Abstract
The growing presence of artificial lighting across the globe presents a number of challenges to human and ecological health despite its societal benefits. Exposure to artificial light at night, a seemingly innocuous aspect of modern life, disrupts behavior and physiological functions. Specifically, light at night induces neuroinflammation, which is implicated in neuropathic and nociceptive pain states, including hyperalgesia and allodynia. Because of its influence on neuroinflammation, we investigated the effects of dim light at night exposure on pain responsiveness in male mice. In this study, mice exposed to four days of dim (5 lux) light at night exhibited cold hyperalgesia. Further, after 28 days of exposure, mice exhibited both cold hyperalgesia and mechanical allodynia. No heat/hot hyperalgesia was observed in this experiment. Altered nociception in mice exposed to dim light at night was concurrent with upregulated interleukin-6 and nerve growth factor mRNA expression in the medulla and elevated μ-opioid receptor mRNA expression in the periaqueductal gray region of the brain. The current results support the relationship between disrupted circadian rhythms and altered pain sensitivity. In summary, we observed that dim light at night induces cold hyperalgesia and mechanical allodynia, potentially through elevated central neuroinflammation and dysregulation of the endogenous opioid system.
Keywords: Light at Night, Pain, Hyperalgesia, Allodynia, Neuroinflammation, Opioid
Introduction
Artificial lighting is an indispensable feature of the 21st-century that provides abundant benefits for human society. Nonetheless, a growing body of evidence has elucidated the detrimental effects of exposure to artificial light at night (LAN), a nearly ubiquitous form of artificial lighting. Most organisms on earth evolved with endogenous circadian systems that rely on light days and dark nights for circadian system coordination (Reppert and Weaver, 2002). As such, LAN can disrupt a range of behavioral and physiological processes that interact with the circadian system, including sleep-wake cycles, metabolism, and immune function (Cho et al., 2015; Russart and Nelson, 2018). Disruption of circadian rhythms is provoked by atypical light signaling from retinal photoreceptors to the suprachiasmatic nucleus (SCN) during the dark phase; the SCN known as the ‘master clock’ of the mammalian circadian system, (Lucas et al., 2012).
LAN exposure has been linked to health issues in both biomedical and ecological contexts. In humans, LAN exposure is correlated with an increased risk for breast and prostate cancers, depression in the elderly population, and obesity (Davis et al., 2001; Kloog et al., 2009; Obayashi et al., 2013; McFadden et al., 2014). Animal studies have demonstrated that dim LAN (~5 lx; dLAN) exposure can result in increased body mass, altered metabolism, disrupted immune function, and disrupted inflammatory signaling in the brain (Bedrosian et al., 2011a; Bedrosian et al., 2011b; Fonken et al., 2013a; Borniger et al., 2014; Hogan et al., 2015). Notably, dLAN augments the central expression of Il-1β in female mice and induces depressive like behavior in both sexes in as few as four nights of exposure (Walker et al., 2019).
dLAN-induced inflammation may play a role in the induction of hyperalgesia or other similar chronic pain states, as pain sensitivity is influenced by the central and peripheral expression of cytokines. For example, pro-inflammatory cytokines heighten pain sensitivity through peripheral and central sensitization (Zhang et al., 2012). Peripherally, inflammation in the dorsal root ganglia (DRG) can lead to sensitization (Martin et al., 2019) and ultimately hyperalgesia (i.e., increased sensitivity of noxious stimuli) and allodynia (i.e., painful reactions to innocuous stimuli). Centrally, inflammation can lead to sensitization and reduced inhibitory signaling in the dorsal horn of the spinal cord (Kawasaki et al., 2008) and in regions of the midbrain or brainstem, such as the periaqueductal grey (PAG) or the rostral ventral medulla (RVM), respectively (Wei et al., 2008; Xu et al., 2018). Typically, the PAG-RVM axis plays a role in the regulation of descending facilitatory and inhibitory nociceptive signaling (Bodnar and Heinricher 2013).
Additionally, dLAN exposure can be characterized as a disruptor of circadian rhythms. Other forms of circadian rhythm disruption, such as night-shift work or sleep disruption have been correlated with altered pain thresholds (Onen et al., 2001; Matre et al., 2017). Pain sensitivity follows circadian patterns in both humans and mice; pain sensitivity is often highest following the end of the active period, likely reflecting rhythmic expression of inflammatory markers and/or opioid receptors in the brain (Kavaliers and Hirst, 1983; Takada et al., 2013). Disruption of these patterns could lead to altered pain sensitivity.
In the present study, we investigated the effects of acute (3–4 nights) dLAN exposure on pain responsiveness and the effects of chronic dLAN exposure (27–28 nights) of dLAN exposure on pain responsiveness and expression of pronociceptive transcripts in male mice. Specifically, we hypothesized that dLAN-mediated circadian rhythm disruption heightens pain responsiveness and perturbs the expression of central and peripheral nociception-related peptides and receptors. Thus, we predicted that dLAN would induce thermal hyperalgesia and mechanical allodynia through increased expression of inflammatory factors.
Experimental Procedures
Mice
Forty adult male CFW mice (~8 weeks old) were ordered from Charles River Laboratories. Upon arrival at the WVU vivarium, mice were given one week to acclimate to light-dark (LD) (14:10 h, ~125:0 lux or ~33.2:0.0 μW/cm2 @ 550 nm) housing conditions. Lights went on/off in the vivarium at 05:00 h and 19:00 h EST, respectively. Throughout the study, mice were individually housed in polypropylene cages (30 X 18 X 14 cm) on corn cob bedding (Envigo 7092; Wisconsin, USA) at a temperature of 22 ± 2 ºC and a relative humidity of 45 ± 5%. Following acclimation, mice were singly housed and pseudorandomly assigned to either LD or dLAN (14:10 h; ~125:5 lux or ~33.2:1.8 μW/cm2 @ 550 nm) housing conditions. dLAN was produced by Luma5 Standard LED light strips (1.5 W/ft, 5000 K “cool white”, 1200 lumens; Hitlights Inc.; Louisiana, USA). Light measurements were taken by placing both a Mavolux 5032C illuminance meter (Nürnberg, Germany) and an Ophir Starbright irradiance meter (Jerusalem, Israel) in the center of an empty cage with the light sensor facing toward the ceiling. For the entire experiment, mice had access to food (Envigo Teklad 2018; Wisconsin, USA) and reverse osmosis water ad libitum.
