Abstract
Seed-borne fungus Penicillium duclauxii was examined in this study to investigate its capability of synthesizing silver nanoparticles (Ag-NPs). In vitro experiments were conducted using corn-grain contaminating fungal isolate. Ag-NPs detection and characterization were assayed by the aid of spectroscopic techniques. Spectroscopy (energy dispersive), X-ray diffraction, transmission electron-microscope and optical absorption dimensions were employed.
Ag-NPs with biosynthesized were used to test invitro against Bipolaris sorghicola; the cause of target leaf spot disease on sorghum plants. The myco-synthesis of Ag NPs using Penicillium duclauxii was proved in this study. Moreover, Bipolaris sorghicola was successfully inhibited by such Ag NPs in vitro.
Keywords: Nanomaterials, Corn, Bipolaris sorghicola, Antimicrobial agents
1. Introduction
A number of biological sources have been recently incorporated into the pure green synthesis of Ag-NPs. Such synthesis involves eco-friendly lowering and capping agents as well as environmentally acceptable solvent. Myco-synthesis of Ag-NPs are more convenient as it is affordable, ecological, informal and quick as equated with other methods (Saxena et al., 2014). Both Yeast and Fungi in large amount will be used in myco-synthesis of Ag-NPs (Mourato et al., 2011). Several species of Penicillium have been frequently reported as Ag-NPs micro-synthesis (Singh et al., 2014). Fusarium solani (Ingle et al., 2009), Penicillium citrinum (Honary et al., 2013), Penicillium fellutanum (Kathiresan et al., 2009), Trichoderma species (Devi et al., 2013), Penicillium brevicompactum (Shaligram et al., 2009) have been reported to synthesize Ag-NPs.
The applications of Ag-NPs in both the crop and food industries along with human health sectors is simultaneously growing since era (Thul et al., 2013). Anti-microbial agent is known to be as one of the major applications in Ag-NPs; which can be applied in managing in plant diseases (Anna et al., 2015, Elgorban et al., 2016a). Proper utilization and complete applications of Ag-NPs in these strategies required to apply in the further fields. In this current study, bio-synthesis of Ag-NPs applying with Penicillium duclauxii was investigated. Obtained activities in Ag-NPs were also patterned towards Bipolaris sorghicola. Former fungus is the seed-borne; originally isolated from corn grains, and the latter is a necrotrophic fungus causes target leaf spot disease of sorghum.
2. Materials and methods
2.1. Biomass
Fungal biomass was obtained after proper growth of P. duclauxii: originally extracts from corn grains. Liquid medium is composed with the combination of KH2PO4, K2HPO4, MgSo4, H2O, (NH4)2SO4, yeast extract and glucose. Inoculation media in the Erlenmeyer flask (100 mL media/250 mL flask) were incubated in 5 days in 180 rpm at 28 ± 2 °C. Unglued fungal mass with Whatmann filter-paper further cleaned with deionized water and transferred in Erlenmeyer flask with 100 mL of deionized water. Further incubation for 72 h in an orbital shaker with 140 rpm was considered. Filtrate solution without cells were separated with filtration to be used in biosynthesis of Ag-NPs (Naveen et al., 2010).
2.2. Biosynthesis
Filtrate solution is added to 1.0 mM AgNO3 solution (1:1). At 200 rpm in rotary shaker, mixture was incubated in the dark at 28 °C. Both positive and negative controls were also added and treatment were triplicates and incubated. Solution were centrifuged at 9000 rpm for 14 min, once the color deviates to Yellow (Naveen et al., 2010). Supernatant was separated and sonicated for 1 h using 740 and 740 X ultrasound sonicator. Further, pellets were dried at 60 °C for 1 day in oven.
2.3. Characterization
Dried pellets and supernatant were used in synthesized classification in Ag-NPs using X-RD and ED-spectroscopy; later was used in optical absorption spectroscopy (OAS) and Transmission Electron Microscope (TEM). X-RD was applied to evaluate metallic nature of biosynthesized Ag-NPs. This assay is achieved through X-pert pro diffractometer used Cu-Kα radiation at 40 KV and 40 mA. Further, X-rays Cu-Kα wavelength is 1.54056 Å; scans were basically performed over a 2θ range from 10°-85° at a speed of 0.02/s (An aperture slit, an anti-scatter slit and a receiving slit was 2-mm and 6-mm and 0.2-mm respectively). Surface morphology of Ag-NPs was investigated by both SEM and EDS (Jeol model JSM-6380). EDS is clubbed with SEM; used to enumerate the compositional analysis of NP. UV–Vis spectrophotometer was used to measure the optical absorption and sonicated supernatant of Ag-NPs, range of the wave-length is 300–900 nm was applied and incident photon flux was normal to the surface. Ag-NPs was lastly checked by loading thin film of supernatant of carbon coated TEM grids, analyzed by TEM.
