Abstract
Background
The prevalence of infections due to carbapenem-resistant Acinetobacter baumannii (CRAB) is on the rise worldwide. Polymyxins are considered as last-resort drugs for CRAB infections, but there is still controversy regarding the efficacy and safety of polymyxins based therapies in CRAB infections. The present systematic review was designed to compare the efficacy and safety of polymyxins based therapies versus non-polymyxins based therapies in CRAB infections.
Methods
We performed a systematic literature search in PubMed, Embase, CINAHL, Cochrane Library, and clinicaltrials.gov to identify eligible studies reporting the clinical outcomes of patients with CRAB infections. The meta-analysis employed a random-effects model to estimate the odds ratio (OR) and standardized mean difference (SMD) with 95% confidence interval (CI). The primary outcome was 1-month mortality for any cause. We also examined clinical response, microbiological response, length of stay in hospital, and adverse events.
Results
Eleven eligible studies were analyzed (1052 patients in total), including 2 randomized clinical trials. Serious risk of bias was found in 8 out of the 11 studies. There was no statistically significant difference between polymyxins based therapies and non-polymyxins based therapies in 1-month mortality for any cause (OR, 0.95; 95% CI, 0.59 to 1.53), microbiological response (OR, 3.83; 95% CI, 0.90 to 16.29) and length of stay in hospital (SMD, 0.24; 95% CI, − 0.08 to 0.56). The pooled OR of clinical response indicated a significant difference in favor of polymyxin based therapies (OR, 1.99; 95% CI, 1.31 to 3.03). The pooled OR of adverse events showed that non-polymyxins based therapies were associated with fewer adverse events (OR, 4.32; 95% CI, 1.39 to 13.48).
Conclusion
The performance of polymyxins based therapies was better than non-polymyxin based therapies in clinical response rate and similar to non-polymyxin based therapies in terms of 1-month mortality and microbiological response in treating CRAB infections. Due to the limitations of our study, we cannot draw a firm conclusion on the optimal treatment of CRAB infections, but polymyxins would be a relatively effective treatment for CRAB infections. Adequate and well-designed large scale randomized controlled trials are required to clarify the relative efficacy of polymyxins based and non-polymyxins based therapies.
Keywords: Polymyxin, Colistin, Carbapenem-resistant Acinetobacter baumannii, Meta-analysis
Background
Acinetobacter baumannii is a gram-negative opportunistic pathogen [1] and a member of the ESKAPE (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter) pathogens. A. baumannii can develop diverse mechanisms of resistance and so is capable of escaping from the effects of the commonly used antibiotics [2]. It may cause a range of nosocomial infections, including pneumonia, bacteremia, wound infection, and post-neurosurgical meningitis, threatening the lives of patients, particularly in the setting of intensive care unit (ICU) [3].
Carbapenems are considered as the first-line agents for treating A. baumannii infections if the isolates are susceptible. But the widespread use of carbapenems since 1990 has provoked the emergence of carbapenem-resistant A. baumannii (CRAB) [4]. Lob et al. reported that only about 8–26% A. baumannii isolates were susceptible to carbapenems worldwide [5]. Alarmingly, the prevalence of CRAB isolates increased from 13.3% in 2004 to 70.5% in 2014 in China [6].
Polymyxins include polymyxin B and polymyxin E (also known as colistin). They were discovered in 1947 [7] but discontinued shortly thereafter due to high nephrotoxicity [8]. In recent years, they are reintroduced into clinical practice for the activity against many multidrug-resistant gram-negative bacilli. Polymyxins may provide a synergistic effect with other antibiotic classes by disrupting the outer membrane of gram-negative bacteria [9].
Currently, polymyxins are the most commonly used agents and often considered as the “last resort” or salvage treatments of CRAB infections [10]. However, there are still many controversies and confusions regarding the efficacy and safety of polymyxins based therapies in treating CRAB infections. While numerous reports showed good therapeutic effects of polymyxins based therapies [11, 12], some reports linked polymyxins based therapies with a higher mortality [13, 14]. Other antibiotics, such as tigecycline and sulbactam, alternative therapeutic options against CRAB, also have shown mixed clinical outcomes [15]. Therefore, it is important to elucidate whether the polymyxins based therapies are more effective than other alternative treatments in patients with CRAB infection.
This meta-analysis was designed to evaluate the efficacy and safety of polymyxins based therapies versus non-polymyxins based therapies in CRAB infections based on all the evidence available in the literature.
Methods
The study design of this systematic review and meta-analysis was consistent with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis Protocols (PRISMA-P 2015) Guidelines [16].
Search strategies
Databases including PubMed, Embase, CINAHL, Cochrane Library and clinicaltrials.gov (www.clinicaltrials.gov) were searched for all the studies reporting the treatment of infections caused by CRAB from their inception to October 2019. We used the following search string: “polymyxin or colistin” and “Acinetobacter baumannii and drug-resistant” or “Acinetobacter baumannii and carbapenem” or “carbapenem-resistant Acinetobacter baumannii” (see Additional file 1 for detailed search strategy). Furthermore, references listed in the identified articles and other reviews were also searched to select relevant studies. No restrictions of language, publication year or publication status were applied.
Selection criteria
Studies for inclusion were based on the following criteria: (1) adult patients with CRAB infection, (2) polymyxins group was polymyxins based therapy and control group was non-polymyxins based therapy, (3) randomized controlled trials (RCTs) or cohort studies or case-control studies (whether retrospective or prospective), (4) at least one active antibiotic was used in treatment groups, and CRAB isolates were sensitive to polymyxins in the polymyxins group, (5) carbapenem resistance was clearly defined as resistant to imipenem and/or meropenem (without limitation on definition of cut off value), (6) 1-month mortality for any cause was reported. Studies for exclusion were based on the following criteria: (1) animals, in vitro, pharmacokinetics/pharmacodynamics (PK/PD) studies or single-arm studies, (2) polymyxins were used in all treatment groups, (3) studies in patients infected with mixed microorganisms, (4) abstracts presented at conferences, editorials, reviews, systematic reviews, and meta-analyses, (5) less than five cases per treatment group were reported. We attempted to contact the authors for details if the data were unclear or missing.
Full-text articles were retrieved for the studies that fulfilled selection criteria. Two authors (CL & YZ) further checked the eligibility of each study independently.
Outcomes
In this study, the primary outcome was 1-month (28–30 days) mortality for any cause (1-month mortality). The secondary outcomes were clinical response, microbiological response, length of stay in hospital and adverse events. Clinical response was defined as complete resolution of at least two signs of infection (such as abnormal temperature, leukocytosis or leukopenia) at the end of treatment. The signs of infection varied due to the site of infection. Clinical judgment was made by the clinician according to local guidelines. The microbiological response was defined as a negative microbiological culture, which was obtained at the end of treatment. Length of stay in hospital was measured from the date of the infection was diagnosed to the date of discharge or death. Adverse events included nephrotoxicity, hepatotoxicity, skin rash, and diarrhea.
