Abstract
Numerous studies have shown that macrophages can orchestrate the microenvironment from the early stage of wound healing to the later stages of scar formation. However, few reviews have highlighted the significance of macrophages during the formation of abnormal scars. The purpose of this review was to outline the polarization of macrophages from early to late stage of pathological scar formation, focusing on spatiotemporal diversity of M1 and M2 macrophages. In this review, the role of macrophages in the formation of hypertrophic scars and keloids is summarized in detail. First, an increased number of M2 cells observed before injuries are significantly associated with susceptibility to abnormal scar pathogenesis. Second, decreased expression of M1 at the early stage and delayed expression of M2 at the late stage results in pathological scar formation. Third, M2 cells are highly expressed at both the margin and the superficial region, which is consistent with the invasive property of keloids. Finally, this review helps to characterize strategies for the prediction and prevention of pathological scar formation.
Keywords: Hypertrophic scar, Keloid, Macrophages, Predisposition, Wound healing
Background
The normal wound healing response can be categorized into haemostasis, inflammation, proliferation and remodeling, and can result in scar formation. This delicate balance of healing processes can be impaired dramatically, resulting in a chronic wound or excessive abnormal scar formation. A persistent inflammatory phase and delayed wound healing lead to the formation of hypertrophic scars (HTS) [1,2]. Keloids may appear directly after wound injury or grow some years later from a mature scar [3]. The complexity of scar formation makes it difficult to summarize the process with a single explanation. The immune system has been shown to regulate atypical fibroblast proliferation, myofibroblast transformation [4] and collagen I accumulation [5] during abnormal scar formation.
The crucial roles of macrophages during skin repair and different healing stages have been well described [6]. Numerous studies have shown that macrophages can orchestrate the microenvironment from the early stage of wound healing to the late stage of scar formation [7]. The depletion of macrophages during different wound healing stages revealed that macrophages have intense impacts on stage-specific healing mechanisms [6, 8]. In a mouse model, macrophage influx at the early stage (0–4 days) of skin repair induces robust vascularized granulation tissue, myofibroblast differentiation and wound contraction [9]. During the intermediate stage (4–8 days) of healing response, macrophages can not stabilize vascular structures and transfer granulation tissue into scar tissue [6]. There is no impact of macrophages at the late stage (8–4 days) of the wound healing process [6].
The classical monocytes, which are CD14++CD16- [8], are derived from bone marrow and circulate in the blood [10]. In response to damage-associated molecular pattern molecules (DAMPs) or pathogen-associated molecular patterns (PAMPs), interleukin-1 (IL-1), IL-6, tumor necrosis factor-α (TNF-α) and chemokine C-C motif ligand (CCL2) [11], circulating monocytes are recruited into tissues. When there is an injury, the presence of inflammatory cytokines, such as TNF-α and interferon-γ (IFN-γ), facilitates the recruitment and adhesion of circulating monocytes to endothelium and translocation into the tissue space [12]. After entering the wound space, CD14+ monocytes transform into macrophages, which not only engulf the pathogens and cellular debris but also produce cytokines and stimulate collagen production and angiogenesis to initiate the healing processes [13, 14].
Depending on different microenvironments, macrophages can polarize into two major phenotypes. Monocytes polarize into classically activated M1 macrophages in the presence of IFN-γ, TNF-α, DAMPs and lipopolysaccharide (LPS) [15, 16]. These pro-inflammatory macrophages secrete cytokines, such as IL-1β, IL-6 and TNF-α [4], which are responsible not only for participating in immune reactions but also stimulating proliferation of fibroblasts and keratinocytes. The function of these M1 macrophages is to remove cellular debris from the wound [17]. Macrophages are considered plastic cells; they can change their phenotypes according to their local cytokine/chemokine microenvironments [18–20]. Generally, activated M2 macrophages can be produced by the stimulation of IL-4, IL-13 or apoptotic neutrophils. After activation, these M2 macrophages produce cytokines—such as platelet-derived growth factor (PDGF), transforming growth factor-β (TGF-β), insulin-like growth factor-1 (IGF-1) and vascular endothelial growth factor (VEGF)—and stimulate proliferation of keratinocytes and fibroblasts [21]. Specifically, M2 macrophages are now classified into four subgroups: M2a, M2b, M2c and M2d [22]. M2a macrophages, also known as wound healing macrophages, are stimulated by IL-4 and/or IL-13 and produce high levels of arginase-1 (Arg-1), PDGF, IGF-1 and other cytokines [23]. The M2a macrophages can produce collagen precursors to stimulate fibroblasts during tissue repair [12, 24]. They are also involved in the extracellular matrix (ECM) formation and angiogenesis [12, 24]. M2b macrophages, also known as regulatory macrophages, can be stimulated by toll-like receptors or IL-1 receptor ligands [12]. M2b macrophages produce high levels of IL-10 to suppress inflammation and secrete IL-6, TNF and different matrix metalloproteinases (MMPs) [7]. M2c macrophages, also known as pro-resolving macrophages due to their matrix remodeling ability [12], are stimulated by glucocorticoids, IL-10 and TGF-β [25]. They can secrete IL-10, IL-1β, MMP9 and TGF-β. M2d macrophages are activated by IL-6 and adenosine receptors and produce high levels of IL-10, TGF-β and VEGF [26]. They can also inhibit pro-inflammatory M1 macrophages by down-regulating TNF-α and IL-12 [27] (Fig. 1).
