Abstract
Endometriosis, a condition in which uterine tissue grows outside the uterus, is a debilitating disease, affecting millions of women and costing the United States approximately $78 billion annually in pain-related disability. It is also the leading cause of chronic pelvic pain (CPP), which is often unresponsive to existing treatments. Adolescent women with the disease are at particular risk as there are often significant diagnostic delays, which in turn can exacerbate pain. Research and treatment guidelines for adolescents with endometriosis are largely based on studies for adult women due to the limited number of studies focusing on adolescents. The current paper critically reviews the literature as it pertains to endometriosis pathophysiology, mechanisms contributing to CPP, and treatment implications and recommendations with a focus on gaps related to adolescents.
Keywords: Endometriosis, Chronic pelvic pain, Inflammation, Nerve, Brain, Psychology, Treatment, Adolescence
1. Introduction
Endometriosis is a debilitating and incurable disease that is characterized by the presence of extrauterine endometrial glands and stroma anywhere outside of the uterine cavity and which affects 1 out of 10 women during the reproductive age (Klein et al., 2014). The subsequent inflammation and fibrosis can result in persistent pain, dyspareunia, infertility, and significant disruption of quality of life (Vercellini et al., 2014). The economic burden associated with endometriosis has been considered to be equivalent to other chronic diseases (e.g., diabetes, Crohn’s disease, Rheumatoid Arthritis (Simoens et al., 2012)) and is associated with lower health-related quality of life (Soliman et al., 2017). Details of the pathogenesis of the disease are considered elsewhere (Burney and Giudice, 2012). However, little is known about this disease in adolescent women, which is problematic given that earlier detection could yield better outcomes and change the trajectory of this debilitating condition from a disease, chronic pain, and quality of life standpoint.
Endometriosis is the leading cause of chronic pelvic pain (CPP), which is defined as noncyclic pain at or below the umbilicus of at least 3–6 months duration that interferes with daily activities (Powell, 2014). One-third of adolescents with CPP have endometriosis (Kontoravdis et al., 1999; Doyle et al., 2009), while prevalence rates for adult women range from 64 to 82% (Parazzini et al., 2017). For women who undergo surgery for endometriosis, up to 30 % of women report no postoperative improvement in pain and the degree of short-term pain improvement among the remaining patients varies (Abbott et al., 2004). Despite its high prevalence and negative effects, little is known about the pathophysiology underlying the development and persistence of CPP in endometriosis. Interestingly, endometriosis lesions are an incidental finding in 7–10 % of abdominal and pelvic surgeries and 20–25 % of women with endometriosis are asymptomatic (Bulletti et al., 2010; Davis et al., 1993; Divasta et al., 2007; Stavroulis et al., 2006; Templeman, 2009; Tandoi et al., 2011) indicating that there is a subset of women with endometriosis who do not experience pelvic pain.
Multiple studies have determined that endometriosis within the adolescent population requires different considerations for treatment as it present differently. Studies on the effects of surgical treatment for adolescents are insufficient, though the treatment may effective for pain reduction. There are few studies that emphasize that complete laparoscopic excision can significantly reduce the recurrence rates of endometriosis in adolescents (Yeung et al., 2017). The largest prospective study of adolescents with endometriosis did not find disease reoccurrence (diagnosed visually or histologically) after complete laparoscopic excision of the disease in teenagers at a repeat laparoscopy for pain (Tandoi et al., 2011). There is no consensus within the field that adjuvant medical treatments are necessary for all adolescent patients nor that long-term problems such as disease recurrence, infertility, or chronic pain may be prevented (Tandoi et al., 2011). The European Society for Human Reproduction and Embryology (ESHRE) has provided guidelines on treatment for clinicians, which include counseling women presenting with endometriosis associated symptoms (e.g. dyspareunia, infertility, CPP), as well as providing known successful treatments of these symptoms, such as hormonal contraceptives, even if the cause is unknown (Dunselman et al., 2014). This is partially due to the invasiveness of laparoscopic surgery, as well as the ease of prescribing hormonal contraceptives, and its dual purpose of preventing pregnancy. This empirical treatment is especially common in adolescents with pelvic pain and dysmenorrhea (Yeung et al., 2017). However, the ESHERA has noted that recommending treatment of the symptoms instead of surgery for young women presenting with endometriosis symptoms may cause longer delays in diagnosis (Dunselman et al., 2014). Approximately 80 % of adolescent girls with CPP not responding to conventional medical therapy have endometriosis (Yeung et al., 2017). As surgery is currently the main procedure for diagnosing, this may result in young women carrying the painful condition into adulthood. Additionally, it has been shown that using hormonal contraceptives in adolescents to treat of primary dysmenorrhea could be indicative of the diagnosis of deep endometriosis in later life (Chapron et al., 2011). The paradox of recommending empirical treatment to symptomatic adolescents might be perpetuating the significant delay in diagnosing endometriosis.
Despite high prevalence and disease burden, the precise pathophysiology of endometriosis, especially the association with CPP, is complex and is a combination of interacting processes that remain a mystery. However, the development of new technologies has provided progress in understanding the many intrinsic molecular mechanisms in the development of endometriosis, with progenitor and stem cells of the eutopic endometrium as the starting players and endometriotic lesions as the final pathomorphological trait. Baranov et al. (2018) hypothesized the existence of an endometriosis development genetic program that govern the origin of endometrium stem cells programmed for endometriosis, their transition (metaplasia) into mesenchymal stem cells, and their invasion of the peritoneum and progression to endometriotic lesions. Complex genomic and epigenetic interactions at different stages of the endometriosis process result in different forms of the disease, with specific features and clinical manifestations (Baranov et al., 2018; Greene et al., 2016; Bulun et al., 2015). Endometriosis and associated pain is complex (Fig. 1). The focus of the current paper is to outline the gaps the current literature on adult women with endometriosis and evaluate future opportunities for research going forward by applying what is known to adolescent women with endometriosis and CPP.
Fig. 1.
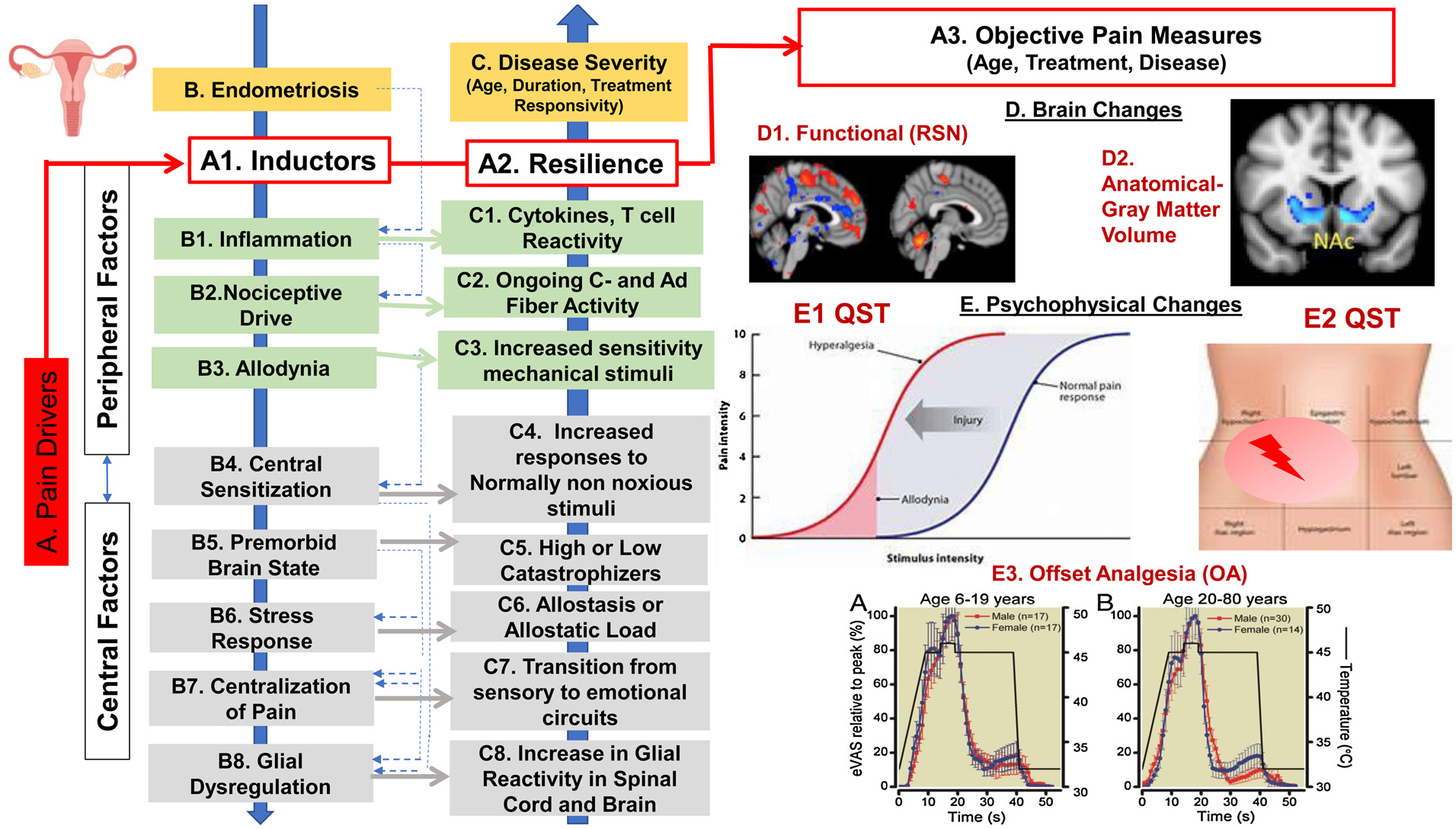
Mechanisms Contributing to Chronic Pain in Patients with Endometriosis.
This figure describes the multiple factors impacting pain in endometriosis, specifically the pain drivers, peripheral and central factors as either inductors or protectors against pain development, brain changes, and psychophysical changes. A. Pain Drivers including inductors of pain and resiliency factors impact and predict objective pain measures (red). B. Disease & C. Disease Severity factors are influenced by both peripheral factors (B1-B3) and central factors (B4-B8) that can be either inductors of pain or protective against the development of chronic pain. D. Brain Changes, which can include assessment of (1) functional – using resting state measures (RSN) (D1) and structural (D2) – gray matter volume (unpublished data from our group). E. Psychophysical Changes- Quantitative Sensory Testing (QST) can be used to produce measures of ongoing Peripheral Nervous System (PNS) and Central Nervous System (CNS) Sensitization (E1-E2). Offset Analgesia (OA) (E3) can be used for measures of CNS Modulatory Responsivity such as normal or intact OA (E3A- in male vs female volunteers ages 6–19 years; including rapid and large decrease in pain levels with a small decrease in pain stimulus, and Disrupted OA (E3B in males vs. female volunteers ages 20–80 years), including minimal decrease in pain levels with a small decrease in pain stimulus (Niesters et al., 2011). Responses for both E3A & E3B are percentage of peak response.
2. Beyond pain – sensation, functioning, fertility, and emerging comorbidities
2.1. The physical pain
While endometriosis is the leading cause of CPP; the distinction between pain that is expected with menstruation, pain that is expected in the context of an inflammatory disease, and pain that is exacerbated due to a myriad of poorly understood factors is unclear and results in diagnostic uncertainty and a poor prognosis.
