Abstract
Within Southern California, east Pacific green sea turtles (Chelonia mydas) forage year-round, taking advantage of diverse food resources, including seagrass, marine algae, and invertebrates. Assessing persistent organic pollutants (POP) in green turtle aggregations in the Seal Beach National Wildlife Refuge (SBNWR, n = 17) and San Diego Bay (SDB, n = 25) can help quantify contamination risks for these populations. Blood plasma was analyzed for polychlorinated biphenyls (PCBs), organochlorinated pesticides (OCPs), and polybrominated diphenyl ethers (PBDEs). PCBs and body size explained much of the separation of turtles by foraging aggregation in a principal component analysis. Turtles from SDB had significantly (p <0.001) higher total PCBs than SBNWR turtles. Most PCBs detected in turtles were non-dioxin-like PCB congeners (153, 138, 99) that are associated with neurotoxicity. Recaptured turtles’ POP levels changed significantly over time indicating significant variation in POP levels through time and space, even among adjacent foraging locations.
Keywords: Chelonia mydas, persistent organic pollutants, polychlorinated biphenyls, organochlorinated pesticides, urbanized habitats, marine turtles
Introduction
Marine species, such as sea turtles, have been negatively impacted by anthropogenic activities, and as a result many sea turtle populations are considered threatened or endangered (Gardner and Oberdorster, 2005; Hamann et al., 2010; Pugh and Becker, 2001). Historically, threats such as overharvesting and habitat loss have severely impacted sea turtle populations, but conservation efforts have initiated recovery for some populations around the world (Hamann et al., 2010; Mazaris et al., 2017; Seminoff et al., 2015). Additional threats, such as pollution, continue to impact marine waterways that sea turtles inhabit (Hamann et al., 2010). Human activities, such as manufacturing, agriculture, and electronic waste produce a variety of anthropogenic contaminants that commonly enter the environment surrounding urban areas that are known to cause cancer, reproductive and immune system impairments across a range of wildlife (Carpenter, 2006; Darnerud et al., 2001; Lohmann et al., 2007; Sindermann, 2005). In particular, large coastal cities can heavily pollute surrounding coastal and estuarine habitats (Sindermann, 2005). Because some sea turtle species, such as green sea turtles (Chelonia mydas), demonstrate high site fidelity and extended residency in foraging habitats (Lutz et al., 2002), sea turtles that inhabit these urbanized coastal foraging grounds have higher risk of exposure to anthropogenic pollutants and can accumulate POPs over time serving as bioindicators for urban habitats (Finlayson et al., 2016; Hamann et al., 2010; Keller, 2013).
Persistent organic pollutants (POPs), one group of anthropogenic contaminants, are made for many industrial and agricultural purposes and are introduced into the environment via urban and agricultural runoff (Flynn and Kleiman, 1997; Gray, 1997; Jones and de Voogt, 1999). POPs include compounds such as polychlorinated biphenyls (PCBs), organochlorinated pesticides (OCPs), and polybrominated diphenyl ethers (PBDEs). Due to their stability in the environment and accumulation in wildlife, PCBs and OCPs are still found in the environment despite being banned in the 1970s (Jones and de Voogt, 1999; Weber et al., 2008). Because of their prevalence and persistence in the environment, there are still numerous questions about the exposure and potential health impacts of POP accumulation in marine species.
Research into the potential effects of PCBs, OCPs, and PBDEs on sea turtle health has increased in recent years (Finlayson et al., 2016; Gardner and Oberdorster, 2005; Keller, 2013; Pugh and Becker, 2001). For example, in vitro DDT exposure has been shown to alter testosterone hormone binding in blood plasma obtained from nesting green sea turtles in Malaysia, suggesting that DDT may disrupt sex steroid-protein binding in green sea turtle blood (Ikonomopoulou et al., 2009). Research on nesting turtles in Malaysia found that hatchlings with higher POP concentrations were smaller in size, which may indicate reduced survival (van de Merwe et al., 2010b). Leatherback sea turtle eggs in Costa Rica found that higher POP concentrations were correlated to lower hatching success (De Andrés et al., 2016). Experiments with an immortal sea turtle testis cell line was found to have increased cytochrome 450 aromatase activity following exposure to POPs, indicating sex steroid alterations (Keller and McClellan-Green, 2004). These studies indicate that POPs may competitively inhibit sex hormone binding, affect hatchling survival, and impair immune function through depressed or increased white blood cell count in sea turtles (De Andrés et al., 2016; Keller and McClellan-Green, 2004; Komoroske et al., 2011; Stewart et al., 2011; van de Merwe et al., 2010b). By quantifying POP accumulation in urban green turtle foraging aggregations, wildlife and endangered species managers can assess potential exposure risks and population recovery strategies.
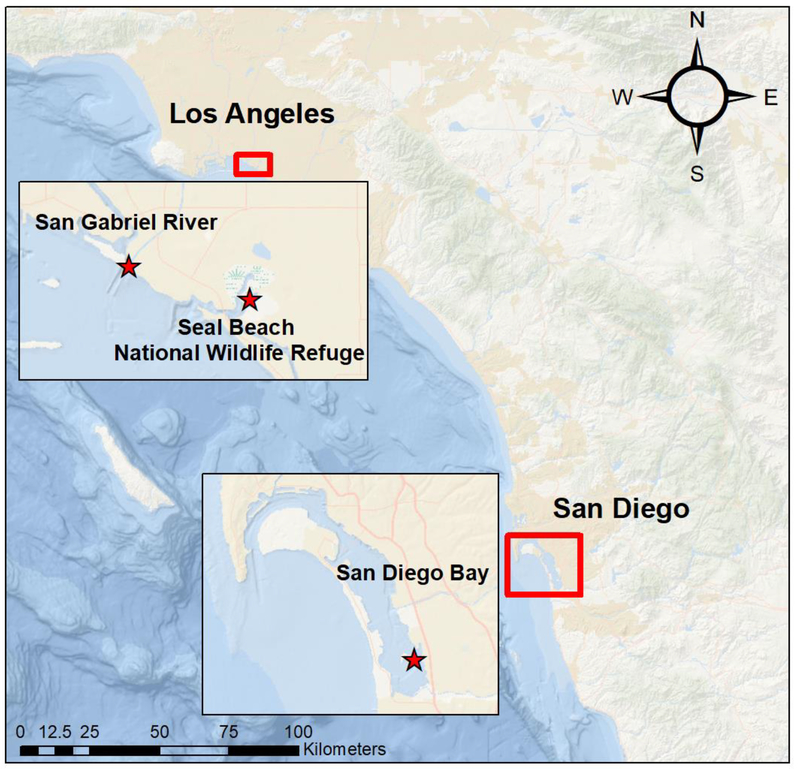
In California, USA, year-round foraging aggregations of green sea turtles are found in several bays and estuaries from Los Angeles to San Diego (Crear et al., 2017, 2016; Eguchi et al., 2010; MacDonald et al., 2012). These locations are highlyurbanized areas known to have POPs present in sediment and wildlife through previous research and sediment analysis (Dodder et al., 2016; Lyons et al., 2014; Lyons and Lowe, 2015; Schiff et al., 2011). Prior research has shown that green sea turtles in San Diego Bay have detectable levels of POPs above the no effect threshold for immunological impairment (e.g., altered lymphocyte production and cytokine gene expression) (Komoroske et al., 2011; Lewison et al., 2011). A growing population of green sea turtles has been identified in the Long Beach/Los Angeles area within the Seal Beach National Wildlife Refuge (SBNWR)and San Gabriel River, which provides heat effluent water allowing year around use of this foraging habitat (Crear et al., 2017, 2016). These SDB and SBNWR green sea turtles provide an opportunity to compare these two subpopulations that inhabit two distinct urban areas.
Considering distance and differences in exposure to pollutants between the two locations, the current study hypothesizes that POPs will differ between green sea turtles from SBNWR and SDB. To assess POP concentration patterns, blood plasma samples will be used to measure POP concentrations in green sea turtles caught in SBNWR and SDB. Previous studies have used lipid-rich tissues, such as liver and adipose, to quantify body burden (Rozman and Klaassen, 2007) of POPs in green sea turtles. However, blood samples have been shown to correlate with POP contamination in lipid-dense organs, albeit at lower concentrations, and thus may provide a non-invasive sampling alternative for measuring POP exposure and bioaccumulation (Finlayson et al., 2016; Gardner and Oberdorster, 2005). This study aims to assess if location-based pollutant signatures are present, how pollutant signatures differ between foraging aggregations, and compare the results with other studies in green turtle POP accumulation.
Methods
Study Sites
San Diego Bay (32° 36’ 54” N, 117° 6’ 4” W; SDB) is a natural bay that contains salt marsh, eelgrass bed, mud/tidal flat habitat. SDB’s coastline is heavily urbanized with homes, military bases, harbors, and shipyard activity throughout the bay (Figure 4). Green sea turtles have inhabited these waters for a long period of time feeding on the eelgrass beds within the bay (MacDonald et al., 2012; McDonald et al., 1994). SDB adult greens nest and are from the genetic stock of the Revillagigedo Islands and Michoacan, Mexico (Dutton et al., 2019). The SDB green turtle foraging aggregation has been studied regularly with capture/recapture and monitoring taking place within the bay since 1990 (Eguchi et al., 2010).
Figure 4:
Capture locations (red stars) for green sea turtles inhabiting the Los Angeles area (top square) and the San Diego area (bottom square) within Southern California, USA. Top square shows the San Gabriel River (left star) and the Seal Beach National Wildlife Refuge (right star); bottom square shows San Diego Bay (bottom star).
