Abstract
Aim:
The humanized anti-CD52 monoclonal antibody alemtuzumab depletes lymphocytes and is currently used to treat relapsing multiple sclerosis. During treatment, anti-alemtuzumab antibodies may develop and reduce effective lymphocyte depletion in future treatment cycles.
Results:
Alemtuzumab–Alexa Fluor 488 conjugate binding to the CHO-CD52 cell surface was inhibited by anti-alemtuzumab antibodies.
Conclusion:
In this proof-of-concept study, a CHO-CD52 cell line has been developed and used to detect the presence of anti-alemtuzumab neutralizing antibodies. This platform provides the basis of an assay for routine screening of serum for neutralizing antibodies from patients treated with alemtuzumab.
Keywords: : alemtuzumab, antidrug antibodies, multiple sclerosis, neutralizing antibodies
METHOD SUMMARY
We developed a competition assay between alemtuzumab and neutralizing antibodies to adherent CD52-expressing cells. The anti-alemtuzumab antibodies inhibit alemtuzumab–Alexa Fluor 488 binding to cell surface CD52. Reduction in the fluorescence signal is proportional the amount of antidrug antibody in the serum sample.
LAY ABSTRACT
Therapeutic monoclonal antibodies are currently used for the treatment of numerous diseases and conditions, including relapsing multiple sclerosis, and are the most advanced targeted therapies available. However, they all have the potential to cause immunogenic reactions and generate antibodies that bind to the drug and reduce its therapeutic efficacy. As a result, patients do not receive the expected benefit from treatment, and the effect is cumulative with repeat dosing. The timely detection of antidrug antibodies has the potential to avoid these major risks. Here we describe a cell-based method for detecting anti-alemtuzumab neutralizing antibodies.
Campath-1 antigen (CD52) [1] is a highly negatively charged glycoprotein of 12 amino acids anchored to glycosylphosphatidylinositol. It is widely expressed on the cell surface of immune cells such as natural killer cells, eosinophils, neutrophils, monocytes/macrophages, dendritic cells and mature lymphocytes, but not on the hematopoietic stem cells. Following T-cell activation, the soluble form of CD52 is released and binds to the sialic acid-binding Ig-like lectin-10 (Siglec-10) receptor on T cells to suppress their function [2]. The soluble CD52 also inhibits Toll-like receptor and TNF receptor signaling to limit activation of NF-κB, suppressing the production of inflammatory cytokines by macrophages, monocytes and dendritic cells [3]. The surface-bound CD52 is the molecular target of a humanized monoclonal antibody, alemtuzumab, also known as Campath-1H or LEMTRADA® [4]. This antibody is used in treating chronic lymphocytic leukemia, for immunosuppression in organ transplantation, and in the treatment of patients with relapsing multiple sclerosis (pwRMS). In May 2014, the marketing authorization for alemtuzumab in pwRMS recommended a dosage of 12 mg/day administered by intravenous infusion for two treatment courses: an initial course lasting five consecutive days, followed 1 year later by a second course of three consecutive days. Recently, a third course of alemtuzumab treatment in pwRMS was approved by the European Medicines Agency [5]. Following two courses of treatment with alemtuzumab, antidrug antibodies (ADA) can be detected in pwRMS. These are binding antibodies in about 85% of patients and the majority of these – approximately 92% – are neutralizing antibodies (Nabs). Failure of lymphocyte depletion has been reported in pwRMS who tested positive for such Nabs [6,7]. To avoid potential treatment failure, it would be desirable to test for alemtuzumab ADA prior to subsequent courses of treatment. In this communication, we describe a novel cell-based ADA assay for the detection of alemtuzumab Nabs. We cloned the CD52 gene from Daudi cells then, using site-specific integration, we generated a stable adherent Chinese hamster ovary (CHO) cell line expressing the CD52 gene and developed an ADA assay to detect Nabs against alemtuzumab in an MS patient’s serum.
