Abstract
Background and Objective:
Periodontal disease (PD) afflicts approximately 50% of the population in the United States and is characterized by chronic inflammation of the periodontium that can lead to loss of the periodontal ligament through collagen degradation, loss of alveolar bone, and to eventual tooth loss. Previous studies have implicated transglutaminase (TG) activity in promoting thin collagen I fiber morphology and decreased mechanical strength in homeostatic PDL. The aim of this study is to determine whether TG activity influenced collagen assembly in PDL in the setting of periodontal disease.
Materials and Methods:
A ligature model was used to induce clinically relevant PD in mice. Mice with ligature were assessed at 5 and 14 days to determine PDL collagen morphology, transglutaminase (TG) activity, and bone loss. The effects of inhibition of TG on PDL was assessed by immunohistochemistry and second harmonic generation (SHG) to visualize collagen fibers in native tissue.
Results:
Ligature placement around the 2nd molar resulted in significant bone loss and a decrease in total collagen content after 5 days of ligature placement. A significant increase in thin over thick fibers was also demonstrated in mice with ligature at 5 day associated with apparent increases in immunoreactivity for TG2 and for TG-mediated N-ε-γ-glutamyl cross-links in PDL. Inhibition of TG activity increased total collagen and thick collagen fiber content over vehicle control in mice with ligature for 5 days. SHG of PDL was used to visualize and quantify the effects of TG inhibition on enhanced collagen fiber organization in unfixed control and diseased PDL.
Conclusion:
These studies support a role of TG in regulating collagen fiber assembly and suggest that strategies to inhibit TG activity in disease might contribute to restoration of PDL tissue integrity.
Keywords: EXTRACELLULAR MATRIX, NONCOLLAGENOUS PROTEINS, DENTAL BIOLOGY, PERIODONTAL DISEASE, SECOND HARMONIC GENERATION IMAGING
Introduction
The collagen fibers of the periodontal ligament (PDL) secure the tooth root into the bone socket. Degradation of PDL collagen fibers in periodontal disease (PD) is a contributing factor to decreases in alveolar bone and eventual tooth loss. Maintaining and restoring PDL collagen content and integrity following disease treatment are essential to periodontal health (1). Currently, therapeutic strategies for regenerating collagen fibers in the PDL are extremely limited and further research is needed to generate new strategies (2).
PDL provides an excellent tissue milieu for investigating mechanisms of collagen processing and assembly during inflammatory disease. Key factors that regulate collagen assembly in the extracellular matrix (ECM) include cross-linking enzymes. Enzymes that catalyze the formation of intra- and intermolecular crosslinks on collagen fibrils facilitate the formation of mature, insoluble collagen fibers (3). Transglutaminases (TGs) are a family of 8 proteins that catalyze extracellular crosslinking via an irreversible covalent acyl exchange; a transamidation reaction, between glutamine and lysine residues resulting in the formation of a γ-glutamyl-ε−lysyl covalent bond (4). TG2, is the most ubiquitously expressed TG family member (4). The crosslinking of ECM proteins by TG is predicted to promote assembly and/or enhance stabilization of collagenous and noncollagenous ECMs (5, 6).
Previously, utilizing transgenic SPARC-null mice that show global reductions in collagen content and fiber thickness, we identified TG activity as a key factor in mediating the collagen phenotype in SPARC-null PDL (7). Increased TG activity in SPARC-null PDL resulted in increased immunoreactivity for TG cross-links and the formation of tighter, thinner collagen fibers. Furthermore, inhibition of TG activity in vivo partially restored collagen content, fiber thickness, and mechanical strength of PDL tissue in SPARC-null mice (8). Herein, experimental evidence, including state of the art methods for viewing collagen architecture by second harmonic generation (SHG), is reported showing TG activity in PD influences levels of collagen and collagen fiber morphology in periodontitis.
Materials and Methods
Animal Use and Care
Male and female C57Bl/6J mice, 3–4 months of age, were randomly assigned within groups. Six-week mice were purchased from Jackson Labs and housed in the Ralph H. Johnson Veteran’s Administration animal facility. Mice were supplied with a standard diet of hard chow. All animal procedures used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of the Ralph H. Johnson Veterans Affairs Medical Center.
Ligature Model of PD
The murine ligature model was based on that described by Abe and Hajishengallis (9). Briefly, 20 mice were individually anesthetized, the mandible, maxilla, and cheeks were retracted and a 0.5 metric silk ligature (Covidien, Mansfield, MA) was placed in the gingival sulcus around the left maxillary second molar. Gingival blanching around the second molar was used as an indicator of correct ligature placement. Right maxillary molars were left untreated and served as same animal controls. Ligatures were left in place for 5- and 14-day time points. Retention of ligature was checked every other day. Ligatures were removed from maxillae at end point to facilitate processing of tissue and to confirm periodontal retention of the ligature until endpoint. Ten mice were used for both micro-computed tomography and immunohistochemistry; 5 each for 5 and 14 day endpoints for each analysis.
