Abstract
Background
The overuse of antibiotics is a serious public health problem in China, causing a high rate of antimicrobial resistance. This study identified the trends of antibiotic consumption in China to provide evidence for further intervention.
Method
The six-year surveillance data on antibiotic sales from 2012 to 2017, which served as a proxy for consumption, were collected from 39 public health care facilities in Shandong province, including three tertiary hospitals, six secondary hospitals, and 30 primary health centers. Based on the Anatomical Therapeutic Chemical (ATC)/DDD methodology, antibiotic consumption was formulated in defined daily doses (DDD) per 1,000 inhabitants per day (DID).
Results
The total antibiotic consumption among all health care settings increased from 16.07 DID in 2012 to a peak of 17.44 DID in 2015 and then decreased to 11.35 DID in 2017 with a 34.90% reduction. J01C (beta-lactam antimicrobials, penicillin), the most frequently used antibiotic class, accounted for 36.32% of the total DID. Consumption of carbapenems increased from 0.029 DID in 2012 to 0.08 DID in 2017. Parenteral antibiotics accounted for nearly 40% of the total consumption. Compared with the 2012 figures, the 2017 consumption showed a small increase in hospital sector that was compensated by the decrease in community care.
Conclusion
A substantial reduction in total antibiotic consumption was observed in China from 2012 to 2017. However, the extensive consumption of broad-spectrum antimicrobials, high proportion of parenteral antibiotic use, and increased use of last-resort antibiotics attracted public health concerns.
Keywords: antibiotic consumption, antimicrobial resistance, antimicrobial stewardship, rational drug use, China
Introduction
Antimicrobial resistance (AMR) driven by antibiotic consumption is a growing threat to global public health (Song, 2018; Zhang et al., 2019). China is among the world’s largest producers and consumers of antibiotics, which are widely used for disease treatment in humans and livestock, as well as prophylaxis and growth promoters for the latter (Qiao et al., 2018). China was estimated to be the second largest consumer of antibiotics in the world in 2010 in terms of the volume of antibiotics sold for human use in retail and hospital pharmacies (Van Boeckel et al., 2014).Antibiotics accounted for nearly 20% of total drug sales in health care facilities (Xiao et al., 2013). Moreover, the percentage of prescriptions that include an antibiotic in China was 41%-60%, which is above the recommended threshold of 30% by the World Health Organization (WHO) (Yin et al., 2013; Wang et al., 2014; Ren et al., 2016). A survey conducted by the Chinese Ministry of Health in 2011 showed that the annual use of antibiotics per capita reached 138 g, which was 10 times more than that of the United States (China News, 2015). In addition, a body of literature revealed that a large proportion of patients received combined therapy with multiple antibiotics (Wang and Chou, 2013). Many reasons can be attributed to the high consumption of antibiotics in China. The main factors affecting misuse of antibiotics lie in physicians’ lack of sufficient knowledge about appropriate use and pressure from patients who believe that antibiotics can quickly alleviate the symptoms of a disease (Xiao et al., 2013). Another widely believed influence factor is the provision of financial compensation for drug sales to medical institutions (Song et al., 2014b). In China, public hospitals can charge 15% on top of wholesale price of medicines. This drug mark-up was originally designed to compensate health care institutions that provide services at below-cost prices. However, this approach can easily result in serious health hazards as physicians tend to over-prescribe unnecessary medicines, including antibiotics.
The increase in AMR triggered a surge of interventions on antibiotic use in China, especially after the implementation of a new health system reform in 2009 (Wang et al., 2016; Tang et al., 2018). In this latest round of health system reform, the prescription authority for antibiotics in primary health care facilities was limited to drugs listed in the Essential Medicines List (EML); these medicines should be sold at zero mark-up to eliminate the benefit chain between facilities, doctors, and medicines (Song et al., 2014a). At present, the zero-markup policy has been extended to all public hospitals. On July 1, 2011, the Chinese Ministry of Health launched a three-year nationwide campaign to control the unreasonable use of antibiotics, mainly targeting public secondary and tertiary hospitals (Chinese Ministry of Health, 2011). In 2012, the Chinese government issued the Administrative Regulations on the Clinical Application of Antimicrobial Agents, which is regarded as the strictest control on antibiotic prescription to date (Xiao and Li, 2013). Antibiotics are categorised into three groups: nonrestricted, restricted, and controlled. Controlled antibiotics are excluded from the EML for primary care. Prescriptions of restricted or controlled antibiotics are subject to strict administrative restrictions. Penalties are applied for violating the rules (Chinese Ministry of Health, 2012). A recently released five-year national action plan aims to fight against AMR and ensure that antibiotics become prescription-only products in retail pharmacies by 2020 (Chinese Ministry of Health, 2016).
Reliable evidence shows the need to reflect on the effects of these policies. Previous studies have focused more on the changes of the number of antibiotic prescriptions and their cost (Liang et al., 2014; Song et al., 2014b; Liu et al., 2015). Few studies have been designed sufficiently to examine the changes in antibiotic consumption based on ATC/DDD methodology, which could demonstrate the epidemiology of antibiotic resistance with a higher resolution (Wushouer et al., 2017; Qu et al., 2018). Moreover, these findings have been mostly limited to tertiary hospitals and thus cannot reflect adequately reflect the actual situation of antibiotic use in China (Wushouer et al., 2017; Qu et al., 2018). Consequently, there is an urgent need for more surveillance data of antibiotic consumption covering various types of health care institutions at different levels and in various regions.
