Key Points
Question
What is the efficacy and safety of clascoterone cream, 1%, in the treatment of facial acne among patients older than 9 years?
Findings
Results of 2 phase 3 randomized clinical trials including 1440 patients demonstrated that patients with acne treated with clascoterone cream, 1%, experienced greater treatment success vs treatment with vehicle, with considerable reductions in absolute noninflammatory and inflammatory lesion counts. Adverse events with use of clascoterone cream, 1%, were predominantly mild and similar to those with use of vehicle.
Meaning
Clascoterone cream, 1%, a novel topical androgen receptor inhibitor, appears to demonstrate a favorable safety profile and improvement in efficacy for acne treatment.
Abstract
Importance
Acne is a common, multifactorial skin condition, and treatments with novel mechanisms have been elusive.
Objective
To assess the safety and efficacy of clascoterone cream, 1%, a novel topical androgen receptor inhibitor, in 2 phase 3 randomized clinical trials (CB-03-01/25 and CB-03-01/26).
Design, Setting, and Participants
Two identical, multicenter, randomized, vehicle-controlled, double-blind, phase 3 studies conducted from November 2015 to April 2018 evaluated the efficacy and safety of use of clascoterone cream, 1%, in males and nonpregnant females 9 years and older with moderate or severe facial acne as scored on the Investigator’s Global Assessment scale. Participants were enrolled if they had 30 to 75 inflammatory lesions and 30 to 100 noninflammatory lesions.
Interventions
Patients were randomized to treatment with clascoterone cream, 1%, or vehicle cream and applied approximately 1 g to the whole face twice daily for 12 weeks.
Main Outcomes and Measures
Treatment success was defined as an Investigator’s Global Assessment score of 0 (clear) or 1 (almost clear), and a 2-grade or greater improvement from baseline and absolute change from baseline in noninflammatory and inflammatory lesion counts at week 12. Safety measures included adverse event frequency and severity.
Results
A total of 1440 patients were randomzied in 2 studies. In CB-03-01/25, 353 participants were randomized to treatment with clascoterone cream, 1% (median [range] age, 18.0 [10-58] years; 221 [62.6%] female), and 355 participants were randomized to treatment with vehicle cream (median [range] age, 18.0 [9-50] years; 215 (60.6%) female); in CB-03-01/26, 369 participants were randomized to treatment with clascoterone cream, 1% (median [range] age, 18.0 [10-50] years; 243 [65.9%] female), and 363 participants were randomized to treatment with vehicle cream (median [range] age, 18.0 [range, 11-42] years; 221 [60.9%] female). At week 12, treatment success rates in CB-03-01/25 and CB-03-01/26 with clascoterone cream, 1%, were 18.4% (point estimate, 2.3; 95% CI, 1.4-3.8; P < .001) and 20.3% (point estimate, 3.7; 95% CI, 2.2-6.3; P < .001) vs 9.0% and 6.5% with vehicle, respectively. At week 12, in both CB-03-01/25 and CB-03-01/26, treatment with clascoterone cream, 1%, resulted in a significant reduction in absolute noninflammatory lesions from baseline to −19.4 (point estimate difference, −6.4; 95% CI, −10.3 to −2.6; P < .001) and −19.4 (point estimate difference, −8.6; 95% CI, −12.3 to −4.9; P < .001) vs −13.0 and −10.8 with vehicle, respectively, as well as a reduction in inflammatory lesions from baseline to −19.3 (point estimate difference, −3.8; 95% CI, −6.4 to −1.3; P < .001) and −20.0 (point estimate difference, −7.4; 95% CI, −9.8 to −5.1; P < .001) vs −15.5 and −12.6 with vehicle, respectively. Adverse events rates were low and mostly mild; the predominant local skin reaction was trace or mild erythema.
Conclusions and Relevance
Use of clascoterone cream, 1%, for acne treatment appears to demonstrate favorable efficacy and safety with low adverse event rates.
Trial Registration
ClinicalTrials.gov Identifiers: NCT02608450 and NCT02608476
This review of 2 randomized clinical trials assesses the safety and efficacy of topical clascoterone cream, 1%, a novel androgen receptor inhibitor for acne treatment.
Introduction
Acne is the eighth most prevalent disease in the world1 and affects more than 640 million people globally.2 The onset of acne often coincides with pubertal hormonal changes,3 and the condition affects approximately 85% of adolescents and young adults aged 12 to 25 years.3 However, acne can also persist into, or develop during, adulthood.4 Novel therapeutic innovations for the treatment of acne have been sparse in recent years, with no new mechanism of action introduced and approved by the US Food and Drug Administration (FDA) since isotretinoin in 1982.5
Acne is a multifactorial condition characterized by excess sebum production, epithelial hyperkeratinization, Cutibacterium acnes colonization in the pilosebaceous unit, and inflammation.6 Current first-line treatments targeting 1 or 2 aspects of acne pathophysiology include benzoyl peroxide, topical retinoids, and topical or oral antibiotics.7,8,9 Antibiotic resistance in acne is a concern.10 Oral isotretinoin, which may be used for more severe cases, affects multiple acnegenic pathways. Although efficacious for the treatment of acne, it is associated with adverse effects and must be used with caution in females of childbearing age owing to known teratogenicity.7,8,9,11 Females with acne can be treated with a combined oral contraceptive (COC) or spironolactone,7,8,9,12 both of which affect androgens.9,12
Androgen receptors (ARs) are expressed throughout the skin and are found in the sebaceous glands, sebocytes, and dermal papilla cells.13 Circulating and locally (skin) synthesized androgens such as testosterone and dihydrotestosterone (DHT) bind to the AR and stimulate sebum production in both males and females.12,13,14
Androgen inhibition is an effective strategy for the treatment of acne in females. Certain COCs (eg, norgestimate, norethindrone) are approved by the FDA to treat acne in females15,16,17; these drugs suppress androgen production, thereby reducing circulating androgens.12,17 Spironolactone is an aldosterone inhibitor and AR blocker12,18 that is used off label to treat acne in females.18,19
Both COCs and spironolactone are associated with systemic adverse effects, are contraindicated in pregnancy, and are unsuitable for use in males with acne.9,12 Other AR inhibitors and/or antiandrogens have not been approved for the treatment of acne in males.
