Abstract
Human microbiota play an important role in the health of their human hosts. Recent studies have demonstrated that microbiota exist in seminal plasma. The current study aims to elucidate whether seminal microbiota exist in patients with different types of dysspermatism and whether bacterial biomarkers can be identified for them. A total of 159 study participants were recruited, including 22 patients with oligoasthenospermia, 58 patients with asthenospermia, 8 patients with azoospermia, 13 patients with oligospermia, and 58 matched healthy controls. Seminal microbiota composition was analyzed using 16S rRNA gene-based sequencing. The results showed that the composition of seminal microbiota of patients with dysspermatism differed from those of healthy controls. Comparison of the microbiota composition in semen samples from patients with different types of dysspermatism showed that microbiota in patients with asthenospermia and oligoasthenospermia were distinct from healthy controls in beta diversity (P < 0.05). Characteristic biomarkers, including Ureaplasma, Bacteroides, Anaerococcus, Finegoldia, Lactobacillus and Acinetobacter lwoffii, were identified based on LEfSe analysis. Inferred functional analysis based on seminal microbiome data further indicated the presence of potential pathogenic biomarkers in patients with asthenospermia and oligoasthenospermia. These results provided profiles of seminal microbiota exhibited in different types of dysspermatism, thus providing new insights into their pathogenesis.
Subject terms: Microbiome, Infertility
Introduction
Human microbiota, with its diverse relationships—commensal, parasitic, mutualistic, and pathogenic—play an important role in human health. Recent studies have reported that microbiota exist in almost every part of human body—even in the endocrine niche, such as in tumors, blood, and synovial fluid1–4. Advances in technology and new research have demonstrated that microbiota are found in seminal plasma, and play an important role in host homeostasis5. It has been demonstrated that the presence of bacteria in sperm is associated with male infertility6. Some bacteria in the urogenital tract may affect spermatogenesis and decrease sperm quality through various means, including decrease in sperm motility, deficiency in DNA integrity, and destruction of mitochondrial function7. Escherichia coli, Mycoplasma genitalium, Ureaplasma urealyticum, Mycoplasma hominis, Staphylococcus aureus, and Chlamydia trachomatis are pathogens associated with male infertility8–12. Several studies using high-throughput sequencing have demonstrated that seminal plasma has a bacterial community, which includes Lactobacillus, Pseudomonas, Prevotella, and Gardnerella, among others13–19.
Approximately 15% of couples worldwide are unable to conceive due to infertility and males contribute to 50% of the infertility cases20,21. There are several causative factors for male infertility, including genetic and environmental factors21–24. Abnormal semen (dysspermatism) is a reason for infertility, which occurs in about 50% of the cases of male infertity25,26. The changes in semen microenvironment could affect the spermatogenesis and motility. Many substances have recently been found in the seminal plasma that affect fertility, such as proteins, metabolites, environmental metals, etc. Compared to healthy controls, caspase-3 and cytochrome C levels were higher, and the total antioxidant capacity (TAC) was lower in seminal plasma of infertile patients27,28. Testosterone and androstenedione vectors correlate with steroids, which may function as biomarkers in patients with endocrine disorders, thus indicating that they may play an important role in sexual maturity29. A study also found that follicle-stimulating hormone deficiency could affect male fertility30. Several studies focused on environmental chemical substances, including perfluoroalkyl compounds, Pb, Cd, Ba, and U, have demonstrated that these substances may adversely affect seminal quality31–33. To summarize, seminal plasma functions not only as a medium to carry, protect, and nourish sperm after ejaculation up to fertilization, but also modulates sperm functions34.
It is critical to identify the bacterial species composition of the microbiota in seminal plasma to better understand the etiology and pathogenesis of urogenital tract infections and their association with infertility. A study that recruited 58 patients with infertility and 19 healthy controls observed bacteria in seminal plasma by gram staining and explored the composition of microbiota. However, the authors did not discover any differences in the microbiota in seminal plasma of patients with infertility and healthy controls13. Another study showed that human testes have microbiota associated with idiopathic non-obstructive azoospermia15. Javurek et al. demonstrated that there is a difference in the composition of seminal microbiota in estrogen receptor-alpha knockout male mice and mice with high-fat diet, which indicates that seminal microbiota could be affected by genetic and environmental factors, thus increasing the risk of disease to the offspring16,17. These studies focused on the microbiota in seminal plasma, but did not find differences between patients with dysspermatism and healthy controls, hence, they remain inconclusive13–15,19,35.
Although previous studies have found microbiota in the seminal plasma of males with infertility, it remains unknown whether characteristic seminal microbiota exist in patients with different types of dysspermatism. This study aspires to improve the understanding of seminal microbiota and explore the potential role of microorganisms, by analyzing the seminal plasma from 159 study participants and characterizing the microbiota profile. KEGG analysis was adopted to predict potential pathways associated with dysspermatism.
Results
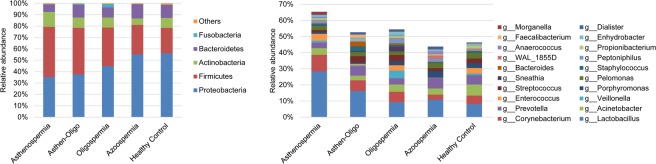
To characterize the features of seminal microbiota, 16S rRNA gene sequencing was done to measure 159 seminal samples, from 22 patients with oligoasthenospermia, 58 patients with asthenospermia, 8 patients with azoospermia, 13 patients with oligospermia, and 58 healthy controls. The clinical characteristics of the study participants are summarized in Table 1. After pre-processing of sequencing data, we obtained 3,871,353 high-quality sequences (Phred ≥ Q30) with an average of 24,348 per sample, yielding 1,065 taxa at a 97% identity cut-off. Five dominant phyla were Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes and Fusobacteria, as shown in Fig. 1.
Table 1.
