Abstract
As training in helminthology has declined in the medical microbiology curriculum, many rare species of zoonotic cestodes have fallen into obscurity. Even among specialist practitioners, knowledge of human intestinal cestode infections is often limited to three genera, Taenia, Hymenolepis and Dibothriocephalus. However, five genera of uncommonly encountered zoonotic Cyclophyllidea (Bertiella, Dipylidium, Raillietina, Inermicapsifer and Mesocestoides) may also cause patent intestinal infections in humans worldwide. Due to the limited availability of summarized and taxonomically accurate data, such cases may present a diagnostic dilemma to clinicians and laboratories alike. In this review, historical literature on these cestodes is synthesized and knowledge gaps are highlighted. Clinically relevant taxonomy, nomenclature, life cycles, morphology of human-infecting species are discussed and clarified, along with the clinical presentation, diagnostic features and molecular advances, where available. Due to the limited awareness of these agents and identifying features, it is difficult to assess the true incidence of these ‘forgotten’ cestodiases as clinical misidentifications are likely to occur. Also, the taxonomic status of many of the human-infecting species of these tapeworms is unclear, hampering accurate species identification. Further studies combining molecular data and morphological observations are necessary to resolve these long-standing taxonomic issues and to elucidate other unknown aspects of transmission and ecology.
Key words: Bertiella, Cestodes, Cyclophyllidea, Dipylidium, Inermicapsifer, Mesocestoides, Raillietina, Zoonoses
Introduction
The order Cyclophyllidea includes the ‘classic’ tapeworms and represents the largest cestode order, with over 3000 named species (Mariaux et al., 2017). Perhaps the most familiar members are the intestinal tapeworms commonly infecting humans; Taenia solium, T. saginata, T. asiatica and Hymenolepis nana. Agents of human cestode infections other than these are seldom discussed, if even covered at all within medical educational curricula and textbooks. However, several other cestode genera exist that can colonize the intestinal tract human hosts and produce patent infections – but these also suffer from a great sparsity of clinical and diagnostic information, research and modern interest. In many clinical settings, even generic identification of tapeworm infections is either never performed or automatically ascribed to well-known agents (e.g. Taenia spp.) without a detailed examination of parasite proglottid, scolex or egg morphology. This further obscures the true diversity and occurrence of these zoonotic cestodiases.
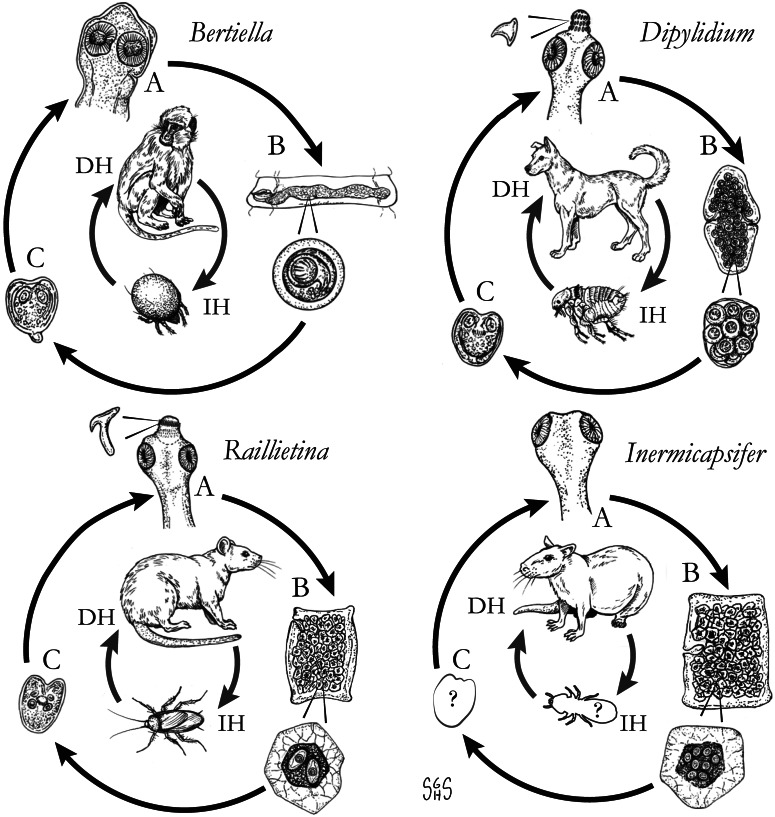
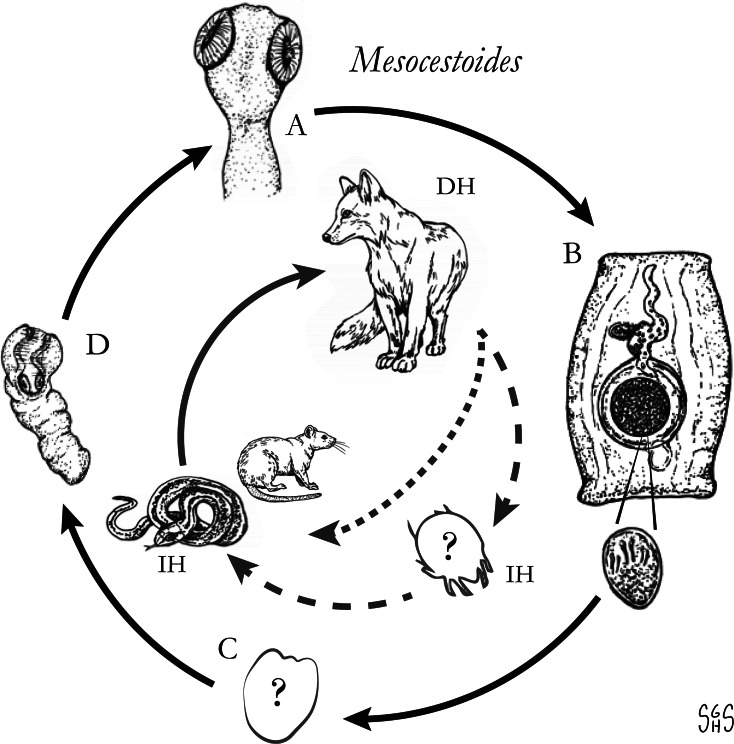
The major zoonotic, non-taeniid and non-hymenolepid cyclophyllidean genera that cause intestinal infections are Bertiella, Dipylidium, Raillietina, Inermicapsifer and Mesocestoides (Table 1) (Belding, 1965; Beaver et al., 1984). All of these genera are normally associated with a variety of mammalian definitive hosts – primates for Bertiella, carnivores for Dipylidium and Mesocestoides, and rodents for Raillietina and Inermicapsifer (Chandler and Pradatsundarasar, 1957; Goldsmid, 1964; James, 1968; Beaver et al., 1984). They undergo indirect life cycles typical of most cestodes, though several aspects of transmission or descriptions of life stages are unknown or poorly understood (Fig. 1). Assessing the true incidence of these infections in humans is difficult due to the relative scarcity of reports and potential misidentifications. Singular reports of other ‘unusual’ cyclophyllidean genera in human also exist [e.g. Drepanidotaenia (Belding, 1965), Mathevotaenia (Lamom and Greer, 1986), Moniezia (El-Shazly et al., 2004)]. However, their validity is not easy to evaluate, so this review will focus on the five genera. For each genus, life history, clinically-relevant taxonomy, morphological features and diagnosis, features of known human cases and treatment will be discussed.
Table 1.
Overview of characteristics of ‘unusual’ zoonotic agents within order Cyclophyllidea
| Bertiella | Dipylidium | Raillietina | Inermicapsifer | Mesocestoides | |
|---|---|---|---|---|---|
| Taxonomic authority | Stiles and Hassall (1902) | Leuckart 1863 | Fuhrmann 1920 | Janicki 1910 | Valliant 1863 |
| Family (Subfamily) | Anoplocephalidae (Anoplocephalinae) |
Dipylidiidae | Davaineidae (Davaineinae) |
Anoplocephalidaea (Inermicapsiferinae) |
Mesocestoididae (Mesocestoidinae) |
| Reported zoonotic species |
B. mucronata B. studeri B. satyri ? |
D. caninum |
R. celebensis R. demerariensis R. siriraji R. madagascarensisb |
I. madagascariensis ( = cubensis) ( = arvicanthidis) |
M. lineatus M. variabilis |
| Usual definitive host(s) | Primates: Old World (B. studeri) and New World (B. mucronata) | Canids and felids; most common in domestic species | Rodents (known zoonotic species)c, rarely primates | Rodents: Natal multimammate rat (Mastomys natalensis), Gambian pouched rat (Cricetomys gambianus); Rock hyrax (Procavia capensis) | Terrestrial carnivores; e.g. canids, felids, procyonids, mustelids, some marsupials |
| Usual intermediate host(s) | Oribatid mites | Fleas (Ctenocephalides spp.), dog louse (Trichodectes canis) | Insects; ants, cockroaches, beetles, etc. | Unknown | First intermediate host unknowna; Second intermediate hosts are numerous mammals, birds, reptiles, amphibians |
| Geographic range | B. mucronata: South America; B. studeri: South East Asia, South Asia, Middle-East, Sub-Saharan Africa, Mauritius, St. Kitts. | Cosmopolitan | Likely cosmopolitan; human infections in Southeast Asia, Australia, Hawaii, South America | Sub-Saharan Africa and West Indies | Cosmopolitan in animal hosts; most human cases from East Asia, USA |
| Scolex | Four large cup shaped suckers; rostellum and hooks absent -B. satyri has a knob-like projection on the apex of its scolex. |
Four round to oval suckers; Rostellum armed with multiple transverse rows of rose thorn-shaped hooks. | Four round suckers with small spines; rostellum armed with alternating small/large hammer-shaped hooks; rostellar sac lined with small accessory spines. | Four round, aspinous suckers; rostellum and hooks absent. | Four large, round suckers; rostellum and hooks absent. |
| Proglottidsd (mm) | 6–15 × 1–3 mm; much broader than tall; lateral genital pore. | 7 × 3 mm; Highly motile and variable in length, elongate, rounded, ‘cucumber seed’ shape; two opposing genital pores situated on the median lateral margins. | 3 × 2 mm; Barrel-shaped, containing numerous egg capsules (100–400), which can obscure genital organs; genital pore on anterior portion of lateral margin. | 3 × 2.5 mm; Numerous egg capsules; genital pore on median lateral margin. | 2.25 × 1.3 mm; Barrel-shaped, with parauterine organ; genital pore opens centrally on ventral surface. |
| Eggs | 35–62 μm; Spherical, with a filamentous vitelline membrane; well-developed pyriform apparatus surrounding oncospheres. | 40–50 μm; Hymenolepis-like, spherical, thin-shelled, colourless. Usually passed in proglottid, bound in packets containing ~10–20 eggs. | Most species 34–60 × 20–45, R. siriraji larger (100–115 × 38–42 μm); Oval, colourless shell with visible vitelline membranes; embryophore tapered to spindle-shaped around oncospheres, Usually passed in proglottid, contained in polygonal capsule with 1–4 eggs (for known zoonotic spp.). | 35 × 50 μm in diameter. Usually passed in proglottid, contained in polygonal capsule with 8–15 eggs. | 40 × 30 μm; Slightly oblong to tapered, colourless, lacking a shell but with a thin embryophore. Passed contained in intact proglottid; dies quickly when released from parauterine organ. |
| Immature stagee | Cysticercoid | Cysticercoid | Cysticercoid | Likely cysticercoid | Tetrathyridium |
Disputed; see relevant section(s) in text.
Not a valid name, but frequently reported in historical literature; most likely represents a combination of R. celebensis, other Raillietina spp. and I. madagascarensis.
Most Raillietina spp. infect avian definitive hosts, though these are not recognized as zoonotic species.
Measurements and descriptions for gravid, posterior proglottids.
Immature stage infective to the definitive host.
Fig. 1.
Generalized life cycles for Bertiella, Dipylidium, Raillietina and Inermicapsifer spp. Cestode stages shown on the outside: (A) scolex of an adult; (B) gravid proglottids with egg(s); (C) cysticercoid; representative definitive hosts (DH) and intermediate hosts (IH) on the inside (Drawings by SGH Sapp).
Bertiella
Generic taxonomy and morphology
Bertiella species are cestodes of primates in both the New and Old Worlds; they also infect rodents, dermopterans and Australian marsupials. This is the only genus of the Anoplocephalidae tapeworms known to infect humans. Blanchard first described the genus as ‘Bertia’ in great apes. Stiles and Hassall (1902) revised the generic name to Bertiella, as the name ‘Bertia’ had already been applied to a group of terrestrial land snails (type strain Bertia cambojiensis) in 1888 by Ancey.
Bertiella spp. scolices are sub-globose with a rudimentary, unarmed rostellum (Fig. 2A) (Belding, 1965). The suckers are large and round; two on the ventral surface and two on the dorsal surface (Schmidt, 1986). The base of the scolex is markedly differentiated from the neck of the worm. Proglottids are craspedote, extended transversely and are far wider than they are long (Belding, 1965; Schmidt, 1986). The longitudinal osmoregulatory canals (excretory ducts) are paired dorsally and ventrally on either side of the proglottid, the ventral duct being much larger than the dorsal duct (Stunkard, 1940; Stunkard et al., 1964). A large coiled transverse canal connects the longitudinal canals (Stunkard, 1940; Beveridge, 1985; Foitová et al., 2011). Mature proglottids possess a single genital pore; this single genital opening irregularly alternates left to right across the length of the strobila (Foitová et al., 2011). The central ovary is fan-shaped and situated on the poral side of the midline (Stunkard et al., 1964), and the ‘C’-shaped vitellarium is dorsal and posterior to the ovary (Stunkard et al., 1964; Beveridge, 1985). A single wide transverse uterus across the centre of the proglottid may extend past the longitudinal osmoregulatory canals. Testes are anterior and dorsal, forming a transverse band which, depending on the species, may or may not extend beyond the longitudinal osmoregulatory canals (Stunkard, 1940; Stunkard et al., 1964; Beveridge, 1985; Galán-Puchades et al., 2000; Foitová et al., 2011). As the proglottids become gravid, the uterus swells with eggs to fill the entirety of the proglottid (Fig. 2B) and the uterus branches into elongate posterior and anterior diverticula as it expands (Beveridge, 1985; Galán-Puchades et al., 2000). The vitellarium, testes and ovary become diminished, but the cirrus sac, vagina and seminal receptacle are retained (Stunkard et al., 1964).
Fig. 2.
Specimens of Bertiella studeri. (A) Carmine-stained scolex; (B) single elongate, gravid proglottid (scale bar = 1 cm), (C) multiple eggs showing pyriform apparati; (D) singular egg, showing oncosphere with hooklets (arrow). Photos courtesy of DPDx, Centers for Disease Control and Prevention.
Ovoid eggs (Fig. 2C) are liberated from the proglottids and shed into host feces (Belding, 1965). These mature eggs have a shell and an inner envelope with an albuminous layer between. The delicate inner envelope contains a distinctive pyriform apparatus (Galán-Puchades et al., 2000). A bicornate protrusion at the apex of the pyriform apparatus is made up of two opposed tubular components and is only visible when viewed along the longitudinal axis of the pyriform apparatus (Beveridge, 1985). Distinct filaments extend out from the two arms of the bicornate protrusion (Beveridge, 1985). Within the pyriform apparatus, a distinctive, round, active oncosphere containing hooklets may be observed (Stunkard, 1940; Beveridge, 1985) (Fig. 2D). When observed in hyperosmotic solutions, such as seen during salt flotation, the eggs may appear flattened on one side or irregular in form, with folds, wrinkles or even vacuolated regions observed in the shell (Stunkard, 1940).
Life cycle and hosts
Currently, there are 29 known species of Bertiella infecting Marsupialia, Rodentia and Dermoptera in Asia, Papua New Guinea and Australia (Beveridge, 1985; Denegri and Perez-Serrano, 1997) and primates from Asia, Africa, South America and some Caribbean and Indian Ocean islands (Denegri and Perez-Serrano, 1997). The adult tapeworms reside within the lower two-thirds of the small intestine of the definitive host, attached by the four suckers present on the scolex (Belding, 1965). In addition to liberated eggs, mature gravid proglottids may also be passed in the feces individually or in strobilar fragments of around 8–16 segments. Based on the activity of the oncosphere, eggs are likely infective to the intermediate host immediately after passage. In dry conditions, oncospheric embryos gradually reduce the activity and die after approximately 1 week. Hot temperatures may also inactivate the eggs. When stored in cool, moist environments, oncospheres remained active after 2–3 months. When stored in water, most died after 5–6 weeks (Stunkard, 1940).
Oribatid mites are the intermediate hosts of Bertiella species and many other Anoplocephalidae (Fig. 1) (Denegri, 1993). These mites consume Bertiella eggs in the environment and oncospheres will hatch within the mite. Cysticercoids begin to form within 9 days; they are pyriform in shape and measure 130–160 × 100–120 μm with a visible invaginated scolex (Stunkard, 1940). In one experiment, infected mites dissected 76 days after exposure contained identifiable cysticercoids, though it could not be determined if these were still viable (Stunkard, 1940). These intermediate host mites live naturally in cool and moist soil and frequently fruit (Stunkard, 1940; Denegri and Perez-Serrano, 1997). Consumption of vegetation, fruit or soil containing mites by primate definitive hosts completes the life cycle (Fig. 1).
Zoonotic species
Only Bertiella studeri and B. mucronata are currently recognized as infecting humans (Denegri and Perez-Serrano, 1997), though B. studeri may in fact represent a species complex that may include some zoonotic members. Following the resurrection of B. satyri and its separation from B. studeri (Foitová et al., 2011), early reports of B. satyri infection of humans may warrant re-investigation (Chandler, 1925). Regardless of the species, primates are the reservoir hosts of all Bertiella species currently recognized as infecting humans.
Bertiella studeri: Blanchard's original description of Bertiella contained two separate species of the new genus of cestode, including Bertia [sic] satyri in a Bornean orangutan (Simia satyrus; now Pongo pygmaeus) and Bertia [sic] studeri from an African chimpanzee (Troglodytes niger; now Pan troglodytes). In 1927, Baer synonymized B. studeri and B. satyri, with B. studeri as the senior synonym (Baer, 1927). However, recent work based on a molecular and morphologic investigation by Foitova et al. has resurrected the species B. satyri (Foitová et al., 2011). This report also suggested that many Old World Bertiella human infections from outside of Africa reported as B. studeri may represent B. satyri or another species of Bertiella (Foitová et al., 2011). Furthermore, investigation of multiple B. studeri-type specimens by Galán-Puchades et al. (2000), expanding on earlier observations by Spasski (1951) suggested that B. studeri should be considered a species complex containing at least four separate types; B. studeri sensu (Stunkard, 1940), B. studeri sensu (Bourquin, 1905), B. studeri sensu (Kagei et al., 1992) and B. studeri sensu (Ando et al., 1996).
Reported B. studeri morphology and morphometrics may be compromised by the possibility raised by Galán-Puchades et al. that this cestode in fact represents a species complex. Further investigation of whether the differences in morphology indeed do represent different species or sub-species, or simply reflect natural variation between different individuals or hosts, is warranted. The difficulties in determining the true number of species comprising ‘B. studeri’ has indicated the urgent need for comparative genotyping of specimens from different geographic locations and hosts, with particular references to primates, including humans, to elucidate the true nature of species within the genus.
Bertiella studeri measure up to 300 mm long by up to 15 mm wide at the widest proglottid and up to 2.5 mm thick (Stunkard, 1940). Mature to post-mature gravid proglottids are 7.8–11.3 mm wide (mean 9.52 mm) by 1.43–2.55 mm long (mean 1.76 mm) (Stunkard, 1940). The adult worm may contain up to 600 proglottids in a single chain. Scolices measurements have been between 475 and 800 μm in diameter with the four oval suckers between 220 and 345 μm in diameter at the widest point and about 200 μm deep (Stunkard, 1940; Stunkard et al., 1964; Bhagwant, 2004). The neck is approximately 2.65–5.0 mm long (Stunkard, 1940). The proglottids increase in length and width with age. Stunkard (1940; Stunkard et al., 1964) described the development of the organs in a B. studeri taken from a rhesus monkey (Macaca mulatta) in captivity and another from a human child, with the development of genital organs starting between 90th and 130th segment distal to the scolex. Between segments 150 and 265, the genital organs appeared fully mature, with the uterus starting to fill with eggs by segments 330–350, and gravid proglottids filled with eggs were seen by segment 366 (Stunkard, 1940). Mature proglottids contained approximately 225–280 testes which may be between 36 and 95 μm in diameter (Stunkard, 1940; Denegri and Perez-Serrano, 1997; Galán-Puchades et al., 2000) but averaged 66 × 83 μm in one specimen (Galán-Puchades et al., 2000). The testes do not extend beyond the longitudinal osmoregulatory canals (Stunkard, 1940; Galán-Puchades et al., 2000) but the enlarged gravid uterus sometimes does. The unarmed cirrus sac measured between 360 and 440 μm in length and 700 and 1100 μm in width in one specimen (Stunkard et al., 1964) and 280–480 × 80–100 in another (Galán-Puchades et al., 2000). The ovary is positioned on the poral side of the midline and has been variously measured in three specimens as between 0.80 and 1.50 mm in diameter (Stunkard, 1940; Stunkard et al., 1964; Bhagwant, 2004). The C-shaped vitellarium is antero-lateral and opens towards the poral side and 500–600 μm wide in one specimen (Stunkard et al., 1964), 230–360 in another (Galán-Puchades et al., 2000). The dorsally situated shell gland is 180–220 μm in diameter. The seminal receptacle measures between 200 and 300 μm long in one specimen (Stunkard et al., 1964) and 340–480 in another (Galán-Puchades et al., 2000). The vagina is 51–110 μm long and 70–90 μm wide (Galán-Puchades et al., 2000).
