Abstract
Purpose
Black women have the highest estimated allostatic load (AL). AL and self-perceived health are strong health predictors and have been linked to racial discrimination. Research suggests that everyday and institution-specific racial discrimination may predict different AL and self-reported health (SRH) outcomes. Furthermore, discrepancies between AL and self-perceived health could widen disparities. We estimated associations between everyday versus institution-specific racial discrimination with AL and SRH.
Methods
Data are from a San Francisco Bay Area community sample of 208 black women aged 30–50 years. Participation involved a questionnaire, self-interview, blood draw, and anthropometric measurements. Adjusted generalized linear regression models estimated associations of racial discrimination with AL and SRH.
Results
After adjusting for age, socioeconomic position, and medication use, institution-specific discrimination was negatively associated with AL (i.e., better health), whereas everyday experiences showed no association. Those reporting very-high (vs. moderate) institution-specific discrimination had lower AL (β = −1.31 [95% CI: −2.41, −0.20]; AL range: 0–15). No racial discrimination—SRH association was found.
Conclusions
For black women, (1) institution-specific racial discrimination may be differentially embodied compared with everyday experiences and (2) institutional racism may contribute to physiologic stress-regulation regardless of self-perceived health status. Potential factors that may contribute to an inverse racial discrimination—AL association, and future research, are discussed.
Keywords: African American, Allostatic load, Black, Minority health, Race/ethnicity, Racial discrimination, Self-reported health, Social determinants of health, Stress, Women’s health
Introduction
Racial discrimination—the process by which members of a racial/ethnic group are treated unfairly based on their race/ethnicity—is significantly associated with a wide variety of adverse health outcomes and unhealthy behaviors [1–8]. The negative health consequences of racial discrimination are theorized to be cumulative over the life course with biological dysfunction emerging by mid- to late-life [9–11], eventually resulting in allostatic load—multisystem physiologic dysregulation due to chronic adaptation to stress [12–14]. Allostatic load can lead to increased risk of numerous chronic diseases such as heart disease and diabetes, and even mortality [15,16].
Blacks consistently show disproportionately higher allostatic load (AL) than other racial/ethnic groups with black women having the highest predicted values [12,17–19]. Researchers suggest that this “weathering” (i.e., physiologic wear and tear) observed among black women may be influenced by lifetime exposure to stressors related to social identity such as gender and racial discrimination [12,20–22]. That is, experiences of social marginalization are likely incorporated biologically, or embodied [23]. Self-reported experiences of racist events have been linked to AL [24,25]. Racial discrimination manifests in many forms, which may have differential impacts on health. However, the effects of different types of discrimination (e.g., interpersonal, institutional) have not been parsed analytically; and racial discrimination associations with AL among black women is underreported.
Scholars propose that institutional experiences of racial discrimination (vs. routine mistreatment) may be a key risk factor for developing chronic health conditions among blacks [10,11,26–30]. Institutional racial discrimination operates within societal organizations (e.g., universities, the workplace) to shape access to health-promoting resources and opportunities, whereas day-to-day experiences are more mundane interpersonal experiences that generally occur in public [31]. Both experiences can vary in frequency (acute vs. chronic). For black women, racial and gender inequalities interact to make avoiding high-risk exposures exceedingly more difficult over the life course regardless of socioeconomic position [19,32–35]. This may help explain, in part, mixed findings between racial discrimination and various health outcomes [2,36–47]. For instance, workplace discrimination was associated with 30% higher breast cancer risk in the Black Women’s Health Study, whereas everyday exposure was weakly associated [44]. Should lifetime experiences within major social institutions be found to elicit deleterious physiological responses leading to elevated AL, interventions addressing institutional racism may be more effective in combatting a variety of health disparities faced by black women and other socially marginalized groups.
Research shows that self-perceived health status is predictive of chronic disease risk and mortality [48–50]. but report discrepancies between self-perceived health status and more objective health indicators. For example, evidence suggests that discrepancies between self-perceived health status and underlying AL levels can widen disparities in disease prevention, detection, and treatment [17,51–53]. Those at higher subclinical disease risk, such as having elevated cholesterol or blood pressure, may not be aware of it. Self-perceived good health contributes to lower health care utilization, such as annual checkups [54,55]. Moreover, discrepancies between perceived and actual health are higher for blacks (vs. whites) [56,57]. This disconnect has considerable implications for the timely clinical detection of AL in black women, the highest at-risk group. Furthermore, associations between self-reported health and racial discrimination are mixed: everyday exposures more consistently predict poor self-reported health than institutional experiences [57–64]. Together these factors suggest a need to assess whether specific racial discrimination exposures are more predictive of health, and predict similar AL and self-perceived health status amid black women.
No studies, to date, have compared associations between everyday versus institution-specific racial discrimination with AL and self-perceived health. To address this gap, our objectives were to examine associations of (1) everyday racial discrimination with both AL and self-reported health and of (2) institution-specific racial discrimination with AL and self-reported health. We hypothesized that (1) everyday racial discrimination would be associated with worse health and that (2) institution-specific racial discrimination would be associated with worse health, and that associations would be stronger than everyday experiences due to the theoretical excess burden related to the embodiment of institutional discrimination [12,21–23].
Materials and methods
Study sample, recruitment, and participation
Data are from the African American Women’s Heart & Health Study, a cross-sectional study examining the association between social-environmental stress and mental and physical health among a sample of 208 midlife black (i.e., African American) women. African American Women’s Heart & Health Study methods for study sample recruitment and participation have been described in more detail elsewhere [25]. Briefly, a community sample was recruited from five San Francisco Bay Area counties using purposive sampling with multiple recruitment strategies to maximize variability across key variables of interest (e.g., racial discrimination, socioeconomic indicators). Participants completed an interviewer-administered questionnaire, computer-assisted self-interview, physical examination, and fasting venous blood draw. Study approval was provided by the Committee for the Protection of Human Subjects at the University of California, Berkeley.
Exposure variables
Everyday discrimination scale
We used a modified version of the everyday discrimination scale (EDS) asking respondents how often they experienced day-to-day unfair treatment based on their race, ethnicity, or skin color in 10 subtle yet routine life situations (e.g., receiving poorer service) (α = 0.95) [65]. Responses were scored on a six-point Likert scale ranging from 1 = “Never” to 6 = “Almost every day”, and were then added across items to generate a summary score (10–60) with higher scores reflecting greater frequency of everyday experiences. Although racial discrimination has been measured continuously to assess health associations [28,60,66], previous evidence suggests a potential curvilinear relationship [25,40]. Therefore the EDS variable was measured both continuously and categorically (5-level qualitative-based) to reflect gradual increases in annual exposure; none (≤20), low = few times/year (21–30), moderate = few times/month (31–40), high = at least once a week (41–50), and very high = ~everyday (51–60) [25].
Experiences of discrimination scale
Respondents were asked whether they have “ever been treated unfairly, judged differently than others, prevented from doing something, been hassled, or made to feel inferior because of their race, ethnicity or skin color” across eight institutional domains (e.g., at work) (α = 0.92) [67]. One survey item from the original experiences of discrimination (EOD) scale (“getting services in a store or restaurant”) was removed to avoid overlap with the same EDS item. Responses were scored on a five-point Likert scale ranging from 1 = “Never” to 5 = “6 or more times” over one’s lifetime. Summary scores across the 8 EOD items ranged from 8 to 40 with higher scores reflecting greater frequency of institution-specific exposure. Like the EDS, summary scores were assessed continuously and as a 5-category qualitative-based variable representing conceptual increases of lifetime discriminatory experiences; never (=8), once (9–16), 2–3× (17–24), 4–5× (25–32), and ≥6× (33–40) [25]. To establish commensurability with the EDS, the EOD categories were then labeled as none, low, moderate, high, and very high.
Outcome variables
Allostatic load
AL comprised 15 biomarkers indicating functioning across four physiologic systems (Table 1). Following Seeman et al. and others [12,14,17,19,25,68–73], we used 75th-percentile distribution-based cut-points for biomarkers without established clinical risk criterion. All other biomarkers were coded according to established cut-points, consistent with the conceptual definition of AL as an indicator of subclinical risk. Each biomarker was coded dichotomously (0 = low risk,1 = high risk), then a summary score was created ranging from 0 to 15 with higher scores reflecting higher AL.
