Abstract
Background
Individuals with osteoarthritis (OA) of the knee can be treated with a knee brace or a foot/ankle orthosis. The main purpose of these aids is to reduce pain, improve physical function and, possibly, slow disease progression. This is the second update of the original review published in Issue 1, 2005, and first updated in 2007.
Objectives
To assess the benefits and harms of braces and foot/ankle orthoses in the treatment of patients with OA of the knee.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE (current contents, HealthSTAR) up to March 2014. We screened reference lists of identified trials and clinical trial registers for ongoing studies.
Selection criteria
Randomised and controlled clinical trials investigating all types of braces and foot/ankle orthoses for OA of the knee compared with an active control or no treatment.
Data collection and analysis
Two review authors independently selected trials and extracted data. We assessed risk of bias using the 'Risk of bias' tool of The Cochrane Collaboration. We analysed the quality of the results by performing an overall grading of evidence by outcome using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach. As a result of heterogeneity of studies, pooling of outcome data was possible for only three insole studies.
Main results
We included 13 studies (n = 1356): four studies in the first version, three studies in the first update and six additional studies (n = 529 participants) in the second update. We included studies that reported results when study participants with early to severe knee OA (Kellgren & Lawrence grade I‐IV) were treated with a knee brace (valgus knee brace, neutral brace or neoprene sleeve) or an orthosis (laterally or medially wedged insole, neutral insole, variable or constant stiffness shoe) or were given no treatment. The main comparisons included (1) brace versus no treatment; (2) foot/ankle orthosis versus no treatment or other treatment; and (3) brace versus foot/ankle orthosis. Seven studies had low risk, two studies had high risk and four studies had unclear risk of selection bias. Five studies had low risk, three studies had high risk and five studies had unclear risk of detection bias. Ten studies had high risk and three studies had low risk of performance bias. Nine studies had low risk and four studies had high risk of reporting bias.
Four studies compared brace versus no treatment, but only one provided useful data for meta‐analysis at 12‐month follow‐up. One study (n = 117, low‐quality evidence) showed lack of evidence of an effect on visual analogue scale (VAS) pain scores (absolute percent change 0%, mean difference (MD) 0.0, 95% confidence interval (CI) ‐0.84 to 0.84), function scores (absolute percent change 1%, MD 1.0, 95% CI ‐2.98 to 4.98) and health‐related quality of life scores (absolute percent change 4%, MD ‐0.04, 95% CI ‐0.12 to 0.04) after 12 months. Many participants stopped their initial treatment because of lack of effect (24 of 60 participants in the brace group and 14 of 57 participants in the no treatment group; absolute percent change 15%, risk ratio (RR) 1.63, 95% CI 0.94 to 2.82). The other studies reported some improvement in pain, function and health‐related quality of life (P value ≤ 0.001). Stiffness and treatment failure (need for surgery) were not reported in the included studies.
For the comparison of laterally wedged insole versus no insole, one study (n = 40, low‐quality evidence) showed a lower VAS pain score in the laterally wedged insole group (absolute percent change 16%, MD ‐1.60, 95% CI ‐2.31 to ‐0.89) after nine months. Function, stiffness, health‐related quality of life, treatment failure and adverse events were not reported in the included study.
For the comparison of laterally wedged versus neutral insole after pooling of three studies (n = 358, moderate‐quality evidence), little evidence was found of an effect on numerical rating scale (NRS) pain scores (absolute percent change 1.0%, MD 0.1, 95% CI ‐0.45 to 0.65), Western Ontario‐McMaster Osteoarthritis Scale (WOMAC) stiffness scores (absolute percent change 0.1%, MD 0.07, 95% CI ‐4.96 to 5.1) and WOMAC function scores (absolute percent change 0.9%, MD 0.94, 95% CI ‐ 2.98 to 4.87) after 12 months. Evidence of an effect on health‐related quality of life scores (absolute percent change 1.0%, MD 0.01, 95% CI ‐0.05 to 0.03) was lacking in one study (n = 179, moderate‐quality evidence). Treatment failure and adverse events were not studied for this comparison in the included studies.
Data for the comparison of laterally wedged insole versus valgus knee brace could not be pooled. After six months' follow‐up, no statistically significant difference was noted in VAS pain scores (absolute percent change ‐2.0%, MD ‐0.2, 95% CI ‐1.15 to 0.75) and WOMAC function scores (absolute percent change 0.1%, MD 0.1, 95% CI ‐7.26 to 0.75) in one study (n = 91, low‐quality evidence); however both groups showed improvement. Stiffness, health‐related quality of life, treatment failure and adverse events were not reported in the included studies for this comparison.
Authors' conclusions
Evidence was inconclusive for the benefits of bracing for pain, stiffness, function and quality of life in the treatment of patients with medial compartment knee OA. On the basis of one laterally wedged insole versus no treatment study, we conclude that evidence of an effect on pain in patients with varus knee OA is lacking. Moderate‐quality evidence shows lack of an effect on improvement in pain, stiffness and function between patients treated with a laterally wedged insole and those treated with a neutral insole. Low‐quality evidence shows lack of an effect on improvement in pain, stiffness and function between patients treated with a valgus knee brace and those treated with a laterally wedged insole. The optimal choice for an orthosis remains unclear, and long‐term implications are lacking.
Keywords: Humans; Orthotic Devices; Braces; Osteoarthritis, Knee; Osteoarthritis, Knee/therapy; Quality of Life; Randomized Controlled Trials as Topic; Shoes
Plain language summary
Braces and orthoses for osteoarthritis of the knee
Research question
This summary of a Cochrane review presents what we know from research about the effects of braces and foot/ankle orthoses in the treatment of patients with osteoarthritis of the knee. We searched for evidence up to March 2014. We found 13 studies (n = 1356) and included in this update six additional studies (n = 529 participants).
Study characteristics
We included studies reporting results in patients with early to severe knee OA (Kellgren & Lawrence grade I‐IV) treated with a knee brace (valgus knee brace, neutral brace, neoprene sleeve) or an orthosis (laterally or medially wedged insole, neutral insole, variable or constant stiffness shoe) or given no treatment.
Background: What is osteoarthritis and what are braces and orthoses?
Osteoarthritis is the most common form of arthritis that can affect the hands, hips, shoulders and knees. In osteoarthritis, the cartilage that protects the ends of bones breaks down, causing pain and swelling. Osteoarthritis can occur in different areas of the knee or can affect the whole knee. Depending on the area, osteoarthritis can change the alignment of joints.
Braces and orthoses are devices that you wear to support your knee joint. Orthoses are insoles that fit comfortably inside your shoes. Braces are made of combinations of metal, foam, plastic, elastic material and straps. A knee brace can be fitted specially for the person wearing it.
Key results
This review shows the following in people with osteoarthritis of the knee.
Wearing a knee brace compared with no brace:
• may result in little or no difference in reducing pain and improving knee function and quality of life after 12 months (low‐quality evidence); and • causes many patients to stop their initial treatment because of lack of effect in both groups.
Stiffness and treatment failure (need for surgery) were not reported.
Wearing a laterally wedged insole compared with no insole:
• may result in little or no difference in reducing pain (low‐quality evidence).
Function, stiffness, health‐related quality of life, treatment failure and side effects were not reported.
Wearing a laterally wedged insole compared with wearing a neutral insole:
• probably results in little or no difference in reducing pain and improving function, stiffness and quality of life after 12 months (moderate‐quality evidence). Treatment failure and side effects were not reported.
Wearing a laterally wedged insole compared with a valgus knee brace:
• may result in little or no difference in reducing pain and improving function after sx months (low‐quality evidence). Stiffness, health‐related quality of life, treatment failure and side effects were not reported
We often do not have precise information about side effects and complications. Side effects may include pain in the back of the knee, low back pain, foot sole pain or skin irritation.
Quality of the evidence
• Low‐quality evidence suggests that people with OA who use a knee brace may have little or no reduction in pain, improved knee function and improved quality of life.
• Moderate‐quality evidence suggests that people with OA of the knee who wear laterally wedged insoles or neutral insoles probably have little or no improvement in pain, function and stiffness.
Summary of findings
Summary of findings for the main comparison. Braces and orthoses for varus medial osteoarthritis of the knee.
| Valgus knee braces and orthoses for varus medial osteoarthritis of the knee | ||||||
|
Patient or population: patients with varus medial osteoarthritis of the knee Settings: general hospital Intervention: valgus knee brace or lateral wedge insole Comparison: no brace or neutral insole | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Intervention | |||||
| Valgus knee brace compared with no brace | ||||||
|
Pain on walking (VAS) Scale from 0 to 10 Follow‐up: 12 months (Higher score is worse) |
Mean pain score in control groups was 5.2 |
Mean pain in intervention groups was equal (0.84 lower to 0.84 higher) | 115 (1 study) |
⊕⊕⊝⊝ Lowa,b | MD = 0.00 (95% CI ‐0.84 to 0.84) Absolute percent change = 0% (95% CI ‐8.4 to 8.4) Relative percent change = 0% (95% CI ‐1.6 to 1.6) NNTB = not statistically significant |
|
|
Knee function (HSS) Scale from 0 to 100 Follow‐up: 12 months (Higher score is better) |
Mean function score in control groups was 69 | Mean function in intervention groups was 1.00 higher (2.98 lower to 4.98 higher) |
110 (1 study) |
⊕⊕⊝⊝ Lowa,b | MD = 1.00 (95% CI ‐2.98 to 4.98) Absolute percent change = 1.0% (95% CI 3.0 to 5.0) Relative percent change = 0.01% (95% CI 0.05 to 0.07) NNTB = not statistically significant |
|
|
Quality of life (EQ‐5D) Scale from 0 to 100 Follow‐up: 12 months (Higher score is better) |
Mean health‐related quality of life score in control groups was 0.6 | Mean health‐related quality of life score in intervention groups was 0.04 lower (0.12 lower to 0.04 higher) | 117 (1 study) |
⊕⊕⊝⊝ Lowa,b | MD = 0.04 (95% CI ‐0.12 to 0.04) Absolute percent change = 0.04% (95% CI ‐0.12 to 0.04) Relative percent change = 0.07% (95% CI ‐0.2 to 0.07) NNTB = not statistically significant |
|
|
Total number of adverse events (withdrawals due to lack of effect)c Follow‐up: 12 months |
Low‐risk population | RR 1.63 (0.94 to 2.82) | 117 (1 study) |
⊕⊕⊝⊝ Lowa,b | Absolute percent change = 15% (95% CI ‐1% to 32%) Relative percent change = 63% (95% CI ‐6% to 182%) NNTB = not statistically significant |
|
| 246 per 1000 | 400 per 1000 (239 to 694) | |||||
| Lateral‐wedge insole compared with neutral insole | ||||||
|
Pain on walking (NRS) Scale from 0 to 10 Follow‐up: mean 12 months (Higher score is worse) |
Mean pain on walking score in control groups was 2.6 | Mean pain on walking in intervention groups was 0.1 higher (0.45 higher to 0.65 lower) |
224 (2 studies) |
⊕⊕⊕⊝ Moderatea | MD = 0.10 (95% CI ‐0.45 to 0.65) Absolute percent change = 1.0% (95% CI 4.5 to ‐6.5) Relative percent change = 3.8% (95% CI 1.7 to ‐25.0) NNTB = not statistically significant |
|
|
Physical function (WOMAC) ‐
12 months Scale from 0 to 100 Follow‐up: mean 12 months (Higher score is better) |
Mean function score in control groups was 36.6 | Mean function in intervention groups was 0.94 higher (2.98 lower to 4.87 higher) | 358 (3 studies) |
⊕⊕⊕⊝ Moderatea | MD = 0.94 (95% CI ‐2.98 to 4.87) Absolute percent change = 0.9% (95% CI ‐3.0 to 4.9) Relative percent change = 2.6% (95% CI ‐8.1 to 13.3) NNTB = not statistically significant |
|
|
Health‐related quality of life (HRQoL) Scale from 0 to 1.0 Follow‐up: 12 months (Higher score is better) |
Mean health‐related quality of life score in control groups was 0.7 |
Mean health‐related quality of life score in intervention groups was 0.01 lower (0.05 lower to 0.03 higher) |
179 (1 study) |
⊕⊕⊕⊝ Moderatea | MD = 0.00 (95% CI ‐0.06 to 0.06) Absolute percent change = 1.0% (95% CI ‐5.0 to 3.0) Relative percent change = 1.4% (95% CI ‐7.1 to 4.3) NNTB = not statistically significant |
|
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; EQ‐5D: EuroQol‐5D; HSS: Hospital for Special Surgery knee score; NNTB: number needed to treat for an additional beneficial outcome; NRS: numerical rating scale; RR: Risk ratio; VAS: Visual analogue scale; WOMAC: Western Ontario‐McMaster Osteoarthritis Scale. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aLimitations in design and implementation of available studies suggest high likelihood of bias.
bImprecision: Results are based on only one study with 117 people.
cMany participants stopped their initial treatment because of lack of effect.
Summary of findings 2. Valgus knee brace compared with no brace for varus medial osteoarthritis of the knee.
| Valgus knee brace compared with no brace for varus medial osteoarthritis of the knee | |||||
| Patient or population: patients with varus medial osteoarthritis of the knee Settings: general hospital Intervention: valgus knee brace Comparison: no brace | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| No brace | Valgus knee brace | ||||
|
Pain
VAS: scale from 0 to 10
Follow‐up: 12 months (Higher score is worse) |
Mean pain score in control groups was 5.2 | Mean pain on walking in intervention groups was equal (0.84 lower to 0.84 higher) |
115 (1 study) |
⊕⊕⊝⊝ Lowa,b |
MD = 0.00 (95% CI ‐0.84 to 0.84) Absolute percent change = 0% (95% CI ‐8.4 to 8.4) Relative percent change = 0% (95% CI ‐1.6 to 1.6) NNTB = not statistically significant |
| Stiffness | See comment | See comment | Not estimablec | See comment | Outcome not reported in included studies |
|
Function HSS: scale from 0 to 100 Follow‐up: 12 months (Higher score is better) |
Mean function score in control groups was 69 |
Mean function in intervention groups was 1.00 higher (2.98 lower to 4.98 higher) |
110 (1 study) |
⊕⊕⊝⊝ Lowa,b |
MD = 1.00 (95% CI ‐2.98 to 4.98) Absolute percent change = 1.0% (95% CI 3.0 to 5.0) Relative percent change = 0.01% (95% CI 0.05 to 0.07) NNTB = not statistically significant |
|
Health‐related quality of life EQ‐5S: scale from 0 to 100 Follow‐up: 12 months (Higher score is better) |
Mean health‐related quality of life score in control groups was 0.6 |
Mean health‐related quality of life score in intervention groups was 0.04 lower (0.12 lower to 0.04 higher) |
117 (1 study) |
⊕⊕⊝⊝ Lowa,b |
MD = 0.04 (95% CI ‐0.12 to 0.04) Absolute percent change = 0.04% (95% CI ‐0.12 to 0.04) Relative percent change = 0.07% (95% CI ‐0.2 to 0.07) NNTB = not statistically significant |
| Treatment failure | See comment | See comment | Not estimablec | See comment | Outcome not reported in included studies |
| Serious adverse events | See comment | See comment | Not estimablec | See comment | Outcome not reported in included studies |
| Total number of adverse events (withdrawals due to lack of effect)d | Study population | 117 (1 study) |
⊕⊕⊝⊝ Lowa,b |
RR = 1.63 (95% CI 0.94 to 2.82) Absolute percent change = 15% (95% CI ‐1% to 32%) Relative percent change = 63% (95% CI ‐6% to 182%) NNTB = not statistically significant |
|
| 400 per 1000 | 246 per 1000 | ||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; NNTB: Number needed to treat for an additional beneficial outcome; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aLimitations in design and implementation of available studies suggest high likelihood of bias.
bImprecision: Results are based on only one study with 117 participants.
cNo useful data were available.
dMany participants stopped their initial treatment because of lack of effect.
