Abstract
Background.
Clinical use of myocardial blood flow (MBF) and flow reserve (MFR) is increasing. Motion correction is necessary to obtain accurate results but can introduce variability when performed manually. We sought to reduce that variability with an automated motion-correction algorithm.
Methods.
A blinded randomized controlled trial of two technologists was performed on the motion correction of 100 dynamic 82Rb patient studies comparing manual motion correction with manual review and adjustment of automated motion correction. Inter-rater variability between technologists for MBF and MFR was the primary outcome with comparison made by analysis of the limits of agreement. Processing time was the secondary outcome.
Results.
Limits of agreements between the two technologists decreased significantly for both MBF and MFR, going from [−0.22, 0.22] mL/min/g and [−0.31, 0.36] to [−0.12, 0.15] mL/min/g and [−0.15, 0.18], respectively (both P<.002). In addition, the average time spent on motion correcting decreased by 1 min per study from 5:21 to 4:21 min (P=.001).
Conclusions.
In this randomized controlled trial, the use of automated motion correction significantly decreased inter-user variability and reduced processing time.
Keywords: PET, Myocardial blood flow, Image analysis
Abstract
Antecedentes.
La utilización clínica del flujo sanguíneo miocárdico (MBF por sus siglas en inglés) y de la reserva de flujo coronario (MFR por sus siglas en inglés) está en aumento. La corrección de movimiento es necesaria para obtener resultados exactos, pero puede introducir variabilidad cuando se realiza manualmente. Nosotros buscamos reducir esa variabilidad con un algoritmo automático de corrección de movimiento.
Métodos.
Se realizó un ensayo controlado aleatorizado ciego de dos tecnólogos sobre la corrección de movimiento de 100 estudios dinámicos de pacientes de rubidio-82, comparando la corrección manual con la revisión y el ajuste de la corrección automática. La variabilidad interobservador entre los tecnólogos para MBF y MFR fue el resultado principal, con la comparación realizada por el análisis de los límites de concordancia. El tiempo de procesamiento fue el resultado secundario.
Resultados.
Límites de concordancia entre los dos tecnólogos disminuyeron significativamente para MBF y MFR, de [− 0,22; 0,22] y [− 0,31; 0,36] a [− 0.12; 0,15] y [− 0,15; 0,18], respectivamente (P < ,002). Adicionalmente, el tiempo promedio de procesamiento disminuyo en 1 min por estudio, de 5:21 a 4:21 min (P = ,001).
Conclusiones.
En este ensayo controlado aleatorizado, la utilización de corrección de movimiento automática disminuyo significativamente la variabilidad entre usarios y redujo el tiempo de procesamiento.
Abstract
Contexte.
L’utilisation clinique du débit sanguin myocardique (DSM) et de la réserve de débit myocardique (RDM) est de plus en plus fréquente. La correction de mouvement durant la séquence d’images dynamiques est nécessaire pour obtenir des résultats justes, mais peut introduire de la variabilité lorsqu’elle est réalisée manuellement. Nous avons cherché à réduire cette variabilité en utilisant un algorithme de correction automatique de mouvement.
Méthodes.
Un essai contrôlé aléatoire masqué a été réalisé pour vérifier l’efficacité de l’algorithme de correction automatique. Utilisant une population de 100 patients référés pour un protocole repos-effort dynamique au 82Rb, l’essai comparait la correction manuelle de mouvement à une rectification manuelle de la correction automatique pour deux opérateurs. La variabilité inter-opérateur du DSM et de la RDM, mesurée en termes d’intervalle de confiance (de niveau 0,95), constitue le résultat majeur de l’étude. Le temps de correction a aussi été analysé comme résultat secondaire.
Résultats.
En utilisant l’algorithme de correction automatique, Les intervalles de confiance pour le DSM et la RDM sont significativement améliorés, allant de [−0,22; 0,22] mL/min/g et [− 0,31; 0,36] à [−0,12; 0,15] mL/min/g et [−0,15; 0,18], respectivement (P<,002). Par ailleurs, le temps moyen de correction de mouvement a diminué d’une minute par patient, passant de 5 min 21 s à 4 min 21 s (P=,001).
Conclusions.