Materials & methods
Cloning CD52 & plasmid construction
Daudi cells (human B lymphoblast cells, which express CD52) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 1% L-glutamine at 37°C in a humidified incubator with 5% CO2. Total RNA was isolated from pelleted (3 × 106) Daudi cells using the RNeasy Mini Kit (Qiagen) and converted to cDNA using the ProtoScript® First Strand cDNA Synthesis Kit (NEB) with Oligo dT primer. Primers were designed to amplify CD52 incorporating a Kozak [8] and overlap sequences for Gibson assembly into the pcDNA5/FRT vector digested with NheI and NotI. The PCR amplification used Q5 polymerase (NEB) and primers: (KzCD52F 5′-TATAGGGAGACCCAAGCTGGCTAGCCACCATGAAGCGCTTCCTC-3′ and CD52R 5′-CGGGCCCTCTAGACTCGAGCGGCCGCTCAACTGAAGCAGAAGAGGTG-3′) with initial denaturing at 98°C for 30 s, then 32 cycles (98°C for 10 s, 65.3°C for 15 s, 72°C for 10 s), then 72°C for 2 min with a final hold at 4°C until analyzed. The plasmid was digested with NheI-HF and NotI-HF in CutSmart buffer (NEB). Both PCR product (241 bp) and vector restriction digest (4.96 kb) were resolved on 1.2% agarose TAE gel, the DNA purified from excised bands, the plasmid assembled by Gibson assembly [9] and the NEB 5α cells transformed. Individual colonies were picked and plasmid isolated from overnight cultures. The sequence was determined by Sanger sequencing with a CMV forward sequencing primer (5′-CGCAAATGGGCGGTAGGCGTG-3′). The plasmid, designated pcDNA5/FRT KzCD52, was used in transfecting CHO cells.
Cell transfection & selection
Prior to transfection, Flp-In™-CHO cells (Invitrogen) were maintained in RPMI medium supplemented with 10% fetal bovine serum, 1% penicillin/streptomycin and 1% L-glutamine with zeocin selection. The day before transfection, the cells were treated with 1% trypsin-EDTA solution and seeded in a 24-well plate (4 × 104 cells per well in 0.5 ml of complete growth medium) without zeocin. The next day, pOG44 plasmid (0.88 μg) and pcDNA5/FRT KzCD52 (0.08 μg) were mixed in 100 μl of Opti-MEM® I Reduced Serum Medium and Lipofectamine® LTX (Invitrogen) added (range 0.75–2.75 μl), mixed gently and incubated for 25 min at room temperature to form DNA–Lipofectamine LTX complexes. The growth medium was removed from the cells and 0.5 ml of complete growth medium added. Aliquots (100 μl) of the DNA–Lipofectamine LTX complexes were added directly to each well containing cells and mixed by gently rocking; the plate was incubated at 37°C in a CO2 incubator for 24 h before initiating hygromycin B (250 μg/ml) selection. The selection medium was changed every 3–4 days until cell foci developed. The cells were detached and subjected to FACS to identify CD52-positive cells via staining with rat anti-CD52 FITC (Bio-Rad, MCA1642FT) and plated with limiting dilution and the process was repeated three times over 6 months to ensure clonal selection. Expression of CD52 was confirmed by staining with rat anti-CD52-FITC, master stocks of CHO-CD52 cells expanded and aliquots stored in vapor phase liquid nitrogen until required.
Alemtuzumab–Alexa Fluor 488 conjugation
Alemtuzumab was labeled using the Rapid Lighting Link® Alexa Fluor® 488 kit following the instructions provided (Expedeon). Briefly, 200 μg of alemtuzumab in 90 μl water was mixed with 10 μl of rapid modifier solution prior to adding to the lyophilized Alexa Fluor 488 reagent. The mixture was incubated at ambient temperature in the dark for 30 min and the coupling reaction terminated by the addition of quench solution (10 μl), then left in the dark for 5 min prior to adding 90 μl phosphate-buffered saline (PBS). The alemtuzumab–Alexa Fluor 488 conjugate (1 μg/μl) was stored in the dark at 4°C until required.