Summary of findings 3. Lateral wedge insole compared with neutral insole for varus medial osteoarthritis of the knee.
| Lateral wedge insole compared with neutral insole for varus medial osteoarthritis of the knee | |||||
| Patient or population: patients with varus medial osteoarthritis of the knee Settings: general hospital Intervention: lateral wedge insole Comparison: neutral insole | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Neutral insole | Lateral wedge insole | ||||
|
Pain
NRS: scale from 0 to 10
Follow‐up: mean 12 months (Higher score is worse) |
Mean pain on walking score in control groups was 2.6 | Mean pain on walking in intervention groups was 0.1 higher (0.45 higher to 0.65 lower) |
224 (2 studies) | ⊕⊕⊕⊝ Moderatea | MD = 0.10 (95% CI ‐0.45 to 0.65) Absolute percent change = 1.0% (95% CI 4.5 to ‐6.5) Relative percent change = 3.8% (95% CI 1.7 to ‐25.0) NNTB = not statistically significant |
|
Stiffness
WOMAC: scale from 0 to 100
Follow‐up: mean 12 months (Higher score is better) |
Mean stiffness score in control groups was 41.6 |
Mean stiffness in intervention groups was 0.07 higher (4.96 lower to 5.1 higher) |
358 (3 studies) |
⊕⊕⊕⊝ Moderatea | MD = 0.07 (95% CI ‐4.96 to 5.10) Absolute percent change = 0.1% (95% CI ‐5.0 to 5.1) Relative percent change = 0.2% (95% CI ‐11.9 to 12.3) NNTB = not statistically significant |
|
Function WOMAC: scale from 0 to 100 Follow‐up: mean 12 months (Higher score is better) |
Mean function score in control groups was 36.6 |
Mean function in intervention groups was 0.94 higher (2.98 lower to 4.87 higher) |
358 (3 studies) |
⊕⊕⊕⊝ Moderatea | MD = 0.94 (95% CI ‐2.98 to 4.87) Absolute percent change = 0.9% (95% CI ‐3.0 to 4.9) Relative percent change = 2.6% (95% CI ‐8.1 to 13.3) NNTB = not statistically significant |
|
Health‐related quality of life HRQoL: scale from 0 to 1.0 Follow‐up: 12 months (Higher score is better) |
Mean health‐related quality of life score in control groups was 0.7 |
Mean health‐related quality of life score in intervention groups was 0.01 lower (0.05 lower to 0.03 higher) |
179 (1 study) |
⊕⊕⊕⊝ Moderateb | MD = 0.00 (95% CI ‐0.06 to 0.06) Absolute percent change = 1.0% (95% CI ‐5.0 to 3.0) Relative percent change = 1.4% (95% CI ‐7.1 to 4.3) NNTB = not statistically significant |
| Treatment failure | See comment | See comment | Not estimablec | See comment | Outcome not reported in included studies |
| Serious adverse events | See comment | See comment | Not estimablec | See comment | Outcome not reported in included studies |
| Total number of adverse events | See comment | See comment | Not estimablec | See comment | Outcome not reported in included studies |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; NNTB: Number needed to treat for an additional beneficial outcome. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded for limitations in design and implementation of available studies suggesting high likelihood of bias.
bDowngraded for imprecision: Results were based on only one study with 179 participants.
cNo useful data were available.
Summary of findings 4. Valgus knee brace compared with lateral wedge insole for varus medial osteoarthritis of the knee.
| Valgus knee brace compared with lateral wedge insole for varus medial osteoarthritis of the knee | |||||
|
Patient or population: patients with varus medial osteoarthritis of the knee Settings: general hospital Intervention: valgus knee brace Comparison: lateral wedge insole | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Lateral wedge insole | Valgus knee brace | ||||
|
Pain VAS: scale from 0 to 10 Follow‐up: 6 months (Higher score is worse) |
Mean pain score in control groups was 4.8 |
Mean pain score in intervention groups was 0.2 lower (1.15 lower to 0.75 higher) |
91 (1 study) | ⊕⊕⊝⊝ Lowa,b |
MD = ‐0.20 (95% CI ‐1.15 to 0.75) Absolute percent change = ‐2.0% (95% CI ‐11.5 to 7.5) Relative percent change = ‐4.2% (95% CI ‐24.0 to 15.6) NNTB = not statistically significant |
| Stiffness | See comment | See comment | Not estimable | See comment | Outcome not reported in included studies |
|
Function WOMAC: scale from 0 to 100 Follow‐up: 6 months (Higher score is better) |
Mean function score in control groups was 50.7 |
Mean function score in intervention groups was 0.1 higher (7.26 lower to 0.75 higher) |
91 (1 study) | ⊕⊕⊝⊝ Lowa,b |
MD = 0.10 (95% CI ‐7.26 to 7.46) Absolute percent change = 0.1% (95% CI ‐7.26 to 0.75) Relative percent change = 0.2% (95% CI ‐14.3 to 1.5) NNTB = not statistically significant |
| Health‐related quality of life | See comment | See comment | Not estimablec | See comment | Outcome not reported in included studies |
| Treatment failure | See comment | See comment | Not estimablec | See comment | Outcome not reported in included studies |
| Serious adverse events | See comment | See comment | Not estimablec | See comment | Outcome not reported in included studies |
| Total number of adverse events | See comment | See comment | Not estimablec | See comment | Outcome not reported in included studies |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; NNTB: number needed to treat for an additional beneficial outcome; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
aDowngraded for imprecision: Results were based on only one study with 91 participants.
bDowngraded for limitations in design and implementation of available studies suggesting high likelihood of bias.
cNo useful data were available.
Background
Description of the condition
Osteoarthritis (OA) of the knee is a common medical condition that is often seen in general practice and causes considerable pain and immobility. In the United States, approximately 9% of individuals aged 60 years and older suffer from knee OA (Losina 2013). The prevalence of symptomatic knee OA has increased substantially over the past 20 years. Aging, obesity and increased awareness of knee pain have accounted for this trend (Nguyen 2011). Risks for a poor functional outcome in individuals with knee OA involve collateral and cruciate ligament laxity, age, body mass index (BMI) and degree of pain (Sharma 2003). In addition to consequences for the patient, OA presents a considerable burden for society because of its chronic course, high costs of interventions and related productivity costs (Healy 2002; Hermans 2012).
Osteoarthritis of the entire knee is distinguished from OA of one compartment (Grelsamer 1995), which generally is caused by a mechanical problem (Brouwer GM 2007; Tetsworth 1994). Individuals with OA of the medial compartment often have a varus alignment, and the mechanical axis and load bearing pass through the medial compartment. Those with OA of the lateral compartment generally have a valgus alignment, and the mechanical axis and load bearing pass through the lateral compartment. Malalignment increases risk and progression of knee OA and predicts decline in physical function (Brouwer GM 2007; Sharma 2001; Tanamas 2009).
Initial treatment for patients with OA of the knee is conservative, consisting of restricted activity, decreased body mass index (BMI), patient education and physical therapy (Foley 2003; Fransen 2001; Fransen 2008; Garner 2005; Goorman 2000; Hoffmann 2001; Huang 2000; Hurley 1998; Zhang 2010). Pharmacological treatments tend to only modify symptoms (e.g. analgesics, anti‐inflammatory drugs); however some are intended to be curative (hyaluronic acids, chondroitin sulphate) (Bellamy 2006; Cepeda 2006; Gibofsky 2003; Karlsson 2002; Leopold 2003; Nuesch 2009; Towheed 2006; Uebelhart 2004; Whittle 2011).
Electro‐acupuncture, transcutaneous electrical nerve stimulation (TENS), braces, foot/ankle orthoses and leech therapy are not standard treatments (Rutjes 2009) but can be effective in symptom reduction (Deshaies 2002; Michalsen 2003; Ng 2003). If symptoms persist, surgical therapy such as high tibial osteotomy or knee arthroplasty can be considered (Brouwer RW 2007; Fletcher 2006; Stukenborg 2001) .
Description of the intervention
A knee brace or a foot/ankle orthosis is defined as "any medical device added to a person's body to support, align, position, immobilize, prevent or correct deformity, assist weak muscles, or improve function" (Deshaies 2002). The general purpose of braces and orthoses is to decrease pain, improve physical function and possibly slow disease progression. Proprioception/stability is a hypothesised but unproven underlying explanatory factor. Lateral wedge insoles and special valgus braces are designed to reduce load in the medial compartment (Hewett 1998; Katsuragawa 1999; Kirkley 1999; Komistek 1999; Lindenfeld 1997; Maillefert 2001; Reeves 2011).
Several types of orthoses are available to treat patients with medial knee OA non‐operatively. This review includes studies comparing the laterally wedged insole, the valgus knee brace, the neutral knee brace, the neoprene sleeve and variable shoe stiffness versus each other or versus no treatment. The valgus knee brace and the laterally wedged insole are used most commonly in the non‐operative treatment of varus medial knee OA.
How the intervention might work
The goal of the interventions is to improve function, reduce symptoms and possibly slow disease progression. The valgus knee brace and the laterally wedged insole are used with the goal of unloading the diseased medial compartment by creating a valgus effect on the knee. Neutral braces and neoprene sleeves are thought to immobilise and stabilise the knee. Neutral insoles, shoes of variable stiffness and lateral wedged insoles could have a cushioning effect (Reeves 2011).
Why it is important to do this review
The literature suggests that patients with varus medial knee OA may benefit from braces and foot/ankle orthoses. However many different types of braces and foot/ankle orthoses are available. It remains unclear which brace or foot/ankle orthosis will provide the greatest benefit or harm to patients treated for varus medial knee OA (Parkes 2013; Reeves 2011; Zhang 2010).
Objectives
To assess the benefits and harms of braces and foot/ankle orthoses in the treatment of patients with OA of the knee.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and controlled clinical trials investigating all types of braces and foot/ankle orthoses for OA of the knee compared with no treatment or other treatment such as restricted activity, patient education, physiotherapy, pharmacological treatment and orthoses or surgical treatment.
Types of participants
Adult patients (> 18 years) with OA of the knee confirmed by radiological investigation (Kellgren & Lawrence (K&L) grade I‐IV).
Types of interventions
All types of braces (rigid knee braces intended to reduce load, knee sleeves/supporters) and foot/ankle orthoses (laterally or medially wedged insoles with or without an ankle support or variable stiffness shoes) for individuals with OA of the knee. The main comparisons were (1) brace versus no treatment; (2) foot/ankle orthosis versus no treatment or other treatment; and (3) brace versus foot/ankle orthosis.
Types of outcome measures
Major outcomes
We considered major outcomes such as pain, function, stiffness, quality of life, treatment failure (need to undergo surgery), serious adverse events and total number of adverse events.
Minor outcomes
We also considered other outcomes such as radiographic scores, compliance and walking distance.
We considered all major outcomes and presented them in the 'Summary of findings' table.
Search methods for identification of studies
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE (current contents, HealthSTAR) until October 2002 in the original review, until May 2007 in the first update and until March 2014 in the second update to identify clinical trials investigating braces and foot/ankle orthoses for OA of the knee. We performed MEDLINE searches for clinical trials using the strategy of The Cochrane Collaboration (Appendix 1, completed March 2014). We applied no language restriction. Moreover we checked the reference lists of included studies and clinical trial registers for ongoing studies.
Data collection and analysis
Selection of studies
Two review authors initially selected trials on the basis of title and abstract. We assessed title, keywords and abstract to establish whether the study met the inclusion criteria regarding diagnosis, design and intervention. For each selected study, we retrieved the full article for final assessment. Next, two review authors independently performed a final selection of trials to be included in the review, using a pretested standardised form. We resolved disagreements on inclusion by discussion.
Data extraction and management
Three review authors independently extracted data on the intervention, types of outcome measures, follow‐up, loss to follow‐up and outcomes using a standardised form. We have presented the various outcome measures separately. We resolved disagreements or discrepancies on data extraction by discussion.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias of included studies. We resolved disagreements in a consensus meeting and when necessary consulted an independent third person. The Cochrane Collaboration recommends a specific tool for assessing risk of bias in each included study. This comprises a description and a judgement for each entry in a 'Risk of bias' table, wherein each entry addresses a specific feature of the study. The judgement for each entry involves providing a response of 'low risk of bias', 'high risk of bias' or 'unclear risk of bias', indicating lack of information or uncertainty about the potential for bias.
Entries used to assess risk of bias include the following (see also 'Risk of bias' table).
-
Random sequence generation (selection bias).
Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence.
-
Allocation concealment (selection bias).
Selection bias (biased allocation to interventions) due to inadequate concealment of allocations before assignment.
-
Blinding (performance bias and detection bias).
Performance bias or detection bias due to knowledge of allocated interventions after assignment.
-
Blinding of participants and personnel (performance bias).
Performance bias due to knowledge of allocated interventions by participants and personnel during the study.
-
Blinding of outcome assessment (detection bias).
Detection bias due to knowledge of allocated interventions by outcome assessors.
-
Incomplete outcome data (attrition bias).
Attrition bias due to quantity, nature or handling of incomplete outcome data.
-
Selective reporting (reporting bias).
Selection of a subset of original variables recorded on the basis of results.
Measures of treatment effect
For dichotomous outcomes, we calculated risk ratios (RRs) with corresponding 95 per cent confidence intervals (95% CIs). For continuous outcomes, we calculated mean differences (MDs) with 95% CIs.
Unit of analysis issues
Not applicable.
Dealing with missing data
It is unclear to us whether we missed outcome data. Many studies have not published a research protocol. Therefore, we analysed only available data.
Data synthesis
We used RevMan 5 software to analyse the data and have presented the various outcomes in analysis graphs. We used both fixed‐effect and random‐effects models. In cases of substantial between‐trial heterogeneity, we used random‐effects analysis instead of a fixed‐effect approach. Pooling of outcomes was possible only for the comparison of lateral wedged insole versus neutral insole. We considered the rest of the trials to be clinically heterogeneous in terms of study population and intervention.
'Summary of findings' table
We created a 'Summary of findings' table for the major outcomes of pain, function, stiffness, health‐related quality of life, treatment failure, serious adverse events and total adverse events.
We analysed the quality of the presenting results by performing an overall grading of evidence by outcome using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (Guyatt 2008a; Guyatt 2008b; Schünemann 2008). We assigned the highest quality rating to randomised trial evidence.
The GRADE approach specifies the following levels of quality.
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Trial evidence can be downgraded to moderate, low or very low quality depending on the presence of the following factors.
Limitations in design and implementation of available studies suggesting high likelihood of bias.
Indirectness of evidence (indirect population, intervention, control, outcomes).
Unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses).
Imprecision of results (wide confidence intervals).
High probability of publication bias.
Quality will fall by one level for each factor, up to a maximum of three levels for all factors. If very severe problems are noted for any one factor (e.g. when assessing limitations in design and implementation, all studies were unconcealed and unblinded and lost more than 50% of participants to follow‐up), the quality of randomised trial evidence may fall by two levels on the basis of that factor alone.
If pooling of study results is not possible, then a single study is included and by definition low‐quality evidence, which can be downgraded according to risk of bias items.
Results
Description of studies
Results of the search
The search strategy (Appendix 1, completed May 2014) yielded a total of 217 records from the following databases: Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE (current contents, HealthSTAR). The search resulted in identification of the citations of 25 reports of potentially eligible studies, for which (where possible) full reports were obtained. We included a total of 13 studies in the review. We needed the opinion of a third review author once (Shakoor 2008) before we could come to a final decision.
Overall, this review consists of 13 included studies, 12 excluded studies, no ongoing studies and no studies awaiting classification (Figure 1). We checked the reference lists of the included studies but identified no further studies.
1.
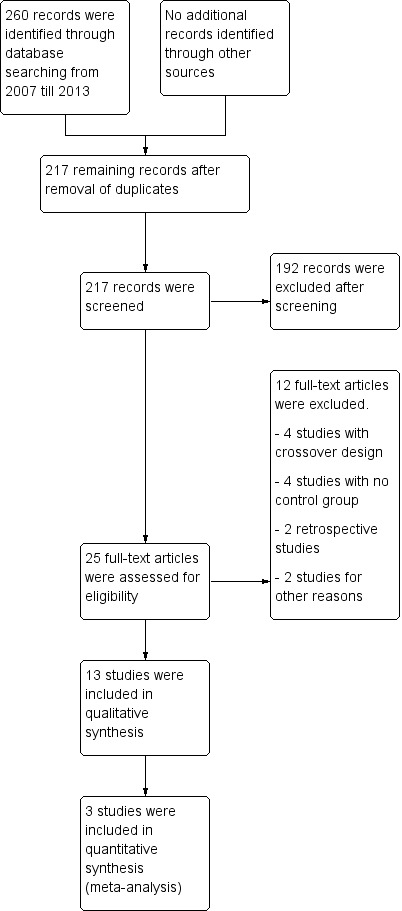
Figure 1 Flow diagram of the study according to the PRISMA statement.
Included studies
We included 13 studies described in 17 publications involving 1356 participants; we included four studies in the first version, added three studies in the first update and added six more studies in this second update. We have described these studies in detail in the Characteristics of included studies table.