Table 1.
Allostatic load biomarker cut-points
| Biomarker | Guideline used | AL cut-points |
|---|---|---|
| Metabolic system | ||
| HDL (mg/dL) | ATPIII | <50 |
| LDL (mg/dL) | ATPIII | ≥100 |
| Waist (in) | ATPIII | >35 |
| Glucose (mg/dL) | ATPIII | ≥100 or <70 |
| HbA1c (mmol/mol) | ADA | ≥5.7 |
| Total cholesterol (mg/dL) | ATPIII | ≥160 |
| Triglycerides (mg/dL) | ATPIII | ≥150 |
| BMI (kg/m2) | ATPIII | ≥25 or <18.5 |
| Cardiovascular system | ||
| Systolic BP (mm Hg) | AHA (JNC 7) | ≥120 |
| Diastolic BP (mm Hg) | AHA (JNC 7) | ≥80 |
| Neuroendocrine system | ||
| *Epinephrine (pg/mL) | n/a | >77.70 |
| *Norepinephrine (pg/mL) | n/a | >686.30 |
| *Cortisol (μg/dL) | n/a | >12.69 |
| Inflammatory system | ||
| *Il-6 (pg/mL) | n/a | >7.85 |
| hsCRP (mg/L) | AHA | >3 |
75th-percentile cut-points used for biomarkers that do not have clinical guidelines; subclinical cut-points used for rest.
Self-reported health
Respondents were asked how they would rate their overall physical health at the present time. Responses were scored on a five-point Likert scale ranging from 1 = “Excellent” to 5 = “Poor” with higher scores representing worse health. Consistent with previous studies [48,62,74–78], self-reported health was assessed dichotomously (0 = “Excellent/Very good/Good”, 1 =“Fair/Poor”) [79].
Covariates
We assessed established empirical and theoretical confounders of the exposure—outcome association; age, education, employment, poverty, and marital/partnership status. Other covariates included medication use (cardiovascular and diabetes) (Information regarding the specific type of cardiovascular and diabetes medications taken by participants was not collected. Without knowing the specific medication and which biomarker(s) are targeted, we cannot account for it in our 15-biomarker AL measure (e.g., antihypertensives lower blood pressure; statins lower cholesterol). To preserve internal validity and account for medication use, we adjusted for medication use. We evaluated potential confounding by indication using stratified and bias analyses, alas cell sizes were too small (see [80,81]). However, stepwise regression showed medication use was significant in final models (P < .05) and that adjusting for it did not introduce bias or diminish the precision of the estimates.) Because research suggests that health behaviors and access to health-promoting resources may be on the pathway between racial discrimination and AL [1,10,19,66,69,82–87], health insurance and behaviors were identified as mediators and thus were not included in the analysis to avoid overcontrolling (see Supplemental Fig. 1). Except for age (measured in years), all other covariates were dichotomized at established risk levels (0 = low, 1 = high) to maximize power. These included 1 = ≤ high school diploma, 1 = not employed, 1 = ≤ 100% federal household poverty threshold, 1 = not married/domestically partnered, and 1 = current medication use. In addition, a composite socioeconomic position (SEP) measure was generated using the four dichotomous SEP variables. SEP summary scores ranged from 0 to 4 with higher values representing worse SEP.
Statistical analysis
STATA SE 13.1 was used for all statistical analyses. Data were missing at random (P > .10) which was ≤5%. Thus, we used multiple imputation (m = 20) to account for missingness [88]. In the final models, one observation had more than one missing variable within the linear combination of predictors and within the AL response variable resulting in computational problems, and was therefore dropped before imputation. Biomarkers were log-transformed before imputation to satisfy assumptions of normality, as needed. Statistical differences between exposure and outcome variables were assessed using bivariate analysis methods (e.g., t test), as applicable. Linear regression was used to estimate EDS and EOD associations with AL, whereas logistic regression was used to estimate self-reported health associated with each exposure, adjusted for covariates significant at P < .10. Similar to previous work [25,40], moderate discrimination was selected as the reference group of categorical regression models given that episodic stress exposure is self-regulatory and considered health-protective [13,89,90]. We conducted model diagnostic tests, as appropriate (e.g., heteroscedasticity). We assessed relative efficiency after imputation to ensure the simulated data did not inflate residual variance [88,91]. Sensitivity and bias analyses were performed on regression models [80,81]. We found no evidence of linear associations for AL or for self-reported health with either discrimination scale, as anticipated; hence, our final models are reported using the qualitative-based EDS and EOD variables described previously. Parameters for power calculations are reported in the final regression table [92].
Results
Sample characteristics
Table 2 presents the sample distribution of sociodemographic characteristics.
Table 2.
Study sample characteristics (n = 207)
| Covariates | n | % |
| Age mean (±SD.) | 41.72 (5.90) | |
| SEP mean (±SD) (range 0–4) | 1.67 (0.96) | |
| Educational attainment | ||
| > High school diploma | 138 | 66.67 |
| ≤ High school diploma | 69 | 33.33 |
| Employment status | ||
| Employed | 114 | 55.07 |
| Not employed | 93 | 44.93 |
| Poverty status | ||
| > 100% federal poverty | 168 | 81.16 |
| ≤ 100% federal poverty | 39 | 18.84 |
| Marital/partnership status | ||
| Married/domestic partnership | 61 | 29.47 |
| Not married/domestic partnership | 146 | 70.53 |
| Cardiovascular medication | ||
| Not currently taking | 164 | 79.23 |
| Currently taking | 43 | 20.77 |
| Diabetes medication | ||
| Not currently taking | 195 | 94.20 |
| Currently taking | 12 | 5.80 |
| Racial discrimination exposures | n | % |
| Everyday discrimination ccale (EDS) | ||
| None (EDS score: less than/equal 20) | 59 | 28.50 |
| Low (EDS score: 21–30) | 65 | 31.40 |
| Moderate (EDS score: 31–40) | 38 | 18.36 |
| High (EDS score: 41–50) | 26 | 12.56 |
| Very high (EDS score: 51–60) | 19 | 9.18 |
| Experiences of discrimination (EOD) | ||
| None (EOD score: 8) | 22 | 10.63 |
| Low (EOD score: 9–16) | 71 | 34.30 |
| Moderate (EOD score: 17–24) | 63 | 30.43 |
| High (EOD score: 25–32) | 29 | 14.01 |
| Very high (EOD score: 33–40) | 22 | 10.63 |
| Health outcomes | ||
| Allostatic load (range 0–15) | ||
| mean (±SD) | 5.96 (2.24) | |
| Self-reported overall physical health | ||
| Excellent/very good/good | 156 | 75.36 |
| Fair/poor | 51 | 24.64 |
SD = standard deviation; SEP = socioeconomic position.
Everyday experiences of discrimination
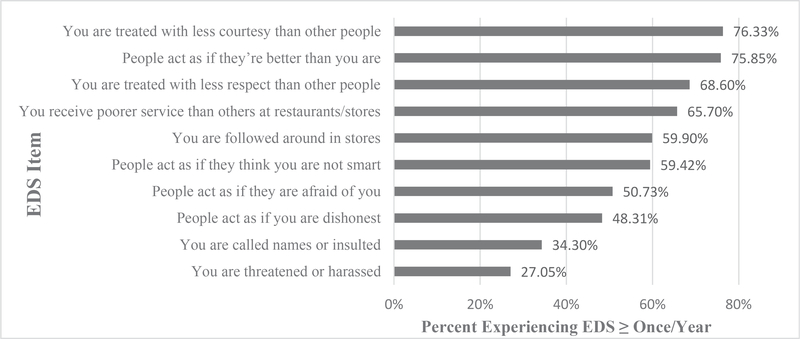
Half of the sample reported low to moderate levels of everyday discrimination (i.e., 1–3×/year) (Table 2). Approximately, 1 in 5 (21%) reported high to very high levels (i.e., at least 1×/week to ~ everyday). Figure 1 shows the distribution of reported occurrences by each scale item. The majority reported racial discrimination in 6 of 10 “everyday” situations.
