Abstract
Patients with well-controlled LDL (low-density lipoprotein) levels still have residual cardiovascular risk associated with elevated triglycerides. Epidemiological studies have shown that elevated fasting triglyceride levels associate independently with incident cardiovascular events, and abundant recent human genetic data support the causality of TGRLs (triglyceride-rich lipoproteins) in atherothrombosis. Omega-3 fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), lower blood triglyceride concentrations but likely exert additional atheroprotective properties at higher doses. Omega-3 fatty acids modulate T-cell differentiation and give rise to various prostaglandins and specialized proresolving lipid mediators that promote resolution of tissue injury and inflammation. The REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) with an EPA-only formulation lowered a composite of cardiovascular events by 25% in patients with established cardiovascular disease or diabetes mellitus and other cardiovascular risk factors. This clinical benefit likely arises from multiple molecular mechanisms discussed in this review. Indeed, human plaques readily incorporate EPA, which may render them less likely to trigger clinical events. EPA and DHA differ in their effects on membrane structure, rates of lipid oxidation, inflammatory biomarkers, and endothelial function as well as tissue distributions. Trials that have evaluated DHA-containing high-dose omega-3 fatty acids have thus far not shown the benefits of EPA alone demonstrated in REDUCE-IT. This review will consider the mechanistic evidence that helps to understand the potential mechanisms of benefit of EPA.
Keywords: eicosapentaenoic acid, fatty acids, inflammation, lipoproteins, triglycerides
Highlights.
Omega-3 fatty acids, such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have multiple biological effects.
In the REDUCE-IT (Reduction of Cardiovascular Events With Icosapent Ethyl–Intervention Trial), a prescription EPA-only formulation called icosapent ethyl reduced cardiovascular events by 25% in high-risk patients with either established cardiovascular disease or diabetes mellitus plus other risk factors.
Cardiovascular outcomes trials that have evaluated mixed (DHA-containing) omega-3 fatty acids on top of contemporary medical therapy have thus far not shown the benefits of icosapent ethyl as seen in REDUCE-IT and the JELIS (Japan EPA Lipid Intervention Study), highlighting the importance of understanding molecular mechanisms of action of specific omega-3 fatty acids.
EPA and DHA differ markedly, with distinct effects on membrane structure, rates of lipid oxidation, inflammatory biomarkers, and endothelial function, as well as tissue distributions.
Ongoing scientific efforts to understand the mechanism of action for EPA will help usher in a new era of cardiovascular therapeutics.
High-Dose Omega-3 Fatty Acid Treatment and Residual Cardiovascular Risk
Despite the success of LDL (low-density lipoprotein)-lowering therapies in reducing cardiovascular risk, individuals with well-controlled LDL still have residual cardiovascular risk associated in part with elevated triglycerides.1,2 The REDUCE-IT (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial) that tested an omega-3 fatty acid (n3-FA)-based therapy of eicosapentaenoic acid (EPA) demonstrated cardiovascular risk reduction in addition to the protection afforded by statins. REDUCE-IT demonstrated that a highly purified ethyl ester of EPA, icosapent ethyl, significantly reduced the risk of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization in at-risk patients with triglycerides above ≈100 mg/dL despite being treated with statins.3–6 First ischemic events fell by 25% (P=0.00000001) and total (first and subsequent) ischemic events by 31% (P=0.0000000004), with consistent benefits across multiple prespecified subgroups, including primary and secondary prevention. The prespecified subgroup of 3146 patients randomized in the United States demonstrated benefits at least as robust as the overall population, with a notable 30% lower rate of mortality (P=0.004) in those randomized to icosapent ethyl.7 The EPA treatment used yielded consistent benefits across baseline levels of triglycerides as well, including in the ≈10% of participants with normal triglycerides.8
The large relative and absolute risk reductions in several different types of end points from REDUCE-IT seemed to exceed that expected by the degree of triglyceride lowering, suggesting that other properties of EPA likely contribute to the benefits, as will be discussed9–11 (Figure 1). Beyond the effect on lowering triglyceride levels, EPA has cell-membrane stabilizing properties that may explain, in part, the significant reductions seen in death from cardiovascular causes (20% reduction), sudden cardiac death (31% reduction), and cardiac arrest (48% reduction). Of note, the results of REDUCE-IT apply to a broad population of at-risk patients.12,13 The findings from REDUCE-IT contrast sharply with previous outcome trials that showed no cardiovascular benefit using EPA/docosahexaenoic acid (DHA) combinations or dietary supplements that may contain oxidized fatty acids and saturated fat.14–18 Further trials with high-dose n3-FAs in various groups will provide additional insight into this question.
Figure 1.
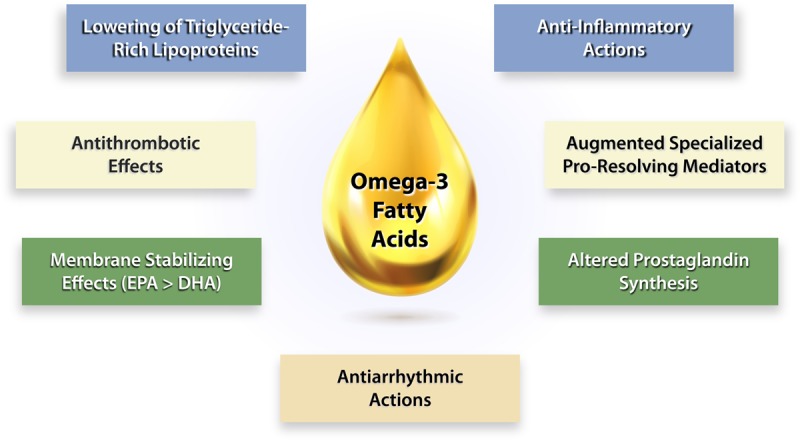
Potential mechanisms of cardioprotection for omega-3 fatty acids. Omega-3 fatty acids may lessen risk of cardiovascular events through a number of mechanisms that contribute to their overall protective actions. Lowering of TGRL (triglyceride-rich lipoprotein) may account for some but certainly not all of the observed benefits (Figure 2). By boosting the production of anti-aggregatory and vasodilatory prostanoids, such as prostacyclin, omega-3 fatty acids may combat thrombosis as well as vasospasm. Omega-3 fatty acids can incorporate into plasma membranes and those of the mitochondria potentially stabilizing them to resist oxidation and confer protection against arrhythmias. Omega-3 fatty acids and certain prostanoids produced from them can exert anti-inflammatory actions. In addition, the omega-3 fatty acids can provide precursors for the synthesis of specialized proresolving mediators that can combat inflammation, perhaps causing less interference with his defenses than direct anti-inflammatory therapies. A combination of these various mechanisms may contribute to the cardiovascular protection associated with omega-3 fatty acid consumption. DHA indicates docosahexaenoic acid; and EPA, eicosapentaenoic acid.
The REDUCE-IT trial followed the JELIS trial (Japan EPA Lipid Intervention Study), which demonstrated that purified EPA (1.8 g/d) reduced the risk of major coronary events in hypercholesterolemic patients receiving statin therapy versus those subjected to statin monotherapy. JELIS did not prespecify a minimum triglyceride level for inclusion into the study.19 This open-label, randomized trial, showed a 19% reduction (P=0.011) in cardiovascular events overall in the trial in the EPA group, with consistent benefits in both secondary and primary prevention. Notably, the median triglyceride level at baseline was only slightly above 150 mg/dL, meaning that approximately half of the patients had normal triglycerides. However, in post hoc analyses, among those subjects with higher triglyceride levels (>150 mg/dL) and low HDL-C (high-density lipoprotein-cholesterol) levels (<40 mg/dL), there was 53% reduction in events with EPA treatment.20
Omega-3 Fatty Acids and Progression of Atherosclerosis in Patients With Elevated Triglycerides
n3-FAs and their bioactive lipid metabolites produce complex and multifactorial biological effects that remain incompletely understood, especially at higher doses, that may reduce the risk of cardiovascular events. A meta-analysis of observational and randomized trials indicated that circulating levels of n3-FAs associated inversely with modestly lower rates of cardiovascular death.21,22 Other studies indicated that the cardiovascular benefits of n3-FAs could link to either EPA or DHA but highlighted the need for more definitive evidence.23 A study of 218 subjects with coronary disease randomized to high-dose EPA and DHA (3.36 g/d) or placebo showed that those with n3-FAs that reached plasma phospholipid levels of at least 4% had a significantly slower progression of coronary plaque as monitored by coronary computed tomographic angiography.24
Clinical studies with high-dose EPA (2–4 g/d) provide some mechanistic insights based on changes in various biomarkers in statin-treated patients with elevated triglycerides that may contribute to the reduction in residual cardiovascular risk due to EPA demonstrated in REDUCE-IT.2,5,6 In people with elevated or very high triglycerides, treatment with a highly purified and quality-controlled preparation of EPA (2–4 g/d) reduced the arachidonic acid-to-EPA ratio in blood, hsCRP (high-sensitivity C-reactive protein), RLP-C (remnant-like particle cholesterol), ApoC-III (apolipoprotein C-III), and oxLDL (oxidized LDL-C) concentrations compared with placebo controls.5,25–32 EPA generally produced these effects in a dose-dependent manner. Further studies of inflammatory and other biomarkers from REDUCE-IT and other trials should lead to additional insights into mechanisms of action of highly purified EPA in individuals with elevated triglycerides and cardiovascular risk.
