We highlight the similarities and differences in primary and lateral root growth, focusing on the differential impact that phytohormones and environmental cues have on these.
Keywords: Lateral root, nutrients, plant hormones, primary root, root system architecture
Abstract
The root system architecture describes the shape and spatial arrangement of roots within the soil. Its spatial distribution depends on growth and branching rates as well as directional organ growth. The embryonic primary root gives rise to lateral (secondary) roots, and the ratio of both root types changes over the life span of a plant. Most studies have focused on the growth of primary roots and the development of lateral root primordia. Comparably less is known about the growth regulation of secondary root organs. Here, we review similarities and differences between primary and lateral root organ growth, and emphasize particularly how external stimuli and internal signals differentially integrate root system growth.
Introduction
The root system’s extensive epidermal surface facilitates the interphase between the plant and soil environment, supplying the plant with water and nutrients (Zürcher and Muller, 2016). A robust root system is key for plant productivity and hence an important agronomical trait (Lynch, 1995). Root systems are comprised of primary roots (PRs), which anchor the system into the soil by growing downwards, and lateral/secondary roots (LRs), which explore the soil through environmentally responsive radial growth and determine the overall root system size.
In both monocots and dicots, the PR develops from an embryonically formed meristem (De Smet et al., 2010) and is the first organ to emerge from the germinating seed. Lateral root primorida (LRPs) originate in monocotyledons and dicotyledons from founder cells in the pericycle, which is the outermost layer of the vascular cylinder (Fig. 1A; Dubrovsky et al., 2000). Interestingly, in most fern species, LRs are derived from the endodermis, while in some water plants they are derived from both the pericycle and endodermis (Lin and Raghavan, 1991; Clowes, 1992; Hou and Hill, 2004). Each LRP undergoes coordinated cell division and cell expansion, leading to LR emergence and meristem activation. Many hormonal and environmental cues affect LRP priming, initiation, and development. Understanding these cues is a highly vibrant research area and we would like to refer to several excellent reviews on this topic (Fukaki and Tasaka, 2009; Péret et al., 2009; Lavenus et al., 2013; Motte et al., 2019). In this review, we will focus on the further development of already emerged LRs.
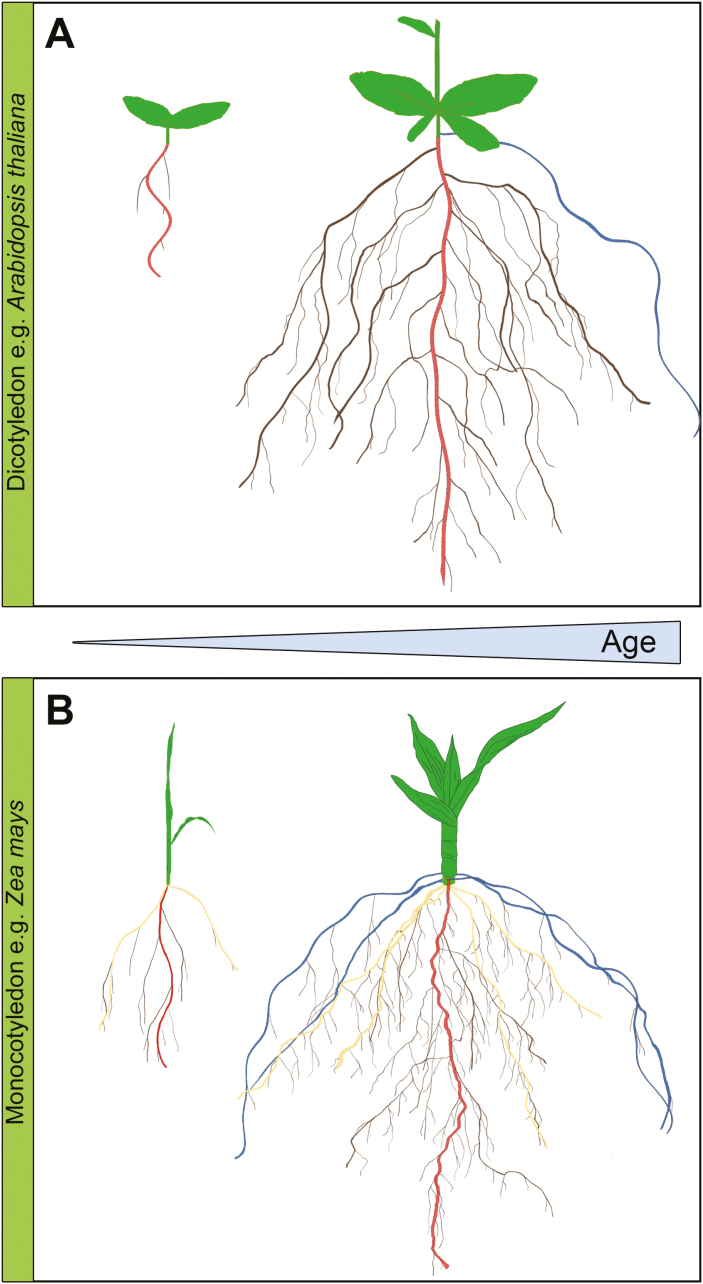
Fig. 1.
The RSA of dicots and monocots changes over time. (A) A typical taproot system of dicots (e.g. Arabidopsis thaliana), consisting of embryonic primary root (PR, red) and branching lateral roots (LRs, brown). Ultimately, the LRs will undergo higher order branching to form secondary and tertiary LRs. Adventitious roots (ARs, blue) form at the shoot–root junction. (B) The fibrous monocot root system of Zea mays consisting of an embryonic primary root (PR, red), embryonic seminal roots (SRs, yellow), which originate close to the top of the PR, crown roots (CRs, blue) that originate from the stem, and LR branching (brown) from the PRs, SRs, and CRs. Ultimately, the LRs will undergo higher order branching to form secondary and tertiary LRs.
In later stages of development, LRs themselves undergo branching to form tertiary and subsequently even higher order LRs (Osmont et al., 2007). Although PR and LR development in monocots, such as rice (Oryza sativa) and maize (Zea mays), seems largely similar to their development in dicots, the overall root system architecture (RSA) is more complex in monocots (Fig. 1B). In later developmental stages, a very fibrous root system is built including embryonic seminal roots (SRs) and various post-embryonic LRs. They later dominate the root system of the adult plant and take over most of the water and nutrient uptake (Hochholdinger, 2004) (Fig. 1B). Additionally, plants readily develop adventitious roots (ARs), which are post-embryonic shoot-borne roots. In monocots, they are sometimes referred to as crown roots when formed below the soil surface and as brace roots when formed above the soil surface (Kovar and Kuchenbuch, 1994). In this review we will mainly focus on LRs of PRs and refer the interested readers to other excellent reviews on ARs (Bellini et al., 2014; Ge et al., 2019; Lakehal and Bellini, 2019).
RSA varies widely both between and within plant species. This natural variation underlines the importance of the RSA for plant adaptation to the environment (Cannon, 1949; Lynch, 1995; Osmont et al., 2007; Hodge et al., 2009; McNear, 2013; Rellán-Álvarez et al., 2015). Because various conditions influence RSA by modulating the angle, rate, and type of individual roots, these aspects of RSA are important target traits for plant breeders (de Dorlodot et al., 2007; Orman-Ligeza et al., 2014). LRP development, LR emergence, organ growth, and periodic branching of higher order LRs are the main processes that increase the size of the root system (Hodge et al., 2009; De Smet et al., 2012). Similar to LRP development, the process and environmental regulation of LR emergence through the overlaying tissues has also been extensively reviewed, mainly focusing on Arabidopsis (Nibau et al., 2008; Bennett and Scheres, 2010; Lavenus et al., 2013; Tian et al., 2014; Motte et al., 2019).
Increasing evidence from several model plants suggests that PRs and LRs display distinct growth programmes modulated by unique molecular signals. In this review, we outline the similarities and differences between PR and LR organ growth (elongation) in response to external stimuli, such as nutrients and other environmental cues, as well as developmentally programmed signals, such as phytohormones.
Plant hormones are key players in differential PR and LR growth
Plant hormones coordinate root growth in response to developmental (internal) and environmental (external) cues. Here we mainly focus on auxin, cytokinin (CK), abscisic acid (ABA), brassinosteroids (BRs), ethylene, gibberellin (GA), salicylic acid (SA), and jasmonate (JA), and their specific synthesis and signal transduction pathways. Extensive crosstalk between these hormone signalling pathways defines the development and growth of LRs and PRs. In the following, we will discuss how these hormones and their interactions determine PR and LR growth.
Auxin’s dual impact on root organ growth
In addition to its central role in PR and LR initiation (reviewed in Péret et al., 2009; Overvoorde et al., 2010), auxin plays a role in defining organ growth rates. Auxin is synthesized in the aerial plant organs, mainly in the shoot apex and young developing leaves (Ljung et al., 2001, 2005) and is transported in the vascular cylinder to the root tip. Additionally, auxin is produced in the root (Chen et al., 2014) and this local biosynthesis is crucial for meristematic activity (Brumos et al., 2018). Auxin is here redirected shootward through epidermal and cortical cells. The current model of auxin flux in the root incorporates the polarized localization of various auxin transporters in the root to facilitate this path of auxin flux through the root. Root growth responses interpret fluctuations in auxin input, which can be modulated by auxin synthesis or transport, into cellular or organ elongation outcomes. Here, we will address how cellular growth outcomes modulated by auxin levels differ between PRs and LRs, and how these differential responses may be regulated by changes in auxin transport or cellular elongation.
Very low auxin concentrations promote PR elongation in Arabidopsis and maize (Gougler and Evans, 1981; Pilet, 1981). Conversely, higher concentrations inhibit PR elongation in several plant species (Thimann, 1939; Chadwick and Burg, 1967; Scott, 1972; Pilet, 1981; Pilet and Saugy, 1987; Debi et al., 2003; Rahman et al., 2007). Auxin has been recently shown to inhibit root growth via a non-transcriptional branch of the canonical auxin receptor TRANSPORT INHIBITOR RESPONSE 1 (TIR1) (Fendrych et al., 2018).
Little is known about the direct effects of auxin concentration on cell division and elongation in LRs. When young Arabidopsis seedlings were transferred to medium containing auxin, the elongation of LRs was inhibited (Debi et al., 2003; Ivanchenko et al., 2010; Giehl et al., 2012). Conversely, Muday and Haworth (1994) reported that auxin promotes the elongation of LRs in tomato (Solanum lycopersicum). This could indicate that there are diversified responses to auxin input in LRs of different species.
Phenotypic analysis of auxin-related mutants may provide further insight into the mechanisms by which endogenous modulation of auxin levels affects growth outcomes in PRs and LRs. The so-called aberrant lateral root formation (alf) mutants display a wide range of lateral root phenotypes (Celenza et al., 1995). ALF1/SUR1 (SUPERROOT1) is the C-S lyase in glucosinolate biosynthesis. In constitutively active alf1/sur1 mutants, there is an overproduction of the endogenous auxin indole-3-acetic acid (IAA) (Mikkelsen et al., 2004). Intriguingly, though PR growth is largely normal in the alf1/sur1 mutant, there is substantial excess LR growth (Boerjan et al., 1995; Celenza et al., 1995). Unfortunately, the causal gene for the alf3-1 mutant phenotype has not yet been mapped. Overall, these results suggest that LRs and PRs execute differential growth responses to endogenous auxin levels. Future work is necessary to elucidate the signalling, synthesis, and transport mechanisms responsible for these differential growth outcomes.
Dynamic relocalization of auxin transporters can modulate auxin flux to facilitate alterations in growth. The main plasma membrane-located auxin transporters are the PIN-FORMED (PIN) proteins (Luschnig et al., 1998; Gälweiler et al., 1998), the ARABIDOPSIS THALIANA ATP-BINDING CASSETTE B (ABCB) subfamily transporters (Noh et al., 2001), and the AUXIN RESISTANT1/LIKE AUX1 (AUX1/LAX) uptake permeases (Maher and Martindale, 1980; Okada and Shimura, 1990; Bennett et al., 1996; Parry et al., 2001). PINs redundantly regulate PR growth (Billou et al., 2005; Vieten et al., 2005), but it is unknown whether they have distinct contributions to growth rates in LRs. Notably, while all canonical PINs are expressed throughout PR development, some PIN genes show transient and stage-dependent expression in LRs (Rosquete et al., 2013; Ruiz Rosquete et al., 2018).
In addition to the PIN family of auxin transporters, the ABCBs 1, 4, 14, 15, and 19 are root-expressed intercellular auxin transporters (Cho and Cho, 2013). Notably, ABCB19/MDR1/PGP19 loss of function does not affect PR growth or LRP development (Wu et al., 2007), but strongly impaired elongation rates in emerged LRs. This relates to distinct acropetal auxin transport in PRs and LRs (Wu et al., 2007).
AUX1/LAX proteins are specifically required for the basipetal transport of auxin through the outer root cell layers (Rashotte et al., 2000; Marchant et al., 2002). Most studies did not detect differences in PR elongation of the wild type and the aux1 mutant (Růzicka et al., 2007; Ivanchenko et al., 2010; Lehotai et al., 2012; Thole et al., 2014; Street et al., 2016), but Li et al. (2016) reported increased PR elongation in aux1 mutants, particularly in alkaline stress conditions. AUX1 plays a prominent role in LRP development and LR emergence (Hobbie and Estelle, 1995; Casimiro et al., 2001; Marchant et al., 2002). Little is known about the role of AUX1 in emerged LRs, but LR length in aux1 mutants is similar to that in the wild type (Linkohr et al., 2002; Giehl et al., 2012). These data suggest that AUX1 does not affect elongation in PRs or LRs under normal growth conditions but that it might modulate PR and LR elongation during environmental stress.