Micro-computed Tomography (μCT) and Bone Volume Fraction Analysis
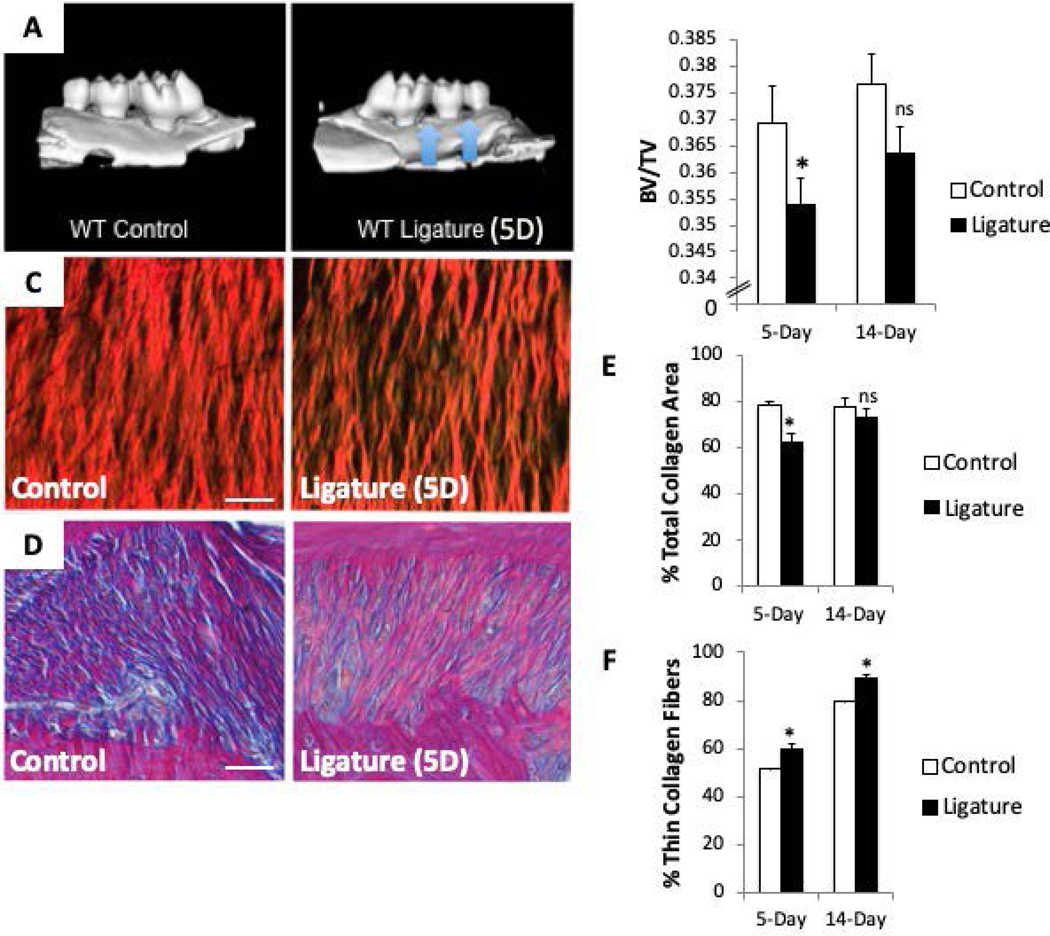
Mandibles and maxillae were harvested from mice (5 mice at 5 days and 5 mice at 14 days) and excess tissue removed. Jaws were incubated overnight in zinc-formalin and scanned using a Scanco Medical μCT 40 system and analyzed with GE microview software. Each scan was reconstructed at a mesh size of 18 × 18 × 18 μm to create a 3D digitized images that were then rotated into a standard orientation. A threshold of 1621 was used to distinguish between mineralized and non-mineralized tissue. The region of interest (ROI) for the BV/TV analysis was based on 3 identifiable anatomical landmarks of each jaw: 1. mesial CEJ of 1st molar to distal CEJ of 3rd molar with jaw oriented in mid-sagittal view, 2. tips of crown cusps, and 3. apical portion of anterior third molar root (Fig. 1). Buccal:palatal aspects of ROI box are resized according to landmarks and 0.9mm are added to the ROI on the Z-axis. ROI analyses were calibrated to exclude tooth roots and PDL space. All μCT scans were blindly assessed and quantified.
Figure 1:
The murine ligature model significantly reduces alveolar bone volume (BV) fraction and periodontal ligament (PDL) collagen content compared to controls. A). Representative μCT images of ligature-induced alveolar bone loss around second maxillary molar (blue arrows). B). BV/Total volume (BV/TV) quantification following 5-day and 14-day ligature. C). Representative Picro Sirius Red (PSR) images of PDL following 5-day ligature. D). Representative images of Herovici-stained PDL following 5-day ligature. Pink/red = thick/mature collagen fibers, blue/purple = thin/young collagen, yellow = cell death, black = nuclei. E). Quantification of total collagen I content of PDL following 5- and 14-day ligature. F). Quantification of thin collagen fibers (green/yellow) from PSR-stained sections following 5- and 14-day ligature. Each image oriented with tooth on top, PDL centered, and alveolar bone on bottom. Images shown are of PDL surrounding second maxillary molar. *p < 0.05 between control and ligature, as determined by Student’s t test. n = 5 mice per group. Scale bars = 25 μm. Error bars = SEM. 5D = 5-day ligature
Histological Analysis of Collagen Morphology and Collagen Volume Fraction
Mandibles and maxillae were dissected, fixed overnight in zinc-formalin, and decalcified in 0.5M EDTA changed daily for 2 weeks (5 mice at 5 days and 5 mice at 14 days). Tissue sections were stained with picrosirius Red (PSR) and quantified as described (10). Slides were visualized with polarized light under which thick collagen fibers appear bright red or red/orange and thin collagen fibers appear green/yellow in color easily differentiated against a black background. Quantitative morphometric analysis of images was conducted using Visiopharm Integrator System (VIS, version 3.2.9.0) with parameters set to measure total area of PDL, background area, total collagen area, thick collagen fiber area (red/orange), and thin collagen fiber area (green/yellow). Herovici stain was performed as per manufacturer’s instructions (American MasterTech). Newly formed collagen fibers or thin fibers stained blue and homeostatic mature collagen or thick fibers stained red/pink were quantified utilizing the same software described above.
