Abstract
Background
Disseminated intravascular coagulation (DIC) is an acquired syndrome characterized by systemic intravascular activation of coagulation, leading to deposition of fibrin in the bloodstream. It may occur in patients with acute and chronic leukemia and is particularly associated with acute promyelocytic leukemia (a subtype of acute myeloid leukemia).
Objectives
To assess the clinical benefits and harms of any pharmacological intervention for treating DIC in patients with acute or chronic leukemia.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2015, Issue 05), MEDLINE (1946 to 7 May 2015), LILACS (1982 to 7 May 2015) and African Index Medicus (7 May 2015). There was no language restrictions. We sought additional randomized controlled trials (RCTs) from the World Health Organization International Clinical Trials Registry Platform and the reference lists of primary studies identified.
Selection criteria
RCTs assessing the clinical benefits and harms of interventions for treating DIC in patients with acute and chronic leukemia.
Data collection and analysis
Two review authors independently performed trial selection, 'Risk of bias' assessment and data extraction. Primary outcomes were overall mortality, in‐hospital mortality from any cause (15‐day and 30‐day) and adverse events.
Main results
In this Cochrane Review update we did not include any new RCT compared with the first review version. Accordingly, four RCTs (388 participants) met the inclusion criteria. These trials evaluated the human activated protein C, recombinant human soluble thrombomodulin, tranexamic acid and dermatan sulphate. Included trials reported data on mortality and bleeding. The studies were conducted in Japan, Italy and the Netherlands. We classified the included trials as: 1) including patients with or without leukemia which did not report data for the leukemia subgroup (366 participants); and 2) only including patients with leukemia (22 participants). Overall, the risk of bias of the included trials was high, since the trial authors did not provide a detailed description about trial design and execution.
According to the GRADE recommendations, we judged the overall quality of the body of evidence for all prefixed outcomes as 'very low', due to methodological limitations and very small sample size.
One trial, including 10 participants with leukemia and comparing dermatan sulphate with heparin, reported no deaths during trial treatment.
In terms of bleeding data, we were unable to pool results from two studies that were only conducted with leukemia patients due to the inconsistency in the measurement and reporting of this outcome. One trial, including 12 participants with leukemia, found very low quality evidence that tranexamic acid can reduce the cumulative hemorrhagic score in participants compared with those assigned to placebo (P = 0.0015, very low quality evidence). On the contrary, there is no evidence that dermatan sulphate compared with placebo reduces new events of hemorrhagic diathesis (1/5 (20%) versus 2/5 (40%); RR 0.50; 95% CI 0.06 to 3.91; P = 0.51, very low quality evidence).
No thromboembolic complications were reported in either trial that included patients with leukemia only (very low quality evidence). The safety profile was inconclusive.
The included trials did not assess overall mortality, resolution of respiratory failure, renal failure or shock.
Authors' conclusions
Due to a lack of new RCTs, our conclusions in this Cochrane Review update are the same as the previous review version. We included four RCTs which reported mortality and bleeding data. It is not possible to determine whether human activated protein C, recombinant human soluble thrombomodulin, tranexamic acid and dermatan sulphate are effective or harmful for patients presenting with DIC related to acute or chronic leukemia. The quality of the evidence was low to very low. Therefore, prescription of these interventions for treating DIC in patients with acute and chronic leukemia can neither be supported nor rejected, unless new evidence from a large high‐quality trial alters this conclusion.
Keywords: Humans, Acute Disease, Anticoagulants, Anticoagulants/therapeutic use, Chronic Disease, Dermatan Sulfate, Dermatan Sulfate/therapeutic use, Disseminated Intravascular Coagulation, Disseminated Intravascular Coagulation/drug therapy, Leukemia, Leukemia/blood, Leukemia/complications, Protein C, Protein C/therapeutic use, Randomized Controlled Trials as Topic, Thrombomodulin, Thrombomodulin/therapeutic use, Tranexamic Acid, Tranexamic Acid/therapeutic use
Plain language summary
Drug therapy for treating systemic intravascular activation of coagulation in patients with leukemia
Review question We reviewed the clinical benefits and harms of anticoagulant and antifibrinolytic therapy for treating disseminated intravascular coagulation (DIC) in patients with acute or chronic leukemia.
Background DIC is a thrombo‐hemorrhagic complication (involving blood clotting or bleeding) characterized by deposition of fibrin (a fibrous protein) in the bloodstream, which occurs in the course of various diseases including acute and chronic leukemia and is particularly associated with acute promyelocytic leukemia (a subtype of acute myeloid leukemia). DIC is never considered as an isolated clinical entity and represents a hematological emergency. Therefore, DIC requires treatment and resolution of the underlying disease in addition to other approaches, such as antibiotic therapy, replacement of blood products and fluid therapy, which are considered 'primary' or 'conventional care' interventions. DIC is accompanied by hyperfibrinolysis (increased dissolution of blood clots) and reduction of natural anticoagulants (proteins of the coagulation cascade). Hence, pharmacological interventions such as tranexamic acid (an antifibrinolytic agent) and heparin (an anticoagulant) have been used for treating patients with this acquired disorder. However, this practice is not considered as standard care and there is a lot of controversy about its clinical benefits.
Study characteristics
We included four trials that had a limited number of patients (388) and assessed four different interventions: human activated protein C, recombinant human soluble thrombomodulin, tranexamic acid and dermatan sulphate with heparin or placebo. Two trials included patients either with or without leukemia. The other two trials only included patients with leukemia. The studies were published between 1989 and 2007, and were conducted in Japan, Italy and the Netherlands. All trials have a high risk of bias.
Key results
There were no deaths reported in a trial comparing dermatan sulphate with heparin. Two small trials which included patients with leukemia only (22 participants) reported bleeding data. These results were not compiled due to inconsistency in the measurement and reporting of that outcome. One trial found very low quality evidence that tranexamic acid compared with placebo reduces bleeding in leukemia patients. On the contrary, there is no evidence that dermatan sulphate compared with placebo reduces new events of hemorrhagic diathesis in patients with leukemia. Saftey profile is inconclusive. No thromboembolic complications were reported in both the trials.
These trials did not report overall mortality, resolution of respiratory failure, renal failure and shock.
Accordingly, the clinical benefits and harms of the human activated protein C, recombinant human soluble thrombomodulin, tranexamic acid and dermatan sulphate are unknown in this population.
Quality of evidence
The confidence in the results of this review is very low. The studies have limitations in the way they were designed and executed. Moreover, the limited number of patients included in the studies led to imprecise results. Well conducted larger studies will provide more information about the effect of human activated protein C, recombinant human soluble thrombomodulin, tranexamic acid and dermatan sulphate for treating DIC in patients with acute or chronic leukemia.
This plain language summary is current as of 7 May 2015.
Search date: 7 May 2015
Summary of findings
Summary of findings for the main comparison. Human activated protein C versus heparin for DIC.
| Human activated protein C versus heparin for DIC | ||||||
| Patient or population: DIC Settings: acute or chronic leukemia patients Intervention: human activated protein C Comparison: heparin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Heparin | Human activated protein C | |||||
| Overall mortality | See comment | See comment | Not estimable | 132 (1 trial) | See comment | Included trial did not measure this outcome |
| Mortality from any cause (15‐day and 30‐day in‐hospital) Follow‐up: 28 days | 400 per 1000 | 204 per 1000 (108 to 388) | RR 0.51 (0.27 to 0.97) | 104 (1 trial) | ⊕⊝⊝⊝ very low1,2,3 | The included trial did not report disaggregated data for patients with leukemia |
| Adverse events Follow‐up: 28 days | 36 per 1000 | 19 per 1000 (2 to 206) | RR 0.53 (0.05 to 5.66) | 107 (1 trial) | ⊕⊝⊝⊝ very low1,2,4 | The included trial did not report disaggregated data for patients with leukemia |
| Bleeding Follow‐up: 28 days | 116 per 1000 | 7 per 1000 (0 to 126) | RR 0.06 (0 to 1.09) | 132 (1 trial) | ⊕⊝⊝⊝ very low1,2,5,6 | The included trial did not report disaggregated data for patients with leukemia. |
| Resolution of respiratory failure | See comment | See comment | Not estimable | — | See comment | Included trial did not measure this outcome |
| Renal failure | See comment | See comment | Not estimable | — | See comment | Included trial did not measure this outcome |
| Shock | See comment | See comment | Not estimable | — | See comment | Included trial did not measure this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; DIC: disseminated intravascular coagulation. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Indirectness: trial included patients with or without leukemia; and this outcome was not discriminated by type of patients. 2Limitations in execution: large loss (21.3%) to follow up could bias the reported estimates. Trial authors reported no reasons for attrition could bias the reported estimates. 3Imprecision: low sample size (104 participants and 32 events) resulting in wide 95% CIs. 4Imprecision: low sample size (107 participants and 3 events) resulting in wide 95% CIs. 5Imprecision: low sample size (132 participants and 8 events) resulting in wide 95% CIs. 6Quality of evidence rated and effect estimates obtained for aggravation of bleeding.
Summary of findings 2. Recombinant human soluble thrombomodulin compared with heparin for DIC.
| Recombinant human soluble thrombomodulin compared with heparin for DIC | ||||||
| Patient or population: DIC Settings: acute or chronic leukemia patients Intervention: recombinant human soluble thrombomodulin Comparison: heparin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Heparin | Recombinant human soluble thrombomodulin | |||||
| Overall mortality | See comment | See comment | Not estimable | 234 (1 trial) | See comment | Included trial did not measure this outcome |
| Mortality from any cause (15‐day and 30‐day in‐hospital) Follow‐up: 28 days | 257 per 1000 | 218 per 1000 (139 to 349) | RR 0.85 (0.54 to 1.36) | 227 (1 trial) | ⊕⊝⊝⊝ very low1,2 | The included trial did not report disaggregated data for patients with leukemia. |
| Adverse events Follow‐up: 28 days | 78 per 1000 | 69 per 1000 (27 to 172) | RR 0.88 (0.35 to 2.2) | 231 (1 trial) | ⊕⊝⊝⊝ very low1,3,4 | The included trial did not report disaggregated data for patients with leukemia. |
| Bleeding Follow‐up: 28 days | See comment | See comment | Not estimable | 234 (1 trial) | ⊕⊝⊝⊝ very low1,4,5 | The included trial did not report disaggregated data for patients with leukemia. |
| Resolution of respiratory failure | See comment | See comment | Not estimable | — | See comment | Included trial did not measure this outcome |
| Renal failure | See comment | See comment | Not estimable | — | See comment | Included trial did not measure this outcome |
| Shock | See comment | See comment | Not estimable | — | See comment | Included trial did not measure this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; DIC: disseminated intravascular coagulation. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Indirectness: trial included patients with or without leukemia; and this outcome was not discriminated by type of patients. 2Imprecision: low sample size (227 participants and 54 events) resulting in wide 95% CIs. 3Imprecision: low sample size (231 participants with 17 events) resulting in wide 95% CIs. 4Author trials measured this outcome either clinical benefit or harm. 5Trial authors reported P = 0.0271 for "disappearance rate of bleeding symptoms at day 7". Results were not discriminated according patients with or without leukemia.
Summary of findings 3. Tranexamic acid compared with placebo for DIC.
| Tranexamic acid compared with placebo for DIC | ||||||
| Patient or population: DIC Settings: acute or chronic leukemia patients Intervention: tranexamic acid Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Tranexamic acid | |||||
| Overall mortality | See comment | See comment | Not estimable | 12 (1 trial) | See comment | Included trial did not measure this outcome |
| Mortality from any cause (15‐day and 30‐day in‐hospital) | See comment | See comment | Not estimable | 12 (1 trial) | See comment | Included trial did not measure this outcome |
| Adverse events Follow‐up: 14 days | See comment | See comment | Not estimable | 12 (1 trial) | ⊕⊝⊝⊝ very low1,2 | Reported no thromboembolic events over course of trial |
| Bleeding Follow‐up: 14 days | See comment | See comment | Not estimable | 12 (1 trial) | ⊕⊝⊝⊝ very low1,2 | Trial reported no number of events |
| Resolution of respiratory failure | See comment | See comment | Not estimable | 12 (1 trial) | See comment | Included trial did not measure this outcome |
| Renal failure | See comment | See comment | Not estimable | 12 (1 trial) | See comment | Included trial did not measure this outcome |
| Shock | See comment | See comment | Not estimable | 12 (1 trial) | See comment | Included trial did not measure this outcome |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; DIC: disseminated intravascular coagulation. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Limitations in design and execution: insufficient information about the random sequence generation, allocation concealment and blinding. High risk for reporting bias. 2Imprecision: low sample size (12 participants).
Summary of findings 4. Dermatan compared with heparin for DIC.
| Dermatan compared with heparin for DIC | ||||||
| Patient or population: DIC Settings: acute or chronic leukemia patients Intervention: dermatan Comparison: heparin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Heparin | Dermatan | |||||
| Overall mortality | See comment | See comment | Not estimable | 10 (1 trial) | See comment | This outcome was not measured in the included study. |
| Mortality from any cause (15‐day and 30‐day in‐hospital) Follow‐up: 9 and 18 days | See comment | See comment | Not estimable | 10 (1 trial) | ⊕⊝⊝⊝ very low1,2 | Trial reported no death during study treatment. |
| Adverse events Follow‐up: 9 and 18 days | See comment | See comment | Not estimable | 10 (1 trial) | ⊕⊝⊝⊝ very low1,2 | It did not report information on safety explicitly. |
| Bleeding Follow‐up: 9 and 18 days | 400 per 1000 | 200 per 1000 (24 to 1000) | RR 0.5 (0.06 to 3.91) | 10 (1 trial) | ⊕⊝⊝⊝ very low2 | |
| Resolution of respiratory failure ‐ not measured | See comment | See comment | Not estimable | — | See comment | This outcome was not measured in the included study. |
| Renal failure | See comment | See comment | Not estimable | — | See comment | This outcome was not measured in the included study. |
| Shock | See comment | See comment | Not estimable | — | See comment | This outcome was not measured in the included study. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; DIC: disseminated intravascular coagulation. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Limitations in design and execution: insufficient information about the random sequence generation, allocation concealment, and blinding. High risk for reporting bias. 2Imprecision: low sample size (10 participants) and low rate of events (3 in total) resulting in wide 95% CIs.
Background
Description of the condition
Hemostasis (blood coagulation) is a self‐defense system to prevent death secondary to massive bleeding after a blood vessel injury (Chu 2004). Blood coagulation and the inflammatory process are interrelated as part of the innate host defense mechanism and form part of the argument for the hemostasis theory (Engelmann 2013; van der Poll 2014).
Disseminated intravascular coagulation (DIC) is an acquired syndrome characterized by systemic intravascular activation of coagulation, leading to deposition of fibrin in the bloodstream, which occurs in the course of several diseases (Dalainas 2008; Franchini 2006; Levi 2013). It has been considered as a loss of homeostasis in hemostasis (Hook 2012).
The morbidity of DIC is related to organ dysfunction and bleeding, which explains the high risk of death associated with this harmful and potentially fatal condition (Levi 2007). Organ dysfunction is explained by intravascular fibrin deposition (ischemic phase); the microvascular thromboses lead to organ damage and failure (Chu 2004; Wada 2014; Williams 2008). The bleeding phase is due to massive and ongoing activation of bloodstream coagulation, which leads to depletion of platelets and activated coagulation factors (Dalainas 2008; Franchini 2006). This explains why DIC is also known as consumption coagulopathy (Levi 2013). It has been suggested that DIC represents up to 26.8% of acquired bleeding disorders (Asthana 2009). However, it has a poorly established incidence and a heterogeneous clinical presentation. Okajima 2000 described a 12.7% incidence of DIC induced by hematologic malignancies in 204 patients with other underlying disorders. In summary, DIC is an acquired clotting catastrophe (DeLoughery 2005) that cannot be considered an isolated clinical entity and it should be managed as a hematological emergency.