Laboratory and clinical studies suggest that EPA also influences vascular functions related to atherosclerosis such as improved endothelial-dependent vasodilatation, membrane stabilization, reduced inflammation, and limiting features of plaques associated with propensity to provoke thrombosis.33–40 In a study of patients who underwent carotid endarterectomy, those treated with n3-FAs enriched with EPA (0.81 g/day of EPA and 0.675 g/day of DHA) had fewer foam cells, features of plaque instability, markers of inflammation, and T-cell content.38 HDL isolated from EPA-only treated individuals exhibited enhanced cholesterol efflux from monocytes and augmented antioxidant and anti-inflammatory actions.41 In cultured human endothelial cells (ECs), EPA-enriched HDL increased levels of specialized proresolving lipid mediators while reducing cytokine-stimulated VCAM-1 (vascular cell adhesion molecule 1) expression.42 EPA partitions into the outer monolayer of the HDL particle and exhibits greater antioxidant function than DHA-loaded HDL.43
In patients with coronary heart disease, a combination EPA with statin therapy significantly reduced coronary plaque volume compared with statin therapy alone in the CHERRY trial (Combination Therapy of Eicosapentaenoic Acid and Pitavastatin for Coronary Plaque Regression Evaluated by Integrated Backscatter Intravascular Ultrasonography).33 In 193 patients who were to undergo percutaneous coronary intervention underwent random allocation to the statin pitavastatin (4 mg/d, n=96) or to a pitavastatin/EPA group (pitavastatin 4 mg/d and EPA 1.8 g/d, n=97), for 6 to 8 months. Integrated backscatter intravascular ultrasound assessed coronary plaque volume and character in nonstented lesions. Total atheroma volume fell significantly in the pitavastatin/EPA but not the pitavastatin alone group. An imaging study in a North American population is currently underway with highly purified EPA (4 g/d) to evaluate changes in atherosclerotic plaque characteristics in statin-treated patients with coronary atherosclerosis, triglyceride levels of 135 to 499 mg/dL, and LDL levels of 40 to 115 mg/dL.44 This study (EVAPORATE [Effect of Vascepa on Improving Coronary Atherosclerosis in People With High Triglycerides Taking Statin Therapy]) is measuring low attenuation plaque volume by multidetector computed tomography angiography in 80 enrolled patients at a final analysis at 18 months. A prespecified interim analysis at 9 months was presented at the 2019 AHA Scientific Sessions (Philadelphia, PA) and showed a significant difference in a secondary end point of total plaque volume progression with EPA versus placebo.
EPA can reduce triglyceride concentrations without raising LDL-C levels at 2-4 g/d, compared with formulations that contain another n3-FA, DHA, at similar doses, in patients with very high triglycerides (>500 mg/dL).25,27,45 The mechanism for this unexpected finding with EPA treatment may relate to reduced production and faster clearance of TGRL (triglyceride-rich lipoproteins; estimated by serum triglyceride measurements) in concert with more rapid clearance of LDL particles and slower production of VLDL (very-low-density lipoprotein) particles.19,27,46 At lower triglyceride levels, mixed n3-FA formulations also do not raise LDL. Through various mechanisms, TGRLs can promote vascular dysfunction and atherosclerosis (Figure 2). Saturated fatty acids, such as palmitate, may promote inflammation through NLRP-3 (NLR family pyrin domain-containing 3) inflammasome activation, leading to activated forms of the proinflammatory cytokines IL (interleukin)-1β and IL-18.47 Clinical trials that have tested other triglyceride-lowering agents (eg, fenofibrate or niacin) have not shown significant cardiovascular benefits when added to statins compared with statin therapy alone.48–52 These trials did not prospectively enroll patients with elevated triglyceride levels, an important limitation.
Figure 2.
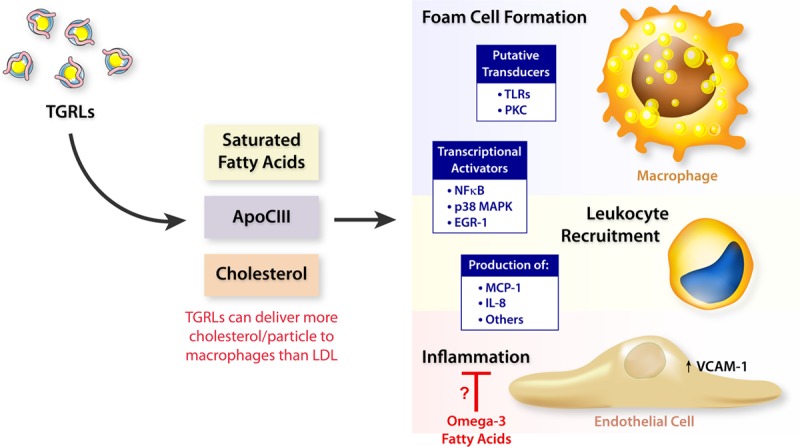
Atherogenic pathways for TGRLs (triglyceride-rich lipoproteins). TGRL (estimated by serum triglyceride measurements) can promote vascular dysfunction and atherosclerosis through a number of mechanisms. Saturated fatty acids, notably palmitate, can promote inflammation, in part, due to activation of the NLRP-3 (NLR family pyrin domain-containing 3) inflammasome, which produces activated forms of the proinflammatory cytokines IL (interleukin)-1β and IL-18.47 ApoC-III (Apolipoprotein CIII) can exert direct proinflammatory effects of cells involved in atherosclerosis, such as macrophages and endothelial cells. Human genetic studies strongly support the causality of ApoC-III in human atherothrombosis. TGRL particles deliver cholesterol effectively to macrophages and can promote foam cell formation. Omega-3 fatty acids may exert some of their apparent protective effect on atherothrombosis by blocking some of these proinflammatory and other deleterious effects of TGRL. EGR-1 indicates early growth response protein 1; LDL, low-density lipoprotein; MAPK, mitogen-activated protein kinase; MCP-1, monocyte chemoattractant protein-1; NF-κB, nuclear factor-κB; PKC, protein kinase C; TLR, toll-like receptors; and VCAM-1, vascular cell adhesion molecule 1.
Beyond their effects on levels of LDL, EPA-, and DHA-containing formulations appear to differ with respect to hsCRP reduction. Prescription EPA reduces hsCRP in patients with elevated or high triglycerides, an effect enhanced by combination with more potent statins.26 In contrast, mixed EPA and DHA treatment at a similar dosage (4 g/d) did not reduce hsCRP concentrations in statin-treated patients.53 The EPA and DHA combination did reduce apoC-III levels as previously observed with purified EPA.26,31,32 A decrease in apoC-III would lower triglyceride levels through several mechanisms, including reduced inhibition of lipoprotein lipase. EPA administration also inhibits platelet activation and aggregation, which, along with reducing mean platelet count, may contribute to antithrombotic effects.54–57 (Figure 1) Finally, both EPA and DHA have effects on hemodynamics, adiponectin, and IL-18 levels as well as platelet function in various clinical and experimental studies.9,58–65 Inconsistent effects of EPA and DHA in such studies may result from differences in the experimental conditions, dosage, and patient characteristics.
EPA and DHA Give Rise to Bioactive Lipids That Modulate Inflammation
Atherosclerosis involves inflammatory mechanisms of both the adaptive and innate immune responses.66,67 The n3-FAs give rise to signaling molecules that can reduce inflammation in different tissues and vascular beds. EPA and DHA form the cardioprotective and antithrombotic metabolites thromboxane A3/prostacyclin. In contrast, n6-FAs form thromboxane A2, a platelet activator that contributes to atherothrombosis.68 The n3-FAs compete with the n6-FA for the COX (cyclooxygenase) enzymes that synthesize the thromboxanes and thus limit the production of these potent proaggregatory and vasoconstrictor mediators.28,69 Despite this link between n6-FAs and proinflammatory signaling molecules, a recent pooled analysis of 30 cohort studies showed higher circulating and tissue levels of linoleic acid associate with lower risk of major cardiovascular events.70 Compared with other FAs, such as saturated FAs, linoleic acid has favorable effects on lipid metabolism.71 Another meta-analysis showed the lower cardiovascular risk was observed with n3-FAs and arachidonic acid but not other n6-FAs.22
Of the various eicosanoids produced by COXes, prostacyclin has particular interest with respect to vascular protection. Produced by healthy ECs, prostacyclin functions through a paracrine signaling pathway mediated by G protein-coupled receptors on nearby platelets and ECs. Receptor binding leads to inhibition of abnormal platelet activation while counteracting the prothrombotic effects of thromboxane. Prostacyclin also promotes smooth muscle relaxation and endothelial-dependent vasodilation.
Additional bioactive lipids that derive from n3-FAs originate from macrophages and neutrophils include the leukotrienes and resolvins.72 The metabolism of n3-FAs produces resolvins, maresins, and protectins. These metabolites, known as specialized proresolving lipid mediators, promote the resolution of inflammation as part of a highly coordinated process that helps to reestablish homeostasis after tissue injury or infection.73 Following acute inflammation, these steps include a reduction in the production of cytokines and extracellular-reactive oxygen species (ROS), along with inhibition of granulocyte trafficking. These bioactive lipids also modulate the inflammatory response by influencing macrophage-mediated clearance of cellular debris.74
Emerging lines of evidence indicate that these metabolites of n3-FAs may also limit chronic inflammation and activation of cells of the adaptive immune system in a coordinated fashion. By modulating membrane lipid dynamics, n3-FAs influence the organization of microdomains or lipid rafts enriched in cholesterol and sphingolipids. These highly ordered lipid assemblies, in turn, control the clustering of proteins required for cell signaling during CD4+ T-lymphocyte activation and differentiation.75 The ability of n3-FAs to reduce inflammation may arise, in part, from favorable changes in the Th1/Th2 balance through polarization of CD4+ T cells toward a Th2 slant as evidenced in vitro.76 Thus, n3-FAs can regulate T-cell function and regulation, such as the T helper (TH)1/TH2 balance, as well as levels of TH1 and TH17.76–78 In general, TH1 or TH17 polarized responses promote inflammation while TH2 and regulatory T cell predominant responses promote repair and resolution. Such findings have important implications for atherosclerosis. In human peripheral blood lymphocytes, resolvins reduced cytokine production by activated CD8+ T cells and CD4+ TH1 while limiting CD4+ T-cell differentiation into TH1 and TH17 cells.79 Mice with genetically impaired ability to synthesize n3-FAs have increased TH1/TH17 cells and decreased regulatory T cells compared with wild-type mice.79
The n3-FAs and their metabolites associate differentially with experimental atherosclerotic plaques. In atherosclerosis-prone mice (lacking apolipoprotein E, Apoe−/−) fed a Western diet supplemented with EPA (1%, w/w) or DHA (1%, w/w) for 3 weeks, EPA treatment reduced plaque volume as compared to DHA.80 EPA and its metabolites, especially 12-hydroxy-EPA, associated preferentially with thin-cap plaques and accumulation of anti-inflammatory M2 macrophages while DHA associated with plaques of various sizes. In the aortic root, total EPA and 12-HETE levels followed a concentration gradient from the vascular endothelium to the media. In addition, n3-FAs can ligate the GPR-120 (G-protein-coupled receptor 120) found on macrophages. Binding these receptors by n3-FAs inhibits the activation of NF-κB (nuclear factor-κB), a key regulator of inflammatory gene transcription.81,82
Comparative Biophysical and Antioxidant Properties of Omega-3 Fatty Acids
EPA and DHA have direct and indirect cellular actions that differ depending on their particular hydrocarbon length and number of double bonds.10 In particular, DHA has an additional double bond (6 total) and 2 more carbons compared with EPA. These structural properties influence the interactions of these 2 fatty acids with surrounding membrane lipids that, in turn, can alter membrane lipid raft formation and signal transduction pathways (Figure 3).36,83–86 Based on its hydrocarbon length and number of double bonds, EPA inserts into lipoprotein particles and cellular membranes in an extended conformation where it can scavenge ROS through stabilization of the unpaired electrons by its multiple conjugated double bonds, a property known as conjugative resonance stabilization.36 Patients with elevated triglycerides treated with prescription EPA (2–4 g/d) have significantly reduced oxLDL levels in plasma compared with placebo.26
Figure 3.
