Abstract
In this study, commercial broilers were experimentally infected with single (classical IBV, variant IBV or AIV-H9N2) or mixed AIV-H9N2 with classical, variant or vaccine strains of IBV. Birds were monitored for clinical and pathological outcomes and virus shedding for 10 days post infection (DPI). Clinical signs were limited to the respiratory tract in all challenged groups and varied from mild to moderate mouth breathing to severe respiratory signs with snorting sound and extended head. Mortalities were only recorded in mixed AIV-H9N2/variant IBV challenge group. AIV-H9N2 challenge caused tracheal petechial hemorrhage that progressed to tracheal congestion and caseation. In mixed AIV-H9N2/IBV vaccine challenge, severe tracheitis with bronchial cast formation was observed. In mixed AIV-H9N2/variant IBV challenge severe congestion of the tracheal mucosa and excessive exudates with a tendency to form tubular casts were observed. Kidney ureate deposition was only observed in variant IBV challenge group. Histopathologically, tracheal congestion, severe degeneration, and deciliation were noticed in all groups of mixed infection. Interestingly, hemorrhage and atrophy were observed in thymus gland of birds challenged with single AIV-H9N2 or mixed AIV-H9N2/IBV. There was no difference in the tracheal shedding level of variant IBV between single and mixed infected groups while classical IBV shedding increased in mixed infection group. Interestingly, the AIV-H9N2 showed constantly high shedding titers till 7DPI with variant or vaccine IBV co-infection. In conclusion, co-infection of IBV and AIV-H9N2 induced severe clinical outcome and high mortality. Also, IBV co-infection increased the shedding of AIV-H9N2 in experimentally infected birds.
Keywords: Avian influenza virus, H9N2, Broiler chickens, Co-infection, IBV
Highlights
-
•
Co-infection of IBV and AIV-H9N2 induced severe clinical outcome and high mortality
-
•
IBV co-infection increased the shedding of AIV-H9N2 in experimentally infected birds
-
•
The synergism between AIV-H9N2 and IBV may explain the failure to control respiratory affection of poultry in Egypt
1. Introduction
Respiratory diseases outbreaks in commercial broiler chicken flocks have increased recently in Egypt causing severe economic losses in the broiler industry. Avian influenza H9N2 and H5N1 subtypes, Infectious bronchitis virus (IBV), and virulent Newcastle disease virus (vNDV) have been frequently isolated from different broiler chicken flocks (Hassan et al., 2016). These pathogens are of major significance and have a great economic impact because they are able to induce disease independently or in association with each other (Roussan et al., 2008).
IBV is causing an acute highly contagious viral respiratory disease of chickens belonged to Coronaviridae. Secondary bacterial infection is the main cause of bird death due to some bacteria gain access to the blood circulation following damage of the respiratory tract caused by IBV (Dwars et al., 2009). Some IBV strains are highly nephropathogenic, able to cause mortality up to 30% in young birds. Regardless of regular vaccinations with Massachusetts (Mass) strains, IBV still has severe and diverse effects on the poultry industry in the country, causing mortality and high condemnations especially with other viral diseases being affecting poultry in Egypt (Abdel-Moneim et al., 2006, Hassan et al., 2016).
Though the H9N2 are low pathogenic viruses, they vary considerably in pathogenicity in different host species. In the Middle East, H9N2 outbreaks were reported to be associated with cast formation in the tracheal bifurcation (Karimi-Madab et al., 2010). Mixed infections of H9N2 AIV with other respiratory pathogens, particularly IBV, Mycoplasma gallisepticum, Staphylococcus aureus, Avibacterium paragallinarum, Escherichia coli, Ornithobacterium rhinotracheale and/or immune suppressive agent can exacerbate H9N2 AIV infection resulting in severe clinical disease and variable mortality (Kishida et al., 2004, Nilli and Asasi, 2003, Perk et al., 2004).
IBV and AIV-H9N2N2 viruses were found to be widely spread in Egyptian poultry with a great economic impact because they are able to induce disease independently or in association with each other (Roussan et al., 2008). The high prevalence of both AIV-H9N2 and IBV determined in field studies highlighted a potential role of IBV in exacerbating the manifestation of AIV-H9N2 infection in broiler chicken with high mortalities (Haghighat-Jahromi et al., 2008, Hassan et al., 2016, Seififi et al., 2010). IBV could provoke ciliostasis in the host‘s airways (Cook et al., 1976, Hassan et al., 2016) and may, therefore, facilitate the opportunity for other related pathogens and aggravate their pathogenicity (Haghighat-Jahromi et al., 2008).
In this study, pathogenesis of both single and mixed AIV-H9N2 and IBV infections under experimental conditions was investigated in commercial broiler chickens. Experimentally infected commercial broilers with classical and variant IBV and vaccine IBV strains in presence or absence of AIV-H9N2N2 infection were monitored for clinical outcomes, virus shedding, postmortem and histopathological lesions.
