Abstract
Background and Purpose
The free fatty acid receptor 1 (FFAR1) plays an important role in glucose‐stimulated insulin secretion making it an attractive anti‐diabetic target. This study characterizes the pharmacological profile of HWL‐088 (2‐(2‐fluoro‐4‐((2′‐methyl‐[1,1′‐ biphenyl]‐3‐yl)methoxy)phenoxy)acetic acid), a novel highly potent FFAR1 agonist in vitro and in vivo. Moreover, we investigated the long‐term effects of HWL‐088 alone and in combination with metformin in diabetic mice.
Experimental Approach
In vitro effects of HWL‐088 on FFAR1 and PPARα/γ/δ were studied in cell‐based assays. Glucose‐dependent insulinotropic effects were evaluated in MIN6 cell line and in rats. Long‐term effects on glucose and lipid metabolism were investigated in ob/ob mice.
Key Results
HWL‐088 is a highly potent FFAR1 agonist (EC50 = 18.9 nM) with moderate PPARδ activity (EC50 = 570.9 nM) and promotes glucose‐dependent insulin secretion in vitro and in vivo. Long‐term administration of HWL‐088 exhibited better glucose control and plasma lipid profiles than those of another FFAR1 agonist, TAK‐875, and synergistic improvements were observed when combined with metformin. Moreover, HWL‐088 and combination therapy improved β‐cell function by up‐regulation of pancreas duodenum homeobox‐1, reduced fat accumulation in adipose tissue and alleviated fatty liver in ob/ob mice. The effect of HWL‐088 involves a reduction in hepatic lipogenesis and oxidative stress, increased lipoprotein lipolysis, glucose uptake, mitochondrial function and fatty acid β‐oxidation.
Conclusion and Implications
These data indicate that long‐term treatment with HWL‐088, a highly potent FFAR1 agonist, improves glucose and lipid metabolism and may be useful for the treatment of diabetes mellitus by mono‐therapy or combination with metformin.
Abbreviations
- ACC1
acetyl‐CoA carboxylase 1
- ALT
alanine aminotransferase
- ANGPTL3
angiopoietin‐like 3
- Apo C‐II
apolipoprotein C‐II
- Apo C‐III
apolipoprotein C‐III
- AS160
TBC1 domain family member 4
- AST
aspartate transaminase
- ATGL
adipose triglyceride lipase
- BAT
brown adipose tissue
- CKMB
creatine kinase‐MB
- CPT1α
carnitine palmitoyl transferase 1α
- FAS
fatty acid synthetase
- FFAR1
free fatty acid receptor 1
- FLIPR
fluorometric imaging plate reader
- G6Pase
glucose‐6‐phosphatase
- GLP‐1
glucagon‐like peptide 1
- GLUT4
glucose transporter 4
- GSK3β
glycogen synthase kinase 3β
- HbA1c
glycosylated haemoglobin
- IP3
inositol triphosphate
- LCAD
long‐chain specific acyl‐CoA dehydrogenase
- LPL
lipoprotein lipase
- OGTT
oral glucose tolerance test
- PCNA
proliferating cell nuclear antigen
- PDase
pyruvate dehydrogenase
- PDX‐1
pancreas duodenum homeobox‐1
- PEPCK
phosphoenol pyruvate carboxykinase
- RT‐PCR
reverse transcription‐PCR
- SAR
structure–activity relationship
- SCD1
stearoyl‐CoA desaturase 1
- SD rats
Sprague–Dawley rats
- SREBP‐1c
sterol regulatory element‐binding protein 1c
- T2DM
type 2 diabetes mellitus
- WAT
white adipose tissue
What is already known
Free fatty acid receptor 1 (FFAR1) plays an important role in the glucose‐stimulated insulin secretion.
HWL‐088 is a novel highly potent FFAR1 agonist identified from comprehensive structure–activity relationship study.
What this study adds
HWL‐088 alone or in combination with metformin improved glucose and lipid metabolism.
HWL‐088 alone or with metformin, improved β‐cell function and reduced fat accumulation by multiple mechanisms.
What is the clinical significance
HWL‐088 may be useful for metabolic disorders involving glucose and lipids.
Combination therapy could be a valuable strategy for the treatment of diabetes mellitus.
1. INTRODUCTION
Type 2 diabetes mellitus (T2DM) is a progressive metabolic disease characterized by increased blood glucose levels, caused by insulin resistance and β‐cell dysfunction (Danaei et al., 2011; DeFronzo, 2009). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4779 is widely used as the initial pharmacological treatment of type 2 diabetes mellitus (Davies et al., 2019). The glucose‐lowering effect of metformin is attributed to its pleiotropic action on glucose metabolism such as, increased glucose disposal, reduced hepatic glucose production and intestinal glucose absorption (Foretz & Viollet, 2014). To obtain the ideal glycaemic control combination therapy with other drugs with different mechanisms of action are often needed. However, most of the anti‐diabetic agents are associated with inadequate glycaemic control, weight gain and hypoglycaemia (Stein, Lamos, & Davis, 2013). Hence, there remains an unmet need for new drugs with improved efficacy and/or safety, which can be used in combination with metformin (Bailey, Tahrani, & Barnett, 2016; Xu et al., 2014).
The free fatty acid receptor 1 (https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=225, also known as GPR40) is a GPCR mainly expressed in pancreatic β‐cells, which plays an important role in the glucose‐stimulated insulin secretion (Briscoe et al., 2003; Itoh et al., 2003). In pancreatic β‐cells, the activation of FFAR1 promotes the dissociation of Gαq protein, which further activates PLC, inducing the inositol triphosphate (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4222) and DAG. IP3 triggers calcium mobilization from endoplasmic reticulum, while DAG could activate PKC and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=1489, which stimulates insulin secretion. In addition, the ATP‐sensitive potassium channel was closed due to glucose metabolism, leading to the depolarization of membrane and pro‐activation of voltage‐gated calcium channels. FFAR1 activation promotes calcium influx by further activating voltage‐gated calcium channels, which increases glucose‐stimulated insulin secretion (Fujiwara, Maekawa, & Yada, 2005; Schnell, Schaefer, & Schoefl, 2007; Shapiro, Shachar, Sekler, Hershfinkel, & Walker, 2005). Besides the low risk of hypoglycaemia, its limited tissue distribution decreases the possibility of target‐related side‐effect in other tissues (Rayasam, Tulasi, Davis, & Bansal, 2007). Based on these advantages FFAR1 was considered an attractive target for the treatment of type 2 diabetes mellitus (Milligan, Shimpukade, Ulven, & Hudson, 2017). Recently, many FFAR1 agonists have been developed, and many clinical trials have also been performed for several candidates, such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6484, https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6485 and LY2881835 (Li et al., 2016; Li, Xu, Huang, & Qian, 2018). In our previous studies, we reported a novel series of phenoxyacetic acid‐based FFAR1 agonists (Li et al., 2015; Wang et al., 2015). In this series, HWL‐088 was selected as it was a highly potent and orally active FFAR1 agonist. More importantly, HWL‐088 has the same phenoxyacetic acid moiety as PPAR agonists (Sznaidman et al., 2003) and has a different chemical structure from that of classical FFAR1 agonists. Whether HWL‐088 had additional beneficial action on glucose and lipid metabolism, which for the classical FFAR1 agonist is unknown.
In the present study, we have evaluated its HWL‐088 biological activity and selectivity against PPAR. Because of the comparable mechanisms of actions between FFAR1 agonists and metformin, we have also explored the long‐term effects of HWL‐088 in combination with metformin on glycaemic control, plasma profiles and tissue morphology in B6/JNju‐Lepem1Cd25/Nju (ob/ob) mice, a type 2 diabetes model that develops obesity and insulin resistance (King, 2012; King & Bowe, 2016).
2. METHODS
2.1. Animals
Male SD rats, ICR mice and C57BL/6 mice were purchased from Guangdong Medical Laboratory Animal Center (Guangdong, China), and 8‐week‐old male ob/ob (B6/JNju‐Lepem1Cd25/Nju) mice were purchased from Model Animal Research Center of Nanjing University (Jiangsu, China). All animals were acclimatized for 1 week before the experiments. The animal room was maintained under a constant 12‐hr light/black cycle with temperature at 23 ± 2°C and relative humidity 50 ± 10% throughout the experimental period. Mice were allowed ad libitum access to standard pellets and water unless otherwise stated and the vehicle used for drug administration in all animal studies was an 0.5% aqueous solution of the sodium salt of carboxy methyl cellulose aqueous solution. The animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny, Browne, Cuthill, Emerson, & Altman, 2010) and the editorial on reporting of animal studies (McGrath & Lilley, 2015) with the recommendations made by the British Journal of Pharmacology. All animal experimental protocols were approved by the ethical committee at Guangdong Pharmaceutical University, conducted according to the Laboratory Animal Management Regulations in China and also adhered to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication NO. 85–23, revised 2011). All experiments were performed and analysed under blinded conditions.
2.2. Materials
HWL‐088, 2‐(2‐fluoro‐4‐((2′‐methyl‐[1,1′‐biphenyl]‐3‐yl)methoxy)phenoxy)acetic acid, was synthesized in our laboratory; see previous reports (Li et al., 2015; Wang et al., 2015). https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2674 (#HY‐13861, CAS Number: 265129‐71‐3), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2686 (#HY‐13928, CAS Number: 317318‐84‐6), https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2414 (#HY‐15206, CAS Number: 10238‐21‐8), and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1056 (#HY‐17386, CAS Number: 122320‐73‐4) were purchased from MedChemExpress (Shanghai, China). TAK‐875 (#BD247920, CAS Number: 1000413‐72‐8) and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4779 (#BD41182, CAS Number: 1115‐70‐4) were purchased from Bidepharm Technology Limited (Shanghai, China).
2.3. FLIPR assay for FFAR1
CHO (RRID:CVCL_0213) cells stably expressing human FFAR1 (accession no. NM_005303) were randomly seeded into 96‐well plates at a density of 15 K cells per well in 10% FBS (Gibco, 10099‐141) of DMEM (Gibco‐BRL, Gaithersburg, MD, USA) without antibiotics and incubated 16 hr at a standard humidified incubator in 5% CO2 at 37°C. After, the culture medium in the wells was removed and washed with 100 μl of Hank's balanced salt solution (Invitrogen, 14025). Then cells were incubated in loading buffer (containing 2.5 μg·ml−1 fluorescent calcium indicator Fluo 4‐AM, 2.5 mmol·L−1 https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4357 and 0.1% fatty acid‐free BSA) for 1 hr at 37°C. Various concentrations of test compounds or https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4710 (Sigma) were added into the cells and the intracellular calcium flux signals were monitored by FLIPR Tetra system (Molecular Devices). The agonistic activities of the test compounds on human FFAR1 were expressed as [(A − B)/(C − B)] × 100% (increase of the intracellular Ca2+ concentration (A) in the test compounds‐treated cells and (B) in vehicle‐treated cell and (C) in 10‐μM γ‐linolenic acid‐treated cells). EC50 value of the test compound was obtained with GraphPad 5 Software (RRID:SCR_002798).