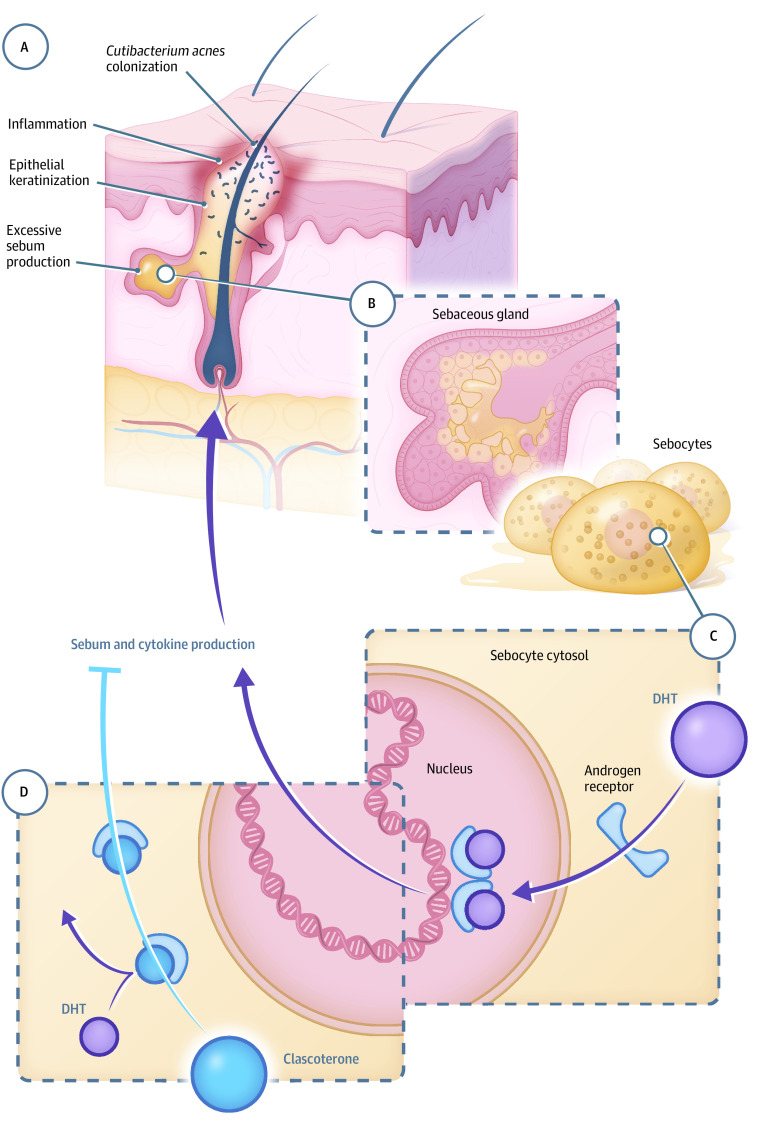
Topical clascoterone cream, 1% (cortexolone 17α-propionate), a new chemical entity, is a novel topical androgen receptor inhibitor under investigation as a first-in-class therapy for the treatment of acne in both males and females.20,21 In vitro studies suggest that clascoterone competes with androgens, specifically DHT, for binding to the androgen receptor, thereby inhibiting downstream signaling of acnegenic pathways.20,21 Reduced transcription of androgen-responsive genes inhibits sebum production and activation of inflammatory pathways, including those involved in proinflammatory cytokine synthesis.21 In this way, clascoterone targets more than 1 acnegenic pathway. Clascoterone targets androgen receptors at the site of application and is quickly metabolized to an inactive form, thus limiting systemic activity.20 The proposed mechanism of action of clascoterone is shown in Figure 1.6,13,20,21,22,23,24
Figure 1. Proposed Mechanism of Action of Clascoterone.
A, Acne is characterized by epithelial hyperkeratinization, excessive sebum production, Cutibacterium acnes colonization of the pilosebaceous unit, and inflammation.6 B, Within the sebaceous gland, sebocytes convert precursor molecules into androgens including dihydrotestosterone (DHT).6,13 C, Within sebocytes, DHT binds to androgen receptors in the cytosol. On binding, the DHT-androgen receptor complex dimerizes and translocates to the nucleus.22 There, it influences transcription of genes involved in acne pathogenesis, including sebum and inflammatory cytokine production.13,21 D, Clascoterone, applied topically to the skin, binds to the androgen receptor with high affinity at the site of application, competing with DHT.20,21,23 Results from in vitro studies suggest it thereby limits the effect of DHT on transcription of genes that modulate sebum production and inflammation.21,24
An early proof-of-concept study25 revealed that topical clascoterone cream, 1%, is well tolerated and clinically more effective than vehicle and tretinoin, 0.05%. Phase 2 studies demonstrated the safety and tolerability of clascoterone cream, 1%, in adolescents and adults with acne vulgaris and established clascoterone cream, 1%, used twice daily as the best regimen for phase 3 development.26,27 Herein, we describe 2 identical phase 3 studies evaluating the efficacy and safety of topical clascoterone cream, 1%, vs vehicle cream applied twice daily for 12 weeks in patients with facial acne vulgaris.