2. Material and methods
2.1. Viruses and antisera
An AIV-H9N2 subtype virus (A/Chicken/Egypt/BSU-BS-K7T/2012, acc. no. KF998212). The virus belongs to group B of G1-like lineage viruses. A variant IBV (IB/Chicken/Egypt/BSU-MN-KB44/2013, acc. no. KR010942), which is closely related to the recent Egyptian variant II IBV (e.g. Eg/BSU-2/2011/acc. no. JX174185) (Abdel-Moneim et al., 2012). The AIV-H9N2N2 and the variant IBV viruses were isolated in Egypt from 26 day-old and 21 day-old broiler chickens in Beni-Suef and Menia governorates respectively (Hassan et al., 2016). A classical locally isolated IBV strain (IB/Chicken/Egypt/MF/2012) was kindly provided by D. Manal A. Afifi, Department of Poultry Diseases, Cairo University, Egypt. All viruses were propagated and titrated in 10-day-old specific pathogen free embryonated chicken eggs (SPF-ECE). Commercial live attenuated 4/91 IBV vaccine (Nobilis® IB 4/91, Intervet International B.V., Boxmeer-Holland) was purchased and used to study the effect of variant IBV vaccines on the pathogenesis of AIV-H9N2 infection. For IBV hemagglutination inhibition (HI) test; purified, concentrated and phospholipase C Type 1 enzyme treated variant (IBV-EG/11539F-201) and classical Mass-like strain (IBV-EG/MEVAC01/201) strains with their positive control antisera were kindly provided by Middle East for Veterinary Vaccines (ME VAC) Co. Research and Development Department.
2.2. Experimental chickens
All experiments were conducted according to Animal Research Ethics Guidelines at the faculty of veterinary medicine, Beni-Suef University, Egypt. One hundred and five-day-old commercial broilers were obtained from a private broiler breeder flock located at El-Badrashin, Giza, Egypt. Chicks were divided into 7 groups (15 chicks/group) in separate isolated experimental. Birds were reared till 20th day old provided with feed and water ad libitum and vaccinated against AIV-H5N1, Newcastle disease virus (at 2 and 9 days old using bivalent MEFLUVAC H5ND®, Middle East for Veterinary Vaccines Co., Egypt) and IBDV (at 14 days old using Bursa-Vac®, Intervet Schering-Plough animal health).
Experimental groups included; Negative control group, Variant IBV strain infected group, Classical IBV strain infected group, AIV-H9N2 infected group, mixed AIV-H9N2/IBV variant strain infected group, mixed AIV-H9N2N2/IBV classical strain infected group, and mixed AIV-H9N2/IBV 4/91 vaccine strain infected group. Blood samples were collected before challenge for hemagglutination inhibition test (HI) for AIV-H9N2N2 and agar precipitation test (AGPT) for IBV. Birds were challenged using intranasal and/or intra-tracheal inoculation of 106 EID50/0.5 ml of titrated viruses for AIV-H9N2N2 and IBV viruses, respectively. In the mixed AIV-H9N2N2/IBV 4/91 vaccine strain infected group, the IBV 4/91 vaccine was administered via eye drop according to the manufacturer instructions in terms of dose and route of administration. Mixed infection groups were challenged simultaneously.
2.3. Sample collection
Serum samples were collected at 20-day old for AIV-H9N2 and IBV serology testing before the challenge. Tracheal swabs were collected from experimentally infected chicks for virus's detection at 2, 5 and 7 DPI. At the same time points, 3 chicks from each group were euthanized and organs (trachea, thymus, and kidney) were collected for histopathological examination (Bancroft and Gamble, 2008). Swab samples were suspended in 2 ml sterile phosphate buffer saline (pH 7.2) and vortexed.
2.4. AIV-H9N2 and IBV post challenge serology
Sera were collected from the remaining chicks at 10 DPI for determination of the serum antibody titer against AIV-H9N2 using HI test (OIE, 2014). The IBV HI tests were conducted as previously described (King and Hopkins, 1983) with minor modifications. Briefly, both tests were done in U-shaped microtiter plates. Serum samples were kaolin-treated before testing (King and Hopkins, 1983). Serial twofold dilutions of treated serum in PBS were done and to each serum dilution 25 μl of the diluted classical and variant IBV HA antigen (8 HA units/25 μl) was added separately. The plates were incubated at room temperature for 20 min then 25 μl of 0.5% chicken RBC's were added to each well, and the plate were mixed and incubated for 40 min at room temperature. The HI titer of a sample was calculated as the reciprocal of the last serum dilution with no HA. Negative control serum and antisera against both classical and variant IBV antigen viruses were included in the test.
2.5. Virus shedding titers
The viral RNA was extracted by Bioflux® viral RNA Mini Spin column kit (Bioflux, China) in accordance with manufacturer's instructions. Verso 1-Step qRT-PCR Kit (Thermo scientific, USA) was used for detection and quantification of AIV-H9N2 (Ben Shabat et al., 2010) and S1 gene of IBV (Callison et al., 2006). The qRT-PCR reaction volume was 25 μl containing 5 μl of extracted RNA, 12.5 μl 2 × One-step RT-PCR ready mix, 1.25 μl RT enhancer, 0.25 μl Verso enzyme mix, 1 μl of 20 pmol of both forward and reverse primers, 0.25 μl of virus specific probe and 3.75 nuclease free water. The thermal profile included a reverse transcription step at 50 °C for 15 min followed by 15 min at 95 °C. The PCR cycling was 40 cycles of denaturation at 95 °C for 15 s, annealing at 60 °C at 60 s, and a final extension at 72 °C for 10 min. To determine AIV-H9N2 and IBV viruses shedding titers, a standard curve for each virus was generated using titrated viruses in SPF-ECE and shedding titers were determined using interpolation (Lee and Suarez, 2004).
2.6. Statistical analysis
The differences in HI antibody titers and virus shedding titers were estimated using One-way ANOVA with Tukey's post-test through GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA, www.graphpad.com).
