Abstract
Objective
The prediction of survival of gastric neuroendocrine tumours (g-NETs) is controversial. Prognostic effects of the metastatic lymph node ratio (LNR) in patients with g-NET were explored, and a nomogram was plotted to predict the survival rates of patients.
Methods
A longitudinal study conducted on the basis of the Surveillance, Epidemiology, and End Results database. The association between LNR and survival were investigated by using Pearson correlation and Cox regression. Overall survival (OS) and cancer-specific survival (CSS) rates were predicted with the help of nomograms.
Results
A total of 315 patients with g-NET diagnosed from 2004 to 2015 were included in this study. LNR was discovered to have a negative correlation with OS and CSS (Pearson correlation coefficients: 0.343 (p<0.001) and 0.389 (p<0.001), respectively). The multivariate analyses indicated age, tumour site, differentiation, T staging, M staging, chemotherapy and LNR to be independent prognostic factors for both OS and CSS. Surgery was also a prognostic determinant for CSS (p=0.003). Concordance indices of the nomograms for OS and CSS were higher than those of the TNM classification (0.772 vs 0.730 and 0.807 vs 0.768, respectively). As per the area under the receiver operating characteristic curve, predictive ability of the nomograms for survival of 1, 3 and 5 years was all better than that of TNM classification.
Conclusions
LNR is an independent predictor of g-NETs. The nomograms plotted in this study have a satisfying predictive ability of survival risks and are capable of guiding tailored treatment strategies for patients with g-NET.
Keywords: gastric neuroendocrine tumor, lymph node ratio, overall survival, cancer-specific survival, SEER database
Key questions.
What is already known about this subject?
Gastric neuroendocrine tumour (g-NET) is a kind of rare tumour, but the incidence rates has become three times higher in the last 30 years. Metastatic lymph node ratio (LNR) has been deemed to be one of the independent prognostic determinants for some cancers. However, the effect of the LNR in patients with g-NET and the prognostic model for patients with g-NET are controversial.
What does this study add?
LNR presented a superior capacity to predict prognosis in patients with g-NET from multiple perspectives. An optimal cut-off point of LNR was obtained by dividing patients into three groups (LNR1: 0, LNR2: 0.001–0.132 and LNR3: 0.133–1.000), which had different prognoses. LNR and treatment strategies (surgery, chemotherapy and radiotherapy) were included in the prognostic analysis and nomograms. The nomograms can predict individualised survival rates and can be used to guide tailored treatment strategies for patients with g-NET.
How might this impact on clinical practice?
LNR is another perspective for clinicians to view the importance of prognosis of lymph node metastasis. Patients can be divided into different groups according to LNR to preliminarily evaluate the survival. Regardless of whether the patient had received treatment or not, the prognostic nomograms established in this study can be used to calculate the probability of surviving for patients with g-NET. The influence of different treatment strategies on prognosis is quantified, which might be used as one of the bases for guiding clinicians to choose treatment strategies.
Introduction
A heterogeneous group of rare tumours known as neuroendocrine tumours (NETs) develop from the diffuse endocrine system.1 Although scattered throughout the body, these tumours largely exist within the gastrointestinal tract.2–4 Peptides and neuroamines are secreted by them, which lead to discrete clinical syndromes, besides carcinoid syndromes.5–7 Although they usually exhibit indolent clinical course, they may become very aggressive and may rapidly turn metastatic.
Gastric neuroendocrine tumours (g-NETs) are progressively prevalent neoplasms with inconsistent biological and clinicopathological characteristics.8 The annual age-adjusted incidence rate increased from 0.03 per 100 000 in 1973–1977 to 0.33 per 100 000 in 2003–2007, perhaps owing to the frequent practice of radiological imaging and endoscopic techniques for investigating the abdominal tumours.9 10 However, owing to the rarity of g-NETs, studies including a defined population specifically focused on g-NETs are deficient. To the best of our knowledge, limited relevant statistics, especially the prognostic models of g-NETs, exist to inform patients surviving for a limited period on being diagnosed with g-NETs of their prognosis at any given time. Therefore, researchers are keen on finding a more effective prediction model.
Recent evidence indicates that the metastatic lymph node ratio (LNR), which was defined as the ratio of positive to examined lymph nodes, is a significant prognostic determinant in malignancies such as gastric, non-small-cell lung, breast and bladder cancers.11–14 The latest study also exhibited the significance of LNR in colorectal cancer.15 Two other new reports have also indicated that an important prognostic indicator for poor cases of survival in NETs is growing LNR.16 17
Therefore, this study sought to investigate the correlation between LNR and survival rates and to develop a new prognostic model to predict overall survival (OS) of 1, 3 and 5 years and cancer-specific survival (CSS) rates on the basis of the Surveillance, Epidemiology, and End Results (SEER) database.
Materials and methods
Patients
The SEER database was used to conduct a retrospective review of patients with g-NET. The cases of g-NETs were screened using the database ‘Incidence-SEER 18 Regs Custom Data (with additional treatment fields), Nov 2017 Sub (1973–2015 varying)’. All cases which were unaware of their age at the time of diagnosis were not included in this study. All cases included were diagnosed in the years starting from 1973 to 2015. To recognise cases of g-NETs, the International Classification of Diseases for Oncology (ICD-O-3) was used. On the basis of ‘Site recode ICD-O-3/WHO 2008’ (Stomach) and ‘ICD-O-3 Hist/bahav’ (8013/3, large-cell neuroendocrine carcinoma; 8153/3, gastrinoma, malignant; 8240/3, carcinoid tumour, NOS; 8241/3, Enterochromaffin cell carcinoid; 8242/3, Enterochromaffin-like cell tumour, malignant; 8246/3, neuroendocrine carcinoma, NOS; 8249/3, atypical carcinoid tumour; and 8574/3, adenocarcinoma with neuroendocrine differentiation). A total of 6373 cases were screened from SEER 18 registries initially. Secondary tumours (n=2144), negative follow-up (n=24) and unknown diagnostic confirmation (n=17) were omitted. Patients were also disqualified if they lacked information regarding the results of regional nodes examined (n=3628), the record of T staging (n=243) and the record of M staging (n=2). Finally, the study group was formed from a total of 315 cases. The T staging were restaged according to the eighth edition of the American Joint Committee on Cancer (AJCC) staging system on the basis of the following codes: derived AJCC T, sixth ed (2004+); derived AJCC T, seventh ed (2010+); and CS tumor size (2004+). The data analysed and used in this study was obtained from SEER database in accordance with the SEER data use agreement (ID: 15243-Nov2018).
