Abstract
The development of executive function is linked to maturation of prefrontal cortex (PFC) in childhood. Childhood obesity has been associated with changes in brain structure, particularly in PFC, as well as deficits in executive functions. We aimed to determine whether differences in cortical structure mediate the relationship between executive function and childhood obesity. We analyzed MR-derived measures of cortical thickness for 2700 children between the ages of 9 and 11 years, recruited as part of the NIH Adolescent Brain and Cognitive Development (ABCD) study. We related our findings to measures of executive function and body mass index (BMI). In our analysis, increased BMI was associated with significantly reduced mean cortical thickness, as well as specific bilateral reduced cortical thickness in prefrontal cortical regions. This relationship remained after accounting for age, sex, race, parental education, household income, birth-weight, and in-scanner motion. Increased BMI was also associated with lower executive function. Reduced thickness in the rostral medial and superior frontal cortex, the inferior frontal gyrus, and the lateral orbitofrontal cortex partially accounted for reductions in executive function. These results suggest that childhood obesity is associated with compromised executive function. This relationship may be partly explained by BMI-associated reduced cortical thickness in the PFC.
Keywords: ABCD, childhood obesity, cortical thickness, executive function, prefrontal cortex
Introduction
Although the rise in incidence of childhood obesity appears to have plateaued in some developed nations, the condition is still estimated to effect 124 million children worldwide (NCD-RisC 2017) or an estimated 1 in 3 children in the US (Ogden et al. 2014). Children who are overweight or obese are more likely to become obese adults, and have an increased risk of poorer health outcomes in later life including diabetes, heart disease, cancer, and overall mortality (Biro and Wien 2010).
Like adult obesity (Graham et al. 2014; Yang et al. 2018), childhood obesity has been linked to impairments in executive functioning (Maayan et al. 2011; Reinert et al. 2013; Yau et al. 2014; Ross et al. 2015; Alarcón et al. 2016; Li et al. 2018), although studies produce conflicting results (Gunstad et al. 2008). Executive function is an umbrella term for several different cognitive dimensions, including inhibitory control, decision-making, working memory, and reward sensitivity, broadly referring to a set of processes that enable planning, problem-solving, flexible reasoning, and regulation of behaviors and emotions. Children who are overweight or obese generally score lower on various measures of executive function (Liang et al. 2014), including working memory (Riggs et al. 2012), reward-sensitivity (Verbeken et al. 2012) and inhibitory control (Guerrieri et al. 2008; Verbeken et al. 2009).
At a biological level, various hypotheses exist relating executive functions to body mass index (BMI). One prominent theory is that the role of executive function in planning and decision-making, response inhibition, and reward evaluation influences food intake (Gluck et al. 2017), contributing to increased BMI. Prospective studies of bariatric patients following surgery support this association (Spitznagel et al. 2013), as do neuroimaging investigations. For example, functional magnetic resonance imaging studies have demonstrated that the dorsolateral prefrontal cortex (DPFC) is differentially activated in obesity in both adults (Le et al. 2006) and children (Davids et al. 2010; Reinert et al. 2013). This region is critical to cognitive control over eating (Gluck et al. 2017), as well as reward-motivated behavior via its links to the mesolimbic and mesocortical regions of the brain. Other regions of the prefrontal cortex (PFC) such as the orbitofrontal cortex (OFC) have also been linked to obesity. The OFC is involved in inhibition and reward processing (Gehring and Willoughby 2002) and has been shown to be differentially activated in lean and obese people depending on the level of satiety (Del Parigi 2010), including in children and adolescents (Holsen et al. 2005; Batterink et al. 2010; Bruce et al. 2010; Stice et al. 2010). This region is also implicated in the response to food stimuli (Killgore and Yurgelun-Todd 2005) in both children (Holsen et al. 2005) and adults (Kringelbach 2005). The ventral lateral PFC, linked to impulsivity, has also been implicated in obesity (Batterink et al. 2010). More generally, children with lower levels of executive abilities are more likely to be sedentary and have higher rates of snack consumption (Liang et al. 2014), and are less likely to benefit from weight-loss interventions (Eichen et al. 2018). While these studies support a link between executive function and BMI, the direction of causality is unclear. For example, there is compelling evidence suggesting that the low-grade inflammatory response that characterizes obesity may have a causal impact on the brain and impair executive function (Shields et al. 2017; Yang et al. 2018). Supporting this causal pathway, studies of postsurgical executive function in bariatric patients have demonstrated that weight loss is correlated with an improvement in cognitive abilities (Alosco et al. 2014a).
In summary, while the direction of causal association between BMI and executive function is not well understood, neuroimaging studies support the hypothesis that cortical structure and function are important to characterizing the relationship between executive function and BMI. Various investigations have linked adolescent obesity with changes in grey matter volume, connectivity, and reduced cortical thickness, commonly in prefrontal regions known to be associated with executive function (Maayan et al. 2011; Alosco et al. 2014b; Yau et al. 2014; Gupta et al. 2015; Ross et al. 2015). In addition, high fat diets have been linked to changes in microRNA expression related to axonal guidance in the PFC of adolescents (Labouesse et al. 2018). Thus, there is a demonstrable association in adolescents between BMI and cortical structure in regions associated with executive function. Whether such structural changes mediate the relationship between BMI and executive abilities is not established. Late childhood and early adolescence is a critical period for the emergence and consolidation of executive function, which is strongly linked to maturation of the PFC (Gray et al. 2003; Frangou et al. 2004; Tamnes et al. 2013). This maturation is characterized by reduced cortical thickness (Sowell et al. 2004; Shaw et al. 2006), the consolidation of regional activity (Durston et al. 2006), and the emergence of more comprehensive and extensive network connections (Ezekiel et al. 2013). An important question therefore is whether childhood obesity is characterized by structural changes in cortical regions important for executive function at this critical developmental period and further, whether these structural changes mediate the link between BMI and differences in executive function observed in childhood.