DIC is one of the major complications of acute myeloid leukemia (Bunjevacki 1978; Dmoszyńska 1976), mainly promyelocytic leukemia (Barbui 2001; Choudhry 2012; Cornell 2012; Dreyfus 1973; Kwaan 2014; Lo‐Coco 2008; Speiser 1990; Stein 2009; Uchiumi 2007; Yanada 2006). However, it can also complicate the clinical course of acute lymphoblastic leukemia (Dixit 2007; Durán Suárez 1980; Higuchi 2005; Sarris 1992), chronic myeloid leukemia (Fujiwara 1997; García de Paredes 1983; German 1976; Gingrich 1979; Hess 2005; Komatsu 1986; Rosenthal 1995; Seligman 1976; Sunder‐Plassmann 1993; Yoshikawa 1980) and chronic lymphocytic leukemia (Kawano 2011). DIC in leukemia is accompanied by hyperfibrinolysis and reduction of natural anticoagulants (protein C and antithrombin III) (Choudhry 2012; Franchini 2010; Hunt 2014; Ikezoe 2012; Kwaan 2014). The main feature of this condition is the enhanced generation of thrombin in vivo (Levi 2009).
The pathogenesis of DIC is based on tissue‐factor‐mediated initiation of systemic coagulation that is insufficiently contained by the physiological anticoagulant pathways, and is amplified by impaired endogenous fibrinolysis (Falanga 2003; Franchini 2010; Ikezoe 2014; Levi 2007; Stein 2009; Wada 2014). The clinical entity is propagated by intravascular release of procoagulant substances, such as tissue thromboplastin, or by damage to vascular endothelium and platelets (Prentice 1985). Guidelines for the diagnosis of DIC have recently been published (Levi 2009). Table 5 shows the International Society of Thrombosis and Haemostasis scoring system for overt DIC.
1. Diagnostic scoring system for DIC (Levi 2009).
|
Scoring system for overt DIC, International Society of Thrombosis and Haemostasis (ISHT) ( Levi 2009) | |||
|
A) Risk assessment Does the patient have any underlying disorder known to be associated with overt DIC? If yes: proceed If no: do not use this algorithm |
B) Order global coagulation tests (prothrombin time, platelet count, fibrinogen, fibrin‐related marker) |
C) Score the test results Platelet count: > 100 x 109/L = 0 < 100 x 109/L = 1 < 50 x 109/L = 2 Elevated fibrin marker: (D‐dimer, fibrin degradation products) (no increase = 0, moderate increase = 2, strong increase = 3) Prolonged PT: (< 3 s = 0; > 3 but < 6 s = 1; > 6 s = 2) Fibrinogen level (> 1 g/L = 0; < 1 g/L =1) |
D) Calculate score ≥ 5 compatible with overt DIC: repeat score daily < 5 suggestive for non‐overt DIC: repeat next 1 to 2 days |
See Appendix 1 for medical terms.
Description of the intervention
Treatment of the underlying disease must be the cornerstone of therapy (Dalainas 2008; Levi 2007; Prentice 1985). Additionally supportive anticoagulant approaches have been used with the aim of resolving coagulation abnormalities. The drugs used include the following:
First‐generation anticoagulants: low molecular weight heparin (Brunton 2008; Iba 2013), unfractionated heparin (Hoyle 1988; Mangal 1984) and danaparoid sodium (Brunton 2008; Iba 2013);
Second‐generation anticoagulants: synthetic protease inhibitor (Tsukagoshi 2000), recombinant hirudin (Saito 1995), human recombinant antithrombin (Iba 2013; Pal 2010), human recombinant activated protein C (Brunton 2008; Iba 2013), recombinant human soluble thrombomodulin (Iba 2013; Ikezoe 2012; Ogawa 2010) and recombinant tissue factor pathway inhibitor (Kaiser 2001);
Antifibrinolytic drugs: tranexamic acid (Levi 2009; Wada 2010). The use of a combination of tranexamic acid and unfractionated heparin has also been reported (Koseki 2007);
Pro‐coagulants: recombinant activated factor VIIa (Abshire 2004).
How the intervention might work
The aim of anticoagulant drugs is to fulfil the role of the natural anticoagulants (protein C and antithrombin systems) which are disrupted during DIC. A close relationship between coagulation and inflammation pathways during DIC has also been identified: "inflammation leads to activation of coagulation and coagulation considerably affects inflammatory activity" (Levi 2008).
1. First generation anticoagulants catalyze the inhibition of several coagulation proteases by antithrombin (a natural anticoagulant) which inhibits activated coagulation factors of the intrinsic and common pathways, including thrombin, factors Xa and IXa. Heparin increases the rate of the thrombin‐antithrombin reaction by at least 1000‐fold (Brunton 2008). Danaparoid sodium is a mixture of non‐heparin glycosaminoglycans isolated from porcine intestinal mucosa (84% heparan sulfate, 12% dermatan sulfate, 4% chondroitin sulfate) and responsible for promoting inhibition of factor Xa by antithrombin (Brunton 2008).
2. Second‐generation anticoagulants: 2.1 Synthetic protease inhibitor is a protease inhibitor with anticoagulant properties (Tsukagoshi 2000); 2.2 Recombinant hirudin is an anticoagulant pharmacodynamically different from heparin (Greinacher 2001) and a product of genetic engineering. It is identical to natural hirudin. It is a direct thrombin inhibitor present in the salivary glands of the medicinal leech (Brunton 2008; Greinacher 2001; Nowak 2002); 2.3 Recombinant human soluble thrombomodulin has an antithrombotic effect (reducing coagulation) by neutralizing the effect of thrombin (Ikezoe 2012); 2.4 Recombinant tissue factor pathway inhibitor is the only endogenous inhibitor of the tissue factor/factor VIIa complex that plays a crucial role in the pathogenesis of thrombotic, vascular and inflammatory disorders (Kaiser 2001); 2.5 Human recombinant activated protein C is a recombinant form of human activated protein C that inhibits coagulation by proteolytic inactivation of factors Va and VIIIa. It also has anti‐inflammatory effects (Brunton 2008).
3. Antifibrinolytic drugs (aminocaproic acid and tranexamic acid) inhibit the conversion to plasminogen and plasmin, blocking the interaction of plasmin with fibrin. This leads to a potent inhibition of fibrinolysis and can reverse states that are associated with excessive fibrinolysis (Brunton 2008);
4. Pro‐coagulant drugs (recombinant activated factor VIIa) bind to the surface of an activated platelet and can directly activate factor X in the absence of tissue factor (Abshire 2004).
Why it is important to do this review
This is the first update of this Cochrane Review, which we performed to update the search strategy (May 2015) to identify new randomized controlled trials (RCTs) on pharmacological interventions to treat DIC in patients with acute and chronic leukemia.
Objectives
The research question of this Cochrane Review is: what are the clinical benefits and harms of anticoagulant and antifibrinolytic therapy for treating DIC in patients with acute or chronic leukemia?
To assess the clinical benefits and harms of pharmacological interventions for treating DIC in patients with acute or chronic leukemia.
Methods
Criteria for considering studies for this review
Types of studies
RCTs irrespective of publication status (unpublished or published as an article, an abstract or a letter), language (no language limitation was applied) and country of origin. We did not apply any limits on the period of follow‐up or diagnostic criteria used for DIC.
Types of participants
Patients of any age with DIC and suffering from acute or chronic leukemia.
Types of interventions
DIC requires resolution of the underlying condition in addition to other approaches, such as antibiotic therapy, replacement of blood products and fluid therapy, which are considered 'primary' or 'conventional care' interventions. Anticoagulant, procoagulant and antifibrinolytic drugs are considered complementary to the conventional care interventions. Thus, for the purposes of this Cochrane Review, eligible RCTs had to compare the same conventional interventions with and without anticoagulants, with and without procoagulants, or with and without antifibrinolytics.
Intervention
Heparins (low molecular weight heparin and unfractionated heparin);
Danaparoid sodium;
Antifibrinolytic drugs (epsilon aminocaproic and tranexamic acid);
Synthetic protease inhibitor and antithrombin;
Human recombinant activated protein C;
Recombinant human soluble thrombomodulin;
Recombinant tissue factor pathway inhibitor;
Recombinant activated factor VIIa;
and Recombinant hirudin and antifibrinolytic drugs.
plus resolution of the underlying condition, conventional care (antibiotic therapy, replacement of blood products, fluid therapy).
Control
Resolution of the underlying condition with conventional care (antibiotic therapy, replacement of blood products, fluid therapy) alone or plus placebo.
Types of outcome measures
Primary outcomes
Overall mortality (Porta 2008);
In‐hospital mortality from any cause (15‐day and 30‐day);
Adverse events: number and type of adverse events defined as patients with any untoward medical occurrence not necessarily having a causal relationship with the treatment. We reported adverse events that led to treatment discontinuation and those that did not lead to treatment discontinuation separately. We have defined serious adverse events according to the International Conference on Harmonisation (ICH) Guidelines (ICH‐GCP 1997) as any event that at any dose results in death, is life‐threatening, requires in‐patient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability, or is a congenital anomaly/birth defect, and any important medical event, which may have jeopardized the patient or requires intervention to prevent it. All other adverse events were considered non‐serious.
Secondary outcomes
Bleeding;
Resolution of respiratory failure;
Resolution of renal failure;
Resolution of shock (defined as the condition associated with circulatory collapse, when the arterial blood pressure is too low to maintain an adequate supply of blood to the tissues) (Concise Medical Dictionary 2011).
Search methods for identification of studies
The MEDLINE search strategy used in the first version of this Cochrane Review was not very sensitive (Marti‐Carvajal 2011) and could have generated publication bias. Therefore, we performed this review update using a new MEDLINE search strategy.
Electronic searches
We searched the following electronic databases to find reports of relevant RCTs:
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2015, Issue 05);
MEDLINE OVID (1946 to 7 May 2015);
LILACS (http://lilacs.bvsalud.org/es/): 1982 to 7 May 2015:
African Index Medicus (http://indexmedicus.afro.who.int/): 7 May 2015:
In the previous review version, we also searched EMBASE (Marti‐Carvajal 2011). For this update, we did not search the EMBASE database.
We used the following keywords in our search: DIC, consumption coagulopathy, acute leukemia and chronic leukemia.
Searching other resources
In addition we searched trials registries via the World Health Organization International Clinical Trials Platform Search Portal (WHO ICTRP).
We also checked the reference lists of all trials identified by the above methods. We contacted trial authors and researchers in the field to obtain further details about unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (Arturo Martí‐Carvajal (AMC) and Vidhu Anand (VA)) independently assessed for inclusion all the potential studies identified from the search strategy. We resolved any disagreements through discussion.
Data extraction and management
In the previous version of this Cochrane Review (Marti‐Carvajal 2011), we used a form to extract data (Zavala 2006). For assessing the eligible studies, two review authors (AMC and VA) extracted the data independently using the agreed form. We entered data into RevMan 2014 and checked them for accuracy. We resolved discrepancies through discussion or, if required, we consulted a third review author (Ivan Solà (IS)).
For this update, if we had included new RCTs, we would have used the same strategy.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias (AMC and VA) of each included trial using the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved discrepancies through discussion or, if necessary , we consulted a third author (IS).
The definitions of each classification is given below:
Sequence generation (checking for possible selection bias)
Low risk (any truly random process, e.g. random number table; computer random number generator);
High risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
Unclear (if the trial was described as randomized, but the method used for the allocation sequence generation was not described).
Allocation concealment (checking for possible selection bias)
Low risk (e.g. telephone or central randomization; consecutively numbered sealed opaque envelopes);
High risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
Unclear risk (if the trial was described as randomized, but the method used to conceal the allocation was not described).
Blinding or masking (checking for possible performance bias)
Low, high or unclear risk for participants;
Low, high or unclear risk for personnel;
Low risk, high or unclear risk for outcome assessors.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
Low risk (the numbers and reasons for dropouts and withdrawals in all intervention groups were described or if it was specified that there were no dropouts or withdrawals);
High risk (the number or reasons for dropouts and withdrawals were not described);
Unclear risk (the report gave the impression that there had been no dropouts or withdrawals, but this was not specifically stated).///
Selective reporting bias
Low risk (where it is clear that all of the trial's pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
High risk (where not all the trial's pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; trial fails to include results of a key outcome that would have been expected to have been reported);
Unclear risk (not all pre‐defined, or clinically relevant and reasonably expected outcomes were reported on, or were not reported fully, or it was unclear whether data on these outcomes were recorded or not).
Other biases
We described for each included RCT any important concerns we had about other possible sources of bias (industry bias, academic bias, bias of the presentation data, etc.):
Low risk (the trial appears to be free of other components that could put it at risk of bias);
High risk (there are other factors in the trial that could put it at risk of bias);
Unclear risk (the trial may or may not be free of other components that could put it at risk of bias).
Overall risk of bias
We considered trials in which we could assess one of the domains as high risk of bias as trials with high risk of bias.
We made explicit judgements about whether studies are at high risk of bias, according to the criteria given in Higgins 2011.
In the previous version (Marti‐Carvajal 2011), AMC entered the data into RevMan 2014 and a second review author checked data for accuracy. In this current version, AMC entered the data into RevMan 2014 and IS checked data for accuracy.
Measures of treatment effect
For dichotomous data, we planned to present results as a summary risk ratio (RR) with 95% confidence intervals (CIs).
Mortality from any cause (15‐day and 30‐day in‐hospital);
Adverse events;
Bleeding;
Resolution of respiratory failure;
Resolution of renal failure;
Resolution of shock.
Unit of analysis issues
The unit of analysis would have been the patients studied. We would have collected and analyzed a single measurement for each outcome from each participant.
Dealing with missing data
We planned to note levels of attrition and explore the impact of high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis.
For all outcomes we would have carried out analysis, as far as possible, on an intention‐to‐treat basis (i.e. we would have attempted to include all participants randomized to each group in the analyses). The denominator for each outcome in each trial would have been the number randomized minus any participants whose outcomes are known to be missing.
For future updates, if possible, we will conduct main analysis based on completers but we will perform sensitivity analysis for per‐protocol, worse and best case scenarios (Hollis 1999).
Assessment of heterogeneity
We planned to use the I² statistic to measure statistical heterogeneity among the trials in each analysis. In the presence of substantial heterogeneity we would have explored this by pre‐specified subgroup analysis. The I² statistic describes the percentage of total variation across trials that is due to heterogeneity rather than sampling error (Higgins 2003). We would have considered a substantial statistical heterogeneity if the I² statistic was greater than 50% (Higgins 2011).
Assessment of reporting biases
If we had suspected reporting bias (see 'Selective reporting bias' section), we would have attempted to contact the trial authors asking them to provide missing outcome data. When this was not possible, and the missing data were thought to introduce serious bias, we would have explored the impact of including such studies in the overall assessment of results by a sensitivity analysis.
We would also have attempted to assess whether the review is subject to publication bias by using a funnel plot to graphically illustrate variability between trials. If asymmetry was detected, we would have explored causes other than publication bias (e.g. selective outcome reporting, poor methodological quality in smaller studies, true heterogeneity) (Higgins 2011). In future updates we will construct a funnel plot if 10 or more RCTs meet the inclusion criteria.
Data synthesis
We planned to out statistical analyses using RevMan 2014 and summarize the findings using a random‐effects model, as differences would have been anticipated in terms of interventions and patients.
Subgroup analysis and investigation of heterogeneity
We anticipated clinical heterogeneity for the following participant and intervention characteristics, and therefore we would have carried out the following subgroup analyses:
Acute versus chronic leukemia;
Type of intervention;
Stage of DIC; and
Unfractionated heparin dose.
We would have restricted subgroup analysis to the review’s primary outcome (Higgins 2011).
Sensitivity analysis
If sufficient trials are identified, we plan to conduct a sensitivity analysis comparing the results using all trials. We would have compared the RCTs with high methodological quality (studies classified as having a 'low risk of bias' versus those identified as having a 'high risk of bias') (Higgins 2011).
We would also have evaluated the risk of attrition bias, as estimated by the percentage of participants lost. We would have excluded trials with a total attrition of more than 20% or where differences between the groups exceeded 10%, or both, from meta‐analysis but would have included them in the review.
