Abstract
Including carbohydrate/fructose-rich foods (predominantly fruit) in the diets of overweight individuals can improve chronic disease risk factors. We hypothesized dried plums (DP) would improve nutrient consumption, total antioxidant capacity (TAC), lipid and adipokine profiles, and would decrease adiposity and inflammation. To test this, we studied the effects of 8-weeks of twice-daily snacking of macronutrient-matched 100 kcal servings of DP or refined carbohydrate-rich snack (low-fat muffins: LFM) on daily energy and nutrient consumption, and chronic disease risk factors in overweight adults. Body weight/composition, waist circumference, blood pressure, plasma glucose, insulin, c-peptide, lipids, TAC, adipokines and inflammation were measured at baseline and throughout the study. Postprandial glucose and insulin were assessed following assigned test foods at baseline and 8-weeks. Repeated measures ANOVAs were undertaken to examine group and time differences. Post-hoc independent and paired samples t-tests were conducted where necessary. DP increased (P < .05) overall intake of dietary fiber and potassium, and TAC, from baseline to 8-weeks. Baseline postprandial glycemia tended (P = .09) to be lower with DP versus LFM, while both groups had a decreased response after 8-weeks. Postprandial insulinemia was lower (P < .05) for DP at both time-points. No differences in body weight/composition, blood pressure, or fasting glucose, insulin, triglycerides, total cholesterol, HDL-C, inflammation or adipokines were detected. Low-density lipoprotein cholesterol (LDL-C) increased (P < .05) throughout the trial following LFM. Overall, DP lessened postprandial insulinemia, improved nutrient consumption and plasma TAC, and maintained plasma LDL-C compared to a macronutrient-matched refined carbohydrate snack, which could decrease chronic disease risk.
Keywords: Snacking, Carbohydrates, Dried Plums, Glucose Metabolism, Total Antioxidant Capacity, Cholesterol
1. Introduction
Consumption of snacks contributes substantially to daily energy intake in the United States. A recent examination of 40-year trends in self-reported eating related behaviors of adults demonstrated an increase in energy from snacks reported between lunch and dinner, as well as other snacks that replaced meals [1]. Although snacks can contribute significantly to energy intake, snacking may actually help to limit overall energy intake throughout the day and effectively alter risk of obesity/weight status of individuals. Researchers examining the relationship between eating patterns and obesity demonstrated that individuals reporting at least four eating episodes per day exhibited a 45% lower obesity risk [2]. However, the type of snack consumed plays an important role and has been shown to differentially impact risk factors for chronic disease such as plasma lipid concentrations [3].
Being overweight increases risk of developing metabolic diseases [4]. Incidence of Type 2 Diabetes Mellitus (T2DM), a key metabolic disease associated with body weight status, is now considered an epidemic, and prevalence is projected to increase considerably over the next few years [4]. Although study results are mixed [5-12], an increase in fruit consumption has been recommended as part of a strategy to prevent the onset of T2DM [6]. Discrepancies among these studies may be due, in part, to the various effects of different fruits on metabolism; thus, it is important to determine the impacts of fruits individually. In a recent analysis of three prospective longitudinal cohort studies, pooled hazard ratios of T2DM were assessed for every three servings per week for twelve different fruits [13]. Results of the analysis revealed that dried plums represented the third lowest pooled hazard ratio (0.89) among the twelve fruits. Comprehensive reviews highlight multiple potential health advantages when dried plums are incorporated within the diet [14,15]. Dried plums are rich in fiber [16] and have a low glycemic index (GI = 29 ± 4) [17]. Considering overweight adults are at increased risk for developing obesity and associated metabolic diseases, such as T2DM, strategies aimed at attenuating the risk are of significant biomedical importance.
The addition of dried plum extract to the water of obese rats decreased body weight and enhanced systemic insulin sensitivity [18]. Dried plums have also been hypothesized to enhance insulin sensitivity due to their high sorbitol (6. 17 g/100 kcals) content [19]. Our laboratory has previously demonstrated that dried plums acutely promote lower postprandial glycemic and insulinemic responses compared to a macronutrient matched low-fat cookie [20].
The nutrient composition of snack foods likely influences the overall impact that snacking has on metabolism and energy balance. Green and colleagues [21] demonstrated that a high-carbohydrate snack is more likely to promote a lower total daily energy intake compared to a high-fat snack. Furthermore, frequent meal consumption including snacks has been shown to produce beneficial effects on blood lipid profiles, suggesting a potential improvement in metabolism and overall dietary quality [22]. Since dried plums are extremely low in fat, a rich source of dietary carbohydrates, a particularly good source of fiber (pectin) and sorbitol, and can lower the glycemic index of the diet as a whole [17], dried plums may be a potentially favorable snack food.
We hypothesize that incorporation of twice daily 100 kcal servings of a whole food carbohydrate-rich snack would improve risk factors for chronic disease relative to a macronutrient-matched refined carbohydrate snack. To test this, we determined the influence of 8 weeks of twice daily consumption of 100 kcal servings of a whole food (dried plums: DP) versus refined carbohydrate snack (macronutrient-matched low-fat muffins: LFM) on risk factors related to chronic disease (waist circumference/adiposity, adipokines, glucose regulation, plasma lipids and blood pressure) as well as antioxidant capacity and overall dietary quality in apparently healthy overweight adults. Additionally, we aimed to determine the acute postprandial glucose and insulin responses to a 240 kcal serving of each snack food at baseline and following the 8-week feeding period. The rationale for the proposed work is that snacks significantly contribute to daily energy intake in the United States and the type of snacks consumed can significantly modulate risk factors for chronic disease. Overweight adults are at significantly greater risk of developing chronic disease relative to age-matched lean adults, and incorporation of carbohydrate/fructose rich foods, primarily fruits, may decrease risk factors of chronic disease. Consistent with this hypothesis, we found that twice daily snacking of 100 kcal servings of dried plums reduced and prevented risk factors for chronic disease compared to a macronutrient-matched refined carbohydrate snack.
2. Methods and materials
2.1. Recruitment/ screening procedures
Subjects were recruited from the community via electronic advertisement, and visits to the laboratory were scheduled via email or by phone. Prior to visiting the laboratory, subjects were screened for food allergies, current levels of physical activity, current medication regimen, and whether or not they had previously been diagnosed with pre-diabetes or metabolic syndrome. If subjects did not have any food allergies to the provided foods of the study, did not participate in greater than two hours per week of moderate to vigorous physical activity as assessed by the rating of perceived exertion [23], did not use metabolically altering medications, or were not previously diagnosed with pre-diabetes or metabolic syndrome, they were invited to the laboratory for further screening. If subjects chose to participate, they were asked to arrive to the laboratory following an overnight fast (10 h). Upon arrival to the laboratory, subjects were asked to read the informed consent and researchers explained the contents. Subjects were advised to ask questions for clarification. After signing the informed consent form, the International Physical Activity Questionnaire (IPAQ) [24] was completed. Researchers assigned subjects to two groups (DP or LFM) based on block randomization and stratified for body fatness. All experimental procedures were approved by the San Diego State University Institutional Review Board (Protocol #1666093) and in accordance with Federal and institutional regulations and policies for the protection of the rights and welfare of human subjects.
