Abstract
Cryopreservation procedures negatively affect the quality traits of sperm, causing certain changes at structural and molecular levels due to thermal, mechanical, osmotic, and oxidative damage. The objective of this study was to examine the potential of canine adipose-derived mesenchymal stem cells (Ad-MSCs) for providing protection to the dog sperm against cryo-damage. Canine Ad-MSCs were selected on the basis of the significantly higher gene expression for different proteins actively involved in the cell repair including annexin 1 (ANX1), histone H3 (H3) and high mobility group B (HMGB) protein compared to skin fibroblasts. Semen was collected from four healthy dogs by digital manipulation. The washed pooled ejaculates were diluted with buffer 2 (extender) supplemented without Ad-MSCs (Control), with 2.5 × 106 Ad-MSCs/mL (Group 1) or with 5 × 106 Ad-MSCs/mL (Group 2). Group 1 exhibited significantly higher post-thaw motility, live sperm, intact plasma membrane and normal acrosomes than the other groups. Additionally, Group 1 showed significantly higher expression levels of genes related to the repair of membranes (ANX1, dysferlin; DYSF, and fibronectin; FN1) and chromatin material (H3 and HMGB). Protein expression of ANX1, H 3, and FN1 was also statistically more in Group 1 than in Control. The results confirm that canine Ad-MSCs can effectively preserve the quality of frozen-thawed sperm by a reduction in cryoinjury. At an appropriate concentration, Ad-MSCs significantly improve the quality of post-thaw dog sperm.
Subject terms: Spermatogenesis, Spermatogenesis, Stem-cell research, Stem-cell research
Introduction
Semen cryopreservation is regarded as the most important step of artificial insemination which is the most widely adopted assisted reproductive technology (ART) in canine practice1. It can facilitate the storage of the genetic material for an extended period, conserve the elite individual’s fertility and serve as a useful tool for preserving the endangered species. In addition, it has greatly benefited the animal-based industry by reducing the cost and the stress associated with transportation, overcoming the quarantine restrictions and breeding issues (e.g., aggressive behavior and size issues)2.
Despite many attempts to improve freezing agents and techniques, reduction in semen quality still remains the major issue associated with freezing procedures. During freezing the quality-related traits of sperm are highly compromised3. Sperm undergoes certain detrimental changes at the structural and molecular levels as a result of thermal, mechanical, osmotic, and oxidative damage4,5. The sperm that survives during freezing procedure suffers a reduction in fertility6 and this has been linked with damage that adversely affects viability, motility, plasma membrane, acrosome and chromatin material7. In addition, the activation of apoptotic pathways results in the fragmentation of sperm cell DNA8. These changes ultimately contribute to an overall reduction in the fertility of sperm.
Current protective modifications employed to minimize the above mentioned damaging effects involve the use of different types of extenders9, variations in the freezing protocols10, and supplementation of extenders with nutrients or antioxidants11–16. However, the rates of whelping using cryopreserved semen (50.0–70.8%) are still lower in comparison with the fresh semen (81.8–83.7%)17. The reasons behind the lower fertility of frozen semen may be the primary or secondary damaged sperm that requires immediate regeneration and recovery. In addition, the chemical additives used for supplementation have been also associated with the issues of cytotoxicity8,18. Therefore, the repair of injured sperm could be a determining factor for improving the fertility of canine using frozen semen.
Mesenchymal stem cells (MSCs) are believed to be an integral part of regenerative medicine. Recently, research has highlighted their potential role in repair mechanisms occurring at cellular level19. The repair of the damaged tissue is believed to be governed by the multipotent nature of MSCs, and the associated paracrine mechanisms involving the secretion of different signaling factors, mainly proteins19,20. These proteins modulate the immune response and facilitate regeneration mechanisms by promoting mitosis and angiogenesis but at the same time suppressing apoptosis and scarring19. These include basic fibroblast growth factor (bFGF), keratinocyte growth factor (KGF), stromal-derived factor-1 (SDF-1), monocyte chemotactic protein-1 (MCP-1), insulin-like growth factor-1 (IGF-1), transforming growth factor-β (TGF-β), platelet-derived growth factor (PDGF), interleukin-8 (IL-8) and vascular endothelial growth factor (VEGF)21,22. In addition, temperature reduction increases the differentiation potential of MSCs along with a reduction in apoptosis and oxidative stress23. The reduced oxidative stress was due to the decrease in the reactive oxygen species, nitric oxide, thiobarbituric acid reactive substances, carbonyl, and lipofuscin production levels23.
The biosynthetic capability of sperm is limited24,25 serving as the main obstacle in the way of self-repair. Many external factors control sperm function by acting through the surface and membrane components26. Therefore, we hypothesized that the use of MSCs in semen cryopreservation may be an effective biological approach to enhance the fertility and viability of sperm by supporting repair mechanisms through the secretion of different proteins. To the best of our knowledge, this is the first study aimed to find out whether canine adipose-derived MSCs (Ad-MSCs) can improve post-thaw fertility and survival of dog sperm. In addition, identification of the factors involved in the repair mechanisms was also attempted.
Results
Comparison of gene expression between canine Ad-MSCs and skin fibroblasts
The expression levels of different genes related to the repair of the membrane (ANX1, FN1, and DYSF) and chromatin material (H3 and HMGB) among canine Ad-MSCs and skin fibroblasts is shown in Fig. 1. The expression levels of ANX1, H3, and HMGB genes were significantly higher in Ad-MSCs than skin fibroblasts. However, the expression level of FN1 and DYSF was not statistically different among the Ad-MSCs and skin fibroblasts.
Figure 1.
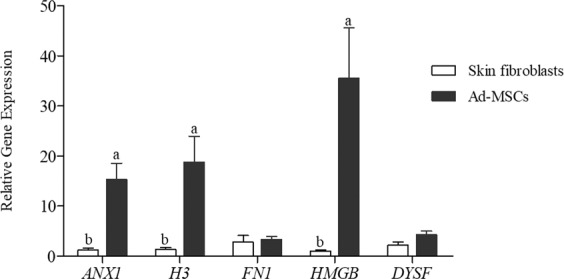
Gene expression of annexin 1 (ANX1), histone H3 (H3), fibronectin (FN1), high mobility group protein B (HMGB) and dysferlin (DYSF) by RT-qPCR in canine Ad-MSCs compared with skin fibroblasts (mean ± SEM). Different lowercase letters a, or b, indicate a significant difference at P < 0.05.