2.4. Antifungal properties
Efficacy of biosynthesized Ag-NPs was patterned against Bipolaris sorghicola. Variable amount of Ag-NPs stock solution was added in PDA medium before hammering in petri-plates to attain 50, 100, 150 and 200 ppm concentrations. Petri-dishes were treated and next inoculate through 2 mm fungal plugs in triplicates after quiet solidification. Treated petri-plates including controls were incubated at 28 ± 2 °C for 7–10 days. The percentage of growth inhibition was calculated from the colony diameters of the tested fungus in Ag-NPs treated media compared with controls.
3. Results
3.1. Detection of Ag NPs
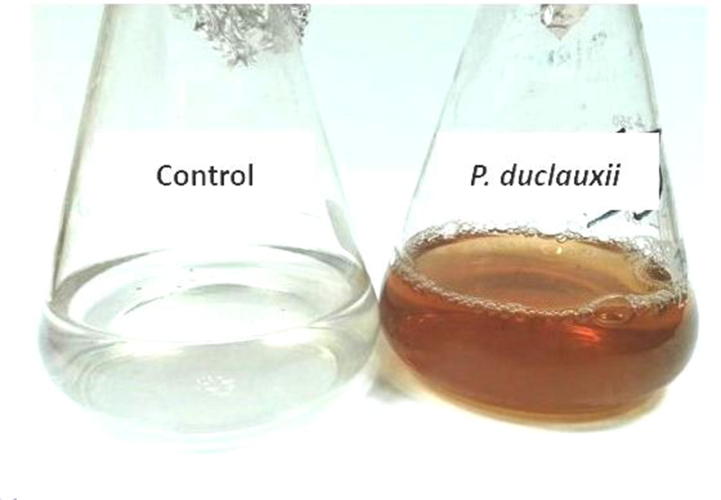
Yellow color was the solution obtained color after couple of days of incubation (Fig. 1). It arises because of AgNO3 reaction and with arrived fungal culture of supernatant. When compared in controls, no color was modified (see Fig. 2).
Fig. 1.
Biosynthesis of Ag NPs by supernatant of P. duclauxii after adding 1 mM AgNO3 showed color change into yellowish. Culture supernatant without 1 mM AgNO3 showed no color change.
Fig. 2.
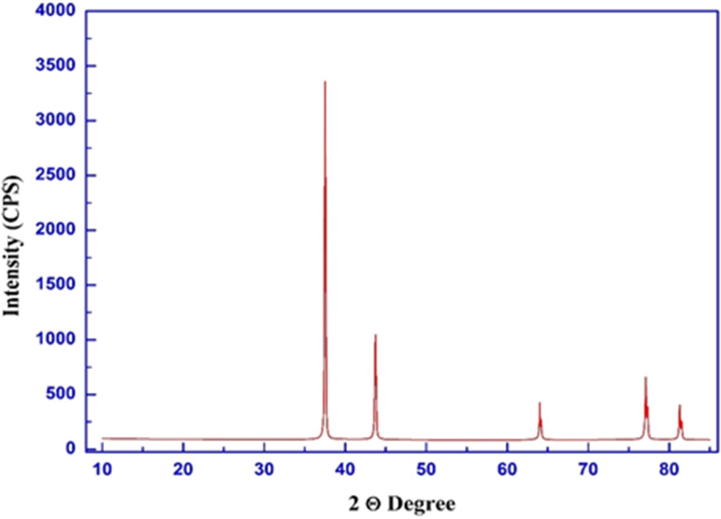
XRD of silver synthesized nanoparticle.
