Abstract
Background
Cardiorespiratory arrest (CA) secondary to traumatic cervical spinal cord injury can occur in minor accidents with low-impact trauma and may be overlooked as the cause of CA in patients admitted in the coronary care unit.
Case summary
We present two patients admitted to the coronary care unit because of suspected CA of cardiac origin. Both patients were found in CA with asystole, one after collapsing in a shopping mall and falling down a few steps and the other in the street next to his bicycle. They underwent early pharmacologically induced coma and hypothermia precluding neurological examination. Both patients remained in coma after rewarming, with preserved brainstem reflexes but absent motor response to pain. One patient had post-anoxic myoclonus in the face without limb involvement. In both patients, median nerve somatosensory evoked potentials demonstrated bilateral absence of thalamocortical N19 responses and abnormal cervicomedullary junction potentials (N13 wave). Extensive diagnostic work-up did not find a cardiac cause of the CA, pulmonary thromboembolism, or intracranial haemorrhage. In both patients, cervical spinal cord injury was diagnosed incidentally 5 and 6 days after CA, when a brain magnetic resonance imaging performed to assess post-anoxic brain injuries detected spinal cord hyperintensities with fracture and luxation of the odontoid. Both patients died 11 and 8 days after CA.
Discussion
Low-impact traumatic cervical spinal cord injury should be considered in the diagnostic work-up of patients with CA of unknown cause.
Keywords: Cardiorespiratory arrest, Traumatic cervical spinal cord injury, Low-impact trauma, Somatosensory evoked potentials, Motor evoked potentials, Post-anoxic coma, Case report
Learning points
Cardiorespiratory arrest (CA) can be associated with traumatic cervical spine injury and in some cases may be the cause of the arrest.
Low-impact trauma occurring in the setting of minor accidents such as falling from a standing position or from the height of a few steps can cause a cervical spinal cord injury leading to subsequent CA.
Traumatic cervical spinal cord injury secondary to low-impact trauma may go unnoticed in patients with CA. Red flags to suspect this condition in patients with CA are: (i) previous history of low-impact trauma; (ii) a brief interval with preserved consciousness occurring between the trauma and CA; (iii) abnormalities of N13 waves in the median nerve somatosensory evoked potentials; and (iv) post-anoxic myoclonus involving the face but not the limbs.
Care should be taken to avoid excessive cervical manipulation during nursing care or neurological examination in patients with CA of unknown cause.
Introduction
Traumatic cervical spinal cord injury, usually at the upper levels of C1 and C2, is an established but rare cause of cardiorespiratory arrest (CA) due to disruption of the sympathetic nervous system pathway.1–5 Only few case reports have documented this mechanism of CA. For example Kim et al. reported a 26-year-old male admitted after a traffic accident with bilateral C6–C7 fractures and spinal cord haemorrhage at C4–C7 level. Cardiac monitoring revealed intermittent bradycardia and on the 22nd day after the accident the patient experienced CA as a consequence of sympathetic hypoactivity.3 This case is descriptive of CA attributable to dysfunction of the autonomic nervous system related to a major traumatic cervical spinal cord injury. CA secondary to spinal cord injury, however, can occur even in minor accidents with low-impact trauma.6 We report two patients admitted in the coronary care unit because of an out-patient CA suspected to be cardiogenic but later found to be related to an unnoticed traumatic cervical spinal cord injury.