Therefore, this study aimed to provide the latest evidence on antibiotic consumption in China by identifying the trends and patterns based on a six-year surveillance in Shandong province. The findings of this study would assist in monitoring the increase in drug resistance and developing appropriate use interventions, as well as provide baseline information for future assessment and regional comparison.
Method
Setting and Data Collection
The surveillance data on antibiotic sales from 39 public health care facilities in Shandong province from 2012 to 2017 were analyzed retrospectively to document the trends in antibiotic consumption in China.
Shandong province is among China’s densely populated and economically developed regions. In 2017, its population was 100.06 million, ranking second among 31 provinces in China; its gross domestic product (GDP) in the same year was CNY 7267.82 billion (Shandong Provincial Bureau of Statistics, 2018). About 4.92% of the GDP in Shandong was spent on health. In 2017, Shandong had 2.65 physicians, 2.93 nurses, and 5.85 beds per 1,000 residents in the health care sector. The share of public health care facilities in this region in terms of patient visit was over 85%. In 2017, the numbers of patients treated in hospitals and primary health care facilities (PHCs) were 225.18 million (34.94%) and 393.08 million (60.99%), respectively. Among these patients, 190.68 million (84.68%) patients were treated in public hospitals (Shandong Health and Family Planning Commission, 2018). In July 2011, all public PHCs in Shandong adopted the EML and drug zero mark-up policy. The zero mark-up policy was fully implemented in county-level public hospitals in 2015 and then secondary and tertiary public hospitals in urban areas in mid-2016.
The 39 public health care facilities in this study were sampled hierarchically from three cities based on geographical and socio-economic factors. The breakdown was three tertiary hospitals, six secondary hospitals, and 30 PHCs. Data were derived from the drug information management system at each facility. The collected information contained generic name, unit strength, pack size, quantity of packs, route of administration, price, amount of sales, and manufacturer information.
Data Analysis
Microsoft Excel 2010 was used for data management and analysis. Sales data were coded in accordance with Anatomical Therapeutic and Chemical (ATC) classification and measured by defined daily dose (DDD) based on recommendations of the WHO Collaborating Center for Drug Statistic Methodology (WHO, 2013). Records of “J01” (antibacterial for systemic use) from 2012 to 2017 were extracted for analysis.
In the ATC classification system, drugs are classified in groups at five different levels. Within the ATC subgroup J01, 98 unique chemical substance names were identified in single or combination antibiotics. They were first aggregated into 24 ATC-4 classes and then into 9 ATC-3 groups. We calculated the DDDs for systemic antibiotics (J01) and its subsequent subgroups, such as penicillin (J01C) and penicillin with extended spectrum (J01CA). A full list of subgroups is displayed in Table 1. These antibiotics were grouped further according to administration route (oral vs. injection) and types of health care institutions (community sectors, secondary hospitals, and tertiary hospitals).
Table 1.
Antibiotic consumption by ATC-4 classifications in Shandong, 2012–2017a.
| ATC classification | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | |
|---|---|---|---|---|---|---|---|
| J01A | TETRACYCLINES | ||||||
| J01AA | Tetracyclines | 0.03 | 0.02 | 0.19 | 0.18 | 0.13 | 0.13 |
| J01C | BETA-LACTAM ANTIBACTERIALS, PENICILLINS | ||||||
| J01CA | Penicillins with extended spectrum | 2.30 | 1.96 | 2.71 | 2.93 | 3.01 | 2.41 |
| J01CE | Beta-lactamase sensitive penicillins | 2.59 | 1.45 | 1.27 | 0.86 | 0.66 | 0.55 |
| J01CF | Beta-lactamase resistant penicillins | 0.16 | 0.22 | 0.19 | 0.17 | 0.11 | 0.09 |
| J01CR | Combinations of penicillins, incl. beta-lactamase inhibitors | 0.43 | 0.50 | 0.61 | 1.14 | 1.18 | 1.08 |
| J01D | OTHER BETA-LACTAM ANTIBACTERIALS | ||||||
| J01DB | First-generation cephalosporins | 1.40 | 2.05 | 1.72 | 1.77 | 1.20 | 0.90 |
| J01DC | Second-generation cephalosporins | 1.12 | 1.29 | 1.54 | 1.31 | 1.04 | 1.07 |
| J01DD | Third-generation cephalosporins | 2.21 | 2.57 | 2.56 | 2.43 | 1.52 | 1.33 |
