Abstract
This study was carried out to determine the biochemical and molecular potential effects of Zn-ONPs sub-lethal toxicity on the hormonal profile of Oreochromis niloticus (O. niloticus). One hundred and fifty O. niloticus juvenile female were used in this experiment; Ninety for determination of LC50 and other 60 fish were divided into 3 groups with 20 fish each (two replicate in each group). Group I used as control group whereas other groups treated with 1/20 and 1/30 of LC50 respectively for 4 days. Serum, pituitary gland, hepatic, pancreatic and muscular tissues were obtained for hormonal and molecular evaluation. Serum growth hormone (GH), thyroid stimulating hormone (TSH), triiodothyronine (T3), tetraiodothyronine (T4), follicular stimulating hormone (FSH), luteinizing hormone (LH), estradiol (E2), testosterone and insulin hormones were significantly decreased with a significant increase in both Adrenocorticosteroid hormone (ACTH) and cortisol levels with no change in serum glucagon levels. On molecular levels there were a significant down regulation in transcriptional levels of GH, Insulin like growth factor I (IGF-I), insulin and Insulin receptor-A (IRA genes. These results suggested that, hormonal and molecular alterations can be used as an early biomarkers for Zn-ONPs toxicity in fish.
Keywords: Zinc oxide nanoparticles, Hormonal alterations, Growth hormone, Insulin like growth factor I, Testosterone
1. Introduction
Water is one of the most important necessities for the preservation of human, plant and animal life. Adequate, safe and accessible supplies must be available to all living organisms, communities and economies. Water toxicity exhibiting a serious health problem for ecosystem especially fish that is very sensitive for water toxicity especially that caused by excess amounts of metals especially heavy metals (Friedman et al., 2017). Nanotechnology is a rapid developing technology produces many commercial products every day. Carbon comes on the head of NP in the commercial product descriptions followed by the Silver nanoparticles, silica, zinc oxide and titanium dioxide nanoparticles (Gupta and Xie, 2018). The wide use of NPs impact on environment, human and animal safety not efficiently studied (Constantin-Marian et al., 2013). Usually NPs are released to aquatic environment from bathing and sewage effluent leading to increase the possibility of humans and other ecosystem exposure to this NPs (Kühr et al., 2018). The NPs toxicity mechanisms are complicated (Sukhanova et al., 2018). NPs stimulate the generation of free radicals by causing damage of the antioxidant system or disrupting cellular metabolism (Pérez-Labrada et al., 2019). Zn-ONPs toxicity especially in ecosystem is related mainly to free radical formation and induction of oxidative stress with influence of size and shape of NPs (Du et al., 2017, Peng et al., 2017). Recent studies have indicated the risks of (Zn-ONPs) to aquatic organisms (Alkaladi et al., 2014, Linhua and Lei, 2012). Several studies reported the toxic effect of Zn-ONPs on ecosystem; Fahmy and Sayed (2017) approved cytotoxicity and genotoxicity of Coelatura aegyptiaca the freshwater molluscan bivalve, also the embryotoxicity of Zn-ONPs to marine medaka, Oryzias melastigma, was explored (Cong et al., 2017). Transcriptional profile alteration in Larval Zebra fish exposed to Zn-ONPs has been also studied (Kim et al., 2017). Zn-ONPs induced its toxic action on juvenile carp through inhibition of antioxidant enzymes activities in addition, the induction of free radicals generation (Linhua and Lei, 2012). Dondero et al. (2006) supposed that, expression level alteration is the earliest sensitive bio-indicators in biological responses against stress. Thus, it can be used as a diagnostic tool to detect and quantify the stresses on the organisms. There are no or scanty studies on the toxic effects of Zn-ONPs, on hormonal profile and their gene expression of Nil Tilapia. In the current study, our hypothesis is that, NPs could induce alterations in freshwater fish hormonal profile on a cellular and molecular levels. These alterations can be used as an early and highly sensitive bio-indicators for monitoring of these NPs pollution in fresh water. In this study we investigated the biochemical changes of hormones levels including pituitary, thyroid, adrenal and gonadal hormones. On the molecular levels we investigated the changes in gene expressions of some hormones genes and hormone receptors as growth hormone, IGF-1, Insulin and insulin receptors.
2. Material and methods
2.1. Experimental fish and protocol
One hundred and fifty juvenile female O. niloticus (10.62 ± 0.24 g BW) were obtained from EL-Sharkia Province, Egypt (Abbassa Fish Hatchery). Juveniles were maintained in a dechlorinated tap water in glass aquaria and kept for acclimatization to the laboratory conditions for two weeks prior to beginning of the experiment. Each aquarium was equipped with an aerator and was rigorously thermostatically controlled. The water in all the aquaria were equilibrated to the same conditions of temperature (25 ± 1.02 °C), pH (6.9 ± 0.1), ammonia (0.035 ± 0.01 mg/l) and dissolved oxygen (7.4 ± 0.34 mg/l); with restricted controlled photoperiod (10 h light : 14 h dark) in the laboratory. The juveniles fed with approximated 3% of their body weight from commercially dry fish pellets throughout all the duration of the experiment.
