Abstract
The facile preparation of three regioisomeric thienopyrrolo[3,2,1-jk]carbazoles applying a convenient C–H activation approach is presented. The incorporation of thiophene into the triarylamine framework significantly impacted the molecular properties in comparison to the analogous indolo[3,2,1-jk]carbazole scaffold. Dependent on the exact substitution pattern, the absorption onsets of the new materials are shifted toward slightly higher wavelengths compared to the analogous indolo[3,2,1-jk]carbazole, whereas the emission maxima of the sulfur derivatives is shifted from 375 to 410 nm. In analogy, the HOMO–LUMO energy gap of the thienopyrrolo[3,2,1-jk]carbazoles is reduced compared to indolo[3,2,1-jk]carbazole. Therefore, the developed thienopyrrolo[3,2,1-jk]carbazoles enrich the family of triarylamine donors and constitute a novel building block for functional organic materials.
The rapid development of functional organic materials and their applications (e.g., organic photovoltaic (OPV),1−4 organic field effect transistors (OFETs),5−9 organic light emitting diodes (OLEDs),1,7,10,11 sensing technology12,13) requires new molecular building blocks for the design and synthesis of novel organic compounds. The possibility to tune molecular properties as well as the macroscopic features (such as crystallization behavior, charge transport properties) by the subtle manipulation of molecular building blocks is essential in the development of new organic materials with tailor-made functionality.1,5,6,14−16
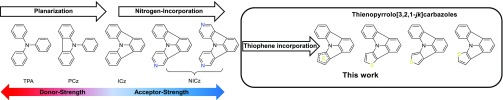
Recently we have introduced indolo[3,2,1-jk]carbazole (ICz) as a new molecular scaffold for the design of host materials for phosphorescent OLEDs.17−19 A convenient synthetic approach toward ICz based on C–H activation renders the widespread application of this molecular building block possible.17,20,21 ICz can be considered a fully planarized derivative of either triphenylamine (TPA) or N-phenylcarbazole (PCz)—triarylamine building blocks which are widely employed as electron donors.22 However, stepwise planarization of TPA decreases the electron-donating properties of the triarylamine, as the lone pair of the central nitrogen is incorporated into the aromatic system of one (PCz) or two (ICz) pyrrole rings (Scheme 1) and induces light electron-accepting properties. Accordingly, ICz can be considered a bipolar building block, and therefore creates opportunities for novel application of this molecular scaffold.17,18,23,24 The versatility of the ICz building block has been demonstrated by its application as a donor in push–pull photosensitizers for dye-sensitized solar cells.25−27 Furthermore, ICz has been employed as weak electron acceptor in thermally activated delayed fluorescent (TADF) emitters.28,29 However, the preparation of deep blue TADF emitters revealed the necessity to further increase the electron accepting properties of the ICz scaffold.29 Therefore, we developed a series of nitrogen substituted ICz derivatives (NICz, Scheme 1) and tuned the energy levels of the highest occupied molecular orbitals (HOMOs) and lowest unoccupied molecular orbitals (LUMOs) over a wide range.21
Scheme 1. Concept of Triarylamine Planarization as well as Replacement of Benzene Rings in the Indolo[3,2,1-jk]carbazole Scaffold with Pyridine or Thiophene.
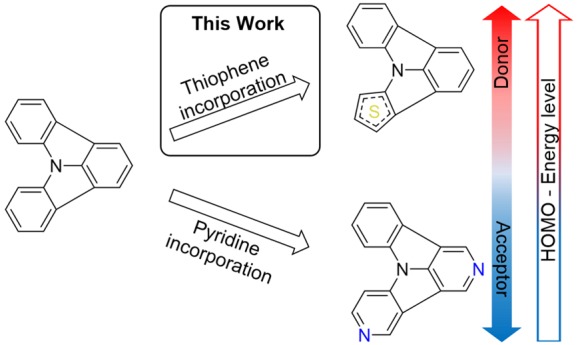
Inspired by these results, we set out to reverse the effect of increased electron deficiency by replacement of one benzene unit of the ICz scaffold with thiophene. Substitution of one benzene ring with thiophene should increase the electron-donating power and destabilize the HOMO level of the annulated system, yet retain planarity, which is an important feature for intermolecular interactions, the overall alignment, and thus interaction of individual molecules in the solid state.25,30 Accordingly, three novel thienopyrrolo[3,2,1-jk]carbazoles were prepared.
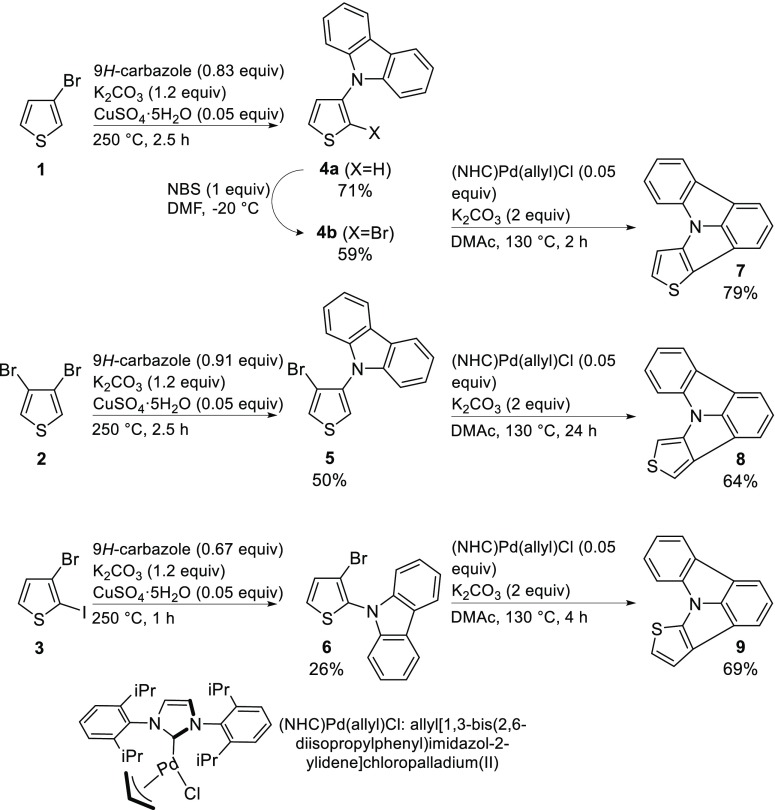
Ring-closing C–H activation has been chosen as the key step in the synthesis of the thienopyrrolo[3,2,1-jk]carbazoles. Therefore, the preparation of the respective precursors was required. These substrates were obtained by attaching a carbazole unit to different halogen substituted thiophenes (Scheme 2). The widespread availability of various halogen substituted thiophenes renders this strategy particularly attractive and provides a certain flexibility in the preparation of the different thienopyrrolo[3,2,1-jk]carbazole regioisomers. Two approaches were considered, employing Buchwald–Hartwig amination and Ullmann condensation.
