In 2016, the proportion of Neisseria gonorrhoeae isolates with reduced susceptibility to azithromycin rose to 3.6%. A phylogenetic analysis of 334 N. gonorrhoeae isolates collected in 2016 revealed a single, geographically diverse lineage of isolates with MICs of 2 to 16 μg/ml that carried a mosaic-like mtr locus, whereas the majority of isolates with MICs of ≥16 μg/ml appeared sporadically and carried 23S rRNA mutations.
KEYWORDS: 23S rRNA, Neisseria gonorrhoeae, antimicrobial resistance, azithromycin, mosaic-like mtrR
ABSTRACT
In 2016, the proportion of Neisseria gonorrhoeae isolates with reduced susceptibility to azithromycin rose to 3.6%. A phylogenetic analysis of 334 N. gonorrhoeae isolates collected in 2016 revealed a single, geographically diverse lineage of isolates with MICs of 2 to 16 μg/ml that carried a mosaic-like mtr locus, whereas the majority of isolates with MICs of ≥16 μg/ml appeared sporadically and carried 23S rRNA mutations. Continued molecular surveillance of N. gonorrhoeae isolates will identify new resistance mechanisms.
TEXT
Neisseria gonorrhoeae, the causative agent of the sexually transmitted disease gonorrhea, has consistently developed resistance to each recommended antibiotic, resulting in its designation as an urgent threat by the Centers for Disease Control and Prevention (CDC) and as a high-priority antibiotic-resistant pathogen by the World Health Organization (1, 2). Increasing numbers of N. gonorrhoeae isolates with reduced susceptibility to azithromycin (AZM) are reported in the United States and internationally, including in China (3), Canada (4), and Europe (5). In the United States, surveillance efforts through the Gonococcal Isolate Surveillance Project (GISP) indicated an increased incidence of reduced susceptibility to AZM from 0.2% in 2012 to 3.6% in 2016 (6). Given this sharp increase, we focused on understanding the genetics of all the isolates from 2016 with reduced susceptibility to AZM, defined as an MIC of ≥2 μg/ml. In a previous report, 117 isolates from 2016 with an AZM MIC of ≥2 μg/ml were genetically analyzed (7). In this report, we expand this analysis to 177 isolates, which represents 95% of all such isolates from 2016.
Isolates with an AZM MIC of <16 μg/ml cluster into a single, diverse clade sharing a common genetic mechanism.
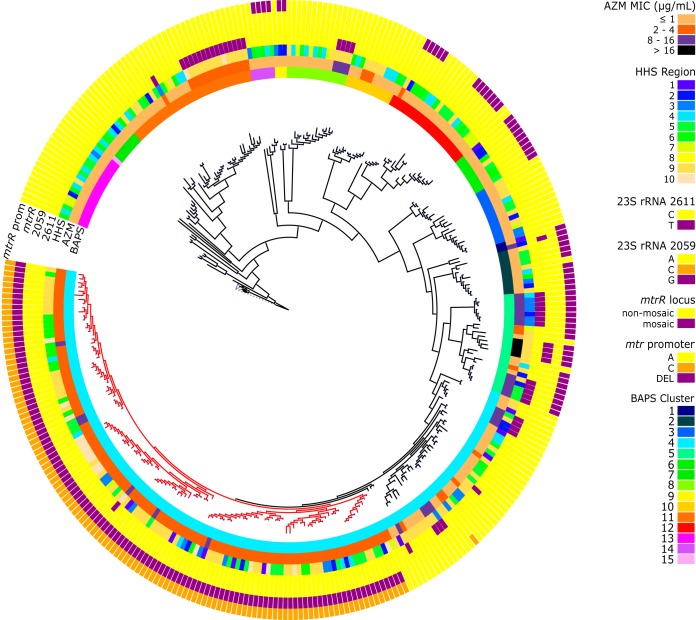
In 2016, 95% (177/186) of all gonococcal isolates collected by GISP with an AZM MIC of ≥2 μg/ml as determined by agar dilution were available for whole-genome sequencing (WGS) on an Illumina MiSeq (8). We investigated the relatedness of the strains by performing sequence typing using MLST and NG-MAST and a core genome single nucleotide polymorphism (SNP) analysis with recombination filtering followed by generation of a maximum-likelihood phylogenetic tree (Fig. 1). We also selected a stratified random sample of 10 isolates from each GISP sentinel site from 2016, of which 157 isolates were available for sequencing and contained mostly susceptible isolates. The distribution of AZM MICs in the sampled susceptible isolates was similar to that of all susceptible isolates in GISP, suggesting that these isolates reflect the circulating U.S. AZM-susceptible gonorrhea population (compare reference 6 and Fig. S1 in the supplemental material). We also investigated alterations in the 23S rRNA alleles or the multiple transferable resistance regulator (mtrR) locus, which contribute to reduced susceptibility to AZM (Tables 1 and 2) using a custom pipeline written in Python (9, 10). A total of 13 clades containing three or more isolates were identified using Bayesian analysis of population structure (BAPS) (11). We identified