Abstract
Purpose of review
The primary purpose of this review is to summarize current literature in the field of vestibular regeneration with a focus on recent developments in molecular and gene therapies.
Recent findings
Since the discovery of limited vestibular hair cell regeneration in mammals in the 1990s, many elegant studies have improved our knowledge of mechanisms of development and regeneration of the vestibular system. A better understanding of the developmental pathways of the vestibular organs has fueled various biological strategies to enhance regeneration, including novel techniques in deriving vestibular hair cells from embryonic and induced pluripotent stem cells. In addition, the identification of specific genetic mutations responsible for vestibular disorders has opened various opportunities for gene replacement therapy.
Summary
Vestibular dysfunction is a significant clinical problem with limited therapeutic options, warranting research on biological strategies to repair/regenerate the vestibular organs to restore function. The use of gene therapy appears promising in animal models of vestibular dysfunction.
Keywords: gene therapy, genetic disorders, regeneration, vestibular, vestibulopathy
INTRODUCTION
Vestibular dysfunction affects approximately 30% of those aged 60 years and older and as high as 50% of those over 85 [1,2]. More than 90 million Americans suffer from vestibular dysfunction, and the prevalence is expected to rise with the aging population [3]. This sensory disorder impairs activities of daily living and contributes to anxiety, depression, and an overall diminished quality of life [4]. One study of over 4000 patients at 618 centers in 13 countries showed that only half of people with vestibular disorders were employed; 70% of those employed had reduced workloads and 63% had lost working days because of their symptoms [5]. Moreover, vestibular dysfunction ranks among the most common reasons for emergency room visits, significantly contributing to the disability burden in the elderly population [5]. Finally, vestibular dysfunction is a strong predictor of falls, which is the leading cause of accidental death in patients [6].
Possible causes of vestibular dysfunction include ototoxins (e.g., aminoglycosides), viral infections, genetic diseases, Meniere’s disease, and benign paroxysmal positional vertigo, with many others being idiopathic and likely linked to aging [7] (Fig. 1). Despite a compensatory process weeks after the onset of vestibular dysfunction, presumably mediated by the central nervous system, disabling symptoms may persist particularly when vestibular insults are bilateral [8,9]. Although vestibular rehabilitation can help alleviate symptoms, there are currently no biological treatments for vestibular dysfunction including hypofunction [10]. Several groups are currently exploring the potential utility of vestibular implants, including a clinical trial that is currently under way (ClinicalTrials.gov Identifier: NCT02725463).