Scheme 2. Synthetic Approach toward Thienopyrrolo[3,2,1-jk]carbazoles 7–9.
Several references regarding Buchwald–Hartwig reactions employing thiophenes can be found in the literature.31−35 However, employing various procedures no product could be obtained using thiophenes 1–3 in combination with carbazole. Thus, Ullmann condensation was explored as potential alternative. After screening various conditions, a modified protocol by Xu et al.36 was employed. Thiophenes 1–3 and carbazole were converted in a solvent-free Cu catalyzed condensation at 250 °C (Scheme 2). Following this approach carbazoles 5 and 6 were obtained. Although the yields were low (50 and 25%), the precursors for C–H activation could be prepared in one single step from readily available starting materials. Notably, the higher reactivity of iodine compared with bromine under the employed Ullmann condition and the favorable 2-position of thiophene enabled the selective preparation of 6 starting from 2-iodo-3-bromothiophene (3). Admittedly, an excess of thiophene had to be employed in the condensation to prevent overreaction, guarantee the formation of a solution and avoid the sublimation of carbazole. Starting from 2-bromo-3-iodothiophene no selectivity could be achieved in the Ullman condensation. Therefore, precursor 4b had to be prepared in two steps. After Ullman condensation of 3-bromothiophene (1) and carbazole, 4a could be selectively brominated to yield 4b.
Two different catalyst systems for the ring closing C–H activation were explored regarding their efficiency. The comparison of the conversion of 9 with (a) Pd(OAc)2 in combination with the N-heterocyclic carbene precursor [NHC]Cl and (b) Pd[NHC](allyl)Cl (Scheme 2) with K2CO3 in DMAc (N,N-dimethylacetamide) at 130 °C19,37,38 revealed the superiority of the preformed catalyst. Using preformed Pd[NHC](allyl)Cl, a yield of 69% was observed, whereas the yield was lower (54%) employing the salt of the ligand. Following this approach, the new thienopyrrolo[3,2,1-jk]carbazoles 7, 8, and 9 could be obtained in good yields of 79, 64, and 69%, respectively.
To investigate the effect of the sulfur incorporation as well as the influence of the fusion position on the molecular properties, photophysical and electrochemical characterizations were performed. Accordingly, UV–vis absorption and emission spectra at room temperature as well as low temperature phosphorescent emission spectra were recorded in deoxygenated DCM (dichloromethane; 5 μM) and toluene/iPrOH (10:1, 1 mg/mL). The results of these investigations are summarized in Figure 1 and compared with plain ICz. Obviously, different absorption characteristics, owing to the fusion position of the thiophene in the molecular framework can be observed, especially regarding π–π* transitions of the conjugated molecular scaffold in the region between 320 and 380 nm. Notably, the most prominent absorption peaks are found below 300 nm. In analogy to ICz (284 nm), 7, 8 and 9 feature distinct absorption peaks at 280, 284, and 283 nm. In structurally related PCz and ICz this transition can be attributed to a π–π* transition with a strong contribution of the lone pair of the central nitrogen atom.18,19 At longer wavelengths the absorption of 8 closely resembles that of ICz. The lowest energy transition of 8 is located at 368 nm with a shoulder at 354 nm. Additionally, one sharp peak can be observed at 314 nm accompanied by a smaller peak at somewhat lower wavelength (301 nm). The according transitions of ICz can be found at 363, 350, 320, and 308 nm. Therefore, it can be concluded that the annulation pattern of 8 does not significantly change the nature of the absorption transition compared to parent ICz but shifts the relative location of the energy levels. In contrast, 7 and 9 with the thiophene fused on the b face display different absorption properties between 320 and 380 nm. In the absorption spectrum of 7 two distinct peaks at 327 and 347 nm can be found, whereas 9 exhibits a weaker, rather broad and unstructured absorption in this region.
Figure 1.