one large clade of highly related isolates with high nodal support (clade A [n = 119]; within-clade difference, 87 ± 58 SNPs) (Fig. 1), which is a subset of BAPS cluster 4. Whereas the most common sequence type (ST) in clade A was ST9363 (100/119), others, including ST11422 and ST8134, were present. Clade A contained 39 total NG-MAST STs, with ST3935 (21/119) being the most common, followed by ST8241 (19/119) and ST12302 (12/119). Clade A is distinguished from the rest of the cluster by the presence of a mosaic-like mtrR locus, which contributes to elevated macrolide MICs by increasing expression of the MtrCDE efflux pump (12). Chi-square analysis showed that a mosaic-like mtrR locus or an A-to-C transversion was highly associated with an MIC range of 2 to 4 μg/ml (χ2 = 204.71, P < 0.0001; or χ2 = 174.98, P < 0.0001, respectively) (Table 1). The success of the organisms in clade A, as suggested by the presence of similar isolates in a genomic analysis of GISP isolates from 2000 to 2013 (13) and whose presence has been continuous since 2014 (7), suggests that the mosaic-like mtrR locus contributes to their ability to survive and be transmitted. In support of this hypothesis, the transformation of a susceptible strain with a mosaic-like mtr locus is sufficient to increase the AZM MIC of the recipient strain (12, 14). Across the data set, the most predominant MLST type was ST9363 (n = 130) (Fig. 1), followed by ST1584 (n = 23), ST11422 (n = 12), ST1893 (n = 11), and ST1579 (n = 10). Some STs, e.g., ST9363, were found across the country, whereas others were more restricted (e.g., ST7371 or ST8154 on the East Coast). For NG-MAST, ST3935 was the most common (n = 23), followed by ST8241 (n = 19), ST7638 (n = 15), and ST12302 (n = 12) (for new NG-MAST STs, see Fig. S3 and Supplementary Methods in the supplemental material).
FIG 1.
Maximum-likelihood phylogeny of Neisseria gonorrhoeae isolates collected in 2016, including all available isolates exhibiting AZM MICs of ≥2 μg/ml in GISP (n = 117). Lineage characterized by reduced susceptibility to AZM (2 to16 μg/ml) is highlighted in red (clade A). Rings from inside to outside are BAPS clusters, AZM MIC values, HHS regions, 23S rRNA C2611T, 23S rRNA A2059G, mosaic-like mtrR presence, and mtrR promoter mutations.
TABLE 1.
Distribution of mtrR mutations
| AZM MIC (μg/ml) | Distribution (no. isolates) of: |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
mtrR locus |
mtrR promoterc
|
MtrR |
|||||||||||||
| Wild type | Mosaic | Wild type | Del A | C SNP | 39A | 39T | 44H | 44R | 45D | 45G | 47L | 47A | Full length | Premature stop | |
| <2.0 | 157a | 0 | 119a | 38 | 0 | 100a | 57 | 26 | 131b | 48 | 109a | 157 | 0 | 28 | 129 |
| 2.0–4.0 | 29 | 113a | 24 | 4 | 114a | 134a | 8 | 6 | 136b | 0 | 142a | 142 | 0 | 19 | 123 |
| 8.0–16.0 | 25 | 6 | 12 | 13 | 6 | 20 | 11 | 4 | 27 | 8 | 23 | 31 | 0 | 0 | 31 |
| >16 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 0 | 4 | 0 | 4 | 4 | 0 | 0 | 4 |
P < 0.0001.
P < 0.005.
Del A, deletion of an adenine in the inverted repeat of the mtrR promoter; C SNP, A-to-C transversion in the inverted repeat of the mtrR promoter.
TABLE 2.
Distribution of 23S rRNA mutant alleles
| AZM MIC (μg/ml) | Distribution (no. isolates) of: |
|||||
|---|---|---|---|---|---|---|
| C2611T |
A2059G |
|||||
| Wild type | 2 mutated loci | 3 mutated loci | 4 mutated loci | Wild type | 4 mutated loci | |
| <2.0 | 156a | 1 | 0 | 0 | 157 | 0 |
| 2.0–4.0 | 126a | 0 | 1 | 15b | 142 | 0 |
| 8.0–16.0 | 6 | 0 | 1 | 24a | 30 | 1 |
| >16.0 | 4 | 0 | 0 | 0 | 0 | 4 |
P < 0.0001.
Not significant.
Isolates with an AZM MIC of ≥16 μg/ml appear sporadically and contain mutations in the 23S rRNA genes.
