Abstract
Garcinia cowa Roxb. ex Choisy (Clusiaceae) is a Thai local edible plant, which has been used for the treatment of diabetes. The aim of this study is to discover and identify bioactive compounds related to antidiabetic properties from the leaf extract of G. cowa. α-Glucosidase inhibitory bioassay-guided isolation of the ethyl acetate extract of the leaves of G.cowa resulted in the isolation and identification of 11 compounds. Of these, a decahydro-1H-xanthene derivative, garciniacowone K (1), was identified as a novel compound. Their structures were characterized by spectroscopic data and by comparison of their NMR spectroscopic data with those previously reported. All compounds were evaluated for their α-glucosidase inhibitory and glucose consumption activities. Compound 2 showed the highest efficacy in inhibiting α-glucosidase enzyme and promoting glucose consumption activity by 3T3-L1 cells, with IC50 values of 0.5 μM and 13.1 μM, respectively, without causing toxicity to cells.
Keywords: Natural product chemistry, Pharmaceutical chemistry, Garcinia cowa, Bioassay-guided isolation, Bioactive compounds, Antidiabetic activity
Natural product chemistry; Pharmaceutical chemistry, Garcinia cowa; Bioassay-guided isolation; Bioactive compounds; Antidiabetic activity.
1. Introduction
Diabetes mellitus is a chronic metabolic disorder characterized by hyperglycemia. There are two principal forms of this disorder: the inability of the pancreas to produce insulin (type 1) and the inability of the body to properly respond to the action of insulin produced (type 2) (Skyler et al., 2017). Over the past three decades, diabetes prevalence has been increasing more rapidly. In 2017, approximately 451 million people worldwide had diabetes mellitus, and this number is expected to rise to 693 million by 2045 (Cho et al., 2010). From this number, 90%–95% of the cases are type 2 or non-insulin-dependent diabetes (ADA, 2009), which, if uncontrolled, will lead to many severe complications (Hippisley-Cox and Coupland, 2016). Thailand is among Asian countries with high prevalence of diabetes (Nanditha et al., 2016). With over 4.2 million cases of diabetes, this disorder is increasingly becoming a major cause of death and the main public health problem in Thailand (Porapakkham et al., 2010).
Although medications to improve glycemic levels are available, patients must spend high cost for the treatment of illness (Chatterjee et al., 2011), and all antidiabetic agents have adverse effects, such as gastrointestinal and cardiovascular adverse effects, infection of the upper respiratory tract, weight gain, and increased risk of bone fracture (Marín-Peñalver et al., 2016). Moreover, drug therapy of diabetes includes not only hypoglycemic agents but also strategies to maintain glycemic control over time, which often requires several drugs with different mechanisms of action (Garber et al., 2016). Accordingly, screening and development of compounds for effective, safe, and well-tolerated treatment of diabetes are still necessary.
Presently, the Thai government has developed a strategy to promote physical activity and balanced diet using traditional herbs to enhance the health of diabetes patients, which has been accepted as a regulation in the public health system (Wiwanitkit, 2011). However, more than a hundred plants used for diabetes treatment in regional healing practices have been subjected to minimal or no industrial development. The information of their chemical profile and pharmacological properties which are necessary for the development of new antidiabetic compounds (Jamshidi-Kia et al., 2018) is still lacking, and desire to perform the public credible in their use is constantly strengthened (Methee and Henrik, 2018).
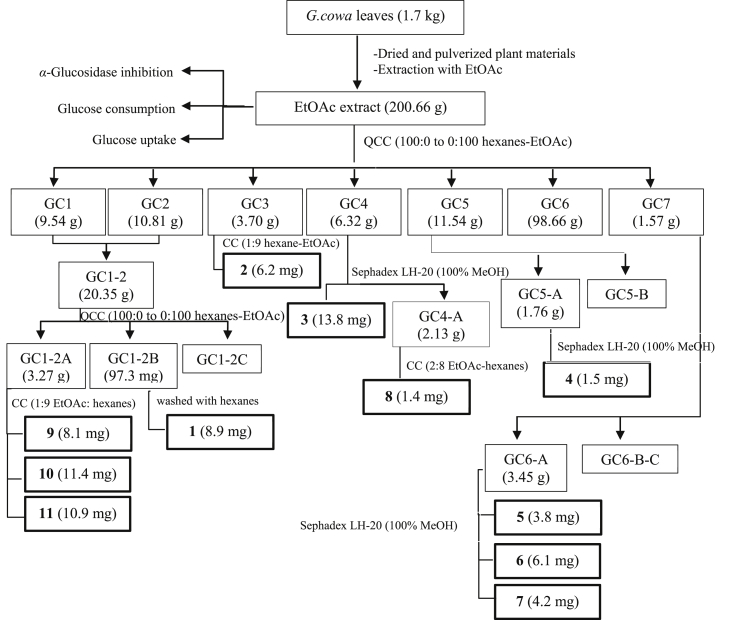
Garcinia cowa Roxb. ex Choisy (Clusiaceae), commonly known as ‘‘Cha muang’’ in Thailand, is a Thai local edible plant used for treating diabetes patients. This plant has medium-sized tree and is distributed in undulating areas and evergreen forests in tropical and subtropical countries (Panthong et al., 2006). The young leaves of G. cowa are used as vegetables and some parts of the plant have been used in the folk medicine for various purposes. The bark has been used as an antipyretic and antimicrobial agent (Mahabusarakam et al., 2005), while the fruits and leaves are used for the improvement of blood circulation, as an expectorant for the treatment of coughs and indigestion and as a laxative (Poomipamorn and Kumkong, 1997). The latex and the root have been used for fever relief (Na Pattalung et al., 1994) and the bark has been used as an antipyresis agent in Thai folk medicine (Na Pattalung et al., 1994). Some biological activities of the leaf extract have been reported for antitumor-promoting and inflammation activities (Mahabusarakam et al., 2005). The chemical constituents of this plant are xanthones and benzophenones (Sriyatep et al., 2015), which are found in various parts of the plants; these substances have shown anticancer (Wahyuni et al., 2015), anti-inflammatory (Wahyuni et al., 2017a, Wahyuni et al., 2017b), antioxidant (Panthong et al., 2009), antibacterial (Sakunpak and Panichayupakaranant, 2012), and α-glucosidase inhibitory (Sriyatep et al., 2015) effects. However, literatures on the investigation of the leaves of this plant are still scarce. Recently, we reported the isolation of two new phloroglucinol benzophenones and three new xanthones from the fresh leaves of G. cowa and their nitric oxide production and α-glucosidase inhibitory activities (Raksat et al., 2020). To continue the chemical study of this plant but different in the plant varieties and the place of collection, the aim of this study is to discover and identify bioactive compounds related to anti-diabetic properties from the ethyl acetate extract of G. cowa leaves (GCEA). We report herein the bioassay-guided isolation (Figure 1) and identification of antidiabetic compounds from G. cowa leaf extract, collected from Tasud Subdistrict, Chiang Rai Province, Thailand.
