Abstract
In this study, 213 infectious bronchitis viruses (IBVs) were isolated from samples collected from 801 flocks suspected to be infected with IBV from January 2016 to December 2017 in China. By using complete nucleotide sequences of S1 gene we determined the phylogeny of these IBV isolates, which in turn allowed us to define six lineages/genotypes, a number of recombinants and a novel variant. The GI-19 lineage was the most frequently isolated type in China in recent years. Although scattered mutations in the S1 gene among the GI-19 lineage viruses were observed, we also noted different sublineages in the GI-19 lineage with unique mutations, suggesting a high degree of S1 gene variation since they were first isolated in the mid-1990s. We also isolated a number of vaccine-like viruses from vaccinated diseased chickens, although more work is needed to differentiate the reisolation of vaccine strains from field strains of the same serotype. One of the important findings in this study is that the prevalence of the TW I type viruses in GI-7 lineage has been increasing in recent years in China. Another important finding is that recombination events occurred between the predominant GI-19 lineage and the commonly used 4/91 vaccine, which gave rise to distinct IBV isolates. In addition, a novel IBV isolate, together with a reference strain in GenBank database, were found to form a novel lineage/genotype that was remarkably distinct from established lineages. The characteristics of the antigenicity, tissue tropism, pathogenicity and complete genome were required for further investigation for the recombinants and the viruses in different sublineages and novel lineages. Meanwhile, permanent monitoring of circulating strains was needed to monitor the emerging viruses and rationally modify vaccination strategies in the field situation.
Keywords: Infectious bronchitis virus, S subunit, Spike protein, Genotype, Lineage
Highlights
-
•
The GI-19 lineage was the most frequently isolated type in China in recent years.
-
•
Different sub-lineages were found in the GI-19 lineage with unique mutations.
-
•
A number of vaccine-like viruses were isolated from vaccinated diseased chickens.
-
•
The TW I type viruses in GI-13 lineage has been increasing in recent years in China.
-
•
A novel IBV isolate was found to form a novel clade that was distinct from established lineages.
1. Introduction
Infectious bronchitis virus (IBV) is a member of the species Avian Coronavirus, genus Gammacoronavirus, and affects the respiratory tract, kidneys, gut and oviduct, and IBV infection is among the most important poultry diseases worldwide (Cavanagh, 2007). In most cases, the birds show respiratory problems such as nasal and/or ocular discharge and other respiratory distress, poor performance, decreased egg production, poor egg shell quality, reduced hatchability, nephritis and sometimes false layers, however, the clinical signs caused by IBV infection can be diverse and nonspecific. The infection is often complicated by secondary opportunistic agents such as Escherichia coli (Dwars et al., 2009; Vandekerchove et al., 2004), Mycoplasma gallisepticum (Georgiades et al., 2001) and Ornithobacterium rhinotracheale (Vandekerchove et al., 2004), and combinations of IBV with other pathogens can seriously increase the level of damage (Haghighat-Jahromi et al., 2008).
IBV has a linear, single-stranded RNA genome of positive polarity of ~27 kb in length and produces enveloped virions. IBV particles consist of four structural proteins: spike (S) glycoprotein, membrane (M), small envelope (E) and nucleocapsid (N) proteins. The S protein is formed by post-translational cleavage of S into two separate polypeptide components, S1 and S2 (Cavanagh, 1983, Cavanagh, 2007). S1 mediates virion attachment to host cells (Casais et al., 2003) and is a target of neutralizing antibodies in chickens (Cavanagh et al., 1986). Mutation and recombination in the genome, especially in the S1 gene, may give rise to variants or new genotypes that may escape from the protection offered by the immunity induced by the classical vaccination programs (Cavanagh, 2007). Hence, genotyping of IBV is associated primarily with changes in the amino acid sequence of the S1 subunit (Cavanagh et al., 1988; Kant et al., 1992), and evolutionary characterization of IBV is mainly based on analysis of the S1 gene (Lee et al., 2003; Wang et al., 1994).
Different IBV genotypes are distributed globally. Some of them are endemic only in particular countries or region, while others circulate worldwide (De Wit et al., 2011). Six genotypes that together comprise 32 distinct viral lineages and a number of interlineage recombinants have been defined worldwide by phylogenetic analysis with the complete nucleotide sequences of the S1 gene (Valastro et al., 2016). Recently, two additional types of IBV that are genetically distinct from those of the 32 lineages and interlineage recombinants were found in China by phylogenetic analysis of complete nucleotide sequences of S1 genes of a number of IBV isolates (Chen et al., 2017; Jiang et al., 2017). They were defined as lineages 28 and 29 in genotype I (GI-28 and GI-29, respectively). This large diversity of lineages/genotypes is a major reason why commercial vaccines often fail or only provide partial protection against IBVs, and therefore, new IB outbreaks continue to occur in vaccinated chicken flocks.
In China, several epidemiological IB surveys have already been conducted and have shown that a variety of IBV genotypes and variants (Liu et al., 2013; Mo et al., 2013; Zhao et al., 2013; Liu et al., 2014; Zhou et al., 2014; Chen et al., 2015; Xu et al., 2016; Zhang et al., 2015; Leghari et al., 2016; Chen et al., 2017; Zhao et al., 2017; Zhou et al., 2017) are circulating and cocirculating. This shows that the Chinese IBV situation is complex and variable, and causes serious economic losses in the Chinese poultry industry. In addition, IB outbreaks also occur in well-vaccinated flocks using different serotype vaccines and vaccination programs. This raises the question of whether new IBV variants, against which vaccines induce an insufficient level of cross-protection, are involved. In our conventional surveillance of IB between January 2016 and December 2017 in China, broilers and layer chickens with suspected IB with associated respiratory problems, nephritis and reduced egg production frequently occurred. In the present study, cloacal swabs and tissue samples were collected from 211 and 590 commercial chicken flocks, respectively. Two hundreds and thirteen IBVs were isolated and the complete nucleotide sequence of S1 genes were sequenced and analyzed. The aims of the study were to gather recent data, update knowledge of the circulating IBV field strains, and obtain a wider picture of IBV genetic diversity to understand better the epidemiology of IBVs circulating in chicken flocks in China in recent years.