What do we know about the origin of pain in endometriosis? One interesting advantage of the disease in humans is that the diagnosis is dependent on biopsy thus conferring a significant opportunity to define pathological processes in the human condition. There are a number of putative processes that include: (1) Ectopic: Endometriosis is found in the peritoneum, ovaries and rectovaginal space; (2) Invasiveness: Endometrial lesions can deeply infiltrate the intestines (Abrao et al., 2015), which may in turn affect nerves resulting in nerve entrapment (Medina and Lebovic, 2009); and (3) Inflammatory: Endometriosis is present in extrauterine locations that is estrogen dependent, thus an inflammatory process may affect normal nerves or damage nerves to produce a state dependent level of peripheral sensitization. Unfortunately, despite understanding these processes, there has been very little research into the mechanisms contributing to CPP associated with endometriosis relative to other diseases associated with chronic pain. Specifically, pain levels are not part of the existing classification systems for endometriosis (Haas et al., 2011), which is a missed opportunity given the clinical presentation of pain with this disease (Andres et al., 2018). Improving the classification system to include pain will help to shift the field to consider this essential component of treatment. One study showed that women with endometriosis appear to have higher pelvic pain when compared to women with other gynecological conditions (Schliep et al., 2015). Symptoms of dyspareunia, dysmenorrhea, dyschezia and pain in the vaginal and abdominopelvic area are regularly reported among women undergoing laparoscopy, even among women with no identified gynecologic pathology. Future research is needed to understand the complex causes of chronic and acute pelvic pain among women who seek out gynecologic care but unidentified gynecologic pathology. Additionally, the investigation of the correlation between the stage of endometriosis and the pelvic pain classification system is needed to better predict outcomes for endometriosis patients with pelvic pain for both surgical and nonsurgical treatment (Schliep et al., 2015). There is a critical need to identify mechanisms contributing to CPP in endometriosis in order to identify those at greatest risk for pain and to identify brain and psychological factors that may predict the potential to undergo pain chronification. Given that most adult patients report symptoms of endometriosis as well as CPP during adolescence, this would be a critical time to intervene. This is especially important in the current opioid epidemic with new research highlighting that 24 % of Obstetrician/Gynecologists prescribe opioids for endometriosis-related pain (Madsen et al., 2018). Understanding the mechanisms that contribute to CPP in adolescent women with endometriosis will shift the field toward prevention and early intervention to allow for treatment before pain becomes disabling.
The guideline for treatment and approaching adolescents with endometriosis is based on studies examining adult women due to the limited number of studies focusing specifically on adolescents. It is recommended that the decision for surgical management of endometriosis should be based on clinical evaluation, imaging modality, and medical treatment response, with restrictive use of laparoscopy for diagnosis (Hwang et al., 2018). Although many clinicians prescribe gonadotropin-releasing hormone agonists to reduce inflammation, blood flow, and adhesions in endometriosis, research has shown that preoperative hormonal treatment may not reduce either endometriosis related pain or recurrence (Dunselman et al., 2014; Rolla, 2019). However, adjuvant hormonal treatment may result in pain relief from the effects of surgical treatment in the short term, and a suitable treatment (≥6 months) to prevent recurrence in the long term (Johnson et al., 2017).
2.2. Psychological distress
While many chronic conditions that result in pain can be psychologically distressing, there are unique considerations in women with endometriosis; for example, endometriosis can result in infertility (Tanbo and Fedorcsak, 2017). A young woman who is diagnosed will not ultimately know if her fertility has been compromised until she tries to become pregnant. This uncertainty can significantly impact quality of life in a way that is unique from other diseases. For example, while cancer treatments can result in infertility, many women are given the option to freeze their eggs prior to starting to treatment (Bouchlariotou et al., 2012), which is also an option for women with endometriosis. As such, it is best to freeze eggs when women are younger and are likely to have improved responses to hormonal stimulation and the disease may not affect their ovaries; however, if there is a delay in diagnosis, as is often the case; this may not be an option.
Psychological factors also assume an important role in determining the severity of symptoms, and women diagnosed with endometriosis report high levels of anxiety, depression and other psychiatric disorders (Lagana et al., 2017). Additionally, it has been demonstrated that high levels of anxiety and depression can amplify the severity of pain (Cavaggioni et al., 2014). Moreover, several studies have described the influence of CPP on the quality of life and well-being of patients with endometriosis (Jia et al., 2012; Vitale et al., 2017; Siedentopf et al., 2008) and it has been shown to significantly influence emotive functioning (Friedl et al., 2015). Population-based studies that examine psychological outcomes for adolescents and young women are scant; however, one study (Rowlands et al., 2016) did find that young women, ages 18–23 years, with endometriosis had a greater risk of moderate to severe psychological distress compared to women without a history of this condition. Another study (Gallagher et al., 2018) of adolescents with endometriosis found that adolescents and young adult women with endometriosis had significantly worse reports of quality of life compared to peers unaffected by endometriosis.
There is a lack of literature on the influence of psychological factors and psychiatric comorbidities on the effectiveness of treatments for endometriosis within both adult and adolescent populations. It is important to evaluate the presence of previous psychiatric diseases to select patient specific treatment approaches. It is unclear if these comorbidities are a result of endometriosis itself or other factors; therefore the relationship between endometriosis and psychological diseases remains a mystery.
2.3. Comorbid conditions
Among adults, the risk of developing a comorbid condition among endometriosis patients was at least twice as large for the following comorbidities: infertility/subfertility, ovarian cyst, uterine fibroids, pelvic inflammatory disorder, interstitial cystitis, irritable bowel syndrome, constipation/dyschezia, ovarian cancer, and endometrial cancer (Surrey et al., 2018). Additionally, the incidence of developing many other comorbidities was significantly higher among endometriosis patients compared with matched women without endometriosis. Two recent and thorough reviews of the epidemiological literature described a number of factors associated with the development of endometriosis, including genetic profile, inflammation, hormonal activity, increased exposure to menstruation, immunological dysfunction, and environmental toxicants (Parazzini et al., 2017; Kvaskoff et al., 2015). Additionally, it was shown that endometriosis is associated with higher rates of a variety of conditions, including ovarian and other gynecological cancers, thyroid cancer, autoimmune diseases, asthma/atopic diseases, and cardiovascular diseases (Kvaskoff et al., 2015). Investigating comorbidities offers multiple benefits, including informing clinical surveillance, helping to develop prevention and early detection guidelines, and improving patient outcomes (Missmer, 2009). It is unknown if women with comorbid conditions experience heightened pain secondary to endometriosis; however, this should be explored when investigating comorbid conditions. Particularly, the association and degree of impact has not been studied or identified among adolescent and young women with endometriosis.
2.3.1. Diagnostic challenges across the ages
Given that endometriosis presents differently in adolescent women compared to adult women both in lesion appearance and pain (e.g., adolescents typically present with cyclic and non-cyclic pelvic pain (Laufer, 2008)) it is surprising that there has not been research exploring age-related differences in this disease. Such insight could result in novel treatments.
2.3.1.1. Prevalence - what about adolescents?.
While endometriosis affects 1 in 10 women, endometriosis is also present in adolescents, with documented cases as young as age eight (Young et al., 2017). One study found that two-thirds of women diagnosed with endometriosis in adulthood presented with symptoms of the disease before age 20 (Stuparich et al., 2017; Sinaii et al., 2002) and it has been shown that endometriosis can occur prior to menarche (M.R. L., 2000).
2.3.1.2. Delay in diagnosis.
The non-cyclic pain presentation in adolescents can make the diagnosis difficult to infer resulting in a delay of an average of seven years between symptom onset and diagnosis (Laufer et al., 2003; Ballweg and Campbell, 2003); thus the incidence of endometriosis in adolescents has been difficult to quantify with estimates varying among different studies (Dessole et al., 2012). Additionally, it has been found that patients who report symptoms as adolescents are evaluated on average by four or more physicians before receiving a diagnosis of endometriosis (Ballweg, 2003). Similarly, the presence of comorbid conditions (e.g., inflammatory, neuroendocrine, chronic pain) further complicating diagnosis (Sinaii et al., 2002; Bulletins–Gynecology ACoP, 2004; Mirkin et al., 2007). Lastly, the delay in the diagnosis is also attributed to the fact that laparoscopy with biopsy is the only way to diagnose endometriosis in the adolescent population, and depends on recognition of atypical manifestations of the disease as lesions in adolescents often appear differently than lesions in adult women (i.e., adolescents tend to have clear, red, white, and/or yellow-brown lesions more frequently than black or blue lesions) and thus may be missed (Laufer et al., 2003). The lack of diagnostic biomarkers is a significant issue likely contributing to the poor long-term pain problems with this population. This delay in diagnosis is problematic as it may predispose women to more chronic pain, as ‘pain predicts pain (Katz and Seltzer, 2009), thus resulting in the development of a sensitized nervous system and a propensity to develop CPP and other chronic pain syndromes.
2.3.1.3. Progression – centralization of pain.
Intermittent or ongoing pain resulting from a disease can produce a sensitized peripheral and central nervous system (peripheral and central sensitization) that becomes maladaptive (centralization of pain). This can be especially true for those adolescent women with the disease who are not diagnosed until adulthood. Once the pain becomes centralized, profound changes in neural networks that involve anxiety, cognition, memory, and normal function of the brains pain inhibitory (descending modulation) systems are thought to take place (Giamberardino et al., 2014; Whitaker et al., 2016; Simis et al., 2015). Most pelvic organs, as well as somatic pelvic tissues to which the pain is referred, share at least part of their central sensory projection. This may trigger the phenomena of cross-sensitization and central sensitization (Jarrell et al., 2014). However, this process is poorly understood as it relates to endometriosis and the secondary pelvic pain, which has resulted in few treatment options for this vulnerable population.
3. The origin of pain in endometriosis
3.1. The lesion
While lesions may be painful, this is not a linear process. Similar to other pain conditions, the presence and severity of pelvic pathology has been shown to not correlate with symptom burden (Dun et al., 2015) and pain frequently recurs even without visible disease at repeat laparoscopy (As-Sanie et al., 2013) so it is unlikely that the lesion is the specific cause of CPP.
3.2. The pain producing processes
Rodent models of endometriosis support the theory that a process of central sensitization likely results in CPP in this population via a viscera-visceral referred hyperalgesia, process in which increased input to the nervous system from one visceral domain can sensitize neurons that receive convergent input from another visceral domain (Berkley et al., 2001). Specifically, in the rat, it has been found that input from the uterus to the spinal cord is mainly by way of hypogastric nerve at the thoracic level, and that from the cervix is by way of both the pelvic and hypogastric nerves suggesting possible routes of referred pain (Berkley et al., 1993a). Neurons within both sets of segments have demonstrated to respond convergently to stimulation of the uterus, colon, and vagina and significant interactions exist between these two separated sets of caudal spinal segments (Berkley et al., 1993a, b). Additionally, the auto-transplanted ectopic endometrial cystic fragments develop their own innervation including sympathetic efferent as well as sensory fibers (Berkley et al., 2004), which may impact the nervous system. These studies support the theory that sensitized afferents innervate regions surrounding the endometrial growths result in central sensitization within the caudal spinal cord that is then referred to the vaginal canal (Berkley et al., 2001), This cross-talk between nerves and blood vessels by which lesions develop a unique local neuronal and vascular supply is referred to as neuroangiogenesis (Asante and Taylor, 2011) and can result in a direct and two-way interaction between the endometrial lesions and the CNS thus resulting in pain (Stratton and Berkley, 2011). Rat models of endometriosis have also revealed vaginal hyperalgesia (Berkley et al., 2001) as well as hyperalgeisa of the paw (Hernandez et al., 2017), a non-pain site, a phenomenon also seen in women with endometriosis (As-Sanie et al., 2013). While mouse models of endometriosis have not been used as extensively as rats, Greaves et al. (2017) found that the peritoneal lesions in mice with endometriosis have an upregulation of prostaglandins as well as changes in gene expression both in the dorsal root ganglia and the CNS, and altered behavior including increased mechanical pain sensitivity suggesting complex biochemical and neurobiological mechanisms that interact to produce localized and referred pain.