Figure 1.
Bioassay-guided isolation of G.cowa leaf extract.
2. Results
2.1. Antidiabetic property of the ethyl acetate extract of the leaves of G. cowa (GCEA)
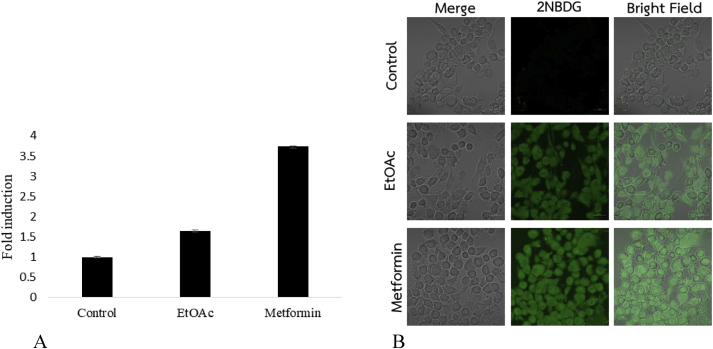
Previous studies had been reported the subacute toxicity of ethyl acetate extract of G. cowa in mice. The extract did not exert any toxicity nor cause mortality during a 21-day study (Wahyuni et al., 2017). For safety analysis, in present study, the antidiabetic potential of GCEA was evaluated using α-glucosidase inhibitory, glucose uptake, and glucose consumption assays, and the results are presented in Table 1. In the α-glucosidase assay, the extract showed an IC50 value of 21.4 μg/mL, which was greater than that of the standard drug (acarbose). Similarly, the results of the glucose consumption and glucose uptake assays also showed that the GCEA induced glucose uptake by cells. GCEA at 100 μg/mL led to the highest cellular glucose uptake of 1.7-fold (Figure 2A) under immuncyto fluorescence image (20×) of glucose uptake expression (Figure 2B) compared with that in the control. The extract increased glucose consumption by 3T3-L1 cells with IC50 value of 39.8 μg/mL. Thus, GCEA may contain active compounds that exert pharmacologic effects.
Table 1.
α-Glucosidase inhibition, glucose consumption, and glucose uptake activities of G. cowa leaf extract (GCEA).
| Sample | α-glucosidase inhibition (IC50, μg/mL) | glucose consumption (IC50, μg/mL) | glucose uptake (fold induction) |
|---|---|---|---|
| GCEA | 21.4 | 39.8 | 1.7 |
| Acarbose | 60.4 | NT | NT |
| Metformin | NT | 6.3 | 3.8 |
Figure 2.
Effects of GCEA on glucose uptake in L6 muscle cells. (A) Scale bar of glucose uptake in differentiated L6 myotube. (B) Immuncyto fluorescence image (20×) measured expression of glucose uptake in differentiated L6 myoblast.
2.2. α-Glucosidase inhibitory activity-guided fractionation of the leaf extract of G. cowa
Bioassay-guided fractionation is used to connect drug discovery screening with biological response-linked instrumental analysis, and it can be performed in many stages to produce different results (Weller, 2012). In this study, GCEA was shown to exert potent inhibitory effect on α-glucosidase (inhibition rate = 99.3 ± 0.84% at a final concentration of 200 μg/mL), and its fractions, GC1-GC6, also showed strong α-glucosidase inhibitory activity (inhibition rate = 74.8–99.9%) (Table 2).These findings suggested that some compounds of these subfractions may act in synergy on a single or multiple target sites (Wagner, 2011).
Table 2.
α-Glucosidase inhibitory activity of crude extract and fractions at 200 μg/mL.