CD52 detection on transfected cells
Flp-In CHO untransfected and Flp-In CHO-CD52 cells (5 × 104) were seeded on to glass coverslips in 24-well plates and left overnight before fixing with 1% paraformaldehyde for 10 min. The cells were washed twice with PBS and blocked with 10% goat serum in PBS for 30 min. The cells were incubated with either alemtuzumab–Alexa Fluor 488 (1:500) or its progenitor, the rat anti-CD52 FITC (1:500) (Bio-Rad MCA1642FT) diluted in 10% goat serum in PBS for 2 h, washed 3x with PBS prior to adding Vectorshield with DAPI before capturing the images on fluorescence microscope (Leica DM5000) and processed using ImageJ.
Inhibition of alemtuzumab–Alexa Fluor 488 binding to Flp-In CHO-CD52 cells using an anti-alemtuzumab monoclonal antibody
Experiments were performed in accordance with the guidelines of the Declaration of Helsinki. The participants gave written informed consent for blood samples for use in research on the institutional NHS consent form. Flp-In CHO-CD52 cells were seeded in Falcon® 96-well Black/Clear Flat Bottom TC-treated Imaging Microplate plates at 3 × 103 cells per well and left for 3 days before fixing with 1% paraformaldehyde for 10 min. The wells were blocked just prior to use, as described above. Initially, sera from patients diagnosed with MS prior to alemtuzumab treatment (n = 2) and from healthy control subjects (n = 2) were diluted 1/10 in human control serum (Merck H4522) and spiked with 1.5 μl anti-alemtuzumab (Bio-Rad HCA199) (1.5 μg/65 μl) or human control serum. Inhibition of alemtuzumab-Alexa Fluor 488 binding to the CHO-CD52 cells was determined. An equal volume of each sample was added to the alemtuzumab–Alexa Fluor 488 conjugate diluted 1:500 in 10% goat serum in PBS (final concentration ∼1 ng alemtuzumab/μl), then the mixture was incubated at room temperature in the dark for 2 h. The serum/alemtuzumab–Alexa Fluor 488 (40 μl/40 ng alemtuzumab–Alexa Fluor 488) was added to the wells in triplicate, then incubated for 2 h before washing with PBS and adding Vectashield with DAPI for 5 min in the dark, washing as before, measuring the total fluorescence on the Clariostar plate reader and the data plotted using GraphPad Prism. In addition, the sera were diluted 1/10 in human control serum (Merck H4522) and spiked with 1.0 μl anti-alemtuzumab (Bio-Rad HCA199) (1.0 μg/100 μl) and serially threefold diluted in human control serum. Inhibition of alemtuzumab–Alexa Fluor 488 binding to the CHO-CD52 cells was determined for each dilution as described.
Intra- & interassay variation
When performing the assay, human control serum aliquots (Merck H4522) with and without the alemtuzumab–Alexa Fluor 488 were used to determine the maximum and minimum fluorescence (background) signal. The inter- and intra-assay coefficients of variation of the maximum and minimum fluorescence were based on values from six replicates on four plates.
Cell-based assay for the detection of anti-alemtuzumab Nabs
Blood samples from consented MS patients treated with alemtuzumab were collected and processed in March 2016 and 2017 and the serum stored at -20°C until required. For experiments on the IN Cell Analyzer (INCA 2200), Flp-In CHO-CD52 cells were seeded into a Falcon 96-well Black/Clear Flat Bottom TC-treated Imaging Microplate; plates were prepared as described above. Serum from a patient treated with alemtuzumab was taken 5 months after the first round of treatment (sample 2016) and the second round of treatment (sample 2017) and assayed for anti-alemtuzumab binding antibodies using an in-house anti-alemtuzumab assay [10]. Human serum (Merck H4522) was used as a blank control and diluent for the patient serum. The patient sera were serially threefold diluted (neat to 1:729) and 65 μl of each dilution added to 65 μl of alemtuzumab–Alexa Fluor 488 conjugate diluted 1:500 in 10% goat serum and incubated at room temperature in the dark for 2 h. The sera/alemtuzumab–Alexa Fluor 488 (40 μl/∼40 ng alemtuzumab) was added to the wells in triplicate, incubated for 2 h before washing with PBS and adding 0.1 ml HCS CellMask™ Deep Red Stain for 90 min in the dark washing, adding Vectashield with DAPI in the dark for 5 min, washing again before capturing the images on INCA2200 (GE). Images were analyzed using the IN Cell Developer Toolbox (GE) to quantify the number of cells with fluorescence foci and the data plotted using GraphPad Prism to calculate the percentage of cells with fluorescence foci and dilution required for 50% inhibition (ID50).