One group (Maillefert 2001) published separately six‐month and two‐year results, another group presented separately six‐month and one‐year results (Erhart‐Hledik 2012) and another group (Toda 2001) published separately eight‐week, six‐month and two‐year results. We have described the 13 selected studies in detail in the Characteristics of included studies table. Four studies (Brouwer 2006; Kirkley 1999; Müller‐Rath 2011; Sattari 2011) investigated knee braces, and eight studies (Barrios 2009; Bennell 2011; Erhart‐Hledik 2012; Maillefert 2001; Sattari 2011; Toda 2001; Toda 2002; Toda 2008) examined foot/ankle orthoses for medial compartment OA of the knee. Two studies (Raaij van 2010; Sattari 2011) compared a knee brace with a foot orthosis. Only two studies (Brouwer 2006; Rodriques 2008) also assessed the benefits of a brace or a foot/ankle orthosis for treating lateral compartment osteoarthritis. No studies assessed the benefits of a brace or an insole for general OA of the knee. In 12 studies the degree of OA was scored according to Kellgren & Lawrence (K&L) (Kellgren 1957), and in one study (Brouwer 2006) according to Ahlback (Ahlback 1968). In two studies osteoarthritic changes were also checked on magnetic resonance imaging (MRI) (Bennell 2011; Erhart‐Hledik 2012). The mean number of participants in the 13 studies was 103 (range 30‐207). Mean participant age was 62 years (range 48‐65 years). In two trials, all participants were females (Toda 2001; Toda 2002). (See also Characteristics of included studies.)
Barrios 2009 published an RCT of 66 participants with symptomatic medial knee OA (K&L grade II‐IV). In this RCT, a treatment group ‐ a full‐length (9.1 degrees (standard deviation (SD) 3.9 degrees)) laterally wedged insole into the shoe (n = 35) ‐ had been compared with a control group ‐ a non‐custom neutral insole into the shoe (n = 31). Block randomisation was performed based on OA grade, gender and age (older or younger than 55 years). Allocation was done by an administrative assistant who was unaware of the methods used. The study included 29 males and 37 females with medial tibiofemoral OA (scored according to K&L), mean age of 62.4 years and mean BMI of 33.0 kg/m2. Baseline characteristics (gender, BMI, OA grade) did not differ between groups. A total of 20/35 (57%) participants remained in the treatment group and 25/31 (81%) in the control group at final one‐year follow‐up. The primary outcome measure was mean Western Ontario‐McMaster Osteoarthritis Scale (WOMAC) subscore (100‐0); secondary outcomes included a six‐minute walking test and a stair negotiation test. Mean and P values were presented, but SD values were missing.
Bennell 2011 reported a double‐blinded RCT of 200 participants with mild to moderately severe medial knee OA and radiological evidence of osteophytes in the medial compartment or medial joint space narrowing on an x‐ray film. In this RCT, a treatment group ‐ a full‐length five‐degree laterally wedged insole (n = 103) ‐ was compared with a control group ‐ a flat insole (n = 97). The randomisation procedure consisted of a computer‐generated block method using sealed envelopes. Participants included 82 men and 118 women; mean age was 64 years. Mean BMI was 29.2. The degree of radiological OA was scored according to K&L on posteroanterior radiographs and on MRI. Mean varus alignment was 181 degrees. Follow‐up was 12 months. Eleven participants in the intervention group and ten in the control group were lost to follow‐up.
Brouwer 2006 published a multi‐centre RCT of 117 participants with symptomatic unicompartmental knee OA (Ahlback > 0). In this RCT, investigators studied the additive effect of a brace intended to reduce load in the conservative treatment of unicompartmental (medial or lateral) knee OA. A total of 60 participants were included in the intervention group (brace and standard conservative treatment) and 57 in the control group (standard conservative treatment alone). The brace is available for right and left knees in four sizes. The brace consists of a thigh shell and a calf shell (both of carbon fibre) connected by titanium hinges on the medial and lateral sides. The adjustable side bar on the medial side of the brace provides valgus (1‐12.5 degrees) with medial unloading, or varus (1‐10 degrees) with lateral unloading. The randomisation procedure consisted of a computer‐generated block method using sealed envelopes. Participants included 59 men and 58 women. Mean age was 59 years. Mean BMI was 29. The degree of OA was scored according to Ahlback. Patients with an Ahlback score of I or II were included. Mean varus alignment was nine degrees. Mean valgus alignment was six degrees (hip‐knee‐ankle (HKA) angle). Follow‐up was 12 months. Four participants in the control group were lost to follow‐up.
Erhart‐Hledik 2012 reported an RCT of 79 participants with symptomatic medial knee OA and osteoarthritic changes on MRI. In this study a treatment group ‐ variable‐stiffness shoe (n = 40) ‐ was compared with a control group ‐ constant stiffness shoe (n = 39). The randomisation procedure was not described. Participants included 42 men and 37 women. Mean age was 60 years. Mean BMI was 27.7. The degree of radiological OA was scored on MRI at baseline. Follow‐up was 12 months. Eight participants in the intervention group and 13 in the control group were lost to follow‐up.
Kirkley 1999 reported an RCT comparing (1) a valgus brace with medical treatment (n = 41); (2) a neoprene sleeve with medical treatment (n = 36); and (3) a control (i.e. medical treatment only) (n = 33). Individuals with OA of the knee and pain localised to the medial compartment were included in this trial. The valgus brace was custom made and consisted of a polyethylene thigh shell connected to a polyethylene calf shell through a polyaxial hinge on the medial side, which allowed application of four degrees valgus. The randomisation procedure consisted of a computer‐generated block method using sealed envelopes. Follow‐up was six months. Nine participants were lost to follow‐up (neoprene sleeve ‐ two/control ‐ seven). Participants included 79 men and 31 women. Mean age was 59 years. Mean varus alignment was nine degrees. Degree of OA of the knee was described only in the unloader brace group. Outcome data were presented as means and P values but without standard deviations; this made pooling impossible. Additional information was obtained from The Kirkley Research Group, but this information was not sufficient for analysis.
Maillefert 2001 presented an RCT of 156 participants with symptomatic medial knee OA (K&L > I). Laterally wedged insoles (n = 82) were compared with neutral insoles (n = 74). Both insoles were made of Ledos material, which consists of pure rubber with cork powder. The laterally elevated insoles were individually modelled, with elevation depending on static pedometer evaluation. The randomisation procedure was not described. Participants included 41 men and 108 women. Mean age was 65 years. Mean BMI was 29. Degree of varus alignment was not measured. After six months' follow‐up, nine participants (four from the wedged insole group) were lost to follow‐up. Two‐year follow‐up results were provided in 2004 (Pham 2004). A total of 106 participants completed the two‐year follow‐up: neutrally wedged insole (n = 51) versus laterally wedged insole (n = 55).
Müller‐Rath 2011 reported a non‐blinded RCT of 33 participants with symptomatic medial knee OA with a minimum of grade II according to the radiographic classification of K&L. Two treatment groups were included: a valgus knee brace (n = 13) and an elastic knee bandage (n = 10). The control group consisted of untreated individuals (n = 10). The randomisation procedure was not described. Participants included 24 men and nine women; mean age was 53.2 years. Mean BMI was 27.2. Mean alignment was 189 degrees of varus femoro‐tibial angle (FTA). The number of participants lost to follow‐up was not reported.
Raaij van 2010 reported a non‐blinded RCT of 91 participants with symptomatic medial knee OA (K&L ≥ I). Participants were block‐randomised to treatment with a 10‐mm laterally full‐length wedged insole (index group, n = 45) or a valgus brace (control group, n = 46). Baseline characteristics were similar regarding mean age (55 years), mean BMI (29 kg/m2), medial and lateral OA grades, analgesic use, mean VAS pain score (5.6 (0‐10 scale)) and mean WOMAC function (47 (0‐100 scale)). Gender differed statistically significantly (index group 65% female vs control group 35% female). At six months, a non‐blinded investigator assessed VAS and WOMAC scores as well as varus alignment correction in the frontal plane using the HKA angle on standardised whole leg films.
Rodriques 2008 randomly assigned 30 consecutive women with bilateral valgus deformity knee OA to two groups: medial insole (insoles with 8‐mm medial elevation at the rearfoot (n = 16)) and neutral insoles (similar insoles without elevation (n = 14)). Both groups also wore ankle supports. The demographic features of both groups were similar regarding mean age (62 years), mean BMI (30 kg/m2), mean disease duration (five years), radiographic osteoarthritis severity (K&L), race distribution and sedentary habits. A blinded examiner assessed VAS, Lequesne and WOMAC scores, along with femorotibial, talocalcaneal and talar tilt angles at baseline and after eight weeks.
Sattari 2011 reported an RCT of 60 participants with knee pain, genu varum and moderate to severe medial knee OA (K&L grade III or IV). Investigators included two treatment groups: a custom‐molded valgus stress knee support (n = 20) and a 1/4‐inch laterally wedged insole (n = 20). The control group (n = 20) received only general management that was universally applied to all three groups, consisting of activity modification, heating agents, straight leg raising, isometric quadriceps home exercises and analgesic use, when needed. The randomisation procedure was computer‐generated. Participants included 22 men and 38 women. Mean age was 48 years. Mean VAS pain score was 6.9. The degree of radiological OA was scored according to K&L. Follow‐up was nine months. Five participants were lost to follow‐up.
Toda 2001 published a prospective trial comparing an elastic subtalar strapped insole (n = 46) versus a traditional lateral wedge insole (n = 44). This study included individuals with symptomatic medial knee OA (K&L II‐IV). The wedge of the strapped insole was made from urethane with elevation of 6.35 mm strapped to an ankle sprain supporter. The traditional insole was a lateral rubber heel wedge with elevation of 6.35 mm. Quasi‐randomisation was performed according to birth date. All participants were female. Mean age was 65 and mean BMI was 25. Follow‐up was eight weeks, and no participant was lost to follow‐up. Standing radiographs of participants with and without their respective insoles were taken before entry into the eight‐week study. Degree of varus was 181 degrees FTA. Six‐month results were published in 2004. A total of 61 participants completed the six‐month follow‐up: subtalar strapped insole (n = 29) versus traditional laterally wedged insole (n = 32). Two‐year results were published in 2006. Only 42 participants completed the two‐year follow‐up: subtalar strapped insole (n = 21) versus traditional laterally wedged insole (n = 21). Analysis was performed without an intention‐to‐treat approach. All results were presented in the original articles as pre/post analysis, not as between‐group differences (Toda 2001). However, for both the original review and the updated review, the study author was contacted for more information; he sent the missing information on between‐group analysis of FTA, VAS and Lequesne index scores.
Toda 2002 published a second trial comparing a subtalar strapped insole (n = 42) with a sock‐type ankle supporter (n = 46). Individuals with symptomatic medial knee OA were included in this trial (K&L II‐IV). The wedge of the strapped insole was made from urethane with elevation of 6.35 mm strapped to an ankle sprain supporter. The sock‐type ankle support extended from malleoli to metatarsals and consisted of a lateral wedged heel insole with elevation of 6.35 mm. The trial took place in the same year (2000) as the first study. The quasi‐randomisation procedure was performed according to birth date. All participants were female. Mean age was 65 and mean BMI was 25. Degree of varus was 181 degrees (FTA). Follow‐up was eight weeks, and no participant was lost to follow‐up. Results were presented as pre/post analysis, not as between‐group differences. Second, the Lequesne index was presented graphically and no exact numbers were given. However, the study author was contacted for more information again, and he provided the missing information on between‐group analysis of the Lequesne index.
Toda 2008 published a third RCT of 227 participants with symptomatic medial knee OA (K&L I‐IV). In this study a placebo ‐ a neutral wedged insole into shoes (n = 45) ‐ was compared with four interventions ‐ a wedged insole with shoes (n = 45), a sock‐type ankle supporter with a wedged insole without shoes (n = 46), a subtalar strapped insole with shoes (n = 45) and a subtalar strapped insole without shoes (n = 46). The randomisation procedure consisted of a computer‐generated block method using sealed envelopes. Baseline characteristics and outcomes were presented only for the 207 participants who completed the 12‐week follow‐up. A total of 20 of 227 participants did not complete the study, which included 24 men and 183 women. Mean age was 65 years. Mean BMI was 25. Degree of OA was scored according to K&L. Degree of varus was 181 degrees (FTA). Most results were presented as pre/post analysis, and only intake of non‐steroidal anti‐inflammatory drugs (NSAIDs) was compared between placebo and different interventions.
Outcome measures included function scores, VAS scores (pain), analgesic/NSAID intake, walking distance, WOMAC scores (pain, function and stiffness), Hospital for Special Surgery knee scores (HSS; function), McMaster Toronto Arthritis score (MACTAR; function), Lequesne index (pain and function), degree of OA (Ahlback and K&L), global patient assessment, quality of life (EQ‐5D; a measure of health status), leg alignment (HKA angle; FTA), compliance and side effects.
Excluded studies
After retrieving the full text for final assessment, the review authors excluded 12 studies (Baker 2007; Birmingham 2001; Horlick 1993; Hunter 2012; Katsuragawa 1999; Kuroyanagi 2007; Matsuno 1997; Rooser 1988; Sasaki 1987; Shakoor 2008; Toda 2002b; Tohyama 1991): two studies (Sasaki 1987; Tohyama 1991) because of a retrospective design, four studies because of a cross‐over design (Baker 2007; Hunter 2012; Kuroyanagi 2007; Shakoor 2008), four studies because of lack of a control group (Birmingham 2001; Horlick 1993; Katsuragawa 1999; Matsuno 1997) and two studies (Rooser 1988; Toda 2002b) because investigators did not report the targeted outcome measure.
Risk of bias in included studies
Further details on risk of bias of each study are available in Figure 2, Figure 3 and the 'Risk of bias' tables (Characteristics of included studies).
2.
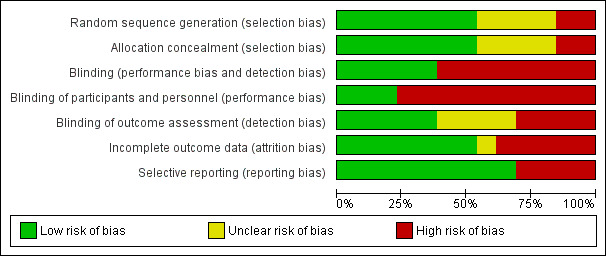
Figure 2 Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
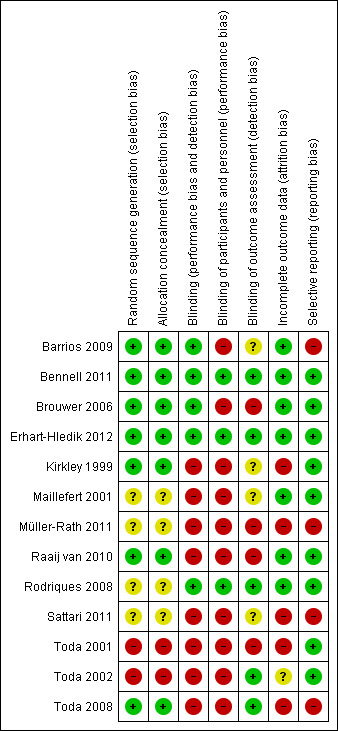
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Randomisation was performed in all studies. However in four studies, the procedure was not clearly described (Maillefert 2001; Müller‐Rath 2011; Rodriques 2008; Sattari 2011). In the other nine studies, the randomised sequence was adequately generated and clearly described (Barrios 2009; Bennell 2011; Brouwer 2006; Erhart‐Hledik 2012; Kirkley 1999; Raaij van 2010; Toda 2001; Toda 2002; Toda 2008). In seven studies, randomisation and concealment of allocations before assignment were adequately generated (Barrios 2009; Bennell 2011; Brouwer 2006; Erhart‐Hledik 2012; Kirkley 1999; Raaij van 2010; Toda 2008).
Blinding
In many studies, blinding procedures for treatment providers, participants and outcome assessors were insufficient. In most trials, blinding procedures for outcome assessors, treatment providers and participants were scored as 'high risk'. In five studies at least one of the outcome assessors was blinded (Bennell 2011; Erhart‐Hledik 2012; Rodriques 2008; Toda 2002; Toda 2008), and in only three of these studies, care providers and participants were also blinded (Bennell 2011; Erhart‐Hledik 2012; Rodriques 2008).
Incomplete outcome data
In six studies, incomplete outcome data were not adequately addressed. These studies with drop‐outs did not include an intention‐to‐treat analysis (Kirkley 1999; Müller‐Rath 2011; Sattari 2011; Toda 2001; Toda 2002; Toda 2008).