Statistical analysis
SPSS V.24.0 for Windows was used to analyse all the enumeration and measurement data. A p value of <0.05 was considered statistically significant. Pearson correlation was performed to ascertain the association between LNR and survival rates. To identify the optimal cut-off point of LNR, the receiver operating characteristic (ROC) curve was adopted. The end points of our study were OS and CSS. Kaplan-Meier test was used to calculate survival probabilities. With the help of the Cox proportional hazards regression model, univariate and multivariate analyses were conducted to estimate the potential predictors in relation to OS and CSS rates.
Nomogram construction and validation
The results of the multivariate analysis were used to plot the nomograms, which integrated all the independent prognostic factors and predicted OS and CSS rates of 1, 3 and 5 years by using the package of ‘rms’ in R software V.3.6.0. Harrell’s C-index, suitable for censored data, was used to assess the performance of predicting outcomes for patients with g-NET.18 The mean predicted survival rates were compared with the mean actual survival rates. After grouping the survival probabilities predicted by nomogram into deciles, Kaplan-Meier test was used to determine the calibration. The accuracy of the nomogram was verified by a bootstrapped resample with 1000 iterations. Furthermore, the area under ROC curve was used to assess the precise survival predictions of 1, 3 and 5 years.
Results
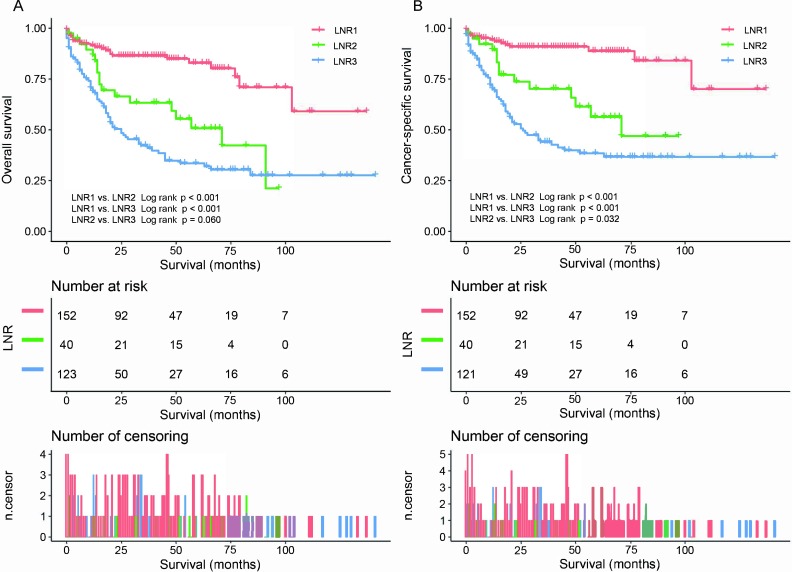
This study encompassed a total of 315 cases, including 160 men and 155 women. The demographic and other study-related characteristics of patients with g-NETs are summarised in table 1. The median age was 61 years (IQR=20), and tumours most frequently occurred in the ‘greater curvature, lesser curvature, and body of stomach (34.9%, 110/315). A total of 306 patients underwent cancer-directed surgery, and 9 patients underwent regional lymph node removal only. The median number of examined lymph nodes was nine (IQR=13). LNR had a negative correlation with OS and CSS (Pearson correlation coefficients: 0.343 (p<0.001) and 0.389 (p<0.001), respectively). The optimal cut-off point of the LNR is related to both the ROC curves and the clinical application. Patients were divided into three groups (LNR1: 0, LNR2: 0.001–0.132 and LNR3: 0.133–1.000). For OS, the numbers of patients in each group were 152, 40 and 123, respectively, while those for CSS were 152, 40 and 121, respectively.
Table 1.