2. Materials and methods
2.1. Samples, virus isolation and purification
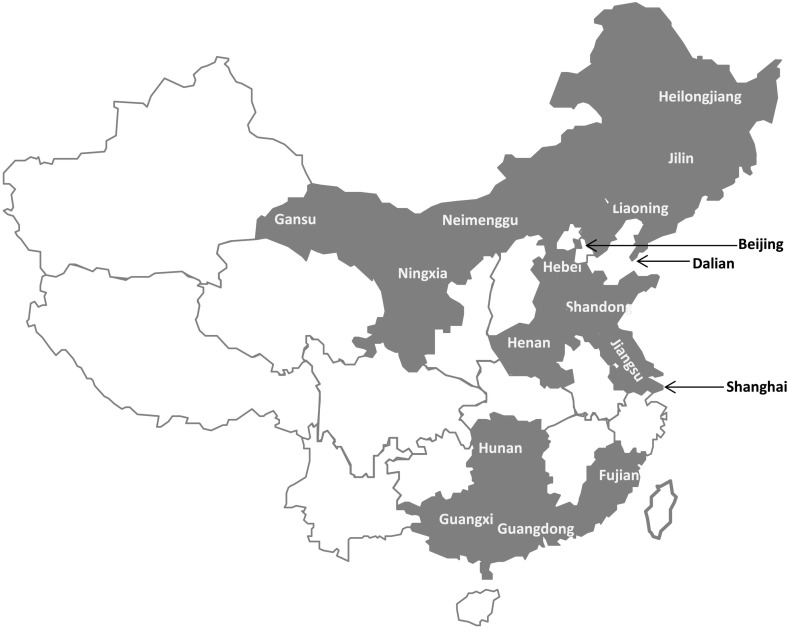
A total of 801 flocks from 17 provinces (cities) were involved in this study (Supplemental Table 1 and Fig. 1 ), during the 2 years of IB surveillance in China. Sampling was conducted from early January 2016 to the end of December 2017. A wide variety of IB vaccination programs had been used in the chickens from which the samples were collected. In all cases, Massachusetts (Mass) type H120 or Ma5 vaccines had been used at 1 day old by coarse spray or oculonasal route. A second vaccination with 4/91 or other vaccine, such as LDT3-A, was also common between 15 and 20 days old. From a given flock on each farm, >20 cloacal swabs were collected. Tissue samples from the trachea, lungs and kidneys were obtained from more than three birds from each flock. The cloacal swabs or tissue samples from the same flock were pooled and used for viral isolation. Hence, a pool of cloacal swabs or tissues represented one sample. The cloacal swabs were placed in 1 ml buffered peptone water and kept at 4 °C until use.
Fig. 1.
The gray regions of the map show Chinese provinces, where the IBV strains were isolated.
The pooled tissue samples were homogenized at a ratio of 1:10 w/v in 1 ml diethyl-pyrocarbonate-treated phosphate-buffered saline in a TissueLyser (Qiagen, Hilden, Germany). Tissue homogenates or cloacal swabs were clarified at 1000 ×g for 10 min at 4 °C. Viral isolation was performed by inoculation of 9-day-old embryonated, specific pathogen-free (SPF) hens' eggs (Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences) via the allantoic cavity route with the supernatant from the homogenates or cloacal swabs. For each sample, three eggs were used for viral isolation. Eggs were incubated at 37 °C and candled daily (Liu and Kong, 2004). Embryos that died within 24 h after inoculation were discarded. On day 3 after inoculation, 100 μl allantoic fluid was collected from each egg, using a 26 gauge needle, through a small hole created in the shell near the air sac (Hewson et al., 2012). The allantoic fluids from the three eggs were pooled and 50 μl pooled allantoic fluids were used to detect IBV using RT-PCR assays with two set of primers targeting two conserved regions in the IBV genome. The remaining allantoic fluids were blind passaged 3–8 times in SPF eggs until the typical embryonic lesions of IBV infection were observed. The eggs were incubated for further 4 days after the allantoic fluids were collected and observed for typical embryonic lesions of IBV infection, such as dwarfing, stunting, and curling, with feather dystrophy.
For the virus-infected allantoic fluids containing inconsistent S1 sequences, each type of virus was isolated by purifying the allantoic fluids using limiting dilution in embryonated SPF eggs. Serial 10-fold dilutions of the allantoic fluids were inoculated into the allantoic cavity of SPF chicken embryos, which were observed for 2 days. Purification of the virus was identified by S1 amplification, cloning and sequencing of at least 10 clones of each virus. The allantoic fluids of the eggs inoculated with the highest dilution that had consistent S1 sequences were used for another two rounds of purification.
2.2. Eggs
White Leghorn SPF fertile chicken eggs were obtained from the Laboratory Animal Center, Harbin Veterinary Research Institute, China. All experimental procedures were approved by the Ethical and Animal Welfare Committee of Heilongjiang province, China.
2.3. RNA extraction and RT-PCR
The virus passages used for RNA extraction were listed in the Supplemental Table 1. RNA was extracted from the allantoic fluids using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The RNA was eluted in 30 μl RNase-free water and was used as the template for RT-PCR amplification.
Two sets of oligonucleotide pairs, one targeting the N genes [N(+) and (N−)] and another targeting a region of the viral genome downstream of the gene 5 and upstream of N (IBV-170 and IBV-171) (Liu et al., 2013), were used for IBV detection in allantoic fluids. The primers, S1Oligo5’ and S1Oligo3′ (Kwon et al., 1993), were used for amplifying the complete S1 genes of the IBV isolates. For the IBV-infected allantoic fluids that were negative for S1Oligo5’ and S1Oligo3’ by RT-PCR, the sequences upstream of S gene and downstream of the cleavage site of S proteins were determined (Zhao et al., 2017). New primers were designed based on the newly determined sequences and used for RT-PCR amplification and subsequent S1 gene sequencing.
The RT-PCR was performed using the One-step RT-PCR kit (Takara Bio, Shiga, Japan) under the following conditions: reverse transcription reaction for 30 min at 50 °C; denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, extension at 72 °C for 2 min, and final extension at 72 °C for 10 min. Upon amplification, 20 μl PCR products were run in 1.5% agarose gel electrophoresis and visualized by GelGreen Nucleic Acid Gel (TaKaRa Bio) staining; bands of the expected size were excised from the gel for sequencing using the Gene Clean Kit (ExoSAP-IT, Affymetrix, Santa Clara, CA, USA). Purified PCR products were cloned into a pMD 18-T vector (TaKaRa Bio). Five to 10 clones were subjected to Sanger sequencing for each of the genes.
2.4. Sequence analysis
The obtained chromatograms were manually checked and edited for unclear base calls. Assembly of consensus nucleotide sequences was conducted using the BioEdit version 7.2.0 (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). The specific identification was primarily conducted by comparing the obtained sequences to on-line available reference sequences by BLAST searching (https://blast.ncbi.nlm.nih.gov/Blast). Sequences of S1 genes from reference IBV strains with different genotypes/lineages were selected and retrieved from NCBI GenBank (https://blast.ncbi.nlm.nih.gov/genbank) based on the results of BLAST searching and the year when the viruses were isolated and the regions where they were isolated. The 34 selected IBV reference strains and their accession numbers are shown in Supplemental Table 2. Nucleotide sequences were aligned using ClustalW and Mega software version 6.0 (http://www.megasoftware.net/). Phylogenetic analysis for the S1 genes were constructed using MEGA 4.0 and the tree construction was generated using the maximum likelihood method based on the Tamura–Nei parameter model with 1000 bootstrap replicates.
The nucleotide and amino acid sequence identities of the S1 genes from IBV isolates were aligned, compared and calculated with those of reference IBV strains retrieved from NCBI GenBank, using the ClustalW multiple alignment method in BioEdit 7.2.0. Mutations and insertions were determined according to the results of pairwise comparisons between the IBV isolates and reference strains.
