Abstract
Background
Birth asphyxia leads to about 4 million neonatal deaths every year around the globe. But, the pooled prevalence of asphyxia was not yet collated in East and Central African countries. Hence, this systematic review and meta-analysis aimed to determine the pooled prevalence of perinatal asphyxia in Central and East Africa.
Methods
PubMed, Google Scholar, Science Direct, Africa Index Medicus, Africa Journal Online, Excerpta Medica Database, and Cochrane Library databases were searched. All necessary data were extracted using a standardized data extraction format. Data were analyzed using STATA 14 statistical software. A heterogeneity of studies was assessed using the I2 statistics. Publication bias was checked by using a funnel plot and Egger's regression test. A random-effect model was computed to estimate the pooled prevalence of perinatal asphyxia.
Results
Thirteen full-text studies were included in the present meta-analysis. The pooled prevalence of perinatal asphyxia in this study was 15.9% (95%CI: 10.8, 21.0% [I2 = 94.6, p = 0.000]). Regional subgroup analysis indicated that the pooled prevalence of perinatal asphyxia was 18.0 % (95%CI:11.4, 26.7% [I2 = 96.00, p = 0.000]) and 9.1 % (95%CI:2.0, 16.2% [I2 = 90.80, P = 0.000]) in East and Central African countries respectively. Similarly, the level of perinatal asphyxia was varied based on asphyxia measuring tools. But the trim fill analysis pointed that there was no difference in the pooled prevalence of perinatal asphyxia in this study.
Conclusion
The pooled prevalence of perinatal asphyxia was high in the current study. It had also substantial variation across the regions and measuring tools. Therefore, there is a call to reduce the high burden of this problem in the region.
Keywords: Public health, Critical care, Pediatrics, Intensive care medicine, Clinical research, Asphyxia, East and Central Africa, Perinatal, Pooled prevalence
Public health, Critical care, Pediatrics, Intensive care medicine, Clinical research, Asphyxia, East and Central Africa, Perinatal, Pooled prevalence.
1. Introduction
Perinatal asphyxia is defined as a condition that leads to progressive hypoxemia, hypercapnia, and metabolic acidosis with multi-organ failure [1]. Perinatal asphyxia is also defined as the inability of a newborn to initiate and sustain adequate respiration after delivery [2]. According to the American College of Obstetricians and Gynecologists, and the American Academy of Pediatrics, a neonate is labelled to be asphyxiated if (a) umbilical cord arterial pH < 7; (b) Apgar score of 0–3 for longer than 5 min; (c) neonatal neurological manifestations (seizures, coma or hypotonia); and (d) multisystem organ dysfunction (cardiovascular, gastrointestinal, hematological, pulmonary or renal system) [3].
Globally, 2 to 10 per 1000 term newborns faced perinatal asphyxia [4]. The report of the World health organization (WHO) indicated that 4 million neonatal deaths occur yearly due to birth asphyxia [5]. The incidence of birth asphyxia in most developed countries accounts less than 0.1% of newborn deaths. But, in developing countries, it ranged from 4.6/1000 to 7–26/1000 live births [6].
More than 25.0% of the world's newborn deaths have occurred in Africa. Of those, birth asphyxia accounts 24.0%. From 20 countries in the world with the highest risk of neonatal death, 75.0 % are in Africa [7]. Birth asphyxia, infections and complications of preterm birth together account 88.0% of newborn deaths in Africa. In Sub-Saharan Africa, birth asphyxia brought 280,000 deaths of the newborn in the first day of life [8]. The incidence of asphyxia in East, Central, and Southern Africa was 22.0% [9].
The common causes of birth asphyxia are umbilical cord problems, ruptured uterus, preeclampsia/eclampsia, placental abruption, placenta previa, anesthesia mistakes, oligohydramnios, premature rupture of the membranes, premature birth, prolonged and arrested labor, uterine hyper-stimulation, fetal stroke, post-maturity syndrome and delayed emergency cesarean section [10, 11].
Hypoxia, hypercarbia, acidosis, hypotension, and ischemia are the immediate result of birth asphyxia. In the long run, it also brings cerebral palsy, seizure disorders, motor disorders, developmental delays, speech delays, learning disabilities, behavioral and emotional disorders, hearing impairments, visual and feeding impairments [12, 13, 14].
The burden of perinatal asphyxia is critical in Sub-Saharan Africa in general, in East and Central Africa in particular. This problem is high in this region due to poor obstetrics coverage, equity and quality because of gaps in local health financing models, inaccessible health facilities, socio-cultural norms, low literacy levels, shortage in health workers and supplies and poor health care spending [15, 16, 17]. Specially, reproductive health services utilization (facility deliveries, skilled delivery assistance and 4+ antenatal visits) was lower in East and Central Africa as compared to the other region of Sub-Saharan Africa countries [18].
Despite the existence of the agreement of the international community to reduce neonatal mortality to at least 12 deaths per 1,000 live births by 2030 [19], the pooled prevalence of perinatal asphyxia was not investigated by researchers in East and Central African countries. Previous studies on this issue were fragmented and inconsistent. Even, the prevalence of perinatal asphyxia varied from 3.1% [20] to 32.8% [21] across this geographical setting and time periods. Therefore, this study aimed to determine the pooled prevalence of perinatal asphyxia in East and Central African counties by using systematic review and meta-analysis.
2. Methods
2.1. Protocol and registration
The results of this review were reported based on the Preferred Reporting Items for Systematic Review and Meta-Analysis statement (PRISMA) guideline [22]. It is not registered in the Prospero database.
2.2. Eligibility criteria
The inclusion criteria were: 1) hospital-based studies, 2) observational (cross-sectional, case control and cohort) studies reported the prevalence of perinatal asphyxia among newborns, 3) studies conducted in East and Central African countries, 4) studies published in English, 5) studies used the Apgar score for asphyxia diagnosis, and 6) studies available at the electronic source before September 2019.
On the other hand, citations without abstract and/or full-text, anonymous reports, editorials, and qualitative studies were excluded from the analysis.
2.3. Information sources
PubMed, Google Scholar, Science Direct, African Index Medicus, Africa Journal Online, EMBASE and Cochrane Library were accessed. Articles with incomplete reported data were handled through contacting corresponding authors.