Characteristics of individuals investigated in this study.
| Oligoastheno-spermia | Astheno-spermia | Azoo-spermia | Oligo-spermia | Healthy control | |
|---|---|---|---|---|---|
| Age (year) | 33.91 ± 6.05 | 31.66 ± 5.97 | 31.25 ± 5.17 | 31.15 ± 5.10 | 30.96 ± 5.11 |
| Sperm density (/ml) | 8.66 ± 7.65 | 52.07 ± 39.99 | N/A | 13.60 ± 10.46 | 68.18 ± 40.50 |
| Sperm count | 59.83 ± 112.45 | 190.47 ± 126.27 | N/A | 66.84 ± 42.68 | 228.46 ± 118.63 |
| pH | 7.28 ± 0.14 | 7.27 ± 0.18 | N/A | 7.31 ± 0.19 | 7.31 ± 0.15 |
| PRa | 12.50 ± 7.77 | 20.63 ± 9.76 | N/A | 22.60 ± 13.29 | 45.88 ± 13.05 |
| NPb | 10.79 ± 8.79 | 15.75 ± 6.99 | N/A | 12.64 ± 6.51 | 19.70 ± 6.42 |
| IMc | 72.31 ± 20.76 | 63.74 ± 14.29 | N/A | 65.81 ± 18.84 | N/A |
aForward motile sperm. bNon-forward motile sperm. cImmobile sperm.
Figure 1.
Relative abundance of taxa among five groups. Comparison of OTUs and relative taxa abundance among asthenospermia, oligospermia, oligoasthenospermia, azoospermia, and healthy controls. (A) At phylum level; (B) at genus level.
Altered seminal microbiota in patients with dysspermatism
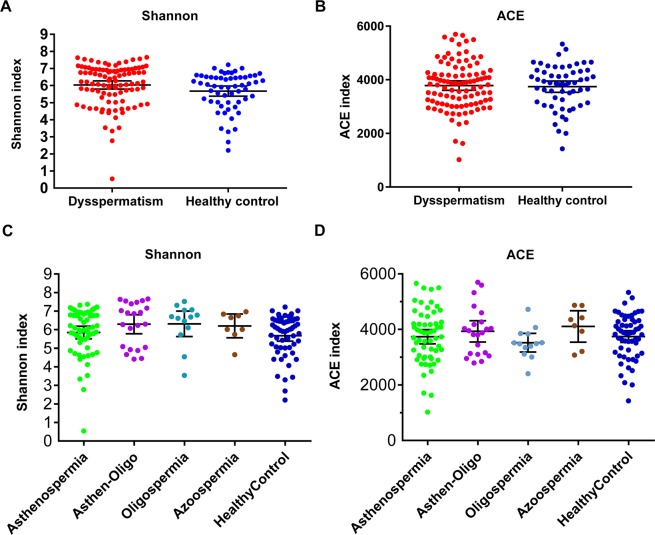
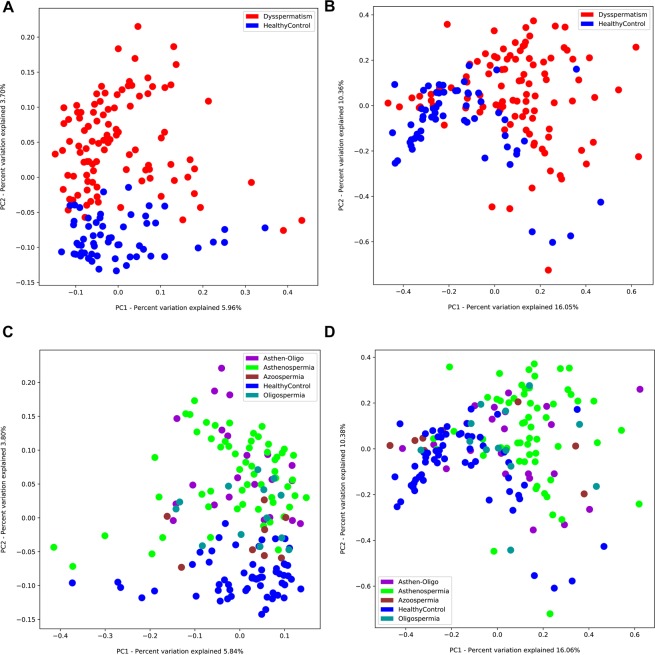
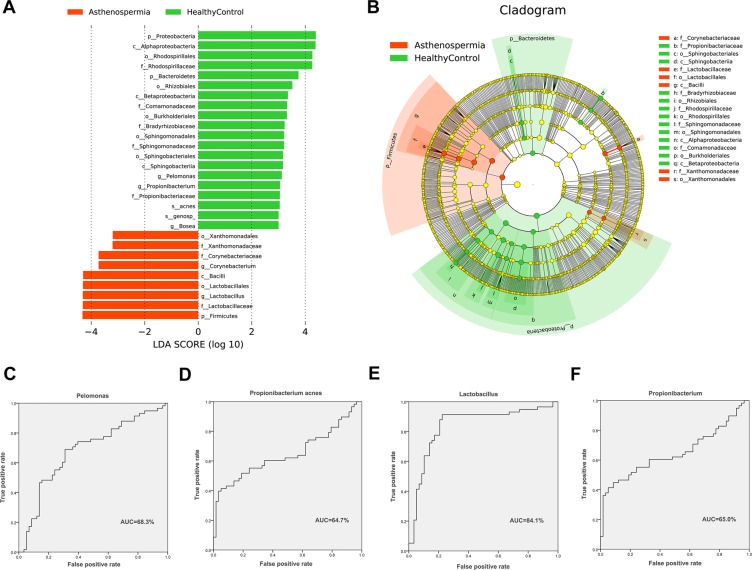
The aim of the analysis of seminal microbiota was to better understand the differences in seminal microbiota between patients with dysspermatism and healthy controls. Although α diversity including ACE index (P > 0.05) and Shannon index (P > 0.05) showed no significant difference in patients with dysspermatism and the healthy control group (Fig. 2A,B), β diversity (based on the unweighted and weighted UniFrac distance) was significantly different between the two groups (r = 0.598, P = 0.001 weighted UniFrac; r = 0.972, P = 0.001, unweighted UniFrac, Fig. 3A,B). A total of 110 significantly different taxa were detected in the two groups. Seminal microbiota in the dysspermatism group was characterized by dominance of genera of Lactobacillus, Bacteroides, Delftia, Sneathia, Enhydrobacter, Anaerococcus, Mycoplana, Finegoldia, Stenotrophomonas, Methylobacterium, Coprobacillus, Aerococcus, Atopobium, Chryseobacterium, Kocuria, Megasphaera, Ralstonia, Achromobacter, Erwinia, Ureaplasma, and Filifactor, and species of Prevotella copri, Saccharopolyspora hirsuta, Kocuria palustris, Prevotella nigrescens, Porphyromonas endodontalis, Lactobacillus coleohominis, Bacteroides barnesiae, and Lactobacillus iners. On the other hand, the microbiota in the healthy control group was dominated by genera of Pelomonas, Propionibacterium, Bosea genosp, Bosea, Afipia, Sphingomonas, Vogesella, Brevibacillus, Xylanimicrobium, Flexispira, Pedomicrobium, Phyllobacterium, Aquimonas, Dietzia, Sediminibacterium, Mycobacterium, and Eikenella, and species of Brevibacterium aureum, Propionibacterium acnes, Corynebacterium simulans, Eubacterium dolichum, and Bacillus thermoamylovorans (P < 0.05, Figs. 4 and S1A). In summary, we found that the composition of seminal microbiota was different between the two groups.
