New drugs or therapeutic combinations are urgently needed against Mycobacterium abscessus. Previously, we demonstrated the potent activity of indole-2-carboxamides 6 and 12 against M. abscessus. We show here that these compounds act synergistically with imipenem and cefoxitin in vitro and increase the bactericidal activity of the β-lactams against M. abscessus. In addition, compound 12 also displays synergism with imipenem and cefoxitin within infected macrophages.
KEYWORDS: Mycobacterium abscessus, indole-2-carboxamide, β-lactam, MmpL3, drug synergism, macrophage, therapeutic activity
ABSTRACT
New drugs or therapeutic combinations are urgently needed against Mycobacterium abscessus. Previously, we demonstrated the potent activity of indole-2-carboxamides 6 and 12 against M. abscessus. We show here that these compounds act synergistically with imipenem and cefoxitin in vitro and increase the bactericidal activity of the β-lactams against M. abscessus. In addition, compound 12 also displays synergism with imipenem and cefoxitin within infected macrophages. The clinical potential of these new drug combinations requires further evaluation.
TEXT
Mycobacterium abscessus is a fast-growing mycobacterial species found particularly frequently in patients with cystic fibrosis (CF), bronchiectasis, and chronic obstructive pulmonary diseases (COPD) (1, 2). In the context of CF and COPD, M. abscessus has emerged as an important opportunistic pathogen responsible for significant mortality (3). However, treatment of M. abscessus lung disease remains particularly challenging, largely due to intrinsic resistance of M. abscessus to most antibiotic classes (1, 2). The typical treatment regimen includes a combination of macrolides, aminoglycosides, and intravenous β-lactams (cefoxitin or imipenem) for at least 12 months (2). There is no reliable therapeutic strategy for the treatment of M. abscessus pulmonary infections, and the lengthy treatment duration and drug toxicity effects are often accompanied by severe undesirable outcomes. Thus, there is an unmet clinical need for new drug regimens with improved efficacy to treat these infections. Along with the development of repurposed drugs, the drug pipeline has recently been fueled with chemical entities acting on new targets in M. abscessus, such as the mycolic acid transporter MmpL3, which is inhibited by a wide range of structurally unrelated small molecules (4). Chemical inhibition of MmpL3 abolishes the export of trehalose monomycolate to the outer membrane, leading to significant bacterial growth inhibition. In M. abscessus, these chemotypes include a piperidinol-based compound (PIPD1) (5), benzimidazoles (6), and indole-2-carboxamide derivatives (7, 8). They exhibit high levels of activity against clinical isolates in vitro, in macrophages, in zebrafish, and in an acute murine model of M. abscessus infection (5–7, 9). Due to their pronounced role in modulating the cell wall architecture and composition, it may be speculated that chemical inhibition of MmpL3 would increase the efficacy of other drugs. Although this has been reported in M. tuberculosis, whereby the indole carboxamides and adamantyl-ureas act synergistically with rifampin, bedaquiline, clofazimine, and β-lactams (10), to date this has not been investigated in M. abscessus.