'Summary of findings' tables
We planned to use The Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2008) to assess the quality of the body of evidence associated with specific outcomes (mortality from any cause (15‐day and 30‐day in‐hospital), overall mortality, bleeding, resolution of respiratory failure, renal failure or shock and adverse events) in this Cochrane Review and construct a 'Summary of findings' table using GRADEpro 2008. The GRADE approach appraises the quality of a body of evidence based on the extent to which one can be confident that an estimate of effect or association reflects the item being assessed. The quality of a body of evidence considers within‐trial risk of bias (methodologic quality), the directness of the evidence, heterogeneity of the data, precision of effect estimates and risk of publication bias.
Results
Description of studies
Results of the search
For this update, we identified 9027 references of potential interest after duplicates were removed. We did not find any new eligible RCTs. Therefore, this Cochrane Review update includes the four RCTs identified in the original review version (Aoki 2002; ART‐123 Trial Group 2007; Avvisati 1989; Cofrancesco 1994). See Figure 1 for search result details.
1.
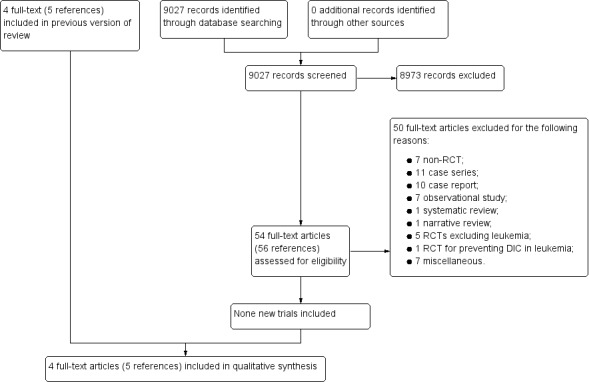
Study flow diagram for this review update.
These RCTs included 388 participants for analysis. We have provided a detailed description of the included trials in the Characteristics of included studies section.
Included studies
We classified the included trials as:
Including patients with or without leukemia (Aoki 2002; ART‐123 Trial Group 2007);
Only including patients with leukemia (Avvisati 1989; Cofrancesco 1994).
Two trials were conducted in 1989 (Avvisati 1989;) and 1994 (Cofrancesco 1994) and the other two trials were conducted during 2002 (Aoki 2002) and 2007 (ART‐123 Trial Group 2007).
Interventions and populations assessed
Each trial investigated a different intervention to treat DIC: human activated protein C (Aoki 2002), recombinant human soluble thrombomodulin (ART‐123 Trial Group 2007), tranexamic acid (Avvisati 1989) and dermatan sulphate (Cofrancesco 1994). All included trials had a parallel‐trial design and compared two arms. The route of administration of the intervention was intravenous in all trials.
Three trials used heparin as comparison group (Aoki 2002; ART‐123 Trial Group 2007;Cofrancesco 1994). One trial used placebo (glucose 5% (w/v) in 10 mL sterile water) (Avvisati 1989).
Two trials had a follow‐up length under 20 days, ranging between nine and 18 days (Aoki 2002; ART‐123 Trial Group 2007). Two trials had a follow‐up length of 28 days (Avvisati 1989; Cofrancesco 1994).
One trial with 132 patients included many types of leukemia, solid tumors, infection and other causes (liver diseases and aortic aneurysm) of DIC. Patients were classified in two groups: leukemic (hematopoietic malignancy and anti‐cancer chemotherapy) and non‐leukemic (infection and other causes such as liver disease and aortic aneurysm) (Aoki 2002). One trial reported results from 234 patients including acute or chronic leukemia, another hematopoietic malignancies, sepsis, pneumonia, biliary tract infection, peritonitis, abscess, viremia, meningitis, cholangitis, enterocolitis and other infections (ART‐123 Trial Group 2007). Two trials were only conducted in patients with leukemia (Avvisati 1989; Cofrancesco 1994). Avvisati 1989 included 12 patients with newly diagnosed acute promyelocytic leukemia. Cofrancesco 1994 considered ten patients with many types of acute leukemia and those who had a blast crisis due to chronic myelogenous leukemia.
Overall, included participants were above 50 years of age and most were male.
Trial location
The included trials were conducted in three countries: two in Japan (Aoki 2002; ART‐123 Trial Group 2007), one trial in Italy (Cofrancesco 1994) and one trial was conducted in Italy and the Netherlands (Avvisati 1989).
Trial methods
Two trials were reported as phase‐III (Aoki 2002; ART‐123 Trial Group 2007), two trials did not report the trial phase (Avvisati 1989; Cofrancesco 1994). Two trials were multicenter (ART‐123 Trial Group 2007; Avvisati 1989) and the site was unclear in two trials (Aoki 2002; Cofrancesco 1994). All trials were conducted using a parallel group trial design. Three trials were conducted without reporting an a priori sample size estimation (Aoki 2002; Avvisati 1989; Cofrancesco 1994). Two trials were designed to include few leukemic patients (fewer than 15 cases): 12 and 10 participants in Avvisati 1989 and Cofrancesco 1994, respectively. The trials including other causes of DIC were conducted with sample sizes of between 55 and 104 participants (Aoki 2002; ART‐123 Trial Group 2007). Two trials did not describe their exclusion criteria (Avvisati 1989; Cofrancesco 1994). One trial is associated with a duplicate publication (Cofrancesco 1994).
Excluded studies
We excluded 50 studies for the following reasons: 10 were case reports (Aviles de la Garza 1982; Koseki 2007; Martínez López 2002; Ogawa 2010; Pizzuto 1969; Sandler 1982; Shindo 2012; Sugawara 1992; Takagi 2011; Yagamuchi 2001), seven were non‐ RCTs (Hoffmann 2004; Iba 2012; Ikezoe 2012; Sakuragawa 1992; Takezako 2014; Wada 1995; Yamato 2013), 11 were case series (Gamba 1979; Gillis 1995; Glarnick 1972; Goldberg 1987; Nieuwenhuis 1986; Nowrousian 1978; Pogliani 1989; Rodríguez Gómez 1993; Saito 1995; Shirahata 2014; Vinazzer 1989), seven were observational studies (Aoki 1994; Kato 2013; Kawano 2011; Matsushita 2014; Sawamura 2009; Yang 2014; Yin 2014), one was a systematic review (Franchini 2007), one was a narrative review (Walia 2012), five were RCTs excluding patients with leukemia (Fourrier 1993; Gando 2013; Hofmann 1997; Nishiyama 2012; Shpilberg 1995), one was a RCT for preventing DIC in leukemia (Chojnowski 2002) and seven were excluded for miscellaneous reasons (Asakura 2014; Göbel 1980; Gross 1982; KYBERSEPT 2006; Liu 2014; Oguma 1990; Vincent 2013).
See the Characteristics of excluded studies table.
Ongoing trials
We found no ongoing RCTs.
Risk of bias in included studies
We have summarized the risk of bias of the included RCTs in Figure 2 and Figure 3.
2.
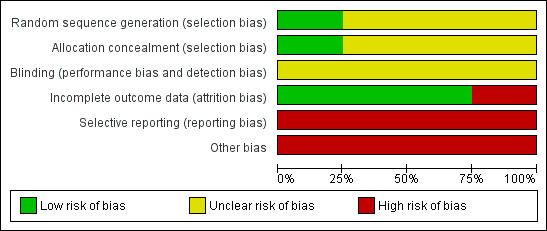
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials.
3.
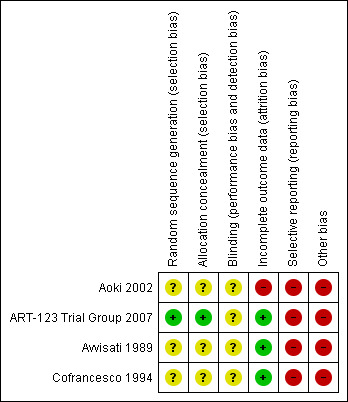
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial.
Allocation
Sequence generation
One RCT was rated as having low risk of bias because the trial authors used computer generation for assigning the participants (ART‐123 Trial Group 2007). Three RCTs reported insufficient information to permit judgment as 'low risk' or 'high risk'. Therefore, we rated them at unclear risk of bias (Aoki 2002; Avvisati 1989; Cofrancesco 1994).
Allocation concealment
One RCT was rated as having low risk of bias (ART‐123 Trial Group 2007). Three RCTs showed unclear risk of bias (Aoki 2002; Avvisati 1989; Cofrancesco 1994).
Blinding
One trial was reported as blinded; however, it was not described how participants and personnel were blinded. Therefore, it was rated as having unclear risk of bias (Avvisati 1989). Three trials did not report any information about this domain; so, were rated as having unclear risk of bias (Aoki 2002; Avvisati 1989; Cofrancesco 1994).
Incomplete outcome data
ART‐123 Trial Group 2007; Avvisati 1989; Cofrancesco 1994 had a low risk of bias for attrition bias. Aoki 2002 lost 21.3% of patients and supplied no details of withdrawals by groups. Hence, this trial was considered as showing high risk of attrition bias.
Selective reporting
Since one or more outcomes of interest were reported incompletely or not at all, Aoki 2002; ART‐123 Trial Group 2007; Avvisati 1989 and Cofrancesco 1994 had high risk of reporting bias.
Other potential sources of bias
Insufficient information on sequence generation could have generated a potential bias of the estimated effect of exposure on an outcome due to baseline differences among exposure groups, which is known as confounding bias (Porta 2008). Therefore, Aoki 2002; ART‐123 Trial Group 2007; Avvisati 1989; Cofrancesco 1994 were rated as having a high risk of bias.
All trials had high risk of bias due to bias in the presentation of data (Aoki 2002; ART‐123 Trial Group 2007; Avvisati 1989; Cofrancesco 1994).
Overall, due to the limitations in the reporting of the methodology and the design for the included trials, we judged them to be at high risk of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We have based the results on 356 analyzed patients out of 388 patients randomized in four included trials (Aoki 2002; ART‐123 Trial Group 2007; Avvisati 1989; Cofrancesco 1994). Each trial investigated a different intervention. Two trials included patients with or without leukemia (Aoki 2002; ART‐123 Trial Group 2007) and two trials included patients with leukemia (Avvisati 1989; Cofrancesco 1994). We compared human activated protein C, recombinant human soluble thrombomodulin, tranexamic acid and dermatan sulphate with placebo or heparin in this review. In future updates, we will also consider any combination of these drugs (if trials were available).
Trials including patients with or without leukemia reported effects of interventions by drugs under research rather than by disease group. Therefore, their results represent all participants in each one of these trials (Aoki 2002; ART‐123 Trial Group 2007).
None of the included trials assessed overall mortality, resolution of respiratory failure, renal failure or shock.
Primary outcomes
Mortality from any cause (15‐day and 30‐day in‐hospital)
Trials including patients with or without leukemia
Human activated protein C versus heparin
Human activated protein C notably reduced mortality from any cause compared with heparin (10/49 (20.4%) versus 22/55 (40%); RR 0.51, 95% 0.27 to 0.97; P = 0.04; Aoki 2002).
Recombinant human soluble thrombomodulin versus heparin
There was not a significant difference in mortality from any cause between recombinant human soluble thrombomodulin versus heparin (25/114 (21.9%) versus 29/113 (25.7%); RR 0.85, 95% 0.54 to 1.36; P = 0.51; ART‐123 Trial Group 2007).
Trials only conducted in patients with leukemia
Dermatan sulphate versus heparin
One trial including 10 participants reported no deaths during trial treatment (Cofrancesco 1994).
Adverse events
Trials including patients with or without leukemia
Human activated protein C versus heparin
Aoki 2002 reported a non‐significant difference between human activated protein C and heparin (1/52 (1.92%) versus 2/55 (3.63%); RR 0.53, 95% CI 0.05 to 5.66; P = 0.60).
Recombinant human soluble thrombomodulin versus heparin
There was not a significant difference between recombinant human soluble thrombomodulin and heparin in terms of serious bleeding‐related adverse events (8/116 (6.9%) versus 9/115 (7.8%); RR 0.88, 95% CI 0.35 to 2.20; P = 0.79; ART‐123 Trial Group 2007).
Regarding non‐related bleeding serious adverse events during the 28 days, there was not a significant difference comparing recombinant human soluble thrombomodulin with heparin (37/116 (31.9%) versus 41/115 (35.6%); RR 0.89, 95% CI 0.62 to 1.28; P = 0.55; ART‐123 Trial Group 2007).
Trials only conducted in patients with leukemia
Tranexamic acid versus placebo
Avvisati 1989 did not report any thromboembolic complications in tranexamic acid or placebo group.
Dermatan sulphate versus heparin
Cofrancesco 1994 did not report information on safety explicitly, however, the trial authors stated that among the patients without hemorraghic diathesis at trial entry, two in the heparin group and one in the dermatan sulphate developed moderate cutaneous bleeding (petechiae, ecchymoses) shortly after the start of anticoagulant treatment and none of the patients had clinically apparent thromboembolic complications.
Secondary outcomes
Bleeding
Trials including patients with or without leukemia
Human activated protein C versus heparin
Aoki 2002 compared human activated protein C versus heparin and did not find a significant difference in terms of aggravation of bleeding (0/63 (0%) versus 8/69 (11.6%); RR 0.06, 95% CI 0.00 to 1.09; P = 0.06). Regarding amelioration of bleeding, Aoki 2002 did not report any significant difference between APC and heparin (35% (22/63) versus 22% (15/69); RR 1.61, 95% CI 0.92 to 2.81; P = 0.10).
Recombinant human soluble thrombomodulin versus heparin
ART‐123 Trial Group 2007 reported the disappearance rate of bleeding symptoms at day seven regarding underlying disease (hematologic malignancy versus infection). Trial authors reported the clinical course of bleeding symptoms in the thrombomodulin group at day seven, which was significantly improved when compared with the heparin group (P = 0.0271).
Trials only conducted in patients with leukemia
Tranexamic acid versus placebo
Avvisati 1989 reported this outcome using a cumulative hemorrhagic score which was composed by three items: skin bleeding, mucosal bleeding and hematuria. During the first trial period (days two to seven), it was two points for patients receiving tranexamic acid versus 31 points for control group patients (P = 0.032). During second period (days 8 to 14) of this trial, the cumulative hemorraghic scores were one point for tranexamic group and 11 points for control group (P = 0.0015). See Characteristics of included studies for cumulative hemorrhagic score details.
Dermatan sulphate versus heparin
Cofrancesco 1994 reported a non‐significant difference between dermatan sulphate and heparin regarding moderate cutaneous bleeding (petechiae, ecchymoses) shortly after the start of anticoagulant treatment (1/5 (20%) versus 2/5 (40%); RR 0.50, 95% CI 0.06 to 3.91; P = 0.51).
Discussion
Summary of main results
In this updated Cochrane Review we found no new RCTs. Hence, the summary of main results comes from the first review version (Marti‐Carvajal 2011).
This review of treatment for DIC in patients with acute and chronic leukemia included four RCTs (388 randomized participants, 356 analyzed) which assessed human activated protein C (Aoki 2002), recombinant human soluble thrombomodulin (ART‐123 Trial Group 2007), tranexamic acid (Avvisati 1989) and dermatan sulphate (Cofrancesco 1994). We classified these included trials as: 1) trials including patients with or without leukemia (Aoki 2002; ART‐123 Trial Group 2007); and 2) trials only including patients with leukemia (Avvisati 1989; Cofrancesco 1994).
Trials that included patients with or without leukemia did not report data for each disease type (Aoki 2002; ART‐123 Trial Group 2007): These trials reported outcomes by drugs under research rather than by disease group. Aoki 2002 assessed symptoms, DIC score and coagulation/fibrinolysis parameters, death from any cause during treatment or within 28 days after treatment, and safety. ART‐123 Trial Group 2007 assessed DIC resolution rate (rate of recovery from DIC) within seven days after the start of infusion (or withdrawal) (primary outcomes) and clinical course of bleeding symptoms and mortality at 28 days after the start of infusion (secondary outcomes). These trials reported safety profile of human activated protein C (Aoki 2002) and recombinant human soluble thrombomodulin (ART‐123 Trial Group 2007);
Trials only including patients with leukemia (Avvisati 1989; Cofrancesco 1994): Avvisati 1989 assessed severity of bleeding, packed red cell transfusion requirements and platelet concentrate transfusion requirements. Furthermore, Avvisati 1989 included thromboembolic complications as safety data. Cofrancesco 1994 did not report their outcomes as primary or secondary, however they described clinically overt bleeding until hospital discharge and type and amount of daily transfusions.