2.2. Chemicals and reagents
Zinc oxide nanoparticles (Zn-ONPs) were purchased from Sigma- Aldrich Chemical Co. (St. Louis, MO, USA) as a colorless to faint yellow viscous liquid.
All other chemicals were obtained commercially from local scientific distributors in Egypt.
2.3. Ethical statement
The experiments were conducted after approval by the local authorities at Cairo University, Egypt and according to the guidelines for the care and use of laboratory animals approved by the National Institutes of Health (NIH).
2.4. Median lethal concentration assessment (96-h LC50) and toxicity symptoms of Nano zinc oxide in juvenile O. niloticus fish
Following range finding tests, Nano zinc oxide in concentrations that ranged from Zero to 9.5 µ/l. A 96-h acute toxicity test was conducted on 90 juvenile O. niloticus fish split into nine groups of 10 fish each. Each group was exposed to varying concentrations of Nano Zinc oxide and the resultant mortalities were counted and recorded at 24, 48, 72 and 96 h intervals. 96-h LC50 value was calculated using the method described by Chinedu et al. (2013) as shown in Table 1.
Table 1.
LC50 of ZnONPs on O. niloticus.
| Fish Groups | Fish No. | Concentration of ZnONPs. µg/l | No. of dead fish after 96 h | a | b | a X b | Σ (a × b) |
|---|---|---|---|---|---|---|---|
| 1 | 10 | Zero | Zero | Zero | Zero | Zero | |
| 2 | 10 | 1 | 1 | 1 | 0.5 | 0.5 | |
| 3 | 10 | 2 | 2 | 1 | 1.5 | 1.5 | |
| 4 | 10 | 3 | 3 | 1 | 2 | 2.5 | |
| 5 | 10 | 4 | 5 | 1 | 4 | 4 | |
| 6 | 10 | 5 | 6 | 1 | 5.5 | 5.5 | |
| 7 | 10 | 6 | 8 | 1 | 7 | 7 | |
| 8 | 10 | 7 | 9 | 1 | 8.5 | 8.5 | |
| 9 | 10 | 8 | 10 | 1 | 9.5 | 9.5 | |
| 39 |
a = Constant factor between two successive doses, b = The mean of dead fish in the groups.
n = Number of fish in each group Σ = The sum of (a × b).
96 LC50 = highest dose - Σ (a × b)/n.
8–39/10 = 8–3.9 = 4.1 µg/l.
2.5. Sub-lethal exposure experiment
The toxic effects of 1/2 and 1/3 96-h LC50 value of Nano zinc oxide on juvenile O. niloticus for an exposure period of four days were assessed. Sixty fish were randomly divided into three groups each of two replicates, comprising of 10 fish each.
Group I (control) fish received no treatment whereas groups II and III were exposed to Zn-ONPs at a concentration of 1/2 and 1/3 96-h LC50 value (2.05 and 1.23 µ/l, respectively). The medium of the aquaria was renewed daily and fresh solutions were spiked so as to maintain water quality with an appropriate DBP level. Throughout the period of exposure, fish were closely observed and clinical signs were rigorously followed, as well as post-mortem lesions of dead fish were monitored.
2.6. Sampling protocol
Blood sampling and serum collection: After the end of the treatment blood samples were taken from the caudal vein of each individual fish, collected and kept for 15 min. then centrifuged at 5000 rmp for 5 min. to obtain serum. The collected serum was then stored at −20 °C till used for biochemical analysis.
Tissue sampling: Pituitary gland, hepatic, pancreatic and muscular tissues were collected as early as possible stored in liquid nitrogen till RNA isolation for molecular determination of GH, IGF-I, Insulin and IRA respectively.
2.7. Hormonal assay
Fish GH, TSH, ACTH, FSH, LH, T3, T4, Estradiol (E2), Testosterone (T), Insulin and Glucagon were determined in the serum of fish using their corresponding ELISA kits from MyBioSource.com for research purpose only with a catalog number (MBS701414) for GH, (MBS282744) for TSH, (MBS705669) for ACTH, (MBS035576) for FSH, (MBS283097) for LH, (MBS2700145) for T3, (MBS701162) for T4, (MBS283228) for Estradiol, (MBS9424420) for testosterone, (MBS017490) for insulin, (MBS034316) for Glucagon. Fish Serum cortisol were also determined using a cortisol ELISA kit from DioMed (Cairo, Egypt).
2.8. Molecular determinations
2.8.1. RNA extraction and cDNA synthesis
Total RNAs were isolated from Pituitary gland, liver, pancreas and muscles using Gene JET RNA purification kit from Fermentas, UK following the manufacture instructions for determination of gene expression of GH, IGF-1, insulin and insulin receptor A respectively. The obtained total RNAs were then quantified in a Nano-Drop® ND-1000 Spectrophotometer (Wilmington, Delaware USA). Only pure samples used for cDNA amplification using reverse transcriptase enzyme (Superscript II RNase H- reverse transcriptase) from (Invitrogen, Carlsbad, CA, USA) in 2720 thermal cycler from applied Bio-system.