Effect of Ad-MSCs on motility and kinetic parameters
Post-thaw motility varied significantly among the groups (Table 1). The post-thaw motility in Group 1 (55.7 ± 1.0%) appeared to be significantly higher than in Group 2 and Control (48.8 ± 1.8% and 43.2 ± 1.0%, respectively). Sperm linearity did not show any statistical variation among the groups, but Group 1 (26.9 ± 0.6%) showed an increasing trend than Group 2 and Control (23.3 ± 0.9% and 23.9 ± 1.8%, respectively). Sperm straightness was not statistically different between the Group 1 and 2 (49.6 ± 1.6% and 49.3 ± 0.6%; respectively) but was significantly higher compared to the Control (45.1 ± 0.5%). The amplitude of lateral head displacement (ALH) was significantly higher in Group 1 (3.4 ± 0.1%) as compared to Control (2.8 ± 0.1%), whereas Group 2 (2.9 ± 0.1%) showed no statistical difference from either of Groups 1 and Control. The post-thaw motility was strongly but positively correlated (P < 0.01) with ALH, membrane integrity, live sperm percentage, normal acrosome integrity, and chromatin integrity (Table 2).
Table 1.
Effects of addition of canine Ad-MSCs in different concentrations (control; without Ad-MSCs, Group 1; 2.5 × 106 cells/mL, Group 2; 5 × 106 cells/mL) on post-thaw semen quality of beagle dogs.
| Group | Motility (%) | Linearity (%) | Straightness (%) | ALH (µm) | Live sperm (%) | Membrane Integrity (%) | Normal acrosome (%) | Normal Chromatin (%) |
|---|---|---|---|---|---|---|---|---|
| Control | 43.2 ± 1.0c | 23.9 ± 1.8 | 45.1 ± 0.5b | 2.8 ± 0.1b | 43.7 ± 1.1b | 45.4 ± 0.8c | 44.3 ± 0.7b | 65.3 ± 0.9b |
| Group 1 | 55.7 ± 1.0a | 26.9 ± 0.6 | 49.6 ± 1.6a | 3.4 ± 0.1a | 52.8 ± 0.8a | 54.6 ± 0.4a | 52.9 ± 0.5a | 72.9 ± 0.7a |
| Group 2 | 48.8 ± 1.8b | 23.3 ± 0.9 | 49.3 ± 0.6a | 2.9 ± 0.1ab | 41.9 ± 1.6b | 48.6 ± 0.9b | 50.1 ± 1.9a | 68.9 ± 1.1b |
ALH, amplitude of lateral head displacement. Values with different superscript letters in a column differ significantly (P < 0.05).
Table 2.
Correlation coefficient between different quality-related parameters of post-thaw semen.
| No. | Variables | Correlation between values | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| 1 | Post-thaw motility (%) | — | 0.28 | 0.50 | 0.94** | 0.88** | 0.74** | 0.78** | 0.78** |
| 2 | Linearity (%) | — | — | −0.00 | 0.289 | 0.48 | 0.54 | 0.21 | 0.28 |
| 3 | Straightness (%) | — | — | — | 0.27 | 0.55 | 0.31 | 0.67* | 0.67* |
| 4 | ALH (µm) | — | — | — | — | 0.76** | 0.74** | 0.62* | 0.60* |
| 5 | Membrane Integrity (%) | — | — | — | — | — | 0.73** | 0.89** | 0.88** |
| 6 | Live sperm (%) | — | — | — | — | — | — | 0.41 | 0.65* |
| 7 | Normal Acrosome (%) | — | — | — | — | — | — | — | 0.82** |
| 8 | Normal Chromatin (%) | — | — | — | — | — | — | — | — |
**Correlation coefficient is significant at the 0.01 level (2-tailed).
*Correlation coefficient is significant at the 0.05 level (2-tailed).
Effect of Ad-MSCs on viability and membrane integrity
The post-thaw live sperm percentage was significantly more in Group 1 (52.8 ± 0.8%) as compared to Group 2 and Control (41.9 ± 1.6% and 43.7 ± 1.1%, respectively; Table 1). However, the latter two groups did not vary statistically. The HOS test results showed the percentage of sperm with an intact plasma membrane differed significantly between the groups, with Group 1 (54.6 ± 0.4%) being statistically superior in comparison with Group 2 (48.6 ± 0.9%) and Control (45.4 ± 0.8%) (Table 1). Live sperm percentage and membrane integrity were strongly and positively correlated (P < 0.01) with each other. In addition, membrane integrity was strongly and positively correlated (P < 0.01) with normal acrosome integrity, and chromatin integrity (Table 2). However, live sperm percentage was positively correlated (P < 0.05) with chromatin integrity (Table 2).
Effect of Ad-MSCs on acrosomal integrity
The results of the post-thaw semen analysis of frozen semen shown in Table 1 indicated that the percentages of sperm with normal acrosomal region differed statistically among the different groups. The post-thaw semen analysis showed significantly higher sperm count with intact acrosome in Group 1 and Group 2 (52.9 ± 0.5% and 50.1 ± 1.9) than Control (44.3 ± 0.7%) (Table 1). The acrosomal integrity was strongly but positively correlated (P < 0.01) with chromatin integrity (Table 2).
Effect of Ad-MSCs on chromatin integrity
Results of the acridine orange test showed that a significantly higher percentage (72.9 ± 0.7%) of frozen-thawed sperm in Group 1 had normal chromatin compared with Group 2 (68.9 ± 1.1%) and Control (65.4 ± 0.9%) (Table 1).
Effect of Ad-MSCs on gene expression
Results indicated a significant enhancement of the expression levels of different genes related to the repair of the membrane (ANX1, and FN1) and chromatin material (H3) in Group 1 sperm as compared with the other groups (Fig. 2). However, no significant difference was witnessed between Group 2 and Control. The expression levels of DYSF and HMGB genes showed a different pattern of expression, Group 2 being statistically similar to both Groups 1 and Control, but the expression in Group 1 was significantly higher than Control.
Figure 2.
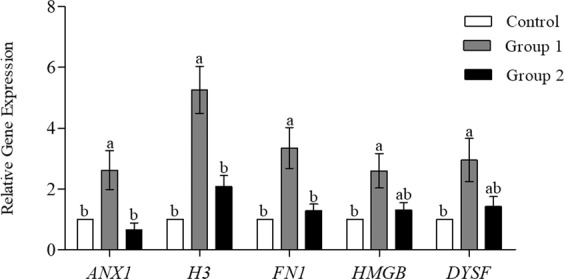
Gene expression of plasma membrane repair related genes annexin 1 (ANX1), fibronectin (FN1), dysferlin (DYSF) and chromatin material repair related genes high mobility group protein B (HMGB), histone H3 (H3) by real time RT-qPCR in Control (without Ad-MSCs), Group 1 (2.5 × 106 cells/mL of buffer 2) and Group 2 (5 × 106 cells/mL of buffer 2) of cryopreserved sperms (mean ± SEM). Different lower case letters a, or b indicate a significant difference at P < 0.05.