The eggs of B. studeri are oval to irregularly ovoid, measuring 33–46 μm in width by 36–65 μm in length (Stunkard, 1940; Stunkard et al., 1964; Beveridge, 1985; Ando et al., 1994; Denegri and Perez-Serrano, 1997; Galán-Puchades et al., 2000; Bhagwant, 2004; Sun et al., 2006; Sharma et al., 2017). Notable size variance has been observed between hosts, which may represent natural variation, or may indicate multiple species within a B. studeri complex. For example, eggs passed by a human, probably infected in Kenya, were 37–51 μm (mean 45 μm) by 37–46 μm (mean 44 μm). The pyriform apparati within these eggs measured 19–28 μm in diameter (mean 23 μm) and the oncospheres in this specimen measured between 12 and 15 μm in diameter (mean 13 μm) (Galán-Puchades et al., 2000). This contrasts with larger eggs recovered from a captive M. mulatta which were 40–60 μm in diameter, containing pyriform apparati measuring 27–35 μm by 20–21 μm and oncospheres measuring 17–18 μm by 19–20 μm (Stunkard, 1940). It should be noted that the morphological features provided here for B. studeri are based on several samples taken from human infections. Recent work on the phylogenetic relationships of Bertiella species (Doležalová et al., 2015) has suggested that multiple species may infect humans in the Old World and thus some of the data summarized here may have been found to include multiple species on which molecular identification had been performed.
Reports exist from a wide variety of Old World primate hosts, including humans (Homo sapiens), chimpanzees (P. troglodytes), baboons (Papio ursinus and Papio doguera) and various monkeys (Chlorocebus sabaeus, M. mulatta, Macaca cynomolgus, Macaca fascicularis, Macaca fuscata, Macaca radiata, Macaca syrichta syrichta, Macaca syrichta fascicularis, Cercopithecus aethiops pygerythrus, Cercopithecus nictitians schmidtii, Cercopithecus neglectus, Cercopithecus mona mona, Cercopithecus sabaensis, Cercopithecus aethiops cyanosusus, Cercopithecus sinicus, Cercopithecus fascicularis) and gibbons (Hyalobates hoolock) (Ando et al., 1994; Denegri and Perez-Serrano, 1997). As noted previously, reports in Bornean orangutans (P. pygmaeus) may represent another species, B. satyri (Chandler, 1925; Foitová et al., 2011). Identified intermediate host oribatid mites of B. studeri are Schlerobates laevigatus and Galumna species (Stunkard, 1940) as well as Scutoverix minutus and Archipeteria coleoptrata. One report of B. studeri in a domestic dog from the Philippines is unexpected and may represent the transient passage of eggs following coprophagy (Africa and Garcia, 1935).
The first human case of B. studeri infection was reported (as B. satyri) from a child in Mauritius by Blanchard (1913). To date, 83 human cases of B. studeri infection have been reported, most acquired in Africa, Mauritius, the Middle East or South East Asia (Table 2). Several cases reported from countries where monkeys are not naturally found have included a history of contact with pet monkeys or zoological gardens (Denegri and Perez-Serrano, 1997). Infections are predominantly seen in children, though adults may also be affected (Denegri and Perez-Serrano, 1997). Humans are accidental hosts and human bertiellosis is most often seen in those with a history of association with monkeys or other non-human primates (Paçô et al., 2003). Some reported eating fruit in areas inhabited by monkeys (Bhagwant, 2004). Most infections have been reported in children (Denegri and Perez-Serrano, 1997). Adult B. studeri infecting humans have a lifespan of at least 2 years (Thompson et al., 1967). Bertiella studeri is generally considered to be restricted to the Old World, except for the Caribbean island of St. Kitts where one human case occurred and was traced back to the local green monkeys (C. sabaeus) which are of West African origin (Cameron, 1929). One puzzling case report of human infection with B. studeri from Brazil has been reported, based on the morphology of the proglottids and eggs. However, the morphometric data reported for these features are also consistent with B. mucronata (Lopes et al., 2015). This case may represent a misidentification of B. mucronata as B. studeri, but as discussed later in this review, the true taxonomic distinction and geographic distribution of these two species are not entirely clear and infections identified as ‘B. studeri’ may represent multiple species. Bertiellosis is traditionally considered to be an innocuous infection in human hosts (Denegri and Perez-Serrano, 1997). However, in several recent cases, various symptoms including abdominal distension, dyspepsia, nausea, diarrhoea, anorexia and perianal itching have been reported (Bhagwant, 2004; Sun et al., 2006; Sharma et al., 2017). Furthermore, the passage of strobilae per rectum either spontaneously or after treatment may cause a psychological disturbance in some patients.
Table 2.
Country of acquisition, age of patient and egg sizes reported for all published human cases of bertiellosis to date
| Geographical location of acquisition | Age (years) |
Reported egg size (μm) | Source | Notes and comments |
|---|---|---|---|---|
| Bertiella studeri | ||||
| Mauritius | 8 | nd | (Blanchard, 1913) | |
| India (Bihar or West Bengal) | 2 | 46–49 | (Chandler, 1925) | Originally reported as ‘B. satyri’* |
| India (West Bengal) | nd | nd | (Mukerji, 1927) | Originally reported as ‘B. satyri’* |
| West Indies (St. Kitts) | ‘young’ | nd | (Cameron, 1929) | St Kitts has monkeys of west African origin |
| India (Meghalaya) | nd | nd | (Sharma, 1930) | Originally reported as ‘B. satyri’* |
| India (Meghalaya) | nd | nd | “ | Originally reported as ‘B. satyri’* |
| India (Meghalaya) | nd | nd | “ | Originally reported as ‘B. satyri’* |
| India (East Bengal) | 8 | 46–49 | (Maplestone, 1930) | |
| Indonesia (Sumatra) | nd | nd | (Joyeux and Dollfus, 1931) | |
| Mauritius | 8 | nd | (Adams and Webb, 1933) | |
| Mauritius | 4 | nd | “ | |
| Mauritius | 7 | nd | (Adams, 1935) | |
| Philippines (Iloilo) | 8 | 55–73 | (Africa and Garcia, 1935) | |
| India (Uttar Pradesh) | 5 | nd | (Maplestone and Riddle, 1936) | |
| Bangladesh | 8 | nd | (Roy, 1938) | |
| Indonesia (Java) | 7 | nd | (Bonne, 1940) | |
| Kenya | 8 | nd | (Buckley and Fairley, 1950) | |
| Indonesia (Java) | 4 | nd | Lie Kian, 1961 (Pers Comm) | |
| Indonesia (Kalimantan) | 3.5 | nd | “ | |
| Singapore | 6 | nd | (Desowitz et al., 1961) | |
| USA (Minnesota) | 5 | 40–60 | (Stunkard et al., 1964) | Patient had close contact with a pet monkey |
| Yemen | nd | nd | (Fogh and Seaton, 1967) | |
| Yemen | 6 | nd | (Thompson et al., 1967) | Diagnosed in Britain in a returned traveller, reported as ‘Bertiella sp.’* |
| Indonesia (Sumatra) | 6.5 | nd | (Kwo and Koh, 1968) | |
| Indonesia (Sumatra) | 7 | nd | “ | |
| Indonesia (Sumatra) | 6 | nd | “ | |
| Indonesia (Sumatra) | 14 | nd | “ | |
| Indonesia (Sumatra) | 5 | nd | “ | |
| Indonesia (Sumatra) | 6 | nd | “ | |
| Democratic Republic of Congo | ‘young’ | nd | “ | |
| Republic of Congo | 2.5 | 45–47 × 48–50 | (Jones et al., 1971) | Diagnosed in Canada in a returned expatriate, reported as ‘Bertiella sp.’* |
| Sri Lanka | ‘pre-school child’ | nd | (Edirisinghe and Cumararajan, 1976) | Reported based on pers. comm. from Wijesundera, M.K. de S. 1975 |
| Sri Lanka | ‘pre-school child’ | nd | “ | Reported based on pers. comm. from Wijesundera, M.K. de S. 1975 |
| Sri Lanka | 4.5 | nd | “ | |
| Yemen | 25 | nd | (Imamkuliev et al., 1983) | Diagnosed in Russia from a Yemeni student |
| India | 29 | nd | (Subbannayya et al., 1984) | Reported as ‘Bertiella sp.’* |
| Thailand | 26 | nd | (Bhaibulaya, 1985) | |
| Saudi Arabia | 28 | nd | (Bolbol, 1985) | Reported as ‘Bertiella sp.’* |
| Gabon | 2 | nd | (Richard-Lenoble et al., 1986) | |
| India (West Bengal) | 9 | 44 × 48 | (Bandyopadhyay and Manna, 1987) | |
| Sri Lanka | ‘child’ | nd | (Weerasooriya et al., 1988) | |
| Sri Lanka | ‘child’ | nd | ‘ | |
| Indonesia (Sumatra) | 8 | nd | (Kosin and Kosin, 1992) | |
| Indonesia (Kalimantan) | 5 | nd | “ | |
| Indonesia (Sumatra) | 3.5 | nd | “ | |
| Indonesia (Sumatra) | ‘child’ | nd | “ | |
| Indonesia (Sumatra) | ‘child’ | nd | “ | |
| Indonesia (Sumatra) | ‘child’ | nd | “ | |
| Indonesia (Sumatra) | ‘child’ | nd | “ | |
| Indonesia (Sumatra) | ‘child’ | nd | “ | |
| Indonesia (Sumatra) | 3 | nd | (Kagei et al., 1992) | |
| Indonesia (Sumatra) | ‘adult’ | nd | “ | |
| Japan | 3 | nd | (Kojima et al., 1992) | |
| Japan | 2 | nd | (Iseki et al., 1993) | |
| India (Orissa) | 4 | 48–62 | (Panda and Panda, 1994) | |
| Kenya | 33 | 37–51 × 37–46 | (Galan-Puchades et al., 1995) | Diagnosed in Spain from a returned traveller |
| Japan | 23 | 47–54 | (Ando et al., 1996) | |
| Equatorial Guinea | 50 | 51–49 | (Galán-Puchades et al., 1997) | |
| Sri Lanka | 10.5 | nd | (Karunaweera et al., 2001) | |
| Vietnam | 4 | 41–50 | (Xuan le et al., 2003) | |
| Sri Lanka | 2.5 | nd | (Gallella et al., 2011) | |
| Saudi Arabia | 40 | nd | (El-Dib et al., 2004) | |
| Mauritius | 3–7 | 50–65 × 30–45 | (Bhagwant, 2004) | |
| Mauritius | 3–7 | nd | “ | |
| Mauritius | 3–7 | nd | “ | |
| Mauritius | 3.5 | nd | “ | |
| Mauritius | 32 | nd | “ | |
| South Africa (Western Cape) | 28 | nd | (Frean and Dini, 2004) | Probably acquired at a private zoo in Cape Town |
| Sri Lanka | 2.5 | nd | (Morawakkorala et al., 2006) | |
| Sri Lanka | 5 | nd | “ | |
| China (Anhui) | 3.5 | 38–50 | (Sun et al., 2006) | |
| Yemen | ‘Man’ | nd | (Achir et al., 2008) | Diagnosed in Algeria from a Yemeni student |
| ‘Africa’ | ‘young’ | 52–61 | (CDC-DPDx, 2009) | Diagnosed from a refugee living in Australia |
| Saudi Arabia (Jizan) | adult | 49–51 | (Al-Mathal et al., 2010) | Diagnosed in Egypt from a recently returned expatriate |
| Equatorial Guinea | 4 | 49–60 | (Lozano Mdel et al., 2010) | |
| Indonesia (Sumatra) | 2.5 | nd | (Anwar and Ghiffari, 2010) | |
| India (Haryana) | 4 | 44–36 | (Malik et al., 2013) | |
| South Africa (Gauteng) | 6 | nd | (Du Plooy, 2014) | |
| South Africa (Gauteng) | 27 | nd | “ | |
| India (Himachal Pradesh) | 2 | 46–65 | (Sharma et al., 2017) | |
| Bertiella mucronata | ||||
| Cuba | ‘young’ | 36–40 | (Cram, 1928) | |
| Brazil | 29 | nd | (Pessoa, 1930) | |
| Argentina | 46 | nd | (Bacigalupo, 1949) | |
| Paraguay | 29 | 38–46 | (d' Alessandro et al., 1963) | |
| Brazil (Minas Gerais) | nd | 38–41 | (de Costa et al., 1967) | |
| Argentina | 45 | nd | (Feldman et al., 1983) | |
| Argentina | 2 | nd | (Garaguso and Mendez, 1983) | |
| Brazil (Goiás) | 2 | 39–41 | (Paçô et al., 2003) | |
| Argentina (Corrientes) | 9 | nd | (Gené et al., 2011) | |
| Brazil (Pará state) | 4 | 42–47 × 41–46 | (Furtado et al., 2012) | |
| Brazil (Minas Gerais) | 8 | 40 × 41 | (da Silva et al., 2011) | |
| Brazil (Minas Gerais) | 2.5 | 42–47 | (Lopes et al., 2015) | Reported as ‘B. studeri’* |
*Note the species categories presented in this table are based on geography (B. studeri from the Old World, B. mucronata from the New World).
The diagnosis of B. studeri infection may be undertaken by the demonstration of distinctive eggs and/or proglottids of the appropriate size and morphology in a patient's stool. These proglottids are active and motile; upon passage and storage overnight at 4°C, they may induce relaxation to allow microscopic examination (Malik et al., 2013). Clearing whole proglottids and staining these with carmine or a similar dye may assist in the detection of identifying internal structures. Travel history should be carefully taken and include any contact with primates from areas where B. studeri is endemic. The recovery of the scolex following treatment may assist in the confirmation of the diagnosis if possible but may not be possible (Denegri and Perez-Serrano, 1997). To definitively confirm species identity and to assist in determining the true nature of B. studeri as a species, collection of proglottids into 70% ethanol or another DNA-supportive fixative (not formalin), followed by submission to a reference laboratory for further molecular identification, is strongly indicated.
Praziquantel and niclosamide have been used successfully for the treatment of several recent cases (Karunaweera et al., 2001; Bhagwant, 2004; Sun et al., 2006; Al-Mathal et al., 2010; Gallella et al., 2011; Malik et al., 2013). However, apparent treatment failure with niclosamide occurred in some recent reports (Al-Mathal et al., 2010; Gallella et al., 2011). In one case, subsequent to niclosamide failure, the patient was successfully treated with 6 days of extract of Myrrh (Commiphora molmol) herbal therapy (Al-Mathal et al., 2010) rather than the conventional praziquantel therapy.
Bertiella mucronata: In 1895, Meyner identified a New World monkey tapeworm, which was initially described from two samples collected by Neumeister in 1888 from black howler monkeys (Mycetes niger, now Alouatta caraya) in Paraguay. At the time, Meyner considered the genus ‘Bertia’ (now Bertiella) to represent only a sub-genus of Taenia and thus this parasite was called ‘Taenia (Bertia) mucronata’ (Meyner, 1895). Stiles corrected this name to ‘Bertia mucronata’ in 1897 and later (with Hassall) (Stiles and Hassall, 1902) revised this genus name to ‘Bertiella’ due to the detected homonymy.
The morphometrics of all reported human cases are summarized, with a comparative table of variations between reported ‘B. studeri’ and B. mucronata, in Table 3. Notable differences between B. mucronata and B. studeri are their respective geographic distributions, a lower number of testes, a smaller cirrus sac diameter and a longer vagina in B. mucronata. Few complete B. mucronata individual specimens have been measured, one from a titi monkey (Callicebinae) in Peru was 256 mm long (Gomez-Puerta et al., 2009), while an infected human in Paraguay passed strobili up to 250 mm in length (d’ Alessandro et al., 1963). The longest complete strobila from one probable B. mucronata from a chimpanzee at a zoo in Cuba (although the primate was originally from Africa, it was considered that the cestode was locally acquired) measured 400 mm (Cram, 1928). Width of the widest mature proglottid has been measured as 7.5–10 mm wide (Cram, 1928; d’ Alessandro et al., 1963; de Costa et al., 1967; Gomez-Puerta et al., 2009), gravid proglottids may measure up to 14 mm wide by 3 mm in thick (Cram, 1928). One mature individual worm from a human in Brazil possessed 700 proglottids (de Costa et al., 1967). The scolex measured 860 μm in diameter in one individual (de Costa et al., 1967) and 340 × 450 μm in another (Gomez-Puerta et al., 2009). The four oval suckers have been reported as either 240 × 260 μm (de Costa et al., 1967) or 210 μm (Gomez-Puerta et al., 2009) in diameter at the widest point. The neck is approximately 200–300 μm long (Cram, 1928). Proglottids contain up to between 120 and 140 testicular follicles, which are variously reported as 38–41 μm (de Costa et al., 1967), 60 μm (d’ Alessandro et al., 1963) and 80–100 μm (Gomez-Puerta et al., 2009) in diameter. The seminal receptacles measured 265 × 130 μm in one specimen (d’ Alessandro et al., 1963), but may grow up to a dimeter of between 350 and 400 μm in mature proglottids (d’ Alessandro et al., 1963; de Costa et al., 1967). The unlobed ovary is located in the centre of the proglottid and is 1.3 × 1.7 mm in diameter (de Costa et al., 1967). The cirrus sac averages 267 μm in length (d’ Alessandro et al., 1963). In mature proglottids, the vagina is 1.03–1.60 mm long, but may reach 2 mm in width when fully gravid (de Costa et al., 1967). Reported egg sizes from human cases of B. mucronata vary between 36 and 47 μm in maximum diameter (Stiles and Hassall, 1902; Cram, 1928; Furtado et al., 2012). Although eggs of B. mucronata are on average smaller than B. studeri, there is a considerable degree of overlap (Cram, 1928; d’ Alessandro et al., 1963; de Costa et al., 1967; Paçô et al., 2003; Gomez-Puerta et al., 2009; da Silva et al., 2011; Lopes et al., 2015). The pyriform apparatus within these eggs has been measured as 21–45 μm in diameter at the widest point and oncospheres as 9–16 μm in diameter (d’ Alessandro et al., 1963; Gomez-Puerta et al., 2009; Furtado et al., 2012; Lopes et al., 2015).
Table 3.
Comparative morphometrics of Bertiella studeri, Bertiella mucronata and Bertiella satyri proglottids from selected references
| B. studeri | B. mucronata | B. satyri | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Bhagwant (2004) | Stunkard (1940) | Stunkard, et al. (1964) | Galan-Puchades et al. (2000) | d'Alessandro et al. (1963) | de Costa, et al. (1967) | Cram (1928) | Chandler (1925) | Foitova et al. (2011) | |
| Homo sapiens | Homo sapiens | Homo sapiens | Homo sapiens | Homo sapiens | Homo sapiens | Homo sapiens | Homo sapiens | Pongo abelli | |
| Gravid proglottid width (mm) | 9.98 | 15 | 6 | 7.8–11.3 | nd | 14 | 14 | 11 | 14 |
| Gravid proglottid length (mm) | nd | 1 | 6.0 | 1.43–2.55 | nd | nd | nd | 1 | 2.9 |
| Gravid proglottid thickness (mm) | 1.82 | 3 | 1.76 | nd | nd | nd | 3 | 1.5 | nd |
| Number of testes | 214–225 | ‘about’ 250 | nd | 280 | 120–140 | 132 | ‘about’ 100 | 75 | 116–124 |
| Diameter of testes (μm) | 76–69 | 36–95 | 70–100 | 68–80 | 60 | 38–41 | 80–100 | 65–70 | 52–111 |
| Maximum diameter of cirrus sac (μm) | 600–900 | 300–500 | 360–440 | 280–480 | 267 | nd | nd | 310 | 322 |
| Maximum diameter of ovary (mm) | 1.15–0.38 | 0.5–0.9 | 0.80–1.5 | 1.54–0.37 | nd | 1.7 | nd | nd | nd |
| Maximum length of seminal receptacle (μm) | 360–900 | 200–300 | 200–300 | 480–670 | 265 | 400 | nd | 360 | nd |
| Maximum width of seminal receptacle (μm) | 200–450 | nd | nd | 310–480 | 130 | 240 | nd | nd | nd |
| Maximum length of vagina (μm) | 330–540 | 400–600 | nd | 1100–1170 | nd | 1600 | nd | >500 | 790–984 |
| Alternation of genital pores | irregular | nd | nd | irregular | nd | nd | irregular | nd | irregular |
New World monkeys are the natural definitive hosts of B. mucronata. Reported species found to be infected are A. caraya, Alouatta guariba clamitans, Callicebus personatus nigrifrons, Callicebus oenanthe, Cebus apella fatuellus, Cebus capucinus and Callithrix sagui (Denegri and Perez-Serrano, 1997; de Souza Júnior et al., 2008; Gomez-Puerta et al., 2009). Recognized intermediate hosts of B. mucronata are Dometorina suramericana and Schlerobates atahualpensis (Denegri, 1993).