| crude extract/fraction | % inhibition at 200 μg/mL |
|---|---|
| GCEA | 99.3 ± 0.84% |
| GC1 | 99.0 ± 0.46% |
| GC2 | 90.4 ± 0.73% |
| GC1-2 | 93.5 ± 0.90% |
| GC1-2A | 79.5 ± 0.71% |
| GC1-2B | 90.8 ± 0.85% |
| GC1-2C | 29.2 ± 1.05% |
| GC3 | 89.0 ± 0.97% |
| GC4 | 88.7 ± 1.12% |
| GC4A | 74.8 ± 0.92% |
| GC5 | 99.0 ± 0.94% |
| GC5A | 84.6 ± 0.70% |
| GC5B | 35.1 ± 0.81% |
| GC6 | 99.9 ± 0.74% |
| GC6A | 96.6 ± 1.14% |
| GC6B | 42.5 ± 0.59% |
| GC6C | 19.7 ± 0.42% |
| Acarbose | 98.9% |
2.3. Structures of the isolated compounds
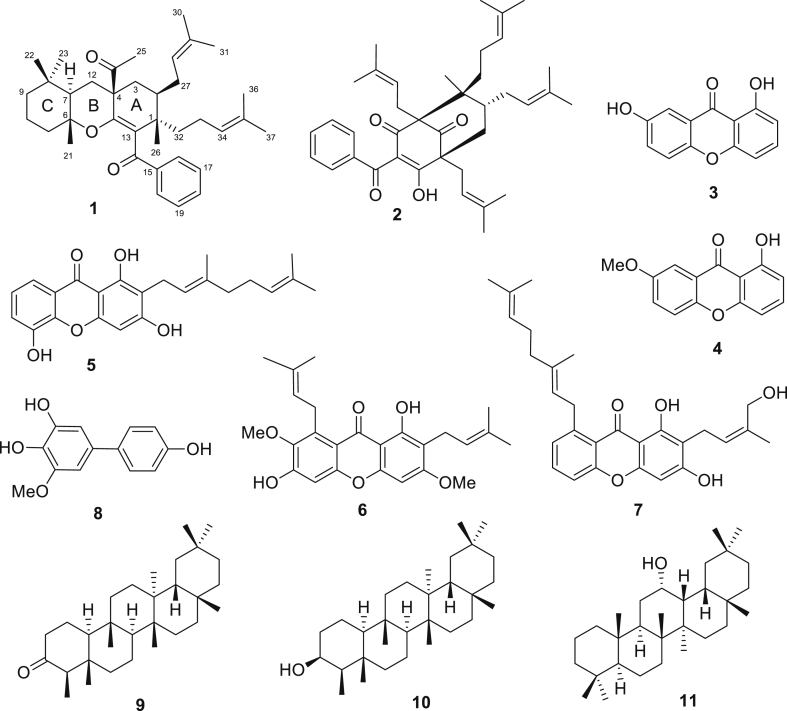
Active fractions of GCEA were obtained using α-glucosidase inhibitory activity-guided fractionation and 11 compounds (1–11) were isolated from the subfractions, as shown in Figure 3. Most compounds were xanthones and benzophenones, which are widely found in plants of the Garcinia genus (Ritthiwigrom et al., 2013). The structures of the isolated compounds were elucidated by spectroscopic data, and a novel compound, garciniacowone K (1), was discovered together with 10 known compounds. The spectroscopic data of the known compounds were compared with those reported and they were identified as guttiferone I (2) (Merza et al., 2006), 1,7-dihydroxyxanthone (3) (Mak et al., 1999), 1-hydroxyl-7 methoxyxanthone (4) (Dharmaratne et al., 2009), mangostinone (5) (Rukachaisirikul et al., 2008), α-mangostin (6) (Nguyen et al., 2017), cowanol (7) (Na pattalung et al., 1994), gacibiphenyl C (8) (Chen et al., 2006), friedelin (9) (Ho et al., 1995), β-friedelinol (10) (Sousa et al., 2012), and oleanane-12-ol (11) (Boar et al., 1977). It should be noted that compounds isolated in this work, except for α-mangostin (6), were totally different from our previously study (Raksat et al., 2020).
Figure 3.
Structures of compounds isolated from G. cowa leaves.
3. Discussion
3.1. Structure elucidation of a novel compound
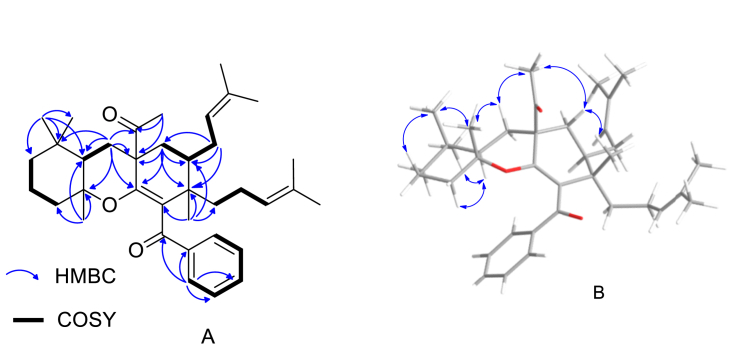
Garciniacowone K (1) was obtained as a white needle solid. Its HRESITOFMS data showed an [M+Na]+ ion at m/z 567.3814, implying a molecular formula of C37H52O3. The IR spectrum showed the presence of two carbonyl groups at 1708 (non-conjugated carbonyl group) and 1658 (conjugated carbonyl group) cm−1, respectively, and the UV spectrum showed maxima absorption band at λmax at 249 and 300 nm. Intensive analysis of the 1H, 13C, DEPT, and 2D NMR spectroscopic data of 1 (Table 3 and Supplementary Material) suggested that the core structure of this compound was a decahydro-1H-xanthene, which were deduced from the following NMR spectroscopic data: δH/δC 1.53 (1H, br d, J = 9.7 Hz, H-2)/36.8, 1.54 (1H, m, H-3a) and 1.99 (1H, m, H-3b)/35.0, 1.20 (1H, m, H-7)/50.4, 1.32 (1H, m, 9a) and 1.12 (1H, m, 9b)/41.2, 1.81 (2H, m, H-10)/23.5, 1.30 (1H, m, 11a) and 1.29 ((1H, m, 11b)/39.6, 2.23 (1H, m, 12a) and 1.44 (1H, m, 12b)/31.2, δC 42.4 (C-1), 55.1 (C-4), 149.6 (C-5), 80.7 (C-6), 33.5 (C-8), and 130.1 (C-13). The 1H and 13C NMR resonances (Table 3) also displayed the characteristic resonances of a benzoyl unit [δH/δC 8.14 (2H, d, J = 6.8 Hz, H-16/H-20)/129.9, 7.44 (1H, m, H-18)/132.2, 7.40 (2H, m, H-17/H-19)/128.0, δC 200.5 (C-14), and 139.1 (C-15)], a 3-methylbut-2-en-1-yl unit [δH/δC 5.01 (1H, m, H-28)/123.2, 2.01 (1H, m, H-27a) and 1.66 (1H, m, H-27b)/27.1, 1.61 (3H, s, H-30)/18.1, 1.71 (3H, s, H-31)/26.0, and δC 133.2 (C-29)], a 4-methylpent-3-en-1-yl unit [δH/δC 1.33 (1H, m, H-32a) and 1.17 (1H, m, H-32b)/19.9, 1.81 (1H, m, H-33a) and 1.15 (1H, m, H-33b)/36.7, 4.39 (1H, t, J = 6.5 Hz, H-34)/124.3, 1.44 (3H, s, H-36)/25.7, 1.25 (3H, s, H-37)/17.4, and δC 130.9 (C-35)], four methyl groups [δH/δC 1.06 (3H, s, H-21)/19.8, 0.71 (3H, s, H-22)/20.7, 0.86 (3H, s, H-23)/31.8, and 1.22 (3H, s, H-26)/23.2 ], and one acetyl group [δH/δC 2.33 (3H, s, H-25)/26.3 and δC 211.7 (C-24)]. The 1H–1H COSY cross peaks of H-2/H2-3/H2-27/H-28 and HMBC correlations of H2-27 with C-1 (δC 42.4), C-2 (δC 36.8), and C-3 (δC 35.0) confirmed the location of the 3-methylbut-2-en-1-yl unit moiety at C-2. The 4-methylpent-3-en-1-yl unit was placed on C-1 due to the HMBC correlation of H2-32 with C-1, C-2, and C-13 (δC 130.1). Four methyl groups were assigned to CH3-21, CH3-22, CH3-23, and CH3-26, respectively, because of their HMBC correlations as shown in Table 1 and Figure 4A. The key HMBC correlations (Figure 4A) between H3-25 and C-4 (δC 55.1) indicated that an acetyl unit was located at C-4 and the benzoyl unit was placed at C-13 by the process of elimination. The relative configuration of compound 1 was assigned by NOESY experiments. The NOESY cross-peaks (Figure 4B) of H3-26/H-3b/H3-25, H3-25/H-12b/H3-21 and H3-21/H-22/H-10b suggesting these protons were β-orientations and the ring junction of B/C ring was trans-orientation. Therefore, garciniacowone K was characterized as 1.
