Abstract
Background
Tropospheric oxidant pollutants may injure the respiratory tract. Cystic fibrosis (CF) respiratory disease involves significant inflammation and excessive oxidative stress, and exposure to air pollutants can magnify the lung damage. The objective of this study was to investigate the association between the short-term variation in the concentration of air pollutants in metropolitan São Paulo, Brazil, and the occurrence of respiratory exacerbations in children and adolescents with CF.
Methods
A longitudinal panel of repeated measurements was obtained from 103 patients attending the outpatient center of our institution from September 6, 2006 through September 4, 2007. Daily concentrations of inhaled particulate matter, sulfur dioxide, nitrogen dioxide, ozone (O3), carbon monoxide, and meteorologic variables, such as the minimum temperature and relative humidity, were evaluated. The generalized estimation equation model for binomial distribution was used to assess the impact of these measurements on the occurrence of acute respiratory exacerbations.
Results
In total, 103 patients with CF (median age, 8.9 years) made 408 visits, with a mean ± SD of 4 ± 1.74 visits per patient (range, 2-9). A respiratory disease exacerbation was diagnosed on 142 visits (38.4%). An interquartile range increase in the O3 concentration (45.62 μg/m3) had a positive, delayed (2 days after exposure) effect on the risk of a respiratory exacerbation (relative risk = 1.86; 95% CI, 1.14-3.02).
Conclusions
This study demonstrates that exposure to short-term air pollution in a large urban center increases the risk of a pulmonary exacerbation in patients with CF.
Abbreviations
- CF
cystic fibrosis
- CO
carbon monoxide
- IQR
interquartile range
- NO2
nitrogen dioxide
- O3
ozone
- PM10
particulate matter < 10 μm in diameter
- RR
relative risk
- SO2
sulfur dioxide
Numerous epidemiologic studies have demonstrated the effect of exposure to air pollutants (particulate matter < 10 μm in diameter [PM10], nitrogen dioxide [NO2], sulfur dioxide [SO2], ozone [O3], and carbon monoxide [CO]) on cardiorespiratory diseases morbidity and premature mortality around the world, with children, the elderly, and those with chronic respiratory disease at greatest risk.1, 2, 3, 4, 5 Our Environmental Epidemiology Study Group, located in São Paulo, Brazil, has demonstrated that exposure to air pollution in the city is a public health hazard.6, 7, 8, 9 Children with cystic fibrosis (CF) are part of a high-risk population whose respiratory tracts may be significantly affected by air pollution.
CF is an autosomal inherited disease characterized by recurrent and chronic respiratory infections10, 11, 12, 13 that ultimately lead to chronic pulmonary inflammation. Airway inflammation has also been reported in the respiratory tracts of infants with CF in the absence of bacterial or viral infections,13, 14 suggesting an alternative proinflammatory pathway in these patients. A number of factors may influence CF severity and outcomes, such as genetic, health care-related, and environmental factors.15, 16, 17 Exposure to air pollution may worsen inflammation in the respiratory tracts of patients with CF and have a substantial clinical impact. The objective of the present study was to investigate the influence of exposure to several tropospheric pollutants on acute respiratory exacerbations in children and adolescents with CF using a longitudinal repeated-measures design.
Materials and Methods
One hundred three patients with CF, attending the outpatient clinic at Children's Institute, Clinics Hospital, University of São Paulo Medical School, were invited to enroll in the study from September 6, 2006 to September 4, 2007, in the city of São Paulo. Parents or caregivers gave informed consent, and children older than 6 years gave assent to be included in the study. The Ethics Committee of Clinics Hospital of University of São Paulo Medical School approved the study (approval code 925/05).
In this longitudinal, observational panel with repeated measures, the data were obtained at scheduled or unscheduled visits to the outpatient clinic. Clinical data were obtained by history and clinical examination; spirometry was performed using a Koko spirometer (nSpire Health, Inc) and predicted values from the Polgar equation.18 Bronchial obstruction was defined when an FEV1/FVC ratio was < 80%.19
Nasopharyngeal aspirates or nasal blow samples were used to identify respiratory viruses, and sputum or oropharyngeal samples were obtained for microbiologic cultures. Investigation of viral infections was performed using specific individual reverse transcription-polymerase chain reaction or polymerase chain reaction targeting respiratory syncytial virus; influenza viruses A and B; human parainfluenza viruses 1, 2, and 3; human coronaviruses; human metapneumovirus; adenovirus; human bocavirus; and picornaviruses. Microbiologic culture of sputum or oropharyngeal samples was performed in selective media, and the identification of any known pathogenic bacteria for patients with CF (Pseudomonas aeruginosa, Burkholderia cepacia complex, Stenotrophomonas maltophilia, Staphylococcus aureus, and so forth) characterized the sample as positive.