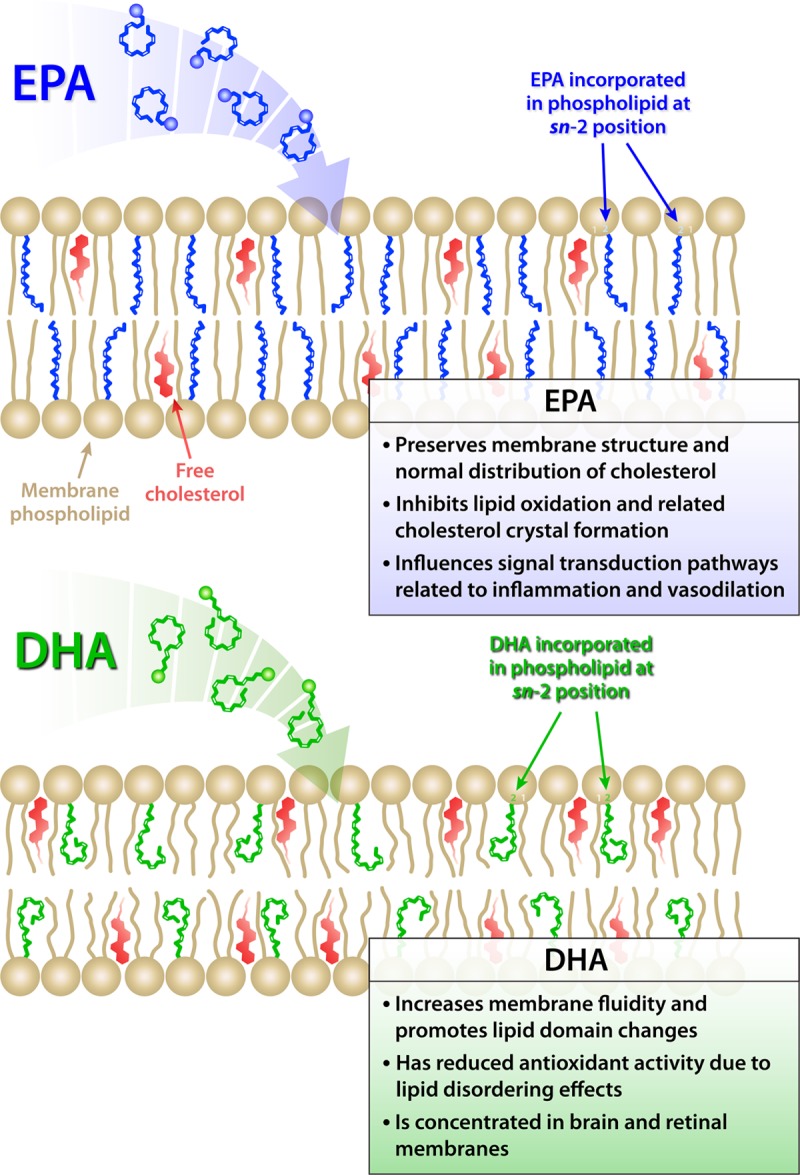
Molecular membrane interactions of omega-3 fatty acids. Schematic illustration of the proposed location and contrasting effects of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) on membrane structure. The insertion of EPA and DHA affect distinct regions of the membrane lipid bilayer due to differences in their hydrocarbon length and number of double bonds. The longer hydrocarbon length of DHA leads to more rapid isomerization and conformational changes that result in increased membrane fluidity and promotion of cholesterol domains. EPA has a more stable and extended structure that contributes to membrane stability as well as inhibition of lipid oxidation and cholesterol domain formation.
Oxidative modification of LDL can favor endothelial dysfunction, vascular inflammation, and other aspects of atherogenesis.87–89 Oxidatively modified LDL, but not native LDL, can foster foam cell formation. Circulating levels of oxLDL and other lipid oxidation products correlate with the severity of acute coronary syndromes and an increased risk for myocardial infarction, vascular procedures, and metabolic syndrome.90–93 In laboratory experiments, EPA had potent antioxidant effects in various apolipoprotein B-containing lipoprotein particles (LDL, VLDL, and small dense LDL) and in model membranes, properties not shared by other agents that lower triglycerides under identical experimental conditions.83,94,95 These observations argue that EPA has a preferred and energetically favorable location in plasma membranes that facilitates ROS scavenging and stabilizes the membrane structure. EPA may also intercalate into other cellular membranes, such as those of the mitochondria, where it could produce similar effects on membrane structure under conditions of oxidative stress. The antioxidant capacity of DHA wanes more rapidly than that of EPA due to its longer carbon chain length and an additional double bond that leads to rapid isomerization and overall increased membrane fluidity rather than stability.36,83,94 In particular, while EPA had more of an extended orientation and conformation within the cell membranes, DHA interacted with the phospholipid head group region with concomitant disorder in the membrane hydrocarbon core.36,83 These differences in membrane dynamics and conformation agree with the greater susceptibility to isomerization observed for DHA by various laboratories.83–85,94,96 The antioxidant effects of EPA in cell membranes in vitro also pertain under conditions of hyperglycemia, a condition of increased generation of ROS and carbonyl species.95 The presence of an active metabolite of atorvastatin that has antioxidant properties in vitro enhanced the antioxidant actions of EPA.94 The atorvastatin metabolite and EPA have complementary locations in the membrane hydrocarbon core that facilitate free radical stabilizing properties in an additive or even synergistic fashion not observed with DHA.94
Omega-3 Fatty Acids and Statins Alter Endothelial Functions
EC vasomotor dysfunction generally involves reduced nitric oxide (NO) bioavailability, and associates with vasoconstriction and early plaque development.97–99 Arterial stiffness correlates with cardiovascular disease and all-cause mortality independent of traditional risk factors.100 EPA produces favorable effects on arterial stiffness in patients with cardiovascular disease or its risk factors, including those with diabetes mellitus or receiving statins.101–104 The ability of EPA to reduce arterial stiffness did not depend on changes in blood pressure or LDL levels but correlated with reduced biomarkers of inflammation and oxidative stress. EPA may also enhance EC vasodilator function when combined with a statin as will be discussed. Mice deficient in the eNOS (endothelial isoform of nitric oxide synthase) show insulin resistance and reduced NO bioavailability.105 Such animals display vascular abnormalities associated with insulin resistance, along with hyperinsulinemia. Previous studies have demonstrated that DHA can suppress the expression of cytokine-induced proatherogenic and proinflammatory proteins in human ECs.106,107 Similar benefits were observed with EPA as it inhibited lipopolysaccharide-induced monocyte adhesion and expression of adhesion molecules both in vitro and in vivo.108
Recent studies investigated the combined effects of n3-FAs and statins on human ECs in culture. The combination of EPA and atorvastatin reduced endothelial dysfunction triggered by either oxLDL or high glucose in a manner not seen with DHA or statin treatment.35 This improvement in endothelial vasodilator function accompanied pronounced increases in the EC ratio of stimulated NO to peroxynitrite (ONOO−, a highly oxidant species) release; the effects did not depend on changes in eNOS protein expression, suggesting an improvement in eNOS activity. The favorable antioxidant interactions between EPA and atorvastatin may relate to common mechanisms.95 The effects of EPA and a statin on EC function also pertained to rodent studies.35 While either EPA or atorvastatin showed separate benefits, their combination augmented NO bioavailability under conditions of high glucose concentrations alone or in combination with exposure to oxLDL.35
Effects of Omega-3 Fatty Acids on Membrane Fluidity, Including Cholesterol Domain and Crystal Formation
Excessive cholesterol accumulation in the membranes of vascular smooth muscle cells and macrophages can promote the formation of distinct lipid domains within the cell membrane consisting of bilayers of cholesterol monohydrate.109,110 Such cholesterol domains may facilitate the formation of extracellular cholesterol crystals, a hallmark of atherosclerotic plaques.111 Oxidative stress and high glucose can also stimulate cholesterol membrane domains independently of lipid changes.112 Such effects depended on glucose concentration and did not apply to iso-osmotic concentrations of another monosaccharide (mannose), likely due to glucose’s ability to promote ROS generation. Cholesterol crystals costimulate inflammasomes, intracellular macromolecular assemblies that contain and regulate caspase-1, the enzyme that processes pro-IL-1β and pro-IL-18 into their active proinflammatory cytokine products.113
In in vitro studies using model membranes, EPA inhibited glucose-induced cholesterol crystalline domain formation at pharmacologically relevant concentrations due to its antioxidant activity.95,114 Neither certain other agents that lower triglycerides nor vitamin E reproduced this action, as they did not inhibit cholesterol domain formation or interfere with oxidative modification of the membrane lipids.95 These findings suggest that EPA has a particular hydrocarbon length and number of double bonds that foster preferential intercalation into the alkyl chain core of the membrane bilayer, where it inhibits cholesterol domain formation. By contrast, the longer hydrocarbon length for DHA promotes rapid isomerization or conformational changes in the membrane,115 while EPA preserves a more ordered membrane structure.36 These conformational differences cause DHA to change the normal distribution of cholesterol and even promote membrane cholesterol domains as compared to EPA.83,116,117 Although these properties might prove deleterious in vascular cells, such changes in the organization of cholesterol and other membrane lipids may contribute essentially to maintaining normal membrane structure and lipid raft organization in nervous tissues and in the retina where DHA is the most common polyunsaturated FA in cellular membranes.118,119
Thus, EPA-based therapeutics appear to offer a major advance in cardiovascular risk reduction that adds to the protection afforded by statins. REDUCE-IT demonstrated that high doses of a highly purified ethyl ester of EPA, icosapent ethyl, provided large relative and absolute risk reductions in cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, and coronary revascularization in at-risk patients with triglycerides above ≈100 mg/dL despite therapy with statins.2,3,5,6,120 Several other medications are in early stages of evaluation, with promising effects on biomarkers such as triglycerides (Figure 4). These compounds may provide similar, lesser, or even greater risk reductions in cardiovascular events than seen in REDUCE-IT, though large, long-term cardiovascular outcome trials will be necessary to establish any clinical benefits. In addition, other trials have focused on inflammation. In patients with previous myocardial infarction, CANTOS (Canakinumab Anti-Inflammatory Thrombosis Outcome Study) found a 15% reduction in MACE (major adverse cardiovascular events) with a targeted anti-inflammatory approach using canakinumab, validating the pivotal role of inflammation in provoking ischemic events.121 COLCOT (Colchicine Cardiovascular Outcomes Trial), using colchicine, confirmed that an anti-inflammatory approach can provide incremental cardiovascular benefit, demonstrating in patients with recent myocardial infarction that there was a 23% reduction in MACE, largely driven by a reduction in coronary revascularization.122 Anti-inflammatory mechanisms of EPA may also contribute to some proportion of cardiovascular benefit in REDUCE-IT, albeit further downstream in the inflammatory process, as evidenced by large reductions in multiple types of end points without increased infection rates.