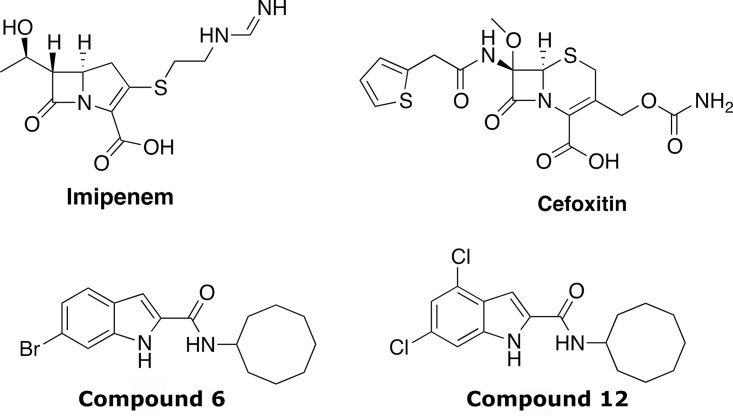
Indole carboxamides 6 and 12 (Fig. 1) present favorable absorption, distribution, metabolism, and excretion (ADME) properties (7, 11), and the ease of obtaining them in high yields prompted us to investigate their interaction profiles with different classes of antibiotics active against M. abscessus and/or used as part of clinical treatment regimens. These include sutezolid, an oxazolidinone that inhibits bacterial translation (12), clofazimine, which affects energy metabolism (13), and particularly, β-lactams (the cephalosporin cefoxitin [FOX] and the carbapenem imipenem [IPM]), which inhibit peptidoglycan biosynthesis and are reported to act in synergy with different drugs against M. abscessus (14, 15) (Fig. 1). The MICs were determined according to the CLSI guidelines (16) in cation-adjusted Mueller-Hinton broth (CaMHB; Sigma-Aldrich). Pair combinations between Cpd6 and Cpd12 with other drugs were tested in CaMHB in a typical checkerboard assay (17) with resazurin reduction as a metabolic readout. This allowed us to establish the fractional inhibitory concentration index (FICI) of each drug combination, where the FICI was determined using the following formula: MICA with B/MICA alone + MICB with A/MICB alone; values ≤0.5 were considered synergistic, those from 0.5 to 4 were considered indifferent, and those ≥4, antagonist (10). While Cpd12 showed a FICI value of ≤0.5 with IPM or FOX, indicative of synergistic interactions, no interaction (indifference) was recorded with clofazimine or sutezolid. A similar interaction profile was observed when combining these drugs with Cpd6 (Table 1).
FIG 1.
Structures of imipenem, cefoxitin, and the lead indole carboxamides 6 and 12 used in this study.
TABLE 1.
Interaction of Cpd6 and Cpd12 with other antibiotics against M. abscessus CIP104536T (smooth strain) assessed by checkerboards REMA in CaMHBa
| Compound | MIC (μg/ml) | Interaction with Cpd12 |
Interaction with Cpd6 |
||||
|---|---|---|---|---|---|---|---|
| FICI (mean) | SD | Outcome | FICI (mean) | SD | Outcome | ||
| Cpd12 | 0.125 | ||||||
| Cpd6 | 0.25 | ||||||
| SUT | 16 | 0.84 | ±0.27 | Indifferent | 0.62 | ±0 | Indifferent |
| IPM | 16 | 0.5 | ±0.18 | Synergistic | 0.5 | ±0 | Synergistic |
| FOX | 64 | 0.45 | ±0.14 | Synergistic | 0.44 | ±0.16 | Synergistic |
| CFZ | 0.5 | 0.88 | ±0.18 | Indifferent | 0.88 | ±0.18 | Indifferent |
Results are the mean of the FICI ± SD of 3 independent experiments. SUT, sutezolid; IPM, imipenem; FOX, cefoxitin; CFZ, clofazimine.
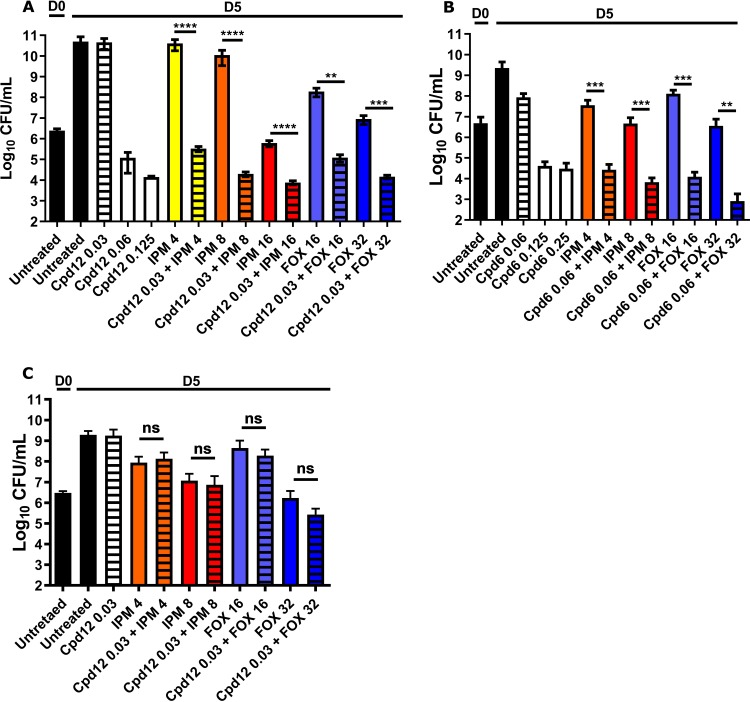