Eleven human cases of B. mucronata infection have been reported in the literature thus far. As with B. studeri, the majority were in children, but four of the infections were reported from adults. All cases have originated in South America (Brazil, Northern Argentina and Paraguay) or Cuba (Table 2) with over half of these reported from Brazil. Non-human primate infections have been reported from Peru (Gomez-Puerta et al., 2009). Many historical case reports did not include clinical history, but one case from Paraguay reported intermittent constipation and diarrhoea (d’ Alessandro et al., 1963), while another from Brazil reported only nocturnal abdominal distension (Furtado et al., 2012). Two cases from Brazil presented with marked symptoms of abdominal pain, combined with intermittent vomiting, dyspepsia, anorexia and diarrhoea (Paçô et al., 2003; da Silva et al., 2011). All cases with clinical history reported the passage of tapeworm proglottids and/or strobilae per rectum (d’ Alessandro et al., 1963; de Souza Júnior et al., 2008; da Silva et al., 2011; Furtado et al., 2012; Lopes et al., 2015). Recently reported cases of bertiellosis due to B. mucronata for which treatment is described have all been successfully treated with praziquantel (Furtado et al., 2012; Lopes et al., 2015).
The most recent Bertiella infection reported from Brazil (Lopes et al., 2015) was identified as B. studeri based on limited features of proglottid morphology, egg size and morphology. While human infections from South America have traditionally been identified as B. mucronata based on geography and egg size, there is a significant variation in the egg diameters reported from these infections (Table 3). Recent molecular work found that a ‘B. mucronata’ isolate from a human in Brazil most closely clustered with B. studeri and B. satyri specimens from non-human primates and a human in Africa and Asia. Based on these findings, it seems likely that multiple species of Bertiella species may infect humans in the New World and the Old World, and that traditional morphologic identification and species assignment to human infections will require future revision in consideration of molecular findings.
Differentiating features of currently reported human-infecting species
Although early reports of B. satyri infection of humans probably represent a misidentification of B. studeri, this species can infect large primates and it remains unclear if some past human infections with this species may have occurred. We include it in this section to allow the correct identification of any potential future B. satyri infections of humans. Comparative morphometrics of Bertiella studeri, Bertiella mucronata and Bertiella satyri proglottids are described in Table 3. Bertiella mucronata is found in the Americas only and has ⩽150 testes per proglottid, whereas as B. studeri has >200 (d’ Alessandro et al., 1963), B. satyri has thus far only reported from the Old World and has approximately 75–124 testes (Chandler, 1925; Foitová et al., 2011). The vagina is much longer in the examples of B. mucronata and B. satyri, extending medially from the edge of the proglottid to the ovary, well past the lateral osmoregulatory canals, whereas the B. studeri vagina does not extend past these canals for any considerable distance (Chandler, 1925; Cram, 1928; Stunkard, 1940; d’ Alessandro et al., 1963; Foitová et al., 2011). The cirrus sac is weakly developed and the vagina is strongly developed and muscular in B. mucronata and B. satyri, whereas in B. studeri, the cirrus sac is strongly developed and muscular, but a weakly developed, amuscular vagina is seen (Cram, 1928; Foitová et al., 2011). Chandler (1925) and Foitova et al. (2011) describe B. satyri as having regularly alternating genital pores whereas B. studeri does not, but the original description of B. satyri by Blanchard (1891; Blanchard, 1913) describes irregularly alternating genital pores. Furthermore, specimens of B. studeri well described by Galan-Puchades et al. (2000) and Bhagwant (2004) possess alternating genital pores. It should be noted that the former specimen had an unusually long vagina for B. studeri and may represent another, as yet unidentified, species of Bertiella (Galán-Puchades et al., 2000). The scolex of B. satyri is easily differentiated from that of B. studeri and B. mucronata by the presence of a knob-like projection (possibly a rudimentary rostellum) on its apex (Chandler, 1925; Foitová et al., 2011).
As noted previously, the taxonomy of Bertiella species was in flux during the late 19th and early 20th centuries. Five separate species names were synonymized by Baer (1927), into B. studeri, based on the opinion that variations in morphometrics in these five Bertiella species in Africa and Asia were not sufficiently pronounced to allow species differentiation. Simply, Old World isolates from humans were B. studeri and New World isolates were B. mucronata (except for those acquired on St. Kitts). Defined anatomical features and measurements may not be entirely reliable for species identification, as variations may be seen based on the stage of sexual maturity of the proglottids and on the degree of muscular contraction at the time of fixation (Stunkard, 1940). Furthermore, there is a wide variation in the reported size of the scolices and suckers and eggs, even within individual species. Finally, for both B. studeri and B. mucronata, very few individual specimens have thus far been thoroughly examined and documented to obtain reliable estimates of the size range of individual anatomical features. The recent resurrection of the human-infecting species B. satyri and the advent of molecular phylogenetics have made this simplistic approach to the taxonomic identification of human-infecting Bertiella species untenable.
Molecular biology
Relatively, few studies on the molecular biology of Bertiella spp. have been conducted. In one study, B. studeri specimens from Mauritius were determined to be monophyletic members of the family Anoplocephalidae based on 18s rRNA gene sequences. When these sequences were analysed by the distance-based neighbour-joining method, Bertiella and other Anoplocephalidae were found to be most closely related to the Hymenolepididae. However, this close relationship was not supported when the maximum parsimony method was employed (Taleb-Hossenkhan and Bhagwant, 2012).
A recent study employed more phylogenetic markers (28S rRNA, partial 5.8S-ITS2 rRNA, cox1 and nad1) to investigate the phylogeny of the genus Bertiella (Doležalová et al., 2015). This study compared the Bertiella sequences from multiple non-human primate and human sources in Africa, Asia and South America. Bertiella species from humans and non-human primates were found to be monophyletic and within the family Anoplocephalidae. However, analysis of 28s rRNA sequences found that the relationship of Bertiella from Australian rodents and marsupials was paraphyletic to other Bertiella species, with the authors suggesting that the latter might be split into a new genus. This work demonstrated a high degree of heterogeneity within the Bertiella spp. analysed. Sequences of the nad1 locus showed close relationships between Bertiella species taken from P. troglodytes in Kenya, H. sapiens in Brazil, Pongo abelii in Indonesia and a human infection acquired in Equatorial Guinea. Analysis of the partial 5.8S-ITS2 rRNA locus showed close phylogenetic relationships between the Bertiella sp. from a human in Brazil, B. satyri from P. abelii in Indonesia, B. studeri from P. troglodytes in Kenya, the Equatorial Guinea-acquired human B. studeri isolate and two ostensibly B. studeri isolates from M. fuscata in Japan. A separate group clustered a B. mucronata taken from Callicebus oecanthe in Peru with isolates from P. troglodytes in Uganda, Gorilla gorilla in the Central African Republic and an Anoplocephala gorillae-like cestode from a Gorilla beringei in Rwanda. The summary of this work is that humans and non-human primates may be infected with multiple species of Bertiella and that future species identification of infecting isolates should be performed using a combination of morphology and standardized molecular techniques.
Dipylidium
Taxonomy and morphology
Known by many names such as the ‘dog tapeworm’, ‘double-pored tapeworm’, ‘flea tapeworm’ and ‘cucumber tapeworm’, Dipylidium caninum is a well-known parasite to pet owners and veterinarians. It is a member of the family Dipylidiidae, which includes various small- to medium-sized tapeworms that are parasitic in carnivorous mammals with retractable, typically armed rostella (Khalil et al., 1994; Hoberg et al., 1999). An important feature of the Dipylidiidae is that the gravid uterus is replaced by multiple egg capsules (Wardle and McLeod, 1952). Several species of Dipylidium have been described over the years, but many names are likely to be invalid. Venard (1937) regarded only three species of Dipylidium as valid; their occurrence in humans is not known and the two other non-D. caninum species are poorly studied. Thus, only D. caninum will be discussed here.
Grossly, D. caninum is a relatively robust tapeworm, measuring 10–70 cm in length and around 3 mm in width, comprised of about 60–175 proglottids. Immature proglottids are trapezoidal in shape, while gravid proglottids shed in feces have a crawling motility and a particular convex shape, often described as resembling cucumber seeds (thus leading to one of its many common names). Dipylidium, unlike many of the other more commonly-encountered human-infecting cyclophyllidea, has two genital pores per proglottid. The genital pores open approximately in the middle of the lateral margins and are arranged directly opposite to each other. The double-pored morphology may be apparent to the naked eye as small, subtle indentations on mature proglottids. There are two sets of reproductive organs within a proglottid, each associated with a genital pore. Bilobed ovaries and a web-like uterus are situated posterior to the opening, and a vas deferens and cirrus sac sit just anterior to the genital pore openings. The testes are numerous and occupy most of the space between the osmoregulatory canals. In fully mature terminal proglottids, the uterus breaks down into egg capsules that fill most of the proglottid. Eggs are present in clusters of usually 10–20 eggs bound by a thin membrane. Individual eggs are 40–50 μm, colourless, spherical and with a thin shell and embryophore (Miyazaki, 1991).
The scolex is 0.25–0.50 mm wide and roughly spade-shaped when the muscular rostellum is extruded. The rostellum is armed with multiple transverse rows of ‘rose-thorn’ hooks. The number of rows is usually reported as three in D. caninum, but the actual number may be difficult to count due to a spiraled arrangement in some specimens (Venard, 1937).
Life cycle and hosts
Intact motile proglottids are shed in the feces of the infected definitive host or may actively crawl out of the anus. Egg capsules are released from the proglottid passively after motility ceases and the tegument dries and disintegrates. Larvae of the flea intermediate host ingest these eggs, and the oncospheres penetrate the digestive tract and enter the haemocoel. The oncosphere continues to undergo developmental changes as the larva pupates, and transformation to a cysticercoid is ultimately completed in the adult flea (about 30 days post infection). Only the larval stages of fleas are capable of ingesting the large egg packets, so infections cannot be initiated in adult fleas which have smaller mouthparts (López-Neyra and Muñoz, 1919; Venard, 1937). The common dog flea (Ctenocephalides canis) and the cat flea (C. felis) are the prototypical intermediate hosts, although the dog louse (Trichodectes canis) and human flea (Pulex irritans) may also reportedly act as intermediate hosts (López-Neyra and Muñoz, 1919; Miyazaki, 1991).
The canid and felid definitive hosts (and humans) become infected by the ingestion of adult fleas containing cysticercoids. The cysticercoid develops into an adult in the small intestine over about 20 days. Historical infection trials suggest that growth is slower in cats than dogs and that mature specimens derived from feline hosts appear more delicate (Venard, 1937). Newer molecular data suggest that genetic differences exist between cat and dog isolates, possibly explaining differences in parasite establishment and development (Beugnet et al., 2018). Apart from anal pruritus from proglottid migration, the natural hosts typically show no complications of infection, except in particularly heavy infections where gastrointestinal irritation can occur from the anchoring of the rostellum (Miyazaki, 1991).
Dipylidium caninum is truly a cosmopolitan parasite and is common in both wild and domestic canid and felid definitive hosts around the world – particularly where flea control is inadequate. However, prevalence is likely underestimated by many fecal flotation methods as egg clusters/proglottids can be too heavy to float and are not distributed evenly in the fecal sample (Blagburn, 2001).
Human infections
Numerous D. caninum infections in humans have been reported worldwide for over a century; however, it is still considered by most clinicians to be an unusual finding. The actual number of confirmed cases is difficult to ascertain as not all have been reported in literature; fewer than 100 case reports exist in English language literature, but within a 4-year span (1973–1977), the Centers for Disease Control received 43 requests for the contemporary drug of choice (niclosamide) in diagnosed cases (Molina et al., 2003). A recent search including Chinese language databases estimated that at least 349 human cases have been reported worldwide (Jiang et al., 2017). It seems a reasonable assumption that the true incidence of dipylidiasis in humans is underestimated, given the ubiquity of D. caninum in pet dogs and cats, and the fact that detailed examination of proglottids in clinical settings is seldom performed.
The vast majority of published infections have occurred in children under about 8 years, with a substantial proportion (estimated one-third) in infants <6 months old (Chappell et al., 1990; Cabello et al., 2011). A recent case series from one clinic reported a mean age of 3.8 years (7 months–10 years) among 10 cases diagnosed over a 2-year period (Portokalidou et al., 2018). Reports in adults are extremely uncommon. Dipylidium caninum was reported in an adult kidney transplant recipient presenting with frequent diarrhoea, but the individual eggs (i.e. not clusters) shown appear to represent Hymenolepis sp. and it is not stated whether proglottids were found (Sahin et al., 2015). A more convincing case exists from a 57-year-old in Australia who passed morphologically consistent proglottids and egg clusters (Jackson et al., 1977).
Transmission to humans occurs through the accidental ingestion of fleas from pet dogs or cats, or possibly by being licked if exposed cysticercoids happen to be present on the tongue of the pet after grooming. Unsurprisingly, many patients lived in or visited households with dogs. Some children were reported to have played ‘games’ with their dogs which could potentially lead to ingestion of fleas/cysticercoids (Turner, 1962; Molina et al., 2003; García-Agudo et al., 2014; Jiang et al., 2017). Close contact between children and dogs is often cited as a reason why the age pattern is observed, although close interaction with dogs is not uncommon among adults. For example, 56% of dog-owning adults report co-sleeping with their dogs (Chomel and Sun, 2011), and 50% allow face-licking (Overgaauw et al., 2009), suggesting that there may be other factors at play (e.g. age-related immunity) besides children's close interaction with pets. Contact with cats is less frequently described in dipylidiasis literature, but their potential contribution to environmental flea infestations and direct infection risk should not be ignored. Flea control strategies and/or infestation status of pets implicated in transmission are not usually reported, but occasionally pets are followed up and D. caninum infections confirmed (García-Agudo et al., 2014).
Typically, very few or no symptoms are reported (Reid et al., 1992; Taylor and Zitzmann, 2011; Portokalidou et al., 2018). Apart from psychological distress of both the child and parent from the passing of motile proglottids, symptoms such as diarrhoea, mild gastrointestinal pain, urticaria, poor appetite and anal pruritis have been reported (Samkari et al., 2008; García-Agudo et al., 2014). Infections are typically light, but occasionally >10 worms are found (Chappell et al., 1990). Note that evaluating the actual infection intensity may be difficult or impossible unless scolices are recovered and counted.
In several instances, proglottids are initially misidentified as fly larvae, rice grains or other helminths (Turner, 1962; Samkari et al., 2008; Szwaja et al., 2011). Infections have been mistaken for the nematode Enterobius vermicularis due to anal pruritis and misidentification of motile proglottids as female pinworms. Laboratory identification is generally straightforward based on the features of proglottids passed in feces (presence of two genital pores, egg clusters, overall size and shape); scolices are also of diagnostic value but are very seldom recovered (Samkari et al., 2008; Taylor and Zitzmann, 2011). Correct identification is important as some anthelmintics (e.g. benzimidazoles) used for treating pinworm infections have no activity against D. caninum (Roberson and Burke, 1982; Taylor and Zitzmann, 2011). As with nearly all intestinal cestodiases, praziquantel and niclosamide are the most widely employed treatments. Of note, apparent resistance to praziquantel has been observed in some dog infections, thus treatment of human cases should be carefully monitored (Chelladurai et al., 2018). Despite the growing concern over the efficacy of praziquantel against D. caninum, reports are still rare and research preliminary. Furthermore, many newer-generation, compounded ectoparasitic preventives (in both spot-on and collar formulations) have proven effective in preventing flea infestations and interrupting D. caninum transmission in pets (Fourie et al., 2012, 2013; Beugnet et al., 2017).
Raillietina
Taxonomy and morphology
The genus Raillietina, one of many taxa named after the pre-eminent helminthologist Alcide Railliet, holds the distinction of being the largest genus of cestodes, comprised of nearly 300 species (Schmidt, 1986). It also represents the only group from the family Davaineidae (subfamily Davaineinae) known to infect humans. As such, this family is largely unknown to the medical parasitological community. The majority are parasites of birds, but some species are associated with mammalian hosts. The known human-infecting species appear to be parasites of rodents (Raillietina celebensis, Raillietina demerariensis, Raillietina siriraji). Davaineinids are characterized by the possession of a short, broad, retractable rostellum, typically armed with small T- or hammer-shaped hooks, and by small spines on the suckers. The subfamily Davaineinae includes members that bear egg capsules instead of uteri (Schmidt, 1986). Conventionally, Raillietina is further divided into several subgenera based on the number of eggs per capsule and the position of the genital pore (Parioniella: one egg/capsule, genital pores unilateral; Skrjabinia: one egg/capsule, genital pores irregularly alternating; Raillietina: multiple eggs/capsule, genital pores unilateral; Fuhrmannetta: multiple eggs/capsule, genital pores irregularly alternating) (Schmidt, 1986). Note that some authors treat these subgeneric divisions as full genera, though the straightforward, cleanly-delineated generic status of these groups does not appear supported by available molecular evidence (Khalil et al., 1994; Littlewood et al., 2008; de Oliveira Simões et al., 2017; Mariaux et al., 2017). Nevertheless, the Raillietina spp. described from human infections all belong to subgenus Raillietina, with unilateral genital pores opening in the anterior portion (usually the anterior quarter, sometimes the anterior third) of the lateral margin of the proglottid (Baer and Sandars, 1956). Specific determination is usually based on the characteristics such as the number of eggs/capsule, length of the cirrus pouch, number of testes, and the number and length of rostellar hooks.
Most Raillietina spp. relevant to zoonotic infections are about 10–51 cm in length and 2–3 mm wide. The scolex possesses a rostellum with numerous hammer-shaped hooks arranged in two circles (usually alternating large and small hooks) and sometimes a collar of minute spines directly behind the rostellum. Proglottids range in shape from broadly rectangular to nearly square with rounded corners further down the strobila, giving the posterior portion a beaded appearance. Egg capsules are apparent in more mature segments and have a polygonal shape (130–180 μm in diameter) with a transparent, parenchymatous exterior zone and a dark, nearly opaque interior.
Life cycle and hosts
While the known zoonotic species are associated with rodent and not avian hosts, most of what is known about the development of Raillietina spp. is from experimental studies of poultry-infecting species (namely Raillietina cesticillus, Raillietina echinobothrida and Raillietina tetragona) (Horsfall, 1938; Reid et al., 1938). There is a lack of published experimental trials on mammal-associated Raillietina spp., so inferences based on avian-associated species should be considered tentative. It is not known whether avian Raillietina spp. are potentially zoonotic or even capable of infecting mammalian hosts (and vice versa). However, the majority of other zoonotic cestodes causing intestinal infections are associated with mammalian definitive hosts (e.g. Dibothriocephalus, Adenocephalus, Spirometra, Dipylidium, Bertiella, Hymenolepis, Taenia, etc.). The differences in digestive anatomy and physiology between mammalian and avian hosts perhaps do not permit the effective establishment of avian definitive host-adapted adult cestodes in humans.
With this caveat in mind, Raillietina spp. appear to follow a life cycle similar to Bertiella and Dipylidium, involving a single intermediate host and a cysticercoid stage (Fig. 1). Proglottids are shed in the feces of infected definitive hosts and are passed in a highly motile state. Migration to the outer surface of the feces has been observed, which seems to aid in its ultimate transmission to the arthropod intermediate host; however, no particular tropism or response to directional stimulus has been identified (Reid et al., 1938). Upon consumption, the oncosphere hatches from the egg and penetrates the intestinal wall of the arthropod. Cysticercoids develop free within the body cavity and are usually infectious after a period of 2–3 weeks. The scolex evaginates upon consumption by the intermediate host, likely in response to digestive enzymes. Early in infection (~5 days), most worms are recovered from the first quarter of the intestine, but in patent infections, the primary site of localization is in the second quarter of the intestine (Gray, 1972). It is not known whether this applies to mammalian hosts, but a similar pattern has been observed in rats infected with Hymenolepis diminuta (Gray, 1972). Prepatent periods are short in avian hosts; gravid R. cesticillus proglottids may be passed by chickens as soon as 13 days post-exposure (Reid et al., 1938). As knowledge of mammalian-infecting species is limited to field studies on naturally-infected hosts, nothing is known on their course of infection.