3.2. Characterization of Ag-NPs
3.2.1. XRD analysis
XRD of biosynthesis Ag NPs is shown in (Fig. 3). Observed and calculated lattice parameters, according to the PDF File no. 040,783 are shown in Table1. D-Average grain size, δ-dislocation density, ε- NPs were shown in Table 2 and calculated with the equations
| (1) |
| (2) |
| (3) |
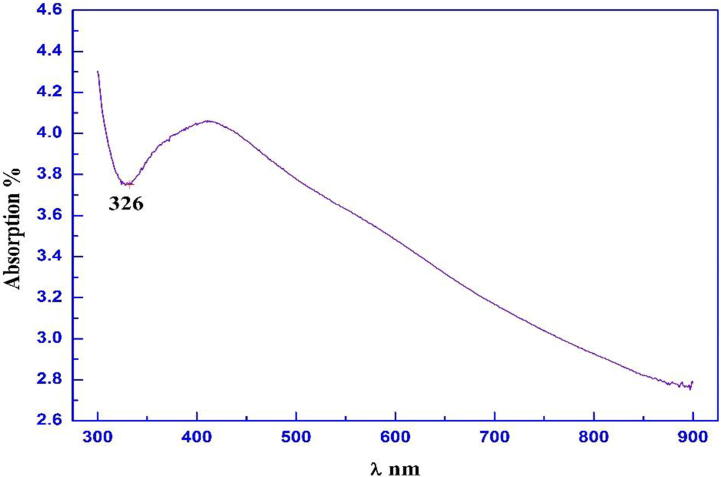
Fig. 3.
Optical absorption of biosynthesized silver nanoparticle.
Table 1.
Calculated lattice parameters, unit cell volume, and space group.
| Silver | ||
|---|---|---|
| a | 4.086 | 4.107 |
| b | 4.086 | 4.107 |
| c | 4.086 | 4.107 |
| α | 90 | 90 |
| β | 90 | 90 |
| γ | 90 | 90 |
| Volume | 68.23 | 69.277 |
| Space Group | Fm3m | Fm3m |
Table 2.
Calculated Average grain size (D), dislocation density (δ), and strain (ε) for biosynthesized Ag Nps.
| Particle size D nm | δ nm−2 | ε |
|---|---|---|
| 54.2 | 3.40 × 10-4 | 6.39 × 10-4 |
Β-full –width at half –maximum of peaks; λ-X-ray wavelength.
Full-prof and Chekcell programs (1) was used for calculation of the Ag NPs lattice parameters and all structure information.
3.2.2. Optical absorption measurements
The optical absorption of Ag NPs using the UV–Visible Spectroscopy was illustrated in Fig. 3. Eequation number 4 shows the calculated particle size from absorption data peak, the calculated r (nm) is 1.637 nm.
| (4) |
3.2.3. Energy dispersive spectrometry
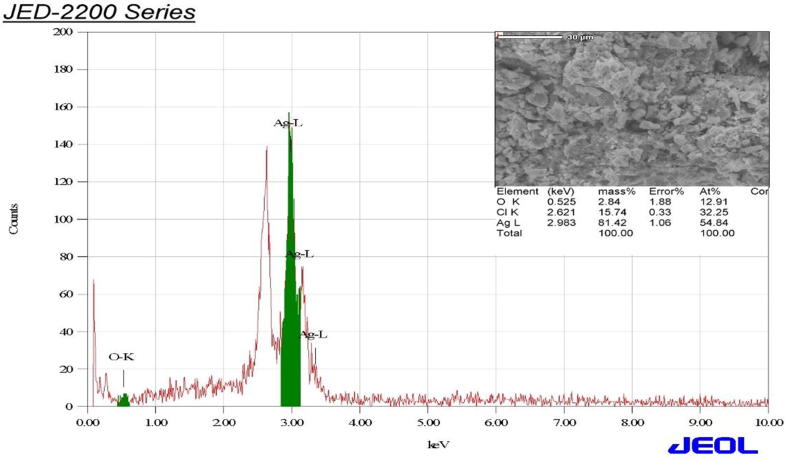
Exhibiting robust Ag signals compared with loose carbon peaks, foundation of pure Ag-NPs might be identified in EDS profile (Fig. 4).
Fig. 4.
Energy dispersive spectrometry (EDS) of biosynthesized silver nanoparticle.