Timeline
| Patient 1 | Patient 2 |
|---|---|
|
Day 0
|
Day 0
|
|
Days 0–3 post-CA
|
Days 0–2 post-CA
|
|
Day 4 post-CA
|
Day 2 post-CA
|
|
Days 3 and 4 post-CA
| |
|
Day 5 post-CA
|
Day 6 post-CA
|
|
Day 11 post-CA
|
Day 8 post-CA
|
Case presentation
Patient 1
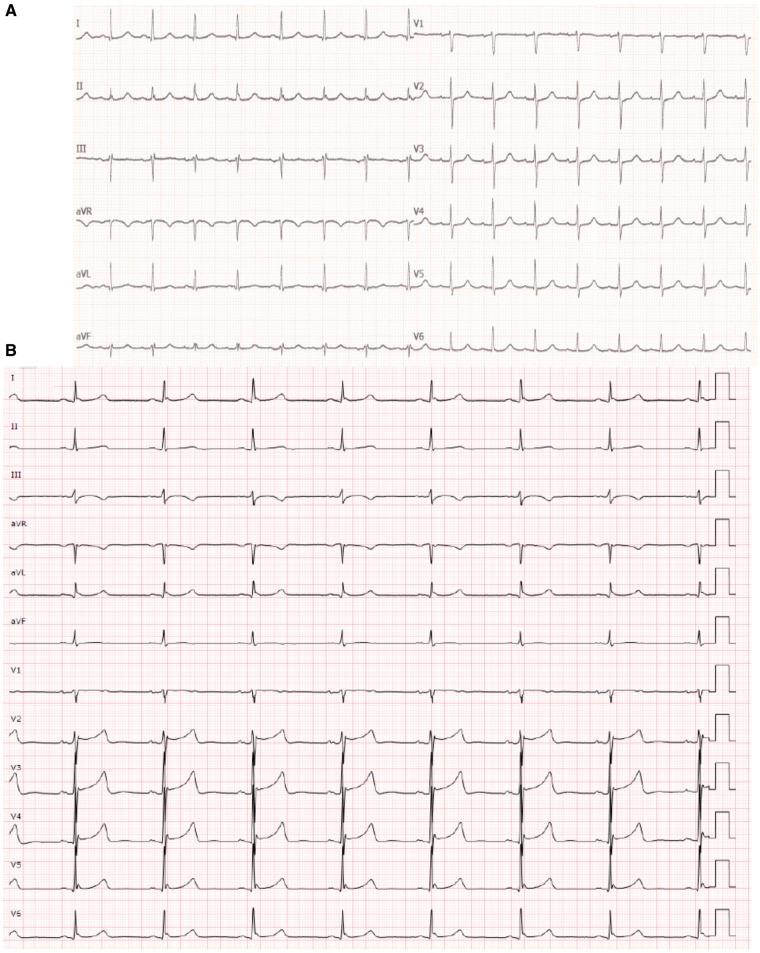
A 76-year-old woman with no relevant past medical history suffered a CA with asystole after collapsing in a shopping mall and falling down a few steps. Spontaneous pulse was recovered after 15 min of advanced resuscitation manoeuvres. She was sedated with propofol, intubated and transferred to the emergency room. The first electrocardiogram (ECG) after hospital admission following adrenaline injection 1 mg was sinus rhythm (Figure 1A). Pupils were symmetric, miotic, and reactive to light. Brainstem reflexes were preserved. Response to pain was absent except for withdrawal in the left foot. Plantar cutaneous responses were neutral.
Figure 1.
First electrocardiogram at hospital admission. (A) Patient 1. Electrocardiogram following adrenaline injection (1 mg). Sinus rhythm. Narrow QRS complex morphology. PR interval 180 msec. Axis 0°. (B) Patient 2. First electrocardiogram at hospital admission following adrenaline injection (2 mg). Sinus bradycardia (heart rate: 45 b.p.m.). PR interval 200 ms. cQT (Rautaharju): 452 ms. Narrow QRS complex with incomplete right bundle block.
The patient was admitted to the coronary care unit because of suspected cardiogenic CA and a hypothermia protocol was initiated. Acute myocardial infarction or other cardiac disorders were not diagnosed as the troponin curve was negative, angiography ruled out significant coronary lesions and echocardiography was normal. A thoracic computed tomography (CT)-scan ruled out pulmonary thromboembolism. There was no intracranial haemorrhage or other abnormalities on brain CT-scan.
On the 4th day post-CA, propofol was stopped but the patient remained in coma. Pupils were miotic and reactive. Other brainstem reflexes were preserved. Motor response to pain was absent. An EEG recorded diffuse delta–theta slowing with frequent irregular bilateral frontal sharp waves every 2–10 s. Median nerve SSEP demonstrated bilateral absence of thalamocortical N19 response and bilateral irregular low-amplitude cervicomedullary junction N13 waves with preserved peripheral potential (Erb point response) (Figure 2A).
Figure 2.