Western blot analysis
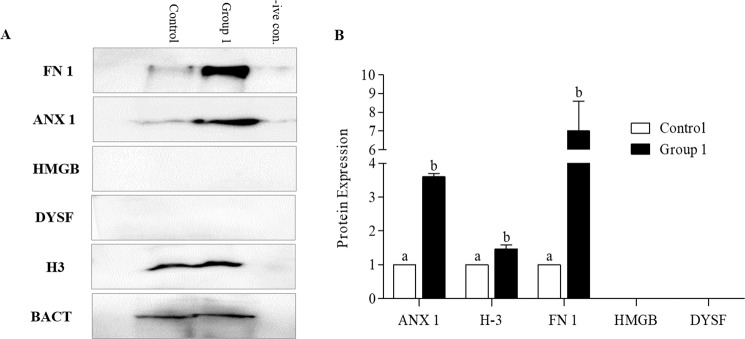
Based on the results obtained for different parameters of semen analysis and expression levels of different genes, protein expression was evaluated among Group 1 with optimal concentration of Ad-MSCs and Control using western blot: proteins related to the repair of membrane (ANX1, FN1, and DYSF) and chromatin material (histone H3, and HMGB). Beta-actin served as an Internal Control. Western blot images of protein expression levels for Group 1 and Control are presented in Fig. 3A and Supplementary Fig. S1. Protein expression was semi-quantitatively evaluated using densitometry (Fig. 3B). Results elucidate that the expression levels of histone H3, FN1 and ANX1 were significantly higher in Group 1 than Control. However, the HMGB and DYSF failed to express in either of the group.
Figure 3.
Expression of proteins analysed by western blot with corresponding antibodies. (A) Representative western blot images of annexin 1 (ANX1), histone H3 (H3), fibronectin (FN1), high mobility group protein B (HMGB) and dysferlin (DYSF) proteins and (B) quantification of ANX1, H3, FN1, HMGB and DYSF protein expression levels in the Control and Ad-MSCs treated cryopreserved canine semen (Group 1). Data obtained by densitometry showing optical densities relative to the expression in Control. Beta actin expression level served as the loading control. Data are expressed as the mean ± SEM. Different lower case letters a, or b, indicate a significant difference at P < 0.05.
Discussion
Studies have demonstrated the potential role of MSCs in various biological processes like contributing to cell generation, survival, and alteration of cellular phenotype27–29. MSCs govern the repair mechanisms occurring at cellular and molecular levels by communication through the release of different factors mostly proteins30. In this study, we investigated the potential role of MSCs in the repair of the plasma membrane and chromatin material of dog sperm following cryopreservation. Canine Ad-MSCs were selected due to their abundant availability, and significantly better gene expression levels as compared to canine skin fibroblasts (Fig. 1). Canine Ad-MSCs show higher expression levels of ANX1, H3, and HMGB due to their un-differentiated nature, multi-lineage differentiation both in vivo & in vitro20, and better proliferation rate as compared to fibroblasts.
This study elucidated the capacity of canine Ad-MSCs to enhance the post-thaw survival rate of dog sperm through ameliorated repair mechanisms, occurring at cellular as well as molecular levels initiated in response to cryoinjury. It is well-known that the variations of temperature during cryopreservation have an inverse effect on function (motility) and structure (plasma membrane, mitochondria, and nucleus) of sperm resulting in impaired fertility31–33. Significant improvements in the post-thaw sperm motility and viability parameters of Group 1, (2.5 × 106 Ad-MSCs per mL of buffer 2). However, the higher concentration of Ad-MSCs in Group 2 resulted in negative impacts on quality-related parameters (Table 1). The sperm in Group 2 suffered serious damage due to increased packing proportion during cryopreservation. As cells become more closely packed, new factors are produced and further protection is required for sustaining cellular viability34–37. Similarly, the post-thaw survival rate of red blood cells34,38, and hepatocytes39, has been negatively influenced by higher concentrations during cryopreservation.
The plasma membrane is the outermost structure of the cell acting as a barrier against foreign agents to maintain the cellular structures and functions. Sperm’s plasma and acrosomal membranes are critically essential for survival following thawing and are termed as the primary sites of change following cryopreservation26. The structural integrity of the plasma membrane is highly correlated with motility and viability of sperm40. Similarly in our study, the Group 1 sperm that showed enhanced integrity of plasma and acrosomal membrane also exhibited significantly higher motility and kinetic parameters (Table 1). The possible reason for improved integrity might be the proteins secreted by Ad-MSCs such as annexin, dysferlin, and fibronectin that are generally involved in repair mechanisms at the cellular level. Annexins have a role in exocytosis, endocytosis, aggregation, membrane fusion41, and Ca+2-dependent membrane binding makes them well suited for repair of membranes42. Annexin 1 has been reported to protect rat retinal ganglion cells against serum-derived apoptosis43. In addition, annexin 1 and 2, along with dysferlin protein have been proposed to be involved in the Ca+2-dependent sarcolemmal repair of skeletal myocytes44,45. Fibronectin regulates cellular processes and the maintenance and repair of damaged tissue46. In addition, it is a seminal plasma protein with a variety of reproductive functions including activation of proteasomes, acrosomal reaction47, capacitation48, gamete interaction49, and embryonic development50. Our assumption that improved membrane integrity was based on high gene expression levels of proteins was well supported by the significantly higher levels of annexin, dysferlin and fibronectin seen in the Group 1 sperm (Fig. 2). We also compared protein expression levels in Group 1 and Control through western analysis, confirming statically higher expression level of annexin and fibronectin proteins in Group 1 (Fig. 3A).
Chromatin integrity appears to be imperative for the proper functioning of sperm51. Excessive damage to sperm DNA compromises fertility52–55 and serves as a predisposing factor for various genetic disorders, birth defects, and early-life cancers in offspring53,56. Histone H3 is one of the core histone proteins, wrapping the DNA of mammalian sperm and its variants have been shown to mediate function like DNA repair57. Similarly, HMGB participates actively in multiple types of nuclear58 and mitochondrial DNA repair59. In our study, Group 1 sperm exhibited a significantly reduced fragmentation of sperm DNA as compared to other groups (Table 1). Quantitative analysis of gene expression among the different groups showed statistically higher expression levels of H3 and HMGB in Group 1 sperm (Fig. 2). The significantly higher expression level of histone H3 protein in Group 1, supported our assumption (Fig. 3B).