Characteristics of patients with gastric neuroendocrine tumours
| Character | n | % |
| Sample size | 315 | 100 |
| Age (years) | ||
| <60 | 140 | 44.4 |
| ≥60 | 175 | 55.6 |
| Sex | ||
| Male | 160 | 50.8 |
| Female | 155 | 49.2 |
| Race | ||
| White | 224 | 71.1 |
| Black | 55 | 17.5 |
| Others | 36 | 11.4 |
| Marital status | ||
| Married | 182 | 57.8 |
| Unmarried | 56 | 17.8 |
| Others | 66 | 21.0 |
| Unknown | 11 | 3.5 |
| Tumour site | ||
| Cardia/fundus | 63 | 20.0 |
| Greater curvature/lesser curvature/body | 110 | 34.9 |
| Pylorus/antrum | 74 | 23.5 |
| Others | 68 | 21.6 |
| Tumour size (cm) | ||
| ≤5 | 209 | 66.3 |
| >5 | 77 | 24.4 |
| Unknown | 29 | 9.2 |
| Differentiation | ||
| Well/moderate | 150 | 47.6 |
| Poor/undifferentiated | 110 | 34.9 |
| Unknown | 55 | 17.5 |
| T staging | ||
| TX | 19 | 6.0 |
| T1 | 54 | 17.1 |
| T2 | 131 | 41.6 |
| T3 | 63 | 20.0 |
| T4 | 48 | 15.2 |
| N staging | ||
| N0 | 150 | 47.6 |
| N1 | 165 | 52.4 |
| M staging | ||
| M0 | 268 | 85.1 |
| M1 | 47 | 14.9 |
| Surgery | ||
| Performed | 306 | 97.1 |
| Not performed | 9 | 2.9 |
| Chemotherapy | ||
| Performed | 65 | 20.6 |
| No/Unknown | 250 | 79.4 |
| Radiotherapy | ||
| Performed | 33 | 10.5 |
| Not performed | 282 | 89.5 |
The univariate and multivariate analyses indicated that age, tumour site, differentiation, T staging, M staging, chemotherapy and LNR were autonomous prognostic determinants for both OS and CSS (tables 2 and 3). Surgery was also an independent prognostic determinant for CSS (p=0.003). OS and CSS were not correlated with gender (p=0.423 and p=0.102, respectively), and surgery was not an independent prognostic determinant for OS (p=0.113). OS and CSS data of different LNR groups are presented in figure 1A, B. The OS rates of 1, 3 and 5 years were observed to be 91.5% vs 86.7% vs 68.7%, 86.4% vs 63.3% vs 42.2%, and 82.9% vs 50.8% vs 33.5%, respectively. Moreover, the CSS rates of 1, 3 and 5 years were recorded to be 94.3% vs 89.4% vs 70.1%, 90.9% vs 70.2% vs 44.0%, and 88.7% vs 56.3% vs 38.4%, respectively. These results showed that the greater the value of LNR, the poorer the prognosis.
Table 2.
Variables associated with overall survival according to the Cox proportional hazards regression model
| Character | Univariable analysis | Multivariable analysis | ||
| HR (95 % CI) | P value | HR (95 % CI) | P value | |
| Age (years) | ||||
| <60 | Reference | – | Reference | – |
| ≥60 | 2.534 (1.689 to 3.803) | <0.001 | 1.860 (1.183 to 2.923) | 0.007 |
| Sex | ||||
| Male | Reference | – | Reference | – |
| Female | 0.620 (0.427 to 0.898) | 0.012 | 0.842 (0.552 to 1.283) | 0.423 |
| Race | 0.768 | |||
| White | Reference | – | ||
| Black | 0.863 (0.519 to 1.437) | 0.572 | ||
| Others | 1.109 (0.629 to 1.955) | 0.720 | ||
| Marital status | 0.947 | |||
| Married | Reference | – | ||
| Unmarried | 1.024 (0.620 to 1.693) | 0.925 | ||
| Others | 1.079 (0.684 to 1.704) | 0.742 | ||
| Tumour site | <0.001 | 0.010 | ||
| Cardia/fundus | Reference | – | Reference | – |
| Greater curvature/lesser curvature/body | 0.470 (0.294 to 0.752) | 0.002 | 0.480 (0.277 to 0.831) | 0.009 |
| Pylorus/antrum | 0.743 (0.461 to 1.197) | 0.222 | 0.877 (0.513 to 1.499) | 0.632 |
| Others | 0.328 (0.180 to 0.599) | <0.001 | 0.353 (0.157 to 0.792) | 0.012 |
| Tumour size (cm) | ||||
| ≤5 | Reference | – | Reference | – |
| >5 | 3.607 (2.461 to 5.288) | <0.001 | 1.176 (0.748 to 1.849) | 0.483 |
| Differentiation | <0.001 | <0.001 | ||
| Well/moderate | Reference | – | Reference | – |
| Poor/undifferentiated | 5.526 (3.544 to 8.617) | <0.001 | 3.814 (2.132 to 6.824) | <0.001 |
| Unknown | 0.915 (0.441 to 1.898) | 0.811 | 1.148 (0.499 to 2.639) | 0.745 |
| T staging | <0.001 | 0.013 | ||
| T1 | Reference | – | Reference | – |
| T2 | 4.888 (1.765 to 13.532) | 0.002 | 1.106 (0.371 to 3.297) | 0.857 |
| T3 | 6.627 (2.319 to 18.941) | <0.001 | 1.300 (0.412 to 4.103) | 0.655 |
| T4 | 14.410 (5.085 to 40.834) | <0.001 | 2.568 (0.823 to 8.016) | 0.104 |
| M staging | ||||
| M0 | Reference | – | Reference | – |
| M1 | 2.922 (1.941 to 4.401) | <0.001 | 1.894 (1.190 to 3.012) | 0.007 |
| Surgery | ||||
| Performed | Reference | – | ||
| Not performed | 2.069 (0.841 to 5.087) | 0.113 | ||
| LNR | <0.001 | <0.001 | ||
| 0 | Reference | – | Reference | – |
| ≤0.132 | 2.898 (1.572 to 5.342) | 0.001 | 2.306 (1.163 to 4.572) | 0.017 |
| >0.132 | 4.605 (2.903 to 7.305) | <0.001 | 3.668 (2.177 to 6.179) | <0.001 |
| Chemotherapy | ||||
| Performed | Reference | – | Reference | – |
| No/unknown | 0.411 (0.280 to 0.602) | <0.001 | 1.855 (1.129 to 3.049) | 0.015 |
| Radiotherapy | ||||
| Performed | Reference | – | Reference | – |
| Not performed | 0.467 (0.293 to 0.745) | 0.001 | 0.765 (0.439 to 1.331) | 0.342 |
LNR, lymph node ratio.