In summary, the phytohormone auxin fulfils multiple roles throughout the development and growth of PRs and LRs. While the responsiveness in PRs and LRs to exogenously applied auxin seems similar, current evidence suggests that endogenous transport and the nuclear signalling rate of auxin are distinct in PRs and LRs. Further work is necessary to better understand the differences in auxin responsiveness of PRs and LRs and how these may be modulated by distinct transport, nuclear signalling, and cell physiology regulatory mechanisms. Moreover, it remains unclear whether cellular auxin responses (e.g. meristematic activity and elongation) are conserved among PRs and LRs.
Cytokinin sensitivity depends on the developmental stage of lateral roots
CK and auxin interact both synergistically and antagonistically during plant development. Like auxin, exogenously applied CK inhibits organ growth of the PR in Arabidopsis (Werner et al., 2001; Werner, 2003; Mason, 2004; Li et al., 2006; Laplaze et al., 2007; Šimášková et al., 2015), maize (Márquez, 2019), and rice (Kudo et al., 2012). Mechanistically, CK negatively regulates quiescence centre (QC) specification (Zhang et al., 2013) and reduces the number of cells in the meristem (Beemster and Baskin, 2000; dello Ioio et al., 2008). The QC is a small group of rarely dividing cells promoting the stem cell status in the root and hence has central importance for providing various cell types (van den Berg et al., 1997; Drisch and Stahl, 2015). CK interferes with auxin signalling in the transition zone, thereby regulating the onset of cellular division, decreasing meristem size (Beemster and Baskin, 2000; Werner, 2003; Li et al., 2006; Šimášková et al., 2015; Di Mambro et al., 2017). Similarly, several publications report that the elongation of LRs is inhibited by CK in Arabidopsis (Werner et al., 2001; Li et al., 2006; Miyawaki et al., 2006), but the cellular mechanism largely remains to be addressed. Additionally, exogenous application or endogenous increase in CK asymmetrically reduces cell proliferation in the meristematic zone and inhibits cell elongation in the elongation zone, thereby determining angular growth in young LRs (Waidmann et al., 2019). However, the interaction between CK and auxin signalling in this response, and whether it mirrors the interaction that occurs in PR growth regulation, remains to be elucidated. Intriguingly, a recent study in maize (Márquez, 2019) showed that the CK effect on LR growth is concentration and tissue dependent, revealing that low CK levels only reduced LR length in the most apical/proximal region of the root system, while at high concentrations CK inhibits the LR elongation along the entire root system but with a more pronounced effect in the apical zones (Márquez, 2019). Hence, the impact of CK on the elongation of LRs could be distinct in developmentally younger and older lateral roots.
It would be interesting to assess how CK signalling, metabolism, transport, and/or distribution mechanisms are distinct in PRs and LRs. In addition, the possibly distinct contributions of environmental stimuli to CK signalling in PRs and LRs remain to be addressed.
Lateral roots are highly sensitive to abscisic acid
Though ABA is mainly known for its role in seed germination and responses to water shortage (Yoshida et al., 2019), it also regualtes root growth. High ABA concentrations reduce PR length in Arabidopsis (Chen et al., 2012; Duan et al., 2013; Thole et al., 2014), medicago (Liang and Harris, 2005; Liang et al., 2007), maize (Pilet and Saugy, 1987), and rice (Tseng et al., 2013; Liu et al., 2015). In Arabidopsis, some evidence suggests that the effect of ABA on PR length is dose dependent (Ephritikhine et al., 1999; De Smet et al., 2003; Barberon et al., 2016). High ABA concentrations act via an ethylene-dependent pathway to inhibit cell division in the QC and the proximal part of the Arabidopsis root meristems (Newton, 1977; Robertson et al., 1990; De Smet et al., 2003; Zhang et al., 2010) by suppressing transcription of cell cycle genes such as CYCLIND3;1 (CYCD3;1) and CYCLIN-DEPENDENT KINASE B1;1 (CDKB1;1) (De Smet et al., 2003). On the other hand, low ABA concentrations promote root growth via an ethylene-independent pathway that requires auxin signalling and PIN2-dependent auxin transport (Li et al., 2017). Additionally, low ABA concentrations act by both promoting QC quiescence and suppressing the differentiation of stem cells and their daughters (Nishimura et al., 2007).
The growth responses of LRs are more sensitive to ABA signalling than those of PRs. Low concentrations of ABA show a quantitatively stronger impact on LR elongation when compared with the PR (De Smet et al., 2003; Xiong et al., 2006; Liang et al., 2007; Chen et al., 2012; Duan et al., 2013). It is unknown why LRs are highly sensitive to ABA, but it probably involves, like in the PR, the down-regulation of cell cycle genes, ultimately arresting the root meristem (De Smet et al., 2003). However, even in the case of persistent stress, LR growth eventually recovers from the ABA-induced inhibition (Zhao et al., 2014).
These data suggest that RSA can be fine-tuned in response to fluctuations in ABA levels. Specifically, increased LR sensitivity to low levels of ABA signalling may allow restructuring of the root system to promote larger root systems that can better access soil water stores during drought conditions.
Sensitivity to brassinosteroid, ethylene, gibberellin, salicylic acid, and jasmonate is distinct in lateral and primary roots
Root growth in response to endogenous and environmental cues is regulated by a diverse array of hormones in addition to auxin, CK, and ABA. Several of these hormones, like auxin, have concentration-dependent effects on PR elongation. In Arabidopsis, low concentrations of either BRs or SA promote PR elongation, while increased concentrations inhibit it. For BRs, this effect is conserved between several plant species (Roddick et al., 1993; Clouse et al., 1996; Müssig et al., 2003; Kartal et al., 2009). However, the effect of SA concentration is reversed in maize, rice (Kusumi et al., 2006), soybean (Gutiérrez-Coronado et al., 1998), Pinus patula (San-Miguel et al., 2003), and Catharanthus roseus (Echevarría-Machado et al., 2007); low SA concentrations induce PR growth.
These hormones probably act by modulating the balance between cell elongation and cell division in the meristem. BR maintains this balance and preserves QC identity by inhibiting or promoting differentiation of distal columella stem cells in a concentration-dependent manner (González-García et al., 2011; Hacham et al., 2011; Fàbregas et al., 2013). Interestingly, in the transition–elongation zone of the root, BRs promote cell elongation, while in the QC and surrounding cells, BR inhibits cell division (Chaiwanon and Wang, 2015; Vragović et al., 2015). Low SA has a similar effect with a distinct mechanism; at concentrations below 50 µM, SA induces distal meristem enlargement by affecting auxin transport proteins, leading to an auxin accumulation in the PR (Pasternak et al., 2019). On the other hand, high SA concentrations inhibit cell proliferation and elongation in the PR (Pasternak et al., 2019).
Unfortunately, little is known about the role of BRs or SA on LR elongation. Only a few studies showed that BRs can enhance the elongation of LRs (Kartal et al., 2009; Vercruyssen et al., 2011). Whether BRs have the same dual function in the meristem of LRs as in PRs and if the response pathway works similarly in both root types remains unknown. Interestingly, application of SA in femtomolar concentrations increased root biomass due to enhanced LR growth in C. roseus (Echevarría-Machado et al., 2007). Together with the enhanced growth of ARs, SA could promote the growth of a shallower root system, helping the plant further explore the surrounding soil. Further work will be necessary to examine how the dual effects of BRs and SA influence physiological responses to local soil conditions.
Current studies have not detected concentration-dependent effects of ethylene, JA, or GAs on PR elongation. Ethylene and JAs both negatively regulate PR growth, while GA enhances it.
First, application of ethylene or its precursor ACC (1-aminocyclopropane-1-carboxylic acid) inhibits the elongation of PRs in monocots and dicots (Le et al., 2001; Swarup et al., 2005; Růzicka et al., 2007; Ma et al., 2013; Li et al., 2015; Barberon et al., 2016; Qin et al., 2017). Ethylene and ACC negatively regulate root meristem size and overall PR length in an auxin-dependent manner (Růzicka et al., 2007; Alarcón et al., 2014; Street et al., 2015; Chen et al., 2018). Interestingly, an additional auxin-independent pathway inhibits cellular elongation of epidermal cells in the PR at low ethylene concentrations (Růzicka et al., 2007; Alarcón et al., 2014). Similarly, JAs inhibit the growth of the PR in Arabidopsis (Staswick et al., 1992; Thines et al., 2007; Chung and Howe, 2009; Mosblech et al., 2011; Shyu et al., 2012; Monte et al., 2014; Thatcher et al., 2016), rice (Hazman et al., 2015), tomato (Tung et al., 1996), and sunflower (Corti Monzón et al., 2011) by inhibiting cell division and elongation (Tung et al., 1996; Raya-González et al., 2012; Gasperini et al., 2015).
Very few studies addressed the influence of ethylene on LR organ growth. Although ethylene responses in the PR and LR appear similar (Tian et al., 2009; Ramaiah et al., 2014), an ethylene-sensitive reporter line suggests different molecular responses in PRs and LRs under high nitrate conditions (Tian et al., 2009), enabling a specific response to environmental cues. On the other hand, JAs have an intriguing dual effect on LR elongation; in Arabidopsis and sunflower LRs, low JA promotes, while high JA inhibits elongation (Corti Monzón et al., 2011; Raya-González et al., 2012). Further work is necessary to understand the mechanism of this dual response to JAs.
While exogenous application of ethylene has no obvious effect on root elongation (Tanimoto, 1987, 1988, 1994; Butcher et al., 1990; Du et al., 2017), application of the GA inhibitor paclobutrazol (PAC) resulted in a reduced PR growth rate (Ubeda-Tomás et al., 2009), similar to GA-deficient mutants in Arabidopsis (Fu and Harberd, 2003; Ubeda-Tomás et al., 2009; Achard et al., 2009). These results suggest that, GAs act in very low concentrations in the root to establish the growth-relevant arrangements of cortical microtubules and cell polarity (Baluška et al., 1993). GA is important for controlling the size of the meristem and mature cell length by promoting mitotic activity in the root meristem (Ubeda-Tomás et al., 2009; Achard et al., 2009).
While nothing is known about the effects of GAs on LR elongation in Arabidopsis, in Populus GA induces a stronger inhibition of LR organ growth when compared with the PR, presumably allowing faster stress responses in LRs (Gou et al., 2010). GA signalling may enable integration of aerial and root development, reducing aerial plant growth and promoting the exploration of the root system under unfavourable growth conditions (Gou et al., 2010).
In summary, the hormone effects on elongation via modulation of cell division and differentiation are more frequently understood in PRs than in LRs. Future work should examine whether the growth response to each hormone is shared between LRs and PRs across species and identify the mechanisms by which the responses are conserved or diverged. In particular, close examination of the concentration-dependent dual effects of hormones may reveal mechanisms by which PR and LR growth are tailored responses to environmental stressors.
Root responses to environmental cues
Mineral nutrients are crucial for all aspects of plant growth and development. In most plant species, these nutrients are almost solely recruited from the soil. Most nutrients are not uniformly distributed throughout different soil layers, but rather form vertical and horizontal gradients (Jackson and Caldwell, 1993; Farley and Fitter, 1999; Lark et al., 2004). In addition, several nutrients display higher mobility in the soil than others (Bray, 1954; Thomas, 1970). Hence, for efficient nutrient mining, plants need to modulate RSA in response to local soil conditions. Additionally, the structure and compactness of the soil can influence the RSA of plants (reviewed in Nawaz et al., 2012). In the following subsections, we first discuss how LR and PR growth is regulated in response to fluctuations in nutrient availability. Then, we highlight how osmotic stress, temperature, light, and gravity define LR expansion and how this may differ from the PR.
Nitrogen sources prominently define growth of lateral roots
The availability of nitrogen (N) to produce amino acids and proteins is one of the key requirements for growth and plays a major role in defining plant productivity. N is primarily taken up through the root system in its inorganic (ammonium and nitrate) forms but can also be taken up in its organic (urea, amino acids, peptides) forms. In several plant species, different responses to nitrate and ammonium have been described.
Ammonium inhibits the growth of PRs in several plant species (Tian et al., 2009; Rogato et al., 2010; Liu et al., 2013), while LR elongation and branching is stimulated by increased local supply of ammonium in barley (Hordeum vulgare) and Arabidopsis (Drew, 1975; Lima et al., 2010; Rogato et al., 2010). This effect is most probably due to the increased expression of the AMMONIUM TRANSPROTER (AMT) in LRs, which provides enhanced transport capacities (Yuan et al., 2007; Lima et al., 2010).