2.8.2. Real time PCR
One μl of synthesized cDNA was mixed with 12.5 μl of PCR Master Mix (2x SYBR® Green from BioRad), 1 μl (10 pmol/μl) of recommended primer and 9.5 μl of RNase free water (Sigma, UK). β-actin gene was used as a controller gene for maintenance and determination of the relative fold modifications using the 2−ΔΔCt method (Livak and Schmittgen 2001).
2.8.3. Primers for experimental genes
Primer sequences and conditions were itemized in (Table 2) and manufactured by Sigma-Aldrich (Steinheim, Germany).
Table 2.
Primer sequences and PCR conditions.
| Gene | Primer sequence (5′→3′) | Gene accession number | Annealing temp °C/cycles | References |
|---|---|---|---|---|
| GH | CTGTCTGTCTGTCTGTCAGTCGT AGAGGAGACGCCCAAACAC |
M26916.1 | 60 °C/40 cycle | Rentier-Delrue et al. (1989) |
| IGF-1 | CCCGAACTTCCTCGACTTGA CCTCAGCCAGACAAGACAAAAA |
EU272149 | 60 °C/40 cycle | Wang et al. (2008) |
| Insulin | ACAACCCCAGGAGAGATGTG AGGGTTTGTGACAGCATTCC |
XM_003458679.5 | 56 °C/50 cycle | Hrytsenko et al. (2007) |
| IRA | AGACGGTGAACGAGTCGGCC TGGCTTACCTCAACGCCAAG |
KC517071.1 | 65 °C/50 cycle | Hrytsenko et al. (2007) |
| β-actin | ACCCACACAGTGCCCATC CAGGTCCAGACGCAGGAT |
EU887951 | 60 °C/40 cycle | Monteiro et al. (2009) |
GH, Growth hormon; IGF-I, Insulin like growth factor I; IRA, Insulin receptor-A.
2.9. Statistical analysis
Data were expressed as mean ± SE. SPSS 18.0 computer program (SPSS) was used for analyzing results. Statistical significance between groups was evaluated using the analysis of variance (one-way ANOVA) test followed by Duncan’s multiple range test for significant difference. P-values <0.05 were accepted as statistically significant.
3. Results
3.1. LC50 value and clinical observation
The 96-h LC50 value for Zn-ONPs in female juvenile O. niloticus was determined to be 4.1 µg/l (Table 1); and it was observed that associated fish mortalities increased with increments in Zn-ONPs concentration when monitored within a 96 h. Throughout the exposure period, no clinical signs were noticed in control fish as well as in fish exposed to the lowest concentration of Nano zinc oxide (<1 µg/l) whereas in those exposed to higher DBP concentrations (>1 µg/l), anomalous clinical signs were noted immediately. The observed impairments included abnormal swimming movements and restlessness followed by convulsions. The fish under study exhibited difficulty in breathing as represented by speedy respiration accompanied by rapid operculum movements and failure to respond to escape reflex. Additionally, a thick layer of mucous associated with a dark discoloration of the skin was also noted. Severe congestion of the internal organs along with excessive slime deposition on the gills was observed during the postmortem investigation.
3.2. Sub-lethal exposure assay
During the 96-h exposure period used in the current study, five mortalities were encountered in the 1/2 96-h LC50 Nano zinc oxide - exposed group while three mortalities were noted in the 1/3 96-h LC50 Nano zinc oxide group.
3.2.1. Hormonal alteration
The results presented in Table 3 showed that, Zn-ONPs administration in a doses equal to 1/2 and 1/3 from LC50 resulted in a significant hormonal alteration appeared as a significant decrease in the hormonal serum levels of GH, TSH, FSH, LH, T3, T4, E2, Testosterone and insulin with no significant change in serum glucagon levels. At the same time there were a significant increase in the hormonal serum levels of ACTH and cortisol hormone. This alteration was higher in a higher dose (1/2 LC50) than that of 1/3 of LC50.
Table 3.
Effect ZnONPs sub-lethal concentrations on some hormone of Oreochromis niloticus.
| Group I (control group) | Group II (1/2 LC50) | Group III (1/3 LC50) | |
|---|---|---|---|
| GH (pg/ml) | 667.4 ± 29.08a | 398 ± 24.2c | 546 ± 26.9b |
| TSH (μIU/ml) | 2.38 ± 0.043a | 1.05 ± 0.02c | 1.8 ± 0.08b |
| ACTH (pg/ml) | 172.8 ± 18.6c | 284.3 ± 6.3a | 205.3 ± 11.3b |
| FSH (mIU/ml) | 4.2 ± 0.85a | 1.1 ± 0.13c | 3.1 ± 0.43b |
| LH (mIU/ml) | 19.6 ± 2.15a | 9.4 ± 1.02c | 13.7 ± 1.32b |
| T3 (pg/mL) | 375.9 ± 32.6a | 186.8 ± 26.8c | 246.3 ± 29.4b |
| T4 (ng/mL) | 168.4 ± 17.4a | 48.6 ± 8.2c | 96.8 ± 13.8b |
| Cortisol (μg/ml) | 5.48 ± 0.67c | 12.25 ± 1.2a | 8.9 ± 0.98b |
| Estradiol (pg/ml) | 32.6 ± 2.31a | 19.4 ± 1.42c | 25.7 ± 1.85b |
| Testosterone (ng/ml) | 6.28 ± 1.12a | 2.25 ± 0.82c | 4.3 ± 0.42b |
| Insulin (µIU/ml) | 5.68 ± 0.88a | 2.15 ± 0.62c | 4.26 ± 0.36b |
| Glucagon (mU/L) | 4.42 ± 0.63a | 4.63 ± 0.44a | 4.83 ± 0.64a |
Means carrying different superscripts are significant at P < 0.05.