Results exhibited that the canine Ad-MSCs have the potential to enhance the quality of cryopreserved dog sperm. Optimal levels of Ad-MSCs positively influenced all the quality-related traits including motility, viability, membrane integrity, acrosomes and chromatin in post-thaw sperm. Canine Ad-MSCs supported repair mechanisms by communicating with the damaged sperm via production of different proteins that serve as an integral part of repair machinery. Further studies would be desirable to determine other factors secreted by Ad-MSCs that may play an active role in preserving sperm quality, and in vivo trials would also help to clarify the usefulness of Ad-MSCs in semen cryopreservation. In addition, the use of Ad-MSCs supplemented semen for artificial insemination may also be helpful in resolving the issues associated with the female reproductive tract due to their regenerative and multipotent nature.
Methods
In this experiment, all chemicals utilized were acquired from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise mentioned.
Animals used
Four healthy mature male beagles, 2–3 years of age, and 7–11 kg weight were utilized for semen collection. A separate indoor cage was used for housing each individual subject, furnished with all the facilities related to animal care and procedures standardized by the Committee for Accreditation of Laboratory Animals at Seoul National University. All procedures were performed according to the guidelines specified in “Guide for the Care and Use of Laboratory Animals at Seoul National University (approval no. SNU-180731–2)”.
Experimental design
In experiment 1, the selection of suitable cell type was performed on the basis of comparison of the relative expression levels of different genes among canine Ad-MSCs and skin fibroblasts through RT-qPCR. In experiment 2, the optimal concentration of canine Ad-MSCs required to preserve the motility and viability of frozen-thawed semen was determined. In experiment 3, the mechanisms responsible for repairing the plasma membrane and chromatin material of the sperm were determined. For this purpose, the expression levels of different genes and proteins in post-thaw sperm were compared among groups without Ad-MSCs (Control) and with an optimal concentration of Ad-MSCs.
Culture of canine Ad-MSCs
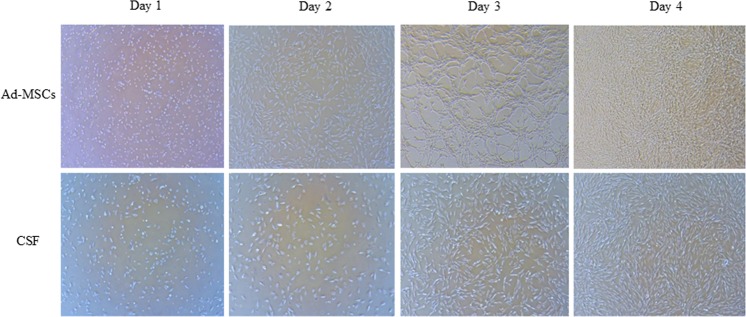
Characterized canine Ad-MSCs60 and AMSC media (the canine Ad-MSCs culture medium) were obtained from Naturecell Co., Ltd (Seoul, Republic of Korea). Ad-MSCs were cultured following the same procedures as previously explained61. Briefly, frozen Ad-MSCs were thawed in a water bath at 37 °C, washed and re-suspended in AMSC media. Following washing, the cells were cultured in a cell culture dish and incubated in a humidified environment of 5% CO2 at 37 °C until 80–90% confluence (Fig. 4). At passage 3, the Ad-MSCs fraction was recovered using 0.05% trypsin-EDTA (Gibco by Life Technologies, Canada), and used in the cryoprocessing of semen.
Figure 4.
Morphology of canine adipose derived mesenchymal stem cells (Ad-MSCs) and canine skin fibroblasts (CSF) after every 24 h of culture up to 80–90% confluence (Magnification: 50×).
Semen collection and preparation
Sperm rich-fractions were collected twice a week. The selection of semen ejaculates for the experiments was based on the same criteria as explained previously61. Briefly, the ejaculates with a sperm count of ≥100 × 106/mL, motile sperm ≥70%, and ≥80% of normal morphology possessing viable sperm were pooled together. Sperm were separated from seminal fluids by the spinning of the pooled ejaculate at 100× g for 1 min at room temperature and recovered the supernatant. The same volume of buffer 1 [24 g/L tris (hydroxymethyl) aminomethane, 14 g/L citric acid, 8 g/L of fructose, and 0.15 g/L kanamycin sulfate in distilled water (pH 6.6, 290 mOsmol)] was added to the recovered supernatant and was subjected to centrifugation at 700× g speed for 5 min. Resuspended sperm pellet using buffer 1 to achieve a concentration of 200 × 106 sperm/mL. The final concentration of 100 × 106 sperm/mL was attained by adding a suitable volume of buffer 2 (6% (v/v) glycerol, 40% (v/v) egg yolk, and 54% (v/v) buffer 1)62, supplemented without Ad-MSCs (Control), with 2.5 × 106 Ad-MSCs/mL (Group 1) or with 5 × 106 Ad-MSCs/mL (Group 2). The mixing of buffer 2 was carried out following a multi-step loading protocol, mixing final sperm fraction with 14%, 19%, 27% and 40% of buffer 2 (total calculated volume) at an interval of 30 sec. The Control and Groups 1 & 2 were prepared and incubated for 45–60 min in a CO2 incubator at 37 °C prior to sperm collection.
Freezing and thawing of sperm
Following extension, the semen was filled into 0.25 mL straws (Minitub, Germany). Sealed straws were kept at 4 °C for 1 h to carry out the equilibration procedure, followed by freezing of semen by horizontally placing the straws 2 cm above liquid nitrogen (LN2) for 15–20 min and finally stored at −192 °C using LN2. Post-thaw evaluations were performed a week after cryopreservation of semen. Thawing of semen was performed in a water bath at 37 °C for 30 sec, then diluted with buffer 1 (1:5, semen: buffer 1) stepwise by 14%, 19%, 27% and finally 40% of the total volume.
Separation of sperm from Ad-MSCs
Post-thaw sperm were separated from the Ad-MSCs using a percoll density gradient procedure, as previously explained63. Briefly, 1 mL of post-thaw semen from each group was pipetted gently onto a double-layered column of 45%/90% Percoll (Pharmacia) in a conical tube of 15 mL capacity. The tubes were then centrifuged at 400 × g for 20 min and the fluid was recovered from phase C, D, and E for further analysis of different parameters. These phases have been reported to possess higher percentages of sperm output, motility and fewer number of Ad-MSCs63.
Assessment of motility and kinetic parameters
Post-thaw motility and kinetic parameters were examined following the same procedure as previously explained61. Briefly, a semen drop (10 µL) was placed on a pre-warmed glass slide and mounted with a cover glass. Sperm were screened in 5 different fields and 200 motile sperm were tracked for the assessment of the kinetic parameters employing a sperm analysis imaging system (FSA2011 premium edition version 2011; Medical Supply, Korea).
