Abstract
Rationale: There have been advances in both cancer and sepsis treatment over the past several decades, yet little is known about trends in sepsis-associated mortality in patients with versus without cancer.
Objectives: To assess trends in sepsis-associated mortality in hospitalized patients with and without cancer using objective clinical criteria to identify sepsis and detailed clinical data to adjust for severity of illness.
Methods: This was a retrospective cohort study at a tertiary referral hospital and cancer center. Adult in-patients with clinical indicators of sepsis (U.S. Centers for Disease Control and Prevention Adult Sepsis Event criteria) were identified between 2003 and 2014. Patients with cancer were identified using diagnosis codes from their hospitalization or the preceding 90 days. Sepsis-associated in-hospital mortality rates were assessed in 3-year intervals. Multivariable logistic regression models were used to adjust for case mix and severity of illness and to test for subgroup interactions in trends.
Results: The cohort included 20,975 patients with sepsis, of whom 7,489 (35.7%) had cancer (61.7% solid and 38.3% hematologic). Sepsis-associated mortality rates in patients with cancer decreased from 31.3% in 2003–2005 to 26.0% in 2012–2014 (absolute decrease, 5.2% [95% confidence interval (CI), 2.3–8.2%]). This mortality reduction persisted after risk adjustment (adjusted odds ratio, 0.53 [95% CI, 0.45–0.63] in 2012–2014 relative to 2003–2005). In contrast, sepsis-associated mortality rates increased in patients without cancer from 20.9% in 2003–2005 to 23.9% in 2012–2014 (absolute increase, 2.1% [95% CI, 0.1–4.1%]), but were stable after risk-adjustment (adjusted odds ratio, 0.90 [95% CI, 0.79–1.03]) (P < 0.001 for comparison of trends between patients with vs. without cancer on both crude and adjusted analysis). Among patients with cancer, declines in risk-adjusted sepsis-associated mortality were observed in both solid and hematologic cancer subgroups, with both community-onset and hospital-onset sepsis, in patients receiving active cancer treatments, and in patients requiring mechanical ventilation at sepsis onset.
Conclusions: Sepsis-associated mortality rates declined significantly over a 12-year period in patients with cancer, but not in patients without cancer. Potential explanations include advances in the management of cancer and/or better sepsis treatments specifically in patients with cancer. Further research is needed to elucidate the reasons for our findings and to assess their generalizability to other hospitals.
Keywords: sepsis, cancer, Adult Sepsis Event, trends
Sepsis affects an estimated 1.7 million adults each year in the United States, ranks among the most costly diseases, and contributes to up to half of all deaths in hospitalized patients (1–4). Although sepsis can affect anyone, most patients who develop sepsis have underlying chronic conditions (5, 6). Cancer in particular is one of the most common predisposing conditions as a consequence of anatomic disruptions from tumor invasion, immunosuppression, frequent hospital stays, medical procedures, and frailty (3, 7, 8).
There has been an increasing focus on early sepsis recognition and treatment over the past few decades; concurrently, major advances in cancer treatment have helped reduce cancer death rates (9, 10). However, relatively little is known about whether and how mortality rates have changed in patients with cancer and sepsis. Prior epidemiologic studies of patients with cancer using administrative data to identify sepsis have suggested that sepsis mortality rates are declining, but comparisons of studies from different time periods are confounded by different administrative definitions as well as improvements in sepsis coding practices over time, leading to the inclusion of less severe cases (8, 11–19).
The aim of this study was to use detailed electronic health record data to compare crude and adjusted sepsis-associated mortality rates in patients with and without cancer over time in a major academic medical center serving a large oncology population. We identified sepsis using the validated U.S. Centers for Disease Control and Prevention (CDC) Adult Sepsis Event surveillance definition, which uses objective clinical markers of presumed infection and concurrent acute organ dysfunction to identify sepsis rather than administrative codes and therefore enables more reliable longitudinal assessments of sepsis epidemiology (1, 20).
Methods
Study Design and Data Source
This was a retrospective cohort study of adults 18 years of age or older who had sepsis during hospitalizations between January 1, 2003, and December 31, 2014 at Brigham and Women’s Hospital, a 779-bed tertiary academic hospital that cares for a large oncology population by serving as the in-patient facility for the Dana Farber Cancer Institute. Our primary data source was the Research Patient Data Registry, a centralized clinical data warehouse containing demographic, administrative, medication, and laboratory data (21). Data on mechanical ventilation were obtained from a database maintained by the hospital’s Respiratory Therapy Department as well as International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (96.7×) or Current Procedural Terminology codes (94002, 94003, or 94004) (17). The study was approved by the Partners Healthcare Institutional Review Board with a waiver of informed consent.
Identifying Sepsis and Cancer
Sepsis cases were identified using CDC Adult Sepsis Event criteria, which identify hospitalizations with clinical indicators of presumed serious infection and concurrent organ dysfunction (20). This definition has high concordance with Sepsis-3 criteria, but is optimized for objective retrospective surveillance using electronic health record data (22). Presumed serious infection is defined as a blood culture order and administration of 4 or more consecutive days of new antibiotics (fewer if death, discharge to hospice, or discharge to another acute care hospital occurs before 4 d) starting from 2 days before through 2 days after the blood culture day. Organ dysfunction includes initiation of vasopressors or mechanical ventilation, lactate 2.0 mmol/L or greater, doubling in baseline creatinine, or decrease in estimated glomerular filtration rate by 50% or greater, doubling in total bilirubin to 2.0 mg/dL or greater, or 50% or greater decrease in platelet count to less than 100 cells/μl. For this analysis, we modified CDC criteria to: 1) exclude the platelet criterion to minimize false positives secondary to chemotherapy; 2) exclude phenylephrine as a qualifying vasopressor, because our dataset does not allow us to distinguish vasopressors given in the operating room, and phenylephrine is rarely a first-line vasopressor for sepsis in our institution; and 3) exclude the lactate criterion, because rates of lactate testing increased substantially over the study timeframe in our institution, which could cause ascertainment bias from identification of milder sepsis cases over time (1, 17, 23).