Table 3.
1H (500 MHz),13C (125 MHz), and HMBC NMR spectroscopic data of compound 1 in CDCl3.
| position | δC | carbon type | δH (J in Hz) | HMBC (1H →13C) |
|---|---|---|---|---|
| 1 | 42.4 | C | - | - |
| 2 | 36.8 | CH | 1.53 (br d, 9.7) | |
| 3 | 35.0 | CH2 | 1.54, 1.99 (m) | C-1, C-2, C-4, C-5, C-24 |
| 4 | 55.1 | C | - | - |
| 5 | 149.6 | C | - | - |
| 6 | 80.7 | C | - | - |
| 7 | 50.4 | CH | 1.20 (m) | C-6, C-9, C-8, C-11, C-12 |
| 8 | 33.5 | C | - | |
| 9 | 41.2 | CH2 | 1.32, 1.12 (m) | - |
| 10 | 23.5 | CH2 | 1.81 (m) | - |
| 11 | 39.6 | CH2 | 1.30, 1.29 (m) | C-9 |
| 12 | 31.2 | CH2 | 2.23, 1.44 (m) | C-4, C-5, C-6, C-7, C-24 |
| 13 | 130.1 | C | - | - |
| 14 | 200.5 | C | - | - |
| 15 | 139.1 | C | - | - |
| 16 | 129.9 | CH | 8.14 (d, 6.8) | C-14, C-18, C-20 |
| 17 | 128.0 | CH | 7.40 (m) | C-15, C-19 |
| 18 | 132.2 | CH | 7.44 (m) | C-16, C-20 |
| 19 | 128.0 | CH | 7.40 (m) | C-15, C-17 |
| 20 | 129.9 | CH | 8.14 (d, 6.8) | C-14, C-16, C-18 |
| 21 | 19.8 | CH3 | 1.06 (s) | C-6, C-7, C-11 |
| 22 | 20.7 | CH3 | 0.71 (s) | C-7, C-8, C-9, C-23 |
| 23 | 31.8 | CH3 | 0.86 (s) | C-7, C-8, C-22 |
| 24 | 211.7 | C | - | - |
| 25 | 26.3 | CH3 | 2.33 (s) | C-4, C-24 |
| 26 | 23.2 | CH3 | 1.22 (s) | C-1, C-2, C-13 |
| 27 | 27.1 | CH2 | 2.01, 1.66 (m) | C-1, C-2, C-4, C-28, C-29 |
| 28 | 123.2 | CH | 5.01 (m) | C-30, C-31 |
| 29 | 133.2 | C | - | - |
| 30 | 18.1 | CH3 | 1.61 (s) | C-28, C-29, C-31 |
| 31 | 26.0 | CH3 | 1.71 (s) | C-28, C-29, C-30 |
| 32 | 19.9 | CH2 | 1.33, 1.17 (m) | C-1, C-13 |
| 33 | 36.7 | CH2 | 1.81, 1.15 (m) | C-1, C-34, C-35 |
| 34 | 124.3 | CH | 4.39 (t, 6.5) | C-36, C-37 |
| 35 | 130.9 | C | - | - |
| 36 | 25.7 | CH3 | 1.44 (s) | C-34, C-35, C-37 |
| 37 | 17.4 | CH3 | 1.25 (s) | C-34, C-35, C-36 |
Figure 4.
Key correlations of compound 1. (A) 1H–1H COSY and HMBC correlations. (B) NOESY correlations.
3.2. α-Glucosidase inhibitory effect of the isolated compounds
Many compounds from the Garcinia genus have antidiabetic properties, according to in vitro studies (Hemshekhar et al., 2011). The compounds isolated from G. cowa leaves (compounds 1–11) and the standard drug acarbose at various concentrations were screened for α-glucosidase inhibitory activity. All samples exhibited a dose-dependent inhibitory effect. The IC50 values are presented in Table 4. The highest α-glucosidase inhibitory activity was shown by compound 2, with IC50 value of 13.1 μM, followed by compound 6 (IC50 = 15.0 μM) and compound 7 (IC50 value = 18.0 μM). The positive control (acarbose) showed IC50 value of 93.3 μM α-Glucosidase enzyme plays an important role in carbohydrate digestion and absorption by catalyzing the conversion of polysaccharides into monosaccharides. Compounds 2, 6, and 7 are the main compound with higher α-glucosidase-inhibitory activity than that of acarbose (the positive control). This finding suggested that these compounds may have antidiabetic efficacy and may be further developed as a drug supplement following additional investigation of their activities and safety (Kim et al., 2017).
Table 4.