In contrast to ammonium, the response of roots to nitrate is more complex. High local concentrations of nitrate inhibit the elongation of LRs but have no effect on the growth of PRs (Zhang and Forde, 1998; Zhang et al., 1999; Tian et al., 2009). Conversely, low local nitrate concentrations (>1 mM) show strong stimulatory effects on LR elongation (Zhang and Forde, 1998; Zhang et al., 1999; Linkohr et al., 2002; Saito et al., 2014) and again have little to no influence on the length of the PR (Zhang and Forde, 1998). On the other hand, l-glutamate, another source of N, inhibits the growth of PRs and stimulates the elongation of LRs (Walch-Liu et al., 2006). These data suggest that local nitrate levels regulate LR elongation, allowing dynamic RSA regulation in response to soil nitrate availability.
The different responses of PRs and LRs in nitrate excess and deficiency is most probably due to crosstalk between nitrate transporters and downstream effects on hormone signalling. NITRATE TRANSPORTER/SENSOR 1.1 (NRT1.1), but not NRT1.2, is also able to transport auxin and has been shown to transport auxin to the tip of LRs under low N availability to promote growth (Krouk et al., 2010; Mounier et al., 2014). Moreover, high levels of nitrate promote the expression of CLE peptides that activate CLAVATA1 (CLV1) signalling, blocking the elongation of LRs and inducing the expression of NRT1.1 and NRT1.2 but having no effect on PRs (Araya et al., 2014). In addition, expression of the AGAMOUSE-LIKE21 (AGL21) transcription factor is induced under low nitrate conditions, modulating downstream expression of auxin biosynthesis genes to stimulate LR but not PR elongation (Yu et al., 2014). BR signalling regulates PR growth responses to low N availability (Jia et al., 2019), but a contribution to LR organ growth was not assessed.
In summary, low local concentrations of N sources promote growth and branching of LRs. This local response is likely to enable the plant to mine more N resources in the soil, tailoring growth in response to N availability. Intriguingly, different N sources elicit opposite LR growth responses: ammonium stimulates LR branching and elongation, while high nitrate causes inhibition. This suggests that root growth may be fine-tuned to the exact local N make-up. Future work examining the molecular and hormone crosstalk signals that regulate these opposite LR growth responses may uncover physiological mechanisms by which the local N composition modulates RSA. Additionally, future work should examine how local N make-up might regulate RSA by determining LR angle. These questions are particularly relevant because it has been shown that plants with a steep and deep root system perform better under low N conditions (Lynch, 2013; Zhan and Lynch, 2015; Colombi and Walter, 2017; Roychoudhry et al., 2017); thus, understanding the molecular control of LR growth by local N sources would inform development of agricultural technology to facilitate robust growth responses to N deficiency.
Phosphate sensitivity of lateral roots is age dependent
Inorganic phosphate (Pi) is a major component of nucleic acids, phospholipids, ATP, and other molecules, and is therefore crucial for plant growth and development. Because phosphate is the only source of phosphorus in the soil, RSA is highly responsive to phosphate starvation. Pi is immobile in soil, resulting in an uneven distribution and low accessibility by plant roots. Additionally, Pi can be bound by soil components or converted to organic phosphate (Yevdokimov et al., 2016), further reducing the amount of plant-available Pi in the soil (Johnston et al., 2014).
The response of roots to low Pi varies strongly between different species and genotypes. Numerous studies have shown a reduction in PR growth in the Arabidopsis Columbia (Col-0) accession during Pi starvation (Williamson et al., 2001; Linkohr et al., 2002; López-Bucio et al., 2002; Sánchez-Calderón et al., 2005, 2006). However, several studies using other Arabidopsis accessions suggested the existence of several diversified strategies in response to low phosphate (Chevalier et al., 2003; Reymond et al., 2006; Kawa et al., 2016). Using quantitative trait locus (QTL) mapping or a genome-wide association study (GWAS), several putative gene candidates were identified, which may control PR growth under low Pi conditions (Reymond et al., 2006; Kawa et al., 2016). In agreement with the genotype-specific responses, the sensitivity of PR growth to low Pi seems to be highly species specific. For example, PRs of rice (O. sativa) show enhanced growth under Pi-deficient conditions (Shimizu et al., 2004; Yi et al., 2005), whereas in maize little to no enhancement was observed (Mollier and Pellerin, 1999; D. Wang et al., 2017; Jia et al., 2018).
Interestingly, the PR response to low Pi is linked to excessive iron accumulation that occurs in these conditions (Svistoonoff et al., 2007). Under Pi starvation, roots secrete protons or organic acids that help to release anionic phosphate from cations in the soil, thereby also releasing and increasing the uptake of iron (Shahbaz et al., 2006; Müller et al., 2015; Balzergue et al., 2017; Mora-Macías et al., 2017). This disrupts the ratio of phosphate to iron in the root and causes apoplastic acidification, leading to peroxidase-dependent cell wall stiffening as well as accumulation of callose in the elongation zone (Balzergue et al., 2017). This results in a reduced cell elongation rate followed by a progressive loss of meristematic cells (Ticconi et al., 2004; Sánchez-Calderón et al., 2006; Lai et al., 2007; Svistoonoff et al., 2007). Other results suggest that accumulation of reactive oxygen species (ROS) under low Pi reduces PR length via cell death in the meristem (Pich et al., 1994; Kampfenkel et al., 1995; Caro and Puntarulo, 1996; Liszkay et al., 2003; Chacón-López et al., 2011). Furthermore, an intensive crosstalk between Pi and aluminium (Al) in plants can influence plant growth and metabolism (Clarkson, 1967). Accordingly, the levels of Pi can ameliorate the inhibitory effects of Al toxicity on root growth (Tan and Keltjens, 1990; Liao et al., 2006; Sun et al., 2008). Pi and Al sensing might use some common signalling mechanism to activate the response to both Pi deficiency and Al toxicity (Belal et al., 2015; Dong et al., 2017). Further studies are needed to better understand how the balance between Pi and metal levels in the meristem affects growth in PRs.
LRs play an important role in the acquisition of Pi in the soil by increasing the root system surface and supporting the solubilization of bound Pi (Lynch, 2007; Pérez-Torres et al., 2008; Kanno et al., 2016). A common response to low Pi of plants is an increase in LR elongation rates (Drew, 1975; Bonser et al., 1996; Williamson et al., 2001; Linkohr et al., 2002; Al-Ghazi et al., 2003; Dai et al., 2012). Under low Pi, dicots such as Arabidopsis and bean (Phaseolus vulgaris), as well as monocots such as barley, rice, and maize display increased branching to form tertiary and higher order LRs (Drew, 1975; Bonser et al., 1996; Ticconi et al., 2004; Negi et al., 2016; Jia et al., 2018). Interestingly, in Arabidopsis, the Pi starvation response is dependent on the age of LRs: in young LRs, the elongation is promoted under low Pi, whereas older LRs respond to Pi deficiency with reduced elongation (Nacry et al., 2005; Sánchez-Calderón et al., 2006). It was suggested that different responses of young and old LRs to low Pi result from a redistribution of auxin in the root system (Nacry et al., 2005); however, the exact mechanism remains elusive. The age-dependent response to Pi is somewhat reminiscent of the positional CK responses in LRs, but its potential interactions need to be assessed. Additionally, low Pi alters LR orientation in Arabidopsis towards a more vertical growth (Bai et al., 2013). Altogether, the reduction of PR elongation and the change in elongation and growth direction of LRs result in a shallower RSA in response to phosphate starvation.
Double mutants of the PHOSPHATE TRANSPORTER 1;1 (PHT1;1) and PHT1;4 showed a faster LR elongation, but slower PR growth under low Pi (Shin et al., 2004), suggesting a model in which the growth responses of LRs and PRs to phosphate deficiency are controlled by distinct pathways. This is supported by the observation that double mutants of the PHOSPHOLIPASE Dζ1 and 2 display enhanced elongation of the PRs and inhibition of the LRs under Pi starvation (Li, 2006). Further work is necessary to better understand how these transcription factors regulate RSA by transcriptional responses to Pi.
Finally, recent work in maize suggests that auxin and BR might play a role in Pi starvation. Altering the levels of the Arabidopsis homologue SERINE/THREONINE PROTEIN PHOSPHATASE 2A (ZmPP2AA1) 1 can change the response of PRs and LRs under phosphate starvation (J. Wang et al., 2017). PP2A is known to control the transport of auxin in Arabidopsis (Rashotte et al., 2001). Additionally, BR signalling has been shown to integrate low Pi and low iron signals to modulate PR growth responses (Singh et al., 2018).These mechanisms should also be investigated in LRs to determine how the balance of Pi and iron regulates LR growth.
Overall, current data suggest that phosphate deficiency alters RSA by decreasing PR elongation and promoting LR elongation through transcriptional, hormonal, and developmental responses. The details of these mechanisms and the interactions between them remain to be elucidated.
Nutrient availability distinctly alters developmental programmes in PRs and LRs
In addition to N and phosphate, the availability of other nutrients influences RSA. In Arabidopsis, sulfur starvation reduces the PR length but leads to slightly longer LRs. However, under prolonged sulfur deficiency, LR growth is also reduced (Gruber et al., 2013). Plants with calcium deficiency produce a very shallow and highly branched root system in which the length of the PR is decreased and the elongation rate of LRs is increased (Gruber et al., 2013). Potassium, manganese, and magnesium deficiency in the soil reduced the length of both PRs and LRs (Gruber et al., 2013; Kellermeier et al., 2014). Boron-deficient plants show reduced PR length, while LR growth is resistant to low boron in the soil (Gruber et al., 2013). Conversely, excess boron leads to boron toxicity, resulting in short PRs and LRs. (Aquea et al., 2012). Finally, mild iron deficiency elicits the opposite growth response, stimulating a slight increase in PR and LR length (Giehl et al., 2012; Lešková et al., 2017). On the other hand, a strong deficiency distinctly reduces the length of the LRs, but has no influence on the elongation of the PR (Lešková et al., 2017). Overall, the accumulating evidence suggests that deficiencies in specific nutrients initiate distinct developmental programmes in PRs and LRs. Future work should address the interaction between these nutrient deficiencies and the transcriptional, hormonal, and cellular mechanisms through which they operate.
The root system is altered in response to osmotic stress
Osmotic stress is here referred to as situations in which water availability for plants is limited, resulting in growth and developmental defects. Such conditions can arise from drought, excessive salt concentrations, chilling, and freezing. When water becomes limited, root systems explore the soil to reach deeper soil layers. A mild water stress can enhance PR growth in Populus, Lespedeza davurica (L.), several oak (Quercus) species, Arabidopsis, and soybean (Glycine max) (Van Der Weele et al., 2000; Bogeat-Triboulot et al., 2007; Yamaguchi et al., 2010; Arend et al., 2011; Kuster et al., 2012; Xu et al., 2012) and deep-rooted genotypes generally grew better under drought stress (Ho et al., 2005). Vicia faba (bean) accessions from drier regions have a larger root system than those from well-irrigated, wet regions (Belachew et al., 2018). Moreover, it has been shown that the PR tip can grow towards wetter soil and away from high osmolarity (Takahashi et al., 2002), a phenomenon described as hydrotropism. Likewise, it has been shown in rice and Arabidopsis that the expression of the DEEPER ROOTING 1 (DRO1)/NEGATIVE GRAVITROPIC RESPONSE OF ROOTS 2 (NGR2)/LAZY 4 (LZY4) gene leads to plants with a steeper root system, thereby enhancing drought tolerance (Uga et al., 2013, 2015; Yoshihara and Spalding, 2017). The exact mechanism whereby roots sense drought and initiate the subsequent downstream signalling is not fully understood. However, it is known that ABA plays an important role in drought-responsive signalling; several studies demonstrated that drought conditions induce ABA production in roots. Interestingly, young PRs accumulate more ABA then older ones (Zhang and Davies, 1989; Zhang and Tardieu, 1996). ABA accumulation alters polar auxin transport rates in the root tip to stimulate cell elongation (Xu et al., 2012; Rowe et al., 2016; T. Wang et al., 2017), suggesting a possible link between drought-induced ABA signalling and changes in auxin that are jointly integrated into RSA remodelling.
In Arabidopsis, elongation of LRs is significantly inhibited by drought (Xiong et al., 2006), though the molecular mechanism of this response remains unclear. Genetic screens have identified the so-called drought inhibition of lateral root growth (dig) mutants with drought-resistant LR elongation (Xiong et al., 2006), but the underlying gene remains to be revealed. Recent work suggests that the MYB96 transcription factor mediates an ABA–auxin crosstalk response specifically in LRs that are exposed to drought stress (Seo et al., 2009).
Hyperosmotic conditions in the soil due to high concentrations of Na+ and other ions can also prevent plant absorption of water and nutrients (Ismail et al., 2014). In Arabidopsis, PR and LR growth is reduced by high salinity, although the elongation of LRs shows stronger inhibition (Burssens et al., 2000; Zolla et al., 2010; Duan et al., 2013; Kawa et al., 2016). Similar to the response to drought stress, high salinity activates the ABA response pathway (Jia et al., 2018), though during salt stress it may be restricted specifically to the endodermis of LRs (Duan et al., 2013). In summary, osmotic stress operates through hormone signalling to modulate PR elongation and growth direction as well as LR growth. Future work should examine the role of osmotic stress in defining RSA via modulation of LR growth direction.