A respiratory exacerbation was defined as the presence of three or more of the following signs or symptoms: fever, increased sputum production or cough intensity, change in sputum color, worsening dyspnea, loss of appetite, > 10% decrease in FEV1, or weight loss.
The Shwachman-Kulczycki score on the last visit was used to determine the disease severity. This score assesses the disease severity using the sum of four criteria: physical activity, physical examination, nutritional status, and chest radiography.
Daily records of the studied pollutants, including O3 (the highest hourly average), SO2 (24-h average), NO2 (the highest hourly average), PM10 (24-h average), and CO (the highest 8-h moving average), were obtained for the entire study period from the São Paulo State Environmental Agency, whose air pollutants monitoring network has 14 automatic stations spread all over the city. Air pollutant levels recorded in each station are highly correlated with the others. Therefore, the average of all stations that measured each pollutant was adopted as an exposure status throughout the city. The daily minimum temperature and mean relative air humidity were obtained from the Institute of Astronomy and Geophysics at the University of São Paulo.
Statistical Analysis
The dependent variable was the diagnosis of a respiratory exacerbation, and the independent variables were the presence of a viral infection, positive sputum or oropharyngeal culture, degree of obstruction by spirometery19 (0 = normal, 1 = mild, 2 = moderate, 3 = moderately severe, 4 = severe, 5 = very severe), Shwachman-Kulczycki score (0 = excellent, 1 = good, 2 = medium, 3 = moderate, 4 = severe), daily mean levels of each pollutant (PM10, SO2, CO, NO2, and O3), minimum temperature, and relative humidity. Descriptive analyses were performed for all of the variables included in the study, and Pearson correlation coefficients were estimated for the air pollutant variables.
We adopted the generalized estimating equations model, considering fixed effects for repeated measurements to estimate the association and effect of pollutants on respiratory exacerbations in patients with CF. S-Plus 2000 Professional Release 3 software (Informer Technologies, Inc) was used, adjusting the model for the independent variables by using an exchangeable correlation as a working matrix, which assumes equal correlation for measurements in each subject.
The lag structure between air pollutant exposure and CF exacerbation was assessed using lags of 0 to 7 days. Single-pollutant models were used for the analysis. However, if more than one pollutant had a significant effect on the outcome, two-pollutant models were adopted. The relative risk of the outcome was reported (with the respective 95% CI) for an interquartile range (IQR) increase in each pollutant.
Results
One hundred three patients with CF with median age of 8.9 years were seen on 408 visits, with a mean ± SD of 4 ± 1.74 visits per patient (range 2-9). Most of the clinical visits were routine consultations, and only 45 visits (11%) were nonscheduled.
Table 1 presents clinical and biodemographic characteristics of the patients. Sex distribution was similar, and children < 10 years old were the majority. The median value of the clinical-radiologic Shwachman-Kulczycki score was 75, and the median BMI was lower than the ideal BMI (range, 11.8- 23.1), indicating a compromised nutritional status in a significant proportion of the patients. Lung function testing results were available from more than two-thirds of the patients with CF, and although median age was relatively low, 31.8% of the patients presented a significant degree of bronchial obstruction (FEV1 < 60% of the predicted value). Increased cough intensity was by far the most frequent symptom that contributed to the diagnosis of respiratory exacerbation. At least two spirometries were performed in 161 visits. A decrease in the FEV1 value > 10% from the baseline (the best result of the year) was observed on 31 occasions (19.3%).
Table 1.
General Characteristics of Patients With Cystic Fibrosis Included in the Study
| Patient Characteristics | Data |
|---|---|
| Sex (N = 103) | |
| Male | 54 (52.4) |
| Age group, y | |
| 0–4 | 23 (22.3) |
| 5–9 | 35 (34.0) |
| 10–14 | 26 (25.2) |
| ≥ 15 | 19 (18.4) |
| Degree of obstruction by FEV1 value (n = 66) | |
| Very severe | 10 (15.2) |
| Severe | 8 (12.1) |
| Moderately severe | 3 (4.5) |
| Moderate | 6 (9.1) |
| Mild | 10 (15.2) |
| Normal | 29 (43.9) |
| Bacteria colonization (n = 101) | |
| Staphylococcus aureus | 91 (90.1) |
| Pseudomonas aeruginosa | 62 (61.4) |
| Median age (IQR), y | 8.9 (8.1) |
| Median Shwachman-Kulczycki score (IQR) | 75 (20) |
| Median BMI (IQR) | 16.0 (3.1) |
| Median % predicted FEV1 (IQR) | 73.8 (45.8) |
| Visits characteristics | |
| Oxygen saturation (n = 398 visits) | |
| < 85% | 7 (1.8) |
| 85%–89% | 37 (9.3) |
| 90%–92% | 42 (10.6) |
| > 92% | 312 (78.4) |
| FEV1 decrease > 10% (n = 161 visits) | 31 (19.3) |
| Signs or symptoms (n = 408 visits) | |
| Fever | 49 (12) |
| Increased sputum production | 139 (34.1) |
| Increased cough intensity | 335 (82.1) |
| Change in sputum | 76 (18.6) |
| Worsening dyspnea | 82 (20.1) |
| Loss of appetite | 54 (13.2) |
Data are presented as No. (%) unless otherwise noted. IQR = interquartile range.