3.3. Psychobiology and endometriosis associated pain
Psychophysiological factors are believed to assume a role in the development of central sensitization. Activation of the stress-response network lowers pain thresholds in both animals and humans, and high levels of anxiety are consistently related to higher pain sensitivity (Hirsh et al., 2008). Many studies have shown a prior history of anxiety, depression, and physical and psychological trauma, to be significantly predictive of the onset of chronic pain later in life (McLean et al., 2005; Talbot et al., 2009; Nicol et al., 2016). This evidence suggests that pre-existing dysregulation of the nervous system may result in high susceptibility to the development of chronic pain via the development of central sensitization, but needs to be explored more specifically in regards to CPP and endometriosis. While there is mounting evidence on psychological factors contributing to CPP (Miller-Matero et al., 2016; Romao et al., 2009; Roth et al., 2011; Savidge and Slade, 1997), there is less known about CPP as it specifically relates to endometriosis. Similar to many other diseases associated with pain and chronic pain states, pain catastrophizing has emerged as an important determining factor in pain-related outcomes and is consistently associated with higher pain levels in women with endometriosis (Carey et al., 2014; Martin et al., 2011; Facchin et al., 2015). Similarly, high levels of anxiety and depression have been found in women with endometriosis (Lagana et al., 2017). One study Souza et al. (2011) found that women with endometriosis and CPP had worse quality of life and mental health compared to women with asymptomatic endometriosis; notably the asymptomatic group was not significantly different on mental health or quality of life variables from the control group, which suggests that the CPP may have a greater impact on psychological functioning than suffering from the actual disease state.
When examining the animal literature, the exploration of psychological factors contributing to CPP in animal models of endometriosis is scarce. While some studies have interestingly examined the role of stress in disease progression (Torres-Reveron et al., 2018; Cuevas et al., 2018), the role of stress on endometriosis-related pain specifically has not been studied extensively. Interestingly, one study (Hernandez et al., 2017) found that in rats with endometriosis that were exposed to stress compared to an endometriosis/no stress group and a sham-no stress group, the endometriosis with stress group had more adverse disease related issues such as increased colonic damage, vesicle mast cell infiltration and more severe vesicles. The endometriosis with stress group also developed significant hyperalgesia but stress interestingly reversed the allodynic effect found in endometriosis, which could be the result of a stress-induced analgesia phenomenon (Cuevas et al., 2018).
Outside of stress, there has been minimal animal research conducted on other salient psychological variables, such as anxiety and depression, on CPP in endometriosis. However, one recent study (Aso and Conrad, 1997), utilized a mouse model to explore the effect of endometriosis on CNS function, electrophysiology, and gene expression specifically to determine whether endometriosis can result in central pain sensitization, anxiety, and depression and to identify the molecular changes in the brain that are mechanistically responsible. Confirming what we know in human females with endometriosis, mice with endometriosis demonstrated more depressive, anxious, and pain behaviors compared to the sham control mice, even after the expected recovery period post-surgery. Microarray analyses revealed that genes involved in anxiety, locomotion and pain were altered; specifically, Gpr88, Glra3 in the insula, Chrnb4, Npas4 in the hippocampus, and Lcn2 in the amygdala were up-regulated, while Lct, Serpina3n in the insula and Nptx2 in the amygdala were down-regulated. Additionally, patch clamp recordings in the amygdala were altered in the mice with endometriosis. The results of this study are important because they suggest that having endometriosis can impact brain electrophysiology and modulate gene expression, which may be in turn result in pain sensitization as well as the anxiety and depressive symptoms frequently reported by women with this disease. Given that endometriosis associated pain is still often conceptualized as peripherally driven, the findings from these animal studies underscore the likely centrally driven mechanisms contributing to CPP in this population, which could impact the development of centrally driven treatments.
3.4. Quantitative sensory testing (QST) - insights into pain sensitization
QST is used to assess responses to standardized noxious stimuli in a controlled laboratory setting and is the only method to assess central sensitization. Research using QST has highlighted variability in pain sensitivity and pain modulation as a putative phenotypic contributor to the risk for development of chronic pain (Rolke et al., 2006; Wilder-Smith, 2011; Shy et al., 2003). Reference values of the face, hand, and foot in sex and age matched healthy adults has been established (Rolke et al., 2006); however, no reference values for QST pelvic pain thresholds exist. CPP secondary to endometriosis has not been extensively explored via the use of QST and has focused on pressure pain. Tu et al. (2008) performed an initial validation and reliability assessment of vaginal pressure-pain thresholds in healthy women through the use of a vaginal pressure algometer and concluded that pressure pain threshold may be a valid and reliable measure of pelvic floor somatic pain sensitivity in healthy women. As a follow-up study utilizing this device, Hellman et al. (2015) found that women with CPP or painful bladder syndrome exhibited enhanced pain sensitivity with lower pressure pain thresholds compared to pain-free participants; however, both the CPP and painful bladder syndrome groups included women with endometriosis and as endometriosis was not the focus of this study the effect of this internal pressure paradigm on women with endometriosis with and without CPP is unknown. As-Sanie et al. (2013); however, did find that that peripheral pressure-pain thresholds were lower in women with endometriosis and CPP and in women with CPP without endometriosis when compared to both women with endometriosis and no CPP and pain-free women suggesting that perhaps the endometrial lesions are not driving the CPP.
There is scant literature on other QST modalities outside of pressure pain as it relates to endometriosis and nothing in adolescent women. A recent study (Grundstrom et al., 2019) did examine thermal and pressure thresholds in 13 women with surgically confirmed endometriosis and 24 women without but who had CPP compared to 55 healthy control women ages 18–40. QST sites included areas of presumed menstrual pain (i.e., abdominal wall) compared to a control site (i.e., dominant leg). Results indicated that women with CPP, regardless of endometriosis status, endorsed significantly lower quality of life as well as significantly greater symptoms of anxiety and depression compared to the control group. Additionally, the CPP group, again regardless of endometriosis diagnosis, had reduced pain thresholds compared to the control women, with duration of pelvic pain significantly negatively correlated with pain threshold. More research conducted on larger samples and other QST modalities such as mechanical pain, temporal summation, and conditioned pain modulation is warranted to further elucidate the relationship between sensory functioning and CPP. Additionally, research on adolescent women is warranted as endometriosis presents differently in this age group (Laufer et al., 2003).
3.5. Brain imaging – peripheral nerve lesions driving central brain changes
Little research on how CPP confers changes to the brain that result in increased resistance to treatment or chronification has been conducted across adolescent or adult populations with endometriosis (i.e., from early onset to major manifestations during development). Some pilot studies conducted in adult women with endometriosis and/or CPP suggest that adult women with CPP have decreases in regional gray matter volume in brain regions associated with pain processing including the thalamus, cingulate gyrus, putamen, and insula (As-Sanie et al., 2012), suggestive of pain amplification related to CNS changes found in other chronic pain conditions such as fibromyalgia (Gracely et al., 2002) and vulvodynia (Harris et al., 2013). Additionally, another study by the same group (As-Sanie et al., 2016) found that women with endometriosis-associated CPP demonstrated increased concentrations of excitatory neurotransmitters in the anterior insula and had greater intrinsic connectivity of the same anterior insula region to the medial prefrontal cortex both known to be regions important in pain processing and reward neurocircuitry. Additionally, increased connectivity between these two regions was positively correlated with anterior insula combined glutamate and glutamine concentrations, as well as with pain intensity, anxiety and depression symptom severity. These studies suggest that pelvic pain is likely not sufficiently explained by the presence of endometriosis and may be due to dysfunction in the CNS pain regulatory system; however, these studies included small samples with only adult participants. How endometriosis and CPP impact the adolescent brain is not understood. Brain correlates utilizing fMRI of these and other changes known to occur in chronic pain have not been previously examined in adolescent or young adult women with endometriosis and are warranted in order to understand the age-related changes that may occur with the disease.
A recent study (Yano et al., 2019) examined pain-related behavior and brain activation in five cynomolgus macaques with naturally occurring endometriosis compared to three healthy female macaques. The macaques underwent pressure algometer pain sensitivity testing of the abdomen after undergoing a single dose of morphine, meloxicam and acetaminophen (a 3-day washout interval was used between drugs). One week following drug testing, brain activation in three of the macaques with endometriosis in response to non-noxious abdominal stimulation and the effect of a single dose of morphine on brain activation were examined with fMRI. For comparison, the effect of non-noxious abdominal stimulation on brain activation in three healthy, control macaques was also assessed compared to the healthy controls. One month following fMRI, treatment stimulation responses were obtained from five macaques with endometriosis. Following baseline determinations, macaques with endometriosis were treated for 8 weeks with dienogest and stimulation-evoked brain activation was assessed with fMRI on the eighth week of dienogest treatment. Response thresholds were measured two and four weeks after cessation of dienogest treatment. Results found that pain response thresholds were significantly less in the macaques with endometriosis compared to the healthy controls. Additionally, the non-noxious abdominal stimulation activated the insula and thalamus, brain regions implicated in pain processing, which was reduced by treatment with morphine and dienogest; however, significant residual non-noxious force-evoked activation of the thalamus was still present at the end of dienogest treatment suggestive of a central sensitization process.
3.5.1. Preemptive processes - getting to the brain before the disease does
3.5.1.1. Brain state and modifying disease trait.
Mechanisms contributing to CPP secondary to endometriosis would be further advanced in research if a common conceptual model was adopted. Specifically, a model that incorporates the complex interplay between centralized pain, psychological impact, environmental influences, and social variables, with more precise language for describing CPP measures. Conceptualizing CPP associated with endometriosis as a peripherally driven phenomenon is a disservice to the millions of women suffering from this disease. Simply removing lesions (often multiple times) is not going to fix the problem of what the evidence suggests is a centralized pain problem and will likely only exacerbate the pain. This is just one component contributing to the extreme delay in diagnosis and treatment for CPP associated with endometriosis - it takes an average of 7 years for a woman to be diagnosed with endometriosis after the onset of symptoms (Arruda et al., 2003). This is in part because CPP is not a monolithic concept but rather an emergent process that involves interactions between individual factors, environmental factors, and psychological and physiological reactivity. A purely physiological approach to pain assumes that pain intensity relates to the degree of tissue damage, but this often cannot explain the wide range of reactions in humans in response to a painful stimulus (Brawn et al., 2014; Sullivan et al., 2001). One aspect that is often neglected in endometriosis research and treatment is the psychological effects of the disease. It has been well documented that the health-related quality of life is impaired in women with endometriosis when compared with healthy control women. Similarly, pain intensity and pain cognition are independent factors influencing the health-related quality of life of women with endometriosis. Patients with endometriosis report more negative pain cognition when compared with controls and also report more pain anxiety, catastrophizing, and hypervigilance toward pain (Sullivan et al., 2001).