We sought to address this question using data from the NIH Adolescent Brain and Cognitive Development (ABCD) dataset (Jernigan and Brown 2018) of n = 2700 children between the ages of 9 and 11 years. Specifically we related measures of cortical thickness to measures of executive function and BMI, and further examined whether cortical thickness confounds the observed relationship between these traits. We chose to focus on measures of cortical thickness as cortical thinning in childhood has been linked to the emergence of executive function (Kharitonova et al. 2013; Bathelt et al. 2018), cortical thickness changes have been linked to childhood obesity (Maayan et al. 2011; Reinert et al. 2013; Alosco et al. 2014b; Yau et al. 2014; Ross et al. 2015; Medic et al. 2016), and previous studies in adulthood have demonstrated that cortical thickness in the PFC mediates the relationship between executive function and BMI (Lavagnino et al. 2016).
Methods and Materials
Subjects
A total of 3923 children aged 9–11 years from the ABCD dataset were initially included (10.15154/1504466). The ABCD dataset is a longitudinal study of over 10 000 children recruited from 21 centers throughout the US, with participants largely recruited through the school system. Sampling plans and recruitment procedures based on considerations of age, gender, race, socio-economic status, and urbanicity were designed to reflect the sociodemographics of the US. Details of recruitment and study design are described elsewhere (Garavan et al. 2018). Details of demographic, physical, and mental health assessments are described elsewhere (Barch et al. 2018).
BMI was based on measures of height and weight, which were taken as the average of up to 3 separate measures. BMI was calculated as weight in lbs divided by height in inches squared, multiplied by 703 (Eq. (1)).
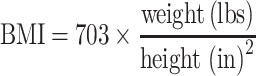 |
(1) |
BMI z-scores (BMIz) were defined using lookup tables from the Center of Disease Control 2001 (CDC Growth Charts 2018), where BMI was adjusted for sex and age. Subjects with a diagnosis of ADHD (n = 536), autism spectrum disorder (n = 49), schizophrenia (n = 2), intellectual disability (n = 2), and diabetes (n = 9) were excluded from analysis.
Additional analyses were carried out using measures of waist circumference and waist-to-height ratio in place of BMI.
Imaging Protocols
Imaging protocols for the ABCD dataset are described elsewhere (Casey et al. 2018), and were harmonized for three 3T scanner platforms (Siemens Prisma, General Electric 750 and Philips) used across the 21 data acquisition sites.
Cortical Reconstruction and Brain Structural Measures
Cortical reconstructions were carried out using FreeSurfer v5.3.0 (Dale et al. 1999; Fischl et al. 1999a, 1999b), as part of the initial baseline processing of the ABCD dataset. Reconstructions were visually inspected for quality control purposes. Only those reconstructions deemed of sufficient quality were included in this study. Based on these surface reconstructions, cortical thickness (Fischl and Dale 2000) values were processed for the Deskian-Killiany atlas (Desikan et al. 2006), with data unavailable for 3/36 regions per hemisphere (omitted regions included “unknown”, “corpus callosum”, and “insula”). Derived results per individual per region were provided as part of the ABCD curated annual release 1.0 (DOI 10.15154/1412097).
Executive Function
Participants involved in the ABCD study participated in a battery of tests designed to test their executive function. An overview of the baseline neurocognition battery is described elsewhere (Luciana et al. 2018). Tests were based on the National Institute of Health (NIH) Toolbox (https://nihtoolbox.desk.com). A composite score of executive function was generated based on results of several tests, namely the Flanker inhibitory control and attention test, the dimensional change card sort test, the picture sequence memory test, the list sorting working memory test, and the pattern comparison processing speed test (Akshoomoff et al. 2013, 2014, 2018). The age-corrected standard scores for each test were based on a normative sample of 2917 children and adolescents (Casaletto et al. 2015). The composite score was derived by averaging the standard scores of each of the measures and then deriving standard scores based on this new distribution. These age-corrected composite scores were used in subsequent analysis. Full data were available for n = 2352 subjects (n = 1802, 352 and 702 for lean, overweight and obese, respectively).
Statistical Analysis
We conducted a mediation analysis to determine whether cortical thickness mediated the relationship between BMI and executive function. As part of this analysis, we determined the following relationships using multivariate methods: regression of executive function on BMI, regression of regional cortical thickness on BMI, and regression of executive function on cortical thickness. Subsequently, we determined whether regional cortical thickness (mediator) was a significant predictor of executive function (the dependent variable) in a model that also included BMI (the independent variable) (Baron and Kenny 1986). We used Mahalanobis distance to identify and remove outliers in all regression models, and false discovery rate (FDR) methods (Benjamini and Hochberg 1995) were used to correct cortical results for multiple comparisons. Standardized regression coefficients were reported.
Mediation was conducted using the “mediation” package in R (Tingley et al. 2014). We assessed the significance of our mediation models using bootstrapping methods to increase power (Hayes 2009), with 1000 bootstrap samples used to generate 95% confidence intervals for the indirect effect. All analyses were conducted in R (v.3.3.3).
Covariates
Puberty is known to influence brain development, and pubertal hormones such as dehydroepiandrosterone (DHEA) have been linked to changes in cortical thickness between the ages of 4 and 13 years (Nguyen et al. 2013). To account for the possible confounding effects of the age of onset of puberty, we included salivary DHEA levels (Uban et al. 2018) as a covariate in our analysis. Birth weight was also included as a nuisance variable, as studies have indicated that this may play a role in intelligence scores at 11 years (Korpela et al. 2018) and has been demonstrated to be significantly predictive of childhood obesity (Biro and Wien 2010; Glavin et al. 2014). We also included estimates of head movement during scanning. Such micromotions have been demonstrated to be genetically correlated with BMI (Hodgson et al. 2017) and associated with biases in MR-derived parameters of cortical structure (Alexander-Bloch et al. 2016). For these reasons, frame-wise displacement (FWD) derived from resting-state data was adopted as an estimate of average head motion and included as a covariate. We also included brain volume and a self-reported measure of physical activity, which was recorded as the number of days in the week prior to interview where the subject had been moderately physically active for more than 60 min. Finally, we included covariates of household income, race, and parental education in our analysis as these have been demonstrated to be associated with BMI (Strauss and Knight 1999).