Figure 1.
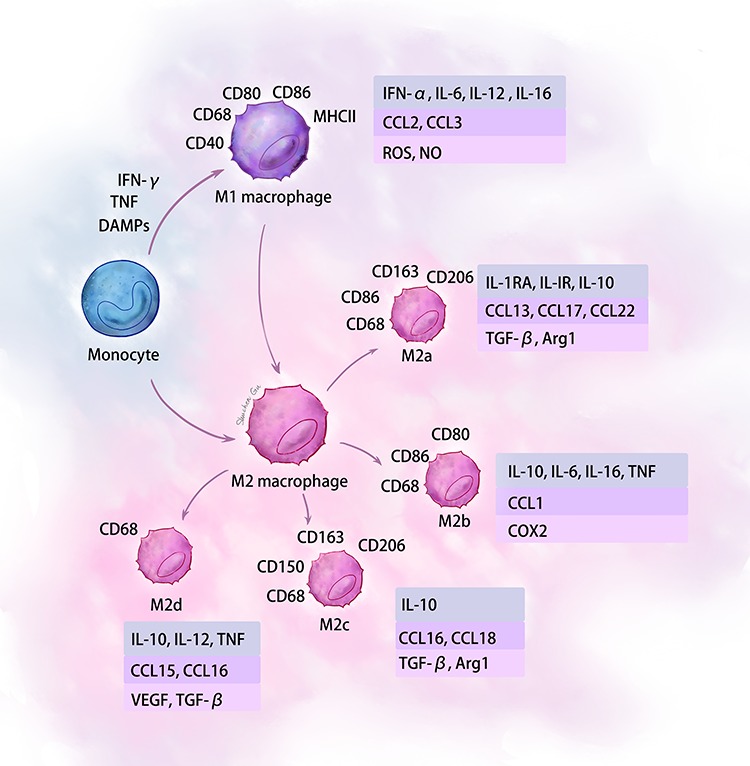
Summary of the polarization states and major cytokines and chemokines produced by macrophages. IFN-γ interferon gamma, DAMPs damage-associated pattern molecules, TNF tumor necrosis factor, IL interleukin, VEGF vascular endothelial growth factor, TGF-β transforming growth factor-beta, MHC major histocompatibility complex, NO nitric oxide, Arg arginase
Previous studies have focused on the different M1/M2 distributions during abnormal scar formation [28, 29]. Increasing evidence shows that M1 is the predominant macrophage population in the early stage of scar formation (inflammatory and early proliferative stage), whereas M2 is the main population in the late stage of scar formation (late proliferative and remodeling stage) [30]. The temporal changes in M1/M2 distribution occur in various tissue repair processes and fibrosis, including skin [30], kidney [31] and liver [32]. After skin injury, Jin et al. demonstrated that M1-associated genes and proteins were less elevated in keloid tissues than M2-associated genes and proteins [33]. Additionally, Li et al. suggested that infiltrated M2 was more commonly present than M1 in keloid tissues [34]. However, simply considering the polarization of M1 to M2 macrophages does not explain the role of macrophages in abnormal scar formation. It is important to consider other characteristics that macrophages show during the formation of abnormal scars, which, in turn, may lead to various clinical strategies.
In this article, we review the characteristics of macrophages during the formation of HTS and keloid. In addition, the significant roles of macrophages in scar predisposition are described. Finally, the polarization of macrophages from the early stage to the late stage of HTS formation and the recent studies examining spatial variances of keloids are summarized.
