Abstract
Common buckwheat (Fagopyrum esculentum Moench 2n = 2x = 16) is an outcrossing crop with heteromorphic self-incompatibility due to its distylous flowers, called pin and thrum. In pin plants, a long style is combined with short stamens and small pollen grains; in thrum plants, a short style is combined with long stamens and large pollen grains. Both the intra-morph self-incompatibility and flower morphology are controlled by a single genetic locus named the S locus; thrum plants are heterozygous (Ss) and pin plants are homozygous recessive (ss) at this locus. Self-incompatibility is an obstacle for establishing pure lines and fixation of agronomically useful genes. Elucidation of the molecular mechanism of heterostylous self-incompatibility of common buckwheat has continued for a quarter of a century. Recent advances in genomic and transcriptomic analyses using next-generation sequencing have made it possible to determine the genomic region harboring the buckwheat S locus and to identify novel genes at this locus. In this review, we summarize the current knowledge on buckwheat heterostyly gained from conventional and molecular genetics and genomics. We also discuss the application of these studies to breeding of common buckwheat.
Keywords: common buckwheat, heteromorphic SI, self-incompatibility
Introduction
Self-incompatibility (SI) prevents self-fertilization. It is widespread in the plant kingdom. SI promotes outbreeding and reduces the likelihood of adverse effects caused by homozygosity of recessive alleles at multiple loci (Charlesworth and Charlesworth 1987, Goldberg et al. 2010). SI can be classified as either homomorphic or heteromorphic. Plants with homomorphic SI have only a single type of flower morphology, whereas plants with heteromorphic SI have two (distyly) or three (tristyly) types. Distyly is common and found in at least 28 angiosperm families including Polygonaceae, Primulaceae, Passifloraceae and Linaceae, whereas tristyly is rare and found in a few angiosperm families such as Oxalidaceae (Barrett 2002, de Nettancourt 2001, Ganders 1979). SI is caused primarily by a reaction between haploid pollen grains or pollen tubes and diploid stigmas or styles, and can be classified as either gametophytic or sporophytic. In gametophytic SI, the SI phenotype of each pollen grain is determined by its own genotype. In sporophytic SI, the SI phenotype of the pollen is determined by the genotype of its diploid parent. Three homomorphic SI systems have been extensively studied at the molecular level (reviewed in Iwano and Takayama 2012): (1) gametophytic SI based on the SLF (SFB)/S-RNase system in Solanaceae, Rosaceae and Plantaginaceae, (2) gametophytic SI based on the PrpS/PrsS system in Papaveraceae, and (3) sporophytic SI based on the SP11 (SCR)/SRK system in Brassicaceae.
Recently, considerable progress in understanding of the molecular basis of heteromorphic SI has been made in heterostylous plant species including common buckwheat (Fagopyrum esculentum) (Li et al. 2015, 2016, Takeshima et al. 2019, Ushijima et al. 2012, Yasui et al. 2012, 2016). Genus Fagopyrum includes both SI and self-compatible (SC) species. Heteromorphic SI was broken down many times independently during diversification of Fagopyrum species (Nishimoto et al. 2003, Ohnishi and Matsuoka 1996, Ohsako and Ohnishi 2000, Yasui and Ohnishi 1998a, 1998b). Two wild Fagopyrum species are SC species with heteromorphic flowers (Ohsako and Ohnishi 1998). Thus, we expect that Fagopyrum species will be well suited for studying the molecular mechanism of heteromorphic SI and its evolutionary history.
SI is important for maintaining genetic diversity, but it is often an obstacle to establishing new pure lines. Common buckwheat needs pollinators such as bees and flies for cross-pollination between pin and thrum plants, and seed production is strongly influenced by pollinator activity. Self-fertilizing SC lines no longer need to attract pollinators. Thus, it is expected that the seed production of SC lines would be more stable than outcrossing varieties. It may also be possible to increase seed yield by reducing the cost of nectar production (Galetto et al. 2018).
Recently, we published a review for genetics of buckwheat heteromorphic SI (Ueno et al. 2016). In addition to the knowledge in the previous review, we compare genes controlling heteromorphic SI among distantly related species, buckwheat, Primula, and Turnera species. We also discuss the application of these studies to breeding of common buckwheat.
Heteromorphic SI and S supergene hypothesis in buckwheat
Buckwheat is a distylous SI plant. It has long-styled flowers with short stamens (so-called pin flowers, Fig. 1A) and short-styled flowers with long stamens (so-called thrum flowers, Fig. 1B) (Darwin 1877, Hilderbrand 1867). Pollen grains of thrum plants (i.e., plants setting thrum flowers) are larger than those of pin plants (Schoch-Bodmer 1934, Tatebe 1953). Intra-morph incompatibility occurs in the style: in crosses between thrum plants, thrum pollen tube growth is inhibited in the upper part of the style with hypertrophy at the tips of pollen tubes, whereas in crosses between pin plants, pin pollen tube growth is inhibited in the middle part of the style with no hypertrophy (Hirose et al. 1995) (Fig. 2). Both SI and flower morphology are controlled by a single locus (S locus); thrum plants are heterozygous (Ss), whereas pin plants are homozygous (ss), and the SS genotype does not exist (Garber and Quisenberry 1927, Lewis and Jones 1992).