FIGURE 1.
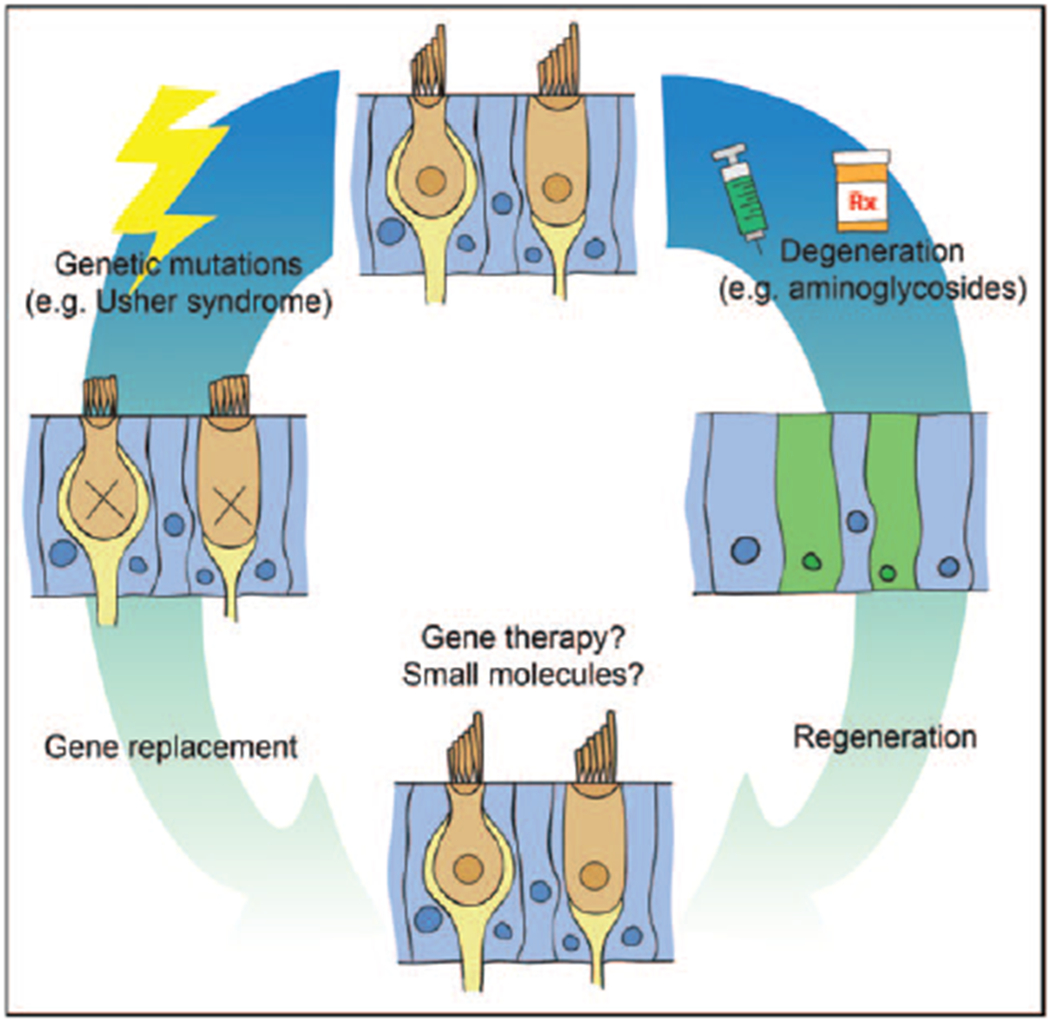
Molecular therapy for vestibular dysfunction. Schematic of possible strategies for molecular therapies for vestibular dysfunction.
In recent years, numerous studies have advanced our understanding of vestibular disorders, particularly those caused by genetic mutations or ototoxin-induced degeneration. Biological strategies consisting of molecular and gene therapies show promising results albeit with associated risks and limitations. Here, we will review these studies and evaluate their potentials as possible therapies.
MOLECULAR THERAPY TO REGENERATE VESTIBULAR ORGANS
The most common underlying abnormality in patients suffering from vestibular dysfunction is hair cell loss [11,12]. Although symptoms of vestibular dysfunction can be partly relieved by central compensation/rehabilitation, vestibular hypofunction is presumed irreversible. Among the five pairs of vestibular organs, the gravity-detecting utricle is the most extensively studied, where a limited degree of hair cell regeneration has been observed in mammals [13–18]. However, the current prevailing notion is that function is not restored. This starkly contrasts nonmammalian sensory organs such as the avian auditory and vestibular organs, which are capable of repairing and restoring function after damage through hair cell regeneration via mitotic and nonmitotic mechanisms [19,20]. Based on these findings, regeneration efforts in mammals have focused on promoting cell cycle reentry and hair cell differentiation.
The basic helix-loop-helix transcription factor Atoh1 is one of the earliest markers for differentiating hair cells and is necessary and sufficient for hair cell specification [21,22]. In the immature cochlea of multiple mammalian species, Atoh1 gene transfer produces extranumerary hair cells, some of which display mechano-sensitive hair bundles and integrate neurally when generated early during development [23–27]. However, the efficacy of Atoh1 in mice is significantly diminished after the onset of hearing, and maturation of these ectopic hair cells appears stunted [24,25,28]. Two studies in the adult guinea pig cochlea have shown that Atoh1 overexpression can promote hair cell formation and partial recovery of hearing after noise damage [26,29]. Unfortunately, other groups found less convincing evidence for either hair cell regeneration or functional restoration [25,26,28,29]. Although an age-related decline in its responsiveness is well accepted, whether Atoh1 alone can induce hair cell regeneration in the mature mammalian cochlea is still debatable.