Immunoreactivity of TG2 and Cross-link Epitope
Immunohistochemistry was performed on maxillary sections following 5-day ligature with same animal controls. Sections were incubated with a polyclonal anti-TG2 primary antibody (pab0024-P, Covalab) or a monoclonal against TG generated cross link epitope: anti-N-(ε−γ-glutamyl)-L-lysine (mab0009-P, Covalab). Secondary antibodies conjugated to biotin were detected with avidin horse-radish peroxidase followed by incubation with 3,3’-diaminobenzidine (Vector Labs). Sections incubated without primary antibody only were used as controls.
In Vivo TG Inhibition with BPA Injection
Twenty-two C57Bl/6J (2–3 months of age) male mice underwent ligature placement. PDL was injected with either 2 μL of 5-(Biotinamido) pentylamine (BPA, 2 mM, solubilized in DMSO) (Sigma-Aldrich, A5348) (n = 5) or 2 μL of DMSO (n = 5) at sites surrounding the 2nd maxillary molar on both the left (ligated) and the right (non-ligated control) side daily for 4 days, a final fifth injection was given 1 hour prior to sacrifice on day 5. Daily injections were used to accommodate the 5-day time point. A second and third set of mice (n = 6: BPA n = 3, DMSO n = 3, for each) were used for second harmonic generation (SHG) imaging and immunohistochemistry.
Second-Harmonic Generation
Non-fixed, non-decalcified maxillae freshly removed from mice were rinsed and then soaked in 50% glycerol for 1 hour (11). Jaws were mounted in optimal cutting temperature (OCT) and sliced to obtain a flat exposed longitudinal slice of PDL using a sledge microtome (Leica Microsystems). Specimens were rinsed in PBS to remove OCT prior to imaging on an Olympus Fluoview 1200 MPA. The excitation laser for SHG was set at a wavelength of 860 nm, and the signal collected in the 420–460 nm range. A 30X lens was used to capture an image resolution of 1024 × 1024 with a field of view of 423 × 423 μm. For z stack images, slices were taken at a range of 0.2 – 0.5 μm/slice for depths up to 100 μm and attenuation compensation was applied to adjust laser power. 2-D discrete frequency Fourier transformation (FFT) was used to bring the image information into the frequency domain along an orthogonal axis. The two-dimensional FFT of each image was computed and the absolute value of the calculated 2D FFT magnitude was displayed. (12). The frequency distribution of the transformed image was quantified as aspect ratio (AR) using the long axis and short axis of each 2D FFT in Matlab software. Three-dimension volume and orthogonal reconstructions of z stack images were creating using Amari (FEI Company, Hillsboro, Oregon) software.
Statistical Analysis
Sample size was based on previous results demonstrating statistically significant differences in collagen volume fraction in treated versus untreated mice (7). A paired student’s t-test was used to calculate p-values within genotypes. For comparisons between genotypes with one condition a student’s t-test was used to calculate p-values. For comparisons between genotypes with more than one condition, ANOVA analysis followed by Tukey test was used to calculate p-values. For all analyses, p < 0.05 was considered statistically significant.
Results
Reduced Alveolar Bone Volume Fraction in Murine Maxillae with Ligature
To mimic human disease pathogenesis a ligature-induced model of PD, initially described by Abe and Hajishengallis (9), was adopted. Maxillary alveolar bone volume/total volume (BV/TV) was assessed by μCT analysis at 5- and 14-day time points following ligature placement (Fig. 1, A&B). Significant decreases in alveolar BV/TV was observed in ligated maxillae over control. As a decrease in BV is a clinical hallmark of PD, the ligature model accurately recapitulated critical characteristics of PD. Whereas significant reductions in alveolar BV/TV was observed at 5 days of ligature placement, differences in BV/TV at 14 days trended toward reduced levels but did not reach statistical significance indicating at least partial recovery from PD at 14 days (Fig. 1B).
Reduced PDL Collagen Content in Mice with Ligature
Picrosirius red (PSR) histological staining of PDL (Fig. 1C) with and without ligature (5- and 14-day time points) was visualized under polarized light and quantified by morphometric analysis (Fig. 1E). Total collagen content was significantly reduced at 5 days of ligature versus control values (Fig. 1E). Similar to BV/TV, PDL collagen content recovered after 14 days of ligature (Fig. 1E). Herovici stain differentiates thin (blue) versus thick (pink/red) collagen fibers. As shown in Fig. 1D, thin collagen fibers (pink) were more apparent in PDL with ligature versus control. Quantification of thin versus thick collagen fibers in PSR-stained tissues revealed a significant increase in thin collagen fibers in mice subjected to ligature at 5 and 14 days indicating increased collagen fiber remodeling in PDL with PD (Fig. 1F) (7).