Empirical studies also indicate that specific psychosocial factors may modulate pain experience, pain-related distress and treatment outcomes. Recently, adverse early life events have been associated with the risk for endometriosis. One study (Liebermann et al., 2018) showed that women with endometriosis, compared to healthy control women reported significantly more often a history of sexual abuse, emotional abuse, and emotional neglect. The associations between abuse and endometriosis were stronger among women presenting without infertility, a group that was more likely to have been symptomatic with respect to pain (Harris et al., 2018). Additionally, severity, chronicity and accumulation of types of abuse were associated with greater risk for endometriosis. A link to maltreatment during childhood needs to be considered during the diagnosis and treatment process of women with endometriosis, as well as the research community. Additionally, research has also suggested a link between exposure to dioxins and dioxin-like chemicals and the development of endometriosis in women (Soave et al., 2015). Dioxin and dioxin-like compounds have long biological half-lives, can accumulate within the organism, and could negatively affect several physiological processes. However, the exact mechanism through which they operate is unclear. It is hypothesized that they could interfere with both immune and endocrine systems and may be an important environmental consideration. Understanding the mechanisms underlying these relations may better define the related pathophysiology of endometriosis and associated pain and should be incorporated into a new conceptualized model of studying the disease.
3.5.2. Treatment approaches
In endometriosis, multidimensional and personalized pain treatment has been elusive. The targets for analgesic treatment fall into the usual categories of prevention or limiting the disease; peripherally acting and centrally acting medications; psychological approaches; and non-invasive procedures such as focused ultrasound. For chronic pain, the target is to reset the brain state using one or a combination of approaches. Below we summarize various therapeutic options.
3.5.2.1. Pharmacological treatments.
Standard treatments for endometrial pain are not specific and few randomized clinical trials for pain have been performed with commonly available medications used for chronic pain. Standard treatments for endometrial pain are not specific and few randomized clinical trials for pain have been performed with commonly available medications used for chronic pain. However, there are a number of trials (n = 165) when searched for interventional studies of endometriosis and pain as listed in clinicaltrials.gov, of which 85 utilize pharmacotherapy. Clinical trials for adolescents resulted in fewer studies (n = 17), of which two utilize pharmacotherapy, are noted in Table 1. New medical treatments for painful endometriosis are summarized in a recent guideline (Dunselman et al., 2014). Considering there are few pharmacological studies specifically targeting adolescent endometriosis, adult studies are also included in Table 1.
Table 1.
Examples of endometriosis and pain pharmacological clinical trials (data collected from clinicaltrails.gov).
| Drug | Phase | Mechanisms of action | Age of participants | ClinicalTrials Identifier |
|---|---|---|---|---|
| Ulipristal (other names: Ulipristal Acetate; Ella) | 4 | Progesterone receptor modulation (Bressler et al., 2017) | 18–50 Years | NCT02213081 |
| Gefapixant (other nameL MK-7264) | 2 | Antagonist P2 × 3 and P2 × 2/3 receptors (Richards et al., 2019) | 18–49 Years | NCT03654326 |
| Relugolix | 1 3 | Active nonpeptide gonadotropin-releasing hormone (GnRH)-receptor antagonist (Markham, 2019) | 1 18–50 Years | 1 NCT03204318 |
| 2 3 | 2 18–51 Years | 2 NCT03654274 | ||
| 3 3 | 3 20+ Years | 3 NCT03931915 | ||
| Estradiol/norethindrone acetate (other names: E2/NETA; Activella) | 1 3 | Hormonal therapy - steroids bind to their receptor, activating hormone response elements and gene transcription; this subsequently activates hormone response proteins that influence cell function and differentiation (Casey and Murray, 2008) | 1 18–50 Years | 1 NCT03204318 |
| 2 3 | 2 18–49 Years | 2 NCT03343067 | ||
| 3 3 | 3 18–51 Years | 3 NCT03654274 | ||
| Lidocaine | 1 NA | Synthetic aminoethylamide with local anesthetic and antiarrhythmic properties (Wickstrom et al., 2013) | 1 18–50 Years | 1 NCT01968694 |
| 2 2 | 2 21+ Years | 2 NCT01329796 | ||
| Quinagolide | 1 2 | non-ergot dopamine agonist (Delgado-Rosas et al., 2011) | 1 18 Years | 1 NCT03692403 |
| 2 2 | 2 18–45 Years | 2 NCT03749109 | ||
| Thalidomide | 1 | anti-angiogenic (Paravar and Lee, 2008) | 18+ Years | NCT01028781 |
| Elagolix (other name: NBI-56418) | 1 3 | Active non-peptidic GnRH antagonist (Vercellini et al., 2019) | 1 18–49 Years | 1 NCT03213457 |
| 2 3 | 2 18–49 Years | 2 NCT03343067 | ||
| 3 3 | 3 18–49 Years | 3 NCT01931670 | ||
| 4 3 | 4 18 to 50Years | 4 NCT01760954 | ||
| 5 3 | 5 18–49 Years | 5 NCT01620528 | ||
| 6 3 | 6 18–50 Years | 6 NCT02143713 | ||
| 7 2 | 7 18–49 Years | 7 NCT00619866 | ||
| 8 2 | 8 18–45 Years | 8 NCT00797225 | ||
| 9 2 | 9 18–45 Years | 9 NCT00109512 | ||
| 10 2 | 10 18–55 Years | 10 NCT00458458 | ||
| Depot-Leuprolide/Norethindrone | 3 | GnRH agonist (Hornstein et al., 1998) | 18 to 52 Years | NCT00229996 |
| Yasmin | 1 3 | Oral contraceptive (Fathizadeh et al., 2010) | 1 18–52 Years | 1 NCT00229996 |
| 2 4 | 2 18–45 Years | 2 NCT02237131 | ||
| Palmitoylethanolamide-polydatin | NA | Endogenous fatty acid amide and Polydatin is a natural precursor of resveratrol (Gugliandolo et al., 2017) | 18–50 Years | NCT02372903 |
| Infliximab | 2 | anti TNFa monoclonal antibody (Ceyhan et al., 2011) | 20–45 Years | NCT00604864 |
| Atorvastatin + oral contraceptive (other name: Atrox 20) | NA | HMG-CoA reductase inhibitors (Simsek et al., 2014) | 18–45 Years | NCT00675779 |
| Mercilon | NA | Oral contraceptive (Anon., 1989) | 18–45 Years | NCT00675779 |
| Linzagolix (other names: OBE2109, KLH-2109) | 1 3 | GnRH antagonist (Greene et al., 2016) | 1 18–49 Years | 1 NCT03986944 |
| 2 2 | 2 18–45 Years | 2 NCT02778399 | ||
| 3 2 | 3 18+ Years | 3 NCT01629420 | ||
| 4 2 | 4 18+ Years | 4 NCT01395940 | ||
| Resveratrol | 4 | Plant-derived polyphenolic phytoalexin (Delgado-Rosas et al., 2011) | 20–50 Years | NCT02475564 |
| Cabergoline | 2 | dopamine agonist (Jouhari et al., 2018) | 15–40 Years | NCT03928288 |
| ASP1707 | 2 | GnRH antagonist (D’Hooghe et al., 2019) | 18–45 Years | NCT01767090 |
| Leuprorelin acetate (other name: Prostap® SR) | 1 2 | synthetic nonapeptide that is a potent GnRHR agonist (Wilson et al., 2007) | 1 18–45 Years | 1 NCT01767090 |
| 2 2 | 2 20+ Years | 2 NCT01458301 | ||
| 3 2 | 3 20+ Years | 3 NCT01452685 | ||
| 4 2 | 4 20+ Years | 4 NCT02778919 | ||
| 5 4 | 5 18–40 Years | 5 NCT02393482 | ||
| Desogestrel (other name: Cerazette) | NA | GnRH inhibitor (Tanmahasamut et al., 2017) | 18–45 Years | NCT01559480 |
| Tanezumab | 2 | Monoclonal antibody against nerve growth factor (Goenka et al., 2017) | 18–49 Years | NCT00784693 |
| Leflutrozole (other names: BGS649) | 1 2 | Aromatase inhibitor (Vercellini et al., 2014) | 1 18–49 Years | 1 NCT01116440 |
| 2 2 | 2 18–40 Years | 2 NCT01190475 | ||
| Letrozole | 1 2 | Non-steroidal aromatase inhibitor (Nothnick, 2011) | 1 18–42 Years | 1 NCT04002141 |
| 2 2 | 2. 18+ Years | 2 NCT00240942 | ||
| depot medroxyprogesterone acetate (DMPA) | 1 4 | Intramuscular injection for long-term contraception (Mishell, 1996) | 1 18–45 Years | 1 NCT01056042 |
| 2 4 | 2 18–45 Years | 2 NCT02534688 | ||
| DLBS1442 (Dismeno) | 2 | Bioactive fraction extracted from the fruit of the native Indonesian plant (Tandrasasmita et al., 2015) | 18–50 Years | NCT01942122 |
| Decapeptyl (other names: Triptorelin acetate) | 1 4 | GnRH agonist (Leone Roberti Maggiore et al., 2014) | 1 18–45 Years | 1 NCT00735852 |
| 2 3 | 2 18–45 Years | 2 NCT03232281 | ||
| Dichloroacetate (other names: DCA) | NA | inhibit pyruvate dehydrogenase kinase (Michelakis et al., 2008) | 18+ Years | NCT04046081 |
| Levonorgestrel (other names: Mirena) | 1 4 | Levonorgestrel releasing intrauterine system - contraceptive (Beatty and Blumenthal, 2009) | 1 18–45 Years | 1 NCT02480647 |
| 2 2 | 2 18+ Years | 2 NCT02203331 | ||
| 3 4 | 3 18–45 Years | 3 NCT02534688 | ||
| BAY1128688 | 2 | AKR1C3 protein inhibitors (Greene et al., 2016) | 18+ Years | NCT03373422 |
| Pravastatin (other name: Pravastatin sodium) | NA | HMG-CoA reductase inhibitors (Sokalska et al., 2019) | 18–38 Years | NCT02079974 |
| Anastrazole | 4 | nonsteroidal inhibitor of aromatase (Chia et al., 2008) | 18–50 Years | NCT01769781 |
| Danazol | 2 | derivative of the synthetic steroid ethisterone (Godin and Marcoux, 2015) | 18–50 Years | NCT00758953 |
| Melatonin | 2 | Hormone produced by pineal - endometriosis mechanisms unknown (Mosher et al., 2019) | 18–55 Years | NCT03782740 |
| Degarelix (other name: firmagon) | 3 | GnRH receptor antagonist (Santen, 1992) | 20–45 Years | NCT01712763 |
| Goserelin (other name: decapeptyle) | 3 | GnRH agonist (Moore et al., 2015) | 20–45 Years | NCT01712763 |
| Anakinra (other name: Kineret) | 1 | Markers of inflammation - nonglycosylated human interleukin-1 receptor antagonist (IL-1Ra) (Stocks et al., 2017) | 18–40 Years | NCT03991520 |
| Dienogest | 1 3 | Orally-active semisynthetic progestogen (Ferrero et al., 2015) | 1 20+ Years | 1 NCT01697111 |
| 2 NA | 2 18 −38 Years | 2 NCT03142035 | ||
| 3 3 | 3 18–45 Years | 3 NCT01822080 | ||
| 4 4 | 4 18–40 Years | 4 NCT03789123 | ||
| 5 2 | 5 12–17 Years | 5 NCT01283724 | ||
| 6 4 | 6 18–51 Years | 6 NCT02385448 | ||
| Rosiglitazone (other name: Avandia) | 2 | Increase insulin sensitivity (de Oliveira et al., 2017) | 18–45 Years | NCT00115661 |
| ERB-041 | 2 | Estrogen receptors (Chaudhary et al., 2014) | 18–45 Years | NCT00318500 |
| Proellex | 12 | Progesterone receptor blocker (Goenka et al., 2017) | 1 18–48 Years | 1 NCT00556075 |
| 2 2 | 2 18–47 Years | 2 NCT01728454 | ||
| 3 2 | 3 18–48 Years | 3 NCT00958412 | ||
| 4 2 | 4 18–47 Years | 4 NCT01961908 | ||
| Vilaprisan (BAY1002670) | 12 | Synthetic and steroidal selective progesterone receptor modulator (Moller et al., 2018) | 1 18+ Years | 1 NCT03573336 |
| 2 3 | 2 18–45 Years | 2 NCT00225186 | ||
| Asoprisnil | 3 2 | Synthetic, steroidal selective progesterone receptor modulator (DeManno et al., 2003) | 1 18–40 Years | 1 NCT00160446 |
| 1 2 | 2 18–40 Years | 2 NCT00160420 | ||
| 2 2 | 3 18–40 Years | 3 NCT00160433 | ||
| Cannabinoid | 2 | Cannabinoid receptor (Bouaziz et al., 2017) | 18–40 Years | NCT03875261 |
| PGL2001 | 2 | Steroid sulfatase inhibitor (Pohl et al., 2014) | 18–45 Years | NCT01631981 |
| Loestrin (other name: microgestin) | 4 | Contraception: norethindrone (a progestin) and ethinyl estradiol (an estrogen) (Greene et al., 2016) | 18–45 Years | NCT02214550 |
While treatments include hormonal agents (e.g., dienogest, aromatase inhibitor (AI), gonadotrophine-releasing hormone (GnRH) antagonist, ant- tumor necrosing factor (TNF)-α, selective estrogen receptor modulator (SERM) (Tosti et al., 2017), & estrogen-progestin and progestins therapies (Vercellini et al., 2016)), novel treatments in the future may focus on the inflammatory response in the diseases. The effects on nerves from endometriosis entail both physical ‘entrapment’ and chemical ‘irritation’. Both activate immune responses. The immune response to tissue damage and its role in pain has been documented in detail elsewhere (Ren and Dubner, 2010; Totsch and Sorge, 2017; Pinho-Ribeiro et al., 2017). In endometriosis, not only may there be a response to tissue damage, but the immune response may be altered and indeed dysfunctional (Herington et al., 2011) rendering a hypersensitivity to pro-inflammatory stimuli or molecules. As such the condition may be responsive to treatments that target specific immune processes (Symons et al., 2018). Such treatments have involved non-specific immune modulators such as ketamine (De Kock et al., 2013; Beilin et al., 2007) to more focused pharmacotherapies (Vercellini et al., 2014) to current development of novel targets.