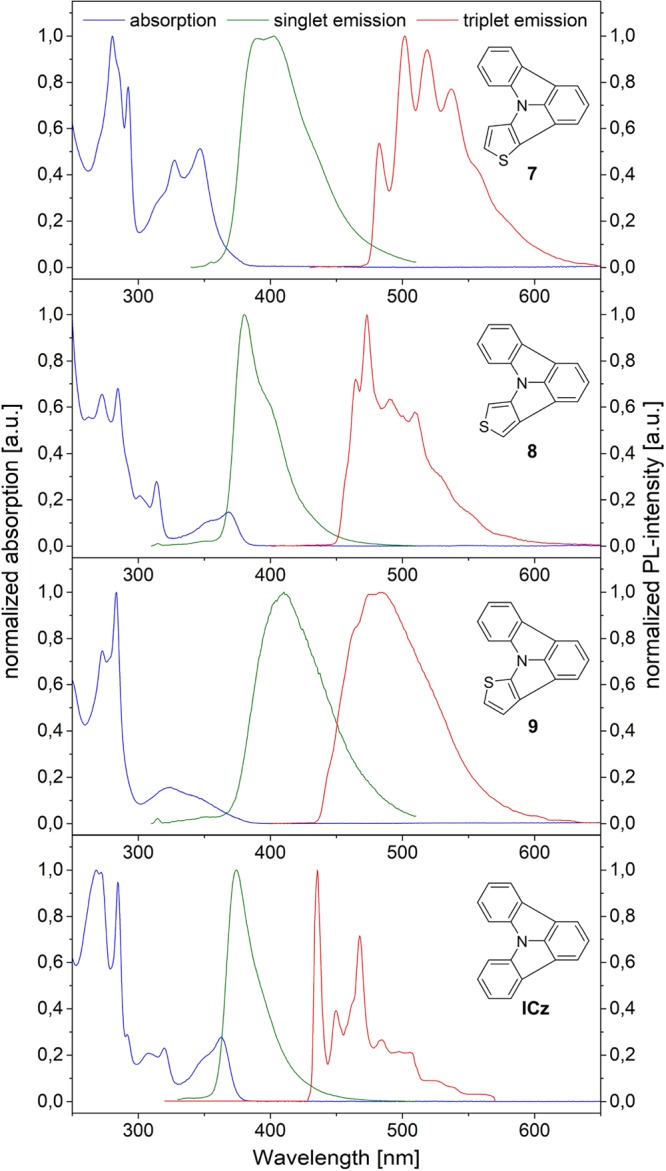
Normalized UV–vis absorption (blue), singlet emission (green), and triplet emission (red) of the target molecules and ICz.
Regarding the HOMO–LUMO energy gaps, thiophene incorporation has similar effects on the thienopyrrolo[3,2,1-jk]carbazoles. In analogy to the absorption profile, 8 exhibits the same HOMO–LUMO energy gap as ICz (3.30 eV). In the case of 7 and 9 the HOMO–LUMO energy gap is shifted toward lower energies of 3.25 and 3.22 eV. Notably, the incorporation of a thiophene subunit decreases the HOMO–LUMO energy gap of the ICz scaffold, which is the opposite effect of nitrogen incorporation.21 Therefore, the presented thienopyrrolo[3,2,1-jk]carbazoles are a valuable addition to the toolbox of ICz based building blocks for functional organic materials broadening the scope of this particular class of materials.
Compared to nonplanarized 3-(N,N-diphenylamino)thiophene39 and 9-(2-thienyl)-9H-carbazole40 the absorption of the developed thienopyrrolo[3,2,1-jk]carbazoles is significantly shifted toward higher wavelengths. 3-(N,N-Diphenylamino)thiophene features one broad absorption band around 290 nm. In contrast the absorption of 9-(2-thienyl)-9H-carbazole is more structured with an absorption maximum at 291 nm and two small bands at 322 and 333 nm, respectively. Notably, the absorption spectrum of planarized congener 9 qualitatively resembles that of 9-(2-thienyl)-9H-carbazole, but the low energy peaks of 9 are distinctively broader and red-shifted. Accordingly, the absorption onset of 9 at 385 nm is red-shifted compared to that of 9-(2-thienyl)-9H-carbazole (345 nm). The occurrence of the red-shifted absorption can be explained by an effective conjugation between the thiophene and carbazole and thus enlarged π-system, due to the planarization of the molecular scaffold. In 9-(2-thienyl)-9H-carbazole this conjugation is significantly decreased due to steric reasons.40
The fluorescence emission maxima of the newly developed compounds are shifted toward higher wavelengths compared with ICz and strictly follow the order of the HOMO–LUMO energy gaps. The emission of 8 resembles that of ICz with a maximum at 380 nm and a shoulder at longer wavelengths. Compound 7 exhibits two approximately equally intense emission peaks at 390 and 402 nm, whereas 9 features broader and unstructured emission with a maximum at 410 nm. In contrast to the singlet emission, 9 shows the highest triplet energy (ET) among the thienopyrrolo[3,2,1-jk]carbazoles. The triplet emission of 9 is broad and relatively unstructured with the highest energy shoulder at 2.79 eV. Red-shifted by about 13 nm, 8 has an ET of 2.71 eV. A substantially lower ET was determined for 7 (2.57 eV). Compared with ICz (2.84 eV), the triplet energies of the novel systems are somewhat lower. Nevertheless, the observed ET values are sufficiently high for potential use in light blue (8 and 9) and green (7) PhOLED devices.
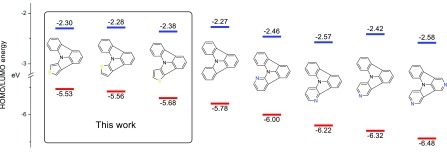
The exact energetic location of the frontier orbitals of organic materials is of enormous importance for charge injection and transport in electronic devices. Thus, the HOMO and LUMO levels of the target molecules were determined via cyclic voltammetry (Table S1). The investigated thienopyrrolo[3,2,1-jk]carbazoles exhibited irreversible oxidation, as typically found for indolo[3,2,1-jk]carbazoles and 9H-carbazole derivatives, owing to the instability of the radical cations formed.18,41 The observed HOMO–LUMO energy gaps are in good agreement with the optical measurements. Notably, the HOMO levels of the three regioisomers are significantly influenced by the position of the sulfur atom. The HOMO levels of 7, 8, and 9 are located at −5.68, −5.56, and −5.53 eV, and therefore are considerably higher compared with that of ICz (−5.78 eV). Strikingly, this tendency constitutes an ideal complementation for the nitrogen substitution, which generally lowers the HOMO energy level (Figure 2).21 Consequently, the development of the thienopyrrolo[3,2,1-jk]carbazole series allows to tune the HOMO energy levels of the ICz based building blocks over a wide range of 0.95 eV, which is of tremendous importance for the design of new materials with tailor-made molecular properties. Owing to a smaller HOMO–LUMO energy gap of the thienopyrrolo[3,2,1-jk]carbazoles, the LUMO levels of the target molecules at −2.38 eV(7), −2.28 eV (8), and −2.30 eV (9) are comparable to that of ICz (−2.27 eV).