Fig. 1.
Percentage reporting EDS more than once per year by item (n = 207).
Experiences of institution-specific discrimination
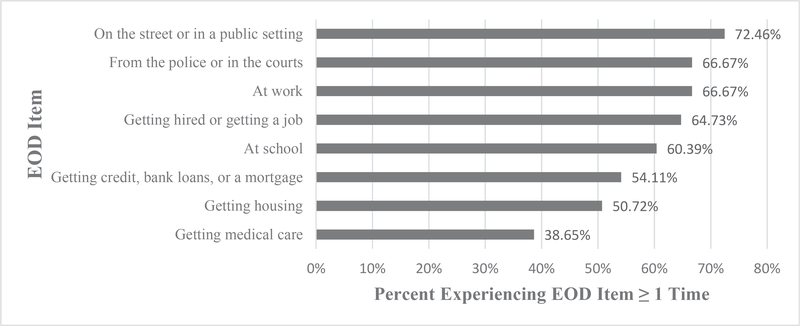
Approximately 2 in 3 women (64%) reported experiencing low to moderate levels of institutional racial discrimination (i.e., 1–3× in ≥1 domain) (Table 2). Figure 2 presents the frequency of reported experiences within each domain. A quarter reported high to very high levels (i.e., ≥4–6× within ≥1 domain), and over half reported at least one racial discrimination experience in 7 of 8 domains.
Fig. 2.
Percentage reporting EOD one or more times by item (n = 207).
Allostatic load
Mean AL score was 5.96 ± 2.24 (range 0–15) (Table 2).
Self-reported health
Precisely 3 in 4 women reported good to excellent health (75%) (Table 2).
Significance of exposure and outcome differences
Bivariate analyses compared exposure-to-exposure, outcome-to-outcome, and exposure-to-outcome differences (see Appendices A.3–4). Significant differences were found between EDS and EOD exposure measures (χ2 = 151; P < .01). Mean AL did not vary by self-reported health status (t = −0.51; P =.69). Estimates showed lower mean AL among “very high” (vs. “moderate”) EOD levels (F = 2.56; P = .04) and no variation for EDS (F = 1.21; P = .31). No differences were found between either EDS or EOD measure with self-reported health (χ2 = 4.05; P = .40 and χ2 = 2.08; 0.72, respectively).
Multivariable linear regression models
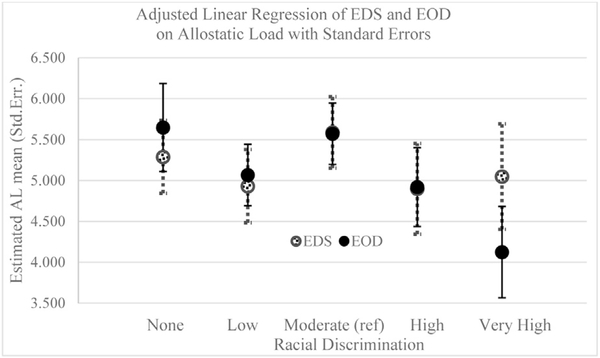
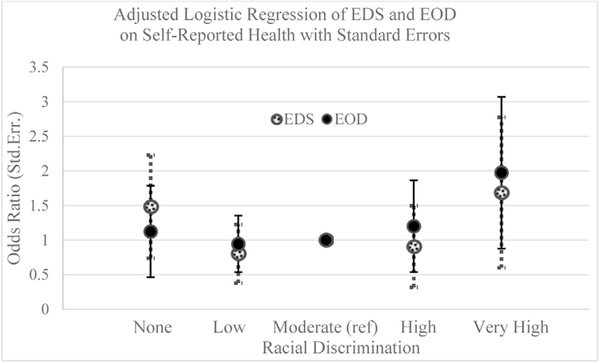
Figures 3 and 4 show the adjusted estimates for AL and self-reported health, respectively, for each EOD and EDS level. Compared with those reporting moderate discrimination (reference), there was a negative association between EOD and AL for those reporting very high discrimination (β = −1.31; 95% confidence interval = −2.41, −0.20) (Table 3). No significant association was found for EDS and AL. Self-reported health was not associated with EDS or EOD.
Fig. 3.
Linear regression of allostatic load by EDS and EOD measures adjusted for age, socioeconomic position, and medication use.
Fig. 4.
Logistic regression of self-reported health by EDS and by EOD measures adjusted for age, socioeconomic position, and medication use.
Table 3.
Linear regression of allostatic load and logistic regression of self-reported health by EDS and by EOD (n = 207)
| Discrimination | Allostatic load |
Self-reported physical health |
||
|---|---|---|---|---|
| EDS |
EOD |
EDS |
EOD |
|
| β* 95% CI | β† 95% CI | OR‡95% CI | OR§ 95% CI | |
| None | −0.166 (1.044, 0.713) | 0.216 (−0.847, 1.279) | 1.483 (0.554, 3.973) | 1.124 (0.354, 3.565) |
| Low | −0.524 (−1.408, 0.360) | −0.364 (−1.104, 0.377) | 0.804 (0.286, 2.256) | 0.945 (0.403, 2.213) |
| Moderate (ref) | ∥5.454 | ∥5.431 | 1.000 | 1.000 |
| High | −0.556 (−1.651, 0.539) | −0.512 (−1.464, 0.441) | 0.907 (0.253, 3.248) | 1.200 (0.405, 3.552) |
| Very high | −0.407 (−1.682, 0.869) | −1.307 (−2.411, −0.203) | 1.686 (0.476, 5.969) | 1.974 (0.664, 5.865) |
Models adjusted for age, socioeconomic position, and medication use. Bolded value represents P < .05.
β = beta coefficient; CI = confidence interval; OR = odds ratio.
(Power) = 1-β = 0.99 (R2 = 0.16, α = 0.05).
1-β = 0.99 (R2 = 0.18, α = 0.05).
1-β = 0.80 (r2 = 0.07, α = 0.10).
1-β = 0.80 (r2 = 0.06, α = 0.10).
estimated mean allostatic load.
Discussion
Summary of findings
In this study, we examined whether self-reported experiences of everyday versus institution-specific racial discrimination showed differing associations with AL and with self-reported health in a community sample of midlife black women. There were four main findings: as hypothesized, we found (1) differential associations between everyday versus institution-specific discrimination and health and (2) divergent associations between institution-specific racial discrimination and AL versus self-reported health. However, contrary to our hypothesis, we found (3) that racial discrimination did not predict self-reported health and (4) a negative association between chronic (vs. moderate) exposure to institutional racial discrimination and AL. Women reporting “very high” levels of EOD had lower levels of AL whereas EDS showed no association. These findings suggest that factors associated with reporting a high burden of institutional racial discrimination may contribute to lower subclinical disease risk for black women, and that the underlying biological manifestation of chronic exposure within major institutions may diverge from associations with health perception.
EOD and AL
Similar to our initial study [25], AL was lower among those reporting chronic institution-specific racial discrimination. Likewise, previous studies have shown inverse associations between adverse life experiences and biological stress reactivity [93–95]. Carpenter et al. demonstrated that childhood maltreatment predicted decreased adrenocorticotropin hormone and cortisol reactivity in adults [94]. Lovallo et al found an inverse dose-dependent effect of adverse life events on cortisol levels and heart rate [93]. We cannot make inferences about stress reactivity patterns because of the cross-sectional nature of our study. However, our finding provides support for the notion that reporting chronic racial discrimination may promote a blunted stress-response for black women.
The paradoxical relationship between reporting high levels of racial discrimination and a reduced stress-response has been previously reported [25,28,40,46]. Krieger and Sidney showed that working-class blacks reporting the highest frequency of EOD had lower risk of elevated systolic blood pressure, and the effect was stronger for women [40]. This buffered response related to racial discrimination has also emerged for cardiovascular disease risk among whites reporting an implicit bias connecting themselves to being a target of racial discrimination [28]. Scholars suggest that positive perceptions of within-group racial identity, as well as attributing negative experiences to systemic racism versus self-blame, may be health-protective [96–102].