Patients with cancer were identified using the Agency for Healthcare Research and Quality’s (AHRQ’s) Clinical Classifications Software, which specifies ICD-9-CM codes for 35 different solid and hematologic malignancies (24). For descriptive purposes, we consolidated the diagnoses into five separate solid cancer categories (breast, gastrointestinal, genitourinary, lung, and other) and three hematologic cancer categories (leukemia, lymphoma, and multiple myeloma) based on clinical assessment of their relatedness and prior literature (25). We identified patients with solid cancer with metastases using ICD-9-CM codes as previously described (25), and stem cell transplants using codes V42.81 or V42.82. Codes from the index sepsis hospitalization or any in-patient or out-patient visit within the preceding 90 days were used. Patients with both hematologic and solid malignancies were categorized as solid cancer if they had solid tumor metastases (n = 73); otherwise, they were classified as having a hematologic malignancy (n = 286). We further identified patients on active cancer therapy during the index sepsis hospitalization or within the prior 90 days (including home medications, out-patient infusions, and targeted molecular agents). All ICD-9-CM codes and cancer medications are shown in Appendices E1 and E2 in the online supplement.
To assess the accuracy of the AHRQ cancer diagnosis codes, one auditor (A.J.C.) reviewed 200 randomly selected sepsis hospitalizations (including patients with and without cancer) using a standardized data abstraction form to ascertain the presence and type of cancer. The AHRQ cancer classifications had good performance relative to medical record review: 85.2% sensitivity (95% confidence interval [CI], 72.9–93.4%) and 90.2% positive predictive value (95% CI, 78.6–96.7%) for solid cancer, and 72.2% sensitivity (95% CI, 54.8–85.8%) and 100% positive predictive value (95% CI, 86.8–100%) for hematologic cancer (Table E1).
Statistical Analyses and Trends in Sepsis-associated Mortality
Descriptive analyses were performed evaluating the characteristics of patients with sepsis with and without cancer. Crude in-hospital mortality rates were calculated for each cancer type, stratified by presence or absence of solid metastases for solid cancer and stem cell transplants for hematologic cancer. Comorbidities were imputed using the Elixhauser method (26). Infectious syndromes were determined using ICD-9-CM codes (Appendix E3).
In patients with and without cancer, we calculated crude sepsis-associated in-hospital mortality rates in 3-year intervals to maximize the precision of our estimates, with a focus on comparing the 2012–2014 time period to the baseline 2003–2005 period. To formally compare trends between patients with versus without cancer, we fit interaction terms between cancer and time (in 3-yr intervals) in a logistic regression model. We then used multivariable logistic regression models to adjust trends for case mix and severity of illness. We did not include any ICD-9-CM–based covariates to minimize any ascertainment bias that might arise from changes in coding practices over time (27, 28). Instead, we adjusted for objective demographic factors (age, sex, and race) and clinical markers of severity of illness (initiation of vasopressors, mechanical ventilation, albumin, anion gap, aspartate aminotransferase, creatinine, hematocrit, sodium, total bilirubin, white blood cell count, and positive blood cultures for noncommensal organisms). Severity of illness was identified using the worst laboratory values from 1 day prior through 1 day after the day of sepsis onset, defined as the first antibiotic administration or blood culture in the window period when CDC criteria were met (20). Laboratory values were dichotomized as normal versus abnormal using clinically relevant cut points (Table E2). Missing covariates for laboratory values were assumed to be normal, as is commonly done for clinical severity-of-illness scores (29). Rates of missing laboratory values were generally low (<2% for complete blood counts and basic chemistries, <15% for liver function tests). Lactate, however, was frequently missing. The rate of lactate missingness diminished over the study period and so was not included as a covariate to minimize ascertainment bias (missing data rates are summarized in Figure E1 in the online supplement).
Among the cancer cohort, we calculated trends in several important subgroups: 1) patients requiring mechanical ventilation at sepsis onset, given recent reports of steadily high mortality in this population (30–32); 2) patients on active cancer treatment, to understand whether mortality trends might be affected by changes in the ratio of patients with active disease versus those in remission; and 3) patients with community-onset and hospital-onset sepsis to understand whether trends might be affected by differential care delivered in the emergency department versus within the hospital. Sepsis was considered hospital onset if the blood culture, first antibiotic day, and organ dysfunction all occurred on Hospital Day 3 or later (33). To explore potential clinical factors that might affect sepsis outcomes in patients with cancer, we also examined trends in the use of targeted molecular cancer drugs, granulocyte-colony–stimulating factor (G-CSF), and antifungal medications that became available during the study period (echinocandins and triazoles).
In a sensitivity analysis, we assessed trends in the combined outcome of both in-hospital death and discharge to hospice, because hospice is an increasingly common end-of-life destination for patients with sepsis and cancer (34). To examine how trends differ when identifying sepsis using administrative codes versus consistent clinical criteria, we analyzed outcomes in hospitalizations meeting modified Angus criteria, which requires concurrent diagnosis codes for infection and acute organ dysfunction, or explicit codes for severe sepsis (995.92) or septic shock (785.52) (7, 14).
All tests of significance used two-sided P values at less than 0.05. Analyses were conducted using SAS version 9.4 (SAS Institute).