2.4. Cell‐based GAL4 transactivation assay for https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=595 and https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=594
For transfection, HEK293 cells (RRID:CVCL_0045) were randomly plated onto 96‐well plates at 5 × 104 cells per well in DMEM (Gibco‐BRL, Gaithersburg, MD, USA) without antibiotics 1 day prior to transfection in a standard humidified incubator in 5% CO2 at 37°C. Transfections were performed using FuGENE HD Transfection Reagent (Promega, E231A) according to the manufacturer's protocol. The cells were transiently transfected with 25 ng per well of pBIND‐PPARδ or PPARγ, 25 ng per well of pG5Luc and 0.15 μl per well of FuGENE HD. After transfection for 24 hr, various concentrations of test compounds or positive controls were added into the cells and incubated for 18 hr. Cells from each well were lysed with 20‐μl lysis buffer, and each well was added 30‐μl Luciferase Assay Reagent II. The firefly and renilla luciferase signals are assayed by Dual Luciferase Reporter Assay System (Promega, E1960). Envision is used as the luminometer. The data values are normalized by dividing the firefly signal with renilla signal. “F/R” means “Firefly/Renilla.” This normalization eliminates the discrepancy of different cell number and transfection efficiency in each well. The %Activation value is calculated by the following equation: %Activation = [(X − Min)/(Max − Min)] × 100% (X is the “F/R” value in each concentration point. Min is the mean “F/R” value from no compound control. Max is the mean “F/R” value from reference compound control). EC50 value of the test compound was obtained with GraphPad 5 Software (RRID:SCR_002798).
2.5. Cell‐based GAL4 transactivation assay for https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=593
For transfection, HepG2 cells (RRID:CVCL_0027) were randomly plated onto 96‐well plates at 6 × 104 cells per well in MEM (Gibco) without antibiotics 1 day prior to transfection. The similar protocol was used as described for the cell‐based GAL4 transactivation assay for PPARγ and PPARδ.
2.6. In vitro insulin secretion assay
MIN6 cells (RRID:CVCL_0431) were randomly pre‐incubated for 2 hr at 37°C with Krebs‐Ringer bicarbonate–HEPES buffer (116‐mM NaCl, 4.7‐mM KCl, 1.17‐mM KH2PO4, 1.17‐mM MgSO4, 25‐mM NaHCO3, 2.52‐mM CaCl2, and 24‐mM HEPES) containing 0.1% BSA. The pre‐incubation buffer was removed and stimulated with 2‐mM glucose, 25‐mM glucose alone or in the presence of HWL‐088 (0.3 and 3 μM) or glibenclamide (3 μM). The plates were incubated for 1 hr at 37°C. The supernatants were collected and measured using insulin elisa kit (Mercodia, 10‐1250‐01, Sweden) according to the manufacturer's instruction. Western blotting analysis was performed to evaluate the expressions of Akt and phosphorylation of Akt (Ser473) in MIN6 cells treated with 2‐mM glucose in the absence or presence of HWL‐088 (3 μM).
2.7. Effects on L6 myoblasts cells
L6 myoblasts cells (RRID:CVCL_0385) were randomly cultured in DMEM, supplemented with 10% (vol/vol) FBS, 1% penicillin, and 100 mg·ml−1 streptomycin in a humidified atmosphere with 5% CO2 at 37°C. Cells were washed three times with PBS and starved for 4 hr in serum‐free DMEM medium before 20 min of incubation without insulin (basal) or with insulin (150 nmol·L−1). After that, cells were treated with HWL‐088 (0.3 μM) or without HWL‐088. Western blotting analysis was performed to evaluate the expressions of Akt, AS160, TBC1D1, and phosphorylation of Akt (Ser473), AS160 (Thr642) and TBC1D1 (Thr590).
2.8. Oral glucose tolerance test in SD rats
Male SD rats were fasted for 12 hr, weighted and randomized into four groups (n = 6 per group). Rats were administrated orally with vehicle, TAK‐875 (10 mg·kg−1) or HWL‐088 (10 mg·kg−1) and subsequently dosed orally with 3 g·kg−1 glucose solution after 1 hr. Another group (HWL‐088 + no glucose group) administrated orally with HWL‐088 (10 mg·kg−1) and subsequently dosed orally with vehicle after 60 min. Blood samples were collected from the lateral tail vein immediately before drug administration (−60 min), before glucose load (0 min) and at 15, 30, 60 and 120 min post dose. The blood glucose was measured by blood glucose test strips (SanNuo ChangSha, China). The level of insulin was measured by a rat insulin elisa kit (Mercodia, 10‐1250‐01, Sweden). At the end of the experiment, rats were killed by exsanguination after https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4233 and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=523 anaesthesia.
2.9. Chronic administration in ob/ob mice
The male ob/ob mice were randomization (n = 6 per group) and dosed twice daily with the vehicle, TAK‐875 (40 mg·kg−1), HWL‐088 (40 mg·kg−1), metformin (100 mg·kg−1) or with combination therapy (HWL‐088 + metformin) by gavage administration for 30 days. Mice were dosed at fixed time daily. The body weights were measured every 5 days and the dosage was adjusted according to the most recent body weight. Non‐fasting and fasting blood glucoses were measured every 5 days. Water and food consumption were measured daily at fixed time intervals. Oral glucose tolerance test (OGTT) was performed on Days 1, 15 and 30 of treatment. Mice were fasted overnight prior to treatment with a single dose of vehicle or tested agents and subsequently dosed orally with glucose aqueous solution (3 g·kg−1) after 30 min. Mice were bled via tail tip immediately before drug administration (−30 min), before glucose challenge (0 min) and at 15, 30, 60 and 120 min post dose, and the blood glucose was measured by blood glucose test strips (SanNuo ChangSha, China).
At the end of treatment, mice were killed by exsanguination after ketamine and xylazine anaesthesia. Tissue and serum samples were collected and processed for histological, serological and expression analysis. Alanine aminotransferase (ALT), aspartate transaminase (AST), total cholesterol, triglyceride, HDL, LDL, urea, creatine kinase‐MB (CKMB) and glycosylated haemoglobin (HbA1c) levels were determined by automatic biochemical analyser (Beckman Coulter, AU5811, Tokyo, Japan). The pancreases, white adipose tissue (WAT), brown adipose tissue (BAT) and the liver of each experimental group were isolated immediately after exsanguination and washed with ice‐cold saline before fixed in 10% (v/v) formalin. The sections were embedded in paraffin after dehydration. Four‐micron sections were cut and stained with haematoxylin–eosin for histopathological assessment at 400× magnification. The adipocytes areas in WAT and BAT were measured using Image‐pro plus 6.0 (Media Cybernetics, Inc., Rockville, MD, USA). Randomly 8–12 images (0.066 mm2) at 400× magnification were selected from each mouse and adipocytes areas were measured. Besides, the number of adipocytes per focus was also counted manually at 400× magnification for each mouse.
The deparaffinized sections of pancreases from each mouse were exposed to guinea pig anti‐insulin antibody (Servicebio, Cat#GB11334, RRID:AB_2811186), rabbit anti‐glucagon antibody (Servicebio, Cat#GB11097, RRID:AB_2811187), rabbit anti‐pancreas duodenum homeobox‐1 (anti‐PDX‐1) antibody (Abcam, Cat#ab47267, RRID:AB_777179), or mouse anti‐ proliferating cell nuclear antigen (PCNA) monoclonal antibody (Servicebio, Cat#GB11010‐1, RRID:AB_2811188) overnight at 4°C. The sections were developed using corresponding genus IgG (HRP, Servicebio, Cat#GB23303, RRID:AB_2811189) and counterstained with haematoxylin. Digital images were obtained with a Model Eclipse Ci‐L (Nikon, Tokyo, Japan). The immuno‐related procedures used comply with the recommendations made by the British Journal of Pharmacology (Alexander et al., 2018).