All experiments were approved by the West Virginia University Institutional Animal Care and Use Committee, and animals were maintained in accordance with NIH Animal Welfare guidelines.
Behavioral Testing
Behavioral testing in this experiment occurred at the following three timepoints: baseline (1 and 2 days prior to experimental housing), acute (days 3 and 4), and chronic (days 27 and 28). Specifically, electric von Frey assessment was performed on the first day of each timepoint; cold and then hot plate tests were performed on the second day with a 1-hour interstimulus interval between tests. All behavioral testing occurred between zeitgeber time (ZT) 06–09. One day prior baseline testing, mice were habituated to the von Frey and Cold/Hot plate chambers for 30 minutes each. Following the baseline tests, mice were pseudorandomly assigned to either the LD or the dLAN group; 20 mice were assigned to each group. For the acute and chronic behavioral tests, the researcher performing the von Frey test and determining withdrawal latencies for the hot and cold plate tests was blind to the experimental condition of the mice. Mice were tested in a random order. Prior to testing, mice were allowed 30 minutes to acclimate to the behavioral room to reduce exploratory behavior on testing days.
Von Frey
Mice were given 15 minutes to acclimate to opaque chambers (80 × 115 × 145 mm; Bioseb; Florida, USA) on top of a wire mesh table prior to the onset of testing. Four mechanical sensitivity measurements were then taken by using an electric von Frey device (Bioseb). The first measurement was excluded, and the final three data were averaged as previously described (Ferrier et al., 2016). A minimum of five minutes passed between each measurement. For each measurement, the probe’s flexible wire tip was pressed gradually upward against the plantar surface of a weight-bearing hind paw until a nocifensive response was elicited. Nocifensive responses were defined as paw lifts, paw shakes, or jumping. Maximum mechanical withdrawal threshold was recorded for each mouse. A decrease in maximum withdrawal threshold in this test was correlated with mechanical allodynia (Martinov et al., 2013).
Cold and Hot Plates
Cold plate testing (IITC Life Science Inc.; California, USA) was performed at 0.0 ºC ± 1.0 ºC, and hot plate testing (IITC Life Science Inc.) was performed at 52.5 ºC ± 0.2 ºC. The interstimulus interval for cold/hot plate testing was set at a minimum of one hour, with the cold plate test strictly occurring first. As soon as mice were placed onto the plates, stopwatches were started and latency to nocifensive behavior was observed and recorded. Mice were immediately removed from the plates as soon as nocifensive behaviors were exhibited, and withdrawal latencies were recorded. Nocifensive behaviors that were considered sufficient for the termination of either test included hind-paw withdrawal, licking, shaking, or jumping. Hind-paw shaking was the most commonly observed behavior in both tests. A maximum cutoff time was set as 30s for each test. In these tests, a decrease in latency to nocifensive behavior was correlated with hyperalgesia (Bannon and Malmberg, 2007).
Tissue Collection, RNA Extraction, and qRT-PCR
Tissue was collected on day 30 to avoid observing potential stress-related physiological effects or acute inflammatory responses that may have been induced by behavioral testing. Thus, from ZT 06–08 on day 30, mice were euthanized with intraperitoneal injections of Euthasol (Virbac; Texas, USA). Following unresponsive toe-pinch tests, transcardiac perfusions were subsequently performed with approximately 20 mL of cold 0.01M PBS (Fisher Scientific; Pennsylvania, USA). All collected tissue samples were immediately submerged into RNALater (Qiagen; Maryland, USA), stored overnight at 4 ºC, and then stored at −80 ºC for up to 3 weeks before dissection. RNA extractions and qRT-PCR were performed as described previously (Walker et al., 2019). RNA was extracted with Trizol Reagent (Invitrogen; California, USA) according to the manufacturer’s instructions and was resuspended in RNase-free water. RNA quality and quantity were determined using a Nanodrop spectrophotometer (Thermo Fisher; Wisconsin, USA), and cDNA was then synthesized using SuperScript IV VILO reverse transcriptase (Invitrogen) following the manufacturer’s protocol. For subsequent PCR, 4 μL (80 ng) of cDNA were combined with 16 μL of a master-mix solution containing: Taqman Fast Advanced Master Mix (Life Technologies; California, USA), an inventoried probe from Applied Biosystems (Life Technologies), a primer-limited probe for the endogenous control 18S, and nuclease-free water (Table 1). All samples were run in duplicate with the following 2-step real-time PCR cycling conditions: 95 ºC for 20 s, followed by 40 cycles of 95 ºC for 3 s, and then 60 ºC for 30 s. Gene expression was quantified using the relative standard curve method as previously described (Walker et al., 2019).
Table 1.
Gene names and assay IDs of the Taqman primers used for this experiment.
| Gene Name | Assay ID |
|---|---|
| Il-6 | Mm00446190_m1 |
| Il-1β | Mm00434228_m1 |
| Tnf-α | Mm00443258_m1 |
| Ngf | Mm00443039_m1 |
| Bdnf | Mm00432069_m1 |
| Mor | Mm01188089_m1 |
| Tac1 | Mm01166996_m1 |
| Trpa1 | Mm01227437_m1 |
| Trpm8 | Mm01299593_m1 |
| 18s | Hs99999901_s1 |
Statistical Analyses
Individual data points having a within-group Z-score > 2 were defined as outliers and were removed prior to analysis. Prior to analysis, data were tested for normality of distribution using the Shapiro-Wilk test. Data that were not normally distributed were log2 transformed. Food consumption and behavioral testing data were analyzed using a repeated measures linear mixed-effects model (LMM); post-hoc comparisons were made using Sidak’s multiple comparisons test. Normally distributed gene expression data were compared using an unpaired Student’s t-test. The Mann-Whitney test was used for group comparison in instances where data were not normally distributed upon log2 transformation. For ease of data visualization, normally distributed data were normalized to the control group (LD) mean, and log2 transformed data were standardized to the control (LD) mean. All data were analyzed using Prism 8 (GraphPad Software; California, USA). Differences between group means were considered statistically significant when p ≦ 0.05.