Selective reporting
In most studies, the selective outcome reporting item was unclear because no study protocol was provided (Kirkley 1999; Müller‐Rath 2011; Sattari 2011; Toda 2001; Toda 2002; Toda 2008).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
We have described comparisons of three main groups, namely, knee brace, foot/ankle orthosis and knee brace versus laterally wedged insole. Below we present the effects of interventions for the main comparisons, and we present the quality of evidence scored by the GRADE approach for each outcome. A 'Summary of findings' table was created using GRADEpro (http://ims.cochrane.org/revman/gradepro) for the three main comparisons, namely, valgus knee brace versus no brace (see Table 1; Table 2), laterally wedged insole versus neutral insole (see Table 1; Table 3) and valgus knee brace versus laterally wedged insole (see Table 4). We have included in our 'Summary of findings' tables the outcomes of pain, stiffness, physical functioning, health‐related quality of life, treatment failure, serious adverse events and total adverse events. Pooling of outcomes was possible only for the comparison of laterally wedged insole versus neutral insole. Data on other comparisons could not be pooled. Almost all studies used different interventions and comparison treatments with a wide variety of outcome measures, often with different follow‐up times.
Valgus knee brace versus no treatment
Four studies described the results of knee braces versus no treatment in OA of the knee (Brouwer 2006; Kirkley 1999; Müller‐Rath 2011; Sattari 2011).
Pain scores
We found four studies that reported pain scores. Brouwer 2006 reported improved VAS pain score after 12 months' follow‐up; however no statistically significant difference was found with no treatment (MD 0, 95% CI ‐0.8 to 0.8). Kirkley 1999 reported significantly better WOMAC pain scores in the brace group compared with the no brace group (P value < 0.001) after six months. Müller‐Rath 2011 reported statistically significantly improved VAS score in a valgus knee brace group after 16 weeks but no improvement in the control group (no treatment). Müller‐Rath 2011 provided no between‐group comparison. In Sattari 2011 the severity of pain decreased statistically significantly more in the knee brace group compared with the no treatment group (MD ‐2.8, 95% CI ‐3.6 to ‐2.0) after nine months (see also Analysis 1.1).
1.1. Analysis.
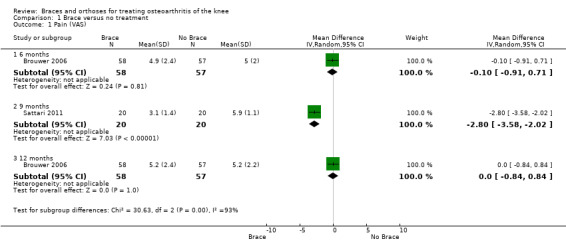
Comparison 1 Brace versus no treatment, Outcome 1 Pain (VAS).
Function
We found three studies that reported function scores. Brouwer 2006 reported statistically non‐significant results or lack of evidence of effect of HSS knee function for patients with a valgus knee brace and no brace after 12 months of follow‐up (MD 1.0, 95% CI ‐3.0 to 5.0). Kirkley 1999 found after six months' follow‐up better WOMAC physical function scores in the brace group than in the no brace group (P value ≤ 0.001). Müller‐Rath 2011 reported improved Tegner, Insal, Lequesne and WOMAC scores in a valgus knee brace group but no improvement in the control group (no treatment). Müller‐Rath 2011 provided no between‐group comparisons (see also Analysis 1.2).
1.2. Analysis.
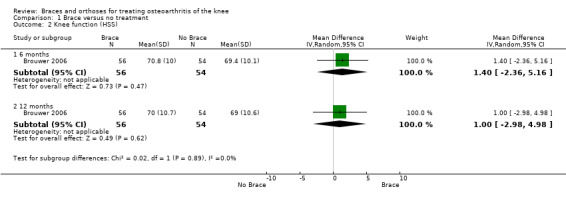
Comparison 1 Brace versus no treatment, Outcome 2 Knee function (HSS).
Stiffness
Stiffness was not reported in the included studies.
Health‐related quality of life
We found two studies that reported health‐related quality of life. Brouwer 2006 found no statistically significant differences in EuroQol score after 12 months between participants with and without a knee brace (MD ‐0.04, 95% CI ‐0.12 to 0.04). Kirkley 1999 found after six months' follow‐up statistically significant improvement in disease‐specific quality of life (P value 0.001) in favour of the brace group (see also Analysis 1.4).
1.4. Analysis.
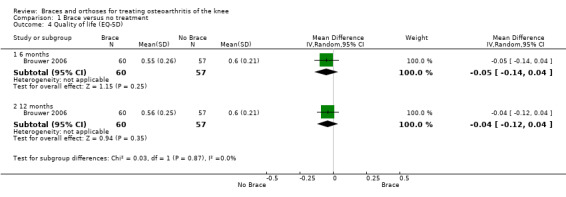
Comparison 1 Brace versus no treatment, Outcome 4 Quality of life (EQ‐5D).
Treatment failure
Treatment failure was not reported in the included studies.
Serious adverse events
Serious adverse events were not reported in the included studies.
Total adverse events
In total, 24 of 60 participants in the brace group and 14 of 57 participants in the control group in Brouwer 2006 stopped their initial treatment, most often because of lack of effect (RR 1.63, 95% CI 0.94 to 2.82) (see also Analysis 1.5). Other reasons for stopping were skin irritation (n = 2) and poor fit (n= 2). Sattari 2011 and Müller‐Rath 2011 reported no side effects in either group.
1.5. Analysis.
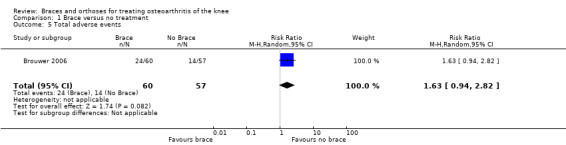
Comparison 1 Brace versus no treatment, Outcome 5 Total adverse events.
Radiographic scores
Radiographic scores were not reported in the included studies.
Compliance
Compliance was not reported in the included studies.
Walking distance
We found two studies that reported walking distance. Brouwer 2006 reported no statistically significant difference in walking distance after 12 months in a brace group compared with a no brace group (MD 0.4, 95% CI ‐0.9 to 1.7). Sattari 2011 reported statistically significantly increased walking distance in the brace group after nine months in contrast to the control group, which received no treatment (MD 1.2, 95% CI 1.0 to 1.5) (see also Analysis 1.3).
1.3. Analysis.
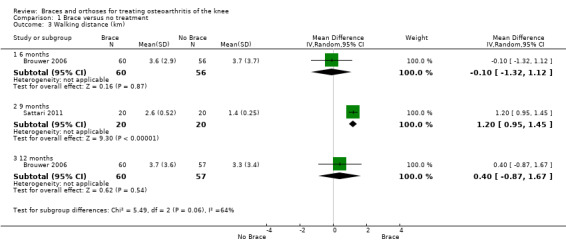
Comparison 1 Brace versus no treatment, Outcome 3 Walking distance (km).
According to the GRADE approach
Low‐quality inconclusive evidence suggests that patients with varus medial knee OA benefit more from brace treatment than from no treatment for the outcomes of pain, function and health‐related quality of life (Guyatt 2008a; Guyatt 2008b; Schünemann 2008).
Foot/Ankle orthosis
Four studies (Barrios 2009; Bennell 2011; Maillefert 2001; Sattari 2011) described the results of a foot/ankle orthosis for medial compartment OA of the knee (foot/ankle orthosis vs no treatment or a neutral insole) (see also Table 3).
Laterally wedged insole versus no treatment
One study (Sattari 2011) described the effects of a laterally wedged insole versus no treatment.
Pain scores
In Sattari 2011, a statistically significantly decreased pain score is described in the insole group compared with the no treatment group (MD ‐1.6, 95% CI ‐2.3 to ‐0.9) (see also Analysis 3.1).
3.1. Analysis.
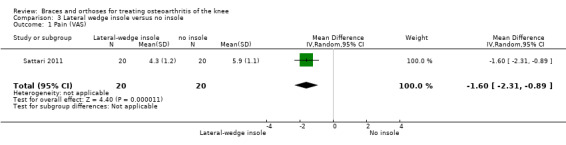
Comparison 3 Lateral wedge insole versus no insole, Outcome 1 Pain (VAS).
Function
Function was not reported in the included study.
Stiffness
Stiffness was not reported in the included study.
Health‐related quality of life
Health‐related quality of life was not reported in the included study.
Treatment failure
Treatment failure was not reported in the included study.
Serious adverse events
Serious adverse events were not reported in the included study.
Total adverse events
Adverse events were not reported in the included study.
Radiographic scores
Radiographic scores were not reported in the included study.
Compliance
Compliance was not reported in the included study.
Walking distance
Sattari 2011 described no statistically significant differences in walking distance after nine months between laterally wedged insole versus no treatment (MD 0.7, 95% CI 0.5 to 0.9) (see also Analysis 3.2).
3.2. Analysis.
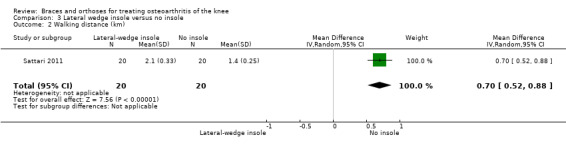
Comparison 3 Lateral wedge insole versus no insole, Outcome 2 Walking distance (km).
Laterally wedged insole versus neutral insole
Three studies (Barrios 2009;Bennell 2011;Maillefert 2001) described the effects of a laterally wedged insole versus a neutral insole.
Pain scores
In Barrios 2009, the WOMAC pain subscale improved statistically significantly in both study groups (neutral insole and laterally wedged insole) compared with baseline at one‐year follow‐up. Between‐group comparisons showed no statistically significant differences (MD ‐2.5, 95% CI ‐13.5 to 8.5). Bennell 2011 showed small mean reductions in pain scores over time in a neutral insole group and in a laterally wedged insole group; however these reductions were smaller than the minimal clinically important difference. Between‐group comparisons did not show a statistically significant difference (MD 1.0, 95% CI ‐3.8 to 5.8). At six months' follow‐up, Maillefert 2001 described a statistically significantly increased WOMAC pain score in a neutral group compared with a laterally wedged insole group (MD 6.4, 95% CI 0.0 to 12.9) (see also Analysis 4.1 and Analysis 4.2).
4.1. Analysis.
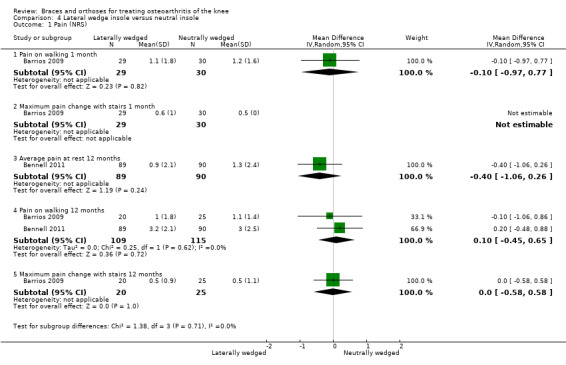
Comparison 4 Lateral wedge insole versus neutral insole, Outcome 1 Pain (NRS).
4.2. Analysis.
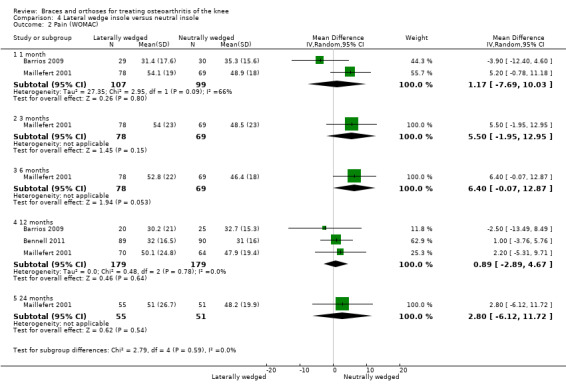
Comparison 4 Lateral wedge insole versus neutral insole, Outcome 2 Pain (WOMAC).
Function
We found three studies that reported function. In Barrios 2009, the WOMAC function subscale score improved statistically significantly in both study groups (neutral insole and laterally wedged insole) compared with baseline at one‐year follow‐up. Between‐group comparisons showed no statistically significant differences (MD 1.4, 95% CI ‐9.2 to 12.0). Bennell 2011 showed in both neutral insole and laterally wedged insole groups small mean reductions in WOMAC function scores over time; however these reductions were smaller than the minimal clinically important difference. Between‐group comparisons did not show a statistically significant difference (MD 1.0, 95% CI ‐4.1 to 6.1). Maillefert 2001 described a non‐statistically significant difference in WOMAC function score after six months in a laterally wedged insole group compared with a neutral insole group (MD 0.6, 95% CI ‐6.9 to 8.1) (see also Analysis 4.4).
4.4. Analysis.
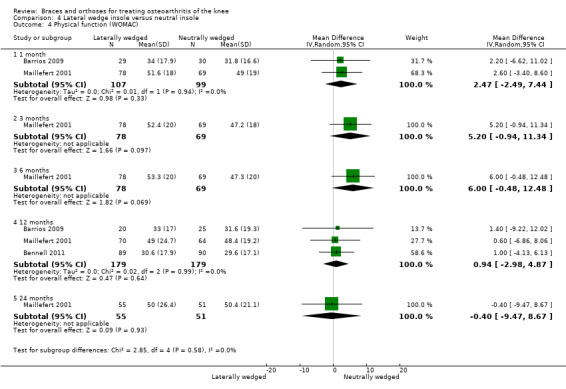
Comparison 4 Lateral wedge insole versus neutral insole, Outcome 4 Physical function (WOMAC).
Stiffness
We found three studies that reported stiffness. In Barrios 2009, the WOMAC stiffness subscale score improved statistically significantly in both study groups (neutral insole and laterally wedged insole) compared with baseline at one‐year follow‐up. Between‐group comparisons showed no statistically significant differences (MD 4.1, 95% CI ‐10.1 to 18.3). Bennell 2011 showed in both neutral insole and laterally wedged insole groups small mean reductions in WOMAC stiffness scores over time; however these reductions were smaller than the minimal clinically important difference. Between‐group comparisons did not show a statistically significant difference (MD 0.0, 95% CI ‐7.3 to 7.3). Maillefert 2001 found at six months' follow‐up no statistically significant difference in WOMAC stiffness in a neutral compared with a wedged insole group (MD ‐1.1, 95% CI ‐9.0 to 6.8) (see also Analysis 4.3).
4.3. Analysis.
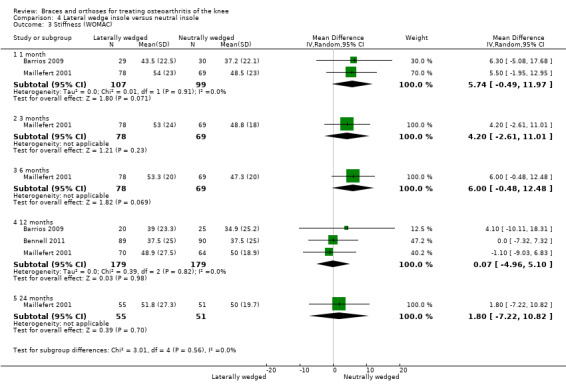
Comparison 4 Lateral wedge insole versus neutral insole, Outcome 3 Stiffness (WOMAC).
Health‐related quality of life
Health‐related quality of life was not reported in the included studies.
Treatment failure
During 12‐month follow‐up, 43% of participants in the lateral wedge group versus 19% of those in the neutral insole group changed their initial treatment in Barrios 2009. Mean duration of insole use in Bennell 2011 was statistically significantly less in the laterally wedged insole group than in the neutral insole group.
Serious adverse events
Serious adverse events were not reported in the included studies.
Total adverse events
Adverse events were not reported in the included studies.
Radiographic scores
Radiographic scores were not reported in the included studies.
Compliance
Maillefert 2001 found statistically significantly better compliance with the laterally wedged insole (87.8%) than with the neutral insole (74.3%).
Walking distance
Walking distance was not reported in the included studies.
According to the GRADE approach
Evidence is lacking to suggest that a laterally wedged insole is more effective than no treatment. Moderate evidence suggests that a laterally wedged insole is as effective as a neutral insole for the outcomes of pain, function and stiffness (Guyatt 2008a; Guyatt 2008b; Schünemann 2008).
Knee brace versus laterally wedged insole
Two studies (Raaij van 2010; Sattari 2011) described the results when a valgus knee brace versus a laterally wedged insole was used for medial compartment OA of the knee (see also Table 4).