3.2.2. Molecular alteration
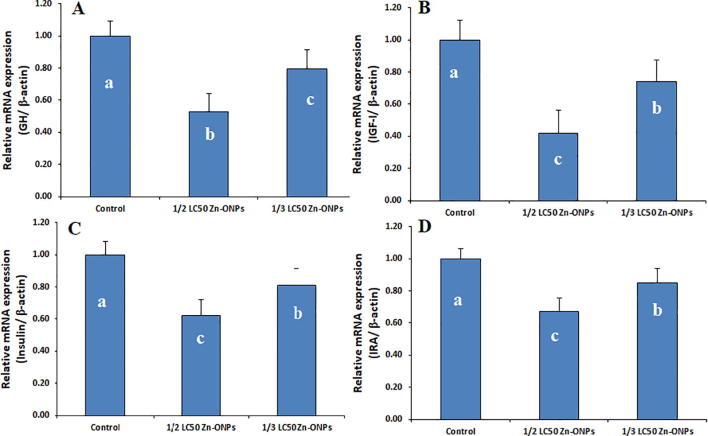
The results showed in Fig. 1 illustrated that, Zn-ONPs administration in a dose equal to 1/2 and 1/3 of LC50 resulted in a significant down regulation in the transcriptional mRNA levels of GH, IGF-I, insulin and IRA which reflects the hormonal and metabolic disturbance induced by Zn-ONPs toxicity.
Fig. 1.
The relative gene expression of Growth hormon (GH) A, IGF-I: Insulin like growth factor I (IGF-I) B, Insulin, C and Insulin receptor (IRA) D.
4. Discussion
Water pollution is the most serious problem possessing a major threat to the safety and security of ecosystem and so millions of people (Inyinbor et al., 2018). Fish is a vital indicator of the aquatic ecosystem because of its vital and environmental role in the aquatic food chain as well as its ability to metabolize, concentrate and assemble minerals (Luoma and Rainbow, 2008, Monteiro et al., 2010). In many toxicological researches, O. niloticus was usually used as a well-established model for determination of toxicity (Abdel-Khalek et al., 2015). Accumulation of metals in water causing a serious problem for aquatic organisms including fish. The enhanced use of Zn-ONPs may increase its environmental concentration during their production, processing and disposal that can results in a massive pollution and toxicity especially for ecosystem (Gupta and Xie, 2018).
LC50 values determination in fish is a very useful method for evaluating the tolerance and safety levels for a particular contaminant or pollutant (Prentera et al., 2004). In our experiment the LC50/96 h recorded were 4.1 µg/l which is considered more toxic than that previously reported for a bulk zinc particles in other experiments confirming that, NPs behaving very differently compared to the bulk particles of the same chemical material (Wigginton et al., 2007), that may be due to smaller size, higher reactivity with higher surface area per unit volume of NPs. Navarro et al. (2008) supposed that, NPs toxicity can resulted from surface reactivity and chemical composition.
Hormonal alteration in fish is very sensitive for environmental pollution especially that caused by metal toxicity (Bakshia and Panigrahi, 2018). Scott and Sloman (2004), reported that, the physiological effects of toxic substances and pollutants include sensory and hormonal disruption with neurological alterations and metabolic disorders producing profound effects on many fish behaviors. Due to this point our experiment was mainly directed for detection of the hormonal alteration that may be produced due to Zn-ONPs toxicity and using it as an early biomarkers for environmental pollution especially that for ecosystem as a first reported trail in this point.
The results obtained from our experiment examining the sub-lethal toxicity of Zn-ONPs were reported in Table 3 representing that, Zn-ONPs treatment in doses equal to 1/2 of LC50 and 1/3 of LC50 (2.05 and 1.23 µ/l, respectively) for frequent 4 days resulted in a significant hormonal alterations in a dose dependent manner appeared as a significant decrease in the hormonal serum levels of GH, TSH, FSH, LH T3, T4, E2, Testosterone, insulin and glucagon with a significant increase in both ACTH and cortisol hormone serum levels.
Internal hormonal imbalance and interfering with biosynthesis, metabolism and normal functions are the major issues contributing to endocrine disrupting chemicals (EDCs) (Iavicoli et al., 2013). The potential endocrine disruption of NPs in the start of this experiment is confirmed by our results that appeared in both cellular and molecular levels.