Results
Characteristics and Outcomes of Patients with Sepsis
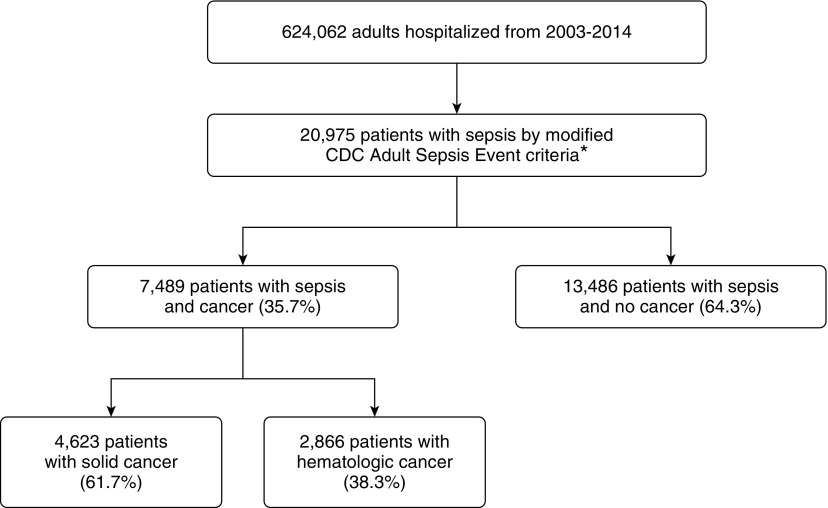
The study cohort included 20,975 hospitalized patients with sepsis; 7,489 (35.7%) had cancer, including 4,623 with solid cancer (2,979 [64.4%] with metastatic disease) and 2,866 with hematologic cancer (413 [14.4%] with stem cell transplants) (Figure 1). Patient characteristics are shown in Table 1.
Figure 1.
Flow diagram for study cohort derivation. *The U.S. Centers for Disease Control and Prevention (CDC) Adult Sepsis Event definition was modified to exclude patients who otherwise would meet organ dysfunction criteria solely based on elevated lactate, decreased platelets, or phenylephrine use.
Table 1.
Characteristics of patients with sepsis with and without cancer, 2003–2014
| Characteristic | Solid Cancer* |
Hematologic Cancer* |
No Cancer |
|---|---|---|---|
| (n = 4,623) | (n = 2,866) | (n = 13,486) | |
| Age, yr, median (IQR) | 64 (55–72) | 58 (46–66) | 62 (49–74) |
| Male sex, n (%) | 2,519 (54.5) | 1,678 (58.6) | 7,381 (54.7) |
| Race | |||
| White, n (%) | 3,725 (80.6) | 2,472 (86.3) | 9,718 (72.1) |
| Black, n (%) | 365 (7.9) | 115 (4.0) | 1,641 (12.2) |
| Other, n (%) | 533 (11.5) | 279 (9.7) | 2,127 (15.8) |
| Comorbidities (Elixhauser) | |||
| Chronic lung disease, n (%) | 517 (11.2) | 182 (6.4) | 1,960 (14.5) |
| Congestive heart failure, n (%) | 492 (10.6) | 299 (10.4) | 3,560 (26.4) |
| Diabetes, n (%) | 431 (9.3) | 184 (6.4) | 2,090 (15.5) |
| Liver disease, n (%) | 179 (3.9) | 79 (2.8) | 709 (5.3) |
| Neurologic disease, n (%) | 245 (5.3) | 97 (3.4) | 1,334 (9.9) |
| Renal failure, n (%) | 304 (6.6) | 181 (6.3) | 1,739 (12.9) |
| Median Elixhauser score (IQR) | 17 (11–25) | 11 (5–17) | 11 (3–17) |
| Hospital-onset sepsis, n (%) | 1,686 (36.5) | 1,469 (51.3) | 4,353 (32.3) |
| Organ dysfunction at sepsis onset, n (%) | |||
| Vasopressors | 2,221 (47.8) | 1,093 (38.1) | 7,972 (59.1) |
| Mechanical ventilation | 1,568 (33.9) | 780 (27.2) | 6,358 (47.2) |
| Creatinine or eGFR | 2,157 (46.7) | 1,330 (46.4) | 5,993 (44.4) |
| Bilirubin | 1,135 (24.6) | 1,366 (47.7) | 2,625 (19.5) |
| Platelet | 794 (17.2) | 758 (26.5) | 2,686 (19.9) |
| WBC <1 × 109/L at sepsis onset, n (%) | 184 (4.0) | 884 (30.8) | 165 (1.2) |
| Infectious syndromes, n (%) | |||
| Pulmonary | 1,819 (39.4) | 1,146 (40.0) | 6,196 (45.9) |
| Urinary | 777 (16.8) | 319 (11.1) | 2,675 (19.8) |
| Intra-abdominal | 959 (20.7) | 400 (14.0) | 1,823 (13.5) |
| Skin/soft tissue | 236 (5.1) | 193 (6.7) | 910 (6.8) |
| Septicemia | 1,787 (38.7) | 1,230 (42.9) | 5,261 (39.0) |
| Bone/joint | 53 (1.2) | 31 (1.1) | 455 (3.4) |
| Central nervous system | 65 (1.4) | 53 (1.9) | 368 (2.7) |
| Obstetric/gynecologic | 13 (0.3) | 6 (0.2) | 104 (0.8) |
| Other | 920 (19.9) | 910 (31.8) | 3,191 (23.7) |
| Unknown source | 816 (17.7) | 552 (19.3) | 2,112 (15.7) |
| Positive blood cultures, n (%)† | 746 (16.1) | 618 (21.6) | 2,012 (14.9) |
| Gram-positive organism, n (%) | 413 (8.9) | 365 (12.7) | 1,274 (9.4) |
| Gram-negative organism, n (%) | 372 (8.0) | 286 (10.0) | 808 (6.0) |
| Fungal organism, n (%) | 75 (1.6) | 66 (2.3) | 205 (1.5) |
| Required ICU admission, n (%) | 2,505 (54.2) | 1,180 (41.2) | 8.623 (64.9) |
| Median hospital LOS, d, n (%) | 12 (7–21) | 21 (10–33) | 14 (8–24) |
| Discharge disposition, n (%) | |||
| Home | 1,692 (36.6) | 1,441 (50.3) | 4,516 (33.5) |
| Facility | 1,371 (29.7) | 528 (18.4) | 5,854 (43.4) |
| Hospice | 242 (5.2) | 70 (2.4) | 124 (0.9) |
| In-hospital death | 1,305 (28.2) | 825 (28.8) | 2,959 (21.9) |
Definition of abbreviations: eGFR = estimated glomerular filtration rate; ICU = intensive care unit; IQR = interquartile range; LOS = length of stay; WBC = white blood cell.