Figure 4.
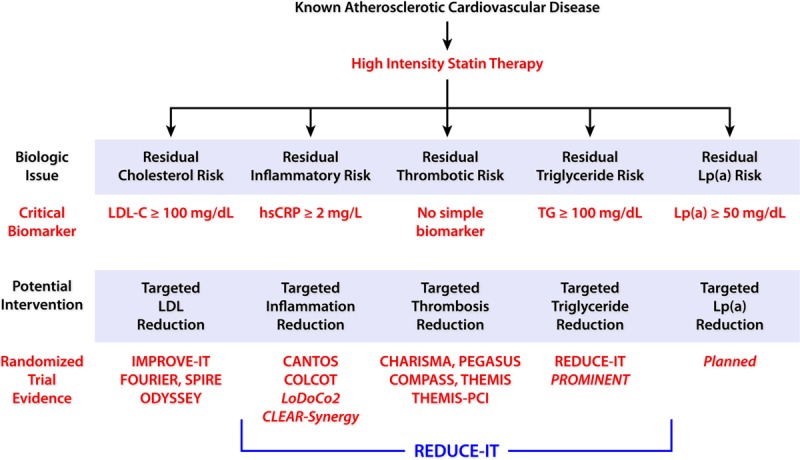
Clinical advances in the management of residual cardiovascular risk. Beyond a plant-based diet and high-intensity statins, further potential strategies to reduce residual cardiovascular risk include those targeting LDL (low-density lipoprotein)-cholesterol, inflammation, thrombosis, triglycerides (TGs), and Lp(a). hsCRP indicates high-sensitivity C-reactive protein; and REDUCE-IT, Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial.
Another trial that tested a mixture of EPA and DHA at 4 g/d, called STRENGTH (A Long-Term Outcomes Study to Assess Statin Residual Risk Reduction With Epanova in High Cardiovascular Risk Patients With Hypertriglyceridemia; URL: https://www.clinicaltrials.gov. Unique identifier: NCT02104817), was terminated due to futility at the recommendation of the independent data-monitoring committee. Thus, currently, the only n3-FA proven to be of cardiovascular benefit in outcome trials remains EPA. The failure to show significant benefit in STRENGTH indicates that the addition of DHA may diminish or even negate certain benefits of EPA, or that the benefits shown in REDUCE-IT accrue from the higher dosage of EPA or the specific formulation. As already discussed, DHA or certain other agents that lower triglyceride tested under identical conditions lack certain atheroprotective mechanisms exerted by EPA, including effects on membrane lipid order and cholesterol crystalline domain formation, along with other important differences. Other potential differences among n3-FAs with respect to mechanisms of atherosclerosis thus merit further study. Several other trials of potent therapies that lower triglycerides are being planned,123 and one with the selective PPAR-α (peroxisome proliferator-activated receptor-alpha) modifying agent pemafibrate is already underway (PROMINENT [Pemafibrate to Reduce Cardiovascular Outcomes by Reducing Triglycerides in Patients With Diabetes]; URL: https://www.clinicaltrials.gov. Unique identifier: NCT03071692).124–126 These trials should help to understand the effects of triglyceride lowering on cardiovascular outcomes. In the meantime, the scientific underpinnings behind the mechanisms of action of n3-FAs such as EPA continue to grow and help inform our understanding of this novel axis of cardiovascular risk reduction.
Acknowledgments
We thank Dr Robert F. Jacob (Elucida Research) for preparation of the figures and Ms Chelsea Swallom (Brigham and Women's Hospital) for editorial assistance.
Sources of Funding
P. Libby receives funding support from the National Heart, Lung, and Blood Institute (R01HL080472 and 1R01HL134892), the American Heart Association (18CSA34080399), and the RRM Charitable Fund.
Disclosures
D.L. Bhatt serves as the Principal Investigator and Study Chair of the REDUCE-IT trial (Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial), funded by Amarin. D.L. Bhatt discloses the following relationships—has served on the Advisory Board of Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Medscape Cardiology, PhaseBio, Plx Pharma, Regado Biosciences; Board of Directors of Boston VA Research Institute, Society of Cardiovascular Patient Care, TobeSoft; Chair of American Heart Association Quality Oversight Committee; Data Monitoring Committees of Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St Jude Medical, now Abbott), Cleveland Clinic (including for the ExCEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria from American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL percutaneous coronary intervention clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/ReachMD (CME steering committees), Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR-ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); research funding from Abbott, Afimmune, Amarin, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, PhaseBio, Pfizer, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties for Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator of Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee for American College of Cardiology; Unfunded Research for FlowCo, Merck, Novo Nordisk, Takeda. Dr Mason discloses the following relationships over the past 12 months—has served on the Advisory Board of Cardax; Consulting and paid lectures for Amarin, Novartis, Pfizer; research funding from Amarin, Cleveland Clinic, Novartis, Pfizer. All funds received from consulting are donated to charity. P. Libby is an unpaid consultant to, or involved in clinical trials for Amgen, AstraZeneca, Esperion Therapeutics, Ionis Pharmaceuticals, Kowa Pharmaceuticals, Novartis, Pfizer, Sanofi-Regeneron, and XBiotech, Inc. P. Libby is a member of scientific advisory board for Amgen, Corvidia Therapeutics, DalCor Pharmaceuticals, IFM Therapeutics, Kowa Pharmaceuticals, Olatec Therapeutics, Medimmune, Novartis, and XBiotech, Inc. P. Libby serves on the Board of XBiotech, Inc. P. Libby’s laboratory has received research funding in the past 2 years from Novartis.
Footnotes
Nonstandard Abbreviations and Acronyms
- ApoC-III
- apolipoprotein C-III
- COX
- cyclooxygenase
- DHA
- docosahexaenoic acid
- EC
- endothelial cell
- eNOS
- endothelial isoform of nitric oxide synthase
- EPA
- eicosapentaenoic acid
- GPR-120
- G-protein-coupled receptor 120
- HDL-C
- high-density lipoprotein-cholesterol
- hsCRP
- high-sensitivity C-reactive protein
- IL
- interleukin
- JELIS
- Japan EPA Lipid Intervention Study
- n3-FAs
- omega-3 fatty acids
- n6-FAs
- omega-6 fatty acids
- NF-κB
- nuclear factor-κB
- NLRP-3
- NLR family pyrin domain-containing 3
- NO
- nitric oxide
- oxLDL
- oxidized LDL-C
- REDUCE-IT
- Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial
- RLP-C
- remnant-like particle cholesterol
- ROS
- reactive oxygen species
- TGRL
- triglyceride-rich lipoproteins
- TH
- T helper
- VCAM-1
- vascular cell adhesion molecule 1
- VLDL
- very-low-density lipoprotein
For Sources of Funding and Disclosures, see page 1143.