Figure 2.
Schematic representation of the experimentally determined energy levels of HOMOs and LUMOs of the developed thienopyrrolo[3,2,1-jk]carbazoles as well as indolo[3,2,1-jk]carbazole and selected azaindolo[3,2,1-jk]carbazoles.21
The facile synthesis of three novel building blocks employing a previously refined C–H activation protocol was described. In addition to a straightforward synthesis, photophysical and electrochemical characterization of the target molecules was performed. It could be shown that molecular properties such as HOMO energy levels and the HOMO–LUMO energy gap could be influenced by the incorporation of sulfur into the parent scaffold. Furthermore, a fine-tuning can be achieved by variation of the substitution position. These novel building blocks serve as a useful addition to an ever-growing toolbox for the development of organic electronics.
Experimental Section
Unless explicitly mentioned otherwise, all reagents from commercial suppliers were used without further purification. Thin layer chromatography (TLC) was performed using TLC-aluminum foil (Merck, silica gel 60 F254). Preparative column chromatography was performed using a Büchi SepacoreTM Flash system. The appropriate PP-cartridges were packed with silica gel (Merck, 40–63 μm). Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Advance III HD 600 MHz spectrometer equipped with a cryoprobe Prodigy at 600.2 MHz (1H) and 150.9 MHz (13C). 1H- and 13C-spectra are given as stated: chemical shift in parts per million (ppm) referenced to the according solvent (1H: CDCl3 δ = 7.26 ppm, CD2Cl2 δ = 5.32 ppm; 13C: CDCl3 δ = 77.2 ppm, CD2Cl2 δ = 53.5 ppm) with tetramethylsilane (TMS) at δ = 0 ppm. Multiplicities of the signals are given as 1H: s = singlet, d = doublet, dd = doublet on doublet, ddd = doublet on doublet on doublet, dt = doublet on triplet t = triplet and m = multiplet. J-Modulated spin echo experiments were conducted to discern quaternary carbons from CH to facilitate characterization since only these two types of carbons are present in the described molecules. For better viewability quaternary carbons are depicted as negative and CH carbons as positive. Cyclic voltammetry was performed using a three-electrode configuration consisting of a Pt working electrode, a Pt counter electrode and a Ag/AgCl reference electrode and a PGSTAT128N potentiostat provided by Metrohm Autolab B.V. Measurements were carried out in a 0.5 mM or saturated (for poorly soluble substances) solution in anhydrous ACN with Bu4NBF4 (0.1 M) as supporting electrolyte. The solutions were purged with nitrogen for 15 min prior to measurement. HOMO and LUMO energy levels were calculated from the onset of oxidation and reduction, respectively. The onset potential was determined by the intersection of two tangents drawn at the background and the rising of oxidation or reduction peaks. Ferrocene was used for calibration. Absorption measurements were conducted using a PerkinElmer Lambda 750 spectrometer with degassed DCM solutions (5 μΜ) Fluorescence and phosphorescence spectra were recorded on a PerkinElmer LS 55 fluorescence spectrometer. For fluorescence measurements 5 μΜ degassed solutions in DCM were used. Phosphorescence spectra of 1 mg/mL solutions in degassed toluene:iPrOH (10:1) were recorded at 77 K. HRESIMS spectra (m/z 50–1900) were obtained on a maXis UHR ESI-Qq-TOF mass spectrometer (Bruker Daltonics, Bremen, Germany) in the positive-ion mode by direct infusion. The sum formulas of the detected ions were determined using Bruker Compass DataAnalysis 4.1 based on the mass accuracy (Δm/z ≤ 5 ppm) and isotopic pattern matching (SmartFormula algorithm).
9-(3-Thienyl)-9H-carbazole (4a)
3-Bromothiophene (1) (2.35 g, 14.4 mmol, 1.2 equiv), 9H-carbazole (2.71 g, 12 mmol, 1 equiv), K2CO3 (2.49 g, 14.4 mmol, 1.2 equiv) and CuSO4·5H2O (150 mg, 0.6 mmol, 0.05 equiv) were put in a reaction vial equipped with a stirring bar and flushed with argon three times. The vial was closed and put on a preheated heating block at 250 °C for 2.5 h. After this time the reaction was cooled to room temperature, the dark brown solid was dissolved in a mixture of DCM and H2O, the phases were separated, and the aqueous phase was extracted three times with DCM. The combined organic phases were washed once with brine, dried over Na2SO4, filtered, and the solvent removed in vacuo. The crude product was purified via column chromatography (LP/DCM 3%) and 4a was obtained as a light brown solid (2.134 g, 71%). 1H NMR (600 MHz, CDCl3) δ 8.15 (d, J = 7.7 Hz, 2H), 7.56 (dd, J = 5.0, 3.2 Hz, 1H), 7.50–7.42 (m, 5H), 7.34 (dd, J = 5.1, 1.4 Hz, 1H), 7.31 (ddd, J = 7.9, 6.7, 1.3 Hz, 2H). 13C NMR (151 MHz, CDCl3, J-MOD) δ 141.1, 136.0, 126.4, 126.2, 125.6, 123.3, 120.4, 120.1, 119.5, 110.1. Melting point 79–80 °C. HRMS (ESI) m/z calcd for C16H12NS+ [M + H]+ 250.0685, found 250.0684.