Conversely, stress theory posits a biopsychosocial mechanism by which one’s appraisal of a recurrent threat, perceived resources, and working memory of previous exposures can result in a reduced (i.e., maladaptive) stress response [13,89,103–105]. Inadequate reactivity to stressors can be health-damaging long term via hyperactivity of supplementary mediators [13]. Indeed, in comparing the distributions of at-risk biomarkers within our sample, women taking cardiometabolic medications were at higher risk than nonmedication users (Appendix A.6). This interpretation proposes a deleterious biopsychosocial pathway. Because black women show the highest predicted AL than other racial/gender groups, an attenuated value could erroneously appear normal or “healthy”, masking the harmful changes in regulatory systems [12,13,89]. Further within-racial group longitudinal studies may help disentangle healthy versus unhealthy stress-response mechanisms associated with racial discrimination.
EDS and AL
Contrary to previous work, we did not find an association between everyday discrimination and AL, which could be explained by our EDS scale explicitly attributing the unfair treatment to one’s race/ethnicity [106]. Four recent publications found positive associations between everyday discrimination and AL using similar versions of the EDS [19,69,107,108]. However, these studies measured general unfair treatment as opposed to experiences of discrimination attributed to race/ethnicity. A meta-analysis including 30 years of studies examining chronic psychosocial factors and acute physiologic responses found that stress reactivity is contingent on the specific nature of the psychosocial exposure [109]. Research shows that most blacks attribute their discrimination experiences to race/ethnicity (vs. other social identities) [28,107,110,111], report racial discrimination as a predominant psychosocial stressor [40,108,110,112,113], and report more chronic experiences (vs. whites) [28,111]. Thus, our result may be partly representing the unique embodiment of routine race-based discrimination for black women as opposed to more general discriminatory exposures. In addition, commonplace stressors become highly predictable and less stressful resulting in a diminished stress response (e.g., military parachute training) [114]. This may help explain why daily racial discrimination showed no association with AL in our study versus studies measuring less predictable unfair treatment, such as getting housing.
EOD, EDS, and self-reported health
Our divergent finding from the literature of a null racial discrimination—self-reported health association for both EOD and EDS could be explained by our restricted sample of middle-aged black women. Racial discrimination levels likely did not vary enough between self-reported health categories to detect adjusted associations. Previous studies have demonstrated positive EDS associations with self-reported health comparing blacks with whites, comparing men with women, and based on estimates adjusted for age and gender [63,65,115]. Findings related to institution-specific racial discrimination are more disparate: EOD has predicted worse self-reported health among U.S. black CARDIA study participants [62] yet showed no association among U.S.-born nor foreign-born blacks in Boston [60]. Subtle differences in study sample composition likely contribute to such varied results. Our finding adds to this literature by demonstrating no within-group differences among Bay Area black women reporting any exposure to everyday or institutional racial discrimination. More importantly, findings suggest that racism-related self-perceived health may differ from actual disease risk, which, if further validated, could contribute substantially to our understanding of racial health disparities.
Limitations and strengths
There are several methodological considerations for this research. First, our cross-sectional design limits causal inference yet provides important evidence that may help inform future work in this area. We recruited a nonprobability sample intended to maximize variability in the exposure. Findings are not generalizable. However, our sample’s distribution of covariates was largely comparable with midlife black women living in the same counties in the 2013 American Community Survey [116]. Next, our utilization of two well-validated, reliable discrimination scales for exposure assessment strengthened the study’s internal validity while also allowing for comparability of our results with other studies. The EDS and EOD measures were highly but not perfectly correlated (r = 0.74) [117] showing that, conceptually, they are capturing different experiences and are not interchangeable (see Appendix A.4). Moreover, collapsing summary scores of discrimination responses into 5 categories across multiple life domains risks misclassifying those with highly frequent experiences in just one or two domains (e.g., being called names “almost everyday”) as low risk when such exposure frequency could be considered chronic. Nevertheless, constructing discrimination categories was supported by our sensitivity analysis comparing continuous and quintile-based exposure variables, which provided evidence that there were no linear or meaningful distribution-based associations (see Supplemental Tables 1–3). Patterns of qualitative-based versus distribution-based EDS and EOD measures showed no agreement (0% and 13%, respectively; see Appendix A.5), suggesting that qualitative categories may better represent conceptual increases in exposure. Furthermore, exposure and outcome misclassification due to poor recall is a fundamental limitation to any observational design using self-report. Finally, we greatly reduced potential misclassification of physical health by using biomarkers for AL assessment.
Conclusion
This study provides preliminary evidence that institutional racial discrimination may contribute to physiologic stress-regulation for midlife black women regardless of self-perceived health status. These findings introduce the potential utility of allostatic load as a clinical tool to assess black women’s underlying health risk. Furthermore, policy and program interventions addressing institutional racism may help mitigate chronic disease disparities. Additional research is needed to elucidate mediators and moderators that buffer the physiologic consequences of racial discrimination, particularly within major social institutions with a focus on black women taking medication to manage high cardiometabolic risk.
Supplementary Material
Acknowledgments
This research was supported by research grants from University of California, Berkeley Hellman Fund, USA; UCB Population Center, USA; UCB Research Bridging Grant, USA; Robert Wood Johnson Health and Society Scholars Program (UCB site), USA; UC Center for New Racial Studies, USA; and the UCB Institute for the Study of Societal Issues (ISSI), USA. M.D.T. was partially supported by NIGMS grant UL1GM118985, USA, and by a Ford Foundation Predoctoral Fellowship administered by the National Academies of Sciences, Engineering, and Medicine; E.K.M. was partially supported by grant GTDR14301469 from the Susan G. Komen Foundation; A.M.A. was also partially supported by NIMHD grant P60MD006902, USA. Study sponsors did not participate in study design, data collection, data analysis, interpretation of study results, or drafting of the manuscript.
The authors wish to thank Holly Berryman Stern from the UCB Tang Center for support related to laboratory specimens, Roger Hoffman of Westportal for in-kind support in building the CASIC platform, Miguel Dorta from StataCorp, Maureen Lahiff for comments on statistical analysis, Kirsten Bibbins-Domingo for comments related to physiologic dysregulation among vulnerable populations, and the many undergraduate, graduate, and postdoctoral students working as volunteer research assistants. They also thank the UCB epidemiology graduate students and members of the HEART Research Group (Health Effects Associated with Racism Threat) at the UCB ISSI for comments regarding the refinement of this article. They would also like to thank the anonymous reviewers for comments on previous drafts of this article.
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Appendix
Table A1.
Frequency (%) of racial discrimination (EDS) among AAWHHS participants (n = 207)
| Everyday discrimination domain | Never | <1/year | Few times/year | Few times/month | ≥1/week | Almost everyday | ≥1/year |
|---|---|---|---|---|---|---|---|
| You are treated with less courtesy than other people | 24 (11.82) | 25 (12.08) | 66 (31.88) | 39 (18.84) | 27 (13.30) | 26 (12.56) | 158 (76.33) |
| You are treated with less respect than other people | 35 (16.91) | 30 (14.49) | 57 (27.54) | 32 (15.46) | 25 (12.08) | 28 (13.53) | 142 (68.60) |
| You receive poorer service than other people at restaurants or stores | 28 (13.53) | 43 (20.77) | 57 (27.54) | 37 (17.87) | 19 (9.18) | 23 (11.11) | 136 (65.70) |
| People act as if they think you are not smart | 49 (23.67) | 35 (16.91) | 48 (23.19) | 21 (10.14) | 28 (13.53) | 26 (12.56) | 123 (59.42) |
| People act as if they are afraid of you | 69 (33.33) | 33 (15.94) | 32 (15.46) | 26 (12.56) | 22 (10.63) | 25 (12.08) | 105 (50.73) |
| People act as if you are dishonest | 64 (30.92) | 43 (20.77) | 38 (18.36) | 20 (9.66) | 14 (6.76) | 28 (13.53) | 100 (48.31) |
| People act as if they are better than you are | 26 (12.56) | 24 (11.59) | 45 (21.74) | 40 (19.32) | 25 (12.08) | 47 (22.71) | 157 (75.85) |
| You are called names or insulted | 95 (45.89) | 41 (19.81) | 34 (16.43) | 12 (5.80) | 12 (5.80) | 13 (6.28) | 71 (34.30) |
| You are threatened or harassed | 118 (57.00) | 33 (15.94) | 23 (11.11) | 10 (4.83) | 9 (4.35) | 14 (6.76) | 56 (27.05) |
| You are followed around in stores | 46 (22.22) | 37 (17.87) | 61 (29.47) | 18 (8.70) | 17 (8.21) | 28 (13.53) | 124 (59.90) |
AAWHHS=African American Women’s Heart & Health Study
Table A2.