Results
Our results support statistically significant associations between BMI and executive function, between BMI and cortical thickness, and between cortical thickness and executive function. Individuals with higher BMI tend to have lower scores on executive function tests and thinner cerebral cortices, while individuals with thinner cerebral cortex tend to have lower scores on executive function tests. The association between BMI and executive function may be mediated by their shared relationship with the thickness of a subset of regions in PFC.
Demographic Variables, BMI, and Executive Function
As expected from prior literature, many demographic and biological variables were related to BMI and executive function, supporting their inclusion as covariates in our statistical model.
There was no association between BMIZ and age; however, males were significantly heavier than females (β = 0.1, t = 2.4, P = 0.02) (see Table 1). Birth weight was significantly associated with BMIZ (β =0.1, t = 5.9, P < 0.001), as was household income (F(2, 2387) = 52, P < 0.001), race (F(3, 3286) = 49, P < 0.001), and level of parental education (F(2, 2387) = 21, P < 0.001) (see Table 1). In line with previous analysis, in-scanner motion was positively associated with BMIZ (β = 0.16, t = 7.6, P < 0.001), while self-reported levels of physical activity were negatively associated with increasing BMIz (β = −0.07, t = 3, P = 0.001). There was no association between BMIZ and total brain volume; however, BMIZ was positively associated with salivary DHEA levels (β =0.17, t = 6, P < 0.001), suggesting that increased BMI was associated with more advanced pubertal stages.
Table 1.
Demographics and variables by BMI class
| Underweight (<5th) | Lean (5th—85th) | Overweight (85th—95th) | Obese (>95th) | |
|---|---|---|---|---|
| N | 127 | 2197 | 472 | 501 |
| Age (months) | 122 | 120 | 120 | 120 |
| Sex (F/M) | 77/50 | 1113/1084 | 225/247 | 231/270 |
| Birth weight (lbs) | 6 (na = 3) | 6.5 (na = 68) | 6.7 (na = 18) | 6.7 (na = 23) |
| Income (lower/middle/higher) | 6/49/64 (na = 8) | 250/748/1045 (na = 154) | 98/167/162 (na = 45) | 123/209/133 (na = 36) |
| Race (white/black/Hispanic/other) | 92/7/14/14 | 1501/199/334/163 | 236/74/135/27 | 218/113/141/29 |
| FWD | 0.19 (na = 4) | 0.24 (na = 164) | 0.28 (na = 54) | 0.31 (na = 51) |
| Parental education (high school/college/postgrad.) | 10/75/42 | 215/1289/693 | 87/283/102 | 102/311/88 |
| DHEA (pg/mL) | 64 (na = 57) | 61 (na = 1044) | 65 (na = 216) | 84 (na = 231) |
| Physical activity (no. days) | 3.7 | 3.8 (na = 1) | 3.5 | 3.5 (na = 2) |
| Executive function (age-corrected) | 99.6 (na = 12) | 99.7 (236) | 95.8 (na = 52) | 93.9 (na = 57) |
| Brain volume (cm3) | 1180 (na = 30) | 1224 (na = 550) | 1216 (na = 131) | 1207 (na = 112) |
BMI was classified using percentile growth charts stratified according to age based on CDC 2001 look up tables (CDC Growth Charts 2018, https://www.cdc.gov/growthcharts/html_charts/bmiagerev.htm). For statistical assessment, household income levels were categorized as less than $35 000, less than $100 000, and greater than $100 000. Race was categorized as white, black, Hispanic and other. Parental education was categorized as up to and included General education diploma (GED), up to and including college or associated degrees, and postgraduate. Mean FWD was used as a measure of head motion during scanning. Physical activity was a self-reported record of number of days in past week where the subject was physically active for more than 60 min/day.
Executive abilities were significantly associated with age (β = 0.09, t = 4.5, P < 0.001) and slightly higher in females (β = 0.1, t = 2.4, P = 0.02). Birth weight was not associated with executive abilities; however, total brain volume (β = 0.07, t = 3.3, P < 0.001), in-scanner motion (β = 0.2, t = 7.5, P < 0.001), physical activity (β = 0.05, t = 2.2, P = 0.03), parental education (F(2, 2387) = 37, P < 0.001), household income (F(2, 2387) = 46, P < 0.001), and race (F(2, 2386) = 27, P < 0.001) were associated. Overall, there was also a positive association between levels of DHEA and executive abilities (β = 0.09, t = 2.9, P = 0.005).
Relationship Between BMI and Executive Function
There was a significant negative relationship between BMIZ and age-corrected executive function (β = −0.05, t = 2.4, P = 0.02, n = 2389) accounting for other variables except cortical thickness and levels of pubertal hormones (see Fig. 1). When levels of DHEA were taken into account, the relationship between BMI and executive function was reduced to trend-level (β = −0.05, t = 1.6, P = 0.1). Because there were far fewer subjects with levels of DHEA (n = 1227), and the effect size is identical, this likely reflects reduced power in the smaller dataset rather than DHEA mediating the relationship between BMI and executive function. Both waist circumference and waist-to-height ratio were also negatively associated with executive function (see Supplementary Materials).
Figure 1.
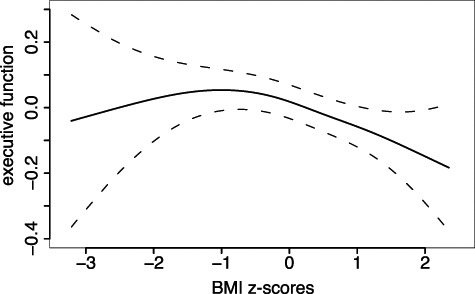
Relationship between BMI and executive function after adjustment for age, sex, race, birth weight, in-scanner motion, parental education, and household income.
Relationship Between BMI and Cortical Thickness
Across individuals with complete data (n = 2668), there was a significant, negative association between BMIZ and mean global cortical thickness (β = −0.5, t = 2.6, P = 0.01) accounting for demographic and other covariates excepting DHEA.