Patients with both hematologic and solid malignancies were categorized as having a solid cancer if they had solid tumor metastases (n = 73); otherwise they were categorized as having a hematologic malignancy (n = 286).
Positive blood cultures include any blood culture taken during a sepsis hospitalization. Some patients had multiple organisms isolated.
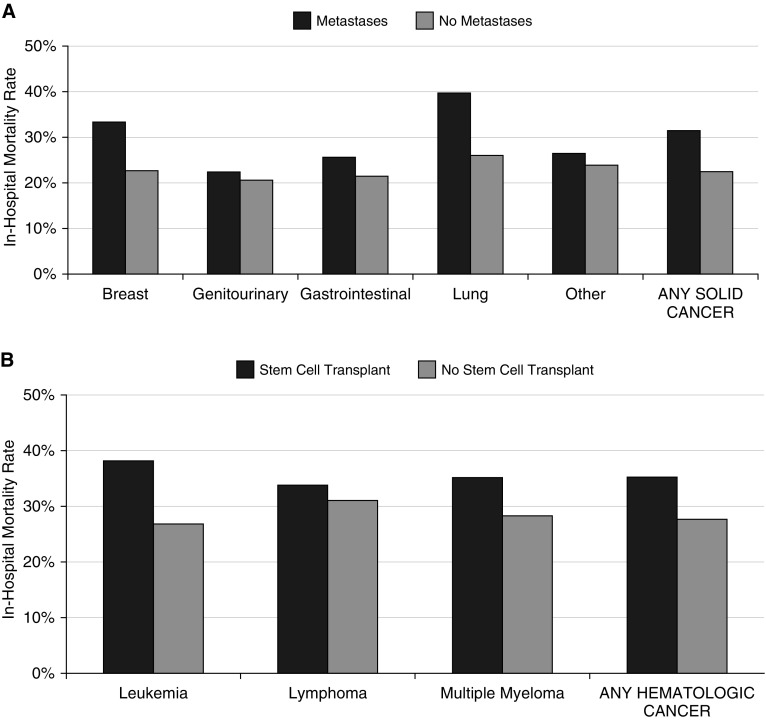
Overall sepsis-associated mortality rates were higher in patients with cancer (2,130/7,489, 28.4%) versus patients without cancer (2,959/13,486, 21.9%) (absolute difference, 6.5% [95% CI, 5.3–7.7%]). Mortality rates were similar with solid (28.2%) and hematologic cancer (28.8%) and higher among patients with solid cancer with versus without metastases (31.5% vs. 22.3%) and patients with hematologic cancer with versus without stem cell transplants (35.4% vs. 27.7%). The highest sepsis-associated mortality rates were seen in patients with metastatic lung cancer (39.6%), patients with leukemia and stem cell transplants (38.3%), and patients with multiple myeloma and stem cell transplants (35.3%) (Figure 2).
Figure 2.
Sepsis-associated in-hospital mortality rates by specific solid and hematologic cancer diagnosis categories. (A) Sepsis-associated mortality rates for patients with solid cancer, stratified by the presence of metastases. Sample size: breast (n = 213 with mets vs. 150 with no mets), genitourinary (n = 298 vs. 298), gastrointestinal (n = 822 vs. 529), lung (n = 848 vs. 143), other (n = 591 vs. 902), and overall (n = 2,979 vs. 1,644). (B) Sepsis-associated mortality rates for patients with hematologic cancer, stratified by presence of stem cell transplant. Sample size: leukemia (230 with stem cell transplant vs. 383 with no transplant), lymphoma (171 vs. 915), multiple myeloma (51 vs. 251), and overall (413 vs. 2,453). mets = metastases.
Trends in Sepsis-associated Mortality in Patients with and without Cancer
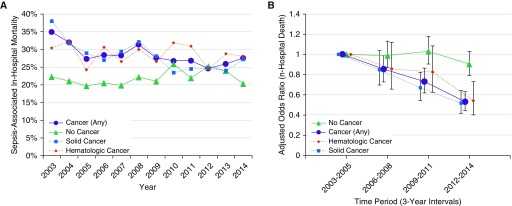
Sepsis-associated mortality rates in patients with cancer decreased from 31.3% in 2003–2005 to 26.0% in 2012–2014 (absolute decrease, 5.2% [95% CI, 2.3–8.2%]; unadjusted odds ratio [OR], 0.77 in 2012–2014 relative to 2003–2005, 95% CI, 0.67–0.89) (Figure 3A and Table E3). This mortality reduction persisted after risk adjustment (adjusted OR, 0.53 [95% CI, 0.44–0.63]); declines in risk-adjusted mortality were observed in both solid and hematologic cancer subgroups (Figure 3B). In contrast, sepsis-associated mortality increased in patients without cancer from 20.9% in 2003–2005 to 23.9% in 2012–2014 (absolute increase, 2.1% [95% CI, 0.1–4.1%]; unadjusted OR, 1.13 [95% CI, 1.01–1.27]), although there was no significant change in risk-adjusted mortality (adjusted OR, 0.91 [95% CI, 0.80–1.04]). Unadjusted and adjusted trends between patients with and without cancer assessed using subgroup interaction tests were significantly different (P < 0.001 for both). The full risk-adjusted model results for patients with and without cancer are shown in Table E2.