Review
Role of macrophages in scar predisposition
The formation of abnormal scars is promoted by systemic factors, including genetics, sex hormones, hypertension and smoking; and by local stimuli, such as mechanical tension and inflammation [35]. Genetics has a strong relation to keloid predisposition [36]. For example, people of darker skin complexion and those with a family history of keloid have a higher predisposition for keloid occurrence [37]. Inflammation plays a critical role in scar formation, which is not only affected by the post-wound microenvironment but also by the number and subtypes of macrophages presenting in the tissue before the injury. In a prospective study [38], the authors took biopsies immediately after the incision and investigated baseline M2 macrophages in the local wound healing milieu. During the follow-up period, the group of patients who developed HTS had higher baseline M2 macrophages (CD68+, CD206+) compared with patients who developed normal scars [38] (Fig. 2a). While studies on preoperative macrophages in keloid formation are still insufficient, infiltrated M2 macrophages have been primarily found in keloid tissues compared to normal skin [29]. The possible explanation for the association between increased preinjury M2 macrophages and HTS formation could be that tissue-resident macrophages altered the immune microenvironment to suppress adaptive immune responses, including M1 macrophages, to favour HTS formation [39]. Tissue-resident macrophages are extremely heterogeneous, which are determined by tissue-specific niche through paracrine signaling, cell-to-cell interaction and local factors, such as inflammation [40]. During wound healing, the fundamental role of tissue-resident macrophages in immune surveillance and induction of inflammation has been well described. However, the heterogeneity of tissue-resident macrophages pre- and post-injury, as well as their corresponding contributions to abnormal scar formation, should be well investigated in the future.
Figure 2.
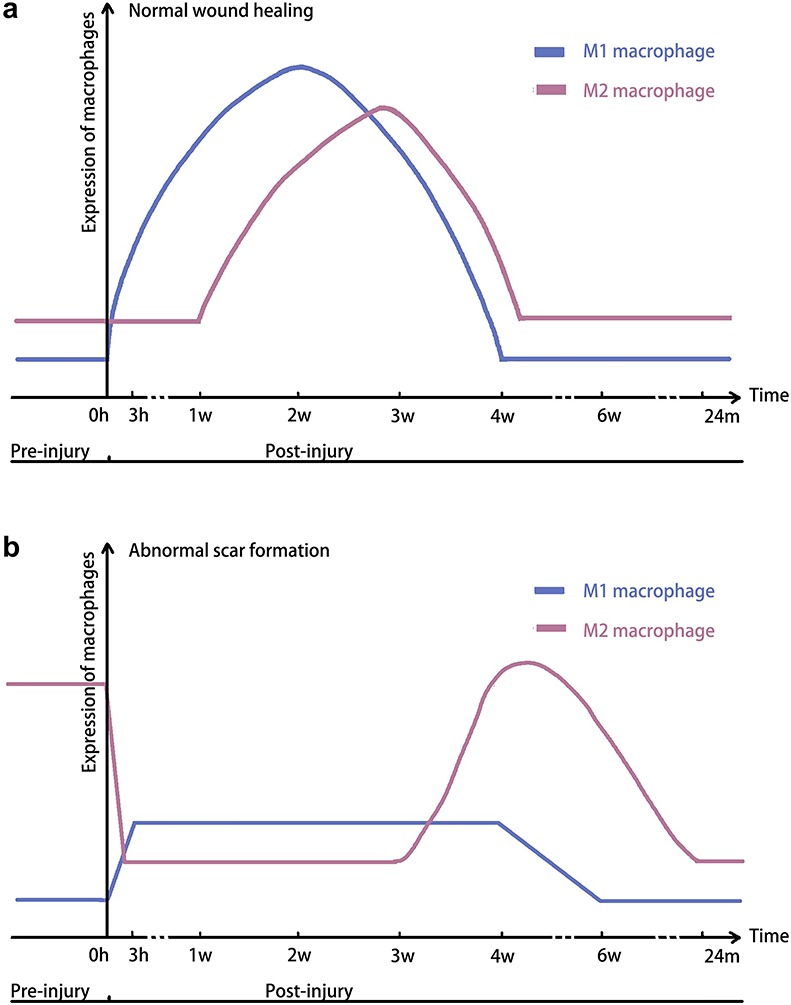
Comparison of macrophage polarization during the wound healing and abnormal scar formation. (a) The number of M1 and M2 macrophages expressed during normal wound healing. (b) The number of M1 and M2 macrophages expressed during abnormal scar formation
Furthermore, the incidence of keloid in different body sites is related to the number and subtypes of macrophages. Previously, Butzelaar et al. measured the macrophages of skin samples from both predilection sites (such as earlobes, mandible, neck and shoulders) and non-predilection sites (such as the upper eyelid, cheek and abdomen) [41]. The results demonstrated that significantly lower numbers of M1 macrophages (CD40+) were observed at the predilection sites of keloid formation, but equal numbers of M2 macrophages (CD163+) were observed at the predilection sites and non-predilection sites [41]. The existence of an anti-inflammatory microenvironment before an injury is one major distinction of predilection and non-predilection sites of keloid formation. Therefore, although the mechanisms underlying scar predisposition have not been thoroughly elucidated, it seems that the increased number of M2 macrophages and decreased/equal number of M1 macrophages play a vital role in scar susceptibility [7, 42, 43]. In the future, the parameters of M1/M2 macrophage balance in normal skin should be established and these parameters could be used as potential predicting factors for risk evaluation for pathological scar formation.