Methods
Trial Design
The CB-03-01/25 and CB-03-01/26 trials were identical, multicenter, randomized, double-blind, vehicle-controlled, parallel-group phase 3 comparison studies of the efficacy and safety of topical clascoterone cream, 1%. These studies were designed according to the FDA’s industry guidelines for establishing effectiveness of drugs intended for treatment of acne vulgaris.28
Study CB-03-01/25 was carried out at 45 sites in the United States (n = 502), 7 sites in Ukraine (n = 132), and 3 sites in the Republic of Georgia (n = 74). Study CB-03-01/26 was conducted at 10 sites in the United States (n = 93), 8 sites in Bulgaria (n = 90), 9 sites in Romania (n = 186), 12 sites in Poland (n = 222), 3 sites in Serbia (n = 39), and 6 sites in the Republic of Georgia (n = 102).
Study approval was obtained from the relevant ethics committees, institutional review boards, and national central authorities for all study sites. The studies were conducted in accordance with the Declaration of Helsinki. Voluntary informed consent/assent forms were signed by every patient and/or their parent or guardian. The protocols were approved by the FDA.
Patients
Male and nonpregnant female patients 9 years or older with moderate to severe facial acne vulgaris (grade 3 or 4 on the Investigator’s Global Assessment [IGA] scale) were eligible for enrollment. Patients had at least 30 to a maximum of 75 inflammatory lesions and at least 30 to a maximum of 100 noninflammatory lesions. Patients must have been on a consistent skin care program for at least 1 month prior to enrollment and agreed to continue this regimen for the entire study.
Key exclusion criteria included more than 2 facial nodules, use of topical antiacne preparations on the face (including over-the-counter acne cleansers or treatments, retinoids, and light treatments), and use of systemic antiacne medications (including corticosteroids, antibiotics, spironolactone, and retinoid therapy). An exclusion criterion of participation in a prior clascoterone clinical trial was added to study CB-03-01/25 owing to oversight in the initial protocol. No other changes in the conduct of the studies occurred. Full inclusion and exclusion criteria are listed in the study protocols (Supplements 1 and 2).
The safety set included all patients who received at least 1 application of the test article. The intent-to-treat (ITT) set included all randomized patients. The per-protocol (PP) set was a subset of the ITT population and included patients who completed the study without any significant protocol deviations. The analysis of safety was conducted on the safety set. The analysis of efficacy was conducted on both the ITT and PP sets, with the ITT set considered as the primary set for statistical analysis
Interventions
The study plan consisted of a screening/baseline visit (visit 1), follow-up after 4 and 8 weeks of treatment (visits 2 and 3), and a final visit (visit 4) at the end of the 12-week treatment period. Patients were instructed to apply approximately 1 g of clascoterone cream, 1%, to the entire face twice daily for 12 weeks.
Eligible patients were randomized using Datatrak One software to receive clascoterone cream, 1%, or vehicle cream in a 1:1 ratio. The randomization sequence was prepared by Datatrak using a permuted block design with a block size of 4. The clinical team, patients, investigator, monitors, and employees of the study sites were blinded to treatment allocation. The vehicle cream was identical to clascoterone cream, 1%, in terms of color, consistency, and smell, and the creams were packed in identical blinded tubes.
Assessments
At all study visits, IGA was performed using a 5-point scale (Supplements 1 and 2). Manual counting of noninflammatory lesions, inflammatory lesions, and nodules was performed. Five digital color photographs of each patient’s face (with the patient looking straight ahead, up, down, left, and right) were taken.
Local and systemic adverse events (AEs) and local skin reactions (LSRs) (ie, telangiectasia, skin atrophy, striae rubrae, erythema, edema, scaling/dryness, stinging/burning, pruritus) were evaluated at each study visit. Investigators assessed LSRs using a 5-point scale (0, none; 1, trace; 2, mild; 3, moderate; 4, severe). Patients rated severity of stinging or burning and pruritus on a 4-point scale (0, none; 1, mild; 2, moderate; 3, severe) at each study visit. Electrocardiograms (ECGs) taken at visits 1 and 4 were assessed by a central reviewer (ie, cardiologist).
Treatment compliance was evaluated at each visit and overall according to the formula: 100 × (number of actual applications/numbers of scheduled applications). Noncompliance was defined as a compliance value less than 80%.
The amount of product used (grams applied) was calculated from the weights of the returned tubes. The mean daily amount of product applied (total amount of product used/number of days of treatment) was calculated for each patient.
Main Outcome Measures
Three coprimary efficacy end points were assessed in hierarchal order: proportion of patients achieving treatment success at week 12, absolute change from baseline in noninflammatory lesion count (NILC), and inflammatory lesion count (ILC) at week 12. Treatment success was defined as at least a 2-point reduction in IGA score from baseline and a score of clear (0) or almost clear (1).
Secondary efficacy end points for both studies included percentage change from baseline in total lesion count (TLC), NILC, ILC at week 12, and absolute change from baseline in TLC at week 12. Safety end points for both studies included local and systemic AEs, LSRs, changes in ECGs at week 12, and results of urine pregnancy tests.