2.5. Recombination analysis
To detect possible recombination events, a multiple alignment of the selected representative of isolates and the potential parental viruses of recombinants based on the results of phylogenetic analysis and BLAST searching was generated using Multiple Alignment with Fast Fourier Transformation (MAFFT) v6 (http://mafft.cbrc.jp/alignment/software/). Furthermore, a SimPlot analysis was performed using SimPlot version 3.5.1 (http://sray.med.som.jhmi.edu/SCRoftware/simplot/) (window size, 40 bp; step, 5 bp) with these viruses. To confirm the recombination events and detect the recombination breakpoints, the representatives of the individual strains were examined using Recombination Detection Program version 4.70 (RDP4 for Windows, Microsoft, Redmond, WA, USA) as previously described (Quinteros et al., 2016).
2.6. GenBank accession numbers
All sequences have been deposited in GenBank database, accession numbers are listed in Supplemental Table 1.
3. Results
3.1. Case history, clinical and epidemiological data
Starting from January 2016 till December 2017, samples from a total of 801 flocks belonging to 17 provinces (cities) in China were collected. Of these samples, 211 cloacal swabs were collected from chickens aged 15–35 days that showed mild to severe IB-like clinical signs. The remaining 590 tissue samples were collected from diseased chickens suspected to be infected by IBV. The IB-like signs varied widely, although respiratory signs were the most prevalent. In all the cases, clinical presentation started with respiratory depression and distress. In breeders and commercial laying hens, decreases in egg production of 2–25% were often seen and the infection was not fatal in most cases. Mortality varied depending on virulence of the infecting serotype, status of immunity, and stress such as cold or secondary bacterial infection from the field experience. In contrast, mortality was obviously higher in commercial broilers and young chickens; sometimes as high as ≥30% in broilers or layer chickens aged <5 weeks. Postmortem examination revealed increased tracheal mucus, congestion, and presence of catarrh in the nasal turbinate and trachea. Kidney lesions were also common in the diseased chickens. Mild to severe colibacillosis was frequently observed, especially in broilers aged ~4 weeks, when postmortem examination was conducted.
3.2. IBV isolation, identification and genotyping
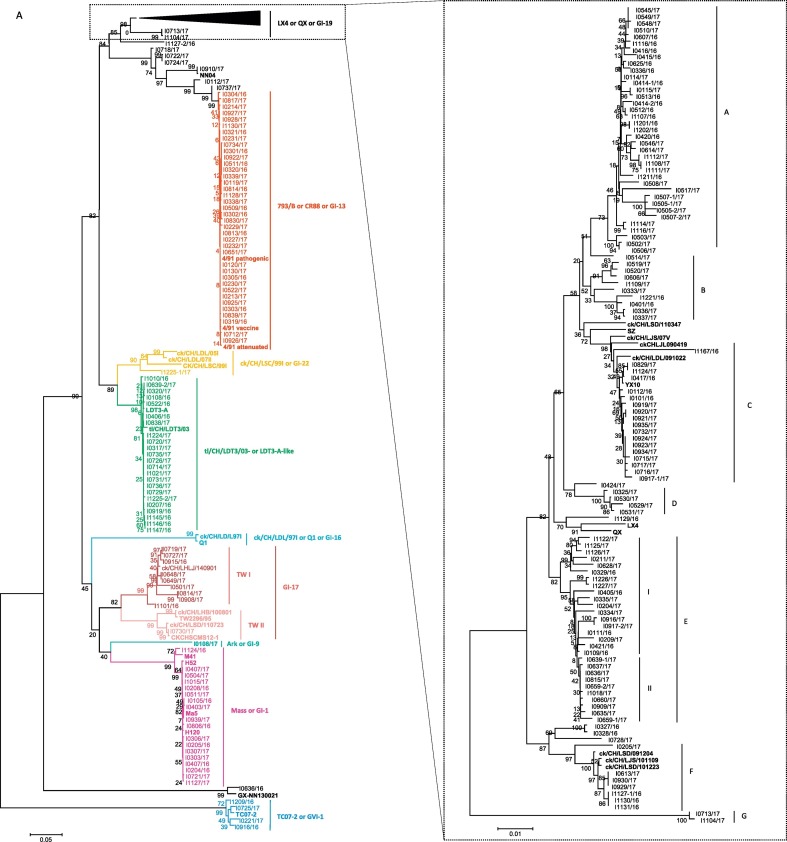
A total of 205 cases were detected as IBV positive from the 801 flocks by virus isolation and subsequent RT-PCR identification. We obtained a total of 213 IBV isolates: 58 strains were isolated from the 211 cloacal swabs; 155 strains were isolated from 590 tissue samples. Two inconsistent sequences of S1 genes were found in the same sample in eight of the tissue samples. We were able to distinguish these 213 IBV isolates based on phylogenetic analysis using sequences of the complete S1 genes. Phylogenic trees were constructed on 247 nucleotide sequences of the S1 gene obtained from 213 IBV isolates and 34 references strains. Our 110 GI-19 lineage (also known as LX4 type or QX-like) isolates and 11 reference strains formed a common branch (Fig. 2A), but they clearly clustered into distinct sublineages within the group. GI-19 lineage isolates were by far the most frequently isolated (110 in 213 flocks, 52.0%), followed by 793/B (also known as CR88 or 4/91 or 1/96 or GI-13), tl/CH/LDT3/03 (LDT3/03)- or LDT3-A-like, Mass (also known as GI-1), TW-I and -II (also known as GI-7), TC07–2 (also known as JP-IV, Keara new cluster 2 or GVI-1), ck/CH/LSC/99I (also known as GI-22), and other variants that were not included in the lineages or genotypes reported so far (Valastro et al., 2016). Strains of these lineages/genotypes accounted for 17.7%, 11.2%, 8.5%, 4.6%, 1.8%, 0.5% and 3.7% of the isolated viruses (Supplemental Fig. 1).