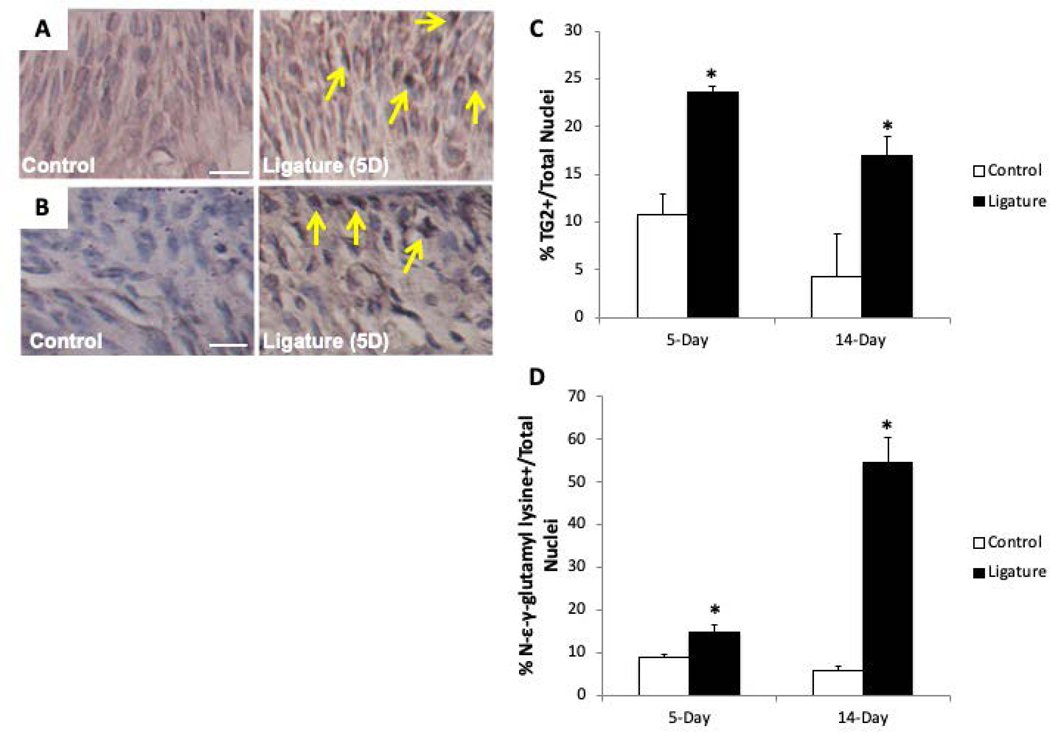
Increased TG Activity in PDL in PD
To assess expression and cross-link formation mediated by TG during inflammatory PD, immunohistochemistry was conducted in PDL following ligature treatment (5- and 14-day time points). Consistent with increases in TG2 expression observed during other inflammatory disease states, TG2 expression significantly increased in PDL following 5 days of ligature compared to control PDL (Fig. 2A & C). Ligated PDL also exhibited apparent increases in TG2 expression at the 14-day time point (Fig. 2C). Using an antibody directed against the epitope formed by the cross-link mediated by TG, more robust staining for TG-mediated cross-links were noted in ligated (5-day) PDL in comparison to control PDL (Fig. 2B & D). At 14 days, statistically significant increases in TG-mediated cross-links were observed in PDL (Fig. 2D).
Figure 2:
Increases in transglutaminase (TG) 2 expression and TG cross-links in PDL after 5- and 14-day ligature time points versus controls. Representative images of anti-TG2 (panel A) and anti-N-ε−γ-glutamyl lysine (panel B) immunohistochemistry (IHC) following 5-day ligature (yellow arrows). C). Quantification of TG2 and N- ε−γ-glutamyl lysine IHC based on the number of nuclei per field following 5- and 14-day ligature. Each image is oriented with tooth on top, PDL centered, and alveolar bone on bottom. Images shown are of PDL surrounding second maxillary molar. *p < 0.05 between control and ligature, as determined by Student’s t test. n = 5 mice per group. Scale bars = 30μm. Error bars = SEM. 5D = 5-day ligature.
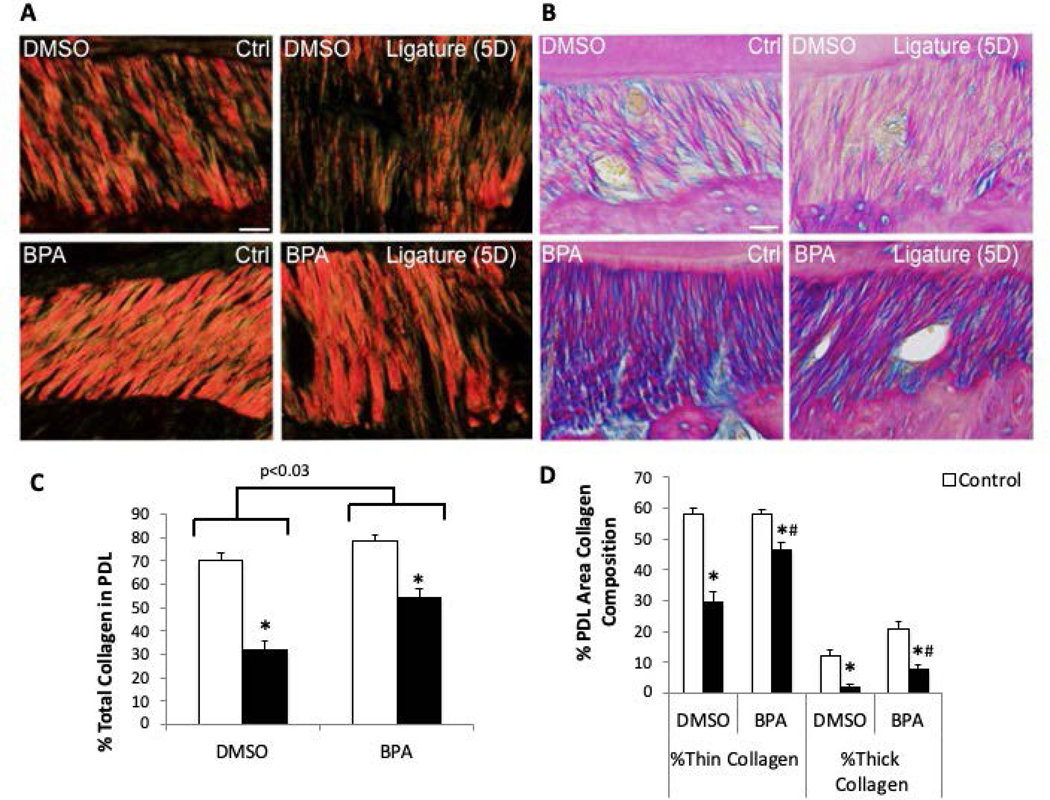
Inhibition of TG Restores PDL Collagen Content Following Ligature
To determine whether TG activity influenced collagen fiber assembly during and after induction of PD, ligatures were placed in mice coincident with injection of a TG inhibitor, 5-(Biotinamido) pentylamine (BPA) or vehicle control (dimethyl sulfoxide, DMSO, BPA is not soluble in aqueous solutions). Compared to control, non-injected PDL, DMSO injection visibly influenced collagen fiber morphology as noted previously in homeostatic PDL indicating that DMSO alone is damaging to PDL collagen (Fig. 1C vs. Fig 3A, DMSO, Crtl)(7). Ligature placement further exacerbated morphological changes in collagen fibers (Fig. 3A, DMSO, Ctrl vs. Ligature(5D)). However, BPA treated PDL demonstrated apparent improvements in collagen fiber morphology over that of DMSO-treated mice in both non-ligature and ligature-induced PD (Fig. 3A, BPA Ctrl, Ligature (5D)). Hence, ligature-induced PD exacerbated the apparent reduction in PDL collagen in all treatment groups versus control tissue (Non-injected (Fig 1A), DMSO, and BPA-treated (Fig 3A)). Importantly, however, injection of BPA improved the appearance of collagen stained fibers over that of DMSO treated with ligature. Comparing BPA-treated ligature mice (Fig. 3A) with non-injected ligature mice (Fig. 1C), BPA-treatment appeared to restore the morphology of damaged collagen to approximate that of non-injected PDL and greater than that of DMSO vehicle.