To determine the optimal concentration of Cpd12 showing no or little activity against M. abscessus CIP104536T (S variant), cultures were exposed to concentrations ranging from 0.03 to 0.125 μg/ml Cpd12 prior to CFU determination at 5 days postexposure. While at the MIC (0.125 μg/ml) there was an ∼6 to 7 log drop in the CFU counts, no decrease was observed at 0.03 μg/ml (Fig. 2A). This concentration was thus chosen to investigate the potential synergistic activity of Cpd12 with β-lactams. IPM was used at 4, 8, and 16 μg/ml, and FOX was used at 16 and 32 μg/ml, corresponding to concentrations 4- and 2-fold lower than their MICs, respectively (Table 1). At these sub-MIC levels, Cpd12 plus IPM decreased CFU counts by ∼4 to 6 log compared to Cpd12 or IPM alone. Similarly, FOX alone at 16 and 32 μg/ml was accompanied by a reduction in the CFU counts, while the addition of 0.03 μg/ml Cpd12 further reduced the CFU by ∼2 to 3 log (Fig. 2A). Comparable results were obtained when assessing the synergistic activity of Cpd6 with IPM or FOX (Fig. 2B). At 0.06 μg/ml and 0.125 μg/ml Cpd6, the CFU were reduced by ∼1 and 5 log, respectively, and no further decrease in the CFU was observed at 0.25 μg/ml. The addition of 4 μg/ml or 8 μg/ml IPM to 0.06 μg/ml Cpd6 resulted in an ∼3 log decrease in the CFU compared to IPM alone. Similarly, the simultaneous addition of 0.06 μg/ml Cpd6 to FOX (at 16 or 32 μg/ml) exacerbated the effect of FOX, leading to an ∼4 log decrease in the CFU compared to FOX alone (Fig. 2B). To assess whether these interactions are due to the chemical inhibition of MmpL3, the CFU killing assay was repeated using a strain highly resistant to both Cpd12 and Cpd6 due to the presence of an A309P missense mutation in MmpL3 (MICCpd12/Cpd6 of 32 μg/ml, [7]). Figure 2C shows that the Cpd12 plus IPM or Cpd12 plus FOX synergistic interactions were abolished, indicating that inhibition of MmpL3 is necessary to establish drug synergism with the β-lactams. This confirms a previous study demonstrating that synergistic interactions between the indole carboxamides NITD-304 and NITD-349 with other clinically relevant drugs are diminished in an MmpL3 mutant of M. tuberculosis resistant to indole carboxamides (10).
FIG 2.
Synergistic activity of indole-2-carboxamide derivatives with IPM and FOX in vitro. CFU counts of Cpd12 (A) and Cpd6 (B) given alone and in combination with imipenem (IPM) or cefoxitin (FOX). M. abscessus cultures were incubated at 30°C in CaMHB for 5 days in the presence of the indicated compounds (μg/ml) and plated on LB agar prior to CFU enumeration. (C) For CFU determination, the M. abscessus mutant A309P (spontaneous resistant strain to Cpd12 carrying the A309P mutation in MmpL3) was exposed to the indicated antibiotics (μg/ml) at 30°C in CaMHB for 5 days. Graphs represent the mean of three independent experiments completed in triplicate. Data are expressed as the mean ± standard deviation (SD). The statistical test used is a nonparametric Mann-Whitney t test in which the combinations were compared to the drugs alone. ns, nonsignificant; **, P ≤ 0.01; ***, P ≤ 0.001.