Results & discussion
Daudi cells were used as a source of human CD52 transcripts. Two specific primers (KzCD52F and CD52R) were designed to amplify CD52 and introduce a Kozak sequence and overlap sequences to facilitate Gibson assembly into pcDNA 5/FRT restricted with NheI and NotI. Following reverse transcription and PCR, a product of 241 bp obtained. Successful cloning was confirmed by Sanger sequencing of the final construct pcDNA5/FRT-CD52. The sequences flanking the CD52 were present and the nucleotide and open reading frame sequences were confirmed by NCBI BLAST search (GenBank X67699.1 and AAH00644.1, respectively). The plasmid pcDNA 5/FRT CD52 sequence is available upon request.
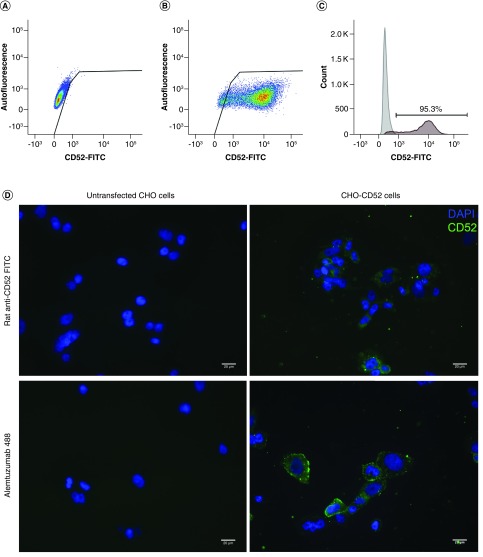
The pcDNA 5/FRT CD52 plasmid was transfected into CHO Flp-In™ cells using Lipofectamine. The cells were maintained on hygromycin B selection, single cell sorted with plating at limiting dilution every 2 months for 6 months until cells derived from a single well were >95% positive in the FACS (Figure 1A–C). Untransfected Flp-In CHO and Flp-In CHO-CD52 cells were seeded on to glass coverslips and probed with rat anti-CD52-FITC and alemtuzumab conjugated to Alexa Fluor 488. As shown in Figure 1D, only the transfected cells exhibited staining with both anti-CD52 antibodies.
Figure 1. . Flow cytometry confirmed the expression of CD52 on transfected Chinese hamster ovary cells.
(A) Untransfected CHO cells. (B) CHO-CD52 cells. (C) Frequency of CHO-CD52 positive cells (95.3%). (D) The presence of CD52 was further confirmed in the transfected cells by staining for CD52 using either the rat anti-CD52-FITC or Alemtuzumab–Alexa Fluor 488. The untransfected controls were included. CD52 expression is in green and the nuclear staining in blue. Scale bar = 20 μm.
CHO: Chinese hamster ovary.