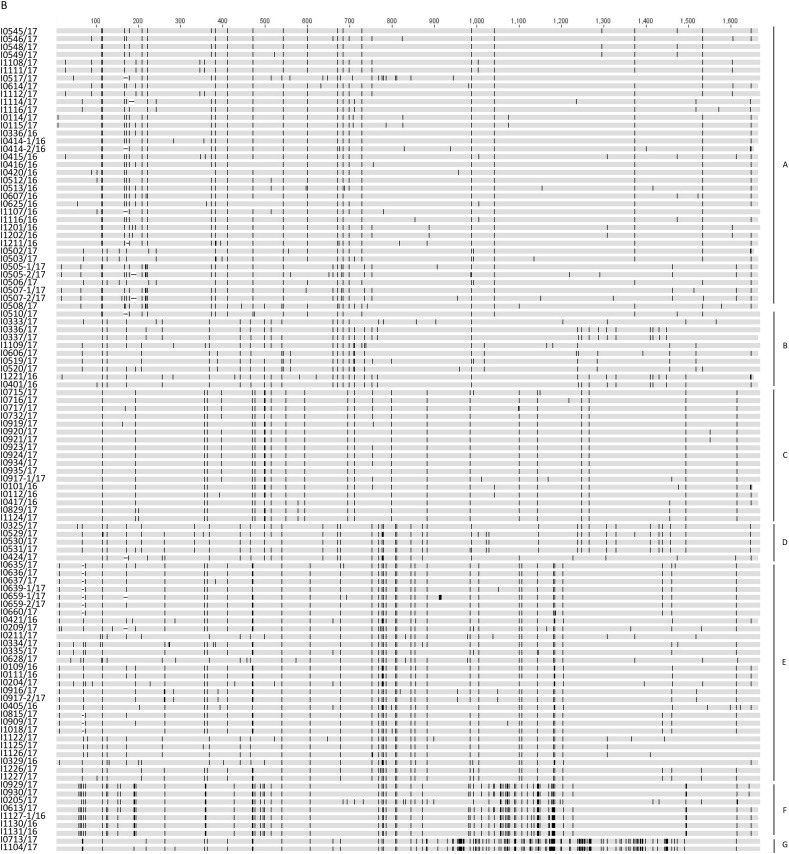
Fig. 2.
Sequence analysis of the complete nucleotide sequences of S1 genes of our 213 IBV isolates. Maximum-likelihood phylogeny was used based on the complete nucleotide sequences of S1 genes of our 213 IBV isolates and 34 reference strains (A). Strains of the same genotypes are coded in the same colors. The reference strains are in bold. Alignment of the complete nucleotide sequences of S1 genes of our 213 IBV isolates performed using MAFFT (B). The nucleotide sequence disagreement at indicated positions is represented in black, while the nucleotide sequence agreements at indicated positions are represented in gray. The GenBank accession numbers for the S1 gene sequences of our 110 isolates and reference strains are listed in Supplemental Tables 1 and 2, respectively.
3.3. GI-19 lineage showed extensive genetic diversity
The S1 gene, starting from the AUG translation start codon to the sequences encoding the cleavage sites of spike protein, of our 110 GI-19 lineage IBV isolates has varying sizes: most of the isolates were 1620 bp; nine isolates were 1617 bp; one was 1614 bp; five were 1611 bp; and four were 1608 bp.
Seven different sublineages (designated A–G) were detected in the GI-19 lineage (Fig. 2A). Viruses of the sublineages A and E are the predominant types circulating in chicken flocks in China. The levels of nucleotide and amino acid sequence identity between our 110 isolates and the QX/LX4 strains was 90.3–96.9% and 89.3–96.1%, respectively, compared to 89.2–100% and 85.4–100% between our isolates. Of those isolates, 37 strains were grouped into sublineage A, 10 into B, 17 into C, five into D, 28 into E, seven into F and two into G, accounting for 33.6%, 9%, 15.5%, 4.5%, 25.5%, 6.4% and 1.8% of the isolated viruses, respectively. In general, isolates between sublineages shared lower levels of nucleotide and amino acid identities compared with isolates in the same sublineages (Table 1 ).
Table 1.
Nucleotide and amino acid identities of the S1 genesa among sublineages of GI-19 lineage viruses.
| Subgroup | A | B | C | D | E | F | G |
|---|---|---|---|---|---|---|---|
| Amino acid identity (%) | |||||||
| A | 95.3–99.8b | 95.3–98.0 | 95.3–97.6 | 94.0–97.6 | 93.8–96.4 | 92.7–94.7 | 87.5–89.7 |
| 96.3–100.0 | |||||||
| B | 96.0–98.2 | 97.1–100.0 | 96.2–97.8 | 96.0–97.6 | 93.1–95.8 | 93.6–95.1 | 88.2–89.7 |
| 98.0–100.0 | |||||||
| C | 96.0–98.2 | 96.9–97.6 | 97.9–100.0 | 94.9–96.5 | 93.8–96.2 | 93.8–94.7 | 85.4–86.4 |
| 98.1–99.9 | |||||||
| D | 95.3–98.0 | 97.0–98.0 | 96.3–97.0 | 97.1–99.6 | 95.6–97.8 | 94.0–94.9 | 89.0–89.9 |
| 97.5–99.8 | |||||||
| E | 95.6–97.0 | 95.1–97.2 | 96.2–97.5 | 95.6–97.8 | 95.5–100.0 | 94.0–95.7 | 88.6–90.4 |
| 97.5–99.8 | |||||||
| F | 93.3–94.7 | 94.1–95.2 | 95.0–95.2 | 94.6–95.3 | 95.0–96.2 | 98.0–100.0 | 89.5–90.1 |
| 98.1–100.0 | |||||||
| G | 89.2–90.6 | 89.7–90.5 | 90.3–90.7 | 90.2–91.2 | 90.8–91.9 | 90.5–90.8 | 99.5 |
| 99.8 | |||||||
| Nucleotide identity (%) | |||||||
Top right, amino acid identity (%); bottom left, nucleotide identity (%).
The first 1653 nucleotides, starting at the AUG translation start codon, of the S1 genes were compared.
Top, amino acid identity (%); bottom, nucleotide identity (%).
Consistent with the results from phylogenetic trees, unique mutations were found in the S1 sequences of each sublineage (Fig. 2B). In addition, deletions were found in the hypervariable region I (HVRI) in S1 genes in some of the viruses in sublineages A and E. Most of them were located in the region of the conformation-dependent neutralizing antigenic site (epitope D) (Supplemental Figs. 2A and 2B). Some couples of viruses with and without deletions in S1 genes were isolated from the same bird. Viruses without the deletions in S1 genes in sublineage E were grouped into the same clade (I), compared to the viruses with deletions in another clade (II) in the phylogenetic tree (Fig. 2A). In addition, the results demonstrated that viruses in sublineages F and G represented unique sublineages compared to other strains in GI-19 lineage and were distinctly related to other strains in this lineage.
3.4. Vaccine-like strains were frequently isolated from diseased chickens
Thirty-eight isolates (17.7%) obtained in this study clustered together and were genetically related to the 4/91 vaccine strain belonging to the GI-13 lineage (Valastro et al., 2016). The differences in nucleotide and amino acid identities between our 38 GI-13 isolates varied from 99.4 to100% and 98.8 to 100%, respectively. The sequences of our 38 isolates had nucleotide and amino acid sequence identities between 99.6 and 99.9%, and 98.7 and 99.9%, respectively, when compared to those of the 4/91 vaccine strain. In addition, half of the GI-13 isolates (n = 19) had a Ser residue at position 95 in the S1 subunit, which is believed to favor virus replication in chickens. The other half had an Ala residue at position 95, which is believed to favor virus replication in embryonated eggs (Cavanagh et al., 2005).