Temperature responses in main and lateral roots are distinct
The growth, development, and productivity of plants are strongly influenced by the ambient temperature. Each plant species has a specific temperature range with optimal growth behaviour (Hatfield et al., 2011; Hatfield and Prueger, 2015), and temperature alterations, particularly strong increases, can have dramatic consequences for plant survival. In most plant species, the rate of PR elongation increases linearly with rising temperature until a certain, species-specific threshold is reached, at which PR growth rate falls sharply as temperature is further increased (Al-ani and Hay, 1983; Sattelmacher et al., 1990; Seiler, 1998; Nagel et al., 2009). Several studies suggest that high temperature affects the PR growth in an auxin-dependent (Hanzawa et al., 2013; Wang et al., 2015; Fei et al., 2017; Feraru et al., 2019) and BR-dependent manner (Martins et al., 2017; Sun et al., 2019, Preprint). In contrast to PRs, LRs show a very wide range of different responses to heat across species. For example, LRs of rapeseed [Brassica napus (L.)] do not show any growth differences when grown under increased temperature (Nagel et al., 2009). In sunflower (Helianthus annuus), temperatures below 20 °C and above 35 °C favour PR growth, while temperatures in the 20–35 °C range favour LR growth (Seiler, 1998). In potato (Solanum tuberosum), PR growth at high temperatures was considerably reduced compared with LR growth (Sattelmacher et al., 1990). Unfortunately, mechanistic insights explaining the distinct responses in PRs and LRs are not yet available.
Light modulates the root system architecture
Altering growth in response to light is key for plant survival. In addition to modulating shoot and flower development, light affects root morphology. Light inhibits PR growth in several mono- and dicotyledon plant species (Burström, 1960; Ohno, 1967; Wain et al., 1968; Wilkins and Wain, 1974; Pilet and Ney, 1978) by reducing cell division activity and inhibiting cellular elongation (Ohno, 1967). This effect is dependent on the intensity and wavelength of the light (Ohno, 1967; Shen-miller, 1978). In Arabidopsis, the transcription factor LONG HYPOCOTYL5 (HY5) promotes the expression of negative regulators of auxin signalling, thereby linking hormone and light signalling pathways (Cluis et al., 2004).
Comparably less is known about the influence of light on the growth of LRs. In Arabidopsis, the LRs of light-grown seedlings are longer when compared with dark-grown seedlings (Moni et al., 2015). This effect seems to be dependent on the blue light receptor PHOTOTROPIN-1 (PHOT1) because LRs of phot1 mutants displayed no difference under these conditions (Moni et al., 2015). The authors suggest that PHOT1 plays a role in the elongation of LRs through the control of an auxin-related signalling pathway (Moni et al., 2015). Future work should address how hormone signalling pathways alter developmental programmes of PRs and LRs in response to light.
Main and lateral roots display distinct gravitropic set point angles
After germination, shoots must orient upwards to access light, while roots grow downward into the soil to access water and nutrients. PRs have an adaptive mechanism to resume downward growth should their orientation relative to the gravity vector be disturbed. After reorientation, starch-filled statoliths sediment to the lower side of columella cells and initiate a signal transduction pathway which harnesses second messengers such as inositol 1,4,5-triphosphate (IP3), Ca2+, and H+ (Su et al., 2017). Through an as yet uncharacterized mechanism, statolith sedimentation and subsequent signalling result in the polarization of PIN-dependent auxin transport to the lower side of the root (Leitz et al., 2009; Baldwin et al., 2013). This process leads to higher auxin accumulation on the lower root flank, resulting in reduced growth on this side and bending towards gravity (Friml et al., 2002; Kleine-Vehn et al., 2010; Ogura et al., 2019).
In contrast to PRs, LRs partially suppress positive gravitropic growth and maintain a gravitropic set point angle (GSA) that allows radial expansion of the root system (plagiotropism) (Digby and Firn, 1995). The hormonal signals modulating asymmetric growth in LRs have been recently elucidated. TIR1/AFB-Aux/IAA-ARF-dependent auxin signalling within the gravity-sensing cells is necessary to establish a GSA in the LRs (Roychoudhry et al., 2013). Like PRs, LRs develop columella cells and amyloplasts in the root tips (Kiss et al., 2002). In recently emerged (so-called stage I) LRs, PIN4 and PIN7 are strongly repressed when compared with the PR, and only PIN3 is transiently expressed in columella cells, presumably limiting the strength of auxin redistribution in (stage II) LRs that are establishing their GSA (Rosquete et al., 2013; Ruiz Rosquete et al., 2018). The subsequent repression of PIN3 after GSA establishment coincides with symmetric auxin signalling at the LR organ flank and symmetric elongation, maintaining the primary GSA (Rosquete et al., 2013). While auxin promotes bending towards gravity during GSA establishment, CK functions as an LR-specific anti-gravitropic component by reducing cellular elongation and proliferation in the upper organ flank of emerged stage II LRs. In this way, CK attenuates LR bending towards gravity and promotes the radial expansion of the root system (Waidmann et al., 2019).
Though hormonal mechanisms tuning angular LR growth have been recently explored, many open questions remain about how developmental and environmental signals modulate hormone signals and transcriptional cascades to modulate angular LR growth. In particular, it will be crucial to uncover how these mechanisms interact to produce distinct directional growth responses in PRs and LRs.
Conclusion
RSA is highly dynamic, responding to external and endogenous cues to adapt to constantly fluctuating conditions (Fig. 2). The mechanisms that control PR and LR growth have been mainly studied separately, reinforcing the incorrect assumption that the mechanisms regulating PR and LR growth are nearly identical. Thanks to the effort of a rapidly growing root community, it becomes increasingly clear that PRs and LRs share common regulatory mechanisms, which are distinctly and combinatorially interpreted to enable distinct growth behaviours. In particular, environmental cues initiate unique growth outcomes in LRs and PRs. This enables plants to dynamically modify RSA to explore the soil and find nutrient-rich patches or reach deeper, water-bearing soil formations.
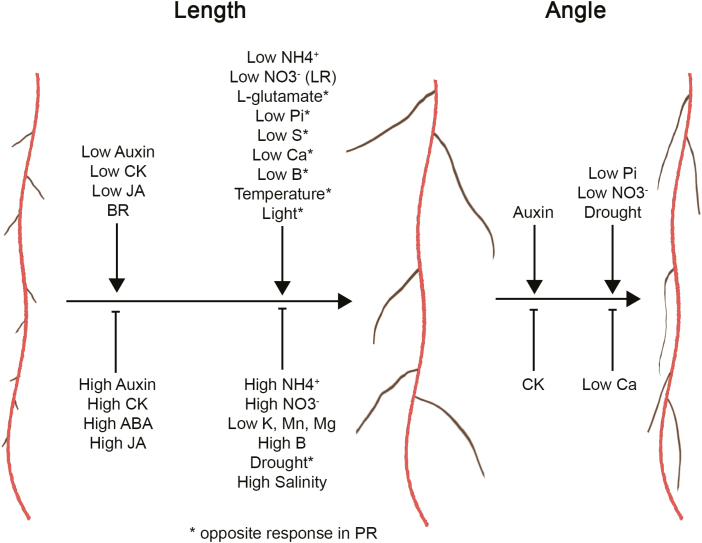
Fig. 2.
Many endogenous and exogenous signals alter lateral root (LR) development in Arabidopsis. Various external and internal stimuli can influence the length and angle of LRs. The asterisks indicate an opposite response in PRs.
Most of the here described responses are often dependent on the developmental context of the root organ. While this may be more apparent when studying LRs, most studies on PRs concentrate on a single time point. Hence, a better understanding of age-dependent responses in primary and lateral roots is needed to substantiate which of the responses of PRs and LRs underlie truly distinct developmental programmes. A challenging step will be to understand the transcriptional and post-translational mechanisms that define the distinct nature of PRs and LRs because they are likely to be key to understanding the distinct hormonal, physiological, and developmental outputs.
Acknowledgements
We apologize to authors whose work we did not cover or cite in this review. This work was supported by the Vienna Research Group (VRG) program of the Vienna Science and Technology Fund (WWTF) (to JK-V), the Austrian Science Fund (FWF) (P29754) (to JK-V), and the European Research Council (ERC) (Starting Grant 639478-AuxinER) (to JK-V).
References
- Achard P, Gusti A, Cheminant S, Alioua M, Dhondt S, Coppens F, Beemster GTS, Genschik P. 2009. Gibberellin signaling controls cell proliferation rate in Arabidopsis. Current Biology 19, 1188–1193. [DOI] [PubMed] [Google Scholar]
- Al-ani MKA, Hay RKM. 1983. The influence of growing temperature on the growth and morphology of cereal seedling root systems. Journal of Experimental Botany 34, 1720–1730. [Google Scholar]
- Alarcón MV, Lloret PG, Salguero J. 2014. Synergistic action of auxin and ethylene on root elongation inhibition is caused by a reduction of epidermal cell length. Plant Signaling & Behavior 9, e28361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Ghazi Y, Muller B, Pinloche S, Tranbarger TJ, Nacry P, Rossignol M, Tardieu F, Doumas P. 2003. Temporal responses of Arabidopsis root architecture to phosphate starvation: evidence for the involvement of auxin signalling. Plant, Cell & Environment 26, 1053–1066. [Google Scholar]
- Aquea F, Federici F, Moscoso C, Vega A, Jullian P, Haseloff J, Arce-Johnson P. 2012. A molecular framework for the inhibition of Arabidopsis root growth in response to boron toxicity. Plant, Cell & Environment 35, 719–734. [DOI] [PubMed] [Google Scholar]
- Araya T, Miyamoto M, Wibowo J, Suzuki A, Kojima S, Tsuchiya YN, Sawa S, Fukuda H, von Wirén N, Takahashi H. 2014. CLE–CLAVATA1 peptide–receptor signaling module regulates the expansion of plant root systems in a nitrogen-dependent manner. Proceedings of the National Academy of Sciences, USA 111, 2029–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arend M, Kuster T, Günthardt-Goerg MS, Dobbertin M. 2011. Provenance-specific growth responses to drought and air warming in three European oak species (Quercus robur, Q. petraea and Q. pubescens). Tree Physiology 31, 287–297. [DOI] [PubMed] [Google Scholar]
- Bai H, Murali B, Barber K, Wolverton C. 2013. Low phosphate alters lateral root setpoint angle and gravitropism. American Journal of Botany 100, 175–182. [DOI] [PubMed] [Google Scholar]
- Baldwin KL, Strohm AK, Masson PH. 2013. Gravity sensing and signal transduction in vascular plant primary roots. American Journal of Botany 100, 126–142. [DOI] [PubMed] [Google Scholar]
- Baluška F, Parker JS, Barlow PW. 1993. A role for gibberellic acid in orienting microtubules and regulating cell growth polarity in the maize root cortex. Planta 191, 149–157. [Google Scholar]
- Balzergue C, Dartevelle T, Godon C, et al. 2017. Low phosphate activates STOP1-ALMT1 to rapidly inhibit root cell elongation. Nature Communications 8, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberon M, Vermeer JEM, De Bellis D, et al. 2016. Adaptation of root function by nutrient-induced plasticity of endodermal differentiation. Cell 164, 447–459. [DOI] [PubMed] [Google Scholar]
- Beemster GT, Baskin TI. 2000. Stunted plant 1 mediates effects of cytokinin, but not of auxin, on cell division and expansion in the root of Arabidopsis. Plant Physiology 124, 1718–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belachew KY, Nagel KA, Fiorani F, Stoddard FL. 2018. Diversity in root growth responses to moisture deficit in young faba bean (Vicia faba L.) plants. PeerJ 6, e4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belal R, Tang R, Li Y, Mabrouk Y, Badr E, Luan S. 2015. An ABC transporter complex encoded by aluminum sensitive 3 and NAP3 is required for phosphate deficiency responses in Arabidopsis. Biochemical and Biophysical Research Communications 463, 18–23. [DOI] [PubMed] [Google Scholar]
- Bellini C, Pacurar DI, Perrone I. 2014. Adventitious roots and lateral roots: similarities and differences. Annual Review of Plant Biology 65, 639–666. [DOI] [PubMed] [Google Scholar]
- Bennett MJ, Marchant A, Green HG, May ST, Ward SP, Millner PA, Walker AR, Schulz B, Feldmann KA. 1996. Arabidopsis AUX1 gene: a permease-like regulator of root gravitropism. Science 273, 948–950. [DOI] [PubMed] [Google Scholar]
- Bennett T, Scheres B. 2010. Root development—two meristems for the price of one? Current Topics in Developmental Biology 91, 67–102. [DOI] [PubMed] [Google Scholar]
- Billou I, Xu J, Wildwater M, et al. 2005. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433, 39–44. [DOI] [PubMed] [Google Scholar]
- Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inzé D. 1995. Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. The Plant Cell 7, 1405–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogeat-Triboulot MB, Brosché M, Renaut J, et al. 2007. Gradual soil water depletion results in reversible changes of gene expression, protein profiles, ecophysiology, and growth performance in Populus euphratica, a poplar growing in arid regions. Plant Physiology 143, 876–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonser AM, Lynch J, Snapp S. 1996. Effect of phosphorus deficiency on growth angle of basal roots in Phaseolus vulgaris. New Phytologist 132, 281–288. [DOI] [PubMed] [Google Scholar]
- Bray RH. 1954. A nutrient mobility concept of soil–plant relationships. Soil Science 78, 9–22. [Google Scholar]
- Brumos J, Robles LM, Yun J, Vu TC, Jackson S, Alonso JM, Stepanova AN. 2018. Local auxin biosynthesis is a key regulator of plant development. Developmental Cell 47, 306–318.e5. [DOI] [PubMed] [Google Scholar]
- Burssens S, Himanen K, van de Cotte B, Beeckman T, Van Montagu M, Inzé D, Verbruggen N. 2000. Expression of cell cycle regulatory genes and morphological alterations in response to salt stress in Arabidopsis thaliana. Planta 211, 632–640. [DOI] [PubMed] [Google Scholar]