Respiratory exacerbation episodes were observed in 60.2% of patients on 142 visits (38.4%). At least one respiratory virus was identified in 203 of the 408 respiratory samples (49.8%); rhinovirus was the most frequent virus and was identified in 139 samples (34.1%).
Microbiological culture was performed in 404 sputum or oropharyngeal samples; four samples were mishandled. The most commonly identified bacteria were S aureus (277 samples, 68.6%) and P aeruginosa (165 samples, 40.9%). Only two patients did not present a positive sputum or oropharyngeal culture during the study period; in most of them (90%) S aureus strains were identified, and in 61% P aeruginosa (62.9% were mucoid strains) was isolated (Table 1).
The humidity and the temperature in São Paulo during the study period exhibited two patterns. One pattern was characterized by hot and wet weather, from November to April, with the average minimum temperature of 14.6°C and average precipitation of 193.27 mm. The other pattern was characterized by cold and dry weather, from May to October, with the average minimum temperature of 7.2°C and average precipitation of 48.48 mm. The range of variation in pollutant concentrations and weather conditions during the study period is presented in Table 2 . On the majority of the days, air quality index for air pollutants was considered acceptable. Ozone was the only pollutant that surpassed the Brazilian national standards for air quality limits (highest average hourly, 160 μg/m3) for 8 days of the study period, with the highest levels occurring during summer (Fig 1 ), as well as the standard adopted by the World Health Organization (WHO) (highest 8-h moving average). On four of the assessed days, daily ozone levels were 160-180 μg/m3, which is considered “unhealthy for sensitive groups.” On the other 4 days, ozone levels surpassed 250 μg/m3, reaching an air quality index considered “unhealthy,” which means everyone's health is prone to suffer harm. This matter may be worse for the most sensitive groups (children, elderly, and people with respiratory diseases), whose health may suffer even more harmful effects.20
Table 2.
Descriptive Statistics of Air Pollutants, Temperature, and Humidity During Study Period
| Measure | No.a | Minimum | Maximum | Mean | SD | IQR | Quartiles 25%–75% |
|---|---|---|---|---|---|---|---|
| O3, μg/m3 | 408 | 17.99 | 254.10 | 76.82 | 36.81 | 45.62 | 52.15–97.67 |
| CO, ppm | 408 | 0.65 | 6.09 | 1.78 | 0.95 | 0.81 | 1.18–1.99 |
| NO2, μg/m3 | 408 | 46.02 | 217.23 | 103.02 | 35.56 | 44.43 | 75.97–120.40 |
| SO2, μg/m3 | 408 | 2.67 | 25.11 | 9.90 | 4.07 | 5.50 | 6.66–12.10 |
| PM10, μg/m3 | 408 | 15.93 | 99.08 | 42.03 | 18.63 | 22.33 | 28.27–50.06 |
| Temperature, °C | 408 | 4.30 | 21.84 | 16.08 | 3.42 | 4.87 | 14.00–18.87 |
| Humidity, % | 408 | 53.79 | 98.02 | 77.04 | 8.92 | 11.96 | 70.72–82.68 |
CO = carbon monoxide; NO2 = nitrogen dioxide; O3 = ozone; PM10 = particulate matter < 10 μm in diameter; ppm = parts per million; SO2 = sulfur dioxide. See Table 1 legend for expansion of other abbreviation.
Number of observations.
Figure 1.
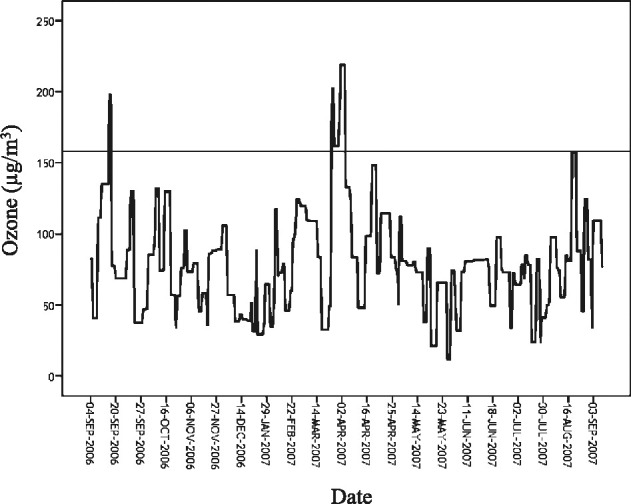
Daily ozone concentration during study period.