The sequences of 18 IBV S1 genes (8.5%) formed a monophyletic group with each other within lineage GI-1 and were closer to those of Mass type H120, Ma5 and H52 vaccine strains and the pathogenic M41 strain (Fig. 2A). Of the 18 isolates, 17 were closer to H120 and Ma5 vaccine strains and isolate I1124/16 was closer to M41 strain. Our 18 GI-1 viruses had 97.7–100% nucleotide identity between each other and shared nucleotide identities between 97.9 and 100% to the H120 and Ma5 vaccine strains, and between 97.3 and 98.3% to the pathogenic M41 strain. We observed 38 mutations that were different between our 17 GI-1 isolates and M41 strain, except for I1124/16 (Table 2 ). Nineteen of the 38 mutations were shared between I1124/16 and the M41 strain, and the other 19 were the same between I1124/16 and H120 strain.
Table 2.
Pairwise comparisons of nucleotide sequences of S1 genes of our 18 Mass type isolates with those of pathogenic M41 and H120 vaccine strains.
| Strain | 41 | 81 | 102 | 112 | 129 | 138 | 147 | 162 | 187 | 197 | 206 | 301 | 349 | 377 | 382 | 385 | 389 | 391 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H120 | C | G | C | G | T | G | T | T | T | C | T | T | C | T | C | C | C | A | ||
| Other 17 isolates | C | G | C | G | T | G | T | T | T | C | T | T | C | T | C | C | C | A | ||
| I1124/16 | C | G | C | A | T | T | A | T | T | C | T | C | C | T | C | C | T | A | ||
| M41 | T | T | T | A | C | T | A | C | C | T | C | C | T | G | A | A | T | T | ||
| 614 | 669 | 737 | 846 | 861 | 1017 | 1063 | 1104 | 1119 | 1136 | 1172 | 1259 | 1428 | 1435 | 1451 | 1551 | 1566 | 1578 | 1625 | 1631 | |
| G | G | C | A | C | T | G | C | C | T | A | A | C | A | A | C | T | C | G | A | |
| G | G | C | A | C | T | C | C | C | T | A | A | C | A | A | C | T | C | G | A | |
| A | A | T | T | T | C | C | T | T | T | A | A | T | G | A | T | C | C | A | C | |
| A | A | T | T | T | C | C | T | T | C | T | G | T | G | C | T | C | T | A | C | |
We compared the nucleotide sequences of S1 genes of our 18 Mass type isolates with those of pathogenic M41 and H120 vaccine strains. Of the 38 mutations, 19 were the same in the pathogenic M41 strain and 19 in the vaccine H120 strain. Nucleotide positions correspond to those in the sequence of the IBVH120 S1 gene. GenBank accession numbers are the same as those shown in Supplemental Table 1.
The 23 IBV isolates (11.2%) clustered together with pathogenic LDT3/03 and LDT3-A vaccine strains, showing nucleotide identities from 98.9 to 100% with each other and 98.4–99.3% to pathogenic LDT3/03 and 98.8–99.8% to LDT3-A vaccine strain (Fig. 2A). Multiple alignments using MAFFT found the mutations scattered across all S1 genes, compared to those of pathogenic LDT3/03 and LDT3-A vaccine strains (Supplemental Fig. 3).
3.5. Three genotypes/lineages of field viruses were circulating in chicken flocks in China
Ten isolates (4.6%) belonged to GI-7 lineage that were previously assigned to two different genetic groups referred to as Taiwan I (TW I) and Taiwan II (TW II) (Wang and Tsai, 1996). Of the 10 isolates, only one (I0730/17) was clustered in the TW II group (>99.6% nucleotide identity) (Fig. 2A). Isolate I0730/17 was more distantly related to representatives of TW II type strains TW2296/95 (94.6% nucleotide identity) (Wang and Tsai, 1996) and ck/CH/LHB/100801 (94.5% nucleotide identity) (Ma et al., 2012). An ACT deletion was found at positions 73–75 in the S1 gene of isolate I0730/17, compared to isolates TW2296/95 and ck/CH/LHB/100801. Nine isolates were assigned to the TW I clade (Fig. 2A) and they shared nucleotide identity between 94.0 and 98.5% with the TW I type strain TW2575/98. Among the nine TW I isolates, I1101/16 showed slight variations from the other eight isolates. In addition, a three-nucleotide insertion and a three-nucleotide deletion were found in the Epitope D regions in S1 genes of isolates I1101/16 and I0730/17, respectively, comparing to other TW I viruses (Supplemental Fig. 4). The complete S1 gene sequences of our nine TW I isolates were 93.2 and 100% homologous to each other but were < 87.1% homologous to the TW II isolate I0730/17.
GI-22 lineage was initially assigned to the ck/CH/LSC/99I-type cluster following the inclusion of the Chinese IBV reference strain ck/CH/LSC/99I isolated in 1999 (Han et al., 2011). In this study, only one GI-22 isolate (I1225–1/17) was obtained and it was distinctly separated from other GI-22 reference strains in the phylogenetic tree (89.3–93.7% nucleotide identity and 83.8–91.4% amino acid identity). In addition, a TTAGCC insertion was found at positions 67–72 in the S1 gene of isolate I1225–1/17, compared to other GI-22 reference strains.
Four (1.8%) of the IBV isolates fell within lineage 1 of genotype VI, along with a previously reported IBV strain TC07–2 (Li et al., 2010). The sequences of the four isolates has nucleotide sequence identity between 97.3 and 98.5% when compared to each other and between 97.8 and 98.8% when compared to the reference TC07–2 strain.