Numerous species have been identified as intermediate hosts for various Raillietina spp., primarily ants and beetles and occasionally terrestrial gastropods (Wardle and McLeod, 1952). Many beetle intermediate hosts are carnivorous or opportunistic species that actively prey upon motile proglottids, such as ground beetles (Carabidae), and also other beetles that feed on animal dung or detritus such as scarab beetles (Scarabaediae) and darkling beetles (Tenebrionidae) (Reid et al., 1938). The actual intermediate host specificity varies widely by Raillietina species, and is not known in many instances (Pradatsundarasar, 1972). In particular, this is poorly characterized for the rodent-associated zoonotic Raillietina spp., including the most commonly reported R. celebensis (Rougier et al., 1981). A preliminary report of zoonotic R. siriraji cysticercoids in cockroaches was eventually shown to be a misidentification of gregarine protozoa (Pradatsundarasar, 1972).
As stated previously, the majority of Raillietina species use avian definitive hosts, and the group includes well-known pathogens of poultry (Dunn, 1978). The zoonotic species have all been identified from various rodents, primarily the two cosmopolitan rat species Rattus norvegicus and Rattus rattus (Miyazaki, 1950; Chandler and Pradatsundarasar, 1957; Niphadkar and Rao, 1969; Tung et al., 2013). Raillietina celebensis has also been recorded from other native murid rodents, including Bandicoot rats (Bandicota spp.) of the Indian subcontinent and Southeast Asia, the Asian house shrew (Suncus murinus) and the trefoil-toothed giant rat (Lenomys meyeri) of Sulawesi (Baer and Sandars, 1956; Tung et al., 2009). The other Old World species R. siriraji has been identified in Polynesian rats (Rattus exulans) and Asian house shrews along with the usual hosts (Chenchittikul et al., 1983; Roberts, 1991). Several surveys of rodents from endemic areas in Southeast Asia simply report Raillietina sp. from a variety of rodents which seem likely to represent either R. celebensis or R. siriraji. Among these, the chestnut white-bellied rat (Niviventer fulvescens), Edward's long-tailed giant rat (Leopoldamys edwardsi), fawn-coloured mouse (Mus cervicolor), red spiny rat (Maxomys surifer), white-toothed rats (Berylmys spp.) and other Rattus spp. (R. losea, R. tanezumi) represent potential hosts (Chaisiri et al., 2010, 2015; Herbreteau and Morand, 2011).
Raillietina demerariensis is more poorly understood, but reports under various synonyms exist from the New World rodents including the Cuban huita (Capromys pilorides) and hystrichomorph rodents (e.g. guinea pigs, capybara, etc.) (Cameron and Reesal, 1951; Baer and Sandars, 1956). The howler monkey Alouatta seneculus has also been described as a host for R. demerariensis and another species R. alouattae, although it is not known whether this represents the normal reservoir host or an aberrant host susceptible to infection (Baer and Sandars, 1956). Unidentified Raillietina sp. possibly representing R. demerariensis are reported from cosmopolitan Rattus spp. in Jamaica and Brazil (Waugh et al., 2006; Simões et al., 2016).
Human infections
Identity of zoonotic species: All reports of Raillietina sp. infections in humans appear to involve mammalian-associated species in the subgenus Raillietina. However, the precise identity and number of Raillietina species involved in human infections is a perplexing and unsettled subject on account of incomplete descriptions, fragmentary reference material, invalid and inconsistent naming, morphological variability and of course the enduring taxonomic ‘lumper/splitter’ dichotomy. The long, complex taxonomic history and debate are discussed at length by both Chandler and Pradatsundarasar (1957) and by Baer and Sandars (1956). In the absence of further modern characterization, the safest tentative conclusion appears to be that three valid species have recovered from humans: R. celebensis in the Old World, R. demerariensis from the New World and R. siriraji from Thailand. These names have an extensive list of synonyms and yet the validity of some species has not been definitively settled (Table 4).
Table 4.
List of names generally regarded as synonyms for Raillietina spp. described from human infections
| Name | Synonyms |
|---|---|
| R. celebensis |
Taenia madagascariensisa Davainea madagascariensisa Davainea celebensis Meggittia celebensis R. asiatica R. formosana R. funebris R. garrisoni R. murium R. madagascariensisa R. sinensis R. sirirajib |
| R. demerariensis |
Taenia demerariensis R. brumpti R. equatoriensis R. quitensis R. leoni R. luisaleoni R. halli |
| R. siriraji | sp. nov. described by Chandler and Pradatsundarasar (1957) |
Of these species, R. celebensis has been most frequently described in human infections, although of course not always under that name (Table 4). Despite the fragmentary nature of descriptions, comparison across reported descriptions of Raillietina spp. from humans and sympatric rodents in the Old World reveals that differences in aspects such as overall dimensions, number of hooks, presence of rostellar spines, number of testes and the length of the cirrus pouch are trivial or too overlapping to warrant specific status (Joyeux and Baer, 1929; Baer and Sandars, 1956). Thus, treating such described species as one entity R. celebensis is probably appropriate. Further genetic studies will help to confirm or refute this.
Adding to the taxonomic upheaval, many historical reports across various countries have used the problematic name R. madagascariensis. The name is rightly regarded by Baer and Sandars (1956) as a species sub judice as it is impossible to determine the true identity of this name due to poor original type material and conflicting historical revisions (Joyeux and Baer, 1929; Baer, 1956). This was first highlighted during the examination of multiple R. madagascariensis-type specimens by Joyeux and Baer (1929), who reported poor states of preservation and misrepresentation; one such scolex was found to be an immature specimen of Taenia saginata. Furthermore, through the years, various workers have proposed revisions, not all of which were universally adopted. For example, Japanese authors synonymized multiple Raillietina spp. and even R. demerariensis of the New World into ‘R. madagascariensis’ (Miyazaki, 1950). However, it is worth nothing that Miyazaki later used R. celebensis in referring to the same works in his textbook nearly 40 years later (Miyazaki, 1991). To make matters even more confusing, Lopez-Neyra proposed synonymizing several African Raillietina spp., North American Raillietina bakeri and Inermicapsifer spp. into R. madagascariensis and placed R. celebensis in a novel genus Meggittia, a view rejected by most subsequent authors (López-Neyra, 1950, 1954) (Table 4).
Overall, it appears ‘R. madagascariensis’ likely represents a number of misidentified Raillietina spp., including several probable examples of R. celebensis. While the term R. madagascariensis appears to have fallen out of favour, a contemporary case report of ‘R. madagascariensis’ surfaced and it is not clear from the details provided whether the authors are treating this as a synonym of Inermicapsifer madagascariensis or as a Raillietina sp. proper (Prosl, 2005). Many other so-called R. madagascariensis cases may also represent I. madagascariensis. The latter misidentification probably stems from a failure to recover scolices and subsequent examination of rostella for hooks, as proglottids can appear similarly (particularly gravid proglottids where genital organs may be obscured by numerous egg capsules).
One other species besides R. celebensis has been described from human infections in Asia. Chandler and Pradatsundarasar (1957) provide a detailed description of multiple specimens reportedly in good condition recovered from two children in Thailand, and found similarities to published reports of R. formosana, R. celebensis and R. madagascarensis. However, the size of the eggs was much larger than typical Raillietina species (100–115 × 38–42 μm vs 34–60 × 20–45 μm), leading them to propose a new species R. siriraji. Thus far, this name appears to be regarded as valid (Matevosyan, 1966), although it is listed as a synonym of R. celebensis by Fain et al. (1977).
New World case reports are fewer in number than those from Asia and Pacific island nations, and all have been attributed to R. demerariensis and its various synonyms (Table 4) (Belding, 1965). While many authors agree that synonymy is appropriate, there has been some argument that the variations among described neotropical Raillietina spp. are more significant than the Old World and that lumping should be approached with caution (Chandler and Pradatsundarasar, 1957).
The sole report of Raillietina in a human from the USA is the most recent at the time of writing, and species identity was not determined. A child in Hawaii passed proglottids containing egg capsules that were repeatedly misidentified as Dipylidium, until re-examined at a reference laboratory and identified as being those of Raillietina sp. (Davis et al., 2019). Few other morphological details were available for subgeneric or specific determination and no scolex was recovered to definitively rule out Inermicapsifer – although the child did not have travel history to Cuba or Sub-Saharan Africa, so Raillietina sp. is most plausible. The authors suggest that this could represent infection with a chicken-associated Raillietina (e.g. R. tetragona), as free-ranging chickens are common in the area. However, all previous reports of zoonotic Raillietina involve rodent-infecting species. The introduced Polynesian rat (R. exulans) is a natural host for R. siriraji (Roberts, 1991), so this cannot be excluded as a possible reservoir host for a known zoonotic Raillietina in Hawaii.
The failure to recover scolices from patients passing proglottids remains a large roadblock in specific identification, and even if scolices are available, interpretation of some features may vary. For example, Margono et al. (1977) reported that viewing rostellar hooks directly or obliquely on the lateral side influenced their appearance enough to appear between R. formosana or R. garrisoni (although now both are regarded as synonyms of R. celebensis). Other factors such as the quality of fixation and anthelmintic treatment may further distort morphology – the former of which is to blame for much of the historical debate over ‘Taenia madagascariensis’. There is obviously a great need to revisit classical morphological examination and augment these with modern molecular studies on potentially zoonotic Raillietina spp. to more definitively resolve long-standing taxonomic issues and elucidate the true number of species and their distribution. These sorts of studies would also be useful in the re-evaluation of the sprawling Raillietina genus as a whole.
Clinical and epidemiological characteristics: While undoubtedly an uncommon event, it is difficult to assess the frequency of zoonotic Raillietina spp. infections with confidence owing to nomenclatural confusion and potential misidentifications with other cestodes. Cases attributed to R. celebensis have been described most commonly from Southeast and East Asia (Indonesia, Malaysia, Vietnam, Thailand, Myanmar, Taiwan, Japan) and the Philippines (Bonne and Mreyen, 1940; Miyazaki, 1950; Baer and Sandars, 1956; Pradatsundarasar, 1960; Fain et al., 1977; Margono et al., 1977). A plurality of reports exist from French Polynesia, including the islands of Tahiti and Mo'orea, although this may not truly represent a focus of infection but rather may reflect an increased awareness of clinical investigators in that region (Fain et al., 1977; Rougier et al., 1980, 1981). Sporadic cases in humans and rats have also been reported from Australia (Queensland) and India (Baer and Sandars, 1956; Niphadkar and Rao, 1969). The endemic range of this cestode may have expanded into the New World as, in 2017, R. celebensis was reported from Brazil during a survey of R. norvegicus (de Oliveira Simões et al., 2017). Detailed morphometrics reported in this case were consistent with R. celebensis, although the 18S gene sequences generated were not useful in definitively confirming species based on existing sequences (which are mostly from avian Raillietina spp.). This again underscores the need for careful molecular characterization of this group, to more accurately determine if this species has truly been introduced to the New World or if this represents a new (or existing) neotropical Raillietina.
Raillietina demerariensis is believed to represent the primary species infecting humans in the New World, although reports are scant compared to R. celebensis and its geographic occurrence has not been recently studied. Zoonotic infections have been recorded primarily from Ecuador, and to a lesser extent British Guyana, Cuba and Honduras (Dollfus, 1939; Joyeux and Baer, 1949; Belding, 1965). The occurrence in rodents is broader and includes Venezuela and Suriname, and numerous species reported as synonyms of R. demerariensis have been identified across the West Indies (Dollfus, 1939; Joyeux and Baer, 1949; Sato et al., 1988).
The final known zoonotic species, R. siriraji, has only been described from Thailand (Chandler and Pradatsundarasar, 1957; Pradatsundarasar, 1960; Charoenlarp and Radomyos, 1973; Chenchittikul et al., 1983), although a re-evaluation of morphometrics from other Raillietina spp. occurring in Southeast Asia is necessary to determine if this species is restricted to Thailand, or if it occurs more broadly. Many published studies do not report sufficient details to make this determination, and surveys of rodents often simply report ‘Raillietina sp.’
Nearly all reports in humans are among children, mostly under 3 years of age (Chandler and Pradatsundarasar, 1957; Pradatsundarasar, 1960; Rougier et al., 1980, 1981). The presumed exposures are via accidental (or intentional) consumption of infected arthropod intermediate hosts. A case series of R. siriraji infections in Thai children highlights that seven out of nine cases had a history of putting cockroaches in the mouth (Pradatsundarasar, 1960). The cockroach has yet to be definitively confirmed as an intermediate host for R. siriraji, but it seems plausible based on other Raillietina spp. Other details on the clinical history and potential routes of exposure among infected children are scanty.
Given the scarcity of case reports, it is difficult to make assumptions about the pathogenicity and true clinical spectrum of Raillietina infections. While likely that most infections are asymptomatic as typical for intestinal cestodiases, many (but not all) existing case reports involve symptomatic patients. Episodic diarrhoea and loose stools are frequently described, although it is difficult to unambiguously establish causality due to other potential enteric infections in the endemic tropical regions (Chandler and Pradatsundarasar, 1957; Rougier et al., 1981). Other symptoms are non-specific and include abdominal discomfort and distention, poor appetite and irritation. Of note is that in veterinary medicine, Raillietina and the closely related Davainea (which formerly included some of the human-infecting Raillietina species) are considered the most pathogenic cestode genera infecting poultry, known to cause substantial intestinal pathology and associated complications, including nodule formation (Horsfall, 1938; Dunn, 1978). Though not necessarily applicable to humans, this demonstrates the pathogenic potential of the genus, particularly if heavy infections are acquired.
Identification and diagnosis to genus level can generally be achieved by morphology owing to the unique appearance of Raillietina vs other human-infecting cestodes. Dissection or forced expression of egg capsules from proglottids should immediately identify the material as belonging to either Raillietina or Inermicapsifer. If Raillietina infection is suspected, every effort should be made to examine the important features of egg capsules and the genital pore of proglottids, particularly for distinguishing proglottids from Inermicapsifer. Scolices provide an unequivocal distinction between these two groups (CDC-DPDx, 2019), though as with many cestode infections, these are not often recovered due to their small size. Though molecular studies are generally lacking for zoonotic Raillietina spp., PCR and sequencing on recovered material could possibly be useful for confirmation of genus. Treatment is not standardized due to the rarity of cases, though single-dose praziquantel has proven effective (Davis et al., 2019).
Inermicapsifer
Taxonomy and morphology
Inermicapsifer is a genus within Anoplocephalidae that includes parasites primarily of rodents, hyraxes and pangolins. This genus is similar in appearance to Raillietina in many respects apart from the scolex, which is unarmed (0.40–0.55 mm) with deep, cup-shaped suckers (Fig. 3). For the single accepted zoonotic species (I. madagascariensis), the maximum length is generally reported around 70–420 mm, varying by the degree of fixation, with around 300–360 segments in fully developed specimens. The proglottids are trapezoidal and prominently craspedote, and mostly wider than long, except for gravid ones which take on a more barrel-shaped appearance similar to Raillietina. For Inermicapsifer spp., the genital pore is mostly unilateral but some specimens may show an irregularly alternating pattern. In I. madagascariensis, the genital pore is situated in the middle point of the lateral margin. The genital ducts pass between osmoregulatory canals. The testes are numerous (usually 48–55 per mature proglottid) and a greater proportion are situated mostly on the aporal side of the female glands. The ovary is fan-shaped (~0.2 mm wide), with the vagina posterior to the small cirrus pouch (0.10–0.15 mm). The uterus in younger segments is visible as a branching structure, but in most segments, it breaks down into egg capsules each containing several eggs (Schmidt, 1986). Mature proglottids shed in feces are replete with such egg capsules, usually around 100–125, which create a mosaic or reticulated pattern (Fig. 3) reminiscent of Raillietina. Like Raillietina, the egg capsules are polygonal in shape and have a dark inner portion and a parenchymatous outer portion (Fig. 3). Each egg capsule generally contains 8–15 eggs, though sometimes fewer. The genital pore is not easily visible in very gravid proglottids (Baylis, 1949; Baer et al., 1950; Baer, 1956).
Fig. 3.
Specimen of Inermicapsifer madagascariensis from Cuba. (A) Gravid proglottid (4 mm long); (B) egg capsule liberated from gravid proglottid (scale bar = 100 μm); (C) portion of strobila, showing median genital pores (arrows) (~30 × magnification); (D) unarmed scolex (scale bar = 200 μm). Photos courtesy of DPDx, Centers for Disease Control and Prevention.
The taxonomic treatment of the genus Inermicapsifer has never been conclusively established and has been debated for decades. When first established by Janicki in 1910, the genus Inermicapsifer was placed in the subfamily Linstowiinae within Anoplocephalidae (Beaver et al., 1984). Originally proposed by Lopez-Neyra, it was later transferred by Spasski to a new subfamily Inermicapsiferinae on the basis of egg capsules containing multiple eggs, and the position of the ovary in the poral half part of the proglottid (Mettrick and Weir, 1963; Khalil et al., 1994). This subfamily is reflected in widely-used cestode identification texts (Yamaguti, 1959; Schmidt, 1986; Khalil et al., 1994), but its validity has not been universally recognized (Baer and Fain, 1955; Stunkard, 1961; Mettrick and Weir, 1963). It should also be noted that apart from the unarmed scolex, Inermicapsifer seems to share more morphological characteristics with Raillietina and other Davaineidae (e.g. uterine development, nearly identical egg capsules, egg morphology) than it does with classical Anoplocephalidae, leading to arguments for its inclusion in Davaineidae instead (Baylis, 1949; Baer and Fain, 1955; Stunkard, 1961; Mettrick and Weir, 1963). However, a familial characteristic of Davaineidae is an armed scolex with hammer-shaped hooks, thus precluding the placement of Inermicapsifer in the family as it stands now (Khalil et al., 1994). In the future, perhaps with supporting molecular phylogenetic studies, the diagnostic features defining these relevant taxa may be further evaluated and revised.
Life cycle and hosts
The life cycle is not completely known, but it probably does not differ substantially from Raillietina and other similar cestodes that involve an intermediate host with a cysticercoid infective to the definitive host (Fig. 1). In contrast to the zoonotic Raillietina spp. which are predominately parasites of the two peridomestic rats (R. norvegicus and R. rattus), I. madagascariensis is very seldom reported from peridomestic rats and instead is a parasite of native African rodents and hyraxes (Goldsmid and Muir, 1972). The two most commonly reported hosts in endemic regions of Sub-Saharan Africa are the Natal multimammate rat (Mastomys natalensis) and Gambian pouched rat (Cricetomys gambianus) (Hira, 1975). Other host species in which I. madagascarensis has been found include the rock hyrax (Procavia capensis), red rock rat (Aethomys chrysophilus), black-tailed tree rat (Thallomys nigricauda), the South African pouched mouse (Saccostomus campestris), the South African vlei rat (Otomys irroratus), the silvery mole-rat (Heliophobius argentocinereus) and grass rats (Arvicanthus spp.) (Ortlepp, 1961). A natural infection in a captive-bred chinchilla (Chinchilla sp.) was also reported (Goldsmid and Muir, 1972).
An important point is that I. madagascariensis has never been found in free-ranging animal definitive hosts in island nations, including Cuba, Madagascar, Mauritius and Comoros (Fain, 1950; Baer, 1956; Hira, 1975). Some authors have suggested that this indicates adaptation to humans as a reservoir in the absence of suitable rodents (Kourí, 1944; Hira, 1975; González Núñez et al., 1996). However, no genetic studies on Inermicapsifer exist and there are no sequences available in GenBank at the time of writing. It is possible that genetic differences may exist between rodent-adapted and human-adapted strains. This pattern has been observed in other helminths classically considered zoonotic, such as the nematode Oesophagostomum bifurcum (Gasser et al., 2006). The sole finding of I. madagascariensis in animal hosts in Cuba is a single report of incidental infections in laboratory-reared ‘white rats’; the route of exposure is unknown nor has there been further evidence for the existence of a zoonotic cycle (Kourí and Kourí, 1952).