Data extraction and quality assessment
The information of first authors, publication years, study years, countries, study designs, patient demographics (including age, gender, resistance profile of the bacteria, disease, and APACHE II score), clinical settings, sample sizes, interventions (including regimen and route of administration) and clinical outcomes were extracted from individual studies. All data extraction was done independently and checked by two authors (CL and YZ) using a pre-defined data extraction form and then compared for verification. The risk of bias of individual studies was assessed by four authors (CL, YZ, XL & JW) using the Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool endorsed by Cochrane Scientific Committee for observational studies [17]. We assessed the risk of bias for seven domains including bias due to confounding, selection of participants into the study, classification of the intervention, deviations from intended interventions, bias due to missing data, measurement of outcomes, and selection of the reported result. The rating of each domain ranged from low, moderate, serious, to critical risk, and no information (NI). The rating of risk of bias was based on the data we used for meta-analysis rather than the data in publications. Any discrepancies were settled by consensus.
Statistical analysis
We performed a meta-analysis using the Mantel-Haenszel method (random-effects model) and inverse variance approach (random-effects model) to estimate the Odd Ratio (OR) and standardized mean difference (SMD) with 95% confidence interval (CI) to compare polymyxins based therapies with non-polymyxins based therapies. Dichotomous variables including 1-month mortality, clinical response, microbiological response, and adverse events were described by OR and CI. Continuous variable, length of stay in hospital, was described by SMD and CI. Heterogeneity between studies was assessed by the Q statistics and I2 tests (P < 0.05 and I2 > 50% suggesting significant heterogeneity). Sensitivity analyses were performed on efficacy outcomes to identify the source of heterogeneity by excluding the studies with serious risk of bias, excluding studies published before 2010, excluding studies with small sample size and excluding studies with inadequate balance in baseline characteristics. The leave-one-out analysis was conducted to ensure that no single study unduly influenced the overall effect size. A funnel plot was used to measure the publication bias. Egger’s test and Peters’ test were used to evaluate the asymmetry of funnel plot [18]. Subgroup analyses were done to compare the efficacy split by infection site, route of administration and region. All the above analyses were performed with Review Manager (RevMan) software, Version 5.3, (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.) except the analysis of publication bias, which was done with STATA software, Version 13.0. (StataCorp LLC, 2013).
Results
The selection of included studies
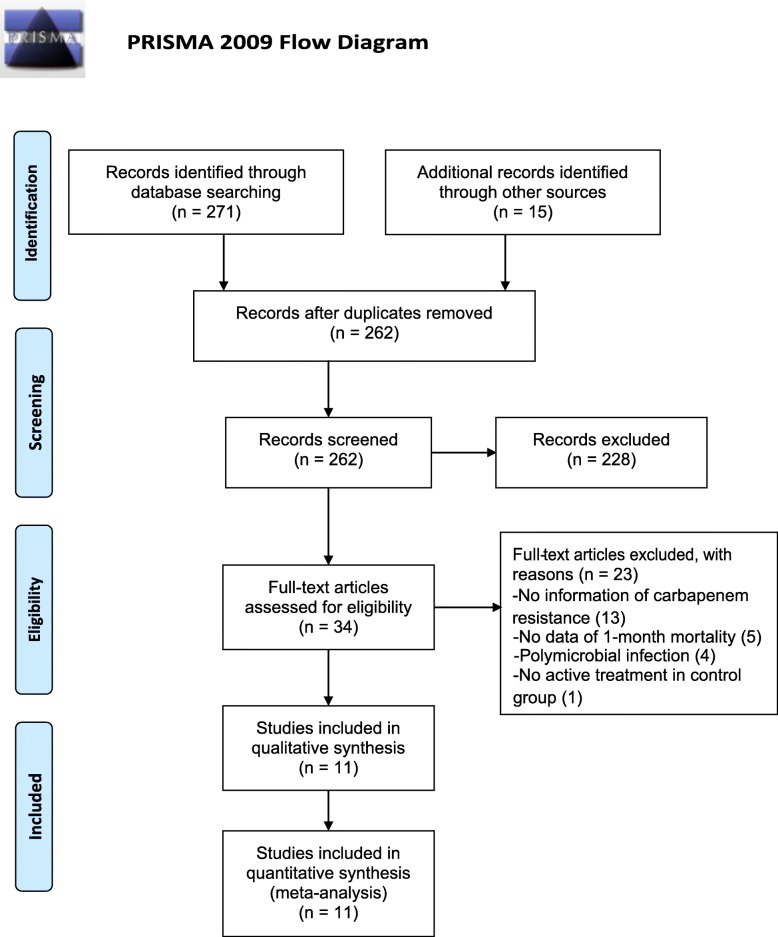
A total of 271 studies were identified through the electronic database search and 15 studies were identified through manual search. After removing the duplicate records between databases, the abstracts of the remaining 262 articles were retrieved for a preliminary screening. Thirty-four studies with full texts were further assessed for eligibility, of which 13 studies were excluded because of insufficient information of carbapenem-resistance, 5 were excluded due to the unavailability of the data of 1-month mortality, 4 were excluded because patients were co-infected with other bacteria and 1 study was excluded because patients in control group did not receive active treatments. Finally, a total of 11 studies were included in our meta-analysis. Additional data were obtained from the author of the paper published by Raz-Pasteur and colleagues in 2019 [19]. The data were adequate for comparing colistin with ampicillin-sulbactam and comparing colistin with trimethoprim-sulfamethoxazole separately in two independent sets of data. Therefore, a total of 12 datasets were analyzed. A flowchart of study selection is shown in Fig. 1.
Fig. 1.
A PRISMA flow chart of study selection
Study characteristics
The meta-analysis was performed with 11 studies [13, 14, 19–27], including a total of 1052 patients (496 patients in polymyxins group and 556 patients in control group). The characteristics of the included studies are listed in Table 1. These 11 studies consisted of 8 retrospective studies [13, 14, 19, 22, 24–27], 2 RCTs [20, 21] and 1 prospective study [23]. The research hospitals are in North America and Europe (USA, Greece, Spain, Turkey, and France), Middle East (Iran, and Israel) and Asia (Taiwan, China, and Thailand). Two studies [25, 26] assessed patients with intracranial infections (such as meningitis). One study [13] reported on patients with intra-abdominal infection. Five studies examined pneumonia [14, 20–22, 24], including hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP). Three studies [19, 23, 27] included more than one site of infection. In polymyxins group, colistin was administered in 10 studies [13, 14, 19–24, 26, 27] and polymyxin B [25] was used in only one study. In the control group, carbapenems, tetracyclines, cephalosporins, β-lactam/β-lactamase inhibitors, quinolones, aminoglycosides and glycylcycline (tigecycline) were the most commonly used alternative antibiotics. In 7 studies [13, 14, 19–21, 23, 24], the intravenous (IV) route of administration was used in both polymyxins and control groups. But in the other 4 studies [22, 25–27], patients in polymyxins group received IV and intrathecal/intracerebral (IT) polymyxins or IV and inhaled (IH) polymyxins, while patients in control group were given IV antibiotics only. One-month mortality was reported in all studies, clinical and microbiological response was reported in 5 studies. The length of stay in hospital and adverse events were reported in 4 studies.