After FDR correction for multiple comparisons, regions of significant cortical thickness reductions bilaterally included lateral OFC, inferior frontal gyrus (parsorbitalis and pars triangularis), and rostral middle frontal and superior frontal cortex. In the left hemisphere, additional significant differences were found in entorhinal cortex, while in the right hemisphere additional changes were found in medial OFC and temporal pole (see Fig. 2, Table 1, Supplementary Material). In all cases, changes took the form of a decrease in cortical thickness associated with BMIZ.
Figure 2.
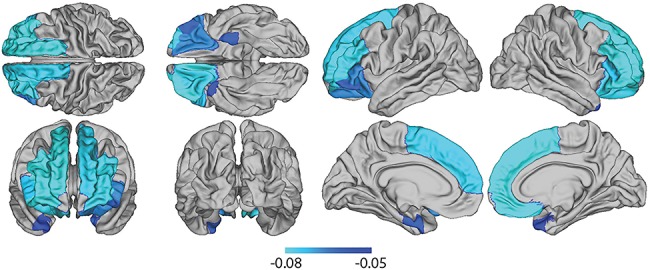
Map of reduced cortical thickness (beta regression coefficients) associated with BMI, adjusted for demographic and other confounder variables.
When we repeated our analysis using waist circumference and waist-to-height ratio in place of BMI, we found that the relationship to cortical thickness was broadly similar to the pattern seen with BMI (see Supplementary Table 1 and Supplementary Materials).
In a separate analysis, there was a negative association between DHEA level and average global cortical thickness (β = −0.06, t = 2.2, P = 0.03) in line with the hypothesis that increases in pubertal hormonal levels are associated with maturation-related cortical thinning in this age range. In a regional analysis of the association between cortical thickness and BMI taking DHEA levels into account, only cortical thickness in rostral middle frontal cortex was associated with BMI (β = −0.13, t = 3.8, P = 0.01). Given that the effect size was not decreased compared with the analysis in the larger dataset, this suggests that DHEA was not a significant confound of the relationship between BMI and cortical thickness, and that differences in results were instead more likely due to a comparative lack of power in the smaller dataset (see Supplementary Table 2 and Supplementary Material).
Relationship Between Cortical Thicknessand Executive Function
Across individuals with complete data (n = 2389), there was a significant negative relationship between mean global cortical thickness and executive abilities (β = −0.07, t = 3.1, P = 0.002), without adjusting for BMIZ. At a local level and after FDR-correction, mean cortical thickness in several regions was predictive of executive function (see Fig. 3), including cuneus, fusiform, lateral occipital, rostral anterior cingulate, rostral middle frontal gyrus, superior and inferior parietal cortex, middle and superior temporal gyrus, pars opercularis, pars triangularis, postcentral, and supramarginal cortices bilaterally, and additionally the caudal anterior cingulate, superior temporal sulcus, caudal middle frontal gyrus, lateral orbitofrontal cortex, pars orbitalis, precuneus, precentral sulcus, posterior cingulate and the superior frontal cortex in the left hemisphere, and lingual region, the precuneus, and the transverse temporal sulcus in the right hemisphere (see Supplementary Table 1 and Supplementary Material). In all regions, this association took the form of a negative relationship between cortical thickness and executive function. This is in line with previous results for this age-range (Shaw et al. 2006).
Figure 3.
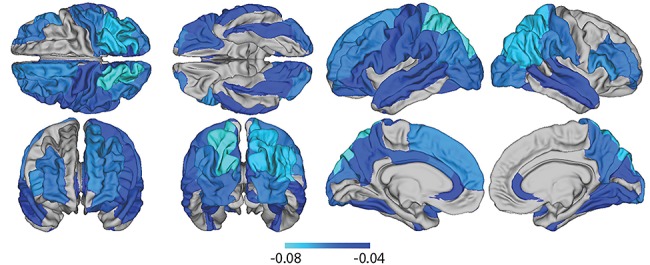
Map of reduced cortical thickness (beta regression coefficients) associated with executive function adjusted for demographic and other confounder variables.
Taking DHEA levels into account in the subset of the sample, the negative relationship between global mean cortical thickness and executive abilities remained (β = −0.08, t = 2.6, P = 0.02). At a regional level, executive function was again associated with reduced cortical thickness in several regions including cuneus and superior parietal cortex in the left hemisphere and pars triangularis and transverse temporal cortex in the right hemisphere. Again, the effect size was not reduced compared to the larger dataset suggesting that DHEA was not a significant confound of the relationship between cortical thickness and executive function (see Supplementary Table 2 and Supplementary Material).
Mediation
Having established a relationship between (1) BMI and cortical thickness, (2) cortical thickness and executive function, and (3) BMI and executive function, we next examined whether cortical thickness was a significant mediator of the relationship between BMIZ and executive function.
Results of analysis revealed that while global mean cortical thickness was not a significant mediator between BMI and executive function, cortical thickness in 11 regions partially mediates the relationship (see Fig. 4, Supplementary Table 3 and Supplementary Material). These regions included the parsorbitalis, pars triangularis, rostral middle frontal and superior frontal cortex bilaterally, and additionally lateral OFC in the left hemisphere, and fusiform and medial OFC in the right hemisphere (see Supplementary Table 3 and Supplementary Material).
Figure 4.
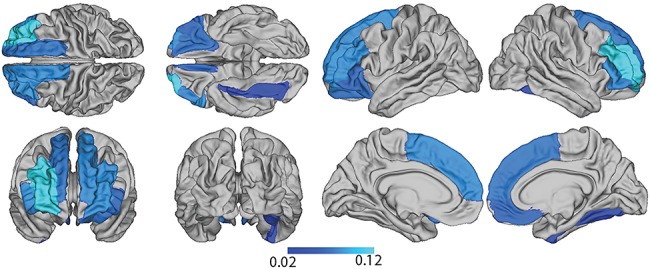
Estimate of mediation effect of regional cortical thickness on the relationship between BMI and executive function.
Discussion
In this study, we investigated the association between BMI, cortical thickness, and executive function in 2700 9–11 year olds recruited as part of the ABCD NIH study. We observed a negative association between executive function and cortical thickness across the cortical surface. Increased BMI was associated with lower scores on a composite measure of executive function. We also found significant BMI-related differences in cortical thickness in line with similar studies (Maayan et al. 2011; Yau et al. 2014; Ross et al. 2015). In particular, reduced cortical thickness was pronounced in orbitofrontal cortex, ventromedial PFC, and DPFC, regions involved in executive functions including decision-making, response inhibition, working memory, and cognitive flexibility.