The Seal Beach National Wildlife Refuge (33° 44’ 07” N, 118° 03’ 52” W; SBNWR) is a wetland area within the Anaheim Bay estuary (Figure 4). SBNWR estuary contains natural habitat as well as restored habitat that was constructed as part of a restoration project. Within the restored habitat are a series of channels and basins that house eelgrass beds, which green sea turtles are known to forage (Crear et al., 2017, 2016). Green sea turtle capture efforts were conducted within a pond that is fed by a culvert which green sea turtles use to enter the pond and forage. This wetland is adjacent to a naval weapons base with green sea turtles travelling between the ponds in the refuge and the San Gabriel River (Crear et al., 2017, 2016).
The San Gabriel River (33° 45’ 15” N, 118° 6’ 13” W; SGR) is a concrete lined river that ends in a 6 km stretch of estuarine habitat (Figure 4). The river acts as a flood control channel with tributaries throughout the Greater Los Angeles area feeding into the river. There are two power plants 3 km from the river’s mouth that use once-through cooling, drawing water from the San Gabriel River to cool steam generators (Crear et al.,2016). As a result, the San Gabriel River’s water temperatures are regularly altered via heated water discharge from once-through cooling. Green sea turtles are increasingly found within SGR in recent years and individuals are known to move regularly between the SGR and adjacent SBNWR (Crear et al., 2016).
Sea Turtle Capture and Sampling
Whole blood samples were collected as described in Barraza et al. (2019). Briefly, green sea turtles were captured, subadults were sexed via testosterone (T) levels in the blood (Allen et al., 2015) and, adult-sized turtles via morphology (Caldwell, 1962), weighed (± 0.1 kg), and measured for curved carapace length (CCL; ± 0.1 cm). Methodology used is from previous research conducted within SDB (32° 36’ 54” N, 117° 6’ 4” W) and the SBNWR (33° 44’ 06.8” N, 118° 03’ 51.9” W)(Crear et al., 2016; Eguchi et al., 2010). Whole blood (3–10 ml) samples were collected and prepared for POP analysis (see below) following a modified National Institute of Standards and Technology (NIST) protocol to reduce the possibility of sample contamination (Keller et al., 2014b). Changes to NIST protocol include: using kilned glass containers with Teflon lids (Thermo Scientific) instead of Teflon containers, and using kilned aluminum instead of hexane rinse aluminum. Blood was collected with powder free nitrile gloves (Kimtech, Roswell, Georgia) into glass sodium-heparinized tubes (Becton Dickson, San Jose, California) and stored in a cooler with ice packs. At the end of each field day, blood samples were centrifuged at 3000 rpm for 10 min to separate plasma. Plasma was transferred into glass vials. Samples were placed at −20°C overnight then transferred to −80°C freezers until POP analysis.
Sea turtles were held for approximately 1 hour for morphometric and blood sample collection; afterward, they were released at their location of capture. Additional plasma samples were provided for POP analyses from previous samples collected using NIST protocols (2009–2014) of green sea turtles in SDB (n = 4) and the San Gabriel River (SGR, n = 6), a river within 4 km of SNBWR. These supplementary samples followed collection methods described in Keller et al. (2014).
POP Analyses
Blood plasma samples were analyzed for 14 PBDEs, 11 OCPs and 54 PCBs (specific analytes listed in Table 2 and Table S4). POPs were extracted using a soxhlet extraction with dichloromethane solvent. Blood plasma was placed directly into sodium sulfate in a thimble to dry and placed through a soxhlet extraction overnight. Extracts were column cleaned with an Alumina-B/Silica column to reduce lipid interference and concentrated with a vacuum-sealed rotary-evaporator followed by nitrogen evaporation. Extracts were analyzed using an Agilent Gas Chromatograph Mass Spectrometer (7890A/5975C) equipped with a J&W 60 meter, 0.25 mm ID, 0.25 μm film thickness DB-5 column via a splitless injection at a temperature of 285°C. The oven temperature profile was programmed from 45°C to 150°C at 20°C/min, and then to 300°C at 2.5°C/min. PCBs and OCPs were analyzed using a mass selective detector (MSD) in Electron Ionization (EI) selected ion monitoring (SIM) mode to scan for PCB and OCP specific ions at 1.67 times/sec. PBDEs were analyzed using the MSD in Negative Ion Chemical Ionization (NCI) mode using methane. A standard curve for all pollutants was based on a 6-point linear regression calibration curve with an R2 value of 0.99. All calibration standards were NIST traceable standards (AccuStandard©). For quality assurance, each sample batch was analyzed with a standard reference material (SRM 1957, NIST), two laboratory blank spikes, and a laboratory blank. Limits of detection in sea turtle plasma were determined using spiked sea turtle plasma with a 6-point linear regression; the standard deviation of spikes was divided by the slope of the regression and multiplied by 3.3. For a conservative approach, the congener with the highest limit of detection for each POP type was used as the limit of detection for all congeners. Recovery surrogates (TCMX, PCB 30, PCB 112, and PCB 198) were added to each sample prior to extraction, and all samples were quantified using the internal standards dibromobiphenyl and tetrabromobiphenyl. Blank spikes, SRM values, and recovery surrogates were analyzed for percent recovery to assess the accuracy and precision of the selected methods (supplementary Tables S2, S3, and S4). To account for lipid content difference, percent lipid was determined via weighing a 25% lipid split of extract and dividing by 25% volume of blood plasma used in the extract.
Table 2.
Summary persistent organic pollutants (PCB and OCP) estimated in blood plasma samples collected from green sea turtles from the Seal Beach National Wildlife Refuge (n = 16) or San Diego Bay (n = 23) using Kalpan-Meier model estimates. NAs are present where the model could not provide an estimate. Mean ± SEM and 95% confidence interval (Conf. Int.) are ng/g blood plasma.
| San Diego Bay | Seal Beach National Wildlife Refuge | |||
|---|---|---|---|---|
| Congener | Mean ± SEM | 95% Conf. Int. | Mean ± SEM | 95% Conf. Int. |
| PCB 3 | 0 | 0 | 0 | 0 |
| PCB 8 | 0 | 0 | 0 | 0 |
| PCB 18 | 0 | 0 | 0 | 0 |
| PCB 28 | 0 | 0 | 0 | 0 |
| PCB 31 | 0 | 0 | 0 | 0 |
| PCB 33 | 0 | 0 | 0 | 0 |
| PCB 37 | 0 | 0 | 0 | 0 |
| PCB 44 | 0 | 0 | 0 | 0 |
| PCB 49 | 0 | 0 | 0 | 0 |
| PCB 52 | 0 | 0 | 0 | 0 |
| PCB 56+60 | 0 | 0 | 0 | 0 |
| PCB 66 | 0.27 ± NA | NA | 0 | 0 |
| PCB 70 | 0 | 0 | 0 | 0 |
| PCB 74 | 0 | 0 | 0 | 0 |
| PCB 77 | 0 | 0 | 0 | 0 |
| PCB 81 | 0 | 0 | 0 | 0 |
| PCB 87 | 0.31 ± NA | NA | 0 | 0 |
| PCB 95 | 0 | 0 | 0 | 0 |
| PCB 97 | 0 | 0 | 0 | 0 |
| PCB 99 | 0.88 ± 0.16 | 0.57 – 1.19 | 0 | 0 |
| PCB 101 | 0 | 0 | 0 | 0 |
| PCB 105 | 0 | 0 | 0 | 0 |
| PCB 110 | 0 | 0 | 0 | 0 |
| PCB 114 | 0.44 ± NA | NA | 0 | 0 |
| PCB 118 | 0.33 ± 0.035 | 0.26 – 0.40 | 0 | 0 |
| PCB 119 | 0 | 0 | 0 | 0 |
| PCB 123 | 0 | 0 | 0 | 0 |
| PCB 126 | 0 | 0 | 0 | 0 |
| PCB 128 | 0.29 ± 0.02 | 0.25 – 0.34 | 0 | 0 |
| PCB 138 | 2.91 ± 0.62 | 1.69 – 4.12 | 0.33 ± 0.02 | 0.28 – 0.40 |
| PCB 141 | 0 | 0 | 0 | 0 |
| PCB 149 | 0.33 ± NA | NA | 0.28 ± NA | NA |
| PCB 151 | 0 | 0 | 0 | 0 |
| PCB 153 | 2.75 ± 0.72 | 1.35 – 4.16 | 0.54 ± NA | NA |
| PCB 156 | 0 | 0 | 0 | 0 |
| PCB 157 | 0 | 0 | 0 | 0 |
| PCB 158 | 0 | 0 | 0 | 0 |
| PCB 167 | 0 | 0 | 0 | 0 |
| PCB 168+132 | 0 | 0 | 0 | 0 |
| PCB 169 | 0 | 0 | 0 | 0 |
| PCB 170 | 0.28 ± 0.002 | 0.28 – 0.28 | 0 | 0 |
| PCB 174 | 0 | 0 | 0 | 0 |
| PCB 177 | 0 | 0 | 0 | 0 |
| PCB 180 | 0.49 ± 0.09 | 0.31 – 0.66 | 0 | 0 |
| PCB 183 | 0.32 ± 0.02 | 0.28 – 0.35 | 0 | 0 |
| PCB 187 | 0.35 ± 0.03 | 0.29 – 0.40 | 0 | 0 |
| PCB 189 | 0 | 0 | 0 | 0 |
| PCB 194 | 0 | 0 | 0 | 0 |
| PCB 195 | 0 | 0 | 0 | 0 |
| PCB 199+200 | 0 | 0 | 0 | 0 |
| PCB 201 | 0.34 ± 0.002 | 0.34 – 0.35 | 0 | 0 |
| PCB 206 | 0 | 0 | 0 | 0 |
| PCB 209 | 0 | 0 | 0 | 0 |
| 4,4′-DDMU | 0 | 0 | 0 | 0 |
| Chlordane-gamma | 0 | 0 | 0 | 0 |
| 2,4′-DDE | 0 | 0 | 0 | 0 |
| Chlordane-alpha | 0 | 0 | 0 | 0 |
| trans-Nonachlor | 0.43 ± 0.005 | 0.42 – 0.44 | 0 | 0 |
| 4,4′-DDE | 0.40 ± NA | NA | 0 | 0 |
| 2,4′-DDD | 0 | 0 | 0.89 ± NA | NA |
| 4,4′-DDD | 0 | 0 | 0 | 0 |
| 2,4′-DDT | 0 | 0 | 0 | 0 |
| cis-Nonachlor | 0 | 0 | 0 | 0 |
| 4,4′-DDT | 0 | 0 | 0 | 0 |
Statistical Analyses
Statistical analyses were done using R (version 3.3.3; R Core Team, 2018), with a significance threshold of alpha = 0.05. To assess current differences in POP levels in SDB and SBNWR turtles, samples collected before 2015 were not included in summary statistics of POPs. Samples collected before 2015 were used to provide data for key habitat locations for which little other data exist, and to provide data for future comparisons. Four blood plasma samples from SDB green sea turtles and six blood plasma samples from individuals in the SGR collected during another study were processes and analyzed separately. Since no PBDEs were detected below or above LOD in any samples, they were not included in the current study’s analyses.