Table 1.
Characteristics of included studies
| Author; Year | Country | Study years | Study design | Setting | CRs MIC /mg/L |
Type of infection | Male/ Female | Age (years) | Treatment regimen | Sample size (PXs vs. Non-PXs) | Route of colistin | APACHE II score (mean) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polymyxins group | Non-polymyxins group | ||||||||||||
| Trottier 2007 [27] | USA | 2004–05 | RS | MIX | NA | Mixb | 83/33 | 17 ~ 90 | COL | AMK; FEP; CPF; DOX; IMP; MNO; TZP | 96 vs. 20 |
IV/IH/IV+ IH/IV + IT |
NR |
| Betrosian 2008 [20] | Greece | NA | RCT | ICU | NA | VAP | 14/14 | adult | COL | SAM | 15 vs. 13 | IV | 14 |
| Lopez-Cortes 2014 [23] | Spain | 2010 | PS | NA | ≥32 | RT/SS/SST UT/IAI/CNS OA/Bm | NA | >18 |
COL; COL+TGC; COL+CRs; COL+SUL; COL+AG; COL+RIF; COL+TGC + AG; COL+TGC+ CRs + AG |
CRs; TGC; SUL; TCY; CRs + TGC; CRs + AG; TGC + RIF; TGC + AG; TGC + CRs + RIF |
52 vs. 12 | IV | NA |
| Ozvatan 2016 [24] | Turkey | 1996–2010 | RS | NA | NA | VAP | NA | ≥18 | COL; COL+OTH | M/I; SAM; CSL; OTH | 29 vs. 187 | IV | NA |
| Zalts 2016 [14] | Israel | 2008–09 | RS | ICU | NA | VAP | 70/28 | >18 | COL | SAM | 66 vs. 32 | IV | 17.5 |
| Pan 2018 [25] | China | 2013–17 | RCS | NA | NA | ICI | 30/31 | >18 | PB+ CTR |
M/I + AMK; M/I + TGC; M/I + CSL; M/I + TGC + CSL; CSL; CSL + AMK; |
23 vs. 38 | IV + IT | 18 |
| Khalili 2018 [21] | Iran | 2015–17 | RCT | ICU | ≥0.08 | VAP | 35/12 | 18 ~ 75 | MEM + COL | MEM + SAM | 24 vs.23 | IV | NA |
| Liang 2018 [22] | Taiwan | 2010–15 | RS | MIX | NA | Pmn | 167/71 | >20 | TGC + COL | TGC; TGC + OTH; SUL; SUL + OTH | 110 vs. 128 | IV/IV+ IH | 23 |
| Sipahi 2018 [26] | Turkey & France | 2007–16 | RS | ICU | NA | Meg | 12/8 | >18 | TGC + COL |
TGC + NET; TGC + AMK; TGC + MEM; TGC |
8 vs. 12 | IV/ IV + IT | NA |
| Chusri 2019 [13] | Thailand | 2012–17 | RS | MIX | ≥16 | IAI | 16/12 | >18 | TGC + COL | TGC | 14 vs. 14 | IV | 15/median |
| Raz-Pasteur 2019 [19] | Israel | 2013–15 | RCS | MIX | NA | Pmn/SST/Bm |
62/21 72/40 |
Any | COL |
SAM; TMP-SMX |
59 vs. 24 59 vs. 53 |
IV | NA |
AG aminoglycosides, AMK amikacin, Bm bacteremia, CNS central nervous system, COL colistin, CPF ciprofloxacin, CRs carbapenems, CSL cefoperazone-sulbactam, CTR ceftriaxone, DOX doxycycline, FEP cefepime, FQs fluoroquinolones, IAI intra-abdominal infection, ICI intracranial infection, ICU intensive care unit, IH inhaled, IMP imipenem, IT intrathecal/intracerebral, IV intravenous, Meg meningitis, MEM meropenem, M/I meropenem/imipenem, MIC minimum inhibitory concentration, MIX intensive care unit and hospital, MNO minocycline, NA not available, NET netilmicin, OA osteoarticular, OTH other antibiotics, PB polymyxin B, PCS prospective cohort study, Pmn pneumonia, PS prospective study, PXs polymyxins, RCS retrospective cohort study, RCT random clinical trial, RIF rifampicin, RS retrospective study, RT respiratory tract, SAM ampicillin-sulbactam, SST skin and soft tissue, SUL sulbactam, TCY tetracycline, TGC tigecycline, TMP-SMX trimethoprim-sulfamethoxazole, TZP piperacillin-tazobactam, UT urinary tract, VAP ventilator-associated pneumonia, vs. versus
Quality assessment
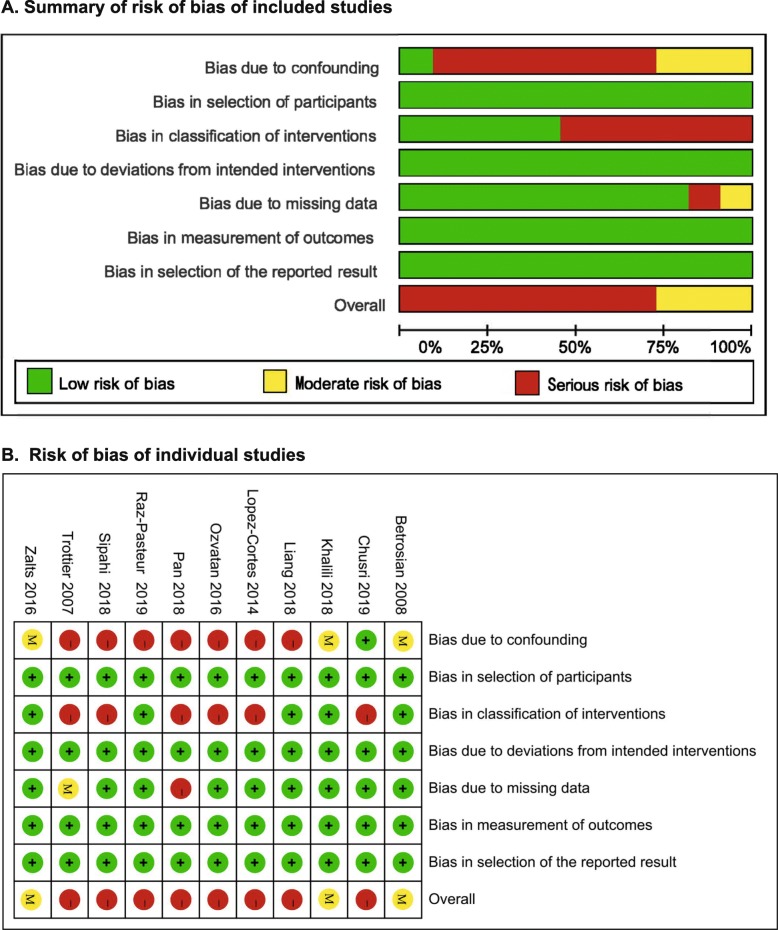
The output of the ROBINS-I tool is summarized in Fig. 2. The risk of bias was rated as moderate for 2 RCTs [20,21] and one [14] retrospective study, serious for the remaining 8 studies. Seven studies [19, 22–27] were at serious risk of bias in the domain of “bias due to confounding”. The common confounding factors were comorbidities (especially immunodeficiency), mixed infection sites, antibiotic susceptibility, severity of disease, and empirical treatment. Six studies [13, 23–27] were judged to be at serious risk in the domain of “bias in classification of interventions” because of lacking records of the dose/loading dose, frequency, and timing of intervention. Only one study was at serious risk of bias due to missing 50 records of patients without explanation [25].