2.4. Searching strategy
The core search terms and phrases were “perinatal”, “birth”, “parturition”, “newborn”, neonate”, “asphyxia”, suffocation”, “respiratory distress syndrome”, “Tanzania”, “Burundi”, “Rwanda”, “Uganda”, “Sudan”, “Ethiopia”, “Eritrea”, “Djibouti”, “Somalia”, “Kenya”, “Angola”, “Cameroon”, “Central African Republic”, “Chad”, “Democratic Republic of the Congo”, “Equatorial Guinea”, “Gabon”, and “Republic of the Congo” were the main key searching terms. “OR” or “AND” were used separately and in combination as Boolean operators. Notably, to fit the advanced PubMed database, the following search strategy was applied (Appendix 1).
2.5. Study selection
Retrieved studies were exported to reference manager software, Endnote version 7, to remove duplicate studies. Four independent reviewers screened the title and abstract. The disagreement was handled based on established article selection criteria. Four independent authors reviewed the abstract and full-text of the articles.
2.6. Data extraction
A standardized data extraction format was adopted from the Joanna Briggs Institute (JBI) data extraction format [23] to extract the data. Four authors (YW, AS, EA, and WA) independently extracted all necessary data using this format. The data extraction format included primary author, publication year, country, region, measuring tool, study design, response rate, sample size, and prevalence.
2.7. Outcome measurement
The outcome variable of study was perinatal asphyxia, which is defined as the inability of a newborn to initiate and sustain respiration, or a new born with an Apgar score of less than 7 at five minutes after delivery (2). The pooled prevalence was calculated by dividing the total number of perinatal asphyxia in all review studies to the total number of involved fetus and neonates in the study and multiplying by 100 [24].
Perinatal asphyxia = (Number of perinatal asphyxia/number of participants) ∗100.
2.8. Quality assessment
The Newcastle-Ottawa Quality Assessment tool [25] was adapted to check the quality of cross-sectional studies in this review [26]. The assessment tool contains 1) representativeness of the sample, 2) sample size, 3) non-respondents and 4) ascertainment of the exposure, 5) independent blind assessment and 6) statistical test) (Appendix 2). Finally, based on this tool, article with a scale of ≥6 out of 10 was considered as good quality.
Each original study was evaluated by four authors independently using this tool. If there were disagreements between those four authors, the consensus was reached by taking the mean score of the four authors. The inter-rater variation of study selection in this study was calculated by using kappa statistics (Appendix 3).
2.9. Statistical analysis
Publication bias was checked by funnel plot and more objectively through Begg's and Egger's regression test [27]. Heterogeneity of studies was quantified using the I-Squared Statistic, in which 25, 50, and 75% represented low, moderate and high heterogeneity respectively [28, 29]. Pooled analysis was conducted using a weighted inverse variance random-effects model [30]. Subgroup analysis was done by the study region, and asphyxia measuring tools. Sensitivity analysis was employed to see the effect of single study on the overall estimation. STATA version 14 statistical software was used for meta-analysis.
3. Results
3.1. Characteristics of reviewed studies
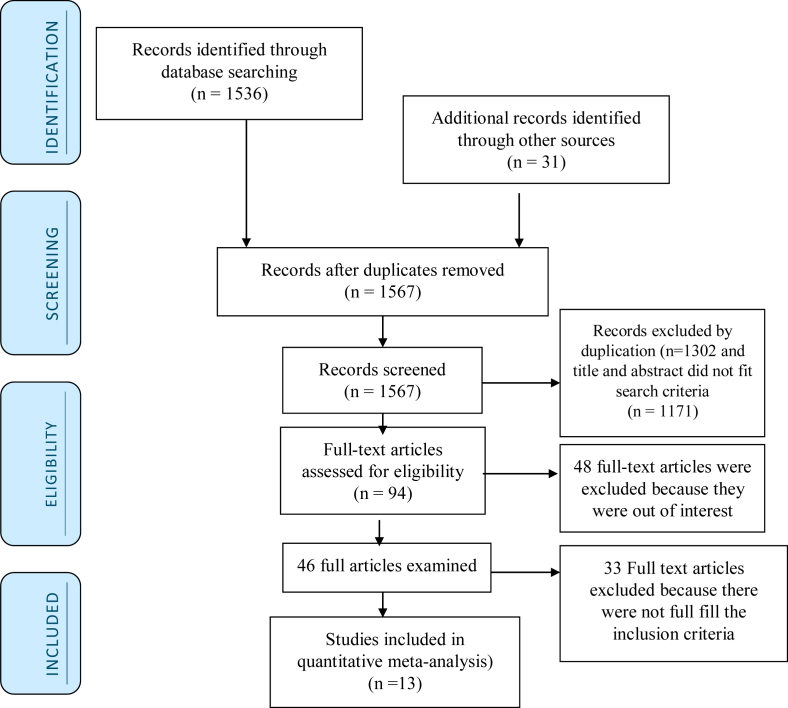
Initially, 1536 records were identified in relation to perinatal asphyxia through PubMed, Google scholar, Africa Index Medicus, Africa Journal Online, EMBASE, and Science Direct databases. Thirty-one records were searched from other sources. From these, 1473 were not considered for further evaluation as a result of duplication and title and abstract did not fit search criteria. From the rest 94 articles, 48 were excluded as a result of not fulfilling our inclusion criteria by reviewing their titles and abstracts. Therefore, 46 full-text articles were accessed and assessed for eligibility based on the inclusion criteria [3, 15, 17, 20, 21, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71]. Finally, 13 studies that fulfilled the eligibility criteria were included in the meta-analysis (Figure 1).
Figure 1.
Flowchart to a selection of studies for a systematic review and meta-analysis of the prevalence of perinatal asphyxia in east and central Africa, 2019.
Data for the thirteen eligible studies were extracted and analysed in this study. The pooled prevalence of perinatal asphyxia was estimated by using 76932 newborns. The prevalence of perinatal asphyxia ranges from 3.1% [20] to 32.9% [24]. From thirteen reviewed articles, ten and three articles were form the three East and two Central African countries respectively. Seven of the studies were from Ethiopia [20, 21, 32, 35, 36, 37, 71], two studies were from Kenya [3, 41], one from Tanzania [40], one from Chad [15] and two from Democratic Republic of Congo [43, 44]. Moreover, all thirteen studies were conducted by cross sectional study design (Table 1).
Table 1.