Table 3.
Variables associated with cancer-specific survival according to the Cox proportional hazards regression model
| Character | Univariable analysis | Multivariable analysis | ||
| HR (95 % CI) | P value | HR (95 % CI) | P value | |
| Age (years) | ||||
| <60 | Reference | – | Reference | – |
| ≥60 | 2.076 (1.341 to 3.212) | 0.001 | 1.651 (1.005 to 2.713) | 0.048 |
| Sex | ||||
| Male | Reference | – | ||
| Female | 0.710 (0.471 to 1.070) | 0.102 | ||
| Race | 0.983 | |||
| White | Reference | – | ||
| Black | 0.957 (0.554 to 1.651) | 0.873 | ||
| Others | 1.024 (0.527 to 1.993) | 0.943 | ||
| Marital status | 0.779 | |||
| Married | Reference | – | ||
| Unmarried | 0.851 (0.473 to 1.532) | 0.591 | ||
| Others | 1.083 (0.659 to 1.778) | 0.753 | ||
| Tumour site | 0.005 | 0.047 | ||
| Cardia/fundus | Reference | – | Reference | – |
| Greater curvature/lesser curvature/body | 0.480 (0.283 to 0.816) | 0.007 | 0.497 (0.271 to 0.912) | 0.024 |
| Pylorus/antrum | 0.775 (0.454 to 1.323) | 0.35 | 0.893 (0.491 to 1.624) | 0.710 |
| Others | 0.369 (0.192 to 0.710) | 0.003 | 0.428 (0.186 to 0.981) | 0.045 |
| Tumour size (cm) | ||||
| ≤5 | Reference | – | Reference | – |
| >5 | 3.947 (2.573 to 6.054) | <0.001 | 1.193 (0.727 to 1.957) | 0.484 |
| Differentiation | <0.001 | <0.001 | ||
| Well/moderate | Reference | – | Reference | – |
| Poor/undifferentiated | 6.546 (3.880 to 11.045) | <0.001 | 3.512 (1.804 to 6.836) | <0.001 |
| Unknown | 1.196 (0.537 to 2.662) | 0.662 | 1.244 (0.493 to 3.134) | 0.644 |
| T staging | <0.001 | 0.004 | ||
| T1 | Reference | – | Reference | – |
| T2 | 7.424 (1.790 to 30.789) | 0.006 | 1.737 (0.392 to 7.689) | 0.467 |
| T3 | 10.689 (2.513 to 45.461) | 0.001 | 1.860 (0.399 to 8.675) | 0.430 |
| T4 | 26.659 (6.350 to 111.923) | <0.001 | 4.406 (0.965 to 20.115) | 0.056 |
| M staging | ||||
| M0 | Reference | – | Reference | – |
| M1 | 3.629 (2.335 to 5.641) | <0.001 | 2.200 (1.318 to 3.674) | 0.003 |
| Surgery | ||||
| Performed | Reference | – | Reference | – |
| Not performed | 2.670 (1.080 to 6.605) | 0.034 | 7.511 (1.982 to 28.462) | 0.003 |
| LNR | <0.001 | <0.001 | ||
| 0 | Reference | – | Reference | – |
| ≤0.132 | 3.618 (1.745 to 7.499) | 0.001 | 3.085 (1.378 to 6.905) | 0.006 |
| >0.132 | 6.533 (3.720 to 11.474) | <0.001 | 4.980 (2.655 to 9.341) | <0.001 |
| Chemotherapy | ||||
| Performed | Reference | – | Reference | – |
| No/unknown | 0.374 (0.246 to 0.570) | <0.001 | 1.795 (1.045 to 3.082) | 0.034 |
| Radiotherapy | ||||
| Performed | Reference | — | Reference | — |
| Not performed | 0.416 (0.253 to 0.684) | 0.001 | 0.647 (0.359 to 1.168) | 0.149 |
LNR, lymph node ratio.
Figure 1.
(A) Overall survival rates for all patients by LNR groups. (B) Cancer-specific survival rates for all patients by LNR groups. Pictures show the number of subjects at risk in each group at 25-month increments. Pictures show the number of censoring in each group at 25-month increments. LNR, lymph node ratio.