Assessment of viability of sperm
Eosin-nigrosin staining procedure was used to examine the percentage of viable sperm as previously explained64,65. Briefly, a semen drop (5–10 µL) was mixed with an equal amount of the stain on a pre-warmed glass slide. A thin smear was made on a new slide using the semen-stain mixture and dried in air. Examined 200 hundred sperm per slide and classified as possessing an intact membrane (white staining) or a non-intact membrane (pink staining).
Assessment of the plasma membrane integrity
The integrity of the sperm plasma membrane was accessed by hypo-osmotic swelling (HOS) assay65, using a HOS solution of 190 mOsmol/kg. Fifty µL of post-thaw semen from each group was mixed with 500 µL of HOS solution and incubated at 37 °C for 30 min. Following incubation, a 5-µL drop of the mixture was placed on a clean, warm glass slide and 200 sperm were examined for their swelling ability using a phase-contrast microscope (Eclipse Ts 2, Nikon). The sperm with intact plasma membrane showed swelling, indicated by the coiled tail.
Assessment of acrosomal membrane integrity
The intactness of sperm acrosome was analyzed using the fluorescein isothiocyanate-conjugated peanut agglutinin (FITC-PNA) staining method. Semen drop (30 µL) was used to prepare smears on glass slides. Smears were air-dried and fixed by dipping the smears in absolute methanol at 20–22 °C for 10 min. After drying the smears, staining was performed by spreading 30 µL of FITC-PNA solution (100 µg/mL) in phosphate buffer saline (PBS). Subsequently, slides were incubated in a dark moist chamber at 37 °C for 30 min. Finally, smears were rinsed using PBS, air-dried and immediately observed using an epifluorescence phase-contrast microscope (Eclipse Ts 2, Nikon). The acrosome-intact sperm were observed with strong green fluorescence and the percentage of fluorescent acrosome-intact sperm was counted in at-least 200 sperms per slide.
Assessment of sperm chromatin quality
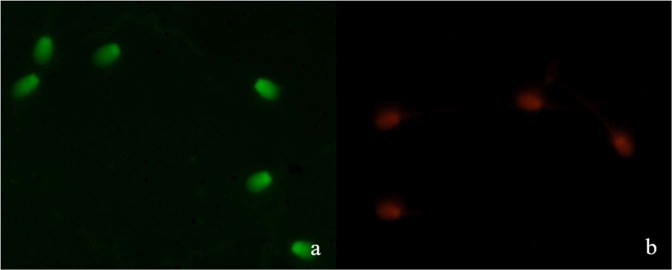
Washed separated sperm were smeared onto a clean glass slide, dried in the air, followed by overnight fixation using Carnoy’s solution (3:1, methanol: glacial acetic acid) and dried again in the air. The slides were dipped into a solution of 0.1 M citric acid solution (pH 2.5) at room temperature for 5 min and then rinsed repeatedly with distilled water. The smeared slides were then stained for 5 min in acridine orange solution (0.2 mg/mL water) and washed with distilled water. Analysis of the slides was carried out fluorescence microscopy at 1,000× magnification of Zeiss microscope and nuclei from 200 sperm were examined. Sperm with normal chromatin material showed green-fluorescence at the head (bicatenary DNA, Fig. 5a), whereas abnormal chromatin material showed red-fluorescence at the head (monocatenary, DNA, Fig. 5b).
Figure 5.
Image of acridine orange-stained sperm showing normal (a) and denatured (b) sperms.
Assessment of gene expression
Quantitative polymerase chain reaction (qPCR) was conducted both for comparison among cells (Ad-MSCs vs. skin fibroblasts) and different semen groups (Control, Group 1, and Group 2) as previously described1. Briefly, RNA was extracted from cells and post-thawed sperm obtained from five pairs of straws from each treatment group and Control. For the assessment of transcript abundance, oligonucleotide primer sequences, (listed in Table 3) were used, employing real-time qPCR (RT-qPCR). Extraction of RNA was conducted using Trizol reagent (Invitrogen, USA), followed by complementary DNA synthesis using Maxime RT PreMix (Intronbio, Korea) following the manufacturer’s protocol. RT-qPCR was utilized to examine the expression of plasma membrane repair-related genes (annexin 1, ANX1; dysferlin, DYSF; and fibronectin 1, FN1) and the chromatin material repair-related genes (high mobility group protein B, HMGB; and histone H3, H3;) using Step One Plus Real-Time PCR System (Applied Biosystems, USA) and the quantification of expression level of each target gene relative to that of the internal gene beta-actin (BACT) was conducted employing the equation, R = 2−[ΔCt sample − ΔCt control].
Table 3.
Primer sequences used for gene expression analysis.
| Gene | Primer sequence (5′-3′) | Product size (bp) | NCBI accession no. |
|---|---|---|---|
| BACT | F: GAGGCATCCTGACTCTGA | 87 | XM_544346.3 |
| R: TCTGGCACCACACTTTCT | |||
| ANX1 | F: GAAGCTCTGAAGAAAGCCC | 128 | NM_001286970.1 |
| R: GTGTCTTCATCAGTTCCAAGG | |||
| H3 | F: CGGTGACTGACACGCGAC | 136 | XM_022404950.1 |
| R: GTTGGAGCAGGCCTTGAACC | |||
| FN1 | F: ATAGCTGGCTGTTACGAC | 74 | XM_022415242.1 |
| R: GCATTTCCCAGGTAGGTG | |||
| HMGB | F: ATATTGCTGCGTACCGAG | 64 | XM_022409535.1 |
| R: TCAGCCTTGACAACTCCC | |||
| DYSF | F: TGGATCAGAGTGGCGTCC | 127 | XM_003432223.4 |
| R: GACAGCAGCTTTCTGGCT |
F, forward; R, reverse. Beta actin (BACT), annexin 1 (ANX1), histone H3 (H3), fibronectin 1(FN 1), high mobility group protein B (HMGB), and dysferlin (DYSF).
