Sodium-glucose cotransporter 2 (SGLT2), encoded by SLC5A2, is primarily responsible for glucose resorption from the proximal tubule of the kidney. Selective inhibitors of SGLT2 (or SGLT2i), such as the gliflozins, are widely used for the treatment of type 2 diabetes (T2D), since they decrease blood glucose levels by increasing excretion of the sugar in the urine (1). These glucose-lowering effects might however be partially offset by the action of SGLT2i to increase circulating glucagon levels (2). Whether SGLT2i influence glucagon secretion via direct (3) or indirect/paracrine (4,5) mechanisms remains keenly debated. A number of studies have shown contrasting effects of SGLT2i on pancreatic α-cells, with increases (3,6–9), decreases (10), or no change (5,10) in glucagon secretion depending on the species, preparation, glucose concentration, and gliflozin used. Moreover, discordant results have been reported by different investigators regarding expression of SGLT2 and Slc5a1/SLC5A1 in mouse, rat, and human islets, as well as sorted cell populations (3,5,8,10).
In this issue of Diabetes, Saponaro et al. (11) shed further light on the complex issue of SGLT2i and glucagon secretion. Using a large number of donors, the authors show that responses of isolated human islets to SGLT2i are highly heterogeneous, with large variation in basal and secreted glucagon levels as well as responsiveness to treatment. This apparent heterogeneity was also reflected at the level of SGLT2/SLC5A2 expression, shown using Western blotting with antibodies validated according to established guidelines, or interrogation of bulk islet data from 207 donors deposited in the Translational Human Pancreatic Islet Genotype Tissue-Expression Resource (TIGER) RNA-seq database. While SGLT2 was found to be strongly colocalized with α-cells, high variability in the number of glucagon-positive/SGLT2i-positive cells, as well as strength of colocalization, was observed between donors and even within different islets of the same donor. As such, the authors conclude that future studies assessing SGLT2i in human islets should take into account the appreciable heterogeneity in SGLT2 expression and glucagon responses. The proposed mechanisms by which SGLT2i influence α-cell function are shown in Fig. 1.
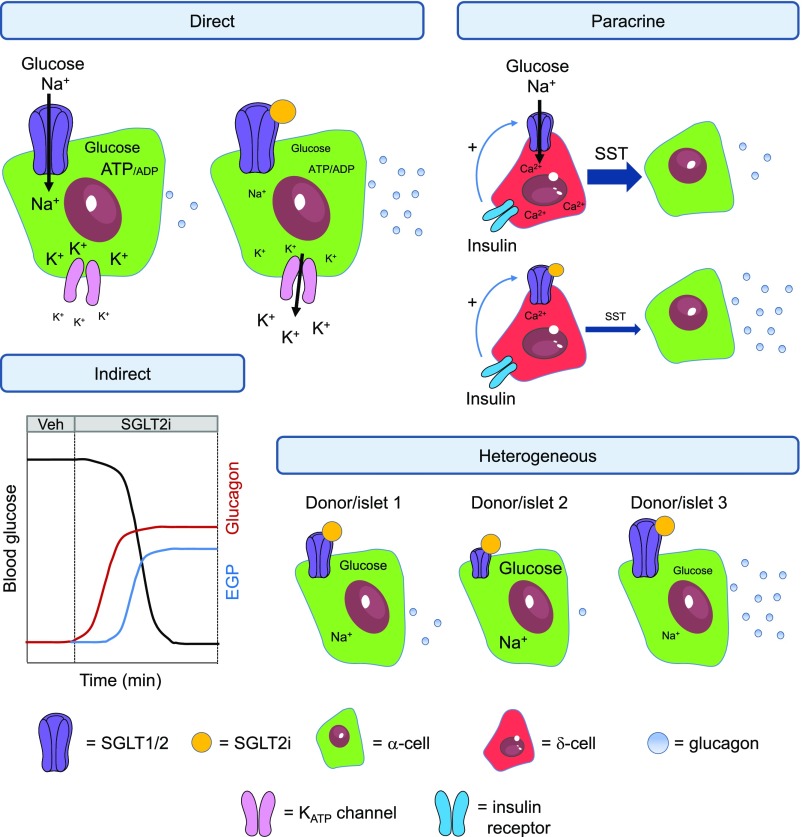
Figure 1.