Fig. 1.

Heteromorphic flowers of common buckwheat. (A) Pin flower; (B) Thrum flower; (C) Long-homostyle flower; (D) Short-homostyle flower.
Fig. 2.
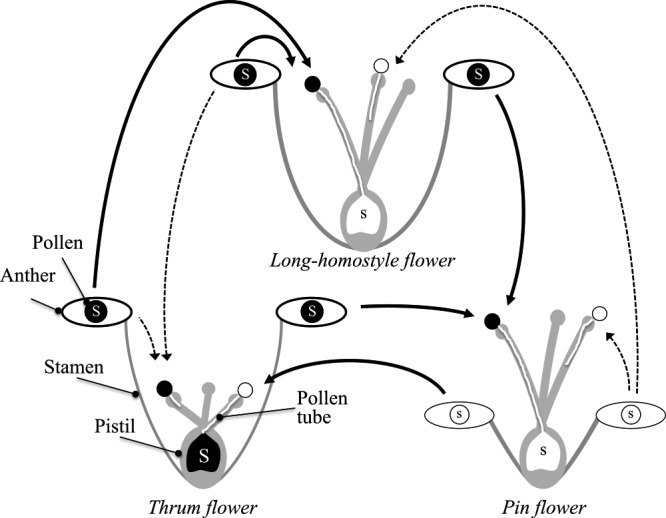
Diagram of cross-compatibility/incompatibility among pin, thrum and long-homostyle plants. Buckwheat is sporophytic SI species, and the SI phenotype of the pollen is determined by the genotype of its diploid parent. Solid arrows, compatible crosses; dashed arrows, incompatible crosses.
Similar phenomenon has been studied in Primula, which has been known as a distylous plant since the Darwin’s era (Darwin 1862). According to Dowrick (1956), the S locus in Primula contains five genes (S supergene complex locus) for the following traits, as suggested by the observed recombinations within the S supergene complex locus: the style length gene (G for short style, g for long style), style incompatibility gene (IS for style incompatibility of short style, is for style incompatibility of long style), pollen incompatibility gene (IP for pollen incompatibility of short anther, ip for pollen incompatibility of long anther), pollen size gene (P for large pollen grain, p for small pollen grain) and anther height gene (A for long anther, and a for short anther). Based on this hypothesis, the S allele would consist of the GISIPPA gene cluster (i.e., haplotype) and the s allele would consist of the gisippa haplotype, and these haplotypes would be inherited without recombination in most cases. Sharma and Boyes (1961) postulated that heteromorphic SI in common buckwheat is also controlled by the S supergene. However, unlike in Primula, recombinations within the supergene complex have not been observed in buckwheat, and the S supergene hypothesis remained unproven for a long time.
Self-compatibility and Sh allele at the S locus of Fagopyrum homotropicum
The S supergene hypothesis was confirmed in common buckwheat by genetic studies that used crosses between common buckwheat and SC Fagopyrum species. An homostylous SC species closely related to common buckwheat, Fagopyrum homotropicum Ohnishi, was found in southern China (Ohnishi 1998), and several researchers have succeeded in transferring the SC property of F. homotropicum to common buckwheat by conducting interspecific crosses between these two species (Aii et al. 1998, Campbell 1995, Matsui et al. 2003b, Woo et al. 1999). It has been proven that the SC property is conferred by the Sh allele at the S locus of F. homotropicum, and that Sh is dominant over the s allele but recessive to the S allele (Woo et al. 1999). Using interspecific crossing and subsequent selfing, Matsui et al. (2003b, 2007) established SC lines that had flowers with long styles, long stamens and large pollen (“long-homostyle flowers”; Fig. 1C). The SC lines had both the male phenotypes of thrum plants and the female phenotypes of pin plants: (1) pollen from SC lines was compatible with long styles of pin plants, but incompatible with short styles of thrum plants, and (2) long styles of SC lines were compatible with pollen from long stamens of thrum plants, but incompatible with pollen from short stamens of pin plants (Fig. 2). On the basis of these observations, Matsui et al. (2003b, 2007) proposed the presence of at least two factors in the S locus, one controlling style incompatibility and style length, and the other one controlling pollen incompatibility, pollen size and stamen height. They also postulated the genotype in the Sh haplotype to be gisIPPA and that this genotype was caused by recombination in the S supergene, as proposed for Primula (Wedderburn and Richards 1992).