2.2. Study participants, design and treatment
Male (n = 19) and female (n = 30) participants between 20–65 years of age with a BMI [weight (kg)/height (m2)] defining them as overweight or obese (≥ 25) were initially recruited (Fig. 1.). Participants underwent an eight-week, parallel arm designed dietary intervention, which consisted of a twice-daily consumption of 100 kcal snacks (one between breakfast and lunch and another between lunch and dinner) along with eight ounces of water. Participants were randomly assigned to consume either DP or a LFM snacks. To ensure participant adherence, daily snack and beverage checklists were provided. Participants were assessed at baseline and after eight weeks of intervention. At those times body composition was assessed and blood was collected. At the four-week time-point, participants arrived at the laboratory to return their snack checklists and to collect the next four weeks of the assigned test food. Participants were advised to maintain their usual levels of physical activity and dietary intake throughout the duration of the study. Two multiple-pass [25] 24-hour recalls were taken at baseline and four to seven subsequent recalls were obtained on random days throughout the intervention to assess nutrient intake. Food records were analyzed using The Food Processor SQL software version 10.12.0, ESHA Research (Salem, OR). A 7-day bowel habit questionnaire [26] was completed during the final week of each intervention. Following the completion of the study, participants filled out the revised dietary restraint scale [27].
Fig. 1 –
Flow chart of sample sizes for subject recruitment, exclusion and final analyses.
2.3. Test foods
Dried plums were provided by Sunsweet Growers, Inc. (Yuba City, CA) and packaged in vacuumed sealed bags. Each bag contained 41.8 ± 0.3 grams (100 kcals) of dried plums. The LFM was designed to provide amounts of protein, carbohydrate and fat similar to those of dried plums, and the nutrient data were assessed using The Food Processor SQL software version 10.12.0, ESHA Research (Salem, OR). Dried Plums and LFM batter were sent to Q Laboratories, Inc. (Cincinnati, Ohio) for validation of protein, carbohydrate and fat content (Table 1.).
Table 1 –
Nutrient composition of 100 Calorie servings of dried plums and low-fat muffins
| Nutrient | Dried Plums | Low-Fat Muffins |
|---|---|---|
| Portion weight (g) | 41.8 | 35.0 |
| Protein (g) | 1.28 | 1.58 |
| Carbohydrates (g) | 29.5 | 23.4 |
| Dietary fiber (g) | 2.8 | 0.7 |
| Soluble fiber (g) | 0.9 | 0.2 |
| Insoluble fiber (g) | 1.6 | 0.5 |
| Sugar (g) | 15.9 | 12.3 |
| Total fat (g) | 0.28 | 0.19 |
| Saturated fat (g) | 0.04 | 0.02 |
| Potassium (mg) | 306 | 19 |
| Riboflavin (mg) | 0.08 | 0.10 |
| Niacin (mg) | 0.79 | 0.9 |
| Calcium (mg) | 18 | 58 |
Nutrient profile determined from DP and LFM batter, via sample analysis by Q laboratories, Inc. (Cincinnati, OH). For dried plums: USDA National Nutrient Database for Standard Reference, Release 21. For LFM: Calculated from recipe ingredients by Food Processor SQL software version 10.12.0.
2.4. Blood pressure
Resting blood pressure (BP) was measured on the right arm using an automatic BP cuff (Omron HEM-712C, Omron Healthcare, Inc. Bannockburn, IL) after the participant had been seated and relaxed for a minimum of 10 minutes with uncrossed legs. BP was measured until 2 readings within 5 mmHg were obtained within one minute and measurements were averaged.
2.5. Body composition testing
Body composition was assessed via air displacement plethysmography (Bod Pod© Gold Standard, Life Measurement, Inc. Concord, CA), multi frequency- bioelectrical impedance analysis (BIA) (InBody 520, Biospace, Inc., Beverly Hills, CA), and by dual x-ray absorptiometry (DEXA) (GE Healthcare, Waukesha, WI) after an overnight fast (10 h) while wearing a swimsuit and following a euhydration protocol (avoiding showering or bathing 12 hours prior to assessment, consuming 32 ounces of water before bed and 24 ounces of water upon waking). Body composition was calculated using a 4-compartment model using the following equations: Fat mass (kg) = 2.747 x Total Body Volume (Bod Pod) − 0.714 x Total Body Water (BIA) + 1.146 X Bone Mineral Content (DXA) − 2.050 x mass [28]. Waist circumference was measured in triplicate, by the same researcher at baseline and 8-weeks for each subject, and averaged.
2.6. Assessment of bowel habits
Subjects rated total number of bowel movements, stool consistency, straining during bowel movement, pain during bowel movement, completeness of evacuation, and overall feeling of constipation using the questionnaire of McRorie et al. (28).
2.7. Acute metabolic responses
At baseline and after 8 weeks, subjects underwent a metabolic response test, following a 12 hour overnight fast, which consisted of consuming 200 kcals of their assigned test food. Fasting arterialized blood samples (1 mL) were collected by fingertip puncture (Clarity Lancets, Diagnostic Test Group, DTG-SL23100, Boca Raton, FL) before the consumption of the test food and at 15 minute intervals for two hours following initiation of consumption. Blood was collected in tubes containing lithium heparin (Ram Scientific, #077220, Yonkers, NY) and centrifuged at 1200 x g for 10 minutes at 4 °C for plasma separation. Plasma samples were used for assessment of glucose and insulin.
2.8. Venous blood collection
Blood samples were collected at baseline and after 4 and 8 weeks. Blood was collected using a 25-gauge butterfly needle and the BD Vacutainer tubes containing EDTA (Fisher Scientific, Pittsburgh, PA). Tubes were centrifuged at 1200 x g for 10 minutes at 4 °C and plasma was extracted. Samples were coded to ensure participant confidentiality and were stored at −80 °C for future batch analysis.