Descriptive summary of 13 studies included in the meta-analysis of the prevalence perinatal asphyxia in East and Central Africa.
| Authors name | Publication Year | Country | Region | Design | Measuring tools | Response rate (%) | Sample size | Prevalence (%) | Quality of study |
|---|---|---|---|---|---|---|---|---|---|
| Sepeku A et al | 2011 | Tanzania | East Africa | cross-sectional | Apgar score <7 | 100.0 | 190 | 21.1 | 7 |
| Belachew et al | 2017 | Ethiopia | East Africa | cross-sectional | Apgar score <7 | 99.0 | 368 | 12.5 | 6 |
| Ibrahim A et al | 2017 | Ethiopia | East Africa | cross-sectional | Apgar score <7 | 100.0 | 9738 | 3.1 | 6 |
| Kibai K et al | 2017 | Kenya | East Africa | cross-sectional | Apgar score <7 | 100.0 | 422 | 29.1 | 7 |
| Demisse G et al | 2017 | Ethiopia | East Africa | cross-sectional | Apgar score <6 | 100.0 | 769 | 12.5 | 8 |
| Gebreheat G et al | 2018 | Ethiopia | East Africa | cross-sectional | Apgar score <7 | 99.7 | 421 | 22.1 | 8 |
| Asfere W et al | 2018 | Ethiopia | East Africa | cross-sectional | Apgar score <7 | 100.0 | 154 | 29.9 | 7 |
| Gichogo M et al | 2018 | Kenya | East Africa | cross-sectional | Apgar score <7 | 100.0 | 237 | 5.1 | 8 |
| Abkika BM et al | 2018 | Chad | Central Africa | cross-sectional | Apgar score <7 | 100.0 | 7254 | 5.1 | 8 |
| Biselele T et al | 2013 | DRC∗ | Central Africa | cross-sectional | Apgar score <6 | 100.0 | 902 | 4.4 | 7 |
| Mande et al | 2018 | DRC∗ | Central Africa | cross-sectional | Apgar score <7 | 100.0 | 612 | 19.4 | 8 |
| Alemu A. et al | 2019 | Ethiopia | East Africa | cross-sectional | Apgar score <7 | 100.0 | 262 | 32.8 | 7 |
| Abdo RA. et al | 2019 | Ethiopia | East Africa | cross-sectional | Apgar score <7 | 100.0 | 279 | 15.1 | 6 |
DRC∗-Democratic Republic of Congo.
3.1.1. Quality appraisal
The Newcastle-Ottawa Scale quality appraisal criteria established for cross-sectional was used. The studies included in this systematic review and meta-analysis had no low quality. Therefore, all thirteen studies were considered (Table 1).
3.2. Meta-analysis
3.2.1. Prevalence of perinatal asphyxia
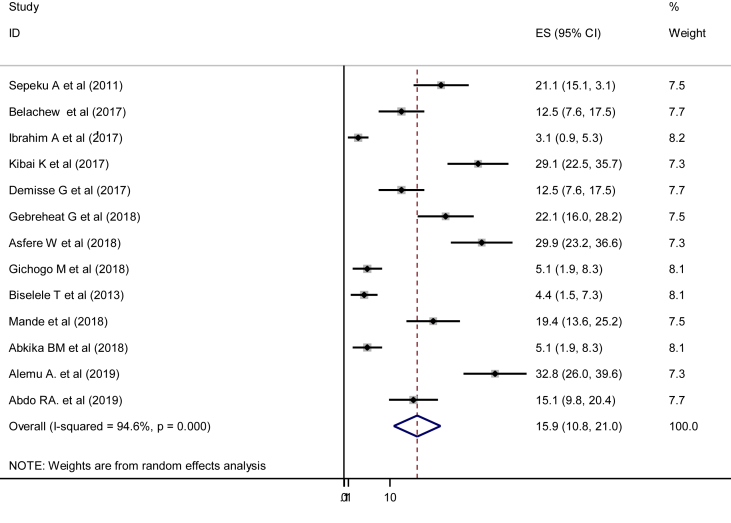
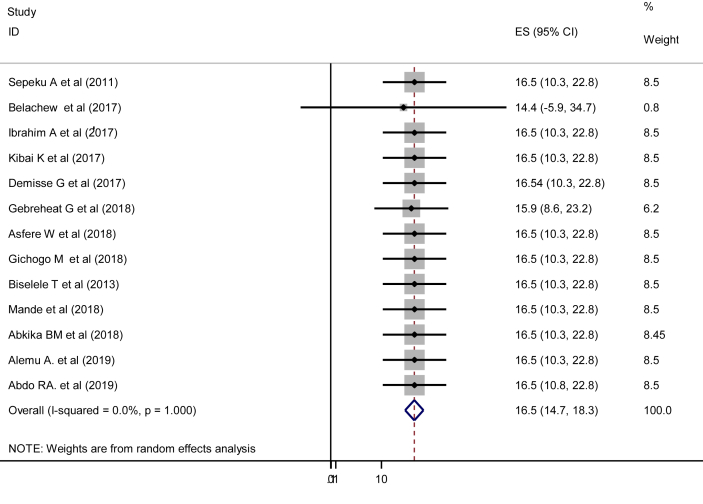
In the current study, the pooled prevalence of perinatal asphyxia was 15.9% (95% CI: 10.8, 21.0%). Severe heterogeneity was observed across the studies (I2 = 94.6, p = 0.000) (Figure 2). The pooled prevalence of perinatal asphyxia with a prediction interval in this study was 16.5% (95% CI:14.7, 18.3%) (Figure 3).
Figure 2.
Pooled prevalence of perinatal asphyxia in East and Central Africa, 2019 (n = 13).
Figure 3.
Forest Plot for pooled prevalence of perinatal asphyxia with prediction interval (n = 13).
3.2.2. Subgroup analysis
As a result of substantial heterogeneity, we performed subgroup analysis based on the region and measuring tools. In this regard, the prevalence was higher in East Africa, 18.0 % (95%CI:11.4, 26.7% [I2 = 96.0, p = 0.000]) as compared with Central Africa, 9.1 % (95%CI:2.0, 16.2% [I2 = 90.8, P = 0.000]). The prevalence of asphyxia, which was determined via Apgar scale of <7 was higher than the prevalence which was measured by Apgar scale of <6 (Table 2).
Table 2.