Figure 3:
Inhibition of TG activity in vivo restores PDL collagen following 5-day (5D) ligature. A). Representative polarized PSR-stained PDL sections of mice with and without 5-day ligature with vehicle only (DMSO) or vehicle with 5-(Biotinamido) pentylamine (BPA) injections. B). Representative Herovici-stained PDL images. Pink/red = thick/mature collagen fibers, blue/purple = thin/young collagen, yellow = cell death, black = nuclei. Each image oriented with tooth on top, PDL centered, and alveolar bone on bottom. Images shown are of PDL surrounding second maxillary molar. Scale bars = 25 μm. n = 5 mice per group. C). PSR quantification of PDL collagen content following 5-day ligature +/− DMSO/BC injections. D). PSR quantification of the percent thick versus thin collagen fiber area in PDL following 5-day ligature +/− BPA injection. *p < 0.05 between control and ligature, #p < 0.05 between DMSO and BPA injections, as determined by Student’s t test. n = 5 mice per group.
PDL stained with Herovici revealed similar differences in collagen fiber morphology as that observed with PSR staining (Fig. 3B). Herovici staining of BPA-injected PDL with ligature revealed increases in both thick and thin (pink and blue, respectively) collagen fibers, and reduced cell death (yellow) compared to ligature PDL injected with vehicle DMSO (Fig. 3B, bottom panels). Similar to PSR-stained tissues, BPA-injected ligature resembled that of non-injected mice with ligature revealed by Herovici stain (Fig. 1D versus 3B).
Quantification of PDL collagen stained with PSR was performed to assess total collagen and the nature of collagen fibers in PD tissue (Fig. 3C and D). The collagen volume fraction in non-disease, non-injected murine PDL is approximately 80% (Fig. 1E). DMSO and BPA injections did not significantly affect the total PDL collagen content as shown in Fig. 3C (DMSO: ~70%; BPA: ~78%). In mice with ligature-induced PD, those injected with DMSO vehicle demonstrated the greatest reduction in total collagen levels (~30%) versus either non-injected (~60%, Fig. 1E) or BPA treated (~50%, Fig. 3C). Hence, inhibition of TG with BPA improved collagen fiber content in mice with PD versus vehicle only. Interestingly, BPA injections increased the incidence of both thin and thick collagen fibers in ligature PDL versus DMSO vehicle (Fig. 3D).
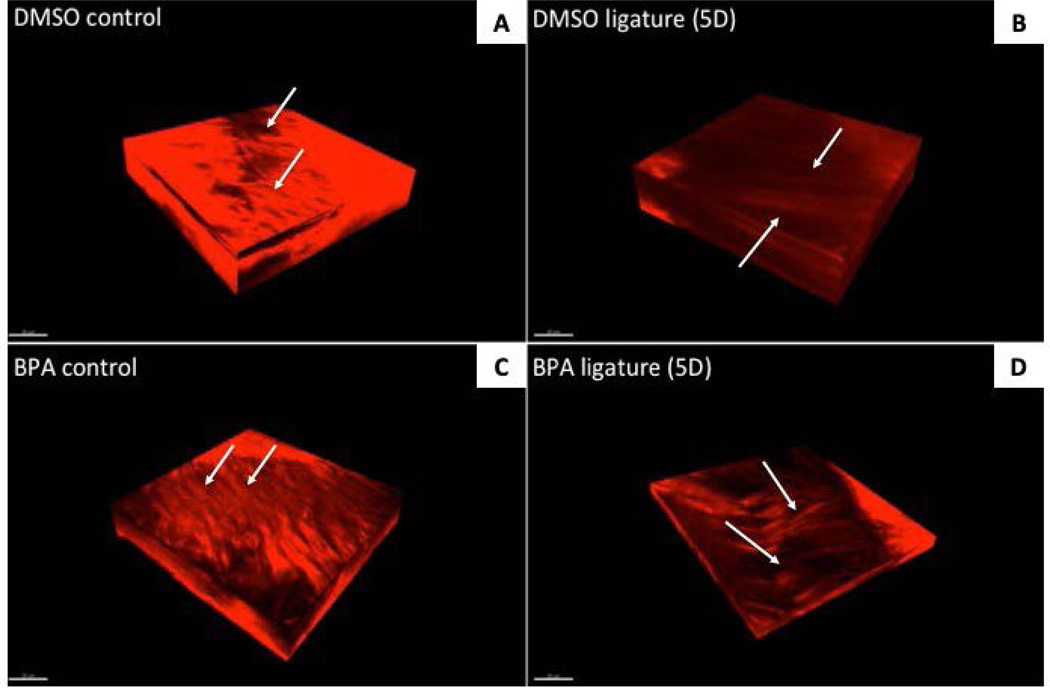
Visualization of PDL Collagen Fibers by Second-Harmonic Generation