Western blot analysis
Western analysis of post-thaw semen was conducted using individual samples from treatment Group 1 and Control (n = 6 per group). Protein extraction from the semen samples was carried out using Protein Extraction Solution (PRO-PREPTM, iNtRON biotechnology, Korea). In each group, a total of 25 µg protein was added in 2X Laemmli sample buffer (Bio-Rad laboratories, USA) in the ratio 1:2. Proper mixing was achieved through gentle pipetting, followed by the boiling of samples for 10 min at 100 °C. Loaded samples into a 10% (w/v) polyacrylamide gel and electrophoresis was carried out at a constant voltage (90 V) for 2 h. Precision Plus ProteinTM Standards Dual Color (Bio-Rad) was utilized as a molecular weight marker. The separated proteins were transferred (15 V for 80 min) to methanol-activated polyvinylidene difluoride (PVDF) membranes (Immobilon-P transfer membranes, Merck KGaA, Darmstadt, Germany) and blocked using 5% (w/v) nonfat dry milk (Bio-Rad) solution at room temperature for 90 min. Membranes were exposed at 4 °C to primary goat polyclonal anti-annexin A1 (Abcam, UK), rabbit polyclonal anti-acetyl-histone H3 (Millipore, USA), rabbit polyclonal anti-fibronectin (Abcam, UK), mouse monoclonal anti-HMG-1 (Sigma, USA) rabbit polyclonal anti-dysferlin, and mouse monoclonal anti-beta actin (Abcam, UK) followed by incubation in respective horseradish peroxidase-conjugated anti-goat, rabbit or mouse (Abcam, UK), at the same temperature for 2 h. Membranes were incubated with ClarityTM Western ECL substrate (Bio-Rad) for 5 min, and the chemiluminescence signals were read using Chemiluminescence imaging-Fusion SOLO software (Vilber Lourmat, France). The quantification of band densities was carried out employing Image J software (Version 1.41; National Institutes of Health, Bethesda, MD, USA).
Statistics
All values were presented as mean ± SEM, and a P-value of <0.05 was taken as an indication of statistical significance. An independent sample t-test was used for comparing the gene expression levels among canine Ad-MSCs and skin fibroblasts. One-way analysis of variance (ANOVA) and Tukey’s Multiple Comparison Test were utilized for comparing the treatments and Control group. The correlation between different parameters was also determined66. One sample t-test was utilized for comparing the results of western blot analysis from the Control and optimal Ad-MSCs concentration group. Analysis of data was conducted using SPSS 21.0 software (SPSS Inc., Chicago, USA).
Ethics approval and consent to participate
This study was approved by the Ethics Committee on Animal Use (approval no. SNU-180731-2-1) of the Seoul National University (Seoul, Republic of Korea).
Supplementary information
Acknowledgements
The authors would like to acknowledge the funding agency; Cooperative Research Program of RDA (CCAR, #PJ013954012019) and the assistance of Feriel Yasmine Mahiddine and Ye Sol Yun.
Author contributions
J.C. and M.J.K. conceived the idea and designed the experiment. A.Y.Q., X.F., and M.J.K. performed the experiments. M.J.K. and A.Y.Q. performed data analysis and manuscript writing. All read final manuscript and approved.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Min Jung Kim, Email: tinia19@snu.ac.kr.
JongKi Cho, Email: cjki@cnu.ac.kr.
Supplementary information
is available for this paper at 10.1038/s41598-020-61803-8.
References
- 1.Abdillah DA, et al. Iodixanol supplementation during sperm cryopreservation improves protamine level and reduces reactive oxygen species of canine sperm. Journal of veterinary science. 2019;20:79–86. doi: 10.4142/jvs.2019.20.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.England GC, Millar KM. The ethics and role of AI with fresh and frozen semen in dogs. Reproduction in domestic animals = Zuchthygiene. 2008;43(Suppl 2):165–171. doi: 10.1111/j.1439-0531.2008.01157.x. [DOI] [PubMed] [Google Scholar]
- 3.Lecewicz M, Strzezek R, Kordan W, Majewska A. Effect of Extender Supplementation with Low-molecular-weight Antioxidants on Selected Quality Parameters of Cryopreserved Canine Spermatozoa. Journal of veterinary research. 2018;62:221–227. doi: 10.2478/jvetres-2018-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holt WV, Head MF, North RD. Freeze-induced membrane damage in ram spermatozoa is manifested after thawing: observations with experimental cryomicroscopy. Biology of reproduction. 1992;46:1086–1094. doi: 10.1095/biolreprod46.6.1086. [DOI] [PubMed] [Google Scholar]
- 5.Sang-Hyoun Park YJ. Il-Jeoung Yu. Effects of antioxidants supplementation in porcine sperm freezing on in vitro fertilization and the glutathione and reactive oxygen species level of presumptive zygotes. Journal of Animal Reproduction and Biotechnology. 2017;32:337–342. doi: 10.12750/JET.2017.32.4.337. [DOI] [Google Scholar]
- 6.LA, J. In In: Johnson, L. A.; Larsson, K. (eds) Deep Freezing Boar Semen: Proc. First Int. Conf. Deep Freezing of Boar Semen Swedish University of Agricultural Sciences, Uppsala, Sweden. 192–222.