Pain scores
We found two studies that reported pain scores. In Raaij van 2010 after six months' follow‐up, VAS pain scores statistically significantly improved in both the insole group and the brace group compared with baseline measurements, but no statistically significant differences were observed between the two study groups for this outcome (MD 0.2, 95% CI ‐1.15 to 0.75). In Sattari 2011, severity of pain decreased statistically significantly in the knee brace group and in the laterally wedged insole group. Investigators reported less pain in the brace group (MD ‐2.8, 95% CI ‐3.6 to ‐2.0) after nine months (see also Analysis 2.3).
2.3. Analysis.
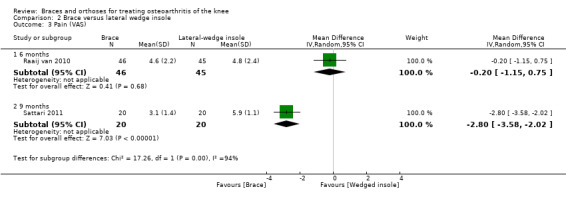
Comparison 2 Brace versus lateral wedge insole, Outcome 3 Pain (VAS).
Function
We found one study that reported function scores. Raaij van 2010 reported statistically significantly improved WOMAC function scores in both the insole group and the brace group compared with baseline measurements but noted no statistically significant differences between the two study groups for this outcome (MD 0.1, 95% CI ‐7.26 to 0.75) (see also Analysis 2.2).
2.2. Analysis.
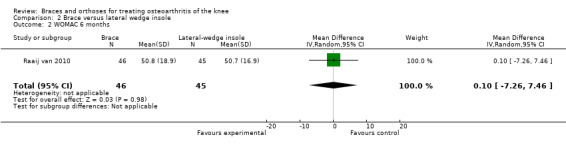
Comparison 2 Brace versus lateral wedge insole, Outcome 2 WOMAC 6 months.
Stiffness
None of the studies reported a specific stiffness score.
Health‐related quality of life
Health‐related quality of life was not reported in the included studies.
Treatment failure
Treatment failure was not reported in the included studies.
Serious adverse events
Serious adverse events were not reported in the included studies.
Total adverse events
Adverse events were not reported in the included studies.
Radiographic scores
Radiographic scores were not reported in the included studies.
Compliance
Compliance was not reported in the included studies.
Walking distance
We found one study that reported walking distance. Sattari 2011 reported an MD of 0.5 km (95% CI 0.23 to 0.77) in favour of the brace group (see also Analysis 2.1).
2.1. Analysis.
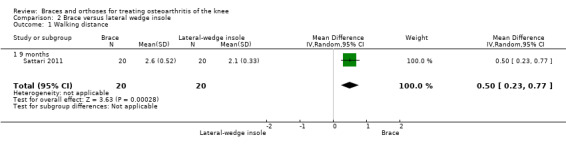
Comparison 2 Brace versus lateral wedge insole, Outcome 1 Walking distance.
According to the GRADE approach
Low‐quality evidence suggests no statistically significant differences in clinical effect between the laterally wedged insole group and the valgus knee brace group for the outcomes of pain and function (Guyatt 2008a; Guyatt 2008b; Schünemann 2008).
Discussion
Summary of main results
We conducted this review to assess the benefits and harms of braces and orthoses for treatment of patients with osteoarthritis (OA) of the knee. We included a total of 13 studies (n = 1356). These studies have reported results for patients with early to severe knee OA (Kellgren & Lawrence (K&L) I‐IV) treated with a valgus knee brace, a laterally wedged insole, a neutral insole or a variable or constant stiffness shoe, or given no treatment.
We found inconclusive evidence for the benefits of a valgus knee brace: Only four controlled trials were published. Kirkley 1999 concluded that in patients with varus knee OA, a brace provides additional beneficial effects in terms of pain and function compared with medical treatment alone. However, baseline characteristics were different between study groups, and the quality of the study was low. Brouwer 2006 concluded that a brace offers little or no additional effect compared with conservative treatment alone in patients with unicompartmental OA. However, many patients do not adhere in the long run to this kind of treatment because the positive effects are too small or because the side effects are too large. Müller‐Rath 2011 reported improved Tegner, Insal, Lequesne, Western Ontario‐McMaster Osteoarthritis Scale (WOMAC) and visual analogue scale (VAS) scores in the knee brace group after 16 weeks of treatment. They reported no improvement in the control group (no treatment) and described no side effects of treatment; however this study was sponsored, and study authors were not able to provide their data because of a server breakdown. Sattari 2011 reported a statistically significantly decreased pain score in the knee brace group compared with the no treatment group after nine months. Walking distance was increased statistically significantly in the brace group in contrast to the no treatment group after nine months. Investigators described no side effects of the brace. All four studies showed some clinical effect; however the methodological quality of these studies was low.
Moderate‐quality evidence shows the benefits of a laterally wedged insole (vs no treatment or a neutral insole) for medial compartment OA: We included seven controlled trials in this review with conflicting evidence. Barrios 2009, Maillefert 2001, Sattari 2011, Toda 2002 and Toda 2008 reported statistically significantly improved patient‐reported outcomes after a laterally wedged insole was worn; however Bennell 2011 and Toda 2001 reported reductions smaller than the minimal clinically important difference.
Conflicting evidence was found for preference of a neutral or a laterally wedged insole. Results reported by Barrios 2009 favoured the laterally wedged insole, Maillefert 2001 favoured the neutral insole and Bennell 2011 reported no statistically significant differences between the two insoles. Pooling of results of three studies comparing laterally wedged and neutral insoles resulted in lack of evidence of an effect on WOMAC pain scores, WOMAC stiffness scores and WOMAC function scores at one month and at 12 months (see also Table 3).
Data for the comparison of laterally wedged insole versus valgus knee brace could not be pooled. After six months' follow‐up, VAS pain scores and WOMAC function scores were improved and did not differ statistically significantly in the two groups (see also Table 4).
Overall completeness and applicability of evidence
Four trials investigated a knee brace and eight studies examined foot/ankle orthoses for medial compartment OA of the knee. It is important to note that the findings of these studies may lack generalisability: In the studies of Toda and Rodriques (Toda 2001; Toda 2002; Rodriques 2008), all participants were female, and in Kirkley 1999 and Sattari 2011, most participants were male. In all studies the age of participants was relatively high (mean 63 years). In the Kirkley 1999 trial, baseline characteristics differed between participants. It is important to present full data: Kirkley 1999 presented change scores without baseline scores and without a standard deviation. Toda 2001 and Toda 2002 presented pre‐analysis and post‐analysis results but did not report between‐group differences. Müller‐Rath 2011 presented their scores only graphically and could not provide their data because of a server breakdown.
Particularly, researchers studied the effects of braces and orthoses for medial compartment OA. Compared with lateral compartment OA of the knee, medial compartment OA has a much higher prevalence because lateral compartment OA is associated with trauma and is less clinically frequent. This is probably why only one randomised controlled trial (RCT) (Brouwer 2006) examined the effect of a brace or an orthosis for lateral compartment or general OA of the knee. Furthermore, varus bracing for lateral OA is probably less effective; the adduction moment at the knee during the stance phase of walking causes mainly medial loading (Johnson 1980). In general OA of the knee, there is no compartment to unload, and perhaps a sleeve or a neutral brace will benefit. No studies compared a brace or an orthosis with operative treatment such as high tibial osteotomy or unicompartmental knee arthroplasty.
Quality of the evidence
Two studies in this review had low risk of any type of bias, six studies had moderate risk and five studies had high risk. The randomisation procedure frequently was not described or was insufficient. Except for the trials of Bennell 2011, Brouwer 2006, Kirkley 1999, Raaij van 2010 and Toda 2008, the randomisation procedure was not described or was inadequate. In most studies, blinding procedures were insufficient, although we realise that when braces are used, blinding is not always possible; for footwear inserts, it is generally less difficult. Results were based on small studies, leading to imprecision.
Potential biases in the review process
One study did not report the number of participants lost to follow‐up. This study was funded by Medi, provider of orthoses. Outcomes were presented only graphically in this publication. Study authors were not able to provide their data on request because of a "server breakdown" (Müller‐Rath 2011).
Agreements and disagreements with other studies or reviews
Other meta‐analyses or systematic reviews were not available for comparison of our results.
Authors' conclusions
Implications for practice.
We conclude that in cases of varus medial compartment knee OA, low‐quality inconclusive evidence shows benefits of bracing for pain, stiffness, function and quality of life in the treatment of medial compartment knee OA. Moderate‐quality evidence suggests that a laterally wedged insole is as effective as a neutral insole. Evidence is lacking to suggest that a laterally wedged insole is more effective than no treatment. Also evidence of low quality suggests no statistically significant difference in clinical effect between the laterally wedged insole and the valgus knee brace.
The optimal choice for an orthosis remains unclear, and long‐term implications are lacking.
Implications for research.
The methodological quality of studies investigating the benefits of braces and orthoses has to be improved, particularly the randomisation procedure and blinding measures. Moreover to improve the generalisability of results, studies should not be limited to female participants.
Short‐term benefit must be established first to justify the considerable resources required by and the ethical implications involved in a lengthy study. Subsequently, a follow‐up period of at least five years is needed because OA is a chronic disease. One general knee score would allow pooling of results. We recommend using the WOMAC because this has been shown to be a valid instrument for measurement of OA (Bellamy 1997). Between‐groups analysis is necessary to show relevant differences. Future studies should provide complete data on outcome measures, including means and standard deviations or 95% confidence intervals.
It is important to score side effects because they influence the patient's compliance with the intervention. This especially concerns braces, which can be obtrusive in many cases. For insoles, bigger and less stylish shoes are needed. New trials should investigate the long‐term benefits of braces and orthoses compared with standard conservative care. More studies are needed to identify predictive factors for the success of brace and insole treatment. If feasible, braces should be compared with ankle/foot orthoses. If braces and orthoses are effective, they should be compared with operative treatment such as high tibial osteotomy or knee arthroplasty for medial compartment OA. It will be interesting to learn for how long surgery can be delayed by this kind of conservative treatment (Gossec 2007).
What's new
| Date | Event | Description |
|---|---|---|
| 1 March 2014 | New search has been performed | New search with 6 new studies |
| 1 March 2014 | New citation required but conclusions have not changed | Change in authorship |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 2 May 2011 | Amended | Converted to new review format. CMSG ID C102‐R |
| 21 August 2007 | New search has been performed | Minor update approved: 8/20/07 New studies found and included or excluded: 5/31/07 See published notes for additional details |
| 17 November 2004 | New citation required and conclusions have changed | Substantive amendments made |
Notes
This is an update of the original review, which was published in Issue 1, 2005. We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE for controlled clinical trials until May 2007. As a result of the search, we have included six new studies in this updated review. The conclusions of this update are consistent with those provided in the original review.
Acknowledgements
The review authors thank Jessie McGowan from the Institute of Population Health University of Ottawa (Canada) for developing the search strategy, and Wichor Bramer from the Erasmus Medical Center Rotterdam (Netherlands) for providing support for the literature search update. We also thank Maria Judd and Lara Maxwell of the Cochrane Musculoskeletal Group for providing support.
Appendices
Appendix 1. Search strategy and summary of results
Database: MEDLINE Ovid SP
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
("osteoarthritis, knee"/ OR ((osteoarthritis/ OR (osteoarthritis OR osteoarthrosis OR "degenerative joint disease" OR "osteo arthritis" OR "osteo arthrosis" OR "degenerative arthritis").ab,ti.) AND ("knee joint"/ OR (knee*).ab,ti.))) AND (exp "Orthotic Devices"/ OR (brace* OR bracing OR orthotic* OR orthoses OR orthosis).ab,ti.)
Database: EMBASE
Search strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
('knee osteoarthritis'/de OR ((osteoarthritis/de OR (osteoarthritis OR osteoarthrosis OR 'degenerative joint disease' OR 'osteo arthritis' OR 'osteo arthrosis' OR 'degenerative arthritis'):ab,ti) AND (knee/de OR (knee*):ab,ti))) AND (orthosis/de OR (brace* OR bracing OR orthotic* OR orthoses OR orthosis):ab,ti) AND [01‐05‐2007]/sd
Database: The Cochrane Library
Search strategy
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
((((osteoarthritis OR osteoarthrosis OR 'degenerative joint disease' OR 'osteo arthritis' OR 'osteo arthrosis' OR 'degenerative arthritis'):ab,ti) AND ((knee*):ab,ti))) AND ((brace* OR bracing OR orthotic* OR orthoses OR orthosis):ab,ti)
| Database and coverage | Search date | Number of references retrieved | Number of references after de‐duplication |
| MEDLINE Ovid SP 2007‐2013 | March 1, 2014 | 82 | 56 |
| EMBASE 2007‐2013 | March 1, 2014 | 167 | 161 |
| The Cochrane Library | March 1, 2014 | 23 | 11 |
| Totals | 272 | 228 |
Data and analyses
Comparison 1. Brace versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (VAS) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 months | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.91, 0.71] |
| 1.2 9 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐3.58, ‐2.02] |
| 1.3 12 months | 1 | 115 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.84, 0.84] |
| 2 Knee function (HSS) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 months | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 1.40 [‐2.36, 5.16] |
| 2.2 12 months | 1 | 110 | Mean Difference (IV, Random, 95% CI) | 1.0 [‐2.98, 4.98] |
| 3 Walking distance (km) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 6 months | 1 | 116 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐1.32, 1.12] |
| 3.2 9 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 1.20 [0.95, 1.45] |
| 3.3 12 months | 1 | 117 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.87, 1.67] |
| 4 Quality of life (EQ‐5D) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 6 months | 1 | 117 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.14, 0.04] |
| 4.2 12 months | 1 | 117 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.12, 0.04] |
| 5 Total adverse events | 1 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.94, 2.82] |
Comparison 2. Brace versus lateral wedge insole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Walking distance | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.23, 0.77] |
| 1.1 9 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.23, 0.77] |
| 2 WOMAC 6 months | 1 | 91 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐7.26, 7.46] |
| 3 Pain (VAS) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 6 months | 1 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐1.15, 0.75] |
| 3.2 9 months | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐3.58, ‐2.02] |
Comparison 3. Lateral wedge insole versus no insole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (VAS) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐2.31, ‐0.89] |
| 2 Walking distance (km) | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.52, 0.88] |
Comparison 4. Lateral wedge insole versus neutral insole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (NRS) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Pain on walking 1 month | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.97, 0.77] |
| 1.2 Maximum pain change with stairs 1 month | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Average pain at rest 12 months | 1 | 179 | Mean Difference (IV, Random, 95% CI) | ‐0.4 [‐1.06, 0.26] |
| 1.4 Pain on walking 12 months | 2 | 224 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.45, 0.65] |
| 1.5 Maximum pain change with stairs 12 months | 1 | 45 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.58, 0.58] |
| 2 Pain (WOMAC) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 1 month | 2 | 206 | Mean Difference (IV, Random, 95% CI) | 1.17 [‐7.69, 10.03] |
| 2.2 3 months | 1 | 147 | Mean Difference (IV, Random, 95% CI) | 5.5 [‐1.95, 12.95] |
| 2.3 6 months | 1 | 147 | Mean Difference (IV, Random, 95% CI) | 6.40 [‐0.07, 12.87] |
| 2.4 12 months | 3 | 358 | Mean Difference (IV, Random, 95% CI) | 0.89 [‐2.89, 4.67] |
| 2.5 24 months | 1 | 106 | Mean Difference (IV, Random, 95% CI) | 2.80 [‐6.12, 11.72] |
| 3 Stiffness (WOMAC) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 1 month | 2 | 206 | Mean Difference (IV, Random, 95% CI) | 5.74 [‐0.49, 11.97] |
| 3.2 3 months | 1 | 147 | Mean Difference (IV, Random, 95% CI) | 4.20 [‐2.61, 11.01] |
| 3.3 6 months | 1 | 147 | Mean Difference (IV, Random, 95% CI) | 6.0 [‐0.48, 12.48] |
| 3.4 12 months | 3 | 358 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐4.96, 5.10] |
| 3.5 24 months | 1 | 106 | Mean Difference (IV, Random, 95% CI) | 1.80 [‐7.22, 10.82] |
| 4 Physical function (WOMAC) | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 1 month | 2 | 206 | Mean Difference (IV, Random, 95% CI) | 2.47 [‐2.49, 7.44] |
| 4.2 3 months | 1 | 147 | Mean Difference (IV, Random, 95% CI) | 5.20 [‐0.94, 11.34] |
| 4.3 6 months | 1 | 147 | Mean Difference (IV, Random, 95% CI) | 6.0 [‐0.48, 12.48] |
| 4.4 12 months | 3 | 358 | Mean Difference (IV, Random, 95% CI) | 0.94 [‐2.98, 4.87] |
| 4.5 24 months | 1 | 106 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐9.47, 8.67] |
| 5 Health‐related quality of life | 1 | 179 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.06, 0.06] |
| 6 Physical activity scale for the elderly | 1 | 179 | Mean Difference (IV, Random, 95% CI) | 15.0 [‐8.45, 38.45] |
| 7 Number of steps taken per day | 1 | 179 | Mean Difference (IV, Random, 95% CI) | 1371.0 [38.53, 2703.47] |
| 8 Global patient assessment at 24 months | 1 | 106 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐7.41, 10.61] |
| 9 Compliance at 6 months | 1 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [1.01, 1.38] |
| 10 Time for negotiation of stairs | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 1 month | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 1.10 [‐1.18, 3.38] |
| 10.2 12 months | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐3.06, 2.46] |
| 11 6‐Minute walk distance | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 1 month | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 23.00 [‐18.61, 64.61] |
| 11.2 12 months | 1 | 45 | Mean Difference (IV, Random, 95% CI) | ‐25.20 [‐77.37, 26.97] |
4.5. Analysis.
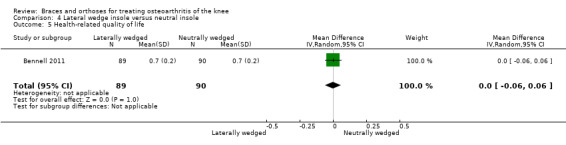
Comparison 4 Lateral wedge insole versus neutral insole, Outcome 5 Health‐related quality of life.