To prevent the formation of pathological scars, changing the polarization of macrophages before or after injuries may be a novel clinical strategy. In malignant tumors, one of the characteristics is the polarization of tumor-associated macrophages from M1-like macrophages (pro-immune) to M2-like macrophages (immune-suppressive). In tumor immunotherapy, macrophages can be polarized into M1 phenotype by reprogramming M0- or M2-like tumor-associated macrophages [44], by targeting micro RNAs that are relevant in macrophage activation and function [45, 46] or by promoting the expression of M1 cytokines which could regulate polarization thorough a feedback loop [47].
The previous study has shown that there were some similarities between tumors, wound and scars [48]. Currently, there is still a lack of clinical trials focusing on converting the polarization of macrophages to interfere with the formation of abnormal scars. In the future, converting the polarization of macrophages from M2 to M1 before surgery may transform the anti-inflammatory microenvironment into a pro-inflammatory milieu to prevent abnormal scar formation.
Role of macrophages in the early stage of pathological scar formation
In the process of wound healing, M1 macrophages are necessary for initiating inflammatory phases by killing pathogens and scavenging debris, while M2 macrophages participate in the proliferation and remodeling stage [49].
During normal tissue repair, the early stage of normal scar formation is marked by high expression of M1 cytokines and biomarkers. In a study, biopsies were taken from human traumatic and burn injury tissues and the number of M1 macrophages started to increase at 0–2 days, peaked at 7–14 days and declined significantly at 14–28 days post-injury [50] (Fig. 2a). However, the imbalance of macrophage polarization during wound healing results in HTS formation [51]. Within the first 3 hours after surgical incision, the levels of M1 inflammatory proteins in HTS, including IL-6 and CCL2, were significantly lower compared to those in normal scar tissue [38]. Additionally, the pro-inflammatory cytokine messenger RNA levels, such as TNF-α, CCL-2 and IL-1β, remained low during HTS formation [52]. Therefore, due to the decreased expression of M1 cytokines, a reduced early inflammation stage may result in the formation of HTS, rather than a normal scar (Fig. 2b).
Role of macrophages in the later stage of pathological scar formation
Considering the different properties of M1 and M2 macrophages, the phases of HTS formation may be divided into the early stage, which is characterized by a low number of M2 macrophages, and the late stage, in which the tissue is heavily infiltrated by M2 macrophages [38, 52].
Previously, one study has shown that the density of M2 macrophages in normal scarring remained low at the early stage (0–14 days) and peaked at 14–28 days [50] (Fig. 2a). Compared to normal scar formation, HTS progression is related to the delayed and prolonged expression of both M2 macrophages and anti-inflammatory cytokines produced by them. Compared with healthy individuals, Liu et al. found that the peripheral blood mononuclear cells, which expressed immature M2 marker (CD204), were highly elevated in the blood of burn patients at 2 weeks [53]. Although these cells can upregulate pro-fibrotic factors, their ability may still differ from mature M2 macrophages. Additionally, van den Broek et al. found that CD163+ M2 macrophages could only be detected at 4–6 weeks post-injury in HTS-forming patients compared to 2 weeks in normal-scar-forming patients [52]. Moreover, as HTS formation progressed, the concentrations of IL-10 and IL-1RN continually reduced and reached normal levels in 6 months. The expression of Arg-1 and CD206 decreased, and the number of M2 macrophages (CD68+ and CMAF+) returned to baseline 24 months after injury [50] (Fig. 2b). These findings are consistent with those of previous studies in animal models. Zhu et al. established a human HTS-like nude mouse model by grafting human skin on the mice [54]. In the xenografted mice, M2 macrophages (F4/80+ and Arg-1+) and the specific cytokines, IL-10 and IL-1α, showed delayed expression compared to the autograft group.