2.9. Biochemical assays
Insulin (Alpco Diagnostics, Salem, NH; 80-INSHU-E011), C-peptide (Alpco Diagnostics, Salem, NH; 80-CPTHU-E01.1), high molecular weight (HMW) adiponectin (ALPCO Diagnostics, Salem, NH; 80-ADPHU-E01), leptin (R&D Systems, Minneapolis, MN; DLP00), and high sensitivity C-reactive protein (hsCRP) (Calbiotech, Spring Valley, CA; CR120C) were assessed using enzyme linked immunosorbent assays (ELISA) according to manufacturer guidelines. The homeostasis model of insulin resistance (HOMA-IR) was calculated as previously described (31). Triglycerides (TG) (Stanbio Laboratory, Boerne, TX; 2 100 430), total cholesterol (TC) (Stanbio Laboratory, Boerne, TX; 1 010 430), high density lipoprotein-cholesterol (HDL-C) (Stanbio Laboratory, Boerne, TX; 1 010 225) and glucose (Stanbio Laboratory, Boerne, TX; 1 070 125) were assessed using colorimetric kits according to manufacturer guidelines. Low density lipoprotein-cholesterol (LDL-C) was calculated using the Friedewald equation [LDL-C = TC - HDLC – TG/ 5] [29]. Total antioxidant capacity was determined with the use of an antioxidant assay kit according to manufacturer guidelines (Sigma, no. CS0790).
2.10. Statistical analyses
Based on previous work from our laboratory [20] in healthy subjects who ate dried plums for a shorter duration (two weeks), and considering changes in plasma triglycerides was a primary outcome of this study, the number of subjects anticipated to detect a difference in plasma triglyceride concentrations utilizing a parallel arm design is 20 per group to achieve 80% power with an alpha of .05. This calculation used conservative estimates of triglyceride difference of 22 ± 29 mg/dL.
Statistical analyses were conducted with Graph Pad Prism version 7.0c (Graph Pad Software; La Jolla, CA, USA) and data were reported as means ± standard deviations. One-way analyses of variance (ANOVA) were used to determine differences in baseline measurements among groups. 2 (snack type) x 3 (time points) repeated measures ANOVA were used to assess differences in body weight, body composition, waist circumference, fasting glucose, fasting insulin, HOMA-IR, fasting C-peptide, fasting hs-CRP, fasting leptin, fasting HMW-adiponectin, and fasting TG, TC, HDL-C and LDL-C, over time between groups, with least significant differences for post hoc analyses when appropriate. Additionally, post hoc 2-tailed, paired t tests were used to determine within group mean differences at various time points when appropriate. Effects of age, sex and baseline values were independently analyzed for all variables, and no effects were detected.
For the acute feeding experiments, area under the curve was calculated from baseline values using the trapezoidal method, subtracting any areas that fell below baseline. and areas under the curves for over time between groups. 2 (snack type) x 2 (time points) repeated measures ANOVA were used to assess glucose and insulin AUC, over time between groups, with least significant differences for post hoc analyses when appropriate. Additionally, post hoc 2-tailed, paired t tests were used to determine within group mean differences. 2 (snack groups) x 7 (time points) repeated measures ANOVA, followed by paired t-tests for post hoc analyses when needed used to assess differences over time for glucose and insulin during the acute feeding experiments. Post-hoc independent samples t-tests were conducted to determine between group differences and paired-samples t-tests were used within groups when appropriate. Alpha was set at 0.05 and significance was determined at P < .05.
3. Results
3.1. Subjects
Two subjects from each group withdrew from the study prior to completion due to personal conflicts. The entire study was completed by 45 subjects (Fig. 1.), which allowed sufficient sample size to power our analyses. The subjects were 37.4 ± 11.9 y of age, and had a mean BMI of 33.0 ± 6.9. Twenty-nine of the 45 subjects were classified as unrestrained eaters and twenty were classified as restrained eaters as determined by the revised restraint scale [27] completed by the subjects at the onset of the study. No differences in responses were detected for restrained versus unrestrained eaters, so data were collapsed for both groups.
3.2. Body weight, body composition, waist circumference and blood pressure
There were no differences over time in either group for weight, percent body fat, fat free mass, waist circumference, or blood pressure (Table 2.).
Table 2 –
Characteristics of subjects at baseline and 8 weeks
| DP |
LFM |
|||
|---|---|---|---|---|
| Baseline | 8 Weeks | Baseline | 8 Weeks | |
| Age (years) | 35.3 ± 12.0 | 39.6 ± 11.1 | ||
| Height (m) | 16.9 ± 1.0 | 16.5 ± 1.0 | ||
| Total Body Weight (kg) | 94.4 ± 21.8 | 95.5 ± 21.4 | 79.3 ± 16.1 | 81.8 ± 17.7 |
| BMI (Body weight (kg)/ Height (m)2) | 33.1 ± 21.8 | 33.4 ± 21.4 | 29.1 ± 16.1 | 30.0 ± 16.1 |
| Fat-Free Mass (kg) | 56.0 ± 12.9 | 55.5 ± 14.0 | 46.3 ± 9.9 | 47.8 ± 12.0 |
| Body Fat (%) | 38.5 ± 13.0 | 38.6 ± 13.4 | 39.2 ± 15.6 | 37.3 ± 18.1 |
| Waist Circumference (cm) | 110.0 ± 19.6 | 111.3 ± 17.8 | 102.6 ± 12.3 | 99.1 ± 12.7 |
| SBP (mm/Hg) | 125.5 ± 14.4 | 125.3 ± 13.7 | 122.4 ± 14.6 | 121.3 ± 19.1 |
| DBP (mm/Hg) | 82.0 ± 12.8 | 80.6 ± 11.0 | 78.0 ± 10.6 | 78.5 ± 12.9 |
BMI: Body Mass Index; SBP: Sytolic Blood Pressure; DBP: Diastolic Blood Pressure. Values are means ± standard deviations. (DP: n = 24 [males: 9; females: 15]; LFM: n = 21 [males:10; females 11]).
3.3. Dietary intake
There were no mean differences between groups in total energy, macronutrient, and micronutrient intake with the exceptions of higher (P < .05) consumption of potassium and fiber for the DP intervention (Table 3). Importantly, dietary intake data were analyzed for a subgroup of subjects (n = 16/ group) due to a low response rate to phone calls and low compliance to self-reporting food recalls.