Second-Harmonic Generation (SHG) microscopy enables the visualization of native (unfixed) PDL collagen in 3-dimensions. Orthogonal reconstruction of z-stacked images of murine PDL injected with either DMSO as vehicle control or BPA following 5 days of ligature were produced to provide qualitative data on collagen morphology, organization, and fiber bundle thickness (Fig. 4). As observed in our previous studies, DMSO injection alone induced morphological changes in collagen organization (Fig. 4A) (7). Five days of ligature further exacerbated collagen fiber morphology and reduced the incidence of discernible thick fiber bundles (Fig. 4B). BPA injection without ligature appeared to increase collagen fiber thickness in PDL when compared to DMSO controls without ligature (Fig. 4C). After induction of PD, the presence of thick collagen bundles was observed in PDL with BPA-mediated inhibition of TG activity (Fig. 4D). The incidence of thick PDL collagen fiber bundles in BPA-treated PDL was further validated, and better appreciated, in 3D motion animations of compiled z-stack images which are presented as a movie in Supplemental Figure 1. Three-dimensional orthogonal volume reconstructions from z-stack motion animations also enable better visualization of BPA-mediated changes to PDL collagen fibers in all planes (Supplemental Figure 2: movie).
Figure 4:
Visualization of native collagen fiber morphology by second harmonic generation (SHG) in PDL treated with inhibitors of TG activity in 5D ligature mice. SHG orthogonal volume images generated from z-stacks taken at 0.2μm/slice for depths of up to 60μm of native PDL +/− DMSO/BPA injections following 5-day ligature treatment. Apparent increases in collagen fiber morphology and organization are observable in BPA-treated PDL (panels C & D) versus those treated with DMSO (A and B). The appearance of thick collagen fiber bundles is apparent in 5D BPA-treated PDL (D) versus vehicle only (B). Arrows, collagen fiber bundles. Scale bar = 20μm.
Quantification of Collagen Organization by SHG Following Inhibition of TG
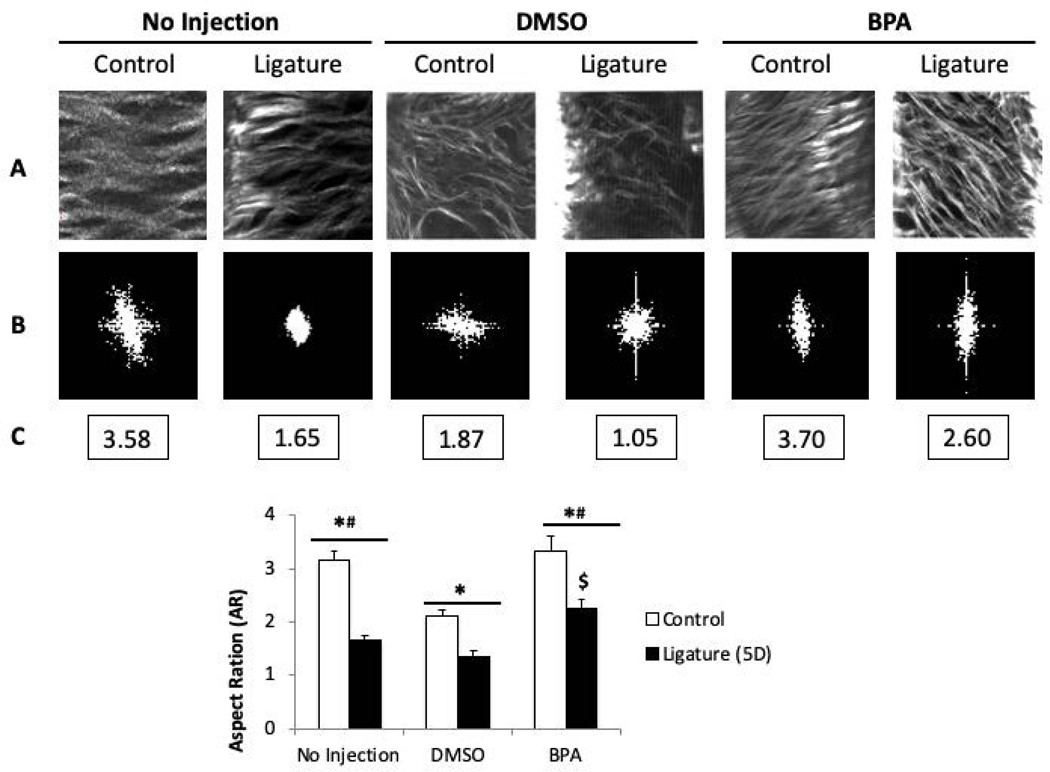
SHG also allows for quantification of collagen fiber morphology. To this end, backward SHG imaging was used to generate two-dimensional compilations of z-stack images (shown in Fig. 5A) that were subjected to Fourier transformation (FFT, Fig. 5B). FFT is a standard method used to quantify SHG images (Matteini 2009). FFT calculates the intensity, geometry, and textures in an image and translates them into a shape which appears in a form ranging from elliptical to circular. The aspect ratio (AR) is calculated as the height of the shape/width of shape. 2D FFT diagrams that transform from circular shapes to elliptical shapes, indicate a transition from random patterns to organized patterns. In our studies, an elliptical FFT is represented with a higher AR indicating a more organized collagen fiber network whereas a circular FFT gives a lower AR representing disorganized PDL collagen (Fig. 5B and C).