The changes that such reduced cortical thickness reflects are unknown. For example, previous studies have suggested that MR-based measures of changes in cortical thickness during childhood may reflect, in part, increases in cortical myelination, particularly in frontal association areas (Croteau-Chonka et al. 2016). Interpreting the results of the current study along these lines, reduced cortical thickness associated with childhood obesity may be a function of an increase in cortical myelination. Future studies may consider more direct measures of myelination with a view to increasing power to detect the extent of structural mediation between BMI and executive function. Moreover, other brain parameters may also be important. For example, relative change in degree of connectivity or consolidation of activity may more closely index the development of executive function and thus may be more sensitive to BMI-related differences (Durston et al. 2006; Ezekiel et al. 2013).
Previous studies have reported a degree of regional-specificity to changes in cortical structure in relation to childhood obesity. The current study, capitalizing on a uniquely large dataset, demonstrates that increased BMI is associated with pervasive reductions in cortical thickness across much of the PFC. While our study does not allow a clear mechanistic interpretation of this predominance of effect in PFC, one possibility is that, since this region is associated with top-down control and inhibitory processes, then BMI-related changes could in turn lead to further difficulties in resisting external drives to consumption and attenuated learning from experience. This could entail a positive feedback in which early detrimental changes to PFC structure and function lead to ensuing behavioral changes that exacerbate weight gain.
More generally, PFC is involved in top-down regulation and inhibitory control as well as emotion and motivation regulation, and changes in this area are convincingly related to risk-taking behavior and substance abuse (Goldstein and Volkow 2011). In addition, the relatively extended maturational trajectory of PFC is thought to subserve experience-dependent learning (Romine and Reynolds 2005). As such, differences in PFC structure during early adolescence may possibly increase the vulnerability of this region to external stressors. Thus, BMI-associated brain changes in PFC may be regarded as a risk factor to the developing brain.
In a complementary analysis, we found that reduced cortical thickness in the PFC partially mediated the relationship between BMI and executive function. This observation is compatible with the idea that elevated BMI causes cortical thinning in turn leading to a reduction in executive function score. The direction of this causality model is supported by some observational studies. For example, in adult populations, a significant number of studies have suggested that obesity may play a causal role in the onset of brain structural changes and cognitive decline (Bruce-Keller et al. 2009; Arnoldussen et al. 2014). It is hypothesized that factors related to increased body mass such as an elevated inflammatory response or neuroendocrine dysfunction might impact on brain structure and cognitive function in a manner akin to neurodegenerative processes observed with aging. Indeed, many studies have associated increased BMI in midlife with increased rates of neurodegeneration and a significant elevated risk of dementia and Alzheimer’s disease in old age (Singh-Manoux et al. 2017). In children, a large-scale longitudinal analysis of early childhood development reported that obesity in very early childhood is a risk factor for reduced cognitive function years later (Li et al. 2018). Important corollaries to these studies are reports of significant improvement of memory and executive function following weight-loss (Gunstad et al. 2011; Veronese et al. 2016), as well as the general neuroprotective effects of severe caloric restriction (Colman et al. 2009).
However, care must be taken when interpreting our results, and we note that the causal model of BMI impacting cortical thickness which then further impacts executive function is just one of a possible six models and it is not possible to distinguish these statistically. For example, our data may also fit a model whereby BMI impacts executive function, which in turn impacts cortical thickness. Alternatively, our data would be equally compatible with a hypothesis that executive function influences BMI, which in turn may influence cortical structure. Indeed, various studies support such a hypothesis. For example, longitudinal studies suggest that the early cognitive environment may be a risk factor for developing obesity in later childhood, with children in lower cognitive stimulation environments at a 2-fold greater risk of developing obesity (Strauss and Knight 1999). Meanwhile, in bariatric patients, executive function has been shown to predict postsurgical weight-loss (Spitznagel et al. 2013).
We also acknowledge the possibility that there is no causal relationship between BMI and executive function. This would be compatible with a model in which cortical structural features drive altered BMI and executive functioning independently. This is feasible given that the genes associated with obesity-risk are predominantly and significantly expressed in the central nervous system and linked to basic functions such as glutamate signaling and synaptic function (Locke et al. 2015) and that BMI, brain structure, and various aspects of cognitive function share common genetic influences (Curran et al. 2013; Hagenaars et al. 2016; Marioni et al. 2016). The finding that BMI shares common genetic influences with various aspects of brain structure and cognition highlights the difficulty in isolating causal associations in noninterventional studies and underscores the importance of more direct studies in nonhumans. In this regard, it may be that BMI and executive function are not causally related, and structural changes associated with each may simply be regarded as an important confound of the relationship (MacKinnon et al. 2000). Indeed, we note that it is not possible to statistically distinguish between a confound and a mediator. In this regard, the results of this study suggest that BMI, cortical thickness or executive function should be included as a potential confound in any future analysis that seeks to investigate the relationship between the other 2 variables.
There were a number of significant limitations in this study. Primarily, due to the cross-sectional nature of our data, we were not able to distinguish between different possible models to determine the causal relationship between BMI, executive function, and cortical thickness. This may be addressed by future studies based on longitudinal data. In addition, our analysis was based on measures of BMI. While BMI is the most commonly used index of adiposity, it is less directly related to cardio-metabolic risks than other metrics such as waist circumference and waist-to-height ratio (Sharma et al. 2015). When we repeated our analysis for waist circumference and waist-to-height ratio, we found that both measures were associated with lower levels of executive function, as well as regional reductions in cortical thickness in a manner similar to what we observed using BMI. However, both measures additionally identified regions where increased waist circumference and waist-to-height ratio were associated with increases in cortical thickness. These results illustrate that BMI may not capture the total variation of cortical structure with increased adiposity. Finally, although we have confined this extensive analysis to the cortical sheet, we acknowledge that subcortical structures have also been implicated in obesity. Therefore, it will be important for future work to extend such analyses to subcortical regions and, critically, to examine covariance relationships between key cortical and subcortical structures in order to more fully characterize the relationship between childhood obesity and brain structure.