References
- 1.Libby P. Triglycerides on the rise: should we swap seats on the seesaw? Eur Heart J. 2015;36:774–776. doi: 10.1093/eurheartj/ehu500. doi: 10.1093/eurheartj/ehu500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganda OP, Bhatt DL, Mason RP, Miller M, Boden WE. Unmet need for adjunctive dyslipidemia therapy in hypertriglyceridemia management. J Am Coll Cardiol. 2018;72:330–343. doi: 10.1016/j.jacc.2018.04.061. doi: 10.1016/j.jacc.2018.04.061. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt DL. Residual cardiovascular risk in statin-treated patients with elevated triglycerides: now we can REDUCE-IT! Eur Heart J. 2019;40:1174–1175. [Google Scholar]
- 4.Bhatt DL, Steg PG, Brinton EA, Jacobson TA, Miller M, Tardif JC, Ketchum SB, Doyle RT, Jr, Murphy SA, Soni PN, et al. REDUCE-IT Investigators. Rationale and design of REDUCE-IT: reduction of cardiovascular events with icosapent ethyl-intervention trial. Clin Cardiol. 2017;40:138–148. doi: 10.1002/clc.22692. doi: 10.1002/clc.22692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowitz C, et al. REDUCE-IT Investigators. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med. 2019;380:11–22. doi: 10.1056/NEJMoa1812792. doi: 10.1056/NEJMoa1812792. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowitz C, et al. REDUCE-IT Investigators. Effects of icosapent ethyl on total ischemic events: from REDUCE-IT. J Am Coll Cardiol. 2019;73:2791–2802. doi: 10.1016/j.jacc.2019.02.032. doi: 10.1016/j.jacc.2019.02.032. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt DL, Miller M, Brinton EA, Jacobson TA, Steg PG, Ketchum SB, Doyle RT, Jr, Juliano RA, Jiao L, Granowitz C, et al. REDUCE-IT USA: results from the 3,146 patients randomized in the United States. Circulation. 2020;141:367–375. doi: 10.1161/CIRCULATIONAHA.119.044440. doi: 10.1161/CIRCULATIONAHA.119.044440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhatt DL, Steg PG, Miller M, Juliano RA, Ballantyne CM. Reply: ischemic event reduction and triglycerides. J Am Coll Cardiol. 2019;74:1849–1850. doi: 10.1016/j.jacc.2019.08.007. doi: 10.1016/j.jacc.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Bhatt DL, Steg PG, Miller M. Cardiovascular risk reduction with icosapent ethyl. reply. N Engl J Med. 2019;380:1678. doi: 10.1056/NEJMc1902165. doi: 10.1056/NEJMc1902165. [DOI] [PubMed] [Google Scholar]
- 10.Mason RP. New insights into mechanisms of action for omega-3 fatty acids in atherothrombotic cardiovascular disease. Curr Atheroscler Rep. 2019;21:2. doi: 10.1007/s11883-019-0762-1. doi: 10.1007/s11883-019-0762-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatt DL, Steg PG, Miller M, Brinton EA, Jacobson TA, Jiao L, Tardif JC, Gregson J, Pocock SJ, Ballantyne CM REDUCE-IT Investigators. Reduction in first and total ischemic events with icosapent ethyl across baseline triglyceride tertiles. J Am Coll Cardiol. 2019;74:1159–1161. doi: 10.1016/j.jacc.2019.06.043. doi: 10.1016/j.jacc.2019.06.043. [DOI] [PubMed] [Google Scholar]
- 12.Lawler PR, Kotrri G, Koh M, Goodman SG, Farkouh ME, Lee DS, Austin PC, Udell JA, Ko DT. Real-world risk of cardiovascular outcomes associated with hypertriglyceridaemia among individuals with atherosclerotic cardiovascular disease and potential eligibility for emerging therapies. Eur Heart J. 2020;41:86–94. doi: 10.1093/eurheartj/ehz767. doi: 10.1093/eurheartj/ehz767. [DOI] [PubMed] [Google Scholar]
- 13.Picard F, Bhatt DL, Ducrocq G, Elbez Y, Ferrari R, Ford I, Tardif JC, Tendera M, Fox KM, Steg PG. Generalizability of the REDUCE-IT trial in patients with stable coronary artery disease. J Am Coll Cardiol. 2019;73:1362–1364. doi: 10.1016/j.jacc.2019.01.016. doi: 10.1016/j.jacc.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 14.Alfaddagh A, Elajami TK, Ashfaque H, Saleh M, Bistrian BR, Welty FK. Effect of eicosapentaenoic and docosahexaenoic acids added to statin therapy on coronary artery plaque in patients with coronary artery disease: a randomized clinical trial. J Am Heart Assoc. 2017;6 pii: e006981 doi: 10.1161/JAHA.117.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mason RP, Sherratt SCR. Omega-3 fatty acid fish oil dietary supplements contain saturated fats and oxidized lipids that may interfere with their intended biological benefits. Biochem Biophys Res Commun. 2017;483:425–429. doi: 10.1016/j.bbrc.2016.12.127. doi: 10.1016/j.bbrc.2016.12.127. [DOI] [PubMed] [Google Scholar]
- 16.Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T, et al. VITAL Research Group. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med. 2019;380:23–32. doi: 10.1056/NEJMoa1811403. doi: 10.1056/NEJMoa1811403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowman L, Mafham M, Wallendszus K, Stevens W, Buck G, Barton J, Murphy K, Aung T, Haynes R, Cox J, et al. ASCEND Study Collaborative Group. Effects of n-3 fatty acid supplements in diabetes mellitus. N Engl J Med. 2018;379:1540–1550. doi: 10.1056/NEJMoa1804989. doi: 10.1056/NEJMoa1804989. [DOI] [PubMed] [Google Scholar]
- 18.Aung T, Halsey J, Kromhout D, Gerstein HC, Marchioli R, Tavazzi L, Geleijnse JM, Rauch B, Ness A, Galan P, et al. Omega-3 Treatment Trialists’ Collaboration. Associations of omega-3 fatty acid supplement use with cardiovascular disease risks: meta-analysis of 10 trials involving 77 917 individuals. JAMA Cardiol. 2018;3:225–234. doi: 10.1001/jamacardio.2017.5205. doi: 10.1001/jamacardio.2017.5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. Japan EPA lipid intervention study (JELIS) Investigators. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369:1090–1098. doi: 10.1016/S0140-6736(07)60527-3. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 20.Saito Y, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Ishikawa Y, Oikawa S, Sasaki J, Hishida H, Itakura H, et al. JELIS Investigators, Japan. Effects of EPA on coronary artery disease in hypercholesterolemic patients with multiple risk factors: sub-analysis of primary prevention cases from the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis. 2008;200:135–140. doi: 10.1016/j.atherosclerosis.2008.06.003. doi: 10.1016/j.atherosclerosis.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCe) ω-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med. 2016;176:1155–1166. doi: 10.1001/jamainternmed.2016.2925. doi: 10.1001/jamainternmed.2016.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG, et al. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Ann Intern Med. 2014;160:398–406. doi: 10.7326/M13-1788. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 23.Mozaffarian D, Wu JH. (n-3) fatty acids and cardiovascular health: are effects of EPA and DHA shared or complementary? J Nutr. 2012;142:614S–625S. doi: 10.3945/jn.111.149633. doi: 10.3945/jn.111.149633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alfaddagh A, Elajami TK, Saleh M, Mohebali D, Bistrian BR, Welty FK. An omega-3 fatty acid plasma index ≥4% prevents progression of coronary artery plaque in patients with coronary artery disease on statin treatment. Atherosclerosis. 2019;285:153–162. doi: 10.1016/j.atherosclerosis.2019.04.213. doi: 10.1016/j.atherosclerosis.2019.04.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ballantyne CM, Bays HE, Kastelein JJ, Stein E, Isaacsohn JL, Braeckman RA, Soni PN. Efficacy and safety of eicosapentaenoic acid ethyl ester (AMR101) therapy in statin-treated patients with persistent high triglycerides (from the ANCHOR study). Am J Cardiol. 2012;110:984–992. doi: 10.1016/j.amjcard.2012.05.031. doi: 10.1016/j.amjcard.2012.05.031. [DOI] [PubMed] [Google Scholar]
- 26.Bays HE, Ballantyne CM, Braeckman RA, Stirtan WG, Soni PN. Icosapent ethyl, a pure ethyl ester of eicosapentaenoic acid: effects on circulating markers of inflammation from the MARINE and ANCHOR studies. Am J Cardiovasc Drugs. 2013;13:37–46. doi: 10.1007/s40256-012-0002-3. doi: 10.1007/s40256-012-0002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bays HE, Ballantyne CM, Kastelein JJ, Isaacsohn JL, Braeckman RA, Soni PN. Eicosapentaenoic acid ethyl ester (AMR101) therapy in patients with very high triglyceride levels (from the Multi-center, plAcebo-controlled, randomized, double-blINd, 12-week study with an open-label extension [MARINE] trial). Am J Cardiol. 2011;108:682–690. doi: 10.1016/j.amjcard.2011.04.015. doi: 10.1016/j.amjcard.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Braeckman RA, Manku MS, Bays HE, Stirtan WG, Soni PN. Icosapent ethyl, a pure EPA omega-3 fatty acid: effects on plasma and red blood cell fatty acids in patients with very high triglyceride levels (results from the MARINE study). Prostaglandins Leukot Essent Fatty Acids. 2013;89:195–201. doi: 10.1016/j.plefa.2013.07.005. doi: 10.1016/j.plefa.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Satoh N, Shimatsu A, Kotani K, Sakane N, Yamada K, Suganami T, Kuzuya H, Ogawa Y. Purified eicosapentaenoic acid reduces small dense LDL, remnant lipoprotein particles, and C-reactive protein in metabolic syndrome. Diabetes Care. 2007;30:144–146. doi: 10.2337/dc06-1179. doi: 10.2337/dc06-1179. [DOI] [PubMed] [Google Scholar]
