Abstract
Forty years after its naming, IL-1 and related cytokines experience a renaissance as central mediators of inflammation and immunity. IL-1 family members, including IL-18, IL-33, IL-36, IL-37, and IL-38, play a key role in the orchestration of the diversity and plasticity of innate and adaptive immune responses. As such, these molecules are involved in homeostasis and in a range of pathologies, ranging from autoimmunity and autoinflammation, to dysmetabolism and cardiovascular disorders, to cancer. Dissection of the complexity and role in immunopathology of the IL-1 family has paved the way to development of trans-disease therapeutic strategies. Therefore, IL-1 serves as a paradigm for immunity and inflammation representing a metanarrative of modern medicine.
1. Introduction
Interleukin-1 (IL-1) was born as a term in 1979 (Aarden et al., 1979) in a pre-gene cloning era, at the intersection between fever, lymphocyte activation, hematopoiesis and more (Dinarello, 2009b; Gabay et al., 2010; Garlanda et al., 2013). Although the term IL-1 hinted to a single molecule, previous work on endogenous pyrogen had already shown the existence of two cytokines with different isoelectric point (pI 5 and 7) (Dinarello et al., 1974). Therefore, early on it was apparent that IL-1 was more than a single molecule, a view vindicated by gene cloning and molecular identification of a complex and diverse family of mediators (IL-1α, IL-1β, IL-18, IL-33, IL-36α, β and γ, IL-37 and IL-38).
The discovery of IL-1 has had far reaching implications beyond its own properties and activities. The concept of a pleiotropic action of cytokines was born with the observation that IL-1 at vanishing low concentrations affected tissues and cells as diverse as T cells and hypothalamus (Dinarello, 2009b). Moreover, as discussed elsewhere (Garlanda et al., 2013), IL-1 and its receptors are upstream of ground-breaking discoveries ranging from Toll-like receptors (TLRs), to the inflammasomes, to decoy receptors.
The IL-1 family is complex with ligands endowed with agonist (6), antagonist (3) or antiinflammatory (one) activity, and 9 receptor chains. IL-1 has long been associated with inflammation and innate immunity (Dinarello, 2009b). It is now apparent that this complex family has a broader role extending beyond classically-defined generic inflammation. IL-1 itself and the related family members IL-33 and IL-18 have been shown to play key differential roles in shaping and orienting innate immunity and inflammation in response to different microbial or environmental challenges (Garlanda et al., 2013). Differentiation and polarization of myeloid cells and innate or adaptive lymphoid cells is driven by IL-1, IL-33 and IL-18. Moreover specialized circuits of homeostasis and defense at mucosal surfaces require IL-1 family members.
Here we will briefly review the complexity of IL-1 family members and their receptors and discuss their involvement in the activation and orientation of innate and adaptive immunity and immunopathology. Previous reviews will provide a framework for the present essay (Dinarello, 2009b; Gabay et al., 2010; Garlanda et al., 2013) which will be focused on more recent developments and newly emerged regulatory pathways such as IL-37 and IL-1R8. The implications of new vistas on the complexity and pathophysiological role of “IL-1 and Friends”1 for human disease and therapeutic targeting will be discussed.
2. Receptors, ligands and processing. Negative regulation (decoys, antagonists, anti-inflammatory cytokines)
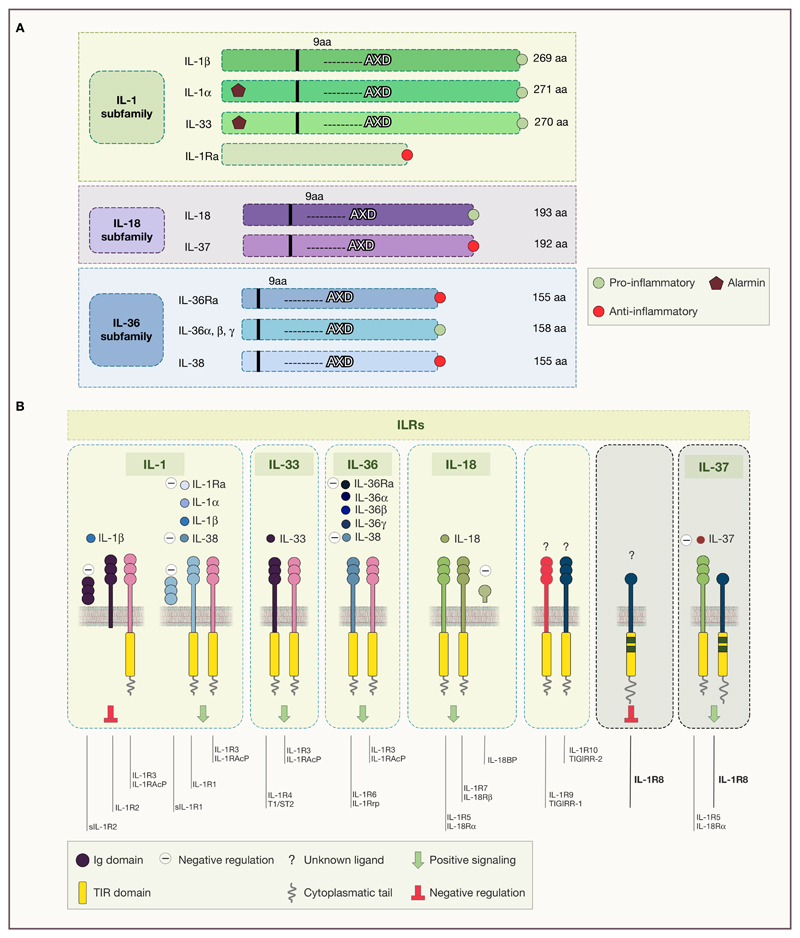
The IL-1 family is composed of 11 soluble molecules and 10 receptors (Garlanda et al., 2013) (Figure 1). IL-1 family-cytokines are divided into three subgroups, based on the IL-1 consensus sequence and the signaling receptor chain and include secreted molecules with agonistic activity (IL-1α, IL-1β, IL-18, IL-33, IL-36α, IL-36β, and IL-36γ), receptor antagonists (IL-1Ra, IL-36Ra, and IL-38), and an anti-inflammatory cytokine (IL-37) (Dinarello, 2018). IL-1-related cytokines are not translated and secreted as bioactive molecules, but they are found in the cytoplasm as precursors. With the exception of IL-1Ra, IL-1 cytokines carry a consensus sequence (AXD), located 9 amino-acids after the cleavage site to reach the optimal bioactivity for the molecule. In case of IL-1β, this is a cleavage site for Caspase-1 (Dinarello, 2018) (Figure 1A). Genomic organization and evolution analysis showed that agonists coevolved with receptor antagonists and anti-inflammatory molecules, since most of them (IL-1β, IL-1Ra, the IL-36 subgroup, IL-38 and IL-37, IL-18) are present in all vertebrates from cartilaginous fish to mammals, thus appearing about 420 million years ago (Rivers-Auty et al., 2018). This suggests the evolutive relevance of balanced responses in the IL-1 system.
Figure 1. Structural organization of IL-1 family member and their receptors.
A) Subfamilies of IL-1 ligands, grouped by structural similarity. The consensus sequence (AXD), located 9 amino-acids after the cleavage site is shown. B) IL-1 receptors and their cognate agonists and antagonists. The ILR subfamily is composed by receptors and accessory proteins (AcPs) for the cytokines of the IL-1 family. A novel nomenclature of ILRs has been recently proposed and it is as follows: IL-1R1 (IL-1RI), IL-1R2 (IL-1RII), IL-1R3 (IL-1RAcP), IL-1R4 (ST2), IL-1R5 (IL-18Rα), IL-1R6 (IL-1Rrp2, IL-36R), IL-1R7 (IL-18Rβ), IL-1R8 (also known as TIR8 or SIGIRR), IL-1R9 (TIGIRR- 2), IL-1R10 (TIGIRR-1).
Interleukin-1 receptor family members (ILRs) are present in all vertebrates, and originated through ancestral gene duplication events, with some exceptions, including IL-1R8 which did not evolve from a common IL-1R ancestral gene (Rivers-Auty et al., 2018). Interestingly, the IL-33 receptor (IL-1RL1) probably originally acted as an orphan receptor or by interacting with other ligands, as suggested by its role in rainbow trout, since IL-33 appeared only in mammals, between 320 and 160 million years ago (Rivers-Auty et al., 2018). ILRs share a common intracellular signaling domain with TLRs, named Toll-IL-1 resistance (TIR) domain, and are characterized by extracellular Ig-like domains (Dinarello, 2018; Garlanda et al., 2013) (Figure 1B). Upon ligand binding, ILRs dimerize through their TIR domains, inducing the recruitment of the TIR domain containing adapter protein MyD88, which couples to downstream protein kinases (e.g. IL-1R associated kinases (IRAKs), and tumor necrosis factor receptor-associated factor 6 (TRAF6)). The signal leads to the activation of key transcription factors associated with inflammatory and immune responses, such as nuclear factor-κB (NFκB), activator protein-1 (AP-1), c-Jun N-terminal kinase (JNK), p38 and other mitogen-associated protein kinases (MAPKs), extracellular signal-regulated kinases (ERKs), and members of the interferon (IFN)-regulatory factor (IRF) (Dinarello, 2009a).
The shared usage of MyD88 by IL-1R family members raises the issue of the mechanism(s) responsible for generation of response specificity. The IL-1R receptor repertoire is a major determinant of specificity. For instance, the IL-18 receptor is highly expressed in NK cells and so is IL-1R8, and IL-18 is a driver of the differentiation and activation of NK cells. Moreover, negative regulators are also differentially expressed and regulated, thus resulting in differential tuning of the response. For instance, IL-4 and IL-13 dampen the response of myelomonocytic cells to IL-1 by upregulating the decoy IL-1R2 (Colotta et al., 1993), but it leaves the responsiveness to IL-33 and expression of type 2 immunity unaffected. Thus, differential expression of receptors and regulatory molecules underlies specificity of action of different members of the IL-1 family.
The strict regulation of this system is essential under physiological and pathological conditions and is controlled by decoys, antagonists and anti-inflammatory cytokines (Garlanda et al., 2013). IL-1R2 exerts regulatory functions acting in membrane bound or released form as a decoy receptor for IL-1, as a dominant negative and a scavenger (Colotta et al., 1993). In addition, IL-1R2 is also present in the cytoplasm where it binds pro-IL-1α, preventing its cleavage and activation (Zheng et al., 2013). IL-1R8, also known as TIR8 or SIGIRR lacks conventional signalling capacities and acts as a negative regulator of the family, acting intracellularly. Available information suggests that IL-1R8 interferes with the association of TIR-containing adaptor molecules to the receptor complex, thus dampening the signalling pathway leading to signal transduction (Molgora et al., 2018). In addition, IL-1R8 is a component of the receptor recognizing the anti-inflammatory cytokine IL-37 (Nold-Petry et al., 2015). IL-37 is an anti-inflammatory cytokine that acts as a natural brake of inflammation and immunity, signalling through IL-1R5/IL-18Rα and IL-1R8 (Nold-Petry et al., 2015). IL-18BP is an extracellular protein that binds IL-18, preventing its interaction with the receptor IL-1R5/IL-18R, and thus neutralizing its activity (Novick et al., 1999). IL-1Ra and IL-36Ra are highly conserved receptor antagonists that bind IL-1R1 and IL-1R6, respectively (Dinarello, 2018), and evolved under strong evolutionary pressure due to specificity of function (Rivers-Auty et al., 2018).
The usage of the same receptor by IL-1α and IL-1β raises the general question as to why two IL-1s, redundancy for robustness versus specialized functions (Mantovani, 2018). Phylogenetic analysis indicates that IL-1α likely arose through a duplication event of the ancestral IL-1β gene, between 320 and 160 million years ago and then IL-1α underwent a divergent evolutionary pressure associated with distinct functions of the pro-domain of the precursor (Rivers-Auty et al., 2018). The IL-1α precursor is present in mesenchymal cells of healthy humans, including keratinocytes, the type 2 epithelial cells of the lung, the entire gastrointestinal tract and in brain astrocytes. IL-1α is also produced by all myeloid cells but is not constitutive as in mesenchymal cells (Table 1). Unlike IL-1β and IL-18, there is no requirement for caspase-1 cleavage of the IL-1α precursor to process and release the active cytokine. In contrast, the IL-1α is active as a precursor whereas the IL-1β precursor is not (Kim et al., 2013). The 31 kDa IL-1α precursor can be cleaved in vitro, particularly in murine cell lines, to a 17 kDa cytokine by unknown proteases. Calcium activated membrane calpains also process the IL-1α precursor, although it is unlikely that this takes place under physiological conditions. In fact, the consistent failure to detect IL-1α in the circulation under severe inflammatory conditions supports the notion that this member of the family is primarily a local mediator in tissues. Recently, pro-IL-1α was shown to be activated by thrombin cleavage at a conserved consensus site, indicating a novel link between coagulation and inflammation (Burzinsky L.C., 2019).
Table 1. Similarities and differences between IL-1α and IL-1β.
| Characteristic | IL-1α | IL-1β |
|---|---|---|
| Constitutive in health | yes | no |
| Distribution | mesenchymal tissues | myeloid only |
| Active in its precursor form | yes | no |
| Caspase-1 processing | no | yes |
| Nuclear function in transcription | yes | no |
| Post-translational myristoylation | yes | no |
| Active integral membrane protein | yes | no |
| Presence in endothelial apoptotic bodies | yes | no |
The most distinctive characteristic of IL-1α is that it acts as an integral membrane protein, particularly on macrophages. Membrane IL-1α activates the IL-1R1 on adjacent cells by a mechanism termed “juxtacrine”. This cell-cell interaction functions when dendritic cells present antigen to T-cells (Dinarello, 2009a). The IL-1α precursor is also constitutively present in endothelial cells and during inflammation, it is released in membrane-bound “apoptotic bodies”, which are biologically active (Berda-Haddad et al., 2011).