3.6. Novel IBV recombinant strains isolated in China
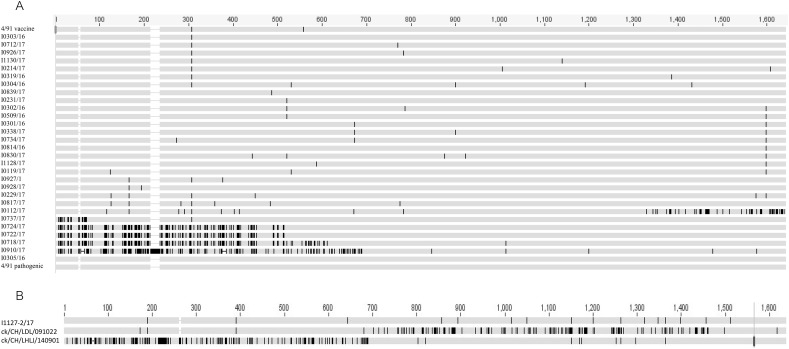
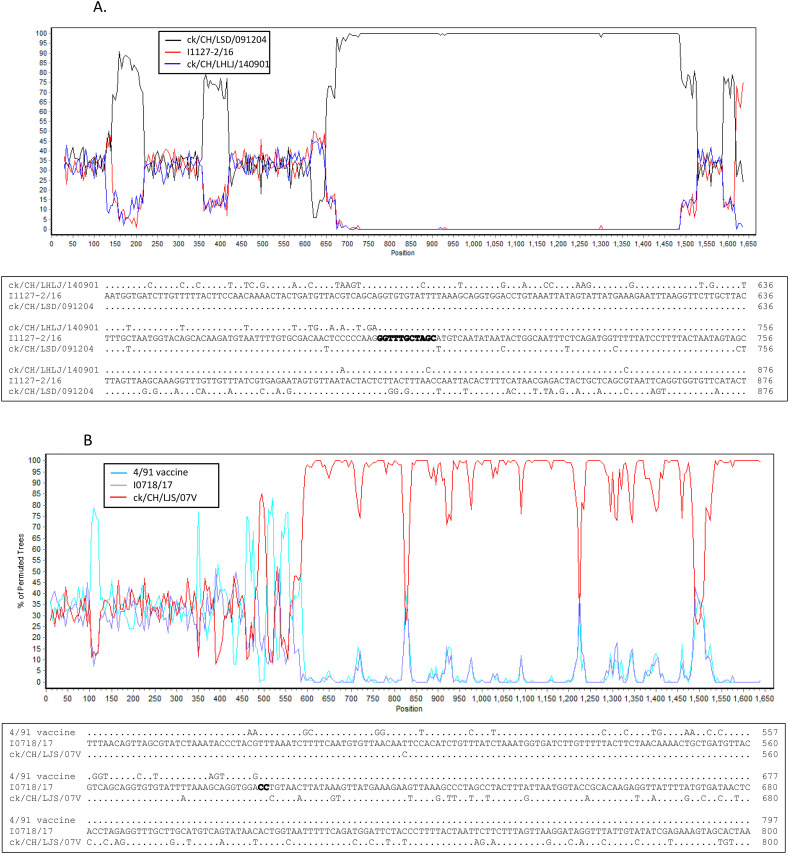
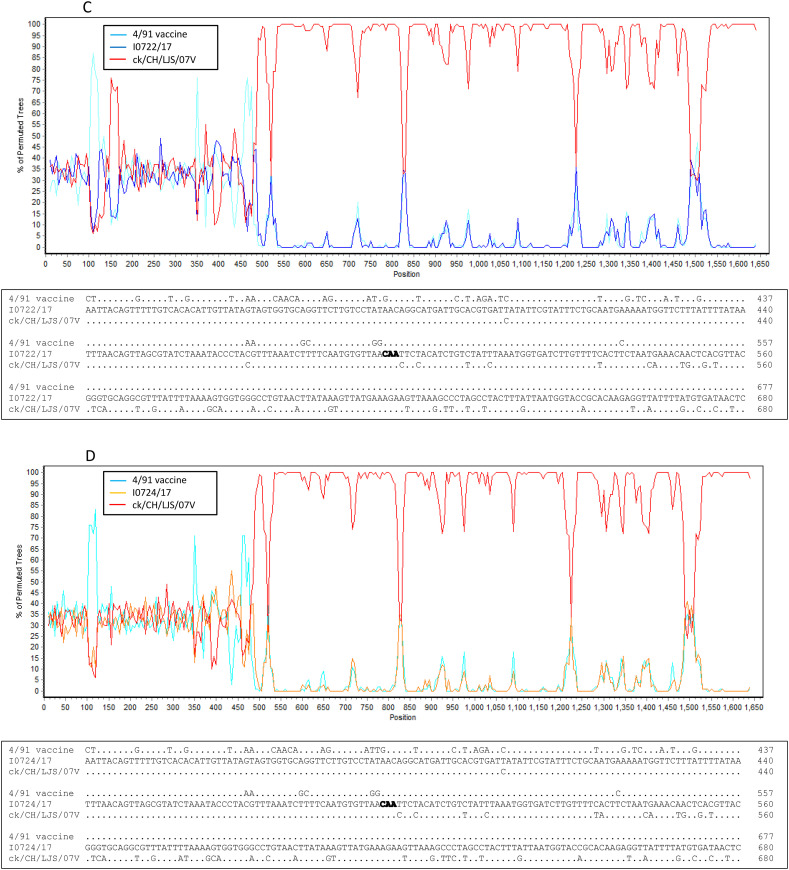
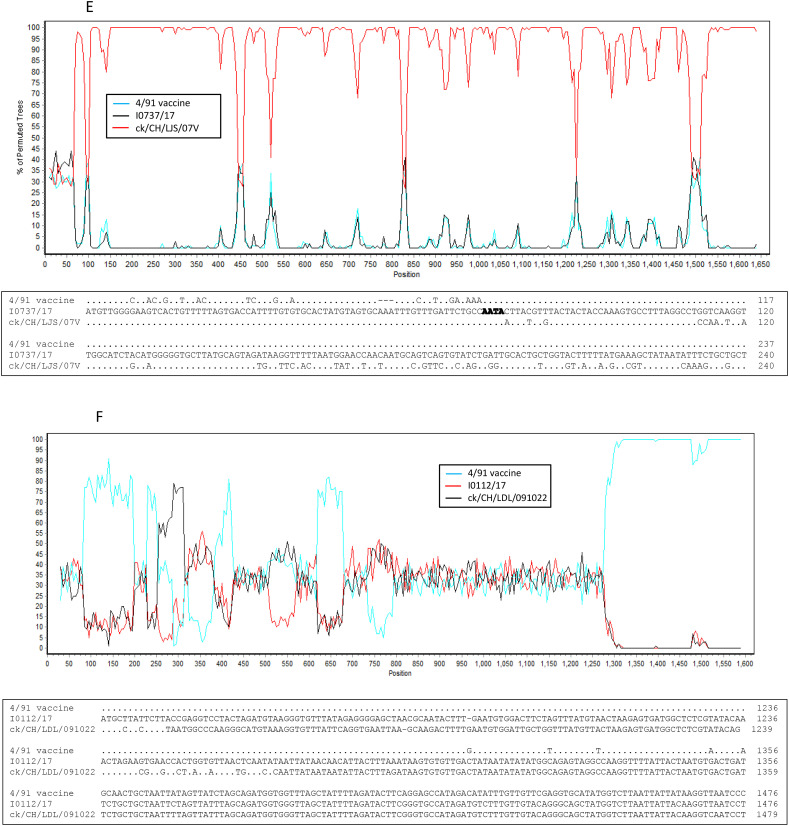
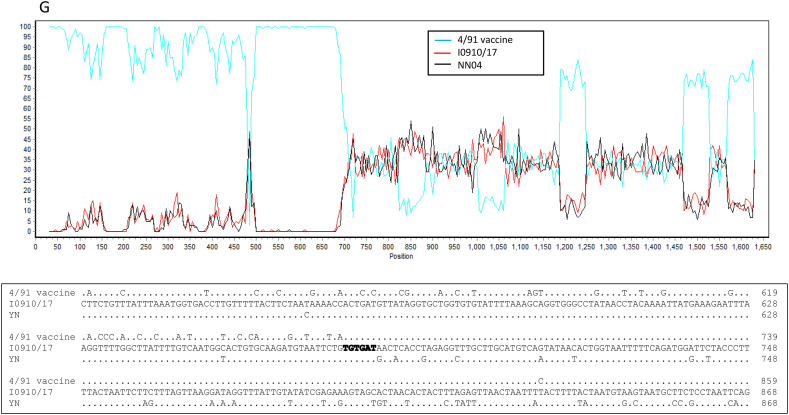
Seven novel IBV isolates, including I2217–2/16, I0718/17, I0722/17, I0724/17, I0910/17, I0112/17 and I0737/17, clustered separately and were found to have different and unique S1 gene sequences from other IBV isolates and reference strains in the phylogenetic tree, although isolate I2217–2/16 was genetically closer to GI-19 isolates and the remaining six isolates were closely related GI-13 viruses (Fig. 2A). Nucleotide sequence alignment suggested that these novel IBV isolates originated from recombination events (Fig. 3A and B). This hypothesis was confirmed by the RDP analysis that showed that isolate I2217–2/16 likely originated from recombination events between ck/CH/LSD/091204-like (GI-19 lineage virus) and ck/CH/LHLJ/140901-like (TW I in GI-7 lineage) viruses (Fig. 4A upper). The remaining six IBV isolates were identified as novel strains that originated from recombination events between viruses in GI-19 and GI-13 lineages (Fig. 4B–G upper).