Fig. 2.
Summary of risk of bias: a Summary of risk of bias of included studies; b Risk of bias in individual studies. The green, yellow and red represent “low risk of bias”, “moderate risk of bias” and “serious risk of bias”, respectively
Meta-analysis for 1-month mortality
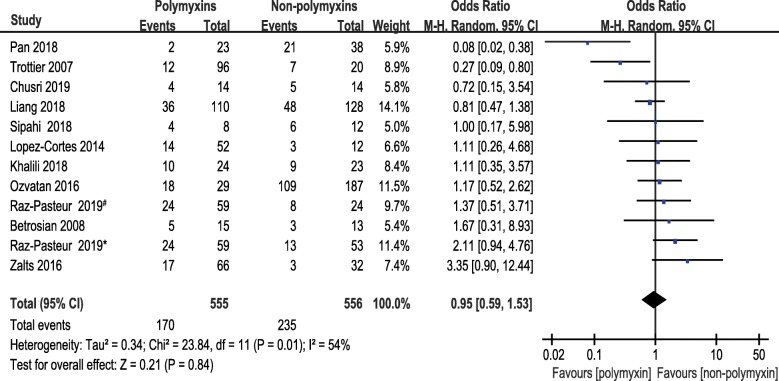
Eleven studies reporting the 1-month mortality of polymyxins based therapies and non-polymyxins based therapies for CRAB infections were included in this meta-analysis. There was no statistically significant difference between the two groups in 1-month mortality (OR, 0.95; 95% CI, 0.59 to 1.53; P = 0.84). Statistically significant heterogeneity (P = 0.01, I2 = 54%) was observed (Fig. 3).
Fig. 3.
The forest plot of studies reporting 1-month mortality. Studies were ranked according to their effect sizes. #Ampicillin–sulbactam was used in non-polymyxins group. *Trimethoprim-sulfamethoxazole was used in non-polymyxins group
Meta-analysis for clinical response
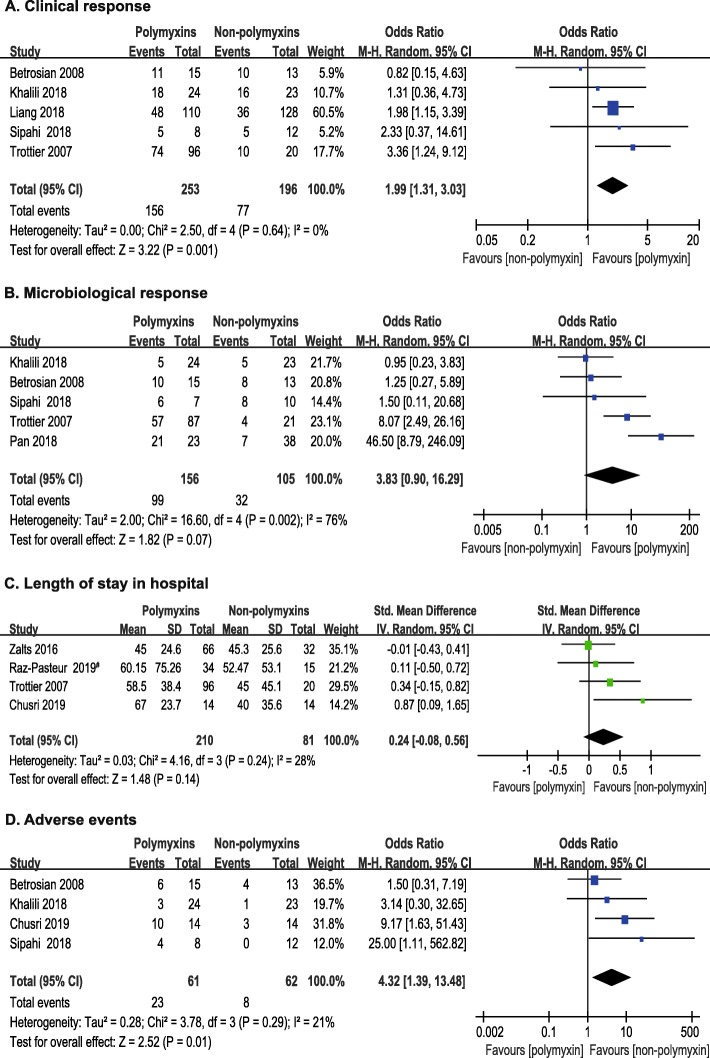
Five studies [20–22, 26, 27] involving 449 patients compared the clinical response of two types of therapies. No statistically significant heterogeneity was observed among these studies (P = 0.64, I2 = 0.0%). The pooled OR of clinical response suggested that polymyxins based therapies may have an advantage over non-polymyxins based therapies (OR, 1.99; 95% CI, 1.31 to 3.03; P = 0.001) (Fig. 4a).
Fig. 4.
The forest plots of secondary outcomes: a clinical response; b microbiological response; c Length of stay in hospital; d Adverse events. Studies were ranked according to their effect sizes. #Ampicillin–sulbactam was used in non-polymyxins group
Meta-analysis for microbiological response
Five studies [20, 21, 25–27] (261 patients) reported the microbiological response. The microbiological response rates favored polymyxins group, but the difference was not statistically significant (OR, 3.83; 95% CI, 0.90 to 16.29; P = 0.07) between the two types of therapies. Statistically significant heterogeneity (P = 0.002, I2 = 76%) was observed among the five studies (Fig. 4b).