Similar to the cochlea, the postnatal mouse utricle also displays a decrease in responsiveness to Atoh1 overexpression with age [30]. In mice younger than 3 weeks old, forced expression of Atoh1 using a transgenic approach induced extranumerary hair cell formation in the central striolar region of the utricle as well as the surrounding nonsensory transitional epithelium. However, Atoh1-induced ectopic hair cell formation was not detected at later ages, in contrast to a previous study where gene transfer of Atoh1-induced hair cell regeneration in the adult utricle [31].
Many Atoh1 targets identified both inside and outside the inner ear are associated with numerous signaling pathways including Notch, Wnt, and Shh [32,33■]. Both Notch and Wnt signaling play critical roles in hair cell formation during development, and their roles during vestibular regeneration are beginning to be revealed in recent years. In the neonatal utricle, damage activated the Wnt target gene Lgr5 in striolar supporting cells, which regenerated hair cells both mitotically and nonmitotically. Furthermore, constitutive activation of the Wnt pathway through stabilization of β-catenin increased mitosis and hair cell regeneration [18]. Similarly, small molecule Wnt activators stimulated supporting cell proliferation, which was further increased when combined with inhibitors of Notch signaling (γ-secretase inhibitors) [34]. Although Notch inhibition has been shown to induce ectopic hair cells in both the neonatal and mature utricle [17,35], its interaction with Wnt signaling to promote amplified proliferation and hair cell formation has only recently been identified in studies of the neonatal mouse utricle and cochlea [34,36,37■]. Although these data suggest that combination therapies to stimulate vestibular hair cell regeneration may be promising, whether they are effective in the mature organ is currently unclear and warrants further examination. Moreover, understanding the genetic landscape of the regenerating utricles from nonmammalian and mammalian species is an area of active investigation and should aid in the discovery of novel candidate genes that promote mammalian hair cell survival and regeneration.
Another major advance in vestibular hair cell regeneration is the in vitro generation of “mini-ears”: inner ear organoids derived from embryonic stem cells, fetal auditory stem cells, and induced pluripotent stem cells [38–40,41■■]. These organoids serve as “inner ears-in-a-dish” that can facilitate the study of inner ear biology, such as potential drug discovery for hair cell regeneration. Hashino and colleagues reported the use of a self-organizing three-dimensional culture system for mouse and human embryonic stem cells [39,41■■,42]. They modulated multiple signaling pathways (TGF, BMP, FGF, and Wnt) to generate multiple organoids over the course of several months in vitro. The inner ear organoids initially resembled developing otic vesicles and subsequently contained cells reminiscent of vestibular hair cells. These newly generated hair cell-like cells had morphological, molecular, and functional properties resembling native vestibular hair cells in the postnatal mice. Importantly, this novel protocol of hair cell induction from stem cells was more efficient than previous methods [38,43], and as such, is capable of accelerating future studies.
Recently, a combination of small molecules was found to stimulate proliferation and organoid formation from supporting cells in neonatal mouse cochleae and, to a limited extent, mature mouse and primate inner ear tissues [44■■]. These clonally expanded organoids generated a much higher yield of hair cells than previous reports using other culture techniques [45,46]. Based on these results, the first in-human study using a combination of small molecules (FX-322) designed to stimulate inner ear regeneration is under way (ClinicalTrials.gov Identifier: NCT03300687). This clinical trial lays the groundwork for future trials in patients suffering from hearing loss by showing first and foremost whether FX-322 is well tolerated at an effective dose to restore hearing in humans.
GENE THERAPY
More than 300 genetic loci have been implicated in hearing loss, about 70 of which have their causative gene identified [47]. Of these, gene therapy can potentially replace missing genes or silence erroneous genes in target cells to restore function. Various delivery methods, viral and nonviral vectors, and target genes have been explored in animal models with potential future clinical applications [48]. Although many genes associated with hearing loss have been identified, only a few are known to cause vestibular dysfunction.