Statistical Analysis
Based on assumptions estimated from the phase 2 dose-escalation study (NCT01631474), at least 350 patients in each treatment arm in each study were required to provide sufficient power (90%) with the selected primary end points. No interim analyses were planned or performed. All statistical analysis was performed using SAS, version 9.3 (SAS Institute).
A logistic regression model with treatment and analysis center as fixed effects was used to compare the proportion of patients achieving treatment success at week 12. Adjusted odds ratio with associated 95% CIs were analyzed. Analysis of the remaining primary and secondary efficacy end points was performed using an analysis of covariance model, with treatment and analysis center as fixed effects and baseline value as the covariate. Adjusted least squares means with associated 95% CIs of difference from the analysis of covariance model were analyzed. Sensitivity analyses were performed to investigate the robustness of the results obtained on the ITT set for the primary efficacy end points. All AEs were coded using the Medical Dictionary for Regulatory Activities, version 18.1.
The multiple imputation method under the missing at random assumption was used to impute missing values for the primary end points and absolute change in total lesions count in the ITT analyses. Sensitivity analyses were performed to investigate the robustness of the results obtained in the ITT set for the primary end points (change from baseline in NILC and ILC and IGA).
Results
Patients
In study CB-03-01/25, the first patient was enrolled on January 21, 2016, and the last patient completed the study on April 11, 2018. In study CB-03-01/26, the first patient was enrolled on November 16, 2015, and the last patient completed the study on February 21, 2018.
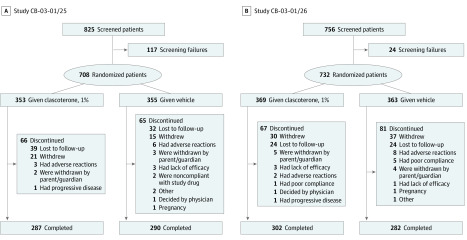
In study CB-03-01/25, 708 patients were randomized; 353 were assigned to treatment with clascoterone cream, 1%, and 355 to vehicle cream. In study CB-03-01/26, 732 patients were randomized; 369 received clascoterone cream, 1%, and 363 received vehicle cream. Figure 2 shows the enrollment and outcomes for participants. All enrolled patients received at least 1 application of product and were included in the ITT and safety sets.
Figure 2. CONSORT Diagrams.
Patient baseline demographic and clinical characteristics are presented in Table 1. Characteristics were balanced between treatment arms in both studies.
Table 1. Baseline Demographic and Clinical Characteristics of Patients in Studies CB-03-01/25 and CB-03-01/26.
| Characteristic | Studies, No. (%) | ||||
|---|---|---|---|---|---|
| CB-01-01/25 | CB-03-01/26 | ||||
| Clascoterone (n = 353) | Vehicle (n = 355) | Clascoterone (n = 369) | Vehicle (n = 363) | ||
| Sex | |||||
| Male | 132 (37.4) | 140 (39.4) | 126 (34.1) | 142 (39.1) | |
| Female | 221 (62.6) | 215 (60.6) | 243 (65.9) | 221 (60.9) | |
| Age, median (range), y | 18.0 (10-58) | 18.0 (9-50) | 18.0 (10-50) | 18.0 (11-42) | |
| Race | |||||
| White | 298 (84.4) | 297 (83.7) | 357 (96.7) | 348 (95.9) | |
| Asian | 9 (2.5) | 10 (2.8) | 0 | 4 (1.1) | |
| Black or African American | 31 (8.8) | 38 (10.7) | 7 (1.9) | 6 (1.7) | |
| Other | 15 (4.2) | 10 (2.8) | 5 (1.4) | 5 (1.4) | |
| Fitzpatrick skin type | |||||
| I | 7 (2.0) | 7 (2.0) | 7 (1.9) | 12 (3.3) | |
| II | 111 (31.4) | 111 (31.3) | 122 (33.1) | 107 (29.5) | |
| III | 122 (34.6) | 121 (34.1) | 170 (46.1) | 166 (45.7) | |
| IV | 63 (17.8) | 64 (18.0) | 57 (15.4) | 54 (14.9) | |
| V | 27 (7.6) | 23 (6.5) | 7 (1.9) | 21 (5.8) | |
| VI | 23 (6.5) | 29 (8.2) | 6 (1.6) | 3 (0.8) | |
| Baseline IGA score | |||||
| 3 (moderate) | 292 (82.7) | 291 (82.0) | 305 (82.7) | 313 (86.2) | |
| 4 (severe) | 61 (17.3) | 64 (18.0) | 64 (17.3) | 50 (13.8) | |
| TLC, mean (SD) | 101.5 (25.12) | 103.6 (26.13) | 105.7 (25.76) | 104.6 (24.18) | |
| NILC, mean (SD) | 59.1 (22.19) | 60.7 (22.09) | 62.8 (21.37) | 63.3 (20.52) | |
| ILC, mean (SD) | 42.4 (11.77) | 42.9 (12.31) | 42.9 (12.20) | 41.3 (10.96) | |
Abbreviations: IGA, Investigator’s Global Assessment; ILC, inflammatory lesion count; NILC, noninflammatory lesion count; TLC, total lesion count.
Treatment Exposure
The majority of patients in both trials were treatment compliant (≥80% of expected cream application at each study visit). In CB-03-01/25, the mean (SD) daily amount of product used in the ITT/safety population was 1.96 (0.60) g of clascoterone cream, 1%, and 1.96 (0.58) g of vehicle; 316 of 353 (89.5%) patients receiving clascoterone cream, 1%, and 310 of 355 (87.3%) patients receiving vehicle were treatment compliant. For CB-03-01/26, the mean (SD) daily amount of product used was 1.97 (0.51) g for clascoterone cream, 1%, and 1.98 (0.51) g for vehicle, with treatment completed in 340 of 369 (92.1%) patients and 313 of 363 (86.2%) patients, respectively.