Table 3 presents Pearson correlation coefficients between pollutants. Primary air pollutants were highly correlated to each other. The lowest ozone correlation was observed with CO, and the highest was with NO2. Since NO2 measurements were performed on a daily basis, on those days with high NO2 concentrations there was a high formation of the secondary pollutant.
Table 3.
Pearson Correlation Coefficients Between Air Pollutants
| Air Pollutants | Pearson Correlation Coefficients |
|||
|---|---|---|---|---|
| O3, μg/m3 | CO, ppm | NO2, μg/m3 | SO2, μg/m3 | |
| CO | 0.13 | |||
| NO2 | 0.64 | 0.65 | ||
| SO2 | 0.37 | 0.56 | 0.57 | |
| PM10 | 0.47 | 0.72 | 0.79 | 0.70 |
All correlations significant at the 0.01 level (two-tailed). See Table 2 legend for expansion of abbreviations.
The estimates of relative risk (RR) of respiratory exacerbation associated with interquartile range increases in daily concentrations of PM10, O3, NO2, SO2, and CO using single-pollutant models are presented in Table 4 . Increase of O3 was associated with a delayed (2 days after exposure) increase in the risk of a respiratory exacerbation in patients with CF. The cumulative effect of lag1 and lag2 (RR = 1.89; 95% CI, 1.10-3.23) was quite similar to that observed for lag2. No statistically significant associations were found between other pollutants and respiratory exacerbation in patients with CF. In two-pollutant or multipollutant models, a lag2 decrease of 11% in the RR of respiratory exacerbation due to O3 increases was observed (RR = 1.65; 95% CI, 0.08-3.09).
Table 4.
RR of Exacerbation of Cystic Fibrosis Associated With an IQR of PM10, O3, NO2, SO2, and CO
| Lag | RR (95% CI) |
||||
|---|---|---|---|---|---|
| PM10 (IQR = 22.33 μg/m3) | O3 (IQR = 45.62 μg/m3) | NO2 (IQR = 44.43 μg/m3) | SO2 (IQR = 5.5 μg/m3) | CO (IQR = 0.81 ppm) | |
| Lag0 | 1.13 (0.62–2.06) | 1.34 (0.92–1.94) | 1.26 (0.76–2.09) | 0.98 (0.58–1.67) | 1.01 (0.76–1.33) |
| Lag1 | 0.92 (0.51–1.67) | 1.48 (0.94–2.33) | 1.36 (0.84–2.19) | 1.09 (0.59–2.01) | 0.90 (0.66–1.23) |
| Lag2 | 1.20 (0.74–1.97) | 1.86 (1.14–3.02) | 1.61 (0.94–2.78) | 1.53 (0.78–2.99) | 1.10 (0.76–1.59) |
| Lag3 | 0.81 (0.50–1.32) | 1.20 (0.76–1.92) | 1.01 (0.63–1.61) | 0.87 (0.47–1.60) | 1.02 (0.76–1.38) |
| Lag4 | 0.86 (0.52–1.44) | 1.26 (0.78–2.01) | 1.29 (0.72–2.33) | 0.78 (0.44–1.38) | 0.94 (0.71–1.24) |
| Lag5 | 0.90 (0.58–1.41) | 1.22 (0.75–1.98) | 1.10 (0.70–1.73) | 1.07 (0.63–1.84) | 0.99 (0.75–1.32) |
| Lag6 | 0.95 (0.65–1.40) | 1.24 (0.72–2.11) | 1.13 (0.66–1.93) | 1.28 (0.73–2.25) | 1.00 (0.81–1.23) |
When a categorical variable representing ozone tertiles was included in the regression model instead of the continuous one, a threshold at the second tertile was observed (65.41-85.0 μg/m3) that represented an increase of 99% (95% CI, 5-194) in CF exacerbations when compared with the first tertile. A similar effect was observed at the third tertile (100%; 95% CI, 4-200).
Discussion
This study demonstrates that an IQR (45.62 μg/m3) increase in ozone concentration can lead to a 52% (from 34% at lag0 to 86% at lag2) increase in the risk of respiratory exacerbation in children and adolescents with CF 48 h after exposure, even after controlling for other risk factors. This means that exposure to air pollutants, such as ozone, is harmful to the respiratory health of this high-risk population.
