Abstract
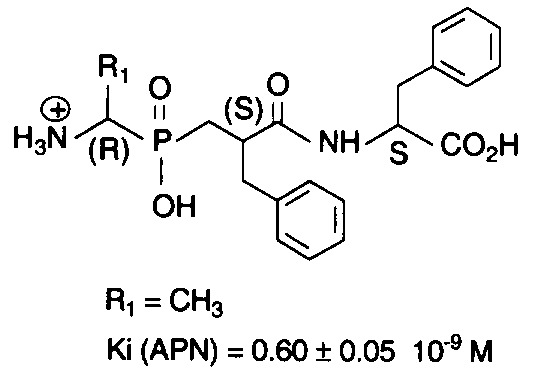
A series of phosphinic compounds mimicking the transition state of substrates hydrolysed by aminopeptidase N (EC 3.4.11.2) were synthesized. These new compounds have potent inhibitory activities with Ki values in the nanomolar range. These derivatives behave as the most potent APN inhibitors designed to date.
Keywords: Enzyme inhibitors / Phosphinic acids and derivatives / Resolution
A series of phosphinic derivatives designed to inhibit aminopeptidase N (EC 3.4.11.2) were synthesized, to act as “transition state analogs”. They have potent inhibitory activities on APN with Ki values in the nanomolar range, and are highly selective versus other metallopeptidases.
References
- 1.Maroux S., Louvard D., Barrati J. Biochem. Biophys. Acta. 1973;321:282–295. doi: 10.1016/0005-2744(73)90083-1. [DOI] [PubMed] [Google Scholar]
- 2.Zini S., Fournié-Zaluski M.C., Chauvel E., Roques B.P., Corvol P., Llorens-Cortes C. 6th Edition. Vol. 93. 1996. pp. 11968–11973. (Proc. Natl. Acad. Sci. U.S.A.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montiel J.L., Cornille F., Roques B.P., Noble F. J. Neurochem. 1997;68:354–361. doi: 10.1046/j.1471-4159.1997.68010354.x. [DOI] [PubMed] [Google Scholar]
- 4.Roques B.P., Noble F., Daugé V., Fournié-Zaluski M.C., Beaumont A. Pharmacol. Rev. 1993;45:87–146. [PubMed] [Google Scholar]
- 5.Look A.T., Ashmun R.A., Shapiro L.H., Peiper S.C. J. Clin. Invest. 1989;83:1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delmas B., Gelfi J., L'Haridon R., Vogel L.K., Sjöström H., Norén O., Laude H. Nature. 1992;357:417–420. doi: 10.1038/357417a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeager C.L., Ashmun R.A., Williams R.K., Cardellichio C.B., Shapiro L.H., Look A.T., Holmes K.V. Nature. 1992;357:420–422. doi: 10.1038/357420a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saiki I., Fujii H., Yoneda J., Abe F., Nakajima M., Tsuruo T., Azuma I. Int. J. Cancer. 1993;54:137–143. doi: 10.1002/ijc.2910540122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menrad A., Speicher D., Wacker J., Herlyn M. Cancer Res. 1993;53:1450–1455. [PubMed] [Google Scholar]
- 10.Wetterholm A., Medina J.F., Radmark O., Shapiro R., Haeggström J., Vallee B.L., Samuelesson B. 6th Edition. Vol. 89. 1992. pp. 9141–9145. (Proc. Natl. Acad. Sci. U.S.A.). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang J., Cooper M. 6th Edition. Vol. 90. 1993. pp. 1222–1226. (Proc. Natl. Acad. Sci. U.S.A.). [Google Scholar]
- 12.Vazeux G., Wang J., Corvol P., Llorens-Cortès C. J. Biol. Chem. 1996;271:9069–9074. doi: 10.1074/jbc.271.15.9069. [DOI] [PubMed] [Google Scholar]
- 13.Matthews B.W. Acc. Chem. Res. 1988;21:333–340. [Google Scholar]
- 14.Umezawa H., Aoyagi T., Suda H., Hamada M., Takeuchi T. J. Antibiot. 1976;29:97–99. doi: 10.7164/antibiotics.29.97. [DOI] [PubMed] [Google Scholar]
- 15.Aoyagi T., Tobe H., Kojima F., Hamada M., Takeuchi T., Umezawa H. J. Antibiot. 1978;31:636–638. doi: 10.7164/antibiotics.31.636. [DOI] [PubMed] [Google Scholar]