Generally, M2 macrophages significantly increase at 4 weeks post-injury and return to baseline at 8 weeks without further recurrence [55]. However, if the number of M2 macrophages remains at a high level, it will lead to either HTS or keloid. Previously, some researchers have held that the concept of HTS and keloids may be the same pathological disease at different temporal points [56]. However, through systematically comparing clinical, histopathologic, biochemical and molecular differences between keloid and HTS, it is becoming more important to recognize differences between keloid and HTS as well as to treat keloid as a separate entity different from HTS [57]. The difference in spatiotemporal manners of macrophages in HTS and keloid need to be further elucidated.
TGF-β, one of the major growth factors produced by M2 macrophages, has been reported to play a significant role in HTS formation. However, the expression of TGF-β did not consistently decrease in parallel with the decrease of M2 macrophages, indicating that M2 macrophages were not the only source of TGF-β secretion [54].
Role of macrophages in different spatial variables of pathological scars
Instead of over-proliferation at the wound center, the keloid is characterized by invasive behavior from the wound margin into surrounding normal skin tissue [58]. Various spatial variables highlight the diversity within keloid. From the macroscopic perspective, the center of keloid tissue is paler and more shrunken compared to its erythematous and swollen margin site [59]. Microscopically, epidermal thickness, collagen ratios and distribution, fibroblast density and the infiltration of inflammatory cells all differ within keloid [60, 61]. Bagabir et al. suggested that keloid should be horizontally divided into three lesional sites: intralesional (center), perilesional (margin) and extralesional (adjacent normal skin) [29]. Vertically, according to histology, keloid was composed of epidermis, superficial dermis, mid-dermis and deep dermis [62]. However, the spatial role of macrophages in different sites both horizontally and vertically should be further studied.
M2 macrophages are thought to secrete profibrotic factors, such as TGF-β, to promote wound fibrosis. Bagabir et al. presented that M2 macrophages (CD163+) were markedly increased in perilesional sites, which was also consistent with the invasive behavior of the keloid margin [29, 63]. In the superficial dermis region, a heavy cellular infiltrate was found, including active fibroblasts, T cells, CD68+ macrophages and CD163+ macrophages [28, 29], together with an increased number of horizontal collagen fibers and microvessels [64]. Perivascular inflammation was observed around the microvessels of the subpapillary and papillary dermis in the keloid lesion [65].
In summary, current studies mainly focus on the distribution and quantity of macrophages. Further exploration of macrophage subtypes is still needed. To find an effective treatment for keloid, future studies should further investigate the M1/M2 macrophage distribution and corresponding cytokine changes in keloids.
Conclusions
This review demonstrated that the number of M2 macrophages presenting in the tissue pre-injury could serve as a local prognostic factor for the formation of pathological scars. This M2-favouring microenvironment before injuries inhibits adaptive immune responses and results in HTS or keloids. After injury occurred, the reduced expression of M1 cytokines in early stage, and the delayed and prolonged expression of both M2 and anti-inflammatory cytokines in later stage may result in the formation of HTS, rather than a normal scar. Other immune cells, such as T cells, B cells, mast cells and neutrophils, were not summarized in this review. There have been few pieces of research focused on the spatiotemporal diversity of these immune cells. Besides, different immune cells might collaborate during abnormal scar formation, which needs to be addressed in future studies. Therefore, future studies should concentrate on the interaction of different immune cells during pathological scar formation.
Abbreviations
HTS, hypertrophic scar; DAMP, damage-associated molecular pattern; PAMP, pathogen-association molecular pattern; IL, interleukin; TNF-α, tumor necrosis factor-α; CCL2, chemokine C-C motif ligand; IFN-γ, interferon-γ; LPS, lipopolysaccharide; PDGF, Platelet-derived growth factor; TGF-β, transforming growth factor-beta; IGF-1, insulin-like growth factors-1; VEGF, vascular endothelial growth factor; Arg-1, argnase-1; ECM, extracellular matrix; MMP, mitochondrial membrane potential
Funding
This work was supported by grants from the Youth Doctor Collaborative Innovation Team Project (QC201803) of Shanghai Ninth People’s Hospital; the Shanghai Jiaotong University School of Medicine; the Shanghai Youth Top-Notch Talent Program (for ZW); the National Natural Science Foundation of China (81772086, 81701901); the Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant (20161424); Shanghai “Rising Stars of Medical Talent” Youth Development Program (Outstanding Youth Medical Talents); and the Shanghai Jiaotong University “Chenxing” Youth Development Program (Associate Professor Type A).
Authors’ contributions
All authors read and approved the final manuscript. Xiangwen Xu and Shuchen Gu contributed equally to the review, and should be viewed as co-first authors.
Conflicts of interest
None declared.