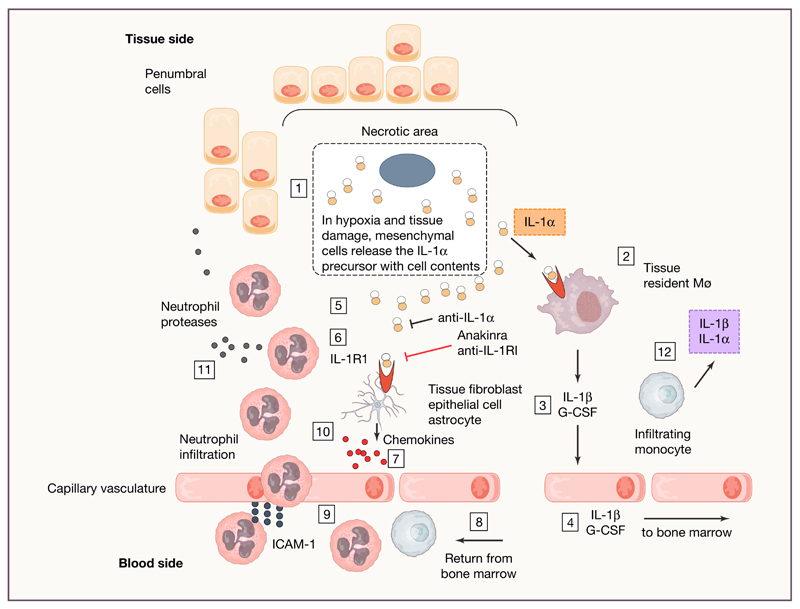
Similar to IL-33 and IL-37, IL-1α is a dual function cytokine in the IL-1 family. Extracellularly, IL-1α binds to the IL-1R1 on the surface of the cell, recruits its co-receptor IL-1R3 and initiates a pro-inflammatory signal, identical to that of IL-1β. With its nuclear localization sequence at the N-terminus, the IL-1α precursor functions in the nucleus as a transcription factor (Dinarello, 2009a). Inside the cell, IL-1α shuttles between the cytosol and the nucleus with amazing rapidity. During natural apoptosis, for example such that takes place in the lining epithelium of the gut or in keratinocytes of the skin, IL-1α leaves the cytosol and binds tightly to chromatin. When that cell dies, there is no inflammation from the cell contents, since IL-1α bound to chromatin does not bind to its cell surface receptor. In contrast, when the cell becomes necrotic, the IL-1α leaves the nucleus and is found in the cytosol. The IL-1α precursor is released with cell contents, where it binds to IL-1R1 on adjacent live cells. Sterile inflammation due to necrotic tissue appears to be IL-1α-mediated and independent of TLR4 (Chen et al., 2007). IL-1α released in this manner has earned the IL-1α precursor the term “alarmin” (Figure 2).
Figure 2. IL-1α as an alarmin in tissue damage.
For instance, ischemia is shown as a prototypic cause of tissue damage. 1. Subjected to low oxygen and increased acidity, cells in the ischemic or damaged tissues lose membrane integrity and the constitutively present IL-1α precursor leaves the cell. 2. IL-1α binds to the IL-1R1 on resident macrophages. 3. IL-1β is released via NLRP3 inflammasome activation with ATP derived from hypoxic cell. Also released is G-CSF. At this point, anakinra, anti-IL-1α and anti-IL-1R1 reduce inflammation. 4. IL-1β and G-CSF enter the venous circulation and into the right ventricle. From the heart, arterial blood reaches the bone-marrow and IL-1β and G-CSF induce the release of neutrophils and monocytes into the venous drainage. 5. The IL-1α precursor accumulates in the extracellular space of the ischemic tissue. 6. IL-1α binds to IL-1R1 on tissue fibroblasts, epithelial cells, astrocytes in the respective tissue type. Anti-IL-1α, anakinra or anti-IL-1R1 reduce the inflammatory process at this point. 7. Production of chemokines such as CCL1. 8. Arterial circulation reaches the ischemic tissue with neutrophils and monocytes from the bone marrow. 9. Neutrophils bind to ICAM-1 and cross the endothelial barrier with the assistance of chemokines. 10. Neutrophils accumulate in the tissue. 11. Neutrophil-mediated damage to the penumbral cells. 12. In addition to IL-1α, infiltrating monocytes produce IL-1β and the ischemic site becomes dominated by IL-1β, which contributes to steps 6-11 sustaining the inflammatory cascade.
Because IL-1α and IL-1β trigger the same IL-1R1 and signal transduction is the same for both cytokines, defining a disease due to IL-1α is, in the strict sense, not possible. However, in mice, the specific deficiency of IL-1α compared to IL-1β can be used as pre-clinical data for neutralizing IL-1α in human diseases. The skin remains an IL-1α “organ”. Anakinra was used to treat hidradenitis suppurativa (Tzanetakou et al., 2016) but a second study in this disease specifically targeting IL-1α revealed a more robust response (Kanni et al., 2018). Other inflammatory neutrophil dermatoses such as pyoderma granulosum appear to be primarily IL-1α driven and in a mouse model, CARD9 signaling was identified as increasing IL-1α (Tartey et al., 2018). In murine neonatal sepsis, IL-1α and not IL-1β drives mortality (Benjamin et al., 2018). IL-1α deficiency after spinal cord injury in the mouse is protective (Bastien et al., 2015).
Although there is no clinical trial of specifically targeting IL-1α in inflammatory bowel disease, studies in mice with DSS colitis clearly implicate IL-1α from the intestinal epithelium as driving the inflammation where IL-1β acts to heal the intestinal barrier (Bersudsky et al., 2014). A similar study also in mice showed that intestinal inflammation from infection with Yersinia enterocolitica is IL-1α and not IL-1β mediated (Dube et al., 2001). As clinical studies using antibodies that specifically neutralize IL-1α increase, it is likely that IL-1α will be identified as a target.
3. Hemopoiesis, ageing and innate memory
Under homeostatic conditions, hematopoietic stem cells (HSC) are capable of constantly replenishing the differentiated cells, undergoing a physiological turnover. In case of infection or any kind of damage associated with the need of increased number of hematopoietic cells, such as sterile acute and chronic inflammation, ageing and blood regeneration after chemotherapy or radiotherapy or bleeding, the system rapidly responds with a specific program generally named “emergency hematopoiesis” (Boettcher and Manz, 2017; Manz and Boettcher, 2014; Takizawa et al., 2012) (Figure 3A). IL-1 was originally discovered as Hemopoietin-1. Steady state levels of IL-1 are dispensable for homeostatic hemopoiesis, whereas acute and chronic IL-1 exposure drives the “emergency” response, leading to a sustained myeloid skewing and a consequent loss and damage of HSCs (Pietras et al., 2016). The transcriptional regulator PU.1, downstream of IL-1R1, was shown to be the key molecule governing IL-1 response in HSCs (Pietras et al., 2016) (Figure 3A). Although IL-1β has been mostly investigated as a systemic stimulus for the emergency response, both IL-1α and IL-1β were locally induced in the bone marrow niche in response to damage (Mitroulis et al., 2018; Pietras et al., 2016), thus suggesting that both contribute to emergency myelopoiesis. HSCs respond to inflammatory mediators and microbial compounds, allowing a rapid immune response but having short- and long- term impact on the stem cell niche preservation (Baldridge et al., 2010; Essers et al., 2009; Liu et al., 2015; Maeda et al., 2009; Sato et al., 2009).
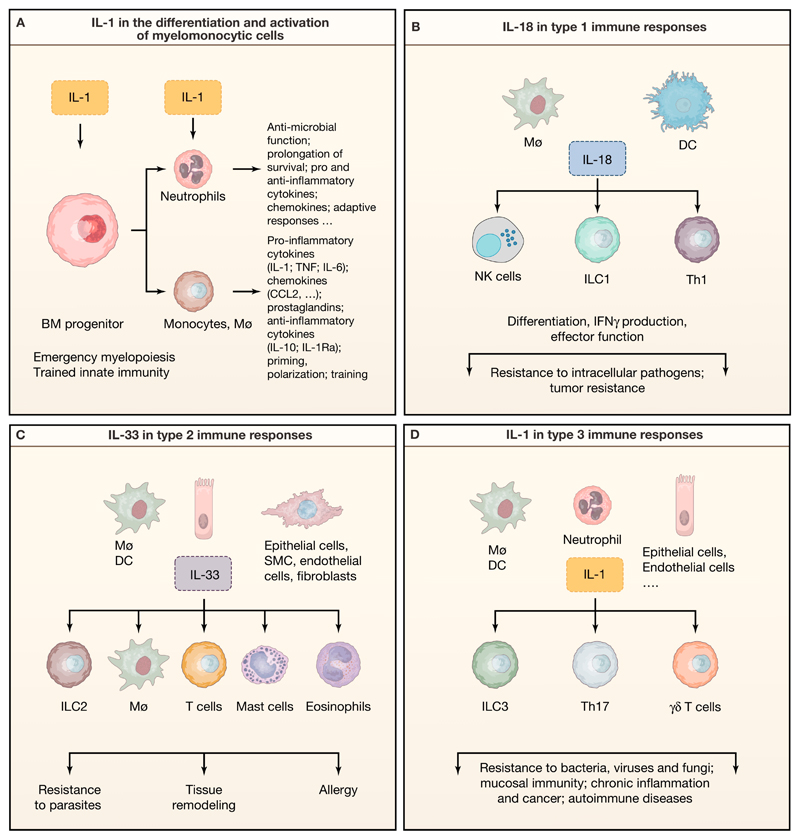
Figure 3. Role of IL-1 family members in the differentiation and function of myelomonocytic cells and in the orchestration of innate and adaptive immune responses.
A) IL-1β regulates emergency hematopoiesis and trained innate immunity acting on bone marrow hematopoietic progenitors, and mature myeloid cell functional activity and survival. B-D) Schematic representation of type 1 (B), type 2 (C) and type 3 immunity (D) activated by IL-18, IL-33 and IL-1, respectively.
In line with this, IL-1 was suggested to be involved in HSC ageing, which is associated with immune senescence and a functional defect in both the innate and adaptive immune response and a consequent increased susceptibility to infections, autoimmune diseases, hematological malignancies and impaired response to vaccines (Bottazzi et al., 2018; Ciabattini et al., 2018; Kovtonyuk et al., 2016). An increased frequency of somatic mutations and the unbalanced expansion of a somatic clone in the hematopoietic lineage, defined as “clonal hematopoiesis of indeterminate potential” (CHIP), often occur in elderly individuals, even in the absence any other hematological disorder (Steensma et al., 2015). Recent studies revealed that CHIP was associated with the risk of coronary hearth disease in humans and atherosclerosis in a murine model (Fuster et al., 2017; Jaiswal et al., 2017). In particular, the expansion of ten-eleven-translocation-2 (TET2)-mutant hematopoietic clones, which represents a common condition in CHIP, augmented the pathology and was suggested to be the major driver of atherosclerosis. IL-1β was shown to be responsible for atherosclerosis promotion in mice with bone marrow TET2-deficiency (Fuster et al., 2017). Indeed, IL-1β production was increased in macrophages in the atherosclerotic plaque upon transplant with 10% TET2-mutant bone marrow and the inhibition of NLRP3 abolished the greater disease severity observed (Fuster et al., 2017).
CHIP, sustained by IL-1 is a feature of ageing. Inflammation is a key feature of senescence at a cellular and organism level. At the cellular level, senescence results in production of a set of cytokines in the so-called senescence-associated secretory phenotype (SASP) (Coppe et al., 2010). SASP includes IL-1α and IL-1β which are major drivers of senscence at a cellular level. As for CHIP, IL-1 has emerged as a key component of inflammation associated with ageing and associated disease manifestations (inflammaging) as also discussed below (Bottazzi et al., 2018; Ciabattini et al., 2018).
Immunological memory has long been considered as a property unique to adaptive immunity. Microbial recognition triggers short (priming; tolerance) and long-term reshaping of the response of myeloid cells to pathogens, a property referred to as memory, adaptive or trained innate immunity (Arts et al., 2016; Bowdish et al., 2007). IL-1β was shown to drive an epigenetic reprogramming in monocytes, similar to that acquired by monocytes trained by β-glucan, BCG and oxLDL (Mitroulis et al., 2018; Moorlag et al., 2018). Moreover, metabolic changes associated with trained immunity were mimicked in monocytes upon treatment with IL-1β, suggesting a key role for IL-1β in trained immunity (Arts et al., 2018; Cheng et al., 2014) (Figure 3A). Importantly, pharmacologic inhibition of IL-1 by Anakinra abolished the effect of β-glucan on HSCs, in terms of cell-cycle modulation, myeloid skewing and metabolic switch (Mitroulis et al., 2018).
The original description of trained immunity was centered on long term education of macrophages in lower organisms and mammals (Kurtz and Franz, 2003; Locati et al., 2013; Netea et al., 2016). More recent results point to a key role of neutrophils and on the effect of IL-1 on hematopoietic precursors (Mitroulis et al., 2018; Moorlag et al., 2018). IL-1 has long been known to promote survival of neutrophils (Colotta et al., 1992). One could speculate that IL-1 mediated prolongation of survival of neutrophils and monocytes-macrophages plays a permissive role in their training.
4. Type 1 immunity (NK cells, ILC1, Th1)
IL-18 is a powerful inducer of type-1 responses in innate and adaptive lymphocytes (Garlanda et al., 2013) (Figure 3B). IL-18 was first described as IFNγ-inducing factor and it is indeed involved in the activation of NK and Th1 cells (Okamura et al., 1995). Similarly to IL-1, it is synthetized as inactive precursor and processed by caspase-1 upon inflammasome activation. It binds its specific receptor (IL-18Rα/IL-1R5), leading to the recruitment of the accessory chain (IL-18Rβ/IL-1R7), myddosome activation and signaling via NFκB.
Since group 1 Innate Lymphoid Cells (ILC1) and NK cells share several functional similarities as well as overlapping identification markers, they were originally considered as both part of the ILC1 group. Recently, a distinct precursor that drives the development of ILC1, but not NK cells, has been characterized. Moreover, phenotypic analysis in different tissues have revealed distinct markers to distinguish NK cells and ILC1s (Colonna, 2018). Both ILC1 and NK cells have emerged as a prime target for IL-18. IL-18, including a membrane-bound form expressed on monocytes-macrophages, cooperates with STAT-inducing cytokines (i.e. IL-12 and/or IL-15) to trigger NK cell effector functions, in terms of IFNγ production, cytotoxicity and FasL expression (Bellora et al., 2014; Bellora et al., 2012; Chaix et al., 2008; Madera and Sun, 2015; Tsutsui et al., 1996) (Figure 3B).
IL-18R-deficient NK cells exhibited an impaired anti-viral response in a CMV infection model and IL-18-MyD88 signaling was necessary for an optimal NK cell activation and differentiation during the infection (Madera and Sun, 2015). Human and murine NK cells treated with IL-18, together with IL-12 and IL-15, acquired a memory-like phenotype and after adoptive transfer were able to persist long term and exhibited increased activation, even though IL-18 signaling was demonstrated to be dispensable for Ly49H-mediated recall response in the mouse model (Cerwenka and Lanier, 2016; Madera and Sun, 2015; Romee et al., 2012).
In a colorectal cancer-derived liver metastasis model (MC38), mice deficient in NLRP3 inflammasome components showed augmented liver disease, which was dependent on a defective IL-18 mediated regulation of NK cells (Dupaul-Chicoine et al., 2015). In particular, in the absence of mature IL-18, NK cell differentiation and FasL-mediated killing was impaired in the liver, causing exacerbated metastasis growth (Dupaul-Chicoine et al., 2015). In a lung metastasis model, the treatment with IL-18 alone and in combination with IL-2 led to NK cell-mediated tumor control, in a FasL- and perforin-dependent manner, but independently of NKG2D (Smyth et al., 2004).
Recently, the inhibitory receptor IL-1R8 was shown to be highly expressed in human and murine NK cells and play a key role in the modulation of NK cell differentiation and effector functions (Molgora et al., 2017). In particular, IL-1R8-deficient NK cells showed unleashed IL-18-driven activation and displayed enhanced maturation, IFNγ production, cytotoxicity and FasL expression. IL-1R8-mediated regulation in NK cells was dependent on IL-18-MyD88 pathway, whereas no involvement of IL-1 signaling was observed. Importantly, Il1r8-/- NK cells were protective in mouse models of hepatocellular carcinoma, sarcoma-derived lung metastasis, colorectal cancer-derived liver metastasis and MCMV infection (Molgora et al., 2017) as discussed below. In parallel, it was found that T regulatory cell (Treg)-derived IL-37 suppressed activation of CD3–CD56dimCD57+FcεRγ+NKG2C– NK cells, but not adaptive (CD3–CD56dimCD57+FcεRγ-NKG2C+) NK cells, which are expanded in CMV seropositive donors. Treg-mediated suppression in canonical NK cells was associated with TIM-3 downregulation and PD-1 and IL-1R8 upregulation and the blockade of IL-37, PD-1 or IL-1R8 rescued the suppression (Sarhan et al., 2018). IL-1R8 therefore emerges as a novel regulator of NK cell anti-tumor and anti-viral potential, inhibiting the IL-18 pathway and promoting IL-37-mediated NK cell suppression.