3.2.4. TEM
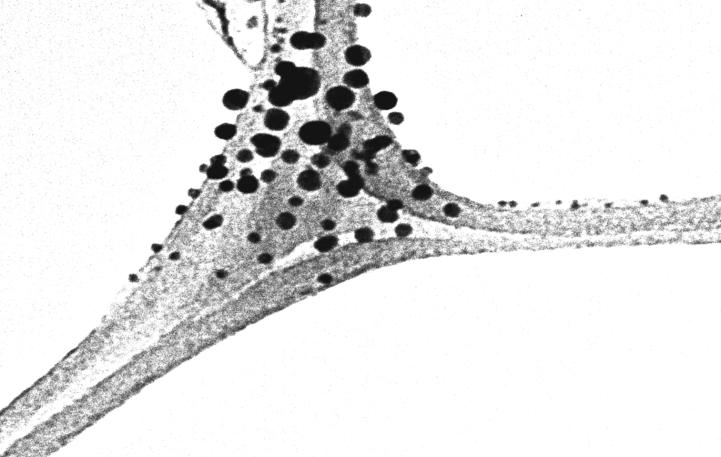
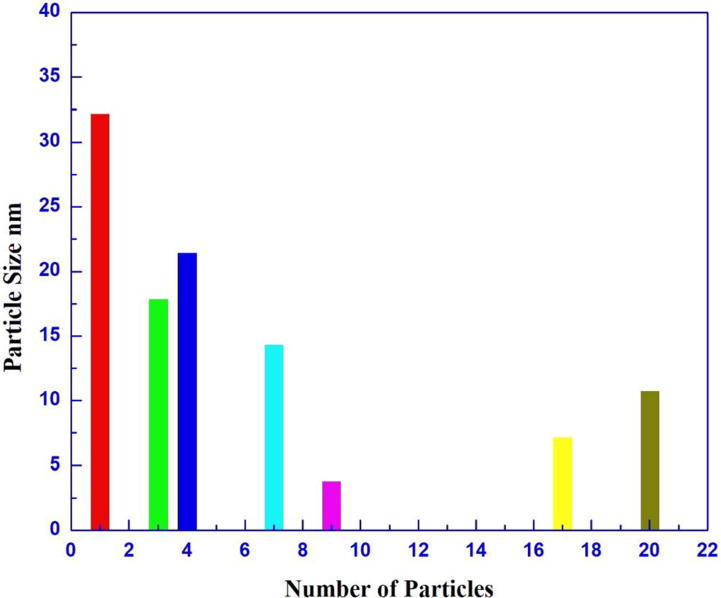
TEM studies stretches further inputs on both morphology and biosynthesized size of Ag-NPs ranges between 3 and 32nms and histogram confirms spherical size of morphology. Fig. 5 confirms clear visible of NPs in varied size and shape majorly appears on spherical. Fig. 6 shows distribution and variation of biosynthesized Ag-NPS.
Fig. 5.
TEM of biosynthesized Ag NPs.
Fig. 6.
Distribution of biosynthesized Ag Np.
3.2.5. Antifungal activity
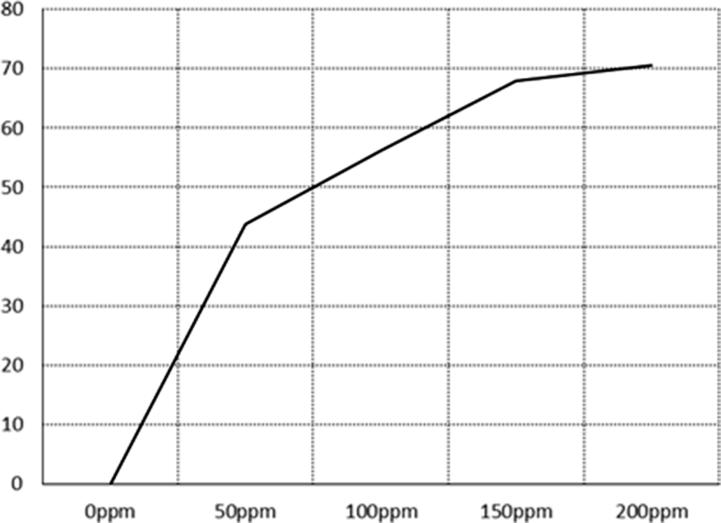
Ag-NPs with biosynthesized by P. duclauxii was invitro examined against fungus with necrotrophic, Bipolaris sorghicola that causes the disease of sorghum. Fig. 7 shows that growth inhibition increases with increasing concentrations of Ag-NPs. 150–200 ppm was the conc. Showed the highest suppression of fungal radial growth. Table 3 shows the efficacy of Ag-NPs ranged from 27.9 to 58.9%. Estimated ED50 and 95 with linear regression was 125.9 and 2191.0 ppm respectively.