The identity of the intermediate host is not known. Various authors have proposed terrestrial arthropods, including ants, beetles and mites (Ortlepp, 1961). Whether or not oribatid mites are involved is an important question from a taxonomic point of view. If they are not competent intermediate hosts, the argument for retaining Inermicapsifer in Anoplocephalidae becomes weaker, as nearly all members of said family require oribatid mite intermediate hosts and this is considered an important taxonomic feature. Inermicapsifer spp. egg capsules are much larger than typical anoplocephalid eggs, which are passed singly, and may prove too large for small oribatid mites to ingest (Baylis, 1949). On the other hand, the capsules are similar in size and character to those of Raillietina spp., which are known to infect ants and beetles as intermediate hosts.
Human infections
Identity and nomenclature of zoonotic species: Only one valid species is recognized in zoonotic infections, I. madagascariensis, but the history of this name and species is fairly involved for the same reasons as the zoonotic Raillietina spp. In 1938, Kourí initially described specimens from human patients in Cuba as R. cubensis. Upon further examination of the unarmed scolices, these specimens were determined not to be Raillietina, so they were re-described as Inermicapsifer cubensis (González Núñez et al., 1996). Fain (1950) later demonstrated that Inermicapsifer arvicanthidis from African rodents was identical to all existing descriptions of I. cubensis, a sentiment also reflected earlier by Baylis (1949) in the first human case report from continental Africa (Kenya). Within 6 years after the synonymizing of I. cubensis with I. arvicanthidis, during an investigation into the taxonomically ambiguous ‘T. madagascariensis’, Baer (1956) proposed the new name I. madagascariensis to unite these names and four other synonyms (while some others reported under the name T. madagascariensis were determined to represent R. celebensis). No other Inermicapsifer spp. (e.g. I. guineensis, I. hyracis) have been identified in human infections.
Epidemiological and clinical aspects: Endemic to the disparate locations of Sub-Saharan Africa, the Malagasy region and the West Indies, it is not entirely clear where I. madagascariensis originated. Generally, it is believed that the parasite was brought from Africa to the West Indies via the African slave trade, and perhaps to Madagascar and peripheral islands via Creole labourers associated with French settlers (Baer, 1956; Goldsmid and Muir, 1972). In continental Sub-Saharan Africa, I. madagascariensis appears to be broadly distributed and cases have been reported from Kenya, Rwanda, Burundi, South Africa, Zimbabwe, Malawi and Zambia (Baylis, 1949; Ortlepp, 1961; Goldsmid, 1964; Nelson et al., 1965; Goldsmid and Muir, 1972; Hira, 1974, 1975). Sporadic records of human infections also exist across the East African Indian Ocean islands (Madagascar, Mauritius, Comoros and Reunion Island) (Bailenger and Carcenac, 1970). Natural infections in rodents have also been found in Senegal though no human cases have been reported from West Africa (Sall-Dramé et al., 2010). In a summary of seven cases from Zimbabwe over about 8 years, it is most common in children between 1 and 3 years of age, but also occurs in 4–5 years old children, similar to previous reports from East Africa (Goldsmid and Muir, 1972). Patients in published reports are almost always of European descent (Nelson et al., 1965; Goldsmid and Muir, 1972). Authors suggest that infections in African children go unreported by parents in rural Africa due to mild symptomology and/or a lack of awareness, and so the infection may be much more common than generally assumed (Nelson et al., 1965; Goldsmid and Muir, 1972).
In Cuba, a hundred or so human cases were reported by Kourí (1944) (as I. cubensis) primarily from Havana and surrounding provinces prior to 1948. Not until 1996 were cases identified and reported in literature again; since then, approximately 45 cases primarily from regions surrounding Havana and Santa Clara have been diagnosed (and reported under the correct name I. madagascariensis) (González Núñez et al., 1996; Mayor and Herrera, 2004; Herrera Valdés et al., 2007). Age ranges and symptomology of patients are generally parallel to those reported in Africa. Among nine cases diagnosed in a single San Jose hospital, all patients were under 3 years old (5/9 between 1and 2 years old), 7/9 were male and 7/9 were from rural regions of the province (Pérez et al., 2009). In every case, it was the passing of motile proglottids by children that prompted parents to seek clinical attention. Anorexia and diarrhoea were also reported in six and four of these cases, respectively; however, a few children also had Giardia coinfections (Pérez et al., 2009). Also similarly to the Sub-Saharan African cases, nearly all cases were diagnosed in children of European descent in these case series.
Nearly all contemporary reports of human I. madagascariensis infections originate from Cuba and not Sub-Saharan Africa, perhaps from a lack of awareness or research interest in the area. A report of ‘Raillietina madagascariensis’ from a child who had travelled to Mauritius likely represents a case of I. madagascariensis (Prosl, 2005). As discussed previously, this name is problematic, and it is not clear whether or not the authors intended this as a synonym of I. madagascariensis or as a ‘true’ Raillietina sp. The proglottids were identified as belonging to Raillietina using Miyazaki's textbook Helminthic Zoonoses, which covers only R. celebensis and not Inermicapsifer (Miyazaki, 1991), and then used a key of Lopez-Neyra (which retains R. madagascarensis as a valid name and regards Inermicapsifer spp. as belonging to Raillietina) to identify species. The authors come to the conclusion that these proglottids are not R. celebensis due to the number of eggs per capsule, but the reported number (5–10 eggs/capsule) is more typical of I. madagascarensis (Hira, 1975). Importantly, the proglottid shown has a genital pore on the median point of the lateral margin (also typical of Inermicapsifer) and not in the anterior portion (as typical of human-infecting Raillietina spp.). As with many cases, the scolex was not recovered, precluding unequivocal identification of this cestode. This problem highlights the need for definitive clarification of taxonomy and nomenclature.
The source of infection is not entirely clear as the intermediate host is not known, though the predominance of young children is similar to other cestodiases transmitted via ingestion of arthropods (e.g. hymenolepiasis, bertielliasis, dipylidiasis). Nelson et al. (1965) note that all cases recorded outside of Africa have been in regions that rely heavily on sugar cane farming, where eating raw cane is a common habit that could lead to accidental ingestion of arthropods. However, it is also suggested that very young children and infants are unlikely to become infected this way as they may be unable to chew raw cane (Hira, 1975).
Most cases do not involve severe symptoms, and presentation is the typical mild collection of symptoms associated with most intestinal cestodiases, or are asymptomatic. Reported symptoms include irritability, abdominal pain, anorexia, gastrointestinal distress and generalized malaise (Hira, 1974; Pérez et al., 2009). Like many of the agents discussed in this review, it is the presence of motile proglottids (often mistaken for rice grains) in the stool that prompts clinical attention. A few cases note weight loss in children which ceased following treatment, suggesting perhaps this was associated with I. madagascariensis infection and not simply other causes in endemic areas (Goldsmid and Muir, 1972).
Diagnosis is based on the examination of the rice grain-like shed proglottids; since egg capsules are released passively after the disintegration of the proglottid, direct smears or flotation of recently-collected patient stool samples will not reveal free egg capsules (Hira, 1974). For the proper identification of I. madagascariensis, care must be taken to distinguish proglottids from those of Raillietina spp. The scolex provides the easiest distinction (unarmed Inermicapsifer vs armed Raillietina), but as discussed throughout, this is seldom recovered from human infections. The position of the genital pore is another useful characteristic (middle of lateral margin for Inermicapsifer; anterior quarter for Raillietina), but this can be difficult to visualize on very gravid proglottids and may require staining (Goldsmid and Muir, 1972). If egg capsules are liberated from proglottids, the number of eggs per capsule may also provide a diagnostic clue (generally 8–15 eggs for Inermicapsifer; 1–4 for known zoonotic Raillietina). Treatment is straightforward using cestodicidal agents such as niclosamide and praziquantel (Goldsmid and Muir, 1972; Mayor & Herrera, 2004). Similar to D. caninum, benzimidazoles appear to lack efficacy; in one case, a child with concurrent E. vermicularis infection was treated with thiabendazole, with no effect on the Inermicapsifer tapeworm (Goldsmid and Muir, 1972).
Mesocestoides
Taxonomy and morphology
Mesocestoididae is an unusual family within Cyclophyllidea, sharing some characteristics with Pseudophyllidean tapeworms. The family contains only two subfamilies, both of which are monogeneric – Mesocestoides is the only genus within Mesocestoidinae (Wardle and McLeod, 1952). Infecting a variety of terrestrial mammalian carnivores, adults are long and slender, measuring about 30–100 cm with a maximum width of 2.5 mm, typically with 600–1000 proglottids at maturity. Proglottids towards the anterior portion of the strobila are somewhat wider than long, and with maturity becoming more long than wide with convex margins (Miyazaki, 1991). Testes are spherical, 20–40 μm in diameter and usually numbered around 60–80 in specimens recovered from humans (Miyazaki, 1991; Fuentes et al., 2003). The uterus is a simple blind tube aligned vertically in the median portion of the proglottid (Wardle and McLeod, 1952). The genital pore does not open on the lateral margin of the proglottid as typical for most Cyclophyllidea; it is located in the centre of the proglottid on the ventral surface, similar to some Pseudophyllidea. The scolex is unremarkable, having four large suckers and no rostellum (Wardle and McLeod, 1952).
The most salient feature of Mesocestoides is the possession of a well-developed parauterine organ, appearing as a dense circular to pear-shaped structure (250–300 μm in diameter) in the posterior portion of the proglottid. This was once considered to be a ‘true’ parauterine organ in contrast to the structures of the same name in some anoplocephalid cestodes, as it was believed to arise independently of uterine tissue (Chandler, 1946; Wardle and McLeod, 1952; James, 1968). However, this assumption was based on the developmental studies using light microscopy and limited specimens, which may be challenging to interpret (Conn, 1987). Ultrastructural analysis of the parauterine organ and the uterine tissues have since confirmed a relationship between the uterine epithelium and the development of the parauterine organ (Conn, 1987). This structure is fully developed in aged proglottids; further down the strobila, eggs move from the uterus into the parauterine organ, which becomes swollen and enlarged in terminal segments. At this point, the uterus will appear empty and the testes degenerate completely (Miyazaki, 1991).
Life cycle and hosts
The life cycle of Mesocestoides spp. is divergent from the more familiar Cyclophyllidea and our understanding remains incomplete. Though debated for some time, it is now generally accepted based on the experimental evidence that not one but two intermediate hosts are involved, although some recent field observations have called this back into question (Fig. 4) (Loos-Frank, 1991; McAllister et al., 2018). Proglottids are passed in the feces of the definitive host; they are highly motile and may crawl actively to the surface of the fecal deposit. The fragile oncospheres die within less than a minute if exposed after breakage of the proglottid, so transmission to the first intermediate host (see below) likely occurs by the ingestion of the entire intact proglottid. The density of the parauterine organ appears to provide some protection, as oncospheres remain motile for 25–40 days within the intact structure (James, 1968; Miyazaki, 1991).
Fig. 4.
Proposed life cycle schemes for Mesocestoides spp., showing two-host (dotted line) and three-host (dashed line) hypotheses. Cestode stages shown on the outside: (A) scolex of an adult; (B) gravid proglottids with oncosphere; (C) unknown cysticercoid or first larval stage; (D) tetrathyridium; representative definitive host (DH) and intermediate hosts (IH) on the inside (Drawings by SGH Sapp).
The role of the first intermediate host and metacestode development therein is the missing link in the complete characterization of the Mesocestoides life cycle. The most popular hypothesis is that this host is an invertebrate that consumes proglottids/eggs found in feces of the infected definitive host, which then supports the development of the oncosphere into a poorly-understood metacestode stage, which probably resembles a procercoid based on in vitro development (Voge and Seidel, 1968; Loos-Frank, 1991). The assumption that an invertebrate serves as a first intermediate host is based both on life cycles of other cestodes and the observation that exposure of vertebrates inoculated with oncospheres/proglottids under many conditions has failed to establish any evidence infection on multiple occasions (Webster, 1949; James, 1968; Loos-Frank, 1991). This seems to suggest that there must be some invertebrate intermediate host despite the failure to identify it. Various authors have proposed terrestrial arthropods such as dung beetles, ants, roaches and mites as potential first intermediate hosts. A singular report of finding Mesocestoides ‘cysticercoids’ in oribatid mites has not been further substantiated in subsequent experimental studies and surveys (James, 1968). Attempts to infect about 50 different species of invertebrates, including several families of arthropods, annelids and mollusks, with the eggs of Mesocestoides spp. have consistently yielded negative results (Webster, 1949; James, 1968). Mesocestoides sp. DNA was detected in 3.1 and 2.4% of Lasius niger and Tapinoma sessile ants, respectively, collected from San Miguel Island (California). However, attempts to infect mice with such ants were not successful (Padgett and Boyce, 2004). Of note, a recent report describes the stages interpreted as transitional, pre-tetrathyridial stages in the body cavity of a ground skink (Scincella lateralis), suggesting that development from the hexacanth embryo to tetrathyridium could possibly be taking place within a single vertebrate host (McAllister et al., 2018). As it stands, the number of hosts involved in the Mesocestoides life cycle remains an outstanding question and the complete life cycle has yet to be demonstrated experimentally.
The second metacestode stage, or tetrathyridium, somewhat resembles a sparganum but has an invaginated, four-suckered scolex (Nelson et al., 1965). It is a solid structure without cystic characteristics, except under aberrant conditions (James, 1968). The second intermediate host presumably becomes infected via consumption of the unknown first intermediate host, which leads to the development of the tetrathyridia in various sites (commonly the peritoneal cavity, mesenteries, also in visceral organs). The host specificity demonstrated by Mesocestoides tetrathyridia is remarkably low, having been recovered from a vast array of mammals, birds, reptiles and amphibians (Miyazaki, 1991).
On rare occasions in nature, tetrathyridia become asexually proliferative which can lead to heavy disseminated infections and death. Instances have nearly all been ascribed to Mesocestoides corti, which was later reidentified as Mesocestoides vogae by Etges (1991). Reports of tetrathyridial asexual budding ascribed to other Mesocestoides spp. exist (Conn, 1990; Crosbie et al., 2000; Galán-Puchades et al., 2002; Conn et al., 2010), though precise species identification is challenging and the presentation varies widely. Some types of tetrathyridial asexual proliferation described involve in the division of the apical extremity resulting in multicephalic forms, budding of the hindbody, and the production of daughter metacestodes from a mother tetrathyridium (Galán-Puchades et al., 2002; Conn et al., 2010; McAllister et al., 2018). The factors influencing the development of these proliferative forms and the mechanism(s) are still not completely understood (Conn et al., 2010). A mechanism involving malignant transformation, also observed with H. nana, has been proposed (Conn et al., 2010; Conn, 2016). High-intensity infections following the ingestion of large numbers of oncospheres are prone to being inaccurately ascribed to asexual proliferation, thus this term should be applied only if abnormal characteristics (e.g. multicephalic or acephalic forms, external budding) can be demonstrated (Conn, 2016).
Definitive hosts are both wild and domestic carnivorous mammals, and adult Mesocestoides spp. may be prevalent in some wildlife populations. In North America, M. variabilis is a common finding in a variety of native mesopredators, such as the raccoon (Procyon lotor), opossum (Didelphis virginiana), striped skunk (Mephitis mephitis), spotted skunk (Spilogale spp.), lynx (Lynx rufus), coyote (Canis latrans), kit fox (Vulpes macrotus) and gray fox (Urocyon cinereoargentus) (Voge, 1955; James, 1968). Other New World Mesocestoides species [e.g. M. vogae ( = corti), M. latus, M. kirbyi] also occur across similar hosts. The primary host of European Mesocestoides spp. (M. lineatus, M. litteratus and unidentified M. sp.) appears to be the red fox (Vulpes vulpes), but infections occur in wolves (Canis lupus), European badgers (Meles meles) and raccoon dogs (Nyctereutes procyonoides) (Thompson, 1976; Jones et al., 1980; Moks et al., 2006; Bagrade et al., 2009; Hrčkova et al., 2011; Bružinskaitė-Schmidhalter et al., 2012). Surveys of Asian wild definitive hosts are less extensive. In Japan, infections in raccoon dogs and Japanese martens (Martes melampus) have been reported (Sato et al., 1999). Wolves, red foxes, corsac foxes (Vulpes corsac) and snow leopards (Panthera uncia) were recently identified as Mesocestoides sp. hosts in Mongolia (Ulziijargal et al., 2020).
As the first intermediate host is unknown and attempts to infect arthropods have never been successful, what is known regarding tetrathyridial and adult stage infections is entirely based on experimental infections involving transferring tetrathyridia derived from naturally-infected hosts among laboratory animals. Prepatent periods following exposure to tetrathyridia have been shown to be highly variable and are probably mostly due to variations in host competence, but inherent species-level differences cannot be ruled out. For example, a range of 16–52 days have been observed for experimentally-infected raccoons, but may as long as 80 days in domestic dogs and over 100 days in domestic cats (James, 1968). Shedding of proglottids may be irregular and vary seasonally (Skarbilovitch, 1945).
Some definitive host species may also develop tetrathyridial infections as second intermediate hosts. This is exemplified by domestic dogs, which may harbour intestinal infections with adult Mesocestoides, or develop potentially life-threatening invasive infections with tetrathyridia (canine peritoneal larval cestodiasis) (Eckert et al., 1969; Speckmann and Webster, 1975; Boyce et al., 2011). Domestic cats have also been shown to serve as both definitive and second intermediate hosts (Mueller, 1930; Eleni et al., 2007).
Human infections
Identity of zoonotic species: The only named species reported from human infections are M. lineatus in Asia and M. variabilis in North America. However, interspecific morphologic plasticity along with other factors complicates specific diagnosis and suggests that current taxonomic divisions in this genus are unsound. Even after examining many specimens from several definitive hosts, Voge (1955) considered these species impossible to distinguish unless the geographic origin was known, and remarked that appearance was greatly influenced by staining methods. Differences in vitellaria and number of testes for M. variabilis were described across its many carnivore hosts. The state of fixation (relaxed vs contracted) was also observed to greatly influence the appearance of some internal structures, including the shape of the parauterine organ and caudal appendage (James, 1968). Further morphological differences in the diameter of the testes, vitellaria, ovaries, position of the cirrus pouch and more are also apparently influenced by age of the proglottids and duration of infection (James, 1968). Differences between the presumed zoonotic species and other sympatric species occurring in natural definitive hosts (e.g. M. latus vs M. variabilis) are also reportedly very slight and subject to intraspecific variation and processing artefacts (Voge, 1955). Given that clinical specimens are typically not recovered under ideal conditions (e.g. brought in by patients after the expulsion, not relaxed prior to fixation, etc.), one may expect that an accurate species identification would be impossible under such circumstances.
Various molecular phylogenetic studies have attempted to further understand species delimitation in Mesocestoides; however, the genus is still far from a formal modern revision. In studies of Mesocestoides spp. isolates from the Western USA, three monophyletic clades were detected (A, B and C) among various hosts including dogs, coyotes, island foxes (Urocyon litteratus) and naturally-infected deer mice (Peromyscus maniculatus) (Crosbie et al., 2000). Morphological assessment of these defined clades revealed extensive overlap in measured characteristics (Padgett et al., 2005). Clade B specimens were most similar to M. vogae (which is possibly synonymous with M. variabilis), but clade A and C specimens could not be matched unambiguously to any existing Mesocestoides species description. None of the three clades was found to be host-specific, although only canid definitive hosts were represented (Padgett et al., 2005). The European picture is clearer than the American one. Among European isolates, M. litteratus and M. lineatus have been found to be distinct species based on some morphological features (number of testes, length of cirrus sac, shape of parauterine organ) and on multiple loci (12s, cox1, nad1) (Literák et al., 2006; Hrčkova et al., 2011; Zaleśny and Hildebrand, 2012). However, studies on ‘M. lineatus’ in East Asia – where it has been reported from human infections – are lacking and thus its specific status and relation to other Mesocestoides spp. are unknown.
There is a clear need for additional Mesocestoides genetic studies as well as conventional comparative studies involving several aspects of complete specimens processed in the same manner. As mentioned, it seems likely that some described species are invalid, whereas other names may represent assemblages of cryptic species. Without further characterization from multiple isolates across different hosts and regions, it is probably most appropriate to report Mesocestoides spp. infections in humans as ‘Mesocestoides sp.’, particularly given that recovering entire specimens from human infections is nearly impossible and the division between M. lineatus and M. variabilis may simply be a geographic assumption at present.
Epidemiology and clinical characteristics: Only about 27 cases of human intestinal infections have been reported; those accessible in English language or translatable medical literature are summarized in Table 5. Over half of these cases are from East Asia (mostly from Japan, fewer from Korea and China) and the remainder from the USA (California, Texas, Missouri, Mississippi, Louisiana, Ohio, New Jersey). Singular reports also exist from Rwanda and a Greenlander living in Denmark, though the latter is based on a personal communication and is not available as a standalone report (Fuentes et al., 2003).
Table 5.