Lors de cette étude, l’utilisation de la correction automatique de mouvement a significativement diminué la variabilité inter-opérateur, ainsi que le temps de correction.
INTRODUCTION
Myocardial blood flow (MBF) and flow reserve (MFR) are increasingly used to improve diagnostic and prognostic assessments in patients with known or suspected coronary artery disease (CAD).1–5 In parallel, there is increasing interest in the variability and reliability of these measurements with several analyses of test-retest variability,6–8 as well as user-induced variability.9 Patient motion affects MBF and MFR values and should be corrected to obtain maximally accurate results.10 Unfortunately, manual motion correction may decrease precision due to inter-user variability. We have previously described a novel automated, image-based, motion-correction algorithm.11 The extent to which this algorithm improves user-related variability in MBF and MFR quantifications is unknown. We performed a randomized controlled trial to evaluate whether automated motion correction reduces inter-user variability in quantification of MBF and MFR from 82Rb PET imaging.
METHODS
Study Design
We conducted a blinded randomized controlled trial of two certified nuclear medicine technologists trained in processing dynamic PET studies. Each technologist motion corrected the dynamic images twice: once with and once without automated motion correction previously applied. To achieve this, each patient study was duplicated, with one copy void of any motion correction, and the other automatically motion corrected with our novel algorithm. For the latter, the image data were saved with the motion correction inherently applied, so it could not be distinguished from a noncorrected patient study. The corrected and noncorrected studies were randomly sorted into two databases, each containing all patient studies, with half corrected and half noncorrected. The technologists only had access to one database at a time so they could not compare duplicates of the same studies.
Study Data
The study was performed using dynamic rest and stress images from a 100-patient subset randomly selected from a set of 225 patients sequentially selected for an earlier study.10 All patients were referred for clinically indicated 82Rb rest and stress scans between June 1, 2017 and July 26, 2017. All subjects provided written informed consent and all exam protocols were approved by the University of Michigan Institutional Review Board.
PET Imaging
Subjects were instructed to avoid caffeine and methylxanthine for a full day prior to their study, as well as to fast overnight. Weight-based doses of 82Rb (12 MBq/kg) were infused into a brachial vein at 50 mL/min over 5 to 25 seconds using a Cardiogen-82 Infusion system (Bracco Diagnostic, Monroe Township, NJ, USA) for both rest and stress. Scans were performed on a 3D PET/CT scanner (Siemens Biograph mCT, Siemens Healthineers, Knoxville, TN, USA), starting with a CT for attenuation correction. List-mode data were acquired over 7 minutes from the start of the radiotracer injection. Stress was induced pharmacologically through an injection of 0.4 mg of regadenoson over 15 seconds followed by a 10-mL saline flush. The tracer injection and stress scan started 60 seconds after the start of the regadenoson injection. High-flow outliers (MBF>5 mL/min/g) were not excluded as they are an occasional occurrence, particularly when the radiotracer cannot be flushed with saline.
Image Processing
Dynamic images were reconstructed from list-mode data using the iterative 3D ordered-subset-expectation-maximization algorithm with point-spread-function and time-of-flight modelling with 21 subsets and 3 iterations. All images were attenuation corrected, along with other standard corrections (randoms, scatter, and prompt gamma). Slices were reconstructed to a 128×128 matrix of 3.18 mm×3.18 mm pixels, with a slice thickness of 3 mm. Dynamic series were made up of 30 frames adding up to 6 minutes 40 seconds of acquisition time, after adding a delay of 20 seconds to the start of the scan, with the following temporal sampling: 16×5 s, 6×10 s, 3×20 s, 4×30 s, and 1×80 s. A summed image, created from the last 4 minutes and 40 seconds of acquisition, was used to create endo- and epicardial surfaces of the left ventricle automatically in Corridor4DM (INVIA Medical Imaging Solutions, Ann Arbor, MI, USA).12 The surfaces underwent quality assurance during clinical workflow and are used in this study.
The motion correction was performed using Corridor4DM. The technologists were provided with three short-axis viewports (apical, mid, and basal) as well as central horizontal-long-axis and vertical-long-axis viewports. For each frame, image volumes could be translated through a click-and-drag action or fine-panning buttons, around the fixed reference myocardial contours. Rotations were not used.