- Burström H. 1960. Influence of iron and gibberellic acid on the light sensitivity of roots. Physiologia Plantarum 13, 597–615. [Google Scholar]
- Butcher DN, Clark JA, Lenton JR. 1990. Gibberellins and the growth of excised tomato roots: comparison of gib-1 mutant and wild type and responses to applied GA3 and 2S,3S paclobutrazol. Journal of Experimental Botany 41, 715–722. [Google Scholar]
- Cannon WC. 1949. A tentative classification of root systems. Ecology 30, 452–458. [Google Scholar]
- Caro A, Puntarulo S. 1996. Effect of in vivo iron supplementation on oxygen radical production by soybean roots. Biochimica et Biophysica Acta 1291, 245–251. [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, et al. 2001. Auxin transport promotes Arabidopsis lateral root initiation. The Plant Cell 13, 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL Jr, Grisafi PL, Fink GR. 1995. A pathway for lateral root formation in Arabidopsis thaliana. Genes & Development 9, 2131–2142. [DOI] [PubMed] [Google Scholar]
- Chacón-López A, Ibarra-Laclette E, Sánchez-Calderón L, Gutiérrez-Alanis D, Herrera-Estrella L. 2011. Global expression pattern comparison between low phosphorus insensitive 4 and WT Arabidopsis reveals an important role of reactive oxygen species and jasmonic acid in the root tip response to phosphate starvation. Plant Signaling & Behavior 6, 382–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chadwick AV, Burg SP. 1967. An explanation of the inhibition of root growth caused by indole-3-acetic acid. Plant Physiology 42, 415–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaiwanon J, Wang ZY. 2015. Spatiotemporal brassinosteroid signaling and antagonism with auxin pattern stem cell dynamics in Arabidopsis roots. Current Biology 25, 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Li Z, Xiong L. 2012. A plant microRNA regulates the adaptation of roots to drought stress. FEBS Letters 586, 1742–1747. [DOI] [PubMed] [Google Scholar]
- Chen H, Ma B, Zhou Y, He SJ, Tang SY, Lu X, Xie Q, Chen SY, Zhang JS. 2018. E3 ubiquitin ligase SOR1 regulates ethylene response in rice root by modulating stability of Aux/IAA protein. Proceedings of the National Academy of Sciences, USA 115, 4513–4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q, Dai X, De-Paoli H, Cheng Y, Takebayashi Y, Kasahara H, Kamiya Y, Zhao Y. 2014. Auxin overproduction in shoots cannot rescue auxin deficiencies in Arabidopsis roots. Plant & Cell Physiology 55, 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier F, Pata M, Nacry P, Doumas P, Rossignol M. 2003. Effects of phosphate availability on the root system architecture: large-scale analysis of the natural variation between Arabidopsis accessions. Plant, Cell & Environment 26, 1839–1850. [Google Scholar]
- Cho M, Cho HT. 2013. The function of ABCB transporters in auxin transport. Plant Signaling & Behavior 8, e22990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HS, Howe GA. 2009. A critical role for the TIFY motif in repression of jasmonate signaling by a stabilized splice variant of the JASMONATE ZIM-domain protein JAZ10 in Arabidopsis. The Plant Cell 21, 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson DT. 1967. Interactions between aluminium and phosphorus on root surfaces and cell wall material. Plant and Soil 27, 347–356. [Google Scholar]
- Clouse SD, Langford M, McMorris TC. 1996. A brassinosteroid-insensitive mutant in Arabidopsis thaliana exhibits multiple defects in growth and development. Plant Physiology 111, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes F. 1992. Regeneration of the discrete root epidermis of Pistia stratiotes L. after perturbation of the meristem. New Phytologist 120, 209–213. [Google Scholar]
- Cluis CP, Mouchel CF, Hardtke CS. 2004. The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. The Plant Journal 38, 332–347. [DOI] [PubMed] [Google Scholar]
- Colombi T, Walter A. 2017. Genetic diversity under soil compaction in wheat: root number as a promising trait for early plant vigor. Frontiers in Plant Science 8, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti Monzón G, Pinedo M, Lamattina L, la Canal de L. 2011. Sunflower root growth regulation: the role of jasmonic acid and its relation with auxins. Plant Growth Regulation 66, 129–136. [Google Scholar]
- Dai X, Wang Y, Yang A, Zhang WH. 2012. OsMYB2P-1, an R2R3 MYB transcription factor, is involved in the regulation of phosphate-starvation responses and root architecture in rice. Plant Physiology 159, 169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debi BR, Mushika J, Taketa S, Miyao A, Hirochika H, Ichii M. 2003. Isolation and characterization of a short lateral root mutant in rice (Oryza sativa L.). Plant Science 165, 895–903. [Google Scholar]
- de Dorlodot S, Forster B, Pagès L, Price A, Tuberosa R, Draye X. 2007. Root system architecture: opportunities and constraints for genetic improvement of crops. Trends in Plant Science 12, 474–481. [DOI] [PubMed] [Google Scholar]
- Dello Ioio R, Nakamura K, Moubayidin L, Perilli S, Taniguchi M, Morita MT, Aoyama T, Costantino P, Sabatini S. 2008. A genetic framework for the control of cell division and differentiation in the root meristem. Science 322, 1380–1384. [DOI] [PubMed] [Google Scholar]
- De Smet I, Lau S, Mayer U, Jürgens G. 2010. Embryogenesis—the humble beginnings of plant life. The Plant Journal 61, 959–970. [DOI] [PubMed] [Google Scholar]
- De Smet I, Signora L, Beeckman T, Inzé D, Foyer CH, Zhang H. 2003. An abscisic acid-sensitive checkpoint in lateral root development of Arabidopsis. The Plant Journal 33, 543–555. [DOI] [PubMed] [Google Scholar]
- De Smet I, White PJ, Bengough AG, et al. 2012. Analyzing lateral root development: how to move forward. The Plant Cell 24, 15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Mambro R, De Ruvo M, Pacifici E, et al. 2017. Auxin minimum triggers the developmental switch from cell division to cell differentiation in the Arabidopsis root. Proceedings of the National Academy of Sciences, USA 114, E7641–E7649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Digby J, Firn RD. 1995. The gravitropic set-point angle (GSA): the identification of an important developmentally controlled variable governing plant architecture. Plant, Cell & Environment 18, 1434–1440. [DOI] [PubMed] [Google Scholar]
- Dong J, Piñeros MA, Li X, Yang H, Liu Y, Murphy AS, Kochian LV, Liu D. 2017. An Arabidopsis ABC transporter mediates phosphate deficiency-induced remodeling of root architecture by modulating iron homeostasis in roots. Molecular Plant 10, 244–259. [DOI] [PubMed] [Google Scholar]
- Drew MC. 1975. Comparison of the effects of a localised supply of phosphate, nitrate, ammonium and potassium on the growth of the seminal root system, and the shoot, in barely. New Phytologist 75, 479–490. [Google Scholar]
- Drisch RC, Stahl Y. 2015. Function and regulation of transcription factors involved in root apical meristem and stem cell maintenance. Frontiers in Plant Science 6, 505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du R, Niu S, Liu Y, Sun X, Porth I, El-Kassaby YA, Li W. 2017. The gibberellin GID1–DELLA signalling module exists in evolutionarily ancient conifers. Scientific Reports 7, 16637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan L, Dietrich D, Ng CH, Chan PM, Bhalerao R, Bennett MJ, Dinneny JR. 2013. Endodermal ABA signaling promotes lateral root quiescence during salt stress in Arabidopsis seedlings. The Plant Cell 25, 324–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubrovsky JG, Doerner PW, Colón-Carmona A, Rost TL. 2000. Pericycle cell proliferation and lateral root initiation in Arabidopsis. Plant Physiology 124, 1648–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echevarría-Machado I, Escobedo-G M RM, Larqué-Saavedra A. 2007. Responses of transformed Catharanthus roseus roots to femtomolar concentrations of salicylic acid. Plant Physiology and Biochemistry 45, 501–507. [DOI] [PubMed] [Google Scholar]
- Ephritikhine G, Fellner M, Vannini C, Lapous D, Barbier-Brygoo H. 1999. The sax1 dwarf mutant of Arabidopsis thaliana shows altered sensitivity of growth responses to abscisic acid, auxin, gibberellins and ethylene and is partially rescued by exogenous brassinosteroid. The Plant Journal 18, 303–314. [DOI] [PubMed] [Google Scholar]
- Fàbregas N, Li N, Boeren S, Nash TE, Goshe MB, Clouse SD, de Vries S, Caño-Delgado AI. 2013. The brassinosteroid insensitive1-like3 signalosome complex regulates Arabidopsis root development. The Plant Cell 25, 3377–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley RA, Fitter AH. 1999. Temporal and spatial variation in soil resources in a deciduous woodland. Journal of Ecology 87, 688–696. [Google Scholar]
- Fei Q, Wei S, Zhou Z, Gao H, Li X. 2017. Adaptation of root growth to increased ambient temperature requires auxin and ethylene coordination in Arabidopsis. Plant Cell Reports 36, 1507–1518. [DOI] [PubMed] [Google Scholar]
- Fendrych M, Akhmanova M, Merrin J, Glanc M, Hagihara S, Takahashi K, Uchida N, Torii KU, Friml J. 2018. Rapid and reversible root growth inhibition by TIR1 auxin signalling. Nature Plants 4, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feraru E, Feraru MI, Barbez E, Waidmann S, Sun L, Gaidora A, Kleine-Vehn J. 2019. PILS6 is a temperature-sensitive regulator of nuclear auxin input and organ growth in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 116, 3893–3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. 2002. Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415, 806–809. [DOI] [PubMed] [Google Scholar]
- Fu X, Harberd NP. 2003. Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421, 740–743. [DOI] [PubMed] [Google Scholar]
- Fukaki H, Tasaka M. 2009. Hormone interactions during lateral root formation. Plant Molecular Biology 69, 437–449. [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. 1998. Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282, 2226–2230. [DOI] [PubMed] [Google Scholar]
- Gasperini D, Chételat A, Acosta IF, Goossens J, Pauwels L, Goossens A, Dreos R, Alfonso E, Farmer EE. 2015. Multilayered organization of jasmonate signalling in the regulation of root growth. PLoS Genetics 11, e1005300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Y, Fang X, Liu W, Sheng L, Xu L. 2019. Adventitious lateral rooting: the plasticity of root system architecture. Physiologia Plantarum 165, 39–43. [DOI] [PubMed] [Google Scholar]
- Giehl RF, Lima JE, von Wirén N. 2012. Localized iron supply triggers lateral root elongation in Arabidopsis by altering the AUX1-mediated auxin distribution. The Plant Cell 24, 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-García MP, Vilarrasa-Blasi J, Zhiponova M, Divol F, Mora-García S, Russinova E, Caño-Delgado AI. 2011. Brassinosteroids control meristem size by promoting cell cycle progression in Arabidopsis roots. Development 138, 849–859. [DOI] [PubMed] [Google Scholar]
- Gou J, Strauss SH, Tsai CJ, Fang K, Chen Y, Jiang X, Busov VB. 2010. Gibberellins regulate lateral root formation in Populus through interactions with auxin and other hormones. The Plant Cell 22, 623–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gougler JA, Evans ML. 1981. Adaptation of corn roots to exogenously applied auxin. Physiologia Plantarum 51, 394–398. [Google Scholar]
- Gruber BD, Giehl RF, Friedel S, von Wirén N. 2013. Plasticity of the Arabidopsis root system under nutrient deficiencies. Plant Physiology 163, 161–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez-Coronado MA, Trejo-López C, Larqué-Saavedra A. 1998. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiology and Biochemistry 36, 563–565. [Google Scholar]
- Hacham Y, Holland N, Butterfield C, Ubeda-Tomas S, Bennett MJ, Chory J, Savaldi-Goldstein S. 2011. Brassinosteroid perception in the epidermis controls root meristem size. Development 138, 839–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzawa T, Shibasaki K, Numata T, Kawamura Y, Gaude T, Rahman A. 2013. Cellular auxin homeostasis under high temperature is regulated through a sorting NEXIN1-dependent endosomal trafficking pathway. The Plant Cell 25, 3424–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield JL, Boote KJ, Kimball BA, Ziska LH, Izaurralde RC, Ort D, Thomson AM, Wolfe D. 2011. Climate impacts on agriculture: implications for crop production. Agronomy Journal 103, 351–370. [Google Scholar]
- Hatfield JL, Prueger JH. 2015. Temperature extremes: effect on plant growth and development. Weather and Climate Extremes 10, 4–10. [Google Scholar]
- Hazman M, Hause B, Eiche E, Nick P, Riemann M. 2015. Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. Journal of Experimental Botany 66, 3339–3352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho MD, Rosas JC, Brown KM, Lynch JP. 2005. Root architectural tradeoffs for water and phosphorus acquisition. Functional Plant Biology 32, 737–748. [DOI] [PubMed] [Google Scholar]
- Hobbie L, Estelle M. 1995. The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. The Plant Journal 7, 211–220. [DOI] [PubMed] [Google Scholar]