In group 1 ILCs, IL-18, together with IL-12, IL-21 and IL-15, induces IFNγ production (Bernink et al., 2015; Cortez et al., 2015; Vivier et al., 2018). IFNγ was not induced in CD127+ and CD103+ ILC1 upon stimulation with IL-15 alone, in contrast to conventional NK cells, whereas IL-12 plus IL-18 activated in all populations an equivalent IFNγ response, which was even greater compared to the treatment with IL-12 plus IL-15 (Bernink et al., 2015). RNAseq analysis revealed that IL-1R8 is expressed in ILC1 cells (Shih et al., 2016). IL-1R8-deficient liver resident ILC1 cells were shown to produce higher levels of IFNγ upon CMV infection in vivo (Molgora et al., 2017).
Finally, IL-18 sustains Th1 and cytotoxic T cell activation (Garlanda et al., 2013; Nakanishi, 2018). IL-18 synergizes with IL-12 and TCR triggering to induce IFNγ and consequent defense against microbes and tumors, being on the other hand involved in autoimmune reactions and tissue damage (Garlanda et al., 2013). Recently, it was reported that the colonization with the commensal protozoan Tritrichomonas musculis led to a substantial IL-18 release in the colon, favouring the expansion of both CD4+IFNγ+ and CD4+IL-17+ T cells and being protective against mucosal bacterial infections. On the other hand, it promoted pathogenic intestinal inflammation and colorectal tumors (Chudnovskiy et al., 2016).
IL-1 enhances antigen-driven response in both CD4 and CD8 T cells, supporting the expansion and activation of specific Th1, Th2, Th17 and Granzyme B+ CD8 T cells in vivo (Ben-Sasson et al., 2009). IL-1 was necessary for naïve CD4+ T cells to overcome Treg-mediated inhibition and memory CD4+ T cells to acquire a fully functional memory phenotype (Schenten et al., 2014). In addition, IL-1β in combination with IL-23 promoted the plasticity of Th17 cells toward a Th1 phenotype, and the generation of CD161+ Th1 subsets, named as “non-classic Th1 cells (Santarlasci et al., 2013).
Finally, IL-1β was shown to enhance Th9 cell function through the IRF1-dependent increase in the production of IL-21, which promoted IFNγ production and antitumor activity of CD8+ and NK cells (Vegran et al., 2014).
Thus, IL-18 produced by macrophages and DCs is a driver of Type 1 immunity by activating NK cells, ILC1 and Th1 cells. Moreover, although IL-1 is an important component of Th17 cell differentiation, it can promote a broad spectrum of T cell responses, including CD8+ cells.
5. Type 2 immunity (ILC2, Th2)
IL-33 is a central cytokine involved in type 2 innate and adaptive immunity and inflammation, modulating ILC2, Th2 and M2 macrophage response, responsible for the control of type 2 infections and tissue repair, as well as harmful allergic responses (Garlanda et al., 2013) (Figure 3C). IL-33 is mainly secreted by unconventional mechanisms or upon cell necrosis, by hematopoietic, stromal and parenchymal cells, and signals via the ST2/IL-1R4 receptor coupled with the accessory protein IL-1RAcP/IL-1R3, inducing MyD88 activation.
IL-33 plays a protective role in the elimination of parasites through the induction of IL-13 in ILCs, which is beneficial for nematode expulsion and control of Toxoplasma gondii encephalitis (Moro et al., 2010; Neill et al., 2010). In helmintic infections, IL-1β inhibits IL-33 and IL-25 production, suppressing the clearance of the pathogen and contributing to infection chronicity (Zaiss et al., 2013).
IL-33 drives the amplification of neutrophil maturation and eosinophilia in vivo, inducing IL-5 in ILC2 (Bouffi et al., 2013; Ikutani et al., 2012; Molofsky et al., 2013; Pecaric-Petkovic et al., 2009). Indeed, in ILC2-deficient mice IL-33-mediated eosinophilic lung inflammation was abolished (Halim et al., 2014). Intranasal injection of IL-33 directly induced ILC proliferation and IL-13/IL-5 secretion and favored basophil production of IL-4, which in turn activated ILC2s (Halim et al., 2014; Motomura et al., 2014). The release of uric acid by allergen-stimulated or damaged epithelial cells was shown to induce IL-33 release in vivo (Enoksson et al., 2011; Shi et al., 2003). IL-33-deficient mice showed defective response to papain intranasal injection, which resembles allergen response, stimulating ILC2s and causing mucus hyperproduction and eosinophilia (Halim et al., 2014). IL-33 was also demonstrated to induce amphiregulin secretion by ILC2s, which promotes tissue healing, suggesting a role of IL-33 in ILC2-mediated tissue repair (Monticelli et al., 2011).
Moreover, dendritic cells activated by IL-33 promoted Th2 response (Besnard et al., 2011). Mouse models of allergic lung inflammation, A. fumigatus airway hyperreactivity and influenza virus revealed a key role of IL-33 as a driver of type 2 responses, in terms of Th2 cell polarization, mucus secretion, eosinophil recruitment and goblet cell hyperplasia (Albacker et al., 2013; Tjota et al., 2013). In humans, single nucleotide polymorphisms (SNPs) have been identified in IL-33 and ST2 genes and are associated with asthma development (Moffatt et al., 2010). In line with this, IL-33 up-regulation occurs in the bronchial mucosa of asthmatic patients and positively correlates with disease severity (Bianchetti et al., 2012; Li et al., 2018).
IL-1β was recently shown to be involved in the regulation of ILC2 function and plasticity, inducing proliferation, cytokine production and promoting the responsiveness to IL-25 and IL-33. Interestingly, IL-1β also induced the expression of T-bet and Th1 associated genes (e.g. IL12RB1, IL12RB2, STAT1, EOMES and NFIL3) in ILC2s and impacted on the ILC2 epigenetic landscape, driving the generation of an hybrid ILC2/1 population and enabling the conversion into ILC1-like cells in response to IL-12 (Ohne et al., 2016). Therefore, IL-1 contributes to the plasticity of ILCs.
6. Type 3 immunity (ILC3, Th17)
IL-17 responses are powerful tools in protective immunity against infections with bacteria, fungi and some viruses, especially at mucosal tissues (Korn et al., 2009; Zhou et al., 2009). On the other hand, IL-17 has a major pathogenic role in several chronic inflammatory and autoimmune diseases. IL-23 was characterized as the fundamental cytokine driving IL-17 production in T cells and both IL-1α and IL-1β synergize with IL-23 to promote IL-17A production by human and murine T cells, either in the presence or the absence of TCR engagement (Langrish et al., 2005; Mills et al., 2013) (Figure 3D). Indeed, IL-1R1-deficient mice lack IL-17A secretion upon IL-23 stimulation and are protected in EAE models, similarly to IL-23-deficient mice (Cua et al., 2003; Sutton et al., 2006).
In line with this, IL-1R8-deficient mice showed an augmented susceptibility to Th17-dependent experimental autoimmune encephalomyelitis (EAE), due to an uncontrolled IL-1 signaling in Th17 cells, leading to increased Th17 proliferation and function (Gulen et al., 2010). Moreover, IL-1 induces IL-6 production in innate immune cells, indirectly supporting T cell differentiation towards IL-17-producing T cells (Acosta-Rodriguez et al., 2007). The IL-23/IL-17 axis is also sustained by both IL-1α and IL-1β in γδ T cells, which express IL-1R1 and similarly to Th17 are pathogenic in several inflammatory diseases (Sutton et al., 2009). Furthermore, γδ T cells express very high levels of IL-18R, and IL-18 together with IL-23 promotes IL-17 production (Lalor et al., 2011). IL-1R8 is highly expressed in γδ T cells and suppresses IL-17A production in γδ T cells. In psoriasis models, IL-1R8-deficient mice showed enhanced γδ T cell infiltration and activation and developed more severe disease, reverted by IL-17A neutralization (Russell et al., 2013). These data highlight the importance of IL-1 and IL-1 regulation in Th17 immunity as well as the IL-17 pathogenic effects.
ILC3s are enriched at mucosal sites, where they control homeostasis and are important components of the early immune response (Vivier et al., 2018). IL-1β supports IL-17A and IL-22 production in ILC3s (Bernink et al., 2015; Cella et al., 2010; Longman et al., 2014). In vitro cultures of sorted ILC3s demonstrated that IL-2, IL-23 and IL-1β preserved the ILC3 phenotype and favoured the acquisition of NKp44. Moreover, IL-23 plus IL-1β were shown to be sufficient for CD127+ILC1 switch towards ILC3, loosing the capacity to produce IFNγ and instead producing IL-22 in vitro and in vivo. In agreement, IL-1, together with IL-2 and IL-23, promoted RORγt upregulation in CD127+ ILC1 induced by retinoic acid (Bernink et al., 2015).
7. T regulatory cells
Arpaia N. et al. showed that selective Treg cell-deficiency in amphiregulin, which promotes tissue repair during organ damage and inflammatory conditions, caused severe lung injury in a model of influenza virus infection (Arpaia et al., 2015). In this context, IL-18 and IL-33 were responsible for amphiregulin secretion by Treg cells, which was independent of TCR triggering (Arpaia et al., 2015). In mice infected with CMV, Treg cells were enriched in the liver and played a key role in controlling liver damage. In this infection, ST2 expression was upregulated in liver Treg cells and IL-33 was fundamental for Treg cell enrichment and function. IL-33 production increased in CMV-infected livers and localized in close proximity with the foci of infection only in F4/80+ cells. In agreement, ST2-deficient mice exhibited increased liver injury and mortality, whereas viral control was not affected. IL-33 administration favoured Treg expansion in the liver, suggesting a promising therapeutic application in CMV-induced hepatitis (Popovic et al., 2017). In contrast, in models of airway inflammation, IL-33 caused a dysregulation of Treg suppression activity, promoting a pathogenic Th2 phenotype and impairing their ability to inhibit effector T cells (Chen et al., 2017).
Single cell-RNAseq analysis of T cells in colorectal cancer, non-small-cell lung cancer and breast carcinoma patients revealed that tumor-infiltrating Treg cells had a marked suppressive phenotype and upregulated immune checkpoint molecules and distinct signature molecules including IL-1R2, which was also validated at the protein level (De Simone et al., 2016; Guo et al., 2018; Plitas et al., 2016). These data suggest that IL-1R2 might be a promising target to inhibit Treg-mediated suppression and pave the way for further studies to address the role of IL-1R2 in Treg in tumors.
8. Selected organs and diseases
IL-1 has been shown to be involved in a wide range of human pathologies ranging from autoinflammatory diseases, to rheumatoid arthritis and IL-1 blocking agents (IL-1Ra, Anakinra; anti-IL-1β mAb, Canakinumab; anti IL-1α, MABp1) have been approved for clinical use or are being evaluated in some of these disorders (Dinarello, 2009b; Gabay et al., 2010; Garlanda et al., 2013; Udalova et al., 2016). Here we will focus on the role of IL-1 family members at selected anatomical sites and related pathologies with emphasis on recent developments.
8.1. Central Nervous System (CNS) and neurodegeneration
IL-1 was originally identified as endogenous pyrogen and has long been known to regulate sleep, appetite and the hypothalamus-pituitary-adrenal axis (Garlanda et al., 2013). IL-1 has functions in the brain unrelated to inflammatory conditions. IL-1 is physiologically expressed in the brain with a circadian rhythm, it activates neurons at much lower concentrations compared to those required for the activation of other cell types (Huang et al., 2011) and regulates several neurophysiological processes, such as sleep, adult neurogenesis, synaptic plasticity and modulation of long-term potentiation (Liu and Quan, 2018). IL-1 modulates perception and learning in a time-and concentration-dependent manner: acute and low levels IL-1 facilitate memory, increase hippocampal-dependent learning and behaviour, whereas chronic or high levels IL-1 reduce sensory function and memory, retard learning, and cause fatigue (del Rey et al., 2013; Liu and Quan, 2018).
IL-1 has also physiological neuroendocrine functions, by inducing adrenocorticotropic hormone (ACTH), corticotropin-releasing hormone (CRH) and glucocorticoids in response to psychological and metabolic stress, and stimulates brain metabolism (Liu and Quan, 2018).
In addition, depending on the concentration, IL-1 can facilitate neuronal survival by promoting the expression of nerve growth factors (NGFs) and other neurotrophic factors, or impair neurogenesis, for instance by favoring the astrocyte rather than neuronal lineage (Garber et al., 2018; Liu and Quan, 2018).
In inflammatory or stress murine models, IL-1β, produced by brain microglia in response to TLR activation, complement components, other cytokines (such as TNFα), and IL-1 itself (Dinarello, 2011), decreased neurogenesis and influenced synaptic plasticity, processes that are vital for the development and retention of spatial memory (Garber et al., 2018; Tong et al., 2012).
The involvement of IL-1 in distinct immunological, neural, and physiological activities in the brain was recently dissected in vivo, and shown to depend on different cell-type-specific IL-1R1 signaling. In particular, endothelial IL-1R1 mediated sickness behavior and drove leukocyte recruitment to the CNS and impaired neurogenesis, ventricular IL-1R1 regulated monocyte recruitment, and the non-inflammatory ventricular, astrocyte and neuronal IL-1R1 mediated neuromodulatory activities (Liu et al., 2019).
Induction of brain proinflammatory cytokine synthesis has been described in different brain pathological conditions associated with neuroinflammation such as acute brain injury, Alzheimer’s disease, Parkinson’s disease, CNS autoimmunity, post-infectious neuropathology, temporal lobe epilepsy, schizophrenia, and febrile convulsions (Khazim et al., 2018; Liu and Quan, 2018). In these neuroinflammatory conditions, microglia inflammasome activation and IL-1 production have been shown to contribute to neuroinflammation and neurodegeneration. For instance, NLRP3 or caspase-1-deficiency protected mice from neuroinflammation and cognitive decline in models of Alzheimer’s disease, or during ageing (Heneka et al., 2013). The contribution of the IL-1 system in neuroinflammation has been demonstrated also through in vitro studies, showing that IL-1 (and TNFα) induces neuronal death directly or indirectly by activating glial production of neurotoxic substances (Liu and Quan, 2018).
In agreement with studies in mice, in selected human ethnic groups, polymorphisms of IL1A allele leading to increased expression of IL-1α protein, have been associated with susceptibility to Alzheimer’s disease, due to IL-1α-dependent production of amyloid precursor protein and further IL-1α and IL-1β production by activated microglia. Similarly, IL1B and IL1RN polymorphisms leading to imbalanced IL-1/IL-1Ra ratio have been proposed to contribute to susceptibility to Alzheimer’s disease and dementia (Khazim et al., 2018).