Figure 2.
Comparison of alpha diversity and relative abundance at phylum level based on the OTUs profile. Box plots depict differences in microbiome diversity based on (A) Shannon index and (B) ACE index between patients with dysspermatism and healthy controls. (C,D) show Shannon index and ACE index among patients with asthenospermia, oligospermia, oligoasthenospermia, and azoospermia, and healthy controls. The p value was calculated using the Wilcoxon rank-sum test.
Figure 3.
PCoA analysis of microbiota between patients with dysspermatism and healthy controls. (A) Unweighted unifrac PCoA; (B) Weighted unifrac PCoA. PCoA analysis of the microbiota among patients with asthenospermia, oligospermia, oligoasthenospermia, and azoospermia, and healthy controls; (C) Unweighted unifrac PCoA; (D) Weighted unifrac PCoA.
Figure 4.
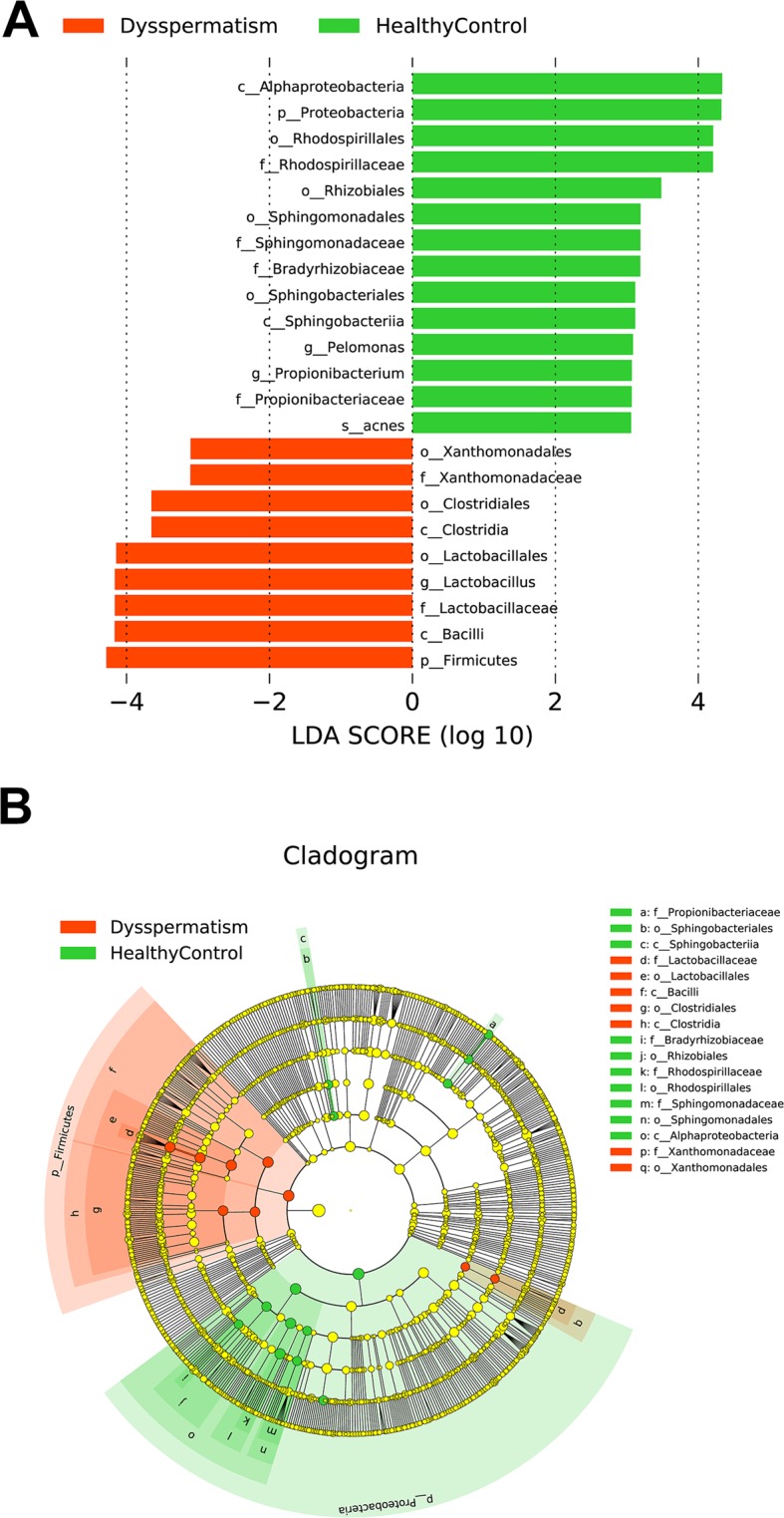
The most differentially abundant taxa between patients with dysspermatism and healthy controls (LDA score above 3) generated using LEfSe analysis.
Patients with asthenospermia or oligoasthenospermia harbor an altered seminal microbiota compared to healthy controls
There are differences in the specific pathogenesis of different types of dysspermatism. To determine the role of seminal microbiota in the different types of dysspermatism, we analyzed the seminal microbiota in patients with four different types of dysspermatism—asthenospermia, oligospermia, oligoasthenospermia, and azoospermia, and compared to healthy controls. There was no significant difference in α diversity (Shannon index and ACE index) among the five groups (P > 0.05, Fig. 2C,D). Analysis of β diversity showed seminal microbiota of patients with oligospermia (r = 0.180, P = 0.044, weighted UniFrac; r = 0.105, P = 0.110, unweighted UniFrac) or azoospermia (r = 0.207, P = 0.073, weighted UniFrac; r = 0.169, P = 0.096, unweighted UniFrac) showed no significant difference when compared to that of healthy controls; whereas seminal microbiota in patients with asthenospermia (r = 0.294, P = 0.0001, weighted UniFrac; r = 0.362, P = 0.0001, unweighted UniFrac) or oligoasthenospermia (r = 0.270, P = 0.001, weighted UniFrac; r = 0.316, P = 0.001, unweighted UniFrac) had significant composition variations compared to that of healthy controls (Fig. 3C,D).