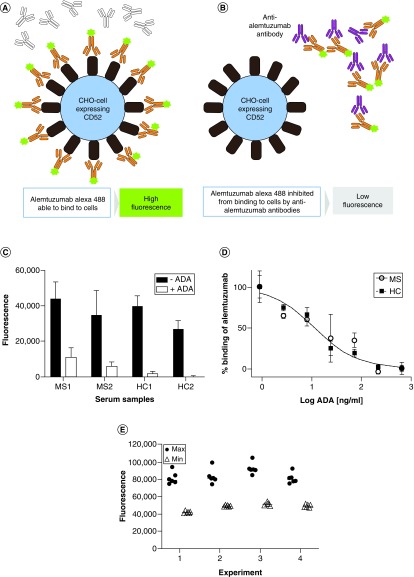
A competitive microtiter plate assay to detect anti-alemtuzumab antibodies was set up as shown on the schematic Figures 2A & B. Briefly, in the absence of Nabs the fluorescence signal would be maximal (Figure 2A) and in the presence of Nabs signal would be reduced (Figure 2B). An anti-alemtuzumab monoclonal antibody was used to spike either pretreatment MS patient sera or healthy control sera and the inhibition of alemtuzumab–Alexa Fluor 488 binding to CD52 determined (Figure 2C). The addition of anti-alemtuzumab reduced the fluorescence signal in both spiked MS and healthy control serums. Moreover, the inhibitory activity could be titrated by serial dilution (Figure 2D). To determine the inter- and intra-assay variation, replicates (n = 6) of the maximum and minimum binding were determined on four experiments (Figure 2E). The intra-assay coefficients of variation were between 10 and 7.2% for the maximum binding, and between 3.5 and 1.6% for the minimum binding. The interassay coefficient of variation was 9.6% for the maximum binding and 7.9% for the minimum binding (Table 1).
Figure 2. . Schematic of the assay format.
(A) In the absence of ADA, Alemtuzumab–Alexa Fluor 488 binds to the cells resulting in a high fluorescence signal. (B) In the presence of neutralizing ADA, the Alemtuzumab–Alexa Fluor 488 is prevented from binding to the cells, resulting in a loss of fluorescence. (C) Serum samples from an MS patient and healthy individuals not treated with alemtuzumab were spiked with anti-alemtuzumab and assayed for inhibiting alemtuzumab–Alexa Fluor 488 binding to CHO-CD52 cells using fluorescence measurement on a Clariostar Plus plate reader and the data plotted using GraphPad Prism. (D) Serum samples from an MS patient and a healthy individual not treated with alemtuzumab were spiked with anti-alemtuzumab, threefold serially diluted and assayed for inhibiting alemtuzumab–Alexa Fluor 488 binding to CHO-CD52 cells using fluorescence measurement on a Clariostar Plus plate reader and the data plotted using GraphPad Prism. (E) The maximum and minimum fluorescence (n = 6) were determined in four experiments, and the data plotted using GraphPad Prism.
ADA: Antidrug antibody; CHO: Chinese hamster ovary; MS: Multiple sclerosis.
Table 1. . Intra- and interassay variance of the anti-alemtuzumab antibody cell-based assay.
| Experiment | Max value mean | Max value SD | % CV | Min value mean | Min value SD | % CV |
|---|---|---|---|---|---|---|
| Intra-1 | 81,067 | 7126 | 8.8 | 41,740 | 1176 | 2.8 |
| Intra-2 | 82,908 | 8325 | 10.0 | 48,841 | 799 | 1.6 |
| Intra-3 | 92,146 | 6628 | 7.2 | 50,883 | 1786 | 3.5 |
| Intra-4 | 80,702 | 6071 | 7.5 | 48,794 | 1587 | 3.3 |
| Inter 1–4 | 84,206 | 8144 | 9.6 | 47,564 | 3771 | 7.9 |
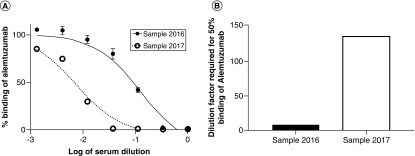
With the alemtuzumab-treated MS patient samples, the reduction in fluorescence could be titrated (Figure 3A) and the ID50 determined (Figure 3B). The neat MS patient sera completely inhibited the binding of alemtuzumab to the CHO-CD52 cell, whereas the control human serum did not. Upon dilution, the 2016 serum sample had ID50 at approximately 1/8 dilution and the 2017 serum sample required further dilution to approximately 1/136 for equivalent inhibition, as shown in Figures 3A & B.