In ischemic or hemorrhagic stroke, NLRP3 inhibition reduced stroke-induced neural damage and functional deficits (Liu and Quan, 2018). Increased inflammasome activation and IL-1 expression have also been described in anxiety disorder, major depression, and autism. NALP3 pharmacological inhibition or deficiency was shown to reduce depressive behavior and anxiety in animal models of these disorders (Liu and Quan, 2018). Along the same line, treatment with IL-1Ra was effective in reducing infarct size in animal models of stroke, stress-induced depression and anxiety (Koo and Duman, 2009), and in improving clinical outcomes in experimental epilepsy (Vezzani et al., 2000).
These preclinical experiments paved the way for the use of IL-1Ra to treat cerebral stroke. A randomized, double-blind, placebo-controlled trial of Anakinra was carried out in patients with acute stroke. Anakinra-treated patients showed reduced systemic inflammation (white blood cells, neutrophil counts, CRP, and IL-6 levels) and cognitive impairment compared to placebo-treated patients (Dinarello, 2011; Wong et al., 2019). In addition, Anakinra was used to treat autoinflammation-associated epilepsy syndrome (DeSena et al., 2018).
In line with findings showing that physiological levels of IL-1R activation are required for correct long-term potentiation, deficiency of IL-1R8 was associated with impaired novel object recognition, spatial reference memory and long-term potentiation, even in the absence of any external inflammatory stimuli (Costello et al., 2011). In addition, it has recently shown that hyperactivation of the IL-1 pathway, due to IL-1R8-deficiency or IL-1 treatment, leads to up-regulation of the mTOR pathway and increased levels of the epigenetic regulator methyl-CpG-binding protein 2 (MeCP2), a synaptopathy protein involved in neurological diseases, causing disruption of dendritic spine morphology, synaptic plasticity and plasticity-related gene expression. Anakinra restored MeCP2 levels and spine plasticity and ameliorated cognitive defects in IL-1R8-deficient mice (Tomasoni et al., 2017).
IL-1 has gained attention for its impact on cognitive function in the context of neuroinflammation during CNS viral infection (Vasek et al., 2016). In West Nile virus neuroinvasive disease (WNND), a condition associated with loss of hippocampal synapses and cognitive dysfunction, astrocytes were shown to be the predominant source of IL-1. In a mouse model of WNND, IL-1R1-deficiency was associated with normal neurogenesis, recovery of presynaptic termini, and resistance to spatial learning defects, indicating that proinflammatory astrocytes impairs neuronal progenitor cell homeostasis via IL-1 overexpression (Garber et al., 2018).
In addition to IL-1, IL-18 and IL-33 play a role in inflammatory diseases of the CNS. Brain resident cells constitutively express IL-18, IL-33 and caspase-1, thus providing a local source of these cytokines. Experimental and clinical studies suggested a crucial role for IL-18-mediated neuroinflammation and neurodegeneration in different conditions, including multiple sclerosis, bacterial meningitis, ischemic stroke and head injury. In contrast, in specific viral infections (e.g. Influenza A), IL-18 was shown to support IFNγ-mediated viral clearance of infected neurons (Felderhoff-Mueser et al., 2005). In Alzheimer disease, IL-18 over-expression has been detected in microglia, astrocytes as well as neurons, and found to be co-localized with both amyloid plaques and tau (Singhal et al., 2014).
Treatment with IL-33 exacerbated EAE, but also promoted the differentiation of M2-like microglia and Treg cells limiting glial scaring in experimental stroke and spinal cord injury (Liew et al., 2016). Treatment with IL-33 reduced synaptic plasticity impairment and cognitive deficits in a mouse model of Alzheimer disease and reduced amyloid plaque deposition by promoting the recruitment and polarization of microglia towards an anti-inflammatory phenotype (Fu et al., 2016). In agreement with results in mice, IL-33 expression was decreased in Alzheimer disease patients’ brain and IL33 and ST2 single nucleotide polymorphisms have been associated with susceptibility to Alzheimer disease (Liew et al., 2016).
The highly homologous orphan receptors, IL-1R9 (TIGIRR-2) and IL-1R10 (TIGIRR-1), and a specific form of the IL-1RAcP, called IL- 1RAcPb, are expressed almost exclusively in the brain. IL-1R9 regulates glutamaterigic synapse formation and stabilization, and IL-1R9 mutations are associated with cognitive impairment, such as mental retardation, autism, and schizophrenia (Garlanda et al., 2013).
Thus, members of the IL-1 family and their receptors are expressed in the CNS and serve physiological functions. Several lines of evidence, including genetic associations, suggest that IL-1 and related cytokines are key mediators of neurodegenerative diseases. These results pave the way to assessing their potential as therapeutic targets in vascular and degenerative diseases of the nervous system.
8.2. Gastrointestinal tract
IL-1 cytokines contribute to maintain the equilibrium between immune tolerance to commensal microbiota and response to intestinal pathogens. The players involved in this process (inflammasomes, IL-1 cytokines, IL-1 receptors and negative regulators) are expressed by epithelial cells or by leukocytes residing in the lamina propria. Several lines of evidence indicate that IL-1 family members, such as IL-1, IL-1Ra, IL-18, and IL-33 possess dual functions depending on the phase of intestinal disease, as well as on their role in initiating vs. sustaining chronic gut inflammation, and finally on the cell type targeted by the cytokine.
Th17 responses have a pro-tumor role in colorectal cancer (Grivennikov et al., 2012). The role of IL-1 in colorectal cancer was recently dissected by using cell type specific IL-1R1-deficient mice. This study showed that IL-1 had pro-tumor effects by promoting Th17-mediated inflammation through IL-17 and IL-22, and epithelial cell-autonomous mechanisms, whereas IL-1 had tumor-suppressive effects by promoting neutrophil-mediated control of bacterial invasion (Dmitrieva-Posocco et al., 2019).
Under homeostatic conditions, NLRP6 and NLRP3 inflammasome activation in epithelial cells drives IL-18 expression, which favours the production of antimicrobial peptides. These are essential for the maintenance of intestinal barrier integrity and normal commensal microbiota. IL-18 was also reported to promote Treg cell-effector functions, and decrease Th17 polarization (Harrison et al., 2015). In contrast, in inflammatory conditions associated with disruption of the epithelial barrier and dysbiosis, activation of inflammasomes in epithelial cells and myeloid cells leads to the release of huge amounts of IL-18 which promotes inflammation and blocks the development of goblet cells, leading to reduced mucus in the colon and presumably increased bacterial access to the intestine surface (Nowarski et al., 2015). The regulation of inflammasome activation and IL-18 secretion were shown to depend on the presence of the microbiota and in particular on specific metabolites such as taurine, histamine, and spermine (Levy et al., 2015).
Accordingly, inflammasome activation and high IL-18 and IL-18BP levels have been detected in the serum and intestinal tissue of Crohn’s disease patients. In addition, NLRC4 mutation leading to high serum IL-18 concentrations and IL-18 transgenic mice are more susceptible to colitis, whereas IL-18 inhibition played a protective effect in experimental models of inflammatory bowel diseases (Kaplanski, 2018; Sivakumar et al., 2002).
IL-33 is constitutively expressed in epithelial cells of the intestine, where it functions as an endogenous alarmin in response to tissue damage. IL-33 is highly expressed in inflamed lesions of IBD patients and it plays dual roles in animal models of intestinal inflammation, depending on the phase of the disease (early and inflammatory or repair and healing) and the inflammatory state (acute or chronic), since IL-33 may influence Th2 responses, Th1 inflammation, mucosal regeneration and fibrosis (Lopetuso et al., 2013). Studies supporting a regulatory role for IL-33 in gut inflammation show that the IL-33 receptor ST2 promotes TGF-β1-mediated differentiation of Treg cells, favouring Treg-cell accumulation and maintenance and induce a regulatory phenotype to Th17 cells recruited in inflammatory conditions (Schiering et al., 2014), or inducing epithelial-derived miR-320 that promotes epithelial repair/restitution and the resolution of inflammation (Lopetuso et al., 2018).
IL-36 cytokines contribute to intestinal inflammation. Indeed, IL-36R-deficiency was associated with reduced innate, inflammatory and Th1 responses in different models of colitis. However, IL-36 signaling is also important for the resolution of mucosal inflammation and healing of mucosal wounds, by promoting IL-22 expression (Medina-Contreras et al., 2016).
Concerning negative regulatory receptors, IL-1R2 was shown to be highly upregulated in epithelial cells in ulcerative colitis remission, compared to active disease, suggesting its involvement in dampening IL-1β proinflammatory activity and favouring the return to mucosal homeostasis (Mora-Buch et al., 2016). In addition, IL-1R8 was shown to be crucial in the modulation of intestinal epithelial cell metabolism, differentiation, cell cycle, and effector functions both in homeostatic conditions and in inflammation, through the regulation of NFκB, JNK and mTOR activation driven by IL-1 or TLR agonists derived from commensal flora in intestinal epithelial cells (Garlanda et al., 2004; Xiao et al., 2007). In experimental models of colitis, IL-1R8 deficiency caused exacerbated intestinal inflammation, in terms of weight loss, intestinal bleeding, local tissue damage, and a reduced survival. This correlated with an increased leukocyte infiltration and higher level of pro- inflammatory cytokines (TNFα, IL-6, IL-1β, IL-12p40, IL-17), chemokines (CXCL1, CCL2), and prostaglandins. In agreement with the protumoral role of chronic inflammation, in different murine models, IL-1R8 was shown to play a key protective role in the pathogenesis of intestinal cancer (Garlanda et al., 2007; Xiao et al., 2007; Zhao et al., 2015). The data suggest that IL-1R8 exerts a tumor suppressor activity by controlling IL-1- and TLR-induced cancer-related inflammation and mTOR-mediated cell cycle progression and consequent genetic instability.
8.3. Cardiovascular pathology
IL-1 affects all components of the vessel wall and cardiomyocytes and, accordingly, it plays an important role in atherosclerosis and its complications including myocardial infarction (Abbate and Dinarello, 2015; Hansson, 2005; Libby, 2017). Early on it was shown that IL-1 activates endothelial cells in a prothrombotic/proinflammatory direction (Mantovani et al., 1992). The proinflammatory program of IL-1 included induction of monocyte-attracting chemokines such as CCL2, procoagulant activity, prostaglandins, platelet activating factor (PAF) and an inhibitor of thrombin dissolution (PAI). A similar set of responses was observed in smooth muscle cells (Libby, 2017), which also are induced to proliferate.
Studies using IL-1R1 or IL-1Ra gene-targeted mice provided strong genetic evidence for IL-1 being a driver of atherosclerosis and its complications. Moreover, IL-1 directly affects the function of cardiomyocytes. The discovery of cholesterol crystal-mediated activation of the NLRP3 inflammasome provided a direct link between lipid metabolism and activation of IL-1β (Duewell et al., 2010). Moreover, IL-1β and IL-1α are stored in platelets which can represent an additional source of this cytokine (Semple et al., 2011). Interestingly platelets also express the negative regulator IL-1R8 and genetic evidence showed that this molecule limits their activation and thrombosis (Anselmo et al., 2016).
IL-1 is a potent inducer of IL-6 which in turn reshapes liver function in the acute phase response including production of C reactive protein (CRP) (Garlanda et al., 2013). IL-6, CRP and a distant relative of CRP, PTX3, induced by IL-1 were found to serve as biomarkers of the severity and risk of cardiovascular pathology (Garlanda et al., 2018; Peri et al., 2000; Ridker et al., 2001).
These preclinical and clinical results set the stage for the CANTOS study aimed at assessing the potential of an anti-IL-1β mAb (Canakinumab) to protect against atherosclerotic disease. 10,061 patients were treated with increasing doses of Canakinumab, with non-fatal myocardial infarction, non-fatal stroke or cardiovascular death as efficacy endpoints (Ridker et al., 2017a). The results of this seminal study showed that anti-IL-1β therapy was effective in preventing recurrence of cardiovascular disease (Ridker et al., 2017a). Interestingly, treatment with Canakinumab also protected against arthritis, gout, osteoarthritis and cancer, as discussed in the following section.
8.4. Cancer
IL-1 family members have complex, divergent roles in the control of carcinogenesis and tumor progression. In general stage of progression and the tissue contexture are important determinants of the impact of IL-1 family members on cancer. As discussed above, IL-18 is a potent inducer of IFNγ in NK cells, ILC1s and Th1 cells. In murine tumors IFNγ is associated with resistance to carcinogenesis and in human cancers IFNγ and related signatures including IL-18 are generally associated with better prognosis (Fridman et al., 2017). In contrast, several lines of evidence link IL-33 driven type 2 immunity to promotion of tumor progression (Hong et al., 2018), although there is evidence for instance in preclinical models of hepatocellular carcinoma of a protective function of this cytokine (Jin et al., 2018). Evidence linking IL-33 to tumor promotion include preclinical models and human prognostic and genetic associations (Amor et al., 2018; Ding et al., 2018; Wen et al., 2018). Interestingly IL-33 was associated with stromal cell driven Treg differentiation in oral squamous cell carcinoma (Wen et al., 2018) and promoted Treg-dependent metastatic mammary tumor growth in the lung (Halvorsen et al., 2019).
The prevailing function of IL-1α and β in cancer is tumor promotion, based on preclinical and clinical data, though early in malignant transformation, dendritic cell membrane IL-1α may play an anti-tumor role as an alarmin acting as an adjuvant for presentation of tumor neoantigens (Marhaba et al., 2008; Song et al., 2003). Early on, it was shown that IL-1β increased tumor progression in mouse tumors (Chirivi et al., 1993; Giavazzi et al., 1990; Vidal-Vanaclocha et al., 1994), a finding confirmed and extended using IL-1β or IL-1R1 gene targeted mice (Voronov et al., 2003). IL-1α and IL-1β are downstream of the intrinsic, oncogene-driven, and the extrinsic, inflammatory disease-driven, pathways linking inflammation and cancer (Mantovani et al., 2008). IL-1α and IL-1β were found to be downstream of the RAS and RET-PTC oncogenes in the intrinsic pathway (Borrello et al., 2005; Cataisson et al., 2012). IL-1β was also found to be a mediator of chronic non-resolving inflammation which increases the risk of developing tumors in the gastrointestinal tract (Garlanda et al., 2013). In a model of skin carcinogenesis IL-1α was shown to have dual functions downstream of RAS, affecting both transformed cells and the tumor microenvironment (TME) (Cataisson et al., 2012). On tumor cells it blocked the expression of differentiation markers. Via NFκB IL-1α orchestrated the construction of an inflammatory microenvironment via induction of cytokines and chemokines.
IL-1-mediated tumor promotion has been identified in different murine and human tumor types, including sarcomas, melanoma, pancreatic ductal adenocarcinoma (Ling et al., 2012; Melisi et al., 2009; Tjomsland et al., 2013;Zhuang et al., 2016), myelomas (Lust et al., 2016), and breast carcinomas (Wu et al., 2018) and involves different mechanisms (Figure 4A). IL-1 has been shown to sustain the expansion and immunosuppressive function of myeloid cells in tumor-bearing mice (Elkabets et al., 2010; Kaplanov et al., 2019; Pan et al., 2017).