Twenty isolates had an AZM MIC of ≥16 μg/ml as determined by agar dilution antimicrobial susceptibility testing. Those were further tested by Etest (bioMérieux), and 4 had an AZM MIC of ≥256 μg/ml. Those isolates were represented by ST1579 (n = 4), ST1901 (n = 4), ST7371 (n = 6), ST9363 (n = 5), and ST7822 (n = 1). The four isolates with MICs of ≥256 μg/ml were part of a previously described cluster of isolates with high-level AZM resistance and reduced susceptibility to ceftriaxone, which was successfully contained (15). Isolates with AZM MICs of ≥16 μg/ml were found throughout the tree and grouped into small subclusters (e.g., BAPS cluster 5 contained three subclusters) (Fig. 1). These isolates all contained mutations in either position 2611 or 2059 (E. coli numbering) of the 23S rRNA, which is the target of macrolides (Fig. 1 and Table 2). In N. gonorrhoeae, there are four copies of the 23S rRNA gene in the genome. Four copies of the 23S rRNA allele carrying the C2611T alteration were associated with an MIC range of 8 to 16 μg/ml (χ2 = 129.65; P < 0.0001) (Table 2). Isolates with four alleles carrying the A2059G alteration were found to have an AZM MIC of ≥256 μg/ml (Fig. 1 and Table 2). When found in clinical isolates or generated by site-directed mutagenesis in a susceptible strain, the A2059G alteration leads to an AZM MIC of ≥256 μg/ml and, potentially, treatment failure (9, 16, 17).
In contrast to the widespread presence of isolates containing the mosaic-like mtrR locus, isolates with the C2611T alteration appear more sporadically. This is consistent with a hypothesis that the 23S rRNA mutations occur spontaneously and carry a fitness cost. The largest clade of these contains 15 isolates (MLST ST1584 and NG-MAST ST7638) and is found only in HHS regions 9 and 10. Many of these smaller clades were geographically isolated (Fig. 1), further suggesting spontaneous mutation followed by clonal expansion.
WGS for N. gonorrhoeae surveillance in 2016 did not include routine selection of enough susceptible isolates to assess when the 23S rRNA mutations arose in the U.S. N. gonorrhoeae population and whether certain strain types are more prone to developing these mutations. Defined selection criteria for routine WGS of GISP isolates were established in late 2017 and include subsets of both susceptible and resistant isolates. These sequences will provide a valuable resource for tracking of known strains with reduced susceptibility to AZM and detection of emerging strains that appear in the United States.
Supplementary Material
ACKNOWLEDGMENTS
We thank David Trees (CDC) for support during the beginning of this study, Brian Raphael (CDC) for helpful comments on the manuscript, and the Biotechnology Core Facility Branch (CDC) for assistance with this study. We thank Jaeyoung Hong and Joseph Kang (CDC) for helpful discussions about the statistical analysis. We also thank partners at the Maryland Department of Health, Texas Department of State Health Services, Washington State Department of Health, and Hawaii Department of Health State Laboratories Division for performing WGS. Finally, we thank the Gonococcal Isolate Surveillance Project (GISP), the GISP Regional Laboratories for antimicrobial susceptibility testing, and participating sentinel sites for their contribution of isolates and epidemiological data used in this study.
The Antimicrobial-Resistant Neisseria gonorrhoeae Working Group includes Catherine Dominguez (Maryland ARLN, Maryland Department of Health), Sopheay Hun (Washington ARLN, Washington State Department of Health), Olusegun O. Soge (Department of Global Health and Medicine, University of Washington), A. Christian Whelen and Pamela O’Brien (Hawaii Department of Health State Laboratories Division), Alesia Harvey (Division of STD Prevention, CDC), and Tara Henning (Division of Healthcare Quality Promotion, CDC).
This work was supported by the CDC and in part by funds made available by the CDC Advanced Molecular Detection and Combating Antibiotic Resistant Bacteria programs.
The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
Supplemental material is available online only.
Contributor Information
Catherine Dominguez, Maryland ARLN, Maryland Department of Health.