α-Glucosidase inhibitory and glucose consumption activities of compounds 1–11.
| compound | α-glucosidase inhibition (IC50, μM) | glucose consumption (IC50, μM) | % cell viability |
|---|---|---|---|
| 1 | >100a | 5.1 | 97.0 |
| 2 | 13.1 | 0.5 | 98.7 |
| 3 | >100a | 56.9 | 105.3 |
| 4 | >100a | 23.2 | 101.7 |
| 5 | >100a | 16.0 | 93.1 |
| 6 | 15.0 | 4.8 | 101.3 |
| 7 | 18.0 | 13.7 | 100.9 |
| 8 | NA | 34.7 | 96.6 |
| 9 | >100a | 18.5 | 101.0 |
| 10 | NA | 25.3 | 101.8 |
| 11 | >100a | 13.5 | 100.8 |
| Acarbose | 93.3 | NT | NT |
| Metformin | NT | 45.4 | 99.7 |
Inactive at > 100 μM.
3.3. Cytotoxicity of the isolated compounds and their effect on glucose consumption by 3T3-L1 cells
Glucose consumption is assessed by measuring the concentration of the compounds that can induce glucose uptake into cells (Yin et al., 2002). As shown in Table 4, compared with the positive control, all samples at low concentration induced glucose uptake by 3T3-L1 cells without causing toxicity. Compound 2 showed the highest efficiency, with IC50 value of 0.5 μM. However, the other compounds also highly induced glucose consumption by 3T3-L1 cells, compared with the positive control (metformin).
4. Conclusions
This was the first report of bioassay-guided isolation antidiabetic compounds from the EtOAc extract of the leaves of G. cowa and their effects on glucose consumption by 3T3-L1 cells and α-glucosidase activity. Investigation of active fractions through bioassay-guided isolation obtained a novel decahydro-1H-xanthene derivative along with 10 known compounds. Guttiferone I showed the highest efficacy inhibiting α-glucosidase enzyme and induced glucose consumption by 3T3-L1 cells without causing toxic effects with IC50 values of 13.1 and 0.5 μM, respectively. Our findings indicated that these bioactive compounds may be developed as a supplement for antidiabetic drugs.
5. Material and methods
5.1. General experimental instruments and chemical reagents
NMR spectra were recorded using an AVANCE NEO 500 and 400 MHz Bruker spectrometer (Bruker, Germany) in acetone-d6 and chloroform-d, with tetramethylsilane (Cambridge Isotope Laboratory, Inc, USA) as an internal standard. High resolution mass spectra were obtained on a Bruker microTOF mass spectrometer (Bruker, Germany). The quick-column chromatography (QCC) system consisted of silica gel C60 (0–20 mm; SiliCycle® Inc., Canada) and silica gel G60 (60–200 mm; SiliCycle® Inc.). Sephadex LH-20 was used for column chromatography (CC). Waters 10 g Sep-Pak's (RP-18, US) was used for reversed-phase flash chromatography. Thin-layer chromatography (TLC) was performed using silica gel GF254 plates (Merck, USA). A multimode microplate reader (Bio Tek, Vermont, USA) was used for measuring and recording absorbance. α-Glucosidase from Saccharomyces cerevisiae and 4-nitrophenyl-α-D-glucopyranoside (p-NPG) were purchased from Sigma Chemical Co. (St Louis, USA). 3T3-L1 and L6 myotube cells were purchased from the American Type Culture Collection (USA).
5.2. Plant material
The leaves of G. cowa (Clusiaceae) were collected from Tasud, Muang, Chiang Rai Province, Thailand (GPS coordinates: 20.0449° N, 99.8943° E). The plant was authenticated by Assoc. Prof. Dr. Nijsiri Ruangrungsi, a botany specialist. A voucher specimen (MFU-NPR0186) was deposited at the Natural Products Research Laboratory, School of Science, Mae Fah Luang University.
5.3. Extraction and purification by bioassay-guided isolation
G. cowa leaves were extracted by maceration. Briefly, dried and pulverized plant materials (1.7 kg) were extracted with EtOAc (3 × 5 L, for 3 days) at room temperature (25 °C). Removal of the solvent under reduced pressure provided EtOAc extract (GCEA, 465.74 g, 27.36% w/w). A portion of GCEA (200.5 g) was subjected to QCC using silica gel eluting with a gradient of hexanes-EtOAc (100:0 to 0:100), yielding seven major fractions (GC1-GC7) based on TLC analysis. All fractions were prepared for α-glucosidase inhibitory assay, and active fractions were further isolated. Fractions GC1 and GC2 were combined (GC1-2, 20.35 g) and was subjected to QCC with silica gel eluting with a gradient of hexanes-EtOAc (100:0 to 0:100) to obtain three fractions (GC1-2A, GC1-2B, and GC1-2C). Fraction GC1-2A (3.27 g) was purified by CC on silica gel using hexanes-EtOAc (1:9) to obtain compounds 9 (8.1 mg), 10 (11.4 mg), and 11 (8.9 mg). Compound 1 (8.9 mg) was obtained from fraction GC1-2B (97.3 mg) by washing with hexanes. Fraction GC3 (3.70 g) was subjected to CC on silica gel using EtOAc-hexanes (1:9) to obtain compound 2 (6.2 mg). Fraction GC4 (6.32 g) was isolated by CC on Sephadex LH-20 using 100% MeOH, yielding compound 3 (13.8 mg) and fraction GC4-A (2.13 g), which was further subjected to CC on silica gel using EtOAc-hexanes (2:8) to obtain compound 8 (1.4 mg). Another active fraction, GC5 (11.54 g), was isolated by reversed-phase chromatography (RP-18; the eluent was MeOH–H2O (4:5) to obtain two fractions (GC5-A and GC5-B). Fraction GC5-A (1.76 g) was further subjected to CC on Sephadex LH-20 using 100% MeOH to obtain compound 4 (1.5 mg). Fraction GC6 (98.66 g) was subjected to QCC with hexanes-EtOAc (100:0 to 0:100), yielding three fractions (GC6A-GC6C). Fraction GC6A (3.45 g) was further subjected to CC on Sephadex LH-20 using 100% MeOH to give compounds 5 (3.8 mg), 6 (6.1 mg), and 7 (4.2 mg). The summary of bioassay-guided isolation was shown in Figure 1.
Garciniacowone K (1): white needle solid; mp 161–162 °C; [α]23D +29 (c 0.1; CHCl3); UV (CHCl3) λmax (log ε): 249 (2.77), 300 (2.69) nm; IR (KBr) υmax: 2961, 1708, 1658, 1455 cm−1; 1H and 13C-NMR spectral data, see Table 3; HRESITOFMS m/z 545.3992, [M + H]+ (calcd for C37H53O3, 545.3989).