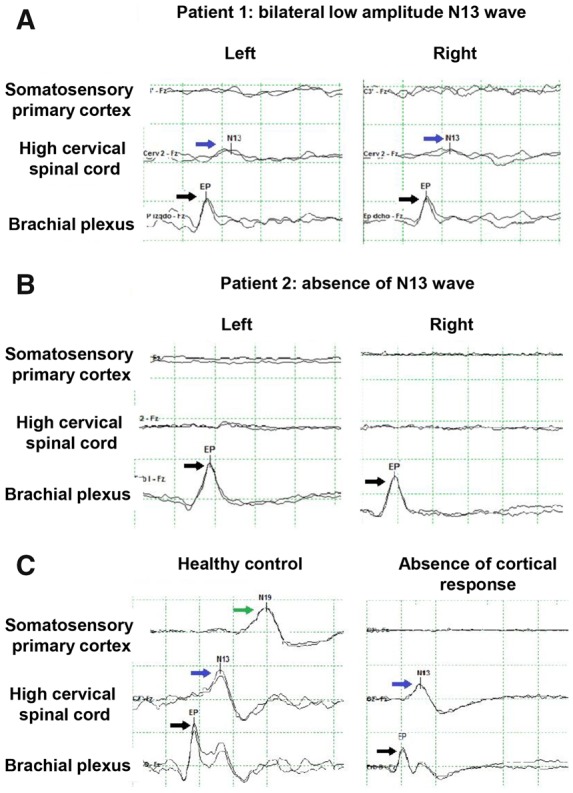
Somatosensory evoked potentials of the median nerve. The evoked electrical response is abbreviated with P or N (meaning positive or negative wave) and the milliseconds of delay after the stimulus. Responses are recorded in brachial plexus or Erb point (N9), high cervical spinal cord response (at the medullary junction, N13), and cortical response (N19). Black arrows point to the N9 wave, blue arrows to the N13, and the green arrow to the N19. (A) Patient 1 with low amplitude N13 response. (B) Patient 2 with absent N13 wave. (C) Healthy control with normal responses and a patient with post-anoxic coma and an absent N19 response but normal N13 wave.
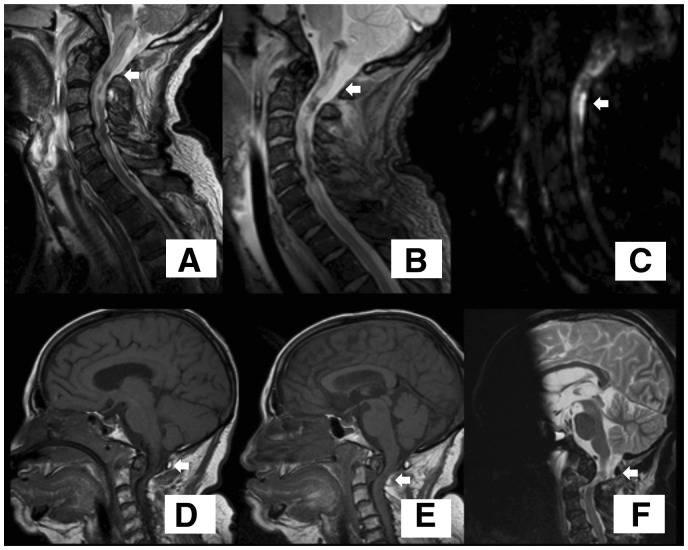
On the 5th day after CA, a brain magnetic resonance imaging (MRI) showed absence of acute hypoxic–ischaemic lesions but an upper cervical spinal cord injury was unexpectedly noticed. A cervical MRI confirmed a fracture with luxation of the odontoid with severe cervical spinal cord hyperintensity (Figure 3A–C). The cervical spinal cord was immobilized and an orthopaedic consultation requested. This traumatic spinal cord injury was considered as the cause of the CA. The clinical history was reviewed and this revealed that after falling down the steps, the patient had preserved consciousness and was able to talk for few seconds with a security guard until she suddenly lost consciousness. The patient died 6 days later (Day 11 post-CA) following refractory severe haemodynamic instability.
Figure 3.