3. Results
3.1. Clinical signs in experimentally infected chickens
Experimentally infected broilers were observed for clinical signs till 10 DPI. Clinical signs varied from mild signs like conjunctivitis, sneezing coughing and head shaking to severe respiratory signs represented in rales and mouth breathing especially with AIV-H9N2 challenged group at 5 DPI, and in mixed AIV-H9N2/IBV vaccine and mixed AIV-H9N2/variant IBV challenged groups at 7 DPI (Table 1 ). Mortality was recorded only in mixed AIV-H9N2/variant IBV challenged group at 9 and 10 DPI (13.3%).
Table 1.
Clinical signs in single or mixed IBV and AIV-H9N2 infected commercial broiler chicks.
| Challenge virus/es | Head shaking & sneezing |
Mouth breathing & rales |
Conjunctivitis |
||||||
|---|---|---|---|---|---|---|---|---|---|
| 2DPIa | 5DPI | 7DPI | 2DPI | 5DPI | 7DPI | 2DPI | 5DPI | 7DPI | |
| G1-negative control | – | – | – | – | – | – | – | – | – |
| G2-variant IBV | 2b | 2 | 2 | – | 1 | 1 | – | 2 | 3 |
| G3-classical IBV | – | 4 | 3 | – | – | – | 1 | 1 | 1 |
| G4-AIV-H9N2 | – | 1 | 2 | – | 1 | 1 | – | 1 | 2 |
| G5-AIV-H9N2 + variant IBV | – | 3 | 3 | – | 2 | 2 | – | 2 | 3 |
| G6-AIV-H9N2 + classical IBV | 2 | 3 | 4 | – | 1 | 1 | 2 | 2 | 3 |
| G7-AIV-H9N2 + 4/91 IBV vaccine | – | 4 | 4 | – | 2 | 2 | – | 2 | 2 |
DPI; days post infection.
Numbers of affected chicks out of 15 inoculated.
3.2. Gross and histopathology lesions
Gross lesions were variable with slight airsaculitis in all groups. Slight mucous was observed in the trachea in AIV-H9N2 and classical IBV single infections, petechial hemorrhage in the trachea of all AIV-H9N2 challenged groups at 2 and 5 DPI. By 7 DPI mucoid plugs at the tracheal bifurcation in single AIV-H9 and mixed AIV-H9N2/variant IBV infected groups were observed (Supplementary Fig. 1).
Histopathologically, the trachea in AIV-H9N2 single infection showed congestion of blood vessels in the submucosa, deciliation, and mononuclear cell infiltration. Variant and classic IBV single infection caused mild degeneration and necrosis of the tracheal mucosal epithelium, mild edema in the submucosa, deciliation and mononuclear cell infiltration. In AIV-H9N2 and IBV mixed infection including the 4/91 strain, severe degeneration, and necrosis of the mucosal epithelium, severe congestion of blood vessels with edema in the submucosa, marked deciliation associated with massive lymphocytic cell infiltrations (Supplementary Fig. 2).
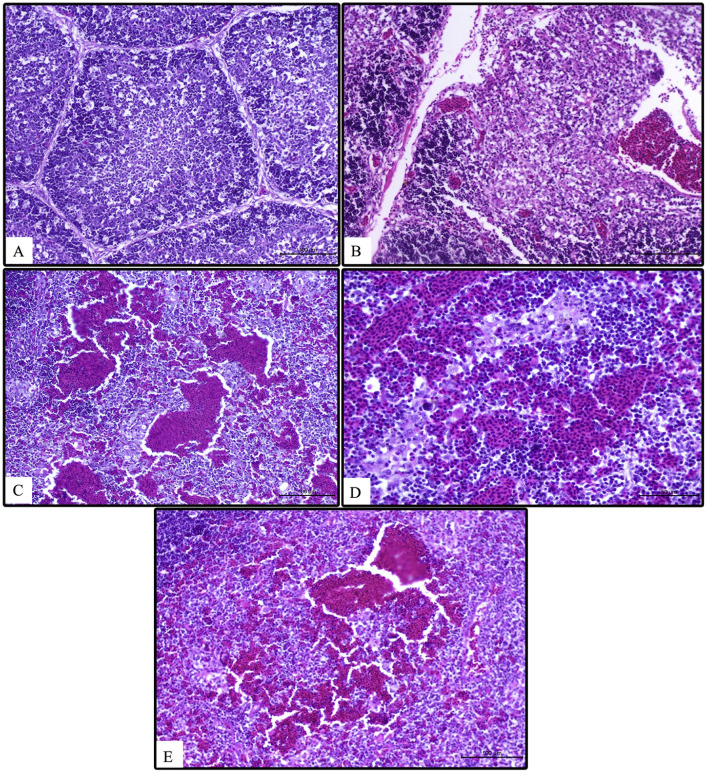
Thymus congestion and petechial hemorrhage were associated with AIV-H9N2 infection as it was only seen in AIV-H9N2 single infection and with variable degrees of severity in mixed AIV-H9N2/IBV infection. Thymus atrophy was observed in AIV-H9N2 single infection by 7 DPI (Fig. 1 ). The negative control, variant and classical IBV challenged groups showed normal thymus histology, while in AIV-H9N2 single infection and AIV-H9N2 with classical, variant IBV or vaccine IBV strain mixed infection the thymus was severely affected and showed congestion and hemorrhage. In some areas, the blood vessels were ruptured leaving blood-filled spaces. Later, the thymus appeared slightly or moderately atrophied (Fig. 2 ).
Fig. 1.