Fig. 3.
Multiple alignments of the nucleotide sequences of S1 genes performed using MAFFT version 6 (http://mafft.cbrc.jp/alignment/software/). The sequences of our 38 GI-13 viruses, 4/91 vaccine, 4/91 pathogenic strain, and six GI-13-related viruses isolated in this study were compared (A). Nine isolates, including I0813/16, I0130/17, I0213/17, I0227/17, I0232/17, I0925/17, I0522/17, I0230/17 and I0120/17, shared the same sequence with that of I0303/16 and hence, only the sequence of I0303/16 is listed in the alignment. Similarly, isolates I0320/16, I0511/16, I0339/17 and I0922/17 had the same sequences as I0301/16 and only the sequence of I0301/16 is listed in the alignment. In addition, isolates I0231/17 and I0321/16, I0305/16 and I0651/17 had the same sequences, respectively, and only I0231/16 and I0305/16 are listed, respectively, in the alignment. The deleted sequences are represented in white. The GenBank accession numbers for these genome sequences are listed in Supplemental Table 2. The sequence of isolate I1127–2/17 was compared with those of reference strains ck/CH/LHLJ/140901 and ck/CH/LDL/091022 (B).
Fig. 4.
Bootscan analysis of S1 gene on the putative recombinant isolate and parental strains (upper parts in each figure), and sequence alignment of the recombination breakpoints and the flanking sequences between the putative recombinant isolate and parental strains (lower parts in each figure). Different reference strains were used as putative parental strains in each figure. Strain ck/CH/LSD/110347 was used as a query strain in Fig. 6A–F and strain ck/CH/LJS/101109 was used as a query strain in Fig. 6G. The y-axis shows the percentage of identity within a window size of 500 bp and a step size of 20 bp. Dots represent same base pairs, while letters show different base pairs. The GenBank accession numbers for these genome sequences are listed in Supplemental Table 2.
4. Discussion
Our study involved 2 years of active and passive surveillance to characterize the diversity of the IBV isolates in recent years in China. A total of 213 IBVs were isolated from vaccinated chickens between January 2016 and December 2017. These 213 IBV isolates were divided into six lineages/genotypes, a number of recombinants, and a novel variant (I0636/1) based on the analysis of the complete S1 genes. In addition to the GI-9 (Arkansas type) viruses reported previously (Han et al., 2018), at least seven IBV lineages/genotypes and a number of recombinants have circulated in vaccinated chicken flocks in recent years in China.
Approximately 52.0% of IBV isolates in this study were identified as GI-19 lineages and GI-19 was the most frequently isolated lineages. Such prevalence is mostly higher compared to that of other lineages/genotypes detected in chicken flocks in China. The GI-19 viruses were first isolated more than two decades ago and were previously established as a new genotype based on the molecular nature of the S1 gene (Liu and Kong, 2004). Currently, these IBV strains have become some of the most widely distributed in many countries (Valastro et al., 2016) that have major poultry production. Viral neutralization studies have revealed that this lineage represents a novel serotype (Gao et al., 2016). In general, outbreaks of IB are associated with immunological escape of antigenically distinct strains that have originated from natural selection (Toro et al., 2006). Mechanisms for the generation of variation in coronaviruses, like IBV, include nucleotide insertions, deletions, or point mutations in the S1 gene made by the viral polymerase (Kusters et al., 1987; Jia et al., 1995), RNA recombination, and small changes in the S2 subunit that can alter the structure of S1 (Callison et al., 1999). In some cases, new IBV genotypes/serotypes can emerge as a result of only a few changes in the amino acid sequences of the S protein. In this study, different sublineages were found within the established GI-19 lineage in the phylogeny, and sublineages A and E were the predominant types of IBV circulating in chicken flocks in China. In general, isolates in the same sublineages shared higher levels of nucleotide and amino acid identities to those of isolates in different sublineages. Multiple point mutations were observed across the S1 gene of viruses in the GI-19 lineage, although unique mutations were found in each sublineage. In addition, deletions were found in some viruses in sublineages A and E and were mainly located in epitope D in HVRI. These results confirmed that GI-19 lineage had evolved into different sublineages and there was a high degree of S1 gene variation among the GI-19 lineage viruses. Amino acid differences of even a few residues can be responsible for different structural predictions that can create different interactions between the S1 and S2 subunits, which may affect the quaternary structure of the spike glycoprotein. The antigenic variation caused by the substitutions and deletions in viruses of GI-19 lineage, especially in viruses in different sub-lineages, requires further investigation.
Vaccines against IBV are often applied to reduce economic losses due to infection with field strains. As in other countries and regions, IBV H120 and Ma5 vaccines are among the commonly used live IBV vaccines in commercial poultry in China. Live IBV vaccine is able to spread extensively within groups of chickens vaccinated at day of hatching or at 15 days of age (Matthijs et al., 2008). In some cases, the IBV vaccine can induce mild signs (such as nasal discharge) of IB shortly after administration (Matthijs et al., 2003, Matthijs et al., 2005, Matthijs et al., 2008), and increases the susceptibility of 4-week-old broilers to colibacillosis to a similar extent as the virulent IBV strain (Matthijs et al., 2005). In addition, the variable virulence of the vaccine viruses when they are coadministered and the ability of some of the combinations to cause severe tracheal damage have been reported (Awad et al., 2016). Most of the diseased broilers in which IBV vaccine-like viruses were isolated in this study showed mild to severe colibacillosis when they were approximately 4 weeks old. Hence, the isolation of IBV vaccine-like (H120, 4/91 and LDT3-A) viruses in this study was not surprising. Although we found that vaccine-like viruses were reisolated in this study, an issue that needs more work is the ability to differentiate the reisolation of vaccine strains from field strains of the same serotype. That is usually done at the level of homology between the sequences of the S gene or a part of it, such as the S1 region, due to the lack of virulence markers.