Figure 3.
Trends in sepsis-associated in-hospital mortality rates in patients with and without cancer. (A) Unadjusted annual trends in in-hospital mortality (all patients with sepsis). (B) 3-year interval mortality rates adjusted for case mix and severity of illness (all patients with sepsis). The numbers of sepsis deaths per 3-year interval in patients with cancer were 571/1,826 (31.3%) in 2003–2005, 580/1,975 (29.4%) in 2006–2008, 517/1,914 (27.0%) in 2009–2011, and 462/1,774 (26.0%) in 2012–2014. The number of sepsis deaths in patients without cancer were 655/3,134 (20.9%) in 2003–2005, 697/3,352 (20.8%) in 2006–2008, 779/3,404 (22.9%) in 2009–2011, and 828/3,596 (23.0%) in 2012–2014. Values presented in B represent the adjusted odds ratios for mortality in each 3-year period (with 95% confidence intervals).
In patients with cancer requiring mechanical ventilation at sepsis onset (n = 2,348, 31.4% of the cancer cohort), crude mortality was high, but also declined over time (54.8% in 2003–2005 vs. 48.1% in 2012–2014; absolute decrease, 6.7% [95% CI, 0.9 to 12.4%]; adjusted OR, 0.67 [95% CI, 0.52 to 0.87]; Figure E2). In patients without cancer requiring mechanical ventilation (n = 6,358, 47.2%), mortality did not significantly change (31.2% in 2003–2005 vs. 31.1% in 2012–2014, absolute decrease, 0.1% [95% CI, −3.1 to 3.4%]; adjusted OR, 0.93 [95% CI, 0.79 to 1.10]).
Within the cancer cohort, similar declines were seen with community-onset sepsis (n = 4,434, 57.9% of the cancer cohort) and hospital-onset sepsis (n = 3,155, 42.1%) (Figure E3). There was no substantial change over time in the proportion of patients with sepsis and cancer who were on active chemotherapy within 90 days before their sepsis hospitalization (Figure E4); within this subset (n = 2,556, 34.1%), trends were similar with a decline in sepsis-associated mortality from 29.2% in 2003–2005 to 25.7% in 2012–2014 (absolute decrease, 3.5% [95% CI, −1.5 to 8.4%]; adjusted OR, 0.51 [95% CI, 0.37 to 0.70]). Examination of care patterns revealed a slight decrease in the proportion of patients with sepsis and cancer who received G-CSF and an increase in the proportion that was receiving targeted cancer therapy, although this latter group was still small in 2012–2014 (6.2%; Figure E4). A higher proportion of patients with sepsis with versus without cancer received echinocandins and triazoles; there was an initial increase in the proportion of patients treated with echinocandins from 2003–2005 to 2006–2008, but this was stable afterwards, whereas the use of triazoles was largely stable throughout the study period (Figure E5).
On sensitivity analyses, the improvements in sepsis-associated outcomes in patients with cancer were attenuated when combining in-hospital death and discharge to hospice, but declines were still present on adjusted analysis (Figure E6). When using the Angus administrative definition of sepsis (n = 20,637 patients, including 7,950 with cancer and 12,687 without cancer), declines in sepsis-associated mortality were more pronounced in both patients with and without cancer, with greater relative improvements in patients with cancer (P < 0.001 for subgroup interaction test) (Figure E7).
Most markers of severity of illness at sepsis onset were higher in the late time period for patients with and without cancer (Tables E4–E6). There was no substantial difference in the quantity of missing laboratory values between the two time periods except for lactates (Figure E1).
Discussion
In this retrospective study using detailed clinical data from an academic hospital that cares for a large oncology population, we found that sepsis-associated mortality rates declined over a 12-year period in patients with cancer, but not in patients without cancer. The improvement in sepsis-associated mortality in patients with cancer was consistent across important subgroups, including those requiring mechanical ventilation at sepsis onset, community and hospital-onset sepsis, and patients receiving active cancer treatment.
Other work has primarily studied the epidemiology of sepsis in patients with cancer using administrative data to identify sepsis (8). Danai and colleagues (11), for example, described a reduction in sepsis mortality from 44.7% in 1979 to 23.8% in 2001 using the National Hospital Discharge Survey. Another study using administrative data from six states in 1999 described a sepsis mortality rate of 37.8%. Most recently, a study using 2013–2014 data from the National Readmissions Database demonstrated in-hospital mortality rates of 27.9% for cancer-related sepsis versus 19.5% in non–cancer-related sepsis (12). The estimates in these various studies, however, are not directly comparable, because each used different administrative definitions of sepsis (35). Furthermore, using administrative data to track sepsis trends is prone to ascertainment bias from increasing sepsis awareness and more diligent documentation and coding practices over time (16, 18, 36, 37). Using CDC’s Adult Sepsis Event definition allows for more confidence in our findings, because this definition uses consistent clinical criteria rather than administrative codes to detect sepsis. Adult Sepsis Events are less susceptible to ascertainment bias than administrative codes, more sensitive than explicit sepsis codes, and more specific than “implicit” administrative definitions that use infection and organ dysfunction codes (1, 17, 38, 39). The importance of objective clinical surveillance is underscored by our sensitivity analysis using the Angus implicit administrative definition, which suggested improvements in sepsis-associated mortality rates in both patients with and without cancer. However, the administrative definition still demonstrated a greater relative improvement in the cancer subgroup.