Thymus lesions in single and mixed infection groups; At 2 normal thymus of control negative group (A), thymus showing severe congestion with petechie in single AIV-H9N2 infection (B), few petechial hemorrhage AIV-H9N2 and variant IBV infection (C), petechial hemorrhage in AIV-H9N2 and classical IBV infection (D), and slight petechial hemorrhage in AIV-H9N2 and IBV vaccine infection (E). At 7 DPI normal thymus of control negative group (F) atrophied hemorrhagic thymus in AIV-H9N2 infection (G), slight petechial hemorrhage in AIV-H9N2 and variant IBV infection (H), atrophy with petechial hemorrhage in AIV-H9N2 and classical IBV (I) and AIV-H9N2 and IBV vaccine strain infection (J).
Fig. 2.
Thymus histopathology in infected broiler chickens; Negative control group (A), severe congestion of medulla blood vessels and hemorrhages, blood vessels were ruptured leaving blood-filled spaces and slight to moderate thymus atrophy in the single AIV-H9N2 (B), AIV-H9N2 and classical IBV (C), AIV-H9N2 and classical IBV (D), AIV-H9N2 and variant IBV (E), and AIV-H9N2 and IBV vaccine (E) infections. Magnification 10 × and scale bar = 100 μM.
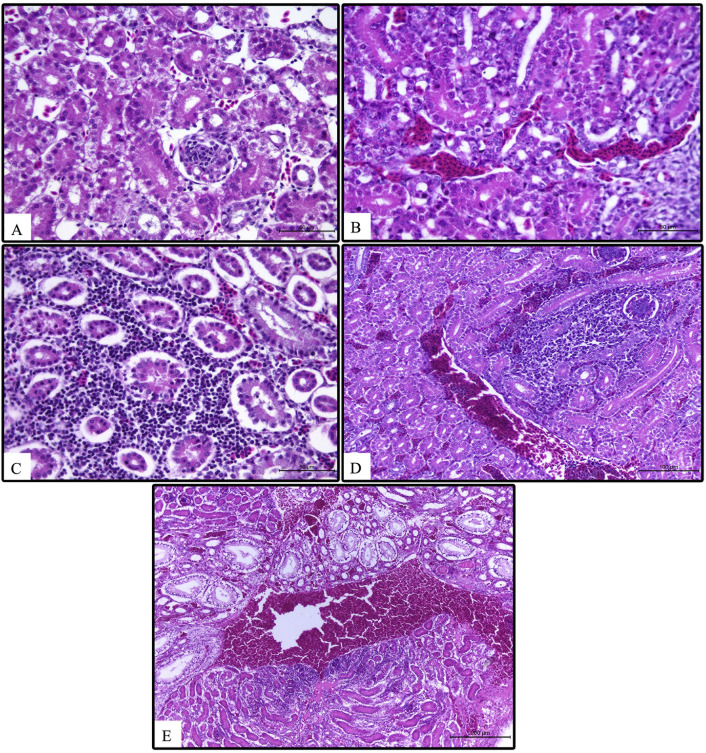
Kidney histopathological examination revealed that kidneys were mostly affected in AIV-H9N2 with variant IBV and in AIV-H9N2 with IBV vaccine strain challenged groups with congested blood vessels, mild hemorrhage, massive interstitial infiltration of leucocytes and tubular degenerative changes. Interstitial nephritis was frequently seen in some birds. Swelling and hypercellularity of the glomeruli were commonly observed (Fig. 3 ).
Fig. 3.
Kidney histopathology in infected broiler chickens. Normal kidney (A), congestion, severe degeneration and necrosis, and interstitial nephritis in variant IBV (B), Massive infiltration of leucocytes blood vessel congestion in AIV-H9N2 and classical IBV (C), severe degenerative changes and hemorrhages in AIV-H9N2 and variant IBV (D), and in AIV-H9N2 and IBV vaccine (E). Magnification 10 × and scale bar = 100 μM.
3.3. Virus shedding titers in both single and mixed AIV-H9N2 and IBV infected groups
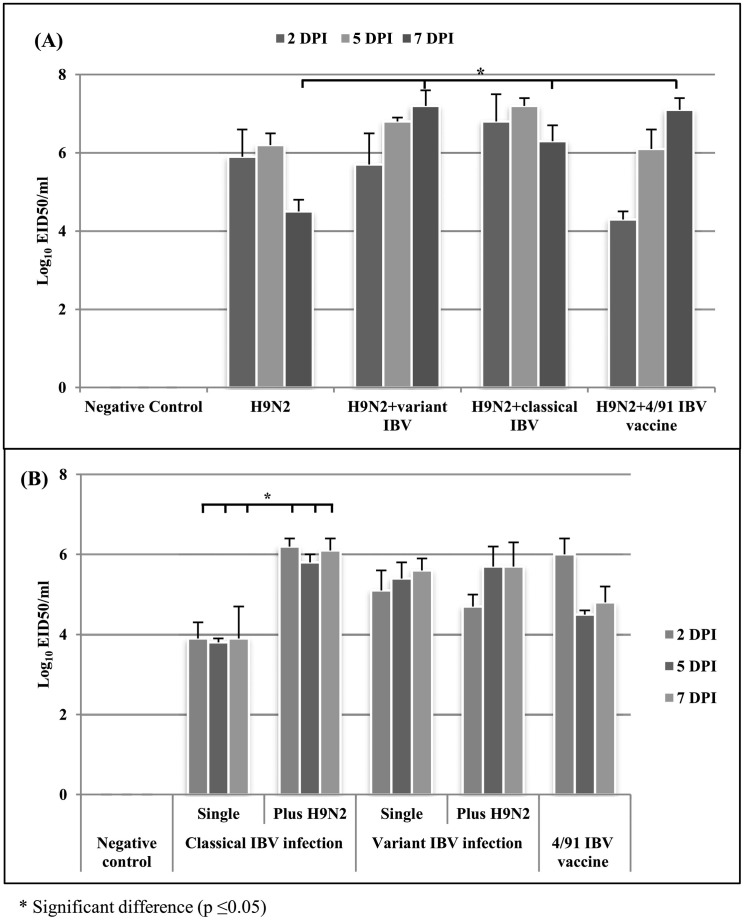
The shedding titers of AIV-H9N2 virus single infection decreased by 7 DPI from 6.2 to 4.5 log10, meanwhile in AIV-H9N2 with IBV mixed infection groups the shedding titer of AIV-H9N2 virus was significantly higher than the AIV-H9N2 single infection group. Similarly, the classical IBV shedding titers in the mixed infection group were significantly higher than those of the classical IBV single infection. On the other hand, variant IBV shedding titers did not show significant differences between either single or combined infection (Fig. 4 ).