Summary of Mesocestoides spp. infections reported from humans
| Location | Species reported | Age/sex | Possible exposure | Source |
|---|---|---|---|---|
| USA (Texas) | M. sp. | 2 yo F | Unknown | CDC-DPDx (unpub. data) 2010 |
| USA (Louisiana) | M. variabilis | 19 mo M | Cajun sausage with game viscera | (Fuentes et al., 2003) |
| USA (Missouri) | M. variabilis | 5 yo M | Unknown | (Gleason and Healy, 1967) |
| USA (California) | M. sp. | 22 mo F | Unknown; exotic pet contact and presence of lizards in playground noted | (Schultz et al., 1992) |
| USA (Mississippi) | M. sp. | 17 mo M | Unknown; noted numerous pets and livestock at home | (Hutchison and Martin, 1980) |
| USA (New Jersey) | M. sp. | 14 mo M | Unknown; geophagy noted | (Gleason et al., 1973) |
| USA (Texas) | M. variabilis | 13 mo F | Unknown | (Chandler, 1942) |
| Japan (Hamamatsu) | M. lineatus | 51 yo F | Raw snake liver | (Ito et al., 1962) |
| Japan (Nagoya) | M. lineatus | 42 yo M | Raw snake liver and blood, eel | (Morisita et al., 1964) |
| Japan (Tokyo) | M. lineatus | 30 yo M | Raw snake blood, heart, and gall bladder | (Kamegai et al., 1967) |
| Japan (Tokyo) | M. lineatus | 57 yo F | Raw snake blood, heart, and gall bladder | ‘ |
| Japan (Toyohashi) | M. lineatus | 36 yo M | Raw snake and eel | (Kosaka, 1942) |
| Japan (Tokyo) | M. lineatus | 31 yo M | Raw snake and snapping turtle | (Hagihara et al., 1964) |
| Japan (Saitama) | M. sp. | 40 yo M | Raw snake | (Miyagi et al., 1965) |
| Japan (Gifu Prefecture) | M. lineatus | 35 yo M | Raw snake liver and blood | (Ohtomo et al., 1983) |
| Japan | M. lineatus | 46 yo M | Raw snake heart, liver, muscle, and blood | (Kagei et al., 1974) |
| Japan | M. lineatus | Unknown | Unknown | (Nagase et al., 1983) |
| South Korea | M. sp. | 45 yo M | Raw snake meat | (Choi et al., 1967) |
| South Korea (Jeju-do) | M. lineatus | 45 yo M | Raw chicken viscera | (Eom et al., 1992) |
| China | M. lineatus | Unknown | Unknown | (Fan, 1988) |
| China (Jilin Province) | M. lineatus | Unknown | Unknown | (Jin et al., 1990) |
| Denmarka | M. variabilis | Unknown | Unknown | Chandler; unpub. [noted in (Gleason and Healy, 1967)] |
| Rwanda/Burundi (Ruanda-Urundi) | M. sp. (nov?) | ‘child’ | Partridge meat | (Fain and Herin, 1954) |
a ‘Greenlander living in Denmark’.
Human mesocestoidiasis is generally regarded as a foodborne zoonosis, caused by the consumption of tetrathyridia in undercooked meat. An intriguing observation is the epidemiological split between known Asian and North American cases, regardless of the species validity questions discussed prior (Table 5). In the Japanese and Korean cases, patients are middle-aged adults, and nearly all were reported to have habitually consumed blood and organs of snakes (particularly Elaphe quadrivirgata and Agkistrodon halys, both known to harbour tetrathyridia) for perceived medicinal properties (Ito et al., 1962; Kamegai et al., 1967; Kagei et al., 1974). This contrasts with North American cases, which have all been documented in children aged 1–5 years (typically between 1 and 2 years). The source of infection is mysterious as raw/undercooked meat consumption is denied in all but one case. Details reported in some North American cases include contact with a variety of domestic animals, exotic pets and geophagy. However, these do not seem plausible sources for exposure to tetrathyridia, the only stage known to be infective to definitive hosts. Inoculation of laboratory mammals with Mesocestoides eggs via proglottids does not establish infection (Webster, 1949). If the theoretical arthropod first intermediate host is eaten, it would lead to a tetrathyridial tissue infection and not an adult intestinal infection based on the currently accepted life cycle scheme. Overall, it is difficult to say whether this confusion is a result of unreliable case histories, an incompletely understood life cycle, or some other factor, but the difference in the pattern of Asian and North American mesocestoidiasis remains striking.
As with most other intestinal cestode infections, infections can be asymptomatic and generally are not severe. Diarrhoea, intermittent abdominal pain, dizziness, poor appetite, weight loss, languor and cramping have been reported (Choi et al., 1967). It is the appearance of motile proglottids in the stool that has in nearly all cases prompted patients to seek clinical attention. The greatest burden ever detected in a human was in a case in Korea, where 32 strobilae were passed following treatment. The patient reportedly ‘habitually ate raw chicken viscera’, a habit which would explain the repeated exposure and accumulation of worms (Eom et al., 1992). The first described human case may have also represented a high burden; the patient reportedly expelled ‘about 35 feet of an unusual, very narrow type of tapeworm’ following administration of a vermifuge (Chandler, 1942). Intact strobilae recovered from human infections may measure from 30to 136 cm, often passed in long, continuous pieces post-treatment (Chandler, 1942; Choi et al., 1967; Kamegai et al., 1967).
Tetrathyridial infections have never been reported in humans. This possibility should not be entirely discounted, given the low host specificity and that tetrathyridial infections have been documented on multiple occasions in non-human primates (Fincham et al., 1995; Di Filippo et al., 2013; Tokiwa et al., 2014). Additionally, the superficial resemblance to spargana and other larval cestodes could possibly confound diagnosis.
Mesocestoidiasis, though rare, could possibly be underestimated and ought to be considered in cases where patients present with motile cestode proglottids and report raw or rare meat or viscera consumption. Diagnosis to genus level is straightforward for trained parasitologists, as the appearance of the proglottids with a true parauterine organ and surficial genital pore is unique to Mesocestoides. Since the proglottid morphology is so distinctive among known zoonotic cestodes, recovery of the scolex is not necessary for confirming identification. Treatment is simple and has been achieved with a variety of cestodicidal agents (e.g. praziquantel, niclosamide, paromomycin) (Gleason and Healy, 1967; Gleason et al., 1973; Hutchison and Martin, 1980; Fuentes et al., 2003).
Conclusion
The diversity of cestodes that can infect humans is broader than generally appreciated – when properly examined, not every motile proglottid in the human stool will prove to be the typical Taenia, Hymenolepis or Diphyllobothriid. Considering the possibility of increasing exposures to rare zoonoses through modern factors such as travel and encroachment on ecosystems, it is likely that the incidence of human infections with these rare Cyclophyllidean tapeworms will increase in the coming decades. Among the unusual Cyclophyllidea discussed, many unanswered questions remain regarding basic taxonomy and nomenclature, life cycles and transmission, ecology in natural hosts and epidemiology in human cases. Modern genetic tools, especially if employed alongside classical morphological investigation, will certainly aid in resolving these issues, some of which have stood unaddressed for many decades. Improving the ‘visibility’ and raising interest among clinicians and laboratory staff will be critical in achieving these goals, as they are on the frontline of case recognition and the subsequent acquisition of material and clinical information from human cases necessary for further investigations. Prevention strategies – beyond proper food safety and sanitation practices – will be improved by a more complete understanding of the transmission and life stages of these parasites. Overall, improving our understanding of these infrequent human intestinal cestodiases will require collaboration between many parties, including morphologists, molecular parasitologists, clinicians, diagnostic laboratory staff, ecologists and the affected patients themselves.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
Not applicable.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Achir I, Zait H and Hamrioui B (2008) Human infection due to Bertiella sp (Cestode: Anoplocephalidae) in a man originating from Yemen in Algeria. Bulletin de la Société de Pathologie Exotique 101, 107–108. [PubMed] [Google Scholar]
- Adams A (1935) A fourth case of human infection with B. studeri (Cestoda) in Mauritius. Annals of Tropical Medicine and Parasitology 29, 361–362. [Google Scholar]
- Adams A and Webb L (1933) Two further cases of human infestation with Bertiella studeri (Blanchard, 1891) Stiles and Hassall, 1902, with some observations on the probable synonymy of the specimens previously recorded from man. Annals of Tropical Medicine and Parasitology 27, 471–475. [Google Scholar]
- Africa CM and Garcia EY (1935) The occurrence of Bertiella in man, monkey and dog in the Philippines. Philippine Journal of Science 56, 1–10. [Google Scholar]
- Al-Mathal E, Saleh N and Morsy T (2010) Human infection with Bertiella studeri (Cestode: Anoplocephalidae) in an Egyptian worker returning back from Saudi Arabia. Journal of the Egyptian Society of Parasitology 40, 89–92. [PubMed] [Google Scholar]
- Ando K, Sato Y, Miura K, Matsuoka H, Chinzei Y and Iwanaka A (1994) The occurrence of Bertiella Studeri (Cestoda: Anoplocephalidae) in Japanese Macaque, Macaca fuscata, from Mie Prefecture, Japan. Japanese Journal of Parasitology 43, 211–218. [Google Scholar]
- Ando K, Ito T, Miura K, Matsuoka H and Chinzei Y (1996) Infection of an adult in Mie Prefecture, Japan by Bertiella studeri. Southeast Asian Journal of Tropical Medicine and Public Health 27, 200–201. [PubMed] [Google Scholar]
- Anwar C and Ghiffari A (2010) Short case report: the first case of child infestation with Bertiella studeri in Pendopo, Talang Ubi, South Sumatera Indonesia. Retrieved from Universitas Sriwijaya Repository: http://eprints.unsri.ac.id/cgi/users/login?target=http%3A%2F%2Feprints.unsri.ac.id%2F4554%2F2%2FThe_First_Case_of_Child_Infestation_with_Bertiella_studeri_in_Pondopo%2C_South_Sumatera_Indonesia.pdf (Accessed 1 May 2018).
- Bacigalupo J (1949) Primer caso humano de Bertiella sp. en Sud América. Revista de la Sociedad Mexicana de Historia Natural 10, 177–183. [Google Scholar]
- Baer JG (1927) Monographie des cestodes de la famille des Anoplocephalidae. Bulletin Biologique de la France et de la Belgique 10, 1–241. [Google Scholar]
- Baer JG (1956) The taxonomic position of Taenia madagascariensis Davaine, 1870, a tapeworm parasite of man and rodents. Annals of Tropical Medicine and Parasitology 50, 152–156. [DOI] [PubMed] [Google Scholar]
- Baer J and Fain A (1955) Les cestodes des pangolins. Bulletin de la Société Neuchâteloise des Sciences Naturelles 78, 38–52. [Google Scholar]
- Baer JG and Sandars DF (1956) The first record of Raillietina (Raillietina) celebensis (Janicki, 1902), (Cestoda) in man from Australia, with a critical survey of previous cases. Journal of Helminthology 30, 173–182. [DOI] [PubMed] [Google Scholar]
- Baer J, Kourí P and Sotolongo F (1950) Anatomy, systematic position and epidemiology of Inermicapsifer cubensis (Kouri 1938) Kouri, 1940, a cestode parasitic in man in Cuba. Revista Kuba de medicina Tropical y Parasitologia 6, 9. [PubMed] [Google Scholar]
- Bagrade G, Kirjušina M, Vismanis K and Ozoliņš J (2009) Helminth parasites of the wolf Canis lupus from Latvia. Journal of Helminthology 83, 63–68. [DOI] [PubMed] [Google Scholar]
- Bailenger J and Carcenac F (1970) A new geographic localization of Inermicapsifer madagascariensis (Davaine 1870) Baer 1956: La Reunion. Bulletin de la Societe de Pathologie Exotique et de ses Filiales 63, 242. [PubMed] [Google Scholar]
- Bandyopadhyay AK and Manna B (1987) The pathogenic and zoonotic potentiality of Bertiella studeri. Annals of Tropical Medicine and Parasitology 81, 465–466. [DOI] [PubMed] [Google Scholar]
- Baylis H (1949) A new human cestode infection in Kenya. Inermicapsifer Arvicanthidis, a parasite of rats. Transactions of the Royal Society of Tropical Medicine and Hygiene 42, 537–542. [DOI] [PubMed] [Google Scholar]
- Beaver P, Jung R and Cupp E (1984) Clinical Parasitology. Philadelphia: Lea & Febiger. [Google Scholar]
- Belding DL (1965) Textbook of Parasitology, 3rd Edn. New York: Meredith Publishing Company. [Google Scholar]
- Beugnet F, Meyer L, Fourie J and Larsen D (2017) Preventive efficacy of NexGard Spectra((R)) against Dipylidium caninum infection in dogs using a natural flea (Ctenocephalides felis) infestation model. Parasite 24, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beugnet F, Labuschagne M, de Vos C, Crafford D and Fourie J (2018) Analysis of Dipylidium caninum tapeworms from dogs and cats, or their respective fleas-Part 2. Distinct canine and feline host association with two different Dipylidium caninum genotypes. Parasite 25, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge I (1985) The genus Bertiella (Cestoda: Anoplocephalidae) from Australasian mammals: new species, new records and redescriptions. Systematic Parasitology 7, 241–289. [Google Scholar]
- Bhagwant S (2004) Human Bertiella studeri (family Anoplocephalidae) infection of probable Southeast Asian origin in Mauritian children and an adult. American Journal of Tropical Medicine and Hygiene 70, 225–228. [PubMed] [Google Scholar]
- Bhaibulaya M (1985) Human infection with Bertiella studeri in Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 16, 505–507. [PubMed] [Google Scholar]
- Blagburn B (2001) Prevalence of canine and feline parasites in the United States. Copendium of Continuing Education for the Practicing Veterinarian 23, 5–10. [Google Scholar]
- Blanchard R (1891) Sur les helminthes de primates antropoides. Mémoires de la Société Zoologique de France 4, 186–196. [Google Scholar]
- Blanchard R (1913) Bertiella satyri de l'Orang-outang, est aussi parasite de l'homme. Bulletin de L'Académie Nationale de Médecine 9, 286–296. [Google Scholar]
- Bolbol A (1985) Bertiella sp. infection in man in Saudi Arabia. Annals of Tropical Medicine and Parasitology 79, 643–644. [DOI] [PubMed] [Google Scholar]
- Bonne C (1940) Over Bertiella studeri (Blanchard 1891). Geneeskundig Tijdschrift voor Nederlandsche-Indie 80, 2222–2230. [Google Scholar]
- Bonne C and Mreyen F (1940) Over Raillietina madagascariensis (Davaine 1869). Geneeskundig Tijdschrift voor Nederlandsche-Indie 80, 1310–1318. [Google Scholar]
- Bourquin J (1905) Cestodes de mammifères: le genre Bertia, Impr. W. Kündig & fils.
- Boyce W, Shender L, Schultz L, Vickers W, Johnson C, Ziccardi M, Beckett L, Padgett K, Crosbie P and Sykes J (2011) Survival analysis of dogs diagnosed with canine peritoneal larval cestodiasis (Mesocestoides spp.). Veterinary Parasitology 180, 256–261. [DOI] [PubMed] [Google Scholar]
- Bružinskaitė-Schmidhalter R, Šarkūnas M, Malakauskas A, Mathis A, Torgerson PR and Deplazes P (2012) Helminths of red foxes (Vulpes vulpes) and raccoon dogs (Nyctereutes procyonoides) in Lithuania. Journal of Parasitology 139, 120–127. [DOI] [PubMed] [Google Scholar]
- Buckley J and Fairley N (1950) Demonstration of a cestode, Bertiella: a rare parasite in man and recorded for the first time as a human infection in Africa. Transactions of the Royal Society of Tropical Medicine and Hygiene 44, 2. [Google Scholar]
- Cabello RR, Ruiz AC, Feregrino RR, Romero LC, Feregrino RR and Zavala JT (2011) Dipylidium caninum infection. BMJ Case Reports 2011, bcr0720114510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron TW (1929) A new record of the occurrence of a tapeworm of the genus Bertiella In man. Journal of Helminthology 7, 231–234. [Google Scholar]
- Cameron TWM and Reesal M (1951) Studies on the endoparasitic fauna of Trinidad mammals: VII. Parasites of hystricomorph rodents. Canadian Journal of Zoology 29, 276–289. [Google Scholar]
- CDC-DPDx (2009) Case 261 – October, 2009. Retrieved from DPDx, Centers for Disease Control and Prevention. Available at https://www.cdc.gov/dpdx/monthlycasestudies/2009/case261.html (Accessed 16 December 2019).
- CDC-DPDx (2019) DPDx: Raillietina infection. Retrieved from DPDx, Centers for Disease Control and Prevention. Available at https://www.cdc.gov/dpdx/raillietina/index.html (Accessed 16 December 2019).