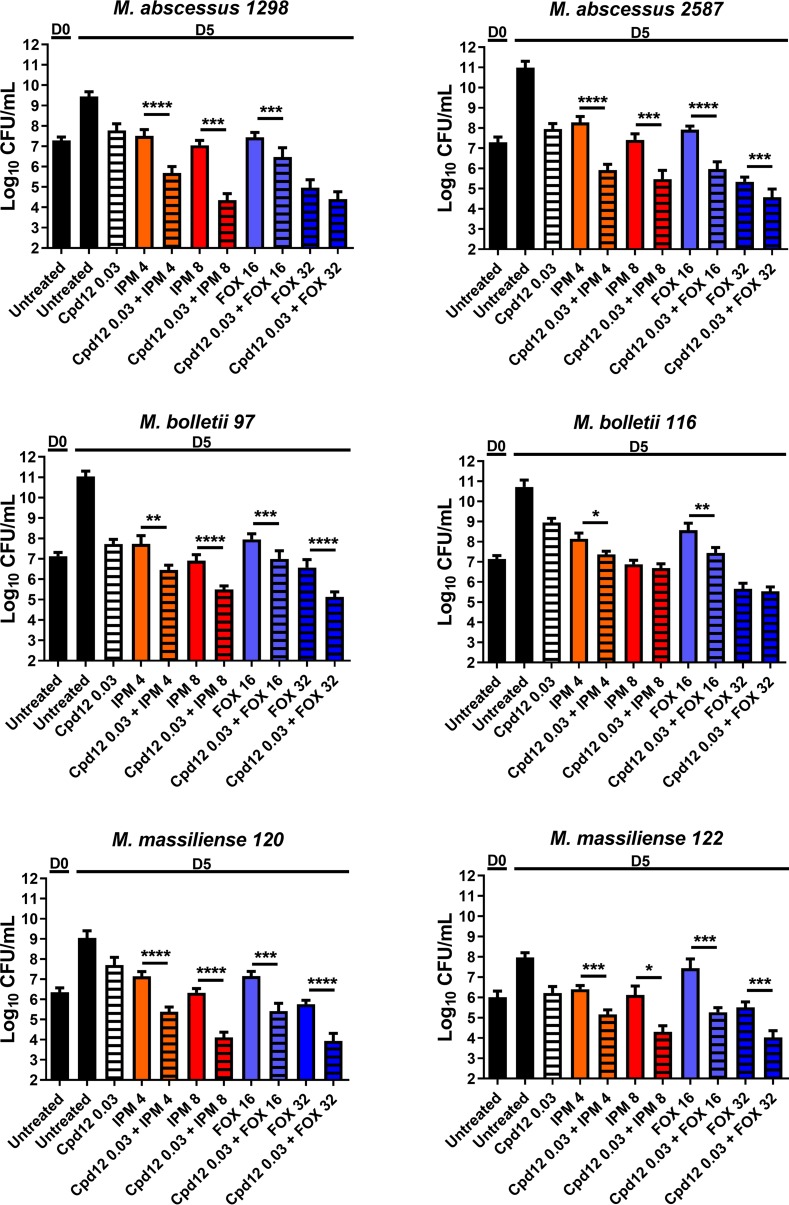
The M. abscessus complex comprises three subspecies, M. abscessus subsp. abscessus, M. abscessus subsp. bolletii, and M. abscessus subsp. massiliense (18), displaying different drug susceptibility profiles. We therefore tested the activity of the Cpd12/β-lactam combinations against a panel of M. abscessus complex clinical isolates (19, 20) by determining the CFU counts of two M. abscessus subsp. abscessus strains (1298 and 2587), two M. abscessus subsp. bolletii strains (97 and 116), and two M. abscessus subsp. massiliense strains (120 and 122). In general, the combination of Cpd12 plus IPM or Cpd12 plus FOX resulted in significantly reduced CFU counts compared to the cultures exposed to Cpd12, IPM, or FOX alone. However, the 6 strains responded differently to each of these drug combinations (Fig. 3). Overall, CFU determination was in direct agreement with the checkerboard results and indicates that low concentrations of Cpd6 and Cpd12 improve the activity of IPM or FOX against the M. abscessus complex in vitro.
FIG 3.
CFU determination of clinical isolates exposed to Cpd12 given alone or in combination with imipenem (IPM) or cefoxitin (FOX). M. abscessus cultures were incubated at 30°C in CaMHB for 5 days in the presence of the indicated compounds (μg/ml) and plated on LB agar prior to CFU enumeration. Data are expressed as the mean ± SD from three independent experiments completed in triplicate. The statistical test used is a nonparametric Mann-Whitney t test in which the combinations were compared to the drugs alone. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P < 0.0001.