Fig. 4.
The AIV-H9N2 (A) and IBV (B) viruses shedding titers in experimentally infected broilers in both single AIV-H9N2 and AIV-H9N2 with IBV mixed infections.
3.4. Post-challenge serology
Pre-challenge AIV-H9N2 and IBV HI antibody titers (20 day-old) confirmed the absence of previous exposure before the challenge. At 10 days post challenge, AIV-H9N2 challenged groups (either single or mixed with IBV) showed high HI antibody titers with the most significant increase in AIV-H9N2 plus classical IBV infection. However, no variation between the titers in the single AIV-H9N2 infected group and the titers in mixed AIV-H9N2/variant IBV and AIV-H9N2/vaccine IBV strain (Table 2 ). Non-significant slight increase in IBV post challenge antibody titers against both classical and variant IBV antigens in AIV-H9N2 and IBV combined infections (table).
Table 2.
Post-challenge Log2 hemagglutination inhibition antibody titers of experimentally infected groups using AIV-H9N2, classical and variant IBV antigens.
| Group | HI antigen used |
||
|---|---|---|---|
| AIV-H9N2 Ag | IBV classical Ag | IBV variant Ag | |
| Negative control | 0.8 ± 0.8 | 0.7 ± 0.8 | 1.1 ± 0.7 |
| Variant IBV | 0.7 ± 0.9 | 7.4 ± 0.5 | 8.4 ± 0.5 |
| Classical IBV | 0.1 ± 0.4 | 8.7 ± 0.5 | 6.6 ± 0.5 |
| AIV-H9N2 | 4.5 ± 1.6 | 0.3 ± 0.5 | 0.6 ± 0.5 |
| AIV-H9N2 + variant IBV | 4.6 ± 1.0 | 7.9 ± 0.4 | 8.9 ± 0.4 |
| AIV-H9N2 + classical IBV | 6.7 ± 0.9a | 8.9 ± 0.4 | 6.9 ± 0.7 |
| AIV-H9N2 + 4/91 IBV vaccine | 4.5 ± 1.4 | 7.0 ± 0.8 | 8.7 ± 0.5 |
Significant difference (P ≤ 0.05).
4. Discussion
Disease outbreaks with variable mortality rates and different clinical manifestations have been increased in Egyptian commercial chicken flocks with respiratory affections being the most common complaint (Hassan et al., 2016). Respiratory affections represent a great problem to the poultry industry because of their multifactorial nature (Bano et al., 2003, Roussan et al., 2008). Studies showed that mixed infection, especially with IB and AIV-H9N2 viruses, was the most common condition in the Egyptian poultry (Hassan et al., 2016).
In this study, clinical manifestations were limited to respiratory signs in all challenged groups under experimental conditions. Mild clinical signs were observed in single infection while severe respiratory distresses were common with mixed infection. Similar results were previously reported (Monne et al., 2013, Nili and Asasi, 2002) with a comparable mortality rate of 10% in mixed AIV-H9N2 and IBV infection. Unlike what is known about low virulence nature of H9N2 subtype, the role of co-infection with other pathogens including IBV, Staphylococcus aureus or Haemophilus paragallinarum (Kishida et al., 2004), and Escherichia coli (Bano et al., 2003) in exacerbating H9N2 virus infection in chickens is of major significance. Such exacerbation possibly occurs by secretion of trypsin-like proteases by bacterial stimulation of host cells to produce or secrete more protease, or destruction of endogenous cell protease inhibitors (Mancini et al., 2005) and suppression of the immune system due to stress (Kishida et al., 2004).
Petechial hemorrhage at trachea was constantly observed and progressed to congestion and trachitis with the presence of mucoid plug at the tracheal bifurcation in group AIV-H9N2 and in AIV-H9N2 plus IBV vaccine challenge. The mucoid plug was previously reported in AIV-H9N2 single infection (Capua and Marangon, 2000, Hooper and Selleck, 1998, Nili and Asasi, 2002, Perk et al., 2006). This finding further support the hypothesis that IBV, even vaccine strains play a role in increasing severity of H9N2 infection (Cook et al., 1976, Haghighat-Jahromi et al., 2008, Nili and Asasi, 2002) possibly through impairment of clearance of bacterial pathogens in the respiratory tract of broilers (Dwars et al., 2009).