Results
dLAN Alters Body Mass and Timing of Food Consumption
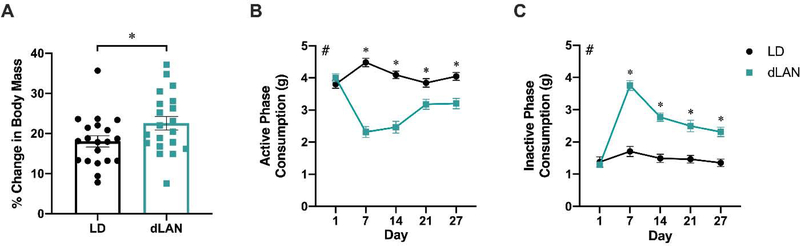
dLAN exposure increased the percent change in body mass relative to baseline (t37 = 2.686, p < 0.05; Figure 1A). dLAN altered the timing of food consumption, driving decreased dLAN group food consumption during the active phase (Figure 1B) and increased consumption during the inactive phase (Figure 1C). A main effect of lighting condition was present in active phase consumption (F1,38 = 58.66, p < 0.0001; Figure 1B) and in inactive phase consumption (F1,38 = 65.16, p < 0.0001; Figure 1C). Food consumption was not altered by one night of dLAN exposure but was continuously altered in each of the following weeks of measurement (p < 0.05; Sidak’s multiple comparisons; Figure 1B, C). These data are consistent with previous reports demonstrating that dLAN induces increased body mass via altered timing of food consumption (Fonken et al., 2010).
Figure 1.
dLAN increases body mass and disrupts timing of food consumption in male mice. Mice housed in dLAN for four weeks had a greater percentage increase in body mass compared to baseline measurements (A). dLAN altered food consumption, resulting in decreased active phase food consumption (B) and increased inactive phase food consumption (C). Error bars represent ± 1 SEM; *p < 0.05; #p < 0.05, Main effect of lighting condition, LMM.
dLAN Induces Cold Hyperalgesia and Mechanical Allodynia
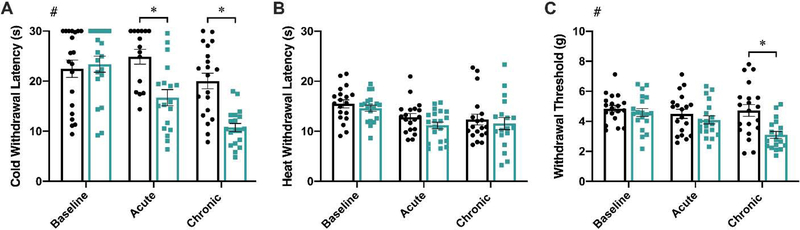
To assess the effects of dLAN on pain responsiveness, three nociceptive tests designed to assess thermal hyperalgesia and mechanical allodynia were performed. A main effect of lighting was present in the cold plate test (F1,38 = 16.46, p < 0.001; Figure 2A), with reduced response latencies observed in the dLAN group at both the acute and chronic time points (p < 0.01 in both cases; Sidak’s multiple comparisons). Similarly, there was a main effect of lighting in the electric von Frey test (F1,108 = 10.50, p < 0.01; Figure 2C). Acute exposure to dLAN did not affect mechanical allodynia (p > 0.05), but chronic exposure reduced withdrawal threshold (p < 0.001; Sidak’s multiple comparisons). Exposure to dLAN did not affect responses to the hot plate at either time point tested (p > 0.05 in all comparisons; Figure 2B)
Figure 2.
dLAN exposure induces cold hyperalgesia and mechanical allodynia. Mice exposed to dLAN displayed reduced cold plate withdrawal latencies after 3–4 nights (acute) and after 27–28 nights (chronic) of dLAN exposure (A). dLAN housing did not alter hot plate withdrawal latencies (B). At the chronic time point, dLAN mice displayed reduced mechanical withdrawal thresholds in the electric von Frey test (C). Error bars represent ± 1 SEM; *p < 0.05; #p < 0.05, Main effect of lighting condition, LMM.