4.6. Analysis.
Comparison 4 Lateral wedge insole versus neutral insole, Outcome 6 Physical activity scale for the elderly.
4.7. Analysis.
Comparison 4 Lateral wedge insole versus neutral insole, Outcome 7 Number of steps taken per day.
4.8. Analysis.
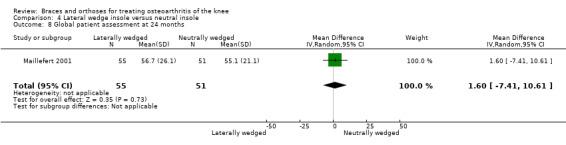
Comparison 4 Lateral wedge insole versus neutral insole, Outcome 8 Global patient assessment at 24 months.
4.9. Analysis.
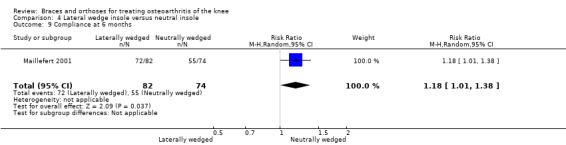
Comparison 4 Lateral wedge insole versus neutral insole, Outcome 9 Compliance at 6 months.
4.10. Analysis.
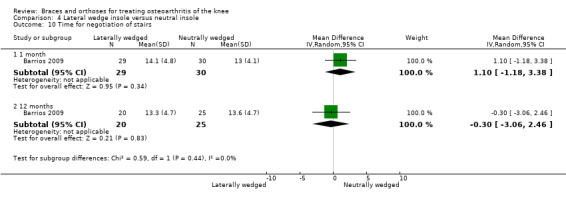
Comparison 4 Lateral wedge insole versus neutral insole, Outcome 10 Time for negotiation of stairs.
4.11. Analysis.
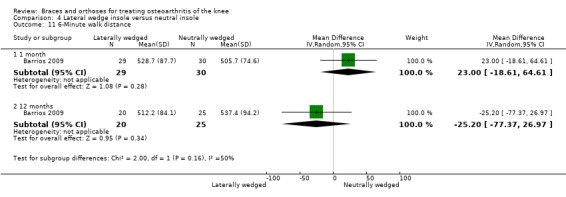
Comparison 4 Lateral wedge insole versus neutral insole, Outcome 11 6‐Minute walk distance.
Comparison 5. Subtalar strapped insole versus inserted lateral wedge insole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (VAS) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 8 weeks | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐9.20 [‐18.28, ‐0.12] |
| 1.2 6 months | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐11.80 [‐22.04, ‐1.56] |
| 1.3 24 months | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐13.34, 9.34] |
| 2 Lequesne index | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 8 weeks | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐2.81, 1.61] |
| 2.2 6 months | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐1.5 [‐4.23, 1.23] |
| 2.3 24 months | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.3 [‐5.45, 0.85] |
| 3 FTA ‐ angle | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 8 weeks | 1 | 90 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐3.45, 0.85] |
| 3.2 6 months | 1 | 61 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐5.84, ‐0.16] |
| 3.3 24 months | 1 | 42 | Mean Difference (IV, Random, 95% CI) | ‐2.70 [‐5.13, ‐0.27] |
| 4 Side effects at 8 weeks | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.74 [0.72, 45.77] |
5.1. Analysis.
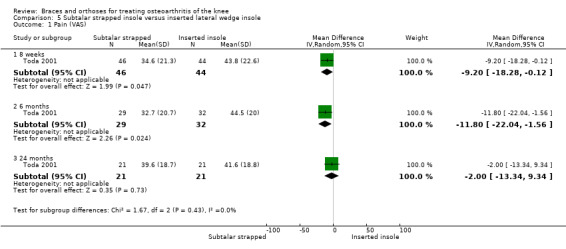
Comparison 5 Subtalar strapped insole versus inserted lateral wedge insole, Outcome 1 Pain (VAS).
5.2. Analysis.
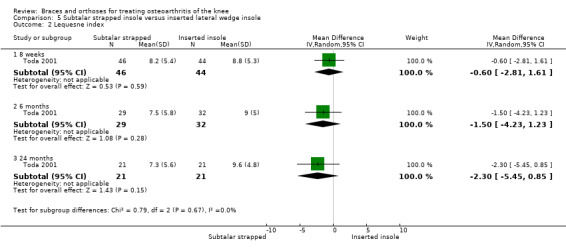
Comparison 5 Subtalar strapped insole versus inserted lateral wedge insole, Outcome 2 Lequesne index.
5.3. Analysis.
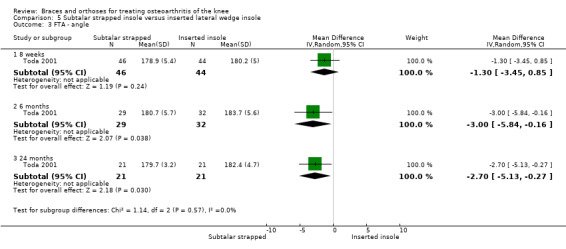
Comparison 5 Subtalar strapped insole versus inserted lateral wedge insole, Outcome 3 FTA ‐ angle.
5.4. Analysis.
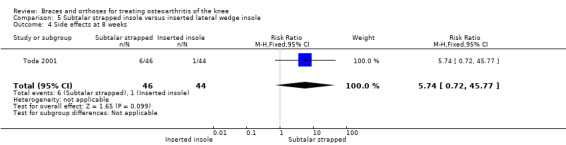
Comparison 5 Subtalar strapped insole versus inserted lateral wedge insole, Outcome 4 Side effects at 8 weeks.
Comparison 6. Subtalar strapped insole versus sock‐type insole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FTA angle | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.89, 1.09] |
| 2 Aggregate score | 1 | 88 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐3.57, 0.77] |
6.1. Analysis.
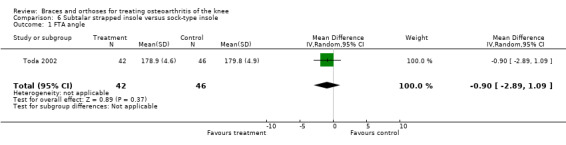
Comparison 6 Subtalar strapped insole versus sock‐type insole, Outcome 1 FTA angle.
6.2. Analysis.
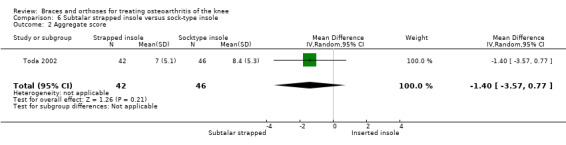
Comparison 6 Subtalar strapped insole versus sock‐type insole, Outcome 2 Aggregate score.
Comparison 7. Medial wedge insole versus neutral insole.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 VAS rest | 1 | 30 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐2.16, 1.36] |
| 2 VAS movement | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐2.2 [‐4.04, ‐0.36] |
| 3 VAS night | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐3.12, 0.12] |
| 4 WOMAC | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐6.70 [‐17.09, 3.69] |
| 5 Lequesne | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐2.40 [‐5.28, 0.48] |
7.1. Analysis.
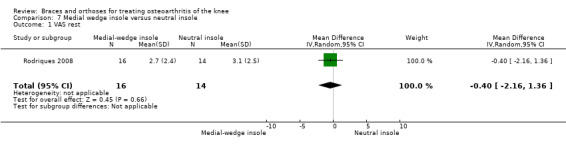
Comparison 7 Medial wedge insole versus neutral insole, Outcome 1 VAS rest.
7.2. Analysis.
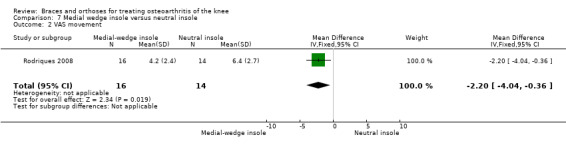
Comparison 7 Medial wedge insole versus neutral insole, Outcome 2 VAS movement.
7.3. Analysis.
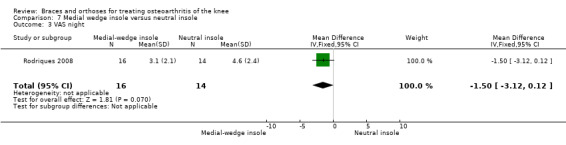
Comparison 7 Medial wedge insole versus neutral insole, Outcome 3 VAS night.
7.4. Analysis.
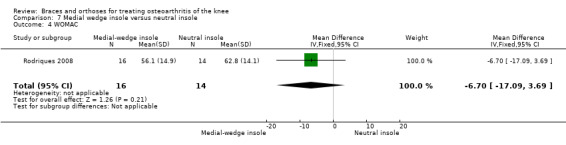
Comparison 7 Medial wedge insole versus neutral insole, Outcome 4 WOMAC.
7.5. Analysis.
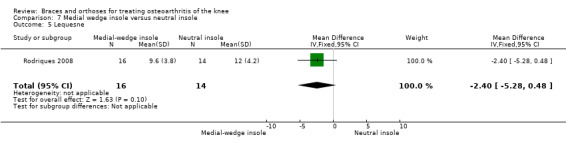
Comparison 7 Medial wedge insole versus neutral insole, Outcome 5 Lequesne.
Comparison 8. Variable stiffness shoe versus constant stiffness shoe.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain (WOMAC) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 6 months | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐8.57, 1.17] |
| 1.2 12 months | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐6.43, 4.23] |
| 2 Stiffness (WOMAC) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 6 months | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐1.90 [‐4.34, 0.54] |
| 2.2 12 months | 1 | 44 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐4.52, 1.72] |
| 3 Physical function (WOMAC) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 6 months | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐6.90 [‐24.14, 10.34] |
| 3.2 12 months | 1 | 55 | Mean Difference (IV, Random, 95% CI) | ‐4.90 [‐24.54, 14.74] |
8.1. Analysis.
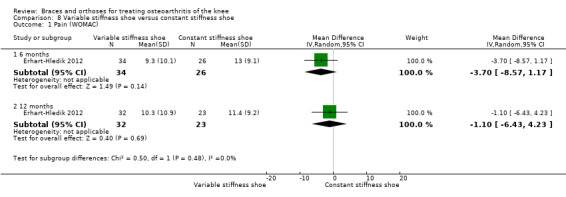
Comparison 8 Variable stiffness shoe versus constant stiffness shoe, Outcome 1 Pain (WOMAC).
8.2. Analysis.
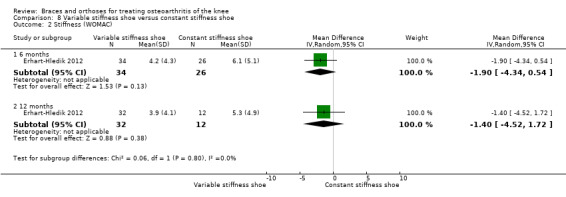
Comparison 8 Variable stiffness shoe versus constant stiffness shoe, Outcome 2 Stiffness (WOMAC).
8.3. Analysis.
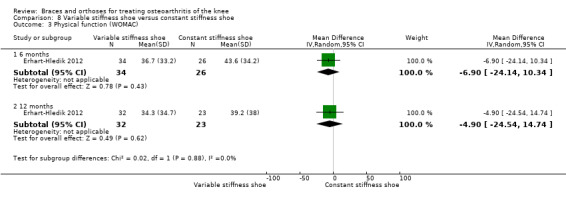
Comparison 8 Variable stiffness shoe versus constant stiffness shoe, Outcome 3 Physical function (WOMAC).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barrios 2009.
| Methods | RCT; block randomisation | |
| Participants | Medial tibiofemoral OA: n = 66 Male/female: 29/37 Mean age (years): 62 Mean BMI (kg/m2): 33 Grade of OA according to Kellgren & Lawrence: II = 27, III = 24, IV = 15 |
|
| Interventions | I = full‐length (9º) wedged insole into shoe (n = 35) vs C = non‐custom neutral insole into shoe (n = 31) Follow‐up: 12 months |
|
| Outcomes | WOMAC, 6‐minute walking test, stair negotiation test | |
| Notes | Mean and P values are presented, but SD values are missing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a block‐randomization was performed based on OA grade, gender, and age (greater or less than 55 years)" |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation was done by an administrative assistant unaware of the methodologies used" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "the subjects were blinded from group assignment" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants assigned to the treatment group were tested to determine the amount of wedging Quote: "subjects who had no pain relieve (after wedging) were excluded from the study" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat |
| Selective reporting (reporting bias) | High risk | Quote: "subjects who had no pain relieve (after wedging) were excluded from the study" |
Bennell 2011.
| Methods | RCT; computer‐generated block randomisation | |
| Participants | Painful medial knee osteoarthritis: n = 200 Male/ female: 82/118 Mean age (years): 64 Mean BMI (kg/m2): 29.2 Mean varus (degrees): 181 Grade of medial OA according to Kellgren & Lawrence: II = 95, III = 105 |
|
| Interventions | I = full‐length (5º) wedged insole into shoe (n = 103) vs C = neutral insole into shoe (n = 97) Follow‐up: 12 months |
|
| Outcomes | NRS scale (pain), WOMAC scale, physical activity scale for the elderly, average number of steps taken per day | |
| Notes | No competing interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were stratified by disease severity (Kellgren and Lawrence grades 2 and 3) and sex and randomly allocated in permuted blocks of 6 to 12" |
| Allocation concealment (selection bias) | Low risk | Quote: "An independent investigator used a computer program to generate the randomisation sequence a priori. Allocation was sealed in opaque and consecutively numbered envelopes held centrally. Envelopes were opened sequentially by an independent person" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "a double blind randomised controlled trial" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants were informed that two types of insoles were being compared but the insoles and study hypotheses were not described" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A blinded examiner assessed the participants at baseline and 12 months" according to patient‐reported outcome measures; participants were blinded as well |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Complete data were reported |
Brouwer 2006.
| Methods | RCT; computer‐generated block randomisation | |
| Participants | Unicompartmental knee OA: n = 117 Male/ female: 69/48 Mean age (years): 59 Mean BMI (kg/m2): 28.5 Varus: n = 95/valgus: n = 22 Mean varus (degrees) = 188 Mean valgus (degrees) = 173 | |
| Interventions | I = Brace intended to reduce load (n = 60) vs C = standard conservative treatment (n = 57) Follow‐up: 12 months | |
| Outcomes | VAS, HSS knee score, walking distance, EuroQol | |
| Notes | No competing interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients were randomised according to a computer‐generated procedure in blocks of 24" |
| Allocation concealment (selection bias) | Low risk | Quote: "the allocation of treatment was concealed until after the patient was included and baseline measurements were executed; sealed envelopes contained the group assignment" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessor of the HSS knee was blinded for allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded; outcome assessor of the HSS knee was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study used patient‐reported outcome measures; participants were not blinded. Functional outcome (HSS knee score) was measured blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Complete data were reported |
Erhart‐Hledik 2012.