Histopathologically, trachea in all mixed infection groups including the AIV-H9N2 with vaccine IBV strain showed severe changes comparable to the milder changes in single classical or variant IBV challenged groups (Purcell et al., 1976). In AIV-H9N2 single infection, congestion is the most predominant lesion with deciliation and leucocytic infiltration, that may explain the formation of mucoid plug by secondary bacterial infections (Nili and Asasi, 2002).
Remarkably, thymus of groups single AIV-H9N2 and AIV-H9N2 plus IBV was greatly affected with severe petechial hemorrhage followed by atrophy as compared to the negative control and single IBV infection groups. In chickens, the presence of AIV-H9N2 infected lymphocytes, during virus dissemination throughout the body via blood or lymph vessels, may explain the thymus pathological lesions (Kwon et al., 2008). However, secondary infection and/or stress due to endogenous glucocorticoid secretion or from the production of specific cytokines cannot be neglected. It was suggested that the immunosuppression predisposing to secondary bacterial infections and subsequent high mortality in field situations in AIV-H9N2 infection could be explained by the atrophy and lymphoid depletion of thymus and probably some other lymphoid organs (Hadipour et al., 2011).
Similar virus tracheal shedding titers were detected in the variant IBV infected birds either single or mixed infections with AIV-H9N2, however, in the current study, the classical IBV shedding significantly increased with AIV-H9N2 mixed infection. Elevated virus shedding titers of AIV-H9N2 in presence of IBV infection was previously reported (Monne et al., 2013). Similarly, in this study, significant increases of AIV-H9N2 virus shedding with classical IBV infection especially at 2 and 5 DPI and with the variant and vaccine IBV strains co-infections up to 7 DPI were observed. However, the only significant elevation of AIV-H9N2 HI antibody titers was recorded with the classical IBV co-infection at 10 DPI. This may be attributed to the earlier and higher replication of both AIV-H9N2 and classical IBV viruses as indicated by higher viruses shedding titers as early as 2 DPI. These results and previous studies results indicate the possible synergistic mechanism between IBV and AIV-H9N2 possibly by trypsin-like proteases encoded by coronaviruses that enhance the AIV-H9N2 hemagglutinin cleavage (Haghighat-Jahromi et al., 2008, Klenk and Garten, 1994, Liu et al., 1995, Ng and Liu, 2000, Perk et al., 2004).
The current study results indicate that the high mortalities observed in AIV-H9N2 under field conditions in Egypt might be partly attributed to mixed infections with widely spread different IBV strains. Further research is needed to elucidate the pathobiological interactions between AIV-H9N2 and IBV viruses in terms of increasing virus replication and pathogenicity.
The following are the supplementary data related to this article.
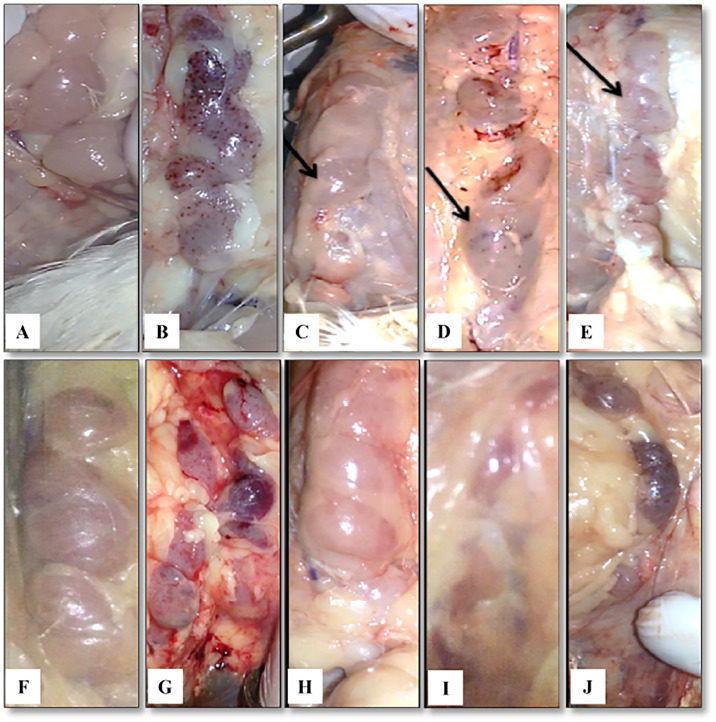
Tracheal lesions in control and challenged broiler chicks. At 2 DPI, Negative control (A) and variant IBV infected group (B), slight mucous in trachea of birds challenged classical IBV (C), petechial hemorrhage in trachea of birds with AIV-H9N2 (D), AIV-H9N2 and variant IBV (E), AIV-H9N2 and classical IBV (F), and AIV-H9N2 and vaccine IBV (G) infections. At 7 DPI, Negative control group (H), mucous in trachea of challenged variant IBV group (I) and classical IBV group (J), petechial hemorrhage in trachea of challenged AIV-H9N2 group (K), severe congestion in the trachea of challenged AIV-H9N2 and variant IBV (L) AIV-H9N2 and classical IBV (M) and AIV-H9N2 and IBV vaccine (N) groups.
Histopathology of trachea in infected commercial broilers. Negative control group (A), mild to moderate mucosal epithelial degeneration and deciliation, mild submucosal edema, and leucocytic infiltration in variant IBV or classical IBV (B) infections. Congested submucosal blood, marked decilliation and sever leucocytic and lymphocytic cell infiltration in AIV-H9N2 (C), AIV-H9N2 and variant IBV (D), AIV-H9N2 and classical IBV (E), and AIV-H9N2 and IBV vaccine (F) infections. Magnification 10 ×.