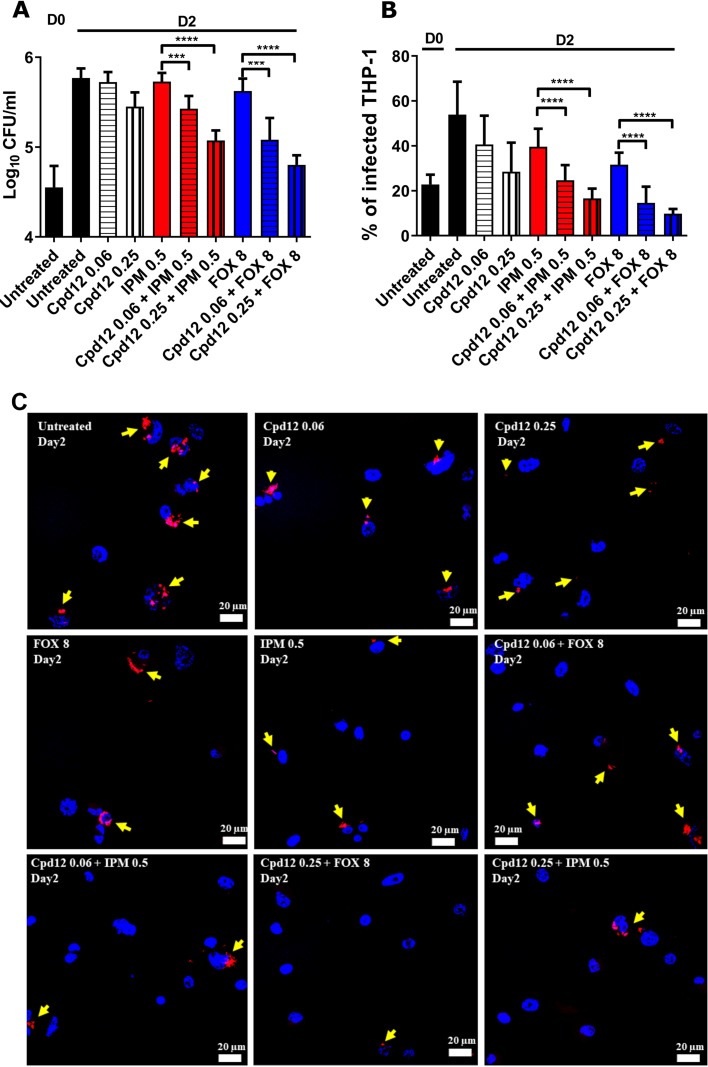
The activity of IPM and FOX alone or in combination with Cpd12 was next evaluated using THP-1 macrophages infected with M. abscessus CIP104536T (S variant) carrying pTEC27, as previously described (6). Infected cells were either left untreated or exposed for 2 days to Cpd12, IPM, or FOX alone or in combination, lysed, and plated for subsequent intracellular bacterial load determination. While IPM and FOX displayed only minor effects at the concentrations tested, the addition of 0.06 μg/ml Cpd12 significantly reduced the bacterial burden by ∼0.5 log (Fig. 4A). This effect was further exacerbated (1 log reduction) when 0.25 μg/ml Cpd12 was used. A microscopy-based infectivity assay reported earlier (6, 21) was subsequently used to quantify the impact of drug treatment on the percentage of infected THP-1 cells. The results confirm the pronounced reduction in the number of infected macrophages treated with Cpd12 plus IPM or Cpd12 plus FOX (∼50% decrease with 0.06 μg/ml Cpd12) compared to cells treated with the drugs alone at day 2 postinfection (Fig. 4B and C). Collectively, these findings suggest that the Cpd12/IPM and Cpd12/FOX combinations are effective on intracellular M. abscessus.
FIG 4.