Meta-analysis for length of stay in hospital
Four studies [13, 14, 19, 27] reported the length of stay in hospital. The heterogeneity observed among 4 studies was not statistically significant (P = 0.24, I2 = 28%). The length of stay in hospital did not differ significantly (SMD, 0.24; 95% CI, − 0.08 to 0.56; P = 0.14) between polymyxins based therapies (210 patients) and non-polymyxins based therapies (81 patients), but with a trend of prolonged hospitalization for patients receiving polymyxins based therapies (Fig. 4c).
Meta-analysis for adverse events
Adverse events were recorded in 4 studies [13, 20, 21, 26] involving 123 patients. The main adverse events of polymyxins based therapies were associated with nephrotoxicity. Twenty nephrotoxicity-related adverse events were seen in the 61 patients with polymyxins based therapies, and 6 in the 62 patients treated with non-polymyxins based therapies. Our result showed that more adverse events occurred in polymyxins group than in control group (OR, 4.32; 95% CI, 1.39 to 13.48; P = 0.01) (Fig. 4d). Statistical heterogeneity was not significant among these studies (P = 0.29, I2 = 21%).
Sensitivity analyses and publication bias analysis
We performed four sensitivity analyses to identify the source of heterogeneity by subgrouping. The planned exclusion was removal of studies with serious risk of bias, removal of studies published before 2010, removal of studies with small sample size, and removal of studies with inadequate balance in the baseline characteristics. The primary outcome and the two secondary outcomes (clinical response and adverse events) did not change substantially after subgrouping, which proved the consistency of our results (Additional file 2: Table S1).
A leave-one-out analysis was also performed to reflect the effect of individual dataset on the pooled ORs. The corresponding pooled ORs of our primary outcome were not changed remarkably (Additional file 2: Table S2).
The funnel plot was constructed to assess the publication bias of the literature (Additional file 3). There was no evidence of publication bias based on Egger’s test (t = − 1.21; P = 0.253) and Peters’ test (t = − 0.18; P = 0.864). The tests could be underpowered due to small sample size.
Subgroup analyses
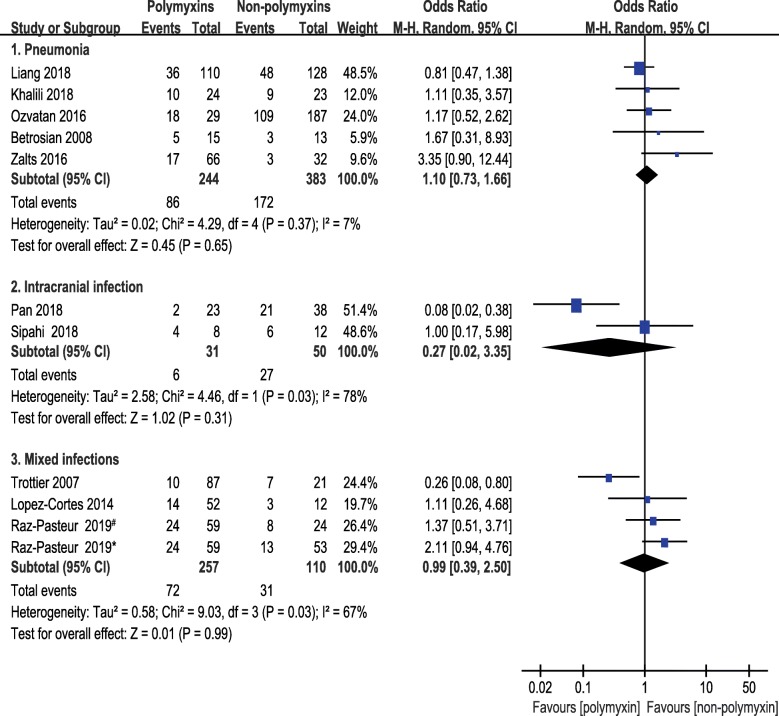
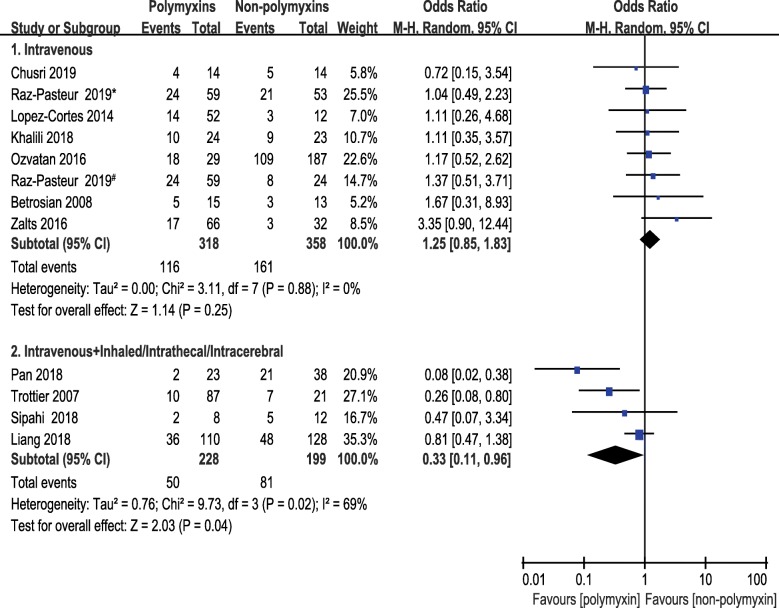
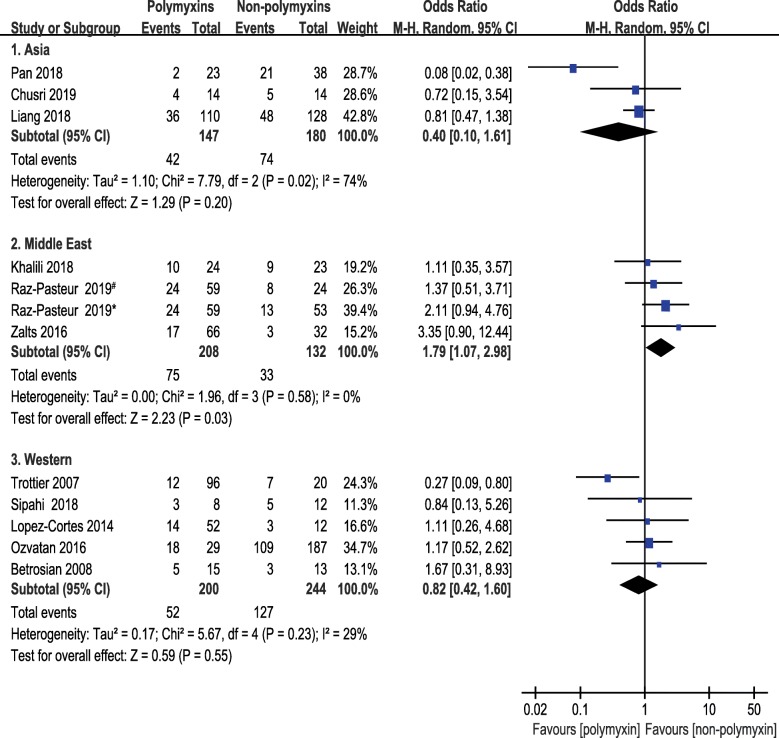
Subgroup analyses of 1-month mortality were planned to split the studies according to infection site, route of administration or region. Studies were classified into 4 subgroups in terms of infection site, i.e., pneumonia, mixed infection, intracranial infection, and intra-abdominal infection. The pooled ORs of 1-month mortality did not differ from the primary analysis remarkably. No significant difference was seen between polymyxins based therapies and non-polymyxins based therapies after subgrouping. The pooled ORs of pneumonia, intracranial infection and mixed infection were 1.10 (95% CI, 0.73 to 1.66), 0.27 (95% CI, 0.02 to 3.35) and 0.99 (95% CI, 0.39 to 2.50), respectively. Only one paper reported the intra-abdominal infection caused by CRAB and the OR was 0.72 with 95% CI (0.15 to 3.54) (Fig. 5). A subgroup analysis was carried out based on route of administration to compare the 1-month mortality between a subset of studies in which antibiotics were given intravenously in both groups and a subset of studies in which polymyxins were given by multiple routes. The pooled OR of studies, in which multiple routes (IV plus IH/IT) were used for polymyxins administration, was 0.33 (95% CI, 0.11 to 0.96). The pooled OR was 1.25 (95% CI, 0.85 to 1.83) in studies that antibiotics were administered intravenously only (Fig. 6). This result indicated that lower mortality was associated with the direct delivery of polymyxins to the focus of infection by intrathecal/intracerebral ventricle injection or inhalation. A subgroup analysis was also carried out according to geographical region in order to understand the regional difference of 1-month mortality. Interestingly, the result showed that studies from Middle East region were in favor of non-polymyxin therapies because of lower mortality rate (OR, 1.79; 95% CI, 1.07 to 2.98) (Fig. 7).
Fig. 5.
The forest plot of studies reporting 1-month mortality grouped by infection site. Studies were ranked according to their effect sizes. #Ampicillin–sulbactam was used in non-polymyxins group. *Trimethoprim-sulfamethoxazole was used in non-polymyxins group
Fig. 6.
The forest plot of studies reporting 1-month mortality grouped by route of administration. Studies were ranked according to their effect sizes. # Ampicillin–sulbactam was used in non-polymyxins group. *Trimethoprim-sulfamethoxazole was used in non-polymyxins group
Fig. 7.
The forest plot of studies reporting 1-month mortality grouped by geographic region. Studies were ranked according to their effect sizes. #Ampicillin–sulbactam was used in non-polymyxins group. *Trimethoprim-sulfamethoxazole was used in non-polymyxins group