Acknowledgments
The authors would like to thank D. Manal A. Afifi for providing classical IBV isolate. The authors also thank Research and Development team at the Middle East for Veterinary Vaccines (ME VAC) Co. for providing purified enzyme treated IBV strains and antisera. This study was supported by the Project Support and Financing Unit at Beni-Suef University, Egypt (Project title: Advanced studies on viral respiratory problems prevalent in broiler chickens).
References
- Abdel-Moneim A.S., El-Kady M.F., Ladman B.S., Gelb J., Jr. S1 gene sequence analysis of a nephropathogenic strain of avian infectious bronchitis virus in Egypt. Virol. J. 2006;3:78. doi: 10.1186/1743-422X-3-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdel-Moneim A.S., Afifi M.A., El-Kady M.F. Emergence of a novel genotype of avian infectious bronchitis virus in Egypt. Arch. Virol. 2012;157:2453–2457. doi: 10.1007/s00705-012-1445-1. [DOI] [PubMed] [Google Scholar]
- Bancroft J., Gamble M. 6th ed. Churchill Livingstone Elsevier; Philadelphia, PA: 2008. Theory and Practice of Histological Techniques. [Google Scholar]
- Bano S., Naeem K., Malik S.A. Evaluation of pathogenic potential of avian influenza virus serotype H9N2 in chickens. Avian Dis. 2003;47:817–822. doi: 10.1637/0005-2086-47.s3.817. [DOI] [PubMed] [Google Scholar]
- Ben Shabat M., Meir R., Haddas R., Lapin E., Shkoda I., Raibstein I., Perk S., Davidson I. Development of a real-time TaqMan RT-PCR assay for the detection of H9N2 avian influenza viruses. J. Virol. Methods. 2010;168:72–77. doi: 10.1016/j.jviromet.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Callison S.A., Hilt D.A., Boynton T.O., Sample B.F., Robison R., Swayne D.E., Jackwood M.W. Development and evaluation of a real-time Taqman RT-PCR assay for the detection of infectious bronchitis virus from infected chickens. J. Virol. Methods. 2006;138:60–65. doi: 10.1016/j.jviromet.2006.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capua I., Marangon S. The avian influenza epidemic in Italy, 1999–2000: a review. Avian Pathol. 2000;29:289–294. doi: 10.1080/03079450050118403. [DOI] [PubMed] [Google Scholar]
- Cook J.K., Darbyshire J.H., Peters R.W. The use of chicken tracheal organ cultures for the isolation and assay of avian infectious bronchitis virus. Arch. Virol. 1976;50:109–118. doi: 10.1007/BF01318005. [DOI] [PubMed] [Google Scholar]
- Dwars R.M., Matthijs M.G., Daemen A.J., van Eck J.H., Vervelde L., Landman W.J. Progression of lesions in the respiratory tract of broilers after single infection with Escherichia coli compared to superinfection with E. coli after infection with infectious bronchitis virus. Vet. Immunol. Immunopathol. 2009;127:65–76. doi: 10.1016/j.vetimm.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Hadipour M.M., Habibi G.H., Golchin P., Hadipourfard M.R., Shayanpour N. The role of avian influenza, newcastle disease and infectious bronchitis viruses during the respiratory disease outbreak in commercial broiler farms of Iran. Int. J. Anim. Vet. Adv. 2011;3:69–72. [Google Scholar]
- Haghighat-Jahromi M., Asasi K., Nili H., Dadras H., Shooshtari A.H. Coinfection of avian influenza virus (H9N2 subtype) with infectious bronchitis live vaccine. Arch. Virol. 2008;153:651–655. doi: 10.1007/s00705-008-0033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan K.E., Shany S.A., Ali A., Dahshan A.H., El-Sawah A.A., El-Kady M.F. Prevalence of avian respiratory viruses in broiler flocks in Egypt. Poult. Sci. 2016;95:1271–1280. doi: 10.3382/ps/pew068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper P., Selleck P. Pathology of low and high virulent influenza virus infections. In: Swayne D.E., Slemons R.D., editors. The Fourth International Symposium on Avian Influenza. Animal Health Association Richmond; Virginia, U.S.: 1998. pp. 134–141. [Google Scholar]
- Karimi-Madab M., Ansari-Lari M., Asasi K., Nili H. Risk factors for detection of bronchial casts, most frequently seen in endemic H9N2 avian influenza infection, in poultry flocks in Iran. Prev. Vet. Med. 2010;95:275–280. doi: 10.1016/j.prevetmed.2010.03.010. [DOI] [PubMed] [Google Scholar]
- King D.J., Hopkins S.R. Evaluation of the hemagglutination-inhibition test for measuring the response of chickens to avian infectious bronchitis virus vaccination. Avian Dis. 1983;27:100–112. [PubMed] [Google Scholar]
- Kishida N., Sakoda Y., Eto M., Sunaga Y., Kida H. Co-infection of Staphyloccocus aureus or Haemophilus paragallinarum exacerbates H9N2 influenza A virus infection in chickens. Arch. Virol. 2004;149:2095–2104. doi: 10.1007/s00705-004-0372-1. [DOI] [PubMed] [Google Scholar]
- Klenk H.D., Garten W. Host cell proteases controlling virus pathogenicity. Trends Microbiol. 1994;2:39–43. doi: 10.1016/0966-842x(94)90123-6. [DOI] [PubMed] [Google Scholar]