Fig. 7.
Inhibition % of biosynthesized Ag NPs on Bipolaris sorghicola.
Table 3.
Efficacy (%) of biosynthesized Ag NPs on Bipolaris sorghicola.
| 50 ppm | 100 ppm | 150 ppm | 200 ppm | ED50 | ED95 | Slope ± SE |
|---|---|---|---|---|---|---|
| 27.96 | 48.63 | 53.50 | 58.97 | 125.9 | 2191.0 | 1.33 ± 5.23 |
ED50 = Median effective dose.
ED95 = The dose required foe desired effect in 95% of the population exposed to it.
SE = The standard error.
Efficacy of Ag-NPs against many micro-organisms has been frequently been ascribed to amendment of cell-wall, cytoplasm and membrane permeability (Manjumeena et al., 2014, Wang et al., 2014, Lysakowska et al., 2015). It has also mentioned as Ag+ treatment could affect fungal DNA, enzymes, cellular proteins and ATP (Feng et al., 2000, Yamanaka et al., 2005, Gajbhiye et al., 2009, Sang et al., 2012).
Disease that affect with target leaf-spot of sorghum in Bipolaris sorghicola was successfully inhibited in myco-synthesized Ag-NPs in this study. Such Ag-NPs may be promising tool in the field of controlling plant disease. Further studies are required to achieve the actual utilization of these nanomaterials in this field.
4. Discussion
4.1. Detection of Ag-NPs
The color of the solution turns into Yellowish color due to presence of AgNO3 solution and with the fungal culture, none of the color modification has been occurred in the control set and our study results were in agreement with Sadowski et al., 2008, Elgorban et al., 2016a who documented as the color changes as an indicator of Ag-NPs biosynthesis. Kumar et al. (2007) indicated as fungal extracellular enzymes may play a role in lowering of Ag ions convert into AgNO3 and legalizes Ag-NPs biosynthesis (Naveen et al., 2010).
4.2. XRD analysis
Crystallographic analysis indicated that Ag NPs are clearly pure and polycrystalline face centered cubic symmetry characterizing the pure silver metal was exhibited by all reflections. Nanda et al. (2015) regulates the crystalline and metallic nature of Ag-NPs applying with XRD analysis. The crystalline nature of Ag-NPs with face centered cubic structures were documented for Ag-NPs synthesis from P. duclauxii. The minimum size of Ag-NPs was 54.2 nm with negative surface charge as well as good stability and conductivity (Sadowski et al., 2008).
4.3. Optical absorption analysis
Strong absorbance and wave-length peaks at 400 nm referred as the appearance of Ag-NPs (Nanda et al., 2015). Nearly 400–420 nm of absorption band have been previously recorded for Ag-NPs produced by P. citrinum (Honary et al., 2013, Goswami et al., 2013).
4.4. Energy dispersive spectrometry analysis
The characterization spectrum of EDS attained for Ag-NPs powder. Visible peaks also authorize the presence of Ag, O2, Cl2 substances in tested samples. Ag was the major element which was about 81% of mass since an intense signal was at 3Kev as per the surface plasmon-resistance (Khan et al., 2013, Mallikarjuna et al., 2014).
4.5. TEM analysis
High resolution of TEM photographs were used for morphological characterization and size disturbance in Ag-NPs. Synthesizing Ag-NPs clearly varied from 3 to 32 nm in size with numerous shapes, mostly visible in spherical in nature (Goswami et al., 2013). The circulation of size of histogram is dynamic light scattering specifies the size of Ag-NPs are 32 nm. Approximately dispersal at low-range of particle size confirms the synthesized particles are also in minor range of particle size.