- Hochholdinger F, Woll K, Sauer M, Dembinsky D. 2004. Genetic dissection of root formation in maize (Zea mays) reveals root-type specific developmental programmes. Annals of Botany 93, 359–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge A, Berta G, Doussan C, Merchan F, Crespi M. 2009. Plant root growth, architecture and function. Plant and Soil 321, 153–187. [Google Scholar]
- Hou G, Hill JP. 2004. Developmental anatomy of the fifth shoot-borne root in young sporophytes of Ceratopteris richardii. Planta 219, 212–220. [DOI] [PubMed] [Google Scholar]
- Ismail A, Takeda S, Nick P. 2014. Life and death under salt stress: same players, different timing? Journal of Experimental Botany 65, 2963–2979. [DOI] [PubMed] [Google Scholar]
- Ivanchenko MG, Napsucialy-Mendivil S, Dubrovsky JG. 2010. Auxin-induced inhibition of lateral root initiation contributes to root system shaping in Arabidopsis thaliana. The Plant Journal 64, 740–752. [DOI] [PubMed] [Google Scholar]
- Jackson RB, Caldwell MM. 1993. The scale of nutrient heterogeneity around individual plants and its quantification with geostatistics. Ecology 74, 612–614. [Google Scholar]
- Jia X, Liu P, Lynch JP. 2018. Greater lateral root branching density in maize improves phosphorus acquisition from low phosphorus soil. Journal of Experimental Botany 69, 4961–4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Giehl RFH, Meyer RC, Altmann T, von Wirén N. 2019. Natural variation of BSK3 tunes brassinosteroid signaling to regulate root foraging under low nitrogen. Nature Communications 10, 2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston AE, Poulton PR, Fixen PE, Curtin D. 2014. Phosphorus. Its efficient use in agriculture . Advances in Agronomy 123, 177–228. [Google Scholar]
- Kampfenkel K, Van Montagu M, Inze D. 1995. Effects of iron excess on Nicotiana plumbaginifolia plants (implications to oxidative stress). Plant Physiology 107, 725–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno S, Arrighi JF, Chiarenza S, et al. 2016. A novel role for the root cap in phosphate uptake and homeostasis. eLife 5, e14577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartal G, Temel A, Arican E, Gozukirmizi N. 2009. Effects of brassinosteroids on barley root growth, antioxidant system and cell division. Plant Growth Regulation 58, 261–267. [Google Scholar]
- Kawa D, Julkowska MM, Sommerfeld HM, Ter Horst A, Haring MA, Testerink C. 2016. Phosphate-dependent root system architecture responses to salt stress. Plant Physiology 172, 690–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermeier F, Armengaud P, Seditas TJ, Danku J, Salt DE, Amtmann A. 2014. Analysis of the root system architecture of Arabidopsis provides a quantitative readout of crosstalk between nutritional signals. The Plant Cell 26, 1480–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss JZ, Miller KM, Ogden LA, Roth KK. 2002. Phototropism and gravitropism in lateral roots of Arabidopsis. Plant & Cell Physiology 43, 35–43. [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J, Ding Z, Jones AR, Tasaka M, Morita MT, Friml J. 2010. Gravity-induced PIN transcytosis for polarization of auxin fluxes in gravity-sensing root cells. Proceedings of the National Academy of Sciences, USA 107, 22344–22349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovar JL, Kuchenbuch RO. 1994. Commercial importance of adventitious rooting to agronomy. In: Davis TD, Haissig BE, eds. Biology of adventitious root formation. Boston, MA: Springer US, 25–35. [Google Scholar]
- Krouk G, Lacombe B, Bielach A, et al. 2010. Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants. Developmental Cell 18, 927–937. [DOI] [PubMed] [Google Scholar]
- Kudo T, Makita N, Kojima M, Tokunaga H, Sakakibara H. 2012. Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-zeatin-O-glucosyltransferase in rice. Plant Physiology 160, 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster TM, Arend M, Günthardt-Goerg MS, Schulin R. 2012. Root growth of different oak provenances in two soils under drought stress and air warming conditions. Plant and Soil 369, 61–71. [Google Scholar]
- Kusumi K, Yaeno T, Kojo K, Hirayama M, Hirokawa D, Yara A, Iba K. 2006. The role of salicylic acid in the glutathione-mediated protection against photooxidative stress in rice. Physiologia Plantarum 128, 651–661. [Google Scholar]
- Lai F, Thacker J, Li Y, Doerner P. 2007. Cell division activity determines the magnitude of phosphate starvation responses in Arabidopsis. The Plant Journal 50, 545–556. [DOI] [PubMed] [Google Scholar]
- Lakehal A, Bellini C. 2019. Control of adventitious root formation: insights into synergistic and antagonistic hormonal interactions. Physiologia Plantarum 165, 90–100. [DOI] [PubMed] [Google Scholar]
- Laplaze L, Benkova E, Casimiro I, et al. 2007. Cytokinins act directly on lateral root founder cells to inhibit root initiation. The Plant Cell 19, 3889–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lark RM, Milne AE, Addiscott TM, Goulding KWT, Webster CP, O’Flaherty S. 2004. Scale- and location-dependent correlation of nitrous oxide emissions with soil properties: an analysis using wavelets. European Journal of Soil Science 55, 611–627. [Google Scholar]
- Lavenus J, Goh T, Roberts I, Guyomarc’h S, Lucas M, De Smet I, Fukaki H, Beeckman T, Bennett M, Laplaze L. 2013. Lateral root development in Arabidopsis: fifty shades of auxin. Trends in Plant Science 18, 450–458. [DOI] [PubMed] [Google Scholar]
- Le J, Vandenbussche F, Van Der Straeten D, Verbelen JP. 2001. In the early response of Arabidopsis roots to ethylene, cell elongation is up- and down-regulated and uncoupled from differentiation. Plant Physiology 125, 519–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehotai N, Kolbert Z, Peto A, Feigl G, Ördög A, Kumar D, Tari I, Erdei L. 2012. Selenite-induced hormonal and signalling mechanisms during root growth of Arabidopsis thaliana L. Journal of Experimental Botany 63, 5677–5687. [DOI] [PubMed] [Google Scholar]
- Leitz G, Kang BH, Schoenwaelder ME, Staehelin LA. 2009. Statolith sedimentation kinetics and force transduction to the cortical endoplasmic reticulum in gravity-sensing Arabidopsis columella cells. The Plant Cell 21, 843–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lešková A, Giehl RFH, Hartmann A, Fargašová A, von Wirén N. 2017. Heavy metals induce iron deficiency responses at different hierarchic and regulatory levels. Plant Physiology 174, 1648–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xu HH, Liu WC, Zhang XW, Lu YT. 2015. Ethylene inhibits root elongation during alkaline stress through AUXIN1 and associated changes in auxin accumulation. Plant Physiology 168, 1777–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. 2006. Double knockouts of phospholipases D 1 and D 2 in Arabidopsis affect root elongation during phosphate-limited growth but do not affect root hair patterning. Plant Physiology 140, 761–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Chen L, Forde BG, Davies WJ. 2017. The biphasic root growth response to abscisic acid in Arabidopsis involves interaction with ethylene and auxin signalling pathways. Frontiers in Plant Science 8, 1154–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Mo X, Shou H, Wu P. 2006. Cytokinin-mediated cell cycling arrest of pericycle founder cells in lateral root initiation of Arabidopsis. Plant & Cell Physiology 47, 1112–1123. [DOI] [PubMed] [Google Scholar]
- Li Z, Waadt R, Schroeder JI. 2016. Release of GTP exchange factor mediated down-regulation of abscisic acid signal transduction through ABA-induced rapid degradation of RopGEFs. PLoS Biology 14, e1002461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y, Harris JM. 2005. Response of root branching to abscisic acid is correlated with nodule formation both in legumes and nonlegumes. American Journal of Botany 92, 1675–1683. [DOI] [PubMed] [Google Scholar]
- Liang Y, Mitchell DM, Harris JM. 2007. Abscisic acid rescues the root meristem defects of the Medicago truncatula latd mutant. Developmental Biology 304, 297–307. [DOI] [PubMed] [Google Scholar]
- Liao H, Wan H, Shaff J, Wang X, Yan X, Kochian LV. 2006. Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system. Plant Physiology 141, 674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima JE, Kojima S, Takahashi H, von Wirén N. 2010. Ammonium triggers lateral root branching in Arabidopsis in an AMMONIUM TRANSPORTER1;3-dependent manner. The Plant Cell 22, 3621–3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin B-L, Raghavan V. 1991. Lateral root initiation in Marsilea quadrifolia. I. Origin and histogenesis of lateral roots. Canadian Journal of Botany 69, 123–135. [DOI] [PubMed] [Google Scholar]
- Linkohr BI, Williamson LC, Fitter AH, Leyser HM. 2002. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. The Plant Journal 29, 751–760. [DOI] [PubMed] [Google Scholar]
- Liszkay A, Kenk B, Schopfer P. 2003. Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extension growth. Planta 217, 658–667. [DOI] [PubMed] [Google Scholar]
- Liu Y, Gao Y, Li T, Zhang Y, Liu M, Yang C. 2015. OsABC1K8, an ABC1-like kinase gene, mediates abscisic acid sensitivity and dehydration tolerance response in rice seedlings. Pakistan Journal of Botany 47, 603–613. [Google Scholar]
- Liu Y, Lai N, Gao K, Chen F, Yuan L, Mi G. 2013. Ammonium inhibits primary root growth by reducing the length of meristem and elongation zone and decreasing elemental expansion rate in the root apex in Arabidopsis thaliana. PLoS One 8, e61031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung K, Bhalerao RP, Sandberg G. 2001. Sites and homeostatic control of auxin biosynthesis in Arabidopsis during vegetative growth. The Plant Journal 28, 465–474. [DOI] [PubMed] [Google Scholar]
- Ljung K, Hull AK, Celenza J, Yamada M, Estelle M, Normanly J, Sandberg G. 2005. Sites and regulation of auxin biosynthesis in Arabidopsis roots. The Plant Cell 17, 1090–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J, Hernández-Abreu E, Sánchez-Calderón L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L. 2002. Phosphate availability alters architecture and causes changes in hormone sensitivity in the Arabidopsis root system. Plant Physiology 129, 244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. 1998. EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes & Development 12, 2175–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. 1995. Root architecture and plant productivity. Plant Physiology 109, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JP. 2007. Roots of the second green revolution. Australian Journal of Botany 55, 493–20. [Google Scholar]
- Lynch JP. 2013. Steep, cheap and deep: an ideotype to optimize water and N acquisition by maize root systems. Annals of Botany 112, 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, He SJ, Duan KX, et al. 2013. Identification of rice ethylene-response mutants and characterization of MHZ7/OsEIN2 in distinct ethylene response and yield trait regulation. Molecular Plant 6, 1830–1848. [DOI] [PubMed] [Google Scholar]
- Maher EP, Martindale SJ. 1980. Mutants of Arabidopsis thaliana with altered responses to auxins and gravity. Biochemical Genetics 18, 1041–1053. [DOI] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G. 2002. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. The Plant Cell 14, 589–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez G. 2019. Cytokinin inhibits lateral root development at the earliest stages of lateral root primordium initiation in maize primary root. Journal of Plant Growth Regulation 38, 83–92. [Google Scholar]
- Martins S, Montiel-Jorda A, Cayrel A, Huguet S, Roux CP, Ljung K, Vert G. 2017. Brassinosteroid signaling-dependent root responses to prolonged elevated ambient temperature. Nature Communications 8, 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Li J, Mathews DE, Kieber JJ, Schaller GE. 2004. Type-B response regulators display overlapping expression patterns in Arabidopsis. Plant Physiology 135, 927–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNear D., Jr 2013. The rhizosphere—roots, soil and everything in between. Nature Education Knowledge 4. [Google Scholar]
- Mikkelsen MD, Naur P, Halkier BA. 2004. Arabidopsis mutants in the C-S lyase of glucosinolate biosynthesis establish a critical role for indole-3-acetaldoxime in auxin homeostasis. The Plant Journal 37, 770–777. [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T. 2006. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis. Proceedings of the National Academy of Sciences, USA 103, 16598–16603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollier A, Pellerin S. 1999. Maize root system growth and development as influenced by phosphorus deficiency. Journal of Experimental Botany 50, 487–497. [Google Scholar]
- Moni A, Lee AY, Briggs WR, Han IS. 2015. The blue light receptor Phototropin 1 suppresses lateral root growth by controlling cell elongation. Plant Biology 17, 34–40. [DOI] [PubMed] [Google Scholar]
- Monte I, Hamberg M, Chini A, Gimenez-Ibanez S, García-Casado G, Porzel A, Pazos F, Boter M, Solano R. 2014. Rational design of a ligand-based antagonist of jasmonate perception. Nature Chemical Biology 10, 671–676. [DOI] [PubMed] [Google Scholar]