Conclusions
In a large, population-based cohort, reduction in PFC cortical thickness was associated with childhood obesity. Higher BMI was also associated with reduced scores on a composite cognitive measure reflecting executive processes, and a complementary mediation analysis was consistent with cortical thickness change mediating the relationship between BMI and executive functioning. The data are consistent with a mechanism whereby PFC changes in childhood obesity may lead to altered regulation of inhibitory control and risk-taking behavior and further difficulties in weight control. However, due to the limitations of our data, care must be taken in interpreting our results and follow-up studies will be critical to establishing causal pathways between BMI, brain structure, and executive function, as well as determining if longitudinal changes in BMI have a measurable impact on these traits.
Funding
The Bernard Wolfe Health Neuroscience Fund and the Wellcome Trust (grant number RNAG/259).
Notes
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children aged 9-10 and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners “under award numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, and U24DA041147”. A full list of supporters is available at https://abcdstudy.org/nih-collaborators. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/principal-investigators.html. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
The ABCD data repository grows and changes over time. The ABCD data used in this report came from the curated annual release 1.0 (DOI 10.15154/1412097). DOIs can be found at https://ndar.nih.gov/study.html?id=500.
Conflict of Interest: P.C.F. has received money in the past for ad hoc consultancy services to GlaxoSmithKline. All other authors declare no competing financial interests.
Supplementary Material
References
- Akshoomoff N, Beaumont JL, Bauer PJ, Dikmen SS, Gershon RC, Mungas D, Slotkin J, Tulsky D, Weintraub S, Zelazo PD et al. 2013. NIH toolbox cognition battery (CB): composite scores of crystallized, fluid, and overall cognition. Monogr Soc Res Child Dev. 78:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akshoomoff NN, Thompson E, McCabe WK, Bloss C, Chang CS, Amarai L, Casey DG, Ernst BJ, Frazier TM, Gruen JA et al. 2014. The NIH toolbox cognition battery: results from a large normative developmental sample (PING). Neuropsychol. 28: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akshoomoff N, Brown TT, Bakerman R, Hagler DJ. 2018. Developmental differentiation of executive functions on the NIH toolbox cognition battery. Neuropsychology. 32:777–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón G, Ray S, Nagel BJ. 2016. Lower working memory performance in overweight and obese adolescents is mediated by white matter microstructure. J Inter Neuropsych Soc. 22:281–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander-Bloch A, Clasen L, Stockman M, Ronan L, Lalonde F, Giedd J, Raznahan A. 2016. Subtle in-scanner motion biases automated measurement of brain anatomy from in vivo MRI. Hum Brain Mapp. 37:2385–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Galioto R, Spitznagel MB, Strain G, Devlin M, Cohen R, Crosby RD, Mitchell JE, Gunstad J. 2014a. Cognitive function after bariatric surgery: evidence for improvement 3 years after surgery. Am J Surg. 207:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alosco ML, Stanek KM, Galioto R, Korgaonkar MS, Grieve SM, Brickman AM, Spitznagel MB, Gunstad J. 2014b. Body mass index and brain structure in healthy children and adolescents. Int J Neurosci. 124:49–55. [DOI] [PubMed] [Google Scholar]
- Arnoldussen IAC, Kiliaan AJ, Gustafson DR. 2014. Obesity and dementia: adipokines interact with the brain. Eur Neuropsychopharmacol. 24:1982–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barch DM, Albaugh MD, Avenevoli S, Chang L, Clark DB, Glantz MD, Hudziak JJ, Jernigan TL, Tapert SF, Yurgelun-Todd D et al. 2018. Demographic, physical and mental health assessments in the adolescent brain and cognitive development study: Rationale and description Dev Cogn Neurosci. 32:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. 1986. The moderator-mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. J Pers Soc Psychol. 51:1173–1182. [DOI] [PubMed] [Google Scholar]
- Bathelt J, Gathercole SE, Johnson A, Astle DE. 2018. Differences in brain morphology and working memory capacity across childhood. Dev Sci. 21:e12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E. 2010. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 52:1696–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. 1995. Controlling the false discovery rate: a practical and powerful approach to multiple testing. JSTOR. 57:289–300. [Google Scholar]
- Biro FM, Wien M. 2010. Childhood obesity and adult morbidities. Am J Clin Nutr. 91:1499S–1505S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Holsen LM, Chambers RJ, Martin LE, Brooks WM, Zarcone JR, Butler MG, Savage CR. 2010. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. Int J Obes. 34:1494–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce-Keller AJ, Keller JN, Morrison CD. 2009. Obesity and vulnerability of the CNS. Biochim Biophys Acta. 1792:395–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Growth Charts 2018. https://www.cdc.gov/growthcharts/clinical_charts.htm (last accessed 30 May 2018).