2.10. Real‐time reverse transcription‐PCR (RT‐PCR)
Total RNA were extracted from liver tissues after homogenization by Trizol Reagent (Invitrogen), and then cDNA was synthesis using ReverTra Ace reverse transcriptase (TOYOBO, Japan, FSQ‐301) according to the manufacturer's protocol. Real‐time RT‐PCR was performed with the SYBR Green Realtime PCR Master Mix (TOYOBO, Japan, QPK‐201) on an iCycler (Bio‐rad) following the manufacturer's instructions. The gene expression levels for the amplification were calculated using the ΔΔCT method and normalized against GAPDH mRNA. The primer sequences were as following:
| Genes | Forward primer | Reverse primer |
|---|---|---|
| https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=255#1263 | GCTGCCCACATCCCATCCAAAC | GCTGACAAGGTGGCGTGAAGG |
| ANGPTL3 | AACAGCAAGACAACAGCATAAG | CTGAGGGTTCTTGAATACCAGT |
| Apoc‐II | TGCCAAGGAGGTTGCCAAAGAC | ATGCCTGCGTAAGTGCTCATGG |
| Apoc‐III | GAAGGGAAGAAACAAAGAGCTG | AAGGATCCCTCTACCTCTTCAG |
| ATGL | TCGCAATCTCTACCGCCTCTCG | TCCTCCACCACAGCAGCTTCC |
| Cpt1α | AGCCAGACTCCTCAGCAGCAG | CACCATAGCCGTCATCAGCAACC |
| FAS | TAAAGCATGACCTCGTGATGAA | GAAGTTCAGTGAGGCGTAGTAG |
| GAPDH | CCTCGTCCCGTAGACAAAATG | TGAGGTCAATGAAGGGGTCGT |
| G6pase | TCCGTGCCTATAATAAAGCAGT | TGGCTTTTTCTTTCCTCGAAAG |
| Gpx1 | GTTTGAGAAGTGCGAAGTGAAT | CGGAGACCAAATGATGTACTTG |
| https://www.guidetopharmacology.org/GRAC/ObjectDisplayForward?objectId=2030 | TGGTAGCATGAAAGTTAGCAGA | CTCTCGGTTCTTAAATCGCTTG |
| LCAD | GGCCGGAAGCTGCATAAGATGG | AGTAAGGCATTAGCTGGCAATCGG |
| LPL | CCTGATGACGCTGATTTTGTAG | CAATGAAGAGATGAATGGAGCG |
| ND1 | CTAATCGCCATAGCCTTCCTAA | GTTGTTAAAGGGCGTATTGGTT |
| PDase | GGTCTGTTTGACATTATACGGC | CGCACAAGATATCCATTCCATC |
| PEPCK | CGCAAGCTGAAGAAATATGACA | GATGACTGTCTTGCTTTCGATC |
| SCD1 | AACATTCAATCCCGGGAGAATA | GAAACTTTCTTCCGGTCGTAAG |
| SREBP‐1c | GCTACCGGTCTTCTATCAATGA | CGCAAGACAGCAGATTTATTCA |
2.11. Western blotting analysis
The cell and tissues were homogenized by using cell lysis buffer (Cell Signaling Technology, Beverly, USA) with 0.5% protease inhibitor cocktail (Sigma, St. Louis, USA). Protein quantification was performed using BCA Protein Assay Kit (Thermo Fisher Scientific, Ref23225, USA). Equal amounts of protein were loaded onto the 12% SDS‐PAGE gels for electrophoresis and transferred to PVDF membranes. After blocking with 5% non‐fatty milk in TBST for 1.5 hr, the membranes were first probed with primary antibodies as follows: anti‐LCAD antibody (Abcam, Cat#ab129711, RRID:AB_11159271), anti‐ATGL antibody (Abcam, Cat#ab109251, RRID:AB_10864772), anti‐PDX‐1 antibody (Abcam, Cat#ab47267, RRID:AB_777179), anti‐p‐Akt (Ser473) antibody (Cell Signaling Technology, Cat#4060, RRID:AB_2315049), anti‐Akt antibody (Cell Signaling Technology, Cat#9272, RRID:AB_329827), anti‐p‐AS160 (Thr642) antibody (Cell Signaling Technology, Cat#8881, RRID:AB_2651042), anti‐AS160 antibody (Cell Signaling Technology, Cat#2447, RRID:AB_2199376), anti‐p‐TBC1D1 (Thr590) antibody (Affinity, AF2422, RRID:AB_2819092), anti‐TBC1D1 antibody (Affinity, AF7908, RRID:AB_2819093), anti‐p‐GSK‐3β (Ser9) antibody (Cell Signaling Technology, Cat#5558, RRID:AB_10013750), anti‐GSK‐3β antibody (Cell Signaling Technology, Cat#12456, RRID:AB_2636978) and anti‐http://www.baidu.com/link?url=sHbaaEh-aFjdGBcCpgR7i1fz1sqEXzjxMh8rX96dmZ5reAxC4qxDAi1yQh6Gclw_0fhul1GI3m5YmieEwDGzEqIoZ7y-q5DqUWidsxvZ28a antibody (Cell Signaling, Cat#2128, RRID:AB_823664). For analysis of LCAD and ATGL, blots were probed with their specific antibodies (diluted with 5% BSA to 1:1000). For analysis of pancreas duodenum homeobox‐1, blots were probed with their specific antibodies (diluted with 5% BSA to 1:3,000). For analysis of http://www.baidu.com/link?url=sHbaaEh-aFjdGBcCpgR7i1fz1sqEXzjxMh8rX96dmZ5reAxC4qxDAi1yQh6Gclw_0fhul1GI3m5YmieEwDGzEqIoZ7y-q5DqUWidsxvZ28a, blots were probed with its antibody (diluted with 5% BSA to 1:1,000). Membranes were probed with HRP‐labelled anti‐rabbit secondary antibody for Cell Signaling (diluted with 5% BSA to 1:1,000). Antibody binding was detected by enhanced chemiluminescence detection kit (Thermo Fisher Scientific, 32016, USA).
2.12. Data and statistical analysis
The data and statistical analysis comply with the recommendations of the British Journal of Pharmacology on experimental design and analysis in pharmacology (Curtis et al., 2015; Curtis et al., 2018). All studies were designed to generate groups of equal size, using randomisation and blinded analysis. The outliers were included in data analysis and presentation. Results are expressed as mean ± SD for the indicated number of independent experiments. Among them, some data were normalized to control unwanted sources of variation according to following methods. First, the data should be normalized to bring all of the variation into proportion with one another. Then the coefficients associated with each variable will scale appropriately to adjust for the disparity in the variable sizes. The number of animals in each group included for statistical tests is shown in the figure legend for analysis. Data normalization was performed to control for sources of variation of baseline parameters. For data transformation, each raw value has been divided by the value of the mean of the control values. Statistical analysis was undertaken only for studies where each group size was at least n = 5. The exploratory in vitro effects of HWL‐088 on FFAR1, PPARα, PPARγ and PPARδ were evaluated by three independent experiments. Statistical analyses were performed using GraphPad 5 Software (RRID:SCR_002798). General effects were analysed by using a one‐way ANOVA with Tukey's multiple‐comparison post hoc test. A value of P < .05 was considered to be significant. Post hoc tests were carried out only if F was significant and there was no variance in homogeneity. The size of these groups was decided by considering the accuracy and reproducibility of the detection method as well as the biological parameters involved. Effect sizes were estimated as odds ratios with corresponding 95% confidence intervals (CI) to quantify the precision of the estimated effects. Each group size is the number of independent values and that statistical analysis was done using these independent values.
2.13. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Harding et al., 2018), and are permanently archived in the Concise Guide to PHARMACOLOGY 2019/20 (Alexander, Christopoulos, Davenport, Kelly, Mathie, Peter J.A. et al., 2019; Alexander, Cidlowski, et al., 2019; Alexander, Fabbro, et al., 2019).
3. RESULTS
3.1. In vitro effects of HWL‐088 on FFAR1 and PPARs
Based on the observations that FFAR1 and PPARs are activated by free fatty acid and play a key role in the regulation of energy metabolism (Monsalve, Pyarasani, Delgado‐Lopez, & Moore‐Carrasco, 2013), we investigasted the preliminary selectivity of HWL‐088 for these receptors. As shown in Figure 1, HWL‐088 had high agonist activity at FFAR1 (EC50 = 18.9 nM) and moderate activity on PPARδ (EC50 = 570.9 nM) without any agonist activity on PPARα and PPARγ.
Figure 1.
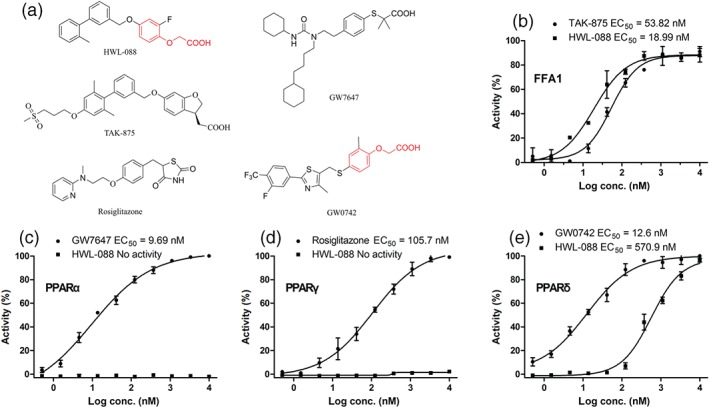
Structure of tested compounds and exploratory in vitro effects of HWL‐088 on FFAR1, PPARα, PPARγ and PPARδ. Results are mean ± SD (n = 3)
3.2. Glucose‐dependent insulinotropic effects of HWL‐088 in MIN6 cells
The glucose‐stimulated insulin secretion action of HWL‐088 was examined in MIN6 cell line with 2‐ or 25‐mM glucose. As shown in Figure 2a, HWL‐088 significantly increased insulin secretion from MIN6 cells at 25 mM but not at 2 mM glucose. In contrast, the insulin secretion by glibenclamide‐treated cells was independent of glucose concentration. Moreover, HWL‐088 revealed a dose‐dependent insulinotropic effect in the presence of 25‐mM glucose. As shown in Figure 2b, there were no significant change in the expressions of Akt and p‐Akt (Ser473) in MIN6 cells treated with 2‐mM glucose in the absence or presence of the high dose of HWL‐088 (3 μM). This result indicated that HWL‐088 does not phosphorylate Akt in the presence of low concentration of glucose (2 mM) and suggesting that it only acts on glucose‐stimulated insulin secretion.
Figure 2.
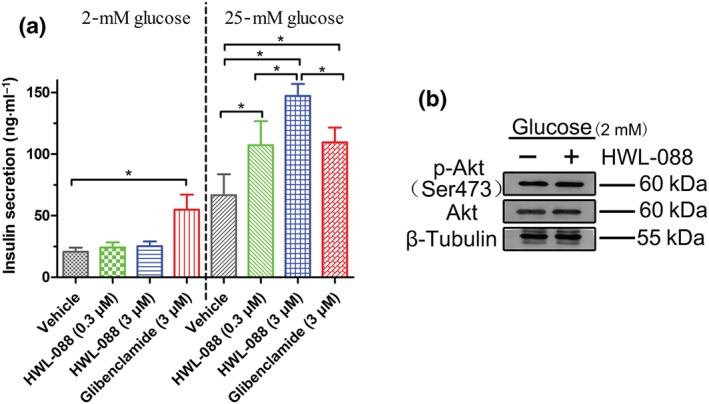
(a) Glucose‐dependent insulinotropic effects of HWL‐088 in MIN6 cells. MIN6 cells were treated with 2‐ or 25‐mM glucose in the absence or presence of HWL‐088 (0.3 and 3 μM) or glibenclamide (3 μM). Results are mean ± SD (n = 5). *P ≤ .05 was analysed using a one‐way ANOVA with Tukey's multiple‐comparison post hoc test. (b) The expressions of Akt and p‐Akt (Ser473) in MIN6 cells treated with 2‐mM glucose in the absence or presence of HWL‐088 (3 μM)
3.3. Glucose‐dependent insulinotropic effects of HWL‐088 in rats
Based on the above results, the insulinotropic effect of HWL‐088 was assessed in rats. As shown in Figure 3, the administration of HWL‐088 significantly improved the glucose tolerance of rats and the blood glucose AUC0−120min, suggesting that the glucose‐lowering effect of HWL‐088 was slightly more effective than TAK‐875 (Figure 3c), the most selective and potent FFAR1 agonist in the field. Moreover, the insulin level was almost unchanged from −60 min to 0 min at fasting condition, while sharply increasing after glucose load at 0 min. Notably, no significant increase of insulin has been observed in HWL‐088 treated group without a glucose load (Figure 3b), further confirming that HWL‐088 just induces a glucose‐dependent insulin secretion.
Figure 3.