S-LOCUS EARLY FLOWERING 3 (S-ELF3) gene in the buckwheat S allele
Factors related to homomorphic SI systems in Brassicaceae, Solanaceae and Papaveraceae have been identified by searching for specific proteins in different S haplotypes, and the finding of proteins segregating with S haplotypes has led to the isolation of S genes (reviewed by Takayama and Isogai 2005).
In distylous plants including buckwheat, the dominance of the S allele over the s allele suggests that a specific gene is expressed only in the flowers of thrum plants. Yasui et al. (2012) isolated mRNAs separately from pistils of thrum and pin plants, and conducted high-throughput sequencing analyses of expressed genes. Four specific transcripts (SSG1–SSG4) were detected in the style of thrum plants. Using more than 1,000 BC1F5 segregating plants and an ion-beam-induced mutant, Yasui et al. (2012) found complete genetic linkage between the S locus and SSG2 or SSG3. SSG3 was found to be a homolog of Arabidopsis EARLY FLOWERING 3 (ELF3) and was named S-LOCUS EARLY FLOWERING 3 (S-ELF3). No homologs were identified for SSG2 (Yasui et al. 2012).
ELF3, a core circadian clock component, forms a complex with ELF4 and LUX ARRHYTHMO (LUX); this complex binds to the promoters of circadian clock genes such as PHYTOCHROME-INTERACTING FACTOR 4 (PIF4), PIF5 and PSEUDO-RESPONSE REGULATOR 9 and regulates their expression in a circadian manner (Helfer et al. 2011, Herrero et al. 2012). Because ELF3 functions in flowering repression (Liu et al. 2001, Zagotta et al. 1996), diurnal control of hypocotyl growth (Nusinow et al. 2011) and thermo-responsive hypocotyl growth (Box et al. 2015, Raschke et al. 2015), S-ELF3 function could be related to the regulation of style length.
Analysis of the S locus based on genome data and comparison of buckwheat and Primula S loci
Yasui et al. (2012) reported that the genomic region harboring S-ELF3 is absent in the s haplotype in a worldwide collection of common buckwheat landraces and in two distantly related Fagopyrum species that have heteromorphic SI. Yasui et al. (2016) assembled the common buckwheat genome and suggested that the region harboring S-ELF3 is present only in the S haplotype, that the S locus in buckwheat is also hemizygous, and that homostyly does not arise by recombination between the S and s alleles but is caused by dysfunction of the G/IS or A/IP gene of the S allele caused by mutations. Yasui et al. (2012) amplified S-ELF3 from Kyushu PL4 (an SC line in which the Sh allele of SC F. homotropicum was incorporated into F. esculentum, Matsui et al. 2008) and showed that a single-nucleotide deletion in this line results in a frameshift in the 5th exon of S-ELF3. Dysfunction of S-ELF3, which is expressed in pistils and could be the G/IS factor, would confer the phenotype of the s haplotype in pistils. Thus, we postulate that Kyushu PL4 possesses the dysfunctional G/IS and functional A/IP factors, giving it both the female phenotypes of pin plants and the male phenotypes of thrum plants (Fig. 3).
Fig. 3.
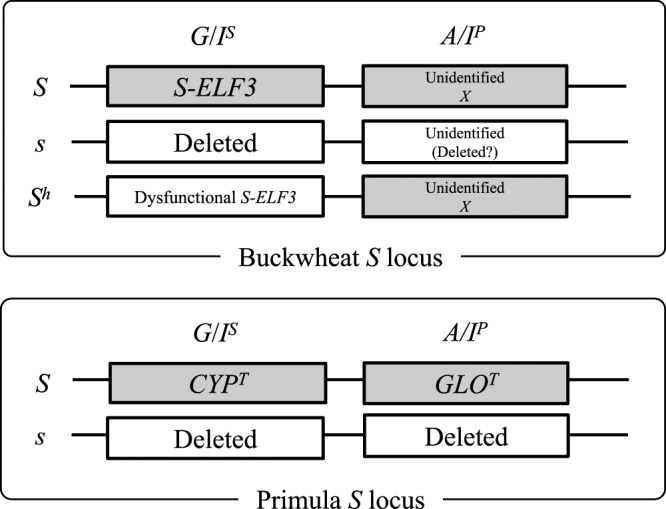
Genes of the buckwheat and Primula S supergene. In thrum plants, the genomic region harboring the S supergene is hemizygous. S-ELF3 is a candidate for G/IS. The gene for A/IP has not yet been identified.