dLAN Upregulates II-6 and Ngf Expression in the Medulla
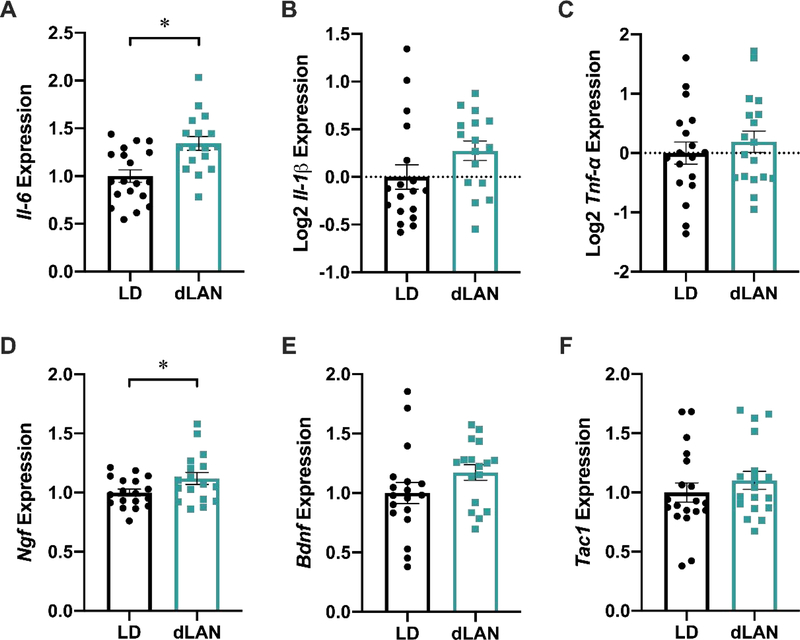
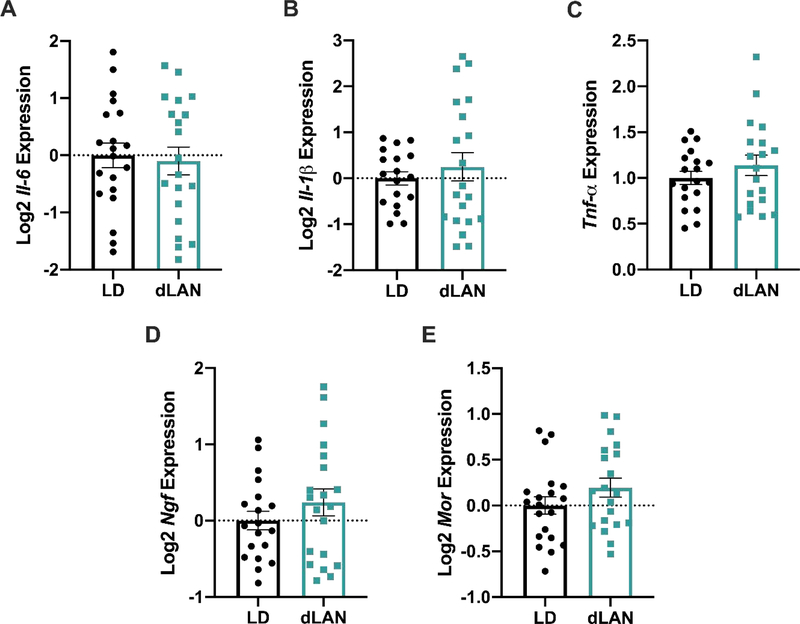
To investigate the link between dLAN exposure and heightened pain responsiveness, levels of the following pronociceptive transcripts were measured: the pro-inflammatory cytokines Il-6, Il-1β, and Tnf-α; the pronociceptive protein substance P’s precursor protein, Tac1; nerve growth factor (Ngf) and brain-derived neurotrophic factor (Bdnf), two neurotrophins that have been implicated in pain states. Il-6 expression was upregulated in the dLAN group (t34 = 3.526, p < 0.01; Figure 3A). Further, Ngf expression was also increased in this region (t33 = 2.089, p < 0.05; Figure 3D). There were no differences between Il-1β, Tnf-α, Bdnf, or Tac1 expression (p > 0.05; Figure 3B, C, E, F).
Figure 3.
dLAN upregulates Il-6 and Ngf relative expression in the medulla. Mice housed in dLAN for four weeks had upregulated levels of Il-6 (A) and Ngf (D) within the medulla. dLAN housing did not alter Tnf-α, Il-1β, Bdnf, or Tac1 relative expression (B, C, E, F). Error bars represent ± 1 SEM; *p < 0.05.
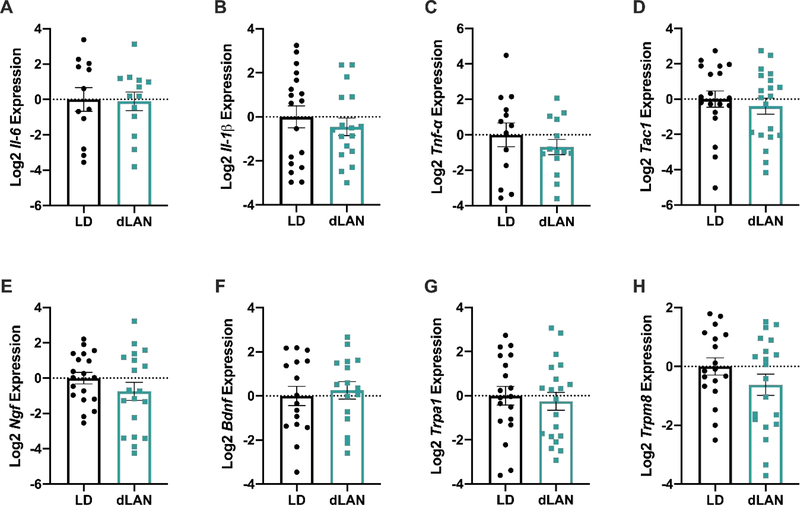
Expression of Tnf-α, Il-1β, Il-6, Bdnf, Ngf, Tac1, Mor, Trpa1, or Trpm8 in the Lumbar Spinal Cord or the L3–5 DRG was unaffected by dLAN (p>0.05 in each case).
To determine the role of the peripheral nervous system in dLAN-induced hyperalgesia, the Lumbar 3–5 DRG were dissected, as these DRG project to the sciatic nerve (Rigaud et al., 2008). No differences in Tnf-α, Il-1β, Il-6, Bdnf, Ngf, Tac1, Mor, Trpa1, or Trpm8 were present in the L3-L5 DRG (p > 0.05 in all instances; Figure 4A-H). Similarly, there were no differences in Tnf-α, Il-1β, Il-6, Ngf, Tac1, or Mor expression in the lumbar region of the spinal cord (p > 0.05 in all instances; Figure 5A-E).
Figure 4.
Cytokine, neurotrophin, and peptide transcripts examined in the L3–5 DRG were not affected by dLAN. Il-6, Il-1β, Tnf-α, Tac1, Ngf, and Bdnf relative expression levels were unaffected (D-F). Gene expression of the ion channel receptors Trpa1 and Trpm8 was unaltered at the time of tissue collection (G, H). Error bars represent ± 1 SEM; *p < 0.05.