One trial assessing tranexamic acid versus placebo did not report information on mortality (Avvisati 1989). Another trial comparing dermatan sulphate versus heparin neither did not mention mortality across trial (Cofrancesco 1994). In terms of bleeding data, we were unable to pool results from studies due to the inconsistency in the measurement and reporting of this outcome. Avvisati 1989 reported significantly reduced bleeding events with tranexamic acid compared with placebo. On the contrary, Cofrancesco 1994 found inconclusive data comparing dermatan sulphate versus heparin. Regarding adverse events, Avvisati 1989 reported no thromboembolic complication as adverse event. Cofrancesco 1994 did not report information on safety profile.
The included trials did not assess overall mortality, resolution of respiratory failure, renal failure or shock as outcomes.
Therefore it is not possible to determine whether human activated protein C (Aoki 2002), recombinant human soluble thrombomodulin (ART‐123 Trial Group 2007), tranexamic acid (Avvisati 1989) and dermatan sulphate (Cofrancesco 1994) are effective or harmful for DIC in patients with acute and chronic leukemia.
Overall completeness and applicability of evidence
The included trials assessed two pre‐fixed primary outcomes of this review (mortality from any cause and adverse events) and one pre‐fixed secondary outcome (bleeding). There is inconsistency in reporting these outcomes.
As mentioned above, two trials included patients with and without leukemia which did not disaggregate data by leukemia (Aoki 2002; ART‐123 Trial Group 2007). It is therefore difficult to estimate the true effect of human activated protein C (Aoki 2002) and recombinant human soluble thrombomodulin (ART‐123 Trial Group 2007) in patients with leukemia affected by DIC.
All included trials had different approaches for describing the bleeding outcome: aggravation of bleeding (Aoki 2002), the disappearance rate of bleeding symptoms at day seven (ART‐123 Trial Group 2007), hemorrhagic score (Avvisati 1989) and hemorrhagic diathesis (Cofrancesco 1994). Furthermore, two trials did not assess this outcome in the leukemic group (Aoki 2002; ART‐123 Trial Group 2007).
Therefore, these important outcomes are difficult to compare, contrast or combine (Hopewell 2010). Recently, the adoption of an agreed minimum core set of outcomes for each medical condition has been suggested (Clarke 2007). This approach would reduce the impact of outcome reporting bias in trials (Kirkham 2010). See Appendix 8 for outcome details.
The impact of outcome reporting bias may be reduced for adopting the recommendations of the Patient‐Centered Outcomes Research Institute (PCORI) (PCORI 2012). PCORI's research is intended to give patients a better understanding of the prevention, treatment and care options available, and the science that supports those options (PCORI 2012).
Three trials included heparin as comparison group (Aoki 2002; ART‐123 Trial Group 2007; Cofrancesco 1994). So, we were interested in pooling data as heparin versus no‐heparin. However, we were unable to pool results from these trials due to the inconsistency in the measurement and reporting of mortality and bleeding data. Aoki 2002 and ART‐123 Trial Group 2007 did not report data for the leukemic group.
This update identified no new trials for reducing the uncertainty of effect of the interventions. The low number of participants carries a risk of random error (play of chance). This update shows a high risk of systematic errors (bias, that is overestimation of benefits ‐ positive false result ‐ and underestimation of harms ‐ negative false result) (Button 2013; Kjaergard 2001; Savović 2012; Thorlund 2011).
Thus, overall completeness and applicability of evidence is poor due to potential spurious findings.
Quality of the evidence
We assessed the quality of evidence as very low due to a overall high risk, and the Cochrane Review results should therefore be treated with great caution.
The main methodological pitfalls found in the four trials included in this review were: firstly, two trials involved either patients with or without leukemia. Both type of patients were not disaggregated which limited the possibility to make conclusions about the efficacy of the treatment of patients with leukemia in this setting regarding mortality from any cause, adverse events and bleeding. One trial assessing human activated protein C, Aoki 2002, showed unclear risk of bias for selection bias and high risk of attrition bias (21.3% loss) and the trial authors reported no reasons for attrition could bias the reported estimates. Furthermore, this trial showed imprecision due low sample size and low events which resulted in wide 95% CIs for mortality from any cause (227 participants and 54 events) and adverse events (231 participants and 17 events). ART‐123 Trial Group 2007 showed indirectness, and imprecision due low sample size and low events which resulted in wide 95% CIs for mortality from any cause (104 participants and 32 events), adverse events (107 participants and three events) and bleeding (132 participants and eight events). Secondly, the other trials only included patients with leukemia. Avvisati 1989, assessing tranexamic acid versus placebo, was rated as very low quality due to limitations in design and execution: insufficient information about random sequence generation, allocation concealment and blinding. This trial was rated as having high risk of reporting bias. Furthermore, this trial was conducted with a very small sample size (12 participants) which resulted in imprecision (i.e. wide 95% CIs). Cofrancesco 1994 compared dermatan versus heparin showed a very low quality due to limitations in design and execution: insufficient information about random sequence generation, allocation concealment and blinding. It was conducted involving 10 participants generating imprecision in bleeding (three events) estimate.
The quality of the evidence of safety profile was rated as very low due to indirectness in two included trials (Aoki 2002; ART‐123 Trial Group 2007) and incomplete or absent reporting of data in Avvisati 1989 and Cofrancesco 1994.
For additional details, please, see Table 1; Table 2; Table 3; Table 4). Our 'Risk of bias' assessment of the included trials has been described in the Risk of bias in included studies section and a summary can be found in Figure 2 and Figure 3.
Potential biases in the review process
In the process of performing a systematic review, there is a group of biases called significance‐chasing biases (Ioannidis 2010). This group includes publication bias, selective outcome reporting bias, selective analysis reporting bias and fabrication bias (Ioannidis 2010). Publication bias represents a major threat to the validity of systematic reviews, particularly in reviews that include small trials.
Potentially, this Cochrane Review has a low risk of publication bias due to the thorough trial search process. We screened more than 8,000 studies from the year 1946. However, we include EMBASE as source in this review update. EMBASE includes more European, non‐English and drug therapy journals (Draper 2014).
Selective outcome reporting bias operates through suppression of information on specific outcomes and has similarities to trial publication bias, in which 'negative' results remain unpublished (Ioannidis 2010). This Cochrane Review includes four trials with a high risk of selective outcome reporting (Aoki 2002; ART‐123 Trial Group 2007; Avvisati 1989; Cofrancesco 1994). As previously mentioned, this Cochrane Review includes two types of trials: one group including patients with or without leukemia (Aoki 2002; ART‐123 Trial Group 2007) and another group only including patients with leukemia (Avvisati 1989; Cofrancesco 1994). The first group did not report information by disease group (Aoki 2002; ART‐123 Trial Group 2007). We did not receive replies from the trial authors in order to get information related with leukemic group. Trial group only including patients with leukemia reported bleeding outcome with inconsistency which prevented compiling the data (Avvisati 1989; Cofrancesco 1994). Furthermore, none of the four included trials assessed overall mortality, resolution of respiratory failure, renal failure or shock as outcomes.
We found two ongoing single and non‐RCTs assessing thrombomodulin for treating DIC in this population (JPRN‐UMIN000008466; JPRN‐UMIN000006738). However, these trials may be useful for improving the knowledge on thrombomodulin harms but not its clinical benefit.
Agreements and disagreements with other studies or reviews
There is a strong uncertainty about the clinical benefits and harms of the interventions used for treating DIC in patients with acute or chronic leukemia. This is due to a lack of RCTs of high methodological quality. The current knowledge is based on retrospective studies (Matsushita 2014; Yang 2014) or case series (Dorantes‐Acosta 2013; Kawano 2011). Therefore, this updated Cochrane Review agrees with other studies, in that there is a paucity of certainty on what is the best scientific advance for treating DIC in patients with leukemia either acute or chronic.
Authors' conclusions
Implications for practice.
This updated Cochrane Review has no new RCTs included. Therefore, the implications for clinical practice from Marti‐Carvajal 2011 still apply.
We included four small RCTs of low methodological quality. It is not possible to determine whether human activated protein C, recombinant human soluble thrombomodulin, tranexamic acid and dermatan sulphate are effective or harmful to patients presenting with DIC related to acute or chronic leukemia, due to the inconsistency in the measurement and reporting of mortality and bleeding data. For the same reason, we were unable to pool results from these trials. Therefore, prescription of these interventions for treating DIC in patients with acute and chronic leukemia can neither be supported nor rejected, unless new evidence from a large high‐quality trial alters this conclusion.
Implications for research.
Further well‐designed, high power RCTs are necessary to evaluate the role of pharmacological interventions for treating DIC in patients with acute and chronic leukemia effectively. Future trials should include the following outcomes: mortality from any cause (15‐day and 30‐day in‐hospital), overall mortality, hemorrhagic diatheses, resolution of respiratory failure, renal failure, shock and safety. The definition of bleeding should be standardized in these patients.
This review update suggests that potential trials should be planned using Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) (Chan 2013) and reported according to the Consolidated Standards of Reporting Trials (CONSORT) statement for improving the quality of reporting of efficacy and of harms in clinical research (Calvert 2013; Schulz 2010). The trials should be conducted according to the PCORI recommendations (PCORI 2012).
What's new
| Date | Event | Description |
|---|---|---|
| 23 June 2015 | New search has been performed | None RCTs were identified. |
| 7 May 2015 | New citation required but conclusions have not changed | We performed a literature search update and updated this Cochrane Review. |
Acknowledgements
We thank the Cochrane Haematological Malignancies Group Editors for their comments which improved the quality of this review. Many thanks to Céline Fournier for her sound and valuable comments given from a consumer's perspective, and to Copy‐Editor Jenny Bellorini. We wish to express our gratitude to Ina Monsef for her valuable endeavor for improving the quality of the search strategy.
The authors of this review update thank Daniel Simancas and Andrés Felipe Cardona, who were review authors of the first review version.
AMC is a PhD student at the Department of Pediatrics, Obstetrics and Gynecology, and Preventive Medicine of the Universitat Autònoma de Barcelona.
Appendices
Appendix 1. Glossary
| Term | Definition | Source |
| Ecchymosis | Extravasation of blood into the skin, resulting in a non‐elevated, rounded or irregular, blue or purplish patch, larger than a petechia. | http://onlinelibrary.wiley.com/cochranelibrary/search/mesh |
| Endothelium | A layer of epithelium that lines the heart, blood vessels (endothelium, vascular), lymph vessels (endothelium, lymphatic), and the serous cavities of the body. | |
| Fibrin | A protein derived from fibrinogen in the presence of thrombin, which forms part of the blood clot. | |
| Fibrinogen | Plasma glycoprotein clotted by thrombin, composed of a dimer of three non‐identical pairs of polypeptide chains (alpha, beta, gamma) held together by disulfide bonds. Fibrinogen clotting is a sol‐gel change involving complex molecular arrangements: whereas fibrinogen is cleaved by thrombin to form polypeptides A and B, the proteolytic action of other enzymes yields different fibrinogen degradation products. | |
| Fibrinolysin (plasmin) | A product of the lysis of plasminogen (profibrinolysin) by plasminogen activators. It is composed of two polypeptide chains, light (B) and heavy (A), with a molecular weight of 75,000. It is the major proteolytic enzyme involved in blood clot retraction or the lysis of fibrin and quickly inactivated by antiplasmins. | |
| Fibrinolysis | The natural enzymatic dissolution of fibrin. | |
| Fibrinolytic system | The fibrinolytic system in mammalian blood plays an important role in the dissolution of blood clots and in the maintenance of a patent vascular system. The fibrinolytic system contains an inactive proenzyme, plasminogen, which can be converted to the active enzyme, plasmin, which degrades fibrin into soluble fibrin degradation products. | Hoffman 2008 |
| Hemorrhage | Bleeding or escape of blood from a vessel. | http://onlinelibrary.wiley.com/cochranelibrary/search/mesh |
| Petechiae | Purplish or brownish red discoloration, easily visible through the epidermis, caused by hemorrhage into the tissues. When the size of the discolorization is > 2 to 3 cm it is generally called ecchymoses (ecchymosis). | |
| Plasminogen | Precursor of plasmin (fibrinolysin). It is a single‐chain beta‐globulin of molecular weight 80,000 to 90,000 kDa found mostly in association with fibrinogen in plasma; plasminogen activators change it to fibrinolysin. It is used in wound debriding and has been investigated as a thrombolytic agent. | |
| Shock | A pathological condition manifested by failure to perfuse or oxygenate vital organs. | |
| Thromboplastin | Constituent composed of protein and phospholipid that is widely distributed in many tissues. It serves as a cofactor with factor VIIa to activate factor X in the extrinsic pathway of blood coagulation. |
Appendix 2. CENTRAL search strategy
#1 MeSH descriptor: [Disseminated Intravascular Coagulation] explode all trees
#2 (intravascul* near/2 dissemin*)
#3 (consumpt* near/2 coagulopath*)
#4 Disseminated Intravascular Coagulation
#5 MeSH descriptor: [Hemorrhage] explode all trees
#6 (hemorrhage or haemorrhage)
#7 bleed*
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
#9 MeSH descriptor: [Anticoagulants] explode all trees
#10 anticoagulant*
#11 synthetic protease inhibitor*
#12 human recombinant activated protein c
#13 recombinant activated factor VIIa
#14 hirudin*
#15 antithrombin*
#16 thrombomodulin*
#17 recombinant tissue factor pathway inhibitor*
#18 tranexamic acid*
#19 low molecular weight heparin*
#20 unfractionated heparin*
#21 danaparoid sodium*
#22 heparin*
#23 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22
#24 MeSH descriptor: [Leukemia] explode all trees
#25 (leukem* or leukaem* or leucem*)
#26 MeSH descriptor: [Leukemia, Lymphoid] explode all trees
#27 MeSH descriptor: [Precursor Cell Lymphoblastic Leukemia‐Lymphoma] explode all trees
#28 lymph* near/3 lymphoblast*
#29 acut* or akut* or agud* or aigu*
#30 lympoblast* or lymphoid* or lymphocyt* or lymphat* or linfoc*tic* or linf*id* or linfoblastic*
#31 leukem* or leukaem* or leucem*
#32 #29 and #30 and #31
#33 philadelphia near/3 positiv*
#34 #24 or #25
#35 #26 or #27 or #28 or #32 or #33
#36 MeSH descriptor: [Leukemia, Promyelocytic, Acute] explode all trees
#37 acut* or akut* or agud* or aigu*
#38 (promyelocyt* or promielocitic* or promyelozyt* or progranulocyt*) and (leukem* or leukaem* or leuc*)
#39 #37 and #38
#40 #36 or #39
#41 MeSH descriptor: [Leukemia, Myeloid, Acute] explode all trees
#42 MeSH descriptor: [Leukemia, Myeloid] explode all trees
#43 MeSH descriptor: [Acute Disease] explode all trees
#44 #42 and #43
#45 acut* or akut* or agud* or aigu*
#46 ((myelo* or mielo* or nonlympho* or granulocytic*) and (leukem* or leukaem* or leuc*))
#47 #45 and #46
#48 aml
#49 #41 or #44 or #47 or #48
#50 #34 or #35 or #40 or #49
#51 #50 and #8 and #23 in Trials
#52 #50 and #8 in Trials
#53 #51 or #52
Appendix 3. MEDLINE OVID search strategy
| 1 | LEUKEMIA/ |
| 2 | (leuk?em$ or leuc?m$).tw,kf,ot. |
| 3 | exp LEUKEMIA, LYMPHOID/ |
| 4 | exp PRECURSOR CELL LYMPHOBLASTIC LEUKEMIA‐LYMPHOMA/ |
| 5 | (lymph$ adj3 lymphoblast$).tw,kf,ot. |
| 6 | (acut$ or akut$ or agud$ or aigu$).tw,kf,ot. |
| 7 | (lympoblast$ or lymphoid$ or lymphocyt$ or lymphat$ or linfoc?tic$ or linf?id$ or linfoblastic$).tw,kf,ot. |
| 8 | (leuk?em$ or leuc?m$).tw,kf,ot. |
| 9 | 6 and 7 and 8 |
| 10 | (philadelphia adj3 positiv$).tw,kf,ot. |
| 11 | or/1‐2 |
| 12 | or/3‐10 |
| 13 | LEUKEMIA, PROMYELOCYTIC, ACUTE/ |
| 14 | (acut$ or akut$ or agud$ or aigu$).tw,kf,ot. |
| 15 | ((promyelocyt$ or promielocitic$ or promyelozyt$ or progranulocyt$) and (leuk?em$ or leuc$)).tw,kf,ot. |
| 16 | 14 and 15 |
| 17 | 13 or 16 |
| 18 | exp LEUKEMIA, MYELOID, ACUTE/ |
| 19 | LEUKEMIA, MYELOID/ |
| 20 | ACUTE DISEASE/ |
| 21 | 19 and 20 |
| 22 | (acut$ or akut$ or agud$ or aigu$).tw,kf,ot. |
| 23 | ((myelo$ or mielo$ or nonlympho$ or granulocytic$) and (leuk?em$ or leuc$)).tw,kf,ot. |
| 24 | 22 and 23 |
| 25 | aml.tw,kf,ot. |
| 26 | 18 or 21 or 24 or 25 |
| 27 | DISSEMINATED INTRAVASCULAR COAGULATION/ |
| 28 | (intravascul$ adj2 dissemin$ adj coagula$).tw,kf,ot. |
| 29 | (consumpt$ adj2 coagulopath$).tw,kf,ot. |
| 30 | Hemorrhage/ |
| 31 | (hemorrhage or haemorrhage).tw,kf,ot. |
| 32 | bleed$.tw,kf,ot. |
| 33 | or/27‐32 |
| 34 | exp ANTICOAGULANTS/ |
| 35 | anticoagulant$.tw,kf,ot. |
| 36 | synthetic protease inhibitor$.tw,kf,ot. |
| 37 | human recombinant activated protein c.tw,kf,ot. |
| 38 | recombinant activated factor VIIa.tw,kf,ot. |
| 39 | hirudin$.tw,kf,nm,ot. |
| 40 | antithrombin$.tw,kf,nm,ot. |
| 41 | thrombomodulin$.tw,kf,nm,ot. |
| 42 | recombinant tissue factor pathway inhibitor$.tw,kf,nm,ot. |
| 43 | tranexamic acid$.tw,kf,nm,ot. |
| 44 | low molecular weight heparin$.tw,kf,nm,ot. |
| 45 | unfractionated heparin$.tw,kf,nm,ot. |
| 46 | danaparoid sodium$.tw,kf,nm,ot. |
| 47 | heparin$.tw,kf,nm,ot. |
| 48 | or/34‐47 |
| 49 | 11 or 12 or 17 or 26 |
| 50 | randomized controlled trial.pt. |
| 51 | controlled clinical trial.pt. |
| 52 | randomi?ed.ab. |
| 53 | placebo.ab. |
| 54 | clinical trials as topic.sh. |
| 55 | randomly.ab. |
| 56 | trial.ti. |
| 57 | or/50‐56 |
| 58 | humans.sh. |
| 59 | 57 and 58 |
| 60 | 49 and 33 and (48 or 59) |
Appendix 4. EMBASE search strategy
1. (random$ or placebo$).ti,ab.