Statistical Analysis
Statistical analysis was performed in MATLAB (MathWorks, Natick, MA, USA) and R 3.5.2 (The R Foundation for Statistical Computing). The results from both technologists were correlated for both cases (non-corrected and auto corrected), along with the creation of Bland-Altman plots. Limits of agreement were compared for statistical significance by fitting a linear mixed-effect model on the absolute MBF difference using fixed effects for automated motion correction, vascular segment, and stress (in the case of MBF), and a random effect for patient. Pearson correlation coefficients were calculated for global MBF and MFR. Coefficients of correlation for segmental MBF and MFR were calculated by fitting a linear mixed-effect model to the data with a fixed effect for automated motion correction and nested random effects for patient, stress (in the case of MBF), vascular segment, and technologist following an example seen in the literature.13 Coefficients of correlation were then compared for statistical significance by using a backtransformed average Fisher’s Z procedure.14
The time spent processing studies was investigated retrospectively by analyzing the time stamps of the results created by the technologists. A two-gaussian model was fitted to the distributions where the second gaussian was used to account for delays unrelated to the processing. The mean of the highest contributing gaussian was then assumed to be the mean processing time.
The amount of motion correction applied by the technologists was analyzed by looking at the number of frames corrected by more than a significant value. We used a cutoff of 3 mm as it relates to the pixel size (3.18 × 3.18 × 3 mm3). The blood pool and tissue phases were separated, as well as the stress and rest datasets, to isolate the effect of automated motion correction on both. The blood pool phase corresponds to the first 2 min of the acquisition (20 frames), while the tissue phase corresponds to the remaining 4 min and 40 s (10 frames). The uncorrected and corrected data were then fit to linear mixed-effect models with singular fixed effects (technologist, rest/stress, blood pool/tissue) and a random effect on patient, to highlight the improvements yielded by the automated motion correction algorithm.
RESULTS