Growth hormone (GH), the peptide hormone of pituitary gland stimulates growth, regeneration and reproduction of human and animal cells. It has a multiple target and diverse effects in vertebrates, mainly as a growth promoter with effect on metabolism (Devesa et al., 2016). Fish GH was reported to alter fish behavior through increasing appetite, aggression, swimming activities with reduction of anti-predator behavior of fish (Jonsson and Bjornsson, 2002), and so, decrease in GH level determined in our experiment due to exposure to Zn-ONPs toxicity may alter these functions affecting fish behavior, food consumption and food conversion efficacy.
In another way fish GH osmo-regulatory function was appeared to be associated with immune function activation through activating immunoglobulin production and increasing ceruloplasmin as an acute phase protein (Yada, 2007). So the resulted down regulation in the transcriptional levels of GH explained in Fig. 1 combined with serum hormonal alteration my results also in immunal function disruption in Tilapia Nilotica.
Thyroid stimulating hormone (TSH), the glycoprotein of anterior pituitary, secreted from thyrotrophic cells stored in secretory granules and released in response to TRH into the circulation regularly stimulating the production of thyroid hormones that modulates multiple metabolic processes that inhibits TSH production (Sarapura et al., 2011). T3 and T4 are the products of thyroid glands in all vertebrates. The early role of T3 and T4 in mammals as well as fish is playing a role in metamorphosis and early development. In fish thyroid hormones play an important role in fish adaptation in response to environmental changes such as changes in temperature and salinity; they also play a significant role in maintenance of cellular and tissue activities as well as biorhythms development. Thyroid hormones accumulation in mature fish eggs playing an extremely important role in fish development, reproduction and embryogenesis (Blanton and Specker, 2007).
In our results TSH production was to be altered by Zn-ONPs exposure leading to alteration in both T3 and T4 serum levels, this may leads to disturbance in general metabolic status producing several metabolic and morphological changes in fish reflecting the inability of fish to respond to environmental changes with a disturbance in fish development and reproduction.
The two fish glycoproteins gonadotropins secreted from pituitary glands, FSH and LH like in all vertebrates play main role in steroidogenesis and gametogenesis (Kazeto et al., 2008). Several studies on FSH and LH in fish species with no exact description to actual function or they relation to reproduction due to the deficient genetic approaches for functional studies in adult fish (Zhang et al., 2015). In fish including Tilapia, FSH is important for early gonadal development and vitellogenesis, it significantly enhance Estradiol (E2) and 11-ketotestosterone (11-KT) secretion from tilapia ovaries and testes respectively (Wang et al., 2018: Aizen et al., 2007). In mammals, LH was documented to stimulate androgen production from leyding cells while FSH directly regulates Sertoli cell functions. However, in fish there is more complex situation as both gonadotropins showing prominent steroidogenic potency (Sambroni et al., 2013, Ohta et al., 2007). After Zn-ONPs toxicity the serum levels of FSH and LH were decreased which may affects gonadal function that represented in down alteration of both Estradiol (E2) and testosterone levels in both 1/2 and 1/3 LC50 treated groups when compared to control group suggesting the deleterious effect occurred by ZnONPs toxicity in Nile Tilapia that may also affecting the reproductive function of fish.
Estrogen (E2) is a steroid hormone with a major role in differentiation and growth of reproductive tissues, fertility and maintaining follicular development (Zubeldia-Brenner et al., 2016). ZnNPs, aluminum exposure, NiNPs toxicities decreased the serum Estradiol levels (Micevych and Kelly, 2012). In another study, administration of NiNPs decrease Estrogen levels in adult rat. Higher levels of Zn-ONPs have a destructive effect on ovary that resulted in depletion of Estradiol levels in serum (Diamanti-Kandarakis et al., 2009).
Adrenocorticotrophic hormone (ACTH) from pituitary gland and its related cortisol hormone (main corticosteroid in fish) from adrenal gland are the main hormones controlling stress situation in fish (Montero et al., 2015). In fish, ACTH release stimulates synthesis of cortisol through many different pathways, the usual cAMP/protein kinase A (cAMP/PKA) pathway and other cAMP independent pathways depending on protein kinase C activation or acetylcholine release stimulating the genes involved in steroidogenesis and activating a cascade of enzymes belongs to the cytochrome P450 family transforming 11-desoxicortisol to cortisol that released into the bloodstream following a stressful situation (Stocco et al. 2005).
ZnONPs sub-lethal toxicity either in high or lower doses induce a state of stress represented as a significant increase in serum levels of ACTH and subsequently serum cortisol level. Cortisol is the primary component of stress response and is relevant to fish welfare, so increase of serum cortisol levels in fish affects brain function and modifying the behavior (Ellis et al., 2012). Cortisol administration was reported to decrease the number of circulation B and T lymphocytes with increase of circulating phagocytes. Cortisol also was reported to induce B cell apoptosis and thus clearing them from blood stream after stress (Pulsforda et al., 1994).