- Casaletto KB, Umlauf A, Beaumont J, Gershon R, Slotkin J, Akshoomoff N, Heaton RK. 2015. Demographically corrected normative standards for the English version of the NIH toolbox cognition battery. J Inter Neuropsych Soc. 21:378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H et al. 2018. The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Dev Cogn Neurosci. 32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW et al. 2009. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 325:201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croteau-Chonka EC, Dean DC, Remer J, Dirks H, O'Muircheartaigh J, Deoni SCL. 2016. Examining the relationships between cortical maturation and white matter myelination throughout early childhood. NeuroImage. 125:413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran JE, McKay DR, Winkler AM, Olvera RL, Carless MA, Dyer TD, Kent JW, Kochunov P, Sprooten E, Knowles EE et al. 2013. Identification of pleiotropic genetic effects on obesity and brain anatomy. Hum Hered. 75:136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. 1999. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 9:179–194. [DOI] [PubMed] [Google Scholar]
- Davids S, Lauffer H, Thoms K, Jagdhuhn M, Hirschfeld H, Domin M, Hamm A, Lotze M. 2010. Increased dorsolateral prefrontal cortex activation in obese children during observation of food stimuli. Int J Obes. 34:94–104. [DOI] [PubMed] [Google Scholar]
- Del Parigi A. 2010. Neuroanatomical correlates of hunger and satiety in lean and obese individuals In: Dube L, Bechara A, Dagher A, Drewnowski A, LeBel J, James P & Yada R, editors. Obesity Prevention: The Role of Brain and Society on Individual Behavior. Elsevier: Academic Press; 1:253–271. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT et al. 2006. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Cassey BJ. 2006. A shift from diffuse to focal cortical activity with development. Dev Sci. 9:1–8. [DOI] [PubMed] [Google Scholar]
- Eichen DM, Brittany EM, Liang J, Strong DR, Rhee K, Boutelle KN. 2018. The relationship between executive functioning and weight loss and maintenance in children and parents participating in family-based treatment for childhood obesity. Behav Res Ther. 105:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezekiel F, Bosma R, Morton JB. 2013. Dimensional change card sort performance associated with age-related differences in functional connectivity of lateral prefrontal cortex. Dev Cogn Neurosci. 5:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. 1999a. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. NeuroImage. 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. 1999b. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou S, Chitins X, Williams SCR. 2004. Mapping IQ and gray matter density in healthy young people. NeuroImage. 23:800–805. [DOI] [PubMed] [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, Jernigan T, Potter A, Thompson W, Zahs D. 2018. Recruiting the ABCD sample: Design considerations and procedures Dev Cogn Neurosci. 32:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. 2002. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 295:2279–2282. [DOI] [PubMed] [Google Scholar]
- Glavin K, Roelants M, Strand BH, Júlíusson PB, Lie KK, Helseth S, Hovengen R. 2014. Important periods of weight development in childhood: a population-based longitudinal study. BMC Public Health. 14:306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluck ME, Viswanath P, Stinson EJ. 2017. Obesity, appetite, and the prefrontal cortex. Curr Obes Rep. 6:380–388. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 12:652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham AL, Gluck ME, Votruba SB, Krakoff J, Thearle MS. 2014. Perseveration augments the effects of cognitive restraint on ad libitum food intake in adults seeking weight loss. Appetite. 82:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Chabris CF, Braver TS. 2003. Neural mechanisms of general fluid intelligence. Nat Neurosci. 6:316–322. [DOI] [PubMed] [Google Scholar]
- Guerrieri R, Nederkoorn C, Jansen A. 2008. The interaction between impulsivity and a varied food environment: its influence on food intake and overweight. Int J Obes. 32:708–714. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Spitznagel MB, Paul RH, Cohen RA, Kohn M, Luyster FS, Clark R, Williams LM, Gordon E. 2008. Body mass index and neuropsychological function in healthy children and adolescents. Appetite. 50:246–251. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Strain G, Devlin MJ, Wing R, Cohen RA, Paul RH, Crosby RD, Mitchell JE. 2011. Improved memory function 12 weeks after bariatric surgery. Surg Obes Relat Dis. 7:465–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mayer EA, Sanmiguel CP, Van Horn JD, Woodworth D, Ellingson BM, Fling C, Love A, Tillisch K, Labus JS. 2015. Patterns of brain structural connectivity differentiate normal weight from overweight subjects. NeuroImage: Clinical. 7:506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagenaars SP, Harris SE, Davies G, Hill WD, Liewald DCM, Ritchie SJ, Marioni RE, Fawns-Ritchie C, Cullen B, Malik R et al. 2016. Shared genetic aetiology between cognitive functions and physical and mental health in UK biobank (N = 112 151) and 24 GWAS consortia. Mol Psychiatry. 21:1624–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. 2009. Beyond Baron and Kenny: statistical mediation analysis in the new millennium. Commun Monograph. 76:408–420. [Google Scholar]
- Hodgson K, Poldrack RA, Curran JE, Knowles EE, Mathias S, Göring HHH, Yao N, Olvera RL, Fox PT, Almasy L et al. 2017. Shared genetic factors influence head motion during MRI and body mass index. Cereb Cortex. 27:5539–5546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holsen LM, Zarcone JR, Thompson TI, Brooks WM, Anderson MF, Ahluwalia JS, Nollen NL, Savage CR. 2005. Neural mechanisms underlying food motivation in children and adolescents. NeuroImage. 27:669–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Brown SA, ABCD consortium coordinators: introduction. 2018Dev Cogn Neurosci. 32:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonova M, Martin RE, Gabrieli JDE, Sheridan MA. 2013. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Dev Cogn Neurosci. 6:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W, Yurgelun-Todd DA. 2005. Body mass predicts orbitofrontal activity during visual presentations of high-calorie foods. NeuroReport. 16:859–863. [DOI] [PubMed] [Google Scholar]
- Korpela S, Nyman A, Munck P, Ahtola A, Matomäki J, Korhonen T, Parkkola R, Haataja L, PIPARI Study Group . 2018. Working memory in very-low-birthweight children at the age of 11 years. Child Neuropsychol. 24:338–353. [DOI] [PubMed] [Google Scholar]
- Kringelbach M. 2005. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 6:691–702. [DOI] [PubMed] [Google Scholar]