Subgroup analysis of prevalence of perinatal asphyxia in East and Central Africa.
| Variables | Characterstics | Estimates (95%CI) | I2 tests with p-value |
|---|---|---|---|
| Region | East Africa | 18.0 % (11.4, 26.7%) | I2 = 96.0, p = 0.000 |
| Central Africa | 9.14 % (2.0, 16.2%) | I2 = 90.8, P = 0.000 | |
| Measuring scale | Apgar score <7 | 17.4 % (11.3, 23.5%) | I2 = 96.0, P = 0.000 |
| Apgar score <6 | 8.2 % (0.3, 16.1%) | I2 = 86.9, p = 0.006 |
A random-effect model was employed to estimate the pooled prevalence of perinatal asphyxia. Different factors associated with the heterogeneity such as publication date, sample size and response rate were investigated using multivariate meta-regression models. From these variables, none of them were statistically significant (Table 3).
Table 3.
Related factors with heterogeneity of perinatal asphyxia prevalence in the current meta-analysis.
| Variables | Coefficient | P-value |
|---|---|---|
| Publication years | 2.9 (0.6, 4.8) | 0.8 |
| Sample size | -0.1 (-1.5, 0.6) | 0.4 |
| Response rate | -9.5 (-14.5, -0.7) | 0.4 |
3.2.2.1. Publication bias
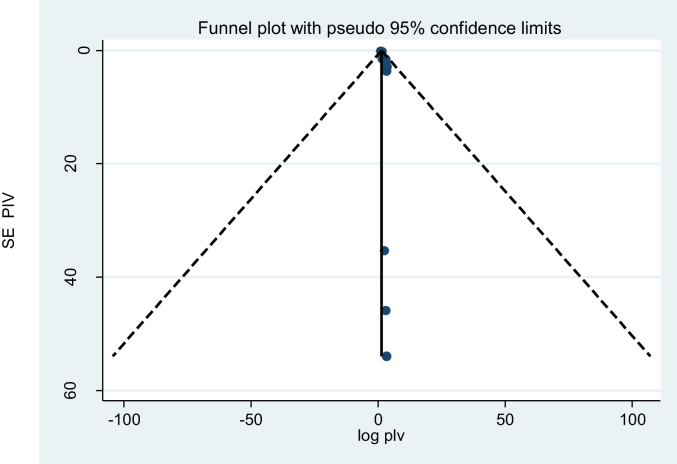
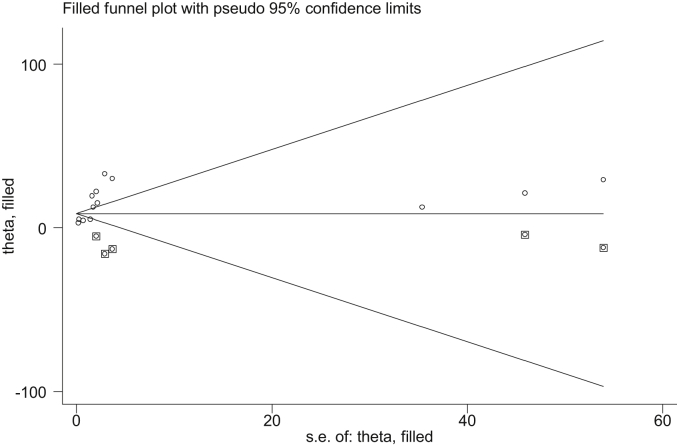
A funnel plot showed asymmetrical distribution (Figure 4). The result of the Egger test was also statistically significant with Bo = 1.9 and p = 0.000. To see publication bias further, trim fill analysis was done, and five studies were filled. In this analysis, the pooled prevalence of perinatal asphyxia was 9.7% (95% CI: 6.2, 12.3%). But the confidence interval indicated that there is no difference in the pooled prevalence of perinatal asphyxia (Figure 5).
Figure 4.
Funnel plot for publication bias, logprop or lnp (log of proportion) represented in the X-Axis and standard error of log proportion in the Y-Axis.
Figure 5.
The trim fill analysis showed the pooled prevalence when the unpublished studies are filled.
3.2.2.2. Sensitivity analysis
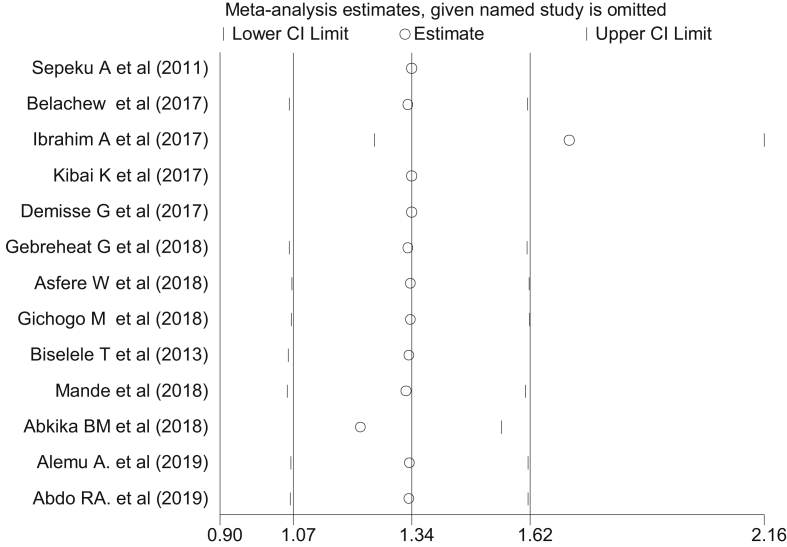
Among all thirteen reviewed studies in the current analysis, the study conducted by Ibrahim et al [20] had shown an impact on the overall estimation (Figure 6). The pooled prevalence of perinatal asphyxia in terms of step by step omission of article is indicated in (Table 4).
Figure 6.
The sensitivity analysis showed the pooled prevalence when the studies omitted step by step.
Table 4.
