogenesis secondary to SGLT2i is additive to insulinopenia. Future studies are necessary to identify islet-independent mechanisms of SGLT2i-induced ketogenesis.
Supplementary Material
Article Information
Acknowledgments. The authors thank Dr. Derek Nunez for thoughtful discussions and review of the manuscript and Quincy D’Alessio for technical help (both from Duke Molecular Physiology Institute, Duke University, Durham, NC).
Funding. M.E.C. received support from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health (NIH) (F32 DK116542). R.W.C. received support from the Endocrine Fellows Foundation. J.E.C. is supported by a career development award from the American Diabetes Association (1-18-JDF-017) and by funding from the NIDDK, NIH (R01 DK123075), and is a Borden Scholar. D.A.D. is supported by the NIDDK, NIH (R01 DK101991). Eli Lilly and Novo Nordisk provided key reagents.
Duality of Interest. B.F. is an employee of Novo Nordisk. K.W.S. is an employee of Eli Lilly. The Campbell laboratory receives research funding from Novo Nordisk and Eli Lilly for projects unrelated to this work. No other potential conflicts of interest relevant to this article were reported.
Author Contributions. M.E.C., R.W.C., M.A.H., D.A.D., and J.E.C. designed and directed the study. M.E.C., R.W.C., J.K., I.I.A., J.B.W., S.E.E., J.D.D., and K.E. performed experiments. B.F. and K.W.S. provided key reagents. M.E.C., D.A.D., and J.E.C. wrote the manuscript. All authors reviewed the manuscript and provided final approval for submission. J.E.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this study were presented in abstract form at the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019.
Footnotes
This article contains Supplementary Data online at https://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db19-1216/-/DC1.
References
- 1.Campbell JE, Drucker DJ. Islet α cells and glucagon--critical regulators of energy homeostasis. Nat Rev Endocrinol 2015;11:329–338 [DOI] [PubMed] [Google Scholar]
- 2.Sandoval DA, D’Alessio DA. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev 2015;95:513–548 [DOI] [PubMed] [Google Scholar]
- 3.Malewiak MI, Griglio S, Kalopissis AD, Le Liepvre X. Oleate metabolism in isolated hepatocytes from lean and obese Zucker rats. Influence of a high fat diet and in vitro response to glucagon. Metabolism 1983;32:661–668 [DOI] [PubMed] [Google Scholar]
- 4.McGarry J, Wright PH, Foster DW. Hormonal control of ketogenesis. Rapid activation of hepatic ketogenic capacity in fed rats by anti-insulin serum and glucagon. J Clin Invest 1975;55:1202–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pégorier JP, Garcia-Garcia MV, Prip-Buus C, Duée PH, Kohl C, Girard J. Induction of ketogenesis and fatty acid oxidation by glucagon and cyclic AMP in cultured hepatocytes from rabbit fetuses. Evidence for a decreased sensitivity of carnitine palmitoyltransferase I to malonyl-CoA inhibition after glucagon or cyclic AMP treatment. Biochem J 1989;264:93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerich JE, Lorenzi M, Bier DM, et al. . Prevention of human diabetic ketoacidosis by somatostatin. Evidence for an essential role of glucagon. N Engl J Med 1975;292:985–989 [DOI] [PubMed] [Google Scholar]
- 7.Mather A, Pollock C. Renal glucose transporters: novel targets for hyperglycemia management. Nat Rev Nephrol 2010;6:307–311 [DOI] [PubMed] [Google Scholar]
- 8.Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 9.Cherney DZ, Perkins BA, Soleymanlou N, et al. . Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation 2014;129:587–597 [DOI] [PubMed] [Google Scholar]
- 10.Taylor SI, Blau JE, Rother KI. SGLT2 inhibitors may predispose to ketoacidosis. J Clin Endocrinol Metab 2015;100:2849–2852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perkins BA, Cherney DZ, Partridge H, et al. . Sodium-glucose cotransporter 2 inhibition and glycemic control in type 1 diabetes: results of an 8-week open-label proof-of-concept trial. Diabetes Care 2014;37:1480–1483 [DOI] [PubMed] [Google Scholar]
- 12.Rosenstock J, Ferrannini E. Euglycemic diabetic ketoacidosis: a predictable, detectable, and preventable safety concern with SGLT2 inhibitors. Diabetes Care 2015;38:1638–1642 [DOI] [PubMed] [Google Scholar]