Early gene therapy work in the inner ear has focused on protection, repair, and regeneration of hair cells and the auditory nerves. In recent years, studies on gene therapy involving the inner ear have mainly focused on mouse models of Usher syndrome, which is the leading cause of blindness, deafness, and vestibular dysfunction and is associated with several defined genetic mutations. Using an adeno-associated virus (AAV) to deliver gene products to vestibular (and cochlear) hair cells in an Usher2d mouse model, Chien and colleagues successfully restored morphology to distorted stereociliary bundles as a result of Whirlin mutations and increased hair cell survival in both the cochlea and utricle [49,50■■]. Remarkably, the improvement in balance function of treated animals lasted months. Emptoz and colleagues took a similar approach in the Usherlg mouse model and also found that replenished gene and protein expression led to improvement in hair cell function and overall vestibular function [51■■]. However, a shortcoming noted with using certain AAVs was limited transfection rates among cochlear hair cells, thus limiting use of this approach as a means to rescue auditory function. Each viral vector has its own characteristic time of onset, duration of gene expression, and cellular tropism, which provides a range of options in terms of use; however, clinical use is limited by toxicity and immunoreactivity.
To overcome the obstacle of limited transfection of cochlear hair cells, Pan and colleagues employed a synthetic adeno-associated viral vector, Anc80L65, for gene transfer in an Usher1c mouse model. They found significantly higher transfection rates of hair cells, resulting in rescue of both vestibular and cochlear hair cells and also balance and auditory functions [52■].
It is important to note that these studies discussed above mainly relied on gene inoculation prior to maturation of the auditory and vestibular systems in the neonatal mice, an age equivalent to the first trimester in humans. In adult mice, AAV introduced via canalostomy transduced primarily inner hair cells and a few outer hair cells, with hearing function and sensory cells preserved [53]. On the other hand, two other groups found that viral vehicles achieved high transduction efficiency in most sensory cell types in the auditory and vestibular organs [54■,55■]. These vehicles may be valuable in testing the efficacy of viral-mediated gene therapy in the mature vestibular system.
Nonviral gene delivery methods have also been explored, which can avoid the potential side-effects of viral vectors including immunoreactivity. Using antisense oligonucleotides to correct defective pre-mRNA splicing in another Usher1c mouse model, Lentz and colleagues rescued vestibular and cochlear hair cells leading to improved vestibular behavioral function [56]. This group subsequently showed that this approach in neonatal mice led to improved vestibular physiology; however, its effectiveness was minimal when administered to juvenile mice, suggesting that the therapeutic window may be rather limited [57■].
Emerging evidence suggests that another promising method is the CRISPR/Cas-based genome-editing technique, which aims to restore wild-type sequences in the mutated genome. With local treatment with a lipid-mediated delivery of Cas9-single guide RNA ribonucleotide protein complexes, hair cell survival and hearing markedly improved in an autosomal dominant single point mutation hearing loss mouse model [58■■].
Although these studies provide strong evidence that biological therapies to treat genetic causes of human deafness and balance disorders are highly feasible, most have found significantly reduced efficacy of gene therapy in both the auditory and vestibular systems of adult mice. Finally, many forms of genetic hearing and vestibular loss affect inner ear nonsensory cell types; thus, another major challenge for the future is to effectively deliver gene products to multiple cell types and to uncover the disease-specific therapeutic window for gene delivery (i.e., before cellular degeneration).
CONCLUSION
The potential therapeutic application of molecular and gene therapies to restore hearing loss and vestibular dysfunction is considerable. In addition to discoveries of potential new therapies to induce new hair cells, there have also been major advances in scientific tools, which will facilitate future studies and our march toward a biological treatment for inner ear diseases.
KEY POINTS.
The immature mouse utricle can mitotically and nonmitotically regenerate hair cells.
The immature mouse utricle is more responsive to several manipulations than the mature organ, including Atoh1 and Wnt activation.
Inner ear organoids contain mechano-sensitive hair cells resembling native vestibular hair cells.