9-(2-Bromo-3-thienyl)-9H-carbazole (4b)
4a (249 mg, 1 mmol, 1 equiv) was dissolved in DMF (12 mL) and cooled to −20 °C with an ice/NaCl bath. While stirring, NBS (178 mg, 1 mmol, 1 equiv) was added slowly, keeping the temperature at −20 °C. After complete addition the solution was warmed to room temperature and stirred overnight. The reaction mixture was poured onto water and extracted three times with DCM. The combined organic phases were washed with brine, dried over Na2SO4, filtered, and the solvent removed under reduced pressure. Purification of the crude product was done by column chromatography (LP/DCM 3%) to yield the product 4b as an off white solid (193 mg, 59%). 1H NMR (600 MHz, CDCl3) δ 8.15 (d, J = 7.8 Hz, 2H), 7.51 (d, J = 5.7 Hz, 1H), 7.44 (ddd, J = 8.2, 7.0, 1.2 Hz, 2H), 7.34–7.29 (m, 2H), 7.23 (d, J = 8.2 Hz, 2H), 7.09 (d, J = 5.7 Hz, 1H). 13C NMR (151 MHz, CDCl3, J-MOD) δ 140.6, 135.6, 126.9, 126.6, 126.1, 123.5, 120.5, 120.3, 110.6, 110.5. Melting point 91–93 °C. HRMS (ESI) m/z calcd for C16H11BrNS+ [M + H]+ 327.9790, found 327.9790.
9-(4-Bromo-3-thienyl)-9H-carbazole (5)
3,4-Dibromothiophene (2) (1.06 g, 4.4 mmol, 1.1 equiv), 9H-carbazole (669 mg, 4 mmol, 1 equiv), K2CO3 (608 mg, 4.4 mmol, 1.1 equiv) and CuSO4·5H2O (50 mg, 0.2 mmol, 0.05 equiv) were put in a reaction vial equipped with a stirring bar and flushed with argon three times. The vial was closed and put on a preheated heating block at 250 °C for 2.5 h. After this time the reaction was cooled to room temperature, the dark brown solid was dissolved in a mixture of DCM and H2O, the phases were separated, and the aqueous phase was extracted three times with DCM. The combined organic phases were washed once with brine, dried over Na2SO4, filtered, and the solvent removed in vacuo. The crude product was purified via column chromatography (LP/DCM 3%) and 5 was obtained as an off-white solid (654 mg, 50%). 1H NMR (600 MHz, CDCl3) δ 8.15 (d, J = 7.8 Hz, 2H), 7.56 (d, J = 3.6 Hz, 1H), 7.53 (d, J = 3.6 Hz, 1H), 7.43 (ddd, J = 8.2, 7.1, 1.2 Hz, 2H), 7.31 (ddd, J = 8.0, 7.2, 1.0 Hz, 2H), 7.19 (d, J = 8.1 Hz, 2H). 13C NMR (151 MHz, CDCl3, J-MOD) δ 141.4, 135.2, 126.1, 124.2, 124.0, 123.4, 120.4, 120.3, 111.9, 110.3. Melting point 87–89 °C. HRMS (ESI) m/z calcd for C16H11BrNS+ [M + H]+ 327.9790, found 327.9789.
9-(3-Bromo-2-thienyl)-9H-carbazole (6)
3-Bromo-2-iodothiophene (3) (866 mg, 3 mmol, 1.5 equiv), 9H-carbazole (334 mg, 2 mmol, 1 equiv), K2CO3 (415 mg, 3 mmol,1.5 equiv) and CuSO4·5H2O (24 mg, 0.1 mmol, 0.05 equiv) were put in a reaction vial equipped with a stirring bar and flushed with Argon three times. The vial was closed and put on a preheated heating block at 250 °C for 1 h. After this time the reaction was cooled to room temperature, the dark brown solid was dissolved in a mixture of DCM and H2O, the phases were separated, and the aqueous phase was extracted three times with DCM. The combined organic phases were washed once with brine, dried over Na2SO4, filtered, and the solvent removed in vacuo. The crude product was purified via column chromatography (LP/DCM 3%) and 6 was obtained as an off-white solid (169 mg, 26%). 1H NMR (600 MHz, CD2Cl2) δ 8.14 (d, J = 7.7 Hz, 2H), 7.51 (d, J = 6.0 Hz, 1H), 7.46 (ddd, J = 8.3, 7.1, 1.2 Hz, 2H), 7.34 (ddd, J = 8.2, 7.2, 1.0 Hz, 2H), 7.25 (d, J = 8.1 Hz, 2H), 7.23 (d, J = 6.0 Hz, 1H). 13C NMR (151 MHz, CD2Cl2, J-MOD) δ 141.4, 133.7, 129.5, 126.4, 126.1, 123.7, 120.9, 120.3, 110.9, 110.4. Melting point 91–93 °C. HRMS (ESI) m/z calcd for C16H11BrNS+ [M + H]+ 327.9790, found 327.9788.
General Procedure for C–H Activation
A round-bottom flask was charged with the precursor (1 equiv), K2CO3 (2 equiv), (NHC)Pd(allyl)Cl (0.05 equiv), flushed with argon three times and the apparatus was assembled under argon counterflow. DMAc with a water content of 1000 ppm was deoxygenated with argon and added to the reaction apparatus under argon counterflow. The mixture was heated to 130 °C and stirred until completion. The reaction mixture was cooled to room temperature and poured onto water. The aqueous phase was extracted 3 times with DCM and the combined organic phases were washed once with brine, dried over Na2SO4, filtered, and the solvent removed in vacuo.
Thieno[2′,3′:4,5]pyrrolo[3,2,1-jk]carbazole (7)
The synthesis of 7 was performed according to the general procedure. Starting from 4b (799 mg, 2.43 mmol, 1 equiv), K2CO3 (672 mg, 4.86 mmol, 2 equiv) and (NHC)Pd(allyl)Cl (70 mg, 0.122 mmol, 0.05 equiv) crude product was obtained after a reaction time of 2h via column chromatography (LP/DCM 4%). The product was further purified by recrystallization from cyclohexane and via HPLC (n-heptane/i-PrOH 0.02% for 1 min to 0.1% over 10 min.) to yield 7 as a white solid (474 mg, 79%). 1H NMR (600 MHz, CDCl3) δ 8.10 (d, J = 7.7 Hz, 1H), 7.94 (d, J = 7.3 Hz, 1H), 7.84 (d, J = 7.5 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.54 (t, J = 7.4 Hz, 1H), 7.53–7.47 (m, 3H), 7.33 (d, J = 7.6 Hz, 1H). 13C NMR (151 MHz, CDCl3, J-MOD) δ 145.2, 141.2, 138.6, 129.6, 128.1, 126.8, 124.0, 123.2, 123.1, 121.8, 119.8, 118.4, 117.8, 117.5, 111.8, 111.5. Melting point 127 °C. HRMS (ESI) m/z calcd. for C16H10NS+ [M + H]+ 248.0528, found 248.0526.