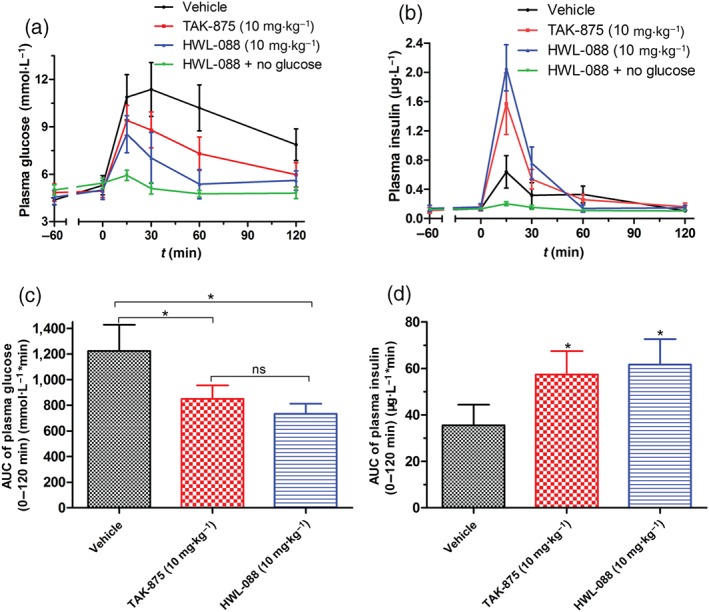
Effects of HWL‐088 in fasting SD rats. (a, b) Time‐dependent changes of plasma glucose and insulin levels after oral administration of HWL‐088, followed by 3 g·kg−1 oral glucose challenge, respectively. (c, d) AUC0−120min of plasma glucose levels and AUC0−120min of insulin levels shown in (a) and (b), respectively. Values are mean ± SD (n = 6). *P ≤ .05 was performed using a one‐way ANOVA with Tukey's multiple‐comparison post hoc test
3.4. The chronic effects of HWL‐088 on glucose tolerance
To investigate the long‐term effects, a chronic administration of HWL‐088 (40 mg·kg−1) alone and combined with metformin was carried out in ob/ob mice, a model of type 2 diabetes that develops obese and insulin resistance (King, 2012; King & Bowe, 2016). TAK‐875, HWL‐08, metformin, HWL‐08 and metformin, or vehicle was orally administered twice daily for 30 days and oral glucose tolerance tests were determined on Days 1, 15 and 30. As shown in Figure 4, the administration of TAK‐875 and HWL‐088 resulted in 18.8% and 28.1% reduction on AUC0−120min of glucose on the first day of treatment. On Days 15 and 30, TAK‐875 or HWL‐088 caused robust glucose‐lowering effects, with a reduction in the AUC0−120min values of 30.7% and 37.3% (Day 30) and 29.1% and 41.1% (Day 59), respectively. These results indicated that the effectiveness of HWL‐088 improved throughout the treatment without desensitization or tachyphylaxis. After chronic administration for 30 days, the glucose tolerance in the combination group (HWL‐088 + metformin) was significantly better than that observed with TAK‐875 or HWL‐088 alone.
Figure 4.
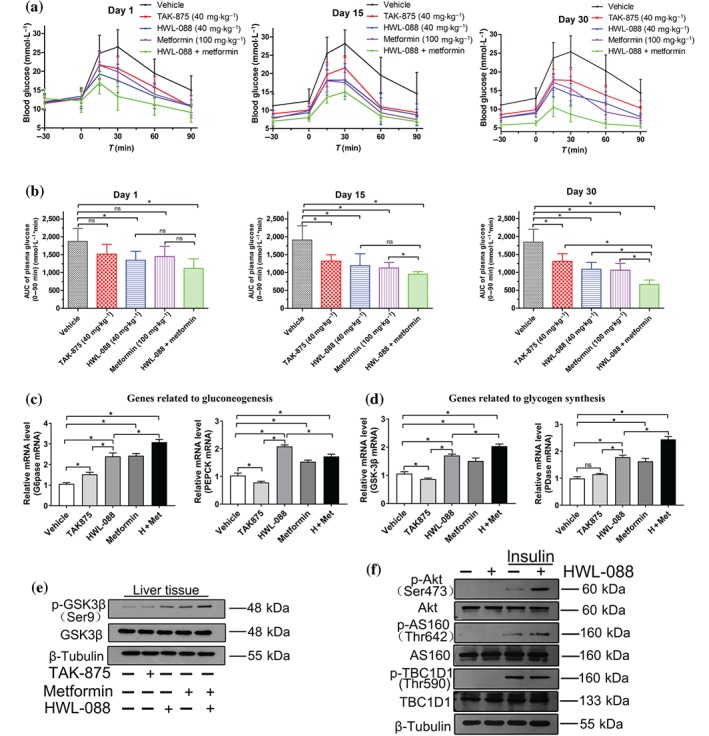
Effects of HWL‐088 on plasma glucose levels (a) and corresponding AUC0–90min (b) of glucose during an OGTT in fasting ob/ob mice. Tested agents or vehicle was orally administered to ob/ob mice twice daily for 30 days, and the OGTTs were determined on treatment days 1, 15, and 30. After long‐term treatment, hepatic expression of genes involved in gluconeogenesis (c) and glycogen synthesis (d) as well as hepatic expression of GSK3β (e). Effects of HWL‐088 on the phosphorylation of AKT, AS160, and TBC1D1 in L6 myoblasts cells (f). Values are mean ± SD (n = 6). *P ≤ .05 was analysed using a one‐way ANOVA with Tukey's multiple‐comparison post hoc test
As shown in Figure 4c, glucose‐6‐phosphatase (G6Pase) and phosphoenol pyruvate carboxykinase (PEPCK), two critical enzymes in gluconeogenesis were observed to be increased by HWL‐088, metformin and the combination of HWL‐088 and metformin. Moreover, two key enzymes involved in glycogen synthesis, glycogen synthase kinase 3β (GSK3β) and pyruvate dehydrogenase (PDase), were also increased in the HWL‐088 and metformin groups (Figure 4d). Accordingly, the phosphorylation of GSK3β (Ser9) was significantly increased in the liver of HWL‐088 and metformin groups (Figure 4e). Although metformin usually inhibits gluconeogenesis, it increases gluconeogenesis in our study. This abnormal phenomenon might be attributed to the relatively low fasting levels of glucose in the treated groups before the mice were killed, which induces gluconeogenesis through a feedback mechanism (involving catecholamines and https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=1136). Because the fasting glucose level of the treated group was lower than that of the vehicle group, the feedback mechanism may be more evident in the treated group (Figure 4c–e). Besides the direct insulinotropic effect, the mechanisms of action of HWL‐088 are also related to https://www.guidetopharmacology.org/GRAC/FamilyDisplayForward?familyId=165#878 translocation and glucose uptake in muscle. As shown in Figure 4f, the expression of p‐Akt (Ser473) and p‐AS160 (Thr642) were up‐regulated in L6 myoblasts cells after incubation of HWL‐088 compared to treatment with insulin alone, which indicated that HWL‐088 promotes GLUT4 translocation and glucose uptake in myoblasts cells.
3.5. Multiple dosing of HWL‐088 improves diabetes status and acts additively with metformin
The long term effects of HWL‐088 on non‐fasting and fasting blood glucose levels, cumulative food and water intake, body weight and the levels of HbA1c were monitored throughout the duration of study. As shown in Figure 5a,b, the non‐fasting and fasting glucose levels of ob/ob mice were significantly higher than those of the wild type control, which were reduced significantly after 5 days of administration of HWL‐088. Thereafter, the levels of plasma glucose remained steady throughout the duration of the treatment. After 15 days of treatment, the non‐fasting blood glucose level in the HWL‐088 and the metformin group was even lower than that of normal control (Figure 5a). Consistent with these levels of plasma glucose HbA1c levels, which reflect long‐term glycaemic control, were significantly decreased after 30 days of treatment compared with the vehicle group (Figure 5f). Interestingly, HWL‐088 alone treated group exhibited significantly lower HbA1c levels than TAK‐875 alone treated group. The cumulative food consumption and water intake were decreased in the HWL‐088 alone and HWL‐088 + metformin group, while there were no significant changes in the TAK‐875 alone and metformin alone treated groups (Figure 5c,d). After 30 days of treatment, no significant changes in body weight were observed in all treated groups (Figure 5e).
Figure 5.
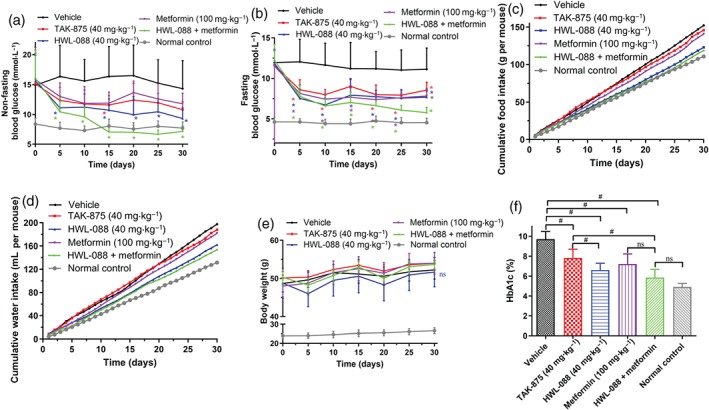
The chronic effects of HWL‐088 on non‐fasting blood glucose levels (a), fasting blood glucose levels (b), cumulative food intake (c) and water intake (d), body weight (e) and the levels of HbA1c (f) in ob/ob diabetic mice. Blood glucose levels, food consumption, water intake, and body weight were measured at fixed time. Values are expressed as mean ± SD (n = 6). *P ≤ .05 compared to vehicle group, # P ≤ .05 was analysed using a one‐way ANOVA with Tukey's multiple‐comparison post hoc test
3.6. Multiple dosing of HWL‐088 improves lipid profile
Diabetic ob/ob mice were hyperlipidaemic with abnormally level of plasma lipid: significantly higher levels of total cholesterol, triglyceride, HDL and LDL compared with normal C57BL/6 mice (Figure 6a–d). After 30 days of treatment with,HWL‐088 the plasma levels of total cholesterol, triglyceride and LDL in ob/ob mice were significantly decreased. In contrast, TAK‐875 treatment did not improve these lipid profiles in ob/ob mice. Moreover, no synergistic effects on the level of triglyceride and LDL were observed in the HWL‐088 + metformin group, despite these lipid profiles being significantly decreased in the metformin alone group. To evaluate the chronic effects on kidney and heart functions, their related biomarkers such as the plasma levels of urea and creatine kinase‐MB were measured (Figure 6e,f). Fortunately, there are no significant changes in these biomarkers in both HWL‐088 alone and in combination with metformin.
Figure 6.