The characteristics of the patient population are displayed in Table 1. Most patients were older, obese, and had hypertension and hypercholesterolemia. In addition, 45 patients had diabetes.
Table 1.
Characteristics of the patient population used in the study
| Characteristics | |
|---|---|
| N | 100 |
| Gender (male) | 55 |
| Age (years) | 61.1±12.5 |
| BMI (kg/m2) | 33.9±8.8 |
| 82Rb dose | 28.6±7.8 |
| Cardiac history/risk factors | |
| Known CAD | 28 |
| Known MI | 13 |
| Hypertension | 80 |
| Hypercholesterolemia | 68 |
| Obesity | 63 |
| Family history | 49 |
| Diabetes | 45 |
| Smoking | 22 |
| Measured MBF | |
| Uncorrected global stress MBF (mL/min/g) | 2.35±1.02 |
| Uncorrected global rest MBF (mL/min/g) | 1.10±0.41 |
| Uncorrected global MFR | 2.20±0.73 |
| ’True’ global stress MBF (mL/min/g) | 2.25±0.93 |
| ’True’ global rest MBF (mL/min/g) | 1.06±0.39 |
| ’True’ global MFR | 2.19±0.70 |
Continuous variables are presented as mean±standard deviation. ‘True’ MBF and MFR values are the average values from the two technologists, who processed with manual adjustments after automated motion correction
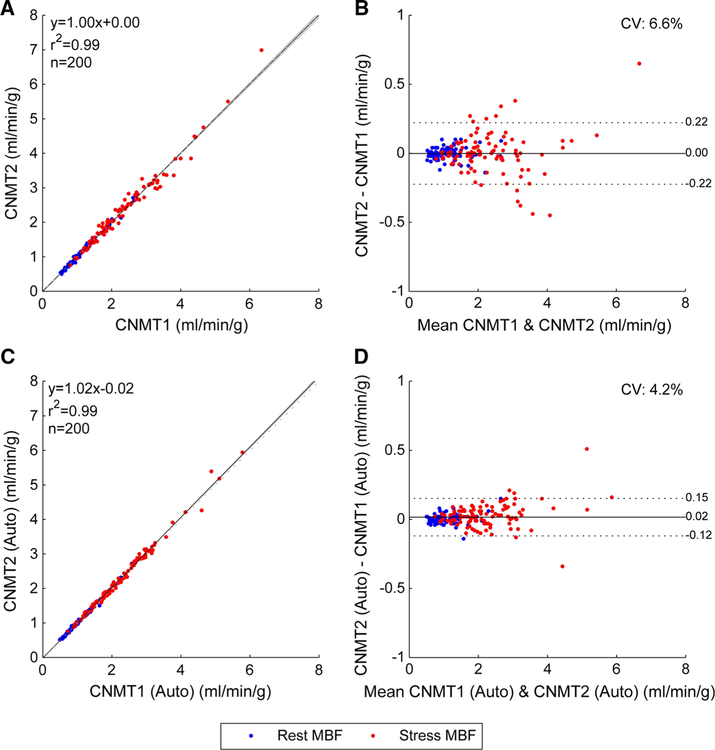
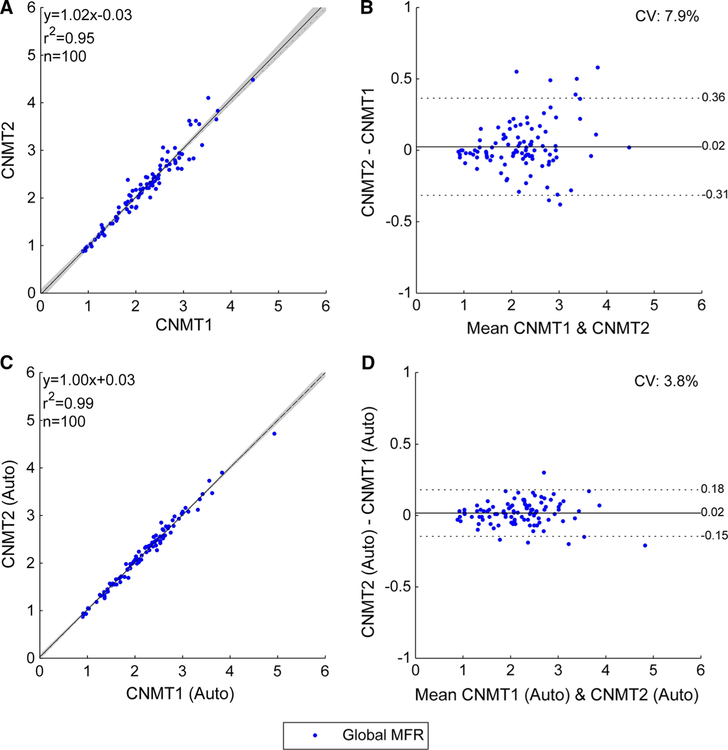
The correlation between the global MBFs obtained by the technologists without motion correction (Figure 1A, B) improved with motion correction (Figure 1C, D). Importantly, the Pearson correlation improved from 0.993 to 0.997 (P<.0001) and the limits of agreement narrowed from [−0.22, 0.22] to [−0.12, 0.15] (p =.002). Correspondingly, correlations between the MFRs obtained by the two technologists without motion correction (Figure 2A, B) also improved with motion correction (Figure 2C, D) with improved Pearson correlation (0.973 vs 0.993, P<.0001) and narrower limits of agreement ([−0.31, 0.36] vs [−0.15, 0.18], P<.0001). Agreement in regional MBF and MFR also improved (Table 2)
Figure 1.
Global MBF correlations between manual motion correction of uncorrected studies (A and B) and manual adjustments of automated motion correction (C and D) from the two technologists. The shaded area on the correlation plots shows the 95% confidence interval of the linear regression.
Figure 2.
Global MFR correlations between manual motion correction of uncorrected studies (A and B) and manual adjustments of automated motion correction (C and D) from the two technologists. The shaded area on the correlation plots shows the 95% confidence interval of the linear regression.
Table 2.