- 16.Chung M.C., Chun H.K., Han K.H., Lee H.J., Lee C.H., Kho Y.H. J. Antibiot. 1996;49:99–102. doi: 10.7164/antibiotics.49.99. [DOI] [PubMed] [Google Scholar]
- 17.Umezawa H., Aoyagi T., Tanaka T., Suda H., Okuyama A., Naganawa H., Hamada M., Takeuchi T. J. Antibiot. 1985;38:1629–1630. doi: 10.7164/antibiotics.38.1629. [DOI] [PubMed] [Google Scholar]
- 18.Baker J.O., Wilker S.H., Bayliss M.E., Prescott J.M. Biochem. J. 1983;22:2089–2103. [Google Scholar]
- 19.Fournié-Zaluski M.C., Coric P., Turcaud S., Bruetschy L., Lucas E., Noble F., Roques B.P. J. Med. Chem. 1992;35:1259–1266. doi: 10.1021/jm00085a013. [DOI] [PubMed] [Google Scholar]
- 20.Shenvi A.B. Biochemistry. 1986;25:1286–1291. doi: 10.1021/bi00354a014. [DOI] [PubMed] [Google Scholar]
- 21.Lejczak B., Kafarski P., Zygmunt J. Biochemistry. 1989;28:3549–3555. doi: 10.1021/bi00434a060. [DOI] [PubMed] [Google Scholar]
- 22.Andersson L., Isley T.C., Wolfenden R. Biochemistry. 1982;21:4177–4180. doi: 10.1021/bi00260a040. [DOI] [PubMed] [Google Scholar]
- 23.Ocain T.D., Rich D.H. J. Med. Chem. 1988;31:2193–2199. doi: 10.1021/jm00119a022. [DOI] [PubMed] [Google Scholar]
- 24.Gordon E.M., Godgrey J.D., Delaney N.G., Asaad M.M., Von Langen D., Cushman D.W. J. Med. Chem. 1988;31:2199–2211. doi: 10.1021/jm00119a023. [DOI] [PubMed] [Google Scholar]
- 25.Harbeson S.L., Rich D.H. J. Med. Chem. 1989;32:1378–1392. doi: 10.1021/jm00126a039. [DOI] [PubMed] [Google Scholar]
- 26.Bouboutou R., Waksman G., Devin J., Fournié-Zaluski M.C., Roques B.P. Life Sci. 1984;35:1023–1030. doi: 10.1016/0024-3205(84)90669-6. [DOI] [PubMed] [Google Scholar]
- 27.Schalk C., d'Orchymont H., Jauch M.F., Tarnus C. Arch. Biochem. Biophy. 1994;31:42–46. doi: 10.1006/abbi.1994.1206. [DOI] [PubMed] [Google Scholar]
- 28.Miyachi H., Kato M., Kato F., Hashimoto Y. J. Med. Chem. 1998;41:263–265. doi: 10.1021/jm970624o. [DOI] [PubMed] [Google Scholar]
- 29.Schechter I., Berger A. Biochem. Biophys. Res. Commun. 1967:157–162. doi: 10.1016/s0006-291x(67)80055-x. [DOI] [PubMed] [Google Scholar]
- 30.Caldwell C.G., Sahoo S.P., Polo S.A., Eversole R.R., Lanza T.J., Mills S.G., Niedzwiecki L.M., Izquierdo-Martin M., Chang B.C., Harrison R.K., Kuo D.W., Lin T.Y., Stein R.L., Durette P.L., Hagmann W.K. Bioorg. Med. Chem. Lett. 1996;6(3):323–328. [Google Scholar]
- 31.Xie J., Soleilhac J.M., Schmidt C., Peyroux J., Roques B.P., Fournié-Zaluski M.C. J. Med. Chem. 1989;32:1497–1503. doi: 10.1021/jm00127a017. [DOI] [PubMed] [Google Scholar]
- 32.Baylis E.K., Campbell C.D., Dingwall J.G. J. Chem. Soc. 1984:2845–2853. [Google Scholar]
- 33.Fournié-Zaluski M.C., Lucas-Soroca E., Devin J., Roques B.P. J. Med. Chem. 1986;29:751–757. doi: 10.1021/jm00155a027. [DOI] [PubMed] [Google Scholar]
- 34.Chen H., Noble F., Coric P., Fournié-Zaluski M.C., Roques B.P. 6th Edition. Vol. 95. 1998. pp. 12028–12033. (Proc. Natl. Acad. Sci. USA). [DOI] [PMC free article] [PubMed] [Google Scholar]