5.4. Structure characterization
The structures of the isolated compounds were characterized by spectroscopic methods, and 1H (500 MHz and 400 MHz) and 13C NMR spectral data were used for comparison with previously reported physical property and NMR spectroscopic data. In addition, the complicated structure was confirmed with 2D NMR data, including COSY, HSQC, HMBC, DEPT-135, and DEPT-90. The results were matched with mass spectral data.
5.5. α-Glucosidase inhibitory assay
The α-glucosidase inhibitory assay procedure followed a previously described method (Kim et al., 2004) with modifications. Briefly, sample solutions at different concentrations (0.1–1000 μg/mL) were dissolved with 5% dimethyl sulfoxide (DMSO) in phosphate buffer (pH 6.8), and then 50 μL of each sample was pipetted and mixed with 50 μL of α-glucosidase enzyme (0.35 U/mL) in a 96-well micro plate. After preincubation at 37 °C for 10 min, 50 μL of 1.5 mM p-NPG was added, and the samples were further incubated at 37 °C for 20 min. Next, 100 μL of Na2CO3 (1 M) was added to terminate the reaction. Acarbose was used as a positive control. The absorbance was measured at 405 nm with a micro plate reader (PerkinElmer, Inc., USA). The process was repeated in triplicate, and the percent inhibition was calculated with the following equation:
| Percent inhibition (%) = [((A – B) − (C – D)) / (A – B)] × 100 |
where,
A is the absorbance of blank reaction containing only 5% DMSO in phosphate buffer,
B is the absorbance of control reaction containing 5% DMSO in phosphate buffer and α-glucosidase enzyme,
C is the absorbance of sample reaction containing sample solution and α-glucosidase enzyme,
D is the absorbance of control sample containing only sample solution.
The concentration of samples that inhibited α-glucosidase activity by 50% was defined as the IC50 value.
5.6. In vitro studies in 3T3-L1 embryonic cells and L6 myoblast cells
5.6.1. Cell culture
3T3-L1 cells were maintained in high-glucose Dulbecco's modified Eagle medium (DMEM) (Thermo Fisher Scientific, USA) supplemented with 10% (v/v) of fetal bovine serum (FBS) (Thermo Fisher Scientific, USA), 2 mM glutamine (Sigma Chemical, USA), streptomycin (Sigma Chemical, USA) (1%), penicillin (Sigma Chemical, USA) (1%), and high glucose concentration (4.5 g/L) at 37 °C in a 5% CO2 incubator. Cells at full confluence on the cell culture flask were subcultured and collected for cytotoxicity test [3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay] and glucose consumption assay.
L6 myoblast cells were incubated at 5% CO2 and 37 °C in high-glucose DMEM containing 10% (v/v) FBS, penicillin, and streptomycin. For glucose uptake assay, the cells were seeded in 24-well plates at a density of 2 × 104 cells/mL. After 48 h, the medium was replaced with high-glucose DMEM containing 2% (v/v) FBS, which was replaced with new medium after 2, 4, and 6 days of culture (Cheng et al., 2006).
5.6.2. Glucose consumption assay
The procedure of the glucose consumption assay has been described in a previous study (Yin et al., 2002; Zhang et al., 2008). A solution including 0.25% trypsin and 1 mM EDTA was used to detach 3T3-L1 cells from the culture flask, and complete culture medium was used to stop trypsin digestion. The cells were washed with phosphate buffer (pH 6.7) for twice and resuspended in low glucose (1.0 mg/mL) detection medium supplemented with 0.05% pluronic F68, 0.2% bactopeptone, and 2 mM glutamine. Next, 100 μL of 3T3-L1 cells (at a density of 1 × 105 mL) were pipetted onto 96-well plates and cultured with various concentration of samples, insulin, or the standard drug metformin (final concentration 0–125 nM) at 37 °C in a 5% CO2 incubator for 4–48 h. After incubation, 10 μL of suspension from each well was transferred to another 96-well plate. The remaining glucose concentration in the suspension was evaluated after 30 min by illuminated reaction, and absorbance was measured at a wavelength 495 nm. Then, glucose concentration in the medium was calculated using a standard curve for glucose. Percent of glucose consumption was calculated according to the following formula, and IC50 value was evaluated.
| Percent of glucose consumption = [(A–B) / (A–C)] × 100 |
where,
A is glucose concentration in the medium of the control group,
B is glucose concentration in the medium of the treatment groups
C is the blank control (the same medium as that of the control group, but without cells)
5.6.3. Glucose uptake assaya
The time-dependent response of L6 myotubes to crude samples was examined following a previously described method (Sharma et al., 2019) with modification. Cells were incubated with samples and metformin for 24 h, washed twice with Kreb-Ringer bicarbonate buffer, incubated with Kreb-Ringer bicarbonate buffer for 1 h, and then starved in serum-free phosphate buffer saline containing 0.2% Bovine serum albumin for 1 h. After that, the cells were further incubated with 2-deoxy-2-[(7-nitro-2, 1, 3-benzoxadiazol-4-yl) amino]-D-glucose for 20 min. Fluorescence intensity was measured at excitation/emission wavelengths of 485/530 nm.
5.6.4. Cytotoxicity analysis (MTT assay)
3T3-L1 cells were cultured at a density of 1 × 104 cells in 96-well plates with insulin (30 nM) in detection medium for 24–48 h at 37 °C in a 5% CO2 incubator. The cells were then subjected to MTT assay (Kang et al., 2013) to evaluate the toxicity of the compounds to cells. Briefly, cells were treated with various doses (0.01–1000 μg/mL) of samples for 72 h at 37 °C in a 5% CO2 incubator, strained by addition of 100 μL MTT solution (0.5 mg/mL in phosphate buffer), and then further incubated for 4 h. After that, the suspension was collected and 150 μL of DMSO was added to each well. Absorbance was measured at 570 nm with a microplate reader. Percentage of cell viability was also determined.