Impact of Cpd12 alone or in combination on intracellular-residing M. abscessus. THP-1 macrophages were infected with M. abscessus S expressing TdTomato (multiplicity of infection [MOI] of 2:1) and treated with the indicated compounds (μg/ml). (A) CFU were determined at day 0 and day 2 postinfection. Data represents the mean ± SD of three independent experiments completed in triplicate. For statistical analysis, a nonparametric Mann-Whitney t test was performed. ***, P ≤ 0.001; ****, P < 0.0001. (B) Percentage of infected THP-1 macrophages at day 0 and day 2 postinfection. Data shown are mean values ± SD for one representative experiment completed in triplicate. One-way analysis of variance (ANOVA) Kruskal-Wallis was used as a statistical test. ****, P < 0.0001. (C) Immunofluorescent fields were taken at day 2 postinfection at magnification 40× (using confocal microscopy) showing the nuclei of macrophages (DAPI in blue) infected with red-fluorescent M. abscessus in the absence or in the presence of the drugs used alone or in combination. Yellow arrows emphasize red-fluorescent M. abscessus (tdTomato) within macrophages. Only intracellular bacteria that were individually observed under the microscope were recorded.
IPM use is usually associated with improved outcome for the treatment of M. abscessus pulmonary disease (22), and IPM combined with other antibiotics exerts a synergistic or additive effect contributing to its success (14, 15). However, resistance to IPM is also emerging, highlighting the limiting application of IPM in the treatment of M. abscessus infections (23, 24). The present results highlight the therapeutic potential of the Cpd12/IPM combination against a panel of clinical M. abscessus complex isolates. This combination may help lower the effective dose of IPM, thus possibly limiting the emergence of IPM-resistant strains. Similarly, the use of indole carboxamides as companion drugs further reduces the effective concentrations of FOX, restricting the eventual emergence of M. abscessus-resistant mutants. A plausible hypothesis explaining this synergistic activity may rely on the fact that the indole carboxamides, through inhibition of mycolic acid transport at the bacterial surface, disorganize and disrupt the mycomembrane, which accelerates the penetration of the β-lactam drugs to reach their targets (the l,d-transpeptidase for IPM and the d,d-transpeptidase for FOX), leading to inhibition of peptidoglycan synthesis. Conversely, inhibition of the peptidoglycan transpeptide linkages by the β-lactams may also facilitate the access of Cpd6 or Cpd12 to their inner membrane target. However, other underlying mechanisms may be responsible for the observed synergistic effects, and further research is required.
In summary, indole-2-caboxamides represent a promising chemotype improving the activity of FOX and IPM, two recommended drugs for the treatment of M. abscessus pulmonary infections (2). Future studies should evaluate whether β-lactamase inhibitors (25, 26) would further improve the observed synergistic interactions. Our results indicate that the Cpd12/β-lactam combinations are highly effective within macrophages by reducing the intracellular bacterial burden and the percentage of infected cells, emphasizing the need for further evaluation in preclinical animal models.
Data availability.
All data are available upon request.
ACKNOWLEDGMENTS
This study was supported by funding from the Association Gregory Lemarchal and Vaincre la Mucoviscidose (RIF20180502320) to L.K. M.D.J. received a postdoctoral fellowship granted by Labex EpiGenMed, an “Investissements d’Avenir” program (ANR-10-LABX-12-01).
The authors have no conflict of interest to declare.