The improvement in sepsis-associated mortality rates in patients with cancer cannot be attributed to a less severely ill population over time, because: 1) the proportion of patients on active cancer treatment was stable over the study period; and 2) patients in the later years of the cohort were older and had more severe disease at sepsis onset. This latter finding explains why the effect estimate for the decline in mortality rates was even more pronounced after adjusting for case mix and severity of illness. One possible explanation for improving outcomes in this population might be greater awareness and more aggressive treatment of sepsis specifically in the cancer population. Other factors that could have contributed to the preferential decrease in sepsis mortality rates in patients with cancer could include advances in anti-infective therapies that are more commonly used in this population, such as more efficacious and less toxic antifungal agents (40–43). This latter hypothesis is supported by the increasing use of echinocandins early on in the study period. We did not, however, observe a higher proportion of patients with sepsis receiving triazoles.
The stable mortality rates in hospitalized patients without cancer suggests that universal trends in sepsis care, such as faster administration of antibiotics and fluids, are probably not the sole explanation for the decrease in sepsis mortality in patients with cancer. The trend may instead be explained by more effective and/or less toxic treatments for cancer (3). In particular, the evolution of more targeted and less toxic treatments may allow patients with cancer to recover from other acute insults more easily and to live longer with their disease (44, 45). In our study, we observed an increase in the proportion of patients with sepsis with cancer on targeted agents, although this only accounted for a small fraction of the cohort. Other studies have suggested that greater use of G-CSF in patients receiving myelotoxic chemotherapy has led to a decreased incidence of febrile neutropenia and infections (46, 47), but we did not observe a higher proportion of patients with sepsis receiving G-CSF.
Patients with cancer and sepsis who required mechanical ventilation had substantially higher mortality rates than patients without cancer (approximately 50% vs. 30%). These findings are consistent with a recent meta-analysis demonstrating short-term mortality rates ranging from 55% to 83% in patients with cancer requiring mechanical ventilation (30). The improvement in mortality rates that we observed in this severely ill subset, however, add to the literature supporting the utility of trials of short-term aggressive care in critically ill patients with cancer (10, 48), and potentially argue against the stigma of cancer as a universally terminal comorbidity in this setting.
The trend toward improving survival was partially attenuated when including discharge to hospice as an outcome, which reflects the increasing use of hospice services at our hospital and nationwide (34). However, discharge to hospice does not guarantee death of the patient within 30 days, 90 days, or even within 6 months (34, 49). In addition, increasing rates of discharge to hospice may actually be in line with patient preferences and arguably represent a more favorable outcome than in-hospital mortality. Regardless, even with including discharge to hospice within our short-term mortality measures, our data demonstrated improvements in risk-adjusted sepsis outcomes.
This study has several strengths, including large sample size, the use of both clinical and administrative data, objective identification of sepsis cases using CDC surveillance criteria, and a validation of the AHRQ Clinical Classifications for cancer diagnosis categories. This study also has several limitations. First, our inclusion of a single center limits generalizability. However, this is a necessary tradeoff to obtain more granular data than is available in most national datasets. Second, we have only limited insight into the mechanisms that might account for the differential improvement in sepsis-associated mortality for patients with versus without cancer. In particular, we were not able to study changes in timeliness of antibiotics and other sepsis-related interventions, because our dataset did have time-stamped medication data. Prior work in our institution has demonstrated increasingly rapid administration of fluids and antibiotics for patients with sepsis in the emergency department during this study period (50), but we do not know if this occurred more in patients with cancer. In addition, we did not have data on all hospitalized patients, and so cannot assess whether improvements in mortality rates extended to all hospitalized patients with cancer. Third, we had incomplete cancer-specific information, including stage of disease. However, we identified patients on active treatment within 90 days of their sepsis hospitalizations and found similar trends in this subgroup. Fourth, although we had a detailed set of clinical measures to adjust for severity of illness, our dataset did not include vital signs, mental status, or other physiologic variables necessary to calculate established scores, such as APACHE (Acute Physiology and Chronic Health Elements) or SOFA (Sequential Organ Failure Assessment). Fifth, because our cohort draws from a hospitalized population rather than a general community cohort, we were not able to draw inferences about the incidence of sepsis in cancer and in various cancer types. Finally, it is possible that increases in intensive care unit capacity and improvements in electronic documentation at our hospital could confound the analysis of changing sepsis mortality over time. However, this would likely have affected both patients with and those without cancer.
In conclusion, although short-term sepsis-associated mortality rates are higher in patients with cancer versus patients without cancer, there is room for optimism, as sepsis-associated mortality declined in patients with cancer over a 12-year period. This is important, as both sepsis and cancer continue to be substantial public health problems. Our findings also suggest that improvements in sepsis outcomes may be explained in part by improvements in the management of comorbid conditions, and/or better sepsis treatment specifically in patients with cancer. Future work should focus on confirming our findings in a nationally representative set of hospitals, studying sepsis prevention strategies in patients with cancer, and better elucidating the factors that improve outcomes for patients with cancer.
Supplementary Material
Footnotes
Supported by U.S. Centers for Disease Control and Prevention grant U54CK000484 and Agency for Healthcare Research and Quality grant K08HS025008 (C.R.).
The content of this article is solely the responsibility of the authors, and does not necessarily represent the official views of the U.S. Centers for Disease Control and Prevention or the Agency for Healthcare Research and Quality.