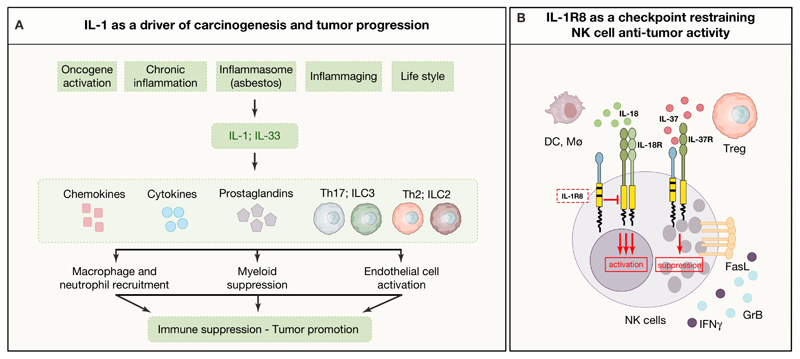
Figure 4. Mechanisms of tumor promotion and control by IL-1 family members.
A) Upstream conditions and downstream cell types and mediators involved in the networks of IL-1 and IL-33-driven tumor promotion and immune suppression. B) Representation of IL-1R8-dependent modulation of IL-18 activity on NK cells, and its role as co-receptor for IL-37.
In mouse and human melanoma, the IL-1R-MyD88 pathway was found to cause upregulation of TET2 in TAM. TET2 is a DNA methylcytosine dioxygenase which skewed macrophage function in an immunosuppression M2-like mode (Pan et al., 2017). TET2 gene targeting changed the polarization state of macrophages and reactivated T cell mediated antitumor resistance.
Vascular cells are a prime target of IL-1 (see above). In the context of neoplasia, IL-1 was shown to promote angiogenesis. Induction of endothelial cell adhesion molecules E-selectin and vascular cell adhesion molecule-1 (VCAM-1) was involved in augmentation of metastasis.
Recent studies have identified an IL-1β signature in the peripheral blood mononuclear cells from patients with metastatic, hormone-negative breast cancer. There is expression of several genes but the most dominant are an IL-1β signature which includes the IL-1R1, MyD88 and IL-1β itself (Wu et al., 2018). When the primary tumor tissue from 145 patients with breast cancer was stimulated in culture, both IL-1β and IL-1α were found in the supernatants. When treated with daily Anakinra for two weeks, each of the IL-1β signature proteins decreased and the reduction was sustained when Anakinra was added to a standard of care chemotherapeutic (Wu et al., 2018). The IL-1β signature includes IL-1 signaling kinases, which are common to both IL-1β and IL-1α. Thus, it is likely that treating patients with Anakinra includes blocking IL-1α. A key player in breast cancer is thymic stromal cell lymphopoietin (TSLP); increased production of TSLP is associated with poor prognosis not only in breast cancer but also in other epithelial cancers (Kuan and Ziegler, 2018). In breast cancer tissue from 145 patients, TSLP levels correlated with IL-1β (Wu et al., 2018). In addition to IL-1β, there is a unique role for IL-1α with TSLP. Breast cancer tumor-derived IL-1α stimulates the production of TSLP from the infiltrating myeloid cells in the microenvironment of breast cancer. The increase in TSLP promotes the survival of the tumor cells via Bcl-2 (Kuan and Ziegler, 2018).
IL-1R8 has recently been shown to serve as a checkpoint for IL-18-driven differentiation and activation of NK cells and ILC1 cells (Molgora et al., 2017). This observation was independently confirmed and it was also found that IL-37, produced by Treg cells, suppressed NK function via IL-1R8 (Sarhan et al., 2018). Blocking IL-1R8 unleashed NK cell-mediated resistance against primary carcinogenesis and metastasis in the liver and lung, two NK cell rich anatomical sites (Molgora et al., 2017). The checkpoint function of IL-1R8 was also observed in human NK cells. These results suggest that IL-1R8 can act as a double edged sword in carcinogenesis. On the one hand it tames tumor promoting inflammation driven by IL-1 or TLRs; on the other hand, it serves as a checkpoint for NK cells which, if unleashed, can mediate anticancer resistance at least at NK cell rich anatomical sites (Figure 4B). Consistently with these results, in breast cancer IL-1R8 expression was found to be associated with an NK cell inflamed molecular signature (Campesato et al., 2017). These results raise the possibility to target the IL-1R8 checkpoint in particular in the context of liver metastasis.
Human genetics is consistent with a role of IL-1 and related molecules in carcinogenesis. Polymorphisms in the IL-1 locus setting the system in a proinflammatory mode were associated with risk of gastric cancer (El-Omar et al., 2000). Along the same line, IL-1α and β and IL-1Ra genetic polymorphisms were associated with risk of developing other tumors including lung cancer (Bhat et al., 2014; Hu et al., 2006; Khazim et al., 2018; Lind et al., 2005; Zienolddiny et al., 2004). Thus, mouse and human genetics is consistent with IL-1 and its regulatory pathways being a driver of tumor promotion.
These results set the stage for therapeutic translation of IL-1 blocking strategies using Anakinra or anti-IL-1α or IL-1β mAb. A 10 year trial of daily Anakinra with weekly low dose dexamethasone in 47 patients with smoldering myeloma resulted in significantly increased in survival (Lust et al., 2009; Lust et al., 2016). Improved survival has been reported when Anakinra is added to the standard of therapy with flurouracil in advanced metastatic colorectal cancer (Isambert et al., 2018), hormone negative breast cancer (Wu et al., 2018) and in advanced pancreatic cancer (Becerra et al., 2018; Whiteley et al., 2016). In preclinical studies, Anakinra also abated CAR-T cell induced cytokine release syndrome and neurotoxicity (Giavridis et al., 2018; Norelli et al., 2018). In a phase I study in patients with end-stage tumors of different types blocking IL-1α reduced cancer cachexia (lean body mass, fatigue, appetite loss) (Hong et al., 2014).
IL-1α had long been known to mediate muscle loss and cachexia (Garlanda et al at 2013). Three trials have administered anti-IL-1α to patients with advanced cancers of various origins (Hong et al., 2014; Hong et al., 2015) as well as patients with colorectal cancer (Hickish et al., 2017). In these trials, end-stage patients were losing lean body mass as a manifestation of cancer cachexia. The most recent trial was a randomized, placebo controlled study in 333 patients with advanced, metastatic colorectal cancer who were treated with an 8 week monotherapy course of neutralizing human anti-human IL-1α (Hickish et al., 2017). The study met its primary and secondary end-points with an increase in lean body mass, improved parameters of quality of life, decreased pain, decreased constitutional symptoms and significantly lower circulating IL-6 and platelet counts. Increased survival was observed in responders compared to non-responders. In this study, levels of circulating IL-1Ra predicted responsiveness to treatment with the IL-1α-targeting antibody (Kurzrock et al., 2019).
There are several lessons derived from these studies besides reduction in cancer cachexia (McDonald et al., 2018). Reducing IL-1α may also reduce inflammation-mediated immunosuppression, both impacting on increased survival and immune-mediated tumor regression (Mantovani et al., 2008).
The wealth of preclinical and clinical data accumulated since 1990 (Giavazzi et al., 1990) set the stage for assessing the impact of blocking IL-1β in human cancer development. In the seminal CANTOS study discussed above, in 10,061 patients with atherosclerosis and high CRP levels, Canakinumab caused a dramatic (>50%) reduction in the incidence and mortality from lung cancer (Ridker et al., 2017a). These impressive results raise questions as to immunological mechanisms and further therapeutic exploitation. The follow-up was relatively short (3.7 years) in relation to the natural history of cancer in humans. We speculate that blocking IL-1-driven recruitment and immunosuppressive function of myeloid cells and unleashing innate and lymphoid cell mediated effector pathways is a determinant of this impressive result.
9. Concluding remarks
Starting in the early ‘70s, over 40 years since its official consensus naming (Aarden et al., 1979), IL-1 has grown into a complex multifaceted family of cytokines with complex regulatory mechanisms, diverse functions and role in immunopathology. In more recent years, largely forgotten ligands (IL-36, IL-37, IL-38) and receptor chains (IL-1R8) have joined the classic stars IL-1, IL-33 and IL-18 as important orchestrators of immunity in different contexts. The role of these molecules and their forerunner IL-1β has extended beyond classic immunopathology, to include degenerative diseases of the CNS and of the cardiovascular system, and cancer. The CANTOS study showing that in over 10,000 patients blocking IL-1β protects against atherosclerosis-driven cardiovascular mortality as well as lung cancer (Ridker et al., 2017a; Ridker et al., 2017b) is revealing of the diversity and yet commonality of disease mechanisms. Indeed, IL-1 represents a paradigm for a metanarrative of 21st century medicine: inflammation and immunity. Dissection of the role of members of this complex family, their regulatory mechanisms and genetic determinants may pave the way to better exploitation of current therapeutic tools and development of novel intervention strategies.
Acknowledgements
The contribution of the European Commission (ERC project PHII-669415 to AM), Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) (project PRIN 2015YYKPNN), Associazione Italiana Ricerca sul Cancro (AIRC IG 19014 to AM and AIRC 5x1000 9962 and 21147), Fondazione Cariplo, and the Italian Ministry of Health (Ricerca Finalizzata, RF-2013-02355470 to CG) is gratefully acknowledged.
Footnotes
“Pavarotti and Friends” were a series of concerts given by tenor Luciano Pavarotti together with Placido Domingo, Josè Carreras and rock stars in Modena.
References
- Aarden LA, Brunner TK, Cerottini J-C, Dayer J-M, de Weck AL, Dinarello CA, Di Sabato G, Farrar JJ, Gery I, Gillis S, et al. Revised Nomenclature for Antigen-Nonspecific T Cell Proliferation and Helper Factors. J Immunol. 1979;123:2928–2929. [PubMed] [Google Scholar]
- Abbate A, Dinarello CA. Anti-inflammatory therapies in acute coronary syndromes: is IL-1 blockade a solution? Eur Heart J. 2015;36:337–339. doi: 10.1093/eurheartj/ehu369. [DOI] [PubMed] [Google Scholar]
- Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–949. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- Albacker LA, Chaudhary V, Chang YJ, Kim HY, Chuang YT, Pichavant M, DeKruyff RH, Savage PB, Umetsu DT. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat Med. 2013;19:1297–1304. doi: 10.1038/nm.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor NG, de Oliveira CE, Gasparoto TH, Vilas Boas VG, Perri G, Kaneno R, Lara VS, Garlet GP, da Silva JS, Martins GA, et al. ST2/IL-33 signaling promotes malignant development of experimental squamous cell carcinoma by decreasing NK cells cytotoxicity and modulating the intratumoral cell infiltrate. Oncotarget. 2018;9:30894–30904. doi: 10.18632/oncotarget.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo A, Riva F, Gentile S, Soldani C, Barbagallo M, Mazzon C, Feruglio F, Polentarutti N, Somma P, Carullo P, et al. Expression and function of IL-1R8 (TIR8/SIGIRR): a regulatory member of the IL-1 receptor family in platelets. Cardiovasc Res. 2016;111:373–384. doi: 10.1093/cvr/cvw162. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Green JA, Moltedo B, Arvey A, Hemmers S, Yuan S, Treuting PM, Rudensky AY. A Distinct Function of Regulatory T Cells in Tissue Protection. Cell. 2015;162:1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts RJ, Joosten LA, Netea MG. Immunometabolic circuits in trained immunity. Semin Immunol. 2016;28:425–430. doi: 10.1016/j.smim.2016.09.002. [DOI] [PubMed] [Google Scholar]
- Arts RJW, Moorlag S, Novakovic B, Li Y, Wang SY, Oosting M, Kumar V, Xavier RJ, Wijmenga C, Joosten LAB, et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23:89–100 e105. doi: 10.1016/j.chom.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Baldridge MT, King KY, Boles NC, Weksberg DC, Goodell MA. Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature. 2010;465:793–797. doi: 10.1038/nature09135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastien D, Bellver Landete V, Lessard M, Vallieres N, Champagne M, Takashima A, Tremblay ME, Doyon Y, Lacroix S. IL-1alpha gene deletion protects oligodendrocytes after spinal cord injury through upregulation of the survival factor Tox3. J Neurosci. 2015;35:10715–10730. doi: 10.1523/JNEUROSCI.0498-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becerra C, Paulson AS, Cavaness K, Hoof PD, Celinski S. Gemcitabine, nabpaclitaxel, cisplatin, and anakinra (AGAP) treatment in patients with non-metastatic pancreatic ductal adenocarcinoma (PDAC). NCT02550327. J Clin Oncol. 2018;36(supplement 4) Abstract 449. [Google Scholar]
- Bellora F, Castriconi R, Dondero A, Pessino A, Nencioni A, Liggieri G, Moretta L, Mantovani A, Moretta A, Bottino C. TLR activation of tumor-associated macrophages from ovarian cancer patients triggers cytolytic activity of NK cells. Eur J Immunol. 2014;44:1814–1822. doi: 10.1002/eji.201344130. [DOI] [PubMed] [Google Scholar]
- Bellora F, Castriconi R, Doni A, Cantoni C, Moretta L, Mantovani A, Moretta A, Bottino C. M-CSF induces the expression of a membrane-bound form of IL-18 in a subset of human monocytes differentiating in vitro toward macrophages. Eur J Immunol. 2012;42:1618–1626. doi: 10.1002/eji.201142173. [DOI] [PubMed] [Google Scholar]
- Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, Dinarello CA, Paul WE. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci U S A. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin JT, Moore DJ, Bennett C, van der Meer R, Royce A, Loveland R, Wynn JL. IL-1alpha and not IL-1beta drives IL-1R1-dependent neonatal murine sepsis lethality. J Immunol. 2018;201:2873–2878. doi: 10.4049/jimmunol.1801089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berda-Haddad Y, Robert S, Salers P, Zekraoui L, Farnarier C, Dinarello CA, Dignat-George F, Kaplanski G. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1alpha. Proc Natl Acad Sci U S A. 2011;108:20684–20689. doi: 10.1073/pnas.1116848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernink JH, Krabbendam L, Germar K, de Jong E, Gronke K, Kofoed-Nielsen M, Munneke JM, Hazenberg MD, Villaudy J, Buskens CJ, et al. Interleukin-12 and -23 Control Plasticity of CD127(+) Group 1 and Group 3 Innate Lymphoid Cells in the Intestinal Lamina Propria. Immunity. 2015;43:146–160. doi: 10.1016/j.immuni.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Bersudsky M, Luski L, Fishman D, White RM, Ziv-Sokolovskaya N, Dotan S, Rider P, Kaplanov I, Aychek T, Dinarello CA, et al. Non-redundant properties of IL-1alpha and IL-1beta during acute colon inflammation in mice. Gut. 2014;63:598–609. doi: 10.1136/gutjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- Besnard AG, Togbe D, Guillou N, Erard F, Quesniaux V, Ryffel B. IL-33-activated dendritic cells are critical for allergic airway inflammation. Eur J Immunol. 2011;41:1675–1686. doi: 10.1002/eji.201041033. [DOI] [PubMed] [Google Scholar]