The sensitivity analysis to estimate of the prevalence of perinatal asphyxia in East and Central African countries.
| Study omitted | Prevalence (95%CI) |
|---|---|
| Sepeku A et al, 2011 | 15.5 (10.3, 20.7) |
| Belachew et al, 2017 | 16.2 (10.8, 21.7) |
| Ibrahim A et al, 2017 | 17.1 (11.6, 22.5) |
| Kibai K et al, 2017 | 14.8 (9.8, 19.8) |
| Demisse G et al, 2017 | 16.2 (10.8, 21.7) |
| Gebreheat G et al, 2018 | 15.4 (10.2, 20.6) |
| Asfere W et al, 2018 | 14.8 (9.8, 19.7) |
| Gichogo M et al, 2018 | 16.9 (11.3, 22.5) |
| Biselele T et al, 2013 | 16.9 (11.4, 22.6) |
| Mande et al, 2018 | 15.6 (10.6, 20.9) |
| Abkika BM et al, 2018 | 16.9 (11.3, 22.5) |
| Alemu A. et al, 2019 | 14.5 (9.7, 19.3) |
| Abdo RA. et al, 2019 | 16.0 (10.6, 21.4) |
4. Discussion
This study revealed that the pooled prevalence of perinatal asphyxia among newborns was 15.9%. This finding was lower than the study conducted in Western Africa (30.0%) [72]. Lower level of asphyxia in the present study as compared with the study conducted in West Africa might be due to variation in study population. In the present study, the study participants were all newborns, but the study participants in West Africa were all admitted neonates. West African countries are better equipped with modern health facilities as compared East and Central Africa countries health facilities. On the contrary of this evidence, the prevalence of perinatal asphyxia is higher in West Africa as compared with East and Central Africa countries [18]. Therefore, further investigation should be done to identify the main reasons for this variation across the regions.
On the other corner, the current pooled prevalence of perinatal asphyxia is higher than the study conducted in South Africa (2.6%) [9]. The lower level of perinatal asphyxia in Southern Africa as compared with the current study is due to the development and implementation of the South African Neonatal Resuscitation programme [73]. This program in South Africa have brought a strong association between the establishment of an effective audit process, and improvement of the quality of maternal health services and perinatal mortality rates [66, 74]. Additional reasons for high level of perinatal asphyxia in the present study as compared with Southern Africa is the presence of low levels of reproductive health services utilization (facility deliveries, skilled delivery assistance and 4+ antenatal visits) in East and Central Africa as compared to South africa [18].
Subgroup analysis pointed out that the prevalence of perinatal asphyxia was 18.0 % in East Africa region and the prevalence of birth asphyxia was 9.1% in Central Africa region. This indicated that substantial heterogenecity of the prevalence of perinatal asphyxia in East and Central Africa countries. Such difference might be due to obstetrics service delivery (coverage, equity and quality) variation across East and Central Africa countries. As the evidence pointed out that obstetrics coverage, equity and quality gaps brings adverse maternal and neonatal outcomes [75, 76]. The other reason for the lower prevalence in Central Africa might be due to only three studies were included in the review process.
As a result of high burden of asphyxia, the researchers should pool the risk factors of perinatal asyphaxia in order to design the preventive mechanism of modifiable factors of perinatal asphyxia. In general, the data in this report will provide useful information for health planners and politicians involved in health care provision.
This study might be subjected to different limitations. The first limitation of the study was only English articles or reports were considered to carry out the analysis. Even though the quality of each study was assessed by using The Newcastle-Ottawa Scale, inter author bias might be occurred on leveling of the scale of each article. Reviewing of different characterstics of involved cases with different sampling methods was also the other limitation of this study. The last but not the least limitation of the current study is availability of limited numbers of the studies.
5. Conclusion
There was a high rate of perinatal asphyxia. It had also sources of heterogeneity in terms of geographical setting and measuring tools. Therefore, there is an alarm for responsible bodies to decrease the high level of this problem in the region.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix.
Appendix 1. search strategy applied to PubMed database in the current review.
| Search # | Query | Items found |
|---|---|---|
| #1 | perinatal | 119521 |
| #2 | (birth) OR parturition | 527962 |
| #3 | (newborn) OR neonate | 249893 |
| #4 | (asphyxia) OR (suffocation) OR respiratory distress syndrome | 88236 |
| #5 | Search (Tanzania AND Burundi AND Rwanda AND Uganda AND Sudan AND Ethiopia AND Eritrea AND Djibouti AND Somalia AND Kenya AND Angola AND Cameroon AND Central African Republic AND Chad AND Democratic Republic of the Congo AND Equatorial Guinea AND Gabon AND Republic of the Congo) | 279 |
| #6 | #1 AND #2 AND #3 AND #4 AND #5 | 11 |
Appendix 2. Scoring of the quality of articles by authors using The Newcastle-Ottawa Quality Assessment tool.
| Selection |
Comparability |
Outcome |
Total score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Study | Quality assessor | Representativeness of sample (∗, ∗) | Sample size (∗) | Non-respondents (∗) | Ascertainment (∗∗) | Study controls for most important factor (∗) | The study control for any additional factor (∗) | Assessment of the outcome (∗) | Statistical test (∗) | |
| Sepeku A et al. | YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| AY | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | ||
| WA | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | ||
| Belachew et al. | YW | 1 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 6 |
| AY | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| WA | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | ||
| Ibrahim A et al. | YW | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 6 |
| AY | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | ||
| EA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| WA | 1 | 0 | 1 | 2 | 1 | 0 | 0 | 1 | ||
| Kibai K et al. | YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| AY | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| EA | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| WA | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | ||
| Demisse G et al. | YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| AY | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 2 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | ||
| WA | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Gebreheat G et al. | YW | 2 | 0 | 1 | 2 | 1 | 1 | 1 | 0 | 8 |
| AY | 2 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | ||
| EA | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| WA | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | ||
| Asfere W et al. | YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| AY | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 0 | ||
| WA | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | ||
| Gichogo M et al. | YW | 2 | 0 | 0 | 2 | 1 | 1 | 1 | 0 | 8 |
| AY | 2 | 1 | 1 | 2 | 1 | 0 | 1 | 1 | ||
| EA | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| WA | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | ||
| Abkika BM et al. | YW | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| AY | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | ||
| WA | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | ||
| Biselele T et al. | YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| AY | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| WA | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | ||
| Mande et al. | YW | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 8 |
| AY | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| EA | 2 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | ||
| WA | 1 | 1 | 1 | 2 | 1 | 1 | 0 | 1 | ||
| Alemu A. et al. | YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| AY | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | ||
| EA | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | ||
| WA | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Abdo RA. et al. | YW | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 6 |
| AY | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | ||
| EA | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | ||
| WA | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||
Appendix 3. Interrater agreement (kappa) for Newcastle-Ottawa Quality Assessment.