Children are particularly susceptible to ambient air pollutants because their lungs and immune systems are not fully developed (complete functional development does not occur before the age of 6 years), they are more active than adults, they breathe more air per unit of body weight, and their peripheral airways are anatomically smaller than those of adults, so inflammation results in proportionally greater airway obstruction.20, 21 Children with CF are part of a high-risk population, because significant and persistent pulmonary inflammation, usually early onset, leads to progressive airway destruction.10, 13, 14, 15, 22 In this state of chronic inflammation and excessive oxidative stress, pollutants can worsen the lung damage as well as increase susceptibility to infections. Several factors, such as viral illnesses, upper respiratory infection, allergens, and air pollution, can disturb the homeostasis between host defenses and the pulmonary inflammation of patients with CF leading to a respiratory exacerbation. At least 35% of patients with CF present one or more episodes of respiratory exacerbation per year.23 Respiratory exacerbations are very harmful for patients with CF, associated with disease progression, decrease in quality of life, and increase in the risk of death.24, 25, 26
Many studies have investigated the association between short-term exposure to air pollution and its adverse effects on the respiratory tracts of children.4, 7, 27, 28, 29 However, little is known about the impact of air pollutants in patients with CF. Accordingly, Kamdar et al22 studied the effect of PM < 2.5 μm on cystic fibrosis epithelium by culturing IB3-1 and S-9 CF human bronchial epithelial cells. The authors demonstrated that PM induces oxidative stress by mediating apoptosis in the CF bronchial epithelium. They suggested that the intense inflammatory response and the impaired mitochondrial function caused by the pollutant have a critical role in inducing cytotoxic effects in the airway epithelium of patients with CF. These processes could probably favor the occurrence of an acute respiratory exacerbation.
Goss et al30 performed another study evaluating the effects of air pollution in patients with CF using patient registry data. The authors evaluated 11,484 patients > 6 years of age enrolled in the Cystic Fibrosis Foundation National Patient Registry in 1999 and 2000. Logistic regression models were used to estimate the odds of two or more pulmonary exacerbations compared with one or zero exacerbations. Increases of 10 μg/m3 in the PM10 and O3 annual averages were associated, respectively, with increases of 9% (95% CI, 2%-17%) and 10% (95% CI, 3%-17%) of the odds of having two exacerbations. Adjusting for PM10 and additional confounders, a 10 μg/m3 increase in O3 was associated with an increase of 1.08 (95% CI, 1.01-1.15) in the OR for pulmonary exacerbations. In our study, when we performed the analysis with an increase of 10 μg/m3 in the ozone concentration, we observed, using a longitudinal repeated-measures design, a risk of pulmonary exacerbation (RR = 1.15; 95% CI, 1.03-1.27) similar to the one found by Goss and colleagues.30
In a recent 5-year retrospective study, Jassal et al31 evaluated residential proximity of roadways and O3 and PM < 2.5 μm levels on rates of pulmonary exacerbations in a cohort of 145 patients seen in the CF center at Children's Hospital Los Angeles. They showed that patients with CF who reside closer to a major road, the more likely they are to experience two or more exacerbations requiring hospital admission and IV antibiotics. Despite the methodologic differences (design, exacerbation criteria, and statistical analysis) between the study by Jassal et al31 and ours, both showed the relevance of automotive fleet-generated air pollution (primarily or secondarily) in respiratory exacerbation of patients with CF.
The city of São Paulo has a population of 10,886,518 people and > 6 million vehicles. These vehicles are the main source of air pollution, and the ozone concentration has progressively increased, mainly during episodes of high temperatures and low humidity. The ozone concentration had frequently exceeded the WHO air quality guidelines (100 μg/m3 for an 8-h daily average)32, 33, 34 when we adopted the highest 8-h moving average as pattern. In São Paulo, because of the relatively high mean temperatures, homes maintain good ventilation in all seasons. Previous studies have shown that personal ambient association for particles and gases was higher for people spending time in places where windows were open than those for people who spend more time in places where windows were closed.35, 36 Therefore, in our study the use of data of fixed monitoring stations was probably representative of indoor exposure.
Our study demonstrated that the exposure to ozone led to an increase in the risk of pulmonary exacerbation of patients with CF. Interestingly, O3 is a strong oxidizing compound that causes damage to the lungs and promotes airway inflammation. The interaction between ozone and lung epithelial lining fluid in patients with CF most likely produces oxidized species that may be responsible for triggering lung inflammation and contribute to acute bronchoconstriction and airway hyperresponsiveness, similar to the changes observed in asthma.37, 38 Furthermore, ozone is able to induce apoptosis, DNA damage, and cytotoxicity on human type 1 alveolar epithelial cells and ciliated airway epithelial cells, which can worsen airway inflammation in patients with CF.39, 40 These processes could be an effect of O3 itself, given its high oxidative capacity, or a collective effect of all pollutants that can be found in the photochemical mixture of air pollution, which is not regularly measured by environmental agencies. The effect observed in two-pollutant or multipollutant models may be a consequence of the high correlation between O3 and primary pollutants. It is quite plausible to assume the effects on respiratory exacerbation as the result of the action of all pollutants.