2. ((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
3. controlled clinical trial$.ti,ab.
4. RETRACTED ARTICLE/
5. or/1‐4
6. (animal$ not human$).sh,tw.
7. 5 not 6
8. leukemia.exp
9. leukemia or acute leukemia or chronic leukemia
10. leukemia.sh,tw.
11. leukemia lymphoblast or lymphoid or lymphocytic or lymphatic or linfoctic or linfoid or linfoblastic
12. leukemia myeloid or myelogenous or myeloproliferative
13. leukemia promyelocyt or promielocitic or progranulocyt
14. or/8‐13
15. disseminated intravascular coagulation.exp
16. blood coagulation disorders.exp
17. purpura fulminans or intravascular coagulation or consumption coagulopathy
18. or/15‐17
19. 7 and 14 and 18
Appendix 5. LILACS search strategy
# 1 Disseminated intravascular coagulation # 2 Leukemia # 3 #1 AND 2
Appendix 6. WHO ICTRP search portal
# 1 Disseminated intravascular coagulation # 2 Leukemia OR Leukaemia # 3 #1 AND 2
Appendix 7. African Index Medicus search strategy
# 1 Disseminated intravascular coagulation # 2 Leukemia # 3 #1 AND 2
Appendix 8. Prefixed outcomes by review authors and assessed outcomes of the included trials
| Prefixed outcomes in this Cochrane Review | Outcomes assessed by the included trials | |||
| Aoki 2002 | ART‐123 Trial Group 2007 | Avvisati 1989 | Cofrancesco 1994 | |
Primary
Secondary
|
|
Primary: DIC resolution rate (rate of recovery from DIC) as assessed at 7 days after the start of infusion (or withdrawal). Secondary: clinical course of bleeding symptoms and mortality at 28 days after the start of infusion (the disappearance rate of bleeding symptoms at day seven). |
Likely: Thromboembolic complications (page 123). |
|
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aoki 2002.
| Methods | Design: parallel (2 groups) Study phase: III Country: Japan Follow‐up period: 28 days after the end of the infusion or until death Randomization unit: patient |
|
| Participants | Randomized: 132
Excluded post‐randomization (likely): 21.2% (28/132)
Analyzed patients for efficacy: 79% (104/132)
Analyzed patients for safety: (107/132)
Age (mean ± SD)
Gender
Patient with leukemia: 1. Activated protein C: Acute promyelocytic leukemia: 6 Non‐leukemias: 17 2. Heparin: Acute promyelocytic leukemia: 6 Non‐leukemias: 26 Inclusion criteria: Patients with DIC diagnosed primarily on the basis of criteria established by the DIC research group of the Ministry of Health and Welfare of Japan. Exclusion criteria:
|
|
| Interventions | Experimental:
Comparison:
The test drug (Human activated protein C, heparin or placebo) was added to 500 mL to 1000 mL of infusion fluid (5% glucose, physiological saline or electrolyte solution) and was administered intravenously at a constant rate for a total duration of 6 days.
|
|
| Outcomes | Symptoms DIC score and coagulation/fibrinolysis parameters Death from any cause during treatment or within 28 days after treatment Safety | |
| Notes | Identifier trial number: not reported Sample size calculation a priori: not declared Conduction time date: no reported Sponsor: not given This trial included 'leukemic group' patients and 'non‐leukemic group'. However, details of deaths in the 'leukemic group' were not given. On 19 August 2010 and 18 February 2014, we sent an e‐mail to the main trial author (aoki.hema@med.tmd.ac.jp), but we were unable to make contact. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk'. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk'. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk'. The trial was described as "double blind", however details were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Lost patients: 21.3%. There were no details of withdrawals by groups. |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. For leukemic patients there is no information on death from any cause. |
| Other bias | High risk |
|
ART‐123 Trial Group 2007.
| Methods | Design: parallel (2 groups) Country: Japan Multicenter: yes (113 sites, Japan) Study phase: III Follow‐up period: 28 days Randomization unit: patient |
|
| Participants | Randomized: 234
Treated: 99% (232/234)
Analyzed: 98% (227/232)
Analyzed for primary endpoint: 96.6% (224/232)
Age (years): Recombinant human soluble thrombomodulin
Heparin
Gender (male)
Type of leukemia: acute leukemia at first diagnosis or relapse Total patients with leukemia: 104 (44.4% of the randomized participants) 1. Recombinant human soluble thrombomodulin (54 patients (52% (54 /104)): Acute myeloblastic leukemia: 36 Acute promyelocytic leukemia: 3 Acute lymphoblastic leukemia: 14
2. Heparin (50 patients (48% (50 /104)): Acute myeloblastic leukemia: 35 Acute promyelocytic leukemia: 1 Acute lymphoblastic leukemia: 9 Chronic myelogenous leukemia: 5 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Experimental: recombinant human soluble thrombomodulin: 0.06 mg kg‐1 was drip infused for 30 minutes once‐daily for 6 consecutive days Comparison: heparin sodium (Fuso Pharmaceutical Industries, Ltd, Osaka, Japan): 8 U kg‐1 h‐1 was drip‐infused 24 hours a day for 6 consecutive days Patients received a continuous infusion of either placebo or tranexamic acid (2000 mg in 500 ml of 5% glucose in water, every 8 hours, total 6 g/day) for the 6 days of anti‐leukemic treatment Co‐intervention: Blood preparations (excluding antithrombin); heparin (≤ 1000 U day‐1) or urokinase (≤ 10 000 U per infusion) to prevent catheter coagulation, and drugs used to treat other conditions |
|
| Outcomes | Primary: DIC resolution rate (rate of recovery from DIC) as assessed at 7 days after the start of infusion (or withdrawal) Secondary: clinical course of bleeding symptoms and mortality at 28 days after the start of infusion |
|
| Notes | Study date: June 2000 and September 2005 Identifier trial number: not given Sample size calculation a priori: yes Sponsor: Asahi Kasei Pharma Role of sponsor: funded. "H. Asakura, M. Nakashima and N. Aoki have received consultancy funding from Asahi Kasei Pharma. Although it was not directly related to the current study, I. Maruyama has received research funding support from Asahi Kasei Pharma." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "were computer generated" (page 32). There is imbalance in age. |
| Allocation concealment (selection bias) | Low risk | — |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "were concealed from the investigators, patients and sponsor" (page 32). Insufficient information to permit judgment of 'low risk' or 'high risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost patients: 4.3% (N = 10); 5 patients from each group. Reasons:
There is no information on loss or withdrawal from the leukemia group |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis. For leukemic patients there is no information on death from any cause. |
| Other bias | High risk | Bias in the presentation of data (Porta 2008). |
Avvisati 1989.
| Methods | Design: parallel (2 groups) Multicenter: yes Country: Italy and the Netherlands Study phase: not given Follow‐up period: 14 days Randomization unit: patient |
|
| Participants | Randomized: 12 Age (median year (range))
Gender: 42% (male) Inclusion criteria:
Exclusion criteria: not given |
|
| Interventions | Experimental: tranexamic acid (1000 mg in 10 mL sterile water (KabiVitrum, Stockholm, Sweden)) Comparison: placebo: glucose 5% in 10 mL sterile water Patients received a continuous infusion of either placebo or tranexamic acid (2000 mg in 500 mL of 5% glucose in water, every 8 hours, total 6 g/day) for the 6 days of anti‐leukemic treatment Co‐intervention:
|
|
| Outcomes |
Likely: Thromboembolic complications (page 123) |
|
| Notes | Identifier trial number: not given Study date: not reported Sample size calculation a priori: no Sponsor: Amstol Foundation (A 584), the Netherlands, the Italian National Academy Research Council and Royal Dutch Academy of Sciences Fellowships Role of sponsor: not given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk'. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk'. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Patients and attending physicians were blinded to the treatment groups" (page 122). Reported no information on how participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data. |
| Selective reporting (reporting bias) | High risk | Comments: trial failed to include results of a key outcome that would have been expected to have been reported, i.e. mortality. |
| Other bias | High risk |
|
Cofrancesco 1994.
| Methods | Design: parallel (2 groups) Multicenter: no Study phase: not described Country: Italy Follow‐up period: 15 days (median), range: 9 to 18 days Randomization unit: patient |
|
| Participants | Randomized: 10 Age (years ± SD): (data calculated by review authors)
Gender: 60% (male) Type of leukemia: acute leukemia at first diagnosis or relapse 1. Dermatan sulphate group (# of patients): Acute myeloblastic leukemia, with granulocytic maturation: 1 Acute promyelocytic leukemia: 2 Acute lymphoblastic leukemia: 1 Crisis blastic‐chronic myelogenous leukemia: 1 2. Heparin group (# of patients): Acute monoblastic leukemia: 2 Acute promyelocytic leukemia: 1 Acute lymphoblastic leukemia: 1 Crisis blastic‐chronic myelogenous leukemia: 1 Inclusion criteria:
Exclusion criteria: not given |
|
| Interventions | Experimental: dermatan sulphate (MF 701, Mediolanum Farmaceutici) by continuous infusion through a percutaneous central vein catheter (fixed rate: 0.3 mg/kg/hour) Comparison: Sodium heparin (Liquemine, Roche) by continuous infusion through a percutaneous central vein catheter (fixed rate: 8.5 U/kg/hour) Co‐intervention:
|
|
| Outcomes | Clinically overt bleeding until hospital discharge
The type and amount of daily transfusions Likely (page 72): These outcomes were reported in the results section: death during the study treatment and clinically apparent thromboembolic complications |
|
| Notes | Identifier trial number: not given Date of study: not given Sample size calculation a priori: no Sponsor: I.R.C.C.S. Ospedale Maggiore, Milan (by a grant 12/1988) Role of sponsor: not given One trial author was associated with the drug company manufacturer of the intervention (Mediolanum Farmaceutici) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk'. "patients were randomly assigned to receive" (page 66). Both groups were not comparable by white blood cell count, platelet count and hemoglobin level at the study entry. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk'. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to permit judgment of 'low risk' or 'high risk'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | — |
| Selective reporting (reporting bias) | High risk | One or more outcomes of interest in the review were reported incompletely so that they could not be entered in a meta‐analysis.Trial authors did not report safety data. |
| Other bias | High risk |
|
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aoki 1994 | Descriptive study. |
| Asakura 2014 | Post‐marketing surveillance study. |
| Aviles de la Garza 1982 | Case series involving patients with leukemia and another clinical conditions. |
| Chojnowski 2002 | A RCT for preventing DIC using low molecular weight heparin. |
| Fourrier 1993 | A RCT excluding patients with leukemia. |
| Franchini 2007 | Systematic review which does not reported inclusion or exclusion of leukemic patients. |
| Gamba 1979 | Case series. |
| Gando 2013 | A RCT excluding patients with history of hematopoietic malignancy. |
| Gillis 1995 | Case series. |
| Glarnick 1972 | Case series. |
| Goldberg 1987 | Case series. |
| Gross 1982 | A RCT that reported no information on inclusion or exclusion patients with leukemia. |
| Göbel 1980 | A RCT that reported no information on inclusion or exclusion patients with leukemia. |
| Hoffmann 2004 | Clinical trial that included no patients with leukemia. |
| Hofmann 1997 | A RCT that included no patients with leukemia. |
| Iba 2012 | Non‐randomized study. It does not report inclusion or exclusion of leukemic patients. |
| Ikezoe 2012 | Trial conducted using historical controls. |
| Kato 2013 | Retrospective cohort study. None information on leukemia. |
| Kawano 2011 | Observational study including patients with leukemia. |
| Koseki 2007 | Case report. |
| KYBERSEPT 2006 | A RCT that reported no information on inclusion or exclusion patients with leukemia. |
| Liu 2014 | A RCT that included no patients with leukemia. |
| Martínez López 2002 | Case report. |
| Matsushita 2014 | Retrospective analysis of an open‐label, multicenter, post‐marketing surveillance study cohort. |
| Nieuwenhuis 1986 | Case series. |
| Nishiyama 2012 | A RCT excluding patients with leukemia. |
| Nowrousian 1978 | Case series. |
| Ogawa 2010 | Case report. |
| Oguma 1990 | Reported no information on leukemia group. |
| Pizzuto 1969 | Case report. |
| Pogliani 1989 | Case series. |
| Rodríguez Gómez 1993 | Case series. |
| Saito 1995 | Case series. |
| Sakuragawa 1992 | Controlled clinical trial. |
| Sandler 1982 | Case report. |
| Sawamura 2009 | Retrospective observational trial included no patients with leukemia. |
| Shindo 2012 | Case report. |
| Shirahata 2014 | Case series. It included only 2 leukemia‐patients. |
| Shpilberg 1995 | RCT involving patients with acute myeloid leukemia without DIC. |
| Sugawara 1992 | Case report. |
| Takagi 2011 | Case report. |
| Takezako 2014 | Retrospective observational study. |
| Vinazzer 1989 | Case series did not report inclusion patients with leukemia. |
| Vincent 2013 | RCT excluded patients receiving chemotherapy. |
| Wada 1995 | Controlled clinical trial. |
| Walia 2012 | Narrative review. |
| Yagamuchi 2001 | Case report. |
| Yamato 2013 | Controlled clinical trial. |
| Yang 2014 | Retrospective observational study. |
| Yin 2014 | Retrospective observational study. It did not included patients with leukemia. |
Differences between protocol and review
We included bleeding as a clinical outcome post hoc at the suggestions of the peer reviewers and Co‐ordinating Editor of the Cochrane Haematological Malignancies Group. It is an important outcome for patients and physicians.