- 30.Satoh-Asahara N, Shimatsu A, Sasaki Y, Nakaoka H, Himeno A, Tochiya M, Kono S, Takaya T, Ono K, Wada H, et al. Highly purified eicosapentaenoic acid increases interleukin-10 levels of peripheral blood monocytes in obese patients with dyslipidemia. Diabetes Care. 2012;35:2631–2639. doi: 10.2337/dc12-0269. doi: 10.2337/dc12-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballantyne CM, Bays HE, Braeckman RA, Philip S, Stirtan WG, Doyle RT, Jr, Soni PN, Juliano RA. Icosapent ethyl (eicosapentaenoic acid ethyl ester): effects on plasma apolipoprotein C-III levels in patients from the MARINE and ANCHOR studies. J Clin Lipidol. 2016;10:635–645.e1. doi: 10.1016/j.jacl.2016.02.008. doi: 10.1016/j.jacl.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Ballantyne CM, Bays HE, Philip S, Doyle RT, Jr, Braeckman RA, Stirtan WG, Soni PN, Juliano RA. Icosapent ethyl (eicosapentaenoic acid ethyl ester): effects on remnant-like particle cholesterol from the MARINE and ANCHOR studies. Atherosclerosis. 2016;253:81–87. doi: 10.1016/j.atherosclerosis.2016.08.005. doi: 10.1016/j.atherosclerosis.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe T, Ando K, Daidoji H, Otaki Y, Sugawara S, Matsui M, Ikeno E, Hirono O, Miyawaki H, Yashiro Y, et al. CHERRY study investigators. A randomized controlled trial of eicosapentaenoic acid in patients with coronary heart disease on statins. J Cardiol. 2017;70:537–544. doi: 10.1016/j.jjcc.2017.07.007. doi: 10.1016/j.jjcc.2017.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Borow KM, Nelson JR, Mason RP. Biologic plausibility, cellular effects, and molecular mechanisms of eicosapentaenoic acid (EPA) in atherosclerosis. Atherosclerosis. 2015;242:357–366. doi: 10.1016/j.atherosclerosis.2015.07.035. doi: 10.1016/j.atherosclerosis.2015.07.035. [DOI] [PubMed] [Google Scholar]
- 35.Mason RP, Dawoud H, Jacob RF, Sherratt SCR, Malinski T. Eicosapentaenoic acid improves endothelial function and nitric oxide bioavailability in a manner that is enhanced in combination with a statin. Biomed Pharmacother. 2018;103:1231–1237. doi: 10.1016/j.biopha.2018.04.118. doi: 10.1016/j.biopha.2018.04.118. [DOI] [PubMed] [Google Scholar]
- 36.Sherratt SCR, Mason RP. Eicosapentaenoic acid and docosahexaenoic acid have distinct membrane locations and lipid interactions as determined by x-ray diffraction. Chem Phys Lipids. 2018;212:73–79. doi: 10.1016/j.chemphyslip.2018.01.002. doi: 10.1016/j.chemphyslip.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 37.Sasaki J, Miwa T, Odawara M. Administration of highly purified eicosapentaenoic acid to statin-treated diabetic patients further improves vascular function. Endocr J. 2012;59:297–304. doi: 10.1507/endocrj.ej11-0394. doi: 10.1507/endocrj.ej11-0394. [DOI] [PubMed] [Google Scholar]
- 38.Cawood AL, Ding R, Napper FL, Young RH, Williams JA, Ward MJ, Gudmundsen O, Vige R, Payne SP, Ye S, et al. Eicosapentaenoic acid (EPA) from highly concentrated n-3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010;212:252–259. doi: 10.1016/j.atherosclerosis.2010.05.022. doi: 10.1016/j.atherosclerosis.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 39.Nishio R, Shinke T, Otake H, Nakagawa M, Nagoshi R, Inoue T, Kozuki A, Hariki H, Osue T, Taniguchi Y, et al. Stabilizing effect of combined eicosapentaenoic acid and statin therapy on coronary thin-cap fibroatheroma. Atherosclerosis. 2014;234:114–119. doi: 10.1016/j.atherosclerosis.2014.02.025. doi: 10.1016/j.atherosclerosis.2014.02.025. [DOI] [PubMed] [Google Scholar]
- 40.Nosaka K, Miyoshi T, Iwamoto M, Kajiya M, Okawa K, Tsukuda S, Yokohama F, Sogo M, Nishibe T, Matsuo N, et al. Early initiation of eicosapentaenoic acid and statin treatment is associated with better clinical outcomes than statin alone in patients with acute coronary syndromes: 1-year outcomes of a randomized controlled study. Int J Cardiol. 2017;228:173–179. doi: 10.1016/j.ijcard.2016.11.105. doi: 10.1016/j.ijcard.2016.11.105. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka N, Ishida T, Nagao M, Mori T, Monguchi T, Sasaki M, Mori K, Kondo K, Nakajima H, Honjo T, et al. Administration of high dose eicosapentaenoic acid enhances anti-inflammatory properties of high-density lipoprotein in Japanese patients with dyslipidemia. Atherosclerosis. 2014;237:577–583. doi: 10.1016/j.atherosclerosis.2014.10.011. doi: 10.1016/j.atherosclerosis.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 42.Tanaka N, Irino Y, Shinohara M, Tsuda S, Mori T, Nagao M, Oshita T, Mori K, Hara T, Toh R, et al. Eicosapentaenoic acid-enriched high-density lipoproteins exhibit anti-atherogenic properties. Circ J. 2018;82:596–601. doi: 10.1253/circj.CJ-17-0294. doi: 10.1253/circj.CJ-17-0294. [DOI] [PubMed] [Google Scholar]
- 43.Sherratt SCR, Mason RP. Eicosapentaenoic acid inhibits oxidation of high density lipoprotein particles in a manner distinct from docosahexaenoic acid. Biochem Biophys Res Commun. 2018;496:335–338. doi: 10.1016/j.bbrc.2018.01.062. doi: 10.1016/j.bbrc.2018.01.062. [DOI] [PubMed] [Google Scholar]
- 44.Budoff M, Muhlestein JB, Le VT, May HT, Roy S, Nelson JR. Effect of Vascepa (icosapent ethyl) on progression of coronary atherosclerosis in patients with elevated triglycerides (200-499 mg/dL) on statin therapy: rationale and design of the EVAPORATE study. Clin Cardiol. 2018;41:13–19. doi: 10.1002/clc.22856. doi: 10.1002/clc.22856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bays HE, Braeckman RA, Ballantyne CM, Kastelein JJ, Otvos JD, Stirtan WG, Soni PN. Icosapent ethyl, a pure EPA omega-3 fatty acid: effects on lipoprotein particle concentration and size in patients with very high triglyceride levels (the MARINE study). J Clin Lipidol. 2012;6:565–572. doi: 10.1016/j.jacl.2012.07.001. doi: 10.1016/j.jacl.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Ballantyne CM, Braeckman RA, Bays HE, Kastelein JJ, Otvos J, Stirtan WG, Soni PN. Effects of icosapent ethyl on lipoprotein particle concentration and the fatty acid desaturation index in statin-treated patients with persistent high triglycerides (the ANCHOR study). J Clin Lipidol. 2013;7:270–271. doi: 10.1016/j.jacl.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Onat UI, Yildirim AD, Tufanli Ö, Çimen I, Kocatürk B, Veli Z, Hamid SM, Shimada K, Chen S, Sin J, et al. Intercepting the lipid-induced integrated stress response reduces atherosclerosis. J Am Coll Cardiol. 2019;73:1149–1169. doi: 10.1016/j.jacc.2018.12.055. doi: 10.1016/j.jacc.2018.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. doi: 10.1056/NEJMoa1107579. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 49.Davidson MH, Rosenson RS, Maki KC, Nicholls SJ, Ballantyne CM, Mazzone T, Carlson DM, Williams LA, Kelly MT, Camp HS, et al. Effects of fenofibric acid on carotid intima-media thickness in patients with mixed dyslipidemia on atorvastatin therapy: randomized, placebo-controlled study (FIRST). Arterioscler Thromb Vasc Biol. 2014;34:1298–1306. doi: 10.1161/ATVBAHA.113.302926. doi: 10.1161/ATVBAHA.113.302926. [DOI] [PubMed] [Google Scholar]
- 50.Ginsberg HN, Elam MB, Lovato LC, Crouse JR, III, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. doi: 10.1056/NEJMoa1001282. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, et al. Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 52.Patel PN, Patel SM, Bhatt DL. Cardiovascular risk reduction with icosapent ethyl. Curr Opin Cardiol. 2019;34:721–727. doi: 10.1097/HCO.0000000000000678. doi: 10.1097/HCO.0000000000000678. [DOI] [PubMed] [Google Scholar]
- 53.Dunbar RL, Nicholls SJ, Maki KC, Roth EM, Orloff DG, Curcio D, Johnson J, Kling D, Davidson MH. Effects of omega-3 carboxylic acids on lipoprotein particles and other cardiovascular risk markers in high-risk statin-treated patients with residual hypertriglyceridemia: a randomized, controlled, double-blind trial. Lipids Health Dis. 2015;14:98. doi: 10.1186/s12944-015-0100-8. doi: 10.1186/s12944-015-0100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clubb FJ, Jr, Schmitz JM, Butler MM, Buja LM, Willerson JT, Campbell WB. Effect of dietary omega-3 fatty acid on serum lipids, platelet function, and atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Arteriosclerosis. 1989;9:529–537. doi: 10.1161/01.atv.9.4.529. doi: 10.1161/01.atv.9.4.529. [DOI] [PubMed] [Google Scholar]
- 55.Nomura S, Inami N, Shouzu A, Omoto S, Kimura Y, Takahashi N, Tanaka A, Urase F, Maeda Y, Ohtani H, et al. The effects of pitavastatin, eicosapentaenoic acid and combined therapy on platelet-derived microparticles and adiponectin in hyperlipidemic, diabetic patients. Platelets. 2009;20:16–22. doi: 10.1080/09537100802409921. doi: 10.1080/09537100802409921. [DOI] [PubMed] [Google Scholar]
- 56.Nomura S, Kanazawa S, Fukuhara S. Effects of eicosapentaenoic acid on platelet activation markers and cell adhesion molecules in hyperlipidemic patients with type 2 diabetes mellitus. J Diabetes Complications. 2003;17:153–159. doi: 10.1016/s1056-8727(02)00172-1. doi: 10.1016/s1056-8727(02)00172-1. [DOI] [PubMed] [Google Scholar]
- 57.Park Y, Harris W. EPA, but not DHA, decreases mean platelet volume in normal subjects. Lipids. 2002;37:941–946. doi: 10.1007/s11745-006-0984-1. doi: 10.1007/s11745-006-0984-1. [DOI] [PubMed] [Google Scholar]
- 58.Lefils-Lacourtablaise J, Socorro M, Géloën A, Daira P, Debard C, Loizon E, Guichardant M, Dominguez Z, Vidal H, Lagarde M, et al. The eicosapentaenoic acid metabolite 15-deoxy-δ(12,14)-prostaglandin J3 increases adiponectin secretion by adipocytes partly via a PPARγ-dependent mechanism. PLoS One. 2013;8:e63997. doi: 10.1371/journal.pone.0063997. doi: 10.1371/journal.pone.0063997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang Y, He J, Yang J. Eicosapentaenoic acid improves polycystic ovary syndrome in rats via sterol regulatory element-binding protein 1 (SREBP-1)/toll-like receptor 4 (TLR4) pathway. Med Sci Monit. 2018;24:2091–2097. doi: 10.12659/MSM.909098. doi: 10.12659/msm.909098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mori TA, Bao DQ, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253–260. doi: 10.1161/01.hyp.34.2.253. doi: 10.1161/01.hyp.34.2.253. [DOI] [PubMed] [Google Scholar]