Summary of the correlation between two technologists
| Manual motion correction only | Manual adjustment of automated motion correction | P value | ||
|---|---|---|---|---|
| Global MBF | r | 0.993 | 0.997 | <.0001 |
| (N=200) | LOA | −0.22 0.22 | −0.12 0.15 | .002 |
| Global MFR | r | 0.973 | 0.993 | <.0001 |
| (N=100) | LOA | −0.31 0.36 | −0.15 0.18 | <.0001 |
| Vascular MBF | r | 0.990 | 0.996 | <.0001 |
| (N=600) | LOA | −0.29 0.28 | −0.15 0.18 | <.0001 |
| Vascular MFR | r | 0.960 | 0.989 | <.0001 |
| (N=300) | LOA | −0.38 0.43 | −0.19 0.22 | <.0001 |
The limits of agreement (LOA) for MBF are in mL/min/g
A couple of discordant data points stand out as seen in Figure 1D, displaying MBF differences of 0.34 and 0.51 mL/min/g (8% and 10% in relative errors, respectively). While these discrepancies are higher than ideal, they are likely to be of limited clinical importance because the MBF values (4.60, 4.26, and 4.88, 5.39 mL/ min/g, respectively) are all well within the normal range.15
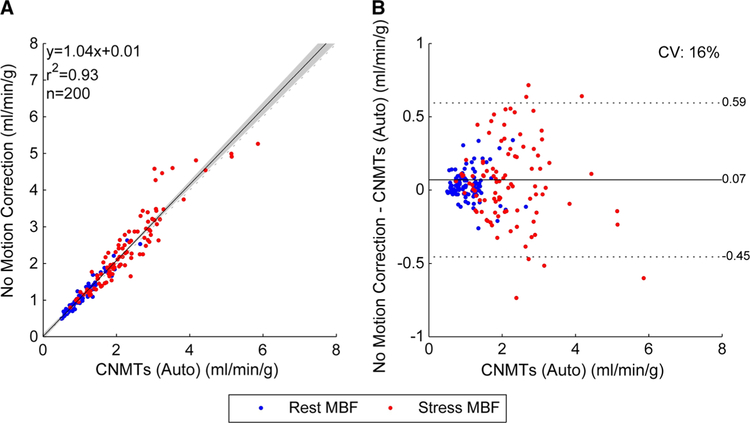
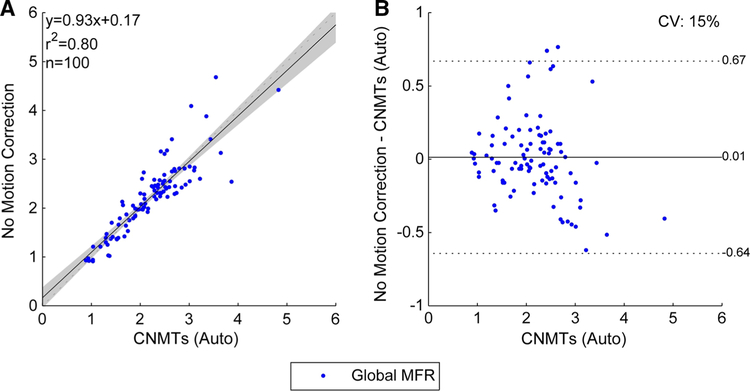
Given the excellent agreement between both technologists, we assumed the ‘true’ MBF and MFR values as the mean of their auto-assisted results. The MBF and MFR values obtained without motion correction are plotted against our ‘true’ values in Figures 3 and 4, respectively. These results highlight the need for dynamic motion correction as we can see the coefficient of variation (CV) on noncorrected MFR is 94% larger than the variability introduced by manual motion correction (P<.0001).
Figure 3.
Global MBF correlations between technologists and no motion correction. The shaded area on the correlation plot shows the 95% confidence interval of the linear regression.
Figure 4.
Global MFR correlations between technologists and no motion correction. The shaded area on the correlation plot shows the 95% confidence interval of the linear regression.
One commonly used threshold on 82Rb MFR used to classify studies as normal or abnormal is 2.0.3,15 For a random patient population matching the distribution of our study population (described in Table 1), the CV measured between our two technologists would result in discordant diagnoses for 6.7% of the patients. Using the automated-motion-correction algorithm prior to performing manual adjustments would reduce the rate of discordance between technologists to 3.2%. (P<.0001).