Two methods were used to summarize POP data. First, all detected PCB and OCP analytes were summed to calculate total POP (∑POPs) concentration detected per turtle and summed by pollutant group to calculate total PCB or total OCPs (∑PCB or ∑OCP) per turtle. Second, a non-parametric Kaplan-Meier model (as described in Helsel, 2012) was used to account for detections below the limit of detection (LOD) and to provide an average value for each PCB and OCP analyte. For a conservative approach to avoid overestimation, and because of resource limitations, the highest congener LOD for PCBs and OCPs, respectively, was used as the cut-off for categorizing values below LOD in the Kaplan-Meier models (Table S1). ∑POPs/PCBs/OCPs for individuals were not calculated using the Kaplan-Meier model because the data were over 70% censored, which previous research has shown to not provide a good estimate of concentrations (Antweiler and Taylor, 2008). As a result, to avoid problems added by substitution (Helsel, 2012) or statistical treatment (Antweiler and Taylor, 2008), ∑PCB and ∑OCP included all detected values (whether above or below LOD), and non-detected analytes (analytes not found at all in scans) were treated as zero. To normalize data and account for individual lipid differences, detected POPs were measured in ng/g blood plasma, converted to ng/g lipid and natural log transformed for all statistical analyses. POPs that were not detected in any plasma samples were omitted from summary tables (Tables 1 and 2) for clarity.
Table 1.
Summary persistent organic pollutants (PCB and OCP) detected in blood plasma samples collected from green sea turtles from the Seal Beach National Wildlife Refuge (n = 16) or San Diego Bay (n = 23). Analytes that were not detected in any green sea turtles sampled are not displayed for clarity, including PBDEs. Non-detects are listed as zeros. Mean ± SE and median (range) are ng/g blood plasma. Limit of detection (LOD) for PCB congeners and OCP analytes is 0.27 and 0.18 ng/g, respectively.
| San Diego Bay | Seal Beach National Wildlife Refuge | |||||
|---|---|---|---|---|---|---|
| Congeners | n > LOD | Median (Range) | Mean ± SE | n > LOD | Median (Range) | Mean ± SE |
| PCB066 | 1 | 0 (0 – 0.27) | 0.01 ± 0.05 | 0 | 0 (0) | 0 |
| PCB087 | 1 | 0 (0 – 0.31) | 0.01 ± 0.06 | 0 | 0 (0) | 0 |
| PCB095 | 11 | 0 (0 – 0.22) | 0.05 ± 0.06 | 1 | 0 (0 – 0.11) | 0.01 ± 0.007 |
| PCB099 | 21 | 0.73 (0 – 3.31) | 0.86 ± 0.68 | 1 | 0 (0 – 0.66) | 0.05 ± 0.04 |
| PCB101 | 1 | 0 (0 – 0.13) | 0.006 ± 0.02 | 1 | 0 (0 – 0.14) | 0.008 ± 0.008 |
| PCB105 | 1 | 0 (0 – 0.22) | 0.01 ± 0.04 | 0 | 0 (0) | 0 |
| PCB110 | 0 | 0 (0) | 0 | 1 | 0 (0 – 0.08) | 0.005 ± 0.004 |
| PCB114 | 1 | 0 (0 – 0.44) | 0.02 ± 0.08 | 0 | 0 (0) | 0 |
| PCB118 | 18 | 0.175 (0 – 0.94) | 0.21 ± 0.19 | 0 | 0 (0 – 0.17) | 0.01 ± 0.01 |
| PCB128 | 11 | 0 (0 – 0.70) | 0.12 ± 0.15 | 0 | 0 (0 – 0.12) | 0.007 ± 0.007 |
| PCB138 | 23 | 2.07 (0.13 – 11.82) | 2.92 ± 2.61 | 16 | 0.15 (0.07 – 2.06) | 0.32 ± 0.12 |
| PCB141 | 0 | 0 (0) | 0 | 1 | 0 (0 – 0.13) | 0.008 ± 0.008 |
| PCB149 | 22 | 0.095 (0 – 0.33) | 0.12 ± 0.06 | 16 | 0.05 (0.02 – 0.28) | 0.07 ± 0.02 |
| PCB151 | 17 | 0.03 (0 – 0.11) | 0.03 ± 0.03 | 6 | 0 (0 – 0.09) | 0.01 ± 0.006 |
| PCB153 | 22 | 1.345 (0 – 11.92) | 2.79 ± 3.01 | 16 | 0.08 (0.03 – 1.32) | 0.18 ± 0.08 |
| PCB156 | 3 | 0 (0 – 0.10) | 0.01 ± 0.02 | 0 | 0 (0) | 0 |
| PCB157 | 3 | 0 (0 – 0.09) | 0.01 ± 0.02 | 0 | 0 (0) | 0 |
| PCB158 | 1 | 0 (0 – 0.20) | 0.01 ± 0.04 | 0 | 0 (0) | 0 |
| PCB167 | 9 | 0 (0 – 0.22) | 0.06 ± 0.07 | 0 | 0 (0) | 0 |
| PCB168+132 | 1 | 0 (0 – 0.12) | 0.01 ± 0.02 | 1 | 0 (0 – 0.13) | 0.008 ± 0.008 |
| PCB170 | 12 | 0.015 (0 – 0.31) | 0.07 ± 0.08 | 1 | 0 (0 – 0.07) | 0.007 ± 0.005 |
| PCB174 | 9 | 0 (0 – 0.10) | 0.02 ± 0.02 | 2 | 0 (0 – 0.14) | 0.01 ± 0.008 |
| PCB177 | 9 | 0 (0 – 0.10) | 0.02 ± 0.03 | 1 | 0 (0 – 0.08) | 0.007 ± 0.005 |
| PCB180 | 23 | 0.21 (0.05 – 2.02) | 0.38 ± 0.41 | 11 | 0.04 (0 – 0.21) | 0.05 ± 0.01 |
| PCB183 | 20 | 0.075 (0 – 0.57) | 0.15 ± 0.14 | 1 | 0 (0 – 0.10) | 0.01 ± 0.006 |
| PCB187 | 23 | 0.26 (0.05 – 0.66) | 0.30 ± 0.15 | 13 | 0.03 (0 –0.35) | 0.06 ± 0.02 |
| PCB189 | 1 | 0 (0 – 0.02) | 0.001 ± 0.003 | 0 | 0 (0) | 0 |
| PCB194 | 5 | 0 (0 – 0.15) | 0.02 ± 0.04 | 0 | 0 (0) | 0 |
| PCB195 | 1 | 0 (0 – 0.04) | 0.002 ± 0.007 | 0 | 0 (0) | 0 |
| PCB201 | 9 | 0 (0 – 0.38) | 0.08 ± 0.10 | 0 | 0 (0 – 0.08) | 0.005 ± 0.005 |
| PCB206 | 4 | 0 (0 – 0.09) | 0.01 ± 0.02 | 0 | 0 (0) | 0 |
| PCB209 | 1 | 0 (0 – 0.04) | 0.002 ± 0.007 | 0 | 0 (0) | 0 |
| Σ PCB | 23 | 5.07 (0.36 – 30.11) | 8.31 ± 7.25 | 16 | 0.32 (0.12 – 5.46) | 0.85 ± 0.33 |
| Chlordane-gamma | 19 | 0.095 (0 – 0.19) | 0.09 ± 0.05 | 14 | 0.1 (0 – 0.2) | 0.10 ± 0.01 |
| Chlordane-alpha | 1 | 0 (0 – 0.09) | 0.004 ± 0.02 | 1 | 0 (0 – 0.08) | 0.005 ± 0.005 |
| Trans-Nonachlor | 19 | 0.09 (0 – 0.51) | 0.12 ± 0.11 | 11 | 0.05 (0 – 0.21) | 0.05 ± 0.01 |
| 4,4′-DDE | 1 | 0 (0 – 0.40) | 0.02 ± 0.07 | 0 | 0 (0) | 0 |
| 2,4′-DDD | 0 | 0 (0) | 0 | 1 | 0 (0 – 0.89) | 0.05 ± 0.05 |
| Cis-Nonachlor | 3 | 0 (0 – 0.14) | 0.01 ± 0.03 | 0 | 0 (0) | 0 |
| Σ OCP | 21 | 0.23 (0 – 0.75) | 0.25 ± 0.16 | 14 | 0.15 (0 – 0.95) | 0.20 ± 0.05 |
| Σ POP | 23 | 5.275 (0.36 – 30.79) | 8.57 ± 7.36 | 16 | 0.44 (0.25 – 5.61) | 1.05 ± 0.34 |
| % Lipid | 23 | 0.5 (0.13 – 2.61) | 0.68 ± 0.10 | 16 | 0.41 (0.16 – 1.06) | 0.47 ± 0.05 |
One SBNWR turtle and three SDB turtles were recaptured and had repeat blood samples taken. Mean POP concentrations of recaptured turtles were used for analyses; no green turtle was captured more than twice. Using the R package vegan (Oksanen et al., 2015), principal component analyses (PCA) were conducted to assess location-based pollutant patterns and included CCL, ∑PCB, and ∑OCP of each individual. Using the R package cluster (Maechler et al., 2016), k-means cluster analyses of the PCA were conducted to assess how individuals clustered by location. Regression analyses were used to find relationships between POP types and CCL. A multivariate analysis of variance (MANOVA) was used to compare ∑PCB and ∑OCP between locations and among size classes. A second MANOVA was conducted that only included turtles of similar size for comparison (Table 2; between 60 and 85 cm CCL), and therefore included similarly aged turtles that are considered sub-adults (Eguchi et al., 2012; Figueroa et al., 1992; Juarez-Ceron et al., 2003).