De novo assembly of the Primula vulgaris genome sequence and comparison of the genomes of pin and thrum plants have revealed that the S supergene is present only in the genome of thrum plants, indicating that thrum plants are hemizygous, not heterozygous, for the S locus (Li et al. 2015, 2016). The nucleotide length of hemizygous genomic region of the S locus is estimated to be 278 Kb (Li et al. 2016). They also suggested that two tightly linked genes in the S haplotype separately control anther and style length, and showed that a transposon insertion into GLOT is associated with loss of anther elevation and that a CYPT allele with a single-base insertion in exon 3 is associated with loss of style length suppression (i.e., exhibiting long style length) (Fig. 3). Yasui et al. (2016) also showed that the length of the S-allele region harboring S-ELF3 is over 5.8 Mb. The genomic region controlling the heteromorphic SI system in buckwheat is longer than that in P. vulgaris. Thus, unlike the short S-allele region of P. vulgaris, the genomic region controlling the heteromorphic SI system is huge in buckwheat. Comparison between the S-allele regions of Primula and Fagopyrum will shed light on the convergent evolution of heteromorphic SI in these distantly related species.
Polygalacturonase as a gene related to heteromorphic SI
Using isoelectric focusing of nondenatured proteins, Athanasiou and Shore (1997) analyzed proteins differentially expressed between pin and thrum plants of Turnera subulata, and detected thrum-specific proteins of the style and pollen. Following two-dimensional (2D)-PAGE analysis, Athanasiou et al. (2003) determined the amino acid sequences of thrum-specific proteins. One protein was a member of the polygalacturonase (PG) family of pectin hydrolytic enzymes involved in the development of pollen and anther, floral morphology (Huang et al. 2009a, 2009b, Zhang and Ren 2008), cell elongation (Babu et al. 2013), seed germination (Sitrit et al. 1996, 1999), pod and anther dehiscence (Ogawa et al. 2009, Xiao et al. 2014) and fruit softening (Kramer and Redenbaugh 1994).
Matsui et al. (2004) surveyed SI responses and flower morphology of Pennline 10, a short-homostyle (Fig. 1D) line established by Marshall (1970), and found that the short pistils of this line have lost the SI response and are compatible with pollen from pin and thrum plants. Matsui et al. (2004) also confirmed that the lack of SI response and the short style length of Pennline 10 are controlled by multiple genes outside the S locus. Recently, Takeshima et al. (2019) compared protein profiles of upper pistils between pin and thrum plants by 2D-PAGE analysis, and detected short style-specific proteins. Amino acid sequence analysis showed that one of these proteins was a truncated form of PG, but the gene encoding this protein, FePG1, is not linked to the S locus. On the basis of FePG1 expression in the styles of short-homostyle plants, Takeshima et al. (2019) suggested that FePG1 is not an S-locus gene but acts downstream of the S locus to control style length. The genus Turnera (Turneraceae) is distantly related to Fagopyrum. Parallel recruitment of PGs in two divergent plant genera, Fagopyrum and Turnera, indicates the important role of PGs in the thrum phenotype in heteromorphic SI. Comparison between downstream networks including PGs will offer a chance to understand the molecular mechanism of convergent evolution of heteromorphic SI in these diversified plants.
Heteromorphic SI in Fagopyrum species
The genus Fagopyrum is composed of two major phylogenetic groups, Cymosum and Urophyllum, and SC species are present in both (Table 1). It is suggested that breakdown of heteromorphic SI occurred many times during Fagopyrum evolution (Nishimoto et al. 2003, Ohnishi and Matsuoka 1996, Ohsako and Ohnishi 2000, Yasui and Ohnishi 1998a, 1998b). Yasui et al. (2012) found that two SC plants, SC line of F. esculentum (Kyushu PL4) and F. tataricum, have nonsense mutations in S-ELF3, and suggested the importance of S-ELF3 in the heteromorphic SI system in Fagopyrum. Determination of the gene structure of S-ELF3 in other SC species will be crucial for elucidating its function in the heteromorphic SI system. Two heteromorphic SC species, F. pleioramosum and F. callianthum, are selfing wild plants sustaining heteromorphic flower morphs (Ohsako and Ohnishi 1998). It will be important to clarify whether they are prototypic heteromorphic SI species, or whether heteromorphic SI in these species has just recently been broken down by recombination within S alleles or mutations in genes that act downstream of those controlling SI.
Table 1.
Mating system and genome size of Fagopyrum speciesa
| Phylogenetic group | Species | Mating systemb | Genome size (Mbp)c |
|---|---|---|---|
| Cymosum group | F. esculentum | Heteromorphic SId | 1,340 |
| F. homotropicum | Homomorphic SCd | 1,080 | |
| F. cymosum | Heteromorphic SI | 1,120 | |
| F. tataricum | Homomorphic SC | 540 | |
| Urophyllum group | F. urophyllum | Heteromorphic SI | 1,850 |
| F. leptopodum | Heteromorphic SI | 690 | |
| F. statice | Heteromorphic SI | 650 | |
| F. gracilipes | Homomorphic SC | 810 | |
| F. pleioramosum | Heteromorphic SC | 1,470 | |
| F. capillatum | Heteromorphic SC | 830 |
a Only species whose genome size was estimated in Nagano et al. (2000) are listed.
b As in Nishimoto et al. (2003).
c As in Nagano et al. (2000).
d SC, self-compatibility; SI, self-incompatibility.