3.5.3. Behavioral based treatments
There is very little evidence for the efficacy of non-pharmacological approaches to the treatment of endometrial pain (Wattier, 2018) and empirically-based, non-pharmacological interventions for the treatment of endometriosis and/CPP are rare. It is, however, well-established in the literature that CPP is highly distressing for women, associated with disability, comorbid with other mental health conditions, and often involves inconclusive and unsatisfactory medical investigations (Dalpiaz et al., 2008; Ghaly and Chien, 2000; Weijenborg et al., 2007). Existing psychologically-based pain treatment interventions, such as Cognitive-Behavioral Therapy (CBT) or Acceptance and Commitment Therapy (ACT), could be modified to address the unique needs facing women with endometriosis and/or CPP. CBT has been established as a valid and effective treatment for chronic pain conditions (Morley et al., 2008; Niv and Devor, 2007), however, CBT studies that investigate interventions specifically targeting endometriosis and/or CPP in women are lacking. Stones and colleagues118 examined a range of behavioral and medical treatments addressing CPP in women and concluded that psychological therapies are shown to be effective for CPP in women; however in practice, treatment recommendations generally come from single studies, and the authors make an urgent call for more research (Stones and Mountfield, 2000) Nevertheless, CBT interventions have been shown to be effective in reducing pain, managing distress, improving sexual function and reducing disability for a range of gynecological conditions that are associated with CPP (Bergeron et al., 2001; Brown et al., 2009; Masheb et al., 2009).
Studies have also shown that endometriosis can adversely affect women and their partners’ general psychological well-being, relationship adjustment and overall quality of life (Aso and Conrad, 1997). Furthermore, women with endometriosis report significantly more sexual dysfunctions compared to healthy women. Research on psychosexual interventions in endometriosis treatment is limited but shows to be effective in reducing endometriosis-related pain and associated psychosexual outcomes. Specifically, an individualized, couple-centered, multimodal approach to care, integrating psychosexual and medical management for endometriosis, is thought to be optimal.
3.5.3.1. Treating endometriosis associated pain in adolescents.
Endometriosis in adolescents requires unique considerations for treatment approaches, as it presents particular challenges in terms of diagnosis, variable presentation and symptoms, and choice of treatment (Laufer, 2008). The evidence of what we know about endometriosis and CPP supports advocating for increased awareness among adolescents and their health care providers about the need for early clinical diagnosis of endometriosis and timely treatment of severe dysmenorrhea/pelvic pain, usually with medical therapy as first line.
Once the disease is diagnosed and treated, these patients have favorable outcomes with hormonal and nonhormonal therapy (Dun et al., 2015); however, for those who do undergo surgery, about 30 % of women still report ongoing pelvic pain after surgery despite taking these medications (Abbott et al., 2004). Therapeutic options have been developed and successfully used to achieve controlling pain (Vercellini et al., 2018), improving quality of life when coupled with surgery (Marqui, 2015), and suppression of the hormonally active endometriotic tissue, although, they can come with unwanted side effects (Rafique and Decherney, 2017). There also is a great need for a specific conceptual model for adolescents with endometriosis; the younger the woman at onset of symptoms, the longer the duration until diagnosis is made (Benagiano et al., 2018). Early diagnosis and treatment is of paramount importance due to the reduced quality of life in young patients and for the possibility of progression that could endanger their reproductive potential.
4. Conclusions
Endometriosis in adolescents is a challenging clinical problem as it may present with a number of clinical and pathological differences versus adult women. Nevertheless, given the chronicity of the disease, the challenge is to avoid a delay in diagnosis, understand the disease and direct effective therapies at an early age. Given that endometriosis and accompanying CPP is a multi-faceted and complex problem there is in desperate need for a new approach from a diagnosis and treatment perspective. This will be accomplished by translational research methods.
While endometriosis can be treated by surgical excision of the lesions and/or hormonal treatment, sometimes combined with anti-inflammatory drugs, medical treatments are not curative and approximately 30 % of women who undergo surgery report ongoing pain after surgical excision of the lesions (Abbott et al., 2004). However, it should be noted that pharmacological treatments, while not curative, can be helpful following surgery and may be an effective strategy to limit the recurrence of the disease (Johnson et al., 2017). By understanding the neural underpinnings of the disease and risk factors for chronification, translational research could provide a basis for evaluating novel treatments and potentially lay the foundation for successful personalized, precision medicine to shorten diagnostic delay and maximize successful pain remediation.
Support
NIH grant K23 GM123372; Boston Center for Endometriosis/Marriott Family Foundation, and a Cathedral Fund grant awarded to CBS; FY18 Program/Investigator-Initiated Research Award with Partnering Option awarded to both CBS and DB by the Department of DefenseW81XWH1910560.