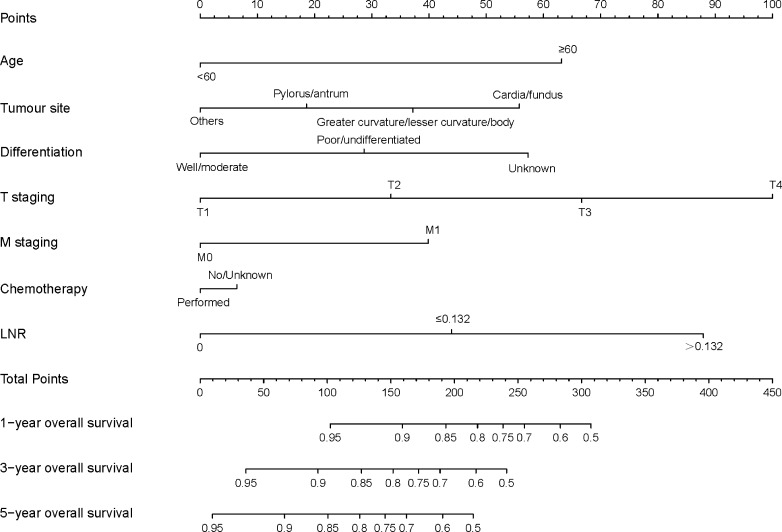
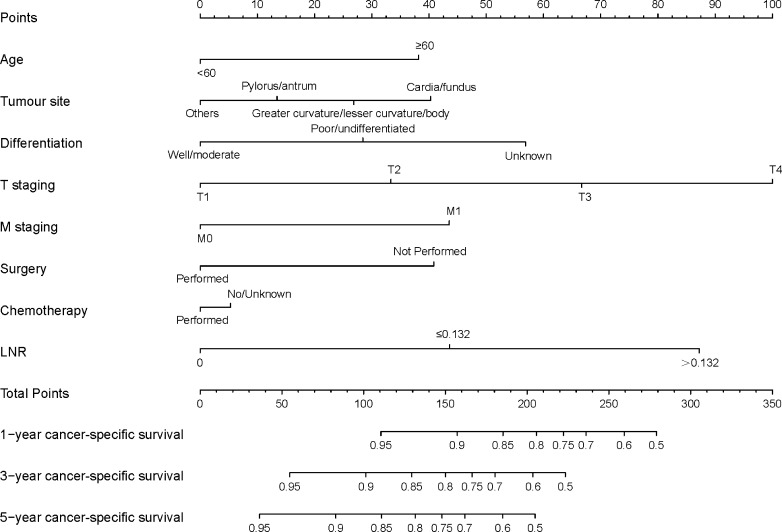
The predictive nomograms, plotted for the OS and CSS rates of 1, 3 and 5 years on the basis of the results of Cox PH regression, are illustrated, respectively, in figures 2 and 3. The discrimination of nomograms was compared with those of the eighth AJCC TNM classification. The C-index for OS was 0.772 (95% CI 0.747 to 0.796), which was higher than that of the eighth AJCC TNM classification (0.730, 95% CI 0.705 to 0.755). The C-index for CSS rates was also enhanced compared with that of the eighth AJCC TNM staging (C-index: 0.807, 95% CI 0.782 to 0.831 vs 0.768, 95% CI 0.743 to 0.793). The accuracy of the nomograms was verified by using bootstrapped resample (1000 iterations).
Figure 2.
Nomogram predicting the OS rates of 1, 3 and 5 years of patients with gastric neuroendocrine tumour. The nomogram summed the points identified on the scale for each variable. The total points projected on the bottom scales indicate the probabilities of OS rates of 1, 3 and 5 years. LNR, lymph node ratio; OS, overall survival.
Figure 3.
Nomogram predicting the CSS rates of 1, 3 and 5 years of patients with g-NETs. The nomogram summed the points identified on the scale for each variable. The total points projected on the bottom scales indicate the probabilities of CSS rates of 1, 3 and 5 years. CSS, cancer-specific survival; LNR, lymph node ratio.
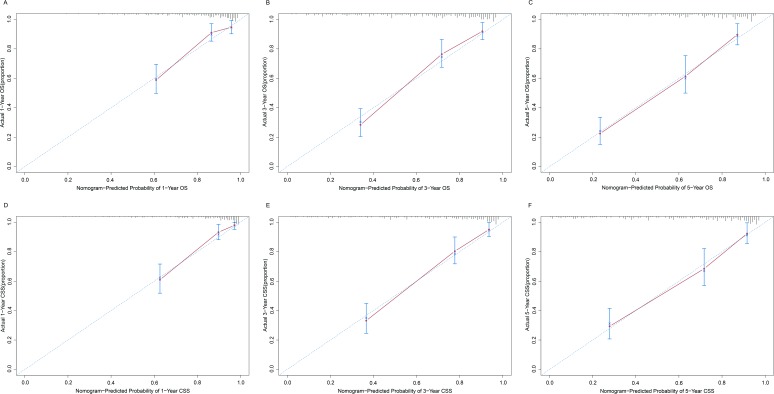
The similarities between the actual survival rates and the survival rates predicted by the nomograms were validated by way of a calibration plot (figure 4A–F). The actual survival rates and survival rates predicted by the nomograms are presented on the x and y axes, respectively, obtained using the Kaplan-Meier method. The predicted OS and CSS rates of 1, 3 and 5 years obtained as a result were consistent with the actual survival rates within a 10% error range signified by the dashed lines.
Figure 4.
Calibrations of the nomograms for predicting survival rates. The x axis represents the nomogram-predicted survival rates, whereas the y axis represents the actual survival rates. 95% CIs were measured via Kaplan-Meier analysis. All predictions lie within a 10% margin of error (within the dashed lines). (A) Calibration of the nomogram for predicting 1-year OS rate. (B) Calibration of the nomogram for predicting 3-year OS rate. (C) Calibration of the nomogram for predicting 5-year OS rate. (D) Calibration of the nomogram for predicting 1-year CSS rate. (E) Calibration of the nomogram for predicting 3-year CCS rate. (F) Calibration of the nomogram for predicting 5-year CSS rate. CSS, cancer-specific survival; OS, overall survival.