- 7.Fraser L, Strzezek J. Effect of different procedures of ejaculate collection, extenders and packages on DNA integrity of boar spermatozoa following freezing-thawing. Animal reproduction science. 2007;99:317–329. doi: 10.1016/j.anireprosci.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Aitken RJ, De Iuliis GN. On the possible origins of DNA damage in human spermatozoa. Molecular human reproduction. 2010;16:3–13. doi: 10.1093/molehr/gap059. [DOI] [PubMed] [Google Scholar]
- 9.Johnson AE, Freeman EW, Wildt DE, Songsasen N. Spermatozoa from the maned wolf (Chrysocyon brachyurus) display typical canid hyper-sensitivity to osmotic and freezing-induced injury, but respond favorably to dimethyl sulfoxide. Cryobiology. 2014;68:361–370. doi: 10.1016/j.cryobiol.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Rodenas C, Parrilla I, Roca J, Martinez EA, Lucas X. Effects of rapid cooling prior to freezing on the quality of canine cryopreserved spermatozoa. The Journal of reproduction and development. 2014;60:355–361. doi: 10.1262/jrd.2014-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michael A, et al. Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine spermatozoa. Theriogenology. 2007;68:204–212. doi: 10.1016/j.theriogenology.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 12.Mokarizadeh A, Rezvanfar MA, Dorostkar K, Abdollahi M. Mesenchymal stem cell derived microvesicles: trophic shuttles for enhancement of sperm quality parameters. Reproductive toxicology (Elmsford, N.Y.) 2013;42:78–84. doi: 10.1016/j.reprotox.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 13.Kim D. Evaluation of antifreeze proteins on miniature pig sperm viability, DNA damage, and acrosome status during cryopreservation. Journal of embryo transfer. 2016;31:355–365. [Google Scholar]
- 14.Sang-Hyoun Park I-JY. Effect of antioxidant supplementation in freezing extender on porcine sperm viability, motility and reactive oxygen species. Journal of Animal Reproduction and Biotechnology. 2017;32:9–15. doi: 10.12750/JET.2017.32.1.9. [DOI] [Google Scholar]
- 15.Sang-Hyoun Park KBO, Ock S-A. Sung June Byun, Hwi-Cheul Lee, Suresh Kumar, Sung Gu Lee, Jae-Seok Woo. Effects of ice-binding protein from Leucosporidium on the cryopreservation of boar sperm. Journal of Animal Reproduction and Biotechnology. 2018;33:185–194. doi: 10.12750/JET.2018.33.3.185. [DOI] [Google Scholar]
- 16.Eun-Ji Kim NAHT, Jeon Y-B, Yu I-J. Effect of K-Carrageenan on sperm quality in cryopreservation of canine semen. Journal of Animal Reproduction and Biotechnology. 2019;34:57–63. doi: 10.12750/JARB.34.1.57. [DOI] [Google Scholar]
- 17.Uchoa DC, Silva TF, Mota Filho AC, Silva LD. Intravaginal artificial insemination in bitches using frozen/thawed semen after dilution in powdered coconut water (ACP-106c) Reproduction in domestic animals = Zuchthygiene. 2012;47(Suppl 6):289–292. doi: 10.1111/rda.12077. [DOI] [PubMed] [Google Scholar]
- 18.Gavella M, Lipovac V, Garaj-Vrhovac V, Gajski G. Protective effect of gangliosides on DNA in human spermatozoa exposed to cryopreservation. Journal of andrology. 2012;33:1016–1024. doi: 10.2164/jandrol.111.015586. [DOI] [PubMed] [Google Scholar]
- 19.Caplan AI, Correa D. The MSC: an injury drugstore. Cell stem cell. 2011;9:11–15. doi: 10.1016/j.stem.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caplan AI. What’s in a name? Tissue engineering. Part A. 2010;16:2415–2417. doi: 10.1089/ten.TEA.2010.0216. [DOI] [PubMed] [Google Scholar]
- 21.Khubutiya MS, Vagabov AV, Temnov AA, Sklifas AN. Paracrine mechanisms of proliferative, anti-apoptotic and anti-inflammatory effects of mesenchymal stromal cells in models of acute organ injury. Cytotherapy. 2014;16:579–585. doi: 10.1016/j.jcyt.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 22.Iwase T, et al. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovascular research. 2005;66:543–551. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Stolzing A, Scutt A. Effect of reduced culture temperature on antioxidant defences of mesenchymal stem cells. Free radical biology & medicine. 2006;41:326–338. doi: 10.1016/j.freeradbiomed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- 24.Hammerstedt RH, Parks JE. Changes in sperm surfaces associated with epididymal transit. Journal of reproduction and fertility. Supplement. 1987;34:133–149. [PubMed] [Google Scholar]
- 25.Amann RP, Hammerstedt RH, Veeramachaneni DN. The epididymis and sperm maturation: a perspective. Reproduction, fertility, and development. 1993;5:361–381. doi: 10.1071/RD9930361. [DOI] [PubMed] [Google Scholar]
- 26.Muino-Blanco T, Perez-Pe R, Cebrian-Perez JA. Seminal plasma proteins and sperm resistance to stress. Reproduction in domestic animals = Zuchthygiene. 2008;43(Suppl 4):18–31. doi: 10.1111/j.1439-0531.2008.01228.x. [DOI] [PubMed] [Google Scholar]
- 27.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews. Immunology. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 28.Valadi H, et al. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature cell biology. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 29.Anand PK. Exosomal membrane molecules are potent immune response modulators. Communicative & integrative biology. 2010;3:405–408. doi: 10.4161/cib.3.5.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo Q, Zhang B, Kuang D, Song G. Role of Stromal-Derived Factor-1 in Mesenchymal Stem Cell Paracrine-Mediated Tissue Repair. Current stem cell research & therapy. 2016;11:585–592. doi: 10.2174/1574888X11666160614102629. [DOI] [PubMed] [Google Scholar]
- 31.Donnelly ET, McClure N, Lewis SE. Cryopreservation of human semen and prepared sperm: effects on motility parameters and DNA integrity. Fertility and sterility. 2001;76:892–900. doi: 10.1016/S0015-0282(01)02834-5. [DOI] [PubMed] [Google Scholar]
- 32.Donnelly ET, Steele EK, McClure N, Lewis SE. Assessment of DNA integrity and morphology of ejaculated spermatozoa from fertile and infertile men before and after cryopreservation. Human reproduction (Oxford, England) 2001;16:1191–1199. doi: 10.1093/humrep/16.6.1191. [DOI] [PubMed] [Google Scholar]
- 33.Spano M, et al. Nuclear chromatin variations in human spermatozoa undergoing swim-up and cryopreservation evaluated by the flow cytometric sperm chromatin structure assay. Molecular human reproduction. 1999;5:29–37. doi: 10.1093/molehr/5.1.29. [DOI] [PubMed] [Google Scholar]
- 34.Mazur P, Cole KW. Influence of cell concentration on the contribution of unfrozen fraction and salt concentration to the survival of slowly frozen human erythrocytes. Cryobiology. 1985;22:509–536. doi: 10.1016/0011-2240(85)90029-X. [DOI] [PubMed] [Google Scholar]
- 35.Mazur P, Cole KW. Roles of unfrozen fraction, salt concentration, and changes in cell volume in the survival of frozen human erythrocytes. Cryobiology. 1989;26:1–29. doi: 10.1016/0011-2240(89)90030-8. [DOI] [PubMed] [Google Scholar]