Thieno[3′,4′:4,5]pyrrolo[3,2,1-jk]carbazole (8)
The synthesis of 8 was performed according to the general procedure. Starting from 5 (581 mg, 1.77 mmol, 1 equiv), K2CO3 (489 mg, 3.54 mmol, 2 equiv) and (NHC)Pd(allyl)Cl (51 mg, 0.089 mmol, 0.05 equiv) crude product was obtained after a reaction time of 24h via filtration over silica. The product was further purified via HPLC (n-heptane/i-PrOH 0.02% for 1 min to 0.1% over 10 min.) to yield 8 as a white solid (282 mg, 64%). 1H NMR (600 MHz, CDCl3) δ 8.11 (d, J = 7.8 Hz, 1H), 7.96 (d, J = 7.5 Hz, 1H), 7.79 (d, J = 7.3 Hz, 1H), 7.73 (d, J = 8.1 Hz, 1H), 7.57 (d, J = 2.2 Hz, 1H), 7.54 (ddd, J = 8.3, 7.5, 1.2 Hz, 1H), 7.46 (t, J = 7.4 Hz, 1H), 7.33 (ddd, J = 8.0, 7.5, 1.1 Hz, 1H), 7.04 (d, J = 2.3 Hz, 1H). 13C NMR (151 MHz, CDCl3, J-MOD) δ 151.7, 141.0, 138.2, 137.7, 128.4, 126.6, 123.0, 122.6, 121.2, 119.6, 119.0, 118.6, 116.3, 113.9, 111.6, 96.6. Melting point 147 °C. HRMS (ESI) m/z calcd for C16H10NS+ [M + H]+ 248.0528, found 248.0527.
Thieno[3′2′:4,5]pyrrolo[3,2,1-jk]carbazole (9)
The synthesis of 9 was performed according to the general procedure. Starting from 6 (488 mg, 1.49 mmol, 1 equiv), K2CO3 (412 mg, 2.98 mmol, 2 equiv) and (NHC)Pd(allyl)Cl (42 mg, 0.074 mmol, 0.05 equiv) crude product was obtained after a reaction time of 4 h via column chromatography (LP/DCM 4%). The product was further purified by recrystallization from cyclohexane and via HPLC (n-heptane/i-PrOH 0.02% for 1 min to 0.1% over 10 min.) to yield 9 as a white solid (255 mg, 69%). 1H NMR (600 MHz, CDCl3) δ 8.11 (d, J = 8.1 Hz, 1H), 7.93 (d, J = 7.3 Hz, 1H), 7.87 (d, J = 7.4 Hz, 1H), 7.71 (d, J = 8.0 Hz, 1H), 7.55 (t, J = 7.4 Hz, 1H), 7.53 (ddd, J = 7.9, 7.3, 1.2 Hz, 1H), 7.49 (d, J = 5.3 Hz, 1H), 7.35 (td, J = 7.6, 1.0 Hz, 1H), 7.08 (d, J = 5.3 Hz, 1H). 13C NMR (151 MHz, CDCl3, J-MOD) δ 146.4, 138.8, 138.2, 131.3, 130.0, 126.9, 123.3, 123.3, 122.2, 119.3, 119.2, 119.2, 118.6, 117.8, 117.4, 111.7. Melting point 124 °C. HRMS (ESI) m/z calcd for C16H10NS+ [M + H]+ 248.0528, found 248.0527.
Acknowledgments
J.F. and P.K. gratefully acknowledge financial support by the Austrian Science Fund (FWF) (grant No. I 2589-N34). This manuscript has been previously published in the ChemRxiv archive.42
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.9b02426.