Table 3 shows there is significantly more motion in the blood pool phase and at stress, as previously shown in the literature.10 Table 4 further highlights the improvements made by the algorithm, as the significant difference between the technologist motion correction on the uncorrected data decreased substantially. The same trend was seen when comparing stress vs rest, and blood pool vs tissue phase, although the difference in motion correction between both phases remained significant.
Table 3.
Number of frames shifted by more than 3 mm by the technologists
| Blood pool phase |
Tissue phase |
||||||
|---|---|---|---|---|---|---|---|
| Manual | Auto | P value | Manual | Auto | P value | ||
| Rest | CNMT1 | 3.95±3.13 | 2.17±2.31 | <.0001 | 0.42±1.33 | 0.44±1.53 | .80 |
| CNMT2 | 4.13±3.71 | 1.49±2.68 | <.0001 | 1.48±3.08 | 1.50±3.23 | .87 | |
| Stress | CNMT1 | 5.27±3.93 | 2.10±2.68 | <.0001 | 0.72±1.58 | 0.40±1.61 | .01 |
| CNMT2 | 6.89±5.26 | 1.92±3.19 | <.0001 | 1.95±3.30 | 1.32±2.96 | .002 | |
Manual refers to the manual motion correction, while Auto refers to the manual adjustments of the automated motion correction
Table 4.
Magnitude and significance of singular fixed effects on the number of frames shifted by more than 3 mm for manual motion correction (manual) and manual adjustments of the automated motion correction (auto)
| Effect | Magnitude | P value |
|---|---|---|
| CNMT1 vs CNMT2 (manual) | −1.02 | <.0001 |
| CNMT1 vs CNMT2 (auto) | −0.28 | .06 |
| Stress vs rest (manual) | 1.21 | <.0001 |
| Stress vs rest (auto) | 0.035 | .81 |
| BP vs tissue (manual) | 3.92 | <.0001 |
| BP vs tissue (auto) | 1.01 | <.0001 |
The results in Table 3 show the automated motion correction significantly reduces the correction made by both technologists, which implies a lesser processing time. From an analysis of the processing times, it was found that the average time per study required declined by 19% from a mean of 5 min 21 s to 4 min 21 s (P =.001), with a consistent effect for both technologists. Table 5 shows the processing time for both technologists, with and without the automated motion correction.
Table 5.
Average time to correct for motion, in minutes:seconds, for the two technologists (CNMT 1 and 2)
| Manual motion correction | Manual adjustment of automated motion correction | P value | |
|---|---|---|---|
| Combined | 5:21±3:12 | 4:21±1:58 | .001 |
| CNMT1 | 5:01±4:21 | 4:03±1:48 | .08 |
| CNMT2 | 5:35±1:49 | 4:30±1:45 | .0001 |
The p-values are calculated from the null hypothesis that the derived average time is unchanged
Table 6 presents the correlation of the technologists and the unadjusted automated motion correction, which yielded similar results to the correlation between both technologists. This result indicates that using the unadjusted automated motion correction should be a viable solution to remove user variability while still obtaining accurate quantification.
Table 6.
Summary of the correlation between the technologists and the unadjusted automated motion correction
| CNMT1 (auto) vs unadjusted automated motion correction | CNMT2 (auto) vs unadjusted automated motion correction | ||
|---|---|---|---|
| Global MBF | r | 0.999 | 0.998 |
| (N=200) | LOA | −0.10 0.08 | −0.12 0.13 |
| Global MFR | r | 0.993 | 0.994 |
| (N=100) | LOA | −0.15 0.18 | −0.12 0.18 |
| Vascular MBF | r | 0.997 | 0.996 |
| (N=600) | LOA | −0.15 0.13 | −0.17 0.18 |
| Vascular MFR | r | 0.986 | 0.988 |
| (N=300) | LOA | −0.22 0.23 | −0.19 0.24 |
The limits of agreement (LOA) for MBF are in mL/min/g
DISCUSSION
This study showed clear improvement in motion-correction reproducibility when using the automated motion correction as a starting point. Limits of agreement narrowed significantly, which would yield a significantly lower discordance rate between technologists. In addition, the time spent motion correcting the studies decreased by one minute, which would quickly add up when processing multiple studies.