Results
From August 2015 to May 2017, whole blood samples were collected from 23 green sea turtles from SDB and 16 green sea turtles from SBNWR. Green sea turtles captured in SDB were significantly larger than individuals from SBNWR (p < 0.001; Figure 1). Of the 79 POPs assessed (14 PBDE congeners, 54 PCB congeners, and 11 OCP analytes), only 32 PCB congeners (LOD = 0.27 ng/g) and 6 OCP analytes (LOD = 0.18 ng/g) were detected at concentrations above the LOD (see Table 1). PCB congeners not detected in any samples included: PCB 3, 8, 18, 28, 31, 33, 37, 44, 49, 52, 56 (60), 70, 74, 77, 81, 97, 119, 123, 126, 169, and 199 (200). OCP analytes not detected in any samples included: 4,4’-DDMU, 2,4’-DDE, 4,4’-DDD, 2,4’-DDT, and 4,4’-DDT. No PBDE analytes (PBDE 17, 28, 47, 66, 71, 85, 99, 100, 138, 153, 154, 183, 190, and 209) were detected (LOD = 0.86 ng/g) in any green turtle blood plasma sample.
Figure 1:
Boxplot of curved carapace length (CCL; cm) of green sea turtles captured from Seal Beach National Wildlife Refuge (SBNWR, n = 16 turtles) and San Diego Bay (SDB, n = 23 turtles). Boxes are the middle 50% quartile with the line representing the median, whiskers are top and bottom 25% quartile. X-axis represents CCL and y-axis capture location of green sea turtles. SDB turtles are significantly (p ≤ 0.001) larger than SBNWR.
PCA k-means cluster analysis grouped green sea turtles from the same location together with some overlap between the different locations (Figure 2). From the PCA, PC1 (∑PCBs) accounted for 58% of the variance, and PC2 (CCL) accounted for 30% of the variance. There were more PCB congeners and OCP analytes detected (Table 1) in SDB turtles (30 PCB congeners; 5 OCPs) than SBNWR turtles (19 PCB congeners; 4 OCPs). Several congeners that displayed differences in the frequency of detection between populations included PCB 180, 187, 149, and 153 (see Table 1 and Table 2). PCB 180 and 187 were detected in all SDB turtles, PCB 99 was only detected in one SBNWR turtle but detected in nearly all SDB turtles, PCB 149 and 153 were detected in all SBNWR turtles (and all but one SDB turtle), and PCB 138 was detected in every turtle captured in the present study. Overall, the concentration of PCBs strongly varied between SDB and SBNWR green turtle plasma samples; in particular, SDB turtles had much higher concentrations of PCB 153, PCB 138, and PCB 99 in their blood plasma. With the exception of moderate levels of PCB 118 in SDB green sea turtles, there were low to non-detectable levels of dioxin-like PCBs (Table 1), such as PCB 77, 126, 169, and 105 in the plasma of all green sea turtles from the current study. The ∑PCBs detected in all turtles made up 95.1% by ng/g lipid of all POPs detected, and ∑OCPs made up 4.9% ng/g lipid of POPs detected. There were no significant differences in ∑PCB and ∑OCP concentrations between male (n = 6) and female green sea turtles (n = 11) regardless of capture location (data not shown). Not all turtles in the current study had sex determined by testosterone measurements; all sex undetermined turtles were juveniles. Two MANOVAs of PCB and OCP concentrations, one including all samples and another including only sub-adults, both showed that location, not CCL or the interaction of CCL and location, was a significant (p < 0.001) factor for PCB concentrations (Table 3).
Figure 2:
Principal component analysis of ∑PCBs and ∑OCPs in blood plasma lipid and curved carapace length (CCL) of green sea turtles from the Seal Beach National Wildlife Refuge (SBNWR; n = 16 green sea turtles) and San Diego Bay (SDB; n = 23 green sea turtles). Vectors indicate the direction that each factor affects principal component scores for each turtle (point). PC1 (x-axis) refers to ∑PCBs in ng/g blood plasma lipid, and PC2 refers to CCL (cm).
Table 3.
Multivariate Analysis of Variance (MANOVA) results of persistent organic pollutant concentrations in green sea turtles inhabiting the Seal Beach National Wildlife Refuge (n = 16) or San Diego Bay (n = 23). “PCBs” are polychlorinated biphenyls, “OCPs” are organochlorinated pesticides, and “CCL” refers to curved carapace length. “All” refers to comparisons that include all turtles captured and analyzed in the current study (n = 39); and “Sub-Adult” refers to comparisons which only include similar sized subadult turtles (n = 23). “Comparison” refers to the dependent and independent variables in the MANOVA.
| Comparison | Mean Squares | F Value | P Value |
|---|---|---|---|
| All PCBs : Location | 55.61 | 60.77 | p < 0.001 |
| All PCBs : CCL | 2.02 | 2.21 | p = 0.14 |
| All PCBs : CCL*Location | 2.32 | 2.53 | p = 0.12 |
| All OCPs : Location | 0.23 | 0.12 | p = 0.73 |
| All OCPs : CCL | 0.39 | 0.2 | p = 0.65 |
| All OCPs : CCL*Location | 0.90 | 0.48 | p = 0.50 |
| Sub-Adult PCBs : Location | 19.66 | 29.93 | p < 0.001 |
| Sub-Adult PCBs : CCL | 1.32 | 2.01 | p = 0.17 |
| Sub-Adult PCBs : CCL*Location | 0.01 | 0.02 | p = 0.89 |
| Sub-Adult OCPs : Location | 0.10 | 0.04 | p = 0.84 |
| Sub-Adult OCPs : CCL | 0.01 | 0.004 | p = 0.94 |
| Sub-Adult OCPs : CCL*Location | 0.24 | 0.10 | p = 0.75 |
The MANOVA including all samples indicated that SDB green sea turtles had significantly more ∑PCBs (7.16, 6.93 ± 0.22 ln ng/g lipid; median, mean ± SE) in their blood plasma than SBNWR (4.33, 4.49 ± 0.22 ln ng/g lipid) green sea turtles (Figure 3A). MANOVA comparing similarly sized turtles (see methods) demonstrated that sub-adults from SDB (6.45, 6.26 ± 0.29 ln ng/g lipid) had significantly more ∑PCBs (4.34, 4.36 ± 0.21 ln ng/g lipid) than SBNWR sub-adult turtles (Figure 3B).
Figure 3:
(A) Natural log transformed ∑PCBs in blood plasma lipid of green sea turtles from Seal Beach National Wildlife Refuge (SBNWR; n = 16 turtles; SBNWR) and San Diego Bay (SDB; n = 23 turtles; SDB). (B) Natural log transformed ∑PCBs in blood plasma lipid of sub-adult (between 60 and 85 cm CCL) green sea turtles from Seal Beach National Wildlife Refuge (SBNWR; n = 14 turtles) and San Diego Bay (SDB; n = 9 turtles). Y-axis represents ng/g lipid ∑PCBs natural log transformed in green turtle plasma samples (corrected for lipid content). X-axis represents location of sea turtles captured. Asterisks indicate significant differences via one-way ANOVA (*** ≤ 0.001). (C) Relationship of natural log ∑PCBs in blood plasma lipid and curved carapace length of green sea turtles from Seal Beach National Wildlife Refuge (SBNWR; n = 16 turtles) and San Diego Bay (SDB; n = 20 turtles). X-axis represents curved carapace length in centimeters of sea turtles captured. Y-axis represents natural log transformed ng/g ∑PCBs in sea turtle blood plasma lipid.
There was no significant difference in ∑OCPs between turtles captured in SDB (3.65, 2.90 ± 0.66 ln ng/g lipid) and SBNWR (3.80, 2.48 ± 0.94 ln ng/g lipid); and there was no relationship between ∑OCPs and turtle CCL (R2= 0.006, p = 0.52). The most commonly-occurring OCPs in green turtle plasma from both locations were chlordanegamma and trans-nonachlor. Most of the DDT metabolites were not detected or were only present in blood plasma from one turtle at each location (Table 1).
Only four green sea turtles were recaptured for the duration of the study, three from SDB and one from SBNWR. Three of the four turtles showed increased (+70.22%, +248.30%, + 367.55% ng/g lipid) ΣPOP concentrations from their initial capture (Table 4). One turtle had decreased (−23.01%) ΣPOPs from its initial capture (Table 4). Changes in ΣPOP concentrations were mainly driven by increases (+74.29%, +240.35%, +383.71%) or decreases (−24.41%) in ΣPCB concentrations.
Table 4.