4.6. Antifungal activity
Green synthesis of Ag-NPs showed highly inhabited the radial growth of Bipolaris sorghicola and generally grown in increasing concentrations of Ag-NPs. These results were in agreement with the prior studies confirmed Ag-NPs has major effect in plant pathogenesis of Bipolaris sorghicola and Colletotrichum species (Kabir et al., 2011, Mishra et al., 2014). Jung et al. (2010) confirmed Ag-NPs were effective for monitoring white rot-disease in onions instigated thru Sclerotium cepivorum. from his study concluded as green Ag-NPs displays significant antifungal activity against Sclerotinia sclerotiorum and Botrytis cinereal in strawberries.
5. Conclusion
Synthesized Ag-NPs were being characterized to be stable without any impurities with minimum size of 13 nm.
Novelty Statement
The silver-Nanoparticles (Ag-NPs) have known as inhibitory effect on microorganism’s in industry and medicine. The most significant application Ag NPs is in medicine such as thematic salve to prevent infection against both open burn and wounds. In this study, we have used the seed borne fungus Penicillium duclauxii to green synthesis of Ag NPs; confirmed by using various characterization techniques.
Declaration of Competing Interest
The authors declared that there is no Conflict of Interest.
Acknowledgment
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group (RGP-306).
Footnotes
Peer review under responsibility of King Saud University.
References
- Anna G., Ewa P., Marek K., Marcin N. Effect of nanosilver in wheat seedlings and Fusarium culmorum culture systems. Eur. J. Plant Pathol. 2015;142:251–261. [Google Scholar]
- Devi T.P., Kulanthaivel S., Kamil D., Borah J.L., Prabhakaran N., Srinivasa N. Biosynthesis of silver nanoparticles from Trichoderma species. Ind. J. Exp. Biol. 2013;51:543–547. [PubMed] [Google Scholar]
- Elgorban A.M., El-Samawaty A.M., Yassin M.A., Sayed S.R., Adil S.F., Elhindi K.M., Marwa B., Khan M. Antifungal silver nanoparticles: synthesis, characterization and biological evaluation. Biotech. Biotechnolog. Equi. 2016;1:56–62. [Google Scholar]
- Feng Q.L., Wu J., Chen G.Q., Cui F.Z., Kim T.N., Kim J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000;52:662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gajbhiye M., Kesharwani J., Ingle A., Gade A., Rai M. Fungus mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine: N.B.M. 2009;5:382–386. doi: 10.1016/j.nano.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Goswami A.M., Sarkar T.S., Ghosh S. An Ecofriendly synthesis of silver nano-bioconjugates by Penicillium citrinum (MTCC9999) and its antimicrobial effect. AMB Express. 2013;3:16–24. doi: 10.1186/2191-0855-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honary S., Barabadi H., Gharaei-Fathabad E., Naghibi F. Green Synthesis of Silver Nanoparticles Induced by the Fungus Penicillium Citrinum. Trop. J. Pharm. Res. 2013;12:7–11. [Google Scholar]
- Ingle A., Gade A., Bawaskar M., Rai M.F. Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. J. Nanopart. Res. 2009;11:2079–2085. [Google Scholar]
- Jung J., Sang-Woo K., Ji-Seon M., Young-Jae K., Kabir L., Kyoung S.K., Youn S.L. The Effect of Nano-Silver Liquid against the White Rot of the Green Onion Caused by Sclerotium cepivorum. Mycobiology. 2010;38:39–45. doi: 10.4489/MYCO.2010.38.1.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabir L., Sang W.K., Jin H.J., Yun S.K., Kyong S.K., Youn S.L. Application of silver nanoparticles for the control of colletotrichum species in vitro and pepper anthracnose disease in field. Mycobiolgy. 2011;39:194–199. doi: 10.5941/MYCO.2011.39.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathiresan K., Manivannan S., Nabeel A.M., Dhivya B. Studies on silver nanoparticles synthesized by a marine fungus Penicillum fellutanum isolated from coastal mangrove sediment. Coll. Surf. B: Biointerf. 2009;71:133–137. doi: 10.1016/j.colsurfb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Khan M., Khan M., Adil S.F., Colloids Surf M., Biointerfaces B., Tahir N., Tremel W., Alkhathlan H.Z., Warthan A., Siddiqui M.R.H. Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int. J. Nanomedicine. 2013;8:1507–1516. doi: 10.2147/IJN.S43309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.S., Abyaneh M.K., Gosavi S.W., Kulkarni S.K., Pasricha R., Ahmad A., Khan M.I. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. 2007. Biotechnol. Lett. 2007;29:439–445. doi: 10.1007/s10529-006-9256-7. [DOI] [PubMed] [Google Scholar]