Patients with asthenospermia harbored unique bacterial biomarkers, which may have potential pathogenicity
LEfSe analysis (Linear discriminant analysis Effect Size) was used to explore the bacterial biomarkers in the semen of patients with asthenospermia. Eighty different taxa in the two groups were chosen based on LDA > 2. Significant increase in the relative abundance of Sneathia, Ralstonia, Ureaplasma, Bacteroides, Chryseobacterium, Aerococcus, Enhydrobacter, Methylobacterium, Anaerococcus, Stenotrophomonas, Mycoplana, Delftia, Finegoldia, Corynebacterium, and Lactobacillus (at the genus level) and Saccharopolyspora hirsute, Acinetobacter lwoffii, and Lactobacillus iners (at the species level) was observed, and a significant reduction in Pelomonas, Propionibacterium, Bosea, Sphingomonas, Phyllobacterium, Pedomicrobium, Xylanimicrobium, Mycobacterium, and Zoogloea (at the genus level) and Propionibacterium acnes and Bosea genosp (at the species level) was observed in the asthenospermia group, compared to the healthy control group (Figs. 5A,B and S1B).
Figure 5.
LEfSe analysis between patients asthenospermia and healthy controls. (A,B) Comparison of the most differentially abundant taxa between patients with asthenospermia and healthy controls (LDA score above 3) generated using LEfSe analysis. We selected 4 biomarkers to predict the probability of patients with asthenospermia. (C–F) These biomarkers are Propionibacterium, Pelomonas, Lactobacillus, and Propionibacterium acnes. The ROC curves as well as the AUC (Area Under the Curve) values were calculated using SPSS.
To evaluate the potential value of the identified bacterial biomarkers for asthenospermia, ROC curves and AUC values were computed. The criteria used for biomarkers are—the genus and species of LDA > 3. Four biomarkers including Propionibacterium (ROC-plot AUC value was 0.650, 95% confidence interval [CI]: 54.6–75.3%), Pelomonas (ROC-plot AUC value was 0.683, 95% CI: 58.4–78.2%), Lactobacillus (ROC-plot AUC value was 0.841, 95% CI: 76.2–92.0%), and Propionibacterium acnes (ROC-plot AUC value was 0.647, 95% CI: 54.4–75.1%) were filtered. Additionally, we evaluated the effects of age and pH of seminal plasma on the four candidate biomarkers, in patients with asthenospermia and those in healthy controls (Fig. 5C–F). None of these factors had significant effect on the selected candidate biomarkers (Table S1).
Unique pathogenic bacteria in patients with oligoasthenospermia
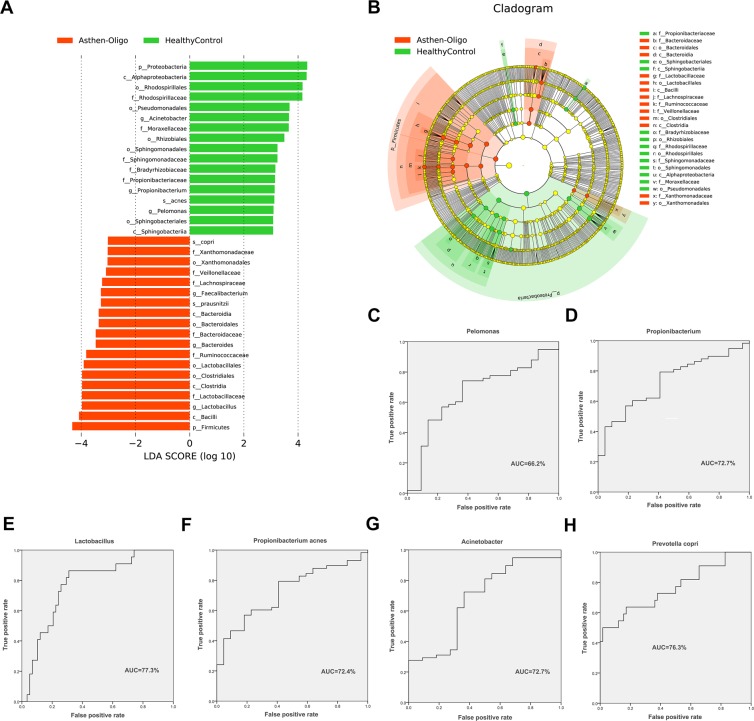
In order to select the biomarkers in oligoasthenospermia and healthy controls, we used LEfSe to analyze the composition of seminal microbiota in both groups. Seminal microbiota in the oligoasthenospermia group were characterized by a dominance of Ralstonia, Oscillospira, Parabacteroides, Lachnospira, Phascolarctobacterium, Chryseobacterium, Zoogloea, Ruminococcus, Stenotrophomonas, Actinoplanes, Mycoplana, Delftia, Sneathia, Megasphaera, Atopobium, Faecalibacterium, Bacteroides, Lactobacillus, Bacteroides uniformis, Stenotrophomonas panacihumi, Bacteroides plebeius, Prevotella copri, and Faecalibacterium prausnitzii, whereas microbiota in healthy controls were dominated by Acinetobacter, Propionibacterium, Pelomonas, Bosea, Deinococcus, Sphingomonas, Sediminibacterium, Pseudomonas, Pedomicrobium, Acinetobacter johnsonii, Streptococcus anginosus, Propionibacterium acnes, and Bosea genosp (Figs. 6A,B and S1C).
Figure 6.
LEfSe analysis between patients with oligoasthenospermia and healthy controls. (A,B) Comparison of the most differentially abundant taxa between patients with oligoasthenospermia and healthy controls (LDA score above 3), were generated using LEfSe analysis. (C–H) These biomarkers are Lactobacillus, Acinetobacter, Propionibacterium, Pelomonas, Prevotella copri, and Propionibacterium acnes. The ROC curves as well as the AUC (Area Under the Curve) value was calculated using SPSS.