Cervical cord magnetic resonance imaging. Odontoid fracture with luxation D’Alonso type II with secondary spinal cord injury due to compression (arrows). (A–C) Cervical magnetic resonance imaging of Patient 1. (A) FLAIR sequence. (B) T2 sequence. (C) DWI sequence. (D–F) Craniocervical magnetic resonance imaging of Patient 2. (D and E) T1 sequence of Patient 2. (F) T2 sequence.
Patient 2
A 65-year-old man without cardiovascular risk factors was found unconscious with an impalpable pulse, lying in the street next to his bicycle. A local policeman started basic resuscitation manoeuvres. Signs of traumatic injury were absent and the patient was still wearing his bike helmet. Fifteen minutes later the medical emergency service initiated advanced cardiopulmonary resuscitation (CPR). The initial rhythm was asystole which reverted to sinus rhythm after 40 min of CPR.
After intubation and sedation with propofol the patient was transferred to the hospital, where body cooling was started. The first ECG at hospital admission following adrenaline injection 2 mg showed sinus bradycardia (heart rate 45 b.p.m., Figure 1B). Neurological examination performed after the patient had received cisatracurium showed symmetric miotic and reactive pupils, absence of corneal and oculocephalic reflexes and no motor response to pain. Troponin analysis showed a slight increase (1.08 ng/mL, normal range < 0.05 ng/mL) 6 h after admission. Echocardiography and coronary angiography demonstrated normal heart motility and absence of coronary lesions. Head CT-scan and thoracic CT angiography were normal.
On the 2nd day post-CA, the patient was rewarmed and the cisatracurium was stopped. Despite sedation with low dose propofol the patient showed intermittent exclusively facial myoclonus. An EEG showed non-reactive diffuse rhythmic delta activity with generalized periodic discharges at 1.5 Hz. After stopping the propofol, the facial myoclonic movements increased in intensity and frequency but remained confined to the face. Pupils were symmetric with preserved pupillary light reflexes. Other brainstem reflexes were present but motor response to pain was absent. Plantar responses were neutral. Serum levels of neuronal specific enolase (NSE) on Day 3 post-CA were markedly increased (195 μg/L, normal range <33 μg/dL). Median nerve SSEP on Day 4 post-CA showed bilateral absence of thalamocortical N19 and cervicomedullary junction N13 responses with normal Erb point potential (Figure 2B).
A brain MRI on Day 6 post-CA revealed severe diffuse FLAIR and DWI signal abnormalities in the cortex and basal ganglia. A cervical spinal cord injury was also detected and cervical MRI confirmed an odontoid fracture with luxation and extensive upper spinal cord hyperintensity (Figure 3D–F). A traumatic cervical spinal cord injury was considered as the cause of the CA and cervical immobilization was applied. Transcranial electrical stimulation was performed to assess the corticospinal tract function and showed motor electrical evoked potentials only in the facial muscles but not in upper or lower limbs, indicating a disruption of the pyramidal tract at the cervical level (Figure 4). The patient died at Day 8 post-CA due to severe sepsis.
Figure 4.
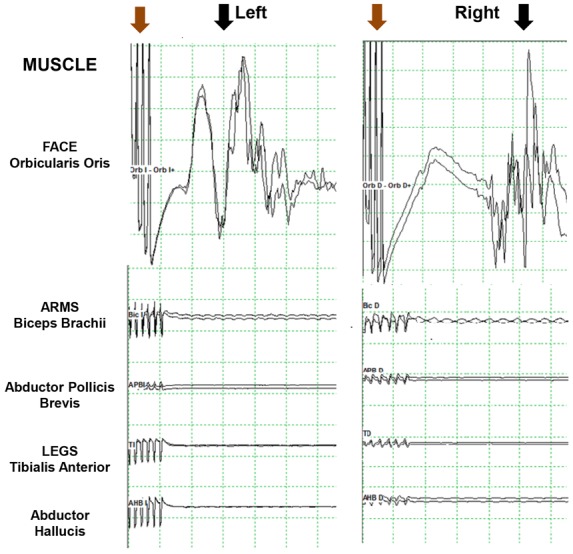
Motor electrical evoked potentials. Train of six electrical stimuli (brown arrow) followed by bilateral response of the facial orbicularis oris muscle (black arrow) but no response in upper and lower limb muscles (biceps brachii, abductor pollicis brevis, tibialis anterior, and abductor hallucis brevis).