Frequency (%) of racial discrimination (EOD scale) among AAWHHS participants (n = 207)
| Experience of discrimination domain | Never | Once | 2–3 times | 4–5 times | ≥6 times | ≥1 experiences |
|---|---|---|---|---|---|---|
| At school | 82 (39.61) | 23 (11.11) | 55 (26.57) | 17 (8.21) | 30 (14.49) | 125 (60.39) |
| Getting hired or getting a job | 73 (35.27) | 33 (15.94) | 52 (25.12) | 22 (10.63) | 27 (13.04) | 134 (64.73) |
| At work | 69 (33.33) | 34 (16.43) | 56 (27.05) | 22 (10.63) | 26 (12.56) | 138 (66.67) |
| Getting housing | 102 (49.28) | 23 (11.11) | 40 (19.32) | 14 (6.76) | 28 (13.53) | 105 (50.72) |
| Getting medical care | 127 (61.35) | 15 (7.25) | 30 (14.49) | 13 (6.28) | 22 (10.63) | 80 (38.65) |
| Getting credit, bank loans, or a mortgage | 95 (45.89) | 15 (7.25) | 47 (22.71) | 13 (6.28) | 37 (17.87) | 112 (54.11) |
| On the street or in a public setting | 57 (27.54) | 32 (15.46) | 70 (33.82) | 17 (8.21) | 31 (14.98) | 150 (72.46) |
| From the police or in the courts | 69 (33.33) | 45 (21.74) | 44 (21.26) | 22 (10.63) | 27 (13.04) | 138 (66.67) |
AAWHHS = African American Women’s Heart & Health Study.
Table A3.
χ2 test of homogeneity for qualitative-based reports of EDS and EOD among AAWHHS participants (n = 207)
| Discrimination | EOD | |||||
|---|---|---|---|---|---|---|
| EDS | None | Low | Moderate | High | Very High | Total |
| None | 19 | 31 | 8 | 1 | 0 | 59 |
| Low | 2 | 31 | 23 | 8 | 1 | 65 |
| Moderate | 0 | 6 | 19 | 8 | 5 | 38 |
| High | 1 | 2 | 9 | 10 | 4 | 26 |
| Very high | 0 | 1 | 4 | 2 | 12 | 19 |
| Total | 22 | 71 | 63 | 29 | 22 | 207 |
Pearson χ2 (16) = 151.5538; P = .000.
AAWHHS=African American Women’s Heart & Health Study.
Table A4.
Bivariate analyses for exposure-to-exposure, outcome-to-outcome, and exposure-to-outcome differences and correlations among AAWHHS participants (n = 207)
| Variable |
Allostatic load |
Self-reported health |
Pearson’s r |
||||
|---|---|---|---|---|---|---|---|
| Discrimination | β 95% CI | P-value | Good n (%) | Not Good n (%) | P-value | AL | EDS |
| EDS | .307 | .400 | −0.0127 | — | |||
| None | −0.126 (−1.048, 0.796) | 41 (26) | 18 (35) | ||||
| Low | −0.839 (−1.738, 0.060) | 52 (33) | 13 (25) | ||||
| Moderate (ref) | — | 30 (19) | 8 (16) | ||||
| High | −0.585 (−1.725, 0.556) | 21 (14) | 5 (10) | ||||
| Very high | −0.595 (−1.841, 0.650) | 12 (8) | 7 (14) | ||||
| EOD | .040 | .721 | −0.0950 | 0.7430 | |||
| None | 0.729 (−0.362, 1.819) | 16 (10) | 6 (12) | ||||
| Low | −0.517 (−1.279, 0.244) | 55 (35) | 16 (31) | ||||
| Moderate (ref) | — | 49 (31) | 14 (27) | ||||
| High | −0.260 (−1.249, 0.728) | 22 (14) | 7 (14) | ||||
| Very High | −1.226 (−2.316, −0.135) | 14 (9) | 8 (16) | ||||
| Self-reported health | (μ) | .694 | |||||
| Good (n = 156) | 5.917 (5.566, 6.268) | — | — | — | — | — | |
| Not good (n = 51) | 6.098 (5.481, 6.715) | — | — | — | — | — | |
AAWHHS = African American Women’s Heart & Health Study; β = beta coefficient; CI = confidence interval; SRH = self-reported health; μ = mean.
Table A5.
Kappa tests for qualitative-based and distribution-based reports of EDS and EOD among AAWHHS participants (n = 207)
| Discrimination | % Agreement | Kappa | P-value |
|---|---|---|---|
| EDS | 0.00 | −0.17 | 1.00 |
| EOD | 12.56 | −0.08 | 1.00 |
AAWHHS = African American Women’s Heart & Health Study.
Table A6.
Sample distribution of at-risk biomarkers by medication (Med) use among AAWHHS participants (n = 207)
| At-risk |
Full (n = 207) |
No Meds (n = 157) |
Meds (n = 50) |
P-value |
|---|---|---|---|---|
| System | n (%) | |||
| CV | ||||
| DBP | 104 (50) | 58 (37) | 46 (92) | .001 |
| SBP | 112 (54) | 66 (42) | 46 (92) | .001 |
| Inflammatory | ||||
| IL6 | 57 (28) | 46 (29) | 11 (22) | .314 |
| CRP | 103 (50) | 72 (46) | 31 (62) | .047 |
| Neuroendocrine | ||||
| Cortisol | 54 (26) | 35 (22) | 19 (38) | .028 |
| Epi | 51 (25) | 40 (25) | 11 (22) | .619 |
| Norepi | 51 (25) | 36 (23) | 16 (32) | .165 |
| Metabolic | ||||
| HDL | 87 (42) | 61 (39) | 19 (38) | .508 |
| LDL | 84 (41) | 62 (40) | 22 (44) | .572 |
| Tri | 15 (7) | 8 (5) | 5 (10) | .388 |
| Cholesterol | 138 (67) | 106 (68) | 33 (66) | .842 |
| BMI | 179 (86) | 135 (86) | 43 (86) | .911 |
| Waist | 151 (73) | 109 (70) | 41 (82) | .098 |
| Glucose | 40 (19) | 23 (15) | 17 (34) | .003 |
| A1c | 40 (19) | 27 (17) | 15 (30) | .028 |
AAWHHS = African American Women’s Heart & Health Study; CV = cardiovascular.
Footnotes
Supplementary data
Supplementary data associated with this article can be found in the online version at https://doi.org/10.1016/j.annepidem.2019.05.002.