In the Urophyllum group, some SI species, such as F. statice and F. leptopodum, have relatively small genomes (Table 1) (Nagano et al. 2000). The S-allele region in common buckwheat consists of 332 small scaffolds, mainly due to the genome complexity caused by the heteromorphic nature of buckwheat and the large genome (1.21 G) (Yasui et al. 2016). DenovoMAGIC 3.0 (Avni et al. 2017) and 10X Chromium technology (Zheng et al. 2016) can be adopted for assembling heteromorphic genome sequences. Species with small genomes are most suitable for obtaining sequences of the S-allele region. Because these species are found only in China, collaboration with Chinese groups would be important for comprehensive understanding of the structure of the S-allele region in Fagopyrum.
Potential application of the knowledge of the S-locus to buckwheat breeding
Buckwheat needs pollination between pin and thrum plants by bees or other insects to produce seeds. Pollination, and therefore yield, is strongly affected by environmental conditions. SI plants maintain high heterogeneity, and fixing agricultural traits is time consuming. Attempts to introduce SC traits from SC species into common buckwheat have been successful with F. homotropicum (Aii et al. 1998, Campbell 1995, Matsui et al. 2003b, Woo et al. 1999). However, plants produced by interspecific crosses have undesirable traits such as shattering and lodging. Matsui et al. (2003a) investigated the inheritance of the shattering habit and removed it by backcrossing with a leading SI common buckwheat cultivar, but more such crosses are needed to produce new cultivars desirable for farmers and consumers.
The Sh allele, conferring the SC trait, is dominant over the s allele but recessive to the S allele. Thus, to obtain F1 plants with the SC trait, pin plants and SC lines need to be crossed. It is easy to determine whether a plant is SC or SI in F2 and later generations from flower morphology, but two genotypes, Sh/Sh homozygous and Sh/s heterozygous, cannot be distinguished by flower morphology. Heterozygous plants would segregate SC and SI plants in the next generation. To accelerate buckwheat breeding, molecular markers to distinguish homo- and heterozygosity at the S locus in seedlings would be beneficial. Aii et al. (1998) developed AFLP-based STS markers linked to the Sh allele, yet the linkage distance was more than 5 cM. Because the S locus including S-ELF3 is present only in thrum plants, it is impossible to develop co-dominant markers for S-ELF3. Developing co-dominant markers ‘tightly linked’ to the S locus would be possible, and the availability of such markers would accelerate breeding programs of buckwheat.
Recently we developed the Buckwheat Genome DataBase (BGDB, http://buckwheat.kazusa.or.jp). This database can be used for the rapid detection of homologues of genes previously identified in other plants, and we’ve detected agronomically useful genes encoding 2S albumin-type allergens and granule-bound starch synthases (Yasui et al. 2016). Now we are screening dysfunctional alleles of these genes from ethyl methanesulfonate (EMS)-induced mutant pools of buckwheat. In near future, we can establish SC lines with highly valuable agricultural traits, such as low-allergen or low amylose buckwheat lines.
Author Contribution Statement
KM wrote the main text and generated figures; YY wrote the main text and generated figures and tables.
Acknowledgments
This study was supported by JSPS KAKENHI Grant Numbers 18H02177 and 18KK0172.