At the level of metabolic hormones, insulin and glucagon hormone secreted from pancreatic tissue in response to various physiological and experimental conditions in fish. Fish insulin and glucagon were reported to be respond to amino acids more likely than glucose just like that reported in carnivorous (Navarro et al., 2002). Serum insulin concentration, insulin, hepatic IGF-I and muscular IRA transcriptional levels in Zn-ONPs groups were significantly lower than that in the control fish, whereas there was no significant alteration in the serum glucagon levels. The impairment of insulin secretion resulted from Zn-ONPs toxicity together with previously illustrated serum decrease in GH and down regulation of its expression levels may affect the expression levels of IGF-I production consequently, fish growth. These results are consistent with the results reported by Plisetskaya and Duan (1994) that connected the down regulation of IGF-I to the reduction of serum insulin levels.
5. Conclusion
From all mentioned before it can be concluded that, Zn-ONPs treatment in a doses equal to 1/2 and 1/3 of LC50 can induce hormonal alteration in fish at both cellular and molecular levels. At cellular levels represented in a significant decrease in serum GH, TSH, T3, T4, FSH, LH, E2, testosterone and insulin hormones with a significant increase in both ACTH and cortisol levels with no change in glucagone levels. At the molecular levels there were a significant down regulation in transcriptional levels of GH, IGF-I, insulin and IRA genes. These results suggested that, hormonal and molecular alterations can be used as an early biomarkers for Zn-ONPs toxicity in fish.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgments
This work was funded by the University of Jeddah, Saudi Arabia, under grant No (UJ-09-18-ICP). The authors, therefore, acknowledge with thanks the University technical and financial support.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdel-Khalek A.A., Kadry M., Hamed A., Marie M.A. Ecotoxicological impacts of zinc metal incomparison to its nanoparticles in Nile tilapia; Oreochromis niloticus. J. Basic Appl. Zool. 2015;72:113–125. [Google Scholar]
- Aizen J., Kasuto H., Golan M., Zakay H., Levavi-Sivan B. Tilapia follicle-stimulating hormone (FSH): immunochemistry, stimulation by gonadotropin-releasing hormone, and effect of biologically active recombinant FSH on steroid secretion. Biol. Reprod. 2007;76 (4)(1):692–700. doi: 10.1095/biolreprod.106.055822. [DOI] [PubMed] [Google Scholar]
- Alkaladi A., Afifi M., Mosleh Y.Y., Abu-Zinada O. Ultra structure alteration of sublethal concentrations of zinc oxide nanoparticals on Nil Tilapia (Oreochromis niloticus) and the protective effects of vitamins C and E. Life Sci. J. 2014;11(10):257–262. [Google Scholar]
- Bakshia A., Panigrahi A.K. A comprehensive review on chromium induced alterations in fresh water fishes. Toxicol Rep. 2018;5:440–447. doi: 10.1016/j.toxrep.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanton M.L., Specker J.L. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction. CRC Crit. Rev. Toxicol. 2007;37(1–2):97–115. doi: 10.1080/10408440601123529. [DOI] [PubMed] [Google Scholar]
- Chinedu E., Arome D., Ameh F.S. A new method for determining acute toxicity in animal models. Toxicol. Int. 2013;20(3):224–226. doi: 10.4103/0971-6580.121674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong Y., Jin F., Wang J., Mu J. The embryotoxicity of ZnO nanoparticles to marine medaka, Oryzias melastigma. Aquat. Toxicol. 2017;185:11–18. doi: 10.1016/j.aquatox.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Constantin-Marian P., Larisa C., Ana-Maria D., Violeta T., Daniela B. Toxicity evaluation by flow cytometric analysis of nanoparticles using the unicellular alga chlorella. Studia Universitatis “Vasile Goldiş”. Seria Ştiinţele Vieţii. 2013;23:381–387. [Google Scholar]
- Devesa J., Almengló C., Devesa P. Multiple effects of growth hormone in the body: is it really the hormone for growth? Clinical medicine insights. Endocrinol. Diabetes. 2016;9:47–71. doi: 10.4137/CMED.S38201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E., Bourguignon J.P., Giudice L.C., Hauser R., Prins G.S., Soto A.M., Zoeller R.T., Gore A.C. Endocrine-disrupting chemicals: an endocrine society scientific statement. Endocr. Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondero F., Dagnino A., Jonsson H., Capri F., Gastaldi L., Viarengo A. Assessing the occurrence of a stress syndrome in mussels (Mytilus edulis) using a combined biomarker/gene expression approach. Aquat. Toxicol. 2006;78:S13–S24. doi: 10.1016/j.aquatox.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Du J., Cai J., Wang S., You H. Oxidative stress and apotosis to zebrafish (Danio rerio) embryos exposed to perfluorooctane sulfonate (PFOS) and ZnO nanoparticles. Int. J. Occup. Med. Environ. Health. 2017;30:213–229. doi: 10.13075/ijomeh.1896.00669. [DOI] [PubMed] [Google Scholar]
- Ellis T., Yildiz H.Y., López-Olmeda J., Spedicato M.T., Tort L.V., Martins C.I. Cortisol and finfish welfare. Fish Physiol. Biochem. 2012;38(1):163–188. doi: 10.1007/s10695-011-9568-y. [DOI] [PubMed] [Google Scholar]
- Fahmy S.R., Sayed D.A. Toxicological perturbations of zinc oxide nanoparticles in the C. aegyptiaca mussel. Toxicol. Ind. Health. 2017;33(7):564–575. doi: 10.1177/0748233716687927. [DOI] [PubMed] [Google Scholar]
- Friedman M.A., Fernandez M., Backer L.C., Dickey R.W., Bernstein J., Schrank K. An updated review of ciguatera fish poisoning: clinical, epidemiological, environmental, and public health management. Mar. Drugs. 2017;15(3):72. doi: 10.3390/md15030072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R., Xie H. Nanoparticles in daily life: applications, toxicity and regulations. J. Environ. Pathol. Toxicol. Oncol. 2018;37(3):209–230. doi: 10.1615/JEnvironPatholToxicolOncol.2018026009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrytsenko O., Wright J.R., Morrison C.M., Pohajdak B. Insulin expression in the brain and pituitary cells of tilapia (Oreochromis niloticus) Brain Res. 2007;1135:31–40. doi: 10.1016/j.brainres.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Iavicoli I., Fontana L., Leso V., Bergamaschi A. The effects of nanomaterials as endocrine disruptors. Int. J. Mol. Sci. 2013;14:16732–16801. doi: 10.3390/ijms140816732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inyinbor, A.A., Adebesin, B.O., Oluyori, A.P., Adelani-Akande, T.A., Dada, A.O., Oreofe, T.A., 2018. Water pollution: effects, prevention, and climatic impact, pp. 33–53.