- Kwon J.S., Lee H.J., Lee D.H., Lee Y.J., Mo I.P., Nahm S.S., Kim M.J., Lee J.B., Park S.Y., Choi I.S., Song C.S. Immune responses and pathogenesis in immunocompromised chickens in response to infection with the H9N2 low pathogenic avian influenza virus. Virus Res. 2008;133:187–194. doi: 10.1016/j.virusres.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Lee C.W., Suarez D.L. Application of real-time RT-PCR for the quantitation and competitive replication study of H5 and H7 subtype avian influenza virus. J. Virol. Methods. 2004;119:151–158. doi: 10.1016/j.jviromet.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Liu D.X., Brierley I., Brown T.D. Identification of a trypsin-like serine proteinase domain encoded by ORF 1a of the coronavirus IBV. Adv. Exp. Med. Biol. 1995;380:405–411. doi: 10.1007/978-1-4615-1899-0_66. [DOI] [PubMed] [Google Scholar]
- Mancini D.A.P., Mendonca R.M.Z., Dias A.L.F., Mendonca R.Z., Pinto J.R. Co-infection between influenza virus and flagellated bacteria. Rev. Inst. Med. Trop. Sao Paulo. 2005;47:275–280. doi: 10.1590/s0036-46652005000500007. [DOI] [PubMed] [Google Scholar]
- Monne I., Hussein H.A., Fusaro A., Valastro V., Hamoud M.M., Khalefa R.A., Dardir S.N., Radwan M.I., Capua I., Cattoli G. H9N2 influenza A virus circulates in H5N1 endemically infected poultry population in Egypt. Influenza Other Respir. Viruses. 2013;7:240–243. doi: 10.1111/j.1750-2659.2012.00399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng L.F., Liu D.X. Further characterization of the coronavirus infectious bronchitis virus 3C-like proteinase and determination of a new cleavage site. Virology. 2000;272:27–39. doi: 10.1006/viro.2000.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nili H., Asasi K. Natural cases and an experimental study of H9N2 avian influenza in commercial broiler chickens of Iran. Avian Pathol. 2002;31:247–252. doi: 10.1080/03079450220136567. [DOI] [PubMed] [Google Scholar]
- Nilli H., Asasi K. Avian influenza H9N2 outbreak in Iran. Avian Dis. 2003;47:828–831. doi: 10.1637/0005-2086-47.s3.828. [DOI] [PubMed] [Google Scholar]
- OIE Avian Influenza, in Manual of diagnostic tests and vaccines for terrestrial animals. [Internet]. World Organisation for Animal Health, pp. 2014. http://www.oie.int/international-standard-setting/terrestrial-manual/access-online/ Available from.
- Perk S., Pokamunski S., Elkin N., Perelman B. Low pathogenicity avian influenza H9N2 in Israel a threat to the poultry industry. In: Hafez H.M., editor. 5th International Symposium on Turkey Diseases. 2004. pp. 72–80. [Google Scholar]
- Perk S., Banet-Noach C., Shihmanter E., Pokamunski S., Pirak M., Lipkind M., Panshin A. Genetic characterization of the H9N2 influenza viruses circulated in the poultry population in Israel. Comp. Immunol. Microbiol. Infect. Dis. 2006;29:207–223. doi: 10.1016/j.cimid.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Purcell D.A., Tham V.L., Surman P.G. The histopathology of infectiousbronchitis in fowls infected with a nephrotropic Tstrain of virus. Aust. Vet. J. 1976;52:85–91. doi: 10.1111/j.1751-0813.1976.tb13864.x. [DOI] [PubMed] [Google Scholar]
- Roussan D.A., Haddad R., Khawaldeh G. Molecular survey of avian respiratory pathogens in commercial broiler chicken flocks with respiratory diseases in Jordan. Poult. Sci. 2008;87:444–448. doi: 10.3382/ps.2007-00415. [DOI] [PubMed] [Google Scholar]
- Seififi S., Asasi K., Mohammadi A. Natural co-infection caused by avian influenza H9 subtype and infectious bronchitis viruses in broiler chicken farms. Veterinarski Archiv. 2010;80:269–281. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tracheal lesions in control and challenged broiler chicks. At 2 DPI, Negative control (A) and variant IBV infected group (B), slight mucous in trachea of birds challenged classical IBV (C), petechial hemorrhage in trachea of birds with AIV-H9N2 (D), AIV-H9N2 and variant IBV (E), AIV-H9N2 and classical IBV (F), and AIV-H9N2 and vaccine IBV (G) infections. At 7 DPI, Negative control group (H), mucous in trachea of challenged variant IBV group (I) and classical IBV group (J), petechial hemorrhage in trachea of challenged AIV-H9N2 group (K), severe congestion in the trachea of challenged AIV-H9N2 and variant IBV (L) AIV-H9N2 and classical IBV (M) and AIV-H9N2 and IBV vaccine (N) groups.
Histopathology of trachea in infected commercial broilers. Negative control group (A), mild to moderate mucosal epithelial degeneration and deciliation, mild submucosal edema, and leucocytic infiltration in variant IBV or classical IBV (B) infections. Congested submucosal blood, marked decilliation and sever leucocytic and lymphocytic cell infiltration in AIV-H9N2 (C), AIV-H9N2 and variant IBV (D), AIV-H9N2 and classical IBV (E), and AIV-H9N2 and IBV vaccine (F) infections. Magnification 10 ×.