Discussion
Although traumatic injury of the high cervical spinal cord is a well-known cause of CA, it may be initially overlooked in patients admitted in the coronary care unit as illustrated by the two patients reported here. We acknowledge the limitations regarding the inference of causality of spinal cord injury and CA, particularly in Case 2 in whom loss of consciousness, collapse and fall were not observed. However, after the extensive diagnostic work-up this mechanism was the most plausible explanation in both patients. Severe acute spinal cord injuries at the cervical level can be complicated with arrhythmias and haemodynamic abnormalities through disruption of the spinal cord sympathetic pathway with preserved parasympathetic outflow of the vagus nerve leading to bradycardia and eventually to asystole and CA. Another potential mechanism that may cause cardiac dysfunction and CA after a traumatic accident can be sudden and massive adrenergic mediators released from sympathetic nerves and suprarenal glands.2,5
Several factors may explain why the cervical spinal cord injury was unnoticed in our patients. The trauma causing the cervical spinal cord injury was not related to a severe accident and there were no external signs of concussion or other traumatic lesions. Patients with CA often collapse and fall down from a standing height without suffering significant traumatic injuries. Our two patients fell down, one a few steps and the other from a bike. In both cases these falls were initially misattributed to a collapse secondary to CA, but the most likely sequence was that these falls were the primary event, a low-impact trauma causing a cervical spinal cord injury with subsequent CA. Clinical history in patients with CA is often limited and information about the trauma can be missed as bystanders can be absent or difficult to interview in the hospital if they are not a patient’s relative, friend or acquaintance. Low-impact trauma equivalent to falling from standing height or a height greater than 1 m or five steps is potentially dangerous and carries a risk of sustaining severe injuries including cervical spine injuries.6 Cervical spinal cord injuries have also been reported to occur in patients collapsing because of cardiogenic CA.7 Another important factor contributing to the oversight of the traumatic spinal cord injury was that the initial diagnostic work-up was focused on cardiovascular causes, particularly suspicion of advanced atrioventricular block, which is a common cause of CA in the elderly. Non-cardiogenic causes of CA, mainly pulmonary thromboembolism and intracranial haemorrhage, are often considered after cardiac evaluation is non-revealing, but traumatic cervical spinal cord injury is rarely considered in the absence of a major trauma. Finally, the pharmacologically induced coma and the use of neuromuscular blocking agents during the hypothermia protocol limited the neurological examination and masked the classical signs of cervical spinal cord injury (e.g. tetraparesis, extensor plantar response, and sensory deficits).8 When anaesthetics and neuromuscular blocking agents are stopped, these signs can be absent due to spinal shock. Lack of motor response to pain can be wrongly considered a sign of bad outcome in the context of a severe post-anoxic brain injury.
Traumatic spinal cord injury in our patients was not diagnosed until brain MRI was performed to assess the post-anoxic brain damage. However, in retrospect, several other findings and signs were indicative of an underlying cervical spinal cord injury and included: (i) occurrence of a traumatic event more significant than collapsing from standing height (e.g. falling several steps or from a bicycle), in the absence of cardiac, pulmonary, or other neurological causes of CA; (ii) A brief interval with preserved consciousness after falling in one patient; (iii) Myoclonus involving only the face in one case as the corticospinal tract disruption at the level of the upper cervical spinal cord precluded the myoclonus to manifest in the limbs; and (iv) In both patients, median nerve SSEP showing abnormalities or absence of the evoked potential at the cervicomedullary junction (N13 wave) despite preservation of a peripheral response in the brachial plexus (Erb point, indicating that the stimulation of the median nerve in the wrists was correct). The SSEP are electrical potentials generated by various portions of the ascending sensory pathways in response to stimulation of peripheral sensory nerves. In patients in coma post-CA, median nerve SSEP represents an important neurological prognostic marker. Bilateral absence of the thalamocortical response (N19 wave) is indicative of an extremely poor neurological outcome,9 although it is important to note that in our patients with a cervical spinal cord injury, the N19 wave did not prove useful as a prognostic marker. In addition, absence of motor response to painful stimulation, another classical sign of poor neurological prognosis, has no value as it can be explained by the spinal cord injury. Motor electrical evoked potentials assess the corticospinal tract integrity and can be helpful in the prognosis related to the spinal cord injury. Neurological prognosis in our patients was poor due to severe spinal cord injury with expected residual tetraplegia in addition to severe post-anoxic brain injury supported by the EEG findings, brain MRI abnormalities, or elevated levels of NSE.