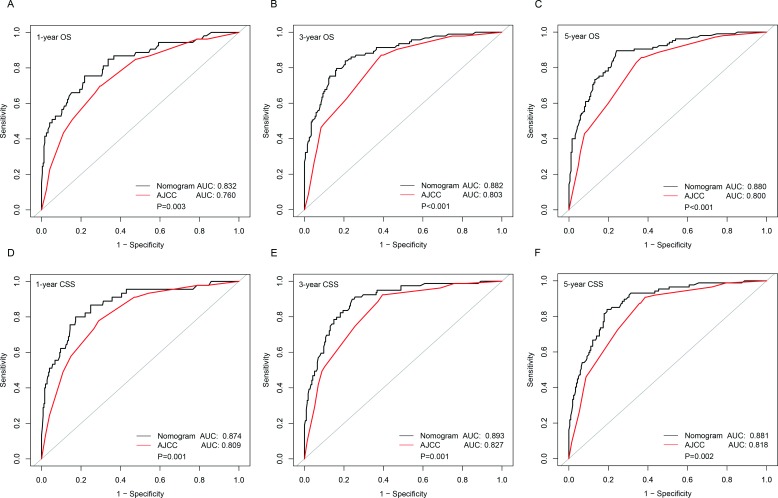
Furthermore, the precision rates of the two predictive models for predicting the OS and CSS rates of 1, 3 and 5 years were compared, respectively, by area under the curve (AUC) models (figure 5A–F). For predicting OS rates of 1, 3 and 5 years, the AUCs of our nomogram were significantly greater than those of the traditional TNM staging system (0.832 vs 0.760 (p=0.003), 0.882 vs 0.803 (p<0.001) and 0.880 vs 0.800 (p<0.001), respectively). For predicting the CSS rates of 1, 3 and 5 years, the AUCs of our nomogram were also significantly greater than those of the traditional TNM staging system (0.874 vs 0.809 (p=0.001), 0.893 vs 0.827 (p=0.001) and 0.881 vs 0.818 (p=0.002), respectively).
Figure 5.
Comparison of the AUCs of the nomogram and eighth AJCC TNM staging system for predicting survival rates. The black lines represent nomogram-predicted survival rates, whereas the red lines represent AJCC TNM stage-predicted survival rates. AUCs of the two models predict OS rates at 1 year (A), 3 years (B) and 5 years (C). AUCs of the two models predict CSS at 1 year (D), 3 years (E) and 5 years (F). AJCC, American Joint Committee on Cancer; AUC, area under the curve; CSS, cancer-specific survival; OS, overall survival.
Discussion
Predicting the outcomes of patients with g-NET is complicated. These tumours are biologically heterogeneous, and outcomes can similarly vary, depending on the status of tumours.16 To the best of our knowledge, for g-NET, this is the first study to include treatment strategies (surgery, chemotherapy and radiotherapy) into prognostic analysis and nomograms. Considering the importance of LNR, we included LNR in the nomogram. Moreover, we restaged the T staging according to the newest eighth edition of the AJCC staging system. Through univariate and multivariate analyses, this study found that LNR is an independent prognostic determinant of OS and CSS, and increasing LNR is associated with poor prognosis, which is similar to the findings of the earlier studies in NETs.16 17
The ROC curves of LNR show their influence on survival at a very low value (cut-off point=0.032 for both OS and CSS). In view of clinical application, we divided the patients into three groups (LNR1: 0, LNR2: 0.001–0.132 and LNR3: 0.133–1.000) based on the specificity and sensitivity of ROC curves. Kaplan-Meier analysis showed that the OS and CSS rates of the LNR1 group are obviously better than those of the LNR2 and LNR3 groups (p<0.001; p<0.001, respectively), and the CSS rate of LNR2 group is better than that of the LNR3 group (p=0.032). The 5-year OS and CSS rates of different LNR groups are 82.9% vs 50.8% vs 33.5%, and 88.7% vs 56.3% vs 38.4%, respectively. The findings of Martin et al16 were similar, indicating that the CSS rate of gastroenteropancreatic NETs was lower with higher LNRs (p<0.0001) and that 10-year CSS rates were 81%, 69%, 55% and 50% for the N0, ≤0.2, >0.2–0.5 and >0.5 LNR groups, respectively. These results indicate yet again that LNR is negatively correlated to survival of g-NETs. Moreover, LNR, being a ratio, is less affected by the number of lymph nodes resected than N staging and may be more suitable for prognosis evaluation.
How is the influence of different prognostic factors on the rate of survival quantified and integrated? Earlier studies have found some prognostic factors of g-NETs, such as age, gender, marital status, size, differentiation and TNM staging.16 17 19–21 Through univariate and multivariate analyses, prognostic factors such as age, tumour site, differentiation, T staging, M staging, chemotherapy and LNR were discovered to be independent for both OS and CSS (all p<0.05). Moreover, surgery was also an independent prognostic determinant for CSS (p=0.003). However, neither the weighting of their influence on survival was assessed nor the survival time of patients with g-NET predicted. Several studies have revealed the predictive abilities of nomograms for predicting NETs with liver metastases,22 and those of the small intestine,23 pancreas24 25 and stomach.20 26 Specific, consistent and clinically applicable nomograms are demonstrated by the results to accurately estimate the prognosis of patients with NETs. Therefore, nomograms have been used to solve the aforementioned problems.
The nomograms demonstrated that LNR is an important prognostic parameter for both OS and CSS rates. According to the nomograms, the weighting of LNR is second only to T staging. The first three factors that affect OS are T staging, LNR and age. Moreover those of CSS are T staging, LNR and differentiation. Nomogram for OS illustrates patients >60 years of age would probably have a poor survival than younger patients. Tumours located in the cardia or fundus of the stomach have a poorer prognosis than those cited in the greater and lesser curvatures, or body of the stomach. In addition, tumours located in the greater and lesser curvatures or body of the stomach have a poorer prognosis than those located in the pylorus or antrum of the stomach. In addition, similar to the findings of two other studies on nomograms regarding NETs conducted by Fang et al19 and Cao et al,20 the nomogram plotted in this study demonstrates poor prognosis as having poor differentiation, increasing T staging and distant metastases. For CSS rates, similar results are obtained in age, tumour site, differentiation, T staging and M staging.
The nomograms also clearly showed the effects of chemotherapy and surgery on survival. Patients without chemotherapy are more likely to pass away than others. Surgery also plays a significant role in CSS rates because patients undergoing a surgery have a better CSS rate than those not undergoing a surgery. This is the first study to include treatment strategies into nomograms. Interestingly, surgery is an independent prognostic determinant for CSS (p=0.003) rate, but it is not an independent prognostic determinant for OS (p=0.113). The effect of chemotherapy is limited in improving OS and CSS rates according to the nomograms. Moreover, no evidence exist indicating that radiotherapy can improve the prognosis.