Discussion
Infections due to CRAB require special attention. The high morbidity/mortality, the potential to cause outbreaks and the spread of antibiotic resistance are all associated with CRAB infections [3]. Polymyxins are the common options for CRAB infections in clinical practice nowadays. Are polymyxins actually the optimal choice for treating CRAB infections? We aim to answer this question by compiling the up-to-date knowledge on the efficacy and safety of polymyxin-based and non-polymyxin-base therapies for CRAB infections.
The goal of all medical treatments is to improve the overall survival of patients. The all-cause mortality is a measurement of overall survival and an objective endpoint which is not subject to bias. That is why we chose 1-month mortality for any cause as the primary efficacy outcome in this meta-analysis. And one-month follow-up period is commonly used in hospitals [28]. One-month mortality for any cause was 30.6% in polymyxins group and 42.3% in non-polymyxins group. Our meta-analysis showed that polymyxins based therapies were not associated with lower 1-month mortality when comparing with non-polymyxins based therapies. Many uncertainties are related to 1-month mortality. Especially in our study, 193 patients were in ICU settings with serious comorbidities and complications. Many unknown and undocumented factors could influence the outcome of 1-month mortality.
To further comprehend the relative efficacy on a more specific level, clinical and microbiological responses were designed as secondary outcomes. Our meta-analysis demonstrated that the performance of polymyxins based therapies (desirable clinical response in 61.7% patients) was better than non-polymyxins based therapies (desirable clinical response in 39.3% patients) in terms of clinical response. The percentage of patients achieving a good microbiological response at the end of treatment was 63.5% in polymyxins group, which was 2 times higher than that in non-polymyxins based group (30.5%). The microbiological response rates favored polymyxins group, but the statistical significance was not reached, probably due to small sample size.
Just like previous reports [29], we found that more adverse events were reported in polymyxins group, especially nephrotoxic events. A total of 23 adverse events were recorded in polymyxins group, of which 20 were associated with nephrotoxicity. We cannot emphasize enough the importance of renal function monitoring and timely dose adjustment for patients receiving polymyxins. The key was to follow the guidelines of polymyxins usage to balance the efficacy and safety [30, 31].
The subgroup analysis based on route of administration showed that direct delivery of polymyxins to the focus of infection helps. This was in agreement with the previous studies reporting the efficacy of IV polymyxins combined with inhaled or intracranial polymyxins, which was significantly superior to IV polymyxins alone for treating CRAB induced pneumonia and meningitis [32–34]. Limited penetration into alveoli and limited ability to cross the blood-brain barrier of polymyxins can partly explain our findings [35, 36].
A previous meta-analysis by Liu et al. in 2014 noted that polymyxins may be effective for treatment of A. baumannii infection [37]. However, the cases included in their analysis did not share a clear definition of carbapenem resistance. They evaluated the treatments of A. baumannii infections showing different types of drug resistance or no resistance. Given the importance of distinguishing drug resistance in antimicrobial treatments, a clear definition of drug resistance in our study selection could reduce the heterogeneity of meta-analysis. Furthermore, only the studies involving adult patients were included in our analysis, while Liu et al. compiled studies of both adult and pediatric patients. Considering the unique physiological and microbiological conditions of pediatric patients, antimicrobial therapy targeting resistant microorganisms should be discussed in pediatric patients separately. This report is therefore a more robust meta-analysis with improved study design.
Several limitations exist in our meta-analysis. First, the quality of the included studies was low. Only 3 of the 11 studies were at moderate risk of bias and the others were at serious risk of bias. Second, the studied population was heterogeneous. The diverse baseline conditions of infections and comorbidities introduced unknown confounding factors. Third, lacking information about drug use for comorbidity may cause the underestimation of unknown drug-drug interaction which affected the clinical outcomes. Furthermore, the sample size was small which could reduce the power of our analysis. Taking together, the findings must be interpreted with caution. Our results may be not representative enough for the whole picture of the polymyxins based and non-polymyxins based therapies against CRAB infections.