REFERENCES
- 1.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ, Winthrop K, ATS Mycobacterial Diseases Subcommittee, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 2.Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann J-L, Nick JA, Noone PG, Bilton D, Corris P, Gibson RL, Hempstead SE, Koetz K, Sabadosa KA, Sermet-Gaudelus I, Smyth AR, van Ingen J, Wallace RJ, Winthrop KL, Marshall BC, Haworth CS. 2016. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary. Thorax 71:88–90. doi: 10.1136/thoraxjnl-2015-207983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brode SK, Daley CL, Marras TK. 2014. The epidemiologic relationship between tuberculosis and non-tuberculous mycobacterial disease: a systematic review. Int J Tuber Lung Dis 18:1370–1377. doi: 10.5588/ijtld.14.0120. [DOI] [PubMed] [Google Scholar]
- 4.Li W, Yazidi A, Pandya AN, Hegde P, Tong W, Calado Nogueira de Moura V, North EJ, Sygusch J, Jackson M. 2018. MmpL3 as a target for the treatment of drug-resistant nontuberculous mycobacterial infections. Front Microbiol 9:1547. doi: 10.3389/fmicb.2018.01547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupont C, Viljoen A, Dubar F, Blaise M, Bernut A, Pawlik A, Bouchier C, Brosch R, Guérardel Y, Lelièvre J, Ballell L, Herrmann J-L, Biot C, Kremer L. 2016. A new piperidinol derivative targeting mycolic acid transport in Mycobacterium abscessus. Mol Microbiol 101:515–529. doi: 10.1111/mmi.13406. [DOI] [PubMed] [Google Scholar]
- 6.Raynaud C, Daher W, Johansen MD, Roquet-Baneres F, Blaise M, Onajole OK, Kozikowski AP, Herrmann JL, Dziadek J, Gobis K, Kremer L. 2020. Active benzimidazole derivatives targeting the MmpL3 transporter in Mycobacterium abscessus. ACS Infect Dis 6:324–337. doi: 10.1021/acsinfecdis.9b00389. [DOI] [PubMed] [Google Scholar]
- 7.Kozikowski AP, Onajole OK, Stec J, Dupont C, Viljoen A, Richard M, Chaira T, Lun S, Bishai W, Raj VS, Ordway D, Kremer L. 2017. Targeting mycolic acid transport by indole-2-carboxamides for the treatment of Mycobacterium abscessus infections. J Med Chem 60:5876–5888. doi: 10.1021/acs.jmedchem.7b00582. [DOI] [PubMed] [Google Scholar]
- 8.Franz ND, Belardinelli JM, Kaminski MA, Dunn LC, Calado Nogueira de Moura V, Blaha MA, Truong DD, Li W, Jackson M, North EJ. 2017. Design, synthesis and evaluation of indole-2-carboxamides with pan anti-mycobacterial activity. Bioorg Med Chem 25:3746–3755. doi: 10.1016/j.bmc.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandya AN, Prathipati PK, Hegde P, Li W, Graham KF, Mandal S, Drescher KM, Destache CJ, Ordway D, Jackson M, North EJ. 2019. Indole-2-carboxamides are active against Mycobacterium abscessus in a mouse model of acute infection. Antimicrob Agents Chemother 63:e02245-18. doi: 10.1128/AAC.02245-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Sanchez-Hidalgo A, Jones V, de Moura VCN, North EJ, Jackson M. 2017. Synergistic interactions of MmpL3 inhibitors with antitubercular compounds in vitro. Antimicrob Agents Chemother 61:e02399-16. doi: 10.1128/AAC.02399-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stec J, Onajole OK, Lun S, Guo H, Merenbloom B, Vistoli G, Bishai WR, Kozikowski AP. 2016. Indole-2-carboxamide-based MmpL3 inhibitors show exceptional antitubercular activity in an animal model of tuberculosis infection. J Med Chem 59:6232–6247. doi: 10.1021/acs.jmedchem.6b00415. [DOI] [PubMed] [Google Scholar]
- 12.Shaw KJ, Barbachyn MR. 2011. The oxazolidinones: past, present, and future. Ann N Y Acad Sci 1241:48–70. doi: 10.1111/j.1749-6632.2011.06330.x. [DOI] [PubMed] [Google Scholar]