- Mora-Macías J, Ojeda-Rivera JO, Gutiérrez-Alanís D, Yong-Villalobos L, Oropeza-Aburto A, Raya-González J, Jiménez-Domínguez G, Chávez-Calvillo G, Rellán-Álvarez R, Herrera-Estrella L. 2017. Malate-dependent Fe accumulation is a critical checkpoint in the root developmental response to low phosphate. Proceedings of the National Academy of Sciences, USA 114, E3563–E3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosblech A, Thurow C, Gatz C, Feussner I, Heilmann I. 2011. Jasmonic acid perception by COI1 involves inositol polyphosphates in Arabidopsis thaliana. The Plant Journal 65, 949–957. [DOI] [PubMed] [Google Scholar]
- Motte H, Vanneste S, Beeckman T. 2019. Molecular and environmental regulation of root development. Annual Review of Plant Biology 70, 465–488. [DOI] [PubMed] [Google Scholar]
- Mounier E, Pervent M, Ljung K, Gojon A, Nacry P. 2014. Auxin-mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant, Cell & Environment 37, 162–174. [DOI] [PubMed] [Google Scholar]
- Muday GK, Haworth P. 1994. Tomato root growth, gravitropism, and lateral development: correlation with auxin transport. Plant Physiology and Biochemistry 32, 193–203. [PubMed] [Google Scholar]
- Müller J, Toev T, Heisters M, Teller J, Moore KL, Hause G, Dinesh DC, Bürstenbinder K, Abel S. 2015. Iron-dependent callose deposition adjusts root meristem maintenance to phosphate availability. Developmental Cell 33, 216–230. [DOI] [PubMed] [Google Scholar]
- Müssig C, Shin GH, Altmann T. 2003. Brassinosteroids promote root growth in Arabidopsis. Plant Physiology 133, 1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacry P, Canivenc G, Muller B, Azmi A, Van Onckelen H, Rossignol M, Doumas P. 2005. A role for auxin redistribution in the responses of the root system architecture to phosphate starvation in Arabidopsis. Plant Physiology 138, 2061–2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel KA, Kastenholz B, Jahnke S, et al. 2009. Temperature responses of roots: impact on growth, root system architecture and implications for phenotyping. Functional Plant Biology 36, 947–949. [DOI] [PubMed] [Google Scholar]
- Nawaz MF, Bourrié G, Trolard F. 2012. Soil compaction impact and modelling. A review. Agronomy for Sustainable Development 33, 291–309. [Google Scholar]
- Negi M, Sanagala R, Rai V, Jain A. 2016. Deciphering phosphate deficiency-mediated temporal effects on different root traits in rice grown in a modified hydroponic system. Frontiers in Plant Science 7, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RJ. 1977. Abscisic acid effects on fronds and roots of Lemna minor L. American Journal of Botany 64, 45–49. [Google Scholar]
- Nibau C, Gibbs DJ, Coates JC. 2008. Branching out in new directions: the control of root architecture by lateral root formation. New Phytologist 179, 595–614. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Yoshida T, Kitahata N, Asami T, Shinozaki K, Hirayama T. 2007. ABA-Hypersensitive Germination1 encodes a protein phosphatase 2C, an essential component of abscisic acid signaling in Arabidopsis seed. The Plant Journal 50, 935–949. [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. 2001. Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. The Plant Cell 13, 2441–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura T, Goeschl C, Filiault D, Mirea M, Slovak R, Wolhrab B, Satbhai SB, Busch W. 2019. Root system depth in Arabidopsis is shaped by EXOCYST70A3 via the dynamic modulation of auxin transport. Cell 178, 400–412.e16. [DOI] [PubMed] [Google Scholar]
- Ohno Y. 1967. Photoinhibition of elongation growth of roots in rice seedlings. Plant & Cell Physiology 8, 141–150. [Google Scholar]
- Okada K, Shimura Y. 1990. Reversible root tip rotation in Arabidopsis seedlings induced by obstacle-touching stimulus. Science 250, 274–276. [DOI] [PubMed] [Google Scholar]
- Orman-Ligeza B, Civava R, de Dorlodot S, Draye X. 2014. Root system architecture . In: Morte A, Varma A, eds. Root engineering. Basic and appplied concepts. Berlin, Heidelberg: Springer Berlin Heidelberg, 39–56. [Google Scholar]
- Osmont KS, Sibout R, Hardtke CS. 2007. Hidden branches: developments in root system architecture. Annual Review of Plant Biology 58, 93–113. [DOI] [PubMed] [Google Scholar]
- Overvoorde P, Fukaki H, Beeckman T. 2010. Auxin control of root development. Cold Spring Harbor Perspectives in Biology 2, a001537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry G, Marchant A, May S, et al. 2001. Quick on the uptake: characterization of a family of plant auxin influx carriers. Journal of Plant Growth Regulation 20, 217–225. [Google Scholar]
- Pasternak T, Groot EP, Kazantsev FV, Teale W, Omelyanchuk N, Kovrizhnykh V, Palme K, Mironova VV. 2019. Salicylic acid affects root meristem patterning via auxin distribution in a concentration-dependent manner. Plant Physiology 180, 1725–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ. 2009. Arabidopsis lateral root development: an emerging story. Trends in Plant Science 14, 399–408. [DOI] [PubMed] [Google Scholar]
- Pérez-Torres CA, López-Bucio J, Cruz-Ramírez A, Ibarra-Laclette E, Dharmasiri S, Estelle M, Herrera-Estrella L. 2008. Phosphate availability alters lateral root development in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor. The Plant Cell 20, 3258–3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pich A, Scholz G, Stephan UW. 1994. Iron-dependent changes of heavy metals, nicotianamine, and citrate in different plant organs and in the xylem exudate of two tomato genotypes. Nicotianamine as possible copper translocator. Plant and Soil 165, 189–196. [Google Scholar]
- Pilet PE. 1981. Some aspects of the control of root growth and georeaction: the involvement of indoleacetic acid and abscisic acid. Plant Physiology 67, 1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet PE, Ney D. 1978. Rapid, localized light effect on root growth in maize. Planta 144, 109–110. [DOI] [PubMed] [Google Scholar]
- Pilet PE, Saugy M. 1987. Effect on root growth of endogenous and applied IAA and ABA: a critical reexamination. Plant Physiology 83, 33–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Zhang Z, Wang J, Chen X, Wei P, Huang R. 2017. The activation of OsEIL1 on YUC8 transcription and auxin biosynthesis is required for ethylene-inhibited root elongation in rice early seedling development. PLoS Genetics 13, e1006955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Bannigan A, Sulaman W, Pechter P, Blancaflor EB, Baskin TI. 2007. Auxin, actin and growth of the Arabidopsis thaliana primary root. The Plant Journal 50, 514–528. [DOI] [PubMed] [Google Scholar]
- Ramaiah M, Jain A, Raghothama KG. 2014. Ethylene response Factor070 regulates root development and phosphate starvation-mediated responses. Plant Physiology 164, 1484–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Brady SR, Reed RC, Ante SJ, Muday GK. 2000. Basipetal auxin transport is required for gravitropism in roots of Arabidopsis. Plant Physiology 122, 481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, DeLong A, Muday GK. 2001. Genetic and chemical reductions in protein phosphatase activity alter auxin transport, gravity response, and lateral root growth. The Plant Cell 13, 1683–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya-González J, Pelagio-Flores R, López-Bucio J. 2012. The jasmonate receptor COI1 plays a role in jasmonate-induced lateral root formation and lateral root positioning in Arabidopsis thaliana. Journal of Plant Physiology 169, 1348–1358. [DOI] [PubMed] [Google Scholar]
- Rellán-Álvarez R, Lobet G, Lindner H, et al. 2015. GLO-Roots: an imaging platform enabling multidimensional characterization of soil-grown root systems. eLife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reymond M, Svistoonoff S, Loudet O, Nussaume L, Desnos T. 2006. Identification of QTL controlling root growth response to phosphate starvation in Arabidopsis thaliana. Plant, Cell & Environment 29, 115–125. [DOI] [PubMed] [Google Scholar]
- Robertson JM, Hubick KT, Yeung EC, Reid DM. 1990. Developmental responses to drought and abscisic acid in sunflower roots: root growth, apical anatomy, osmotic adjustment. Journal of Experimental Botany 41, 325–327. [Google Scholar]
- Roddick JG, Rijnenberg AL, Ikekawa N. 1993. Developmental effects of 24-epibrassinolide in excised roots of tomato grown in vitro. Physiologia Plantarum 87, 453–458. [Google Scholar]
- Rogato A, D’Apuzzo E, Barbulova A, Omrane S, Parlati A, Carfagna S, Costa A, Lo Schiavo F, Esposito S, Chiurazzi M. 2010. Characterization of a developmental root response caused by external ammonium supply in Lotus japonicus. Plant Physiology 154, 784–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosquete MR, von Wangenheim D, Marhavý P, Barbez E, Stelzer EH, Benková E, Maizel A, Kleine-Vehn J. 2013. An auxin transport mechanism restricts positive orthogravitropism in lateral roots. Current Biology 23, 817–822. [DOI] [PubMed] [Google Scholar]
- Rowe JH, Topping JF, Liu J, Lindsey K. 2016. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytologist 211, 225–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roychoudhry S, Del Bianco M, Kieffer M, Kepinski S. 2013. Auxin controls gravitropic setpoint angle in higher plant lateral branches. Current Biology 23, 1497–1504. [DOI] [PubMed] [Google Scholar]
- Roychoudhry S, Kieffer M, Del Bianco M, Liao CY, Weijers D, Kepinski S. 2017. The developmental and environmental regulation of gravitropic setpoint angle in Arabidopsis and bean. Scientific Reports 7, 42664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz Rosquete M, Waidmann S, Kleine-Vehn J. 2018. PIN7 auxin carrier has a preferential role in terminating radial root expansion in Arabidopsis thaliana. International Journal of Molecular Sciences 19, 1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růzicka K, Ljung K, Vanneste S, Podhorská R, Beeckman T, Friml J, Benková E. 2007. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. The Plant Cell 19, 2197–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito A, Tanabata S, Tanabata T, Tajima S, Ueno M, Ishikawa S, Ohtake N, Sueyoshi K, Ohyama T. 2014. Effect of nitrate on nodule and root growth of soybean (Glycine max (L.) Merr.). International Journal of Molecular Sciences 15, 4464–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Cruz-Ramírez A, Nieto-Jacobo F, Dubrovsky JG, Herrera-Estrella L. 2005. Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant & Cell Physiology 46, 174–184. [DOI] [PubMed] [Google Scholar]
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Gutiérrez-Ortega A, Hernández-Abreu E, Herrera-Estrella L. 2006. Characterization of low phosphorus insensitive mutants reveals a crosstalk between low phosphorus-induced determinate root development and the activation of genes involved in the adaptation of Arabidopsis to phosphorus deficiency. Plant Physiology 140, 879–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San-Miguel R, Gutiérrez M, Larqué-Saavedra A. 2003. Salicylic acid increases the biomass accumulation of Pinus patula. Southern Journal of Applied Forestry 27, 52–54. [Google Scholar]
- Sattelmacher B, Marschner H, Kühne R. 1990. Effects of the temperature of the rooting zone on the growth and development of roots of potato (Solanum tuberosum). Annals of Botany 65, 27–36. [Google Scholar]
- Scott TK. 1972. Auxins and roots. Annual Review of Plant Physiology 23, 235–258. [Google Scholar]
- Seiler GJ. 1998. Influence of temperature on primary and lateral root growth of sunflower seedlings. Environmental and Experimental Botany 40, 135–146. [Google Scholar]
- Seo PJ, Xiang F, Qiao M, Park JY, Lee YN, Kim SG, Lee YH, Park WJ, Park CM. 2009. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis. Plant Physiology 151, 275–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbaz AM, Oki Y, Adachi T, Murata Y, Khan MHR. 2006. Phosphorus starvation induced root-mediated pH changes in solubilization and acquisition of sparingly soluble P sources and organic acids exudation by Brassica cultivars. Soil Science and Plant Nutrition 52, 623–633. [Google Scholar]
- Shen-miller J. 1978. Spectral response of corn (Zea mays) in root geotropism. Plant & Cell Physiology 19, 445–452. [Google Scholar]
- Shimizu A, Yanagihara S, Kawasaki S, Ikehashi H. 2004. Phosphorus deficiency-induced root elongation and its QTL in rice (Oryza sativa L.). Theoretical and Applied Genetics 109, 1361–1368. [DOI] [PubMed] [Google Scholar]
- Shin H, Shin HS, Dewbre GR, Harrison MJ. 2004. Phosphate transport in Arabidopsis: Pht1;1 and Pht1;4 play a major role in phosphate acquisition from both low- and high-phosphate environments. The Plant Journal 39, 629–642. [DOI] [PubMed] [Google Scholar]
- Shyu C, Figueroa P, Depew CL, Cooke TF, Sheard LB, Moreno JE, Katsir L, Zheng N, Browse J, Howe GA. 2012. JAZ8 lacks a canonical degron and has an EAR motif that mediates transcriptional repression of jasmonate responses in Arabidopsis. The Plant Cell 24, 536–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimášková M, O’Brien JA, Khan M, et al. 2015. Cytokinin response factors regulate PIN-FORMED auxin transporters. Nature Communications 6, 8717. [DOI] [PubMed] [Google Scholar]
- Singh AP, Fridman Y, Holland N, Ackerman-Lavert M, Zananiri R, Jaillais Y, Henn A, Savaldi-Goldstein S. 2018. Interdependent nutrient availability and steroid hormone signals facilitate root growth plasticity. Developmental Cell 46, 59–72. [DOI] [PubMed] [Google Scholar]