Gene replacement therapy can successfully repair auditory and vestibular hair cells and preserve organ function in genetic mouse models.
Acknowledgements
We thank T. Jan and P. Atkinson for insightful comments.
Financial support and sponsorship
This work is supported by Stanford Medical Scientist Training Program and NIH/NIDCD F30DC015698 (Z.N.S.), AAOHNS resident research grant (G.S.K.), T32DC015209, California Institute in Regenerative Medicine RN3-06529, and NIDCD/NIHRO1DC013910 (A.G.C.).
A.G.C serves on the scientific advisory board of Decibel.
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
■ of special interest
■■ of outstanding interest
- 1.Jonsson R, Sixt E, Landahl S, Rosenhall U. Prevalence of dizziness and vertigo in an urban elderly population. J Vestib Res 2004; 14:47–52. [PubMed] [Google Scholar]
- 2.Barin K, Dodson EE. Dizziness in the elderly. Otolaryngol Clin North Am 2011; 44:437–454. [DOI] [PubMed] [Google Scholar]
- 3.Dillon CF, Gu Q, Hoffman HJ, Ko CW. Vision, hearing, balance, and sensory impairment in Americans aged 70 years and over: United States, 1999–2006. NCHS Data Brief 2010; 31:1–8. [PubMed] [Google Scholar]
- 4.Agrawal Y, Pineault KG, Semenov YR. Health-related quality of life and economic burden of vestibular loss in older adults. Laryngoscope Investig Otolaryngol 2018; 3:8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burt CW, Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1999–2000. Vital Health Stat 13 2004; 157:1–70. [PubMed] [Google Scholar]
- 6.Agrawal Y, Carey JP, Della Santina CC, et al. Disorders of balance and vestibular function in US adults: data from the National Health and Nutrition Examination Survey, 2001–2004. Arch Intern Med 2009; 169: 938–944. [DOI] [PubMed] [Google Scholar]
- 7.van de Berg R, van Tilburg M, Kingma H. Bilateral vestibular hypofunction: challenges in establishing the diagnosis in adults. ORL J Otorhinolaryngol Relat Spec 2015; 77:197–218. [DOI] [PubMed] [Google Scholar]
- 8.Dutia MB. Mechanisms of vestibular compensation: recent advances. Curr Opin Otolaryngol Head Neck Surg 2010; 18:420–424. [DOI] [PubMed] [Google Scholar]
- 9.Gillespie MB, Minor LB. Prognosis in bilateral vestibular hypofunction. Laryngoscope 1999; 109:35–41. [DOI] [PubMed] [Google Scholar]
- 10.Baloh RW. Vertigo in older people. Curr Treat Options Neurol 2000; 2:81–89. [DOI] [PubMed] [Google Scholar]
- 11.Rauch SD. Vestibular histopathology of the human temporal bone. What can we learn? Ann N Y Acad Sci 2001; 942:25–33. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji K, Velázquez-Villaseñor L, Rauch SD, et al. Temporal bone studies of the human peripheral vestibular system. Meniere’s disease. Ann Otol Rhinol Laryngol Suppl 2000; 181:26–31. [DOI] [PubMed] [Google Scholar]
- 13.Forge A, Li L, Corwin JT, Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science 1993; 259:1616–1619. [DOI] [PubMed] [Google Scholar]
- 14.Warchol ME, Lambert PR, Goldstein BJ, et al. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science 1993; 259:1619–1622. [DOI] [PubMed] [Google Scholar]
- 15.Kawamoto K, Izumikawa M, Beyer LA, et al. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear Res 2009; 247:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burns JC, Cox BC, Thiede BR, et al. In vivo proliferative regeneration of balance hair cells in newborn mice. J Neurosci 2012; 32:6570–6577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin V, Golub JS, Nguyen TB, et al. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci 2011; 31:15329–15339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Chai R, Kim GS, et al. Lgr5+ cells regenerate hair cells via proliferation and direct transdifferentiation in damaged neonatal mouse utricle. Nat Commun 2015; 6:6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carey JP, Fuchs AF, Rubel EW. Hair cell regeneration and recovery of the vestibuloocular reflex in the avian vestibular system. J Neurophysiol 1996; 76:3301–3312. [DOI] [PubMed] [Google Scholar]
- 20.Ryals BM, Rubel EW. Hair cell regeneration after acoustic trauma in adult Coturnix quail. Science 1988; 240:1774–1776. [DOI] [PubMed] [Google Scholar]