Summary of persistent organic pollutants (ΣPOPs, ΣPCB, ΣOCP) concentrations in recaptured turtles, as well as their change in POP concentrations since their first capture. No turtle was caught more than twice. N.D. signifies that no analytes were detected. ΣPOPs, ΣPCB, and ΣOCP concentrations are in ng/g blood plasma lipid. (%Δ) Refers to the percent change in analyte concentrations from first capture to recapture.
| ID | Location | Date Captured | ΣPCBs | ΣOCPs | ΣPOPs | %ΔPCBs | %ΔOCPs | %ΔPOPs | Δ Time |
|---|---|---|---|---|---|---|---|---|---|
| GK-33 | SBNWR | 25/08/2015 | 37.31 | 16.42 | 53.73 | 383.71 | 330.82 | 367.55 | 1 year, 4 months |
| GK-33 | SBNWR | 7/12/2016 | 180.49 | 70.73 | 251.22 | ||||
| GK-23 | SDB | 1/06/2016 | 635.56 | 37.78 | 673.33 | 74.29 | 1.81 | 70.22 | 5 months |
| GK-23 | SDB | 10/11/2016 | 1107.69 | 38.46 | 1146.15 | ||||
| LB-326 | SDB | 15/12/2015 | 3645.21 | 38.36 | 3683.56 | −24.41 | 110.02 | −23.01 | 6 months |
| LB-326 | SDB | 15/06/2016 | 2755.56 | 80.56 | 2836.11 | ||||
| LB-337 | SDB | 27/08/2015 | 173.58 | N.D. | 173.58 | 240.35 | NA | 248.30 | 1 year, 3 months |
| LB-337 | SDB | 2/11/2016 | 590.80 | 13.79 | 604.60 | ||||
Samples from previous SDB green turtle blood collections had significantly (p = 0.002) higher ΣPCBs (8.95, 8.75 ± 0.30 ln ng/g lipid) and significantly (p = 0.001) higher ΣOCPs (5.66, 5.63 ± 0.18 ln ng/g lipid) than SDB green sea turtles measured in the current study. Green sea turtles from SGR had significantly (p = 0.004) higher ΣPCBs (7.99, 7.52 ± 0.64 ln ng/g lipid) but similar ΣOCPs (5.09, 5.18 ± 0.49 ln ng/g lipid) than green sea turtles from SBNWR. Results from additional samples are provided the supplementary section for future comparisons (Table S4).
Percent recovery of SRM, plasma, blank, and blank spikes TCMX recovery surrogate accuracy was 67.71–99.95% and precision was ± 1.79–6.88% (see Table S2). In all samples, accuracy of PCB 30 was 77.23–100.80%, and precision was 1.38–6.75%. Detection of PCB 112 had an accuracy of 92.17–105.65% and a precision of 1.75–6.38%. Accuracy of PCB 198 was 70.42–100.56% in all samples, and precision of PCB 198 in all samples was ± 0.49–8.16%. Detection of FPBDE had an accuracy of 76.55–88.01% and precision of 3.44–8.77 %. Detection of DFPBDE had an accuracy of 52.35–74.75% and precision of 2.26–6.70%. There was no outside contamination detected in any blanks analyzed.
Discussion
Location Pollutant Signatures
As expected, there were unique location-specific pollutant signatures in green sea turtles inhabiting SBNWR and SDB. Green sea turtles from SBNWR had similar PBDE and OCP concentrations to SDB green sea turtles; however, the majority of POPs detected in green turtle blood plasma were PCBs. As a result, PCBs constituted the highest proportion of green turtle POP loads by ng/g lipid and were the strongest factor in the separation of green sea turtles by location in the PCA loading plot (Figure 2). These patterns are reflected in the higher concentration and variety of PCBs in SDB green turtle blood plasma relative to SBNWR green sea turtles, which suggests that SDB green sea turtles experience higher PCB exposure than SBNWR green sea turtles (Table 1; Table 2).
Differences Between Sample Locations
The current study measured PCB concentrations green sea turtles from two locations, approximately 160 km apart. The two green sea turtle aggregations have some biological factors (e.g., size and sex) that could influence their PCB accumulation patterns. Previous research has shown that SDB green sea turtles’ POPs correlate with CCL (Komoroske et al., 2011); therefore, it could be expected that larger, older individuals would have higher PCB concentrations as seen in other sea turtle species (Finlayson et al., 2016; Pugh and Becker, 2001). SDB green sea turtles were larger than green sea turtles caught in SBNWR (Figure 1), and PCB differences could be due to longer exposure times. Although bioaccumulation with age/size has been found in turtles from other locations (Finlayson et al., 2016; Gardner and Oberdorster, 2005) and the data often trended with CCL (Figure 2, 3C), the current study did not find significant relationships between size (CCL) and PCB levels (Table 3). However, the current study captured only a small size range of SBNWR green sea turtles (50.8–82.6 cm CCL); thus, future capture of an increased range in size of individuals from SBNWR would help determine if there is a relationship between size and POP levels. It is possible that SBNWR currently represents a relatively new year-round expansion of green turtle foraging habitat, utilized primarily by smaller juvenile individuals that will take some time to grow into adults. It is also possible that adult turtles could not fit into the culverts that leads into the capture location within SBNWR as there were adult turtles caught within the SGR in previous sampling years. However, one adult turtle was seen in SBNWR, indicating some adults could access the ponds inside SBNWR.
Sex is another biological factor that may influence pollutant load (Keller, 2013). Green sea turtles from SDB are mostly female (>75%) and of adult-size (>85cm CCL), whereas turtles collected from SBNWR are also mostly female, but smaller than adult-size (<85 cm CCL)(Allen et al., 2016, 2015). Female green sea turtles can maternally offload POPs by metabolizing fat reserves that transfer lipids, and therefore POPs, to their eggs (Munoz and Vermeiren, 2019; van de Merwe et al., 2010b). As a result, there is potential for adult female green sea turtles to exhibit lower blood plasma POPs due to maternal offloading, yet, the data did not support that conclusion as female and male adult turtles in this study had similar POP concentrations. The current study had only six adult males, and all but one was from SDB, which is insufficient to observed sex-based POP differences.
Green sea turtles in SBNWR have access to anthropogenically-warmed waters from power plants within SGR (Crear et al., 2017, 2016). In contrast, SDB green sea turtles in the present study live under more natural conditions—although it should be noted that data from Komoroske et al. (2011) in SDB were from a period when waters were warmed from the south San Diego Bay power plant. Anthropogenically warmed waters could explain why SDB green sea turtles had lower PCB levels when the power plant was active (since monitoring began) than after power plant closure in December 2010. When SDB green sea turtles had access to anthropogenically warmed waters, their growth rate was similar to green sea turtles that inhabit tropical waters (Eguchi et al.,2012). While not confirmed, it is postulated that SBNWR/SGR green sea turtles have an accelerated growth rate due to their access to anthropogenically-warmed waters. Depending on pollutant availability, accelerated growth rates could be diluting SBNWR green sea turtles’ PCB concentrations through increased mass from growth and the opposite for SDB green sea turtles currently inhabiting non-warmed waters. While it can be argued that warmer waters can increase the rate at which green sea turtles feed on PCB contaminated food, thereby increasing their potential PCB exposure, results indicate that overall, SBNWR turtles have lower PCB concentrations than SDB green sea turtles.
Comparison to Previous Research
Previous research by Komoroske et al. (2011) examined POPs in SDB green sea turtles and captured a size range of green sea turtles similar to those captured in the current study. In the current study, green sea turtles from SDB and SBNWR had lower chlordane gamma and chlordane alpha than previously assessed in SDB, possibly indicating a reduction in bioavailable pesticides or exposure (Komoroske et al., 2011; Lewison et al., 2011). It is also important to note that Komoroske et al. (2011) had different LODs for each of the POPs analyzed than the current study, with LODs lower in the current study for PCBs and OCPs but higher for PBDEs. Unlike Komoroske et al (2011), the current study did not find any PBDEs in blood plasma samples. These findings could indicate a decrease in PBDE contamination at both sites samples were last taken, especially since PBDEs concentrations are decreasing throughout the Southern California Bight (Dodder et al., 2016, 2012). The current study had a higher LOD and recovery surrogates were relatively low compared to other analytes, but SRM measurements were accurate and precise. There is a possibility that the higher LOD would miss low PBDE concentrations in green turtle blood plasma; however, there was no indication of any PBDE concentrations at or below the LOD in gas chromatography mass spectrometry scans.
SDB turtles have among the highest ΣPCBs detected in blood plasma compared to previous SDB samples and other turtles worldwide, with studies detecting ΣPCBs as low as 2.84 pg/g blood plasma and as high as 5.38 ng/g blood plasma compared to 30.11 ng/g ΣPCBs found in an SDB turtle (Camacho et al., 2014; Keller et al., 2014a; Komoroske et al., 2011; Lewison et al., 2011; Swarthout et al., 2010; van de Merwe et al., 2010b, 2010a). Conversely, SBNWR green sea turtles had similar or lower ΣPCB levels in their blood than other studies worldwide (Keller, 2013). Additional samples collected in 2011– 2013 from green sea turtles inhabiting the SGR had higher PCB concentrations than SBNWR turtles. Sediment samples from previous research found very low (0 – 1.77 ug/kg dw) PCB concentrations near the SGR and SBNWR (Dodder et al., 2016), possibly due to differences in food items available between the locations (eelgrass in SBNWR and algae in the SGR)(Crear et al., 2016). Green sea turtles sampled from the SGR were similar in size to the current study’s SBNWR green sea turtles, except for one adult turtle (93 cm, CCL), which had PCB levels analogous to other adult turtles from SDB samples. While the sample size was low for SGR turtles (n = 6) and the foraging locations are within 8 km of each other, the current study’s data indicate that turtles from SGR may have higher PCB exposure and ensuing risk of PCB accumulation than turtles from SBNWR. Overall, the current study’s results indicate that PCB exposure risk in SDB turtles may have increased since previous studies and that the SDB location may represent one of the highest PCB-contaminated green turtle populations studied to date (Finlayson et al., 2016; Gardner and Oberdorster, 2005; Keller, 2013). Nonetheless, previous research has shown that blood plasma PCB concentrations can vary with time and between samples (Lewison et al., 2011).