| J01DE | Fourth-generation cephalosporins | 0.16 | 0.09 | 0.06 | 0.05 | 0.03 | 0.03 |
| J01DF | Monobactams | 0.09 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 |
| J01DH | Carbapenems | 0.03 | 0.03 | 0.04 | 0.05 | 0.06 | 0.08 |
| J01E | SULFONAMIDES AND TRIMETHOPRIM | ||||||
| J01EE | Combinations of sulfonamides and trimethoprim, incl. derivatives | 0.04 | 0.05 | 0.00 | 0.00 | 0.00 | 0.00 |
| J01F | MACROLIDES, LINCOSAMIDES, AND STREPTOGRAMINS | ||||||
| J01FA | Macrolides | 2.01 | 2.47 | 2.32 | 1.93 | 1.64 | 1.38 |
| J01FF | Lincosamides | 0.25 | 0.16 | 0.11 | 0.24 | 0.20 | 0.28 |
| J01G | AMINOGLYCOSIDE ANTIBACTERIALS | ||||||
| J01GA | Streptomycins | 0.01 | 0.01 | 0.01 | 0.05 | 0.00 | 0.00 |
| J01GB | Other aminoglycosides | 0.55 | 0.88 | 0.65 | 0.69 | 0.42 | 0.25 |
| J01M | QUINOLONE ANTIBACTERIALS | ||||||
| J01MA | Fluoroquinolones | 1.24 | 1.42 | 1.43 | 1.50 | 1.20 | 0.97 |
| J01MB | Other quinolones | 0.82 | 1.09 | 1.01 | 1.46 | 0.64 | 0.36 |
| J01X | OTHER ANTIBACTERIALS | ||||||
| J01XA | Glycopeptide antibacterials | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| J01XD | Imidazole derivatives | 0.45 | 0.53 | 0.56 | 0.62 | 0.42 | 0.38 |
| J01XE | Nitrofuran derivatives | 0.12 | 0.15 | 0.02 | 0.00 | 0.00 | 0.00 |
| J01XX | Other antibacterials | 0.03 | 0.05 | 0.04 | 0.02 | 0.03 | 0.04 |
Expressed in DDDs per 1,000 inhabitants and per day.
Antibiotics categorized under J01BA and J01XC are negligible and thus not listed herein.
The antibiotic sales were finally converted into DDD per 1,000 inhabitants per day (DID) at the level of the active substance. As there was no direct access to the exact number of population covered by the sample facilities, we calculated the weighted population as a proxy based on the following equation. This calculation process was under two assumptions: no significant difference existed in (1) the distribution of the sample facilities and (2) the population distribution covered by the sample facilities (Tang et al., 2018).
| (1) |
Y: Covered inhabitants in a given year;
Pi: Total population in a given year;
ni: Number of sample facilities;
Ni: Number of total facilities.
We collected the relevant census data for calculating inhabitants from the Shandong Health Statistics Yearbook (Shandong Health and Family Planning Commission, 2018) and Shandong Statistics Yearbook (Shandong Provincial Bureau of Statistics, 2018).
Results
Total Antibiotic Use
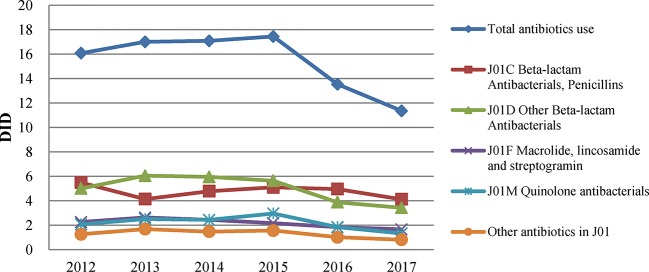
The total consumption of antibiotics among all health care settings in Shandong increased from 16.07 DID in 2012 to a peak of 17.44 DID in 2015 and then decreased to 11.35 DID in 2017, with a 34.90% reduction (Figure 1). Total consumption had the sharpest drop in 2016. The proportion of antibiotics in total pharmaceutical expenditure continued to decline, from 16.23% in 2012 to 12.87% in 2017. Total pharmaceutical expenditure in Shandong increased at an average rate of 15.66% in the first 4 years and then decreased by 25.96% in 2017.
Figure 1.
Antibiotic consumption in Shandong, China, 2012–2017.
Antibiotic Use by Class
In 2017, the most commonly used antibiotic class in Shandong was J01C (beta-lactam antimicrobials, penicillins), accounting for 36.32% of total consumption, followed by J01D (other beta-lactam antimicrobials; 30.16%), J01F (macrolides, lincosamides, and streptogramins; 14.62%) and J01M (quinolone antibacterials; 11.74%).
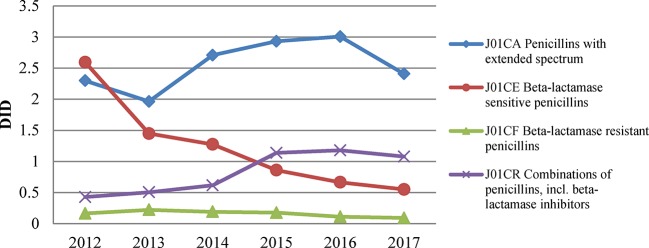
During the study period, penicillin consumption (J01C) was volatile, as illustrated in Figure 1. It showed an obvious upward trend from 4.13 DID in 2013 to 5.10 DID in 2015, and then declined afterwards. Such a trend mainly shaped the changes in total antibiotic use over the period. Of the different types of penicillin, extended-spectrum penicillin (J01CA) was the most used, accounting for 21.21% of the total antibiotic consumption in 2017. After a sustained increase in the use of extended-spectrum penicillin from 1.96 DID in 2013 to 3.01 DID in 2016, there was a significant decline in 2017, with a decrease of about 20% (Figure 2). Usage of narrow-spectrum penicillin (J01CE) was reduced consistently between 2012 and 2017, from 2.59 to 0.55 DID. However, the combined use of penicillin (J01CR, mainly piperacillin and enzyme inhibitor) soared the most in all antibiotics during this period, with an increase of 0.65DID (Figure 2).