- Jonsson E., Bjornsson T.B. Physiological functions of growth hormone in fish with special reference to its influence on behavior. Fish. Sci. 2002;68(1):742–748. [Google Scholar]
- Kazeto Y., Kohara M., Miura T., Miura C., Yamaguchi S., Trant J.M., Adachi S., Yamauchi K. Japanese eel follicle-stimulating hormone (Fsh) and luteinizing hormone (Lh): production of biologically active recombinant Fsh and Lh by Drosophila S2 cells and their differential actions on the reproductive biology. Biol. Reprod. 2008;79(5):938–946. doi: 10.1095/biolreprod.108.070052. [DOI] [PubMed] [Google Scholar]
- Kim R.O., Choi J.S., Kim B.C., Kim W.K. Comparative analysis of transcriptional profile changes in larval zebrafish exposed to zinc oxide nanoparticles and zinc sulfate. Bull. Environ. Contam. Toxicol. 2017;98:183–189. doi: 10.1007/s00128-016-1995-0. [DOI] [PubMed] [Google Scholar]
- Kühr S., Schneider S., Meisterjahn B., Schlich K., Hund-Rinke K., Schlechtriem C. Silver nanoparticles in sewage treatment plant effluents: chronic effects and accumulation of silver in the freshwater amphipod Hyalella azteca. Environ. Sci. Eur. 2018;30(1):7. doi: 10.1186/s12302-018-0137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhua H., Lei C. Oxidative stress responses in different organs of carp (Cyprinus carpio) with exposure to ZnO nanoparticles. Ecotoxicol. Environ. Saf. 2012;80:103–110. doi: 10.1016/j.ecoenv.2012.02.017. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen K.D. Analysis of relative gene expression data using real time quantitative PCR and the 2 (-Delta Delta C (T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Luoma S.N., Rainbow P.S. Metal Contamination in Aquatic Environments: Science and Lateral Management. Cambridge University Press; Cambridge: 2008. Sources and cycles of trace metals; pp. 47–66. [Google Scholar]
- Micevych P.E., Kelly M.J. Membrane estrogen receptor regulation of hypothalamic function. Neuroendocrinology. 2012;96:103–110. doi: 10.1159/000338400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro D., Rantin F., Kalinin A. Inorganic mercury exposure: toxicological effects, oxidative stress biomarkers and bioaccumulation in the tropical freshwater fish matrinxã, Brycon amazonicus (Spix and Agassiz, 1829) Ecotoxicology. 2010;19:105–123. doi: 10.1007/s10646-009-0395-1. [DOI] [PubMed] [Google Scholar]
- Monteiro S.M., Dos Santos N.M., Calejo M., Fontainhas-Fernandes A., Sousa M. Copper toxicity in gills of the teleost fish, Oreochromis niloticus: Effects in apoptosis induction and cell proliferation. Aquat. Toxicol. 2009;94:219–228. doi: 10.1016/j.aquatox.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Montero D., Terova G., Rimoldi S., Tort L., Negrin D., Zamorano M.J., Izquierdo M. Modulation of adrenocorticotrophin hormone (ACTH)-induced expression of stress-related genes by PUFA in inter-renal cells from European sea bass (Dicentrarchus labrax) J. Nutr. Sci. 2015;4(16):1–13. doi: 10.1017/jns.2015.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro E., Baun A., Behra R., Hartmann N.B., Filser J., Quigg A., Santschi P.T., Sigg L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants and fungi. Ecotoxicology. 2008;17(5):372–386. doi: 10.1007/s10646-008-0214-0. [DOI] [PubMed] [Google Scholar]
- Navarro I., Rojas P., Capilla E., Albalat A., Castillo J., Montserrat N., Codina M., Gutiérrez J. Insights into insulin and glucagon responses in fish. Fish Physiol. Biochem. 2002;27(3–4):205–216. [Google Scholar]
- Ohta T., Miyake H., Miura C., Kamei H., Aida K. Follicle-stimulating hormone induces spermatogenesis mediated by androgen production in Japanese eel, Anguilla japonica. Biol. Reprod. 2007;77:970–977. doi: 10.1095/biolreprod.107.062299. [DOI] [PubMed] [Google Scholar]