Our protocol did not describe the intention to present a 'Summary of findings' table, whereas the review states this intention.
We considered that the 'Adverse events' are clinical relevant outcomes for patients and physicians. Therefore, for this update it appears as a primary outcome.
In the previously published review, Marti‐Carvajal 2011, we searched EMBASE. For this review update, we did not conduct a search using that database.
Contributions of authors
AMC wrote the first draft of this review update with subsequent input from VA and IS. AMC is guarantor for the review.
Sources of support
Internal sources
Any institution funded this Cochrane Review update, Venezuela.
External sources
-
Iberoamerican Cochrane Centre, Spain.
Academic
-
Cochrane Haematological Malignancies Group, Germany.
Academic
Declarations of interest
In 2004 and 2007 AMC was employed by Eli Lilly to run a four‐hour workshop on 'How to critically appraise clinical trials on osteoporosis and how to teach this'. This activity was not related to his work with Cochrane or any Cochrane Review. VA: none known. IS: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aoki 2002 {published data only}
- Aoki N, Matsuda T, Saito H, Takatsuki K, Okajima K, Takahashi H, et al. A comparative double‐blind randomized trial of activated protein C and unfractionated heparin in the treatment of disseminated intravascular coagulation. International Journal of Hematology 2002;75(5):540‐7. [PUBMED: 12095157] [DOI] [PubMed] [Google Scholar]
ART‐123 Trial Group 2007 {published data only}
- Aikawa N, Shimazaki S, Yamamoto Y, Saito H, Maruyama I, Ohno R, et al. Thrombomodulin alfa in the treatment of infectious patients complicated by disseminated intravascular coagulation: subanalysis from the phase 3 trial. Shock 2011;35(4):349‐54. [PUBMED: 21068698] [DOI] [PubMed] [Google Scholar]
- Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART‐123) in disseminated intravascular coagulation (DIC): results of phase III randomized, double‐blind, clinical trial [Abstract No. 576]. Blood 2006;108(11 Part 1):174. [CN‐00624858] [DOI] [PubMed] [Google Scholar]
- Saito H, Maruyama I, Shimazaki S, Yamamoto Y, Aikawa N, Ohno R, et al. Efficacy and safety of recombinant human soluble thrombomodulin (ART‐123) in disseminated intravascular coagulation: results of a phase III, randomized, double‐blind clinical trial. Journal of Thrombosis and Hemostasis 2007;5(1):31‐41. [PUBMED: 17059423] [DOI] [PubMed] [Google Scholar]
Avvisati 1989 {published data only}
- Avvisati G, Cate JW, Büller HR, Mandelli F. Tranexamic acid for control of haemorrhage in acute promyelocytic leukaemia. Lancet 1989;2(8655):122‐4. [PUBMED: 2567893] [DOI] [PubMed] [Google Scholar]
Cofrancesco 1994 {published data only}
- Cofrancesco E, Boschetti C, Leonardi P, Cortellaro M. A randomized, heparin (H)‐controlled, pilot study of dermatan sulfate (DS) in the treatment of disseminated intravascular coagulation in acute leukemia [abstract]. Thrombosis Research 1993;70(Suppl 1):74. [DOI] [PubMed] [Google Scholar]
- Cofrancesco E, Boschetti C, Leonardi P, Cortellaro M. Dermatan sulphate in acute leukaemia. Lancet 1992;339(8802):1177‐8. [PUBMED: 1349404] [DOI] [PubMed] [Google Scholar]
- Cofrancesco E, Boschetti C, Leonardi P, Gianese F, Cortellaro M. Dermatan sulphate for the treatment of disseminated intravascular coagulation (DIC) in acute leukemia: a randomised, heparin‐controlled pilot study. Thrombosis Research 1994;74(1):65‐75. [PUBMED: 8029809] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aoki 1994 {published data only}
- Aoki N, Hirosawa S, Kurosawa S, Matsuda T, Saito H, Takamatsu J, et al. Clinical evaluation of low molecular weight heparin preparation (LHN‐1) on disseminated intravascular coagulation (DIC): multicenter phase II clinical study. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1994;10(12):2629‐65. [Google Scholar]
Asakura 2014 {published data only}
- Asakura H, Takahashi H, Tsuji H, Matsushita T, Ninomiya H, Honda G, et al. Post‐marketing surveillance of thrombomodulin alfa, a novel treatment of disseminated intravascular coagulation ‐ safety and efficacy in 1,032 patients with hematologic malignancy. Thrombosis Research 2014;133(3):364‐70. [DOI] [PubMed] [Google Scholar]
Aviles de la Garza 1982 {published data only}
- Aviles de la Garza R, Perez Tamayo R, Becker Fause I, Linares Ponton G, Anaya Galindo R. Intravascular disseminated coagulation. Critical analysis of a 5 years experience at the Instituto Nacional de la Nutrición Salvador Zubirán (Mexico) [Coagulación intravascular diseminada. Análisis crítico de la experiencia del Instituto Nacional de la Nutricion Salvador Zubirán en 5 años]. Revista de Investigación Clínica 1982;34(4):307‐12. [13075 (LILACS)] [PubMed] [Google Scholar]
Chojnowski 2002 {published data only}
- Chojnowski K, Trelinski J, Wawrzyniak E, Robak T. The influence of low molecular weight heparin on the intravascular activation of the coagulation system in patients with acute leukemia during induction chemotherapy‐‐report of a prospective randomized study. Leukemia & Lymphoma 2002;43(5):1021‐8. [PUBMED: 12148881] [DOI] [PubMed] [Google Scholar]
Fourrier 1993 {published data only}
- Fourrier F, Chopin C, Huart J J, Runge I, Caron C, Goudemand J. Double‐blind, placebo‐controlled trial of antithrombin III concentrates in septic shock with disseminated intravascular coagulation. Chest 1993;104(3):882‐8. [PUBMED: 8365305] [DOI] [PubMed] [Google Scholar]
Franchini 2007 {published data only}
- Franchini M, Manzato F, Salvagno GL, Lippi G. Potential role of recombinant activated factor VII for the treatment of severe bleeding associated with disseminated intravascular coagulation: a systematic review. Blood Coagulation & Fibrinolysis 2007;18(7):589‐93. [PUBMED: 17890943] [DOI] [PubMed] [Google Scholar]
Gamba 1979 {published data only}
- Gamba G, Grignani G, Biancardi M, Geroldi D, Ascari E. Acute leukemias and D.I.C.: effectiveness of treatment with low doses of heparin. Haematologica 1979;64(3):322‐30. [PUBMED: 113300] [PubMed] [Google Scholar]
Gando 2013 {published data only}
- Gando S, Saitoh D, Ishikura H, Ueyama M, Otomo Y, Oda S, et al. A randomized, controlled, multicenter trial of the effects of antithrombin on disseminated intravascular coagulation in patients with sepsis. Critical Care 2013;17(6):R297. [PUBMED: 24342495] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillis 1995 {published data only}
- Gillis S, Dann EJ, Eldor A. Low molecular weight heparin in the prophylaxis and treatment of disseminated intravascular coagulation in acute promyelocytic leukemia. European Journal of Hematology 1995;54(1):59‐60. [PUBMED: 7859878] [DOI] [PubMed] [Google Scholar]
Glarnick 1972 {published data only}
- Gralnick HR, Bagley J, Abrell E. Heparin treatment for the hemorrhagic diathesis of acute promyelocytic leukemia. American Journal of Medicine 1972;52(2):167‐74. [PUBMED: 4500954] [DOI] [PubMed] [Google Scholar]
Göbel 1980 {published data only}
- Göbel U, Voss H, Jurgens H, Petrich C, Pothmann R, Sprock I, et al. Efficiency of heparin in the treatment of newborn infants with respiratory distress syndrome and disseminated intravascular coagulation. European Journal of Pediatrics 1980;133(1):47‐9. [PUBMED: 7353570] [DOI] [PubMed] [Google Scholar]
Goldberg 1987 {published data only}
- Goldberg MA, Ginsburg D, Mayer RJ, Stone RM, Maguire M, Rosenthal DS, et al. Is heparin administration necessary during induction chemotherapy for patients with acute promyelocytic leukemia?. Blood 1987;69(1):187‐91. [PUBMED: 3466655] [PubMed] [Google Scholar]
Gross 1982 {published data only}
- Gross SJ, Filston HC, Anderson JC. Controlled study of treatment for disseminated intravascular coagulation in the neonate. Journal of Pediatrics 1982;100(3):445‐8. [PUBMED: 7038076] [DOI] [PubMed] [Google Scholar]
Hoffmann 2004 {published data only}
- Hoffmann JN, Mühlbayer D, Jochum M, Inthorn D. Effect of long‐term and high‐dose antithrombin supplementation on coagulation and fibrinolysis in patients with severe sepsis. Critical Care Medicine 2004;32(9):1851‐9. [PUBMED: 15343012] [DOI] [PubMed] [Google Scholar]
Hofmann 1997 {published data only}
- Hofmann M, Rest A, Hafner G, Tanner B, Brockerhoff P, Weilemann LS. Evaluation of coagulation and fibrinolytics parameters in the therapeutic management of disseminated intravascular coagulation with sepsis by low molecular weight/heparin [German]. Der Anaesthesist 1997;46(8):689‐96. [PUBMED: 9382207] [DOI] [PubMed] [Google Scholar]
Iba 2012 {published data only}
- Iba T, Saito D, Wada H, Asakura H. Efficacy and bleeding risk of antithrombin supplementation in septic disseminated intravascular coagulation: a prospective multicenter survey. Thrombosis Research 2012;130(3):e129‐33. [PUBMED: 22542365] [DOI] [PubMed] [Google Scholar]
Ikezoe 2012 {published data only}
- Ikezoe T, Takeuchi A, Isaka M, Arakawa Y, Iwabu N, Kin T, et al. Recombinant human soluble thrombomodulin safely and effectively rescues acute promyelocytic leukemia patients from disseminated intravascular coagulation. Leukemia Research 2012;36(11):1398‐402. [PUBMED: 22917769] [DOI] [PubMed] [Google Scholar]
Kato 2013 {published data only}
- Kato T, Sakai T, Kato M, Hagihara M, Hasegawa T, Matsuura K, et al. Recombinant human soluble thrombomodulin administration improves sepsis‐induced disseminated intravascular coagulation and mortality: a retrospective cohort study. Thrombosis Journal 2013;11(1):3. [PUBMED: 23414216] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kawano 2011 {published data only}
- Kawano N, Kuriyama T, Yoshida S, Yamashita K, Ochiai H, Nakazaki S, et al. Clinical features and treatment outcomes of six patients with disseminated intravascular coagulation resulting from acute promyelocytic leukemia and treated with recombinant human soluble thrombomodulin at a single institution. Internal Medicine 2013;52(1):55‐62. [PUBMED: 23291674] [DOI] [PubMed] [Google Scholar]
- Kawano N, Tasaki A, Kuriyama T, Tahara Y, Yoshida S, Ono N, et al. Effects of recombinant human soluble thrombomodulin treatment for disseminated intravascular coagulation at a single institution‐‐an analysis of 62 cases caused by infectious diseases and 30 cases caused by hematological diseases. Internal Medicine 2014;53(3):205‐13. [PUBMED: 24492688] [DOI] [PubMed] [Google Scholar]
- Kawano N, Yoshida S, Ono N, Himeji D, Nagahiro Y, Sayaka K, et al. Clinical features and outcomes of 35 disseminated intravascular coagulation cases treated with recombinant human soluble thrombomodulin at a single institution. Journal of Clinical and Experimental Hematopathology 2011;51(2):101‐7. [PUBMED: 22104308] [DOI] [PubMed] [Google Scholar]
Koseki 2007 {published data only}
- Koseki M, Asada N, Uryu H, Takeuchi M, Asakura H, Matsue K. Successful combined use of tranexamic acid and unfractionated heparin for life‐threatening bleeding associated with intravascular coagulation in a patient with chronic myelogenous leukemia in blast crisis. International Journal of Hematology 2007;86(5):403‐6. [PUBMED: 18192107] [DOI] [PubMed] [Google Scholar]
KYBERSEPT 2006 {published data only}
- Kienast J, Juers M, Wiedermann CJ, Hoffmann JN, Ostermann H, Strauss R, et al. Treatment effects of high‐dose antithrombin without concomitant heparin in patients with severe sepsis with or without disseminated intravascular coagulation. Journal of Thrombosis and Haemostasis 2006;4(1):90‐7. [PUBMED: 16409457] [DOI] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu XL, Wang XZ, Liu XX, Hao D, Jaladat Y, Lu F, et al. Low‐dose heparin as treatment for early disseminated intravascular coagulation during sepsis: A prospective clinical study. Experimental and Therapeutic Medicine 2014;7(3):604‐8. [PUBMED: 24520253] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martínez López 2002 {published data only}
- Martínez López I, Jiménez Armada J, Ballmajó Real M, Sánchez García RW, Solano Rolando M. Fulminant disseminated intravascular coagulation in a patient with acute promyelocytic leukaemia [Coagulación intravascular diseminada fulminante en una paciente con leucemia promielocítica aguda]. Revista Cubana de Medicina 2002;4(1):303‐7. [354369 (LILACS)] [Google Scholar]
Matsushita 2014 {published data only}
- Matsushita T, Watanabe J, Honda G, Mimuro J, Takahashi H, Tsuji H, et al. Thrombomodulin alfa treatment in patients with acute promyelocytic leukemia and disseminated intravascular coagulation: a retrospective analysis of an open‐label, multicenter, post‐marketing surveillance study cohort. Thrombosis Research 2014;133(5):772‐81. [PUBMED: 24636871] [DOI] [PubMed] [Google Scholar]
Nieuwenhuis 1986 {published data only}
- Nieuwenhuis HK, Sixma JJ. Treatment of disseminated intravascular coagulation in acute promyelocytic leukemia with low molecular weight heparinoid Org 10172. Cancer 1986;58(3):761‐4. [PUBMED: 2425925] [DOI] [PubMed] [Google Scholar]
Nishiyama 2012 {published data only}
- Nishiyama T, Kohno Y, Koishi K. Effects of antithrombin and gabexate mesilate on disseminated intravascular coagulation: a preliminary study. American Journal of Emergency Medicine 2012;30(7):1219‐23. [PUBMED: 22204993] [DOI] [PubMed] [Google Scholar]
Nowrousian 1978 {published data only}
- Nowrousian MR, Hilgard P. Low‐dose heparin in the management of acute myelocytic leukaemia. Blut 1978;37(6):341‐3. [PUBMED: 281971] [DOI] [PubMed] [Google Scholar]
Ogawa 2010 {published data only}