- Chaisiri K, Chaeychomsri W, Siruntawineti J, Ribas A, Herbreteau V and Morand S (2010) Gastrointestinal helminth infections in Asian house rats (Rattus Tanezumi) from northern and northeastern Thailand. Journal of Tropical Medicine and Parasitology 33, 29–35. [PubMed] [Google Scholar]
- Chaisiri K, Siribat P, Ribas A and Morand S (2015) Potentially zoonotic helminthiases of murid rodents from the Indo-Chinese peninsula: impact of habitat and the risk of human infection. Vector-Borne and Zoonotic Disease 15, 73–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler AC (1925) New records of Bertiella Satyri (Cestoda) in man and apes. Parasitology 17, 421–425. [Google Scholar]
- Chandler AC (1942) First record of a case of human infection with tapeworms of the genus Mesocestoides. American Journal of Tropical Medicine and Hygiene 1, 493–497. [Google Scholar]
- Chandler AC (1946) Observations on the anatomy of Mesocestoides. Journal of Parasitology 32, 242–246. [PubMed] [Google Scholar]
- Chandler AC and Pradatsundarasar A (1957) Two cases of Raillietina Infection in infants in Thailand, with a discussion of the taxonomy of the species of Raillietina (Cestoda) in man, rodents and monkeys. Journal of Parasitology 43, 81–88. [PubMed] [Google Scholar]
- Chappell CL, Enos JP and Penn HM (1990) Dipylidium caninum, an underrecognized infection in infants and children. Pediatric Infectious Diseases Journal 9, 745–747. [PubMed] [Google Scholar]
- Charoenlarp P and Radomyos P (1973) Treatment of Raillietina Siriraji with atabrine. Southeast Asian Journal of Tropical Medicine and Public Health 4, 288. [PubMed] [Google Scholar]
- Chelladurai JJ, Kifleyohannes T, Scott J and Brewer MT (2018) Praziquantel resistance in the zoonotic cestode Dipylidium caninum. American Journal of Tropical Medicine and Hygiene 99, 1201–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenchittikul M, Daengpium S, Hasegawa M, Itoh T and Phanthumachinda B (1983) A study of commensal rodents and shrews with reference to the parasites of medical importance in Chanthaburi Province, Thailand. Southeast Asian Journal of Tropical Medicine and Public Health 14, 255–259. [PubMed] [Google Scholar]
- Choi WY, Kim BC and Choi HS (1967) The first case of human infection with tapeworms of the genus Mesocestoides In Korea. Korean Journal of Parasitology 5, 60–64. [DOI] [PubMed] [Google Scholar]
- Chomel BB and Sun B (2011) Zoonoses in the bedroom. Emerging Infectious Diseases 17, 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn DB (1987) Fine structure, development, and senescence of the uterine epithelium of Mesocestoides lineatus (Cestoda: Cyclophyllidea). Transactions of the American Microscopical Society 106, 63–73. [Google Scholar]
- Conn DB (1990) The rarity of asexual reproduction among Mesocestoides tetrathyridia (Cestoda). Journal of Parasitology 76, 453–455. [PubMed] [Google Scholar]
- Conn DB (2016) Malignant transformation of Hymenolepis nana in a human host (Comment). The New England Journal of Medicine 374, 1293–1293. [DOI] [PubMed] [Google Scholar]
- Conn DB, Galán-Puchades MT and Fuentes MV (2010) Interactions between anomalous excretory and tegumental epithelia in aberrant Mesocestoides Tetrathyridia from Apodemus sylvaticus in Spain. Parasitology Research 106, 1109–1115. [DOI] [PubMed] [Google Scholar]
- Cram EB (1928) A species of the cestode genus Bertiella in man and the chimpanzee in Cuba. American Journal of Tropical Medicine and Hygiene 1, 339–344. [Google Scholar]
- Crosbie PR, Nadler SA, Platzer EG, Kerner C, Mariaux J and Boyce WM (2000) Molecular systematics of Mesocestoides Spp.(Cestoda: Mesocestoididae) from domestic dogs (Canis familiaris) and coyotes (Canis latrans). Journal of Parasitology 86, 350–357. [DOI] [PubMed] [Google Scholar]
- d’ Alessandro B, Beaver P and Pallares RM (1963) Bertiella infection in man in Paraguay. American Journal of Tropical Medicine and Hygiene 12, 193–198. [DOI] [PubMed] [Google Scholar]
- da Silva AVM, Arruda FCS, Costa GA and dos Santos HA (2011) Bertielose humana: segundo relato em Minas Gerais, Brasil. Revista De Patologia Tropical 40, 185–190. [Google Scholar]
- Davis RE, Mathison BA and Couturier MR (2019) Raillietiniaisis in a toddler from Hawaii; a case of mistaken tapeworm identity. Clinical Infectious Diseases 69, 1053–1055. [DOI] [PubMed] [Google Scholar]
- de Costa HM, Correa L and Brener Z (1967) A new human case of a parasitic infection caused by Bertiella mucronata (Meyner, 1895) Stiles & Hassal, 1902 (Cestoda-anoplocephalidae). Revista do Instituto de Medicina Tropical de Sao Paulo 9, 95–97. [PubMed] [Google Scholar]
- Denegri GM (1993) Review of oribatid mites as intermediate hosts of tapeworms of the Anoplocephalidae. Experimental Applied Acarology 17, 567–580. [Google Scholar]
- Denegri GM and Perez-Serrano J (1997) Bertiellosis in man: a review of cases. Revista do Instituto de Medicina Tropical de Sao Paulo 39, 123–128. [DOI] [PubMed] [Google Scholar]
- de Oliveira Simões R, Simões SBE, Luque JL, Iñiguez AM and Júnior AM (2017) First record of Raillietina celebensis (Cestoda: Cyclophyllidea) in South America: redescription and phylogeny. Journal of Parasitology, 103, 359–365. [DOI] [PubMed] [Google Scholar]
- de Souza Júnior J, Greinert Goulart J, Varnier S, Denegri G, da Silva Filho H, Braga Hirano ZM and de Avila-Pires FD (2008) Bertiellosis in Brazilian non-human primates: natural infection in Alouatta Guariba clamitans (Cabrera, 1940) (primates: atelidae) in Santa Catarina state, Brazil. Revista De Patologia Tropical 37, 48–56. [Google Scholar]
- Desowitz R, Wong H and Fernando M (1961) The first record of human infection with Bertiella studeri in Malaya. Journal of Helminthology 35, 207–208. [DOI] [PubMed] [Google Scholar]
- Di Filippo MM, Meoli R, Cavallero S, Eleni C, De Liberato C, Berrilli FJI and Evolution Ga (2013) Molecular identification of Mesocestoides sp. metacestodes in a captive gold-handed tamarin (Saguinus midas). Infection, Genetics, and Evolution 65, 399–405. [DOI] [PubMed] [Google Scholar]
- Doležalová J, Vallo P, Petrželková KJ, Foitova I, Nurcahyo W, Mudakikwa A, Hashimoto C, Jirků M, Lukeš J and Scholz T (2015) Molecular phylogeny of anoplocephalid tapeworms (Cestoda: Anoplocephalidae) infecting humans and non-human primates. Parasitology 142, 1278–1289. [DOI] [PubMed] [Google Scholar]
- Dollfus R-P (1939) Cestodes du genre Raillietina Trouvés chez l'homme en Amérique intertropicale. Annales de Parasitologie Humaine et Comparée 17, 415–442. [Google Scholar]
- Dunn AM (1978) Veterinary Helminthology. Portsmouth, New Hampshire: William Heinemann Medical Books Ltd. [Google Scholar]
- Du Plooy I (2014) Results of Routine Examinations for Parasitic Infections of Humans from Laboratory-Submitted Samples in Gauteng, North West and Mpumalanga Provinces between 2009 and 2010 (PhD thesis). University of Pretoria, Pretoria, Gauteng, South Africa. [Google Scholar]
- Eckert J, von Brand T and Voge M (1969) Asexual multiplication of Mesocestoides corti (Cestoda) in the intestine of dogs and skunks. Journal of Parasitology 55, 241–249. [PubMed] [Google Scholar]
- Edirisinghe JS and Cumararajan SM (1976) The first record of Bertiella studeri infection in a child from Sri Lanka. Ceylon Medical Journal 21, 137–140. [PubMed] [Google Scholar]
- El-Dib NA, Al-Rufaii A, El-Badry AA, Al-Zoheiry AA and El-Aall A (2004) Human infection with Bertiella species in Saudi Arabia. Saudi Pharmaceutical Journal 12, 168–169. [Google Scholar]
- El-Shazly A, Morsy T and Dawoud H (2004) Human Monieziasis expansa: the first Egyptian parastic zoonosis. Journal of the Egyptian Society of Parasitology 34, 515–518. [PubMed] [Google Scholar]
- Eleni C, Scaramozzino P, Busi M, Ingrosso S, D'amelio S and De Liberato C (2007) Proliferative peritoneal and pleural cestodiasis in a cat caused by metacestodes of Mesocestoides sp. Anatomohistopathological findings and genetic identification. Parasite 14, 71–76. [DOI] [PubMed] [Google Scholar]
- Eom KS, Kim S-H and Rim H-L (1992) Second case of human infection with Mesocestoides lineatus. Korean Journal of Parasitology 30, 147–150. [DOI] [PubMed] [Google Scholar]
- Etges FJ (1991) The proliferative tetrathyridium of Mesocestoides vogae sp. n. (Cestoda). Journal of the Helminthological Society of Washington 58, 181–185. [Google Scholar]
- Fain A (1950) Inermicapsifer cubensis (Kouri, 1938). Présence du cestode I. cubensis Synonyme de Inermicapsifer arvicanthidis (Kofend, 1917) chez un enfant indigène et chez un rat (Rattus r. rattus L.) au Ruanda-Urundi (Congo Belge). Bulletin de la Société de Pathologie Exotique 43, 438–443. [Google Scholar]
- Fain A and Herin V (1954) Human infestation with Mesocestoides at Astrida, Ruanda-Urundi. Presence of this tapeworm in a cat and evidence of the larvae in various vertebrates. Annales de la Societe Belge de Medecine Tropicale 34, 893–900. [PubMed] [Google Scholar]
- Fain A, Limbos P, Van Ros G, De Mulder P and Herin A (1977) Présence du cestode Raillietina (R.) celebensis (Janicki, 1902) chez un enfant originaire de Tahiti. Annales de la Société belge de Médecine tropicale 57, 137–142. [PubMed] [Google Scholar]
- Fan S (1988) First case of Mesocestoides lineatus infection in China. Chinese Journal of Parasitology and Parasitic Diseases 6, 310. [Google Scholar]
- Feldman R, Denegri G, Avolio J and Cantu N (1983) Nuevo caso humano de teniasis por Bertiella mucronata (Cestoda Anaplocephalidae), Meyner, 1895, en la Argentina. I. Diagnostico y tratamiento. Acta Bioquímica Clínica Latinoamericana 17, 571–578. [Google Scholar]
- Fincham J, Seier J, Verster A, Rose A, Taljaard J, Woodroof C and Rutherfoord G (1995) Pleural Mesocestoides and cardiac shock in an obese vervet monkey (Cercopithecus aethiops). Veterinary Pathology 32, 330–333. [DOI] [PubMed] [Google Scholar]
- Fogh S and Seaton DR (1967) A case of infection with Bertiella studeri from the Yemen. Transactions of the Royal Society of Tropical Medicine and Hygiene 61, 19–19. [Google Scholar]
- Foitová I, Mašová Š, Tenora F, Koubková B, Hodová I, Vyskočilová M, Baruš V and Nurcahyo W (2011) Redescription and resurrection of Bertiella satyri (Cestoda, Anoplocephalidae) parasitizing the orangutan (Pongo abelii) in Indonesia. Parasitology Research 109, 689–697. [DOI] [PubMed] [Google Scholar]
- Fourie JJ, Crafford D, Horak IG and Stanneck D (2012) Prophylactic treatment of flea-infested cats with an imidacloprid/flumethrin collar to forestall infection with Dipylidium caninum. Parasites and Vectors 5, 151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie JJ, Crafford D, Horak IG and Stanneck D (2013) Prophylactic treatment of flea-infested dogs with an imidacloprid/flumethrin collar (Seresto(R), Bayer) to preempt infection with Dipylidium caninum. Parasitology Research 112(suppl. 1, 33–46. [DOI] [PubMed] [Google Scholar]
- Frean J and Dini L (2004) Unusual anoplocephalid tapeworm infections in South Africa. Annals of the Australasian College of Tropical Medicine 5, 8–11. [Google Scholar]
- Fuentes MV, Galan-Puchades MT and Malone JB (2003) A new case report of human Mesocestoides infection in the United States. American Journal of Tropical Medicine and Hygiene 68, 566–567. [DOI] [PubMed] [Google Scholar]
- Furtado AP, Batista EDJO, Gonçalves EC, Silva AMHO, Melo FTV, Giese EG and Santos JN (2012) Human bertielliasis in Amazonia: case report and challenging diagnosis. PLoS Neglected Tropical Diseases 6, e1580–e1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán-Puchades MT, Fuentes MV and Mas-Coma S (1995) Human Bertiella studeri in Spain, probably of African origin. American Journal of Tropical Medicine and Hygiene 56, 610–612. [DOI] [PubMed] [Google Scholar]
- Galán-Puchades M, Fuentes M, Simarro P and Mas-Coma S (1997) Human Bertiella studeri in equatorial Guinea. Transactions of the Royal Society of Tropical Medicine and Hygiene 91, 680. [DOI] [PubMed] [Google Scholar]
- Galán-Puchades MT, Fuentes MV and Mas-Coma S (2000) Morphology of Bertiella studeri (Blanchard, 1891) sensu Stunkard (1940) (Cestoda: Anoplocephalidae) of human origin and a proposal of criteria for the specific diagnosis of bertiellosis. Folia Parasitologica 47, 23–28. [DOI] [PubMed] [Google Scholar]
- Galán-Puchades MT, Fuentes MV and Conn DB (2002) A new type of endogenous asexual proliferation in cyclophyllidean metacestodes. Acta Parasitologica 47, 288–293. [Google Scholar]
- Gallella S, Gunawardena G and Karunaweera N (2011) Bertiella studeri infection: resistance to niclosamide. Ceylon Medical Journal 49, 65. [PubMed] [Google Scholar]
- Garaguso P and Mendez O (1983) Primer caso argentino de parasitismo humano por Bertiella mucronata (Meyner, 1895). Congreso Latinoamericano de Parasitología 6, 232. [Google Scholar]
- García-Agudo L, García-Martos P and Rodríguez-Iglesias M (2014) Dipylidium caninum infection in an infant: a rare case report and literature review. Asian Pacific Journal of Tropical Biomedicine 4, S565–S567. [Google Scholar]
- Gasser R, De Gruijter J and Polderman AJP (2006) Insights into the epidemiology and genetic make-up of Oesophagostomum bifurcum from human and non-human primates using molecular tools. Parasitology 132, 453–460. [DOI] [PubMed] [Google Scholar]
- Gené C, Rea M, Borda C, Mosqueda L and Benítez O (2011) Parasitosis intestinal infantil en la provincia de Corrientes. Argentina. Biomédica 31, 209–421.22159537 [Google Scholar]
- Gleason NN and Healy GR (1967) Report of a case of Mesocestoides (Cestoda) in a child in Missouri. Journal of Parasitology 53, 83–84. [PubMed] [Google Scholar]
- Gleason N, Kornblum R and Walzer P (1973) Mesocestoides (Cestoda) in a child in New Jersey treated with niclosamide (Yomesan®). American Journal of Tropical Medicine and Hygiene 22, 757–760. [DOI] [PubMed] [Google Scholar]
- Goldsmid J (1964) Infestation of man by the genera Inermicapsifer and Raillietina (Cestoda) in Rhodesia. Central African Journal of Medicine 10, 410–411. [PubMed] [Google Scholar]
- Goldsmid J and Muir M (1972) Inermicapsifer madagascariensis (Davaine, 1870), Baer, 1956 (Platyhelminthes: Cestoda) as a parasite of man in Rhodesia. Central African Journal of Medicine 18, 205–207. [PubMed] [Google Scholar]
- Gomez-Puerta LA, Lopez-Urbina MT and Gonzalez AE (2009) Occurrence of tapeworm Bertiella mucronata (Cestoda: Anoplocephalidae) in the Titi monkey Callicebus oenanthe from Peru: new definitive host and geographical record. Veterinary Parasitology 163, 161–163. [DOI] [PubMed] [Google Scholar]
- González Núñez I, Díaz Jid M and Núñez Fernández F (1996) Infección por Inermicapsifer madagascariensis (Davaine, 1870); Baer 1956.: Presentación de 2 casos. Revista Cubana de Medicina Tropical 48, 224–226. [PubMed] [Google Scholar]
- Gray J (1972) Studies on the course of infection of the poultry cestode Raillietina cesticillus (Molin, 1858) in the definitive host. Journal of Parasitology 65, 243–250. [DOI] [PubMed] [Google Scholar]
- Hagihara T, Amaki K, Okayasu D, Nakashima A, Iwata A, Higo T, Sugihara K, Ohata N and Kono K (1964) 2 Cases of curious helminthiasis (Mesocetoideases and Mansoniasis). Japan Medical Journal 2088, 24–27. [Google Scholar]
- Herbreteau V and Morand S (2011) Gastrointestinal helminth fauna in rodents from Loei Province. Thailand. Srinakharinwirot Science Journal 26, 111–126. [Google Scholar]
- Herrera Valdés NE, Díaz García ME, Sandoval Acosta M and García Batista N (2007) Inermicapsifer madagascariensis. Revista Cubana de Medicina Militar 36, 28–29. [Google Scholar]
- Hira P (1974) Infestation of man by the cestode Inermicapsifer madagascariensis in Lusaka, Zambia. Medical Journal of Zambia 8, 35–38. [Google Scholar]
- Hira P (1975) Human and rodents infection with the cestode Inermicapsifer madagascariensis (Davaine, 1870) Baer, 1956 in Zambia. Annales de la Société Belge de Médecine Tropicale 55, 321–325. [PubMed] [Google Scholar]
- Hoberg EP, Jones A and Bray R (1999) Phylogenetic analysis among the families of the Cyclophyllidea (Eucestoda) based on comparative morphology, with new hypotheses for co-evolution in vertebrates. Systematic Parasitology 42, 51–73. [DOI] [PubMed] [Google Scholar]
- Horsfall MW (1938) Observations on the life history of Raillietina echinobothrida and of R. tetragona (Cestoda). Journal of Parasitology 24, 409–421. [Google Scholar]
- Hrčkova G, Miterpakova M, O'connor A, ŠnÁbel V and Olson PD (2011) Molecular and morphological circumscription of Mesocestoides tapeworms from red foxes (Vulpes vulpes) in central Europe. Journal of Parasitology 138, 638–647. [DOI] [PubMed] [Google Scholar]
- Hutchison WF and Martin JB (1980) Mesocestoides (Cestoda) in a child in Mississippi treated with paromomycin sulfate (Humatin®). American Journal of Tropical Medicine and Hygiene 29, 478–479. [DOI] [PubMed] [Google Scholar]
- Imamkuliev KD, Alekseeva MI, Gorbunova Iu P and Belikova GG (1983) Bertielliasis: a description of a case brought into the USSR. Meditsinskaia Parazitologiia (Mosk.) 4, 77–78. [PubMed] [Google Scholar]
- Iseki M, Uni S and Kimata L (1993) Infection of a child in Japan by Bertiella studeri. Japanese Journal of Parasitology 42, 129. [Google Scholar]
- Ito J, Honda J and Ishiguro M (1962) The second record of a case of human infection with Mesocestoides lineatus in Japan (Cestoda). Japanese Journal of Parasitology 11, 71–75. [Google Scholar]
- Jackson D, Crozier W, Andersen S, Giles W and Bowen T (1977) Dipylidiasis in a 57-year-old woman. Medical Journal of Australia 2, 740–741. [DOI] [PubMed] [Google Scholar]
- James HA (1968) Studies on the genus Mesocestoides (Cestoda: Cyclophyllidea) (PhD thesis). Ames, Iowa, USA: Iowa State University. [Google Scholar]
- Jiang P, Zhang X, Liu RD, Wang ZQ and Cui J (2017) A human case of zoonotic dog Tapeworm, Dipylidium caninum (Eucestoda: Dilepidiidae), in China. Korean Journal of Parasitology 55, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Yi S and Liu Z (1990) The first case of human infection with Mesocestoides lineatus (Goeze, 1782) in Jilin Province. Journal of the Norman Bethune University of Medical Sciences 4, 360–361. [Google Scholar]
- Jones R, Hunter H and Van Rooyen C (1971) Bertiella infestation in a Nova Scotia child formerly resident in Africa. Canadian Medical Association Journal 104, 612. [PMC free article] [PubMed] [Google Scholar]
- Jones G, Neal C and Harris E (1980) The helminth parasites of the badger (Meles meles) in Cornwall. Mammal Review 10, 163–164. [Google Scholar]
- Joyeux C and Baer J (1929) Les cestodes parasites de l ‘home. Bulletin de la Société de Pathologie Exotique 22, 114–136. [Google Scholar]
- Joyeux C and Baer J (1949) The normal host of Raillietina (R.) demerariensis in Netherlands Guiana. Acta Tropica 6, 141–144. [PubMed] [Google Scholar]
- Joyeux C and Dollfus R (1931) Un nov cas de B. studeri (R. Bl) chez l'homme. Comptes rendus des séances de la Société de Biologie et de ses Filiales 107, 35–36. [Google Scholar]
- Kagei N, Kihata M, Shimizu S, Urabe M and Ishii A (1974) The 10th case of human infection with Mesocestoides lineatus (Cestoda: Cyclophyllidea) in Japan. Japanese Journal of Parasitology 23, 376–382. [Google Scholar]
- Kagei N, Purba Y and Sakamoto O (1992) Two cases of human infection with Bertiella studeri in North Sumatra, Indonesia. Japanese Journal of Tropical Medicine and Hygiene 20, 165–168. [Google Scholar]
- Kamegai S, Ichihara A, Nonobe H and Machida M (1967) The 6th and 7th records of human infection with Mesocestoides lineatus (Cestoda) in Japan. Research Bulletin of the Meguro Parasitological Museum 1, 1–7. [Google Scholar]
- Karunaweera N, Ihalamulla R, Wickramathanthri H and Lamahewage A (2001) Bertiella studeri: a case of human infection. Ceylon Journal of Medical Science 44, 23–42. [Google Scholar]
- Khalil L, Jones A and Bray R (1994) Keys to the Cestode Parasites of Vertebrates. Oxford, United Kingdom: CAB International. [Google Scholar]