- Bhat IA, Naykoo NA, Qasim I, Ganie FA, Yousuf Q, Bhat BA, Rasool R, Aziz SA, Shah ZA. Association of interleukin 1 beta (IL-1beta) polymorphism with mRNA expression and risk of non small cell lung cancer. Meta Gene. 2014;2:123–133. doi: 10.1016/j.mgene.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchetti L, Marini MA, Isgro M, Bellini A, Schmidt M, Mattoli S. IL-33 promotes the migration and proliferation of circulating fibrocytes from patients with allergen-exacerbated asthma. Biochem Biophys Res Comm. 2012;426:116–121. doi: 10.1016/j.bbrc.2012.08.047. [DOI] [PubMed] [Google Scholar]
- Boettcher S, Manz MG. Regulation of Inflammation- and Infection-Driven Hematopoiesis. Trends Immunol. 2017;38:345–357. doi: 10.1016/j.it.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Borrello MG, Alberti L, Fischer A, Degl'innocenti D, Ferrario C, Gariboldi M, Marchesi F, Allavena P, Greco A, Collini P. Induction of a proinflammatory program in normal human thyrocytes by the RET/PTC1 oncogene. Proc Natl Acad Sci USA. 2005;102:14825–14830. doi: 10.1073/pnas.0503039102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottazzi B, Riboli E, Mantovani A. Aging, inflammation and cancer. Semin Immunol. 2018;40:74–82. doi: 10.1016/j.smim.2018.10.011. [DOI] [PubMed] [Google Scholar]
- Bouffi C, Rochman M, Zust CB, Stucke EM, Kartashov A, Fulkerson PC, Barski A, Rothenberg ME. IL-33 markedly activates murine eosinophils by an NF-kappaB-dependent mechanism differentially dependent upon an IL-4-driven autoinflammatory loop. J Immunol. 2013;191:4317–4325. doi: 10.4049/jimmunol.1301465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowdish DM, Loffredo MS, Mukhopadhyay S, Mantovani A, Gordon S. Macrophage receptors implicated in the “adaptive” form of innate immunity. Microbes Infect. 2007;9:1680–1687. doi: 10.1016/j.micinf.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Burzinsky LC, M H, Pyrillou K, Wiggins KA, Chan JNE, Figg N, Kitt LK, Summers C, Tatham KC, Martin PB, Bennet MR, Clarke MCH. The coagulation and immune system are fundamentally linked through the activation of interleukin-1a by thrombin. Immunity. 2019 doi: 10.1016/j.immuni.2019.03.003. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campesato LF, Silva APM, Cordeiro L, Correa BR, Navarro FCP, Zanin RF, Marcola M, Inoue LT, Duarte ML, Molgora M, et al. High IL-1R8 expression in breast tumors promotes tumor growth and contributes to impaired antitumor immunity. Oncotarget. 2017;8:49470–49483. doi: 10.18632/oncotarget.17713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataisson C, Salcedo R, Hakim S, Moffitt BA, Wright L, Yi M, Stephens R, Dai RM, Lyakh L, Schenten D, et al. IL-1R-MyD88 signaling in keratinocyte transformation and carcinogenesis. J Exp Med. 2012;209:1689–1702. doi: 10.1084/jem.20101355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cella M, Otero K, Colonna M. Expansion of human NK-22 cells with IL-7, IL-2, and IL-1beta reveals intrinsic functional plasticity. Proc Natl Acad Sci U S A. 2010;107:10961–10966. doi: 10.1073/pnas.1005641107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16:112–123. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- Chaix J, Tessmer MS, Hoebe K, Fuseri N, Ryffel B, Dalod M, Alexopoulou L, Beutler B, Brossay L, Vivier E, Walzer T. Cutting edge: Priming of NK cells by IL-18. J Immunol. 2008;181:1627–1631. doi: 10.4049/jimmunol.181.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Kobayashi T, Iijima K, Hsu FC, Kita H. IL-33 dysregulates regulatory T cells and impairs established immunologic tolerance in the lungs. J Allergy Clin Immunol. 2017;140:1351–1363 e1357. doi: 10.1016/j.jaci.2017.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CJ, Kono H, Golenbock D, Reed G, Akira S, Rock KL. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med. 2007;13:851–856. doi: 10.1038/nm1603. [DOI] [PubMed] [Google Scholar]
- Cheng SC, Quintin J, Cramer RA, Shepardson KM, Saeed S, Kumar V, Giamarellos-Bourboulis EJ, Martens JH, Rao NA, Aghajanirefah A, et al. mTOR- and HIF-1alpha-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345 doi: 10.1126/science.1250684. 1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirivi RG, Garofalo A, Padura IM, Mantovani A, Giavazzi R. Interleukin 1 receptor antagonist inhibits the augmentation of metastasis induced by interleukin 1 or lipopolysaccharide in a human melanoma/nude mouse system. Cancer Res. 1993;53:5051–5054. [PubMed] [Google Scholar]
- Chudnovskiy A, Mortha A, Kana V, Kennard A, Ramirez JD, Rahman A, Remark R, Mogno I, Ng R, Gnjatic S, et al. Host-Protozoan Interactions Protect from Mucosal Infections through Activation of the Inflammasome. Cell. 2016;167:444–456 e414. doi: 10.1016/j.cell.2016.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D. Vaccination in the elderly: The challenge of immune changes with aging. Semin Immunol. 2018;40:83–94. doi: 10.1016/j.smim.2018.10.010. [DOI] [PubMed] [Google Scholar]
- Colonna M. Innate Lymphoid Cells: Diversity, Plasticity, and Unique Functions in Immunity. Immunity. 2018;48:1104–1117. doi: 10.1016/j.immuni.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Re F, Muzio M, Bertini R, Polentarutti N, Sironi M, Giri JG, Dower SK, Sims JE, Mantovani A. Interleukin-1 type II receptor: a decoy target for IL-1 that is regulated by IL-4. Science. 1993;261:472–475. doi: 10.1126/science.8332913. [DOI] [PubMed] [Google Scholar]
- Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortez VS, Robinette ML, Colonna M. Innate lymphoid cells: new insights into function and development. Curr Opin Immunol. 2015;32:71–77. doi: 10.1016/j.coi.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costello DA, Watson MB, Cowley TR, Murphy N, Murphy Royal C, Garlanda C, Lynch MA. Interleukin-1alpha and HMGB1 mediate hippocampal dysfunction in SIGIRR-deficient mice. J Neurosci. 2011;31:3871–3879. doi: 10.1523/JNEUROSCI.6676-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- De Simone M, Arrigoni A, Rossetti G, Gruarin P, Ranzani V, Politano C, Bonnal RJP, Provasi E, Sarnicola ML, Panzeri I, et al. Transcriptional Landscape of Human Tissue Lymphocytes Unveils Uniqueness of Tumor-Infiltrating T Regulatory Cells. Immunity. 2016;45:1135–1147. doi: 10.1016/j.immuni.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey A, Balschun D, Wetzel W, Randolf A, Besedovsky HO. A cytokine network involving brain-borne IL-1beta, IL-1ra, IL-18, IL-6, and TNFalpha operates during long-term potentiation and learning. Brain Behav Immun. 2013;33:15–23. doi: 10.1016/j.bbi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- DeSena AD, Do T, Schulert GS. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J Neuroinflammation. 2018;15:38. doi: 10.1186/s12974-018-1063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009a;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1beta and the autoinflammatory diseases. N Engl J Med. 2009b;360:2467–2470. doi: 10.1056/NEJMe0811014. [DOI] [PubMed] [Google Scholar]
- Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117:3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev. 2018;281:8–27. doi: 10.1111/imr.12621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello CA, Goldin NP, Wolff SM. Demonstration and characterization of two distinct human leukocytic pyrogens. J Exp Med. 1974;139:1369–1381. doi: 10.1084/jem.139.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding L, Ren J, Zhang D, Li Y, Huang X, Hu Q, Wang H, Song Y, Ni Y, Hou Y. A novel stromal lncRNA signature reprograms fibroblasts to promote the growth of oral squamous cell carcinoma via LncRNA-CAF/interleukin-33. Carcinogenesis. 2018;39:397–406. doi: 10.1093/carcin/bgy006. [DOI] [PubMed] [Google Scholar]
- Dmitrieva-Posocco O, Dzutsev A, Posocco DF, Hou V, Yuan W, Thovarai V, Mufazalov IA, Gunzer M, Shilovskiy IP, Khaitov MR, et al. Cell-Type-Specific Responses to Interleukin-1 Control Microbial Invasion and Tumor-Elicited Inflammation in Colorectal Cancer. Immunity. 2019;50:166–180 e167. doi: 10.1016/j.immuni.2018.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube PH, Revell PA, Chaplin DD, Lorenz RG, Miller VL. A role for IL-1 alpha in inducing pathologic inflammation during bacterial infection. Proc Natl Acad Sci U S A. 2001;98:10880–10885. doi: 10.1073/pnas.191214498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duewell P, Kono H, Rayner KJ, Sirois CM, Vladimer G, Bauernfeind FG, Abela GS, Franchi L, Nunez G, Schnurr M, et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature. 2010;464:1357–1361. doi: 10.1038/nature08938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupaul-Chicoine J, Arabzadeh A, Dagenais M, Douglas T, Champagne C, Morizot A, Rodrigue-Gervais IG, Breton V, Colpitts SL, Beauchemin N, Saleh M. The Nlrp3 Inflammasome Suppresses Colorectal Cancer Metastatic Growth in the Liver by Promoting Natural Killer Cell Tumoricidal Activity. Immunity. 2015;43:751–763. doi: 10.1016/j.immuni.2015.08.013. [DOI] [PubMed] [Google Scholar]
- El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, et al. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- Elkabets M, Ribeiro VS, Dinarello CA, Ostrand-Rosenberg S, Di Santo JP, Apte RN, Vosshenrich CA. IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol. 2010;40:3347–3357. doi: 10.1002/eji.201041037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoksson M, Lyberg K, Moller-Westerberg C, Fallon PG, Nilsson G, Lunderius-Andersson C. Mast cells as sensors of cell injury through IL-33 recognition. J Immunol. 2011;186:2523–2528. doi: 10.4049/jimmunol.1003383. [DOI] [PubMed] [Google Scholar]
- Essers MA, Offner S, Blanco-Bose WE, Waibler Z, Kalinke U, Duchosal MA, Trumpp A. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature. 2009;458:904–908. doi: 10.1038/nature07815. [DOI] [PubMed] [Google Scholar]
- Felderhoff-Mueser U, Schmidt OI, Oberholzer A, Buhrer C, Stahel PF. IL-18: a key player in neuroinflammation and neurodegeneration? Trends Neurosci. 2005;28:487–493. doi: 10.1016/j.tins.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Fridman WH, Zitvogel L, Sautes-Fridman C, Kroemer G. The immune contexture in cancer prognosis and treatment. Nat Rev Clin Oncol. 2017;14:717–734. doi: 10.1038/nrclinonc.2017.101. [DOI] [PubMed] [Google Scholar]
- Fu AK, Hung KW, Yuen MY, Zhou X, Mak DS, Chan IC, Cheung TH, Zhang B, Fu WY, Liew FY, Ip NY. IL-33 ameliorates Alzheimer's disease-like pathology and cognitive decline. Proc Natl Acad Sci U S A. 2016;113:E2705–2713. doi: 10.1073/pnas.1604032113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JJ, MacLauchlan S, Zuriaga MA, Polackal MN, Ostriker AC, Chakraborty R, Wu CL, Sano S, Muralidharan S, Rius C, et al. Clonal hematopoiesis associated with TET2 deficiency accelerates atherosclerosis development in mice. Science. 2017;355:842–847. doi: 10.1126/science.aag1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- Garber C, Vasek MJ, Vollmer LL, Sun T, Jiang X, Klein RS. Astrocytes decrease adult neurogenesis during virus-induced memory dysfunction via IL-1. Nat Immunol. 2018;19:151–161. doi: 10.1038/s41590-017-0021-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Bottazzi B, Magrini E, Inforzato A, Mantovani A. PTX3, a Humoral Pattern Recognition Molecule, in Innate Immunity, Tissue Repair, and Cancer. Physiol Rev. 2018;98:623–639. doi: 10.1152/physrev.00016.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Riva F, Polentarutti N, Buracchi C, Sironi M, De Bortoli M, Muzio M, Bergottini R, Scanziani E, Vecchi A, et al. Intestinal inflammation in mice deficient in Tir8, an inhibitory member of the IL-1 receptor family. Proc Natl Acad Sci U S A. 2004;101:3522–3526. doi: 10.1073/pnas.0308680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlanda C, Riva F, Veliz T, Polentarutti N, Pasqualini F, Radaelli E, Sironi M, Nebuloni M, Zorini EO, Scanziani E, Mantovani A. Increased susceptibility to colitis-associated cancer of mice lacking TIR8, an inhibitory member of the interleukin-1 receptor family. Cancer Res. 2007;67:6017–6021. doi: 10.1158/0008-5472.CAN-07-0560. [DOI] [PubMed] [Google Scholar]
- Giavazzi R, Garofalo A, Bani MR, Abbate M, Ghezzi P, Boraschi D, Mantovani A, Dejana E. Interleukin 1-induced augmentation of experimental metastases from a human melanoma in nude mice. Cancer Res. 1990;50:4771–4775. [PubMed] [Google Scholar]
- Giavridis T, van der Stegen SJC, Eyquem J, Hamieh M, Piersigilli A, Sadelain M. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat Med. 2018;24:731–738. doi: 10.1038/s41591-018-0041-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov SI, Wang K, Mucida D, Stewart CA, Schnabl B, Jauch D, Taniguchi K, Yu GY, Osterreicher CH, Hung KE, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491:254–258. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulen MF, Kang Z, Bulek K, Youzhong W, Kim TW, Chen Y, Altuntas CZ, Sass BakJensen K, McGeachy MJ, Do JS, et al. The receptor SIGIRR suppresses Th17 cell proliferation via inhibition of the interleukin-1 receptor pathway and mTOR kinase activation. Immunity. 2010;32:54–66. doi: 10.1016/j.immuni.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X, Zhang Y, Zheng L, Zheng C, Song J, Zhang Q, Kang B, Liu Z, Jin L, Xing R, et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat Med. 2018;24:978–985. doi: 10.1038/s41591-018-0045-3. [DOI] [PubMed] [Google Scholar]
- Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity. 2014;40:425–435. doi: 10.1016/j.immuni.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorsen EC, Franks SE, Wadsworth BJ, Harbourne BT, Cederberg RA, Steer CA, Martinez-Gonzalez I, Calder J, Lockwood WW, Bennewith KL. IL-33 increases ST2(+) Tregs and promotes metastatic tumour growth in the lungs in an amphiregulin-dependent manner. Oncoimmunology. 2019;8:e1527497. doi: 10.1080/2162402X.2018.1527497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. The New England journal of medicine. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- Harrison OJ, Srinivasan N, Pott J, Schiering C, Krausgruber T, Ilott NE, Maloy KJ. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3(+) Treg cell function in the intestine. Mucosal Immunol. 2015;8:1226–1236. doi: 10.1038/mi.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D, Remus A, Tzeng TC, et al. NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493:674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickish T, Andre T, Wyrwicz L, Saunders M, Sarosiek T, Kocsis J, Nemecek R, Rogowski W, Lesniewski-Kmak K, Petruzelka L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2017;18:192–201. doi: 10.1016/S1470-2045(17)30006-2. [DOI] [PubMed] [Google Scholar]