Table 3 –
Dietary intake before and after 8 weeks of twice daily snacking of 100 Calories of DP or LFM
| Nutrient |
DP |
LFM |
||
|---|---|---|---|---|
| Daily Intake | Baseline | 8 Weeks | Baseline | 8 Weeks |
| Total energy (kcal) | 1911 ± 695 | 1940 ± 703 | 1903 ± 671 | 1933 ± 674 |
| Protein (g) | 77.4 ± 35.9 | 78.1 ± 36.4 | 76.9 ± 36.4 | 77.2 ± 35.7 |
| Carbohydrates (g) | 234 ± 95 | 238 ± 95.0 | 234 ± 91 | 239 ± 93 |
| Dietary fiber (g) | 16.3 ± 7.3 | 22.12 ± 8.3* | 17.2 ± 7.3 | 18.0 ± 8.3 |
| Sugar (g) | 93.8 ± 48.3 | 94.9 ± 49.7 | 94.2 ± 49.5 | 94.3 ± 48.3 |
| Total fat (g) | 75.5 ± 38.7 | 76.7 ± 39.6 | 74.8 ± 38.6 | 75.8 ± 38.1 |
| Saturated Fat (g) | 23.8 ± 14.9 | 24.1 ± 15.3 | 23.7 ± 15.3 | 23.5 ± 14.9 |
| MUFAa (g) | 17.8 ± 14.2 | 18.5 ± 14.3 | 16.8 ± 13.5 | 17.22 ± 13.4 |
| PUFAb (g) | 9.1 ± 7.9 | 9.5 ± 8.0 | 8.8 ± 7.6 | 9.1 ± 7.5 |
| Cholesterol (mg) | 266 ± 163 | 265 ± 160 | 262 ± 164 | 256 ± 164 |
| Vitamin A (IU) | 6914 ± 10 172 | 6778 ± 10 271 | 7079 ± 10 472 | 7053 ± 10 223 |
| Thiamin (mg) | 0.9 ± 0.6 | 0.9 ± 0.6 | 0.9 ± 0.6 | 0.9 ± 0.6 |
| Riboflavin (mg) | 1.1 ± 0.8 | 1.1 ± 0.8 | 1.1 ± 0.8 | 1.1 ± 0.8 |
| Niacin (mg) | 15.5 ± 10.3 | 15.8 ± 10.0 | 15.5 ± 9.9 | 15.8 ± 10.0 |
| Vitamin B6 (mg) | 11.7 ± 8.2 | 12.0 ± 7.9 | 12.0 ± 7.9 | 12.2 ± 8.0 |
| Vitamin B12 (μg) | 3.0 ± 2.8 | 2.9 ± 2.3 | 3.1 ± 2.6 | 3.2 ± 2.7 |
| Vitamin C (mg) | 57.7 ± 83.2 | 67.0 ± 85.8 | 58.1 ± 50.7 | 59.33 ± 50.2 |
| Vitamin D (IU) | 62.7 ± 83.0 | 62.3 ± 67.7 | 65.8 ± 79.3 | 68.6 ± 83.0 |
| Vitamin E (IU) | 7.2 ± 6.2 | 7.5 ± 6.2 | 7.0 ± 5.8 | 7.3 ± 5.9 |
| Folate (μg) | 230 ± 162 | 232 ± 142 | 233 ± 149 | 241 ± 160 |
| Vitamin K (μg) | 52.2 ± 48.9 | 51.8 ± 50.0 | 51.4 ± 49.9 | 51.7 ± 49.1 |
| Calcium (mg) | 682 ± 327 | 681 ± 318 | 687 ± 311 | 695 ± 315 |
| Iron (mg) | 11.9 ± 6.8 | 12.0 ± 5.8 | 11.9 ± 6.1 | 12.4 ± 6.6 |
| Magnesium (mg) | 166 ± 96 | 172 ± 94 | 166 ± 86 | 170 ± 89 |
| Manganese (mg) | 1.5 ± 0.9 | 1.5 ± 0.9 | 1.4 ± 0.8 | 1.5 ± 0.9 |
| Phosphorus (mg) | 674 ± 414 | 686 ± 423 | 660 ± 409 | 659 ± 400 |
| Potassium (mg) | 1435 ± 570 | 2157 ± 641* | 1722 ± 1330 | 1347 ± 593 |
| Zinc (mg) | 6.2 ± 4.8 | 6.3 ± 4.6 | 6.3 ± 4.6 | 6.5 ± 4.8 |
MUFA: Monounsaturated fatty acid
PUFA: Polyunsaturated fatty acid. Values with (*) represent significant (P < .05) same group, between time-point differences. Values are means ± standard deviations. (DP: n = 16; LFM: n = 16).
3.4. Biochemical analyses
C-peptide was ~20% higher after 8 weeks in the DP group (P < .05). No differences in C-peptide were detected between the DP and LFM groups, or over time in the LFM group. No differences in fasting plasma insulin, glucose, HOMA-IR, high-sensitivity C-reactive protein, hsCRP, leptin, HMW-adiponectin, triglycerides, total cholesterol or HDL-C were observed between or within groups across time points (Table 4). No differences were detected for time or group for leptin, hsCRP, insulin, HOMA-IR, and HMW-adiponectin. LDL-C was ~25% (P < .05) and ~30% (P < .05) greater at 4 and 8 weeks, respectively, in the LFM group compared to DP (Table 4.). Fasting total antioxidant capacity was increased from baseline to 8 weeks in the DP group, while no difference was detected in the LFM group (Table 4.).
Table 4 –
Plasma biochemical profiles at baseline, and 4 weeks and 8 weeks following twice daily snacking of 100 Calories of DP or LFM
| Variable | Baseline | DP |
Baseline | LFM |
||
|---|---|---|---|---|---|---|
| 4 Weeks | 8 Weeks | 4 Weeks | 8 Weeks | |||
| Insulin (μIU/mL) | 2.09 ± 0.55 | 2.18 ± 0.48 | 2.39 ± 1.65 | 2.07 ± 0.61 | 2.08 ± 0.58 | 1.95 ± 0.31 |
| Glucose (mg/dL) | 94.6 ± 14.4 | 99.0 ± 11.1 | 95.7 ± 11.8 | 95.1 ± 10.8 | 91.5 ± 13.7 | 96.6 ± 16.8 |
| HOMA-IR | 0.50 ± 0.22 | 0.54 ± 0.15 | 0.56 ± 0.38 | 0.50 ± 0.20 | 0.51 ± 0.21 | 0.47 ± 0.13 |
| C-Peptide (ng/mL) | 6.54 ± 3.12a | 7.19 ± 3.27ab | 8.10 ± 4.23b | 6.47 ± 3.66 | 8.09 ± 4.46 | 8.18 ± 5.06 |
| HS-CRP (mg/L) | 5.01 ± 1.99 | 4.99 ± 2.22 | 4.75 ± 2.19 | 3.70 ± 1.71 | 3.53 ± 1.40 | 3.53 ± 1.35 |
| Leptin (pg/mL) | 374.7 ± 173.6 | 367.9 ± 161.9 | 426.8 ± 216.8 | 396.8 ± 254.4 | 437.6 ± 200.1 | 414.7 ± 251.4 |
| HMW- Adiponectin (ng/mL) | 3.55 ± 1.48 | 3.43 ± 1.7 | 3.33 ± 1.36 | 4.54 ± 2.55 | 4.56 ± 2.45 | 4.22 ± 2.20 |
| Triglycerides (mg/dL) | 139.3 ± 22.5 | 137.4 ± 26.9 | 136.7 ± 31.9 | 138.7 ± 21.0 | 135.7 ± 30.0 | 140.2 ± 27.2 |
| Total Cholesterol (mg/dL) | 144.1 ± 27.9 | 146.8 ± 26.1 | 140.9 ± 26.9 | 163.1 ± 28.6 | 164.1 ± 31.9 | 162.6 ± 38.3 |
| HDL-C (mg/dL) | 45.1 ± 10.6 | 41.0 ± 11.0 | 44.3 ± 11.3 | 50.2 ± 13.3 | 44.8 ± 10.8 | 48.1 ± 17.2 |
| LDL-C (mg/dL) | 78.3 ± 22.2 | 71.1 ± 23.4# | 66.8 ± 14.5# | 78.8 ± 23.8 | 97.5 ± 36.8 | 91.3 ± 40.5 |
| LDL-C: HDL-C | 1.6 ± 0.7a | 1.9 ± 0.8#b | 1.5 ± 0.6#a | 1.6 ± 0.8a | 2.2 ± 1.1b | 1.9 ± 2.2b |
| Total Antioxidant Capacity (mM) | 1.40 ± 0.5a | N/A | 1.90 ± 0.4b | 1.5 ± 0.4 | N/A | 1.6 ± 0.4 |
HOMA-IR: Homeostatic model assessment of insulin resistance; HS-CRP: High sensitivity- C reactive protein; HMW- Adiponectin: High molecular weight- Adiponectin; HDL-C: High density lipoprotein-Cholesterol; LDL-C: Low density lipoprotein- Cholesterol. Values with (#) represent same time-point, between group differences (P < .05). Values with different letter superscripts represent same group, between time-point differences (P < .05). Values are means ± standard deviations. (DP: n = 24; LFM: n = 21).