Efficacy
Both trials met the primary efficacy end points. Considerably more patients receiving clascoterone cream, 1%, vs vehicle achieved treatment success at week 12 (CB-03-01/25, 18.4% vs 9.0%; and CB-03-01/26, 20.3% vs 6.5%, respectively). The absolute change from baseline in NILC and ILC at week 12 were also substantially greater with use of clascoterone cream, 1% vs vehicle (Table 2).
Table 2. Efficacy and Safety Results From Studies CB-03-01/25 and CB-03-01/26.
| Category | Studies, No. (%) | |||
|---|---|---|---|---|
| CB-03-01/25 | CB-03-01/26 | |||
| Clascoterone (n = 353) | Vehicle (n = 355) | Clascoterone (n = 369) | Vehicle (n = 363) | |
| Efficacy | ||||
| Treatment success at week 12 | 57 (16.1) | 25 (7.0) | 69 (18.7) | 17 (4.7) |
| Adjusted proportions, treatment success at week 12, %a | 18.4 | 9.0 | 20.3 | 6.5 |
| Point estimate (95% CI) | 2.3 (1.4 to 3.8) | NA | 3.7 (2.2 to 6.3) | NA |
| 2-sided P value for treatment effect | <.001 | NA | <.001 | NA |
| Absolute change from baseline in NILC at week 12 | −19.4 | −13.0 | −19.4 | −10.8 |
| Difference, point estimate (95% CI) | −6.4 (−10.3 to −2.6) | NA | −8.6 (−12.3 to −4.9) | NA |
| 2-sided P value for treatment effect | <.001 | NA | <.001 | NA |
| Absolute change from baseline in ILC at week 12 | −19.3 | −15.5 | −20.0 | −12.6 |
| Difference, point estimate (95% CI) | −3.8 (−6.4 to −1.3) | NA | −7.4 (−9.8 to −5.1) | NA |
| 2-sided P value for treatment effect | .003 | NA | <.001 | NA |
| Absolute change in TLC from baseline at week 12 | −39.1 | −28.8 | −40.0 | −23.6 |
| Difference, point estimate (95% CI) | −10.3 (−15.7 to −4.9) | NA | −16.4 (−21.8 to −11.0) | NA |
| 2-sided P value for treatment effect | <.001 | NA | <.001 | NA |
| Change in TLC from baseline at week 12, % | −37.0 | −28.4 | −37.3 | −22.1 |
| Difference, point estimate (95% CI) | −8.6 (−13.9 to −3.3) | NA | −15.2 (−20.5 to −9.9) | NA |
| 2-sided P value for treatment effect | .001 | NA | <.001 | NA |
| Change in NILC from baseline at week 12, % | −30.6 | −21.6 | −29.3 | −15.6 |
| Difference, point estimate (95% CI) | −9.0 (−15.8 to −2.2) | NA | −13.7 (−19.9 to −7.6) | NA |
| 2-sided P value for treatment effect | .009 | NA | <.001 | NA |
| Change in ILC from baseline at week 12, % | −44.8 | −36.5 | −46.9 | −29.6 |
| Difference, point estimate (95% CI) | −8.3 (−14.2 to −2.4) | NA | −17.2 (−22.9 to −11.6) | NA |
| 2-sided P value for treatment effect | .005 | NA | <.001 | NA |
| Safety | ||||
| Patients experiencing ≥1 TEAE | 40 (11.3) | 41 (11.5) | 42 (11.4) | 50 (13.8) |
| Patients experiencing TEAE by severity | ||||
| Mild | 31 (8.8) | 24 (6.8) | 32 (8.7) | 33 (9.1) |
| Moderate | 9 (2.5) | 15 (4.2) | 10 (2.7) | 16 (4.4) |
| Severe | 0 | 2 (0.6) | 0 | 1 (0.3) |
| Patients experiencing TEAEs | ||||
| Serious | 0 | 1 (0.3) | 0 | 1 (0.3) |
| Related to study drug | 4 (1.1) | 9 (2.5) | 8 (2.2) | 13 (3.6) |
| Leading to study drug discontinuation | 3 (0.8) | 4 (1.1) | 2 (0.5) | 8 (2.2) |
| Most frequent TEAEs | ||||
| Nasopharyngitis | 6 (1.7) | 13 (3.7) | 4 (1.1) | 7 (1.9) |
| Headache | 2 (0.6) | 1 (0.3) | 4 (1.1) | 3 (0.8) |
| Oropharyngeal pain | 2 (0.6) | 1 (0.3) | 4 (1.1) | 4 (1.1) |
| Vomiting | 2 (0.6) | 2 (0.6) | 2 (0.5) | 1 (0.3) |
Abbreviations: IGA, Investigator’s Global Assessment; ILC, inflammatory lesion count; NA, not applicable; NILC, noninflammatory lesion count; TEAE, treatment-emergent adverse event; TLC, total lesion count.
Adjusted proportion of patients with treatment success defined as at least a 2-point reduction in IGA vs baseline and an IGA score of 0 or 1 at week 12 (logistic regression multiple imputation under missing at random). A logistic regression model with treatment and pooled analysis centers as fixed effects was used to compare the proportion of subjects with at least a 2-point reduction in IGA compared with baseline and an IGA score of 0 or 1 at week 12.