Figure 2.
Four types of penicillin consumed in Shandong, China, 2012–2017.
Overall, the use of cephalosporins (J01D) declined over the years, from a peak of 6.05 DID in 2013 to 3.42 DID in 2017 (Figure 1). The cephalosporin consumption was mainly in the third generations. The sales of first, second, and third generations of cephalosporin contributed to 26.29%, 31.26%, and 38.85% of the total consumption of cephalosporin, respectively. Similarly, their consumption also declined over the years (Table 1).
A similar downward trend was observed in J01F (macrolides, lincosamides, and streptogramins) consumption from 2.63 DID in 2013 to 1.66DID in 2017. Macrolides and lincosamides accounted for 83.13% and 16.87% of the total J01F consumption in 2017, respectively. The consumption of quinolone antibacterials (J01M) started at 2.07 DID in 2012, increased to 2.96 DID in 2015, and then decreased to 1.33 DID in 2017. Fluoroquinolones (J01MA) were the most commonly used quinolone products in Shandong, accounting for 72.93% of the total consumption of quinolones.
The consumption of carbapenems increased steadily between 2012 and 2017, from 0.029 to 0.08 DID, whereas that of glycopeptides remained stable. These two classes of antibiotics were only used in hospitals (Table 1).
The number of antibiotic agents consumed in Shandong decreased from 86 in 2012 to 73 in 2017. Table 2 presents the top 10 antibiotics consumed in each year. Among them, seven antibacterial drugs, namely, benzylpenicillin, amoxicillin, ceftriaxone, cephalexin, azithromycin, levofloxacin, and cefuroxime, could be always observed. The five most used antibiotics in 2017 were amoxicillin (2.21 DID), amoxicillin and beta-lactamase inhibitor (0.78 DID), levofloxacin (0.77 DID), azithromycin (0.58 DID), and benzylpenicillin (0.55DID).
Table 2.
Top 10 most used antibiotics in Shandong, 2012–2017.
| Rank | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ATC5 | DID | ATC5 | DID | ATC5 | DID | ATC5 | DID | ATC5 | DID | ATC5 | DID | |
| 1 | J01CE01 | 2.59 | J01CA04 | 1.66 | J01CA04 | 2.34 | J01CA04 | 2.70 | J01CA04 | 2.79 | J01CA04 | 2.21 |
| 2 | J01CA04 | 2.03 | J01CE01 | 1.45 | J01CE01 | 1.27 | J01MB04 | 1.46 | J01MA12 | 0.87 | J01CR02 | 0.78 |
| 3 | J01DD04 | 1.12 | J01DB01 | 1.33 | J01DD04 | 1.23 | J01MA12 | 1.10 | J01CR02 | 0.78 | J01MA12 | 0.77 |
| 4 | J01MB04 | 0.82 | J01DD04 | 1.31 | J01DB01 | 1.03 | J01DD04 | 0.92 | J01FA10 | 0.71 | J01FA10 | 0.58 |
| 5 | J01DB01 | 0.81 | J01MB04 | 1.09 | J01MB04 | 1.01 | J01CE01 | 0.86 | J01DB01 | 0.67 | J01CE01 | 0.55 |
| 6 | J01FA06 | 0.68 | J01FA06 | 0.95 | J01MA12 | 1.00 | J01FA10 | 0.73 | J01CE01 | 0.66 | J01DB01 | 0.50 |
| 7 | J01FA10 | 0.66 | J01MA12 | 0.93 | J01FA06 | 0.78 | J01DB01 | 0.71 | J01MB04 | 0.64 | J01DC02 | 0.43 |
| 8 | J01MA12 | 0.66 | J01FA10 | 0.67 | J01DC02 | 0.71 | J01CR02 | 0.61 | J01DD04 | 0.58 | J01DD04 | 0.41 |
| 9 | J01FA09 | 0.51 | J01FA09 | 0.63 | J01FA09 | 0.65 | J01DC02 | 0.59 | J01DC04 | 0.42 | J01DC04 | 0.41 |
| 10 | J01DC02 | 0.48 | J01DC02 | 0.60 | J01FA10 | 0.62 | J01DB04 | 0.58 | J01DC02 | 0.37 | J01FA06 | 0.37 |
Antibiotic Use by Administration Route
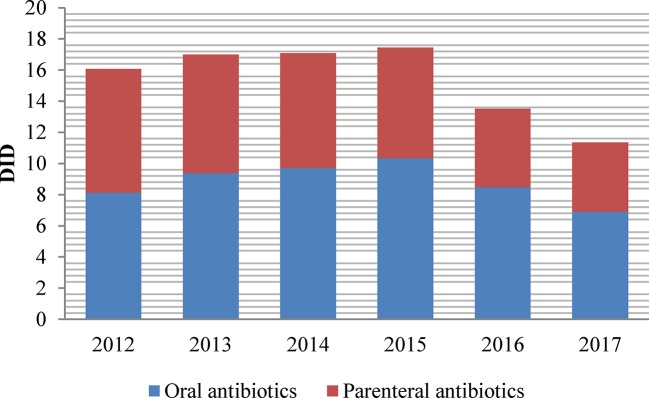
Oral antibiotics were more prevalent over the entire study period (Figure 3). In 2017, the total use of parenteral antibiotics in Shandong accounted for 39.22% of the total DID, representing 89.74% of total drug expenditure. In 2017, the proportion of drugs used in parenteral format was lowest in PHCs (33.77%) and higher in secondary (47.67%) and tertiary (45%) hospitals. Parenteral antibiotics consumption continuously decreased from 7.94 DID in 2012 to 4.45 DID in 2017, by 11% per year on average. The consumption of oral preparations of antibiotics started at 8.13 DID in 2012, increased to 10.34 DID in 2015, and then decreased to 6.90 DID in 2017.