- 13.Richard M, Gutiérrez AV, Viljoen A, Rodriguez-Rincon D, Roquet-Baneres F, Blaise M, Everall I, Parkhill J, Floto RA, Kremer L. 2018. Mutations in the MAB_2299c TetR regulator confer cross-resistance to clofazimine and bedaquiline in Mycobacterium abscessus. Antimicrob Agents Chemother 63:e01316-18. doi: 10.1128/AAC.01316-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miyasaka T, Kunishima H, Komatsu M, Tamai K, Mitsutake K, Kanemitsu K, Ohisa Y, Yanagisawa H, Kaku M. 2007. In vitro efficacy of imipenem in combination with six antimicrobial agents against Mycobacterium abscessus. Int J Antimicrob Agents 30:255–258. doi: 10.1016/j.ijantimicag.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 15.Le Run E, Arthur M, Mainardi J-L. 2018. In vitro and intracellular activity of imipenem combined with rifabutin and avibactam against Mycobacterium abscessus. Antimicrob Agents Chemother 62:e00623-18. doi: 10.1128/AAC.00623-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woods GL, Brown-Elliott BA, Conville PS, Desmond EP, Hall GS, Lin G, Pfyffer GE, Ridderhof JC, Siddiqi SH, Wallace RJ. 2011. Susceptibility testing of mycobacteria, nocardiae and other aerobic actinomycetes: approved standard. 2nd ed M24-A2 Clinical and Laboratory Standards Institute, Wayne, PA. [PubMed] [Google Scholar]
- 17.Odds FC. 2003. Synergy, antagonism, and what the chequerboard puts between them. J Antimicrob Chemother 52:1. doi: 10.1093/jac/dkg301. [DOI] [PubMed] [Google Scholar]
- 18.Adekambi T, Sassi M, van Ingen J, Drancourt M. 2017. Reinstating Mycobacterium massiliense and Mycobacterium bolletii as species of the Mycobacterium abscessus complex. Int J Syst Evol Microbiol 67:2726–2730. doi: 10.1099/ijsem.0.002011. [DOI] [PubMed] [Google Scholar]
- 19.Singh S, Bouzinbi N, Chaturvedi V, Godreuil S, Kremer L. 2014. In vitro evaluation of a new drug combination against clinical isolates belonging to the Mycobacterium abscessus complex. Clin Microbiol Infect 20:O1124–1127. doi: 10.1111/1469-0691.12780. [DOI] [PubMed] [Google Scholar]
- 20.Halloum I, Viljoen A, Khanna V, Craig D, Bouchier C, Brosch R, Coxon G, Kremer L. 2017. Resistance to thiacetazone derivatives active against Mycobacterium abscessus involves mutations in the MmpL5 transcriptional repressor MAB_4384. Antimicrob Agents Chemother 61:e02509-16. doi: 10.1128/AAC.02509-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viljoen A, Raynaud C, Johansen MD, Roquet-Banères F, Herrmann J-L, Daher W, Kremer L. 2019. Verapamil improves the activity of bedaquiline against Mycobacterium abscessus in vitro and in macrophages. Antimicrob Agents Chemother 63:e00705-19. doi: 10.1128/AAC.00705-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kwak N, Dalcolmo MP, Daley CL, Eather G, Gayoso R, Hasegawa N, Jhun BW, Koh W-J, Namkoong H, Park J, Thomson R, van Ingen J, Zweijpfenning SMH, Yim J-J. 2019. Mycobacterium abscessus pulmonary disease: individual patient data meta-analysis. Eur Respir J 54:1801991. doi: 10.1183/13993003.01991-2018. [DOI] [PubMed] [Google Scholar]
- 23.Lee M-C, Sun P-L, Wu T-L, Wang L-H, Yang C-H, Chung W-H, Kuo A-J, Liu T-P, Lu J-J, Chiu C-H, Lai H-C, Chen N-Y, Yang J-H, Wu T-S. 2017. Antimicrobial resistance in Mycobacterium abscessus complex isolated from patients with skin and soft tissue infections at a tertiary teaching hospital in Taiwan. J Antimicrob Chemother 72:2782–2786. doi: 10.1093/jac/dkx212. [DOI] [PubMed] [Google Scholar]
- 24.Li B, Yang S, Chu H, Zhang Z, Liu W, Luo L, Ma W, Xu X. 2017. Relationship between antibiotic susceptibility and genotype in Mycobacterium abscessus clinical isolates. Front Microbiol 8:1739. doi: 10.3389/fmicb.2017.01739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubée V, Bernut A, Cortes M, Lesne T, Dorchene D, Lefebvre A-L, Hugonnet J-E, Gutmann L, Mainardi J-L, Herrmann J-L, Gaillard J-L, Kremer L, Arthur M. 2015. β-Lactamase inhibition by avibactam in Mycobacterium abscessus. J Antimicrob Chemother 70:1051–1058. doi: 10.1093/jac/dku510. [DOI] [PubMed] [Google Scholar]
- 26.Kaushik A, Ammerman NC, Lee J, Martins O, Kreiswirth BN, Lamichhane G, Parrish NM, Nuermberger EL. 2019. In vitro activity of the new β-lactamase inhibitors relebactam and vaborbactam in combination with β-lactams against Mycobacterium abscessus complex clinical isolates. Antimicrob Agents Chemother 63:e02623-18. doi: 10.1128/AAC.02623-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon request.