- Labouesse MA, Polesel M, Clementi E, Müller F, Markkanen E, Mouttet F, Cattaneo A, Richetto J. 2018. MicroRNA expression profiling in the prefrontal cortex: putative mechanisms for the cognitive effects of adolescent high fat feeding. Sci Rep. (1):14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavagnino L, Mwangi B, Bauer IE, Cao B, Selvaraj S, Prossin A, Soares JC. 2016. Reduced inhibitory control mediates the relationship between cortical thickness in the right superior frontal Gyrus and body mass index. Neuropsychopharmacology. 41:2275–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le DSNT, Pannacciulli N, Chen K, Parigi AD, Salbe A, Reiman EM, Krakoff J. 2006. Less activation of the left dorsolateral prefrontal cortex in response to a meal: a feature of obesity. Am J Clin Nutr. 84:725–731. [DOI] [PubMed] [Google Scholar]
- Li N, Yolton K, Lanphear BP, Chen A, Kalkwarf HJ, Braun JM. 2018. Impact of early-life weight status on cognitive abilities in children. Obesity. 26:1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Matheson BE, Kaye WH, Boutelle KN. 2014. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes. 38:494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locke AE, Kahali B, Berndt SI, Justice AE, Pers TH, Day FR, Powell C, Vedantam S, Buchkovich ML, Yang J et al. 2015. Genetic studies of body mass index yield new insights for obesity biology. Nature. 518:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luciana M, Bjork JM, Nagel BJ, Barch DM, Gonzalez R, Nixon SJ, Banich MT. 2018. Adolescent neurocognitive development and impacts of substance use: overview of the adolescent brain cognitive development (ABCD) baseline neurocognition battery. Dev Cogn Neurosci. 32:67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maayan L, Hoogendoorn C, Sweat V, Convit A. 2011. Disinhibited eating in obese adolescents is associated with orbitofrontal volume reductions and executive dysfunction. Obesity. 19:1382–1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM. 2000. Equivalence of the mediation. Confounding and suppression effect. Prev Sci. 1:173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marioni RE, Yang J, Dykiert D, Mõttus R, Campbell A, CHARGE Cognitive Working Group, Davies G, Hayward C, Porteous DJ, Visscher PM et al. 2016. Assessing the genetic overlap between BMI and cognitive function. Mol Psychiatry. 21:1477–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medic N, Ziauddeen H, Erche KD, Bullmore ET, Nathan PJ, Ronan L, Fletcher PC. 2016. Increased body mass index is associated with specific regional alterations in brain structure. Int J Obes. 40:1177–1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCD Risk Factor Collaboration (NCD-RisC) 2017. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 390:2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T-V, McCracken JT, Ducharme S, Cropp BF, Botteron KN, Evans AC, Karama S. 2013. Interactive effects of dehydroepiandrosterone and testosterone on cortical thickness during early brain development. J Neurosci. 33:10840–10848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. 2014. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 311:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinert KRS, Po’e EK, Barkin SL. 2013. The relationship between executive function and obesity in children and adolescents: a systematic literature review. J Obes. 820956–820910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs NR, Huh J, Chou CP, Spruijt-Metz D, Pentz AM. 2012. Executive function and latent classes of childhood obesity risk. J Behav Med. 35:642–650. [DOI] [PubMed] [Google Scholar]
- Romine CB, Reynolds CR. 2005. A model of the development of frontal lobe functioning: findings from a meta-analysis. Appl Neuropsychol. 12:190–201. [DOI] [PubMed] [Google Scholar]
- Ross N, Yau PL, Convit A. 2015. Obesity, fitness and brain integrity in adolescence. Appetite. 93:44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma AK, Metzger DL, Daymont C, Hadjiyannakis S, Rodd CJ. 2015. LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5-19 y in NHANES III: association with cardio-metabolic risks. Pediatr Res. 78:723–729. [DOI] [PubMed] [Google Scholar]
- Shaw P, Greenstein D, Lerch J, Clasen L, Lenroot R, Gogtay N, Evans A, Rapoport J, Giedd J. 2006. Intellectual ability and cortical development in children and adolescents. Nature. 440:676–679. [DOI] [PubMed] [Google Scholar]
- Shields GS, Moons WG, Slavich GM. 2017. Inflammation, self-regulation, and health: an immunologic model of self-regulatory failure. Perspect Psychol Sci. 8:588–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A, Dugravot A, Shipley M, Brunner EJ, Elbaz A, Sabia S, Kivimaki M. 2017. Obesity trajectories and risk of dementia: 28 years of follow-up in the Whitehall II study. Alzheimers Dement. 14:178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW. 2004. Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 24:8223–8231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel MB, Alosco M, Strain G, Devlin M, Cohen R, Paul R, Crosby RD, Mitchell JE, Gunstad J. 2013. Cognitive function predicts 24-month weight loss success after bariatric surgery. Surg Obes Relat Dis. 9:765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. 2010. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage. 50:1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss RS, Knight J. 1999. Influence of the home environment on the development of obesity in children. Pediatrics. 103:e85. [DOI] [PubMed] [Google Scholar]
- Tamnes CK, Walhovd KB, Grydeland H, Holland D, Østby Y, Dale AM, Fjell AM. 2013. Longitudinal working memory development is related to structural maturation of frontal and parietal cortices. J Cog Neurosci. 25:1611–1623. [DOI] [PubMed] [Google Scholar]
- Tingley D, Yamamoto T, Hirose K, Keele L, Imai K. 2014. Mediation: R package for causal mediation analysis. J Stat Softw. 59:1–38.26917999 [Google Scholar]
- Uban KA, Horton MK, Jacobus J, Heyser C, Thompson WK, Tapert SF, Madden PAF, Sowell ER, Adolescent Brain Cognitive Development Study . 2018. Biospecimens and the ABCD study: rationale, methods of collection, measurement and early data. Dev Cogn Neurosci. 32:97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbeken S, Braet C, Claus L, Nederkoorn C, Oosterlaan J. 2009. Childhood obesity and impulsivity: an investigation with performance-based measures. Behav Change. 26:153–167. [Google Scholar]
- Verbeken S, Braet C, Lammertyn J, Goossens L, Moens E. 2012. How is reward sensitivity related to bodyweight in children? Appetite. 58:478–483. [DOI] [PubMed] [Google Scholar]
- Veronese N, Facchini S, Stubbs B, Luchini C, Solmi M, Manzato E, Sergi G, Maggi S, Cosco T, Fontana L. 2016. Weight loss is associated with improvements in cognitive function among overweight and obese people: a systematic review and meta-analysis. Neurosci Biobehav Rev. 72:87–94. [DOI] [PubMed] [Google Scholar]
- Yang Y, Shields GS, Guo C, Liu Y. 2018. Executive function performance in obesity and overweight individuals: a meta-analysis and review. Neurosci Biobehav Rev. 84:225–244. [DOI] [PubMed] [Google Scholar]
- Yau PL, Kang EH, Javier DC, Convit A. 2014. Preliminary evidence of cognitive and brain abnormalities in uncomplicated adolescent obesity. Obesity. 22:1865–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