Collaborators: Catherine Dominguez, Catherine Dominguez, Sopheay Hun, Olusegun O. Soge, A. Christian Whelen, Pamela O'Brien, Alesia Harvey, and Tara Henning
REFERENCES
- 1.World Health Organization. 2017. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. World Health Organization, Geneva. [Google Scholar]
- 2.Centers for Disease Control and Prevention. 2019. Antibiotic resistance threats in the United States, 2019. U.S. Department of Health and Human Services, Atlanta, GA: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf. [Google Scholar]
- 3.Wan C, Li Y, Le WJ, Liu YR, Li S, Wang BX, Rice PA, Su XH. 2018. Increasing resistance to azithromycin in Neisseria gonorrhoeae in eastern Chinese cities: resistance mechanisms and genetic diversity among isolates from Nanjing. Antimicrob Agents Chemother 62:e02499-17. doi: 10.1128/AAC.02499-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin I, Sawatzky P, Liu G, Allen V, Lefebvre B, Hoang L, Drews S, Horsman G, Wylie J, Haldane D, Garceau R, Ratnam S, Wong T, Archibald C, Mulvey MR. 2016. Decline in decreased cephalosporin susceptibility and increase in azithromycin resistance in Neisseria gonorrhoeae, Canada. Emerg Infect Dis 22:65–67. doi: 10.3201/eid2201.151247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole MJ, Spiteri G, Jacobsson S, Woodford N, Tripodo F, Amato-Gauci AJ, Unemo M, Euro-GASP Network. 2017. Overall low extended-spectrum cephalosporin resistance but high azithromycin resistance in Neisseria gonorrhoeae in 24 European countries, 2015. BMC Infect Dis 17:617. doi: 10.1186/s12879-017-2707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2017. Sexually transmitted disease surveillance 2016. U.S. Department of Health and Human Services, Atlanta, GA: https://www.cdc.gov/std/stats16/default.htm. [Google Scholar]
- 7.Thomas JC, Seby S, Abrams AJ, Cartee J, Lucking S, Vidyaprakash E, Schmerer M, Pham CD, Hong J, Torrone E, Cyr SS, Shafer WM, Bernstein K, Kersh EN, Gernert KM, Antimicrobial-Resistant Neisseria gonorrhoeae Working Group. 2019. Evidence of recent genomic evolution in gonococcal strains with decreased susceptibility to cephalosporins or azithromycin in the United States, 2014–2016. J Infect Dis 220:294–305. doi: 10.1093/infdis/jiz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NCBI. 2016. BioProject: GCWGS_SL Neisseria gonorrhoeae genome sequencing. https://www.ncbi.nlm.nih.gov/bioproject/PRJNA317462.
- 9.Chisholm SA, Dave J, Ison CA. 2010. High-level azithromycin resistance occurs in Neisseria gonorrhoeae as a result of a single point mutation in the 23S rRNA genes. Antimicrob Agents Chemother 54:3812–3816. doi: 10.1128/AAC.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wadsworth CB, Sater MRA, Bhattacharyya RP, Grad YH. 2019. Impact of species diversity on the design of RNA-based diagnostics for antibiotic resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother 63:e00549-19. doi: 10.1128/AAC.00549-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng L, Connor TR, Siren J, Aanensen DM, Corander J. 2013. Hierarchical and spatially explicit clustering of DNA sequences with BAPS software. Mol Biol Evol 30:1224–1228. doi: 10.1093/molbev/mst028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rouquette-Loughlin CE, Reimche JL, Balthazar JT, Dhulipala V, Gernert KM, Kersh EN, Pham CD, Pettus K, Abrams AJ, Trees DL, St Cyr S, Shafer WM. 2018. Mechanistic basis for decreased antimicrobial susceptibility in a clinical isolate of Neisseria gonorrhoeae possessing a mosaic-like mtr efflux pump locus. mBio 9:e02281-18. doi: 10.1128/mBio.02281-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grad YH, Harris SR, Kirkcaldy RD, Green AG, Marks DS, Bentley SD, Trees D, Lipsitch M. 2016. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J Infect Dis 214:1579–1587. doi: 10.1093/infdis/jiw420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wadsworth CB, Arnold BJ, Sater MRA, Grad YH. 2018. Azithromycin resistance through interspecific acquisition of an epistasis-dependent efflux pump component and transcriptional regulator in Neisseria gonorrhoeae. mBio 9:e01419-18. doi: 10.1128/mBio.01419-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papp JR, Abrams AJ, Nash E, Katz AR, Kirkcaldy RD, O'Connor NP, O'Brien PS, Harauchi DH, Maningas EV, Soge OO, Kersh EN, Komeya A, Tomas JE, Wasserman GM, Kunimoto GY, Trees DL, Whelen AC. 2017. Azithromycin resistance and decreased ceftriaxone susceptibility in Neisseria gonorrhoeae, Hawaii, USA. Emerg Infect Dis 23:830–832. doi: 10.3201/eid2305.170088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gose SO, Soge OO, Beebe JL, Nguyen D, Stoltey JE, Bauer HM. 2015. Failure of azithromycin 2.0 g in the treatment of gonococcal urethritis caused by high-level resistance in California. Sex Transm Dis 42:279–280. doi: 10.1097/OLQ.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, van der Veen S. 2018. Neisseria gonorrhoeae 23S rRNA A2059G mutation is the only determinant necessary for high-level azithromycin resistance and improves in vivo biological fitness. J Antimicrob Chemother 74:407–415. doi: 10.1093/jac/dky438. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.