3.5. Acute metabolic responses
Area under the curve for postprandial glycemic response tended (P = .09) to be greater at baseline for the LFM group (Fig. 2, A). Glycemic response area under the curve was decreased (P < .05) after eight weeks of adaptation to consuming either DP or LFM (Fig. 2, A-C). Postprandial insulin responses were lower (P < .05) for DP versus LFM at baseline and after 8 weeks (Fig. 2, D-F).
Fig. 2 –
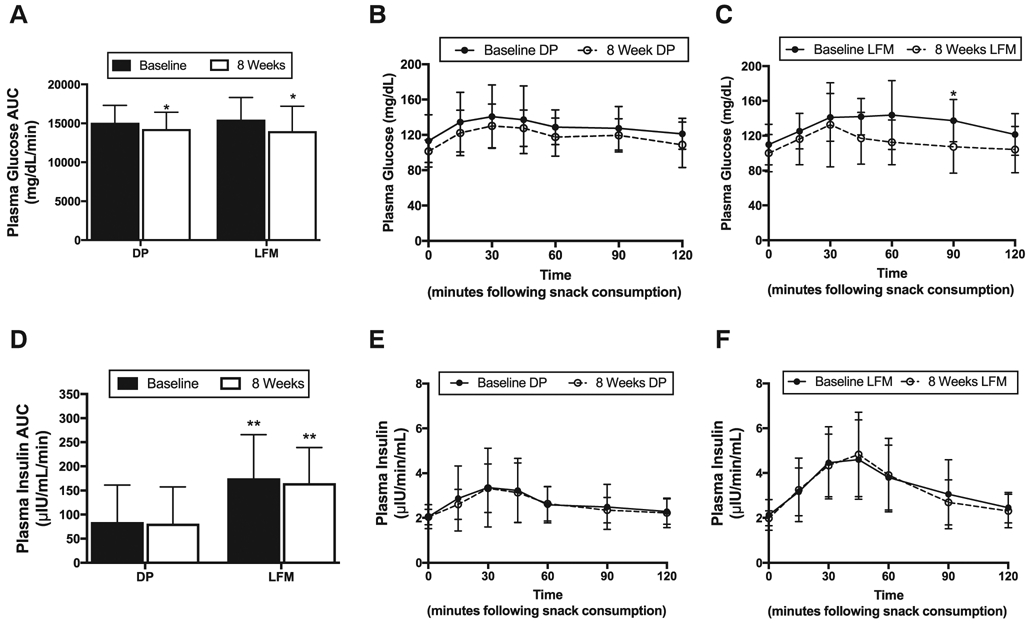
(A) Plasma glucose AUC at baseline (closed bars) and 8 weeks (open bars) in the DP and LFM groups. (B) Glucose responses to dried plums at baseline (•) and 8-weeks (°), and (C) low-fat muffins at baseline (•) and 8-weeks (°). (D) Plasma insulin AUC at baseline (closed bars) and 8 weeks (open bars) in the DP and LFM groups. (E) Plasma insulin responses to dried plums at baseline (•) and 8-weeks (°), and (F) low-fat muffins at baseline (•) and 8-weeks (°). Significant (P < .05) within group differences are indicated by a single asterisk (*) and significant between group differences are indicated by two asterisks (**). Values are means ± SD. Error bars reflect standard deviations. (DP: n = 24; LFM: n = 21).
3.6. Bowel habits
No mean differences in bowel habit data were detected for total bowel movements, stool consistency, straining during bowel movement, pain during bowel movement, completeness of evacuation, or overall feeling of constipation (Fig. 3. A-E).
Fig. 3 –
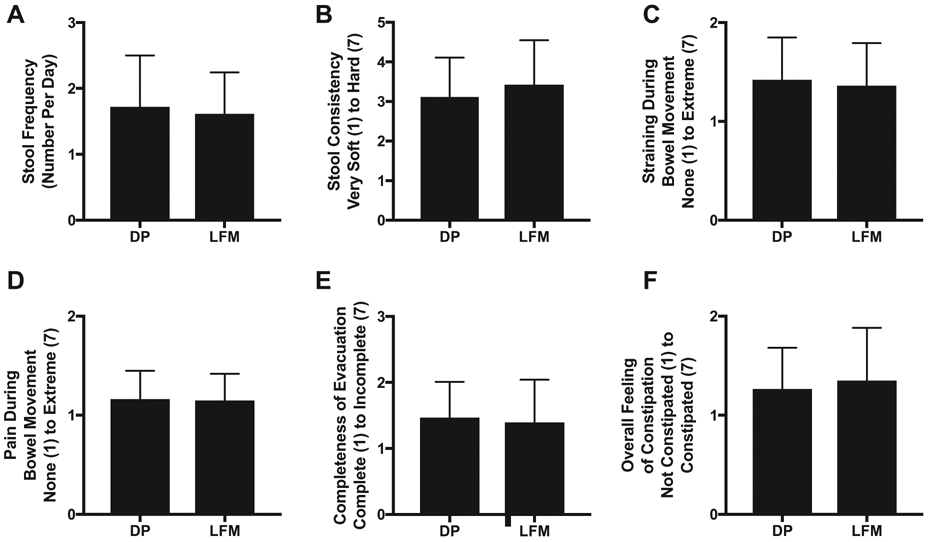
Bowel habit ratings during the final week (7 days recorded) of the 8-week intervention of consumption of either DP or LFM. (A) Stool Frequency, (B) Stool Consistency, (C) Straining During Bowel Movement, (D) Pain During Bowel Movement, (E) Completeness of Evacuation and (F) Overall Feeling of Constipation were rated on 7-point scales. Values are means ± SD. Error bars reflect standard deviations. (DP: n = 24; LFM: n = 21).