- Lysakowska M.E., Ciebiada-Adamiec A., Klimek L., Sienkiewicz M. The activity of silver nanoparticles (Axonnite) on clinical and environmental strains of Acinetobacter spp. Burns. 2015;41:364–371. doi: 10.1016/j.burns.2014.07.014. [DOI] [PubMed] [Google Scholar]
- Mallikarjuna K., Sushma N.J., Narasimha G., Manoj L., Raju B.D.P. Phytochemical fabrication and characterization of silver nanoparticles by using pepper leaf broth. Arabian J. Chem. 2014;7:1099–1103. [Google Scholar]
- Manjumeena R., Duraibabu D., Sudha J., Kalaichelvan P.T. Biogenic nanosilver incorporated reverse osmosis membrane for antibacterial and antifungal activities against selected pathogenic strains: An enhanced eco-friendly water disinfection approach. J. Environ. Sci. Health A Toxic Hazard. Subst. Environ. Eng. 2014;49:1125–1133. doi: 10.1080/10934529.2014.897149. [DOI] [PubMed] [Google Scholar]
- Mishra S., Singh B.R., Singh A., Keswani C., Naqvi A.H., Singh H.B. Biofabricated Silver Nanoparticles Act as a Strong Fungicide against Bipolaris sorokiniana Causing spot blotch disease in wheat. PLoS ONE. 2014;9:e97881. doi: 10.1371/journal.pone.0097881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourato A., Gadanho M., Lino A.R., Tenreiro R. Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorg. Chem. Appl. 2011:546074. doi: 10.1155/2011/546074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda A., Majeed S., Abdullah M.S., Nayak B.K., Rizvi E.H. Efficacy of nanosilver from soil fungus enhancing the antisepticactivity of ciprofloxacin against pathogenic bacteria. Der Pharma Chemica. 2015;7:141–146. [Google Scholar]
- Naveen H.K.S., Kumar G., Karthik L., Rao B.K.V. Extracellular biosynthesis of silver nanoparticles using filamentous fungus Penicillium sp. Arch. Appl. Sci. Res. 2010;2:161–167. [Google Scholar]
- Sadowski Z., Maliszewska I.H., Grochowalska B., Polowczyk I., Kozlecki T. Synthesis of silver nanoparticles using microorganisms. Mater. Sci.- Poland. 2008;26:419–424. [Google Scholar]
- Sang W.K., Jin H.J., Kabir L., Yun S.K., Ji S.M., Youn S.L. Antifungal effects of silver nanoparticles (Ag NPs) against various plant pathogenic fungi. Mycobiology. 2012;40:415–427. doi: 10.5941/MYCO.2012.40.1.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena J., Sharma M.M., Gupta S., Singh A. Emerging role OF fungi IN nanoparticle synthesis and their applications. World J. Pharm. Pharmaceut. Sci. 2014;3:1586–1613. [Google Scholar]
- Shaligram N.S., Bule M., Bhambure R., Singhal R.S., Singh S.K., Szakac S.G., Pandey A. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Proc. Biochem. 2009;44:939–943. [Google Scholar]
- Singh D., Rathod V., Ninganagouda S., Hiremath J., Singh A.K., Mathew J. Optimization and characterization of silver nanoparticle by endophytic fungi Penicillium sp. isolated from Curcuma longa (Turmeric) and application studies against MDR E. coli and S. aureus. Bioinorg. Chem. Appl. 2014:408021. doi: 10.1155/2014/408021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thul S.T., Sarangi B.K., Panday R.A. Nanotechnology in Agro-ecosystem: Implications on plant productivity and its soil environment. Expert. Opin. Environ. Biol. 2013;2:1. [Google Scholar]
- Wang C., Huang X., Deng W., Chang C., Hang R., Tang B.A. A nano-silver composite based on the ion-exchange response for the intelligent antibacterial applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2014;41:134–141. doi: 10.1016/j.msec.2014.04.044. [DOI] [PubMed] [Google Scholar]
- Yamanaka M., Hara K., Kudo J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005;71:7589–7593. doi: 10.1128/AEM.71.11.7589-7593.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]