Figure 3.
Consumption of oral and parenteral antibiotics in Shandong, China, 2012–2017.
Antibiotic Use by Type of Health Care Institution
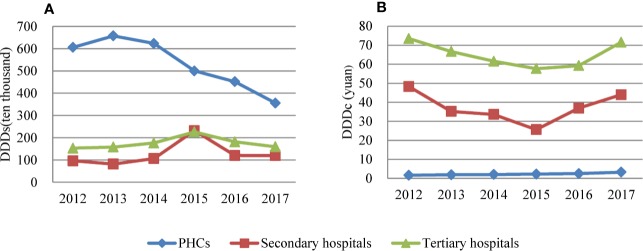
The pattern of antibiotic consumption in different types of health care institutions is demonstrated in Figure 4. In PHCs, antibiotic use showed a decreasing pattern and total consumption fell by 41% from 2012 to 2017. In hospitals, antibiotic consumption began to decline after reaching its peak in 2015. Both secondary and tertiary hospitals showed a similar varying trend. Compared with 2012, the 2017 consumption showed a small increase in hospital, but then it was compensated by a decrease in community care. The average daily cost of antibiotics in hospitals was much higher than that in PHCs, although the daily cost of antibiotics in PHCs increased yearly. In 2017, the average daily cost of antibiotics was CNY 3.28 in PHCs, CNY 43.98 in secondary hospitals, and CNY 71.53 in tertiary hospitals.
Figure 4.
(A) Consumption of antibiotics and (B) cost of antibiotics at different levels of health care facility in Shandong, China, 2012–2017.
Discussion
Our study quantified the antibiotic consumption in China in a six-year span, which would be informing for decision-makers, health care providers, as well as the public.
The results of this study showed that Shandong province reported a lower antibiotic consumption in China in 2017, compared with the global average (15.7 in 2015) (Klein et al., 2018). Over the study period, the percentage of total pharmaceutical expenditures on antibiotics steadily dropped and total antibiotic consumption presented a substantial decreasing trend after 2015. These changes coincided with the multiple interventions that regulate the use of antibiotics in China, including the 2012 Administrative Rules for the Clinical Use of Antibiotics issued by the Ministry of Health. This administration intervention appeared to work better according to the findings of this study and others (Zhang et al., 2019). In addition to persuasive guidelines, penalties for violating these administrative rules and measures, such as downgraded accreditation and service fee levels and dismissal of managers, were applied. Medical workers involved may lose permission to prescribe antibiotics, or even have their medical registration revoked.
In addition, a comprehensive reform of public hospitals has been carried out nationwide since 2015 (General Office of the State Council, 2015). The core content of this reform was to expand the zero mark-up drug policy to secondary and tertiary public hospitals, with the goal of completely eliminating the harmful incentives for drug abuse. Previous studies have indicated that the zero mark-up drug policy could help reduce the prescription rate of antibiotics (Wei et al., 2017). Therefore, this reform may have interacted with the antimicrobial stewardship programs and played an important role in improving antibiotic use. Public hospitals in Shandong province contribute to more than 85% of the total number of patients admitted to all medical institutions and more than 90% of the total health expenditure (Shandong Health and Family Planning Commission, 2018). The mixed effects of these two interventions could be an explanation for the dramatic decline in antibiotic consumption in 2015, as shown in this study.
Penicillin was found to be the most consumed antibiotic in this study. This is in line with the results found in Europe and the United States (Centers for Disease Control and Prevention, 2015; European Centre for Disease Prevention and Control, 2016). However, the findings of the present study regarding major antibiotic use were different from previous studies based on Chinese tertiary hospitals. This may be related to restrictions on the use of antibiotics by different levels of health care institutions (Wushouer et al., 2017; Qu et al., 2018). In addition, the widespread use of broad-spectrum antibiotics in China is noteworthy. In Shandong, the consumption of penicillin with extended spectrum was much higher than that of other subgroups. The combined use of penicillin, commonly defined as extended-spectrum antibiotics, showed an upward pattern and increased the most over 6 years. The third-generation cephalosporins, which has a more extended-spectrum, accounted for more than half of the total cephalosporin consumption. A distinct advantage of broad-spectrum antibiotics is that they require minimal recognition of infectious pathogens prior to treatment compared with narrow-spectrum antibiotics. However, the patient’s normal flora will be affected by them, and antibiotic resistance might be accelerated (Kaye et al., 2001). Therefore, necessary measures should be taken to further standardize the prescription of antibiotics, targeting at avoiding the abuse of broad-spectrum antibiotics.