References
- [1]. Gangemi EN, Gregori D, Berchialla P, Zingarelli E, Cairo M, Bollero D, et al. Epidemiology and risk factors for pathologic scarring after burn wounds. Arch Facial Plast Surg. 2008;10:93–102. [DOI] [PubMed] [Google Scholar]
- [2]. Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN. Hypertrophic scarring: The greatest unmet challenge after burn injury. Lancet 2016;388:1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up-to-date approach to manage keloids and hypertrophic scars: A useful guide. Burns 2014;40:1255–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. van der Veer WM, Bloemen MCT, Ulrich MMW, Molema G, van Zuijlen PP, Middelkoop E, et al. Potential cellular and molecular causes of hypertrophic scar formation. Burns 2009;35:15–29. [DOI] [PubMed] [Google Scholar]
- [5]. Trace AP, Enos CW, Mantel A, Keloids HVM. Hypertrophic scars: A Spectrum of clinical challenges. Am J Clin Dermatol. 2016;17:201–223. [DOI] [PubMed] [Google Scholar]
- [6]. Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Müller W, et al. Differential roles of macrophages in diverse phases of skin repair. 2010;184:3964–3977. [DOI] [PubMed] [Google Scholar]
- [7]. Hesketh M, Sahin KB, West ZE, Murray RZ. Macrophage phenotypes regulate scar formation and chronic wound healing. Int J Mol Sci. 2017;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Zhu Z, Ding J, Ma Z, Iwashina T, Tredget EE. Systemic depletion of macrophages in the subacute phase of wound healing reduces hypertrophic scar formation. Wound Repair Regen. 2016;24:644–656. [DOI] [PubMed] [Google Scholar]
- [9]. Mirza R, DiPietro LA, Koh TJ. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175:2454–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Jakubzick CV, Randolph GJ, Henson PM. Monocyte differentiation and antigen-presenting functions. Nat Rev Immuno. 2017;17:349–362. [DOI] [PubMed] [Google Scholar]
- [11]. Vishwakarma A, Bhise NS, Evangelista MB, Rouwkema J, Dokmeci MR, Ghaemmaghami AM, et al. Engineering Immunomodulatory biomaterials to tune the inflammatory response. Trends Biotechnol. 2016;34:470–482. [DOI] [PubMed] [Google Scholar]
- [12]. Krzyszczyk P, Schloss R, Palmer A, Berthiaume F. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol. 2018;1:1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Baum CL, Arpey CJ. Normal cutaneous wound healing: Clinical correlation with cellular and molecular events. Dermatol Surg. 2005;31:674–686. [DOI] [PubMed] [Google Scholar]
- [14]. Limandjaja GC, Waaijman T, Roffel S, Niessen FB, Gibbs S. Monocytes co-cultured with reconstructed keloid and normal skin models skew towards M2 macrophage phenotype. Arch Dermatol Res 2019;311:615–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Martin P, Leibovich SJ. Inflammatory cells during wound repair: The good, the bad and the ugly. Trends Cell Biol 2005;15:599–607. [DOI] [PubMed] [Google Scholar]
- [16]. Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–870. [DOI] [PubMed] [Google Scholar]
- [17]. Vogel DYS, Glim JE, Stavenuiter AWD, Breur M, Heijnen P, Amor S, et al. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology. 2014;219:695–703. [DOI] [PubMed] [Google Scholar]
- [18]. Boyette LB, Macedo C, Hadi K, Elinoff BD, Walters JT, Ramaswami B, et al. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS One. 2017;12:e0176460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Amini-Nik S, Cambridge E, Yu W, Guo A, Whetstone H, Nadesan P, et al. β-Catenin-regulated myeloid cell adhesion and migration determine wound healing. J Clin Invest. 2014;124:2599–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Sinha M, Sen CK, Singh K, Das A, Ghatak S, Rhea B, et al. Direct conversion of injury-site myeloid cells to fibroblast-like cells of granulation tissue. Nat Commun. 2018;9:936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Verreck FAW, de Boer T, Langenberg DML, van der Zanden L, Ottenhoff THM. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-gamma- and CD40L-mediated costimulation. J Leukoc Biol 2006;79:285–293. [DOI] [PubMed] [Google Scholar]
- [22]. Wilgus TA. Immune cells in the healing skin wound: Influential players at each stage of repair. Pharmacol Res. 2008;58:112–116. [DOI] [PubMed] [Google Scholar]