Our study has some limitations, however. Individual data on potential confounders, such as socioeconomic status and exposure to cigarette smoke, were not available, although some published studies have shown inconsistencies in associating poorer clinical outcomes in patients with CF with secondhand smoke exposure.41, 42, 43, 44, 45 All of these studies did not evaluate the influence of air pollution.
In contrast to the WHO air quality guidelines for PM, O3, NO2, and SO2 32, in which it is stated that the adverse effects of ozone present a dose-response pattern, we showed that, in the relation between ozone and CF exacerbation, it is possible to assume that there is a level at which risk of harm is minimum (< 65.4 μg/m3). Moreover, we showed that whatever the standard adopted is (US Environmental Protection Agency, WHO, European Environment Agency, or Brazilian National Environment Council), this standard is settled above this level of minimum effect. Based on our results, the WHO Air Quality Guidelines32 (the most restrictive) could be lowered by at least 25% to minimize risks of CF exacerbations.
Conclusion
This study has demonstrated that, in a large urban center, acute exposure to air pollution (until 2 days after the increase of ozone concentration) increases the risk of pulmonary exacerbation in patients suffering from severe chronic respiratory diseases, such as CF. Oxidant pollutants may have an important role in making the damage to these patients' lungs even worse. Also, air quality standards for ozone are far from assuring smaller risks of respiratory exacerbations in sensitive groups like patients with CF.
Acknowledgments
Authors contributions: Dr Farhat takes responsibility for the integrity of the work as a whole.
Dr Farhat: contributed to designing the study, analyzing the data, writing the article, and revising the final version.
Dr Almeida: contributed to designing the study, acquiring the data, writing the manuscript, and revising the final version.
Dr Silva-Filho: contributed to designing the study, acquiring the data, writing the manuscript, and revising the final version.
Ms Farhat: contributed to acquiring and analyzing the data and writing the article.
Dr Rodrigues: contributed to designing the study, acquiring the data, writing the manuscript, and revising the final version.
Dr Braga: contributed to designing the study, analyzing the data, writing the article, and revising the final version.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank the São Paulo Environmental State Agency (CETESB) for support on air pollution data gathering and interpretation and Institute of Astronomy and Geophysics of the University of São Paulo for providing meteorologic data. We also thank Jesuíno Romano from CETESB and Lourdes Conceição Martins, PhD, from Environmental Epidemiology Study Group, Laboratory of Experimental Air Pollution, University of São Paulo Faculty of Medical Sciences, for their assistance in the reply to reviewers.
Footnotes
For editorial comment see page 1093
Funding/Support: This study was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo [Grant 05/01625-8].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Bell ML, Dominici F, Samet JM. A meta-analysis of time-series studies of ozone and mortality with comparison to the national morbidity, mortality, and air pollution study. Epidemiology. 2005;16(4):436–445. doi: 10.1097/01.ede.0000165817.40152.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Delfino RJ, Gillen DL, Tjoa T. Electrocardiographic ST-segment depression and exposure to traffic-related aerosols in elderly subjects with coronary artery disease. Environ Health Perspect. 2011;119(2):196–202. doi: 10.1289/ehp.1002372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hůnová I, Malý M, Rezáčová J, Braniš M. Association between ambient ozone and health outcomes in Prague. Int Arch Occup Environ Health. 2013;86(1):89–97. doi: 10.1007/s00420-012-0751-y. [DOI] [PubMed] [Google Scholar]
- 4.Fraga J, Botelho A, Sá A, Costa M, Quaresma M. The lag structure and the general effect of ozone exposure on pediatric respiratory morbidity. Int J Environ Res Public Health. 2011;8(10):4013–4024. doi: 10.3390/ijerph8104013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brook RD, Rajagopalan S, Pope CA, III, American Heart Association Council on Epidemiology and Prevention, Council on the Kidney in Cardiovascular Disease, and Council on Nutrition, Physical Activity and Metabolism Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 6.Lin CA, Amador Pereira LA, de Souza Conceição GM. Association between air pollution and ischemic cardiovascular emergency room visits. Environ Res. 2003;92(1):57–63. doi: 10.1016/s0013-9351(02)00054-3. [DOI] [PubMed] [Google Scholar]