References
- Abbott J, Hawe J, Hunter D, Holmes M, Finn P, Garry R, 2004. Laparoscopic excision of endometriosis: a randomized, placebo-controlled trial. Fertil. Steril 82 (4), 878–884. [DOI] [PubMed] [Google Scholar]
- Abrao MS, Petraglia F, Falcone T, Keckstein J, Osuga Y, Chapron C, 2015. Deep endometriosis infiltrating the recto-sigmoid: critical factors to consider before management. Hum. Reprod. Update 21 (3), 329–339. [DOI] [PubMed] [Google Scholar]
- Andres MP, Borrelli GM, Abrao MS, 2018. Endometriosis classification according to pain symptoms: can the ASRM classification be improved? Best Pract. Res. Clin. Obstet. Gynaecol 51, 111–118. [DOI] [PubMed] [Google Scholar]
- Anon, 1989. Mercilon–a new low-dose combined oral contraceptive. Drug Ther. Bull 27 (13), 51–52. [PubMed] [Google Scholar]
- Arruda MS, Petta CA, Abrao MS, Benetti-Pinto CL, 2003. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum. Reprod 18 (4), 756–759. [DOI] [PubMed] [Google Scholar]
- Asante A, Taylor RN, 2011. Endometriosis: the role of Neuroangiogenesis. Annu. Rev. Physiol 73 (1), 163–182. [DOI] [PubMed] [Google Scholar]
- Aso T, Conrad MN, 1997. Molecular cloning of DNAs encoding the regulatory subunits of elongin from Saccharomyces cerevisiae and Drosophila melanogaster. Biochem. Biophys. Res. Commun 241 (2), 334–340. [DOI] [PubMed] [Google Scholar]
- As-Sanie S, Harris RE, Napadow V, et al. , 2012. Changes in regional gray matter volume in women with chronic pelvic pain: a voxel-based morphometry study. Pain. 153 (5), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- As-Sanie S, Harris RE, Harte SE, Tu FF, Neshewat G, Clauw DJ, 2013. Increased pressure pain sensitivity in women with chronic pelvic pain. Obstet. Gynecol 122 (5), 1047–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- As-Sanie S, Kim J, Schmidt-Wilcke T, et al. , 2016. Functional connectivity is associated with altered brain chemistry in women with endometriosis-associated chronic pelvic pain. J. Pain 17 (1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballweg ML, 2003. Big picture of endometriosis helps provide guidance on approach to teens: comparative historical data show endo starting younger, is more severe. J. Pediatr. Adolesc. Gynecol 16 (3 Suppl), S21–26. [DOI] [PubMed] [Google Scholar]
- Ballweg ML, Campbell PF, 2003. Psychosocial aspects of teen endo. J. Pediatr. Adolesc. Gynecol 16 (3 Suppl), S13–15. [DOI] [PubMed] [Google Scholar]
- Baranov V, Malysheva O, Yarmolinskaya M, 2018. Pathogenomics of endometriosis development. Int. J. Mol. Sci 19 (7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty MN, Blumenthal PD, 2009. The levonorgestrel-releasing intrauterine system: safety, efficacy, and patient acceptability. Ther. Clin. Risk Manag 5 (3), 561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilin B, Rusabrov Y, Shapira Y, et al. , 2007. Low-dose ketamine affects immune responses in humans during the early postoperative period. Br. J. Anaesth 99 (4), 522–527. [DOI] [PubMed] [Google Scholar]
- Benagiano G, Guo SW, Puttemans P, Gordts S, Brosens I, 2018. Progress in the diagnosis and management of adolescent endometriosis: an opinion. Reprod. Biomed. Online 36 (1), 102–114. [DOI] [PubMed] [Google Scholar]
- Bergeron S, Binik YM, Khalife S, et al. , 2001. A randomized comparison of group cognitive–behavioral therapy, surface electromyographic biofeedback, and vestibulectomy in the treatment of dyspareunia resulting from vulvar vestibulitis. Pain 91 (3), 297–306. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Robbins A, Sato Y, 1993a. Functional differences between afferent fibers in the hypogastric and pelvic nerves innervating female reproductive organs in the rat. J. Neurophysiol 69 (2), 533–544. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Hubscher CH, Wall PD, 1993b. Neuronal responses to stimulation of the cervix, uterus, colon, and skin in the rat spinal cord. J. Neurophysiol 69 (2), 545–556. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Cason A, Jacobs H, Bradshaw H, Wood E, 2001. Vaginal hyperalgesia in a rat model of endometriosis. Neurosci. Lett 306 (3), 185–188. [DOI] [PubMed] [Google Scholar]
- Berkley KJ, Dmitrieva N, Curtis KS, Papka RE, 2004. Innervation of ectopic endometrium in a rat model of endometriosis. Proc. Natl. Acad. Sci. U. S. A 101 (30), 11094–11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaziz J, Bar On A, Seidman DS, Soriano D, 2017. The clinical significance of endocannabinoids in endometriosis pain management. Cannabis Cannabinoid Res. 2 (1), 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchlariotou S, Tsikouras P, Benjamin R, Neulen J, 2012. Fertility sparing in cancer patients. Minim. Invasive Ther. Allied Technol 21 (4), 282–292. [DOI] [PubMed] [Google Scholar]
- Brawn J, Morotti M, Zondervan KT, Becker CM, Vincent K, 2014. Central changes associated with chronic pelvic pain and endometriosis. Hum. Reprod. Update 20 (5), 737–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressler LH, Bernardi LA, Snyder MA, Wei JJ, Bulun S, 2017. Treatment of endometriosis-related chronic pelvic pain with Ulipristal Acetate and associated endometrial changes. HSOA J Reprod Med Gynaecol Obstet. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown CS, Wan J, Bachmann G, Rosen R, 2009. Self-management, amitriptyline, and amitripyline plus triamcinolone in the management of vulvodynia. J Womens Health (Larchmt). 18 (2), 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulletins–Gynecology ACoP, 2004. ACOG practice bulletin No. 51. Chronic pelvic pain. Obstet. Gynecol 103 (3), 589–605. [PubMed] [Google Scholar]
- Bulletti C, Coccia ME, Battistoni S, Borini A, 2010. Endometriosis and infertility. J. Assist. Reprod. Genet 27 (8), 441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulun SE, Monsivais D, Kakinuma T, et al. , 2015. Molecular biology of endometriosis: from aromatase to genomic abnormalities. Semin. Reprod. Med 33 (3), 220–224. [DOI] [PubMed] [Google Scholar]
- Burney RO, Giudice LC, 2012. Pathogenesis and pathophysiology of endometriosis. Fertil. Steril 98 (3), 511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey ET, Martin CE, Siedhoff MT, Bair ED, As-Sanie S, 2014. Biopsychosocial correlates of persistent postsurgical pain in women with endometriosis. Int. J. Gynaecol. Obstet 124 (2), 169–173. [DOI] [PubMed] [Google Scholar]
- Casey CL, Murray CA, 2008. HT update: spotlight on estradiol/norethindrone acetate combination therapy. Clin. Interv. Aging 3 (1), 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaggioni G, Lia C, Resta S, et al. , 2014. Are mood and anxiety disorders and alexithymia associated with endometriosis? A preliminary study. Biomed Res. Int 2014, 786830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceyhan ST, Onguru O, Fidan U, et al. , 2011. Comparison of aromatase inhibitor (letrozole) and immunomodulators (infliximab and etanercept) on the regression of endometriotic implants in a rat model. Eur. J. Obstet. Gynecol. Reprod. Biol 154 (1), 100–104. [DOI] [PubMed] [Google Scholar]
- Chapron C, Souza C, Borghese B, et al. , 2011. Oral contraceptives and endometriosis: the past use of oral contraceptives for treating severe primary dysmenorrhea is associated with endometriosis, especially deep infiltrating endometriosis. Hum. Reprod 26 (8), 2028–2035. [DOI] [PubMed] [Google Scholar]
- Chaudhary SC, Singh T, Talwelkar SS, et al. , 2014. Erb-041, an estrogen receptor-beta agonist, inhibits skin photocarcinogenesis in SKH-1 hairless mice by down-regulating the WNT signaling pathway. Cancer Prev. Res. Phila. (Phila) 7 (2), 186–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia S, Gradishar W, Mauriac L, et al. , 2008. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J. Clin. Oncol 26 (10), 1664–1670. [DOI] [PubMed] [Google Scholar]
- Cuevas M, Cruz ML, Ramirez AE, et al. , 2018. Stress during development of experimental endometriosis influences nerve growth and disease progression. Reprod. Sci 25 (3), 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Hooghe T, Fukaya T, Osuga Y, et al. , 2019. Efficacy and safety of ASP1707 for endometriosis-associated pelvic pain: the phase II randomized controlled TERRA study. Hum. Reprod 34 (5), 813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpiaz O, Kerschbaumer A, Mitterberger M, Pinggera G, Bartsch G, Strasser H, 2008. Chronic pelvic pain in women: still a challenge. BJU Int. 102 (9), 1061–1065. [DOI] [PubMed] [Google Scholar]
- Davis GD, Thillet E, Lindemann J, 1993. Clinical characteristics of adolescent endometriosis. J. Adolesc. Health 14 (5), 362–368. [DOI] [PubMed] [Google Scholar]
- De Kock M, Loix S, Lavand’homme P, 2013. Ketamine and peripheral inflammation. CNS Neurosci. Ther 19 (6), 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira KMH, Garlet GP, De Rossi A, et al. , 2017. Effects of rosiglitazone on the outcome of experimental periapical lesions in mice. J. Endod 43 (12), 2061–2069. [DOI] [PubMed] [Google Scholar]
- Delgado-Rosas F, Gomez R, Ferrero H, et al. , 2011. The effects of ergot and non-ergot-derived dopamine agonists in an experimental mouse model of endometriosis. Reproduction. 142 (5), 745–755. [DOI] [PubMed] [Google Scholar]
- DeManno D, Elger W, Garg R, et al. , 2003. Asoprisnil (J867): a selective progesterone receptor modulator for gynecological therapy. Steroids. 68 (10–13), 1019–1032. [DOI] [PubMed] [Google Scholar]
- Dessole M, Melis GB, Angioni S, 2012. Endometriosis in adolescence. Obstet. Gynecol. Int 2012, 869191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divasta AD, Laufer MR, Gordon CM, 2007. Bone density in adolescents treated with a GnRH agonist and add-back therapy for endometriosis. J. Pediatr. Adolesc. Gynecol 20 (5), 293–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle JO, Missmer SA, Laufer MR, 2009. The effect of combined surgical-medical intervention on the progression of endometriosis in an adolescent and young adult population. J. Pediatr. Adolesc. Gynecol 22 (4), 257–263. [DOI] [PubMed] [Google Scholar]
- Dun EC, Kho KA, Morozov VV, Kearney S, Zurawin JL, Nezhat CH, 2015. Endometriosis in adolescents. JSLS 19 (2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunselman GA, Vermeulen N, Becker C, et al. , 2014. ESHRE guideline: management of women with endometriosis. Hum. Reprod 29 (3), 400–412. [DOI] [PubMed] [Google Scholar]
- Facchin F, Barbara G, Saita E, et al. , 2015. Impact of endometriosis on quality of life and mental health: pelvic pain makes the difference. J. Psychosom. Obstet. Gynaecol 36 (4), 135–141. [DOI] [PubMed] [Google Scholar]
- Fathizadeh N, Bafghi RK, Boroumandfar K, Savabi M, 2010. Comparing the effects of Yasmin(R) and LD (low-dose estrogen) as contraceptive methods on menstrual cycle changes in women referred to the health care centers of Isfahan. Iran. J. Nurs. Midwifery Res 15 (4), 252–258. [PMC free article] [PubMed] [Google Scholar]
- Ferrero S, Remorgida V, Venturini PL, Bizzarri N, 2015. Endometriosis: the effects of dienogest. BMJ Clin. Evid 2015. [PMC free article] [PubMed] [Google Scholar]
- Friedl F, Riedl D, Fessler S, et al. , 2015. Impact of endometriosis on quality of life, anxiety, and depression: an Austrian perspective. Arch. Gynecol. Obstet 292 (6), 1393–1399. [DOI] [PubMed] [Google Scholar]
- Gallagher JS, DiVasta AD, Vitonis AF, Sarda V, Laufer MR, Missmer SA, 2018. The impact of endometriosis on quality of life in adolescents. J. Adolesc. Health 63 (6), 766–772. [DOI] [PubMed] [Google Scholar]