- 21.Bermingham NA, Hassan BA, Price SD, et al. Math1: an essential gene for the generation of inner ear hair cells. Science 1999; 284:1837–1841. [DOI] [PubMed] [Google Scholar]
- 22.Woods C, Montcouquiol M, Kelley MW. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat Neurosci 2004; 7:1310–1318. [DOI] [PubMed] [Google Scholar]
- 23.Gubbels SP, Woessner DW, Mitchell JC, et al. Functional auditory hair cells produced in the mammalian cochlea by in utero gene transfer. Nature 2008; 455:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelly MC, Chang Q, Pan A, et al. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci 2012; 32:6699–6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Z, Dearman JA, Cox BC, et al. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci 2012; 32:6600–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawamoto K, Ishimoto S, Minoda R, et al. Math1 gene transfer generates new cochlear hair cells in mature guinea pigs in vivo. J Neurosci 2003; 23:4395–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci 2000; 3:580–586. [DOI] [PubMed] [Google Scholar]
- 28.Atkinson PJ, Wise AK, Flynn BO, et al. Hair cell regeneration after ATOH1 gene therapy in the cochlea of profoundly deaf adult guinea pigs. PLoS One 2014; 9:e102077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izumikawa M, Minoda R, Kawamoto K, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med 2005; 11:271–276. [DOI] [PubMed] [Google Scholar]
- 30.Gao Z, Kelly MC, Yu D, et al. Spatial and age-dependent hair cell generation in the postnatal mammalian utricle. Mol Neurobiol 2016; 53:1601–1612. [DOI] [PubMed] [Google Scholar]
- 31.Schlecker C, Praetorius M, Brough DE, et al. Selective atonal gene delivery improves balance function in a mouse model of vestibular disease. Gene Ther 2011; 18:884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klisch TJ, Xi Y, Flora A, et al. In vivo Atoh1 targetome reveals how a proneural transcription factor regulates cerebellar development. Proc Natl Acad Sci U S A 2011; 108:3288–3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.■.Xie WR, Jen HI, Seymour ML, et al. An Atoh1-S193A phospho-mutant allele causes hearing deficits and motor impairment. J Neurosci 2017; 37:8583–8594. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study elegantly describes Atoh1 target genes and also the importance of Atoh1 dosage in preserving hearing function.
- 34.Wu J, Li W, Lin C, et al. Co-regulation of the Notch and Wnt signaling pathways promotes supporting cell proliferation and hair cell regeneration in mouse utricles. Sci Rep 2016; 6:29418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collado MS, Thiede BR, Baker W, et al. The postnatal accumulation of junctional E-cadherin is inversely correlated with the capacity for supporting cells to convert directly into sensory hair cells in mammalian balance organs. J Neurosci 2011; 31:11855–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Wu J, Yang J, et al. Notch inhibition induces mitotically generated hair cells in mammalian cochleae via activating the Wnt pathway. Proc Natl Acad Sci U S A 2014; 112:166–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.■.Atkinson PJ, Dong Y, Gu S, et al. Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea. J Clin Invest 2018; 128: 1641–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a previously unrecognized interaction with Sox2, damage and Wnt signaling in the neonatal mouse cochlea.
- 38.Oshima K, Shin K, Diensthuber M, et al. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell 2010; 141: 704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu XP, Koehler KR, Mikosz AM, et al. Functional development of mechanosensitive hair cells in stem cell-derived organoids parallels native vestibular hair cells. Nat Communs 2016; 7:11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W, Jongkamonwiwat N, Abbas L, et al. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature 2012; 490:278–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.■■.Koehler KR, Nie J, Longworth-Mills E, et al. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat Biotechnol 2017; 35:583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides important information on generating hair cell-bearing organoids derived from human pluipotent stem cells.