Literature Cited
- Aii J., Nagano M., Penner G., Campbell G.C. and Adachi T. (1998) Identification of RAPD markers linked to the Homostylar (Ho) gene in buckwheat. Breed. Sci. 48: 59–62. [Google Scholar]
- Athanasiou A. and Shore J.S. (1997) Morph-specific proteins in pollen and styles of distylous Turnera (Turneraceae). Genetics 146: 669–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou A., Khosravi D., Tamari F. and Shore J.S. (2003) Characterization and localization of short-specific polygalacturonase in distylous Turnera subulata (Turneraceae). Am. J. Bot. 90: 675–682. [DOI] [PubMed] [Google Scholar]
- Avni R., Nave M., Barad O., Baruch K., Twardziok S.O., Gundlach H., Hale I., Mascher M., Spannagl M., Wiebe K. et al. (2017) Wild emmer genome architecture and diversity elucidate wheat evolution and domestication. Science 357: 93–97. [DOI] [PubMed] [Google Scholar]
- Babu Y., Musielak T., Henschen A. and Bayer M. (2013) Suspensor length determines developmental progression of the embryo in Arabidopsis. Plant Physiol. 162: 1448–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett S.C. (2002) The evolution of plant sexual diversity. Nat. Rev. Genet. 3: 274–284. [DOI] [PubMed] [Google Scholar]
- Box M.S., Huang B.E., Domijan M., Jaeger K.E., Khattak A.K., Yoo S.J., Sedivy E.L., Jones D.M., Hearn T.J., Webb A.A.R. et al. (2015) ELF3 controls thermoresponsive growth in Arabidopsis. Curr. Biol. 25: 194–199. [DOI] [PubMed] [Google Scholar]
- Campbell, C. (1995) Inter-specific hybridization in the genus Fagopyrum. In: Matano, T. and A. Ujihara (eds.) Proceedings of the 6th International Symposium on Buckwheat, Shinshu University Press, Ina, pp. 255–263. [Google Scholar]
- Charlesworth D. and Charlesworth B. (1987) The effect of investment in attractive structures on allocation to male and female functions in plants. Evolution 41: 948–968. [DOI] [PubMed] [Google Scholar]
- Darwin C. (1862) On the two forms or dimorphic condition in the species of Primula, and on their remarkable sexual relations. Bot. J. Linn. Soc. 6: 77–96. [Google Scholar]
- Darwin, C. (1877) The different forms of flowers on plants of the same species. Murray, London. [Google Scholar]
- de Nettancourt, D. (2001) Incompatibility and incongruity in wild and cultivated plants. Springer, Berlin. [Google Scholar]
- Dowrick V.P.J. (1956) Heterostyly and homostyly in Primula obconica. Heredity 10: 219–236. [Google Scholar]
- Galetto L., Araujo F.P., Grilli G., Amarilla L.D., Torres C. and Sazima M. (2018) Flower trade-offs derived from nectar investment in female reproduction of two Nicotiana species (Solanaceae). Acta Bot. Brasilica 32: 473–478. [Google Scholar]
- Ganders F.R. (1979) The biology of heterostyly. N.Z. J. Bot. 17: 607–635. [Google Scholar]
- Garber R.J. and Quisenberry K.S. (1927) The inheritance of length of style in buckwheat. J. Agric. Res. 34: 181–183. [Google Scholar]
- Goldberg E.E., Kohn J.R., Lande R., Robertson K.A., Smith S.A. and Igic B. (2010) Species selection maintains self-incompatibility. Science 330: 493–495. [DOI] [PubMed] [Google Scholar]
- Helfer A., Nusinow D.A., Chow B.Y., Gehrke A.R., Bulyk M.L. and Kay S.A. (2011) LUX ARRHYTHMO encodes a night time repressor of circadian gene expression in the Arabidopsis core clock. Curr. Biol. 21: 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrero E., Kolmos E., Bujdoso N., Yuan Y., Wang M.M., Berns M.C., Uhlworm H., Coupland G., Saini R., Jaskolski M. et al. (2012) EARLY FLOWERING4 recruitment of EARLY FLOWERING3 in the nucleus sustains the Arabidopsis circadian clock. Plant Cell 24: 428–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilderbrand, F. (1867) Die Geschlechter-vertheilung bei den pelanzen und das gesetz der vermiedene und unvortheilhaften stetigen selbstbefruchtung. Verlag von Wilhelm Engelmann, Leipzig. [Google Scholar]
- Hirose T., Ujihara A., Kitabayashi H. and Minami M. (1995) Pollen tube behavior related to self-incompatibility in interspecific crosses of Fagopyrum. Breed. Sci. 45: 65–70. [Google Scholar]
- Huang L., Cao J.S., Zhang A.H., Ye Y.Q., Zhang Y.C. and Liu T.T. (2009a) The polygalacturonase gene BcMF2 from Brassica campestris is associated with intine development. J. Exp. Bot. 60: 301–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Ye Y.Q., Zhang Y.C., Zhang A.H., Liu T.T.T. and Cao J.S. (2009b) BcMF9, a novel polygalacturonase gene, is required for both Brassica campestris intine and exine formation. Ann. Bot. 104: 1339–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwano M. and Takayama S. (2012) Self/non-self discrimination in angiosperm self-incompatibility. Curr. Opin. Plant Biol. 15: 78–83. [DOI] [PubMed] [Google Scholar]