As previously described, 6 biomarkers were selected in patients with oligoasthenospermia and healthy controls, which contained Lactobacillus (ROC-plot AUC value was 0.773, 95% CI: 66.2–88.4%), Acinetobacter (ROC-plot AUC value was 0.679, 95% CI: 54.5–81.3%), Propionibacterium (ROC-plot AUC value was 0.727, 95% CI: 61.3–84.1%), Pelomonas (ROC-plot AUC value was 0.662, 95% CI: 52.9–79.6%), Prevotella copri (ROC-plot AUC value was 0.763, 95% CI: 63.2–89.3%), and Propionibacterium acnes (ROC-plot AUC value was 0.724, 95% CI: 61.0–83.9%) (Fig. 6C–H). We also evaluated these candidate biomarkers by using confounding factors and found that there were no confounding factors that could affect these biomarkers (Table S2).
Predicted metagenome functions in patients with asthenospermia and healthy controls, and patients with oligoasthenospermia and healthy controls
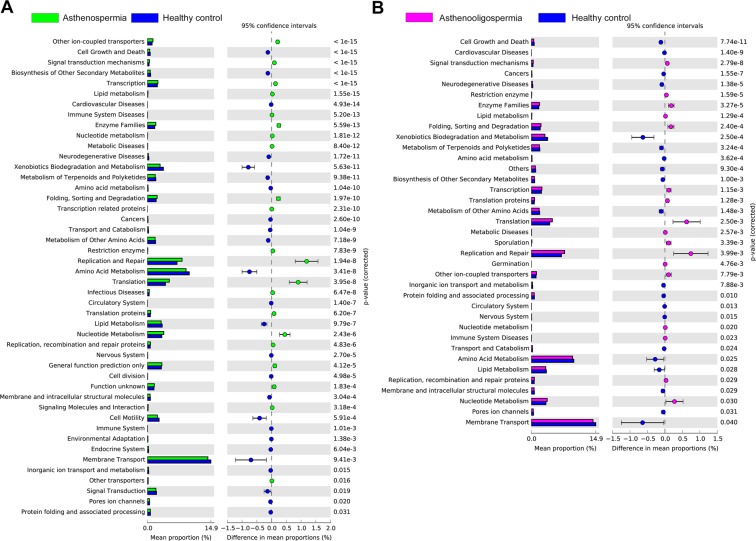
Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) was used to predict the different KEGG pathways in the two groups, and to discuss potential mechanisms of seminal microbiota in asthenospermia and oligoasthenospermia groups. Forty different KEGG pathways were displayed in patients with asthenospermia; these exhibited increased activities in some disease pathways such as cell growth and death, lipid metabolism, enzyme families, infectious diseases, cell division, cell motility, etc. There are 32 different KEGG pathways in patients with oligoasthenospermia and healthy controls, which showed increased pathways in cell growth and death, lipid metabolism, and metabolic diseases in patients with oligoasthenospermia (Fig. 7).
Figure 7.
Predicted metagenome function based on KEGG pathway analysis. Extended error bar plot showed significantly different KEGG pathways between (A) patients with asthenospermia and healthy controls; (B) between patients with oligoasthenospermia and healthy controls.
Discussion
In this study, high-throughput sequencing technology was used to analyze and measure the microbiota found in seminal plasma. Our study demonstrated that patients with different types of dysspermatism (oligoasthenospermia or asthenospermia) had significantly different composition of microbiota when compared to healthy controls. This pilot study also explored the opportunistic pathogens in the seminal plasma, which could be potential pathogens that increase the risk of dysspermatism.
Technological advances in recent years have led to several studies on the relationship between microbiota and human health. A large number of studies have shown that disorders of human microbial groups can lead to diseases, such as type II diabetes36. Recent studies have shown that bacteria also exist in the synovial fluid of patients with arthritis and play an important role in the occurrence of arthritis3. More interestingly, there are microorganisms in the blood4. This evidence indicates that different niches of the human body environment harbor distinct and potentially functional microbiota. In recent years, high throughput sequencing has been used to measure bacteria in seminal plasma, by which Lactobacillus, Pseudomonas, Prevotella Streptococcus, and Gardnerella were revealed to exist in seminal samples of patients and healthy controls13–15,19,35. These studies also found potentially pathogenic bacteria, such as Finegoldia and Anaerococcus13,14. We analyzed the composition of microbiota in the seminal plasma at the phylum level and found five dominant phyla: Proteobacteria, Firmicutes, Actinobacteria, Bacteroidetes, and Fusobacteria, which is consistent with the findings of a previous study15. We also analyzed major genera in the seminal plasma. The top 10 relatively abundant genera found commonly in the male seminal plasma were: Lactobacillus, Corynebacterium, Acinetobacter, Prevotella, Enterococcus, Veillonella, Streptococcus, Porphyromonas, Sneathia, and Pelomonas37. Based on all current studies, microbiota exist in the seminal plasma and share similar microbial community composition13–18,35.
Variation in the composition of microbiota could affect patients with asthenospermia or oligoasthenospermia. We found most of the taxa that were found in increased concentration in the seminal fluid of patients with asthenospermia and oligoasthenospermia were gram-negative bacteria containing lipopolysaccharide (LPS) in their cell walls. LPS can upregulate cytokines causing inflammation38. A previous study indicated that inflammatory mediators can directly cause DNA fragmentation in ejaculated spermatozoa, which ultimately limits the fertilization abilities of the germ cells39.
The relative abundance of Ureaplasma, Bacteroides, Anaerococcus, Finegoldia, Lactobacillus, and Acinetobacter lwoffii was significantly higher in asthenospermia, and Lactobacillus was notably abundant in oligoasthenospermia. Ureaplasma is the smallest prokaryote between bacteria and viruses, mainly found in the genitourinary tract of the human body. Bacteroides ureolyticus is a species of Bacteroides that could impair sperm structure and function by diminishing motility and causing sperm membrane injury, especially to the lipid bilayers as shown in in vitro tests29,40,41. Another study using 16S rRNA sequencing demonstrated that genus Anaerococcus could be a biomarker for predicting male infertility13. We also found this bacterium in patients with asthenospermia. Lactobacillus is a gram-positive bacterium, which produces SCFA (short-chain fatty acids). Recent studies have shown that SCFA are beneficial to human health. However, seminal pH is generally 7.242. Significant increase of Lactobacillus in asthenospermia may change the pH of semen and result in male infertility. Although our results found Lactobacillus in human semen, the mechanism remains to be determined, however, it provides new insights into male infertility.