References
- [1].Borrell LN, Kiefe CI, Diez-Roux AV, Williams DR, Gordon-Larsen P. Racial discrimination, racial/ethnic segregation, and health behaviors in the CARDIA study. Ethn Health 2013;18(3):227–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Dolezsar CM, McGrath JJ, Herzig AJM, Miller SB. Perceived racial discrimination and hypertension: a comprehensive systematic review. Health Psychol 2014;33(1):20–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Alhusen JL, Bower KM, Epstein E, Sharps P. Racial discrimination and adverse birth outcomes: an integrative review. J Midwifery Women Health 2016;61(6):707–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brondolo E, Ver Halen NB, Pencille M, Beatty D, Contrada RJ. Coping with racism: a selective review of the literature and a theoretical and methodological critique. J Behav Med 2009;32(1):64–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Hall WJ, Chapman MV, Lee KM, Merino YM, Thomas TW, Payne BK, et al. Implicit racial/ethnic bias among health care professionals and its influence on health care outcomes: a systematic review. Am J Public Health 2015;105(12):e60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Paradies Y, Ben J, Denson N, Elias A, Priest N, Pieterse A, et al. Racism as a determinant of health: a systematic review and meta-analysis. PloS one 2015;10(9):e0138511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pieterse AL, Todd NR, Neville HA, Carter RT. Perceived racism and mental health among Black American adults: a meta-analytic review. J Couns Psychol 2012;59:1–9. [DOI] [PubMed] [Google Scholar]
- [8].Priest N, Paradies Y, Trenerry B, Truong M, Karlsen S, Kelly Y.A systematic review of studies examining the relationship between reported racism and health and wellbeing for children and young people. Soc Sci Med 2013;95:115–27. [DOI] [PubMed] [Google Scholar]
- [9].Krieger N. Discrimination and health inequities. Int J Health Serv 2014;44(4): 643–710. [DOI] [PubMed] [Google Scholar]
- [10].Phelan JC, Link BG. Is racism a fundamental cause of inequalities in health? Annu Rev Sociol 2015;41:311–30. [Google Scholar]
- [11].Williams DR, Mohammed SA. Racism and health I: pathways and scientific evidence. Am Behav Sci 2013;57(8):1152–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health 2006;96(5):826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].McEwen BS. Protective and damaging effects of stress mediators: central role of the brain In: Mayer EA, Saper CB, editors. Biological basis for mind body interactions, vol. 122 Amsterdam: Elsevier Science Publ B V; 2000. p. 25–34. [DOI] [PubMed] [Google Scholar]
- [14].Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk: MacArthur studies of successful aging. Proc Natl Acad Sci U S A 2001;98(8):4770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Beckie TM. A systematic review of allostatic load, health, and health disparities. Biol Res Nurs 2012;14(4):311–46. [DOI] [PubMed] [Google Scholar]
- [16].McEwen BS. Stress, adaptation, and disease: allostasis and allostatic load. Ann N Y Acad Sci 1998;840(1):33–44. [DOI] [PubMed] [Google Scholar]
- [17].Chyu L, Upchurch DM. Racial and ethnic patterns of allostatic load among adult women in the United States: findings from the national health and nutrition examination survey 1999–2004. J Womens Health 2011;20(4): 575–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Deuster PA, Kim-Dorner SJ, Remaley AT, Poth M. Allostatic load and health status of African Americans and Whites. Am J Health Behav 2011;35(6):641–53. [DOI] [PubMed] [Google Scholar]
- [19].Upchurch DM, Stein J, Greendale GA, Chyu L, Tseng CH, Huang MH, et al. A longitudinal investigation of race, socioeconomic status, and psychosocial mediators of allostatic load in midlife women: findings from the study of women’s health across the nation. Psychosom Med 2015;77(4):402–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Jackson FM, Phillips MT, Hogue CJR, Curry-Owens TY. Examining the burdens of gendered racism: implications for pregnancy outcomes among college-educated African American women. Matern Child Health J 2001;5(2): 95–107. [DOI] [PubMed] [Google Scholar]
- [21].Bowleg L The problem with the phrase women and minorities: intersectionality—an important theoretical framework for public health. Am J Public Health 2012;102(7):1267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Crenshaw K Mapping the margins: intersectionality, identity politics, and violence against women of color. Stanf Law Rev 1991;43:1241–99. [Google Scholar]
- [23].Krieger N Theories for social epidemiology in the 21st century: an ecosocial perspective. Int J Epidemiol 2001;30(4):668–77. [DOI] [PubMed] [Google Scholar]
- [24].Brody GH, Lei MK, Chae DH, Yu T, Kogan SM, Beach SRH. Perceived discrimination among African American adolescents and allostatic load: a longitudinal analysis with buffering effects. Child Dev 2014;85(3):989–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Allen AM, Thomas MD, Michaels EK, Reeves AN, Okoye U, Price MM, et al. Racial discrimination, educational attainment, and biological dysregulation among midlife African American women. Psychoneuroendocrinology 2019;99:225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gee GC, Ford CL. Structural racism and health inequities: old issues, new directions. Du Bois Rev 2011;8(1):115–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Geronimus AT, Pearson JA, Linnenbringer E, Schulz AJ, Reyes AG, Epel ES, et al. Race-ethnicity, poverty, urban stressors, and telomere length in a detroit community-based sample. J Health Soc Behav 2015;56(2):199–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Krieger N, Waterman PD, Kosheleva A, Chen JT, Smith KW, Carney DR, et al. Racial discrimination & cardiovascular disease risk: my body my story study of 1005 US-born black and white community health center participants (US). PLoS One 2013;8(10):e77174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sabo S, Shaw S, Ingram M, Teufel-Shone N, Carvajal S, de Zapien JG, et al. Everyday violence, structural racism and mistreatment at the US-Mexico border. Soc Sci Med 2014;109:66–74. [DOI] [PubMed] [Google Scholar]
- [30].Mendez DD, Hogan VK, Culhane JF. Stress during pregnancy: the role of institutional racism. Stress Health 2013;29(4):266–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bastos JL, Celeste RK, Faerstein E, Barros AJD. Racial discrimination and health: a systematic review of scales with a focus on their psychometric properties. Soc Sci Med 2010;70(7):1091–9. [DOI] [PubMed] [Google Scholar]
- [32].Olshansky SJ, Antonucci T, Berkman L, Binstock RH, Boersch-Supan A, Cacioppo J, et al. Differences in life expectancy due to race and educational differences are widening, and many may not catch up. Health Aff (Millwood) 2012;31(8):1803–13. [DOI] [PubMed] [Google Scholar]
- [33].Geronimus AT, Hicken MT, Pearson JA, Seashols SJ, Brown KL, Cruz TD. Do US black women experience stress-related accelerated biological aging? Hum Nat 2010;21(1):19–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Gee GC, Walsemann KM, Brondolo E. A life course perspective on how racism may be related to health inequities. Am J Public Health 2012;102(5):967–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hayward MD, Miles TP, Crimmins EM, Yang Y. The significance of socioeconomic status in explaining the racial gap in chronic health conditions. Am Soc Rev 2000;1:910–30. [Google Scholar]
- [36].Hickson DA, Lewis TT, Liu JK, Mount DL, Younge SN, Jenkins WC, et al. The associations of multiple dimensions of discrimination and abdominal fat in African American adults: the jackson heart study. Ann Behav Med 2012;43(1):4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Lewis TT, Kravitz HM, Janssen I, Powell LH. Self-reported experiences of discrimination and visceral fat in middle-aged African-American and caucasian women. Am J Epidemiol 2011;173(11):1223–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Moody DLB, Brown C, Matthews KA, Bromberger JT. Everyday discrimination prospectively predicts inflammation across 7-years in racially diverse midlife women: study of women’s health across the nation. J Soc Issues 2014;70(2): 298–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Krieger N, Carney D, Lancaster K, Waterman PD, Kosheleva A, Banaji M. Combining explicit and implicit measures of racial discrimination in health research. Am J Public Health 2010;100(8):1485–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Krieger N, Sidney S. Racial discrimination and blood pressure: the CARDIA study of young black and white adults. Am J Public Health 1996;86(10): 1370–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Cardarelli R, Cardarelli KM, Fulda KG, Espinoza A, Cage C, Vishwanatha J, et al. Self-reported racial discrimination, response to unfair treatment, and coronary calcification in asymptomatic adults - the North Texas Healthy Heart study. BMC Public Health 2010;10:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lewis TT, Williams DR, Tamene M, Clark CR. Self-reported experiences of discrimination and cardiovascular disease. Curr Cardiovasc Risk Rep 2014;8(1):365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Wagner JA, Tennen H, Finan PH, Ghuman N, Burg MM. Self-reported racial discrimination and endothelial reactivity to acute stress in women. Stress Health 2013;29(3):214–21. [DOI] [PubMed] [Google Scholar]