In addition, the nomograms plotted in this study may help clinicians in making a decision. Generally, all clinicians face the problem of choosing treatment strategies. In this study, chemotherapy was found to be mainly performed in poor/undifferentiated or TNM stage III patients (online supplementary 1), and had a potential ability to improve the OS and CSS rates. Therefore, if patients have a poorly differentiated, advanced disease and can tolerate the side effects of chemotherapy, chemotherapy is recommendable. Surgery might be capable of improving the CSS rate. Thus, doctors might calculate the total points for patients based on nomograms, evaluate the value of surgery and then perform surgery on patients with long life expectancy. However, further research is needed to investigate the effect of radiotherapy on the rate of survival of patients with g-NET. With the development of medical technology and in-depth research, patients may gain more benefits from medical treatments.
esmoopen-2019-000632supp001.pdf (14.6KB, pdf)
Furthermore, the nomograms plotted in this study more significantly predicted OS and CSS rates than the eighth AJCC TNM staging, with a C-index of 0.772 (95% CI 0.747 to 0.796) vs 0.730 (95% CI 0.705 to 0.755) and 0.807 (95% CI 0.782 to 0.831) vs 0.768 (95% CI 0.743 to 0.793). Using the bootstrapped resample (1000 iterations), the calibration plot reveals that the predicted OS and CSS rates nearly correspond with the actual survival rates on the basis of the Kaplan-Meier method. Moreover, the ROC curves revealed that our nomograms showed comparatively better predictive ability than the eighth AJCC staging in predicting OS and CSS rates of 1, 3 and 5 years (all p<0.05) rates. All consistently indicated the nomograms based on the LNR were superior in estimating the results for patients with g-NET compared with those of the traditional TNM staging system.
This study has some limitations. First, only limited patient cohorts were included because of excluding the patients diagnosed with secondary NET (n=2144) or with incomplete information about the regional nodes examined (n=3628). Given that g-NET is a special disease with low incidence, the number of patients enrolled in this study after necessary screening was relatively small. In the future, more cases would be accumulated and the research collaborated with other institutions to gradually expand the number of patients. Another limitation is that we only used the SEER database to discover the influence of LNR and to establish prognostic models. Through accumulating more cases, we will verify our findings and further improve the g-NETs prognosis model by using external database. The third limitation is that our nomograms contain limited clinicopathological variables. Cives et al27 showed that g-NETs may be subdivided into types I–III, with different pathogenesis, pathology, treatment and prognosis. The Ki-67 proliferation index is also a potential prognostic indicator. However, owing to the limitation of SEER database, g-NETs could not be divided into types I–III, and information on Ki-67% could not be obtained. As molecular and gene detection techniques become increasingly mature, additional prognostic factors (eg, genes or biological markers) will be found. Nevertheless, these variables were excluded from the nomogram because of the current unavailability of them on the SEER database, and more research is needed to explore their effects on prognosis. The nomograms are likely to improve in the future treatment therapies. The last limitation is that information on resection status (R0/R1/R2) could not be obtained from the database, so the clinicopathological features of different resection statuses could not be further analysed.
To conclude, the present study identified that LNR is an independent prognostic determinant for g-NETs. We established nomograms based on the SEER database, which displayed a comparatively better prognostic discrimination and accuracy of prediction of the OS and CSS rates than the eighth AJCC TNM staging to predict the prognosis of patients with g-NETs. These nomograms can predict individualised survival rates and can be used to guide tailored treatment strategies for patients with g-NET.
Acknowledgments
The authors sincerely thank the Surveillance, Epidemiology, and End Results (SEER) programme for the efforts in establishing the SEER database. This paper has been accepted by ESMO Congress, Barcelona, Spain, 2019.
Footnotes
JL and YL contributed equally.
Contributors: All authors helped to perform the research; JL wrote the manuscript and performed procedures; YL drafted conception and wrote the manuscript; YW and HL contributed to writing the manuscript and performing data analysis; FL, QZ and XL contributed to drafting conception and data analysis; JW contributed to drafting conception, writing the manuscript and design. All authors approved the final manuscript.
Funding: This work was supported by the Fujian Province Natural Science Foundation (grant number 2017J01260); and Joint Funds for the Innovation of Science and Technology, Fujian Province (grant number 2017Y9074).
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The data analysed and used in this study was obtained from Surveillance, Epidemiology, and End Results (SEER) database in accordance with the SEER data use agreement (ID: 15243-Nov2018). Therefore, this study did not require approval of ethical board.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available in a public, open access repository. This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0.