Schematic showing effects of SGLT2i on α-cell function. SGLT2i have been proposed to influence glucagon release through direct, paracrine, and indirect effects. Direct: Binding of SGLT2i might alter intracellular glucose and Na+ concentration, leading to changes in α-cell metabolism and membrane potential. Glucagon is decreased through poorly defined and complex mechanisms involving α-cell repolarization. Paracrine: Insulin binds to the insulin receptor on δ-cells to increase SGLT1/2 activity, leading to Ca2+ release from intracellular stores and stimulation of somatostatin release, which tonically inhibits glucagon secretion. SGLT2i block this effect by binding to either SGLT1 or SGLT2 on the δ-cell membrane, decreasing somatostatin secretion and releasing α-cells from tonic inhibition (but note that Saponaro et al. [11] did not detect presence of SGLT2 in δ-cells, unlike what has been reported by others [4]). Indirect: SGLT2i stimulate glycosuria, which lowers blood glucose levels. α-Cells respond to hypoglycemia by releasing glucagon, which increases endogenous glucose production. Heterogeneous: SGLT2/SLC5A2 expression is highly variable between donors and even islets of the same individuals. Some individuals/islets respond to SGLT2i, whereas others are less responsive, unresponsive, or even inhibited. If studies are underpowered, and depending on the samples examined (i.e., responsive, nonresponsive, or inhibited), effects of SGLT2i are likely to be reported as either: 1) positive, 2) negative, or 3) absent. EGP, endogenous glucose production; KATP channel, ATP-sensitive potassium channel; SST, somatostatin; Veh, vehicle. Adapted from Servier Medical Art under a CC BY3.0 license (https://creativecommons.org/licenses/by/3.0/).
These findings corroborate earlier studies showing that SGLT2i induce glucagon secretion from isolated human islets (3,6), possibly via direct effects on SGLT2 expressed in α-cells. Moreover, the studies further suggest that heterogeneity observed between human islet preparations might contribute to some of the discrepancies previously reported in the literature. Without a large number of donors, an experiment is unlikely to be adequately powered to reliably detect differences in SLC5A2 or glucagon secretion, giving rise to conflicting results depending on whether the samples received respond positively, negatively, or not at all to SGLT2i.
The study by Saponaro et al. raises a number of interesting questions and avenues of future exploration concerning SGLT2i and glucagon secretion. What are the stimulus-secretion coupling mechanisms by which SGLT2i affects α-cells? Based on its mode of action (inhibiting a sodium-glucose cotransporter), SGLT2i can be expected to repolarize α-cells by dual mechanisms. The reduction of glucose uptake and metabolism will lower the cytoplasmic ATP/ADP ratio and increase KATP channel activity (12). However, SGLT2i will also remove the depolarizing effect of Na+ influx down its electrochemical gradient (9). How these effects influence glucagon secretion is difficult to predict given the complex relationship between membrane potential and secretion in α-cells (12).
It is also important to elucidate why SGLT2 protein expression is so variable between donors. Does this relate to SLC5A2 transcript or mRNA abundance in the same donor, or is SGLT2 regulated at the posttranscriptional/translational level in islets? To elucidate whether this heterogeneity is an α-cell–intrinsic trait or reflects changes in other cell types, further analyses of sorted populations should be performed as new data sets become available. Suggesting that the methodology used might also influence data, SLC5A2 is readily detected in purified fetal and adult human α-cells and at levels comparable to those of GLUT1 and GLUT3 (SLC2A1 and SLC2A3) (13) but appears virtually absent from single-cell RNA-seq data sets (5).
The localization of SGLT2 also poses a conundrum. Given its role to transport glucose and Na+ across the membrane, SGLT2 would be expected to be present on the cell surface. However, despite extensive antibody validation, SGLT2 appears to be localized primarily within the cytoplasm of human α-cells. While the authors show that SGLT2 might translocate to the cytoplasm depending on glucose concentration, it should be noted that G-protein–coupled receptors such as GLP1R only appear in the cytosol upon prolonged stimulation with orthosteric ligand (14). Novel chemical probes against SGLT2 might be helpful in clarifying whether SGLT2 compartmentalization represents a real phenomenon or, alternatively, reflects the fixation protocol and antibody used.
It is also assumed that SGLT2i are highly selective for SGLT2. However, off-target effects cannot be completely excluded. Indeed, the SGLT2i canagliflozin has been reported to activate AMP kinase by inhibiting mitochondrial function (15). The finding that SGLT2i decrease cardiovascular mortality risk in patients with T2D (16) and heart failure (17) might be attributed to such off-target effects, since SGLT2/SLC5A2 is expressed at low levels in cardiomyocytes (18), being ∼1% of that found in in the kidney (9).
Lastly, what is the relative contribution of direct (i.e., α-cell–centric) and indirect (i.e., via somatostatin or hypoglycemia) SGLT2i actions on glucagon secretion in vivo in humans? As for in vitro experiments, studies have shown opposing effects of SGLT2i in volunteers: under isoglycemic/euglycemic hyperinsulinemic conditions, glucagon has been reported to either increase (19) or remain unchanged (2,20). To this end, preclinical mouse studies using Cre-Lox, or high-fidelity reporter approaches (21), might help to untangle some of the complexity of SGLT2i action in the α-cell on a more homogenous background.
In summary, Saponaro et al. provide new insight into SGLT2i action in the islet, by showing the presence of considerable heterogeneity between donors in terms of SGLT2 expression and ligand responsiveness. Going forward, it will be important not only to account for this heterogeneity but also for researchers to work together, using well-validated reagents, standardized protocols, and adequately powered experiments, to see whether or not these findings will impact treatment of patients with SGLT2i.