Author Contributions: study concept and design—A.J.C., S.P.K., B.E.G., M.K., R.M.B., and C.R.; acquisition of data—A.J.C., C.C., M.K., and C.R.; analysis and interpretation of data—A.J.C., C.C., and C.R.; drafting of the manuscript—A.J.C. and C.R.; critical revision of the manuscript for important intellectual content—all authors; final approval of the manuscript for publication—all authors; responsibility for data integrity—C.R.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of the CDC Prevention Epicenters Program
References
- 1.Rhee C, Dantes R, Epstein L, Murphy DJ, Seymour CW, Iwashyna TJ, et al. CDC Prevention Epicenter Program. Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009–2014. JAMA. 2017;318:1241–1249. doi: 10.1001/jama.2017.13836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu V, Escobar GJ, Greene JD, Soule J, Whippy A, Angus DC, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA. 2014;312:90–92. doi: 10.1001/jama.2014.5804. [DOI] [PubMed] [Google Scholar]
- 3.Rhee C, Jones TM, Hamad Y, Pande A, Varon J, O’Brien C, et al. Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program. Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open. 2019;2:e187571. doi: 10.1001/jamanetworkopen.2018.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paoli CJ, Reynolds MA, Sinha M, Gitlin M, Crouser E. Epidemiology and costs of sepsis in the United States—an analysis based on timing of diagnosis and severity level. Crit Care Med. 2018;46:1889–1897. doi: 10.1097/CCM.0000000000003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sinapidis D, Kosmas V, Vittoros V, Koutelidakis IM, Pantazi A, Stefos A, et al. Progression into sepsis: an individualized process varying by the interaction of comorbidities with the underlying infection. BMC Infect Dis. 2018;18:242. doi: 10.1186/s12879-018-3156-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novosad SA, Sapiano MR, Grigg C, Lake J, Robyn M, Dumyati G, et al. Vital signs: epidemiology of sepsis: prevalence of health care factors and opportunities for prevention. MMWR Morb Mortal Wkly Rep. 2016;65:864–869. doi: 10.15585/mmwr.mm6533e1. [DOI] [PubMed] [Google Scholar]
- 7.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, et al. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004;8:R291–R298. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 10.Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. 2002;360:1131–1135. doi: 10.1016/S0140-6736(02)11199-8. [DOI] [PubMed] [Google Scholar]
- 11.Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129:1432–1440. doi: 10.1378/chest.129.6.1432. [DOI] [PubMed] [Google Scholar]
- 12.Hensley MK, Donnelly JP, Carlton EF, Prescott HC. Epidemiology and outcomes of cancer-related versus non–cancer-related sepsis hospitalizations. Crit Care Med. 2019;47:1310–1316. doi: 10.1097/CCM.0000000000003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jolley RJ, Sawka KJ, Yergens DW, Quan H, Jetté N, Doig CJ. Validity of administrative data in recording sepsis: a systematic review. Crit Care. 2015;19:139. doi: 10.1186/s13054-015-0847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwashyna TJ, Odden A, Rohde J, Bonham C, Kuhn L, Malani P, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the angus implementation of the international consensus conference definition of severe sepsis. Med Care. 2014;52:e39–e43. doi: 10.1097/MLR.0b013e318268ac86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rhee C, Kadri SS, Danner RL, Suffredini AF, Massaro AF, Kitch BT, et al. Diagnosing sepsis is subjective and highly variable: a survey of intensivists using case vignettes. Crit Care. 2016;20:89. doi: 10.1186/s13054-016-1266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhee C, Murphy MV, Li L, Platt R, Klompas M Centers for Disease Control and Prevention Epicenters Program. Comparison of trends in sepsis incidence and coding using administrative claims versus objective clinical data. Clin Infect Dis. 2015;60:88–95. doi: 10.1093/cid/ciu750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhee C, Kadri S, Huang SS, Murphy MV, Li L, Platt R, et al. Objective sepsis surveillance using electronic clinical data. Infect Control Hosp Epidemiol. 2016;37:163–171. doi: 10.1017/ice.2015.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rhee C, Gohil S, Klompas M. Regulatory mandates for sepsis care—reasons for caution. N Engl J Med. 2014;370:1673–1676. doi: 10.1056/NEJMp1400276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadri SS, Rhee C, Strich JR, Morales MK, Hohmann S, Menchaca J, et al. Estimating ten-year trends in septic shock incidence and mortality in United States academic medical centers using clinical data. Chest. 2017;151:278–285. doi: 10.1016/j.chest.2016.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Hospital toolkit for adult sepsis surveillance. 2018 Mar 23 [accessed 2019 Nov 1]. Available from: https://www.cdc.gov/sepsis/pdfs/sepsis-surveillance-toolkit-mar-2018_508.pdf.
- 21.Murphy SNCH, Chueh HC. A security architecture for query tools used to access large biomedical databases. Proc AMIA Symp. 2002:552–556. [PMC free article] [PubMed] [Google Scholar]
- 22.Rhee C, Zhang Z, Kadri SS, Murphy DJ, Martin GS, Overton E, et al. CDC Prevention Epicenters Program. Sepsis surveillance using adult sepsis events simplified eSOFA criteria versus sepsis-3 sequential organ failure assessment criteria. Crit Care Med. 2019;47:307–314. doi: 10.1097/CCM.0000000000003521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee C, Murphy MV, Li L, Platt R, Klompas M Centers for Disease Control and Prevention Prevention Epicenters Program. Lactate testing in suspected sepsis: trends and predictors of failure to measure levels. Crit Care Med. 2015;43:1669–1676. doi: 10.1097/CCM.0000000000001087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agency for Healthcare Quality and Research Healthcare Cost and Utilization Project. Clinical Classifications Software for ICD-9-CM. 2018 Nov 3 [accessed 2019 Aug 1]. Available from: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp.