- Ghaly AF, Chien PW, 2000. Chronic pelvic pain: clinical dilemma or clinician’s nightmare. Sex. Transm. Infect 76 (6), 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamberardino MA, Tana C, Costantini R, 2014. Pain thresholds in women with chronic pelvic pain. Curr. Opin. Obstet. Gynecol 26 (4), 253–259. [DOI] [PubMed] [Google Scholar]
- Godin R, Marcoux V, 2015. Vaginally administered danazol: an overlooked option in the treatment of rectovaginal endometriosis? J. Obstet. Gynaecol. Can 37 (12), 1098–1103. [DOI] [PubMed] [Google Scholar]
- Goenka L, George M, Sen M, 2017. A peek into the drug development scenario of endometriosis - A systematic review. Biomed. Pharmacother 90, 575–585. [DOI] [PubMed] [Google Scholar]
- Gracely RH, Petzke F, Wolf JM, Clauw DJ, 2002. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 46 (5), 1333–1343. [DOI] [PubMed] [Google Scholar]
- Greaves E, Horne AW, Jerina H, et al. , 2017. EP2 receptor antagonism reduces peripheral and central hyperalgesia in a preclinical mouse model of endometriosis. Sci. Rep 7, 44169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene AD, Lang SA, Kendziorski JA, Sroga-Rios JM, Herzog TJ, Burns KA, 2016. Endometriosis: where are we and where are we going? Reproduction 152 (3), R63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundstrom H, Gerdle B, Alehagen S, Bertero C, Arendt-Nielsen L, Kjolhede P, 2019. Reduced pain thresholds and signs of sensitization in women with persistent pelvic pain and suspected endometriosis. Acta Obstet. Gynecol. Scand 98 (3), 327–336. [DOI] [PubMed] [Google Scholar]
- Gugliandolo E, Fusco R, Biundo F, et al. , 2017. Palmitoylethanolamide and Polydatin combination reduces inflammation and oxidative stress in vascular injury. Pharmacol. Res 123, 83–92. [DOI] [PubMed] [Google Scholar]
- Haas D, Chvatal R, Habelsberger A, Wurm P, Schimetta W, Oppelt P, 2011. Comparison of revised American Fertility Society and ENZIAN staging: a critical evaluation of classifications of endometriosis on the basis of our patient population. Fertil. Steril 95 (5), 1574–1578. [DOI] [PubMed] [Google Scholar]
- Harris RE, Napadow V, Huggins JP, et al. , 2013. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology 119 (6), 1453–1464. [DOI] [PubMed] [Google Scholar]
- Harris HR, Wieser F, Vitonis AF, et al. , 2018. Early life abuse and risk of endometriosis. Hum. Reprod 33 (9), 1657–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman KM, Patanwala IY, Pozolo KE, Tu FF, 2015. Multimodal nociceptive mechanisms underlying chronic pelvic pain. Am. J. Obstet. Gynecol 213 (6), e821–829 827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herington JL, Bruner-Tran KL, Lucas JA, Osteen KG, 2011. Immune interactions in endometriosis. Expert Rev. Clin. Immunol 7 (5), 611–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez S, Cruz ML, Seguinot II, Torres-Reveron A, Appleyard CB, 2017. Impact of psychological stress on pain perception in an animal model of endometriosis. Reprod. Sci 24 (10), 1371–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsh AT, George SZ, Bialosky JE, Robinson ME, 2008. Fear of pain, pain catastrophizing, and acute pain perception: relative prediction and timing of assessment. J. Pain 9 (9), 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein MD, Surrey ES, Weisberg GW, Casino LA, 1998. Leuprolide acetate depot and hormonal add-back in endometriosis: a 12-month study. Lupron Add-Back Study Group. Obstet. Gynecol 91 (1), 16–24. [DOI] [PubMed] [Google Scholar]
- Hwang H, Chung YJ, Lee SR, et al. , 2018. Clinical evaluation and management of endometriosis: guideline for Korean patients from Korean Society of Endometriosis. Obstet. Gynecol. Sci 61 (5), 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell J, Ross S, Robert M, et al. , 2014. Prediction of postoperative pain after gynecologic laparoscopy for nonacute pelvic pain. Am. J. Obstet. Gynecol 211 (4), e361–368 360. [DOI] [PubMed] [Google Scholar]
- Jia SZ, Leng JH, Shi JH, Sun PR, Lang JH, 2012. Health-related quality of life in women with endometriosis: a systematic review. J. Ovarian Res 5 (1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NP, Hummelshoj L, Adamson GD, et al. , 2017. World Endometriosis Society consensus on the classification of endometriosis. Hum. Reprod 32 (2), 315–324. [DOI] [PubMed] [Google Scholar]
- Jouhari S, Mohammadzadeh A, Soltanghoraee H, et al. , 2018. Effects of silymarin, cabergoline and letrozole on rat model of endometriosis. Taiwan. J. Obstet. Gynecol 57 (6), 830–835. [DOI] [PubMed] [Google Scholar]
- Katz J, Seltzer Z, 2009. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev. Neurother 9 (5), 723–744. [DOI] [PubMed] [Google Scholar]
- Klein S, D’Hooghe T, Meuleman C, Dirksen C, Dunselman G, Simoens S, 2014. What is the societal burden of endometriosis-associated symptoms? A prospective Belgian study. Reprod. Biomed. Online 28 (1), 116–124. [DOI] [PubMed] [Google Scholar]
- Kontoravdis A, Hassan E, Hassiakos D, Botsis D, Kontoravdis N, Creatsas G, 1999. Laparoscopic evaluation and management of chronic pelvic pain during adolescence. Clin. Exp. Obstet. Gynecol 26 (2), 76–77. [PubMed] [Google Scholar]
- Kvaskoff M, Mu F, Terry KL, et al. , 2015. Endometriosis: a high-risk population for major chronic diseases? Hum. Reprod. Update 21 (4), 500–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagana AS, La Rosa VL, Rapisarda AMC, et al. , 2017. Anxiety and depression in patients with endometriosis: impact and management challenges. Int. J. Womens Health 9, 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufer MR, 2008. Current approaches to optimizing the treatment of endometriosis in adolescents. Gynecol. Obstet. Invest 66 (Suppl. 1), 19–27. [DOI] [PubMed] [Google Scholar]
- Laufer MR, Sanfilippo J, Rose G, 2003. Adolescent endometriosis: diagnosis and treatment approaches. J. Pediatr. Adolesc. Gynecol 16 (3 Suppl), S3–11. [DOI] [PubMed] [Google Scholar]
- Leone Roberti Maggiore U, Scala C, Remorgida V, et al. , 2014. Triptorelin for the treatment of endometriosis. Expert Opin. Pharmacother 15 (8), 1153–1179. [DOI] [PubMed] [Google Scholar]
- Liebermann C, Kohl Schwartz AS, Charpidou T, et al. , 2018. Maltreatment during childhood: a risk factor for the development of endometriosis? Hum. Reprod [DOI] [PubMed] [Google Scholar]
- L MR, 2000. Premenarcheal endometriosis without an associated obstructive anomaly: presentation, diagnosis, and treatment. Fertil. Steril 74 (3). [Google Scholar]
- Madsen AM, Stark LM, Has P, Emerson JB, Schulkin J, Matteson KA, 2018. Opioid knowledge and prescribing practices among obstetrician-gynecologists. Obstet. Gynecol 131 (1), 150–157. [DOI] [PubMed] [Google Scholar]
- Markham A, 2019. Relugolix: first global approval. Drugs. 79 (6), 675–679. [DOI] [PubMed] [Google Scholar]
- Marqui AB, 2015. Evaluation of endometriosis-associated pain and influence of conventional treatment: a systematic review. Rev. Assoc. Med. Bras. (1992) 61 (6), 507–518. [DOI] [PubMed] [Google Scholar]
- Martin CE, Johnson E, Wechter ME, Leserman J, Zolnoun DA, 2011. Catastrophizing: a predictor of persistent pain among women with endometriosis at 1 year. Hum. Reprod 26 (11), 3078–3084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masheb RM, Kerns RD, Lozano C, Minkin MJ, Richman S, 2009. A randomized clinical trial for women with vulvodynia: cognitive-behavioral therapy vs. Supportive psychotherapy. Pain 141 (1–2), 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean SA, Clauw DJ, Abelson JL, Liberzon I, 2005. The development of persistent pain and psychological morbidity after motor vehicle collision: integrating the potential role of stress response systems into a biopsychosocial model. Psychosom. Med 67 (5), 783–790. [DOI] [PubMed] [Google Scholar]
- Medina MG, Lebovic DI, 2009. Endometriosis-associated nerve fibers and pain. Acta Obstet. Gynecol. Scand 88 (9), 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelakis ED, Webster L, Mackey JR, 2008. Dichloroacetate (DCA) as a potential metabolic-targeting therapy for cancer. Br. J. Cancer 99 (7), 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller-Matero LR, Saulino C, Clark S, Bugenski M, Eshelman A, Eisenstein D, 2016. When treating the pain is not enough: a multidisciplinary approach for chronic pelvic pain. Arch. Womens Ment. Health 19 (2), 349–354. [DOI] [PubMed] [Google Scholar]
- Mirkin D, Murphy-Barron C, Iwasaki K, 2007. Actuarial analysis of private payer administrative claims data for women with endometriosis. J. Manag. Care Pharm 13 (3), 262–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell DR Jr., 1996. Pharmacokinetics of depot medroxyprogesterone acetate contraception. J. Reprod. Med 41 (5 Suppl), 381–390. [PubMed] [Google Scholar]
- Missmer SA, 2009. Commentary: endometriosis–epidemiologic considerations for a potentially ‘high-risk’ population. Int. J. Epidemiol 38 (4), 1154–1155. [DOI] [PubMed] [Google Scholar]
- Moller C, Bone W, Cleve A, et al. , 2018. Discovery of vilaprisan (BAY 1002670): a highly potent and selective progesterone receptor modulator optimized for gynecologic therapies. ChemMedChem. 13 (21), 2271–2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore HC, Unger JM, Phillips KA, et al. , 2015. Goserelin for ovarian protection during breast-cancer adjuvant chemotherapy. N. Engl. J. Med 372 (10), 923–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley S, Williams A, Hussain S, 2008. Estimating the clinical effectiveness of cognitive behavioural therapy in the clinic: evaluation of a CBT informed pain management programme. Pain 137 (3), 670–680. [DOI] [PubMed] [Google Scholar]
- Mosher AA, Tsoulis MW, Lim J, et al. , 2019. Melatonin activity and receptor expression in endometrial tissue and endometriosis. Hum. Reprod 34 (7), 1215–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol AL, Sieberg CB, Clauw DJ, Hassett AL, Moser SE, Brummett CM, 2016. The association between a history of lifetime traumatic events and pain severity, physical function, and affective distress in patients with chronic pain. J. Pain 17 (12), 1334–1348. [DOI] [PubMed] [Google Scholar]
- Niesters M, Hoitsma E, Sarton E, Aarts L, Dahan A, 2011. Offset analgesia in neuropathic pain patients and effect of treatment with morphine and ketamine. Anesthesiology 115, 1063–1071 In press. [DOI] [PubMed] [Google Scholar]
- Niv D, Devor M, 2007. European Federation of IC. Position paper of the European Federation of IASP Chapters (EFIC) on the subject of pain management. Eur. J. Pain 11 (5), 487–489. [DOI] [PubMed] [Google Scholar]
- Nothnick WB, 2011. The emerging use of aromatase inhibitors for endometriosis treatment. Reprod. Biol. Endocrinol 9, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paravar T, Lee DJ, 2008. Thalidomide: mechanisms of action. Int. Rev. Immunol 27 (3), 111–135. [DOI] [PubMed] [Google Scholar]
- Parazzini F, Esposito G, Tozzi L, Noli S, Bianchi S, 2017. Epidemiology of endometriosis and its comorbidities. Eur. J. Obstet. Gynecol. Reprod. Biol 209, 3–7. [DOI] [PubMed] [Google Scholar]
- Pinho-Ribeiro FA, Verri WA Jr., Chiu IM, 2017. Nociceptor sensory neuron-immune interactions in pain and inflammation. Trends Immunol. 38 (1), 5–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl O, Bestel E, Gotteland JP, 2014. Synergistic effects of E2MATE and norethindrone acetate on steroid sulfatase inhibition: a randomized phase I proof-of-principle clinical study in women of reproductive age. Reprod. Sci 21 (10), 1256–1265. [DOI] [PubMed] [Google Scholar]