NMR spectra; cyclic voltammograms; HRMS spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Cinar M. E.; Ozturk T. Thienothiophenes, Dithienothiophenes, and Thienoacenes: Syntheses, Oligomers, Polymers, and Properties. Chem. Rev. 2015, 115, 3036–3140. 10.1021/cr500271a. [DOI] [PubMed] [Google Scholar]
- Lin Y.; Li Y.; Zhan X. Small molecule semiconductors for high-efficiency organic photovoltaics. Chem. Soc. Rev. 2012, 41, 4245–4272. 10.1039/c2cs15313k. [DOI] [PubMed] [Google Scholar]
- Mishra A.; Bäuerle P. Small Molecule Organic Semiconductors on the Move: Promises for Future Solar Energy Technology. Angew. Chem., Int. Ed. 2012, 51, 2020–2067. 10.1002/anie.201102326. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-J.; Yang S.-H.; Hsu C.-S. Synthesis of Conjugated Polymers for Organic Solar Cell Applications. Chem. Rev. 2009, 109, 5868–5923. 10.1021/cr900182s. [DOI] [PubMed] [Google Scholar]
- Mei J.; Diao Y.; Appleton A. L.; Fang L.; Bao Z. Integrated Materials Design of Organic Semiconductors for Field-Effect Transistors. J. Am. Chem. Soc. 2013, 135, 6724–6746. 10.1021/ja400881n. [DOI] [PubMed] [Google Scholar]
- Jiang W.; Li Y.; Wang Z. Heteroarenes as high performance organic semiconductors. Chem. Soc. Rev. 2013, 42, 6113–6127. 10.1039/c3cs60108k. [DOI] [PubMed] [Google Scholar]
- Anthony J. E. Functionalized Acenes and Heteroacenes for Organic Electronics. Chem. Rev. 2006, 106, 5028–5048. 10.1021/cr050966z. [DOI] [PubMed] [Google Scholar]
- Anthony J. E. The Larger Acenes: Versatile Organic Semiconductors. Angew. Chem., Int. Ed. 2008, 47, 452–483. 10.1002/anie.200604045. [DOI] [PubMed] [Google Scholar]
- Wang C.; Dong H.; Hu W.; Liu Y.; Zhu D. Semiconducting π-Conjugated Systems in Field-Effect Transistors: A Material Odyssey of Organic Electronics. Chem. Rev. 2012, 112, 2208–2267. 10.1021/cr100380z. [DOI] [PubMed] [Google Scholar]
- Tao Y.; Yang C.; Qin J. Organic host materials for phosphorescent organic light-emitting diodes. Chem. Soc. Rev. 2011, 40, 2943–2970. 10.1039/c0cs00160k. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Mao Z.; Xie Z.; Zhang Y.; Liu S.; Zhao J.; Xu J.; Chi Z.; Aldred M. P. Recent advances in organic thermally activated delayed fluorescence materials. Chem. Soc. Rev. 2017, 46, 915–1016. 10.1039/C6CS00368K. [DOI] [PubMed] [Google Scholar]
- Li J.; Zhang Q. Linearly Fused Azaacenes: Novel Approaches and New Applications Beyond Field-Effect Transistors (FETs). ACS Appl. Mater. Interfaces 2015, 7, 28049–28062. 10.1021/acsami.5b00113. [DOI] [PubMed] [Google Scholar]
- Someya T.; Dodabalapur A.; Huang J.; See K. C.; Katz H. E. Chemical and Physical Sensing by Organic Field-Effect Transistors and Related Devices. Adv. Mater. 2010, 22, 3799–3811. 10.1002/adma.200902760. [DOI] [PubMed] [Google Scholar]
- Narita A.; Wang X.-Y.; Feng X.; Mullen K. New advances in nanographene chemistry. Chem. Soc. Rev. 2015, 44, 6616–6643. 10.1039/C5CS00183H. [DOI] [PubMed] [Google Scholar]
- Stȩpień M.; Gońka E.; Żyła M.; Sprutta N. Heterocyclic Nanographenes and Other Polycyclic Heteroaromatic Compounds: Synthetic Routes, Properties, and Applications. Chem. Rev. 2017, 117, 3479–3716. 10.1021/acs.chemrev.6b00076. [DOI] [PubMed] [Google Scholar]
- Uzelac E. J.; McCausland C. B.; Rasmussen S. C. Pyrrolo[2,3-d:5,4-d′]bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-Ring Bithiophenes. J. Org. Chem. 2018, 83, 664–671. 10.1021/acs.joc.7b02570. [DOI] [PubMed] [Google Scholar]
- Kautny P.; Lumpi D.; Wang Y.; Tissot A.; Bintinger J.; Horkel E.; Stöger B.; Hametner C.; Hagemann H.; Ma D.; Fröhlich J. Oxadiazole based bipolar host materials employing planarized triarylamine donors for RGB PHOLEDs with low efficiency roll-off. J. Mater. Chem. C 2014, 2, 2069–2081. 10.1039/C3TC32338B. [DOI] [Google Scholar]
- Kautny P.; Wu Z.; Eichelter J.; Horkel E.; Stöger B.; Chen J.; Ma D.; Fröhlich J.; Lumpi D. Indolo[3,2,1-jk]carbazole based planarized CBP derivatives as host materials for PhOLEDs with low efficiency roll-off. Org. Electron. 2016, 34, 237–245. 10.1016/j.orgel.2016.04.036. [DOI] [Google Scholar]
- Zhao C.; Schwartz T.; Stöger B.; White F. J.; Chen J.; Ma D.; Fröhlich J.; Kautny P. Controlling excimer formation in indolo[3,2,1-jk]carbazole/9H-carbazole based host materials for RGB PhOLEDs. J. Mater. Chem. C 2018, 6, 9914–9924. 10.1039/C8TC03537G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J.; Liu Q.; Tang J.; Perdih F.; Kranjc K. A facile synthesis of indolo[3,2,1-jk]carbazoles via palladium-catalyzed intramolecular cyclization. Tetrahedron Lett. 2012, 53, 5248–5252. 10.1016/j.tetlet.2012.07.093. [DOI] [Google Scholar]
- Kader T.; Stöger B.; Fröhlich J.; Kautny P. Azaindolo[3,2,1-jk]carbazoles: New Building Blocks for Functional Organic Materials. Chem. - Eur. J. 2019, 25, 4412. 10.1002/chem.201805578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirota Y.; Kageyama H. Charge carrier transporting molecular materials and their applications in devices. Chem. Rev. 2007, 107, 953–1010. 10.1021/cr050143+. [DOI] [PubMed] [Google Scholar]