Variance from Lipid Mobilization
Previous research has shown that the mobilization of adipose or recent dietary exposure could also temporarily increase POP concentrations in blood plasma, indicating that POP concentrations can vary depending on whether green sea turtles are captured before or after breeding migrations that require fasting or recent feeding events (Hamann et al., 2002; Keller et al., 2004). The current study’s ΣPCB, ΣOCPs, and ΣPOPs concentrations were lipid-normalized to account for these lipid mobilization events. Four green sea turtles were recaptured, three from SDB and one from SBNWR, with three out of the four turtles having increased ΣPOPs (+74%, +240%, + 383% ng/g lipid) since their initial capture (Table 4). Of note, three out for four recaptured green sea turtles had higher ΣPOPs in ng/g lipid in the fall/winter months than the spring/summer months, suggesting that these green sea turtles POP concentrations continually change even when accounting for increased lipid in blood plasma. Previous research that has repeated sampling of individuals in SDB found that POP concentrations can vary with time of sampling and even within samples of the same individual collected on the same day (Lewison et al., 2011). The results are also supported by additional blood samples collected from 2011–2013, which further indicate that SDB green turtle POP concentrations continuously fluctuate over time. These samples had higher PCB and OCP concentrations compared to current (2015–2017) SDB turtle samples and those found in 2009 (Komoroske et al., 2011). Only four SDB turtles were analyzed as recaptures, which makes it difficult to suggest that the whole aggregation is reflected in the analysis. However, these samples and trends may suggest that time of year and/or temperature may be affecting ΣPOP concentrations found in the blood of these green sea turtles.
Future Directions
Overall, both populations of green sea turtles in the current study had low to non-detectable levels of dioxin-like PCBs, a heavily studied group of PCBs (Domingo and Bocio, 2007; Srogi, 2008). Rather, the most abundant PCB congeners detected were nondioxin-like PCBs (Table 1), such as PCB 138, 153, 187, and 180. These PCBs are known to activate the ryanodine receptor or alter dopaminergic-signaling pathways (Holland et al., 2017; Kenet et al., 2007; Pessah et al., 2006; Wigestrand et al., 2013; Yang et al., 2009). Non-dioxin-like PCB actions through these pathways have been related to induced muscle impairment, altered neuronal growth, and impaired learning and memory (Pessah et al., 2010; Wayman et al., 2012; Wigestrand et al., 2013; Yang et al., 2009). Although these previous studies were conducted in mammalian species, their findings suggest that green sea turtles could be at risk for the induction of neurotoxicity due to PCB burdens. Juvenile/hatchling green sea turtles born from SDB green sea turtles may receive high PCB burdens through maternal transfer of non-dioxin-like PCBs, potentially negatively impacting their early life stages. Given that the majority of PCBs detected in SDB and SBNWR turtles were non-dioxin-like PCBs, and the current lack of research into neurotoxic non-dioxin-like PCBs in reptiles, it would be beneficial to investigate these possible effects in the SBNWR and SDB green sea turtles (Finlayson et al., 2016; Gardner and Oberdorster, 2005). The current study did not include many other pollutants (e.g., PAHs, plastics) that could be accumulating within southern California green sea turtles, which future research could help determine risk and possible mixture interactions. New research into POPs include: health panels for investigating health effects (Banerjee et al., 2019; Keller et al., 2014a; Komoroske et al., 2011), non-targeted extraction methods to evaluate mixture effects and monitor multiple compound types (Dogruer et al., 2018; Heffernan et al., 2017; Vijayasarathy et al., 2019), and combining non-targeted extractions with cell line bioassays as measurements of contamination and toxicity (Allan et al., 2017; Finlayson et al., 2019b, 2019c, 2019a; Jin et al., 2015). These new methods and tools help link POP concentrations to various health effects and assess the effects of multiple pollutant types; and while the body of research is growing, pollutant physiological tipping points and pollutant mixture interactions are still not well understood (Cortes-Gomez et al., 2017; Finlayson et al., 2016). Until more research is completed, it is uncertain whether the amount POPs detected are high enough to have a detrimental effect on SDB and SBNWR green sea turtles’ health.
Conclusions
Overall, evidence was found that green sea turtles from SDB accumulated higher PCB levels than green sea turtles from SBNWR. While factors such as size, and lipid mobilization events can change PCB levels in the short term (6 months to 1.5 years), the results indicate greater PCB levels in green sea turtles in SDB than those found in other parts of the world (Keller, 2013). The current study’s results suggest that green sea turtles foraging within SGR are at greater risk of PCB accumulation than SBNWR turtles and similar PCB levels to SDB turtles. The most common PCBs accumulated were nondioxin-like PCBs, indicating opportunities for future research to investigate the possible effects of non-dioxin-like PCBs impacts on green turtle physiology. Overall, considering the disparity and fluctuations in PCB accumulation patterns found, additional monitoring of turtles within the Los Angeles and San Diego areas may be necessary. Even green sea turtles foraging within proximate habitats (SBNWR vs. SGR) exhibited different PCB accumulation patterns. While the health effects of these PCBs are currently unknown, green sea turtles will continue to inhabit these urban areas for the foreseeable future due to increasingly warm waters and population recovery (Seminoff et al., 2015). Urban populations, such as the green sea turtles inhabiting critical habitat within southern California, provide clues and opportunities to elucidate how green sea turtles are affected by anthropogenic pollutants.
Supplementary Material
Highlights.
Green sea turtles accumulate PCBs and OCPs based on foraging location
Green turtles from San Diego Bay have higher PCB levels than other studies
Results indicate that POP levels in blood plasma continuously change over time
Most PCBs detected in green turtle plasma were non-dioxin-like PCBs
Acknowledgements
Financial support was provided by the National Oceanic and Atmospheric Administration’s National Marine Fisheries Services; specifically, the West Coast Regional Office, Long Beach CA, and the Southwest Fisheries Science Center, La Jolla CA. Research reported in this publication was also supported financially by the National Institute of General Medical Sciences of the National Institute of Health under Award Number R25GM071638. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We would like to thank the CSULB Shark Lab, particularly Connor White, Echelle Burns, and Emily Meese for their assistance with statistical analyses and figure construction. We thank US Fish and Wildlife manager Kirk Gilligan, veterinary staff of Aquarium of the Pacific Lance Adams, DVM, and Juli Barron, RVT, Joel Schumacher, Erin LaCasella, and all the volunteers and NOAA-NMFS team that assisted in sea turtle capture and handling. Special thank you to Dr. Jennifer Lynch for donating additional samples to this research. All research and animal handling were carried out under National Marine Fisheries Service Research Permits #16803, 14510, and 16803.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
We have no conflicts of interest to report.