Figure 3. . The serum samples from a patient were threefold serially diluted and assayed from neat to 1/729 and the images were analyzed using the GE Developer Toolbox to quantify the number of cells with fluorescence foci and the data plotted using GraphPad Prism (A) to calculate the percentage of cells with fluorescence foci and the ID50 (B).
ID50: Dilution required for 50% inhibition.
Alemtuzumab mediates depletion of lymphocytes in vivo by antibody-dependent cellular cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC) [11–15] and is used in the treatment of RMS [16]. In Phase III studies, alemtuzumab treatment beyond the initial two courses was offered if a relapse occurred or if a patient accumulated two or more unique MRI lesions (either gadolinium-enhancing lesions and/or new or enlarging T2 lesions). If patients have developed Nabs, the efficacy of the third and subsequent rounds of alemtuzumab treatment may be compromised. Retreatment with alemtuzumab beyond the initial two courses of therapy has been approved by the EMA [5]. A test to determine the presence and relative levels of neutralizing ADA may help to stratify patients before proceeding with further treatment with alemtuzumab.
The target for alemtuzumab, CD52, has been cloned and expressed in Jurkat and CHO cell line by several groups for a variety of functional studies [17], for use as an antigen and for soluble CD52 production [18], and in NS0 cells for use as an immunogen and in a cell-based ELISA [19]. Here we generated a stable recombinant adherent CHO cell line expressing human CD52 for use with alemtuzumab–Alexa 488 in a competitive binding assay to detect ADA Nabs. For proof of concept, we determined whether: a commercially available anti-alemtuzumab monoclonal antibody could inhibit the binding to the CHO-CD52 cells when spiked into serum and this inhibitory activity could be titrated. The addition of anti-alemtuzumab reduced the fluorescence signal in both MS and healthy sera by between 70 and 100% (Figure 2C). Moreover, spiking MS patient and healthy control serum with ADA and assaying serial dilutions demonstrated that the inhibitory activity titrated out with 50% inhibition at 1 ng/ml ADA, equimolar with the alemtuzumab–Alexa 488 concentration used in the assay (Figure 2D). The maximum and minimum fluorescence signals within and across experiments were determined (Figure 2E). The maximum signal intra-assay coefficients of variation were 7.2–10% with an interassay value of 9.6%. The minimum intra-assay signal was found to be 1.6–3.5% with an interassay value of 7.9% (Table 1). These values are within the acceptable range.
In this study, we investigated sera from a patient who had received two rounds of treatment following the approved schedule. We had previously determined that the serum sample contained binding ADA against the variable heavy (VH) and light (VL) domains of alemtuzumab. The appearance of ADA following the second round of treatment is common [7]; however, due to the sensitivity of this assay, we could detect the presence of Nabs 5 months after the first infusion – albeit at a lower titer, but significantly above the level of detection. Furthermore, the titer increases 17-fold following the second infusion of drug (Figure 2F & G). Whether 5 months postinfusion is the optimal point for sampling remains to be determined.
Ideally, for pwRMS on alemtuzumab to be considered for further treatment, the levels of ADA 1 month before the proposed treatment should be determined. If detectable levels are present, the treatment should be delayed and the ADA titers monitored (to fall below detection) prior to treatment. Alternatively, patients may benefit from plasma exchange to lower the ADA prior to drug administration. Although speculative, such an approach could extend the therapeutic utility of alemtuzumab. The availability of a cell-based assay for the rapid detection of anti-alemtuzumab Nabs that requires 65 μl of serum provides an opportunity to screen patients in advancement of treatment, to monitor ADA levels and determine the appropriate timing for drug administration for maximum patient benefit.
Adherent cell lines expressing the therapeutic antibody targets provide a general cost-effective, rapid, quantitative data collection platform for developing assays to detect Nabs on the INCA 2200 instrument or the Clariostar Plus plate reader. The approach described here could be adapted for the detection of ADA Nabs against other therapeutic antibodies used in MS that target cell surface antigens such as CD20 and CD49d.