Bacteria that were filtered out by factors such as age may be potentially pathogenic. The characteristic of asthenospermia is that the activity of spermatozoa is weakened. We found an increase in Lactobacillus and decrease in Propionibacterium, Pelomonas, and Propionibacterium acnes in the seminal plasma of patients with asthenospermia, which indicated that these bacteria may affect sperm activity. Oligoasthenospermia features both lesser and weaker sperm activity. Our results indicated an increase in Lactobacillus and Prevotella copri and decrease in Propionibacterium, Pelomonas, Acinetobacter, and Propionibacterium acnes, which may correlate with sperm formation and activity.
PICRUSt was used to predict potential metabolic pathways. We found that the lipid metabolism was significantly different in the three experimental groups. The essence of sex hormones is steroids. Previous studies have demonstrated that disorders of sex hormones result in male infertility. For instance, changes in FSH, LH, and T levels are associated with damage to the testes, impeding of spermatogenesis and maturation, and result in decreased sperm motility and activity, leading to infertility43–45. This finding also exhibits strong correlation with the change in microbiota.
To summarize, our results suggest that dysspermatism is associated with seminal microbiota, and also show that seminal microbiota in patients with asthenospermia and oligoasthenospermia are different from those in healthy controls. Biomarkers were screened and the KEGG metabolic pathways predicted. These results are beneficial for clinical diagnosis and could be further used to develop new treatment for patients with dysspermatism. Although we performed a bacterial culture experiment on semen samples, the pathogens were not cultured because of their low content. To better understand the mechanism of dysspermatism, future studies are needed in combination with metabolomics and culturomics.
Methods
Study participants
A total of 159 study participants from Shandong Health Center for Women & Children were recruited, including 22 patients with oligoasthenospermia (having both characteristics of asthenospermia and oligospermia), 58 patients with asthenospermia (the total number of sperm less than 39 × 106/ml), 8 patients with azoospermia (the absence of sperm or very low sperm content in semen), 13 patients with oligospermia (the proportion of progressive motility is less than 32%), and 58 healthy controls42. The study participants were about 31.65 ± 6.01 years old, and there was no significant difference among these groups. The diagnostic criteria of dysspermatism followed the 5th WHO laboratory manual for examination and processing of human semen42. Signed informed consent was taken and clinical indexes were also collected. All study participants met the following criteria: (1) study participants hadn’t taken antibiotics for three months prior to study enrollment, (2) study participants and their family members have no known genetic disease, (3) no history of sexually transmitted diseases, and (4) no history of corticosteroid use. All study procedures were approved by the Medical Ethical Committee of Maternal and Child Health Care Hospital of Shandong Province (#IRB: 2017-03). All methods and experimental protocols in this study were performed in accordance with relevant guidelines and standard operating procedures.
Collection of seminal plasma samples
In order to prevent contamination, stringent criteria and procedures were adopted to collect seminal plasma samples by masturbation. All participants abstained from sexual activity for 3 to 7 days before sample collection. Before sampling, hands were washed thoroughly with soap 2 to 3 times. The penis, especially the glans and coronal sulcus, was washed with warm soapy water and then wiped 2 to 3 times with 75% alcohol. The seminal plasma was injected directly into sterile glass containers, avoiding skin contact with the interior wall of the container. Fresh semen was used for routine seminal plasma clinical testing, and the remaining seminal samples were transferred to sterile microcentrifuge tubes and stored at −80 °C, within 2 hours of collection.
Isolation of seminal fluid microbial DNA and 16S rRNA sequencing
About 400 μl of seminal specimens were used for genomic DNA extraction, which was extracted using the CTAB (Cetyl trimethylammonium bromide) method46. Nanodrop 2000 (Thermo Scientific) spectrophotometer was used to determine the concentration of extracted DNA. The V1–V2 regions of 16S rRNA gene were amplified and sequenced on an Illumina HiSeq. 2500 system. PCR was conducted using bacterial universal primers 27F (5′ -(6FAM) AGA GTT TGA TCC TGG CTC AG 3′) and 355R (5′ GCT GCC TCC CGT AGG AGT 3′). Each PCR reaction contained 12.5 ul Q5 Hot Start High-Fidelity 2X Master Mix (BioLabs), 1.25 μl 10 μM Forward Primer, 1.25 ul 10 μM Reverse Primer, and 10 μl DNA template in a total volume of 25 μl. The following experiments were carried out as per the sequencing manual.
rRNA sequencing analysis
The 16S sequence paired-end data set was joined and qualitatively filtered using the Laser FLASH method described by Magoč and Salzberg47. All sequences were analyzed using the Quantitative Insights into Microbial Ecology (QIIME, version 1.9.1) software suite48, according to the QIIME tutorial (http://qiime.org/) with a few modified methods. Chimeric sequences, where a single organism has distinct genotypes, were removed using Metagenomics tool—usearch61 with denovo models49. Sequences were clustered against the 2013 Greengenes (13_5 release) ribosomal database’s 97% reference data set (http://greengenes.secondgenome.com/downloads). Sequences that remained unmatched with any of the entries in this reference were subsequently clustered into de novo OTUs at 97% similarity, using UCLUST algorithm. Taxonomy was assigned to all OTUs using the RDP classifier50 within QIIME and the Greengenes reference data set. Rarefaction and rank abundance curves were calculated from OTU tables using alpha diversity and rank abundance scripts within the QIIME pipeline. The hierarchical clustering based on population profiles of most common and abundant taxa was performed using UPGMA clustering (Unweighted Pair Group Method with Arithmetic mean, also known as average linkage) on the distance matrix of OTU abundance. This resulted in a Newick-formatted tree, obtained utilizing the QIIME package. Furthermore, QIIME was used to analyze alpha diversity (Shannon, ACE), beta diversity (weighted UniFrac, Principal Coordinate Analysis (PCoA)), Linear discriminant analysis (LDA) and Effect Size (LEfSe). SPSS (version 23) was used to calculate the receiver operating characteristic (ROC) curve and the area under the curve (AUC) values.
Data
All sequencing data were submitted to the NCBI SRA database (accession number: PRJNA534354).
Statistical analysis
The clinical characteristics of the study participants are represented as the mean ± SD, which were determined using Mann-Whitney U test. The diversity categorization of alpha and beta diversity is defined in the OTU table to a sequencing depth of 1,000 per sample. Moreover, alpha diversity was determined using Mann-Whitney U test and beta diversity was acquired using ANOSIM (Analysis of Similarities). LEfSe combines Kruskal-Wallis test or pair wise Wilcoxon rank sum test with linear discriminant analysis (LDA), whose threshold value on the logarithmic LDA score equals to 2.0. Analyses were performed using the SPSS statistical package (version 23). P values <0.05 were considered statistically significant.