- 25.Gupta A, Das A, Tariq R, Bhulani N, Premnath N, Solanky D, et al. Trends in outcomes of patients with metastatic cancer undergoing intubation and mechanical ventilation: results of the national hospital discharge survey. J Natl Compr Canc Netw. 2018;16:286–292. doi: 10.6004/jnccn.2017.7053. [DOI] [PubMed] [Google Scholar]
- 26.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Nimptsch U. Disease-specific trends of comorbidity coding and implications for risk adjustment in hospital administrative data. Health Serv Res. 2016;51:981–1001. doi: 10.1111/1475-6773.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindenauer PK, Lagu T, Shieh MS, Pekow PS, Rothberg MB. Association of diagnostic coding with trends in hospitalizations and mortality of patients with pneumonia, 2003–2009. JAMA. 2012;307:1405–1413. doi: 10.1001/jama.2012.384. [DOI] [PubMed] [Google Scholar]
- 29.Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:762–774. doi: 10.1001/jama.2016.0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soares M, Depuydt PO, Salluh JI. Mechanical ventilation in cancer patients: clinical characteristics and outcomes. Crit Care Clin. 2010;26:41–58. doi: 10.1016/j.ccc.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 31.Taccone FS, Artigas AA, Sprung CL, Moreno R, Sakr Y, Vincent JL. Characteristics and outcomes of cancer patients in European ICUs. Crit Care. 2009;13:R15. doi: 10.1186/cc7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pène F, Percheron S, Lemiale V, Viallon V, Claessens YE, Marqué S, et al. Temporal changes in management and outcome of septic shock in patients with malignancies in the intensive care unit. Crit Care Med. 2008;36:690–696. doi: 10.1097/CCM.0B013E318165314B. [DOI] [PubMed] [Google Scholar]
- 33.Rhee C, Wang R, Zhang Z, Fram D, Kadri SS, Klompas M. CDC Prevention Epicenters Program. Epidemiology of hospital-onset versus community-onset sepsis in U.S. hospitals and association with mortality: a retrospective analysis Using electronic clinical data. Crit Care Med. 2019;47:1169–1176. doi: 10.1097/CCM.0000000000003817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aldridge MD, Canavan M, Cherlin E, Bradley EH. Has hospice use changed? 2000–2010 utilization patterns. Med Care. 2015;53:95–101. doi: 10.1097/MLR.0000000000000256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaieski DF, Edwards JM, Kallan MJ, Carr BG. Benchmarking the incidence and mortality of severe sepsis in the United States. Crit Care Med. 2013;41:1167–1174. doi: 10.1097/CCM.0b013e31827c09f8. [DOI] [PubMed] [Google Scholar]
- 36.Rhee C, Murphy MV, Li L, Platt R, Klompas M Centers for Disease Control and Prevention Epicenters Program. Improving documentation and coding for acute organ dysfunction biases estimates of changing sepsis severity and burden: a retrospective study. Crit Care. 2015;19:338. doi: 10.1186/s13054-015-1048-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gohil SK, Cao C, Phelan M, Tjoa T, Rhee C, Platt R, et al. Impact of policies on the rise in sepsis incidence, 2000–2010. Clin Infect Dis. 2016;62:695–703. doi: 10.1093/cid/civ1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simpson SQ. Surveillance for adult sepsis events: an idea whose time has come. Crit Care Med. 2019;47:467–468. doi: 10.1097/CCM.0000000000003561. [DOI] [PubMed] [Google Scholar]
- 39.Simpson SQ. Sepsis epidemiology from administrative data: going, going…. Crit Care Med. 2019;47:739–740. doi: 10.1097/CCM.0000000000003690. [DOI] [PubMed] [Google Scholar]
- 40.Nagappan V, Deresinski S. Reviews of anti-infective agents: posaconazole: a broad-spectrum triazole antifungal agent. Clin Infect Dis. 2007;45:1610–1617. doi: 10.1086/523576. [DOI] [PubMed] [Google Scholar]
- 41.Chandrasekar PH, Sobel JD. Micafungin: a new echinocandin. Clin Infect Dis. 2006;42:1171–1178. doi: 10.1086/501020. [DOI] [PubMed] [Google Scholar]
- 42.Greer ND. Voriconazole: the newest triazole antifungal agent. Proc Bayl Univ Med Cent. 2003;16:241–248. doi: 10.1080/08998280.2003.11927910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vallabhaneni S, Baggs J, Tsay S, Srinivasan AR, Jernigan JA, Jackson BR. Trends in antifungal use in US hospitals, 2006–12. J Antimicrob Chemother. 2018;73:2867–2875. doi: 10.1093/jac/dky270. [DOI] [PubMed] [Google Scholar]
- 44.Carelle N, Piotto E, Bellanger A, Germanaud J, Thuillier A, Khayat D. Changing patient perceptions of the side effects of cancer chemotherapy. Cancer. 2002;95:155–163. doi: 10.1002/cncr.10630. [DOI] [PubMed] [Google Scholar]
- 45.Joo WD, Visintin I, Mor G. Targeted cancer therapy—are the days of systemic chemotherapy numbered? Maturitas. 2013;76:308–314. doi: 10.1016/j.maturitas.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goyal RK, Tzivelekis S, Rothman KJ, Candrilli SD, Kaye JA. Time trends in utilization of G-CSF prophylaxis and risk of febrile neutropenia in a Medicare population receiving adjuvant chemotherapy for early-stage breast cancer. Support Care Cancer. 2018;26:539–548. doi: 10.1007/s00520-017-3863-9. [DOI] [PubMed] [Google Scholar]
- 47.Wang L, Baser O, Kutikova L, Page JH, Barron R. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2015;23:3131–3140. doi: 10.1007/s00520-015-2686-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azoulay E, Afessa B. The intensive care support of patients with malignancy: do everything that can be done. Intensive Care Med. 2006;32:3–5. doi: 10.1007/s00134-005-2835-6. [DOI] [PubMed] [Google Scholar]
- 49.LeSage K, Borgert AJ, Rhee LS. Time to death and reenrollment after live discharge from hospice: a retrospective look. Am J Hosp Palliat Care. 2015;32:563–567. doi: 10.1177/1049909114535969. [DOI] [PubMed] [Google Scholar]
- 50.Hume PS, Varon J, Englert JA, Hurwitz S, Klompas M, Baron RM, et al. Trends in “usual care” for septic shock. Infect Control Hosp Epidemiol. 2018;39:1125–1126. doi: 10.1017/ice.2018.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.