- [23]. Ogle ME, Segar CE, Sridhar S, Botchwey EA. Monocytes and macrophages in tissue repair: Implications for immunoregenerative biomaterial design. Exp Biol Med. 2016;241:1084–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Garash R, Bajpai A, Marcinkiewicz BM, Spiller KL. Drug delivery strategies to control macrophages for tissue repair and regeneration. Exp Biol Med (Maywood). 2016;241:1054–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Ferrante CJ, Leibovich SJ. Regulation of macrophage polarization and wound healing. Adv Wound Care. 2012;1:10–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Sunakawa Y, Stintzing S, Cao S, Heinemann V, Cremolini C, Falcone A, et al. Variations in genes regulating tumor-associated macrophages (TAMs) to predict outcomes of bevacizumab-based treatment in patients with metastatic colorectal cancer: Results from TRIBE and FIRE3 trials. Ann Oncol. 2015;26:2450–2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Shaker SA, Ayuob NN, Hajrah NH. Cell talk: A phenomenon observed in the keloid scar by immunohistochemical study. Appl Immunohistochem Mol Morphol. 2011;19:153–159. [DOI] [PubMed] [Google Scholar]
- [29]. Bagabir R, Byers R, Chaudhry I, Müller W, Paus R, Bayat A. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br J Dermatol. 2012;167:1053–1066. [DOI] [PubMed] [Google Scholar]
- [30]. Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol 2013;93:875–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi B-S, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22:317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Tacke F, Zimmermann HW. Macrophage heterogeneity in liver injury and fibrosis. J Hepatol. 2014;60:1090–1096. [DOI] [PubMed] [Google Scholar]
- [33]. Jin Q, Gui L, Niu F, Yu B, Lauda N, Liu J, et al. Macrophages in keloid are potent at promoting the differentiation and function of regulatory T cells. Exp Cell Res. 2018;362:472–476. [DOI] [PubMed] [Google Scholar]
- [34]. Li X, Wang Y, Yuan B, Yang H, Qiao L. Status of M1 and M2 type macrophages in keloid. Int J Clin Exp Pathol. 2017;10:11098–11105. [PMC free article] [PubMed] [Google Scholar]
- [35]. Keloid OR. Hypertrophic scars are the result of chronic inflammation in the reticular dermis. Int J Mol Sci. 2017;18:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Shih B, Bayat A. Genetics of keloid scarring. Arch Dermatol Res. 2016;25:319–339. [DOI] [PubMed] [Google Scholar]
- [37]. Ii DAG. Current understanding of the genetic causes of Keloid formation. J Invest Dermatol. 2017;18:S50–S53. [DOI] [PubMed] [Google Scholar]
- [38]. Butzelaar L, Schooneman DPM, Soykan EA, Talhout W, Ulrich MMW, van den Broek LJ, et al. Inhibited early immunologic response is associated with hypertrophic scarring. Exp Dermatol. 2016;25:797–804. [DOI] [PubMed] [Google Scholar]
- [39]. Rodero MP, Khosrotehrani K. Skin wound healing modulation by macrophages. Int J Clin Exp Pathol. 2010;3:643–653. [PMC free article] [PubMed] [Google Scholar]
- [40]. Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41]. Butzelaar L, Niessen FB, Talhout W, Schooneman DPM, Ulrich MM, Beelen RHJ, et al. Different properties of skin of different body sites: The root of keloid formation? Wound Repair Regen. 2017;25:758–766. [DOI] [PubMed] [Google Scholar]
- [42]. Delavary B, Glim J, Niessen F, Middelkoop E, van Egmond M, Beelen R. Macrophage phenotype and function in hypertrophic and normotrophic scars. Wound Repair Regen. 2011;19:A85. [Google Scholar]
- [43]. Prasai A, El Ayadi A, Jay J, Herndon DN, Finnerty CC. Changes in macrophage phenotype in hypertrophic scarring population. Wound Repair Regen. 2018;26:A3. [Google Scholar]
- [44]. Xie L, Yang Y, Meng J, Wen T, Liu J, Xu H. Cationic polysaccharide spermine-pullulan drives tumor associated macrophage towards M1 phenotype to inhibit tumor progression. Int J Biol Macromol 2019;15:1012–1019. [DOI] [PubMed] [Google Scholar]
- [45]. Ma S, Liu M, Xu Z, Li Y, Guo H, Ge Y, et al. A double feedback loop mediated by microRNA-23a/27a/24-2 regulates M1 versus M2 macrophage polarization and thus regulates cancer progression. Oncotarget. 2016;7:13502–13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Moore CS, Rao VTS, Durafourt BA, Bedell BJ, Ludwin SK. Bar-or a, et al. MiR-155 as a multiple sclerosis-relevant regulator of myeloid cell polarization. Ann Neurol. 2013;74:709–720. [DOI] [PubMed] [Google Scholar]