- 13.Kelmenson DA, Burr K, Azhar Y, Reynolds P, Baker CA, Rasouli N. Euglycemic Diabetic Ketoacidosis With Prolonged Glucosuria Associated With the Sodium-Glucose Cotransporter-2 Canagliflozin. J Investig Med High Impact Case Rep 2017;5:2324709617712736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown F, McColl T. Euglycemic diabetic ketoacidosis secondary to dapagliflozin use: a case report. J Emerg Med 2018;54:109–111 [DOI] [PubMed] [Google Scholar]
- 15.Chou YM, Seak CJ, Goh ZNL, Seak JC, Seak CK, Lin CC. Euglycemic diabetic ketoacidosis caused by dapagliflozin: a case report. Medicine (Baltimore) 2018;97:e11056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma PV, Jobanputra YB, Lewin K, Bagatell S, Lichtstein DM. Diabetic ketoacidosis in patients with type 2 diabetes on sodium-glucose cotransporter-2 inhibitors - a case series. Rev Recent Clin Trials 2018;13:156–160 [DOI] [PubMed] [Google Scholar]
- 17.Bonner C, Kerr-Conte J, Gmyr V, et al. . Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015;21:512–517 [DOI] [PubMed] [Google Scholar]
- 18.Saponaro C, Gmyr V, Thevenet J, et al. . The GLP1R agonist liraglutide reduces hyperglucagonemia induced by the SGLT2 inhibitor dapagliflozin via somatostatin release. Cell Rep 2019;28:1447–1454.e4 [DOI] [PubMed] [Google Scholar]
- 19.Kuhre RE, Ghiasi SM, Adriaenssens AE, et al. . No direct effect of SGLT2 activity on glucagon secretion. Diabetologia 2019;62:1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGarry JD, Foster DW. Hormonal control of ketogenesis. Biochemical considerations. Arch Intern Med 1977;137:495–501 [PubMed] [Google Scholar]
- 21.Olivieri MC, Botelho LH. Synergistic inhibition of hepatic ketogenesis in the presence of insulin and a cAMP antagonist. Biochem Biophys Res Commun 1989;159:741–747 [DOI] [PubMed] [Google Scholar]
- 22.Unger RH, Orci L. Physiology and pathophysiology of glucagon. Physiol Rev 1976;56:778–826 [DOI] [PubMed] [Google Scholar]
- 23.McGarry JD, Foster DW. Ketogenesis and its regulation. Am J Med 1976;61:9–13 [DOI] [PubMed] [Google Scholar]
- 24.Perry RJ, Rabin-Court A, Song JD, et al. . Dehydration and insulinopenia are necessary and sufficient for euglycemic ketoacidosis in SGLT2 inhibitor-treated rats. Nat Commun 2019;10:548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Capozzi ME, Svendsen B, Encisco SE, et al. . β Cell tone is defined by proglucagon peptides through cAMP signaling. JCI Insight 2019;4:e126742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capozzi ME, Wait JB, Koech J, et al. . Glucagon lowers glycemia when β-cells are active. JCI Insight 2019;4:e129954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svendsen B, Larsen O, Gabe MBN, et al. . Insulin secretion depends on intra-islet glucagon signaling. Cell Rep 2018;25:1127–1134.e2 [DOI] [PubMed] [Google Scholar]
- 28.Zhu L, Dattaroy D, Pham J, et al. . Intra-islet glucagon signaling is critical for maintaining glucose homeostasis. JCI Insight 2019;4:e127994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schäffer L, Brand CL, Hansen BF, et al. . A novel high-affinity peptide antagonist to the insulin receptor. Biochem Biophys Res Commun 2008;376:380–383 [DOI] [PubMed] [Google Scholar]
- 30.Chambers AP, Sorrell JE, Haller A, et al. . The role of pancreatic preproglucagon in glucose homeostasis in mice. Cell Metab 2017;25:927–934.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cahill GF Jr, Herrera MG, Morgan AP, et al. . Hormone-fuel interrelationships during fasting. J Clin Invest 1966;45:1751–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Merovci A, Solis-Herrera C, Daniele G, et al. . Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferrannini E, Muscelli E, Frascerra S, et al. . Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Millar P, Pathak N, Parthsarathy V, et al. . Metabolic and neuroprotective effects of dapagliflozin and liraglutide in diabetic mice. J Endocrinol 2017;234:255–267 [DOI] [PubMed] [Google Scholar]
- 35.Blackard WG, Nelson NC, Andrews SS. Portal and peripheral vein immunoreactive glucagon concentrations after arginine or glucose infusions. Diabetes 1974;23:199–202 [DOI] [PubMed] [Google Scholar]
- 36.Jun LS, Millican RL, Hawkins ED, et al. . Absence of glucagon and insulin action reveals a role for the GLP-1 receptor in endogenous glucose production. Diabetes 2015;64:819–827 [DOI] [PubMed] [Google Scholar]
- 37.Korytko AI, Millican RL. Glucagon receptor antagonists. U.S. patent US7968686 B2. 28 June 2011.