- Hong DS, Hui D, Bruera E, Janku F, Naing A, Falchook GS, Piha-Paul S, Wheler JJ, Fu S, Tsimberidou AM, et al. MABp1, a first-in-class true human antibody targeting interleukin-1alpha in refractory cancers: an open-label, phase 1 dose-escalation and expansion study. Lancet Oncol. 2014;15:656–666. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- Hong DS, Janku F, Naing A, Falchook GS, Piha-Paul S, Wheler JJ, Fu S, Tsimberidou AM, Stecher M, Mohanty P, et al. Xilonix, a novel true human antibody targeting the inflammatory cytokine interleukin-1 alpha, in non-small cell lung cancer. Invest New Drugs. 2015;33:621–631. doi: 10.1007/s10637-015-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J, Kim S, Lin PC. Interleukin-33 and ST2 Signaling in Tumor Microenvironment. J Interferon Cytokine Res. 2018;39:61–71. doi: 10.1089/jir.2018.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Shao M, Chen Y, Zhou J, Qian J, Xu L, Ma H, Wang X, Xu Y, Lu D, Shen H. Allele 2 of the interleukin-1 receptor antagonist gene (IL1RN*2) is associated with a decreased risk of primary lung cancer. Cancer Lett. 2006;236:269–275. doi: 10.1016/j.canlet.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Huang Y, Smith DE, Ibanez-Sandoval O, Sims JE, Friedman WJL. Neuron-specific effects of interleukin-1beta are mediated by a novel isoform of the IL-1 receptor accessory protein. J Neurosci. 2011;31:18048–18059. doi: 10.1523/JNEUROSCI.4067-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikutani M, Yanagibashi T, Ogasawara M, Tsuneyama K, Yamamoto S, Hattori Y, Kouro T, Itakura A, Nagai Y, Takaki S, Takatsu K. Identification of innate IL-5-producing cells and their role in lung eosinophil regulation and antitumor immunity. J Immunol. 2012;188:703–713. doi: 10.4049/jimmunol.1101270. [DOI] [PubMed] [Google Scholar]
- Isambert N, Hervieu A, Rebe C, Hennequin A, Borg C, Zanetta S, Chevriaux A, Richard C, Derangere V, Limagne E, et al. Fluorouracil and bevacizumab plus anakinra for patients with metastatic colorectal cancer refractory to standard therapies (IRAFU): a single-arm phase 2 study. Oncoimmunology. 2018;7:e1474319. doi: 10.1080/2162402X.2018.1474319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiswal S, Natarajan P, Silver AJ, Gibson CJ, Bick AG, Shvartz E, McConkey M, Gupta N, Gabriel S, Ardissino D, et al. Clonal Hematopoiesis and Risk of Atherosclerotic Cardiovascular Disease. N Engl J Med. 2017;377:111–121. doi: 10.1056/NEJMoa1701719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z, Lei L, Lin D, Liu Y, Song Y, Gong H, Zhu Y, Mei Y, Hu B, Wu Y, et al. IL-33 Released in the Liver Inhibits Tumor Growth via Promotion of CD4(+) and CD8(+) T Cell Responses in Hepatocellular Carcinoma. J Immunol. 2018;201:3770–3779. doi: 10.4049/jimmunol.1800627. [DOI] [PubMed] [Google Scholar]
- Kanni T, Argyropoulou M, Spyridopoulos T, Pistiki A, Stecher M, Dinarello CA, Simard J, Giamarellos-Bourboulis EJ. MABp1 Targeting IL-1alpha for Moderate to Severe Hidradenitis Suppurativa Not Eligible for Adalimumab: A Randomized Study. J Invest Dermatol. 2018;138:795–801. doi: 10.1016/j.jid.2017.10.030. [DOI] [PubMed] [Google Scholar]
- Kaplanov I, Carmi Y, Kornetsky R, Shemesh A, Shurin GV, Shurin MR, Dinarello CA, Voronov E, Apte RN. Blocking IL-1beta reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci U S A. 2019;116:1361–1369. doi: 10.1073/pnas.1812266115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplanski G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunol Rev. 2018;281:138–153. doi: 10.1111/imr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazim K, Azulay EE, Kristal B, Cohen I. Interleukin 1 gene polymorphism and susceptibility to disease. Immunol Rev. 2018;281:40–56. doi: 10.1111/imr.12620. [DOI] [PubMed] [Google Scholar]
- Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S, Dinarello CA. The interleukin-1alpha precursor is biologically active and is likely a key alarmin in the IL-1 family of cytokines. Front Immunol. 2013;4:391. doi: 10.3389/fimmu.2013.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Evidence for IL-1 receptor blockade as a therapeutic strategy for the treatment of depression. Curr Opin Investig Drugs. 2009;10:664–671. [PMC free article] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Kovtonyuk LV, Fritsch K, Feng X, Manz MG, Takizawa H. Inflamm-Aging of Hematopoiesis, Hematopoietic Stem Cells, and the Bone Marrow Microenvironment. Front Immunol. 2016;7:502. doi: 10.3389/fimmu.2016.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuan EL, Ziegler SF. A tumor-myeloid cell axis, mediated via the cytokines IL-1alpha and TSLP, promotes the progression of breast cancer. Nat Immunol. 2018;19:366–374. doi: 10.1038/s41590-018-0066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz J, Franz K. Innate defence: evidence for memory in invertebrate immunity. Nature. 2003;425:37–38. doi: 10.1038/425037a. [DOI] [PubMed] [Google Scholar]
- Kurzrock R, Hickish T, Wyrwicz L, Saunders M, Wu Q, Stecher M, Mohanty P, Dinarello CA, Simard J. Interleukin-1 receptor antagonist levels predict favorable outcome after bermekimab, a first-in-class true human interleukin-1alpha antibody, in a phase III randomized study of advanced colorectal cancer. Oncoimmunology. 2019;8 doi: 10.1080/2162402X.2018.1551651. 1551651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalor SJ, Dungan LS, Sutton CE, Basdeo SA, Fletcher JM, Mills KH. Caspase-1-processed cytokines IL-1beta and IL-18 promote IL-17 production by gammadelta and CD4 T cells that mediate autoimmunity. J Immunol. 2011;186:5738–5748. doi: 10.4049/jimmunol.1003597. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Thaiss CA, Zeevi D, Dohnalova L, Zilberman-Schapira G, Mahdi JA, David E, Savidor A, Korem T, Herzig Y, et al. Microbiota-Modulated Metabolites Shape the Intestinal Microenvironment by Regulating NLRP6 Inflammasome Signaling. Cell. 2015;163:1428–1443. doi: 10.1016/j.cell.2015.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wang W, Lv Z, Li Y, Chen Y, Huang K, Corrigan CJ, Ying S. Elevated Expression of IL-33 and TSLP in the Airways of Human Asthmatics In Vivo: A Potential Biomarker of Severe Refractory Disease. J Immunol. 2018;200:2253–2262. doi: 10.4049/jimmunol.1701455. [DOI] [PubMed] [Google Scholar]
- Libby P. Interleukin-1 Beta as a Target for Atherosclerosis Therapy: Biological Basis of CANTOS and Beyond. J Am Coll Cardiol. 2017;70:2278–2289. doi: 10.1016/j.jacc.2017.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- Lind H, Zienolddiny S, Ryberg D, Skaug V, Phillips DH, Haugen A. Interleukin 1 receptor antagonist gene polymorphism and risk of lung cancer: a possible interaction with polymorphisms in the interleukin 1 beta gene. Lung Cancer. 2005;50:285–290. doi: 10.1016/j.lungcan.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Ling J, Kang Y, Zhao R, Xia Q, Lee DF, Chang Z, Li J, Peng B, Fleming JB, Wang H, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Wang Y, Ding Y, Baez I, Payne KJ, Borghesi L. Cutting Edge: Hematopoietic Stem Cell Expansion and Common Lymphoid Progenitor Depletion Require Hematopoietic-Derived, Cell-Autonomous TLR4 in a Model of Chronic Endotoxin. J Immunol. 2015;195:2524–2528. doi: 10.4049/jimmunol.1501231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Nemeth DP, McKim DB, Zhu L, DiSabato DJ, Berdysz O, Gorantla G, Oliver B, Witcher KG, Wang Y, et al. Cell-Type-Specific Interleukin 1 Receptor 1 Signaling in the Brain Regulates Distinct Neuroimmune Activities. Immunity. 2019 doi: 10.1016/j.immuni.2018.12.012. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Quan N. Microglia and CNS Interleukin-1: Beyond Immunological Concepts. Front Neurol. 2018;9:8. doi: 10.3389/fneur.2018.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locati M, Mantovani A, Sica A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv Immunol. 2013;120:163–184. doi: 10.1016/B978-0-12-417028-5.00006-5. [DOI] [PubMed] [Google Scholar]
- Longman RS, Diehl GE, Victorio DA, Huh JR, Galan C, Miraldi ER, Swaminath A, Bonneau R, Scherl EJ, Littman DR. CX(3)CR1(+) mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Exp Med. 2014;211:1571–1583. doi: 10.1084/jem.20140678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopetuso LR, Chowdhry S, Pizarro TT. Opposing Functions of Classic and Novel IL-1 Family Members in Gut Health and Disease. Front Immunol. 2013;4:181. doi: 10.3389/fimmu.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopetuso LR, De Salvo C, Pastorelli L, Rana N, Senkfor HN, Petito V, Di Martino L, Scaldaferri F, Gasbarrini A, Cominelli F, et al. IL-33 promotes recovery from acute colitis by inducing miR-320 to stimulate epithelial restitution and repair. Proc Natl Acad Sci U S A. 2018;115:E9362–E9370. doi: 10.1073/pnas.1803613115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lust JA, Lacy MQ, Zeldenrust SR, Dispenzieri A, Gertz MA, Witzig TE, Kumar S, Hayman SR, Russell SJ, Buadi FK, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84:114–122. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lust JA, Lacy MQ, Zeldenrust SR, Witzig TE, Moon-Tasson LL, Dinarello CA, Donovan KA. Reduction in C-reactive protein indicates successful targeting of the IL-1/IL-6 axis resulting in improved survival in early stage multiple myeloma. Am J Hematol. 2016;91:571–574. doi: 10.1002/ajh.24352. [DOI] [PubMed] [Google Scholar]
- Madera S, Sun JC. Cutting edge: stage-specific requirement of IL-18 for antiviral NK cell expansion. J Immunol. 2015;194:1408–1412. doi: 10.4049/jimmunol.1402001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Malykhin A, Teague-Weber BN, Sun XH, Farris AD, Coggeshall KM. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6.Sle1.Yaa animals. Blood. 2009;113:4534–4540. doi: 10.1182/blood-2008-12-192559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A. Redundancy and robustness versus division of labour and specialization in innate immunity. Semin Immunol. 2018;36:28–30. doi: 10.1016/j.smim.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Bussolino F, Dejana E. Cytokine regulation of endothelial cell function. FASEB J. 1992;6:2591–2599. doi: 10.1096/fasebj.6.8.1592209. [DOI] [PubMed] [Google Scholar]
- Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302–314. doi: 10.1038/nri3660. [DOI] [PubMed] [Google Scholar]
- Marhaba R, Nazarenko I, Knofler D, Reich E, Voronov E, Vitacolonna M, Hildebrand D, Elter E, Apte RN, Zoller M. Opposing effects of fibrosarcoma cell-derived IL-1 alpha and IL-1 beta on immune response induction. Int J Cancer. 2008;123:134–145. doi: 10.1002/ijc.23503. [DOI] [PubMed] [Google Scholar]
- McDonald JJ, McMillan DC, Laird BJA. Targeting IL-1alpha in cancer cachexia: a narrative review. Curr Opin Support Palliat Care. 2018;12:453–459. doi: 10.1097/SPC.0000000000000398. [DOI] [PubMed] [Google Scholar]
- Medina-Contreras O, Harusato A, Nishio H, Flannigan KL, Ngo V, Leoni G, Neumann PA, Geem D, Lili LN, Ramadas RA, et al. Cutting Edge: IL-36 Receptor Promotes Resolution of Intestinal Damage. J Immunol. 2016;196:34–38. doi: 10.4049/jimmunol.1501312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melisi D, Niu J, Chang Z, Xia Q, Peng B, Ishiyama S, Evans DB, Chiao PJ. Secreted interleukin-1alpha induces a metastatic phenotype in pancreatic cancer by sustaining a constitutive activation of nuclear factor-kappaB. Mol Cancer Res. 2009;7:624–633. doi: 10.1158/1541-7786.MCR-08-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KH, Dungan LS, Jones SA, Harris J. The role of inflammasome-derived IL-1 in driving IL-17 responses. J Leukoc Biol. 2013;93:489–497. doi: 10.1189/jlb.1012543. [DOI] [PubMed] [Google Scholar]
- Mitroulis I, Ruppova K, Wang B, Chen LS, Grzybek M, Grinenko T, Eugster A, Troullinaki M, Palladini A, Kourtzelis I, et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell. 2018;172:147–161 e112. doi: 10.1016/j.cell.2017.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, von Mutius E, Farrall M, Lathrop M, Cookson W, Consortium, G A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–1221. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgora M, Bonavita E, Ponzetta A, Riva F, Barbagallo M, Jaillon S, Popovic B, Bernardini G, Magrini E, Gianni F, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature. 2017;551:110–114. doi: 10.1038/nature24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molgora M, Supino D, Mantovani A, Garlanda C. Tuning inflammation and immunity by the negative regulators IL-1R2 and IL-1R8. Immunol Rev. 2018;281:233–247. doi: 10.1111/imr.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AB, Nussbaum JC, Liang HE, Van Dyken SJ, Cheng LE, Mohapatra A, Chawla A, Locksley RM. Innate lymphoid type 2 cells sustain visceral adipose tissue eosinophils and alternatively activated macrophages. J Exp Med. 2013;210:535–549. doi: 10.1084/jem.20121964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monticelli LA, Sonnenberg GF, Abt MC, Alenghat T, Ziegler CG, Doering TA, Angelosanto JM, Laidlaw BJ, Yang CY, Sathaliyawala T, et al. Innate lymphoid cells promote lung-tissue homeostasis after infection with influenza virus. Nat Immunol. 2011;12:1045–1054. doi: 10.1031/ni.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorlag S, Roring RJ, Joosten LAB, Netea MG. The role of the interleukin-1 family in trained immunity. Immunol Rev. 2018;281:28–39. doi: 10.1111/imr.12617. [DOI] [PubMed] [Google Scholar]
- Mora-Buch R, Dotti I, Planell N, Calderon-Gomez E, Jung P, Masamunt MC, Llach J, Ricart E, Batlle E, Panes J, Salas A. Epithelial IL-1R2 acts as a homeostatic regulator during remission of ulcerative colitis. Mucosal Immunol. 2016;9:950–959. doi: 10.1038/mi.2015.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moro K, Yamada T, Tanabe M, Takeuchi T, Ikawa T, Kawamoto H, Furusawa J, Ohtani M, Fujii H, Koyasu S. Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature. 2010;463:540–544. doi: 10.1038/nature08636. [DOI] [PubMed] [Google Scholar]