Figure 5:
Quantification of images taken with Second-Harmonic Generation (SHG) methodology demonstrates an increase in PDL collagen fiber organization following inhibition of TG. A). Representative stack of SHG images (1024×1024 μm2 ROIs) taken from PDL of mice +/−DMSO/BPA injections following 5-day ligature. B). The frequency Fourier transformation (FFT) diagram of each representative region of interes (ROI). C). The calculated value of the aspect ratio (AR) of the ellipse approximating the FFT magnitude of representative ROIs. DMSO = vehicle control; BPA = 5-(Biotinamido) pentylamine. D). Quantification of the aspect ratio (AR) of SHG z-stack images of native PDL following ligature +/− DMSO/BPA injections at 5D of ligature with treatment. *p < 0.01 between control and ligature, #p < 0.01 between DMSO and BPA, $p < 0.01 between no injection and BPA injection, as determined by Student’s t test. n = 7 mice per group. Error bars = SEM. DMSO = vehicle control; BPA = 5-(Biotinamido) pentylamine.
Accordingly, the control non-injected PDL demonstrated an AR value significantly greater than non-injected PDL with ligature demonstrating collagen disorganization coincident with ligature-induced PD disease and verification of the method (No injection, Fig. 5C and 5D). DMSO (vehicle) injection alone significantly reduced PDL organization from non-injected control that was further reduced in mice with ligature. Notably, injection of BPA (solubilized in DMSO) significantly improved the AR for PDL collagen fiber organization in comparison to DMSO (vehicle alone) in both control and ligature conditions. Importantly, the AR value for BPA-treated PDL with ligature was greater than that of non-injected PDL with ligature and DMSO-treated ligature. We conclude that inhibition of TG activity during conditions of PD significantly improves collagen organization in the PDL.
Discussion
Our previous studies demonstrated TG activity played a critical role in PDL collagen assembly by virtue of the fact that deficits associated with collagen architecture characeteristic of SPARC-null PDL was rescued by inhibition of TG activity (7). Hence, whether TG activity might play an essential role in collagen assembly during PD merited investigation given that levels of TG2 activity are anticipated to increase with inflammation (8). To our knowledge, the findings reported here are the first examples of morphological and functional rescue of PDL collagen via manipulation of TG activity following disease and confirmed by SHG.
First, we validated the ligature-model of PD in our mice and showed significant reductions in both alveolar bone volume fraction and a reduction in PDL collagen content after 5-days of ligature. Unexpectedly, we found that WT mice recovered from measurable bone loss at 14 days of ligature despite persistent ligature placement (confirmed at end point). Interestingly, we found that SPARC-null mice, unlike WT mice, had persistent reductions in alveolar bone volume fraction after 14 days of ligature (Rosset et al., unpublished). We conclude that, in our hands, although persistent bone loss is not detected in WT mice at day 14, transgenic mice with abrogated SPARC expression and concomitant deficits in collagen deposition, have sustained bone loss at this time point. Generally, mice are known to be inherently resistant to developing chronic persistent PD which has led to the development of multiple rodent PD models attempting to recapitulate, at least in part, human disease (13). Strain variation, age, and laboratory procedures might contribute to differential development of PD bone loss using the ligature model (14).
Under conditions of induced PD, DMSO (vehicle) treated PDL demonstrated the most severe aberrations in PDL collagen morphology. Every attempt to minimize the volume and limit the toxicity of the DMSO vehicle was undertaken. Nonetheless, DMSO was damaging to PDL collagen. However, BPA (in DMSO) clearly improved the morphology, content, and organization of PDL collagen. Future experiments to test inhibitors of TG soluble in non-toxic vehicle are paramount in testing the capacity of TG inhibitors to improve PDL repair. Experimental results reported herein suggest a therapeutic benefit to TG inhibition to improve repair following PD.
TG family members catalyze ECM cross-linking, a function that is implicated in stabilizing and cross-linking proteins. In addition to forming insoluble protein complexes, a critical function of TG in ECM collagen fiber assembly in vivo was established by our laboratory. Previous reports have shown that the expression and activity of TG is increased in various tissues during inflammatory disease states (15). TG2 expression increases in tissues during states of inflammation (neuroinflammation, colitis, celiac disease, PD) and has been implicated in the cross-linking of anti-inflammatory molecules and protein aggregates (16–20). In oral tissues, Curro et al. reported increased TG2 mRNA levels in gingival epithelium of patients with chronic PD compared to healthy controls (18). Clinical studies assessing gingival overgrowth in patients with PD reported significant increases in TG2 expression in gingival biopsies compared to healthy patients, implicating a role of TG2 in periodontal matrix remodeling (16, 17).
As thinner collagen fibers are indicative of remodeling ECM, we ascribe the increase in thin collagen fibers in BPA-treated versus DMSO-treated PDL to reflect a general enhancement of collagen fiber formation under conditions of TG inhibition. That an increase in thick collagen fibers was also observed in BPA-treated PDL is consistent with our previous results in which TG-inhibition ameliorated the formation of thicker collagen fibers (7). The more robust immunostaining for TG2 and for N-ε-γ glutamyl lysine, the cross-link catalyzed by TG observed in PDL with PD suggests that TG activity is active participant in collagen fiber assembly in PD. Importantly, visualization of native collagen morphology by SHG imaging exhibited restoration of PDL collagen organization in BPA-treated PDL above that of untreated and vehicle treated PDL. Our data provide evidence for a function of TG in fibrillar collagen regeneration in vivo following destruction induced by PD.