4. Discussion
Results of this study only partially support our key hypotheses; therefore, we partially reject the original hypotheses. Consuming a DP snack twice daily for eight weeks resulted in greater total daily intake of fiber and potassium and improved fasting total antioxidant capacity compared to LFM. Furthermore, DP resulted in lower postprandial insulin responses compared to LFM at baseline and 8 weeks, but there no differences in the postprandial insulin response over time within each group. Interestingly, however, these data indicate that regular consumption of a carbohydrate-rich snack, whether DP or LFM, tends to improve acute glycemic responses to that snack over eight weeks. Moreover, DP lowered plasma LDL-C at four and eight weeks, which resulted in lower LDL-C:HDL-C, relative to LFM, throughout the trial. Lastly, data collected from the Bowel Habits Questionnaire support our hypothesis, and previous work from our laboratory collected in adult women [3], that dried plums do not significantly influence bowel habits. Our hypotheses were not supported, in that dried plums did not influence plasma TG, daily fat and cholesterol intake, body weight, body composition, and concentration of plasma inflammatory markers, or adipokines.
Although we did not detect between group differences for total energy, macronutrient, or micronutrient intake, we did observe a significant increase in potassium intake in the DP group throughout the 8-week intervention. The increase in dietary potassium observed in the present study is in agreement with previous research from our laboratory [3]. Interestingly, dietary intake of potassium may be associated with the decreased insulin response observed in the DP group [30,31]. Thus, incorporating twice daily consumption of dried plums into one’s typical dietary regimen may help decrease chronic disease risk through improved potassium intake.
As described in the analysis of data from NHANES, 1999–2010 by Grooms et al. [32], an increase in dietary fiber is associated with a decreased risk for metabolic disease, primarily due to regulation of insulin and blood lipids [32]. The portion of dried plums used for the current study provided approximately 6 g of fiber per day (2.67 g/ 100 kcals), whereas the LFM provided approximately 1.5 g of fiber per day (0.7 g/100 kcals). Dietary fiber has been suggested to regulate postprandial glycemia and insulinemia by slowing the rates of gastric emptying and absorption of macronutrients, which results in a slower appearance of glucose into the blood stream, decreasing the potential for lipogenesis and storage of glucose as triglycerides [33], which may be particularly favorable for overweight adults who are at greater risk of developing metabolic diseases relative to lean [34]. The difference in postprandial insulin responses observed between the DP and LFM groups at both baseline and after the 8-week intervention may support the idea that increased dietary fiber supports regulation of blood insulin concentration [32]. Furthermore, the low fiber composition of the LFM snack may have contributed to the elevated LDL:C-HDL:C, as low fiber diets are inversely associated with LDL-C [35]. Dried plums also contain other nutrients such as sorbitol and phytonutrients that may be at least partially responsible for the differences in postprandial insulinemia observed in this study.
The sugar composition of dried plums are glucose (45%), fructose (25%), sucrose (1%) and sorbitol (29%) [15]. Sorbitol is a sugar alcohol found naturally in many fruits and constitutes a large portion of the sugar composition of dried plums [15]. Sorbitol is poorly absorbed [36] and the consumption of sorbitol with fructose further decreases the absorption of both sugars [37]; thus, the fructose and sorbitol contents of dried plums may potentially decrease absorption of carbohydrate resulting in a more stable insulin response. Furthermore, the high composition of chlorogenic acid found in dried plums (23% of total phenolic compounds) [15] may contribute to the insulin response observed in the current study. Chlorogenic acid has been shown to inhibit hepatic glycogenolysis and gluconeogenesis, thus resulting in decreased appearance of glucose in the blood [38]
According to the international table of glycemic index (GI) [17] dried plums should be considered a low-GI food (GI = 29 ± 4). In a review of the chemical composition and potential health effects of dried plums [15], it was suggested that the fiber, fructose, and sorbitol contents of dried plums are likely responsible for considering dried plums a low-GI food. The low-GI of dried plums likely explains the trend for a lower postprandial glycemic response at baseline relative to the LFM group and the lower insulin response detected at both time-points in the present study. Interestingly, however, we found that twice daily snacking of DP or LFM for eight weeks resulted in an improved postprandial glycemic response. Previous reports, although in children, have shown that metabolic flexibility can occur across various high carbohydrate diets, resulting in a metabolic adaptation to those diets, specifically when high carbohydrate diets do not vary in macronutrient content [39], which is likely what occurred in the present study. We observed an increase in plasma C-peptide at the end of the 8-week intervention in the DP group, while no differences were detected in the LFM group. Although the production of C-peptide and insulin are tightly coupled, C-peptide is excreted at a more constant rate over a longer period of time [40], which may explain the discrepancy between the fasting C-peptide and insulin data in the current study.
Despite the potential for adaptation to consistently consuming a carbohydrate rich food, consumption of high glycemic index (GI) carbohydrates appears to be a greater risk factor for metabolic disease compared to energy matched low GI carbohydrates [41]. Low GI foods have been shown to promote greater satiety and lower postprandial insulinemia compared to high-GI foods, demonstrating the potential role of eating low-GI foods as a strategy for attenuating progression of metabolic disease [15]. Thus, low-GI snacks rich in nutrients, such as dried plums, may be a more favorable option than high-GI snacks, as a means of regulating blood glucose and insulin concentrations between meals.
Polyphenols, key nutrients in dried plums (Neochlorogenic acid, Chlorogenic acid, Caffeic acid, Coumaric acid and Rutin) and their metabolites are known to not only act as antioxidants themselves, but also activate endogenous antioxidant signaling pathways [42-44]. Furthermore, increased abundance of oxygen-free radicals in the blood have been associated with marked elevated levels of plasma LDL-C [45]. Considering dried plums are a concentrated source of polyphenols, frequent consumption may elevate total antioxidant capacity in the blood, and over time could attenuate the development of metabolic disease, and prevent oxidation of LDL-C [46]. The increase in fasting plasma total antioxidant capacity in the present study supports previous in vitro studies [47] suggesting polyphenol-rich dried plums may be capable of reducing oxidative stress. Diets rich in antioxidants are associated with lower risk of developing metabolic disease [48], thus implementing dried plums into a daily snacking regimen may help to prevent the onset of metabolic disease, and specifically dyslipidemia, given the LDL-C lowering effects of dried plums in the present study.
There are several possible limitations in the current study. First, the population was restricted to overweight adults, which limits extrapolation to other populations. Second, the study was not designed to evaluate metabolic altering effects of specific bioactive compounds present in dried plum vs. the low-fat muffin, and future studies should be designed to evaluate this. Third, completely accurate food intake data is very difficult to obtain. We attempted to minimize inaccuracies by using the multi-pass method of food intake assessment. Fourth, all snack intake, despite the acute feeding experiments, occurred out of the laboratory, the self-reported compliance of snack consumption might not be completely reliable.