In conclusion, low-impact traumatic injury of the upper cervical spinal cord can be an overlooked cause of non-cardiogenic CA. Early identification of this potential cause of CA is important to prompt early cervical immobilization and fixation. Clues to suspect a traumatic cervical spinal cord injury in a patient with CA include a prior fall or injury that is more significant than collapsing from standing height, a brief interval with preserved consciousness between the fall and CA, abnormalities of the N13 wave in the median nerve SSEP, and post-anoxic myoclonus involving the face but not the limbs. Neuroimaging with MRI and/or CT including the cervical spine is essential to detect this rare potential cause of CA.
Lead author biography

Dr Mayà-Casalprim is a neurology resident at Hospital Clínic de Barcelona (2016–2020), and a collaborator of the Neurological Tissue Bank at University of Barcelona. He is member of the Catalan Society of Neurology, the Spanish Society of Neurology and the International Movement Disorder Society. He is the former coordinator of the Neurology Residents Study Group of the Catalan Society of Neurology (2017-2019).
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Ethical permissions and approvals
The publication of these two cases has been approved by the Hospital Clinic Ethical Committee/Institutional Review Board, Barcelona, Spain (certificate number 14/2019).
Supplementary Material
Acknowledgements
We would like to thanks Dr Myrna Rosenfeld for the critical review of the manuscript.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case series including image(s) and associated text has been obtained from the patients in line with COPE guidance.
Conflict of interest: none declared.
References
- 1. Teasell RW, Arnold JMO, Krassioukov A, Delaney GA.. Cardiovascular consequences of loss of supraspinal control of the sympathetic nervous system after spinal cord injury. Arch Phys Med Rehab 2000;81:506–516. [DOI] [PubMed] [Google Scholar]
- 2. Grigorean VT, Sandu AM, Popescu M, Iacobini MA, Stoian R, Neascu C, Strambu V, Popa F.. Cardiac dysfunctions following spinal cord injury. J Med Life 2009;2:133–145. [PMC free article] [PubMed] [Google Scholar]
- 3. Kim SW, Park CJ, Kim K, Kim YC.. Cardiac arrest attributable to dysfunction of the autonomic nervous system after traumatic cervical spinal cord injury. Chin J Traumatol 2017;20:118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miyata K, Mikami T, Koyanagi I, Mikuni N, Narimatsu E.. Cervical spinal cord injuries associated with resuscitation from fatal circulatory collapse. Acute Med Surg 2016;3:86–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lehmann KG, Lane JG, Piepmeier JM, Batsford WP.. Cardiovascular abnormalities accompanying acute spinal cord injury in humans: Incidence, time course and severity. J Am Coll Cardiol 1987;10:46–52. [DOI] [PubMed] [Google Scholar]
- 6. Clayton JL, Harris MB, Weintraub SL, Marr AB, Timmer J, Stuke LE, McSwain NE, Duchesne JC, Hunt JP.. Risk factors for cervical spine injury. Injury 2012;43:431–435. [DOI] [PubMed] [Google Scholar]
- 7. Desroziers M, Mole S, Jost D, Tourtier JP.. The need to immobilise the cervical spine during cardiopulmonary resuscitation and electric shock administration in out-of-hospital cardiac arrest. BMJ Case Rep 2016;doi: 10.1136/bcr-2016-214659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brown EN, Lydic R, Schiff ND.. General anesthesia, sleep, and coma. N Engl J Med 2010;363:2638–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rossetti AO, Rabinstein AA, Oddo M.. Neurological prognostication of outcome in patients in coma after cardiac arrest. Lancet Neurol 2016;15:597–609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.