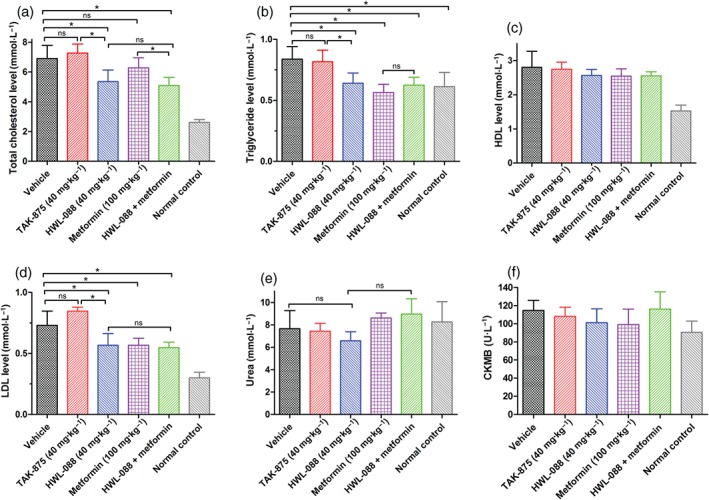
The chronic effects of HWL‐088 on the levels of total cholesterol (a), triglyceride (b), HDL (c) and LDL (d), urea (e), and creatine kinase‐MB (f) in ob/ob diabetic mice after 30 days of treatment. Values are expressed as mean ± SD (n = 6). *P ≤ .05 was analysed using a one‐way ANOVA with Tukey's multiple‐comparison post hoc test
3.7. Chronic effects of HWL‐088 on pancreatic β‐cell
After 30 days treatment, the pancreas were isolated from each experimental group and analysed by haematoxylin–eosin staining or immunostaining with anti‐insulin, anti‐glucagon, anti‐pancreas duodenum homeobox‐1 (PDX‐1) and anti‐proliferating cell nuclear antigen (PCNA) antibodies (Figure 7). The haematoxylin–eosin staining and immunostaining for insulin suggested that the pancreatic β‐cell in vehicle‐treated group was enlarged and disorganized with diffusion into the surrounding tissue compared with that of normal C57BL/6 mice. Moreover, widely scattered glucagon‐positive α‐cells and decreased pancreas duodenum homeobox‐1 (a key transcriptional factor of β‐cell function) expression in the pancreas islet were observed in vehicle‐treated ob/ob mice. Chronic treatment with TAK‐875 did not cause any detectable improvement in the morphology of islet or in the expression of pancreas duodenum homeobox‐1 and proliferating cell nuclear antigen (a marker of cell proliferation) compared to vehicle‐treated group. In contrast, in ob/ob mice treated with HWL‐088, many insulin‐positive cells displayed relatively dense arrangement compared with those in the vehicle or TAK‐875 treated mice. Interestingly, relatively high expressions of pancreas duodenum homeobox‐1 were observed in islets from HWL‐088 treated mice in both immunohistochemical analysis and western blot. Further, immunostaining of proliferating cell nuclear antigen exhibited elevated numbers of proliferating cell nuclear antigen‐positive cell in the HWL‐088 treated group compared with the vehicle or TAK‐875 treated groups.
Figure 7.
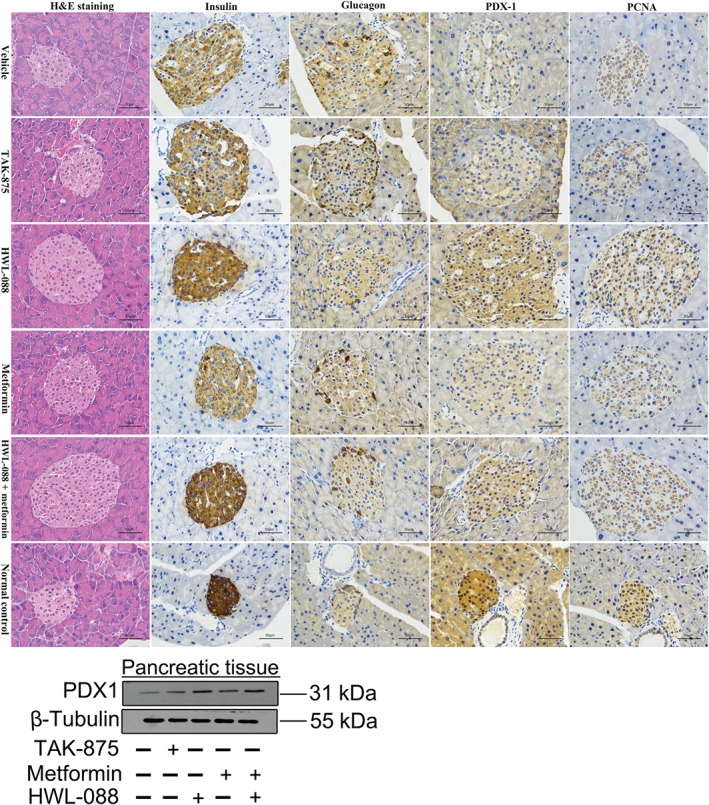
Effects of HWL‐088 on pancreatic β‐cell (H&E staining), insulin staining, glucagon staining, pancreas duodenum homeobox‐1 (PDX‐1) expression, and proliferating cell nuclear antigen (PCNA)‐positive cell numbers in pancreatic islets from ob/ob mice after 30 days of treatment. The pancreas were isolated and haematoxylin–eosin staining, or immunostained with anti‐insulin, anti‐glucagon, anti‐PDX‐1 and anti‐PCNA antibodies at 400× magnification. Representative images for each group are shown. Scale bar = 50 μm
In the metformin‐treated group, the morphology of islet was improved and the insulin secretion was decreased, while there was no notable improvement in the expression of pancreas duodenum homeobox‐1 and proliferating cell nuclear antigen compared to vehicle‐treated group (Figure 7). Interestingly, combination of HWL‐088 with metformin dramatically improved the morphology of islet cells and increased insulin‐positive cells even more than that of HWL‐088 alone. Moreover, immunohistochemical analysis revealed elevated expression of pancreas duodenum homeobox‐1 and raised numbers of proliferating cell nuclear antigen‐positive cell in the combination (HWL‐088 + metformin) treated group. Also, the increases in protein levels of pancreas duodenum homeobox‐1 were observed in HWL‐088 alone and in the combination group.
3.8. Chronic effects of HWL‐088 on adipose tissue
Histological analysis of the adipose tissue exhibited that the adipocytes size was markedly increased in WAT and BAT of ob/ob mice, which was reduced by chronic administration of HWL‐088 alone or the combination of HWL‐088 and metformin for 30 days (Figure 8). Moreover, quantitative measurement of WAT and BAT indicated that the adipocytes area or size was significantly increased in ob/ob mice due to fat accumulation, whereas HWL‐088 or the combination of HWL‐088 + metformin markedly reduced the fat accumulation in both WAT and BAT compared to that of vehicle group. In addition, the administration of HWL‐088 alone enhanced the number of adipocytes/unit area, which decreased in ob/ob mice due to adipocytes hypertrophy (Figure 8).
Figure 8.
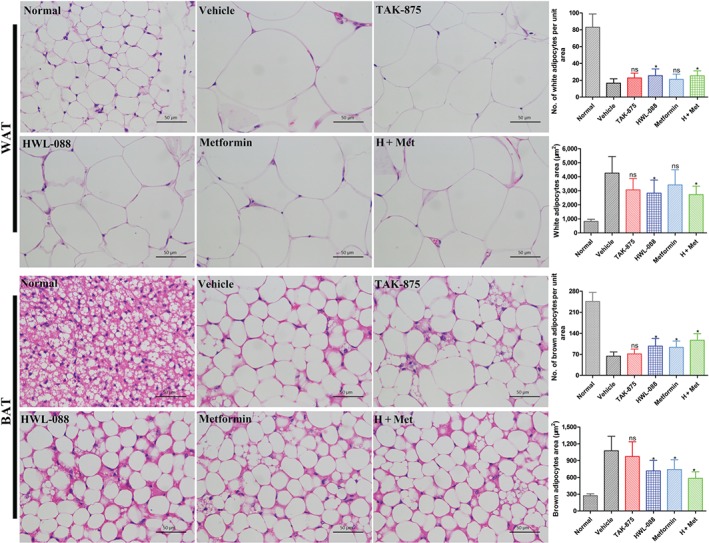
Effect of HWL‐088 on the histological alterations and fat accumulation in white adipose tissue (WAT) and brown adipose tissue (BAT) after 30 days of treatment. WAT and BAT were isolated and haematoxylin–eosin staining; the photomicrographs of histological alterations such as fat accumulation and hypertrophy of adipocytes were observed at 400× magnification. Accumulated fat was washed out in xylene during the tissue processing. Histograms show the quantitative analyses including the number of adipocytes/unit area and adipocytes area in WAT and BAT. All the values are expressed as mean ± SD (n = 6). *P ≤ .05 compared to vehicle group was analysed using a one‐way ANOVA with Tukey's multiple‐comparison post hoc test
3.9. Chronic effects of HWL‐088 on liver
Histological analysis exhibited that there was remarkable vesicular steatosis, fat accumulation, inflammation and ballooning in the liver of ob/ob mice. TAK‐875 treatment slightly reduced the vesicular steatosis and inflammation, while there was no significant change observed on liver ballooning. Treatment with HWL‐088 significantly reduced the vesicular steatosis, inflammation and ballooning in the liver compared to the vehicle and TAK‐875 treated groups (Figure 9). The liver ballooning almost completely disappeared in the metformin treated group and the tissue slice showed significant improvements of vesicular steatosis, inflammation and ballooning in liver from the combination (HWL‐088 + metformin) group compared with the treated groups of HWL‐088 or metformin alone. Similar to the histological improvement in the liver, the plasma levels of AST and ALT were significantly reduced in HWL‐088, metformin, and the HWL‐88 + metformin groups, but not in TAK‐875 group (Figure 9).
Figure 9.