Overall, the inclusion of a low glycemic, high fiber, dried plum snack significantly increased total dietary intake of fiber and potassium, and resulted in a lower postprandial insulin response, following acute feeding compared to a more refined, carbohydrate-rich snack. Furthermore, lower plasma concentrations of LDL-C were detected in as few as 4 weeks after consuming DP in comparison to a refined snack, which was also reflected in the LDL-C:HDL-C ratio. While the sorbitol, fiber and polyphenol contents of DP likely explain the majority of their differing metabolic responses, future research may be needed to determine if other components are responsible. Taken together, this research suggests that a whole dried fruit snack appears to be a modestly better choice than a more highly refined snack with a similar macronutrient profile and could help to attenuate the onset of metabolic disease in overweight adults.
Acknowledgment
Authors would like to thank Steffani Nelson, Nancy Snyder, Benjamin Reiter, Jonathan Blair and Brookell White for help with the study. The views expressed in this manuscript do not reflect the official policy or position of the Department of the Army, Department of Defense or the U.S. Government. Authors express no conflict of interest. This work was supported by a grant from the California Dried Plum Board.
Abbreviations:
- BMI
Body Mass Index
- DP
Dried Plums
- LFM
Low-Fat Muffins
- TAC
Total Antioxidant Capacity
- ANOVA
Analysis of Variance
- TC
Total Cholesterol
- HDL-C
High Density Lipoprotein-Cholesterol
- LDL-C
Low Density Lipoprotein-Cholesterol
- TG
Triglycerides
- T2DM
Type 2 Diabetes Mellitus
- GI
Glycemic Index
- GLUT
Glucose Transporter
- Kcal
kilocalorie
- IPAQ
International Physical Activity Questionnaire
- mg/dL
milligrams per deciliter
- BP
Blood Pressure
- BIA
Bioelectrical Impedance Analysis
- EDTA
Ethylenediaminetetraacetic acid
- HMW
High Molecular Weight
- hsCRP
High-Sensitivity C-Reactive Protein
- HOMA-IR
Homeostasis Model of Insulin Resistance
- AUC
Area Under the Curve
- NHANES
National Health and Nutrition Examination Survey
REFERENCES
- [1].Kant AK, Graubard BI. 40-year trends in meal and snack eating behaviors of American adults. J Acad Nutr Diet 2015; 115:50–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ma Y, Bertone ER, Stanek EJ, Reed GW, Hebert JR, Cohen NL, et al. Association between eating patterns and obesity in a free-living US adult population. Am J Epidemiol 2003;158: 85–92. [DOI] [PubMed] [Google Scholar]
- [3].Howarth L, Petrisko Y, Furchner-Evanson A, Nemoseck T, Kern M. Snack selection influences nutrient intake, triglycerides, and bowel habits of adult women: a pilot study. J Am Diet Assoc 2010;110:1322–7. [DOI] [PubMed] [Google Scholar]
- [4].Bullard KM, Cowie CC, Lessem SE, Saydah SH, Menke A, Geiss LS, et al. Prevalence of diagnosed diabetes in adults by diabetes type - United States, 2016. MMWR Morb Mortal Wkly Rep 2018;67:359–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Carter P, Gray LJ, Troughton J, Khunti K, Davies MJ. Fruit and vegetable intake and incidence of type 2 diabetes mellitus: systematic review and meta-analysis. BMJ 2010;341:c4229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cooper AJ, Sharp SJ, Lentjes MA, Luben RN, Khaw KT, Wareham NJ, et al. A prospective study of the association between quantity and variety of fruit and vegetable intake and incident type 2 diabetes. Diabetes Care 2012;35:1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Cooper AJ, Forouhi NG, Ye Z, Buijsse B, Arriola L, Balkau B, et al. Fruit and vegetable intake and type 2 diabetes: EPIC-InterAct prospective study and meta-analysis. Eur J Clin Nutr 2012;66:1082–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kurotani K, Nanri A, Goto A, Mizoue T, Noda M, Kato M, et al. Vegetable and fruit intake and risk of type 2 diabetes: Japan public health center-based prospective study. Br J Nutr 2013; 109:709–17. [DOI] [PubMed] [Google Scholar]
- [9].Liu S, Serdula M, Janket SJ, Cook NR, Sesso HD, Willett WC, et al. A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care 2004;27:2993–6. [DOI] [PubMed] [Google Scholar]
- [10].Meyer KA, Kushi LH, Jacobs DR, Slavin J, Sellers TA, Folsom AR. Carbohydrates, dietary fiber, and incident type 2 diabetes in older women. Am J Clin Nutr 2000;71:921–30. [DOI] [PubMed] [Google Scholar]
- [11].Montonen J, Järvinen R, Heliövaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr 2005;59:441–8. [DOI] [PubMed] [Google Scholar]
- [12].Villegas R, Shu XO, Gao YT, Yang G, Elasy T, Li H, et al. Vegetable but not fruit consumption reduces the risk of type 2 diabetes in Chinese women. J Nutr 2008;138:574–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Muraki I, Imamura F, Manson JE, Hu FB, Willett WC, van Dam RM, et al. Fruit consumption and risk of type 2 diabetes: results from three prospective longitudinal cohort studies. BMJ 2013;347:f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Igwe EO, Charlton KE. A systematic review on the health effects of plums (Prunus domestica and Prunus salicina). Phytother Res 2016;30:701–31. [DOI] [PubMed] [Google Scholar]
- [15].Stacewicz-Sapuntzakis M Dried plums and their products: composition and health effects–an updated review. Crit Rev Food Sci Nutr 2013;53:1277–302. [DOI] [PubMed] [Google Scholar]
- [16].Miller JC. Importance of glycemic index in diabetes. Am J Clin Nutr 1994;59:747S–52S. [DOI] [PubMed] [Google Scholar]
- [17].Foster-Powell K, Holt SH, Brand-Miller JC. International table of glycemic index and glycemic load values: 2002. Am J Clin Nutr 2002;76:5–56. [DOI] [PubMed] [Google Scholar]
- [18].Utsunomiya H, Yamakawa T, Kamei J, Kadonosono K, Tanaka S. Anti-hyperglycemic effects of plum in a rat model of obesity and type 2 diabetes, Wistar fatty rat. Biomed Res 2005;26:193–200. [DOI] [PubMed] [Google Scholar]
- [19].Fryer LG, Hajduch E, Rencurel F, Salt IP, Hundal HS, Hardie DG, et al. Activation of glucose transport by AMP-activated protein kinase via stimulation of nitric oxide synthase. Diabetes 2000;49:1978–85. [DOI] [PubMed] [Google Scholar]
- [20].Furchner-Evanson A, Petrisko Y, Howarth L, Nemoseck T, Kern M. Type of snack influences satiety responses in adult women. Appetite 2010;54:564–9. [DOI] [PubMed] [Google Scholar]