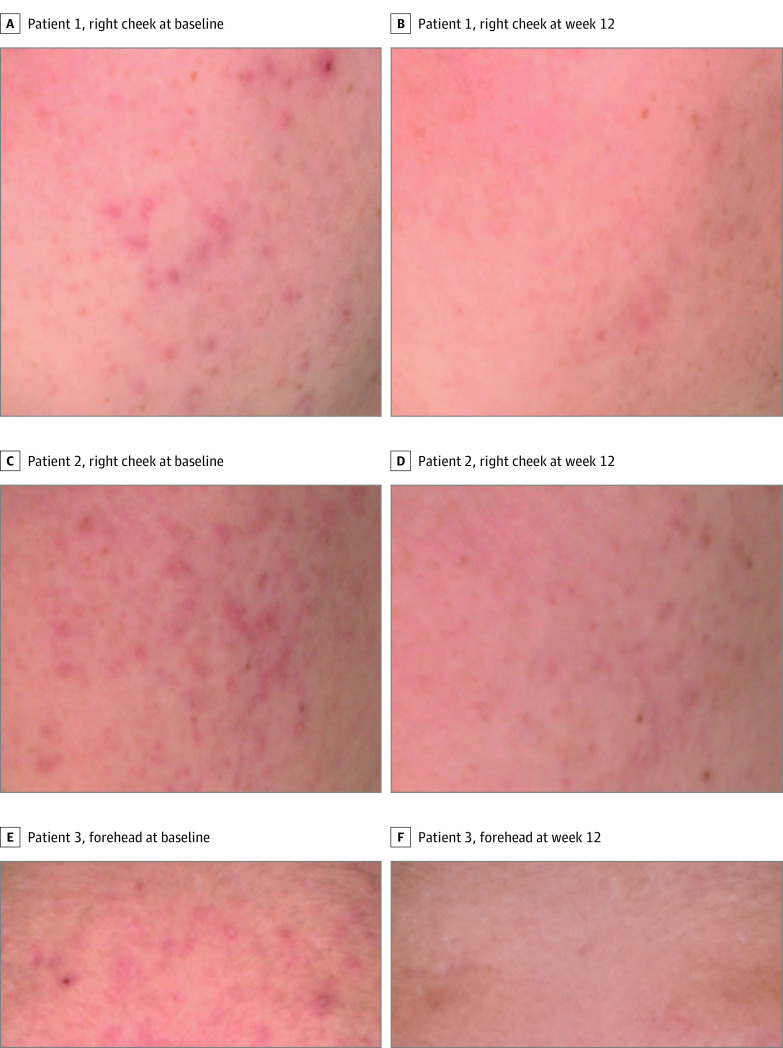
Secondary efficacy end points were also met, with considerably greater absolute change from baseline to week 12 in TLC, and percentage change from baseline to week 12 in NILC, ILC, and TLC (Table 2). Representative patient photographs at baseline (Figure 3A, C, and E) and week 12 (Figure 3B, D, and F) are shown.
Figure 3. Improvement of Acne in Patients Treated With Clascoterone Topical Cream, 1%, Twice Daily for 12 Weeks.
Representative photographs of 3 patients, both male and female, at baseline and week 12.
Planned sensitivity analyses were performed to investigate the robustness of the results obtained from the ITT population for the 3 primary efficacy end points. The PP population included patients who completed the studies without major protocol deviation (270 patients assigned to clascoterone cream, 1%, and 260 to vehicle in CB-03-01/25, and 286 patients assigned to clascoterone cream, 1%, and 268 to vehicle in CB-03-01/26). Statistical significance for all 3 primary efficacy end points was retained in both trials when tested in the PP population, and all other sensitivity analyses also confirmed the results obtained in the ITT population.
Safety
Overall, clascoterone was well tolerated and demonstrated a similar safety profile to that of vehicle (Table 2). The most common treatment emergent AEs (TEAEs) in studies CB-03-01/25 and CB-03-01/26 were nasopharyngitis, headache, and oropharyngeal pain.
TEAEs (n = 4) considered related to use of clascoterone cream, 1%, in study CB-03-01/25 were application site pain, oropharyngeal pain, application site dryness, and application site hypersensitivity (all mild in severity). In study CB-03-01/26, TEAEs (n = 9) considered related to clascoterone cream were application site dryness, application site erythema, application site hypertrichosis, acne, dermatitis contact, hair color changes, eye irritation, peritonsillar abscess, and headache.
The majority of TEAEs in both studies were mild or moderate in severity. Two patients receiving vehicle cream in CB-03-01/25 experienced severe TEAEs of pneumonia and application site acne, and 1 patient receiving vehicle cream in CB-03-01/26 experienced a severe TEAE of contusion. There were no severe TEAEs in patients treated with clascoterone.
One patient in each study assigned to receive vehicle cream experienced a serious TEAE (severe hematoma of the right thigh in CB-03-01/26 and severe pneumonia in CB-03-01/25), neither of which was considered related to study treatment or led to study discontinuation. No deaths were reported in either study.
Seventeen patients discontinued due to TEAEs: 5 receiving clascoterone cream, 1% (CB-03-01/25, 3 of 353 [0.8%]; and CB-03-01/26, 2 of 369 [0.5%]), and 12 receiving vehicle cream (CB-03-01/25, 4 of 353 [1.1%]; and CB-03-01/26, 8 of 369 [2.2%]). TEAEs leading to discontinuation of clascoterone use included application site hypersensitivity, oropharyngeal pain, sebaceous hyperplasia, facial acute contact dermatitis, and depigmented hair on the nose (n = 1 for each); all were mild in severity.