Fitting the motion-correction variability within the MBF repeatability values reported in the literature is particularly difficult, given that most articles make no mention of dynamic motion correction. Not knowing how motion was dealt with prevents us from combining variances and calculating an estimate of the overall uncertainty in MBF repeatability with, and without automated motion correction. A simple comparison of LOA range does however show that the motion correction is an important component of the overall variability. A recent study focused on optimizing the 82rubidium MBF repeatability shows an LOA range of 0.40 for MFR 6 which indicates that the reduced inter-user variability from the automatically corrected data would still be one of the main components of the overall variability with a range of 0.33.
The clinical implementation of the automated motion correction should prove more successful than this study, as users will have a visual cue notifying them that the data have been automatically motion corrected. This should incite users to perform fewer shifts, thus reducing variability and processing time, without hurting quantification accuracy given the excellent agreement between the unadjusted automated motion correction and the adjustments made by the technologists.
Particular attention was given to isolating the motion-correction step of the standard processing of a study, as well as avoiding any type of bias the technologists could get from knowing which studies went through automated motion correction. There was no evidence that the technologists identified which studies were corrected based on the number of frames adjusted. Therefore, the main limitation of this study is its limited scope, as only the inter-user variability was investigated. Intra-user variability would be a useful metric to tally, but it would require a technologist to go through all the duplicated datasets once more and could start introducing bias from knowing which datasets require less motion correction.
Another limitation lies on the secondary analysis of the data, looking at the processing time. The processing time information is inherently flawed as the technologists were tasked with processing the studies in an office environment rather than a clinical environment where their processing of each study is awaited by physicians. As such, noise in the data is a common occurrence and could not be easily accounted for. While tasking the technologists with timing their processing would have solved this issue, it also would have likely skewed the results.
Further review of the outlier and discordant datasets revealed studies with limited diagnostic potential, as two of the three high-flow outliers and both discordant studies presented noisy input time activity curves with low amplitudes. Given that the study used data acquired on a 3D PET scanner, these results could be an indicator of a limitation of the algorithm for 2D data, which will require further investigation.
In depth analysis of the manual adjustments from the technologists will yield information on what frames are not properly corrected by the automated algorithm, which in turn could indicate what features the algorithm does not process correctly. Improvements to the algorithm could then be assessed by repeating this trial.
CONCLUSION
A randomized controlled trial was performed to analyze the effect of automated motion correction on the variability in dynamic motion correction between users. The results display significant improvement in limits of agreement between the two users, warranting the use of the algorithm. In addition, the use of the algorithm resulted in a modest reduction of the processing time.
NEW KNOWLEDGE GAINED
Automated motion correction significantly reduces the inter-user variability introduced by dynamic motion correction, as well as the time spent motion correcting. It could also be used without user adjustments to fully remove user variability while maintaining accurate quantification of MBF and MFR.
Supplementary Material
Acknowledgements
The authors would like to thank Chanée Nelson and Kevin Fischio for their efforts in processing the datasets in this study.
V.L. Murthy receives research support and funding from the INVIA Medical Imaging Solutions; research grants and lecture honoraria from the Siemens Medical Imaging; an expert witness testimony payment on behalf of the Jubilant Draximage; and advisory board payments from the Curium and Ionetix. V.L. Murthy has stock in General Electric and Cardinal Health, and stock options in Ionetix.
Abbreviations
- PET
Positron emission tomography
- CT
Computed tomography
- Rb
Rubidium
- MBF
Myocardial blood flow
- mfr
Myocardial flow reserve
- LOA
Limits of agreement
- CV
Coefficient of variation
Footnotes
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s12350–019-01911–9) contains supplementary material, which is available to authorized users.
The authors of this article have provided a PowerPoint file, available for download at SpringerLink, which summarises the contents of the paper and is free for re-use at meetings and presentations. Search for the article DOI on SpringerLink.com.
Disclosure
A. Poitrasson-Rivière, J.B. Moody, and T. Hagio are employees of the INVIA Medical Imaging Solutions. R.L. Weinberg has no conflicts of interest to disclose. J.R. Corbett and E.P. Ficaro are owners of the INVIA Medical Imaging Solutions.