| Methods | RCT; randomisation procedure not described; outcome assessment partially blinded | |
| Participants | Medial compartment knee OA: n = 79 Male/female: 42/37 Mean age (years): 60 Mean BMI (kg/m2): 27.7 | |
| Interventions | I = Variable‐stifness shoe (n = 40) vs C = constant stiffness shoe (n = 39) Follow‐up = 6 and 12 months |
|
| Outcomes | WOMAC | |
| Notes | 6‐Month results were presented earlier. Patients were included on the basis of MRI. Anteroposterior radiograph was used during follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "using a random number generator" |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization code was revealed to the study coordinator in charge of subject recruitment and in contact with the subjects regarding WOMAC scores, once recruitment, data collection, and analyses were completed" |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Subjects were blinded to their shoe type" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Subjects were blinded to their shoe type (The researcher performing the gait analysis was not blinded to the shoe type)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Subjects were blinded to their shoe type" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Complete data were reported; study author provided additional data for this review |
Kirkley 1999.
| Methods | RCT; computer‐generated blocked randomisation scheme with use of sealed envelopes; blinding of outcome assessment not described | |
| Participants | Varus arthrosis: n = 119 Male/female: 79/31 Mean age (years): 59 Mean varus (degrees): 189 | |
| Interventions | I = unloader brace (n = 41) vs C1 = neoprene brace (n = 36) vs C2 = medical treatment only (n = 33) Follow‐up: 6 months | |
| Outcomes | WOMAC and MACTAR scores Function assessed with use of the 6‐minute walking and the 30‐second stair climbing test | |
| Notes | No competing interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a computer‐generated blocked randomisation scheme" |
| Allocation concealment (selection bias) | Low risk | Quote: "with use of sealed envelopes" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded to the intervention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study used patient‐reported outcomes. Outcome assessor of patient‐reported outcome measures was not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Complete data were reported |
Maillefert 2001.
| Methods | RCT; randomisation procedure not described; outcome assessment partially blinded | |
| Participants | Painful medial knee osteoarthritis: n = 156
Male/female: 41/108
Mean age (years): 65
Mean BMI (kg/m2): 29 Grade of OA according to Kellgren & Lawrence: II = 69, III = 60, IV = 18 |
|
| Interventions | I = laterally wedged insole (n = 78) vs C = neutrally wedged insole (n = 69); follow‐up: 1, 3, 6 months | |
| Outcomes | WOMAC, concomitant treatment, compliance | |
| Notes | No competing interests | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence generation procedure was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure was not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were blinded to randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The practitioner nor the patient was blinded to the randomization"; however the research nurse was blinded to allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Any missing data were collected by a research nurse, unaware of the randomisation by telephone" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Analysis was made using an intention‐to‐treat approach" |
| Selective reporting (reporting bias) | Low risk | Complete data were reported |
Müller‐Rath 2011.
| Methods | RCT; randomisation procedure and blinding of outcome assessment not described | |
| Participants | Symptomatic varus knee OA: n = 33 Male/female: 24/9 Mean age (years): 53 Mean BMI (kg/m2): 27.2 | |
| Interventions | I = valgus knee brace or elastic knee bandage vs C = no treatment Follow‐up: 16 weeks |
|
| Outcomes | Tegner, Insall, Lequesne, WOMAC, HSS, VAS | |
| Notes | Number of participants lost to follow‐up is not reported. Study is funded by Medi, provider of orthoses. Study authors could not provide their data because of a "server breakdown" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No lost participants were reported; study authors could not provide their data because of a "server breakdown" |
| Selective reporting (reporting bias) | High risk | No intention‐to‐treat |
Raaij van 2010.
| Methods | RCT; computer‐generated blocked randomisation | |
| Participants | Medial knee OA: n = 91
Male/female: 46/45
Mean age (years): 55
Mean BMI (kg/m2): 29
Mean varus (degrees) = 187 Degree of medial OA according to Kellgren & Lawrence (n): I = 37, II = 17, III = 35, IV = 2 Degree of lateral OA according to Kellgren & Lawrence (n): 0 = 67, I = 22, II = 2 |
|
| Interventions | I = 10‐mm laterally full‐length wedged insole (n = 45) vs C = valgus brace (n = 46) | |
| Outcomes | VAS (pain), WOMAC, degree of varus (hip‐knee‐ankle angle) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomised according to a computer‐generated procedure (block randomisation, with variable sizes of the blocks)" |
| Allocation concealment (selection bias) | Low risk | Quote: "the randomizations codes were held by an independent observer to ensure masked blocking" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded trial |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "completely unblinded" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "one non‐blinded investigator, a trained orthopedic surgeon, assessed the follow‐up measurements" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "by intention‐to‐treat" |
| Selective reporting (reporting bias) | Low risk | Complete data were reported |
Rodriques 2008.
| Methods | RCT; randomisation procedure not described; outcome assessment blinded | |
| Participants | Bilateral valgus deformity knee OA: N = 30 All female Mean age (years): 62 Mean BMI (kg/m2): 30 Degree of OA lateral compartment according to Kellgren & Lawrence: II = 16, III = 8, IV = 6 |
|
| Interventions | I = medially wedged insole (n = 16) vs C = neutral insole (n = 14) Follow‐up: 2 months |
|
| Outcomes | VAS pain (night, rest, movement), Lequesne index score, WOMAC | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure is not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "patients of both groups received the same new shoe and were blind to insole use" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "outcomes were administered at baseline and after 8 weeks by a blinded examiner" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete data were reported |
| Selective reporting (reporting bias) | Low risk | Complete data were reported |
Sattari 2011.
| Methods | RCT; computer‐generated randomisation | |
| Participants | Varus knee OA: n = 60 Male/female: 22/38 Mean age (years): 48 Mean BMI (kg/m2): not reported Degree of OA medial compartment according to Kellgren & Lawrence: III = 39, IV = 21 |
|
| Interventions | I = custom‐molded valgus stress knee support (n = 20) or 1/4‐inch lateral wedged insole (n = 20) vs C = no intervention Follow‐up: 9 months |
|
| Outcomes | VAS (pain), Lequesne index (walking distance) | |
| Notes | Conflicts of interest are not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation sequence procedure is not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment procedure is not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded; outcome assessors were blinded; study used patient‐reported outcome measures |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "5 patients were removed from the study because of absence from follow‐up. They were substituted with new patients, to maintain 20 patients in each group" |
| Selective reporting (reporting bias) | High risk | No intention‐to‐treat |
Toda 2001.
| Methods | RCT; randomisation performed by date of birth. Blinded assessments of level of pain according to VAS, Lequesne index, radiographic outcome | |
| Participants | American College of Rheumatology criteria for knee osteoarthritis (n = 90)
All female
Mean age (years): 65
Mean varus (FTA; degrees): 181 Degree of OA according to Kellgren & Lawrence: II = 55, III = 27, IV = 8 |
|
| Interventions | I = strapped insole (n = 46) vs C = lateral wedge insole (n = 44) Follow‐up: 8 weeks, 6 months and 2 years | |
| Outcomes | VAS, Lequesne (pain) index score, radiographic changes | |
| Notes | In Table 3 of the first publication, median value of final VAS score in strapped insole group is incorrect No between‐groups analysis was performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Randomisation was performed by date of birth" |
| Allocation concealment (selection bias) | High risk | Quote: "Randomisation was performed by date of birth" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded; outcome assessors were blinded; study used patient‐reported outcome measures; radiographic changes were measured blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to the intervention. Research nurse was blinded to objectives of the study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study used patient‐reported outcome measures (PROMs). Outcome assessor of PROMS, namely, the participant, was not blinded in this study. However participant and research nurse were blinded to objectives of the study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Complete data were reported |
Toda 2002.
| Methods | RCT; randomisation performed by date of birth | |
| Participants | American College of Rheumatology criteria for knee OA: n = 88 All female Mean age (years): 65 Mean BMI (kg/m2): 25 Degree of varus (FTA; degrees): 181 | |
| Interventions | I = subtalar strapped support (n = 44) vs C = sock‐type support (n = 46) Follow‐up: 8 weeks | |
| Outcomes | Lequesne (pain) index, radiographic changes | |
| Notes | Scores were shown in figures; no exact numbers were given No between‐groups analysis was performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Randomisation was performed by date of birth" |
| Allocation concealment (selection bias) | High risk | Quote: "Randomisation was performed by date of birth" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded; outcome assessment was blinded; study used patient‐reported outcome measures. Radiographic changes were measured blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "in this study, participants were not blinded to the treatment. However, participants were not told whether the method of fixation at ankle joint, belt or sock‐type, was thought to be important" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All other items were assessed by physical therapists who were uninformed of the objective of the study when patients presented the OOC" "The radiographic assessment was completed by 3 orthopedic surgeons prior to being informed of the category of the patients" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Scores were shown in figures; no exact numbers were given |
| Selective reporting (reporting bias) | Low risk | Complete data were reported |
Toda 2008.
| Methods | RCT; computer‐generated blocked randomisation | |
| Participants | Patients with medial compartment OA of the knee according to American College of Rheumatology criteria and a criterion stipulating standing femorotibial angle greater than 176 degrees: n = 207 Male/female: 24/183 Mean age (years): 65 Mean BMI (kg/m2): 25 Grade of OA according to Kellgren & Lawrence: I = 17, II = 133, III = 35, IV = 22 Varus: 181 degrees (FTA) |
|
| Interventions | I = wedged insole with shoes (n = 45), sock‐type ankle supporter with wedged insole without shoes (n = 46), subtalar strapped insole with shoes (n = 45) and subtalar strapped insole without shoes (n = 46) vs C = neutral wedged insole into shoes (n = 45) | |
| Outcomes | Lequesne index VAS pain NSAID intake |
|
| Notes | Baseline characteristics and outcomes (differences compared with baseline) were presented only for the 207 participants who completed 12‐week follow‐up Most results were presented as pre/post analysis, and only NSAID intake was compared between placebo and the different interventions |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation procedure for the allocation was a computer‐generated block method using sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "In the initial visit, clinicians were given randomly generated treatment allocations with sealed opaque envelopes in a series of blocks of 10" |
| Blinding (performance bias and detection bias) All outcomes | High risk | Participants were not blinded to the intervention; study used patient‐reported outcome measures |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to the intervention; research nurse was blinded to objectives of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "a research nurse who was blind to the objectives of the study asked the participants to assess the Lequesne index and the VAS for subjective knee pain at baseline and 12‐weeks assessments"; however participants were not blinded to the intervention, and patient‐reported outcome measures were used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Baseline characteristics and outcomes (differences compared with baseline) were presented for only the 207 participants who completed 12‐week follow‐up |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baker 2007 | Cross‐over design |
| Birmingham 2001 | No control group |
| Horlick 1993 | Participants are their own controls |
| Hunter 2012 | Cross‐over design |
| Katsuragawa 1999 | No control group |
| Kuroyanagi 2007 | Cross‐over design |
| Matsuno 1997 | No control group |
| Rooser 1988 | Rheumatoid arthritis after total knee arthroplasty Healthy controls |
| Sasaki 1987 | Retrospective trial |
| Shakoor 2008 | Cross‐over design |
| Toda 2002b | Correlation study |
| Tohyama 1991 | Retrospective study |
Differences between protocol and review
No major differences exist between the protocol and the review.
Contributions of authors
Reinoud Brouwer (RB) proposed the review. RB, Sita Bierma‐Zeinstra (SB) and Arianne Verhagen (AV) wrote the protocol. RB, Tom van Raaij (TR) and Tijs Duivenvoorden (TD) selected the studies following review of the abstracts. RB, TD and TR made the final selection after reading the full articles. RB, TD, SB and Jan Verhaar (JV) assessed methodological quality. RB, TD and AV performed data extraction. With contributions from all co‐authors, RB wrote the review.