5.6.5. Calculation
All data were expressed as the mean of triplicate experiments ±standard deviation. Dose-response curves were plotted using the percentages of α-glucosidase inhibition or glucose consumption and concentration (μg/mL). The concentration of treatment that inhibited α-glucosidase enzyme or promoted glucose consumption by 50% (IC50) were determined from regression analysis using Microsoft Excel (Microsoft Corporation, USA) and Graph Pad Prism version 6 (Graphpad Software Inc., USA).
Declarations
Author contribution statement
Surat Laphookhieo: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Wisanu Maneerat: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Piyaporn Phukhatmuen: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Achara Raksat, Rawiwan Charoensup, Thidarat Duangyod: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by the Agricultural Research Development Agency (Public Organization) of Thailand (grant number CRP6105021500), the Royal Golden Jubilee Ph.D. Program, Thailand Science Research and Innovation (DBG6280007), and Mae Fah Luang University.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank Assoc. Prof. Dr. Nijsiri Ruangrungsi for plant identification. Mae Fah Luang University and University of British Columbia are also acknowledged for laboratory facilities.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- American Diabetes Association (ADA) Diagnosis and classification of diabetes mellitus. Diabetes Care. 2009;32:62–67. [Google Scholar]
- Boar R.B., Joukhadar L., Luque M.D., Mcghie J.F. On the reported transformation of β-amyrin into oleanolic acid. J. Chem. Soc., Perkin Trans. 1977;9:2104–2109. [Google Scholar]
- Chatterjee S., Riewpaiboon A., Piyauthakit P., Riewpaiboon W. Cost of informal care for diabetic patients in Thailand. Prim. Care Diabetes. 2011;5:109–115. doi: 10.1016/j.pcd.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Chen J.J., Peng C.F., Huang H.Y., Chen I.S. Benzophyrans, biphenyls and xanthones from the root of Garcinia linii and their activity against mycobacterium tuberculosis. Planta Med. 2006;72:473–477. doi: 10.1055/s-2005-916253. [DOI] [PubMed] [Google Scholar]
- Cheng Z., Pang T., Gu M., Gao A.H., Xie C.M., Li J.Y., Nan F.J., Li J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim. Biophys. Acta. 2006;1760:1682–1689. doi: 10.1016/j.bbagen.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Cho N.H., Shaw J.E., Karuranga S., Huang Y., Rocha J.D., Ohlrogge A.W., Malanda B. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2010;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Dharmaratne H.R.W., Napagoda M.T., Tennakoon S.B. Xanthones from roots of Calophyllum thwaitesii and their bioactivity effects of phytochemicals against diabetes. Nat. Prod. Res. 2009;23:539–545. doi: 10.1080/14786410600899118. [DOI] [PubMed] [Google Scholar]
- Garber A.J., Abrahamson M.J., Barzilay J.I., Blonde L., Bloomgarden Z.T., Bush M.A., Dagogo Jack S., De Fronzo R.A., Einhorn D., Fonseca V.A. Consensus statement by the American association of clinical endocrinologists and American college of endocrinology on the comprehensive type 2 diabetes management algorithm 2016 executive summary. Endocr. Pract. 2016;22:84–113. doi: 10.4158/EP151126.CS. [DOI] [PubMed] [Google Scholar]
- Hemshekhar M., Sunitha K., Sebastin Santhosh M., Devaraja S., Kemparaju K., Vishwanath B.S., Niranjana S.R., Girish K.S. An overview on genus Garcinia: phytochemical and therapeutical aspects. Phytochemistry Rev. 2011;10:325–351. [Google Scholar]
- Hippisley-Cox J., Coupland C. Diabetes treatments and risk of amputation, blindness, severe kidney failure, hyperglycaemia, and hypoglycaemia: open cohort study in primary care. Br. Med. J. 2016;352:1–13. doi: 10.1136/bmj.i1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamshidi-Kia F., Lorigooini Z., Amini-Khoei H. Medicinal plants: past history and future perspective. J. Herbmed. Pharmacol. 2018;7:1–7. [Google Scholar]
- Kang J.W., Nam D., Kim K.H., Huh J.E., Lee J.D. Effect of gambisan on the inhibition of adipogenesis in 3T3-L1 adipocytes. Evid. Based Complementary Altern. Med. 2013;2013:11. doi: 10.1155/2013/789067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D.J., Kang Y.H., Kim K.K., Kim T.W., Park J.B., Choe M. Increased glucose metabolism and alpha-glucosidase inhibition in Cordyceps militaris water extract-treated HepG2 cells. Nutr. Res. Pract. 2017;11:180–189. doi: 10.4162/nrp.2017.11.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.M., Wang M.H., Rhee H.I. A novel α-glucosidase inhibitor from pine bark. Carbohydr. Res. 2004;339:715–717. doi: 10.1016/j.carres.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Mahabusarakam W., Chairerk P., Taylor W.C. Xanthones from Garcinia cowa Roxb. latex. Phytochemistry. 2005;66:1148–1153. doi: 10.1016/j.phytochem.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Mak N.K., Kui W., Zhang M., Wong N.S.R., Tai L.S., Yung K.K.L., Leung H.W. Effects of euxanthone on neuronal differentiation. Life Sci. 1999;66:347–354. doi: 10.1016/s0024-3205(99)00596-2. [DOI] [PubMed] [Google Scholar]
- Marín-Peñalver J.J., Martín-Timón I., Sevillano-Collantes C., Cañizo-Gómez F.J.D. Update on the treatment of type 2 diabetes mellitus. World J. Diabetes. 2016;7:354–395. doi: 10.4239/wjd.v7.i17.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merza J., Mallet S., Litaudon M., Dumontet V., Seraphin D., Richomme P. New cytotoxic guttiferone analogues Garcinia virgate from New Caledonia. Planta Med. 2006;72:87–89. doi: 10.1055/s-2005-873185. [DOI] [PubMed] [Google Scholar]