- Kramer M.G. and Redenbaugh K. (1994) Commercialization of a tomato with an antisense polygalacturonase gene: The FLAVR SAVRTM tomato story. Euphytica 79: 293–297. [Google Scholar]
- Lewis, D. and D.A. Jones (1992) The genetics of heterostyly. In: Barrett, S.C.H. (ed.) Evolution and Function of Heterostyly. Springer-Verlag, Berlin Heidelberg, pp. 129–150. [Google Scholar]
- Li J.H., Webster M.A., Wright J., Cocker J.M., Smith M.C., Badakshi F., Heslop-Harrison P. and Gilmartin P.M. (2015) Integration of genetic and physical maps of the Primula vulgaris S locus and localization by chromosome in situ hybridization. New Phytol. 208: 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J.H., Cocker J.M., Wright J., Webster M.A., McMullan M., Dyer S., Swarbreck D., Caccamo M., van Oosterhout C. and Gilmartin P.M. (2016) Genetic architecture and evolution of the S locus supergene in Primula vulgaris. Nat. Plants 2: 16188. [DOI] [PubMed] [Google Scholar]
- Liu X.L., Covington M.F., Fankhauser C., Chory J. and Wanger D.R. (2001) ELF3 encodes a circadian clock-regulated nuclear protein that functions in an Arabidopsis PHYB signal transduction pathway. Plant Cell 13: 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H.G. (1970) Registration of ‘Pennline 10’ buckwheat. Crop Sci. 10: 726. [Google Scholar]
- Matsui K., Tetsuka T. and Hara T. (2003a) Two independent gene loci controlling non-brittle pedicels in buckwheat. Euphytica 134: 203–208. [Google Scholar]
- Matsui K., Tetsuka T., Nishio T. and Hara T. (2003b) Heteromorphic incompatibility retained in self-compatible plants produced by a cross between common and wild buckwheat. New Phytol. 159: 701–708. [DOI] [PubMed] [Google Scholar]
- Matsui K., Nishio T. and Tetsuka T. (2004) Genes outside the S supergene suppress S functions in buckwheat (Fagopyrum esculentum). Ann. Bot. 94: 805–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui K., Nishio T. and Tetsuka T. (2007) Use of self-compatibility and modifier genes for breeding and genetic analysis in common buckwheat (Fagopyrum esculentum). Jpn. Agric. Res. Q. 41: 1–5. [Google Scholar]
- Matsui K., Tetsuka T., Hara T. and Morishita T. (2008) Breeding and characterization of a new self-compatible common buckwheat parental line, “Buckwheat Norin-PL1”. Bull. Natl. Agric. Res. Cent. Kyushu Okinawa Reg. 49: 1–17. [Google Scholar]
- Nagano M., Aii J., Campbell C.G., Kawasaki S. and Adachi T. (2000) Genome size analysis of the genus Fagopyrum. Fagopyrum 17: 35–39. [Google Scholar]
- Nishimoto Y., Ohnishi O. and Hasegawa M. (2003) Topological incongruence between nuclear and chloroplast DNA trees suggesting hybridization in the urophyllum group of the genus Fagopyrum (Polygonaceae). Genes Genet. Syst. 78: 139–153. [DOI] [PubMed] [Google Scholar]
- Nusinow D.A., Helfer A., Hamilton E.E., King J.J., Imaizumi T., Schultz T.F., Farre E.M. and Kay S.A. (2011) The ELF4-ELF3-LUX complex links the circadian clock to diurnal control of hypocotyl growth. Nature 475: 398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa M., Kay P., Wilson S. and Swain S.M. (2009) ARABIDOPSIS DEHISCENCE ZONE POLYGALACTURONASE1 (ADPG1), ADPG2, and QUARTET2 are polygalacturonases required for cell separation during reproductive development in Arabidopsis. Plant Cell 21: 216–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi O. and Matsuoka Y. (1996) Search for the wild ancestor of buckwheat II. Taxonomy of Fagopyrum (Polygonaceae) species based on morphology, isozymes and cpDNA variability. Genes Genet. Syst. 71: 383–390. [Google Scholar]
- Ohnishi O. (1998) Search for the wild ancestor of buckwheat III. The wild ancestor of cultivated common buckwheat, and of tatary buckwheat. Econ. Bot. 52: 123–133. [Google Scholar]
- Ohsako T. and Ohnishi O. (1998) New Fagopyrum species revealed by morphological and molecular analyses. Genes Genet. Syst. 73: 85–94. [Google Scholar]
- Ohsako T. and Ohnishi O. (2000) Intra- and interspecific phylogeny of wild Fagopyrum (Polygonaceae) species based on nucleotide sequences of noncoding regions in chloroplast DNA. Am. J. Bot. 87: 573–582. [PubMed] [Google Scholar]