Publisher’s Note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, et al. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med 2014;55:248–55. 10.2967/jnumed.113.121442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, et al. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–24. 10.1161/CIRCULATIONAHA.111.050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murthy VL, Bateman TM, Beanlands RS, Berman DS, Borges-Neto S, Chareonthaitawee P, et al. Clinical quantification of myocardial blood flow using pet: Joint position paper of the SNMMI cardiovascular council and the ASNC. J Nucl Cardiol 2017;59:273–93. 10.1007/s12350-017-1110-x. [DOI] [PubMed] [Google Scholar]
- 4.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740–8. 10.1016/j.jacc.2011.01.065. [DOI] [PubMed] [Google Scholar]
- 5.Farhad H, Dunet V, Bachelard K, Allenbach G, Kaufmann PA, Prior JO. Added prognostic value of myocardial blood flow quantitation in rubidium-82 positron emission tomography imaging. Eur Hear J 2013;14:1203–10. 10.1093/ehjci/jet068. [DOI] [PubMed] [Google Scholar]
- 6.Klein R, Ocneanu A, Renaud JM, Ziadi MC, Beanlands RSB, deKemp RA. Consistent tracer administration profile improves test-retest repeatability of myocardial blood flow quantification with 82Rb dynamic PET imaging. J Nucl Cardiol 2016;25:929–41. 10.1007/s12350-016-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Efseaff M, Klein R, Ziadi MC, Beanlands RS, deKemp RA. Short-term repeatability of resting myocardial blood flow measurements using rubidium-82 PET imaging. J Nucl Cardiol 2012;19:997–1006. 10.1007/s12350-012-9600-3. [DOI] [PubMed] [Google Scholar]
- 8.Manabe O, Yoshinaga K, Katoh C, Naya M, deKemp RA, Tamaki N. Repeatability of rest and hyperemic myocardial blood flow measurements with 82Rb dynamic PET. J Nucl Med 2009;50:68–71. 10.2967/jnumed.108.055673. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Renaud JM, Ziadi MC, Thorn SL, Adler A, Beanlands RS, et al. Intra- and inter-operator repeatability of myocardial blood flow and myocardial flow reserve measurements using rubidium-82 pet and a highly automated analysis program. J Nucl Cardiol 2010;17:600–16. 10.1007/s12350-010-9225-3. [DOI] [PubMed] [Google Scholar]
- 10.Lee BC, Moody JB, Poitrasson-Rivière A, Melvin AC, Weinberg RL, Corbett JR, et al. Blood pool and tissue phase patient motion effects on82rubidium PET myocardial blood flow quantification. J Nucl Cardiol 2018. 10.1007/s12350-018-1256-1. [DOI] [PMC free article] [PubMed]
- 11.Lee BC, Moody JB, Poitrasson-Rivière A, Melvin AC, Weinberg RL, Corbett JR, et al. Automated dynamic motion correction using normalized gradient fields for 82rubidium PET myocardial blood flow quantification. J Nucl Cardiol 2018. 10.1007/s12350-018-01471-4. [DOI] [PMC free article] [PubMed]
- 12.Ficaro EP, Lee BC, Kritzman JN, Corbett JR. Corridor4DM: The Michigan method for quantitative nuclear cardiology. J Nucl Cardiol 2007;14:455–65. 10.1016/j.nuclcard.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Hamlett A, Ryan L, Serrano-Trespalacios P, Wolfinger R. Mixed models for assessing correlation in the presence of replication. J Air Waste Manag Assoc 2003;53:442–50. 10.1080/10473289.2003.10466174. [DOI] [PubMed] [Google Scholar]
- 14.Silver NC, Hittner JB, May K. Testing dependent correlations with nonoverlapping variables: A Monte Carlo simulation. J Exp Educ 2004;73:53–69. 10.3200/JEXE.71.1.53-70. [DOI] [Google Scholar]
- 15.Anagnostopoulos C, Almonacid A, El Fakhri G, Curillova Z, Sitek A, Roughton M, et al. Quantitative relationship between coronary vasodilator reserve assessed by 82Rb PET imaging and coronary artery stenosis severity. Eur J Nucl Med Mol Imaging 2008;35:1593–601. 10.1007/s00259-008-0793-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.