Figure 5.
Cytokine, neurotrophin, and peptide transcripts in the lumbar region of the spinal cord were not affected by dLAN. Il-6, Il-1β, Tnf-α, Ngf, and Bdnf levels were not different between groups at the time of tissue collection (A-E). Error bars represent ± 1 SEM; *p < 0.05.
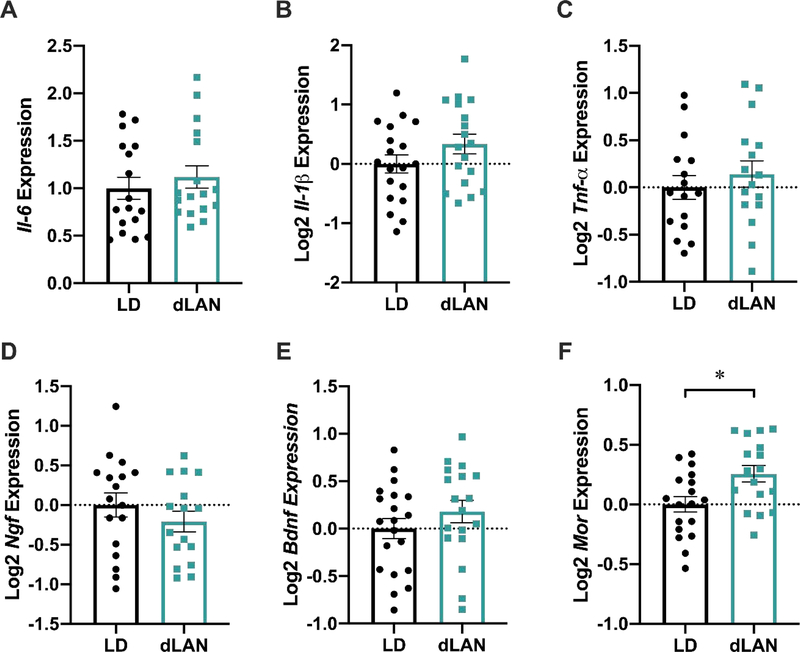
dLAN Upregulates Mor Expression in the PAG
No differences were detected in PAG expression of Tnf-α, Il-1β, Il-6, Bdnf, Ngf, or Tac1 (p > 0.05; Figure 6B-F). To further investigate the presence of cold but not heat hyperalgesia in dLAN mice, we examined the potential effect of dLAN on the endogenous opioid system by measuring μ-opioid receptor (Mor) expression. Expression of Mor was increased in the dLAN group (t33 = 2.704, p < 0.05; Figure 6F).
Figure 6.
dLAN increases Mor relative expression in the PAG. Il-6, Il-1β, and Tnf-α levels were not different in the PAG of dLAN mice (A-C). Expression of Ngf and Bdnf was unaffected in the PAG (D, E). Mor expression was upregulated in dLAN mice (F). Error bars represent ± 1 SEM; *p < 0.05.
Discussion
In this experiment, we examined the effects of acute (3–4 nights) and chronic (28–29 nights) dLAN exposure on both pain responsiveness and the gene expression of several pronociceptive peptides. We observed that male mice exposed to dLAN experienced cold hyperalgesia and mechanical allodynia at acute and chronic time points. In contrast, mice did not exhibit heat hyperalgesia at either assessed time point. Further, dLAN exposure upregulated levels of Il-6 and Ngf in the medulla and increased Mor expression in the PAG.
A common theme of many maladaptive pain states is the presence of central and/or peripheral inflammation. Central inflammation leads to sensitization and hyperalgesia via glial activation and modulation of synaptic activity in pain circuitry (Ji et al., 2018). Specifically, many chronic pain states are correlated with increased central and peripheral levels of Il-6 and other pro-inflammatory cytokines (Kawasaki et al., 2008). Il-6 is a pleiotropic cytokine that is suspected to play a major role in the development and maintenance of several chronic and neuropathic pain states (Zhou et al., 2016). For example, a study using a model of cancer-induced bone pain demonstrated that descending facilitatory signaling from the RVM can be activated by the accumulation of pro-inflammatory cytokines, such as Il-6, in supra-spinal regions of the CNS (Liu et al., 2012). Additionally, Il-6 inhibitors delivered intrathecally can alleviate mechanical allodynia associated with peripheral nerve injury (Arruda et al., 2000). As such, the upregulated supra-spinal expression of Il-6 in dLAN mice (Figure 3A) likely is associated with their heightened pain responsiveness. Notably, a potential source for these increased levels may be activated microglia (Ji and Suter, 2007).
Another mechanism underlying dLAN-induced hyperalgesia may be peripheral inflammation. Previous data demonstrate that LAN disrupts metabolic function and increases peripheral inflammation (Fonken et al., 2013b). LAN exposure is also positively correlated with obesity, a condition associated with chronic pro-inflammatory states (McFadden et al., 2014). Obesity-induced peripheral inflammation can drive peripheral sensitization and heightened pain sensitivity (Hitt et al., 2007; Ray et al., 2011; Iannitti et al., 2012). This peripheral sensitization can induce central sensitization, which leads to the activation of descending facilitatory signaling from the RVM (Urban and Gebhart, 1999; Schaible et al., 2011). Though no correlations were found between these factors in this study (p > 0.05; correlations not shown), we did not specifically examine the effects of dLAN on peripheral inflammation. Accordingly, peripheral inflammation should still be considered as a candidate link between dLAN and altered pain responsiveness. More investigation is needed to elucidate the potential relationship among these variables.