Ethical approval and informed consent
All study procedures were approved by the Medical Ethical Committee of Maternal and Child Health Care Hospital of Shandong Province (#IRB: 2017-03). All study participants signed informed consent forms and were from the same geographical area. Data was collected using a standardized questionnaire that included basic information, medical history, and examination results.
Supplementary information
Acknowledgements
This study was supported by the Shandong Provincial Key Research and Development Program under contract No. 2018CXGC1219 and No. 2016YYSP009; the National Natural Science Foundation of China under contract No. 31471202; the Weihai Technique Extension Project under contract No. 2016GNS023. Lei Zhang is also supported by the TaiShan Scholars Program of Shandong Province (No. tshw20120206) and TaiShan Industrial Experts Program (No. tscy20190612).
Author contributions
L.Z. and H.Y. designed the study. J.Z. performed measurements and data analysis. Z.X., J.Y., C.Z., L.L., W.Y. and D.Y. obtained the samples. J.Z. and J.Y. wrote the manuscript and L.Z. revised the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Huijun Yang and Jiaming Zhang.
These authors jointly supervised this work: Junjie Yang and Lei Zhang.
Contributor Information
Junjie Yang, Email: microbiota@foxmail.com.
Lei Zhang, Email: microbiome@foxmail.com.
Supplementary information
is available for this paper at 10.1038/s41598-020-63787-x.
References
- 1.Spadoni I, et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science. 2015;350:830–834. doi: 10.1126/science.aad0135. [DOI] [PubMed] [Google Scholar]
- 2.Cavarretta I, et al. The Microbiome of the Prostate Tumor Microenvironment. Eur. Urol. 2017;72:625–631. doi: 10.1016/j.eururo.2017.03.029. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, et al. Effect of Acrylamide on Oocyte Nuclear Maturation and Cumulus Cells Apoptosis in Mouse In Vitro. Plos One. 2015;10:e0135818. doi: 10.1371/journal.pone.0135818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharyya M, Ghosh T, Shankar S, Tomar N. The conserved phylogeny of blood microbiome. Mol. Phylogenet Evol. 2017;109:404–408. doi: 10.1016/j.ympev.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Altmae S, Franasiak JM, Mandar R. The seminal microbiome in health and disease. Nat. Rev. Urol. 2019;16:703–721. doi: 10.1038/s41585-019-0250-y. [DOI] [PubMed] [Google Scholar]
- 6.Merino G, et al. Bacterial infection and semen characteristics in infertile men. Arch. Androl. 1995;35:43–47. doi: 10.3109/01485019508987852. [DOI] [PubMed] [Google Scholar]
- 7.La Vignera S, Vicari E, Condorelli RA, D’Agata R, Calogero AE. Male accessory gland infection and sperm parameters (review) Int. J. Androl. 2011;34:e330–347. doi: 10.1111/j.1365-2605.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 8.Ahmadi MH, Mirsalehian A, Gilani MAS, Bahador A, Talebi M. Improvement of semen parameters after antibiotic therapy in asymptomatic infertile men infected with Mycoplasma genitalium. Infection. 2018;46:31–38. doi: 10.1007/s15010-017-1075-3. [DOI] [PubMed] [Google Scholar]
- 9.Kaur K, Prabha V. Spermagglutinating Escherichia coli and its role in infertility: in vivo study. Microb. Pathog. 2014;69-70:33–38. doi: 10.1016/j.micpath.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Esmailkhani A, et al. Assessing the prevalence of Staphylococcus aureus in infertile male patients in Tabriz, northwest Iran. Int. J. Reprod. Biomed. 2018;16:469–474. [PMC free article] [PubMed] [Google Scholar]
- 11.Berjis K, Ghiasi M, Sangy S. Study of seminal infection among an infertile male population in Qom, Iran, and its effect on sperm quality. Iran. J. Microbiol. 2018;10:111–116. [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmadi MH, Mirsalehian A, Sadighi Gilani MA, Bahador A, Talebi M. Asymptomatic Infection With Mycoplasma hominis Negatively Affects Semen Parameters and Leads to Male Infertility as Confirmed by Improved Semen Parameters After Antibiotic Treatment. Urology. 2017;100:97–102. doi: 10.1016/j.urology.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 13.Hou D, et al. Microbiota of the seminal fluid from healthy and infertile men. Fertil. Steril. 2013;100:1261–1269. doi: 10.1016/j.fertnstert.2013.07.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weng SL, et al. Bacterial communities in semen from men of infertile couples: metagenomic sequencing reveals relationships of seminal microbiota to semen quality. Plos One. 2014;9:e110152. doi: 10.1371/journal.pone.0110152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alfano M, et al. Testicular microbiome in azoospermic men-first evidence of the impact of an altered microenvironment. Hum. Reprod. 2018;33:1212–1217. doi: 10.1093/humrep/dey116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Javurek AB, et al. Discovery of a Novel Seminal Fluid Microbiome and Influence of Estrogen Receptor Alpha Genetic Status. Sci. Rep. 2016;6:23027. doi: 10.1038/srep23027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Javurek AB, et al. Consumption of a high-fat diet alters the seminal fluid and gut microbiomes in male mice. Reprod. Fertil. Dev. 2017;29:1602–1612. doi: 10.1071/RD16119. [DOI] [PubMed] [Google Scholar]
- 18.Mandar R, et al. Complementary seminovaginal microbiome in couples. Res. Microbiol. 2015;166:440–447. doi: 10.1016/j.resmic.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 19.Baud D, et al. Sperm Microbiota and Its Impact on Semen Parameters. Front. Microbiol. 2019;10:234. doi: 10.3389/fmicb.2019.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alahmar AT. The effects of oral antioxidants on the semen of men with idiopathic oligoasthenoteratozoospermia. Clin. Exp. Reprod. Med. 2018;45:57–66. doi: 10.5653/cerm.2018.45.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnici J, Fenech A, Muscat C, Calleja-Agius J. The role of seminal fluid in infertility. Minerva Ginecol. 2017;69:390–401. doi: 10.23736/S0026-4784.17.04049-7. [DOI] [PubMed] [Google Scholar]