- 36.Mazur P, Rigopoulos N. Contributions of unfrozen fraction and of salt concentration to the survival of slowly frozen human erythrocytes: influence of warming rate. Cryobiology. 1983;20:274–289. doi: 10.1016/0011-2240(83)90016-0. [DOI] [PubMed] [Google Scholar]
- 37.Pegg DE, Diaper MP. The “unfrozen fraction” hypothesis of freezing injury to human erythrocytes: a critical examination of the evidence. Cryobiology. 1989;26:30–43. doi: 10.1016/0011-2240(89)90031-X. [DOI] [PubMed] [Google Scholar]
- 38.Pegg DE. The effect of cell concentration on the recovery of human erythrocytes after freezing and thawing in the presence of glycerol. Cryobiology. 1981;18:221–228. doi: 10.1016/0011-2240(81)90092-4. [DOI] [PubMed] [Google Scholar]
- 39.De Loecker W, Koptelov VA, Grischenko VI, De Loecker P. Effects of cell concentration on viability and metabolic activity during cryopreservation. Cryobiology. 1998;37:103–109. doi: 10.1006/cryo.1998.2106. [DOI] [PubMed] [Google Scholar]
- 40.Hammerstedt RH, Graham JK, Nolan JP. Cryopreservation of mammalian sperm: what we ask them to survive. Journal of andrology. 1990;11:73–88. [PubMed] [Google Scholar]
- 41.Gerke V, Moss SE. Annexins: from structure to function. Physiological reviews. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 42.Draeger A, Monastyrskaya K, Babiychuk EB. Plasma membrane repair and cellular damage control: the annexin survival kit. Biochemical pharmacology. 2011;81:703–712. doi: 10.1016/j.bcp.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 43.Sun J, et al. Annexin 1 protects against apoptosis induced by serum deprivation in transformed rat retinal ganglion cells. Molecular biology reports. 2012;39:5543–5551. doi: 10.1007/s11033-011-1358-1. [DOI] [PubMed] [Google Scholar]
- 44.Han R, Campbell KP. Dysferlin and muscle membrane repair. Current opinion in cell biology. 2007;19:409–416. doi: 10.1016/j.ceb.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lennon NJ, et al. Dysferlin interacts with annexins A1 and A2 and mediates sarcolemmal wound-healing. The Journal of biological chemistry. 2003;278:50466–50473. doi: 10.1074/jbc.M307247200. [DOI] [PubMed] [Google Scholar]
- 46.To WS, Midwood KS. Plasma and cellular fibronectin: distinct and independent functions during tissue repair. Fibrogenesis & tissue repair. 2011;4:21. doi: 10.1186/1755-1536-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Diaz ES, Kong M, Morales P. Effect of fibronectin on proteasome activity, acrosome reaction, tyrosine phosphorylation and intracellular calcium concentrations of human sperm. Human reproduction (Oxford, England) 2007;22:1420–1430. doi: 10.1093/humrep/dem023. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-Leon E, et al. Fibronectin stimulates human sperm capacitation through the cyclic AMP/protein kinase A pathway. Human reproduction (Oxford, England) 2015;30:2138–2151. doi: 10.1093/humrep/dev154. [DOI] [PubMed] [Google Scholar]
- 49.Thys M, et al. Expression and putative function of fibronectin and its receptor (integrin alpha(5)beta(1)) in male and female gametes during bovine fertilization in vitro. Reproduction (Cambridge, England) 2009;138:471–482. doi: 10.1530/rep-09-0094. [DOI] [PubMed] [Google Scholar]
- 50.Chen D, et al. Fibronectin signals through integrin alpha5beta1 to regulate cardiovascular development in a cell type-specific manner. Developmental biology. 2015;407:195–210. doi: 10.1016/j.ydbio.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Human reproduction update. 2003;9:331–345. doi: 10.1093/humupd/dmg027. [DOI] [PubMed] [Google Scholar]
- 52.Evenson DP, Darzynkiewicz Z, Melamed MR. Relation of mammalian sperm chromatin heterogeneity to fertility. Science (New York, N.Y.) 1980;210:1131–1133. doi: 10.1126/science.7444440. [DOI] [PubMed] [Google Scholar]
- 53.Aitken RJ, Baker MA, Sawyer D. Oxidative stress in the male germ line and its role in the aetiology of male infertility and genetic disease. Reproductive biomedicine online. 2003;7:65–70. doi: 10.1016/S1472-6483(10)61730-0. [DOI] [PubMed] [Google Scholar]
- 54.Carrell DT, et al. Sperm DNA fragmentation is increased in couples with unexplained recurrent pregnancy loss. Archives of andrology. 2003;49:49–55. doi: 10.1080/01485010290099390. [DOI] [PubMed] [Google Scholar]
- 55.Henkel R, et al. Influence of deoxyribonucleic acid damage on fertilization and pregnancy. Fertility and sterility. 2004;81:965–972. doi: 10.1016/j.fertnstert.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 56.Fraga CG, Motchnik PA, Wyrobek AJ, Rempel DM, Ames BN. Smoking and low antioxidant levels increase oxidative damage to sperm DNA. Mutation research. 1996;351:199–203. doi: 10.1016/0027-5107(95)00251-0. [DOI] [PubMed] [Google Scholar]
- 57.Malik HS, Henikoff S. Phylogenomics of the nucleosome. Nature structural biology. 2003;10:882–891. doi: 10.1038/nsb996. [DOI] [PubMed] [Google Scholar]
- 58.Yuan F, Gu L, Guo S, Wang C, Li GM. Evidence for involvement of HMGB1 protein in human DNA mismatch repair. The Journal of biological chemistry. 2004;279:20935–20940. doi: 10.1074/jbc.M401931200. [DOI] [PubMed] [Google Scholar]
- 59.Ito H, et al. HMGB1 facilitates repair of mitochondrial DNA damage and extends the lifespan of mutant ataxin-1 knock-in mice. EMBO molecular medicine. 2015;7:78–101. doi: 10.15252/emmm.201404392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han SH, et al. Effect of ectopic OCT4 expression on canine adipose tissue-derived mesenchymal stem cell proliferation. Cell biology international. 2014;38:1163–1173. doi: 10.1002/cbin.10295. [DOI] [PubMed] [Google Scholar]
- 61.Qamar AY, Fang X, Kim MJ, Cho J. Improved post-thaw quality of canine semen after treatment with exosomes from conditioned medium of adipose-derived mesenchymal stem cells. Animals. 2019;9:865. doi: 10.3390/ani9110865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nugraha Setyawan EM, et al. Corrigendum to “Maintaining canine sperm function and osmolyte content with multistep freezing protocol and different cryoprotective agents” [Cryobiol. 71 (2015) 344–349] Cryobiology. 2016;73:446. doi: 10.1016/j.cryobiol.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 63.Phillips TC, Dhaliwal GK, Verstegen-Onclin KM, Verstegen JP. Efficacy of four density gradient separation media to remove erythrocytes and nonviable sperm from canine semen. Theriogenology. 2012;77:39–45. doi: 10.1016/j.theriogenology.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 64.Kim S, Lee Y, Yang H, Kim YJ. Rapid freezing without cooling equilibration in canine sperm. Animal reproduction science. 2012;130:111–118. doi: 10.1016/j.anireprosci.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 65.Root Kustritz MV. The value of canine semen evaluation for practitioners. Theriogenology. 2007;68:329–337. doi: 10.1016/j.theriogenology.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 66.Steel, R. G. D., Torrie, J., Dickey, D. A. Principle and procedures of statistics. A biochemical approach 3rd edition McGraw Hill Book Co.Inc, New York, USA. (1997).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.