- Staswick PE, Su W, Howell SH. 1992. Methyl jasmonate inhibition of root growth and induction of a leaf protein are decreased in an Arabidopsis thaliana mutant. Proceedings of the National Academy of Sciences, USA 89, 6837–6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street IH, Aman S, Zubo Y, Ramzan A, Wang X, Shakeel SN, Kieber JJ, Schaller GE. 2015. Ethylene inhibits cell proliferation of the Arabidopsis root meristem. Plant Physiology 169, 338–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street IH, Mathews DE, Yamburkenko MV, Sorooshzadeh A, John RT, Swarup R, Bennett MJ, Kieber JJ, Schaller GE. 2016. Cytokinin acts through the auxin influx carrier AUX1 to regulate cell elongation in the root. Development 143, 3982–3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su SH, Gibbs NM, Jancewicz AL, Masson PH. 2017. Molecular mechanisms of root gravitropism. Current Biology 27, R964–R972. [DOI] [PubMed] [Google Scholar]
- Sun L, Feraru E, Feraru MI, Wabnik K, Kleine-Vehn J. 2019. PIN-LIKES coordinate brassinosteroid signalling with nuclear auxin input in Arabidopsis thaliana. bioRxiv doi: 10.1101/646489. [Preprint]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun QB, Shen RF, Zhao XQ, Chen RF, Dong XY. 2008. Phosphorus enhances Al resistance in Al-resistant Lespedeza bicolor but not in Al-sensitive L. cuneata under relatively high Al stress. Annals of Botany 102, 795–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T. 2007. Root tip contact with low-phosphate media reprograms plant root architecture. Nature Genetics 39, 792–796. [DOI] [PubMed] [Google Scholar]
- Swarup R, Kramer EM, Perry P, Knox K, Leyser HM, Haseloff J, Beemster GT, Bhalerao R, Bennett MJ. 2005. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. Nature Cell Biology 7, 1057–1065. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Goto N, Okada K, Takahashi H. 2002. Hydrotropism in abscisic acid, wavy, and gravitropic mutants of Arabidopsis thaliana. Planta 216, 203–211. [DOI] [PubMed] [Google Scholar]
- Tan K, Keltjens WG. 1990. Interaction between aluminium and phosphorus in sorghum plants. Plant and Soil 124, 15–23. [Google Scholar]
- Tanimoto E. 1987. Gibberellin-dependent root elongation in Lactuca sativa: recovery from growth retardant-suppressed elongation with thickening by low concentration of GA3. Plant & Cell Physiology 28, 963–973. [Google Scholar]
- Tanimoto E. 1988. Gibberellin regulation of root growth with change in galactose content of cell walls in pisum sativum. Plant & Cell Physiology 29, 269–280. [Google Scholar]
- Tanimoto E. 1994. Interaction of gibberellin A3 and ancymidol in the growth and cell-wall extensibility of dwarf pea roots. Plant & Cell Physiology 35, 1019–1029. [Google Scholar]
- Thatcher LF, Cevik V, Grant M, Zhai B, Jones JD, Manners JM, Kazan K. 2016. Characterization of a JAZ7 activation-tagged Arabidopsis mutant with increased susceptibility to the fungal pathogen Fusarium oxysporum. Journal of Experimental Botany 67, 2367–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann KV. 1939. Auxin and the inhibition of plant growth. Biological Reviews 14, 314–337. [Google Scholar]
- Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J. 2007. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448, 661–665. [DOI] [PubMed] [Google Scholar]
- Thole JM, Beisner ER, Liu J, Venkova SV, Strader LC. 2014. Abscisic acid regulates root elongation through the activities of auxin and ethylene in Arabidopsis thaliana. G3 4, 1259–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas GW. 1970. Soil and climatic factors which affect nutrient mobility . In: Engelstad OP, ed. Nutrient mobility in soils: accumulation and losses. Madison, WI: Soil Science Society of America, 1–20. [Google Scholar]
- Tian H, De Smet I, Ding Z. 2014. Shaping a root system: regulating lateral versus primary root growth. Trends in Plant Science 19, 426–431. [DOI] [PubMed] [Google Scholar]
- Tian QY, Sun P, Zhang WH. 2009. Ethylene is involved in nitrate-dependent root growth and branching in Arabidopsis thaliana. New Phytologist 184, 918–931. [DOI] [PubMed] [Google Scholar]
- Ticconi CA, Delatorre CA, Lahner B, Salt DE, Abel S. 2004. Arabidopsis pdr2 reveals a phosphate-sensitive checkpoint in root development. The Plant Journal 37, 801–814. [DOI] [PubMed] [Google Scholar]
- Tseng IC, Hong CY, Yu SM, Ho TH. 2013. Abscisic acid- and stress-induced highly proline-rich glycoproteins regulate root growth in rice. Plant Physiology 163, 118–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung P, Hooker TS, Tampe PA, Reid DM, Thorpe TA. 1996. Jasmonic acid: effects on growth and development of isolated tomato roots cultured in vitro. International Journal of Plant Sciences 157, 713–721. [Google Scholar]
- Ubeda-Tomás S, Federici F, Casimiro I, Beemster GT, Bhalerao R, Swarup R, Doerner P, Haseloff J, Bennett MJ. 2009. Gibberellin signaling in the endodermis controls Arabidopsis root meristem size. Current Biology 19, 1194–1199. [DOI] [PubMed] [Google Scholar]
- Uga Y, Kitomi Y, Ishikawa S, Yano M. 2015. Genetic improvement for root growth angle to enhance crop production. Breeding Science 65, 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uga Y, Sugimoto K, Ogawa S, et al. 2013. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nature 45, 1097–1102. [DOI] [PubMed] [Google Scholar]
- van den Berg C, Willemsen V, Hendriks G, Weisbeek P, Scheres B. 1997. Short-range control of cell differentiation in the Arabidopsis root meristem. Nature 390, 287–289. [DOI] [PubMed] [Google Scholar]
- van der Weele CM, Spollen WG, Sharp RE, Baskin TI. 2000. Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. Journal of Experimental Botany 51, 1555–1562. [DOI] [PubMed] [Google Scholar]
- Vercruyssen L, Gonzalez N, Werner T, Schmülling T, Inzé D. 2011. Combining enhanced root and shoot growth reveals cross talk between pathways that control plant organ size in Arabidopsis. Plant Physiology 155, 1339–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieten A, Vanneste S, Wisniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml J. 2005. Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 132, 4521–4531. [DOI] [PubMed] [Google Scholar]
- Vragović K, Sela A, Friedlander-Shani L, Fridman Y, Hacham Y, Holland N, Bartom E, Mockler TC, Savaldi-Goldstein S. 2015. Translatome analyses capture of opposing tissue-specific brassinosteroid signals orchestrating root meristem differentiation. Proceedings of the National Academy of Sciences, USA 112, 923–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waidmann S, Ruiz Rosquete M, Schöller M, et al. 2019. Cytokinin functions as an asymmetric and anti-gravitropic signal in lateral roots. Nature Communications 10, 3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wain RL, Taylor HF, Intarakosit P, Shannon TGD. 1968. Halogen derivatives of 4-hydroxybenzoic acid as root growth stimulants and the importance of light in this response. Nature 217, 870–871. [Google Scholar]
- Walch-Liu P, Ivanov II, Filleur S, Gan Y, Remans T, Forde BG. 2006. Nitrogen regulation of root branching. Annals of Botany 97, 875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Lv S, Jiang P, Li Y. 2017. Roles, regulation, and agricultural application of plant phosphate transporters. Frontiers in Plant Science 8, 798–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Pei L, Jin Z, Zhang K, Zhang J. 2017. Overexpression of the protein phosphatase 2A regulatory subunit a gene ZmPP2AA1 improves low phosphate tolerance by remodeling the root system architecture of maize. PLoS One 12, e0176538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Zhang Y, Kieffer M, Yu H, Kepinski S, Estelle M. 2015. HSP90 regulates temperature-dependent seedling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1. Nature Communications 7, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Li C, Wu Z, Jia Y, Wang H, Sun S, Mao C, Wang X. 2017. Abscisic acid regulates auxin homeostasis in rice root tips to promote root hair elongation. Frontiers in Plant Science 8, 1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T. 2003. Cytokinin-deficient transgenic arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. The Plant Cell 15, 2532–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Motyka V, Strnad M, Schmülling T. 2001. Regulation of plant growth by cytokinin. Proceedings of the National Academy of Sciences, USA 98, 10487–10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins H, Wain RL. 1974. The root cap and control of root elongation in Zea mays L. seedlings exposed to white light. Planta 121, 1–8. [DOI] [PubMed] [Google Scholar]
- Williamson LC, Ribrioux SP, Fitter AH, Leyser HM. 2001. Phosphate availability regulates root system architecture in Arabidopsis. Plant Physiology 126, 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Lewis DR, Spalding EP. 2007. Mutations in Arabidopsis multidrug resistance-like ABC transporters separate the roles of acropetal and basipetal auxin transport in lateral root development. The Plant Cell 19, 1826–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Wang RG, Mao G, Koczan JM. 2006. Identification of drought tolerance determinants by genetic analysis of root response to drought stress and abscisic acid. Plant Physiology 142, 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu BC, Niu FR, Duan DP, Xu WZ, Bot JHPJ. 2012. Root morphological characteristics of Lespedeza davurica (L.) intercropped with Bothriochloa ischaemum (L.) Keng under water stress and P application. Pakistan Journal of Botany 44, 1857–1864. [Google Scholar]
- Yamaguchi M, Valliyodan B, Zhang J, Lenoble ME, Yu O, Rogers EE, Nguyen HT, Sharp RE. 2010. Regulation of growth response to water stress in the soybean primary root. I. Proteomic analysis reveals region-specific regulation of phenylpropanoid metabolism and control of free iron in the elongation zone. Plant, Cell & Environment 33, 223–243. [DOI] [PubMed] [Google Scholar]
- Yevdokimov I, Larionova A, Blagodatskaya E. 2016. Microbial immobilisation of phosphorus in soils exposed to drying–rewetting and freeze–thawing cycles. Biology and Fertility of Soils 1–12. [Google Scholar]
- Yi K, Wu Z, Zhou J, Du L, Guo L, Wu Y, Wu P. 2005. OsPTF1, a novel transcription factor involved in tolerance to phosphate starvation in rice. Plant Physiology 138, 2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Christmann A, Yamaguchi-Shinozaki K, Grill E, Fernie AR. 2019. Revisiting the basal role of ABA—roles outside of stress. Trends in Plant Science 24, 625–635. [DOI] [PubMed] [Google Scholar]
- Yoshihara T, Spalding EP. 2017. LAZY genes mediate the effects of gravity on auxin gradients and plant architecture. Plant Physiology 175, 959–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu LH, Miao ZQ, Qi GF, Wu J, Cai XT, Mao JL, Xiang CB. 2014. MADS-box transcription factor AGL21 regulates lateral root development and responds to multiple external and physiological signals. Molecular Plant 7, 1653–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Loqué D, Kojima S, Rauch S, Ishiyama K, Inoue E, Takahashi H, von Wirén N. 2007. The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. The Plant Cell 19, 2636–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan A, Lynch JP. 2015. Reduced frequency of lateral root branching improves N capture from low-N soils in maize. Journal of Experimental Botany 66, 2055–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Forde BG. 1998. An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279, 407–409. [DOI] [PubMed] [Google Scholar]
- Zhang H, Han W, De Smet I, Talboys P, Loya R, Hassan A, Rong H, Jürgens G, Paul Knox J, Wang MH. 2010. ABA promotes quiescence of the quiescent centre and suppresses stem cell differentiation in the Arabidopsis primary root meristem. The Plant Journal 64, 764–774. [DOI] [PubMed] [Google Scholar]
- Zhang H, Jennings A, Barlow PW, Forde BG. 1999. Dual pathways for regulation of root branching by nitrate. Proceedings of the National Academy of Sciences, USA 96, 6529–6534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Davies WJ. 1989. Abscisic acid produced in dehydrating roots may enable the plant to measure the water status of the soil. Plant, Cell & Environment 12, 73–81. [Google Scholar]
- Zhang J, Tardieu F. 1996. Relative contribution of apices and mature tissues to ABA synthesis in droughted maize root systems. Plant & Cell Physiology 37, 598–605. [Google Scholar]
- Zhang W, Swarup R, Bennett M, Schaller GE, Kieber JJ. 2013. Cytokinin induces cell division in the quiescent center of the Arabidopsis root apical meristem. Current Biology 23, 1979–1989. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Xing L, Wang X, Hou YJ, Gao J, Wang P, Duan CG, Zhu X, Zhu JK. 2014. The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77-dependent transcription of auxin-responsive genes. Science Signaling 7, ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolla G, Heimer YM, Barak S. 2010. Mild salinity stimulates a stress-induced morphogenic response in Arabidopsis thaliana roots. Journal of Experimental Botany 61, 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zürcher E, Müller B. 2016. Cytokinin synthesis, signaling, and function—advances and new insights. International Review of Cell and Molecular Biology 324, 1–38. [DOI] [PubMed] [Google Scholar]