References
- 1.Partelli S, Maurizi A, Tamburrino D, et al. GEP-NETS update: a review on surgery of gastro-entero-pancreatic neuroendocrine tumors. Eur J Endocrinol 2014;171:R153–62. 10.1530/EJE-14-0173 [DOI] [PubMed] [Google Scholar]
- 2.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer 2003;97:934–59. 10.1002/cncr.11105 [DOI] [PubMed] [Google Scholar]
- 3.Maggard MA, O'Connell JB, Ko CY. Updated population-based review of carcinoid tumors. Ann Surg 2004;240:117–22. 10.1097/01.sla.0000129342.67174.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crocetti E, Paci E. Malignant carcinoids in the USA, seer 1992-1999. An epidemiological study with 6830 cases. Eur J Cancer Prev 2003;12:191–4. 10.1097/00008469-200306000-00004 [DOI] [PubMed] [Google Scholar]
- 5.Modlin IM, Kidd M, Latich I, et al. Current status of gastrointestinal carcinoids. Gastroenterology 2005;128:1717–51. 10.1053/j.gastro.2005.03.038 [DOI] [PubMed] [Google Scholar]
- 6.Frilling A, Akerström G, Falconi M, et al. Neuroendocrine tumor disease: an evolving landscape. Endocr Relat Cancer 2012;19:R163–85. 10.1530/ERC-12-0024 [DOI] [PubMed] [Google Scholar]
- 7.Halperin DM, Shen C, Dasari A, et al. Frequency of carcinoid syndrome at neuroendocrine tumour diagnosis: a population-based study. Lancet Oncol 2017;18:525–34. 10.1016/S1470-2045(17)30110-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klöppel G, Perren A, Heitz PU. The gastroenteropancreatic neuroendocrine cell system and its tumors: the who classification. Ann N Y Acad Sci 2004;1014:13–27. 10.1196/annals.1294.002 [DOI] [PubMed] [Google Scholar]
- 9.Lawrence B, Gustafsson BI, Chan A, et al. The epidemiology of gastroenteropancreatic neuroendocrine tumors. Endocrinol Metab Clin North Am 2011;40:1–18. 10.1016/j.ecl.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 10.Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063–72. 10.1200/JCO.2007.15.4377 [DOI] [PubMed] [Google Scholar]
- 11.Nitti D, Marchet A, Olivieri M, et al. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol 2003;10:1077–85. 10.1245/ASO.2003.03.520 [DOI] [PubMed] [Google Scholar]
- 12.Jonnalagadda S, Arcinega J, Smith C, et al. Validation of the lymph node ratio as a prognostic factor in patients with N1 nonsmall cell lung cancer. Cancer 2011;117:4724–31. 10.1002/cncr.26093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voordeckers M, Vinh-Hung V, Van de Steene J, et al. The lymph node ratio as prognostic factor in node-positive breast cancer. Radiother Oncol 2004;70:225–30. 10.1016/j.radonc.2003.10.015 [DOI] [PubMed] [Google Scholar]
- 14.Herr HW. Superiority of ratio based lymph node staging for bladder cancer. J Urol 2003;169:943–5. 10.1097/01.ju.0000032474.22093.06 [DOI] [PubMed] [Google Scholar]
- 15.Zhang C-H, Li Y-Y, Zhang Q-W, et al. The prognostic impact of the metastatic lymph nodes ratio in colorectal cancer. Front Oncol 2018;8:628. 10.3389/fonc.2018.00628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin JA, Warner RRP, Aronson A, et al. Lymph node metastasis in the prognosis of gastroenteropancreatic neuroendocrine tumors. Pancreas 2017;46:1214–8. 10.1097/MPA.0000000000000921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim MK, Warner RRP, Ward SC, et al. Prognostic significance of lymph node metastases in small intestinal neuroendocrine tumors. Neuroendocrinology 2015;101:58–65. 10.1159/000371807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 1996;15:361–87. [DOI] [PubMed] [Google Scholar]
- 19.Fang C, Wang W, Feng X, et al. Nomogram individually predicts the overall survival of patients with gastroenteropancreatic neuroendocrine neoplasms. Br J Cancer 2017;117:1544–50. 10.1038/bjc.2017.315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao L-L, Lu J, Lin J-X, et al. Incidence and survival trends for gastric neuroendocrine neoplasms: an analysis of 3523 patients in the seer database. Eur J Surg Oncol 2018;44:1628–33. 10.1016/j.ejso.2018.01.082 [DOI] [PubMed] [Google Scholar]
- 21.Song W, Tian C. The effect of marital status on survival of patients with gastrointestinal stromal tumors: a seer database analysis. Gastroenterol Res Pract 2018;2018:5740823 10.1155/2018/5740823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruzzenente A, Bagante F, Bertuzzo F, et al. A novel nomogram to predict the prognosis of patients undergoing liver resection for neuroendocrine liver metastasis: an analysis of the Italian neuroendocrine liver metastasis database. J Gastrointest Surg 2017;21:41–8. 10.1007/s11605-016-3228-6 [DOI] [PubMed] [Google Scholar]
- 23.Modlin IM, Gustafsson BI, Pavel M, et al. A nomogram to assess small-intestinal neuroendocrine tumor ('carcinoid') survival. Neuroendocrinology 2010;92:143–57. 10.1159/000319784 [DOI] [PubMed] [Google Scholar]
- 24.Ellison TA, Wolfgang CL, Shi C, et al. A single institution's 26-year experience with nonfunctional pancreatic neuroendocrine tumors: a validation of current staging systems and a new prognostic nomogram. Ann Surg 2014;259:204–12. 10.1097/SLA.0b013e31828f3174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye L, Ye H, Zhou Q, et al. A retrospective cohort study of pancreatic neuroendocrine tumors at single institution over 15 years: new proposal for low- and high-grade groups, validation of a nomogram for prognosis, and novel follow-up strategy for liver metastases. Int J Surg 2016;29:108–17. 10.1016/j.ijsu.2016.03.036 [DOI] [PubMed] [Google Scholar]
- 26.Cao L-L, Lu J, Lin J-X, et al. Nomogram based on tumor-associated neutrophil-to-lymphocyte ratio to predict survival of patients with gastric neuroendocrine neoplasms. World J Gastroenterol 2017;23:8376–86. 10.3748/wjg.v23.i47.8376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cives M, Strosberg JR, Tumors GN. Gastroenteropancreatic neuroendocrine tumors.. CA Cancer J Clin 2018;68:471–87. 10.3322/caac.21493 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
esmoopen-2019-000632supp001.pdf (14.6KB, pdf)