- 61.Grimsgaard S, Bønaa KH, Hansen JB, Myhre ES. Effects of highly purified eicosapentaenoic acid and docosahexaenoic acid on hemodynamics in humans. Am J Clin Nutr. 1998;68:52–59. doi: 10.1093/ajcn/68.1.52. doi: 10.1093/ajcn/68.1.52. [DOI] [PubMed] [Google Scholar]
- 62.Allaire J, Couture P, Leclerc M, Charest A, Marin J, Lépine MC, Talbot D, Tchernof A, Lamarche B. A randomized, crossover, head-to-head comparison of eicosapentaenoic acid and docosahexaenoic acid supplementation to reduce inflammation markers in men and women: the comparing EPA to DHA (ComparED) study. Am J Clin Nutr. 2016;104:280–287. doi: 10.3945/ajcn.116.131896. doi: 10.3945/ajcn.116.131896. [DOI] [PubMed] [Google Scholar]
- 63.Terano T, Hirai A, Hamazaki T, Kobayashi S, Fujita T, Tamura Y, Kumagai A. Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis. 1983;46:321–331. doi: 10.1016/0021-9150(83)90181-8. doi: 10.1016/0021-9150(83)90181-8. [DOI] [PubMed] [Google Scholar]
- 64.Woodman RJ, Mori TA, Burke V, Puddey IB, Barden A, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic acid and docosahexaenoic acid on platelet, fibrinolytic and vascular function in hypertensive type 2 diabetic patients. Atherosclerosis. 2003;166:85–93. doi: 10.1016/s0021-9150(02)00307-6. doi: 10.1016/s0021-9150(02)00307-6. [DOI] [PubMed] [Google Scholar]
- 65.Cicero AF, Ertek S, Borghi C. Omega-3 polyunsaturated fatty acids: their potential role in blood pressure prevention and management. Curr Vasc Pharmacol. 2009;7:330–337. doi: 10.2174/157016109788340659. doi: 10.2174/157016109788340659. [DOI] [PubMed] [Google Scholar]
- 66.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med. 2013;368:2004–2013. doi: 10.1056/NEJMra1216063. doi: 10.1056/NEJMra1216063. [DOI] [PubMed] [Google Scholar]
- 67.Ketelhuth DF, Hansson GK. Adaptive response of T and B cells in atherosclerosis. Circ Res. 2016;118:668–678. doi: 10.1161/CIRCRESAHA.115.306427. doi: 10.1161/CIRCRESAHA.115.306427. [DOI] [PubMed] [Google Scholar]
- 68.Mitchell JA, Kirkby NS. Eicosanoids, prostacyclin and cyclooxygenase in the cardiovascular system. Br J Pharmacol. 2019;176:1038–1050. doi: 10.1111/bph.14167. doi: 10.1111/bph.14167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arterburn LM, Hall EB, Oken H. Distribution, interconversion, and dose response of n-3 fatty acids in humans. Am J Clin Nutr. 2006;83(6 suppl):1467S–1476S. doi: 10.1093/ajcn/83.6.1467S. doi: 10.1093/ajcn/83.6.1467S. [DOI] [PubMed] [Google Scholar]
- 70.Marklund M, Wu JHY, Imamura F, Del Gobbo LC, Fretts A, de Goede J, Shi P, Tintle N, Wennberg M, Aslibekyan S, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Fatty Acids and Outcomes Research Consortium (FORCE) Biomarkers of dietary omega-6 fatty acids and incident cardiovascular disease and mortality. Circulation. 2019;139:2422–2436. doi: 10.1161/CIRCULATIONAHA.118.038908. doi: 10.1161/CIRCULATIONAHA.118.038908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M, et al. Effects of n-6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. 2012;95:1003–1012. doi: 10.3945/ajcn.111.030114. doi: 10.3945/ajcn.111.030114. [DOI] [PubMed] [Google Scholar]
- 72.Larsson SC, Kumlin M, Ingelman-Sundberg M, Wolk A. Dietary long-chain n-3 fatty acids for the prevention of cancer: a review of potential mechanisms. Am J Clin Nutr. 2004;79:935–945. doi: 10.1093/ajcn/79.6.935. doi: 10.1093/ajcn/79.6.935. [DOI] [PubMed] [Google Scholar]
- 73.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–1197. doi: 10.1038/ni1276. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 74.Fredman G, Tabas I. Boosting inflammation resolution in atherosclerosis: the next frontier for therapy. Am J Pathol. 2017;187:1211–1221. doi: 10.1016/j.ajpath.2017.01.018. doi: 10.1016/j.ajpath.2017.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hou TY, McMurray DN, Chapkin RS. Omega-3 fatty acids, lipid rafts, and T cell signaling. Eur J Pharmacol. 2016;785:2–9. doi: 10.1016/j.ejphar.2015.03.091. doi: 10.1016/j.ejphar.2015.03.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang P, Smith R, Chapkin RS, McMurray DN. Dietary (n-3) polyunsaturated fatty acids modulate murine Th1/Th2 balance toward the Th2 pole by suppression of Th1 development. J Nutr. 2005;135:1745–1751. doi: 10.1093/jn/135.7.1745. doi: 10.1093/jn/135.7.1745. [DOI] [PubMed] [Google Scholar]
- 77.Monk JM, Hou TY, Turk HF, Weeks B, Wu C, McMurray DN, Chapkin RS. Dietary n-3 polyunsaturated fatty acids (PUFA) decrease obesity-associated Th17 cell-mediated inflammation during colitis. PLoS One. 2012;7:e49739. doi: 10.1371/journal.pone.0049739. doi: 10.1371/journal.pone.0049739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monk JM, Hou TY, Turk HF, McMurray DN, Chapkin RS. n3 PUFAs reduce mouse CD4+ T-cell ex vivo polarization into Th17 cells. J Nutr. 2013;143:1501–1508. doi: 10.3945/jn.113.178178. doi: 10.3945/jn.113.178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiurchiù V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, Serhan CN. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci Transl Med. 2016;8:353ra111. doi: 10.1126/scitranslmed.aaf7483. doi: 10.1126/scitranslmed.aaf7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sato T, Horikawa M, Takei S, Yamazaki F, Ito TK, Kondo T, Sakurai T, Kahyo T, Ikegami K, Sato S, et al. preferential incorporation of administered eicosapentaenoic acid into thin-cap atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2019;39:1802–1816. doi: 10.1161/ATVBAHA.119.313093. doi: 10.1161/ATVBAHA.119.313093. [DOI] [PubMed] [Google Scholar]
- 81.Oh DY, Talukdar S, Bae EJ, Imamura T, Morinaga H, Fan W, Li P, Lu WJ, Watkins SM, Olefsky JM. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell. 2010;142:687–698. doi: 10.1016/j.cell.2010.07.041. doi: 10.1016/j.cell.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamada H, Umemoto T, Kakei M, Momomura SI, Kawakami M, Ishikawa SE, Hara K. Eicosapentaenoic acid shows anti-inflammatory effect via GPR120 in 3T3-L1 adipocytes and attenuates adipose tissue inflammation in diet-induced obese mice. Nutr Metab (Lond) 2017;14:33. doi: 10.1186/s12986-017-0188-0. doi: 10.1186/s12986-017-0188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mason RP, Jacob RF, Shrivastava S, Sherratt SCR, Chattopadhyay A. Eicosapentaenoic acid reduces membrane fluidity, inhibits cholesterol domain formation, and normalizes bilayer width in atherosclerotic-like model membranes. Biochim Biophys Acta. 2016;1858:3131–3140. doi: 10.1016/j.bbamem.2016.10.002. doi: 10.1016/j.bbamem.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 84.Shaikh SR. Biophysical and biochemical mechanisms by which dietary N-3 polyunsaturated fatty acids from fish oil disrupt membrane lipid rafts. J Nutr Biochem. 2012;23:101–105. doi: 10.1016/j.jnutbio.2011.07.001. doi: 10.1016/j.jnutbio.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Williams JA, Batten SE, Harris M, Rockett BD, Shaikh SR, Stillwell W, Wassall SR. Docosahexaenoic and eicosapentaenoic acids segregate differently between raft and nonraft domains. Biophys J. 2012;103:228–237. doi: 10.1016/j.bpj.2012.06.016. doi: 10.1016/j.bpj.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shaikh SR, Wassall SR, Brown DA, Kosaraju R. N-3 Polyunsaturated fatty acids, lipid microclusters, and vitamin E. Curr Top Membr. 2015;75:209–231. doi: 10.1016/bs.ctm.2015.03.003. doi: 10.1016/bs.ctm.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 87.Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet. 1994;344:793–795. doi: 10.1016/s0140-6736(94)92346-9. doi: 10.1016/s0140-6736(94)92346-9. [DOI] [PubMed] [Google Scholar]
- 88.Steinberg D. Lewis A. Conner Memorial Lecture. Oxidative modification of LDL and atherogenesis. Circulation. 1997;95:1062–1071. doi: 10.1161/01.cir.95.4.1062. doi: 10.1161/01.cir.95.4.1062. [DOI] [PubMed] [Google Scholar]
- 89.Chisolm GM, Steinberg D. The oxidative modification hypothesis of atherogenesis: an overview. Free Radic Biol Med. 2000;28:1815–1826. doi: 10.1016/s0891-5849(00)00344-0. doi: 10.1016/s0891-5849(00)00344-0. [DOI] [PubMed] [Google Scholar]
- 90.Ehara S, Ueda M, Naruko T, Haze K, Itoh A, Otsuka M, Komatsu R, Matsuo T, Itabe H, Takano T, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103:1955–1960. doi: 10.1161/01.cir.103.15.1955. doi: 10.1161/01.cir.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 91.Holvoet P, Kritchevsky SB, Tracy RP, Mertens A, Rubin SM, Butler J, Goodpaster B, Harris TB. The metabolic syndrome, circulating oxidized LDL, and risk of myocardial infarction in well-functioning elderly people in the health, aging, and body composition cohort. Diabetes. 2004;53:1068–1073. doi: 10.2337/diabetes.53.4.1068. doi: 10.2337/diabetes.53.4.1068. [DOI] [PubMed] [Google Scholar]