- Ogawa E, Yagasaki H, Kato M, Shichino H, Chin M, Mugishima H. Successful treatment of disseminated intravascular coagulation in a child with acute myelogenous leukaemia using recombinant thrombomodulin. British Journal of Haematology 2010;149(6):911‐2. [PUBMED: 20346017] [DOI] [PubMed] [Google Scholar]
Oguma 1990 {published data only}
- Oguma Y, Sakuragawa N, Maki M, Hasegawa H, Nakagawa M. Treatment of disseminated intravascular coagulation with low molecular weight heparin. Research Group of FR‐860 on DIC in Japan. Seminars in Thrombosis and Hemostasis 1990;16 Suppl:34‐40. [PUBMED: 1962902] [PubMed] [Google Scholar]
- Oguma Y, Sakuragawa N, Maki M, Nakagawa M, Hasegawa H. Clinical effect of low molecular weight heparin (fragmin) on DIC: a multicenter cooperative study in Japan. Thrombosis Research 1990;59(1):37‐49. [PUBMED: 2169078] [DOI] [PubMed] [Google Scholar]
Pizzuto 1969 {published data only}
- Pizzuto J, Gonzalez Llaven J, Uribe Cortes JA. Heparin as treatment for hypercoagulability syndrome in a case of acute promyelocytic leukemia [Spanish]. Sangre 1969;14(3):363‐70. [PUBMED: 5288844] [PubMed] [Google Scholar]
Pogliani 1989 {published data only}
- Pogliani EM, Salvatore M, Masera G, Corneo G. Antifibrinolytic therapy in acute promyelocytic leukemia. Acta Haematologica 1989;81(1):58‐9. [PUBMED: 2494837] [DOI] [PubMed] [Google Scholar]
Rodríguez Gómez 1993 {published data only}
- Rodríguez Gómez M, López Fernández MF, Batlle J. Therapy of disseminated intravascular coagulation in acute promyelocytic leukemias. Apropos of 19 cases [Terapéutica de la coagulación intravascular diseminada en las leucemias agudas promielocíticas. A propósito de 19 casos]. Sangre 1993;38(6):449‐53. [PUBMED: 8171380] [PubMed] [Google Scholar]
Saito 1995 {published data only}
- Saito M, Asakura H, Jokaji H, Uotani C, Kumabashiri I, Morishita E, et al. Recombinant hirudin for the treatment of disseminated intravascular coagulation in patients with haematological malignancy. Blood Coagulation & Fibrinolysis 1995;6(1):60‐4. [PUBMED: 7540878] [DOI] [PubMed] [Google Scholar]
Sakuragawa 1992 {published data only}
- Sakuragawa N, Hasegawa H, Maki M, Nakagawa M, Nakashima M. Clinical evaluation of low molecular weight heparin (FR‐860) on disseminated intravascular coagulation (DIC): a multicenter cooperative double‐blind trial in comparison with heparin. Rinsho Iyaku (Journal of Clinical Therapeutics and Medicines) 1992;8(2):423‐52. [Google Scholar]
- Sakuragawa N, Hasegawa H, Maki M, Nakagawa M, Nakashima M. Clinical evaluation of low‐molecular‐weight heparin (FR‐860) on disseminated intravascular coagulation (DIC) ‐ a multicenter co‐operative double‐blind trial in comparison with heparin. Thrombosis Research 1993;72(6):475‐500. [PUBMED: 8128454] [DOI] [PubMed] [Google Scholar]
Sandler 1982 {published data only}
- Sandler RM, Liebman HA, Patch MJ, Teitelbaum A, Levine AM, Feinstein DI. Antithrombin III and anti‐activated factor X activity in patients with acute promyelocytic leukemia and disseminated intravascular coagulation treated with heparin. Cancer 1982;50(10):2106‐10. [PUBMED: 6957257] [DOI] [PubMed] [Google Scholar]
- Sandler RM, Liebman HA, Patch MJ, Teitelbaum A, Levine AM, Feinstein DI. Antithrombin III and anti‐activated factor X activity in patients with acute promyelocytic leukemia and disseminated intravascular coagulation treated with heparin. Cancer 1983;51(4):681‐5. [PUBMED: 6571800] [DOI] [PubMed] [Google Scholar]
Sawamura 2009 {published data only}
- Sawamura A, Gando S, Hayakawa M, Hoshino H, Kubota N, Sugano M. Effects of antithrombin III in patients with disseminated intravascular coagulation diagnosed by newly developed diagnostic criteria for critical illness. Clinical and Applied Thrombosis/Hemostasis 2009;15(5):561‐6. [PUBMED: 18840625] [DOI] [PubMed] [Google Scholar]
Shindo 2012 {published data only}
- Shindo M, Ikuta K, Addo L, Ito S, Fujiya M, Torimoto Y, et al. Successful control of disseminated intravascular coagulation by recombinant thrombomodulin during arsenic trioxide treatment in relapsed patient with acute promyelocytic leukemia. Case Reports in Hematology 2012;2012:908196. [PUBMED: 23008787] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shirahata 2014 {published data only}
- Shirahata A, Mimuro J, Takahashi H, Kitajima I, Tsuji H, Eguchi Y, et al. Recombinant soluble human thrombomodulin (thrombomodulin alfa) in the treatment of neonatal disseminated intravascular coagulation. European Journal of Pediatrics 2014;173(3):303‐11. [PUBMED: 24005342] [DOI] [PubMed] [Google Scholar]
Shpilberg 1995 {published data only}
- Shpilberg O, Blumenthal R, Sofer O, Eldor A, Ben Bassat I. A controlled trial of tranexamic acid (TA) treatment for reduction of bleeding during acute myeloid leukemia (AML) induction and consolidation. Blood 1993;82(10 Suppl 1):547a. [CN‐00461734] [DOI] [PubMed] [Google Scholar]
- Shpilberg O, Blumenthal R, Sofer O, Katz Y, Chetrit A, Ramot B, et al. A controlled trial of tranexamic acid therapy for the reduction of bleeding during treatment of acute myeloid leukemia. Leukemia & Lymphoma 1995;19(1‐2):141‐4. [PUBMED: 8574160] [DOI] [PubMed] [Google Scholar]
Sugawara 1992 {published data only}
- Sugawara T, Okuda M, Yamaguchi Y, Endo K, Yoshinaga K. Successful treatment with tranexamic acid for severe bleeding in acute promyelocytic leukemia. Acta Haematologica 1992;87(1‐2):109. [PUBMED: 1585765] [DOI] [PubMed] [Google Scholar]
Takagi 2011 {published data only}
- Takagi K, Tasaki T, Yamauchi T, Iwasaki H, Ueda T. Successful administration of recombinant human soluble thrombomodulin (Recomodulin) for disseminated intravascular coagulation during induction chemotherapy in an elderly patient with acute monoblastic leukemia involving the t(9;11)(p22;q23) MLL/AF9 translocation. Case Reports in Hematology 2011;2011:273070. [PUBMED: 22937304] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takezako 2014 {published data only}
- Takezako N, Sekiguchi N, Noto S, Ogino Y, Miwa A, Ikezoe T. Recombinant human thrombomodulin in the treatment of acute myeloid leukemia patients complicated by disseminated intravascular coagulation: Results of a multicenter, retrospective epidemiologic study in Japan. Blood. 2014; Vol. 124:2875. [CN‐01049686]
Vinazzer 1989 {published data only}
- Vinazzer H. Therapeutic use of antithrombin III in shock and disseminated intravascular coagulation. Seminars in Thrombosis and Hemostasis 1989;15(3):347‐52. [PUBMED: 2688106] [DOI] [PubMed] [Google Scholar]
Vincent 2013 {published data only}
- Vincent JL, Ramesh MK, Ernest D, LaRosa SP, Pachl J, Aikawa N, et al. A randomized, double‐blind, placebo‐controlled, Phase 2b study to evaluate the safety and efficacy of recombinant human soluble thrombomodulin, ART‐123, in patients with sepsis and suspected disseminated intravascular coagulation. Critical Care Medicine 2013;41(9):2069‐79. [PUBMED: 23979365] [DOI] [PubMed] [Google Scholar]
Wada 1995 {published data only}
- Wada H, Wakita Y, Nakase T, Shimura M, Hiyoyama K, Nagaya S, et al. Outcome of disseminated intravascular coagulation in relation to the score when treatment was begun. Mie DIC Study Group. Thrombosis and Haemostasis 1995;74(3):848‐52. [PUBMED: 8571309] [PubMed] [Google Scholar]
Walia 2012 {published data only}
- Walia SS, Walia HS. Therapeutic interventions in endotoxin‐induced disseminated intravascular coagulation. Journal of Natural Science, Biology, and Medicine 2012;3(1):108‐9. [PUBMED: 22690065] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yagamuchi 2001 {published data only}
- Yamaguchi M, Murata R, Mizutani T, Kawamura Y, Ueda M. Acute myelogenous leukemia associated with disseminated intravascular coagulation and acute myocardial infarction at relapse [Japanese]. Rinsho Ketsueki (Japanese Journal of Clinical Haematology) 2001;42(9):716‐8. [PUBMED: 11680986] [PubMed] [Google Scholar]
Yamato 2013 {published data only}
- Yamato M, Minematsu Y, Fujii J, Mori K, Minato T, Miyagawa S, et al. Effective combination therapy of polymyxin‐B direct hemoperfusion and recombinant thrombomodulin for septic shock accompanied by disseminated intravascular coagulation: a historical controlled trial. Therapeutic Apheresis and Dialysis 2013;17(5):472‐6. [PUBMED: 24107274] [DOI] [PubMed] [Google Scholar]
Yang 2014 {published data only}
- Yang H, Zhu CY, Wang QS, Niu JH, Zhang Q, Zhu HY, et al. Analysis of empirical treatment for newly diagnosed acute promyelocytic leukemia combined with disseminated intravascular coagulation [Chinese]. Zhongguo Shi Yan Xue Ye Xue Za Zhi/Zhongguo Bing li Sheng li Xue hui/Journal of Experimental Hematology/Chinese Association of Pathophysiology 2014;22(2):315‐22. [PUBMED: 24762998] [DOI] [PubMed] [Google Scholar]
Yin 2014 {published data only}
- Yin Q, Li C. Treatment effects of xuebijing injection in severe septic patients with disseminated intravascular coagulation. Evidence‐based Complementary and Alternative Medicine 2014;2014:949254. [PUBMED: 24778706] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abshire 2004
- Abshire T, Kenet G. Recombinant factor VIIa: review of efficacy, dosing regimens and safety in patients with congenital and acquired factor VIII or IX inhibitors. Journal of Thrombosis and Haemostasis 2004;2(6):899‐909. [PUBMED: 15140125] [DOI] [PubMed] [Google Scholar]
Asthana 2009
- Asthana B, Sharma P, Ranjan R, Jain P, Aravindan A, Chandra Mishra P, et al. Patterns of acquired bleeding disorders in a tertiary care hospital. Clinical and Applied Thrombosis/Hemostasis 2009;15(4):448‐53. [PUBMED: 19520680] [DOI] [PubMed] [Google Scholar]
Barbui 2001
- Barbui T, Falanga A. Disseminated intravascular coagulation in acute leukemia. Seminars in Thrombosis and Hemostasis 2001;27(6):593‐604. [PUBMED: 11740683] [DOI] [PubMed] [Google Scholar]
Brunton 2008
- Brunton L, Parker K, Blumenthal D, Buxton I. Blood coagulation and anticoagulant, thrombolytic, and antiplatelet drugs. In: Brunton L, Parker K, Blumenthal D, Buxton I editor(s). Goodman & Gilman's Manual of Pharmacology and Therapeutics. 11th Edition. New York: McGraw‐Hill Companies, Inc, 2008:949‐66. [DOI: 10.1036/0071443436] [DOI] [Google Scholar]
Bunjevacki 1978
- Bunjevacki G, Sultan Y, Izraël V. [Disseminated intravascular coagulation (D.I.C.) and fibrinolysis in patients with acute leukemia (author's transl)] [Coagulation intravasculaire disséminée et fibrinolyse au cours des leucemies aiguës]. Nouvelle Revue Française d'Hématologie 1978;20(4):575‐84. [PUBMED: 752151] [PubMed] [Google Scholar]
Button 2013
- Button KS, Ioannidis JP, Mokrysz C, Nosek BA, Flint J, Robinson ES, et al. Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews. Neuroscience 2013;14(5):365‐76. [PUBMED: 23571845] [DOI] [PubMed] [Google Scholar]
Calvert 2013
- Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD. Reporting of patient‐reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013;309(8):814‐22. [PUBMED: 23443445] [DOI] [PubMed] [Google Scholar]
Chan 2013
- Chan AW, Tetzlaff JM, Gøtzsche PC, Altman DG, Mann H, Berlin JA, at al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. [PUBMED: 23303884] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choudhry 2012
- Choudhry A, DeLoughery TG. Bleeding and thrombosis in acute promyelocytic leukemia. American Journal of Hematology 2012;87(6):596‐603. [PUBMED: 22549696] [DOI] [PubMed] [Google Scholar]
Chu 2004
- Chu AJ. Biochemical strategies to anticoagulation: a comparative overview. Current Vascular Pharmacology 2004;2(3):199‐228. [PUBMED: 15320820] [DOI] [PubMed] [Google Scholar]
Clarke 2007
- Clarke M. Standardising outcomes for clinical trials and systematic reviews. Trials 2007;8:39. [PUBMED: 18039365] [DOI] [PMC free article] [PubMed] [Google Scholar]
Concise Medical Dictionary 2011
- Martin E. Concise Medical Dictionary. 8th Edition. New York: Oxford University Press, 2011. [Google Scholar]
Cornell 2012
- Cornell RF, Palmer J. Adult acute leukemia. Disease‐a‐Month 2012;58(4):219‐38. [PUBMED: 22449370] [DOI] [PubMed] [Google Scholar]
Dalainas 2008
- Dalainas I. Pathogenesis, diagnosis, and management of disseminated intravascular coagulation: a literature review. European Review for Medical and Pharmacological Sciences 2008;12(1):19‐31. [PUBMED: 18401969] [PubMed] [Google Scholar]
DeLoughery 2005
- DeLoughery TG. Critical care clotting catastrophies. Critical Care Clinics 2005;21(3):531‐62. [PUBMED: 15992672] [DOI] [PubMed] [Google Scholar]
Dixit 2007
- Dixit A, Chatterjee T, Mishra P, Kannan M, Choudhry DR, Mahapatra M, et al. Disseminated intravascular coagulation in acute leukemia at presentation and during induction therapy. Clinical and Applied Thrombosis/Hemostasis 2007;13(3):292‐8. [PUBMED: 17636191] [DOI] [PubMed] [Google Scholar]
Dmoszyńska 1976
- Dmoszyńska A. Intravascular disseminated coagulation syndrome in patients with acute leukemias [Zespol rozsianego krzepniecia srodnaczyniowego u chorych na ostre bialaczki]. Acta Haematologica Polonica 1976;7(2):139‐46. [PUBMED: 1065178] [PubMed] [Google Scholar]
Dorantes‐Acosta 2013
- Dorantes‐Acosta E, Medina‐Sanson A, Jaimes‐García Y, López‐Martínez B. Clinical features and treatment outcomes of pediatric acute promyelocytic leukemia in a Mexican pediatric hospital. Revista de Investigación Clínica 2013;65(5):392‐8. [PUBMED: 24687338] [PubMed] [Google Scholar]
Draper 2014
- Draper M. Which database should I use?. https://www.adelaide.edu.au/library/guide/med/wd.html (accessed 29 September 2014).