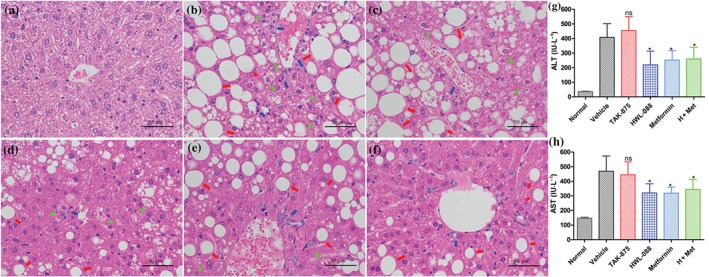
Effect of HWL‐088 on the histological alterations and fat accumulation in liver of ob/ob mice after 30 days of treatment. Representative photomicrographs of histological alterations in liver stained with haematoxylin–eosin at 400× magnification (a: normal control; b: vehicle group; c: TAK‐875 treated group; d: HWL‐088 treated group; e: metformin treated group; f: HWL‐088 and metformin treated group). Empty/hollow spaces show fat globules or accumulation (macro‐vesicular steatosis). Steatosis: red arrow, inflammation: blue arrow, ballooning: luminous yellow arrow. Histograms show the plasma levels of Alanine transaminase (ALT, g) and aspartate transaminase (AST, h). All the values are expressed as mean ± SD (n = 6). *P ≤ .05 versus vehicle were analysed using a one‐way ANOVA with Tukey's multiple‐comparison post hoc test
3.10. Chronic effects of HWL‐088 on mRNA and protein expression
To elucidate the mechanism behind the ability of HWL‐088 to normalise plasma lipid profiles and fatty liver, the expression levels of the lipid metabolism‐related gene were determined. Lipoprotein lipase (LPL) is a key enzyme involved in lipoprotein lipolysis, which is regulated by apolipoprotein C‐II (Apo C‐II), apolipoprotein C‐III (Apo C‐III) and angiopoietin‐like 3 (ANGPTL3). As shown in Figure 10a, HWL‐088 up‐regulates Apo C‐II and down‐regulates the expression of Apo C‐III and ANGTPL3, inducing the expression of LPL. Moreover, a significantly increased gene and protein expressions of adipose triglyceride lipase (ATGL) were observed in HWL‐088 and metformin groups compared with that of the TAK‐875 group. The combination (HWL‐088 + metformin) group further increased expression of Apo C‐II, LPL and ATGL compared with HWL‐088 group (Figure 10a). In contrast, no significant difference on the expressions of ANGPTL3, Apo C‐II, Apo C‐III, LPL and ATGL was observed in TAK‐875 group compared to vehicle group.
Figure 10.
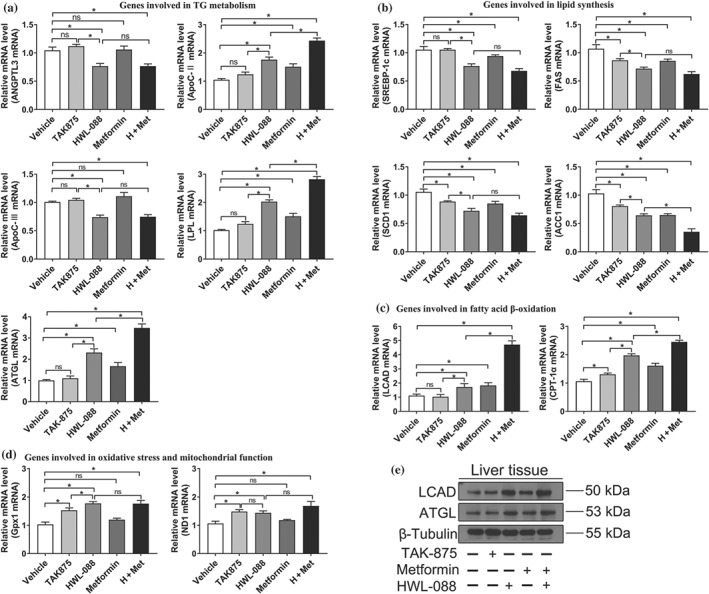
Effect of HWL‐088 on the hepatic expression of mRNA and protein. HWL‐088 altered expression of genes involved in TG metabolism (a), lipid synthesis (b), fatty acid β‐oxidation (c), oxidative stress, and mitochondrial function (d). (e) The changes in protein expression of LCAD and ATGL. Values are mean ± SD (n = 6). *P ≤ .05 was analysed using a one‐way ANOVA with Tukey's multiple‐comparison post hoc test
The expressions of sterol regulatory element‐binding protein 1c (SREBP‐1c) and its downstream genes fatty acid synthetase (FAS), stearoyl‐CoA desaturase 1 (SCD1) and acetyl‐CoA carboxylase 1 (ACC1) were significantly decreased in HWL‐088 group (Figure 10b). Moreover, the HWL‐088+ metformin group further reduced expression of ACC1 compared with HWL‐088 alone group (Figure 10b). The results suggested that HWL‐088 inhibited hepatic lipogenesis by down‐regulating expression of the related genes.
To further clarify the mechanism of lipid‐lowering effect of HWL‐088, the expressions of genes involved in fatty acid β‐oxidation was evaluated. The expressions of carnitine palmitoyl transferase 1α (CPT1α) and long‐chain specific acyl‐CoA dehydrogenase (LCAD) were increased in HWL‐088 group (Figure 10c,e). In addition, a dramatically synergistic effect was observed in the HWL‐088+ metformin group on inducing the expressions of CPT1α and LCAD. In conclusion, these results suggested that HWL‐088 promoted lipid metabolism through regulating the expression of genes associated with lipoprotein lipolysis, lipogenesis and fatty acid β‐oxidation. These effects were additive with metformin and significantly larger than the highly selective FFAR1 agonist TAK‐875.
The progress of fatty liver is also closely related to the increased cellular oxidative stress. The expression of GPx1, which quenches H2O2, was significantly increased in HWL‐088 group (Figure 10d). Since mitochondria are directly associated with ROS production, the marker of mitochondrial function (ND1) was also quantified. The expression of ND1 was significantly improved in HWL‐088 group and further increased in the HWL‐088+ metformin group.
4. DISCUSSION AND CONCLUSIONS
Recently, FFAR1 has been recognized as an attractive target for the treatment of type 2 diabetes mellitus. The activation of FFAR1 promotes insulin secretion in a glucose‐dependent manner and its limited tissue distribution decreases the possibility of target‐related side‐effect in other tissues (Rayasam et al., 2007). Besides the low risk of hypoglycaemia, previous studies also indicated that long‐term administration of FFAR1 agonists exerted beneficial effects on the function of β‐cell (Ito et al., 2013; Tsujihata et al., 2011). Sulphonylureas, the widely used insulinotropic drugs, are associated with hypoglycaemia, weight gain and β‐cell exhaustion resulting from glucose‐independent insulin secretion. Therefore, FFAR1 agonists would be expected to have some advantages.
We focused on developing a novel highly potent, selective and orally available FFAR1 agonist. After comprehensive structure–activity relationship studies, HWL‐088 was identified as a highly potent FFAR1 agonist. Indeed, HWL‐088 significantly increased insulin secretion only in high level of glucose both in vitro and in vivo, indicating a low risk of hypoglycaemia. Because FFAR1 and PPARs are activated by free fatty acid and their pharmacophoric features are very similar (Helal, Darwish, & Hammad, 2014; Li et al., 2019), we explored the selectivity profiles of HWL‐088 for PPARα, PPARγ and PPARδ. The results indicated that HWL‐088 has moderate agonistic activity on PPARδ, exhibiting high selectivity for PPARδ over PPARα and PPARγ. PPARδ activation produces multiple pharmacological effects, such as insulin sensibilization, adipocyte differentiation, anti‐inflammatory and lipid metabolism (Chen, Montagner, Tan, & Wahli, 2018; Lee et al., 2006; Luquet et al., 2005; Palomer et al., 2018). Moreover, the activation of PPARδ protects β‐cells from apoptosis (Daoudi et al., 2011), up‐regulating GLP‐1 receptor (Yang et al., 2014) and increasing mitochondrial function in β‐cells (Tang et al., 2013). Therefore, the unique mechanism of HWL‐088 might provide more advantages for the treatment of type 2 diabetes mellitus over that of traditional FFAR1 agonists.
To evaluate the long‐term effects of HWL‐088 in a diabetic model, we administered it orally, twice daily, to ob/ob mice for 30 days. Compared to the highly selective FFAR1 agonist TAK‐875, HWL‐088 exhibited many additional beneficial actions, such as more stable glucose control, lesser food and water intake and lower lipid levels after long‐term treatment. TBC1 domain family member 4 (AS‐160) is expressed in many tissues including the brain, kidney, liver and muscle (Kane et al., 2002). The insulin‐stimulated phosphorylation of AS160 is crucial to GLUT4 translocation (Peck et al., 2009) and is reduced in some type 2 diabetes patients (Karlsson et al., 2005). Besides direct insulinotropic effect, HWL‐088 increases GLUT4 translocation and glucose uptake in muscle by up‐regulating the expression of p‐Akt (Ser473) and p‐AS160 (Thr642) and acts additively with insulin. On the one hand, HWL‐088 promotes glucose‐dependent insulin secretion and insulin increases GLUT4 translocation and glucose uptake in muscle. On the other hand, HWL‐088 acts additively with insulin in up‐regulating the expression of p‐Akt (Ser473) and p‐AS160 (Thr642). These two synergistic mechanisms might be contributing to the robust glucose‐lowering effect of HWL‐088 in vivo. These unique pharmacological effects might be attributed to the dual potencies of HWL‐088 by simultaneous activation of FFAR1 and PPARδ. Indeed, PPARδ play a key role in insulin sensibilization and lipid regulation, which could act synergistic effects with FFAR1 in glucose and lipid metabolism (Chen et al., 2018; Luquet et al., 2005; Palomer et al., 2018).
The anti‐diabetic effects of metformin are mediated by pleiotropic mechanisms, such as increased glucose disposal, reduced glucose absorption and hepatic glucose production (Hundal & Inzucchi, 2003). As expected from the complementary mechanisms of action of metformin and FFAR1 agonist, the chronic administration of HWL‐088 and metformin in combination lead to a more stable glucose control compared to mono‐therapy. HWL‐088 alone or combination administration improves pancreatic β‐cell function, while the TAK‐875 group did not show any detectable improvement in the morphology of islet or in the expression of pancreas duodenum homeobox‐1 and proliferating cell nuclear antigen, which was consistent with a previous observations (Ito et al., 2013). The mechanism of the additional benefit on β‐cells by chronic administration of HWL‐088 might be multifaceted. Firstly, the lipotoxic effect is an important factor resulting in the progression of β‐cell dysfunction (Robertson, Harmon, Tran, & Poitout, 2004). HWL‐088 reduced the lipid levels and thereby the lipotoxic effect is alleviated to some extent. Secondly, the glucotoxicity on β‐cells is usually associated with diabetes due to the progressive and sustaining hyperglycaemia. Therefore, the improvement in β‐cell function in the treated groups of HWL‐088 or with combination administration with metformin may be mediated, at least in part, by the decrease in the levels of glucose. Moreover, PPARδ activation protects β‐cells from apoptosis (Daoudi et al., 2011) and increases mitochondrial function in β‐cells (Tang et al., 2013). These factors might be also responsible for the improvement in β‐cell function as HWL‐088 has moderate agonistic activity on PPARδ.