In addition to pro-inflammatory cytokines, a number of other neurotrophins and peptides, including BDNF, NGF, and substance P, also play a role in the modulation of nociceptive signaling in the central and peripheral nervous systems (Basbaum et al., 2009). Although the role of NGF in peripheral sensitization is well defined, its modulation of nociception in the central nervous system is less clear (McMahon, 1996). Several experiments have reported on the nociceptive effects of spinal NGF, but evidence is conflicting as to whether or not upregulated NGF in the spinal cord induces pro- or anti-nociception (Malcangio et al., 2000; Cirillo et al., 2010; Khan and Smith, 2015). To our knowledge, no previous reports have characterized the effects of supra-spinal NGF on nociception. Here, we noted upregulated levels of Ngf in the medulla (Figure 3D), which may indicate that NGF modulates nociceptive synaptic plasticity in the brainstem as it does in the spinal cord. Alternatively, the increased levels of Ngf may not be biologically relevant in this context. Additional studies are needed to confirm the role of supra-spinal NGF in hyperalgesia.
It is understood that cold and heat hyperalgesia are regulated by different classes of neuronal receptors (Zimmermann et al., 2007; Julius, 2013; Lippoldt et al., 2016). Accordingly, we analyzed transcript levels of the cold-sensing ion channel receptors Trpm8 and Trpa1 in the L3-L5 DRG (McKemy, 2005). No differences were seen between groups, suggesting that these receptors may not play a role in dLAN-mediated cold hyperalgesia (Figure 4G, H). However, differences in the expression or sensitization of TRPA1 or TRPM8 may occur at earlier time points of dLAN exposure and then persist in a chronic manner.
Few disease states or pain conditions have been characterized where thermal hyperalgesia is differentially affected, i.e., the presence of cold but not heat hyperalgesia. Evidence suggests that night-shift work and sleep deprivation may affect cold and heat hyperalgesia differently, but no mechanism has been proposed (Ødegård et al., 2015; Matre et al., 2017; Pieh et al., 2018). Differential thermal hyperalgesia is also reported in the context of opioid-induced hyperalgesia (OIH). In OIH, reduced cold pain thresholds and tolerances are coincident for both opioid treated and withdrawal patients; this effect is likely mediated by the MOR and TRPM8 receptors (Gong and Jasmin, 2017). For example, exogenous opioid treatment can produce cold, but not heat, hyperalgesia (Chu et al., 2006) and can increase cold sensitivity in the absence of other forms of nociception (Doverty et al., 2001a; Doverty et al., 2001b). It should be noted that OIH can also produce heat hyperalgesia and other forms of hyperalgesia and allodynia in a range of contexts, yet there is currently a limited understanding as to why pain is inconsistently affected by OIH (Angst and Clark, 2006; Roeckel et al., 2017).
To investigate the differential thermal hyperalgesia phenomenon in the context of the endogenous opioid system, Mor expression was examined in the PAG and was found to be upregulated in dLAN mice (Figure 6F). The presence of thermal hyperalgesia despite upregulated levels of MOR in the spinal cord has been noted previously (Zaringhalam et al., 2008). Additionally, MOR antagonists attenuate the effects of stress-induced hyperalgesia, indicating that MORs may play a role in the induction of hyperalgesia (Suarez-Roca et al., 2006). Finally, as MOR expression in both the PAG and the brain stem fluctuate in a circadian manner, heightened Mor expression after exposure to dLAN suggests that circadian rhythm disruption may affect Mor expression (Yoshida et al., 2005; Takada et al., 2013). Although the endogenous opioid system canonically functions to inhibit pain signaling (Glaum et al., 1994), the present data indicate that dLAN exposure alters MOR expression and function.
In this study, we have demonstrated that dLAN exposure augments pain responsiveness in mice. We report that dLAN-induced hyperalgesia can occur in as few as four nights of dLAN exposure. Three molecular candidates that may play a role in heightened pain responsiveness after chronic dLAN exposure are Il-6, NGF, and MOR. Taken together, these findings indicate that dLAN may be an important factor for physicians and patients alike to consider when treating and managing pain symptoms. Reducing pain levels, and potentially prescribed opioids, may require the consideration of night-time lighting as a mitigating factor.
Highlights.
Dim light at night exposure (~5 lux) induces cold hyperalgesia and mechanical allodynia in male mice.
These nociceptive symptoms are potentially mediated by upregulated Il-6 and Ngf expression in the medulla.
Dim light at night exposure upregulates Mor expression in the periaqueductal gray.
These data suggest that there may be a relationship between circadian disruption and altered pain sensitivity.
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 5U54GM104942–04. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. We thank Terri Poling for her excellent care of the animals during this study. Special appreciation is given to Alexandra Richmond and Jordan Pascoe for their valuable assistance with data collection.
Footnotes
Data Availability
Data related to this article are available upon reasonable request to the corresponding author.