Conclusion
In this proof-of-concept study, an adherent cell-based method for assaying for the presence of anti-alemtuzumab Nabs in sera from a patient treated with alemtuzumab was developed. The method utilizes a stable adherent CHO cell line expressing human CD52, and a competition assay between the antidrug antibody and alemtuzumab labeled with Alexa Fluor 488. The method successfully detected the presence of Nabs that inhibited the binding of alemtuzumab to the CHO-CD52 cells. The assay should be used to monitor patients on this drug for the emergence of Nabs and to correlate the inhibitory activity with the lymphocyte counts as an indicator of drug efficacy.
Future perspective
Over the next decade, many more therapeutics – either as human monoclonal antibodies or based on human monoclonal binding sites (i.e., CAR-T cell therapies) – will enter the clinic for unmet needs. The frequent administration of the biologic agent required for some chronic conditions will trigger antibody responses in some individuals, reducing the drug efficacy. Testing for the emergence of ADA, and in particular neutralizing ADA, will be imperative. The testing may be further developed for use in point-of-care devices or for monitoring at home.
Summary points.
Retreatment with alemtuzumab beyond the initial two courses of therapy has been approved by the EMA.
Humanization of antibodies does not prevent the emergence of antidrug antibodies in humans.
Following two courses of treatment with alemtuzumab, neutralizing antidrug antibodies can be detected.
Using a stable adherent cell line expressing CD52, it is possible to detect alemtuzumab-neutralizing antibodies.
Acknowledgments
The authors thank G Warnes for assistance with the flow cytometry.
Footnotes
Author contributions
AS Kang conceived the concept; AS Kang and G Saxena constructed the plasmid. L Ali, GR Leisegang developed the cell line. AS Kang, G Saxena prepared the reagents, AS Kang, G Saxena, M Jones and L Gammon developed and applied the assay on the INCA 2200. S Gnanapavan, K Schmierer and G Giovannoni provided the clinical samples. D Baker assisted in the interpretation of the data. All assisted with evaluating the data and preparing the manuscript. L Ali and G Saxena contributed equally to this paper.
Financial & competing interests disclosure
L Ali sincerely thanks NUMS for the award of a visiting fellowship in collaboration with QMUL. G Giovannoni, S Gnanapavan, K Schmierer have received fees for consultancy, meetings and grant support (S Gnanapavan) from Sanofi Genzyme within the last 3 years, otherwise none are considered relevant. However, SG has received travel support, consultancy fees or grant support from Biogen, Novartis, Teva, Pfizer and Takeda. D Baker has received consultancy and presentation fees from Canbex Therapeutics, Japan Tobacco, Merck and Roche. K Schmierer has received consultancy and presentation fees from Biogen, Bayer HealthCare, Lipomed, Medday, Novartis, Roche and Teva. G Giovannoni has received consultancy, presentation fees or grants from AbbVie Biotherapeutics, Bayer Healthcare, Biogen, Canbex, Celgene, Ironwood, Japan Tobacco, Novartis, Roche, Sanofi-Genzyme, Synthon, Takeda, Teva and Vertex. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The participants gave written informed consent for blood samples to be used for research on the BartsHealth NHS consent form. Blood from consented MS patients at the Barts and the London treated with alemtuzumab was collected and processed in March 2016 and 2017.
Data sharing statement
Sequences will be made available upon request and the cell line made available using a standard MTA agreement and a nominal fee to cover administration and technical resource required to maintain, expand and provide the cells. Data of interest will be available on request.
Open access
This work is licensed under the Attribution-NonCommercial-NoDerivatives 4.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/4.0/
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Hale G, Bright S, Chumbley G. et al. Removal of T cells from bone marrow for transplantation: a monoclonal antilymphocyte antibody that fixes human complement. Blood 62(4), 873–882 (1983). [PubMed] [Google Scholar]
- 2.Bandala-Sanchez E, Bediaga N G, Goddard-Borger ED. et al. CD52 glycan binds the proinflammatory B box of HMGB1 to engage the Siglec-10 receptor and suppress human T cell function. Proc. Natl Acad. Sci. USA 115(30), 7783–7788 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rashidi M, Bandala-Sanchez E, Lawlor KE. et al. CD52 inhibits Toll-like receptor activation of NF-kappaB and triggers apoptosis to suppress inflammation. Cell Death Differ. 25(2), 392–405 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riechmann L, Clark M, Waldmann H, Winter G. Reshaping human antibodies for therapy. Nature 332(6162), 323–327 (1988). [DOI] [PubMed] [Google Scholar]; • Describes the first humanized monoclonal antibody Campath-1H (alemtuzumab) and the rationale for the humanization.