- [44].Taylor TR, Williams CD, Makambi KH, Mouton C, Harrell JP, Cozier Y, et al. Racial discrimination and breast cancer incidence in US black women - The Black Women’s Health Study. Am J Epidemiol 2007;166(1):46–54. [DOI] [PubMed] [Google Scholar]
- [45].Gregoski MJ, Buxbaum SG, Kapuku G, Dong Y, Zhu H, Davis M, et al. Interactive influences of ethnicity, endothelin-1 gene, and everyday discrimination upon nocturnal ambulatory blood pressure. Ann Behav Med 2013;45(3):377–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Everage NJ, Gjelsvik A, McGarvey ST, Linkletter CD, Loucks EB. Inverse associations between perceived racism and coronary artery calcification. Ann Epidemiol 2012;22(3):183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Wagner J, Lampert R, Tennen H, Feinn R. Exposure to discrimination and heart rate variability reactivity to acute stress among women with diabetes. Stress Health 2015;31(3):255–62. [DOI] [PubMed] [Google Scholar]
- [48].Ganna A, Ingelsson E. 5 year mortality predictors in 498 103 UK Biobank participants: a prospective population-based study. Lancet 2015;386(9993): 533–40. [DOI] [PubMed] [Google Scholar]
- [49].Goldberg P, Gueguen A, Schmaus A, Nakache J, Goldberg M. Longitudinal study of associations between perceived health status and self reported diseases in the French Gazel cohort. J Epidemiol Community Health 2001;55(4):233–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kaplan GA, Goldberg DE, Everson SA, Cohen RD, Salonen R, Tuomilehto J, et al. Perceived health status and morbidity and mortality: evidence from the Kuopio ischaemic heart disease risk factor study. Int J Epidemiol 1996;25(2): 259–65. [DOI] [PubMed] [Google Scholar]
- [51].Crimmins EM, Johnston M, Hayward M, Seeman T. Age differences in allostatic load: an index of physiological dysregulation. Exp Gerontol 2003;38(7):731–4. [DOI] [PubMed] [Google Scholar]
- [52].Nobel L, Roblin DW, Becker ER, Druss BG, Joski PI, Allison JJ. Index of cardiometabolic health: a new method of measuring allostatic load using electronic health records. Biomarkers 2017;22(5):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mosca L, Barrett-Connor E, Kass Wenger N. Sex/gender differences in cardiovascular disease prevention: what a difference a decade makes. Circulation 2011;124(19):2145–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Bertakis KD, Azari R, Helms JL, Callahan EJ, Robbins JA. Gender differences in the utilization of health care services. J Fam Pract 2000;49:147–52. [PubMed] [Google Scholar]
- [55].Miilunpalo S, Vuori I, Oja P, Pasanen M, Urponen H. Self-rated health status as a health measure: the predictive value of self-reported health status on the use of physician services and on mortality in the working-age population. J Clin Epidemiol 1997;50(5):517–28. [DOI] [PubMed] [Google Scholar]
- [56].Loprinzi PD. Factors influencing the disconnect between self-perceived health status and actual health profile: implications for improving self-awareness of health status. Prev Med 2015;73:37–9. [DOI] [PubMed] [Google Scholar]
- [57].Harris R, Tobias M, Jeffreys M, Waldegrave K, Karlsen S, Nazroo J. Racism and health: the relationship between experience of racial discrimination and health in New Zealand. Soc Sci Med 2006;63(6):1428–41. [DOI] [PubMed] [Google Scholar]
- [58].Karlsen S, Nazroo JY. Relation between racial discrimination, social class, and health among ethnic minority groups. Am J Public Health 2002;92(4): 624–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Gee GC, Ryan A, Laflamme DJ, Holt J. Self-reported discrimination and mental health status among African descendants, Mexican Americans, and other Latinos in the New Hampshire REACH 2010 Initiative: the added dimension of immigration. Am J Public Health 2006;96(10):1821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Krieger N, Kosheleva A, Waterman PD, Chen JT, Koenen K. Racial discrimination, psychological distress, and self-rated health among US-born and foreign-born Black Americans. Am J Public Health 2011;101(9):1704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Brondolo E, Hausmann LR, Jhalani J, Pencille M, Atencio-Bacayon J, Kumar A, et al. Dimensions of perceived racism and self-reported health: examination of racial/ethnic differences and potential mediators. Ann Behav Med 2011;42(1):14–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Borrell LN, Kiefe CI, Williams DR, Diez-Roux AV, Gordon-Larsen P. Self-reported health, perceived racial discrimination, and skin color in African Americans in the CARDIA study. Soc Sci Med 2006;63(6):1415–27. [DOI] [PubMed] [Google Scholar]
- [63].Carty DC, Kruger DJ, Turner TM, Campbell B, DeLoney EH, Lewis EY. Racism, health status, and birth outcomes: results of a participatory community-based intervention and health survey. J Urban Health 2011;88(1):84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Paradies Y A systematic review of empirical research on self-reported racism and health. Int J Epidemiol 2006;35(4):888–901. [DOI] [PubMed] [Google Scholar]
- [65].Williams DR, Yan Y, Jackson JS, Anderson NB. Racial differences in physical and mental health: socio-economic status, stress and discrimination. J Health Psychol 1997;2(3):335–51. [DOI] [PubMed] [Google Scholar]
- [66].Ikram UZ, Snijder MB, Agyemang C, Schene AH, Peters RJ, Stronks K, et al. Perceived ethnic discrimination and the metabolic syndrome in ethnic minority groups: the healthy life in an urban setting study. Psychosom Med 2017;79(1):101–11. [DOI] [PubMed] [Google Scholar]
- [67].Krieger N, Smith K, Naishadham D, Hartman C, Barbeau EM. Experiences of discrimination: validity and reliability of a self-report measure for population health research on racism and health. Soc Sci Med 2005;61(7): 1576–96. [DOI] [PubMed] [Google Scholar]
- [68].Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 2010;35(1): 2–16. [DOI] [PubMed] [Google Scholar]
- [69].Tomfohr LM, Pung MA, Dimsdale JE. Mediators of the relationship between race and allostatic load in African and white Americans. Health Psychol 2016;35(4):322–32. [DOI] [PubMed] [Google Scholar]
- [70].Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation–allostatic load and its health consequences: MacArthur studies of successful aging. Arch Intern Med 1997;157(19):2259–68. [PubMed] [Google Scholar]
- [71].Robinette JW, Charles ST, Almeida DM, Gruenewald TL. Neighborhood features and physiological risk: an examination of allostatic load. Health Place 2016;41:110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Tan M, Mamun A, Kitzman H, Mandapati SR, Dodgen L. Neighborhood disadvantage and allostatic load in African American women at risk for obesity-related diseases. Prev Chronic Dis 2017;14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Zilioli S, Imami L, Ong AD, Lumley MA, Gruenewald T. Discrimination and anger control as pathways linking socioeconomic disadvantage to allostatic load in midlife. J Psychosom Res 2017;103:83–90. [DOI] [PubMed] [Google Scholar]
- [74].Finch BK, Hummer RA, Kol B, Vega WA. The role of discrimination and acculturative stress in the physical health of Mexican-origin adults. Hispanic J Behav Sci 2001;23(4):399–429. [Google Scholar]
- [75].Kawachi I, Kennedy BP, Glass R. Social capital and self-rated health: a contextual analysis. Am J Public Health 1999;89(8):1187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Kennedy BP, Kawachi I, Glass R, Prothrow-Stith D. Income distribution, socioeconomic status, and self rated health in the United States: multilevel analysis. Bmj 1998;317(7163):917–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Subramanian S, Kawachi I, Kennedy BP. Does the state you live in make a difference? Multilevel analysis of self-rated health in the US. Soc Sci Med 2001;53(1):9–19. [DOI] [PubMed] [Google Scholar]