- [47]. Lin L, Lin H, Wang L, Wang B, Hao X, Shi Y. MiR-130a regulates macrophage polarization and is associated with non-small cell lung cancer. Oncol Rep. 2015;34:3088–3096. [DOI] [PubMed] [Google Scholar]
- [48]. Gal P, Varinska L, Faber L, Novak S, Szabo P, Mitrengova P, et al. How Signaling molecules regulate tumor microenvironment: Parallels to wound repair. Molecules. 2017;22:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RHJ. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–762. [DOI] [PubMed] [Google Scholar]
- [50]. Chen L, Wang J, Li S, Yu Z, Liu B, Song B, et al. The clinical dynamic changes of macrophage phenotype and function in different stages of human wound healing and hypertrophic scar formation. Int Wound J 2019;16:360–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Boyce DE, Ciampolini J, Ruge F, Murison MS, Harding KG. Inflammatory-cell subpopulations in keloid scars. Br J Plast Surg. 2001;54:511–516. [DOI] [PubMed] [Google Scholar]
- [52]. van den Broek LJ, van der Veer WM, de Jong EH, Gibbs S, Niessen FB. Suppressed inflammatory gene expression during human hypertrophic scar compared to normotrophic scar formation. Exp Dermatol. 2015;24:623–629 [DOI] [PubMed] [Google Scholar]
- [53]. Liu H, Ding J, Ma Z, Zhu Z, Shankowsky HA, Tredget EE. A novel subpopulation of peripheral blood mononuclear cells presents in major burn patients. Burns 2015;41:988–1007. [DOI] [PubMed] [Google Scholar]
- [54]. Zhu Z, Ding J, Ma Z, Iwashina T, Tredget EE. The natural behavior of mononuclear phagocytes in HTS formation. Wound Repair Regen. 2016;24:14–25. [DOI] [PubMed] [Google Scholar]
- [55]. Ud-Din S, McGeorge D, Bayat A. Immune cell activity in human cutaneous wound healing and skin scarring: A multiple time point immunohistochemical study. Wound Repair Regen 2019;27:A16. [Google Scholar]
- [56]. Adeyemi-Doro HO. Keloids: The natural history. Afr J Med Med Sci. 1976;5:93–100. [PubMed] [Google Scholar]
- [57]. Kose O, Waseem A. Keloids and hypertrophic scars: Are they two different sides of the same coin? Dermatol Surg. 2008;34:336–346. [DOI] [PubMed] [Google Scholar]
- [58]. Mari W, Alsabri SG, Tabal N, Younes S, Sherif A, Simman R. Novel insights on understanding of Keloid scar: Article review. J Am Coll Clin Wound Spec. 2015;7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59]. Jumper N, Hodgkinson T, Paus R, Bayat A. Site-specific gene expression profiling as a novel strategy for unravelling keloid disease pathobiology. PloS one. 2017;12:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60]. Syed F, Ahmadi E, Iqbal SA, Singh S, McGrouther DA, Bayat A. Fibroblasts from the growing margin of keloid scars produce higher levels of collagen i and III compared with intralesional and extralesional sites: Clinical implications for lesional site-directed therapy. Br J Dermatol. 2011;164:83–96. [DOI] [PubMed] [Google Scholar]
- [61]. Huang C, Akaishi S, Hyakusoku H, Ogawa R. Can the clinically separated hypertrophic scar and keloid be successive under the microscope? - a fibroproliferative skin disorder hypothesis. Wound Repair Regen. 2011;19:A27. [Google Scholar]
- [62]. Jiao H, Zhang T, Fan J, Xiao R. The superficial dermis may initiate keloid formation: Histological analysis of the keloid dermis at different depths. Front Physiol. 2017;7:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63]. Bagabir R, Byers R, Chaudhry I, Muller W, Paus R, Bayat A. Site-specific immunophenotyping of keloid disease reveals the presence of keloid-associated lymphoid tissue (KALT). Wound Repair Regen. 2012;20:1053–1066. [DOI] [PubMed] [Google Scholar]
- [64]. Huang C, Akaishi S, Hyakusoku H, Ogawa R. Are keloid and hypertrophic scar different forms of the same disorder? A fibroproliferative skin disorder hypothesis based on keloid findings. Int Wound J. 2014;11:517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65]. Bux S, Madaree A. Keloids show regional distribution of proliferative and degenerate connective tissue elements. Cells Tissues Organs. 2010;191:213–234. [DOI] [PubMed] [Google Scholar]