- Kojima S, Hata H and Watanabe K (1992) Bertiella sp. (cestode) infection in a child in Mie prefecture. Japanese Journal of Parasitology 41, 211–218. [Google Scholar]
- Kosaka S (1942) The first record of human infection with Mesocestoides lineatus in Japan. Jikken Shokakibyogaku Zasshi 17, 405–408. [Google Scholar]
- Kosin E and Kosin L (1992) Helminthic zoonosis in Indonesia. Yonsei Reports of Tropical Medicine 23, 81–88. [Google Scholar]
- Kourí P (1944) Third communication on Inermicapsifer cubensis. Revista de Medicina Tropical y Parasitologia 10, 107–112. [Google Scholar]
- Kourí P and Kourí J (1952) Hallazgo del Inermicapsifer cubensis en la rata blanca. Nota previa Kuba 8, 27. [PubMed] [Google Scholar]
- Kwo E and Koh I (1968) Six more cases of human infection with Bertiella studeri in Sumatra, Indonesia. Yonsei Reports of Tropical Medicine 23, 81–88. [Google Scholar]
- Lamom C and Greer GJ (1986) Human infection with an anoplocephalid tapeworm of the genus Mathevotaenia. American Journal of Tropical Medicine and Hygiene 35, 824–826. [DOI] [PubMed] [Google Scholar]
- Literák I, Tenora F, Letkova V, Goldova M, Torres J and Olson P (2006) Mesocestoides litteratus (Batsch, 1786) (Cestoda: Cyclophyllidea: Mesocestoididae) from the red fox: morphological and 18S rDNA characterization of European isolates. Helminthologia 43, 191–195. [Google Scholar]
- Littlewood DTJ, Waeschenbach A and Nikolov PN (2008) In search of mitochondrial markers for resolving the phylogeny of cyclophyllidean tapeworms (Platyhelminthes, Cestoda) – a test study with Davaineidae. Acta Parasitologica 53, 133–144. [Google Scholar]
- Loos-Frank B (1991) One or two intermediate hosts in the life cycle of Mesocestoides (Cyclophyllidea, Mesocestoididae)? Parasitology Research 77, 726–728. [DOI] [PubMed] [Google Scholar]
- Lopes VV, Dos Santos HA, Da Silva AVM, Fontes G, Vieira GL, Ferreira AC and Da Silva ES (2015) First case of human infection by Bertiella studeri (Blanchard, 1891) Stunkard, 1940 (Cestoda; Anoplocephalidae) in Brazil. Revista do Instituto de Medicina Tropical de Sao Paulo 57, 447–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Neyra C (1950) T. madagascariensis, Davaine 1869 and I. cubensis (Kouri, 1939) are one species of the genus Raillietina (Fuhrmann, 1920) Lopez-Neyra, 1934. Revista Iberica de Parasitologia 10, 187–203. [Google Scholar]
- López-Neyra C (1954) Anoplocephalidae. Revista Iberica de Parasitologia 14, 13–130. [Google Scholar]
- López-Neyra C and Muñoz M (1919) Study on the development of certain species of the genus Dipylidium, -Leuckart. Boletin de la Real Sociedad Espanola de Historia Natural, Madrid 19, 494–506. [Google Scholar]
- Lozano Mdel C, Garcia-Martos P, Garcia-Tapia A and Fernandez C (2010) Bertiella studeri infection in a girl from Equatorial Guinea. Enfermedades Infecciosas y Microbiología Clínica 28, 652–653. [DOI] [PubMed] [Google Scholar]
- Malik S, Srivastava V and Samantaray J (2013) Human bertiellosis from north India. The Indian Journal of Pediatrics 80, 258–260. [DOI] [PubMed] [Google Scholar]
- Maplestone PA (1930) A new case of Bertiella studeri in a human being. Indian Medical Gazette 65, 258–260. [PMC free article] [PubMed] [Google Scholar]
- Maplestone PA and Riddle JS (1936) Infection with Bertiella studeri. Indian Medical Gazette 71, 81. [PMC free article] [PubMed] [Google Scholar]
- Margono S, Handojo I, Hadidjaja P and Mahfudin H (1977) Raillietina infection in children in Indonesia. Southeast Asian Journal of Tropical Medicine and Public Health 8, 195–199. [PubMed] [Google Scholar]
- Mariaux J, Tkach VV, Vasileva GP, Waeschenbach A, Beveridge I, Dimitrova Y, Haukisalmi V, Greiman S, Littlewood D and Makarikov A (2017) Cyclophyllidea van Beneden in Braun, 1900. In Caira J and Jensen K (eds), Planetary Biodiversity Inventory: Tapeworms from Vertebrate Bowels of the Earth. Lawrence, KS: University of Kansas, Natural History Museum, Special Publication, pp. 77–148. [Google Scholar]
- Matevosyan E (1966) On the identity of species of Raillietina From man, monkeys and rodents. Tematicheskii Sbornik Rabot po Gel'mintologii (Trudy VIGIS) 12, 11–20. [Google Scholar]
- Mayor VM and Herrera JCS (2004) Primer caso de Inermicapsifer madagascariensis (Davaine, 1870; Baer, 1956) informado en la provincia de Santiago de Cuba. Medisan 8, 26–29. [Google Scholar]
- McAllister CT, Tkach VV and Conn DB (2018) Morphological and molecular characterization of post-larval pre-tetrathyridia of Mesocestoides sp. (Cestoda: Cyclophyllidea) from ground skink, Scincella lateralis (Sauria: Scincidae), from southeastern Oklahoma. Journal of Parasitology 104, 246–254. [DOI] [PubMed] [Google Scholar]
- Mettrick D and Weir J (1963) Studies on the genus Inermicapsifer Janicki, 1910 with notes on some genera in the subfamilies Inermicapsiferinae, Linstowiinae, and Davaineinae. Proceedings of the Helminthological Society of Washington 30, 199–205. [Google Scholar]
- Meyner R (1895) Anatomie und Histologie zweier neuer Taenien-Arten des Subgenus Bertia. Zeitschrift für Naturforschung Leipzig 68, 1–106. [Google Scholar]
- Miyagi T, Hurusawa K, Oshima T, Wakeshima T, Ozu S, Eda C, Kano R, Kaneko K, Shinonaga T, Miyamoto K and Takae S (1965) A case of human infection with Mesocestoides. Japanese Journal of Parasitology 14, 613–614. [Google Scholar]
- Miyazaki I (1950) Raillietina madágascariensis found in Kyushu, Japan. Kyushu Memoirs of Medical Sciences 1, 1–6. [Google Scholar]
- Miyazaki I (1991) An Illustrated Book of Helminthic Zoonoses. Tokyo, Japan: International Medical Foundation of Japan Tokyo. [Google Scholar]
- Moks E, Jõgisalu I, Saarma U, Talvik H, Järvis T and Valdmann H (2006) Helminthologic survey of the wolf (Canis lupus) in Estonia, with an emphasis on Echinococcus granulosus. Journal of Wildlife Diseases 42, 359–365. [DOI] [PubMed] [Google Scholar]
- Molina CP, Ogburn J and Adegboyega P (2003) Infection by Dipylidium caninum in an infant. Archives of Pathology and Laboratory Medicine 127, e157–e159. [DOI] [PubMed] [Google Scholar]
- Morawakkorala R, Senarathna A, de Alwis A and Abeywardana S (2006) Two cases of monkey tapeworm (Bertiella studeri) infestation from Sabaragamuwa Province. Sri Lanka Journal of Child Health 35, 34–35. [Google Scholar]
- Morisita T, Kobayashi M, Goto M, Eguchi T, Moriyama K and Ohashi M (1964) The third record of a case of human infection with Mesocestoides lineatus (Cestoda) in Japan. Japanese Journal of Parasitology 13, 1–4. [Google Scholar]
- Mueller JF (1930) Cestodes of the genus Mesocestoides from the opossum and the cat. American Midland Naturalist 12, 81–90. [Google Scholar]
- Mukerji A (1927) The incidence of helminthic infections in the Carmichael hospital for tropical diseases, Calcutta. The Indian Medical Gazette 62, 695–696. [PMC free article] [PubMed] [Google Scholar]
- Nagase K, Kani A, Totani T, Hamamoto T and Torikai K (1983) Report of a human case of Mesocestoides lineatus and preliminary investigation into infective sources. Japanese Journal of Parasitology 32, 18. [Google Scholar]
- Nelson G, Pester F and Rickman R (1965) The significance of wild animals in the transmission of cestodes of medical importance in Kenya. Transactions of the Royal Society of Tropical Medicine and Hygiene 59, 507–524. [DOI] [PubMed] [Google Scholar]
- Niphadkar S and Rao S (1969) On the occurrence of Raillietina (R.) celebensis (Janicki, 1902) in rats in Bombay with special reference to its zoonotic importance. Indian Veterinary Journal 46, 816–818. [PubMed] [Google Scholar]
- Ohtomo H, Hioki A, Ito A, Kajita K, Ishizuka T, Okuyama M, Miura K, Kagei N and Hayashi S (1983) Therapeutic effect of paromomycin sulfate on the 13th case of Mesocestoides lineatus infection found in Japan. Japanese Journal of Antibiotics 36, 632–637. [PubMed] [Google Scholar]
- Ortlepp R (1961) A record of three cases of human infection in Southern Africa with a common tapeworm of rats. South African Medical Journal 35, 873–879. [PubMed] [Google Scholar]
- Overgaauw PA, van Zutphen L, Hoek D, Yaya FO, Roelfsema J, Pinelli E, van Knapen F and Kortbeek LM (2009) Zoonotic parasites in fecal samples and fur from dogs and cats in The Netherlands. Vetereinary Parasitology 163, 115–122. [DOI] [PubMed] [Google Scholar]
- Paçô JM, Campos D and Araújo JLDB (2003) Human bertiellosis in Goiás, Brazil: a case report on human infection by Bertiella sp.(Cestoda: Anoplocephalidae). Revista do Instituto de Medicina Tropical de Sao Paulo 45, 159–161. [DOI] [PubMed] [Google Scholar]
- Padgett KA and Boyce WM (2004) Life-history studies on two molecular strains of Mesocestoides (Cestoda: Mesocestoididae): identification of sylvatic hosts and infectivity of immature life stages. Journal of Parasitology 90, 108–113. [DOI] [PubMed] [Google Scholar]
- Padgett KA, Nadler SA, Munson L, Sacks B and Boyce WM (2005) Systematics of Mesocestoides (Cestoda: Mesocestoididae): evaluation of molecular and morphological variation among isolates. Journal of Parasitology 91, 1435–1443. [DOI] [PubMed] [Google Scholar]
- Panda DN and Panda MR (1994) Record of Bertiella Studeri (Blanchard, 1891), an anoplocephalid tapeworm, from a child. Annals of Tropical Medicine and Parasitology 88, 451–452. [DOI] [PubMed] [Google Scholar]
- Pérez RRG, Hernandez ER and Martínez YH (2009) Inermicapsifer madagascariensis: características clínicas y epidemiológicas. Medimay 15, 40–43. [Google Scholar]
- Pessoa S (1930) Sobre um case de parasitismo humano por cestoide anoplocephalideo do genero Bertiella. Boletim da Sociedade Médica de Cirurgiões, 14, 158–162. [Google Scholar]
- Portokalidou S, Gkentzi D, Stamouli V, Varvarigou A, Marangos M, Spiliopoulou I and Dimitriou G (2018) Dipylidium caninum infection in children: clinical presentation and therapeutic challenges. Pediatric Infectious Disease Journal 38, e158–e159. [DOI] [PubMed] [Google Scholar]
- Pradatsundarasar A (1960) Nine cases of Raillietina infection in Bangkok. Journal of the Medical Association of Thailand 43, 56–58. [Google Scholar]
- Pradatsundarasar A (1972) Preliminary observations of the larval stage of the tapeworm Raillietina siriraji in cockroaches. Southeast Asian Journal of Tropical Medicine and Public Health 3, 146. [Google Scholar]
- Prosl H (2005) Holiday dreams with a downside: uncommon tapeworm infection in an infant. Wiener Klinische Wochenschrift 117, 56–59. [DOI] [PubMed] [Google Scholar]
- Reid W, Ackert JE and Case AA (1938) Studies on the life history and biology of the fowl tapeworm Raillietina cesticillus (Molin). Transactions of the American Microscopical Society 57, 65–76. [Google Scholar]
- Reid C, Perry F and Evans N (1992) Dipylidium caninum in an infant. European Journal of Pediatrics 151, 502–503. [DOI] [PubMed] [Google Scholar]
- Richard-Lenoble D, Kombila M, Maganga M and Affre G (1986) Bertiella infection in a Gabon-born girl. American Journal of Tropical Medicine and Hygiene 35, 134. [DOI] [PubMed] [Google Scholar]
- Roberson EL and Burke TM (1982) Evaluation of granulated fenbendazole as a treatment for helminth infections in dogs. Journal of the American Veterinary Association 180, 53–55. [PubMed] [Google Scholar]
- Roberts M (1991) The parasites of the Polynesian rat within and beyond New Zealand. International Journal for Parasitology 21, 777–783. [DOI] [PubMed] [Google Scholar]
- Rougier Y, Legros F, Durand J and Cordoliani Y (1980) Three cases of infection with a rare cestode in French Polynesia. Bulletin de la Société de Pathologie Exotique 73, 86–89. [PubMed] [Google Scholar]
- Rougier Y, Legros F, Durand J and Cordoliani Y (1981) Four cases of parasitic infection by Raillietina (R.) celebensis (Kanicki, 1902) in French Polynesia. Transactions of the Royal Society of Tropical Medicine and Hygiene 75, 121. [DOI] [PubMed] [Google Scholar]
- Roy SC (1938) Bertiella Studeri, a natural tape-worm parasite of monkeys, in a hindu child. The Indian Medical Gazette 73, 346–346. [PMC free article] [PubMed] [Google Scholar]
- Sahin I, Koz S, Atambay M, Kayabas U, Piskin T and Unal B (2015) A rare cause of diarrhea in a kidney transplant recipient: Dipylidium caninum. Transplant Proceedings 47, 2243–2244. [DOI] [PubMed] [Google Scholar]
- Sall-Dramé R, Brouat C, Bâ C and Duplantier J-M (2010) Variation in cestode assemblages of Mastomys And Arvicanthis species (rodents: Muridae) from Lake Retba in Western Senegal. Journal of Parasitology 96, 675–681. [DOI] [PubMed] [Google Scholar]
- Samkari A, Kiska DL, Riddell SW, Wilson K, Weiner LB and Domachowske JB (2008) Dipylidium caninum mimicking recurrent Enterobius vermicularis (pinworm) infection. Journal of Clinical Pediatrics 47, 397–399. [DOI] [PubMed] [Google Scholar]
- Sato H, Okamoto M, Ohbayashi M and Basanez MG (1988) A new cestode, Raillietina (Raillietina) oligocapsulata N. sp., and R.(R.) demerariensis (Daniels, 1895) from Venezuelan mammals. Japanese Journal of Veterinary Research 36, 31–45. [PubMed] [Google Scholar]
- Sato H, Ihama Y, Inaba T, Yagisawa M and Kamiya H (1999) Helminth fauna of carnivores distributed in north-western Tohoku, Japan, with special reference to Mesocestoides paucitesticulus and Brachylaima tokudai. Journal of Veterinary Science 61, 1339–1342. [DOI] [PubMed] [Google Scholar]
- Schmidt GD (1986) CRC Handbook of Tapeworm Identification. Boca Raton, Florida, USA: CRC Press, Inc. [Google Scholar]
- Schultz LJ, Roberto RR, Rutherford GW III, Hummert B and Lubell I (1992) Mesocestoides (Cestoda) infection in a California child. Pediatric Infectious Disease Journal 11, 332–333. [DOI] [PubMed] [Google Scholar]
- Sharma A (1930) Helminthic infections in Shillong. The Indian Medical Gazette 65, 200–203. [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Menon J, Lal S and Thapa BR (2017) Bertiella studeri infection – a rare cause of chronic abdominal pain in a child from North India. Journal of Tropical Pediatrics 64, 348–351. [DOI] [PubMed] [Google Scholar]
- Simões R, Luque J, Gentile R, Rosa M, Costa-Neto S and Maldonado A (2016) Biotic and abiotic effects on the intestinal helminth community of the brown rat Rattus norvegicus from Rio de Janeiro, Brazil. Journal of Helminthology 90, 21–27. [DOI] [PubMed] [Google Scholar]
- Skarbilovitch T (1945) Seasonal destrobilization in the cestode Mesocestoides lineatus, parasitic of silver-black fox. Doklady Akademii nauk SSSR 46, 171–172. [Google Scholar]
- Spasski A (1951) Anoplocephalata-tapeworms of domestic and wild animals. In Skrjabin K (ed.), Principles of Cestodology, vol. I. Moscow, Russia: Izdatelstvo Akademii Nauk SSSR Moscow, USSR, p. 735. [Google Scholar]
- Speckmann G and Webster W (1975) Natural infection and treatment of a dog with Mesocestoides tapeworms. The Canadian Veterinary Journal 16, 26. [PMC free article] [PubMed] [Google Scholar]
- Stiles CHW and Hassall A (1902) Bertiella New name for this cestode genus Bertia Blanchard, 1891. Science 16, 434. [DOI] [PubMed] [Google Scholar]
- Stunkard HW (1940) The morphology and life history of the cestode, Bertiella studeri. American Journal of Tropical Medicine and Hygiene 1, 305–333. [Google Scholar]
- Stunkard HW (1961) Cycloskrjabinia taborensis (Loewen, 1934), a cestode from the red bat, Lasiurus borealis (Müller, 1776), and a review of the Family Anoplocephalidae. Journal of Parasitology 47, 847–856. [PubMed] [Google Scholar]
- Stunkard HW, Koivastik T and Healy GR (1964) Infection of a child in Minnesota by Bertiella studeri (Cestoda: Anoplocephalidae). American Journal of Tropical Medicine and Hygiene 13, 402–409. [DOI] [PubMed] [Google Scholar]
- Subbannayya K, Achyutha RK, Shivananda P, Kundaje G, Adams L and Healy G (1984) Bertiella infection in an adult male in Karnataka. A case report. Indian Journal of Pathology and Microbiology 27, 269. [PubMed] [Google Scholar]
- Sun X, Fang Q, Chen X-Z, Hu S-F, Xia H and Wang X-M (2006) Bertiella studeri infection, China. Emerging Infectious Diseases 12, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szwaja B, Romański L and Zabczyk M (2011) A case of Dipylidium caninum infection in a child from the southeastern Poland. Wiadomosci Parazytologiczne 57, 175–178. [PubMed] [Google Scholar]
- Taleb-Hossenkhan N and Bhagwant S (2012) Molecular characterization of the parasitic tapeworm Bertiella studeri from the island of Mauritius. Parasitology Research 110, 759–768. [DOI] [PubMed] [Google Scholar]
- Taylor T and Zitzmann MB (2011) Dipylidium caninum in a 4-month old male. Journal of Clinical Laboratory Science 24, 212. [PubMed] [Google Scholar]
- Thompson R (1976) The occurrence of Mesocestoides sp. in British wild red foxes (Vulpes vulpes crucigera). Journal of Helminthology 50, 91–94. [DOI] [PubMed] [Google Scholar]
- Thompson CD, Jellard CH and Buckley JJ (1967) Human infection with a tapeworm, Bertiella sp., probably of African origin. British Medical Journal 3, 659–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokiwa T, Taira K, Yamazaki M, Kashimura A and Une Y (2014) The first report of peritoneal tetrathyridiosis in squirrel monkey (Saimiri sciureus). Parasitology International 63, 705–707. [DOI] [PubMed] [Google Scholar]
- Tung K-C, Hsiao F-C, Yang C-H, Chou C-C, Lee W-M, Wang K-S and Lai C-H (2009) Surveillance of endoparasitic infections and the first report of Physaloptera sp. and Sarcocystis Spp. in farm rodents and shrews in central Taiwan. Journal of Veterinary Medical Science 71, 43–47. [DOI] [PubMed] [Google Scholar]
- Tung K-C, Hsiao F-C, Wang K-S, Yang C-H and Lai C-H (2013) Study of the endoparasitic fauna of commensal rats and shrews caught in traditional wet markets in Taichung City, Taiwan. Journal of Microbiology, Immunology, and Infection 46, 85–88. [DOI] [PubMed] [Google Scholar]
- Turner JA (1962) Human dipylidiasis (dog tapeworm infection) in the United States. Journal of Pediatrics 61, 763–768. [DOI] [PubMed] [Google Scholar]
- Ulziijargal G, Yeruult C, Khulan J, Gantsetseg C, Wandra T, Yamasaki H and Narankhajid M (2020) Molecular identification of Taenia hydatigena and Mesocestoides species based on copro-DNA analysis of wild carnivores in Mongolia. International Journal for Parasitology: Parasites and Wildlife 11, 72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venard CE (1937) Morphology, bionomics, and taxonomy of the cestode Dipylidium caninum. Annals of the New York Academy of Sciences 37, 273–328. [Google Scholar]
- Voge M (1955) North American cestodes of the genus Mesocestoides. University of California Publications in Zoology 59, 125–156. [Google Scholar]
- Voge M and Seidel JS (1968) Continuous growth in vitro of Mesocestoides (Cestoda) from oncosphere to fully developed tetrathyridium. Journal of Parasitology 54, 269–271. [PubMed] [Google Scholar]
- Wardle RA and McLeod JA (1952) The Zoology of Tapeworms. Minneapolis, Minnesota, USA: University of Minnesota Press. [Google Scholar]
- Waugh CA, Lindo JF, Foronda P, Ángeles-Santana M, Lorenzo-Morales J and Robinson RD (2006) Population distribution and zoonotic potential of gastrointestinal helminths of wild rats Rattus rattus and R. norvegicus From Jamaica. Journal of Parasitology 92, 1014–1018. [DOI] [PubMed] [Google Scholar]
- Webster JD (1949) Fragmentary studies on the life history of the cestode Mesocestoides latus. Journal of Parasitology 35, 83–90. [PubMed] [Google Scholar]
- Weerasooriya MV, de Silva DDS and Mendis DH (1988) Bertiella studeri infections in children-report of cases. In Proceedings of the 48th Annual Sessions of the Galle Clinical Society. p. 17.
- Xuan le T, Anantaphruti MT, Tuan PA, Tu le X and Hien TV (2003) The first human infection with Bertiella Studeri in Vietnam. Southeast Asian Journal of TropicalMedicine and Public Health 34, 298–300. [PubMed] [Google Scholar]
- Yamaguti S (1959) Systema Helminthum. Volume II. The cestodes of Vertebrates. New York: Interscience Publishers. [Google Scholar]
- Zaleśny G and Hildebrand J (2012) Molecular identification of Mesocestoides Spp. from intermediate hosts (rodents) in central Europe (Poland). Parasitology Research 110, 1055–1061. [DOI] [PubMed] [Google Scholar]