- Motomura Y, Morita H, Moro K, Nakae S, Artis D, Endo TA, Kuroki Y, Ohara O, Koyasu S, Kubo M. Basophil-derived interleukin-4 controls the function of natural helper cells, a member of ILC2s, in lung inflammation. Immunity. 2014;40:758–771. doi: 10.1016/j.immuni.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Nakanishi K. Unique Action of Interleukin-18 on T Cells and Other Immune Cells. Front Immunol. 2018;9:763. doi: 10.3389/fimmu.2018.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Joosten LA, Latz E, Mills KH, Natoli G, Stunnenberg HG, O'Neill LA, Xavier RJ. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352:aaf1098. doi: 10.1126/science.aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nold-Petry CA, Lo CY, Rudloff I, Elgass KD, Li S, Gantier MP, Lotz-Havla AS, Gersting SW, Cho SX, Lao JC, et al. IL-37 requires the receptors IL-18Ralpha and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat Immunol. 2015;16:354–365. doi: 10.1038/ni.3103. [DOI] [PubMed] [Google Scholar]
- Norelli M, Camisa B, Barbiera G, Falcone L, Purevdorj A, Genua M, Sanvito F, Ponzoni M, Doglioni C, Cristofori P, et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat Med. 2018;24:739–748. doi: 10.1038/s41591-018-0036-4. [DOI] [PubMed] [Google Scholar]
- Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- Nowarski R, Jackson R, Gagliani N, de Zoete MR, Palm NW, Bailis W, Low JS, Harman CC, Graham M, Elinav E, Flavell RA. Epithelial IL-18 Equilibrium Controls Barrier Function in Colitis. Cell. 2015;163:1444–1456. doi: 10.1016/j.cell.2015.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohne Y, Silver JS, Thompson-Snipes L, Collet MA, Blanck JP, Cantarel BL, Copenhaver AM, Humbles AA, Liu YJ. IL-1 is a critical regulator of group 2 innate lymphoid cell function and plasticity. Nat Immunol. 2016;17:646–655. doi: 10.1038/ni.3447. [DOI] [PubMed] [Google Scholar]
- Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Pan W, Zhu S, Qu K, Meeth K, Cheng J, He K, Ma H, Liao Y, Wen X, Roden C, et al. The DNA Methylcytosine Dioxygenase Tet2 Sustains Immunosuppressive Function of Tumor-Infiltrating Myeloid Cells to Promote Melanoma Progression. Immunity. 2017;47:284–297 e285. doi: 10.1016/j.immuni.2017.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pecaric-Petkovic T, Didichenko SA, Kaempfer S, Spiegl N, Dahinden CA. Human basophils and eosinophils are the direct target leukocytes of the novel IL-1 family member IL-33. Blood. 2009;113:1526–1534. doi: 10.1182/blood-2008-05-157818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peri G, Introna M, Corradi D, Iacuitti G, Signorini S, Avanzini F, Pizzetti F, Maggioni AP, Moccetti T, Metra M, et al. PTX3, A prototypical long pentraxin, is an early indicator of acute myocardial infarction in humans. Circulation. 2000;102:636–641. doi: 10.1161/01.cir.102.6.636. [DOI] [PubMed] [Google Scholar]
- Pietras EM, Mirantes-Barbeito C, Fong S, Loeffler D, Kovtonyuk LV, Zhang S, Lakshminarasimhan R, Chin CP, Techner JM, Will B, et al. Chronic interleukin-1 exposure drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nat Cell Biol. 2016;18:607–618. doi: 10.1038/ncb3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plitas G, Konopacki C, Wu K, Bos PD, Morrow M, Putintseva EV, Chudakov DM, Rudensky AY. Regulatory T Cells Exhibit Distinct Features in Human Breast Cancer. Immunity. 2016;45:1122–1134. doi: 10.1016/j.immuni.2016.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic B, Golemac M, Podlech J, Zeleznjak J, Bilic-Zulle L, Lukic ML, Cicin-Sain L, Reddehase MJ, Sparwasser T, Krmpotic A, Jonjic S. IL-33/ST2 pathway drives regulatory T cell dependent suppression of liver damage upon cytomegalovirus infection. PLoS Pathog. 2017;13:e1006345. doi: 10.1371/journal.ppat.1006345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017a;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin-1beta inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet. 2017b;390:1833–1842. doi: 10.1016/S0140-6736(17)32247-X. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM., Jr Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- Rivers-Auty J, Daniels MJD, Colliver I, Robertson DL, Brough D. Redefining the ancestral origins of the interleukin-1 superfamily. Nat Commun. 2018;9 doi: 10.1038/s41467-018-03362-1. 1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood. 2012;120:4751–4760. doi: 10.1182/blood-2012-04-419283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell SE, Stefanska AM, Kubica M, Horan RM, Mantovani A, Garlanda C, Fallon PG, Walsh PT. Toll IL-1R8/Single Ig IL-1-Related Receptor Regulates Psoriasiform Inflammation through Direct Inhibition of Innate IL-17A Expression by gammadelta T Cells. J Immunol. 2013;191:3337–3346. doi: 10.4049/jimmunol.1300828. [DOI] [PubMed] [Google Scholar]
- Santarlasci V, Cosmi L, Maggi L, Liotta F, Annunziato F. IL-1 and T Helper Immune Responses. Front Immunol. 2013;4:182. doi: 10.3389/fimmu.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarhan D, Hippen KL, Lemire A, Hying S, Luo X, Lenvik T, Curtsinger J, Davis Z, Zhang B, Cooley S, et al. Adaptive NK Cells Resist Regulatory T-cell Suppression Driven by IL37. Cancer Immunol Res. 2018;6:766–775. doi: 10.1158/2326-6066.CIR-17-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Onai N, Yoshihara H, Arai F, Suda T, Ohteki T. Interferon regulatory factor-2 protects quiescent hematopoietic stem cells from type I interferon-dependent exhaustion. Nat Med. 2009;15:696–700. doi: 10.1038/nm.1973. [DOI] [PubMed] [Google Scholar]
- Schenten D, Nish SA, Yu S, Yan X, Lee HK, Brodsky I, Pasman L, Yordy B, Wunderlich FT, Bruning JC, et al. Signaling through the adaptor molecule MyD88 in CD4+ T cells is required to overcome suppression by regulatory T cells. Immunity. 2014;40:78–90. doi: 10.1016/j.immuni.2013.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiering C, Krausgruber T, Chomka A, Frohlich A, Adelmann K, Wohlfert EA, Pott J, Griseri T, Bollrath J, Hegazy AN, et al. The alarmin IL-33 promotes regulatory T-cell function in the intestine. Nature. 2014;513:564–568. doi: 10.1038/nature13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11:264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- Shi Y, Evans JE, Rock KL. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425:516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- Shih HY, Sciume G, Mikami Y, Guo L, Sun HW, Brooks SR, Urban JF, Jr, Davis FP, Kanno Y, O'Shea JJ. Developmental Acquisition of Regulomes Underlies Innate Lymphoid Cell Functionality. Cell. 2016;165:1120–1133. doi: 10.1016/j.cell.2016.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhal G, Jaehne EJ, Corrigan F, Toben C, Baune BT. Inflammasomes in neuroinflammation and changes in brain function: a focused review. Front Neurosci. 2014;8:315. doi: 10.3389/fnins.2014.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50:812–820. doi: 10.1136/gut.50.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth MJ, Swann J, Kelly JM, Cretney E, Yokoyama WM, Diefenbach A, Sayers TJ, Hayakawa Y. NKG2D recognition and perforin effector function mediate effective cytokine immunotherapy of cancer. J Exp Med. 2004;200:1325–1335. doi: 10.1084/jem.20041522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Voronov E, Dvorkin T, Fima E, Cagnano E, Benharroch D, Shendler Y, Bjorkdahl O, Segal S, Dinarello CA, Apte RN. Differential effects of IL-1 alpha and IL-1 beta on tumorigenicity patterns and invasiveness. J Immunol. 2003;171:6448–6456. doi: 10.4049/jimmunol.171.12.6448. [DOI] [PubMed] [Google Scholar]
- Steensma DP, Bejar R, Jaiswal S, Lindsley RC, Sekeres MA, Hasserjian RP, Ebert BL. Clonal hematopoiesis of indeterminate potential and its distinction from myelodysplastic syndromes. Blood. 2015;126:9–16. doi: 10.1182/blood-2015-03-631747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC. A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med. 2006;203:1685–1691. doi: 10.1084/jem.20060285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Takizawa H, Boettcher S, Manz MG. Demand-adapted regulation of early hematopoiesis in infection and inflammation. Blood. 2012;119:2991–3002. doi: 10.1182/blood-2011-12-380113. [DOI] [PubMed] [Google Scholar]
- Tartey S, Gurung P, Samir P, Burton A, Kanneganti TD. Dysregulated CARD9 signaling in neutrophils drives inflammation in a mouse model of neutrophilic dermatoses. J Immunol. 2018;201:1639–1644. doi: 10.4049/jimmunol.1800760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjomsland V, Bojmar L, Sandstrom P, Bratthall C, Messmer D, Spangeus A, Larsson M. IL-1alpha expression in pancreatic ductal adenocarcinoma affects the tumor cell migration and is regulated by the p38MAPK signaling pathway. PLoS One. 2013;8:e70874. doi: 10.1371/journal.pone.0070874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjota MY, Williams JW, Lu T, Clay BS, Byrd T, Hrusch CL, Decker DC, de Araujo CA, Bryce PJ, Sperling AI. IL-33-dependent induction of allergic lung inflammation by FcgammaRIII signaling. J Clin Invest. 2013;123:2287–2297. doi: 10.1172/JCI63802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasoni R, Morini R, Lopez-Atalaya JP, Corradini I, Canzi A, Rasile M, Mantovani C, Pozzi D, Garlanda C, Mantovani A, et al. Lack of IL-1R8 in neurons causes hyperactivation of IL-1 receptor pathway and induces MECP2-dependent synaptic defects. Elife. 2017;6:e21735. doi: 10.7554/eLife.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Prieto GA, Kramar EA, Smith ED, Cribbs DH, Lynch G, Cotman CW. Brain-derived neurotrophic factor-dependent synaptic plasticity is suppressed by interleukin-1beta via p38 mitogen-activated protein kinase. J Neurosci. 2012;32:17714–17724. doi: 10.1523/JNEUROSCI.1253-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Nakanishi K, Matsui K, Higashino K, Okamura H, Miyazawa Y, Kaneda K. IFN-gamma-inducing factor up-regulates Fas ligand-mediated cytotoxic activity of murine natural killer cell clones. J Immunol. 1996;157:3967–3973. [PubMed] [Google Scholar]
- Tzanetakou V, Kanni T, Giatrakou S, Katoulis A, Papadavid E, Netea MG, Dinarello CA, van der Meer JW, Rigopoulos D, Giamarellos-Bourboulis EJ. Safety and efficacy of anakinra in severe hidradenitis suppurativa: a randomized clinical trial. JAMA Dermatol. 2016;152:52–59. doi: 10.1001/jamadermatol.2015.3903. [DOI] [PubMed] [Google Scholar]
- Udalova IA, Mantovani A, Feldmann M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat Rev Rheumatol. 2016;12:472–485. doi: 10.1038/nrrheum.2016.91. [DOI] [PubMed] [Google Scholar]
- Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, Yu J, Perez-Torres C, Frouin A, Wilton DK, et al. A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016;534:538–543. doi: 10.1038/nature18283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegran F, Berger H, Boidot R, Mignot G, Bruchard M, Dosset M, Chalmin F, Rebe C, Derangere V, Ryffel B, et al. The transcription factor IRF1 dictates the IL-21-dependent anticancer functions of TH9 cells. Nat Immunol. 2014;15:758–766. doi: 10.1038/ni.2925. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Moneta D, Conti M, Richichi C, Ravizza T, De Luigi A, De Simoni MG, Sperk G, Andell-Jonsson S, Lundkvist J, et al. Powerful anticonvulsant action of IL-1 receptor antagonist on intracerebral injection and astrocytic overexpression in mice. Proc Natl Acad Sci U S A. 2000;97:11534–11539. doi: 10.1073/pnas.190206797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal-Vanaclocha F, Amezaga C, Asumendi A, Kaplanski G, Dinarello CA. Interleukin-1 receptor blockade reduces the number and size of murine B16 melanoma hepatic metastases. Cancer Res. 1994;54:2667–2672. [PubMed] [Google Scholar]
- Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, et al. Innate Lymphoid Cells: 10 Years On. Cell. 2018;174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Natl Acad Sci USA. 2003;100:2645–2650. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YH, Lin HQ, Li H, Zhao Y, Lui VWY, Chen L, Wu XM, Sun W, Wen WP. Stromal interleukin-33 promotes regulatory T cell-mediated immunosuppression in head and neck squamous cell carcinoma and correlates with poor prognosis. Cancer Immunol Immunother. 2019;68:221–232. doi: 10.1007/s00262-018-2265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley A, Becerra C, McCollum D, Paulson AS, Goel A. A pilot, non-randomized evaluation of the safety of anakinra plus FOLFIRINOX in metastatic pancreatic ductal adenocarcinoma patients. J Clin Oncol. 2016;34(suppl):e165750. [Google Scholar]
- Wong R, Lenart N, Hill L, Toms L, Coutts G, Martinecz B, Csaszar E, Nyiri G, Papaemmanouil A, Waisman A, et al. Interleukin-1 mediates ischaemic brain injury via distinct actions on endothelial cells and cholinergic neurons. Brain Behav Immun. 2019;76:126–138. doi: 10.1016/j.bbi.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T-C, Xu K, Martinek J, Young RR, Banchereau R, George J, Turner J, KIM KI, Zurawski S, Wang X, et al. IL-1 receptor antagonist controls transcriptional signature of inflammation in patients with metastatic breast cancer. Cancer Research. 2018;78:5243–5258. doi: 10.1158/0008-5472.CAN-18-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H, et al. The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity. 2007;26:461–475. doi: 10.1016/j.immuni.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Zaiss MM, Maslowski KM, Mosconi I, Guenat N, Marsland BJ, Harris NL. IL-1beta suppresses innate IL-25 and IL-33 production and maintains helminth chronicity. PLoS Pathog. 2013;9:e1003531. doi: 10.1371/journal.ppat.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bulek K, Gulen MF, Zepp JA, Karagkounis G, Martin BN, Zhou H, Yu M, Liu X, Huang E, et al. Human Colon Tumors Express a Dominant-Negative Form of SIGIRR That Promotes Inflammation and Colitis-Associated Colon Cancer in Mice. Gastroenterology. 2015;149:1860–1871 e1868. doi: 10.1053/j.gastro.2015.08.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1alpha, controlling necrosis-induced sterile inflammation. Immunity. 2013;38:285–295. doi: 10.1016/j.immuni.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Zhuang Z, Ju HQ, Aguilar M, Gocho T, Li H, Iida T, Lee H, Fan X, Zhou H, Ling J, et al. IL1 receptor antagonist inhibits pancreatic cancer growth by abrogating NF-kappaB activation. Clin Cancer Res. 2016;22:1432–1444. doi: 10.1158/1078-0432.CCR-14-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zienolddiny S, Ryberg D, Maggini V, Skaug V, Canzian F, Haugen A. Polymorphisms of the interleukin-1 beta gene are associated with increased risk of non-small cell lung cancer. Int J Cancer. 2004;109:353–356. doi: 10.1002/ijc.11695. [DOI] [PubMed] [Google Scholar]