Conclusion
Although our study cannot provide an answer for the optimal treatment of CRAB infection, our results did show that polymyxin could be a relatively effective therapy for CRAB infection. More randomized trials are needed to address the exact efficacy of different anti-CRAB treatments. Renal function monitoring to adjust the dose of polymyxin accordingly could largely improve the safety of polymyxin treatment [31]. Stewardship of polymyxin use also helps to prevent the emergence of polymyxin-resistant bacteria. More importantly, all healthcare providers should work closely to keep prioritizing research and development of new antibiotics.
Supplementary information
Additional file 2: Table S1. Summary of sensitivity analyses. Table S2. Summary of leave-one-out analysis on primary outcome.
Additional file 3. Funnel plot showing publication bias of 1-month mortality.
Acknowledgments
We thank Baoxia Zhang, Ye Cao and Chang Liu for assistance with meta-analysis methodology and text editing that greatly improved the manuscript. We would also like to show our gratitude to the Prof. Paul at The Ruth and Bruce Rappaport Faculty of Medicine, Prof. Trottier at Leonard Miller School of Medicine, Prof. Lopez-Cortes at Hospitales Universitarios Virgen Macarena y Virgen del Rocío, Prof. Akalin at Uludag University, and Prof. Zhang at Zhejiang University School of Medicine for sharing their additional data with us during the course of this study.
Abbreviations
- CI
Confidence interval
- CRAB
Carbapenem-resistant Acinetobacter baumannii
- ESKAPE
Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp
- HAP
Hospital-acquired pneumonia
- ICU
Intensive care unit
- IH
Inhaled
- IT
Intrathecal/intracerebral
- IV
Intravenous
- NI
No information
- OR
Odds ratio
- PK/PD
Pharmacokinetics/pharmacodynamics
- RCT
Randomized controlled trial
- ROBINS-I
Risk of Bias In Non-randomized Studies of Interventions
- SMD
Standardized mean difference
- VAP
Ventilator-associated pneumonia
Authors’ contributions
This study was designed, coordinated and supervised by JZ as the corresponding author. CL and YZ were involved in the study design, data extraction, quality assessment, and data analysis. XL and JW provided technical assistance for quality assessment. The manuscript was written by CL and YZ. All authors have read and approved this manuscript. CL and YZ contributed equally to this work.
Funding
This work was supported by the National Major Scientific and Technological Special Project for “Significant New Drugs Development” during the Thirteenth Five-year Plan Period of China (2017ZX09304005). This funder had no role in the study design, data analyses, result interpretation and decision to submit this paper.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cheng Lyu and Yuyi Zhang contributed equally to this work.
Contributor Information
Cheng Lyu, Email: levvy.lv@aliyun.com.
Yuyi Zhang, Email: zhangyuyi@shphc.org.cn.
Xiaofen Liu, Email: liuxiaofen227@163.com.
Jufang Wu, Email: 13816357099@163.com.
Jing Zhang, Email: zhangj_fudan@aliyun.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12879-020-05026-2.
References
- 1.Howard A, O'Donoghue M, Feeney A, Sleator RD. Acinetobacter baumannii: an emerging opportunistic pathogen. Virulence. 2012;3:243–250. doi: 10.4161/viru.19700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santajit S, Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int. 2016;2016:2475067. doi: 10.1155/2016/2475067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maragakis LL, Perl TM. Acinetobacter baumannii: epidemiology, antimicrobial resistance, and treatment options. Clin Infect Dis. 2008;46:1254–1263. doi: 10.1086/529198. [DOI] [PubMed] [Google Scholar]
- 4.Asif M, Alvi IA, Rehman SU. Insight into Acinetobacter baumannii: pathogenesis, global resistance, mechanisms of resistance, treatment options, and alternative modalities. Infect Drug Resist. 2018;11:1249–1260. doi: 10.2147/IDR.S166750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lob SH, Hoban DJ, Sahm DF, Badal RE. Regional differences and trends in antimicrobial susceptibility of Acinetobacter baumannii. Int J Antimicrob Agents. 2016;47:317–323. doi: 10.1016/j.ijantimicag.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 6.Gao L, Lyu Y, Li Y. Trends in drug resistance of Acinetobacter baumannii over a 10-year period: Nationwide data from the China surveillance of antimicrobial resistance program. Chin Med J. 2017;130:659–664. doi: 10.4103/0366-6999.201601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Storm DR, Rosenthal KS, Swanson PE. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- 8.Ordooei Javan A, Shokouhi S, Sahraei Z. A review on colistin nephrotoxicity. Eur J Clin Pharmacol. 2015;71:801–810. doi: 10.1007/s00228-015-1865-4. [DOI] [PubMed] [Google Scholar]
- 9.Li J, Nation RL, Turnidge JD, Milne RW, Coulthard K, Rayner CR, et al. Colistin: the re-emerging antibiotic for multidrug-resistant gram-negative bacterial infections. Lancet Infect Dis. 2006;6:589–601. doi: 10.1016/S1473-3099(06)70580-1. [DOI] [PubMed] [Google Scholar]
- 10.Karaiskos I, Lagou S, Pontikis K, Rapti V, Poulakou G. The "old" and the "new" antibiotics for MDR gram-negative pathogens: for whom, when, and how. Front Public Health. 2019;7:151. doi: 10.3389/fpubh.2019.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assimakopoulos SF, Karamouzos V, Lefkaditi A, Sklavou C, Kolonitsiou F, Christofidou M, et al. Triple combination therapy with high-dose ampicillin/sulbactam, high-dose tigecycline and colistin in the treatment of ventilator-associated pneumonia caused by pan-drug resistant Acinetobacter baumannii: a case series study. Infez Med. 2019;27:11–16. [PubMed] [Google Scholar]
- 12.Cascio A, Conti A, Sinardi L, Iaria C, Angileri FF, Stassi G, et al. Post-neurosurgical multidrug-resistant Acinetobacter baumannii meningitis successfully treated with intrathecal colistin. A new case and a systematic review of the literature. Int J Infect Dis. 2010;14:e572–e579. doi: 10.1016/j.ijid.2009.06.032. [DOI] [PubMed] [Google Scholar]
- 13.Chusri S, Singkhamanan K, Wanitsuwan W, Suphasynth Y, Kositpantawong N, Panthuwong S, et al. Adjunctive therapy of intravenous colistin to intravenous tigecycline for adult patients with non-bacteremic post-surgical intra-abdominal infection due to carbapenem-resistant Acinetobacter baumannii. J Infect Chemother. 2019;25:681–686. doi: 10.1016/j.jiac.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 14.Zalts R, Neuberger A, Hussein K, Raz-Pasteur A, Geffen Y, Mashiach T, et al. Treatment of Carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia: retrospective comparison between intravenous Colistin and intravenous ampicillin-Sulbactam. Am J Ther. 2016;23:e78–e85. doi: 10.1097/MJT.0b013e3182a32df3. [DOI] [PubMed] [Google Scholar]