Declarations of Interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Angst MS, Clark JD (2006) Opioid-induced hyperalgesia: A qualitative systematic review. Anesthesiol 104:570–587. [DOI] [PubMed] [Google Scholar]
- Arruda JL, Sweitzer S, Rutkowski MD, DeLeo JA (2000) Intrathecal anti-il-6 antibody and igg attenuates peripheral nerve injury-induced mechanical allodynia in the rat: Possible immune modulation in neuropathic pain. Brain Res 879:216–225. [DOI] [PubMed] [Google Scholar]
- Bannon AW, Malmberg AB (2007) Models of nociception: Hot plate, tail flick, and formalin tests in rodents. Curr Protoc Neurosci 41:8.9.1–8.9.16. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D (2009) Cellular and molecular mechanisms of pain. Cell 139:267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Walton JC, Haim A, Nelson RJ (2011a) Dim light at night provokes depression-like behaviors and reduces ca1 dendritic spine density in female hamsters. Psychoneuroendocrinology 36:1062–1069. [DOI] [PubMed] [Google Scholar]
- Bedrosian TA, Fonken LK, Walton JC, Nelson RJ (2011b) Chronic exposure to dim light at night suppresses immune responses in siberian hamsters. Biology Letters 7:468–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar R, Heinricher MM (2013) Central mechanisms of pain suppression Neuroscience in the 21st Century, pp 2595–2619. New York, NY: Springer New York. [Google Scholar]
- Borniger JC, Maurya SK, Periasamy M, Nelson RJ (2014) Acute dim light at night increases body mass, alters metabolism, and shifts core body temperature circadian rhythms. Chronobiol Int 31:917–925. [DOI] [PubMed] [Google Scholar]
- Cho Y, Ryu S-H, Lee BR, Kim KH, Lee E, Choi J (2015) Effects of artificial light at night on human health: A literature review of observational and experimental studies applied to exposure assessment. Chronobiol Int 32:1294–1310. [DOI] [PubMed] [Google Scholar]
- Chu LF, Clark DJ, Angst MS (2006) Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: A preliminary prospective study. J Pain Res 7:43–48. [DOI] [PubMed] [Google Scholar]
- Cirillo G, Cavaliere C, Bianco MR, De Simone A, Colangelo AM, Sellitti S, Alberghina L, Papa M (2010) Intrathecal ngf administration reduces reactive astrocytosis and changes neurotrophin receptors expression pattern in a rat model of neuropathic pain. Cell Mol Neurobiol 30:51–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Mirick DK, Stevens RG (2001) Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst 93:1557–1562. [DOI] [PubMed] [Google Scholar]
- Doverty M, Somogyi AA, White JM, Bochner F, Beare CH, Menelaou A, Ling W (2001a) Methadone maintenance patients are cross-tolerant to the antinociceptive effects of morphine. Pain 93:155–163. [DOI] [PubMed] [Google Scholar]
- Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W (2001b) Hyperalgesic responses in methadone maintenance patients. Pain 90:91–96. [DOI] [PubMed] [Google Scholar]
- Ferrier J, Marchand F, Balayssac D (2016) Assessment of mechanical allodynia in rats using the electronic von frey test. Bio Protoc 6:e1933. [Google Scholar]
- Fonken LK, Aubrecht TG, Meléndez-Fernández OH, Weil ZM, Nelson RJ (2013a) Dim light at night disrupts molecular circadian rhythms and increases body weight. Journal of biological rhythms 28:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonken LK, Lieberman RA, Weil ZM, Nelson RJ (2013b) Dim light at night exaggerates weight gain and inflammation associated with a high-fat diet in male mice. Endocrinology 154:3817–3825. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A, Nelson RJ (2010) Light at night increases body mass by shifting the time of food intake. Proc Natl Acad Sci U S A 107:18664–18669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ, Hammond DL (1994) Inhibitory actions of delta 1-, delta 2-, and mu-opioid receptor agonists on excitatory transmission in lamina ii neurons of adult rat spinal cord. J Neurosci 14:4965–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong K, Jasmin L (2017) Sustained morphine administration induces trpm8-dependent cold hyperalgesia. J Pain Res 18:212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG (2007) Comorbidity of obesity and pain in a general population: Results from the southern pain prevalence study. J Pain Res 8:430–436. [DOI] [PubMed] [Google Scholar]
- Hogan MK, Kovalycsik T, Sun Q, Rajagopalan S, Nelson RJ (2015) Combined effects of exposure to dim light at night and fine particulate matter on c3h/henhsd mice. Behav Brain Res 294:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannitti T, Graham A, Dolan S (2012) Increased central and peripheral inflammation and inflammatory hyperalgesia in zucker rat model of leptin receptor deficiency and genetic obesity. Exp Physiol 97:1236–1245. [DOI] [PubMed] [Google Scholar]
- Ji RR, Nackley A, Huh Y, Terrando N, Maixner W (2018) Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiol 129:343–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Suter MR (2007) P38 mapk, microglial signaling, and neuropathic pain. Mol Pain 3:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius D (2013) Trp channels and pain. Annu Rev Cel Dev Bi 29:355–384. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Hirst M (1983) Daily rhythms of analgesia in mice: Effects of age and photoperiod. Brain Res 279:387–393. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng J-K, Ji R-R (2008) Cytokine mechanisms of central sensitization: Distinct and overlapping role of interleukin-1β, interleukin-6, and tumor necrosis factor-α in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci 28:5189–5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan N, Smith MT (2015) Neurotrophins and neuropathic pain: Role in pathobiology. Molecules 20:10657–10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog I, Haim A, Stevens RG, Portnov BA (2009) Global co-distribution of light at night (lan) and cancers of prostate, colon, and lung in men. Chronobiol Int 26:108–125. [DOI] [PubMed] [Google Scholar]