After long‐term administration, HWL‐088 or combination with metformin markedly reduced the fat accumulation in both WAT and BAT. Moreover, HWL‐088 significantly alleviated vesicular steatosis, inflammation and ballooning in the liver of ob/ob mice compared to that of TAK‐875. Besides, the biomarkers AST and ALT were significantly reduced in the treated groups, except for TAK‐875. HWL‐088 has at least three different mechanism by which causes the improvement of fatty liver. The first is to reduce hepatic lipogenesis by inhibition‐related genes expressions. The second is to increase lipid metabolism by promoting lipoprotein lipolysis and fatty acid β‐oxidation. The third is to reduce oxidative stress and increase mitochondrial function in liver. These beneficial effects of HWL‐088 might be also attributed to its agonistic activity at PPARδ, which has been reported to decrease hepatic fat content and the markers of liver malfunction in humans (Bays et al., 2011). Indeed, lipoprotein lipolysis was significantly improved in HWL‐088 group by up‐regulating Apo C‐II, LPL and ATGL and down‐regulating Apo C‐III and ANGTPL3. However, no significant change in the expression of these genes was observed with the highly selective FFAR1 agonist TAK‐875, indicating that lipoprotein lipolysis of HWL‐088 can be, at least in part, attributed to the activation of PPARδ. Moreover, HWL‐088 reduced hepatic lipogenesis by down‐regulating the expression of SREBP‐1c, FAS, SCD1 and ACC1, which was similar to that previously reported FFAR1 agonist GW9508 (Ou et al., 2014).
Oxidative stress and mitochondrial dysfunction are two important factors for fatty liver progression (Begriche, Igoudjil, Pessayre, & Fromenty, 2006; Kim, Wei, & Sowers, 2008). There are two possible mechanisms which could explain the reduced oxidative stress. The first involves a cellular antioxidant enzyme and the second relates to the improved mitochondrial function, which directly reduces the production of ROS. Our results indicate that the expressions of antioxidant enzyme (GPx1) and mitochondrial function marker (ND1) were significantly increased in HWL‐088 group. These results suggest that HWL‐088 and combination therapy with metformin might be potential a treatment of non‐alcoholic fatty liver disease (NAFLD), the most common liver disease frequently observed in obesity or diabetes (Cheung & Sanyal, 2010).
In conclusion, the present study indicates that HWL‐088 is a highly potent FFAR1 agonist with moderate potency on PPARδ. The unique mechanism of HWL‐088 provides more favourable effects on glucose homeostasis, lipid metabolism, pancreatic β‐cell and liver function than that of TAK‐875, the leading FFAR1 agonist. Our findings also demonstrate that combination therapy with HWL‐088 and metformin may be a valuable strategy for the treatment of type 2 diabetes mellitus and non‐alcoholic fatty liver disease.
AUTHOR CONTRIBUTIONS
Z.L., L.Z., and Y.C. participated in research design. Z.L., Y.C., Q.R., Z.Z., and L.D. conducted experiments. Z.L., Y.C., and L.H. performed data analysis. Z.L. and L.Z. wrote or contributed to the writing of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
DECLARATION OF TRANSPARENCY AND SCIENTIFIC RIGOUR
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research as stated in the BJP guidelines for https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14207, https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14208, and https://bpspubs.onlinelibrary.wiley.com/doi/full/10.1111/bph.14206, and as recommended by funding agencies, publishers and other organisations engaged with supporting research.
ACKNOWLEDGEMENTS
This study was supported by the National Natural Science Foundation of China (Grant 81803341), the Natural Science Foundation of Guangdong Province, China (Grant 2018A030313445), the Guangdong Basic and Applied Basic Research Foundation (Grant 2019A1515011036), the innovative strong school project of Guangdong Pharmaceutical University (Grant 2018KTSCX111), the projects of Guangzhou Key Laboratory of Construction and Application of New Drug Screening Model Systems (201805010006) and Key Laboratory of New Drug Discovery and Evaluation of Ordinary Universities of Guangdong Province (2017KSYS002), and the Innovation Team Projects in Universities of Guangdong Province (2018KCXTD016).
Chen Y, Ren Q, Zhou Z, et al. HWL‐088, a new potent free fatty acid receptor 1 (FFAR1) agonist, improves glucolipid metabolism and acts additively with metformin in ob/ob diabetic mice. Br J Pharmacol. 2020;177:2286–2302. 10.1111/bph.14980
Contributor Information
Luyong Zhang, Email: lyzhang@cpu.edu.cn.
Zheng Li, Email: li.zheng.sky@163.com.
REFERENCES
- Alexander, S. P. H. , Christopoulos, A. , Davenport, A. P. , Kelly, E. , Mathie, A. , Peter, J. A. , … CGTP Collaborators (2019). The concise guide to pharmacology 2019/2020: G protein‐coupled receptors. British Journal of Pharmacology, 176, S21–S141. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Cidlowski, J. A. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … GTP colaborators (2019). The concise guide to pharmacology 2019/2020: Nuclear hormones receptors. British Journal of Pharmacology, 176, S229–S246. 10.1111/bph.14748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Fabbro, D. , Kelly, E. , Mathie, A. , Peters, J. A. , Veale, E. L. , … CGTP collaborators (2019). The concise guide to pharmacology 2019/20: Enzymes. British Journal of Pharmacology, 176, S297–S396. 10.1111/bph.14572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, S. P. H. , Roberts, R. E. , Broughton, B. R. S. , Sobey, C. G. , George, C. H. , Stanford, S. C. , … Ahluwalia, A. (2018). Goals and practicalities of immunoblotting and immunohistochemistry: A guide for submission to the British Journal of Pharmacology . British Journal of Pharmacology, 175(3), 407–411. 10.1111/bph.14112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, C. J. , Tahrani, A. A. , & Barnett, A. H. (2016). Future glucose‐lowering drugs for type 2 diabetes. The Lancet Diabetes and Endocrinology, 4(4), 350–359. 10.1016/S2213-8587(15)00462-3 [DOI] [PubMed] [Google Scholar]
- Bays, H. E. , Schwartz, S. , Littlejohn, T. III , Kerzner, B. , Krauss, R. M. , Karpf, D. B. , … Roberts, B. K. (2011). MBX‐8025, a novel peroxisome proliferator receptor‐δ agonist: Lipid and other metabolic effects in dyslipidemic overweight patients treated with and without atorvastatin. The Journal of Clinical Endocrinology and Metabolism, 96(9), 2889–2897. 10.1210/jc.2011-1061 [DOI] [PubMed] [Google Scholar]
- Begriche, K. , Igoudjil, A. , Pessayre, D. , & Fromenty, B. (2006). Mitochondrial dysfunction in NASH: Causes, consequences and possible means to prevent it. Mitochondrion, 6(1), 1–28. 10.1016/j.mito.2005.10.004 [DOI] [PubMed] [Google Scholar]
- Briscoe, C. P. , Tadayyon, M. , Andrews, J. L. , Benson, W. G. , Chambers, J. K. , Eilert, M. M. , … Muir, A. I. (2003). The orphan G protein‐coupled receptor GPR40 is activated by medium and long chain fatty acids. The Journal of Biological Chemistry, 278(13), 11303–11311. 10.1074/jbc.M211495200 [DOI] [PubMed] [Google Scholar]
- Chen, J. , Montagner, A. , Tan, N. S. , & Wahli, W. (2018). Insights into the role of PPARβ/δ in NAFLD. International Journal of Molecular Sciences, 19(7), pii: E1893. https://doi.og/10.3390/ijms19071893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung, O. , & Sanyal, A. J. (2010). Recent advances in nonalcoholic fatty liver disease. Current Opinion in Gastroenterology, 26(3), 202–208. 10.1097/MOG.0b013e328337b0c4 [DOI] [PubMed] [Google Scholar]
- Curtis, M. J. , Alexander, S. , Cirino, G. , Docherty, J. R. , George, C. H. , Giembycz, M. A. , … Ahluwalia, A. (2018). Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. British Journal of Pharmacology, 175(7), 987–993. 10.1111/bph.14153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis, M. J. , Bond, R. A. , Spina, D. , Ahluwalia, A. , Alexander, S. P. , & Giembycz, M. A. (2015). Experimental design and analysis and their reporting: New guidance for publication in BJP (vol 172, pg 3461, 2015). British Journal of Pharmacology, 172(18), 4600–4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danaei, G. , Finucane, M. M. , Lu, Y. , Singh, G. M. , Cowan, M. J. , Paciorek, C. J. , … Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose) (2011). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: Systematic analysis of health examination surveys and epidemiological studies with 370 country‐years and 2.7 million participants. Lancet, 378(9785), 31–40. 10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- Daoudi, M. , Hennuyer, N. , Borland, M. G. , Touche, V. , Duhem, C. , Gross, B. , … Lestavel, S. (2011). PPARβ/δ activation induces enteroendocrine L cell GLP‐1 production. Gastroenterology, 140(5), 1564–1574. 10.1053/j.gastro.2011.01.045 [DOI] [PubMed] [Google Scholar]
- Davies, M. J. , D'Alessio, D. A. , Fradkin, J. , Kernan, W. N. , Mathieu, C. , Mingrone, G. , … Buse, J. B. (2019). Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia, 62(5), 873–873. 10.1007/s00125-019-4845-x [DOI] [PubMed] [Google Scholar]
- DeFronzo, R. A. (2009). From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes, 58(4), 773–795. 10.2337/db09-9028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foretz, M. , & Viollet, B. (2014). New promises for metformin: Advances in the understanding of its mechanisms of action. M S‐Medecine Science, 30(1), 82–92. [DOI] [PubMed] [Google Scholar]
- Fujiwara, K. , Maekawa, F. , & Yada, T. (2005). Oleic acid interacts with GPR40 to induce Ca2+ signaling in rat islet β‐cells: Mediation by PLC and L‐type Ca2+ channel and link to insulin release. American Journal of Physiology. Endocrinology and Metabolism, 289(4), E670–E677. 10.1152/ajpendo.00035.2005 [DOI] [PubMed] [Google Scholar]