- 15.De Pascale G, Montini L, Pennisi M, Bernini V, Maviglia R, Bello G, et al. High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care. 2014;18:R90. doi: 10.1186/cc13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;350:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed]
- 17.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raz-Pasteur A, Liron Y, Amir-Ronen R, Abdelgani S, Ohanyan A, Geffen Y, et al. Trimethoprim-sulfamethoxazole vs. colistin or ampicillin-sulbactam for the treatment of carbapenem-resistant Acinetobacter baumannii: a retrospective matched cohort study. J Glob Antimicrob Resist. 2019;17:168–172. doi: 10.1016/j.jgar.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Betrosian AP, Frantzeskaki F, Xanthaki A, Douzinas EE. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. J Inf Secur. 2008;56:432–436. doi: 10.1016/j.jinf.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 21.Khalili H, Shojaei L, Mohammadi M, Beigmohammadi MT, Abdollahi A, Doomanlou M. Meropenem/colistin versus meropenem/ampicillin-sulbactam in the treatment of carbapenem-resistant pneumonia. J Comp Eff Res. 2018;7:901–911. doi: 10.2217/cer-2018-0037. [DOI] [PubMed] [Google Scholar]
- 22.Liang CA, Lin YC, Lu PL, Chen HC, Chang HL, Sheu CC. Antibiotic strategies and clinical outcomes in critically ill patients with pneumonia caused by carbapenem-resistant Acinetobacter baumannii. Clin Microbiol Infect. 2018;24:908.e1–908.e7. doi: 10.1016/j.cmi.2017.10.033. [DOI] [PubMed] [Google Scholar]
- 23.López-Cortés LE, Cisneros JM, Fernández-Cuenca F, Bou G, Tomás M, Garnacho-Montero J, et al. GEIH/REIPI-Ab2010 group. Monotherapy versus combination therapy for sepsis due to multidrug-resistant Acinetobacter baumannii: analysis of a multicentre prospective cohort. J Antimicrob Chemother. 2014;69:3119–3126. doi: 10.1093/jac/dku233. [DOI] [PubMed] [Google Scholar]
- 24.Özvatan T, Akalın H, Sınırtaş M, Ocakoğlu G, Yılmaz E, Heper Y, et al. Nosocomial Acinetobacter pneumonia: treatment and prognostic factors in 356 cases. Respirology. 2016;21:363–369. doi: 10.1111/resp.12698. [DOI] [PubMed] [Google Scholar]
- 25.Pan S, Huang X, Wang Y, Li L, Zhao C, Yao Z, et al. Efficacy of intravenous plus intrathecal/intracerebral ventricle injection of polymyxin B for post-neurosurgical intracranial infections due to MDR/XDR Acinectobacter baumannii: a retrospective cohort study. Antimicrob Resist Infect Control. 2018;7:8. doi: 10.1186/s13756-018-0305-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sipahi OR, Mermer S, Demirdal T, Ulu AC, Fillatre P, Ozcem SB, et al. Tigecycline in the treatment of multidrug-resistant Acinetobacter baumannii meningitis: results of the Ege study. Clin Neurol Neurosurg. 2018;172:31–38. doi: 10.1016/j.clineuro.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 27.Trottier V, Namias N, Pust DG, Nuwayhid Z, Manning R, Marttos AC, Jr, et al. Outcomes of Acinetobacter baumannii infection in critically ill surgical patients. Surg Infect. 2007;8:437–443. doi: 10.1089/sur.2006.029. [DOI] [PubMed] [Google Scholar]
- 28.Fleischman RJ, Adams AL, Hedges JR, Ma OJ, Mullins RJ, Newgard CD. The optimum follow-up period for assessing mortality outcomes in injured older adults. J Am Geriatr Soc. 2010;58:1843–1849. doi: 10.1111/j.1532-5415.2010.03065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aggarwal R, Dewan A. Comparison of nephrotoxicity of Colistin with Polymyxin B administered in currently recommended doses: a prospective study. Ann Clin Microbiol Antimicrob. 2018;17:15. doi: 10.1186/s12941-018-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nation RL, Li J, Cars O, Couet W, Dudley MN, Kaye KS, et al. Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis. 2015;15:225–234. doi: 10.1016/S1473-3099(14)70850-3. [DOI] [PubMed] [Google Scholar]
- 31.Tsuji BT, Pogue JM, Zavascki AP, Paul M, Daikos GL, Forrest A, et al. International Consensus Guidelines for the Optimal Use of the Polymyxins: Endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP) Pharmacotherapy. 2019;39:10–39. doi: 10.1002/phar.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Valachis A, Samonis G, Kofteridis DP. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia: a systematic review and metaanalysis. Crit Care Med. 2015;43:527–533. doi: 10.1097/CCM.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 33.Jang JY, Kwon HY, Choi EH, Lee WY, Shim H, Bae KS. Efficacy and toxicity of high-dose nebulized colistin for critically ill surgical patients with ventilator-associated pneumonia caused by multidrug-resistant Acinetobacter baumannii. J Crit Care. 2017;40:251–256. doi: 10.1016/j.jcrc.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 34.Falagas ME, Bliziotis IA, Tam VH. Intraventricular or intrathecal use of polymyxins in patients with gram-negative meningitis: a systematic review of the available evidence. Int J Antimicrob Agents. 2007;29:9–25. doi: 10.1016/j.ijantimicag.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 35.Boisson M, Jacobs M, Grégoire N, Gobin P, Marchand S, Couet W, et al. Comparison of intrapulmonary and systemic pharmacokinetics of colistin methanesulfonate (CMS) and colistin after aerosol delivery and intravenous administration of CMS in critically ill patients. Antimicrob Agents Chemother. 2014;58:7331–7339. doi: 10.1128/AAC.03510-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Markantonis SL, Markou N, Fousteri M, Sakellaridis N, Karatzas S, Alamanos I, et al. Penetration of colistin into cerebrospinal fluid. Antimicrob Agents Chemother. 2009;53:4907–4910. doi: 10.1128/AAC.00345-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Q, Li W, Feng Y, Tao C. Efficacy and safety of polymyxins for the treatment of Acinectobacter baumannii infection: a systematic review and meta-analysis. PLoS One. 2014;9:e98091. doi: 10.1371/journal.pone.0098091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 2: Table S1. Summary of sensitivity analyses. Table S2. Summary of leave-one-out analysis on primary outcome.
Additional file 3. Funnel plot showing publication bias of 1-month mortality.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.