Historically, increases in collagen cross-links have been associated with increases in tissue integrity and tissue stiffness (3). Hence, the finding that inhibition of TG activity was associated with increased collagen content and improved ECM organization might appear counterintuitive. We speculate that smaller collagen fibers, mediated by increased TG activity, might be more prone to inflammatory degradation, thus exacerbating the loss of PDL collagen indicative of PD. Future studies to elucidate the molecular mechanisms by which TG activity controls collagen fiber morphology and organization will be useful, including identification of the site on fibrillar collagen modified by TG activity. In addition, development of TG2-specific inhibitors has the potential to lead to a directed therapy for controlling tissue remodeling in inflammation and in fibrotic disease states.
Supplementary Material
Acknowledgments
This work was supported by NIH F30DE027210 (EMR), Veteran’s Administration Merit Award 1I01BX001385-01A1 (ADB), and NIH R01DE021134 (HY).
Footnotes
Additional supporting information may be found in the online version of this article at the publisher’s website.
Disclosures
All authors state that they have no conflicts of interest.
References
- (1).Beertsen W, McCulloch CA, Sodek J. The periodontal ligament: a unique, multifunctional connective tissue. Periodontol 2000 1997; 13: 20–40. [DOI] [PubMed] [Google Scholar]
- (2).Rios HF, Lin Z, Oh B, Park CH, Giannobile WV. Cell- and gene-based therapeutic strategies for periodontal regenerative medicine. J Periodontol 2011; 82: 1223–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Eyre DR, Paz MA, Gallop PM. Cross-linking in collagen and elastin. Annu Rev Biochem 1984; 53: 717–748. [DOI] [PubMed] [Google Scholar]
- (4).Collighan RJ, Griffin M. Transglutaminase 2 cross-linking of matrix proteins: biological significance and medical applications. Amino Acids 2009; 36: 659–670. [DOI] [PubMed] [Google Scholar]
- (5).Akimov SS, Belkin AM. Cell-surface transglutaminase promotes fibronectin assembly via interaction with the gelatin-binding domain of fibronectin: a role in TGFbeta-dependent matrix deposition. Journal of cell science 2001; 114: 2989–3000. [DOI] [PubMed] [Google Scholar]
- (6).Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. The Journal of cell biology 2000; 148: 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Trombetta-eSilva J, Rosset EA, Hepfer RG, et al. Decreased Mechanical Strength and Collagen Content in SPARC-Null Periodontal Ligament is Reversed by Inhibition of Transglutaminase Activity. J Bone Miner Res 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Trombetta-eSilva J, Rosset EA, Hepfer RG, et al. Decreased Mechanical Strength and Collagen Content in SPARC-Null Periodontal Ligament Is Reversed by Inhibition of Transglutaminase Activity. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research 2015; 30: 1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Abe T, Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods 2013; 394: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Trombetta-Esilva J, Yu H, Arias DN, Rossa C Jr., Kirkwood KL, Bradshaw AD. LPS induces greater bone and PDL loss in SPARC-null mice. J Dent Res 2011; 90: 477–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Shi C, Cisewski SE, Bell PD, Yao H. Measurement of three-dimensional anisotropic diffusion by multiphoton fluorescence recovery after photobleaching. Annals of biomedical engineering 2014; 42: 555–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Matteini P, Ratto F, Rossi F, et al. Photothermally-induced disordered patterns of corneal collagen revealed by SHG imaging. Optics express 2009; 17: 4868–4878. [DOI] [PubMed] [Google Scholar]
- (13).Graves DT, Kang J, Andriankaja O, Wada K, Rossa C Jr., Animal models to study host-bacteria interactions involved in periodontitis. Front Oral Biol 2012; 15: 117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Lin J, Bi L, Yu X, et al. Porphyromonas gingivalis exacerbates ligature-induced, RANKL-dependent alveolar bone resorption via differential regulation of Toll-like receptor 2 (TLR2) and TLR4. Infection and immunity 2014; 82: 4127–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Kim SY. Transglutaminase 2 in inflammation. Frontiers in bioscience : a journal and virtual library 2006; 11: 3026–3035. [DOI] [PubMed] [Google Scholar]
- (16).Asioli S, Righi A, Cardone P, et al. Transglutaminase 2 expression is significantly increased in cyclosporine-induced gingival overgrowth. Histology and histopathology 2011; 26: 1399–1404. [DOI] [PubMed] [Google Scholar]
- (17).Ceruti P, Asioli S, Mussano F, et al. Transglutaminase 2 May Be Associated with Peri-implant Gingival Overgrowth: Preliminary Assessments. The International journal of prosthodontics 2015; 28: 615–620. [DOI] [PubMed] [Google Scholar]
- (18).Curro M, Matarese G, Isola G, et al. Differential expression of transglutaminase genes in patients with chronic periodontitis. Oral diseases 2014; 20: 616–623. [DOI] [PubMed] [Google Scholar]
- (19).Di Sabatino A, Lenti MV, Giuffrida P, Vanoli A, Corazza GR. New insights into immune mechanisms underlying autoimmune diseases of the gastrointestinal tract. Autoimmunity reviews 2015; 14: 1161–1169. [DOI] [PubMed] [Google Scholar]
- (20).Ientile R, Curro M, Caccamo D. Transglutaminase 2 and neuroinflammation. Amino acids 2015; 47: 19–26. [DOI] [PubMed] [Google Scholar]
- (21).Fessel G, Li Y, Diederich V, et al. Advanced glycation end-products reduce collagen molecular sliding to affect collagen fibril damage mechanisms but not stiffness. PLoS One 2014; 9: e110948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.