- 22.Akinsal EC, Baydilli N, Dundar M, Ekmekcioglu O. The frequencies of Y chromosome microdeletions in infertile males. Turk. J. Urol. 2018;44:389–392. doi: 10.5152/tud.2018.73669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li, W. et al. Biallelic mutations of CFAP251 cause sperm flagellar defects and human male infertility. J Hum Genet, 10.1038/s10038-018-0520-1 (2018). [DOI] [PubMed]
- 24.Adelman CA, Petrini JH. ZIP4H (TEX11) deficiency in the mouse impairs meiotic double strand break repair and the regulation of crossing over. Plos Genet. 2008;4:e1000042. doi: 10.1371/journal.pgen.1000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dohle GR, et al. EAU guidelines on male infertility. Eur. Urol. 2005;48:703–711. doi: 10.1016/j.eururo.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Pant N, et al. Lead and cadmium concentration in the seminal plasma of men in the general population: correlation with sperm quality. Reprod. Toxicol. 2003;17:447–450. doi: 10.1016/S0890-6238(03)00036-4. [DOI] [PubMed] [Google Scholar]
- 27.Emokpae MA, Chima HN. Effect of senescence on some apoptosis and oxidative stress markers in infertile normozospermic and oligospermic men: A cross-sectional study. Int. J. Reprod. Biomed. 2018;16:435–442. doi: 10.29252/ijrm.16.7.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du Plessis SS, Agarwal A, Halabi J, Tvrda E. Contemporary evidence on the physiological role of reactive oxygen species in human sperm function. J. Assist. Reprod. Genet. 2015;32:509–520. doi: 10.1007/s10815-014-0425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zufferey F, et al. Steroid profiles in both blood serum and seminal plasma are not correlated and do not reflect sperm quality: Study on the male reproductive health of fifty young Swiss men. Clin. Biochem. 2018;62:39–46. doi: 10.1016/j.clinbiochem.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Ratnayake GM, Weerathunga PN, Ruwanpura LP, Wickramasinghe A, Katulanda P. Isolated follicle stimulated hormone deficiency in male: case report. BMC Res. Notes. 2018;11:24. doi: 10.1186/s13104-017-3109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Nisio, A. et al. Endocrine disruption of androgenic activity by perfluoroalkyl substances: clinical and experimental evidence. J Clin Endocrinol Metab, 10.1210/jc.2018-01855 (2018). [DOI] [PubMed]
- 32.Sukhn, C., Awwad, J., Ghantous, A. & Zaatari, G. Associations of semen quality with non-essential heavy metals in blood and seminal fluid: data from the Environment and Male Infertility (EMI) study in Lebanon. J Assist Reprod Genet, 10.1007/s10815-018-1236-z (2018). [DOI] [PMC free article] [PubMed]
- 33.McDiarmid, M. A., Gucer, P., Centeno, J. A., Todorov, T. & Squibb, K. S. Semen Uranium Concentrations in Depleted Uranium Exposed Gulf War Veterans: Correlations with Other Body Fluid Matrices. Biol Trace Elem Res, 10.1007/s12011-018-1527-3 (2018). [DOI] [PubMed]
- 34.Camargo M, Intasqui P, Bertolla RP. Understanding the seminal plasma proteome and its role in male fertility. Basic. Clin. Androl. 2018;28:6. doi: 10.1186/s12610-018-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monteiro C, et al. Characterization of microbiota in male infertility cases uncovers differences in seminal hyperviscosity and oligoasthenoteratozoospermia possibly correlated with increased prevalence of infectious bacteria. Am. J. Reprod. Immunol. 2018;79:e12838. doi: 10.1111/aji.12838. [DOI] [PubMed] [Google Scholar]
- 36.Qin J, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 37.Younes JA, et al. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018;26:16–32. doi: 10.1016/j.tim.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Lewkowicz P, Lewkowicz N, Sasiak A, Tchorzewski H. Lipopolysaccharide-activated CD4+CD25+ T regulatory cells inhibit neutrophil function and promote their apoptosis and death. J. Immunol. 2006;177:7155–7163. doi: 10.4049/jimmunol.177.10.7155. [DOI] [PubMed] [Google Scholar]
- 39.Fraczek M, Szumala-Kakol A, Dworacki G, Sanocka D, Kurpisz M. In vitro reconstruction of inflammatory reaction in human semen: effect on sperm DNA fragmentation. J. Reprod. Immunol. 2013;100:76–85. doi: 10.1016/j.jri.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Fraczek M, Szumala-Kakol A, Jedrzejczak P, Kamieniczna M, Kurpisz M. Bacteria trigger oxygen radical release and sperm lipid peroxidation in in vitro model of semen inflammation. Fertil. Steril. 2007;88:1076–1085. doi: 10.1016/j.fertnstert.2006.12.025. [DOI] [PubMed] [Google Scholar]
- 41.Fraczek M, et al. Fertilizing potential of ejaculated human spermatozoa during in vitro semen bacterial infection. Fertil. Steril. 2014;102:711–719 e711. doi: 10.1016/j.fertnstert.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Organization, W. H. WHO laboratory manual for the examination and processing of human semen. (2010). [PubMed]
- 43.Liu, W. et al. Correlation of genetic results with testicular histology, hormones and sperm retrieval in nonobstructive azoospermia patients with testis biopsy. Andrologia49, 10.1111/and.12705 (2017). [DOI] [PubMed]
- 44.Jurewicz J, et al. Human Semen Quality, Sperm DNA Damage, and the Level of Reproductive Hormones in Relation to Urinary Concentrations of Parabens. J. Occup. Env. Med. 2017;59:1034–1040. doi: 10.1097/JOM.0000000000001106. [DOI] [PubMed] [Google Scholar]
- 45.Garolla A, et al. FSH treatment in infertile males candidate to assisted reproduction improved sperm DNA fragmentation and pregnancy rate. Endocrine. 2017;56:416–425. doi: 10.1007/s12020-016-1037-z. [DOI] [PubMed] [Google Scholar]
- 46.Wang X, et al. Gut microbiota dysbiosis is associated with Henoch-Schonlein Purpura in children. Int. immunopharmacology. 2018;58:1–8. doi: 10.1016/j.intimp.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Magoc T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caporaso JG, et al. QIIME allows analysis of high-throughput community sequencing data. Nat. methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 50.Cole JR, et al. The Ribosomal Database Project: improved alignments and new tools for rRNA analysis. Nucleic acids Res. 2009;37:D141–145. doi: 10.1093/nar/gkn879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.