- 38.Sloop KW, Cao JX, Siesky AM, et al. . Hepatic and glucagon-like peptide-1-mediated reversal of diabetes by glucagon receptor antisense oligonucleotide inhibitors. J Clin Invest 2004;113:1571–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mu J, Jiang G, Brady E, et al. . Chronic treatment with a glucagon receptor antagonist lowers glucose and moderately raises circulating glucagon and glucagon-like peptide 1 without severe alpha cell hypertrophy in diet-induced obese mice. Diabetologia 2011;54:2381–2391 [DOI] [PubMed] [Google Scholar]
- 40.Gu W, Winters KA, Motani AS, et al. . Glucagon receptor antagonist-mediated improvements in glycemic control are dependent on functional pancreatic GLP-1 receptor. Am J Physiol Endocrinol Metab 2010;299:E624–E632 [DOI] [PubMed] [Google Scholar]
- 41.Coore HG, Randle PJ. Regulation of insulin secretion studied with pieces of rabbit pancreas incubated in vitro. Biochem J 1964;93:66–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malaisse W, Malaisse-Lagae F, Wright PH, Ashmore J. Effects of adrenergic and cholinergic agents upon insulin secretion in vitro. Endocrinology 1967;80:975–978 [DOI] [PubMed] [Google Scholar]
- 43.Damond N, Thorel F, Moyers JS, et al. . Blockade of glucagon signaling prevents or reverses diabetes onset only if residual β-cells persist. eLife 2016;5:e13828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth Flach RJ, Danai LV, DiStefano MT, et al. . Protein kinase mitogen-activated protein kinase kinase kinase kinase 4 (MAP4K4) promotes obesity-induced hyperinsulinemia. J Biol Chem 2016;291:16221–16230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu MS, Jeng CY, Hollenbeck CB, Chen YD, Jaspan J, Reaven GM. Does glucagon increase plasma free fatty acid concentration in humans with normal glucose tolerance? J Clin Endocrinol Metab 1990;70:410–416 [DOI] [PubMed] [Google Scholar]
- 46.Jensen MD, Heiling VJ, Miles JM. Effects of glucagon on free fatty acid metabolism in humans. J Clin Endocrinol Metab 1991;72:308–315 [DOI] [PubMed] [Google Scholar]
- 47.Gravholt CH, Møller N, Jensen MD, Christiansen JS, Schmitz O. Physiological levels of glucagon do not influence lipolysis in abdominal adipose tissue as assessed by microdialysis. J Clin Endocrinol Metab 2001;86:2085–2089 [DOI] [PubMed] [Google Scholar]
- 48.Galsgaard KD, Pedersen J, Knop FK, Holst JJ, Wewer Albrechtsen NJ. Glucagon receptor signaling and lipid metabolism. Front Physiol 2019;10:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaughan M, Steinberg D. Effect of hormones on lipolysis and esterification of free fatty acids during incubation of adipose tissue in vitro. J Lipid Res 1963;4:193–199 [PubMed] [Google Scholar]
- 50.Coppack SW, Jensen MD, Miles JM. In vivo regulation of lipolysis in humans. J Lipid Res 1994;35:177–193 [PubMed] [Google Scholar]
- 51.Riddle MC, Cefalu WT. SGLT inhibitors for type 1 diabetes: an obvious choice or too good to be true? Diabetes Care 2018;41:2444–2447 [DOI] [PubMed] [Google Scholar]
- 52.Burke KR, Schumacher CA, Harpe SE. SGLT2 inhibitors: a systematic review of diabetic ketoacidosis and related risk factors in the primary literature. Pharmacotherapy 2017;37:187–194 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.