- Peng C., Zhang W., Gao H., Li Y., Tong X., Li K., Zhu X., Wang Y., Chen Y. Behavior and potential impacts of metal-based engineered nanoparticles in aquatic environments. Nanomaterials (Basel) 2017;7(21):1–33. doi: 10.3390/nano7010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Labrada F., López-Vargas E.R., Ortega-Ortiz H., Cadenas-Pliego G., Benavides-Mendoza A., Juárez-Maldonado A. Responses of tomato plants under saline stress to foliar application of copper nanoparticles. Plants. 2019;8:151. doi: 10.3390/plants8060151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plisetskaya E.M., Duan C. Insulin and insulin-like growth factor I in coho salmon Oncorhynchus kisutch injected with streptozotocin. Am. J. Physiol. 1994;267(5 Pt 2):R1408–R1412. doi: 10.1152/ajpregu.1994.267.5.R1408. [DOI] [PubMed] [Google Scholar]
- Prentera J., MacNeila C., Dicka J.T.A., Riddella G.E., Dunnb A.M. Lethal and sublethal toxicity of ammonia to native, invasive and parasitized freshwater amphipods. Water Res. 2004;38:2850–12847. doi: 10.1016/j.watres.2004.03.042. [DOI] [PubMed] [Google Scholar]
- Pulsforda A.L., Lemaire-Gony S., Tomlinsonc M., Collingwood N., Glynn P.J. Effects of acute stress on the immune system of the dab. Limanda limanda. 1994;109(2):129–139. [Google Scholar]
- Rentier-Delrue F., Swennen D., Philippart J.C., L’Hoir C., Lion M., Benrubi O., Martial J.A. Tilapia growth hormone: molecular cloning of cDNA and expression in Escherichia coli. DNA. 1989;8:271–278. doi: 10.1089/dna.1.1989.8.271. [DOI] [PubMed] [Google Scholar]
- Sambroni E., Lareyre J.J., Le Gac F. FSH controls gene expression in fish both independently of and through steroid mediation. PLoS ONE. 2013;8(10) doi: 10.1371/journal.pone.0076684. e76684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarapura V.D., Gordon D.F., Samuels M.H. Thyroid-stimulating hormone. Pituitary. 2011 doi: 10.1016/B978-0-12-380926-1.10006-9. [DOI] [Google Scholar]
- Scott G.R., Sloman K.A. The effects of environmental pollutants on complex fish behavior: integrating behavioral and physiological indicators of toxicity. Aquat. Toxicol. 2004;68:369–392. doi: 10.1016/j.aquatox.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Stocco D.M., Wang X., Jo Y. Multiple signaling pathways regulation steroidogenesis and steroidogenic acute regulatory protein expression: more complicated than we thought. Mol. Endocrinol. 2005;19:2647–2659. doi: 10.1210/me.2004-0532. [DOI] [PubMed] [Google Scholar]
- Sukhanova A., Bozrova S., Sokolov P., Berestovoy M., Karaulov A., Nabiev I. Dependence of nanoparticle toxicity on their physical and chemical properties. Nanoscale Res. Lett. 2018;13:44. doi: 10.1186/s11671-018-2457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.S., Jiao B., Hu C., Huang X., Liu Z., Cheng C.H. Discovery of a gonad-specifi c IGF subtype in teleost. Biochem. Biophys. Res. Commun. 2008;367:336–341. doi: 10.1016/j.bbrc.2007.12.136. [DOI] [PubMed] [Google Scholar]
- Wang R., Song B., Wu J., Zhang Y., Chen A., Shao L. Potential adverse effects of nanoparticles on the reproductive system. Int. J. Nanomed. 2018;13:8487–8506. doi: 10.2147/IJN.S170723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigginton N.S., Hausa K.L., Hochella M.F. Aquatic environmental nanoparticles. J. Environ. Monit. 2007;9:1306–1316. doi: 10.1039/b712709j. [DOI] [PubMed] [Google Scholar]
- Yada T. Growth hormone and fish immune system. Gen. Comp. Endocrinol. 2007;152:353–358. doi: 10.1016/j.ygcen.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Zhu B., Ge W. Genetic analysis of zebrafish gonadotropin (FSH and LH) functions by TALEN-mediated gene disruption. Mol. Endocrinol. 2015;29(1):76–98. doi: 10.1210/me.2014-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubeldia-Brenner L., Roselli C.E., Recabarren S.E., Gonzalez M.C., Deniselle, Lara H.E. Developmental and functional effects of steroid hormones on the neuroendocrine axis and spinal cord. J. Neuroendocrinol. 2016;28(7) doi: 10.1111/jne.12401. [DOI] [PMC free article] [PubMed] [Google Scholar]