- [78].Yen IH, Kaplan GA. Poverty area residence and changes in depression and perceived health status: evidence from the Alameda County Study. Int J Epidemiol 1999;28(1):90–4. [DOI] [PubMed] [Google Scholar]
- [79].Idler EL, Benyamini Y. Self-rated health and mortality: a review of twentyseven community studies. J Health Soc Behav 1997;38:21–37. [PubMed] [Google Scholar]
- [80].Psaty BM, Koepsell TD, Lin D, Weiss NS, Siscovick DS, Rosendaal FR, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc 1999;47(6):749–54. [DOI] [PubMed] [Google Scholar]
- [81].Shapiro S Confounding by indication? Epidemiology 1997;8(1):110. [PubMed] [Google Scholar]
- [82].Brodish AB, Cogburn CD, Fuller-Rowell TE, Peck S, Malanchuk O, Eccles JS. Perceived racial discrimination as a predictor of health behaviors: the moderating role of gender. Race Soc Probl 2011;3(3):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Feagin J, Bennefield Z. Systemic racism and US health care. Soc Sci Med 2014;103:7–14. [DOI] [PubMed] [Google Scholar]
- [84].Forsyth JM, Schoenthaler A, Ogedegbe G, Ravenell J. Perceived racial discrimination and adoption of health behaviors in hypertensive black Americans: the CAATCH trial. J Health Care Poor Underserved 2014;25(1):276–91. [DOI] [PubMed] [Google Scholar]
- [85].Johnson P, Risica PM, Gans KM, Kirtania U, Kumanyika SK. Association of perceived racial discrimination with eating behaviors and obesity among participants of the SisterTalk study. J Natl Black Nurses Assoc 2012;23(1):34. [PMC free article] [PubMed] [Google Scholar]
- [86].Lee C, Ayers SL, Kronenfeld JJ. The association between perceived provider discrimination, health care utilization, and health status in racial and ethnic minorities. Ethn Dis 2009;19(3):330. [PMC free article] [PubMed] [Google Scholar]
- [87].Gibbons FX, Kingsbury JH, Weng C-Y, Gerrard M, Cutrona C, Wills TA, et al. Effects of perceived racial discrimination on health status and health behavior: a differential mediation hypothesis. Health Psychol 2014;33(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rubin DB. Multiple imputations in sample surveys-a phenomenological Bayesian approach to nonresponse. In: Paper presented at: Proceedings of the survey research methods section of the American Statistical Association; 1978. [Google Scholar]
- [89].Lovallo WR. Stress and health: biological and psychological interactions, 3rd ed. Thousand Oaks, CA: Sage publications; 2015. [Google Scholar]
- [90].Seplaki CL, Goldman N, Weinstein M, Lin YH. Measurement of cumulative physiological dysregulation in an older population. Demography 2006;43(1):165–83. [DOI] [PubMed] [Google Scholar]
- [91].Rubin DB. Multiple imputation for nonresponse in surveys, vol. 81 Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- [92].Soper D Post-hoc statistical power for multiple regression. https://www.danielsoper.com/statcalc/calculator.aspx?id=9. [Accessed 8 January 2018].
- [93].Lovallo WR, Farag NH, Sorocco KH, Cohoon AJ, Vincent AS. Lifetime adversity leads to blunted stress axis reactivity: studies from the Oklahoma family health patterns project. Biol Psychiatry 2012;71(4):344–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Carpenter LL, Carvalho JP, Tyrka AR, Wier LM, Mello AF, Mello MF, et al. Decreased adrenocorticotropic hormone and cortisol responses to stress in healthy adults reporting significant childhood maltreatment. Biol Psychiatry 2007;62(10):1080–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Carpenter LL, Shattuck TT, Tyrka AR, Geracioti TD, Price LH. Effect of childhood physical abuse on cortisol stress response. Psychopharmacology 2011;214(1):367–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chae DH, Lincoln KD, Adler NE, Syme SL. Do experiences of racial discrimination predict cardiovascular disease among African American men? The moderating role of internalized negative racial group attitudes. Soc Sci Med 2010;71(6):1182–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Chae DH, Nuru-Jeter AM, Adler NE. Implicit racial bias as a moderator of the association between racial discrimination and hypertension: a study of Midlife African American men. Psychosom Med 2012;74(9):961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Crocker J, Major B. Social stigma and self-esteem: the self-protective properties of stigma. Psychol Rev 1989;96(4):608. [Google Scholar]
- [99].LaVeist TA, Sellers R, Neighbors HW. Perceived racism and self and system blame attribution: consequences for longevity. Ethn Dis 2001;11(4):711–21. [PubMed] [Google Scholar]
- [100].Sellers RM, Caldwell CH, Schmeelk-Cone KH, Zimmerman MA. Racial identity, racial discrimination, perceived stress, and psychological distress among African American young adults. J Health Soc Behav 2003;1:302–17. [PubMed] [Google Scholar]
- [101].Mossakowski KN. Coping with perceived discrimination: does ethnic identity protect mental health? J Health Soc Behav 2003;44(3):318–31. [PubMed] [Google Scholar]
- [102].Tull ES, Sheu Y-T, Butler C, Cornelious K. Relationships between perceived stress, coping behavior and cortisol secretion in women with high and low levels of internalized racism. J Natl Med Assoc 2005;97(2):206. [PMC free article] [PubMed] [Google Scholar]
- [103].Suls JM, Luger T, Martin R. The biopsychosocial model and the use of theory in health psychology In: Suls JM, Davidson KW, Kaplan GA, editors. Handbook of health psychology and behavioral medicine. New York: Guilford Press; 2010. p. 15–27. [Google Scholar]
- [104].Lazarus R, Folkman S. Stress, coping and appraisal. New York: Springer; 1984. [Google Scholar]
- [105].Psychoneuroimmunology Ader R. - conditioning and stress (Vol 44, PG 77, YR 1993). Annu Rev Psychol 1994;45:U8. [DOI] [PubMed] [Google Scholar]
- [106].Kressin NR, Raymond KL, Manze M. Perceptions of race/ethnicity-based discrimination: a review of measures and evaluation of their usefulness for the health care setting. J Health Care Poor Underserved 2008;19(3):697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ong AD, Williams DR, Nwizu U, Gruenewald TL. Everyday unfair treatment and multisystem biological dysregulation in African American adults. Cult Divers Ethn Minor Psychol 2017;23(1):27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Fuller-Rowell TE, Evans GW, Ong AD. Poverty and health: the mediating role of perceived discrimination. Psychol Sci 2012;23(7):734–9. [DOI] [PubMed] [Google Scholar]
- [109].Chida Y, Hamer M. Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: a quantitative review of 30 years of investigations: Psychological Bulletin 2008;134(6):829. [DOI] [PubMed] [Google Scholar]
- [110].Nuru-Jeter A, Dominguez TP, Hammond WP, Leu J, Skaff M, Egerter S, et al. “It’s the skin you’re in”: African-American women talk about their experiences of racism. An exploratory study to develop measures of racism for birth outcome studies. Matern Child Health J 2009;13(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Williams DR, John DA, Oyserman D, Sonnega J, Mohammed SA, Jackson JS. Research on discrimination and health: an exploratory study of unresolved conceptual and measurement issues. Am J Public Health 2012;102(5):975–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Cunningham TJ, Seeman TE, Kawachi I, Gortmaker SL, Jacobs DR, Kiefe C, et al. Racial/ethnic and gender differences in the association between self-reported experiences of racial/ethnic discrimination and inflammation in the CARDIA cohort of 4 US communities. Soc Sci Med 2012;75(5):922–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lewis TT, Aiello AE, Leurgans S, Kelly J, Barnes LL. Self-reported experiences of everyday discrimination are associated with elevated C-reactive protein levels in older African-American adults. Brain Behav Immun 2010;24(3):438–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Sapolsky RM. Why is psychological stress stressful? In: Sapolsky RM, editor. Why zebra’s don’t get ulcers. 3rd ed. New York: WH Freeman; 1994. p. 252–70. [Google Scholar]
- [115].Nuru-Jeter AM, Michaels EK, Thomas MD, Reeves AN, Thorpe RJ, LaVeist TA. Relative roles of race versus socioeconomic position in studies of health inequalities: a matter of interpretation. Annu Rev Public Health 2018;39:169–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].United States Census Bureau. General demographic characteristics. 2018.
- [117].Mukaka MM. A guide to appropriate use of correlation coefficient in medical research. Malawi Med J 2012;24(3):69–71. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.