| Item | Kappa | SEM | 95% CI |
|---|---|---|---|
| Representativeness of sample | 0.37 | 0.07 | (1.00, 1.30) |
| Sample size | 0.03 | 0.06 | (0.69, 0.91) |
| Non-respondents | 0.16 | 0.05 | (0.77, 0.96) |
| Ascertainment | 0.17 | 0.07 | (1.00, 1.30) |
| Study controls for most important factor | 0.02 | 0.02 | (0.94, 1.02) |
| The study control for any additional factor | 0.01 | 0.06 | (0.65, 0.89) |
| Assessment of the outcome | 0.02 | 0.05 | (0.74, 0.95) |
| Statistical test | 0.16 | 0.06 | (0.56, 0.82) |
| Overall | 0.30 | 0.22 | (0.17, 0.63) |
References
- 1.Lawn J.E., Cousens S., Zupan J., Team L.N.S.S. 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 2.L. Je, S. Cousens, J. Zupan, Million neonatal deaths: when, Lancet Mar. 4 5–11. [DOI] [PubMed]
- 3.Kriebs J.M. Guidelines for perinatal care: by the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists. J. Midwifery Wom. Health. 2010;55(2) e37-e. [Google Scholar]
- 4.Barkovich A.J., Truwit C.L. Brain damage from perinatal asphyxia: correlation of MR findings with gestational age. Am. J. Neuroradiol. 1990;11(6):1087–1096. [PMC free article] [PubMed] [Google Scholar]
- 5.Aslam H.M., Saleem S., Afzal R., Iqbal U., Saleem S.M., Shaikh M.W.A. Risk factors of birth asphyxia. Ital. J. Pediatr. 2014;40(1):94. doi: 10.1186/s13052-014-0094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhutta Z.A. Elsevier; 2014. Paediatrics in the Tropics. Manson's Tropical Infectious Diseases; pp. 1197–1214. e2. [Google Scholar]
- 7.Guo A., Bowling J.M., Bartram J., Kayser G. Water, sanitation, and hygiene in rural health-care facilities: a cross-sectional study in Ethiopia, Kenya, Mozambique, Rwanda, Uganda, and Zambia. Am. J. Trop. Med. Hyg. 2017;97(4):1033–1042. doi: 10.4269/ajtmh.17-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unicef . eSocialSciences; 2015. Committing to Child Survival: a Promise Renewed. [Google Scholar]
- 9.Kinoti S. Asphyxia of the newborn in east, central and southern Africa. East Afr. Med. J. 1993;70(7):422–433. [PubMed] [Google Scholar]
- 10.Boo N.-Y., Cheah I.G.-S. The burden of hypoxic-ischaemic encephalopathy in Malaysian neonatal intensive care units. Singap. Med. J. 2016;57(8):456. doi: 10.11622/smedj.2016137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Graham E.M., Ruis K.A., Hartman A.L., Northington F.J., Fox H.E. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am. J. Obstet. Gynecol. 2008;199(6):587–595. doi: 10.1016/j.ajog.2008.06.094. [DOI] [PubMed] [Google Scholar]
- 12.Thornberg E., Thiringer K., Odeback A., Milsom I. Birth asphyxia: incidence, clinical course and outcome in a Swedish population. Acta Paediatr. 1995;84(8):927–932. doi: 10.1111/j.1651-2227.1995.tb13794.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee A.C., Kozuki N., Blencowe H., Vos T., Bahalim A., Darmstadt G.L. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr. Res. 2013;74(S1):50. doi: 10.1038/pr.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chau V., Poskitt K.J., Miller S.P. Advanced neuroimaging techniques for the term newborn with encephalopathy. Pediatr. Neurol. 2009;40(3):181–188. doi: 10.1016/j.pediatrneurol.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Pell C., Meñaca A., Were F., Afrah N.A., Chatio S., Manda-Taylor L. Factors affecting antenatal care attendance: results from qualitative studies in Ghana, Kenya and Malawi. PloS One. 2013;8(1) doi: 10.1371/journal.pone.0053747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Waters D., Adeloye D., Woolham D., Wastnedge E., Patel S., Rudan I. Global birth prevalence and mortality from inborn errors of metabolism: a systematic analysis of the evidence. J. Global Health. 2018;8(2) doi: 10.7189/jogh.08.021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serour G.I. Healthcare workers and the brain drain. Int. J. Gynecol. Obstet. 2009;106(2):175–178. doi: 10.1016/j.ijgo.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 18.Abekah-Nkrumah G. Trends in utilisation and inequality in the use of reproductive health services in Sub-Saharan Africa. BMC Publ. Health. 2019;19(1):1541. doi: 10.1186/s12889-019-7865-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Organization W.H. 2016. Global Health Observatory Data, Under-five Mortality. 2018. [Google Scholar]
- 20.Ibrahim N., Muhye A., Abdulie S. Prevalence of birth asphyxia and associated factors among neonates delivered in Dilchora Referral Hospital. Dire Dawa, Eastern Ethiopia Clinics. Mother Child Health. 2017;14(279):2. [Google Scholar]
- 21.Alemu A., Melaku G., Abera G.B., Damte A. Prevalence and associated factors of perinatal asphyxia among newborns in Dilla University referral hospital, Southern Ethiopia–2017. Pediatr. Health Med. Therapeut. 2019;10:69. doi: 10.2147/PHMT.S196265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sherbourne C.D., Stewart A.L. The MOS social support survey. Soc. Sci. Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 23.Brugha T., Bebbington P., Tennant C., Hurry J. The List of Threatening Experiences: a subset of 12 life event categories with considerable long-term contextual threat. Psychol. Med. 1985;15(1):189–194. doi: 10.1017/s003329170002105x. [DOI] [PubMed] [Google Scholar]
- 24.Noordzij M.D.F., Zoccali C., Jager K.J. Measures of disease frequency: prevalence and incidence. Nephron Clin. Pract. 2010;115(1):c17–c20. doi: 10.1159/000286345. [DOI] [PubMed] [Google Scholar]