- Lippoldt EK, Ongun S, Kusaka GK, McKemy DD (2016) Inflammatory and neuropathic cold allodynia are selectively mediated by the neurotrophic factor receptor gfrα3. Proc Natl Acad Sci U S A 113:4506–4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Bu H, Liu C, Gao F, Yang H, Tian X, Xu A, Chen Z et al. (2012) Inhibition of glial activation in rostral ventromedial medulla attenuates mechanical allodynia in a rat model of cancer-induced bone pain. J Huazhong Univ Sci Technolog Med Sci 32:291–298. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Lall GS, Allen AE, Brown TM (2012) How rod, cone, and melanopsin photoreceptors come together to enlighten the mammalian circadian clock Progress in brain research, vol. 199, pp 1–18. Amsterdam: Elsevier. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Ramer MS, Boucher TJ, McMahon SB (2000) Intrathecally injected neurotrophins and the release of substance p from the rat isolated spinal cord. Eur J Neurosci 12:139–144. [DOI] [PubMed] [Google Scholar]
- Martin SL, Reid AJ, Verkhratsky A, Magnaghi V, Faroni A (2019) Gene expression changes in dorsal root ganglia following peripheral nerve injury: Roles in inflammation, cell death and nociception. Neural Regen Res 14:939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinov T, Mack M, Sykes A, Chatterjea D (2013) Measuring changes in tactile sensitivity in the hind paw of mice using an electronic von frey apparatus. JoVE - J Vis Exp e51212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matre D, Knardahl S, Nilsen KB (2017) Night-shift work is associated with increased pain perception. Scand J Work Env Hea 43:260–268. [DOI] [PubMed] [Google Scholar]
- McFadden E, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ (2014) The relationship between obesity and exposure to light at night: Cross-sectional analyses of over 100,000 women in the breakthrough generations study. Am J Epidemiol 180:245–250. [DOI] [PubMed] [Google Scholar]
- McKemy DD (2005) How cold is it? Trpm8 and trpa1 in the molecular logic of cold sensation. Mol Pain 1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB (1996) Ngf as a mediator of inflammatory pain. Philos T R Soc B 351:431–440. [DOI] [PubMed] [Google Scholar]
- Obayashi K, Saeki K, Iwamoto J, Ikada Y, Kurumatani N (2013) Exposure to light at night and risk of depression in the elderly. J Affect Disorders 151:331–336. [DOI] [PubMed] [Google Scholar]
- Ødegård SS, Omland PM, Nilsen KB, Stjern M, Gravdahl GB, Sand T (2015) The effect of sleep restriction on laser evoked potentials, thermal sensory and pain thresholds and suprathreshold pain in healthy subjects. Clin Neurophysiol 126:1979–1987. [DOI] [PubMed] [Google Scholar]
- Onen SH, Alloui A, Gross A, Eschallier A, Dubray C (2001) The effects of total sleep deprivation, selective sleep interruption and sleep recovery on pain tolerance thresholds in healthy subjects. J Sleep Res 10:35–42. [DOI] [PubMed] [Google Scholar]
- Pieh C, Jank R, Waiß C, Pfeifer C, Probst T, Lahmann C, Oberndorfer S (2018) Night-shift work increases cold pain perception. Sleep Med 45:74–79. [DOI] [PubMed] [Google Scholar]
- Ray L, Lipton RB, Zimmerman ME, Katz MJ, Derby CA (2011) Mechanisms of association between obesity and chronic pain in the elderly. Pain 152:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR (2002) Coordination of circadian timing in mammals. Nature 418:935–941. [DOI] [PubMed] [Google Scholar]
- Rigaud M, Gemes G, Barabas M-E, Chernoff DI, Abram SE, Stucky CL, Hogan QH (2008) Species and strain differences in rodent sciatic nerve anatomy: Implications for studies of neuropathic pain. Pain 136:188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeckel LA, Utard V, Reiss D, Mouheiche J, Maurin H, Robé A, Audouard E, Wood JN et al. (2017) Morphine-induced hyperalgesia involves mu opioid receptors and the metabolite morphine-3-glucuronide. Sci Rep 7:10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russart KLG, Nelson RJ (2018) Light at night as an environmental endocrine disruptor. Physiol Behav 190:82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible H-G, Ebersberger A, Natura G (2011) Update on peripheral mechanisms of pain: Beyond prostaglandins and cytokines. Arthritis Res Ther 13:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Roca H, Silva JA, Arcaya JL, Quintero L, Maixner W, Pinerua-Shuhaibar L (2006) Role of mu-opioid and nmda receptors in the development and maintenance of repeated swim stress-induced thermal hyperalgesia. Behav Brain Res 167:205–211. [DOI] [PubMed] [Google Scholar]
- Takada T, Yamashita A, Date A, Yanase M, Suhara Y, Hamada A, Sakai H, Ikegami D et al. (2013) Changes in the circadian rhythm of mrna expression for μ opioid receptors in the periaqueductal gray under a neuropathic pain like state. Synapse 67:216–223. [DOI] [PubMed] [Google Scholar]
- Urban MO, Gebhart GF (1999) Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci U S A 96:7687–7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker WHII, Borniger JC, Gaudier-Diaz MM, Hecmarie Meléndez-Fernández O, Pascoe JL, Courtney DeVries A, Nelson RJ (2019) Acute exposure to low-level light at night is sufficient to induce neurological changes and depressive-like behavior. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Guo W, Zou S, Ren K, Dubner R (2008) Supraspinal glial–neuronal interactions contribute to descending pain facilitation. J Neurosci 28:10482–10495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Zhao H, Gao H, Zhao H, Liu D, Li J (2018) Participation of pro-inflammatory cytokines in neuropathic pain evoked by chemotherapeutic oxaliplatin via central gabaergic pathway. Mol Pain 14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Koyanagi S, Matsuo A, Fujioka T, To H, Higuchi S, Ohdo S (2005) Glucocorticoid hormone regulates the circadian coordination of micro-opioid receptor expression in mouse brainstem. J Pharmacol Exp Ther 315:1119–1124. [DOI] [PubMed] [Google Scholar]
- Zaringhalam J, Manaheji H, Mghsoodi N, Farokhi B, Mirzaiee V (2008) Spinal μ -opioid receptor expression and hyperalgesia with dexamethasone in chronic adjuvant induced arthritis in rats. Clin Exp Pharmacol P 35:1309–1315. [DOI] [PubMed] [Google Scholar]
- Zhang J, Li H, Teng H, Zhang T, Luo Y, Zhao M, Li YQ, Sun ZS (2012) Regulation of peripheral clock to oscillation of substance p contributes to circadian inflammatory pain. Anesthesiol 117:149–160. [DOI] [PubMed] [Google Scholar]
- Zhou YQ, Liu Z, Liu ZH, Chen SP, Li M, Shahveranov A, Ye DW, Tian YK (2016) Interleukin-6: An emerging regulator of pathological pain. J Neuroinflamm 13:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann K, Leffler A, Babes A, Cendan CM, Carr RW, Kobayashi J, Nau C, Wood JN et al. (2007) Sensory neuron sodium channel nav1.8 is essential for pain at low temperatures. Nature 447:855–858. [DOI] [PubMed] [Google Scholar]