In both studies, the proportion of subjects with each LSR was similar for both treatment groups throughout the study. The majority of patients in each treatment group remained free of most LSRs throughout both studies.
The most frequently observed new or worsening LSRs for both CB-03-01/25 and CB-03-01/26 were erythema (clascoterone arm: 38 of 335 [11.3%]) and 46 of 352 [13.1%], respectively; vehicle arm: 52 of 332 [15.7%] and 49 of 330 [14.8%], respectively), and scaling/dryness (clascoterone arm: 41 of 335 [12.2%]) and 31 of 352 [8.8%], respectively; vehicle arm: 43 of 332 [13.0%] 25 of 330 [7.6%]), respectively), with most being minimal-mild in severity (clascoterone arm: 34 of 335 [10.1%] and 39 of 352 [11.1%], respectively; vehicle arm: 45 of 332 [13.6%] and 43 of 330 [13.0%], respectively). There was 1 case of severe new or worsening erythema in the vehicle group in CB-03-01/25. Minimal-mild scaling/dryness occurred among arms in CB-03-01/25 and CB-03-01/26 (clascoterone arm: 39 of 335 [11.6%] and 31 of 352 [8.8%], respectively; vehicle arm: 43 of 332 [13.0%] and 25 of 330 [7.6%], respectively). There were no severe cases of scaling/dryness; however, there were 2 cases of moderate scaling/dryness in the CB-03-01/25 clascoterone arm and 1 in the vehicle arm of CB-03-01/26. Occurrence of mild pruritus was similar in both CB-03-01/25 and CB-03-01/26 (clascoterone arm: 17 of 335 [5.1%] and 19 of 352 [5.4%], respectively; vehicle arm: 20 of 332 [6.0%] and 17 of 330 [5.2%]), respectively). One severe pruritus case occurred in each study clascoterone arm, and 3 occurred in the vehicle arms (1 in CB-03-01/25 and 2 in CB-03-01/26). Moderate pruritus also occurred in CB-03-01/25 and CB-03-01/26 (clascoterone arm: 4 of 335 [1.2%] and 10 of 352 [2.8%], respectively; vehicle arm: 6 of 332 [1.8%] and 9 of 330 [2.7%], respectively). New or worsening telangiectasia, skin atrophy, edema, striae rubrae, and stinging/burning occurred at lower frequencies than erythema, scaling/dryness and pruritus, and if present, were predominantly trace-mild or minimal.
No substantial changes in ECGs were observed in either treatment group in either study. In each study, 1 patient assigned to vehicle became pregnant. In CB-03-01/25, a woman receiving vehicle had a positive pregnancy test at visit 3. She was discontinued from the study and lost to follow-up. In CB-03-01/26, a woman received vehicle treatment for 9 days, then discontinued the treatment before pregnancy confirmation. The neonate was born preterm but “fine” at 35 weeks. No other details were available.
Discussion
The results of these 2 identical phase 3 studies demonstrate that clascoterone cream, 1%, applied topically twice daily for 12 weeks, is considerably more effective than application of vehicle cream at achieving IGA success and reducing NILC and ILC in patients with facial acne vulgaris. No safety concerns were noted during these studies, and clascoterone cream, 1%, was well tolerated.
Together, these 2 studies enrolled 1440 patients with acne between the ages of 9 and 58 years. Treatment adherence was approximately 90% for patients applying clascoterone cream, 1%, which suggests that the treatment regimen is easy to follow and suitable for general clinical practice. Results of the studies showed positive efficacy outcomes for clascoterone cream, 1%, compared with vehicle, with statistically significant improvements in all primary and secondary efficacy end points.
There is currently no approved topical antiandrogen treatment for acne. While effective, available therapies targeting the androgen pathway may be associated with systemic adverse effects and are not suitable for all patients with acne.9,12 The safety profile of clascoterone cream, 1%, in the 2 present studies was similar to that of the vehicle cream, with most TEAEs and LSRs being mild in severity. No systemic adverse effects were observed, which is consistent with the results of in vivo studies showing clascoterone has only local, not systemic, antiandrogenic activity.20,25,26,27
Limitations
Limitations of these phase 3 studies include the small sample sizes available for subgroup analyses, such as race and age, which limit the conclusions that can be drawn for subpopulations. Additionally, concomitant acne therapies were not used, thus limiting the understanding of optimal topical combination therapeutic strategies. While these studies evaluated 12-week treatment with clascoterone cream, 1%, further studies are needed to evaluate long-term safety. Patient-reported outcomes were not included as a secondary end point, but they could be considered for future studies to better understand the influence of treatment on quality of life.
Conclusions
In conclusion, clascoterone cream, 1%, is a topical androgen receptor inhibitor with a novel mechanism of action for acne treatment. The 2 phase 3 trials assessed here demonstrate the safety and efficacy of clascoterone cream, 1%, in patients 9 years and older who have facial acne vulgaris. Clascoterone cream, 1%, is under consideration as a first-in-class therapeutic agent for acne treatment, potentially providing an alternative to antibiotics and/or offering an adjunct treatment to existing combination acne therapies, including retinoids.
Trial Protocol.
Trial Protocol.
Data Sharing Statement.