- [21].Green SM, Wales JK, Lawton CL, Blundell JE. Comparison of high-fat and high-carbohydrate foods in a meal or snack on short-term fat and energy intakes in obese women. Br J Nutr 2000;84:521–30. [PubMed] [Google Scholar]
- [22].Farshchi HR, Taylor MA, Macdonald IA. Beneficial metabolic effects of regular meal frequency on dietary thermogenesis, insulin sensitivity, and fasting lipid profiles in healthy obese women. Am J Clin Nutr 2005;81:16–24. [DOI] [PubMed] [Google Scholar]
- [23].Utter AC, Nieman DC, Dumke CL, McAnulty SR, Kang J, McAnulty LS. Ratings of perceived exertion during intermittent and continuous exercise. Percept Mot Skills 2007;104: 1079–87. [DOI] [PubMed] [Google Scholar]
- [24].Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the international physical activity questionnaire short form (IPAQ-SF): a systematic review. Int J Behav Nutr Phys Act 2011;8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA automated multiple-pass method accurately estimates group total energy and nutrient intake. J Nutr 2006;136:2594–9. [DOI] [PubMed] [Google Scholar]
- [26].McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther 1998;12:491–7. [DOI] [PubMed] [Google Scholar]
- [27].Williamson DA, Martin CK, York-Crowe E, Anton SD, Redman LM, Han H, et al. Measurement of dietary restraint: validity tests of four questionnaires. Appetite 2007;48:183–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wilson JP, Strauss BJ, Fan B, Duewer FW, Shepherd JA. Improved 4-compartment body-composition model for a clinically accessible measure of total body protein. Am J Clin Nutr 2013;97:497–504. [DOI] [PubMed] [Google Scholar]
- [29].Warnick GR, Knopp RH, Fitzpatrick V, Branson L. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin Chem 1990;36:15–9. [PubMed] [Google Scholar]
- [30].Stone MS, Martyn L, Weaver CM. Potassium intake, bioavailability, hypertension, and glucose control. Nutrients 2016;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ekmekcioglu C, Prohaska C, Pomazal K, Steffan I, Schernthaner G, Marktl W. Concentrations of seven trace elements in different hematological matrices in patients with type 2 diabetes as compared to healthy controls. Biol Trace Elem Res 2001;79:205–19. [DOI] [PubMed] [Google Scholar]
- [32].Grooms KN, Ommerborn MJ, Pham DQ, Djoussé L, Clark CR. Dietary fiber intake and cardiometabolic risks among US adults, NHANES 1999–2010. Am J Med 2013;126 [1059–67.e1–4]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Nilsson AC, Ostman EM, Holst JJ, Björck IM. Including indigestible carbohydrates in the evening meal of healthy subjects improves glucose tolerance, lowers inflammatory markers, and increases satiety after a subsequent standardized breakfast. J Nutr 2008;138:732–9. [DOI] [PubMed] [Google Scholar]
- [34].Meigs JB, Wilson PW, Fox CS, Vasan RS, Nathan DM, Sullivan LM, et al. Body mass index, metabolic syndrome, and risk of type 2 diabetes or cardiovascular disease. J Clin Endocrinol Metab 2006;91:2906–12. [DOI] [PubMed] [Google Scholar]
- [35].Ho HVT, Jovanovski E, Zurbau A, Blanco Mejia S, Sievenpiper JL, Au-Yeung F, et al. A systematic review and meta-analysis of randomized controlled trials of the effect of konjac glucomannan, a viscous soluble fiber, on LDL cholesterol and the new lipid targets non-HDL cholesterol and apolipoprotein B. Am J Clin Nutr 2017;105:1239–47. [DOI] [PubMed] [Google Scholar]
- [36].Corazza GR, Strocchi A, Rossi R, Sirola D, Gasbarrini G. Sorbitol malabsorption in normal volunteers and in patients with coeliac disease. Gut 1988;29:44–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rumessen JJ, Gudmand-Høyer E. Malabsorption of fructose-sorbitol mixtures. Interactions causing abdominal distress. Scand J Gastroenterol 1987;22:431–6. [DOI] [PubMed] [Google Scholar]
- [38].Hemmerle H, Burger HJ, Below P, Schubert G, Rippel R, Schindler PW, et al. Chlorogenic acid and synthetic chlorogenic acid derivatives: novel inhibitors of hepatic glucose-6-phosphate translocase. J Med Chem 1997;40: 137–45. [DOI] [PubMed] [Google Scholar]
- [39].Treuth MS, Sunehag AL, Trautwein LM, Bier DM, Haymond MW, Butte NF. Metabolic adaptation to high-fat and high-carbohydrate diets in children and adolescents. Am J Clin Nutr 2003;77:479–89. [DOI] [PubMed] [Google Scholar]
- [40].Leighton E, Sainsbury CA, Jones GC. A practical review of C-peptide testing in diabetes. Diabetes Ther 2017;8:475–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schwingshackl L, Hoffmann G. Long-term effects of low glycemic index/load vs. high glycemic index/load diets on parameters of obesity and obesity-associated risks: a systematic review and meta-analysis. Nutr Metab Cardiovasc Dis 2013;23:699–706. [DOI] [PubMed] [Google Scholar]
- [42].Kayano S, Kikuzaki H, Fukutsuka N, Mitani T, Nakatani N. Antioxidant activity of prune (Prunus domestica L.) constituents and a new synergist. J Agric Food Chem 2002;50: 3708–12. [DOI] [PubMed] [Google Scholar]
- [43].Nakatani N, Kayano S, Kikuzaki H, Sumino K, Katagiri K, Mitani T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J Agric Food Chem 2000;48:5512–6. [DOI] [PubMed] [Google Scholar]
- [44].Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol 2006;72:1439–52. [DOI] [PubMed] [Google Scholar]
- [45].Frisard M, Ravussin E. Energy metabolism and oxidative stress: impact on the metabolic syndrome and the aging process. Endocrine 2006;29:27–32. [DOI] [PubMed] [Google Scholar]
- [46].Kay CD, Gebauer SK, West SG, Kris-Etherton PM. Pistachios increase serum antioxidants and lower serum oxidized-LDL in hypercholesterolemic adults. J Nutr 2010;140:1093–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hooshmand S, Kumar A, Zhang JY, Johnson SA, Chai SC, Arjmandi BH. Evidence for anti-inflammatory and antioxidative properties of dried plum polyphenols in macrophage RAW 264.7 cells. Food Funct 2015;6:1719–25. [DOI] [PubMed] [Google Scholar]
- [48].Gregório BM, De Souza DB, de Morais Nascimento FA, Pereira LM, Fernandes-Santos C. The potential role of antioxidants in metabolic syndrome. Curr Pharm Des 2016;22:859–69 [DOI] [PubMed] [Google Scholar]