- Methee P., Henrik B. Thai ethnomedicinal plants used for diabetes treatment. OBM Integr. Complementary Med. 2018;3:1–20. [Google Scholar]
- Nanditha A., Ma R.C.W., Ramachandran A., Snehalatha C., Chan J.C.N., Chia K.S., Shaw J.E., Zimmet P.Z. Diabetes in asia and the pacific: implications for the global epidemic. Diabetes Care. 2016;39:472–485. doi: 10.2337/dc15-1536. [DOI] [PubMed] [Google Scholar]
- Na-Pattalung P., Thongtheeraparp W., Wiriyachitra P., Taylor W.C. Xanthones of Garcinia cowa. Planta Med. 1994;60:365–368. doi: 10.1055/s-2006-959502. [DOI] [PubMed] [Google Scholar]
- Nguyen N.K., Truong X.A., Bui T.Q., Bui D.N., Nguyen H.X., Tran P.T., Nguyen L.H.D. α-Glucosidase inhibitory xanthones from the roots of Garcinia fusca. Chem. Biodivers. 2017;14:1–8. doi: 10.1002/cbdv.201700232. [DOI] [PubMed] [Google Scholar]
- Panthong K., Hutadilok-Towatana N., Panthong A. Cowaxanthone F, a new tetraoxygenated xanthone, and other anti-inflammatory and antioxidant compounds from Garcinia cowa. Can. J. Chem. 2009;87:1636–1640. [Google Scholar]
- Panthong K., Pongcharoen W., Phongpaichit S. Tetraoxygenated xanthones from the fruits of Garcinia cowa. Phytochemistry. 2006;67:999–1004. doi: 10.1016/j.phytochem.2006.02.027. [DOI] [PubMed] [Google Scholar]
- Porapakkham Y., Rao C., Pattaraarchachai J., Polprasert W., Vos M., Adair T., Lopez A.D. Estimated causes of death in Thailand, 2005: implications for health policy. Popul. Health Metrics. 2010;8:1–14. doi: 10.1186/1478-7954-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poomipamorn S., Kumkong A. FaungFa Printing; Bangkok: 1997. Edible Multipurpose Tree Species; p. 486. (in Thai) [Google Scholar]
- Raksat A., Phukhatmuen P., Yang J., Maneerat W., Charoensup R., Andersen R.J., Wang Y.A., Pyne S.P., Laphookhieo S. Phloroglucinol benzophenones and xanthones from the leaves of Garcinia cowa and their nitric oxide production and α-glucosidase inhibitory activities. J. Nat. Prod. 2020;83(1):164–168. doi: 10.1021/acs.jnatprod.9b00849. [DOI] [PubMed] [Google Scholar]
- Ritthiwigrom T., Laphookhieo S., Pyne S.G. Chemical constituents and biological activities of Garcinia cowa Roxb. Maejo Int. J. Sci. Technol. 2013;7:212–231. [Google Scholar]
- Rukachaisirikul V., Trisuwan K., Sukpondma Y., Phongpaichit S. A new benzoquinone derivative from the leaves of Garcinia parvifolia. Arch Pharm. Res. (Seoul) 2008;31:17–20. doi: 10.1007/s12272-008-1114-9. [DOI] [PubMed] [Google Scholar]
- Sakunpak A., Panichayupakaranant P. Antibacterial activity of Thai edible plants against gastrointestinal pathogenic bacteria and isolation of a new broad spectrum antibacterial polyisoprenylated benzophenone, chamuangone. Food Chem. 2012;130:826–831. [Google Scholar]
- Sharma B.R., Park C.M., Kim H., Kim H.J., Rhyu D.Y. Tinospora cordifolia preserves pancreatic beta cells and enhances glucose uptake in adipocytes to regulate glucose metabolism in diabetic rats. Phytother Res. 2019;33:2744–2765. doi: 10.1002/ptr.6462. [DOI] [PubMed] [Google Scholar]
- Skyler S.J., Bakris L.G., Bonifacio E., Darsow T., Eckel H.,R., Groop L., Handelsman Y., Insel A.R., Mathieu C., McElvaine A.T., Palmer J.P., Pugliese A., Schatz D.A., Sosenko J.,M., Wilding J.P.H., Ratner R.E. Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes. 2017;66:241–255. doi: 10.2337/db16-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa G.F., Duarte L.P., Alcantara A.F.C., Silva G.D.F., Vieira-Filho S.A., Silva R.R., Oliveira D.M., Takahashi J.A. New triterpenes from Maytenus robusta: structural elucidation based on NMR experimental data and theoretical calculations. Molecules. 2012;17:13439–13456. doi: 10.3390/molecules171113439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sriyatep T., Siridechakorn I., Maneerat W., Pansanit A., Ritthiwigrom T., Andersen R.J. Bioactive prenylated xanthones from the young fruits and flowers of Garcinia cowa. J. Nat. Prod. 2015;78:265–271. doi: 10.1021/np5008476. [DOI] [PubMed] [Google Scholar]
- Wiwanitkit V. Thai ethnopharmacological herbs for diabetes treatment: data collection and informatics tracing for therapeutic property. Diabetes Metab. Syndr. 2011;5:103–104. doi: 10.1016/j.dsx.2012.02.005. [DOI] [PubMed] [Google Scholar]
- Wagner H. Synergy research: approaching a new generation of phytopharmaceutical. Fitoterapia. 2011;82:34–37. doi: 10.1016/j.fitote.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Wahyuni F.S., Ali D.A.I., Lajis N.H., Dachriyanus Anti-inflammatory activity of isolated compounds from the stem bark of Garcinia cowa Roxb. Pharmacogn. J. 2017;9:55–57. [Google Scholar]
- Wahyuni F.S., Arisanty D., Hayaty N.F., Juwita D.A., Almahdy Sub-acute cytoxicity study of the ethyl acetate fraction of Asam Kandis rinds (Garcinia cowa Roxb.) on the liver and renal function in mice. Pharmacogn. J. 2017;9:345–349. [Google Scholar]
- Wahyuni F.S., Shaari K., Stanslas J., Lajis N., Hamidi D. Cytotoxic compounds from the leaves of Garcinia cowa Roxb. J. Appl. Pharmaceut. Sci. 2015;5:6–11. [Google Scholar]
- Yin J., Hu R., Chen M., Tang J., Li F., Yang Y., Chen J. Metabolism effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51:1439–1443. doi: 10.1053/meta.2002.34715. [DOI] [PubMed] [Google Scholar]
- Zhang L., Hu J.J., Du G.H. Establishment of a cell-based assay to screen insulin-like hypo glycemic drugs. Drug Discov. Ther. 2008;2(4):229–233. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.