References
- 1.Tan JK, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol. 2015;172(suppl 1):3-12. doi: 10.1111/bjd.13462 [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163-2196. doi: 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lynn DD, Umari T, Dunnick CA, Dellavalle RP. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13-25. doi: 10.2147/AHMT.S55832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rocha MA, Bagatin E. Adult-onset acne: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2018;11:59-69. doi: 10.2147/CCID.S137794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leyden JJ, Del Rosso JQ, Baum EW. The use of isotretinoin in the treatment of acne vulgaris: clinical considerations and future directions. J Clin Aesthet Dermatol. 2014;7(2)(suppl):S3-S21. [PMC free article] [PubMed] [Google Scholar]
- 6.Moradi Tuchayi S, Makrantonaki E, Ganceviciene R, Dessinioti C, Feldman SR, Zouboulis CC. Acne vulgaris. Nat Rev Dis Primers. 2015;1:15029. doi: 10.1038/nrdp.2015.29 [DOI] [PubMed] [Google Scholar]
- 7.Hauk L. Acne vulgaris: treatment guidelines from the AAD. Am Fam Physician. 2017;95(11):740-741. [PubMed] [Google Scholar]
- 8.Thiboutot DM, Dréno B, Abanmi A, et al. Practical management of acne for clinicians: an international consensus from the Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2018;78(2)(suppl 1):S1-S23.e1. [DOI] [PubMed] [Google Scholar]
- 9.Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945-73.e33. doi: 10.1016/j.jaad.2015.12.037 [DOI] [PubMed] [Google Scholar]
- 10.Adler BL, Kornmehl H, Armstrong AW. Antibiotic resistance in acne treatment. JAMA Dermatol. 2017;153(8):810-811. doi: 10.1001/jamadermatol.2017.1297 [DOI] [PubMed] [Google Scholar]
- 11.Layton A. The use of isotretinoin in acne. Dermatoendocrinol. 2009;1(3):162-169. doi: 10.4161/derm.1.3.9364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elsaie ML. Hormonal treatment of acne vulgaris: an update. Clin Cosmet Investig Dermatol. 2016;9:241-248. doi: 10.2147/CCID.S114830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dart DA. Androgens have forgotten and emerging roles outside of their reproductive functions, with implications for diseases and disorders. J Endocr Disord. 2014;1(1):1005. [Google Scholar]
- 14.Lai JJ, Chang P, Lai KP, Chen L, Chang C. The role of androgen and androgen receptor in skin-related disorders. Arch Dermatol Res. 2012;304(7):499-510. doi: 10.1007/s00403-012-1265-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estrostep Fe. Prescribing information. Allergan Inc; 2017.
- 16.Ortho-cyclen and Ortho tri-cyclen. Prescribing information. Janssen Pharmaceutical Inc; 2017.
- 17.Huber J, Walch K. Treating acne with oral contraceptives: use of lower doses. Contraception. 2006;73(1):23-29. doi: 10.1016/j.contraception.2005.07.010 [DOI] [PubMed] [Google Scholar]
- 18.Aldactone. Prescribing information. Pfizer Inc; 2018.
- 19.Layton AM, Eady EA, Whitehouse H, Del Rosso JQ, Fedorowicz Z, van Zuuren EJ. Oral spironolactone for acne vulgaris in adult females: a hybrid systematic review. Am J Clin Dermatol. 2017;18(2):169-191. doi: 10.1007/s40257-016-0245-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferraboschi P, Legnani L, Celasco G, Moro L, Ragonesi L, Colombo D. A full conformational characterization of antiandrogen cortexolone-17α-propionate and related compounds through theoretical calculations and nuclear magnetic resonance spectroscopy. MedChemComm. 2014;5:904-914. doi: 10.1039/C4MD00049H [DOI] [Google Scholar]
- 21.Rosette C, Agan FJ, Mazzetti A, Moro L, Gerloni M. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18(5):412-418. [PubMed] [Google Scholar]
- 22.Tan MH, Li J, Xu HE, Melcher K, Yong EL. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacol Sin. 2015;36(1):3-23. doi: 10.1038/aps.2014.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Celasco G, Moro L, Bozzella R, et al. Biological profile of cortexolone 17alpha-propionate (CB-03-01), a new topical and peripherally selective androgen antagonist. Arzneimittelforschung. 2004;54(12):881-886. [DOI] [PubMed] [Google Scholar]
- 24.Rosette C, Rosette N, Mazzetti A, Moro L, Gerloni M. Cortexolone 17α-propionate (clascoterone) is an androgen receptor antagonist in dermal papilla cells in vitro. J Drugs Dermatol. 2019;18(2):197-201. [PubMed] [Google Scholar]
- 25.Trifu V, Tiplica GS, Naumescu E, Zalupca L, Moro L, Celasco G. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris: a pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165(1):177-183. doi: 10.1111/j.1365-2133.2011.10332.x [DOI] [PubMed] [Google Scholar]
- 26.Mazzetti A, Moro L, Gerloni M, Cartwright M. Pharmacokinetic profile, safety, and tolerability of clascoterone (cortexolone 17-alpha propionate, CB-03-01) topical cream, 1% in subjects with facial acne vulgaris: an open-label phase 2a study. J Drugs Dermatol. 2019;18(6):563-568. [PubMed] [Google Scholar]
- 27.Mazzetti A, Moro L, Gerloni M, Cartwright M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18(6):570-575. [PubMed] [Google Scholar]
- 28.US Food and Drug Administration Center for Drug Evaluation and Research . Guidance for industry: acne vulgaris: developing drugs for treatment. September 19, 2005. Accessed March 23, 2020. https://www.federalregister.gov/documents/2005/09/19/05-18512/draft-guidance-for-industry-on-acne-vulgaris-developing-drugs-for-treatment-availability.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol.
Trial Protocol.
Data Sharing Statement.