- 42.Koehler KR, Mikosz AM, Molosh AI, et al. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature 2013; 500: 217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ronaghi M, Nasr M, Ealy M, et al. Inner ear hair cell-like cells from human embryonic stem cells. Stem Cells Dev 2014; 23:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.■■.McLean WJ, Yin X, Lu L, et al. Clonal expansion of Lgr5-Positive cells from mammalian cochlea and high-purity generation of sensory hair cells. Cell Rep 2017; 18:1917–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterizes a growth factor cocktail that can effectively expand colonies of progenitor cells derived from the neonatal mouse cochlea.
- 45.Oshima K, Grimm CM, Corrales CE, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. J Assoc Res Otolaryngol 2007; 8:18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chai R, Kuo B, Wang T, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci U S A 2012; 109:8167–8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morton CC, Nance WE. Newborn hearing screening: a silent revolution. N Engl J Med 2006; 354:2151–2164. [DOI] [PubMed] [Google Scholar]
- 48.Ahmed H, Shubina-Oleinik O, Holt JR. Emerging gene therapies for genetic hearing loss. J Assoc Res Otolaryngol 2017; 18:649–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chien WW, Isgrig K, Roy S, et al. Gene therapy restores hair cell stereocilia morphology in inner ears of deaf whirler mice. Mol Ther 2016; 24:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.■■.Isgrig K, Shteamer JW, Belyantseva IA, et al. Gene therapy restores balance and auditory functions in a mouse model of usher syndrome. Mol Ther 2017; 25:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors used a virus-mediated gene replacement approach to preserve cochlear and vestibular haircells and auditory and vesitbular function in an Usher mouse model.
- 51.■■.Emptoz A, Michel V, Lelli A, et al. Local gene therapy durably restores vestibular function in a mouse model of Usher syndrome type 1G. Proc Natl Acad Sci U S A 2017; 114:9695–9700. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a gene therapy approach to treat inner ear hair cell loss and dysfunction causing by Usher syndrome.
- 52.■.Pan B, Askew C, Galvin A, et al. Genetherapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat Biotechnol 2017; 35:264–272. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes a gene therapy approach that successfully preserved hair cell integrity and also auditory and vestibular function in an Usher mouse model.
- 53.Tao Y, Huang M, Shu Y, et al. Delivery of adeno-associated virus vectors in adult mammalian inner-ear cell subtypes without auditory dysfunction. Hum Gene Ther 2018; 29:492–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.■.Suzuki J, Hashimoto K, Xiao R, et al. Cochlear gene therapy with ancestral AAV in adult mice: complete transduction of inner hair cells without cochlear dysfunction. Sci Rep 2017; 7:45524. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterizes viral vehicles that efficiently transduced inner ear cell types in the mature mouse cochlear and vestibular systems.
- 55.■.Yoshimura H, Shibata SB, Ranum PT, Smith RJ. Enhanced viral-mediated cochlear gene delivery in adult mice by combining canal fenestration with round window membrane inoculation. Sci Rep 2018; 8:2980. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors applied viruses that successfully transfected the mature cochlear and vestibular systems.
- 56.Lentz JJ, Jodelka FM, Hinrich AJ, et al. Rescue of hearing and vestibular function by antisense oligonucleotides in a mouse model of human deafness. NatMed 2013; 19:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.■.Vijayakumar S, Depreux FF, Jodelka FM, et al. Rescue of peripheral vestibular function in Usher syndrome mice using a splice-switching antisense oligonucleotide. Hum Mol Genet 2017; 26:3482–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study describes an age-dependent preservation of vestibular function by oligonucleotide treatment in the neonatal mice.
- 58.■■.Gao X, Tao Y, Lamas V, et al. Treatment of autosomal dominant hearing loss by in vivo delivery of genome editing agents. Nature 2018; 553:217–221. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study characterizes a genome editing approach that successfully improved hair cell survival and hearing function.