The most used antibiotic agent was amoxicillin, followed by amoxicillin and beta-lactamase inhibitor, levofloxacin, azithromycin, and benzylpenicillin. These results are comparable to those of a study conducted in 48 primary health care facilities sampled from six provinces in China (Wang et al., 2014) and consistent with those from other Asian countries, such as Malaysia and India (Sharma et al., 2012; Shamsuddin et al., 2016).
Findings in this study showed a declining antibiotic use. This may be partly attributed to the fact that antibiotics are forced into three categories based on doctors’ prescription rights (unrestricted, restricted, and specialist use only), which was part of the antibiotic stewardship campaign in 2012 (Wushouer et al., 2017). This measure made antibiotics more difficult to be prescribed. A similar decline in antibiotic use in China can also be observed in other studies (Bao et al., 2015; Wushouer et al., 2017).
The use of carbapenems and glycopeptides, which are often regarded as the last-line treatment for multidrug-resistant bacteria, remains at a very low level in Shandong, because their use is highly restricted and requires pre-authorization. Notably, however, carbapenems consumption more than doubled during the study period. The surveys in other regions in China had a similar finding and indicated that imipenem, meropenem, and biapenem were the mainly used agents (Xu and Jia, 2015). Its increase may be a response to the rising prevalence of MRSA and ESBL-producing bacteria, which were identified in epidemiological surveillance studies (Xiao et al., 2013).
Several studies have indicated a very high level of antibiotics consumption in primary health care facilities in China (Wang et al., 2014; Sun et al., 2015). In this study, the consumption in PHCs displayed a declining trend over six years, indicating a positive effect of a series of restrictive measures, which have been imposed in national essential medicines policies since 2009, on antibiotic prescribing practices. These measures include limiting the availability of antibiotics and setting a zero-markup for sales of medicines in primary care. Comparatively, the obvious reduction of antibiotic consumption in hospitals was observed after 2015. As discussed above, the downward trend may be caused by the zero mark-up policy implemented in secondary and tertiary hospitals in Shandong. Moreover, the cost of antibiotics used in hospitals was much higher than that in PHCs; this could be a key to medical cost control.
Although there has been a decline of 3.49 DID since 2012, the parenteral antibiotics presented nearly 40% of total consumption and more than 80% of total expenditure in 2017. The high prevalence of antibiotics and injections may be related to the inertia in a physician’s practice and high demands of patients (Yang et al., 2014). In many cases, patients prefer antibiotics and injections for rapid recovery; therefore, antibiotics are often prescribed in combination with injections (Li, 2014). Health education and interventions are supposed to be set up for the public to promote the proper use of antibiotics (Ye et al., 2017).
The importance of this study lies providing up-to-date evidence on antibiotics consumption in China by different levels of health care facilities in Shandong province. Valuable information could be provided to national and provincial governments for developing more clearly targeted antibiotic stewardship policies. The limitations of this study are as follows. First, the antibiotic consumption data were only collected from 39 public health care institutions. Total consumption of antibiotics in Shandong province might be under-estimated. However, the trends and patterns of antibiotic use can be correctly reflected. Second, the population denominator used in this study did not consider patient flow between regions; this study may have underestimated antibiotic consumption. Finally, this study analyzed antibiotic sales data, which do not directly reflect the actual use of medicines and types of infections requiring antibiotics. The appropriateness of antibiotic prescribing practices was also not evaluated. Although the method used is internationally accepted, the resulting DDD and DID data cannot be followed back to the demand of the individual patient. In the future, prescription analysis and AMR monitoring must be combined to assess antibiotic consumption based on population, which can inform better the decision-making and improve clinical significance.
Conclusion
This study revealed a substantial decline in antibiotic consumption in China in 2012-2017, and a consistent preference for penicillins, cephalosporins, macrolides, and fluoroquinolones. The large consumption of broad-spectrum antimicrobials, high proportion of parenteral antibiotic use, and increased use of last-resort antibiotics attracted public health concerns. Using antibiotics in secondary and tertiary hospitals may be the key to controlling medical costs. Continued efforts are needed to obtain more evidence to inform policies and thereby optimize antibiotic prescribing and minimize antibiotic resistance.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.
Author Contributions
YS and TZ contributed to the conception and design of the study. YS organized the database. YS, ZH, and KS conducted the statistical analysis. YS finished the first draft of the manuscript. All authors made contributions to manuscript revision and read and approved the submitted version.
Funding
This study was funded by the National Natural Science Foundation of China (grant number 71503149), the Innovation Project of Shandong Academy of Medical Sciences, and Academic promotion programme of Shandong First Medical University. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank the Shandong Health and Family Planning Commission for their support and cooperation. Parts of the work were presented in a poster at ISPOR Asia Pacific 2018 and published in the form of abstract in Value in Health.