Dreyfus 1973
- Dreyfus B, Varet B, Heilmann‐Gouault M, Sultan C, Reyes F, Gluckman E, et al. The treatment of disseminated intravascular coagulation in 18 cases of promyelocytic acute leukaemia (author's translation) [Traitement dans 18 observations de leucémies aiguës promyélocytaires de la coagulation intravasculaire disséminée]. Nouvelle Revue Française d'Hématologie 1973;13(6):761‐70. [PUBMED: 4280042] [PubMed] [Google Scholar]
Durán Suárez 1980
- Durán Suárez JR, Juncá Piera J, Zuazu Nagore J, Triginer Boixeda JM. Disseminated intravascular coagulation as the presenting feature of lymphoblastic leukemia. Study of a case [Coagulación intravascular diseminada como forma de presentación de leucosis linfoblástica. Estudio de un caso]. Medicina Clínica 1980;75(9):391‐3. [PUBMED: 6936572] [PubMed] [Google Scholar]
Engelmann 2013
- Engelmann B, Massberg S. Thrombosis as an intravascular effector of innate immunity. Nature Reviews. Immunology 2013;13(1):34‐45. [PUBMED: 23222502] [DOI] [PubMed] [Google Scholar]
Falanga 2003
- Falanga A, Rickles FR. Pathogenesis and management of the bleeding diathesis in acute promyelocytic leukaemia. Best Practice & Research. Clinical Haematology 2003;16(3):463‐82. [PUBMED: 12935963] [DOI] [PubMed] [Google Scholar]
Franchini 2006
- Franchini M, Lippi G, Manzato F. Recent acquisitions in the pathophysiology, diagnosis and treatment of disseminated intravascular coagulation. Thrombosis Journal 2006;4:4. [PUBMED: 16504043] [DOI] [PMC free article] [PubMed] [Google Scholar]
Franchini 2010
- Franchini M, Minno MN, Coppola A. Disseminated intravascular coagulation in hematologic malignancies. Seminars in Thrombosis and Hemostasis 2010;36(4):388‐403. [PUBMED: 20614391] [DOI] [PubMed] [Google Scholar]
Fujiwara 1997
- Fujiwara H, Takahashi N, Tada J, Higuchi T, Harada H, Mori H, et al. Blast crisis accompanied by severe DIC of Ph negative chronic myeloid leukemia showing t(9;16) and positive M‐BCR/ABL rearrangement [Japanese]. Rinsho Ketsueki 1997;38(8):663‐8. [PUBMED: 9311272] [PubMed] [Google Scholar]
García de Paredes 1983
- García de Paredes ML, González Larriba JL, Ordoñez Gallego A, Martínez Martínez B, Montero García JM. Disseminated intravascular coagulation as the 1st manifestation of a chronic myeloid leukemia [Spanish]. Medicina Clínica (Barc) 1983;81(8):369‐70. [PUBMED: 6580521] [PubMed] [Google Scholar]
German 1976
- German HJ, Smith JA, Lindenbaum J. Chronic intravascular coagulation associated with chronic myelocytic leukemia. Use of heparin in connection with a surgical procedure. American Journal of Medicine 1976;61(4):547‐52. [PUBMED: 1067753] [DOI] [PubMed] [Google Scholar]
Gingrich 1979
- Gingrich RD, Burns CP. Disseminated coagulopathy in chronic myelomonocytic leukemia. Cancer 1979;44(6):2249‐53. [PUBMED: 292510] [DOI] [PubMed] [Google Scholar]
GRADEpro 2008 [Computer program]
- Brozek J, Oxman A, Schünemann H. Grading of Recommendations Assessment, Development and Evaluation (GRADE). Version 3.2. Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group, 2008.
Greinacher 2001
- Greinacher A, Lubenow N. Recombinant hirudin in clinical practice. Circulation 2001;103(10):1479‐84. [PUBMED: 11245656] [DOI] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, and GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [PUBMED: 18436948] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hess 2005
- Hess EP, Sztajnkrycer MD. Images in emergency medicine. Acute leukemia with blast crisis, disseminated intravascular coagulation, and intraparenchymal hemorrhage. Annals of Emergency Medicine 2005;46(4):314, 322. [PUBMED: 16187462] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higuchi 2005
- Higuchi T, Toyama D, Hirota Y, Isoyama K, Mori H, Niikura H, et al. Disseminated intravascular coagulation complicating acute lymphoblastic leukemia: a study of childhood and adult cases. Leukemia & Lymphoma 2005;46(8):1169‐76. [PUBMED: 16085558] [DOI] [PubMed] [Google Scholar]
Hoffman 2008
- Hoffman R. Hematology. Basic Principles and Practice. 5th Edition. Philadelphia: Churchill Livingstone, 2008. [ISBN: 978‐0‐443‐06715‐0] [Google Scholar]
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [PUBMED: 10480822] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hook 2012
- Hook KM, Abrams CS. The loss of homeostasis in hemostasis: new approaches in treating and understanding acute disseminated intravascular coagulation in critically ill patients. Clinical and Translational Science 2012;5(1):85‐92. [PUBMED: 22376264] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hopewell 2010
- Hopewell S, Clarke M, Higgins JPT (editors). Core reporting of outcomes in effectiveness trials. Cochrane Methods. Cochrane Database of Systematic Reviews 2010;Suppl 1:1‐29. [ISSN 2044‐4702] [Google Scholar]
Hoyle 1988
- Hoyle CF, Swirsky DM, Freedman L, Hayhoe FG. Beneficial effect of heparin in the management of patients with APL. British Journal of Haematology 1988;68(3):283‐9. [PUBMED: 3162683] [DOI] [PubMed] [Google Scholar]
Hunt 2014
- Hunt BJ. Bleeding and coagulopathies in critical care. New England Journal of Medicine 2014;370(9):847‐59. [PUBMED: 24571757] [DOI] [PubMed] [Google Scholar]
Iba 2013
- Iba T, Nagaoka I, Boulat M. The anticoagulant therapy for sepsis‐associated disseminated intravascular coagulation. Thrombosis Research 2013;131(5):383‐9. [PUBMED: 23566533] [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice 1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Ikezoe 2014
- Ikezoe T. Pathogenesis of disseminated intravascular coagulation in patients with acute promyelocytic leukemia, and its treatment using recombinant human soluble thrombomodulin. International Journal of Hematology 2014;100(1):27‐37. [PUBMED: 24217998] [DOI] [PubMed] [Google Scholar]
Ioannidis 2010
- Ioannidis JP. Meta‐research: The art of getting it wrong. Research Synthesis Methods 2010;1(3‐4):169‐84. [DOI] [PubMed] [Google Scholar]
JPRN‐UMIN000006738
- Tokuhira M. Clinical efficacy of thrombomodulin alfa in hematological malignancy with disseminated intravascular coagulation. http://apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000006738 (accessed 29 September 2014).
JPRN‐UMIN000008466
- Yokoyama H. Clinical study on the efficacy of recombinant thrombomodulin for DIC complicated with acute leukemia. http://www.umin.ac.jp/ctr/index.htm (accessed 29 September 2014).
Kaiser 2001
- Kaiser B, Hoppensteadt DA, Fareed J. Tissue factor pathway inhibitor: an update of potential implications in the treatment of cardiovascular disorders. Expert Opinion on Investigational Drugs 2001;10(11):1925‐35. [PUBMED: 11772296] [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [PUBMED: 20156912] [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [PUBMED: 11730399] [DOI] [PubMed] [Google Scholar]
Komatsu 1986
- Komatsu N, Yoshida N, Tsuboyama A, Sato Y, Sakamoto S, Miura Y. Promyelocytic crisis of chronic myelogenous leukemia: coagulopathy similar to atypical disseminated intravascular coagulation in acute promyelocytic leukemia. Nippon Ketsueki Gakkai Zasshi 1986;49(4):894‐9. [PUBMED: 3464156] [PubMed] [Google Scholar]
Kwaan 2014
- Kwaan HC. The unique hemostatic dysfunction in acute promyelocytic leukemia. Seminars in Thrombosis and Hemostasis 2014;40(3):332‐6. [PUBMED: 24590422] [DOI] [PubMed] [Google Scholar]
Levi 2007
- Levi M. Disseminated intravascular coagulation. Critical Care Medicine 2007;35(9):2191‐5. [PUBMED: 17855836] [DOI] [PubMed] [Google Scholar]
Levi 2008
- Levi M, Poll T. The role of natural anticoagulants in the pathogenesis and management of systemic activation of coagulation and inflammation in critically ill patients. Seminars in Thrombosis and Hemostasis 2008;34(5):459‐68. [PUBMED: 18956286] [DOI] [PubMed] [Google Scholar]
Levi 2009
- Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. British Journal of Haematology 2009;145(1):24‐33. [PUBMED: 19222477] [DOI] [PubMed] [Google Scholar]
Levi 2013
- Levi M, Poll T. Disseminated intravascular coagulation: a review for the internist. Internal and Emergency Medicine 2013;8(1):23‐32. [PUBMED: 23015284] [DOI] [PubMed] [Google Scholar]
Lo‐Coco 2008
- Lo‐Coco F, Ammatuna E, Montesinos P, Sanz MA. Acute promyelocytic leukemia: recent advances in diagnosis and management. Seminars in Oncology 2008;35(4):401‐9. [PUBMED: 18692690] [DOI] [PubMed] [Google Scholar]
Mangal 1984
- Mangal AK, Grossman L, Vickars L. Disseminated intravascular coagulation in acute monoblastic leukemia: response to heparin therapy. Canadian Medical Association Journal 1984;130(6):731‐3. [PUBMED: 6582992] [PMC free article] [PubMed] [Google Scholar]
Nowak 2002
- Nowak G. Pharmacology of recombinant hirudin. Seminars in Thrombosis and Hemostasis 2002;28(5):415‐24. [PUBMED: 12420236] [DOI] [PubMed] [Google Scholar]
Okajima 2000
- Okajima K, Sakamoto Y, Uchiba M. Heterogeneity in the incidence and clinical manifestations of disseminated intravascular coagulation: a study of 204 cases. American Journal of Hematology 2000;65(3):215‐22. [PUBMED: 11074538] [DOI] [PubMed] [Google Scholar]
Pal 2010
- Pal N, Kertai MD, Lakshminarasimhachar A, Avidan MS. Pharmacology and clinical applications of human recombinant antithrombin. Expert Opinion on Biological Therapy 2010;10(7):1155‐68. [PUBMED: 20528611] [DOI] [PubMed] [Google Scholar]
PCORI 2012
- Patient‐Centered Outcomes Research Institute (PCORI). Preliminary draft methodology report: "Our questions, our decisions: Standards for patient‐centered outcomes research". http://www.pcori.org/assets/Preliminary‐Draft‐Methodology‐Report.pdf (accessed 1 April 2013):1‐61.
Porta 2008
- Porta M. A Dictionary of Epidemiology. 5th Edition. New York: Oxford University Press, 2008. [Google Scholar]
Prentice 1985
- Prentice CR. Acquired coagulation disorders. Clinics in Haematology 1985;14(2):413‐42. [PUBMED: 3899441] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rosenthal 1995
- Rosenthal NS, Knapp D, Farhi DC. Promyelocytic blast crisis of chronic myelogenous leukemia. A rare subtype associated with disseminated intravascular coagulation. American Journal of Clinical Pathology 1995;103(2):185‐8. [PUBMED: 7856560] [DOI] [PubMed] [Google Scholar]
Sarris 1992
- Sarris AH, Kempin S, Berman E, Michaeli J, Little C, Andreeff M, et al. High incidence of disseminated intravascular coagulation during remission induction of adult patients with acute lymphoblastic leukemia. Blood 1992;79(5):1305‐10. [PUBMED: 1536954] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones H, Altman D, Harris R, Jüni P, Pildal J. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [PUBMED: 22945832] [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomized trials. Annals of Internal Medicine 2010;152(11):726‐32. [PUBMED: 20335313 ] [DOI] [PubMed] [Google Scholar]
Seligman 1976
- Seligman BR, Rosner F, Solomon RB. Chronic myelogenous leukemia. Disseminated intravascular coagulation and chloromas containing sea‐blue histiocytes. New York State Journal of Medicine 1975;75(8):1271‐4. [PUBMED: 1055903] [PubMed] [Google Scholar]
Speiser 1990
- Speiser W, Pabinger‐Fasching I, Kyrle PA, Kapiotis S, Kottas‐Heldenberg A, Bettelheim P, et al. Hemostatic and fibrinolytic parameters in patients with acute myeloid leukemia: activation of blood coagulation, fibrinolysis and unspecific proteolysis. Blut 1990;61(5):298‐302. [PUBMED: 2271776] [DOI] [PubMed] [Google Scholar]
Stein 2009
- Stein E, McMahon B, Kwaan H, Altman JK, Frankfurt O, Tallman MS. The coagulopathy of acute promyelocytic leukaemia revisited. Best Practice & Research. Clinical Haematology 2009;22(1):153‐63. [PUBMED: 19285282] [DOI] [PubMed] [Google Scholar]
Sunder‐Plassmann 1993
- Sunder‐Plassmann G, Speiser W, Korninger C, Stain M, Bettelheim P, Pabinger‐Fasching I, et al. Disseminated intravascular coagulation and decrease in fibrinogen levels induced by vincristine/prednisolone therapy of lymphoid blast crisis of chronic myeloid leukemia. Annals of Hematology 1991;62(5):169‐73. [PUBMED: 2049463] [DOI] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Imberger G, Walsh M, Chu R, Gluud C, Wetterslev J, et al. The number of patients and events required to limit the risk of overestimation of intervention effects in meta‐analysis‐‐a simulation study. PLoS One 2011;6(10):e25491. [PUBMED: 22028777] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tsukagoshi 2000
- Tsukagoshi S. Pharmacokinetics studies of nafamostat mesilate (FUT), a synthetic protease inhibitor, which has been used for the treatments of DIC and acute pancreatitis, and as an anticoagulant in extracorporeal circulation [Japanese]. Gan to Kagaku Ryoho. Cancer and Chemotherapy 2000;27(5):767‐74. [PUBMED: 10832450] [PubMed] [Google Scholar]
Uchiumi 2007
- Uchiumi H, Matsushima T, Yamane A, Doki N, Irisawa H, Saitoh T, et al. Prevalence and clinical characteristics of acute myeloid leukemia associated with disseminated intravascular coagulation. International Journal of Hematology 2007;86(2):137‐42. [PUBMED: 17875527] [DOI] [PubMed] [Google Scholar]
van der Poll 2014
- Poll T, Herwald H. The coagulation system and its function in early immune defense. Thrombosis and Haemostasis 2014;112(4):640‐8. [PUBMED: 24696161] [DOI] [PubMed] [Google Scholar]
Wada 2010
- Wada H, Asakura H, Okamoto K, Iba T, Uchiyama T, Kawasugi K, et al. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thrombosis Research 2010;125(1):6‐11. [PUBMED: 19782389] [DOI] [PubMed] [Google Scholar]
Wada 2014
- Wada H, Matsumoto T, Yamashita Y, Hatada T. Disseminated intravascular coagulation: testing and diagnosis. Clinica Chimica Acta 2014;436:130‐4. [PUBMED: 24792730] [DOI] [PubMed] [Google Scholar]
Williams 2008
- Williams J, Mozurkewich E, Chilimigras J, Ven C. Critical care in obstetrics: pregnancy‐specific conditions. Best Practice & Research. Clinical Obstetrics & Gynaecology 2008;22(5):825‐46. [PUBMED: 18775679] [DOI] [PubMed] [Google Scholar]
Yanada 2006
- Yanada M, Matsushita T, Suzuki M, Kiyoi H, Yamamoto K, Kinoshita T, et al. Disseminated intravascular coagulation in acute leukemia: clinical and laboratory features at presentation. European Journal of Haematology 2006;77(4):282‐7. [PUBMED: 16856920] [DOI] [PubMed] [Google Scholar]
Yoshikawa 1980
- Yoshikawa H, Maruo K. Blastic crisis of a chronic myelogenous leukemia with disseminated intravascular coagulation, with special reference to "AAAP" therapy (author's translation) [Japanese]. Rinsho Ketsueki [Japanese Journal of Clinical Hematology] 1980;21(1):52‐8. [PUBMED: 6929365] [PubMed] [Google Scholar]
Zavala 2006 [Computer program]
- Zavala D, Martí‐Carvajal A, Peña‐Martí G, Comunián G. Data extraction sheet to help manage the characteristics of included studies in Cochrane reviews. Valencia, Venezuela: Universidad de Carabobo, 2006.
References to other published versions of this review
Marti‐Carvajal 2011
- Marti‐Carvajal AJ, Simancas D, Cardona AF. Treatment for disseminated intravascular coagulation in patients with acute and chronic leukemia. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD008562.pub2] [DOI] [PubMed] [Google Scholar]