- 5.NHS. Urgent Clinical Commisioning Policy Statement: Alemtuzumab for treating relapsing remitting multiple sclerosis – third cycle (all ages). https://www.england.nhs.uk/wp-content/uploads/2018/08/Alemtuzumab-for-treating-relapsing-remitting-multiple-sclerosis--third-cycle-all-ages.pdf (2018).
- 6.Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol. 74(8), 961–969 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Interpreted how the lymphocyte reconstituted after treatment. The hyper-repopulation of immature B cells in the absence of CD4 or CD8 regulatory cells provided the ideal setting for the generation of autoantibodies and for antidrug antibodies against residual alemtuzumab.
- 7.Dubuisson N, Baker D, Kang AS. et al. Alemtuzumab depletion failure can occur in multiple sclerosis. Immunology 154(2), 253–260 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Identified that alemtuzumab depletion failure can occur in multiple sclerosis, suggesting the presence of neutralizing antibodies.
- 8.Kozak M. Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc. Natl Acad. Sci. USA 87(21), 8301–8305 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibson DG, Young L, Chuang RY, Venter JC, Hutchison CA, 3rd, Smith HO. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6(5), 343–345 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Saxena GK, Theocharopoulos I, Aziz NT. et al. GloBody technology: detecting anti-drug antibody against VH/VL domains. Sci. Rep. 10, 1860 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu Y, Turner MJ, Shields J. et al. Investigation of the mechanism of action of alemtuzumab in a human CD52 transgenic mouse model. Immunology 128(2), 260–270 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao SP, Sancho J, Campos-Rivera J. et al. Human peripheral blood mononuclear cells exhibit heterogeneous CD52 expression levels and show differential sensitivity to alemtuzumab mediated cytolysis. PLoS ONE 7(6), e39416 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ginaldi L, De Martinis M, Matutes E. et al. Levels of expression of CD52 in normal and leukemic B and T cells: correlation with in vivo therapeutic responses to Campath-1H. Leuk. Res. 22(2), 185–191 (1998). [DOI] [PubMed] [Google Scholar]
- 14.Bugelski PJ, Martin PL. Concordance of preclinical and clinical pharmacology and toxicology of therapeutic monoclonal antibodies and fusion proteins: cell surface targets. Br. J. Pharmacol. 166(3), 823–846 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demko S, Summers J, Keegan P, Pazdur R. FDA drug approval summary: alemtuzumab as single-agent treatment for B-cell chronic lymphocytic leukemia. Oncologist 13(2), 167–174 (2008). [DOI] [PubMed] [Google Scholar]
- 16.Havrdova E, Horakova D, Kovarova I. Alemtuzumab in the treatment of multiple sclerosis: key clinical trial results and considerations for use. Ther. Adv. Neurol. Disord. 8(1), 31–45 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hale C, Bartholomew M, Taylor V, Stables J, Topley P, Tite J. Recognition of CD52 allelic gene products by CAMPATH-1H antibodies. Immunology 88(2), 183–190 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tati K, Yazdanpanah-Samani M, Ramezani A, Mahmoudi Maymand E, Ghaderi A. Establishment a CHO cell line expressing human CD52 molecule. Rep. Biochem. Mol. Biol. 5(1), 56–61 (2016). [PMC free article] [PubMed] [Google Scholar]
- 19.Holgate RG, Weldon R, Jones TD, Baker MP. Characterisation of a novel anti-CD52 antibody with improved efficacy and reduced immunogenicity. PLoS ONE 10(9), e0138123 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]