- 92.Walter MF, Jacob RF, Jeffers B, Ghadanfar MM, Preston GM, Buch J, Mason RP PREVENT study. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: a longitudinal analysis of the PREVENT study. J Am Coll Cardiol. 2004;44:1996–2002. doi: 10.1016/j.jacc.2004.08.029. doi: 10.1016/j.jacc.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 93.Walter MF, Jacob RF, Bjork RE, Jeffers B, Buch J, Mizuno Y, Mason RP PREVENT Investigators. Circulating lipid hydroperoxides predict cardiovascular events in patients with stable coronary artery disease: the PREVENT study. J Am Coll Cardiol. 2008;51:1196–1202. doi: 10.1016/j.jacc.2007.11.051. doi: 10.1016/j.jacc.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 94.Mason RP, Sherratt SC, Jacob RF. Eicosapentaenoic acid inhibits oxidation of ApoB-containing lipoprotein particles of different size in vitro when administered alone or in combination with atorvastatin active metabolite compared with other triglyceride-lowering agents. J Cardiovasc Pharmacol. 2016;68:33–40. doi: 10.1097/FJC.0000000000000379. doi: 10.1097/FJC.0000000000000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mason RP, Jacob RF. Eicosapentaenoic acid inhibits glucose-induced membrane cholesterol crystalline domain formation through a potent antioxidant mechanism. Biochim Biophys Acta. 2015;1848:502–509. doi: 10.1016/j.bbamem.2014.10.016. doi: 10.1016/j.bbamem.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 96.Shaikh SR, Kinnun JJ, Leng X, Williams JA, Wassall SR. How polyunsaturated fatty acids modify molecular organization in membranes: insight from NMR studies of model systems. Biochim Biophys Acta. 2015;1848(1 pt B):211–219. doi: 10.1016/j.bbamem.2014.04.020. doi: 10.1016/j.bbamem.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 97.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rees DD, Palmer RM, Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989;86:3375–3378. doi: 10.1073/pnas.86.9.3375. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res. 1999;43:562–571. doi: 10.1016/s0008-6363(99)00169-8. doi: 10.1016/s0008-6363(99)00169-8. [DOI] [PubMed] [Google Scholar]
- 100.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 101.Haiden M, Miyasaka Y, Kimura Y, Tsujimoto S, Maeba H, Suwa Y, Iwasaka T, Shiojima I. Effect of eicosapentaenoic acid on regional arterial stiffness: assessment by tissue Doppler imaging. World J Cardiol. 2012;4:256–259. doi: 10.4330/wjc.v4.i8.256. doi: 10.4330/wjc.v4.i8.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ito R, Satoh-Asahara N, Yamakage H, Sasaki Y, Odori S, Kono S, Wada H, Suganami T, Ogawa Y, Hasegawa K, et al. An increase in the EPA/AA ratio is associated with improved arterial stiffness in obese patients with dyslipidemia. J Atheroscler Thromb. 2014;21:248–260. doi: 10.5551/jat.19976. doi: 10.5551/jat.19976. [DOI] [PubMed] [Google Scholar]
- 103.Takaki A, Umemoto S, Ono K, Seki K, Ryoke T, Fujii A, Itagaki T, Harada M, Tanaka M, Yonezawa T, et al. ELIA study group. Add-on therapy of EPA reduces oxidative stress and inhibits the progression of aortic stiffness in patients with coronary artery disease and statin therapy: a randomized controlled study. J Atheroscler Thromb. 2011;18:857–866. doi: 10.5551/jat.7260. doi: 10.5551/jat.7260. [DOI] [PubMed] [Google Scholar]
- 104.Mita T, Watada H, Ogihara T, Nomiyama T, Ogawa O, Kinoshita J, Shimizu T, Hirose T, Tanaka Y, Kawamori R. Eicosapentaenoic acid reduces the progression of carotid intima-media thickness in patients with type 2 diabetes. Atherosclerosis. 2007;191:162–167. doi: 10.1016/j.atherosclerosis.2006.03.005. doi: 10.1016/j.atherosclerosis.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 105.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342–345. doi: 10.1161/01.cir.104.3.342. doi: 10.1161/01.cir.104.3.342. [DOI] [PubMed] [Google Scholar]
- 106.De Caterina R, Cybulsky MI, Clinton SK, Gimbrone MA, Jr, Libby P. The omega-3 fatty acid docosahexaenoate reduces cytokine-induced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arterioscler Thromb. 1994;14:1829–1836. doi: 10.1161/01.atv.14.11.1829. doi: 10.1161/01.atv.14.11.1829. [DOI] [PubMed] [Google Scholar]
- 107.Weber C, Erl W, Pietsch A, Danesch U, Weber PC. Docosahexaenoic acid selectively attenuates induction of vascular cell adhesion molecule-1 and subsequent monocytic cell adhesion to human endothelial cells stimulated by tumor necrosis factor-alpha. Arterioscler Thromb Vasc Biol. 1995;15:622–628. doi: 10.1161/01.atv.15.5.622. doi: 10.1161/01.atv.15.5.622. [DOI] [PubMed] [Google Scholar]
- 108.Yamada H, Yoshida M, Nakano Y, Suganami T, Satoh N, Mita T, Azuma K, Itoh M, Yamamoto Y, Kamei Y, et al. In vivo and in vitro inhibition of monocyte adhesion to endothelial cells and endothelial adhesion molecules by eicosapentaenoic acid. Arterioscler Thromb Vasc Biol. 2008;28:2173–2179. doi: 10.1161/ATVBAHA.108.171736. doi: 10.1161/ATVBAHA.108.171736. [DOI] [PubMed] [Google Scholar]
- 109.Tulenko TN, Chen M, Mason PE, Mason RP. Physical effects of cholesterol on arterial smooth muscle membranes: evidence of immiscible cholesterol domains and alterations in bilayer width during atherogenesis. J Lipid Res. 1998;39:947–956. [PubMed] [Google Scholar]
- 110.Mason RP, Walter MF, Day CA, Jacob RF. Active metabolite of atorvastatin inhibits membrane cholesterol domain formation by an antioxidant mechanism. J Biol Chem. 2006;281:9337–9345. doi: 10.1074/jbc.M513000200. doi: 10.1074/jbc.M513000200. [DOI] [PubMed] [Google Scholar]
- 111.Grebe A, Latz E. Cholesterol crystals and inflammation. Curr Rheumatol Rep. 2013;15:313. doi: 10.1007/s11926-012-0313-z. doi: 10.1007/s11926-012-0313-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Self-Medlin Y, Byun J, Jacob RF, Mizuno Y, Mason RP. Glucose promotes membrane cholesterol crystalline domain formation by lipid peroxidation. Biochim Biophys Acta. 2009;1788:1398–1403. doi: 10.1016/j.bbamem.2009.04.004. doi: 10.1016/j.bbamem.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 113.Karasawa T, Takahashi M. Role of NLRP3 inflammasomes in atherosclerosis. J Atheroscler Thromb. 2017;24:443–451. doi: 10.5551/jat.RV17001. doi: 10.5551/jat.RV17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Braeckman RA, Stirtan WG, Soni PN. Pharmacokinetics of eicosapentaenoic acid in plasma and red blood cells after multiple oral dosing with icosapent ethyl in healthy subjects. Clin Pharmacol Drug Dev. 2014;3:101–108. doi: 10.1002/cpdd.84. doi: 10.1002/cpdd.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soubias O, Gawrisch K. Docosahexaenoyl chains isomerize on the sub-nanosecond time scale. J Am Chem Soc. 2007;129:6678–6679. doi: 10.1021/ja068856c. doi: 10.1021/ja068856c. [DOI] [PubMed] [Google Scholar]
- 116.Wassall SR, Stillwell W. Docosahexaenoic acid domains: the ultimate non-raft membrane domain. Chem Phys Lipids. 2008;153:57–63. doi: 10.1016/j.chemphyslip.2008.02.010. doi: 10.1016/j.chemphyslip.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 117.Soni SP, LoCascio DS, Liu Y, Williams JA, Bittman R, Stillwell W, Wassall SR. Docosahexaenoic acid enhances segregation of lipids between: 2H-NMR study. Biophys J. 2008;95:203–214. doi: 10.1529/biophysj.107.123612. doi: 10.1529/biophysj.107.123612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Innis SM. Dietary (n-3) fatty acids and brain development. J Nutr. 2007;137:855–859. doi: 10.1093/jn/137.4.855. doi: 10.1093/jn/137.4.855. [DOI] [PubMed] [Google Scholar]
- 119.Uauy R, Dangour AD. Nutrition in brain development and aging: role of essential fatty acids. Nutr Rev. 2006;64(5 pt 2):S24–33. doi: 10.1301/nr.2006.may.s24-s33. discussion S72. doi: 10.1301/nr.2006.may.s24-s33. [DOI] [PubMed] [Google Scholar]
- 120.Boden WE, Bhatt DL, Toth PP, Ray KK, Chapman MJ, Luscher TF. Profound reductions in first and total cardiovascular events with icosapent ethyl in the REDUCE-IT trial: why these results usher in a new era in dyslipidaemia therapeutics. Eur Heart J. 2019;pii:ehz778. doi: 10.1093/eurheartj/ehz778. doi: 10.1093/eurheartj/ehz778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, et al. CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 122.Tardif JC, Kouz S, Waters DD, Bertrand OF, Diaz R, Maggioni AP, Pinto FJ, Ibrahim R, Gamra H, Kiwan GS, et al. Efficacy and safety of low-dose colchicine after myocardial infarction. N Engl J Med. 2019;381:2497–2505. doi: 10.1056/NEJMoa1912388. doi: 10.1056/NEJMoa1912388. [DOI] [PubMed] [Google Scholar]
- 123.Qamar A, Libby P, Bhatt DL. Targeting RNA to lower triglycerides: long strides from short molecules. Eur Heart J. 2019;40:2797–2800. doi: 10.1093/eurheartj/ehz321. doi: 10.1093/eurheartj/ehz321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Nicholls SJ, Lincoff AM, Bash D, Ballantyne CM, Barter PJ, Davidson MH, Kastelein JJP, Koenig W, McGuire DK, Mozaffarian D, et al. Assessment of omega-3 carboxylic acids in statin-treated patients with high levels of triglycerides and low levels of high-density lipoprotein cholesterol: rationale and design of the STRENGTH trial. Clin Cardiol. 2018;41:1281–1288. doi: 10.1002/clc.23055. doi: 10.1002/clc.23055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pradhan AD, Paynter NP, Everett BM, Glynn RJ, Amarenco P, Elam M, Ginsberg H, Hiatt WR, Ishibashi S, Koenig W, et al. Rationale and design of the pemafibrate to reduce cardiovascular outcomes by reducing triglycerides in patients with diabetes (PROMINENT) study. Am Heart J. 2018;206:80–93. doi: 10.1016/j.ahj.2018.09.011. doi: 10.1016/j.ahj.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 126.Fruchart JC, Santos RD, Yamashita S, Libby P International Atherosclerosis Society/R3i Foundation Consensus Panel. Residual vascular risk in diabetes - will the SPPARM alpha concept hold the key? Diabetes Metab Syndr. 2019;13:2723–2725. doi: 10.1016/j.dsx.2019.05.034. doi: 10.1016/j.dsx.2019.05.034. [DOI] [PubMed] [Google Scholar]