- Harding, S. D. , Sharman, J. L. , Faccenda, E. , Southan, C. , Pawson, A. J. , Ireland, S. , … NC‐IUPHAR (2018). The IUPHAR/BPS guide to pharmacology in 2018: Updates and expansion to encompass the new guide to immunopharmacology. Nucleic Acids Research, 46(D1), D1091–D1106. 10.1093/nar/gkx1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helal, M. A. , Darwish, K. M. , & Hammad, M. A. (2014). Homology modeling and explicit membrane molecular dynamics simulation to delineate the mode of binding of thiazolidinediones into FFAR1 and the mechanism of receptor activation. Bioorganic & Medicinal Chemistry Letters, 24(22), 5330–5336. 10.1016/j.bmcl.2014.07.043 [DOI] [PubMed] [Google Scholar]
- Hundal, R. S. , & Inzucchi, S. E. (2003). Metformin—New understandings, new uses. Drugs, 63(18), 1879–1894. 10.2165/00003495-200363180-00001 [DOI] [PubMed] [Google Scholar]
- Ito, R. , Tsujihata, Y. , Matsuda‐Nagasumi, K. , Mori, I. , Negoro, N. , & Takeuchi, K. (2013). TAK‐875, a GPR40/FFAR1 agonist, in combination with metformin prevents progression of diabetes and β‐cell dysfunction in Zucker diabetic fatty rats. British Journal of Pharmacology, 170(3), 568–580. 10.1111/bph.12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, Y. , Kawamata, Y. , Harada, M. , Kobayashi, M. , Fujii, R. , Fukusumi, S. , … Fujino, M. (2003). Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature, 422(6928), 173–176. 10.1038/nature01478 [DOI] [PubMed] [Google Scholar]
- Kane, S. , Sano, H. , Liu, S. C. , Asara, J. M. , Lane, W. S. , Garner, C. C. , & Lienhard, G. E. (2002). A method to identify serine kinase substrates. Akt phosphorylates a novel adipocyte protein with a Rab GTPase‐activating protein (GAP) domain. The Journal of Biological Chemistry, 277(25), 22115–22118. 10.1074/jbc.C200198200 [DOI] [PubMed] [Google Scholar]
- Karlsson, H. K. , Zierath, J. R. , Kane, S. , Krook, A. , Lienhard, G. E. , & Wallberg‐Henriksson, H. (2005). Insulin‐stimulated phosphorylation of the Akt substrate AS160 is impaired in skeletal muscle of type 2 diabetic subjects. Diabetes, 54(6), 1692–1697. 10.2337/diabetes.54.6.1692 [DOI] [PubMed] [Google Scholar]
- Kilkenny, C. , Browne, W. , Cuthill, I. C. , Emerson, M. , & Altman, D. G. (2010). Animal research: Reporting in vivo experiments: The ARRIVE guidelines. British Journal of Pharmacology, 160(7), 1577–1579. 10.1111/j.1476-5381.2010.00872.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J.‐A. , Wei, Y. , & Sowers, J. R. (2008). Role of mitochondrial dysfunction in insulin resistance. Circulation Research, 102(4), 401–414. 10.1161/CIRCRESAHA.107.165472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, A. , & Bowe, J. (2016). Animal models for diabetes: Understanding the pathogenesis and finding new treatments. Biochemical Pharmacology, 99, 1–10. 10.1016/j.bcp.2015.08.108 [DOI] [PubMed] [Google Scholar]
- King, A. J. F. (2012). The use of animal models in diabetes research. British Journal of Pharmacology, 166(3), 877–894. 10.1111/j.1476-5381.2012.01911.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, C. , Kang, K. , Mehl, I. , Nofsinger, R. , Alaynick, W. , Chong, L. , … Evans, R. M. (2006). Peroxisome proliferator‐activated receptor δ promotes very low‐density lipoprotein‐derived fatty acid catabolism in the macrophage. National Academy of Sciences of the USA, 103(7), 2434–2439. 10.1073/pnas.0510815103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Chen, Y. , Zhou, Z. , Deng, L. , Xu, Y. , Hu, L. , … Zhang, L. (2019). Discovery of first‐in‐class thiazole‐based dual FFA1/PPARδ agonists as potential anti‐diabetic agents. European Journal of Medicinal Chemistry, 164, 352–365. 10.1016/j.ejmech.2018.12.069 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Qiu, Q. Q. , Geng, X. Q. , Yang, J. Y. , Huang, W. L. , & Qian, H. (2016). Free fatty acid receptor agonists for the treatment of type 2 diabetes: Drugs in preclinical to phase II clinical development. Expert Opinion on Investigational Drugs, 25(8), 871–890. 10.1080/13543784.2016.1189530 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Wang, X. , Xu, X. , Yang, J. , Xia, W. , Zhou, X. , … Qian, H. (2015). Design, synthesis and biological activity of phenoxyacetic acid derivatives as novel free fatty acid receptor 1 agonists. Bioorganic & Medicinal Chemistry, 23(22), 7158–7164. 10.1016/j.bmc.2015.10.011 [DOI] [PubMed] [Google Scholar]
- Li, Z. , Xu, X. , Huang, W. , & Qian, H. (2018). Free fatty acid receptor 1 (FFAR1) as an emerging therapeutic target for type 2 diabetes mellitus: Recent progress and prevailing challenges. Medicinal Research Reviews, 38(2), 381–425. 10.1002/med.21441 [DOI] [PubMed] [Google Scholar]
- Luquet, S. , Gaudel, C. , Holst, D. , Lopez‐Soriano, J. , Jehl‐Pietri, C. , Fredenrich, A. , & Grimaldi, P. A. (2005). Roles of PPARδ in lipid absorption and metabolism: A new target for the treatment of type 2 diabetes. Biochimica et Biophysica Acta, 1740(2), 313–317. 10.1016/j.bbadis.2004.11.011 [DOI] [PubMed] [Google Scholar]
- McGrath, J. C. , & Lilley, E. (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): New requirements for publication in BJP . British Journal of Pharmacology, 172(13), 3189–3193. 10.1111/bph.12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan, G. , Shimpukade, B. , Ulven, T. , & Hudson, B. D. (2017). Complex pharmacology of free fatty acid receptors. Chemical Reviews, 117(1), 67–110. 10.1021/acs.chemrev.6b00056 [DOI] [PubMed] [Google Scholar]
- Monsalve, F. A. , Pyarasani, R. D. , Delgado‐Lopez, F. , & Moore‐Carrasco, R. (2013). Peroxisome proliferator‐activated receptor targets for the treatment of metabolic diseases. Mediators of Inflammation, 2013, 1–18. 10.1155/2013/549627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, H.‐Y. , Wu, H.‐T. , Lu, F.‐H. , Su, Y.‐C. , Hung, H.‐C. , Wu, J.‐S. , … Chang, C. J. (2014). Activation of free fatty acid receptor 1 improves hepatic steatosis through a p38‐dependent pathway. Journal of Molecularl Enodcrinol, 53(2), 165–174. 10.1530/JME-14-0003 [DOI] [PubMed] [Google Scholar]
- Palomer, X. , Barroso, E. , Pizarro‐Delgado, J. , Pena, L. , Botteri, G. , Zarei, M. , … Vázquez‐Carrera, M. (2018). PPARβ/δ: A key therapeutic target in metabolic disorders. International Journal of Molecular Sciences, 19(3), 1‐14. 10.3390/ijms19030913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck, G. R. , Chavez, J. A. , Roach, W. G. , Budnik, B. A. , Lane, W. S. , Karlsson, H. K. , … Lienhard, G. E. (2009). Insulin‐stimulated phosphorylation of the Rab GTPase‐activating protein TBC1D1 regulates GLUT4 translocation. The Journal of Biological Chemistry, 284(44), 30016–30023. 10.1074/jbc.M109.035568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayasam, G. V. , Tulasi, V. K. , Davis, J. A. , & Bansal, V. S. (2007). Fatty acid receptors as new therapeutic targets for diabetes. Expert Opinion on Therapeutic Targets, 11(5), 661–671. 10.1517/14728222.11.5.661 [DOI] [PubMed] [Google Scholar]
- Robertson, R. P. , Harmon, J. , Tran, P. O. T. , & Poitout, V. (2004). β‐cell glucose toxicity, lipotoxicity, and chronic oxidative stress in type 2 diabetes. Diabetes, 53, 119–124. [DOI] [PubMed] [Google Scholar]
- Schnell, S. , Schaefer, M. , & Schoefl, C. (2007). Free fatty acids increase cytosolic free calcium and stimulate insulin secretion from β‐cells through activation of GPR40. Molecular and Cellular Endocrinology, 263(1–2), 173–180. 10.1016/j.mce.2006.09.013 [DOI] [PubMed] [Google Scholar]
- Shapiro, H. , Shachar, S. , Sekler, I. , Hershfinkel, M. , & Walker, M. D. (2005). Role of GPR40 in fatty acid action on the β cell line INS‐1E. Biochemical and Biophysical Research Communications, 335(1), 97–104. 10.1016/j.bbrc.2005.07.042 [DOI] [PubMed] [Google Scholar]
- Stein, S. A. , Lamos, E. M. , & Davis, S. N. (2013). A review of the efficacy and safety of oral antidiabetic drugs. Expert Opinion on Drug Safety, 12(2), 153–175. 10.1517/14740338.2013.752813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sznaidman, M. L. , Haffner, C. D. , Maloney, P. R. , Fivush, A. , Chao, E. , Goreham, D. , … Sternbach, D. D. (2003). Novel selective small molecule agonists for peroxisome proliferator‐activated receptor delta (PPARδ)—Synthesis and biological activity. Bioorganic & Medicinal Chemistry Letters, 13(9), 1517–1521. 10.1016/s0960-894x(03)00207-5 [DOI] [PubMed] [Google Scholar]
- Tang, T. , Abbott, M. J. , Ahmadian, M. , Lopes, A. B. , Wang, Y. , & Sul, H. S. (2013). Desnutrin/ATGL activates PPARδ to promote mitochondrial function for insulin secretion in islet β cells. Cell Metabolism, 18(6), 883–895. 10.1016/j.cmet.2013.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujihata, Y. , Ito, R. , Suzuki, M. , Harada, A. , Negoro, N. , Yasuma, T. , … Takeuchi, K. (2011). TAK‐875, an orally available G protein‐coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose‐dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. The Journal of Pharmacology and Experimental Therapeutics, 339(1), 228–237. 10.1124/jpet.111.183772 [DOI] [PubMed] [Google Scholar]
- Wang, X. , Zhao, T. , Yang, B. , Li, Z. , Cui, J. , Dai, Y. , … Qian, H. (2015). Synthesis and biological evaluation of phenoxyacetic acid derivatives as novel free fatty acid receptor 1 agonists. Bioorganic & Medicinal Chemistry, 23(1), 132–140. 10.1016/j.bmc.2014.11.016 [DOI] [PubMed] [Google Scholar]
- Xu, X. , Wang, G. , Zhou, T. , Chen, L. , Chen, J. , & Shen, X. (2014). Novel approaches to drug discovery for the treatment of type 2 diabetes. Expert Opinion on Drug Discovery, 9(9), 1047–1058. [DOI] [PubMed] [Google Scholar]
- Yang, Y. , Tong, Y. , Gong, M. , Lu, Y. , Wang, C. , Zhou, M. , … Tong, N. (2014). Activation of PPARβ/δ protects pancreatic β cells from palmitate‐induced apoptosis by upregulating the expression of GLP‐1 receptor. Cellular Signalling, 26(2), 268–278. 10.1016/j.cellsig.2013.11.019 [DOI] [PubMed] [Google Scholar]