Article Information
Funding. D.J.H. was supported by the Medical Research Council (MR/N00275X/1 and MR/S025618/1) and Diabetes UK (17/0005681) project grants. This project has received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation programme (Starting Grant 715884 to D.J.H.). Work in Oxford was supported by the Wellcome Trust, Diabetes UK, and the European Foundation for the Study of Diabetes.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
See accompanying article, p. 902.
References
- 1.Hsia DS, Grove O, Cefalu WT. An update on SGLT2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2017;24:73–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrannini E, Muscelli E, Frascerra S, et al. . Metabolic response to sodium-glucose cotransporter 2 inhibition in type 2 diabetic patients. J Clin Invest 2014;124:499–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonner C, Kerr-Conte J, Gmyr V, et al. . Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015;21:512–517 [DOI] [PubMed] [Google Scholar]
- 4.Vergari E, Knudsen JG, Ramracheya R, et al. . Insulin inhibits glucagon release by SGLT2-induced stimulation of somatostatin secretion. Nat Commun 2019;10:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuhre RE, Ghiasi SM, Adriaenssens AE, et al. . No direct effect of SGLT2 activity on glucagon secretion. Diabetologia 2019;62:1011–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pedersen MG, Ahlstedt I, El Hachmane MF, Göpel SO. Dapagliflozin stimulates glucagon secretion at high glucose: experiments and mathematical simulations of human A-cells. Sci Rep 2016;6:31214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saponaro C, Gmyr V, Thévenet J, et al. . The GLP1R agonist liraglutide reduces hyperglucagonemia induced by the sglt2 inhibitor dapagliflozin via somatostatin release. Cell Reports 2019;28:1447–1454.e4 [DOI] [PubMed] [Google Scholar]
- 8.Solini A, Sebastiani G, Nigi L, Santini E, Rossi C, Dotta F. Dapagliflozin modulates glucagon secretion in an SGLT2-independent manner in murine alpha cells. Diabetes Metab 2017;43:512–520 [DOI] [PubMed] [Google Scholar]
- 9.Knudsen JG, Hamilton A, Ramracheya R, et al. . Dysregulation of glucagon secretion by hyperglycemia-induced sodium-dependent reduction of ATP production. Cell Metab 2019;29:430–442.e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suga T, Kikuchi O, Kobayashi M, et al. . SGLT1 in pancreatic α cells regulates glucagon secretion in mice, possibly explaining the distinct effects of SGLT2 inhibitors on plasma glucagon levels. Mol Metab 2019;19:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saponaro C, Mühlemann M, Acosta-Montalvo A, et al. . Interindividual heterogeneity of SGLT2 expression and function in human pancreatic islets. Diabetes 2020;69:902–914 [DOI] [PubMed] [Google Scholar]
- 12.Zhang Q, Ramracheya R, Lahmann C, et al. . Role of KATP channels in glucose-regulated glucagon secretion and impaired counterregulation in type 2 diabetes. Cell Metab 2013;18:871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blodgett DM, Nowosielska A, Afik S, et al. . Novel observations from next-generation RNA sequencing of highly purified human adult and fetal islet cell subsets. Diabetes 2015;64:3172–3181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buenaventura T, Bitsi S, Laughlin WE, et al. . Agonist-induced membrane nanodomain clustering drives GLP-1 receptor responses in pancreatic beta cells. PLoS Biol 2019;17:e3000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawley SA, Ford RJ, Smith BK, et al. . The Na+/glucose cotransporter inhibitor canagliflozin activates AMPK by inhibiting mitochondrial function and increasing cellular AMP levels. Diabetes 2016;65:2784–2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zinman B, Wanner C, Lachin JM, et al.; EMPA-REG OUTCOME Investigators . Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–2128 [DOI] [PubMed] [Google Scholar]
- 17.McMurray JJV, Solomon SD, Inzucchi SE, et al.; DAPA-HF Trial Committees and Investigators . Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008 [DOI] [PubMed] [Google Scholar]
- 18.Zhou L, Cryan EV, D’Andrea MR, Belkowski S, Conway BR, Demarest KT. Human cardiomyocytes express high level of Na+/glucose cotransporter 1 (SGLT1). J Cell Biochem 2003;90:339–346 [DOI] [PubMed] [Google Scholar]
- 19.Merovci A, Solis-Herrera C, Daniele G, et al. . Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. J Clin Invest 2014;124:509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundkvist P, Pereira MJ, Kamble PG, et al. . Glucagon levels during short-term SGLT2 inhibition are largely regulated by glucose changes in patients with type 2 diabetes. J Clin Endocrinol Metab 2019;104:193–201 [DOI] [PubMed] [Google Scholar]
- 21.Richards P, Parker HE, Adriaenssens AE, et al. . Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 2014;63:1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]