- 25.Liberati A., Altman D., Tetzlaff J., Mulrow C., Gøtzsche P., Ioannidis J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions. Br. Med. J. 2009;339 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Modesti P.A., Reboldi G., Cappuccio F.P., Agyemang C., Remuzzi G., Rapi S. Panethnic differences in blood pressure in Europe: a systematic review and meta-analysis. PloS One. 2016;11(1) doi: 10.1371/journal.pone.0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ioannidis J. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract. 2008;14(5):951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 29.Higgins J., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 30.Borenstein M., Hedges L.V., Higgins J., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 31.Roba A., Diro D. Morbidities, rate and time trends of neonatal mortality in dilchora referral hospital, Dire Dawa, Ethiopia, 2012-2017. Austin Med. Sci. 2017;2(1) [Google Scholar]
- 32.Zaat T.R., van Steijn M.E., de Haan-Jebbink J.M., Olff M., Stramrood C.A., van Pampus M.G. Posttraumatic stress disorder related to postpartum haemorrhage: a systematic review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018 Jun 1;225:214–220. doi: 10.1016/j.ejogrb.2018.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Tasew H., Zemicheal M., Teklay G., Mariye T., Ayele E. Risk factors of birth asphyxia among newborns in public hospitals of Central Zone, Tigray, Ethiopia 2018. BMC Res. Notes. 2018;11(1):496. doi: 10.1186/s13104-018-3611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wosenu L., Worku A.G., Teshome D.F., Gelagay A.A. Determinants of birth asphyxia among live birth newborns in University of Gondar referral hospital, northwest Ethiopia: a case-control study. PloS One. 2018;13(9) doi: 10.1371/journal.pone.0203763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gebreheat G., Tsegay T., Kiros D., Teame H., Etsay N., Welu G. Prevalence and associated factors of perinatal asphyxia among neonates in general hospitals of Tigray, Ethiopia. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/5351010. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Demisse A.G., Alemu F., Gizaw M.A., Tigabu Z. Patterns of admission and factors associated with neonatal mortality among neonates admitted to the neonatal intensive care unit of University of Gondar Hospital, Northwest Ethiopia. Pediatr. Health Med. Therapeut. 2017;8:57. doi: 10.2147/PHMT.S130309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belachew T., Joseph J. Birth asphyxia and associated factors among newborns delivered in Jimma zone public hospitals, Southwest Ethiopia: a cross-sectional study. J. Midwifery Reproductive Health. 2018;6(2):1289–1295. [Google Scholar]
- 38.Simiyu I.N., Mchaile D.N., Katsongeri K., Philemon R.N., Msuya S.E. Prevalence, severity and early outcomes of hypoxic ischemic encephalopathy among newborns at a tertiary hospital, in northern Tanzania. BMC Pediatr. 2017;17(1):131. doi: 10.1186/s12887-017-0876-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Athumani J. Prevalence and immediate outcomes of hypoxic Ischaemic encephalopathy (hIe) among infants with birth asphyxia admitted at the neonatal ward of Muhimbili national hospital in Dar es salaam, Tanzania. Dar Es Salaam Medical Students' J. 2008;15(1):17–19. [Google Scholar]
- 40.Sepeku A., Kohi T. Treatment outcomes of neonatal asphyxia at a national hospital in Dar es Salaam, Tanzania. Afr. J. Nurs. Midwifery. 2011;13(2):43–56. [Google Scholar]
- 41.Gichogo D.M., Murila F., Matiang'i M., Ndege W., Bosire K. Prevalence of asphyxia and readiness for neonatal resuscitation in Kenya. Afr. J. Midwifery Women's Health. 2018;12(1):21–27. [Google Scholar]
- 42.Chiabi A., Nguefack S., Evelyne M., Nodem S., Mbuagbaw L., Mbonda E. Risk factors for birth asphyxia in an urban health facility in Cameroon. Iran. J. Child Neurol. 2013;7(3):46. [PMC free article] [PubMed] [Google Scholar]
- 43.Biselele T., Naulaers G., Bunga Muntu P., Nkidiaka E., Kapepela M., Mavinga L. A descriptive study of perinatal asphyxia at the university hospital of Kinshasa (democratic republic of Congo) J. Trop. Pediatr. 2013;59(4):274–279. doi: 10.1093/tropej/fmt011. [DOI] [PubMed] [Google Scholar]
- 44.Mande B., Muyobela K., Hasivirwe V., Batoko L. Clinical features and outcome of birth asphyxia in hôpital du Cinquantenaire of Kisangani: a cross-sectional study. Asian J. Pediatr. Res. 2018:1–6. [Google Scholar]
- 45.Walana W., Acquah Ekuban K., Abdul-Mumin A., Naafu B., Aruk E. Pattern, causes and treatment outcomes of neonatal admission in the tamale teaching hospital. Clin. Mother Child Health. 2016;13(252):2. [Google Scholar]
- 46.Ekwochi U., Asinobi N.I., Osuorah C.D., Ndu I.K., Ifediora C., Amadi O.F. Incidence and predictors of mortality among newborns with perinatal asphyxia: a 4-year prospective study of newborns delivered in health care facilities in Enugu, South-East Nigeria. Clin. Med. Insights Pediatr. 2017;11 doi: 10.1177/1179556517746646. 1179556517746646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aliyu I., Lawal T.O., Onankpa B. Prevalence and outcome of perinatal asphyxia: our experience in a semi-urban setting. Trop. J. Med. Res. 2017;20(2):161. [Google Scholar]
- 48.Ilah B.G., Aminu M.S., Musa A., Adelakun M.B., Adeniji A.O., Kolawole T. Prevalence and risk factors for perinatal asphyxia as seen at a specialist hospital in Gusau, Nigeria. Sub-Saharan Afr. J. Med. 2015;2(2):64. [Google Scholar]
- 49.West B., Opara P. Perinatal asphyxia in a specialist hospital in Port Harcourt, Nigeria. Niger. J. Paediatr. 2013;40(3):206–210. [Google Scholar]
- 50.Onyiriuka A.N. Birth asphyxia in a Nigerian mission hospital in Benin city. Trop. J. Obstet. Gynaecol. 2009;26(1):80–88. [Google Scholar]