Arthur Barraza (on behalf of all co-authors)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allan HL, van de Merwe JP, Finlayson KA, O’Brien JW, Mueller JF, Leusch FDL, 2017. Analysis of sugarcane herbicides in marine turtle nesting areas and assessment of risk using in vitro toxicity assays. Chemosphere 185, 656–664. 10.1016/j.chemosphere.2017.07.029 [DOI] [PubMed] [Google Scholar]
- Allen CD, Bell IP, Seminoff JA, 2016. Sex ratio of green turtle foraging grounds in the Pacfic: establishing baselines for climate change research, in: Mangel JC, Rees A, Pajuelo M, et al. (Eds) Proceedings of the Thirty-Sixth Annual Symposium on Sea Turtle Biology and Conservation International Sea Turtle Society, p. NOAA Technical Memorandum NMFS-SEFSC-734, p 1. [Google Scholar]
- Allen CD, Robbins MN, Eguchi T, Owens DW, Meylan AB, Meylan PA, Kellar NM, Schwenter JA, Nollens HH, LeRoux RA, Dutton PH, Seminoff JA, 2015. First assessment of the sex ratio for an east pacific green sea turtle foraging aggregation: Validation and application of a testosterone ELISA. PLoS One 10, e0138861 10.1371/journal.pone.0138861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antweiler RC, Taylor HE, 2008. Evaluation of Statistical Treatments of Left-Censored Environmental Data using Coincident Uncensored Data Sets: I. Summary Statistics. Environ. Sci. Technol. 42, 3732–3738. 10.1021/es071301c [DOI] [PubMed] [Google Scholar]
- Banerjee SM, Allen CD, Schmitt T, Cheng BS, Seminoff JA, Eguchi T, Komoroske LM, 2019. Baseline Health Parameters of Eastern Pacific Green Turtles at Southern California Foraging Grounds. Chelonian Conserv. Biol. [Google Scholar]
- Barraza AD, Komoroske LM, Allen C, Eguchi T, Gossett R, Holland E, Lawson DD, Leroux RA, Long A, Seminoff JA, Lowe CG, 2019. Trace metals in green sea turtles (Chelonia mydas) inhabiting two southern California coastal estuaries. Chemosphere 223, 342–350. 10.1016/j.chemosphere.2019.01.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell DK, 1962. Carapace Length: Body Weight Relationship and Size and Sex Ratio of the Northeastern Pacific Green Sea Turtle, Chelonia mydas carrinegra. Los Angeles County Museum, Los Angeles. [Google Scholar]
- Camacho M, Boada LD, Oros J, Lopez P, Zumbado M, Almeida-Gonzalez M, Luzardo OP, 2014. Monitoring organic and inorganic pollutants in juvenile live sea turtles: Results from a study of Chelonia mydas and Eretmochelys imbricata in Cape Verde. Sci. Total Environ. 481, 303–310. 10.1016/j.scitotenv.2014.02.051 [DOI] [PubMed] [Google Scholar]
- Carpenter DO, 2006. Polychlorinated biphenyls (PCBs): routes of exposure and effects on human health. Rev. Environ. Health 21, 1–24. [DOI] [PubMed] [Google Scholar]
- Cortes-Gomez AA, Romero D, Girondot M, 2017. The current situation of inorganic elements in marine turtles: A general review and meta-analysis. Environ. Pollut. 229, 567–585. 10.1016/j.envpol.2017.06.077 [DOI] [PubMed] [Google Scholar]
- Crear DP, Lawson DD, Seminoff JA, Eguchi T, LeRoux RA, Lowe CG, 2017. Habitat use and behavior of the east pacific green turtle, Chelonia mydas, in an urbanized system. Bull. South. Calif. Acad. Sci. 116, 17–32. [Google Scholar]
- Crear DP, Lawson DD, Seminoff JA, Eguchi T, LeRoux RA, Lowe CG, 2016. Seasonal shifts in the movement and distribution of green sea turtles, Chelonia mydas, in response to anthropogenically altered water temperatures. Mar. Ecol. Prog. Ser. 548, 219–232. 10.3354/meps11696 [DOI] [Google Scholar]
- Darnerud PO, Eriksen GS, Jóhannesson T, Larsen PB, Viluksela M, 2001. Polybrominated diphenyl ethers: Occurrence, dietary exposure, and toxicology. Environ. Health Perspect. 109, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Andrés E, Gómara B, González-Paredes D, Ruiz-Martín J, Marco A, 2016. Persistent organic pollutant levels in eggs of leatherback turtles (Dermochelys coriacea) point to a decrease in hatching success. Chemosphere 146, 354–361. 10.1016/j.chemosphere.2015.12.021 [DOI] [PubMed] [Google Scholar]
- Dodder N, Schiff K, Latker A, Tang C-L, 2016. Southern California Bight 2013 Regional Monitoring Program: Volume IV Sediment Chemistry. Southern California Coastal Water Research Project, Costa Mesa. [Google Scholar]
- Dodder NG, Maruya KA, Lauenstein GG, Ramirez J, Ritter KJ, Schiff KC, 2012. Distribution and sources of polybrominated diphenyl ethers in the Southern California Bight. Environ. Toxicol. Chem. 31, 2239–2245. [DOI] [PubMed] [Google Scholar]
- Dogruer G, Weijs L, Tang JY, Hollert H, Kock M, Bell I, Madden CA, Gaus C, 2018. Effect-based approach for screening of chemical mixtures in whole blood of green turtles from the Great Barrier Reef. Sci. Total Environ. 612, 321–329. 10.1016/j.scitotenv.2017.08.124 [DOI] [PubMed] [Google Scholar]
- Domingo JL, Bocio A, 2007. Levels of PCDD/PCDFs and PCBs in edible marine species and human intake: A literature review. Environ. Int. 33, 397–405. 10.1016/j.envint.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Dutton PH, LeRoux RA, LaCasella EL, Seminoff JA, Eguchi T, Dutton DL, 2019. Genetic analysis and satellite tracking reveal origin of the green turtles in San Diego Bay. Mar. Biol. 166, 1–13. 10.1007/s00227-018-3446-4 [DOI] [Google Scholar]
- Eguchi T, Seminoff JA, LeRoux RA, Dutton PH, Dutton DL, 2010. Abundance and survival rates of green turtles in an urban environment: coexistence of humans and an endangered species. Mar. Biol. 157, 1869–1877. 10.1007/s00227-010-1458-9 [DOI] [Google Scholar]
- Eguchi T, Seminoff JA, LeRoux RA, Prosperi D, Dutton DL, Dutton PH, 2012. Morphology and growth rates of the green sea turtle (Chelonia mydas) in a northernmost temperate foraging ground. Herpetologica 68, 76–87. [Google Scholar]
- Figueroa A, Alvarado-Diaz J, Hernandez F, Rodriguez G, Robles J, 1992. Population recovery of the sea turtles of Michoacan, Mexico: an integrated conservaton approach. [Google Scholar]
- Finlayson KA, Leusch FDL, Limpus CJ, van de Merwe JP, 2019a. Towards the development of standardised sea turtle primary cell cultures for toxicity testing. Ecotoxicol. Environ. Saf. 173, 63–70. 10.1016/j.ecoenv.2019.01.117 [DOI] [PubMed] [Google Scholar]
- Finlayson KA, Leusch FDL, van de Merwe JP, 2019b. Cytotoxicity of organic and inorganic compounds to primary cell cultures established from internal tissues of Chelonia mydas. Sci. Total Environ. 664, 958–967. 10.1016/j.scitotenv.2019.02.052 [DOI] [PubMed] [Google Scholar]
- Finlayson KA, Leusch FDL, van de Merwe JP, 2019c. Primary green turtle (Chelonia mydas) skin fibroblasts as an in vitro model for assessing genotoxicity and oxidative stress. Aquat. Toxicol. 207, 13–18. 10.1016/j.aquatox.2018.11.022 [DOI] [PubMed] [Google Scholar]
- Finlayson KA, Leusch FDL, van de Merwe JP, 2016. The current state and future directions of marine turtle toxicology research. Environ. Int. 94, 113–123. 10.1016/j.envint.2016.05.013 [DOI] [PubMed] [Google Scholar]
- Flynn LT, Kleiman CF, 1997. Public Health Concerns About Environmental Polychlorinated Biphenyls (PCBs). American Council on Science and Health, New York. [DOI] [PubMed] [Google Scholar]
- Gardner SCM, Oberdorster E, 2005. Toxicology of Reptiles. CRC Press, Boca Raton. [Google Scholar]
- Gray JS, 1997. Marine biodiversity: Patterns, threats and conservation needs. Biodivers. Conserv. 6, 153–175. 10.1023/A:1018335901847 [DOI] [Google Scholar]
- Hamann M, Godfrey MH, Seminoff JA, Arthur K, Barata PCR, Bjorndal KA, Bolten AB, Broderick AC, Campbell LM, Carreras C, Casale P, Chaloupka M, Chan SKF, Coyne MS, Crowder LB, Diez CE, Dutton PH, Epperly SP, FitzSimmons NN, Formia A, Girondot M, Hays GC, Cheng IS, Kaska Y, Lewison R, Mortimer JA, Nichols WJ, Reina RD, Shanker K, Spotila JR, Tomás J, Wallace BP, Work TM, Zbinden J, Godley BJ, 2010. Global research priorities for sea turtles: Informing management and conservation in the 21st century. Endanger. Species Res. 11, 245–269. 10.3354/esr00279 [DOI] [Google Scholar]
- Hamann M, Limpus CJ, Whittier JM, 2002. Patterns of lipid storage and mobilisation in the female green sea turtle (Chelonia mydas). J Comp Physiol B 172, 485–493. 10.1007/s00360-002-0271-2 [DOI] [PubMed] [Google Scholar]
- Heffernan AL, Gómez-Ramos MM, Gaus C, Vijayasarathy S, Bell I, Hof C, Mueller JF, Gómez-Ramos MJ, 2017. Non-targeted, high resolution mass spectrometry strategy for simultaneous monitoring of xenobiotics and endogenous compounds in green sea turtles on the Great Barrier Reef. Sci. Total Environ. 599–600, 1251–1262. 10.1016/j.scitotenv.2017.05.016. [DOI] [PubMed] [Google Scholar]
- Helsel DR, 2012. Statistics for Censored Environmental Data [Google Scholar]
- Holland EB, Feng W, Zheng J, Dong Y, Li X, Lehmler HJ, Pessah IN, 2017. An Extended Structure-Activity Relationship of Nondioxin-Like PCBs Evaluates and Supports Modeling Predictions and Identifies Picomolar Potency of PCB 202 Towards Ryanodine Receptors. Toxicol. Sci. 155, 170–181. 10.1093/toxsci/kfw189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomopoulou MP, Olszowy H, Hodge M, Bradley AJ, 2009. The effect of organochlorines and heavy metals on sex steroid-binding proteins in vitro in the plasma of nesting green turtles, Chelonia mydas. J. Comp. Physiol. B 179, 653–662. [DOI] [PubMed] [Google Scholar]