Declarations of interest
Conflict of interest is possible because two included studies (Brouwer 2006; Raaij van 2010) were conducted by four of the authors (RB, TR, SB, JV) of this systematic review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barrios 2009 {published data only}
- Barrios JA, Crenshaw JR, Royer TD, Davis IS. Walking shoes and laterally wedged orthoses in the clinical management of medial tibiofemoral osteoarthritis: a one‐year prospective controlled trial. The Knee 2009;16:136‐42. [DOI] [PubMed] [Google Scholar]
Bennell 2011 {published data only}
- Bennell KL, Bowles K, Payne CP, Cicuttini F, Williamson E, Forbes A, et al. Lateral wedge insoles for medial knee osteoarthritis: 12 month randomised controlled trial. British Medical Journal 2011;342:2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brouwer 2006 {published data only}
- Brouwer RW, Raaij TM, Verhaar JAN, Coene LNJEM, Bierma‐Zeinstra SMA. Brace treatment for osteoarthritis of the knee: a prospective randomized multi‐centre‐trial. Osteoarthritis and Cartilage 2006;14(8):777‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Erhart‐Hledik 2012 {published data only}
- Erhart‐Hledik JC, Elspas B, Giori NJ, Andriacchi TP. Effect of variable‐stiffness walking shoes on knee adduction moment, pain, and function in subjects with medial compartment knee osteoarthritis after 1 year. Journal of Orthopaedic Research 2012;April:514‐21. [DOI] [PubMed] [Google Scholar]
Kirkley 1999 {published data only}
- Kirkley A, Webster‐Bogaert S, Litchfield R, Amendola A, MacDonald S, McCalden R, et al. The effect of bracing on varus gonarthrosis. Journal of Bone and Joint Surgery [American] 1999;81(4):539‐47. [DOI] [PubMed] [Google Scholar]
Maillefert 2001 {published data only}
- Maillefert JF, Hudry C, Baron G, Kieffert P, Bourgeois P, Lechevalier D, et al. Laterally elevated wedged insoles in the treatment of medial compartment osteoarthritis: a prospective randomized controlled trial. Osteoarthritis and Cartilage / OARS Osteoarthritis Research Society 2001;9:738‐45. [DOI] [PubMed] [Google Scholar]
- Pham T, Maillefert JF, Hudry C, Kieffert P, Lechevalier D, Dougados M. Laterally elevated wedged insoles in the treatment of medial knee osteoarthritis. Osteoarthritis and Cartilage 2004;12(1):46‐55. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Müller‐Rath 2011 {published data only}
- Müller‐Rath R, Cho HY, Siebert CH, Miltner O. Clinical and gait analytical investigation of valgus knee bracing in therapy for medial degenerative joint disease of the knee [Klinische und ganganalytische Untersuchungeiner valgisierenden Kniegelenkentlastungsorthese in der Therapie der medialen Gonarthrose]. Zeitschrift für Orthopädie und Unfallchirurgie 2011;149:160‐5. [DOI] [PubMed] [Google Scholar]
Raaij van 2010 {published data only}
- Raaij TM, Reijman M, Brouwer RW, Bierma‐Zeinstra SMA, Verhaar JAN. Medial knee osteoarthritis treated by insoles or braces. Clinical Orthopaedics and Related Research 2010;468:1926‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rodriques 2008 {published data only}
- Rodriques PT, Ferreira AF, Pereira RMTR, Bonfa E, Borba EF, Fuller R. Effectiveness of medial‐wedge insole treatment for valgus knee osteoarthritis. Arthritis and Rheumatism 2008;59(5):603‐8. [DOI] [PubMed] [Google Scholar]
Sattari 2011 {published data only}
- Sattari S, Ashraf A. Comparison of the effects of 3‐point valgus knee support and lateral wedge insoles in medial compartment knee osteoarthritis. Iranian Red Crescent Medical Journal 2011;13:xx‐xx. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toda 2001 {published and unpublished data}
- Toda Y, Segal N, Kato A, Yamamoto S, Irie M. Effect of a novel insole on the subtalar joint of patients with medial compartment osteoarthritis of the knee. The Journal of Rheumatology 2001;28(12):2705‐10. [PubMed] [Google Scholar]
- Toda Y, Tsukimura N. A 2‐year follow up of a study to compare the efficiency of lateral wedged insoles with subtalar strapping and in‐shoe lateral wedged insoles in patients with varus deformity osteoarthritis of the knee. Osteoarthritis and Cartilage 2006;14(3):231‐7. [DOI] [PubMed] [Google Scholar]
- Toda Y, Tsukimura N. A six‐month follow up of a randomised trial comparing the efficiency of a lateral‐wedge insole with subtalar strapping and in‐shoe lateral‐wedge insole in patients with varus deformity osteoarthritis of the knee. Arthritis and Rheumatism 2004;50(10):3129‐36. [DOI] [PubMed] [Google Scholar]
Toda 2002 {published data only}
- Toda Y, Segal N. Usefulness of an insole with subtalar strapping for analgesia in patients with medial compartment osteoarthritis of the knee. Arthritis and Rheumatism 2002;5(15):468‐73. [DOI] [PubMed] [Google Scholar]
Toda 2008 {published data only}
- Toda Y, Tsukimura N. Influence of concomitant healed footwear when wearing a lateral wedged insole for medial compartment osteoarthritis of the knee. Osteaoarthritis and Cartilage 2008;16:244‐53. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baker 2007 {published data only}
- Baker K, Goggins J, Xie H, Szumowski K, LaValley M, Hunter DJ, et al. A randomized crossover trial of a wedged insole for treatment of knee osteoarthritis. Arthritis and Rheumatism 2007;56(4):1198‐203. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Birmingham 2001 {published data only}
- Birmingham TB, Kramer JF, Kirkley A, Inglis JT, Spaulding SJ, Vandervoort AA. Knee bracing for medial compartment osteoarthritis: effects on proprioception and postural control. Rheumatology 2001;40(3):285‐9. [DOI] [PubMed] [Google Scholar]
Horlick 1993 {published data only}
- Horlick SG, Loomer RL. Valgus knee bracing for medial gonarthrosis. Clinical Journal of Sports Medicine 1993;3(4):251‐5. [Google Scholar]
Hunter 2012 {published data only}
- Hunter D, Gross KD, McCree P, Ling L, Hirko K, Harvey WF. Realignment treatment for medial tibiofemoral osteoarthritis: randomised trial. Annals of Rheumatic Diseases 2012;71:1658‐65. [DOI] [PubMed] [Google Scholar]
Katsuragawa 1999 {published data only}
- Katsuragawa Y, Funkui N, Nakamura K. Change of bone mineral density with valgus bracing. International Orthopaedics 1999;23(3):164‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kuroyanagi 2007 {published data only}
- Kuroyanagi Y, Nagura T, Matsumoto H, Otani T, Suda Y, Nakamura T, et al. The lateral wedged insole with subtalar strapping significantly reduces dynamic knee load in the medial compartment. Gait analysis on patients with medial knee osteoarthritis. Osteoarthritis and Cartilage 2007;15(8):932‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Matsuno 1997 {published data only}
- Matsuno H, Kadowaki KM, Tsuji H. Generation II knee bracing for severe osteoarthritis of the knee. Archives of Physical Medicine and Rehabilitation 1997;78(7):745‐9. [DOI] [PubMed] [Google Scholar]
Rooser 1988 {published data only}
- Rooser B, Ekbladh R, Lidgren L. The shock‐absorbing effect of soles and insoles. International Orthopaedics 1988;12(4):335‐8. [DOI] [PubMed] [Google Scholar]
Sasaki 1987 {published data only}
- Sasaki T, Yasuda K. Clinical evaluation of the treatment of osteoarthritic knee using a newly designed wedge insole. Clinical Orthopaedics and Related Research 1987;221:181‐7. [PubMed] [Google Scholar]
Shakoor 2008 {published data only}
- Shakoor N, Lidtke RH, Sengupta M, Fogg LF, Block JA. Effects of specialized footwear on joint loads in osteoarthritis of the knee. Arthritis and Rheumatism 2008;59(9):1214‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Toda 2002b {published data only}
- Toda Y, Segal N, Kato A, Yamamoto S, Irie M. Correlation between body composition and efficiency of lateral wedged insole for medial compartment osteoarthritis of the knee. Journal of Rheumatology 2002;29(3):541‐5. [PubMed] [Google Scholar]
Tohyama 1991 {published data only}
- Tohyama H, Yasuda K, Kaneda K. Treatment of osteoarthritis of the knee with heel wedges. International Orthopaedics 1991;15(1):31‐3. [DOI] [PubMed] [Google Scholar]
Additional references
Ahlback 1968
- Ahlback S. Osteoarthrosis of the knee. A radiographic investigation. Acta Radiologica Diagnosis 1968;Suppl 277:7‐72. [MEDLINE: ] [PubMed] [Google Scholar]
Bellamy 1997
- Bellamy N, Kirwan J, Boers M. Recommendations for a core set of outcome measures for future phase III clinical trials in knee, hip and hand osteoarthritis. Consensus development at OMERACT III. Rheumatology 1997;24:700‐802. [PubMed] [Google Scholar]
Bellamy 2006
- Bellamy N, Campbell J, Robinson V, Gee T, Bourne R, Wells G. Viscosupplementation for the treatment of osteoarthritis. Cochrane Database of Systematic Reviews 2006;19(2):CD005321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brouwer GM 2007
- Brouwer GM, Tol AW, Bergink AP, Belo JN, Bernsen RM, Reijman M, et al. Association between valgus and varus alignment and the development and progression of radiographic osteoarthritis of the knee. Arthritis and Rheumatism 2007;56(4):1204‐11. [DOI] [PubMed] [Google Scholar]
Brouwer RW 2007
- Brouwer RW, Raaij TM, Bierma‐Zeinstra SMA, Verhagen AP, Jakma TTSC, Verhaar JAN. Osteotomy for treating knee osteoarthritis. Cochrane Database of Systematic Reviews 2007;18(3):CD004019. [Google Scholar]
Cepeda 2006
- Cepeda MS, Camargo F, Zea C, Valencia L. Tramadol for osteoarthritis. Cochrane Database of Systematic Reviews 2006;19(3):CD005522. [DOI] [PubMed] [Google Scholar]
Deshaies 2002
- Deshaies LD. Upper extremity orthoses. In: Trombly CA, Radomski MV editor(s). Occupational Therapy for Physical Dysfunction. 5th Edition. Baltimore, MD: Lippincott, Williams & Wilkins, 2002:313‐49. [Google Scholar]
Fletcher 2006
- Fletcher X, Parratte S, Aubaniac JM, Argenson JN. A 12‐28‐year follow up study of closing wedge high tibial osteotomy. Clinical Orthopaedics and Related Research 2006;452:91‐6. [DOI] [PubMed] [Google Scholar]
Foley 2003
- Foley A, Halbert J, Hewitt T, Cotty M. Does hydrotherapy improve strength and physical function in patients with osteoarthritis: a randomised controlled trial comparing a gym based and a hydrotherapy based strengthening programme. Annals of the Rheumatic Diseases 2003;62(12):1162‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fransen 2001
- Fransen M, Crosbie J, Edmonds J. Physical therapy is effective for patients with osteoarthritis of the knee: a randomized controlled clinical trial. Journal of Rheumatology 2001;28(1):156‐64. [PubMed] [Google Scholar]
Fransen 2008
- Fransen M, McConell S. Exercise for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2008;8(4):CD004376. [DOI] [PubMed] [Google Scholar]
Garner 2005
- Garner SE, Fidan DD, Frankisch R, Maxwell L. Rofecoxib for osteoarthritis. Cochrane Database of Systematic Reviews 2005;25(1):CD005115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibofsky 2003
- Gibofsky A, Williams GW, McKenna F, Fort JG. Comparing the efficiency of cyclooxygenase 2‐specific inhibitors treating osteoarthritis: appropriate trial design considerations and results of a randomized, placebo‐controlled trial. Arthritis and Rheumatism 2003;48(11):3102‐11. [DOI] [PubMed] [Google Scholar]
Goorman 2000
- Goorman SD, Watanabe TK, Miller EH, Perry C. Functional outcome in knee osteoarthritis after treatment with hylan G‐F 20: a prospective study. Archives of Physical Medicine and Rehabilitation 2000;81(4):479‐83. [DOI] [PubMed] [Google Scholar]
Gossec 2007
- Gossec L, Hawker G, Davis AM, Maillefert JF, Lohmander LS, Altman R, et al. OMERACT/OARSI initiative to define states of severity and indication for joint replacement in hip and knee osteoarthritis. Journal of Rheumatology 2007;34(6):1432‐5. [PubMed] [Google Scholar]
Grelsamer 1995
- Grelsamer RP. Unicompartmental osteoarthritis of the knee. Journal of Bone and Joint Surgery [American] 1995;77(2):278‐92. [DOI] [PubMed] [Google Scholar]
Guyatt 2008a
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008b
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Healy 2002
- Healy WL, Iorio R, Ko J, Appleby D, Lemos DW. Impact of cost reduction programs on short‐term patient outcome and hospital cost of total knee arthroplasty. Journal of Bone and Joint Surgery [American] 2002;84(3):348‐53. [DOI] [PubMed] [Google Scholar]
Hermans 2012
- Hermans J, Koopmanschap MA, Bierma‐Zeinstra SM, Linge JH, Verhaar JA, Reijman M, et al. Productivity costs and medical costs among working patients with knee osteoarthritis. Arthritis Care and Research (Hoboken) 2012;64(6):853‐61. [DOI] [PubMed] [Google Scholar]
Hewett 1998
- Hewett TE, Noyes FR, Barber‐Westin SD, Heckmann TP. Decrease in knee joint pain and increase in function in patients with medial compartment arthrosis: a prospective analysis of valgus bracing. Orthopedics 1998;21(2):131‐8. [DOI] [PubMed] [Google Scholar]
Hoffmann 2001
- Hoffmann S, Theiler R. Physiotherapy in osteoarthritis, a review of literature on conservative therapy of knee and hip osteoarthritis. Therapeutische Umschau Revue Therapeutique 2001;58(8):480‐6. [DOI] [PubMed] [Google Scholar]
Huang 2000
- Huang MH, Chen CH, Chen TW, Weng MC, Wang WT, Wang YL. The effect of weight reduction on the rehabilitation of patients with knee osteoarthritis and obesity. Arthritis Care and Research 2000;13(6):398‐405. [DOI] [PubMed] [Google Scholar]
Hurley 1998
- Hurley MV, Scott DL. Improvement in quadriceps sensorimotor function and disability of patients with knee osteoarthritis following a clinically practicable exercise regime. British Journal of Rheumatology 1998;37(11):1181‐7. [DOI] [PubMed] [Google Scholar]
Johnson 1980
- Johnson F, Leitl S, Waugh W. The distribution of load across the knee. Journal of Bone and Joint Surgery [British] 1980;62‐B(3):346‐9. [DOI] [PubMed] [Google Scholar]
Karlsson 2002
- Karlsson J, Sjogren LS, Lohamnder LS. Comparison of two hyaluronan drugs and placebo in patients with knee osteoarthritis. A controlled, randomized, double‐blind, parallel design multicentre study. Rheumatology (Oxford) 2002;41(11):1240‐8. [DOI] [PubMed] [Google Scholar]
Kellgren 1957
- Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Annals of the Rheumatic Diseases 1957;16(4):494‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Komistek 1999
- Komistek RD, Dennis DA, Northcut EJ, Wood A, Parker AW, Traina SM. An in vivo analysis of the effectiveness of osteoarthritic knee bracing during heel‐strike of gait. Journal of Arthroplasty 1999;14(6):738‐42. [DOI] [PubMed] [Google Scholar]
Leopold 2003
- Leopold SSL, Redd BB, Warme WJ, Wehrle PA, Pettis PD, Shott S. Corticosteroid compared with hyaluron acid injections for the treatment of osteoarthritis of the knee. A prospective randomized trial. Journal of Bone and Joint Surgery [American] 2003;85‐A(7):1197‐2003. [DOI] [PubMed] [Google Scholar]
Lindenfeld 1997
- Lindenfeld TN, Hewett TE, Andriacchi TP. Joint loading with valgus bracing in patients with varus gonarthrosis. Clinical Orthopaedics and Related Research 1997;344:290‐7. [PubMed] [Google Scholar]
Losina 2013
- Losina E, Weinstein AM, Reichmann WM, Burbine SA, Solomon DH, Daigle ME, et al. Lifetime risk and age at diagnosis of symptomatic knee osteoarthritis in the US. Arthritis Care and Research (Hoboken) 2013;65(5):703‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Michalsen 2003
- Michalsen A, Klotz S, Ludtke R, Moebus S, Spahn G, Dobos GJ. Effectiveness of leech therapy in osteoarthritis of the knee: a randomized controlled trial. Annals of Internal Medicine 2003;4(139):724‐30. [DOI] [PubMed] [Google Scholar]
Ng 2003
- Ng MM, Leung MC, Poon DM. The effects of electro‐acupuncture and transcutaneous electrical stimulation on patients with painful osteoarthritic knees: a randomized controlled trial with follow‐up evaluation. Journal of Alternative & Complementary Medicine 2003;9(5):641‐9. [DOI] [PubMed] [Google Scholar]
Nguyen 2011
- Nguyen US, Zhang Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Annals of Internal Medicine 2011;155(11):725‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nuesch 2009
- Nuesch E, Rutjes AW, Husni E, Welch V, Juni P. Oral or transdermal opioids for osteoarthritis of the knee or hip. Cochrane Database of Systematic Reviews 2009;7(4):CD007321. [DOI] [PubMed] [Google Scholar]
Parkes 2013
- Parkes MJ, Maricar N, Lunt M, LaValley MP, Johnes RK, Segal NA, et al. Lateral wedge insoles as a conservative treatment for pain in patients with medial knee osteoarthritis. JAMA 2013;310(7):722‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2011
- Reeves ND, Bowling FL. Conservative biomechanical strategies for knee osteoarthritis. Nature Reviews Rheumatology 2011;7:113‐22. [DOI] [PubMed] [Google Scholar]
Rutjes 2009
- Rutjes AW, Nuesch E, Sterchi R, Kalichman L, Hendriks E, Osiri M, et al. Transcutaneous electrostimulation for osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009;7(4):CD002823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2008
- Schünemann HJ, Oxman AD, Brozek J, Glasziou P, Jaeschke R, Vist GE, et al. Grading quality of evidence and strength of recommendations for diagnostic tests and strategies. BMJ 2008;336(7653):1106‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharma 2001
- Sharma L, Song J, Felson DT, Cahue S, Shamiweh E, Dunlop DD. The role of knee alignment in disease progression and functional decline in knee osteoarthritis. JAMA 2001;11(286):188‐95. [DOI] [PubMed] [Google Scholar]
Sharma 2003
- Sharma L, Cahue S, Song J, Hayes K, Pai YC, Dunlop D. Physical functioning over three years in knee osteoarthritis: role of psychosocial, local, mechanical, and neuromuscular factors. Arthritis and Rheumatism 2003;48(12):3359‐70. [DOI] [PubMed] [Google Scholar]
Stukenborg 2001
- Stukenborg‐Colsman C, Wirth CJ, Lazovic D, Wefer A. High tibial osteotomy versus unicompartmental joint replacement in unicompartmental knee joint osteoarthritis: 7‐10‐ year follow‐up prospective randomised study. Knee 2001;8(3):187‐94. [DOI] [PubMed] [Google Scholar]
Tanamas 2009
- Tanamas S, Hanna FS, Cicuttini FM, Wluka AE, Berry P, Urquhart DM. Does knee malalignment increase the risk of development and progression of knee osteoarthritis? A systematic review. Arthritis and Rheumatism 2009;61(4):459‐67. [DOI] [PubMed] [Google Scholar]
Tetsworth 1994
- Tetsworth K, Paley D. Malalignment and degenerative arthropathy. The Orthopedic Clinics of North America 1994;25(3):367‐77. [PubMed] [Google Scholar]
Towheed 2006
- Towheed TE, Maxwell L, Judd MG, Catton M, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database of Systematic Reviews 2006;25(1):CD004257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uebelhart 2004
- Uebelhart D, Malaise M, Marcolongo R, DeVathaire F, Piperno M, Mailleux E, et al. Intermittent treatment of knee osteoarthritis with oral chondroitin sulfate: a one‐year, randomized, double‐blind, multicenter study versus placebo. Osteoarthritis and Cartilage 2004;12(4):269‐76. [DOI] [PubMed] [Google Scholar]
Whittle 2011
- Whittle SL, Richards BL, Husni E, Buchbinder R. Opioid therapy for treating rheumatoid arthritis pain. Cochrane Database of Systematic Reviews 2011;9(11):CD003113. [DOI] [PubMed] [Google Scholar]
Zhang 2010
- Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part III. Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010;18:476‐99. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
2005 Initial review
- Brouwer RW, Jakma TS, Verhagen AP, Verhaar JA, Bierma‐Zeinstra SM. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004020] [DOI] [PubMed] [Google Scholar]
2009 First update
- Brouwer RW, Raaij TM, Jakma TS, Verhagen AP, Verhaar JAN, Bierma‐Zeinstra SMA. Braces and orthoses for treating osteoarthritis of the knee. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004020.pub2] [DOI] [PubMed] [Google Scholar]