- Raschke A., Ibanez C., Ullrich K.K., Anwer M.U., Becker S., Glockner A., Trenner J., Denk K., Saal B., Sun X.D. et al. (2015) Natural variants of ELF3 affect thermomorphogenesis by transcriptionally modulating PIF4-dependent auxin response genes. BMC Plant Biol. 15: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch-Bodmer H. (1934) Zum Heterostylieproblem: Griffelbeschaffenheit und Pollenschlauchwachstum bei Fagopyrum esculentum. Planta 22: 149–152. [Google Scholar]
- Sharma K.D. and Boyes J.W. (1961) Modified incompatibility of buckwheat following irradiation. Can. J. Bot. 39: 1241–1246. [Google Scholar]
- Sitrit Y., Downie B., Bennett A.B. and Bradford K.J. (1996) A novel exo-polygalacturonase is associated with radicle protrusion in tomato (Lycopersicon esculentum) seeds. Plant Physiol. 111: 752–752. [Google Scholar]
- Sitrit Y., Hadfield K.A., Bennett A.B., Bradford K.J. and Downie A.B. (1999) Expression of a polygalacturonase associated with tomato seed germination. Plant Physiol. 121: 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama S. and Isogai A. (2005) Self-incompatibility in plants. Annu. Rev. Plant Biol. 56: 467–489. [DOI] [PubMed] [Google Scholar]
- Takeshima R., Nishio T., Komatsu S., Kurauchi N. and Matsui K. (2019) Identification of a gene encoding polygalacturonase expressed specifically in short styles in distylous common buckwheat (Fagopyrum esculentum). Heredity (Edinb) 123: 492–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatebe T. (1953) Physiological research on the fertility of the buckwheat. III. On the self-fertile, long-styled plants. Japan. J. Breed. 2: 240–244. [Google Scholar]
- Ueno, M., Y. Yasui, J. Aii, K. Matsui, S. Sato and T. Ota (2016) Genetic analyses of the heteromorphic self-incompatibility (S) locus in buckwheat. In: Zhou, M. (ed.) Molecular breeding and nutritional aspects of buckwheat. Academic Press, pp. 411–422. [Google Scholar]
- Ushijima K., Nakano R., Bando M., Shigezane Y., Ikeda K., Namba Y., Kume S., Kitabata T., Mori H. and Kubo Y. (2012) Isolation of the floral morph-related genes in heterostylous flax (Linum grandiflorum): the genetic polymorphism and the transcriptional and post-transcriptional regulations of the S locus. Plant J. 69: 317–331. [DOI] [PubMed] [Google Scholar]
- Wedderburn F.M. and Richards A.J. (1992) Secondary homostyly in Primula L.; evidence for the model of the S supergene. New Phytol. 121: 649–655. [Google Scholar]
- Woo S.H., Adachi T., Jong S.K. and Campbell C.G. (1999) Inheritance of self-compatibility and flower morphology in an inter-specific buckwheat hybrid. Can. J. Plant Sci. 79: 483–490. [Google Scholar]
- Xiao C., Somerville C. and Anderson C.T. (2014) POLYGALACTURONASE INVOLVED IN EXPANSION1 functions in cell elongation and flower development in Arabidopsis. Plant Cell 26: 1018–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y. and Ohnishi O. (1998a) Interspecific relationships in Fagopyrum (Polygonaceae) revealed by the nucleotide sequences of the rbcL and accD genes and their intergenic region. Am. J. Bot. 85: 1134–1142. [PubMed] [Google Scholar]
- Yasui Y. and Ohnishi O. (1998b) Phylogenetic relationships among Fagopyrum species revealed by nucleotide sequences of the ITS region of the nuclear rRNA gene. Genes Genet. Syst. 73: 201–210. [DOI] [PubMed] [Google Scholar]
- Yasui Y., Mori M., Aii J., Abe T., Matsumoto D., Sato S., Hayashi Y., Ohnishi O. and Ota T. (2012) S-LOCUS EARLY FLOWERING 3 is exclusively present in the genomes of short-styled buckwheat plants that exhibit heteromorphic self-incompatibility. PLoS ONE 7: e31264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y., Hirakawa H., Ueno M., Matsui K., Katsube-Tanaka T., Yang S.J., Aii J., Sato S. and Mori M. (2016) Assembly of the draft genome of buckwheat and its applications in identifying agronomically useful genes. DNA Res. 23: 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta M.T., Hicks K.A., Jacobs C.I., Young J.C., Hangarter R.P. and Meeks-Wagner D.R. (1996) The Arabidopsis ELF3 gene regulates vegetative photomorphogenesis and the photoperiodic induction of flowering. Plant J. 10: 691–702. [DOI] [PubMed] [Google Scholar]
- Zhang X.H. and Ren Y. (2008) Floral morphology and development in Sargentodoxa (Lardizabalaceae). Int. J. Plant Sci. 169: 1148–1158. [Google Scholar]
- Zheng G.X., Lau B.T., Schnall-Levin M., Jarosz M., Bell J.M., Hindson C.M., Kyriazopoulou-Panagiotopoulou S., Masquelier D.A., Merrill L., Terry J.M. et al. (2016) Haplotyping germline and cancer genomes with high-throughput linked-read sequencing. Nat. Biotechnol. 34: 303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]