- 7.Farhat SC, Paulo RL, Shimoda TM. Effect of air pollution on pediatric respiratory emergency room visits and hospital admissions. Braz J Med Biol Res. 2005;38(2):227–235. doi: 10.1590/s0100-879x2005000200011. [DOI] [PubMed] [Google Scholar]
- 8.Santos UP, Terra-Filho M, Lin CA. Cardiac arrhythmia emergency room visits and environmental air pollution in Sao Paulo, Brazil. J Epidemiol Community Health. 2008;62(3):267–272. doi: 10.1136/jech.2006.058123. [DOI] [PubMed] [Google Scholar]
- 9.Olmo NR, Saldiva PH, Braga AL, Lin CA, Santos U de P, Pereira LA. A review of low-level air pollution and adverse effects on human health: implications for epidemiological studies and public policy. Clinics (Sao Paulo) 2011;66(4):681–690. doi: 10.1590/S1807-59322011000400025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratjen F, Döring G. Cystic fibrosis. Lancet. 2003;361(9358):681–689. doi: 10.1016/S0140-6736(03)12567-6. [DOI] [PubMed] [Google Scholar]
- 11.da Silva Filho LV, Zerbinati RM, Tateno AF. The differential clinical impact of human coronavirus species in children with cystic fibrosis. J Infect Dis. 2012;206(3):384–388. doi: 10.1093/infdis/jis274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Almeida MB, Zerbinati RM, Tateno AF. Rhinovirus C and respiratory exacerbations in children with cystic fibrosis. Emerg Infect Dis. 2010;16(6):996–999. doi: 10.3201/eid1606.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dakin CJ, Numa AH, Wang H, Morton JR, Vertzyas CC, Henry RL. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165(7):904–910. doi: 10.1164/ajrccm.165.7.2010139. [DOI] [PubMed] [Google Scholar]
- 14.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151(4):1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 15.Rottner M, Freyssinet JM, Martínez MC. Mechanisms of the noxious inflammatory cycle in cystic fibrosis. Respir Res. 2009;10:23. doi: 10.1186/1465-9921-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kraemer R, Baldwin DN, Ammann RA, Frey U, Gallati S. Progression of pulmonary hyperinflation and trapped gas associated with genetic and environmental factors in children with cystic fibrosis. Respir Res. 2006;7:138. doi: 10.1186/1465-9921-7-138. 10.1186/1465-9921-7-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schechter MS. Nongenetic influences on cystic fibrosis outcomes. Curr Opin Pulm Med. 2011;17(6):448–454. doi: 10.1097/MCP.0b013e32834ba899. [DOI] [PubMed] [Google Scholar]
- 18.Polgar G, Weng TR. The functional development of the respiratory system from the period of gestation to adulthood. Am Rev Respir Dis. 1979;120(3):625–695. doi: 10.1164/arrd.1979.120.3.625. [DOI] [PubMed] [Google Scholar]
- 19.Pellegrino R, Viegi G, Brusasco V. Interpretative strategies for lung function tests. Eur Respir J. 2005;26(5):948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 20.Bateson TF, Schwartz J. Children's response to air pollutants. J Toxicol Environ Health A. 2008;71(3):238–243. doi: 10.1080/15287390701598234. [DOI] [PubMed] [Google Scholar]
- 21.Grigg J. Particulate matter exposure in children: relevance to chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6(7):564–569. doi: 10.1513/pats.200905-026RM. [DOI] [PubMed] [Google Scholar]
- 22.Kamdar O, Le W, Zhang J, Ghio AJ, Rosen GD, Upadhyay D. Air pollution induces enhanced mitochondrial oxidative stress in cystic fibrosis airway epithelium. FEBS Lett. 2008;582(25–26):3601–3606. doi: 10.1016/j.febslet.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cystic Fibrosis Foundation Patient Registry . 2009 Annual Data Report. Cystic Fibrosis Foundation; Bethesda, MD: 2011. [Google Scholar]
- 24.Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest. 2002;121(1):64–72. doi: 10.1378/chest.121.1.64. [DOI] [PubMed] [Google Scholar]
- 25.Sanders DB, Bittner RC, Rosenfeld M. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol. 2011;46(4):393–400. doi: 10.1002/ppul.21374. [DOI] [PubMed] [Google Scholar]
- 26.de Boer K, Vandemheen KL, Tullis E. Exacerbation frequency and clinical outcomes in adult patients with cystic fibrosis. Thorax. 2011;66(8):680–685. doi: 10.1136/thx.2011.161117. [DOI] [PubMed] [Google Scholar]