- 51.Ugwu G.M., Abedi H., Ugwu E. Incidence of birth asphyxia as seen in central hospital and GN children’s clinic both in Warri Niger Delta of Nigeria: an eight year retrospective review. Global J. Health Sci. 2012;4(5):140. doi: 10.5539/gjhs.v4n5p140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Egharevba O., Kayode-Adedeji B., Alikah S. Perinatal asphyxia in a rural Nigerian hospital: incidence and determinants of early outcome. J. Neonatal Perinat. Med. 2018;(Preprint):1–5. doi: 10.3233/NPM-1759. [DOI] [PubMed] [Google Scholar]
- 53.Tewesa E., Chirwa E., Majamanda M.D., Maluwa A., Chimwaza A. Associative factors for birth asphyxia at queen Elizabeth central hospital—Malawi. J. Biosci. Med. 2017;5:22. (05) [Google Scholar]
- 54.Yadav S., Shah G.S., Poudel P., Mishra O.P. Risk factors for adverse outcome in asphyxiated new born in Eastern Nepal. Int. J. Community Med. Public Health. 2017;3(6):1419–1423. [Google Scholar]
- 55.Gane B., Bhat V., Rao R. Antenatal and intrapartum risk factors for perinatal asphyxia: a case control study. Curr. Pediatr. Res. 2013 [Google Scholar]
- 56.Yelamali B., Panigatti P., Pol R., Talawar K., Naik S., Badakali A. Outcome of newborn with birth asphyxia in tertiary care hospital-a retrospective study. Med. Innov. 2014;3(2):59–64. [Google Scholar]
- 57.Pattar R., Raj A., Yelamali B. Incidence of multiorgan dysfunction in perinatal asphyxia. Int. J. Contemp. Pediatric. 2015;2(4):428–432. [Google Scholar]
- 58.Boskabadi H., Ashrafzadeh F., Doosti H., Zakerihamidi M. Assessment of risk factors and prognosis in asphyxiated infants. Iran. J. Pediatr. 2015;25(4) doi: 10.5812/ijp.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tabassum F., Rizvi A., Ariff S., Soofi S., Bhutta Z.A. Risk factors associated with birth asphyxia in rural district Matiari, Pakistan: a case control study. Int. J. Clin. Med. 2014;5(21):1430. [Google Scholar]
- 60.Mukhtar-Yola M., Audu L.I., Olaniyan O., Akinbi H.T., Dawodu A., Donovan E.F. Decreasing birth asphyxia: utility of statistical process control in a low-resource setting. BMJ Open Qual. 2018;7(3) doi: 10.1136/bmjoq-2017-000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Theoneste N., Angelique M. Factors contributing to birth asphyxia as the major complication among newborns delivered at gitwe district hospital, southern province, Rwanda. Int. J. Public Health Clin. Sci. 2017;4(3):142–151. [Google Scholar]
- 62.Nyishime M., Borg R., Ingabire W., Hedt-Gauthier B., Nahimana E., Gupta N. A retrospective study of neonatal case management and outcomes in rural Rwanda post implementation of a national neonatal care package for sick and small infants. BMC Pediatr. 2018;18(1):353. doi: 10.1186/s12887-018-1334-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rule A.R., Maina E., Cheruiyot D., Mueri P., Simmons J.M., Kamath-Rayne B.D. Using quality improvement to decrease birth asphyxia rates after ‘Helping Babies Breathe’training in Kenya. Acta Paediatr. 2017;106(10):1666–1673. doi: 10.1111/apa.13940. [DOI] [PubMed] [Google Scholar]
- 64.Bruckmann E.K., Velaphi S. Intrapartum asphyxia and hypoxic ischaemic encephalopathy in a public hospital: incidence and predictors of poor outcome. S. Afr. Med. J. 2015;105(4):298–303. doi: 10.7196/samj.9140. [DOI] [PubMed] [Google Scholar]
- 65.Buchmann E., Pattinson R., Nyathikazi N. Intrapartum-related birth asphyxia in South Africa lessons from the first national perinatal care survey. S. Afr. Med. J. 2002;92(11):897–901. [PubMed] [Google Scholar]
- 66.Padayachee N. Johannesburg Academic Hospital; 2015. Outcomes of Neonates with Perinatal Asphyxia at Charlotte Maxeke. (CMJAH) from 2007-2011. [Google Scholar]
- 67.Elhassan E.M., Hassanb A.A., Mirghani O.A., Adam I. Morbidity and mortality pattern of neonates admitted into nursery unit in Wad medani hospital, Sudan. Sudan J. Med. Sci. 2010;5(1) [Google Scholar]
- 68.Kaye D. Antenatal and intrapartum risk factors for birth asphyxia among emergency obstetric referrals in Mulago Hospital, Kampala, Uganda. East Afr. Med. J. 2003;80(3):140–143. doi: 10.4314/eamj.v80i3.8683. [DOI] [PubMed] [Google Scholar]
- 69.Mwanza N. 2014. Determinants of Admission for Birth Asphyxia Among Term Babies Admitted to NICU at Zambia university Teaching Hospital. [Google Scholar]
- 70.Halloran D., McClure E., Chakraborty H., Chomba E., Wright L., Carlo W. Birth asphyxia survivors in a developing country. J. Perinatol. 2009;29(3):243. doi: 10.1038/jp.2008.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Abdo RA, Halil HM, Kebede BA, Anshebo AA, Gejo NG. Birth Asphyxia and its Contributing Factors Among the Neonates Delivered at Negest Eleni Mohammed Memorial Teaching Hospital, Southern Ethiopia: A Cross-Sectional Study. [DOI] [PMC free article] [PubMed]
- 72.Enweronu-Laryea C.C., Adedemy J.-D., Okonkwo I.R., Lee A.C., Robertson N.J. International perspectives: birth-associated neonatal encephalopathy: postresuscitation care in West african newborns. NeoReviews. 2018;19(9):e507–e515. [Google Scholar]
- 73.Dmu P.D.B., Adhikari P.M., Bolton P.K., Cooper P.P., Ducasse D.G., Coovadia P.A. Association SAP; 2004. South African Handbook of Rescusitation of the Newborn. editor. [Google Scholar]
- 74.Kidanto H.L., Mogren I., van Roosmalen J., Thomas A.N., Massawe S.N., Nystrom L. Introduction of a qualitative perinatal audit at muhimbili national hospital, dar es salaam, Tanzania. BMC Pregnancy Childbirth. 2009;9(1):45. doi: 10.1186/1471-2393-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Munjanja S.P., Magure T., Kandawasvika G. 2012. 11 Geographical Access, Transport and Referral Systems. Maternal and Perinatal Health in Developing Countries; p. 139. [Google Scholar]
- 76.Målqvist M., Sohel N., Do T.T., Eriksson L., Persson L.-Å. Distance decay in delivery care utilisation associated with neonatal mortality. A case referent study in northern Vietnam. BMC Publ. Health. 2010;10(1):762. doi: 10.1186/1471-2458-10-762. [DOI] [PMC free article] [PubMed] [Google Scholar]