- Powell J, 2014. The approach to chronic pelvic pain in the adolescent. Obstet. Gynecol. Clin. North Am 41 (3), 343–355. [DOI] [PubMed] [Google Scholar]
- Rafique S, Decherney AH, 2017. Medical management of endometriosis. Clin. Obstet. Gynecol 60 (3), 485–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K, Dubner R, 2010. Interactions between the immune and nervous systems in pain. Nat. Med 16 (11), 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards D, Gever JR, Ford AP, Fountain SJ, 2019. Action of MK-7264 (gefapixant) at human P2X3 and P2X2/3 receptors and in vivo efficacy in models of sensitisation. Br. J. Pharmacol 176 (13), 2279–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolke R, Baron R, Maier C, et al. , 2006. Quantitative sensory testing in the German Research Network on Neuropathic Pain (DFNS): standardized protocol and reference values. Pain. 123 (3), 231–243. [DOI] [PubMed] [Google Scholar]
- Rolla E, 2019. Endometriosis: advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Res. 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romao AP, Gorayeb R, Romao GS, et al. , 2009. High levels of anxiety and depression have a negative effect on quality of life of women with chronic pelvic pain. Int. J. Clin. Pract 63 (5), 707–711. [DOI] [PubMed] [Google Scholar]
- Roth RS, Punch MR, Bachman JE, 2011. Psychological factors and chronic pelvic pain in women: a comparative study with women with chronic migraine headaches. Health Care Women Int. 32 (8), 746–761. [DOI] [PubMed] [Google Scholar]
- Rowlands IJ, Teede H, Lucke J, Dobson AJ, Mishra GD, 2016. Young women’s psychological distress after a diagnosis of polycystic ovary syndrome or endometriosis. Hum. Reprod 31 (9), 2072–2081. [DOI] [PubMed] [Google Scholar]
- Santen RJ, 1992. Clinical review 37: endocrine treatment of prostate cancer. J. Clin. Endocrinol. Metab 75 (3), 685–689. [DOI] [PubMed] [Google Scholar]
- Savidge CJ, Slade P, 1997. Psychological aspects of chronic pelvic pain. J. Psychosom. Res 42 (5), 433–444. [DOI] [PubMed] [Google Scholar]
- Schliep KC, Mumford SL, Peterson CM, et al. , 2015. Pain typology and incident endometriosis. Hum. Reprod 30 (10), 2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shy ME, Frohman EM, So YT, et al. , 2003. Quantitative sensory testing: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 60 (6), 898–904. [DOI] [PubMed] [Google Scholar]
- Siedentopf F, Tariverdian N, Rucke M, Kentenich H, Arck PC, 2008. Immune status, psychosocial distress and reduced quality of life in infertile patients with endometriosis. Am J Reprod Immunol. 60 (5), 449–461. [DOI] [PubMed] [Google Scholar]
- Simis M, Reidler JS, Duarte Macea D, et al. , 2015. Investigation of central nervous system dysfunction in chronic pelvic pain using magnetic resonance spectroscopy and noninvasive brain stimulation. Pain Pract. 15 (5), 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoens S, Dunselman G, Dirksen C, et al. , 2012. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum. Reprod 27 (5), 1292–1299. [DOI] [PubMed] [Google Scholar]
- Simsek Y, Gul M, Yilmaz E, Ozerol IH, Ozerol E, Parlakpinar H, 2014. Atorvastatin exerts anti-nociceptive activity and decreases serum levels of high-sensitivity C-reactive protein and tumor necrosis factor-alpha in a rat endometriosis model. Arch. Gynecol. Obstet 290 (5), 999–1006. [DOI] [PubMed] [Google Scholar]
- Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P, 2002. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum. Reprod 17 (10), 2715–2724. [DOI] [PubMed] [Google Scholar]
- Soave I, Caserta D, Wenger JM, Dessole S, Perino A, Marci R, 2015. Environment and Endometriosis: a toxic relationship. Eur. Rev. Med. Pharmacol. Sci 19 (11), 1964–1972. [PubMed] [Google Scholar]
- Sokalska A, Hawkins AB, Yamaguchi T, Duleba AJ, 2019. Lipophilic statins inhibit growth and reduce invasiveness of human endometrial stromal cells. J. Assist. Reprod. Genet 36 (3), 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman AM, Coyne KS, Zaiser E, Castelli-Haley J, Fuldeore MJ, 2017. The burden of endometriosis symptoms on health-related quality of life in women in the United States: a cross-sectional study. J. Psychosom. Obstet. Gynaecol 38 (4), 238–248. [DOI] [PubMed] [Google Scholar]
- Souza CA, Oliveira LM, Scheffel C, et al. , 2011. Quality of life associated to chronic pelvic pain is independent of endometriosis diagnosis–a cross-sectional survey. Health Qual. Life Outcomes 9, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavroulis AI, Saridogan E, Creighton SM, Cutner AS, 2006. Laparoscopic treatment of endometriosis in teenagers. Eur. J. Obstet. Gynecol. Reprod. Biol 125 (2), 248–250. [DOI] [PubMed] [Google Scholar]
- Stocks MM, Crispens MA, Ding T, Mokshagundam S, Bruner-Tran KL, Osteen KG, 2017. Therapeutically targeting the inflammasome product in a chimeric model of endometriosis-related surgical adhesions. Reprod. Sci 24 (8), 1121–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stones RW, Mountfield J, 2000. Interventions for treating chronic pelvic pain in women. Cochrane Database Syst. Rev (4), CD000387. [DOI] [PubMed] [Google Scholar]
- Stratton P, Berkley KJ, 2011. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum. Reprod. Update 17 (3), 327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuparich MA, Donnellan NM, Sanfilippo JS, 2017. Endometriosis in the adolescent patient. Semin. Reprod. Med 35 (1), 102–109. [DOI] [PubMed] [Google Scholar]
- Sullivan MJ, Thorn B, Haythornthwaite JA, et al. , 2001. Theoretical perspectives on the relation between catastrophizing and pain. Clin. J. Pain 17 (1), 52–64. [DOI] [PubMed] [Google Scholar]
- Surrey ES, Soliman AM, Johnson SJ, Davis M, Castelli-Haley J, Snabes MC, 2018. Risk of developing comorbidities among women with endometriosis: a retrospective matched cohort study. J Womens Health (Larchmt). 27 (9), 1114–1123. [DOI] [PubMed] [Google Scholar]
- Symons LK, Miller JE, Kay VR, et al. , 2018. The immunopathophysiology of endometriosis. Trends Mol. Med 24 (9), 748–762. [DOI] [PubMed] [Google Scholar]
- Talbot NL, Chapman B, Conwell Y, et al. , 2009. Childhood sexual abuse is associated with physical illness burden and functioning in psychiatric patients 50 years of age and older. Psychosom. Med 71 (4), 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanbo T, Fedorcsak P, 2017. Endometriosis-associated infertility: aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand 96 (6), 659–667. [DOI] [PubMed] [Google Scholar]
- Tandoi I, Somigliana E, Riparini J, Ronzoni S, Vigano P, Candiani M, 2011. High rate of endometriosis recurrence in young women. J. Pediatr. Adolesc. Gynecol 24 (6), 376–379. [DOI] [PubMed] [Google Scholar]
- Tandrasasmita OM, Sutanto AM, Arifin PF, Tjandrawinata RR, 2015. Anti-inflammatory, antiangiogenic, and apoptosis-inducing activity of DLBS1442, a bioactive fraction of Phaleria macrocarpa, in a RL95–2 cell line as a molecular model of endometriosis. Int. J. Womens Health 7, 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanmahasamut P, Saejong R, Rattanachaiyanont M, Angsuwathana S, Techatraisak K, Sanga-Areekul N, 2017. Postoperative desogestrel for pelvic endometriosis-related pain: a randomized controlled trial. Gynecol. Endocrinol 33 (7), 534–539. [DOI] [PubMed] [Google Scholar]
- Templeman C, 2009. Adolescent endometriosis. Obstet. Gynecol. Clin. North Am 36 (1), 177–185. [DOI] [PubMed] [Google Scholar]
- Torres-Reveron A, Rivera LL, Flores I, Appleyard CB, 2018. Environmental manipulations as an effective alternative treatment to reduce endometriosis progression. Reprod. Sci 25 (9), 1336–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosti C, Biscione A, Morgante G, Bifulco G, Luisi S, Petraglia F, 2017. Hormonal therapy for endometriosis: from molecular research to bedside. Eur. J. Obstet. Gynecol. Reprod. Biol 209, 61–66. [DOI] [PubMed] [Google Scholar]
- Totsch SK, Sorge RE, 2017. Immune system involvement in specific pain conditions. Mol. Pain 13, 1744806917724559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu FF, Fitzgerald CM, Kuiken T, Farrell T, Norman Harden R, 2008. Vaginal pressure-pain thresholds: initial validation and reliability assessment in healthy women. Clin. J. Pain 24 (1), 45–50. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Vigano P, Somigliana E, Fedele L, 2014. Endometriosis: pathogenesis and treatment. Nat. Rev. Endocrinol 10 (5), 261–275. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Buggio L, Berlanda N, Barbara G, Somigliana E, Bosari S, 2016. Estrogen-progestins and progestins for the management of endometriosis. Fertil. Steril 106 (7), 1552–1571 e1552. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Buggio L, Frattaruolo MP, Borghi A, Dridi D, Somigliana E, 2018. Medical treatment of endometriosis-related pain. Best Pract. Res. Clin. Obstet. Gynaecol 51, 68–91. [DOI] [PubMed] [Google Scholar]
- Vercellini P, Vigano P, Barbara G, Buggio L, Somigliana E, 2019. Luigi Mangiagalli’ Endometriosis Study G. Elagolix for endometriosis: all that glitters is not gold. Hum. Reprod 34 (2), 193–199. [DOI] [PubMed] [Google Scholar]
- Vitale SG, La Rosa VL, Rapisarda AMC, Lagana AS, 2017. Impact of endometriosis on quality of life and psychological well-being. J. Psychosom. Obstet. Gynaecol 38 (4), 317–319. [DOI] [PubMed] [Google Scholar]
- Wattier JM, 2018. Conventional analgesics and non-pharmacological multidisciplinary therapeutic treatment in endometriosis: CNGOF-HAS Endometriosis Guidelines. Gynecol. Obstet. Fertil. Senol 46 (3), 248–255. [DOI] [PubMed] [Google Scholar]
- Weijenborg PT, Greeven A, Dekker FW, Peters AA, Ter Kuile MM, 2007. Clinical course of chronic pelvic pain in women. Pain 132 (Suppl. 1), S117–123. [DOI] [PubMed] [Google Scholar]
- Whitaker LH, Reid J, Choa A, et al. , 2016. An exploratory study into objective and reported characteristics of neuropathic pain in women with chronic pelvic pain. PLoS One 11 (4), e0151950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom K, Bruse C, Sjosten A, Spira J, Edelstam G, 2013. Quality of life in patients with endometriosis and the effect of pertubation with lidocaine - a randomized controlled trial. Acta Obstet. Gynecol. Scand 92 (12), 1375–1382. [DOI] [PubMed] [Google Scholar]
- Wilder-Smith OH, 2011. Chronic pain and surgery: a review of new insights from sensory testing. J. Pain Palliat. Care Pharmacother 25 (2), 146–159. [DOI] [PubMed] [Google Scholar]
- Wilson AC, Meethal SV, Bowen RL, Atwood CS, 2007. Leuprolide acetate: a drug of diverse clinical applications. Expert Opin. Investig. Drugs 16 (11), 1851–1863. [DOI] [PubMed] [Google Scholar]
- Yano M, Matsuda A, Natsume T, et al. , 2019. Pain-related behavior and brain activation in cynomolgus macaques with naturally occurring endometriosis. Hum. Reprod 34 (3), 469–478. [DOI] [PubMed] [Google Scholar]
- Yeung P, Gupta S, Gieg S, 2017. Endometriosis in adolescents: a systematic review. J. Endometr. Pelvic Pain Disord 9 (1), 17–29. [Google Scholar]
- Young K, Fisher J, Kirkman M, 2017. Clinicians’ perceptions of women’s experiences of endometriosis and of psychosocial care for endometriosis. Aust. N. Z. J. Obstet. Gynaecol 57 (1), 87–92. [DOI] [PubMed] [Google Scholar]