- 27.Dockery DW, Cunningham J, Damokosh AI. Health effects of acid aerosols on North American children: respiratory symptoms. Environ Health Perspect. 1996;104(5):500–505. doi: 10.1289/ehp.96104500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Poon R, Chen L. Acute effects of air pollution on pulmonary function, airway inflammation, and oxidative stress in asthmatic children. Environ Health Perspect. 2009;117(4):668–674. doi: 10.1289/ehp11813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strickland MJ, Darrow LA, Klein M. Short-term associations between ambient air pollutants and pediatric asthma emergency department visits. Am J Respir Crit Care Med. 2010;182(3):307–316. doi: 10.1164/rccm.200908-1201OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goss CH, Newsom SA, Schildcrout JS, Sheppard L, Kaufman JD. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am J Respir Crit Care Med. 2004;169(7):816–821. doi: 10.1164/rccm.200306-779OC. [DOI] [PubMed] [Google Scholar]
- 31.Jassal MS, Yu AM, Bhatia R, Keens TG, Davidson Ward SL. Effect of residential proximity to major roadways on cystic fibrosis exacerbations. Int J Environ Health Res. 2013;23(2):119–131. doi: 10.1080/09603123.2012.708917. 10.1080/09603123.2012.708917. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization Air quality guidelines. Global update 2005. Particulate matter, ozone, nitrogen dioxide and sulfur dioxide. Cap 7.2 ozone. WHO website. http://www.euro.who.int/en/what-we-publish/abstracts/air-quality-guidelines.-global-update-2005.-particulate-matter,-ozone,-nitrogen-dioxide-and-sulfur-dioxide Accessed May 27, 2012. [PubMed]
- 33.CETESB Rede de Monitoramento. CETESB website. http://sistemasinter.cetesb.sp.gov.br/ar/ar_automatica.asp Accessed February 2012.
- 34.Sérgio Chiarelli P, Amador Pereira LA, Nascimento Saldiva PH. The association between air pollution and blood pressure in traffic controllers in Santo André, São Paulo, Brazil. Environ Res. 2011;111(5):650–655. doi: 10.1016/j.envres.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Sarnat SE, Coull BA, Schwartz J, Gold DR, Suh HH. Factors affecting the association between ambient concentrations and personal exposures to particles and gases. Environ Health Perspect. 2006;114(5):649–654. doi: 10.1289/ehp.8422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rojas-Bracho L, Suh HH, Catalano PJ, Koutrakis P. Personal exposures to particles and their relationships with personal activities for chronic obstructive pulmonary disease patients living in Boston. J Air Waste Manag Assoc. 2004;54(2):207–217. doi: 10.1080/10473289.2004.10470897. [DOI] [PubMed] [Google Scholar]
- 37.Song S, Wu Y, Jiang J, Yang L, Cheng Y, Hao J. Chemical characteristics of size-resolved PM2.5 at a roadside environment in Beijing, China. Environ Pollut. 2012;161:215–221. doi: 10.1016/j.envpol.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 38.Mudway IS, Kelly FJ. Ozone and the lung: a sensitive issue. Mol Aspects Med. 2000;21(1–2):1–48. doi: 10.1016/s0098-2997(00)00003-0. [DOI] [PubMed] [Google Scholar]
- 39.Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008;26(4):339–362. doi: 10.1080/10590500802494538. [DOI] [PubMed] [Google Scholar]
- 40.Kosmider B, Loader JE, Murphy RC, Mason RJ. Apoptosis induced by ozone and oxysterols in human alveolar epithelial cells. Free Radic Biol Med. 2010;48(11):1513–1524. doi: 10.1016/j.freeradbiomed.2010.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beydon N, Amsallem F, Bellet M. Pulmonary function tests in preschool children with cystic fibrosis. Am J Respir Crit Care Med. 2002;166(8):1099–1104. doi: 10.1164/rccm.200205-421OC. [DOI] [PubMed] [Google Scholar]
- 42.Rubin BK. Exposure of children with cystic fibrosis to environmental tobacco smoke. N Engl J Med. 1990;323(12):782–788. doi: 10.1056/NEJM199009203231203. [DOI] [PubMed] [Google Scholar]
- 43.Smyth A, O'Hea U, Williams G, Smyth R, Heaf D. Passive smoking and impaired lung function in cystic fibrosis. Arch Dis Child. 1994;71(4):353–354. doi: 10.1136/adc.71.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smyth A, O'Hea U, Feyerabend C, Lewis S, Smyth R. Trends in passive smoking in cystic fibrosis, 1993-1998. Pediatr Pulmonol. 2001;31(2):133–137. doi: 10.1002/1099-0496(200102)31:2<133::aid-ppul1021>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 45.Collaco JM, Vanscoy L, Bremer L. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA. 2008;299(4):417–424. doi: 10.1001/jama.299.4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]