- Jin L, Escher BI, Limpus CJ, Gaus C, 2015. Coupling passive sampling with in vitro bioassays and chemical analysis to understand combined effects of bioaccumulative chemicals in blood of marine turtles. Chemosphere 138, 292–299. 10.1016/j.chemosphere.2015.05.055 [DOI] [PubMed] [Google Scholar]
- Jones KC, de Voogt P, 1999. Persistent organic pollutants (POPs): State of the science. Environ. Pollut. 100, 209–221. 10.1016/s02697491(99)00098-6 [DOI] [PubMed] [Google Scholar]
- Juarez-Ceron JA, Sarti-Martinez AL, Dutton P, 2003. First study of the green/black turtles of the Revillagigedo Archipelago: a unique nesting stock in the Eastern Pacific. NOAA Tech. Memo. NMFS-SEFSC 503, 70–73. [Google Scholar]
- Keller JM, 2013. Exposure to and Effects of Persistent Organic Pollutants, in: The Biology of Sea Turtles Vol III pp. 285–328. [Google Scholar]
- Keller JM, Balazs GH, Nilsen F, Rice M, Work TM, Jensen BA, 2014a. Investigating the Potential Role of Persistent Organic Pollutants in Hawaiian Green Sea Turtle Fibropapillomatosis. Environ. Sci. Technol. 48, 7807–7816. 10.1021/es5014054 [DOI] [PubMed] [Google Scholar]
- Keller JM, Kucklick JR, Harms CA, Clellan Mc Green PD, 2004. Organochlorine contaminants in sea turtles: Correlations between whole blood and fat. Environ. Toxicol. Chem. 23, 726–738. [DOI] [PubMed] [Google Scholar]
- Keller JM, McClellan-Green P, 2004. Effects of organochlorine compounds on cytochrome P450 aromatase activity in an immortal sea turtle cell line. Mar Env. Res 58, 347–351. 10.1016/j.marenvres.2004.03.080 [DOI] [PubMed] [Google Scholar]
- Keller JM, Pugh RS, Becker PR, 2014b. Biological and Environmental Monitoring and Archival of Sea Turtle Tissues (BEMAST): Rationale, Protocols, and Initial Collections of Banked Sea Turtle Tissues. National Institute of Standards and Technology, Gaithersburg, Maryland: 10.6028/nist.ir.7996 [DOI] [Google Scholar]
- Kenet T, Froemke RC, Schreiner CE, Pessah IN, Merzenich MM, 2007. Perinatal exposure to a noncoplanar polychlorinated biphenyl alters tonotopy, receptive fields, and plasticity in rat primary auditory cortex. Proc. Natl. Acad. Sci. USA 104, 7646–7651. 10.1073/pnas.0701944104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komoroske LM, Lewison RL, Seminoff JA, Deheyn DD, Dutton PH, 2011. Pollutants and the health of green sea turtles resident to an urbanized estuary in San Diego, CA. Chemosphere 84, 544–552. 10.1016/j.chemosphere.2011.04.023 [DOI] [PubMed] [Google Scholar]
- Lewison R, Lung C-T, Seminoff J, Deheyn D, 2011. Chemical Analysis of Threatened and Endangered Species in San Diego, San Diego: Port of San Diego. [Google Scholar]
- Lohmann R, Breivik K, Dachs J, Muir D, 2007. Global fate of POPs: Current and future research directions. Environ. Pollut. 150, 150–165. 10.1016/j.envpol.2007.06.051 [DOI] [PubMed] [Google Scholar]
- Lutz PL, Musick JA, Wyneken J, 2002. The biology of sea turtles. CRC press. [Google Scholar]
- Lyons K, Lavado R, Schlenk D, Lowe CG, 2014. Bioaccumulation of organochlorine contaminants and ethoxyresorufin-O-deethylase activity in southern California round stingrays (Urobatis halleri) exposed to planar aromatic compounds. Environ. Toxicol. Chem. 33, 1380–1390. 10.1002/etc.2564 [DOI] [PubMed] [Google Scholar]
- Lyons K, Lowe CG, 2015. Organochlorine contaminants and maternal offloading in the lecithotrophic Pacific angel shark (Squatina californica) collected from southern California. Mar. Pollut. Bull. 97, 518–522. 10.1016/j.marpolbul.2015.05.019 [DOI] [PubMed] [Google Scholar]
- MacDonald BD, Lewison RL, Madrak SV, Seminoff JA, Eguchi T, 2012. Home ranges of East Pacific green turtles Chelonia mydas in a highly urbanized temperate foraging ground. Mar. Ecol. Prog. Ser. 461, 211–221. 10.3354/meps09820 [DOI] [Google Scholar]
- Maechler M, Rousseeuw P, Struyf A, Hubert M Hornik K, 2016. cluster: Cluster Analysis Basics and Extensions. R package version 2.0.5. [Google Scholar]
- Mazaris AD, Schofield G, Gkazinou C, Almpanidou V, Hays GC, 2017. Global sea turtle conservation successes. Sci. Adv. 3, e1600730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald D, Dutton P, Mayer D, Merkel K, 1994. Review of the Green Turtles of South San Diego Bay in Relation to the Operations of the SDG&E South Bay Power Plant. San Diego Gas and Electric Company, San Diego. [Google Scholar]
- Munoz CC, Vermeiren P, 2019. Maternal Transfer of Persistent Organic Pollutants to Sea Turtle Eggs: A Meta-Analysis Addressing Knowledge and Data Gaps Towards an Improved Synthesis of Research Outputs. Environ. Toxicol. Chem. 0–3. 10.1002/etc.4585 [DOI] [PubMed] [Google Scholar]
- Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, Hara RBO, Simpson GL, Solymos P, Stevens MHH, Wagner H, 2015. Community Ecology Package ‘vegan’. R version 23–1. Available: https://cran.rproject.org/web/packages/vegan/. [Google Scholar]
- Pessah IN, Cherednichenko G, Lein PJ, 2010. Minding the calcium store: Ryanodine receptor activation as a convergent mechanism of PCB toxicity. Pharmacol. Ther. 125, 260–285. 10.1016/j.pharmthera.2009.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessah IN, Hansen LG, Albertson TE, Garner CE, Ta TA, Do Z, Kim KH, Wong PW, 2006. Structure− activity relationship for noncoplanar polychlorinated biphenyl congeners toward the ryanodine receptor-Ca2+ channel complex type 1 (RyR1). Chem. Res. Toxicol. 19, 92–101. [DOI] [PubMed] [Google Scholar]
- Pugh RS, Becker PR, 2001. Sea Turtle Contaminants: A Review with Annotated Bibliography. US Department of Commerce, National Institute of Standards and Technology, Gaithersburg. [Google Scholar]
- Rozman KK, Klaassen CD, 2007. Casarett and Doull’s toxicology: The basic science of poisons. [Google Scholar]
- Schiff K, Gossett R, Ritter K, Tiefenthaler L, Dodder N, Lao W, K. M, 2011. Southern California Bight 2008 Regional Monitoring Program: III: Sediment Chemistry. Costa Mesa: Southern California Coastal Water Research Project. [Google Scholar]
- Seminoff JA, Allen CD, Balazs GH, Dutton PH, Eguchi T, Haas H, Hargrove SA, Jensen Mi.P., Klemm DL, Lauritsen AM, 2015. Status Review of the Green Turtle (Chelonia mydas) Under the Engangered Species Act. NOAA, La Jolla, CA. [Google Scholar]
- Sindermann CJ, 2005. Coastal Pollution: Effects on Living Resources and Humans. CRC Press, Boca Raton. [Google Scholar]
- Srogi K, 2008. Levels and congener distributions of PCDDs, PCDFs and dioxin-like PCBs in environmental and human samples: A review. Environ. Chem. Lett. 6, 1–28. 10.1007/s10311-007-0105-2 [DOI] [Google Scholar]
- Stewart KR, Keller JM, Templeton R, Kucklick JR, Johnson C, 2011. Monitoring persistent organic pollutants in leatherback turtles (Dermochelys coriacea) confirms maternal transfer. Mar. Pollut. Bull. 62, 1396–1409. 10.1016/j.marpolbul.2011.04.042 [DOI] [PubMed] [Google Scholar]
- Swarthout RF, Keller JM, Peden-Adams M, Landry AM, Fair PA, Kucklick JR, 2010. Organohalogen contaminants in blood of Kemp’s ridley (Lepidochelys kempii) and green sea turtles (Chelonia mydas) from the Gulf of Mexico. Chemosphere 78, 731–741. 10.1016/j.chemosphere.2009.10.059 [DOI] [PubMed] [Google Scholar]
- van de Merwe JP, Hodge M, Olszowy HA, Whittier JM, Lee SY, 2010a. Using blood samples to estimate persistent organic pollutants and metals in green sea turtles (Chelonia mydas). Mar. Pollut. Bull. 60, 579–588. 10.1016/j.marpolbul.2009.11.006 [DOI] [PubMed] [Google Scholar]
- van de Merwe JP, Hodge M, Whittier JM, Ibrahim K, Lee SY, 2010b. Persistent organic pollutants in the green sea turtle Chelonia mydas: Nesting population variation, maternal transfer, and effects on development. Mar. Ecol. Prog. Ser. 403, 269–278. 10.3354/meps08462 [DOI] [Google Scholar]
- Vijayasarathy S, Baduel C, Hof C, Bell I, del Mar Gómez Ramos M, Ramos MJG, Kock M, Gaus C, 2019. Multi-residue screening of non-polar hazardous chemicals in green turtle blood from different foraging regions of the Great Barrier Reef. Sci. Total Environ. 652, 862–868. 10.1016/j.scitotenv.2018.10.094 [DOI] [PubMed] [Google Scholar]
- Wayman GA, Yang D, Bose DD, Lesiak A, Ledoux V, Bruun D, Pessah IN, Lein PJ, 2012. PCB-95 promotes dendritic growth via ryanodine receptordependent mechanisms. Env. Heal. Perspect 120, 997–1002. 10.1289/ehp.1104832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber R, Gaus C, Tysklind M, Johnston P, Forter M, Hollert H, Heinisch E, Holoubek I, Lloyd-Smith M, Masunaga S, Moccarelli P, Santillo D, Seike N, Symons R, Torres JP, Machado, Verta M, Varbelow G, Vijgen J, Watson A, Costner P, Woelz J, Wycisk P, Zennegg M, 2008. Dioxin- and POPcontaminated sites--Contemporary and future relevance and challenges. Environ. Sci. Pollut. Res. Int. 15, 363–393. [DOI] [PubMed] [Google Scholar]
- Wigestrand MB, Stenberg M, Walaas SI, Fonnum F, Andersson PL, 2013. Nondioxin-like PCBs inhibit [(3)H]WIN-35,428 binding to the dopamine transporter: A structure-activity relationship study. Neurotoxicology 39, 18–24. 10.1016/j.neuro.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Yang D, Kim KH, Phimister A, Bachstetter AD, Ward TR, Stackman RW, Mervis RF, Wisniewski AB, Klein SL, Kodavanti PR, Anderson KA, Wayman G, Pessah IN, Lein PJ, 2009. Developmental exposure to polychlorinated biphenyls interferes with experience-dependent dendritic plasticity and ryanodine receptor expression in weanling rats. Environ. Health Perspect. 117, 426–435. 10.1289/ehp.11771 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.