References
- Bao L., Peng R., Wang Y., Ma R., Ren X., Meng W., et al. (2015). Significant reduction of antibiotic consumption and patients’ costs after an action plan in China 2010-2014. PloS One 10, e0118868. 10.1371/journal.pone.0118868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2015). Outpatient antibiotic prescriptions - Unitied States, 2014. Atlanta, GA: US Department of Health and Human Services, CDC; [Online], Available: https://www.cdc.gov/antibiotic-use/community/pdfs/Annual-ReportSummary_2014.pdf. [Google Scholar]
- China News (2015). China has become a major country in the use of antibiotics. Beijing, China: CNTV; [Online], Available: http://news.cntv.cn/2015/11/19/ARTI1447899899337552.shtml. [Google Scholar]
- Chinese Ministry of Health (2011). The announcement of carrying out intensive nationwide intervention on antimicrobial clinical use. Beijing, China: Available: http://www.nhc.gov.cn/zwgkzt/wsbysj/201104/51376.shtml [Google Scholar]
- Chinese Ministry of Health (2012). The Measures for the Management of the Clinical Application of Antibacterial Drugs. Beijing, China: [Online]. Available: http://www.gov.cn/gongbao/content/2012/content_2201890.htm. [Google Scholar]
- Chinese Ministry of Health (2016). National Action Plan to Contain Antimicrobial Resistance (2016-2020). Beijing, China: [Online], Available: http://www.gov.cn/xinwen/2016-08/25/content_5102348.htm [Google Scholar]
- European Centre for Disease Prevention and Control (2016). Country overview of antimicrobial consumption. Stockholm,Sweden: ECDC; [Online]. Available: https://www.ecdc.europa.eu/en/antimicrobial-consumption/database/country-overview. [Google Scholar]
- General Office of the State Council (2015). Opinions on the implementation of comprehensive reform of county-level public hospitals. Beijing, China: [Online], Available: http://www.gov.cn/zhengce/content/2015-05/08/content_9710.htm [Google Scholar]
- Kaye K. S., Cosgrove S., Harris A., Eliopoulos G. M., Carmeli Y. (2001). Risk factors for emergence of resistance to broad-spectrum cephalosporins among Enterobacter spp. Antimicrob. Agents Chemother. 45, 2628–2630. 10.1128/AAC.45.9.2628-2630.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein E. Y., Boeckel T. P. V., Martinez E. M., Pant S., Gandra S., Levin S. A., et al. (2018). Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. U.S.A. 115, 3463–3470. 10.1073/pnas.1717295115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. (2014). China’s misuse of antibiotics should be curbed. BMJ 348, g1083. 10.1136/bmj.g1083 [DOI] [PubMed] [Google Scholar]
- Liang X., Xia T., Zhang X., Jin C. (2014). Governance structure reform and antibiotics prescription in community health centres in Shenzhen, China. Family Pract. 31, 311–318. 10.1093/fampra/cmu001 [DOI] [PubMed] [Google Scholar]
- Liu C., Zhang X., Wan J. (2015). Public reporting influences antibiotic and injection prescription in primary care: a segmented regression analysis. J. Eval. Clin. Pract. 21, 597–603. 10.1111/jep.12343 [DOI] [PubMed] [Google Scholar]
- Qiao M., Ying G. G., Singer A. C., Zhu Y. G. (2018). Review of antibiotic resistance in China and its environment. Environ. Int. 110, 160–172. 10.1016/j.envint.2017.10.016 [DOI] [PubMed] [Google Scholar]
- Qu X., Yin C., Sun X., Huang S., Li C., Dong P., et al. (2018). Consumption of antibiotics in Chinese public general tertiary hospitals, (2011-2014): Trends, pattern changes and regional differences. PloS One 13, e0196668. 10.1371/journal.pone.0196668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren N., Zhou P., Wen X., Li C., Huang X., Guo Y., et al. (2016). Point prevalence survey of antimicrobial use in Chinese hospitals in 2012. Am. J. Infection Control 44, 332–339. 10.1016/j.ajic.2015.10.008 [DOI] [PubMed] [Google Scholar]
- Shamsuddin S., Akkawi M. E., Zaidi S. T. R., Long C. M., Manan M. M. (2016). Antimicrobial drug use in primary healthcare clinics: a retrospective evaluation. Int. J. Infect. Dis. 52, 16–22. 10.1016/j.ijid.2016.09.013 [DOI] [PubMed] [Google Scholar]
- Shandong Health and Family Planning Commission (2018). “Shandong Health statistics Yearbook (2012-2017)”. Beijing: China Book Press. [Google Scholar]
- Shandong Provincial Bureau of Statistics (2018). “Shandong Statistics Yearbook (2012-2017)”. Beijing: China Statistics Press. [Google Scholar]