- Im Y.; Han S. H.; Lee J. Y. Dibenzothiophene and indolocarbazole cored bipolar hosts for blue phosphorescent organic light-emitting diodes. Org. Electron. 2018, 62, 560–565. 10.1016/j.orgel.2018.06.031. [DOI] [Google Scholar]
- Im Y.; Han S. H.; Lee J. Y. Bipolar type indolocarbazole host for green phosphorescent organic light-emitting diodes. J. Ind. Eng. Chem. 2018, 66, 381–386. 10.1016/j.jiec.2018.06.004. [DOI] [Google Scholar]
- Hinkel F.; Kim Y. M.; Zagraniarsky Y.; Schlütter F.; Andrienko D.; Müllen K.; Laquai F. Efficiency-limiting processes in cyclopentadithiophene-bridged donor-acceptor-type dyes for solid-state dye-sensitized solar cells. J. Chem. Phys. 2018, 148, 044703 10.1063/1.4999136. [DOI] [PubMed] [Google Scholar]
- Luo C.; Bi W.; Deng S.; Zhang J.; Chen S.; Li B.; Liu Q.; Peng H.; Chu J. Indolo[3,2,1-jk]carbazole Derivatives-Sensitized Solar Cells: Effect of π-Bridges on the Performance of Cells. J. Phys. Chem. C 2014, 118, 14211–14217. 10.1021/jp503455m. [DOI] [Google Scholar]
- Cao W.; Fang M.; Chai Z.; Xu H.; Duan T.; Li Z.; Chen X.; Qin J.; Han H. New D-π-A organic dyes containing a tert-butyl-capped indolo[3,2,1-jk]carbazole donor with bithiophene unit as π-linker for dye-sensitized solar cells. RSC Adv. 2015, 5, 32967–32975. 10.1039/C5RA02720A. [DOI] [Google Scholar]
- Seo J.-A.; Im Y.; Han S. H.; Lee C. W.; Lee J. Y. Unconventional Molecular Design Approach of High-Efficiency Deep Blue Thermally Activated Delayed Fluorescent Emitters Using Indolocarbazole as an Acceptor. ACS Appl. Mater. Interfaces 2017, 9, 37864–37872. 10.1021/acsami.7b09351. [DOI] [PubMed] [Google Scholar]
- Im Y.; Han S. H.; Lee J. Y. Deep blue thermally activated delayed fluorescent emitters using CN-modified indolocarbazole as an acceptor and carbazole-derived donors. J. Mater. Chem. C 2018, 6, 5012–5017. 10.1039/C8TC00546J. [DOI] [Google Scholar]
- Kader T.; Stoger B.; Frohlich J.; Kautny P. The phase transitions of 4-aminopyridine-based indolocarbazoles: twinning, local- and pseudo-symmetry. Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater. 2019, 75, 97–106. 10.1107/S2052520618017341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M.; Yamamoto T.; Nishiyama M. Synthesis of novel (bis)(diarylamino)thiophenes via palladium-catalysed reaction of (di)bromothiophenes with diarylamines. Chem. Commun. 2000, 133–134. 10.1039/a908195j. [DOI] [Google Scholar]
- Ogawa K.; Radke K. R.; Rothstein S. D.; Rasmussen S. C. Synthesis of Secondary and Tertiary Aminothiophenes via Palladium-Catalyzed Amination. J. Org. Chem. 2001, 66, 9067–9070. 10.1021/jo016195q. [DOI] [PubMed] [Google Scholar]
- Luker T. J.; Beaton H. G.; Whiting M.; Mete A.; Cheshire D. R. Palladium catalysed amination of electron deficient halothiophenes. Tetrahedron Lett. 2000, 41, 7731–7735. 10.1016/S0040-4039(00)01307-1. [DOI] [Google Scholar]
- Kamimoto N.; Schollmeyer D.; Mitsudo K.; Suga S.; Waldvogel S. R. Palladium-Catalyzed Domino C–H/N–H Functionalization: An Efficient Approach to Nitrogen-Bridged Heteroacenes. Chem. - Eur. J. 2015, 21, 8257–8261. 10.1002/chem.201500897. [DOI] [PubMed] [Google Scholar]
- Hooper M. W.; Utsunomiya M.; Hartwig J. F. Scope and Mechanism of Palladium-Catalyzed Amination of Five-Membered Heterocyclic Halides. J. Org. Chem. 2003, 68, 2861–2873. 10.1021/jo0266339. [DOI] [PubMed] [Google Scholar]
- Xu H.; Yin K.; Huang W. Highly Improved Electroluminescence from a Series of Novel EuIII Complexes with Functional Single-Coordinate Phosphine Oxide Ligands: Tuning the Intramolecular Energy Transfer, Morphology, and Carrier Injection Ability of the Complexes. Chem. - Eur. J. 2007, 13, 10281–10293. 10.1002/chem.200700678. [DOI] [PubMed] [Google Scholar]
- Marion N.; Navarro O.; Mei J.; Stevens E. D.; Scott N. M.; Nolan S. P. Modified (NHC)Pd(allyl)Cl (NHC = N-Heterocyclic Carbene) Complexes for Room-Temperature Suzuki–Miyaura and Buchwald–Hartwig Reactions. J. Am. Chem. Soc. 2006, 128, 4101–4111. 10.1021/ja057704z. [DOI] [PubMed] [Google Scholar]
- Navarro O.; Nolan S. P. Large-Scale One-Pot Synthesis of N-Heterocyclic Carbene-Pd(allyl)Cl Complexes. Synthesis 2006, 2006, 366–367. 10.1055/s-2005-918497. [DOI] [Google Scholar]
- Lin J.; Ni X. Synthesis, structures, and electrochromic behaviors of poly(triarylamine)s based on 3-substituted thiophene derivatives. RSC Adv. 2015, 5, 14879–14886. 10.1039/C4RA16079G. [DOI] [Google Scholar]
- Kato S.-i.; Shimizu S.; Taguchi H.; Kobayashi A.; Tobita S.; Nakamura Y. Synthesis and Electronic, Photophysical, and Electrochemical Properties of a Series of Thienylcarbazoles. J. Org. Chem. 2012, 77, 3222–3232. 10.1021/jo202625p. [DOI] [PubMed] [Google Scholar]
- Mondal E.; Hung W.-Y.; Dai H.-C.; Wong K.-T. Fluorene-Based Asymmetric Bipolar Universal Hosts for White Organic Light Emitting Devices. Adv. Funct. Mater. 2013, 23, 3096–3105. 10.1002/adfm.201202889. [DOI] [Google Scholar]
- Bader D.; Fröhlich J.; Kautny P. Thienopyrrolocarbazoles: New Building Blocks for Functional Organic Materials. ChemRxiv 2019, 10.26434/chemrxiv.7777343.v1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.