- Sharma M., Bo E., Marrone G., Dhaneria S., Lundborg C. S. (2012). Antibiotic prescribing in two private sector hospitals; one teaching and one non-teaching: A cross-sectional study in Ujjain, India. BMC Infect. Dis. 12, 155. 10.1186/1471-2334-12-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Bian Y., Petzold M., Li L., Yin A. (2014. a). Effects of the National Essential Medicine System in reducing drug prices: an empirical study in four Chinese provinces. J. Pharm. Policy Pract. 7, 12. 10.1186/2052-3211-7-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y., Bian Y., Petzold M., Li L., Yin A. (2014. b). The impact of China’s national essential medicine system on improving rational drug use in primary health care facilities: an empirical study in four provinces. BMC Health Serv. Res. 14, 507. 10.1186/s12913-014-0507-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y. (2018). “Trends and Patterns of Antibiotic Consumption in China: Evidence From A 5 Year Surveillance in Shandong Province, 2012-2016”, in ISPOR Asia Pacific 2018, 2018 ed. (Japan: Value in Health; ). [Google Scholar]
- Sun Q., Dyar O. J., Zhao L., Tomson G., Nilsson L. E., Grape M., et al. (2015). Overuse of antibiotics for the common cold - attitudes and behaviors among doctors in rural areas of Shandong Province, China. BMC Pharmacol. Toxicol. 16, 6. 10.1186/s40360-015-0009-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Liu C., Zhang Z., Zhang X. (2018). Effects of prescription restrictive interventions on antibiotic procurement in primary care settings: a controlled interrupted time series study in China. Cost Eff. Resour. Alloc. 16, 1. 10.1186/s12962-018-0086-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boeckel T. P., Gandra S., Ashok A., Caudron Q., Grenfell B. T., Levin S. A., et al. (2014). Global antibiotic consumption 2000 to 2010: an analysis of national pharmaceutical sales data. Lancet Infect. Dis. 14, 742–750. 10.1016/S1473-3099(14)70780-7 [DOI] [PubMed] [Google Scholar]
- Wang J. F., Chou K. C. (2013). Metallo-beta-lactamases: structural features, antibiotic recognition, inhibition, and inhibitor design. Curr. Top. Med. Chem. 13, 1242–1253. 10.2174/15680266113139990011 [DOI] [PubMed] [Google Scholar]
- Wang J., Wang P., Wang X., Zheng Y., Xiao Y. (2014). Use and prescription of antibiotics in primary health care settings in China. JAMA Intern. Med. 174, 1914–1920. 10.1001/jamainternmed.2014.5214 [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang X., Liang X., Bloom G. (2016). Addressing antimicrobial resistance in China: policy implementation in a complex context. Globalization Health 12, 1–9. 10.1186/s12992-016-0167-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Yin J., Walley J. D., Zhang Z., Hicks J. P., Zhou Y., et al. (2017). Impact of China’s essential medicines scheme and zero-mark-up policy on antibiotic prescriptions in county hospitals: a mixed methods study. Trop. Med. Int. Health 22, 1166–1174. 10.1111/tmi.12922 [DOI] [PubMed] [Google Scholar]
- WHO (2013). “Anatomical Therapeutic Chemical (ATC) Classification System: Guidelines for ATC Classification and DDD Assignment” (Oslo: WHO Collaborating Centre for Drug Statisitcs Methodology; ). [Google Scholar]
- Wushouer H., Tian Y., Guan X. D., Han S., Shi L. W. (2017). Trends and patterns of antibiotic consumption in China’s tertiary hospitals: Based on a 5 year surveillance with sales records 2011-2015. PloS One 12, e0190314. 10.1371/journal.pone.0190314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Li L. (2013). Legislation of clinical antibiotic use in China. Lancet Infect. Dis. 13, 189–191. 10.1016/S1473-3099(13)70011-2 [DOI] [PubMed] [Google Scholar]
- Xiao Y., Zhang J., Zheng B., Zhao L., Li S., Li L. (2013). Changes in Chinese policies to promote the rational use of antibiotics. PloS Med. 10, e1001556. 10.1371/journal.pmed.1001556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. Z., Jia C. (2015). Recent trend of carbapenem consumption in 34 hospitals of Nanjing. Chin. J. Antibiot. 40, 451–458. 1001-8689(2015)06-0451-08 [Google Scholar]
- Yang L., Liu C., Wang L., Yin X., Zhang X. (2014). Public reporting improves antibiotic prescribing for upper respiratory tract infections in primary care: a matched-pair cluster-randomized trial in China. Health Res. Policy Syst. 12, 61. 10.1186/1478-4505-12-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye D., Chang J., Yang C., Yan K., Ji W., Aziz M. M., et al. (2017). How does the general public view antibiotic use in China? Result from a cross-sectional survey. Int. J. Clin. Pharm. 39, 1–8. 10.1007/s11096-017-0472-0 [DOI] [PubMed] [Google Scholar]
- Yin X., Song F., Gong Y., Tu X., Wang Y., Cao S., et al. (2013). A systematic review of antibiotic utilization in China. J. Antimicrob. Chemother. 68, 2445. 10.1093/jac/dkt223 [DOI] [PubMed] [Google Scholar]
- Zhang X., Cui Y., Liu C., Zuo K., Tang Y. (2019). Antibiotic Sales in Primary Care in Hubei Province, China: An Analysis of 2012-2017 Procurement Records. Int. J. Environ. Res. Public Health 16, 3376. 10.3390/ijerph16183376 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets generated for this study are included in the article/supplementary material.