Genotypes TW I and TW II first emerged in the early 1990s in Taiwan and were considered to be of distinct genotypes (Wang and Tsai, 1996). The two genotypes were grouped into the same lineage in genotype I as GI-7 (Valastro et al., 2016). A novel TW I type (nrTW I), as an emerging genotype that has been reported in Mainland China, was considered to have originated from recombination events between viruses of GI-19 lineage and TW I (Xu et al., 2016). In this study, nine TW I-like isolates were obtained although we could not determine whether they were TW I or nrTW I because the switch sites of nrTW I were beyond the S1 gene. However, mounting evidence shows that the prevalence of the TW I-like strains has been increasing in recent years in China (Feng et al., 2017). These strains have caused severe economic losses because they cause false layers as well as death (Gao et al., 2016).
In 2007, an IBV strain (designated as TC07–2) that represents a genetically distinct lineage was isolated from broilers with respiratory illness in Guangdong Province, China (Li et al., 2010). Two years later, TC07–2-like viruses were also isolated in Japan and Korea, and designated as JP-IV (Mase et al., 2010) and new cluster 2 (Lim et al., 2012) genotypes, respectively. Later, TC07–2-like viruses were designated as GVI-1 by phylogenetic analysis using complete nucleotide sequences of the S1 gene from 13 IBV isolates in China and Korea between 2007 and 2012 (Valastro et al., 2016). We isolated four GVI-1 viruses in this study from four provinces in China between 2016 and 2017. Although the origin of GVI-1 viruses in China is not clear and it is not the main type of IBV in China, GVI-1 IBVs have been isolated from vaccinated chickens from different regions in different years in China, indicating that this IBV lineage is still circulating in chicken flocks and is endemic in China. The characteristics of the viral evolution, antigenicity, tissue tropism and pathogenicity of this IBV lineage remain poorly understood and require further investigation.
It is believed that recombination as a consequence of mixed infections by different IBV field strains, and widespread use of various vaccines made from heterotypic IBVs in the fields, may play important roles in the emergence of novel genetic IBV variants (Liu et al., 2013; Chen et al., 2015; Zhang et al., 2015; Leghari et al., 2016; Chen et al., 2017). We isolated and identified seven novel isolates in this study that were genetically distinct from those of other IBV strains isolated in this study and circulated in other parts of the world, and demonstrated that they originated from recombination events. It was not surprising to find that six of the viruses originated from recombination events between 4/91 vaccine and GI-19 strains because the commonly used 4/91 vaccine could not provide enough protection against the predominant GI-19 lineage circulating in China. This could make the two lineages coexist in some chicken flocks and would make such recombination events possible (Han et al., 2017). In addition, it is important to take into consideration that recombination between distantly related clades (lineages) is easier to detect than between closely related viruses, for which there are few phylogenetic signals. Therefore, it was likely that recombination events between more closely related viruses, i.e., within a lineage, expectedly remained undetected in this study. Nevertheless, we can conclude that the presence of some isolates in this study might have been caused, at least partially, by mutations of the S1 gene associated with recombination events between the field and vaccine strains, although the biological characteristics of these recombinants requires to be elucidated.
Different subpopulations of viruses have been identified in the same sample of coronaviruses, such as avian IBVs, through deep sequencing or virus isolation and subsequent sequencing of the viral genomes (de Wit et al., 2017). In this study, we identified two different IBVs and isolated the viruses in the same tissue sample from each of the eight flocks. It is possible that multiple IBV populations/types in the same bird (some were the vaccine strains often used in chicken flocks) could lead to high genetic diversity and frequent recombination, which suggests the potential for emergence of divergent and novel IBV coronaviruses. It is reported that co-infection of IBV with other respiratory pathogens, such as avian influenza subtype H9, can increase the level of damage to birds (Georgiades et al., 2001; Haghighat-Jahromi et al., 2008). However, the effects of co-infection by multiple populations/types of IBV strains on the birds are unknown and should be investigated further.
Despite the large sample size, our study did not detect and isolate any of the GI-16 (also known as Q1 or ck/CH/LDL/97I) (Liu et al., 2007), GI-28 (Chen et al., 2017) and GI-29 (Jiang et al., 2018) lineages that have previously been reported in China. This was possibly because our study was not designed as a case–control or cohort study. In addition, a longitudinal study in a fixed chicken farm would be beneficial to understand further the dynamics of evolution and infection of IBV over time. The results justify permanent monitoring of circulating strains in order to monitor the emerging viruses and modify vaccination strategies to take account of the evolving field situation.
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (2017YFD0500105), the China Agriculture Research Systerm (No. CARS-40-K18), National “Twelfth Five-Year” Plan for Science & Technology Support (2015BAD12B03) and the Provincial Supported Science Foundation of Heilongjiang Province for The National Key Technology R&D Program (GX16B003). The authors declare that they have no competing interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.meegid.2018.09.018.
Appendix A. Supplementary data
Supplementary ma terial 1
Supplementary material 2
References
- Awad F., Hutton S., Forrester A., Baylis M., Ganapathy K. Heterologous live infectious bronchitis virus vaccination in day-old commercial broiler chicks: clinical signs, ciliary health, immune responses and protection against variant infectious bronchitis viruses. Avian Pathol. 2016;45:169–177. doi: 10.1080/03079457.2015.1137866. [DOI] [PubMed] [Google Scholar]
- Callison S.A., Jackwood M.W., Hilt D.A. Infectious bronchitis virus S2 gene sequence variability may affect S1 subunit specific antibody binding. Virus Genes. 1999;19:143–151. doi: 10.1023/A:1008179208217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casais R., Dove B., Cavanagh D., Britton P. Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism. J. Virol. 2003;77:9084–9089. doi: 10.1128/JVI.77.16.9084-9089.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus IBV: structural characterization of the spike protein. J. Gen. Virol. 1983;64:2577–2583. doi: 10.1099/0022-1317-64-12-2577. [DOI] [PubMed] [Google Scholar]
- Cavanagh D. Coronavirus avian infectious bronchitis virus. Vet. Res. 2007;38:281–297. doi: 10.1051/vetres:2006055. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Darbyshire J.H., Peters R.W. Coronavirus IBV: virus retaining spike glycopolypeptide S2 but not S1 is unable to induce virus-neutralizing or haemagglutination-inhibiting antibody, or induce chicken tracheal protection. J. Gen. Virol. 1986;67:1435–1442. doi: 10.1099/0022-1317-67-7-1435. [DOI] [PubMed] [Google Scholar]
- Cavanagh D., Davis P.J., Mockett A.P. Amino acids within hypervariable region 1 of avian coronavirus IBV (Massachusetts serotype) spike glycoprotein are associated with neutralization epitopes. Virus Res. 1988;11:141–150. doi: 10.1016/0168-1702(88)90039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh D., Picault J.P., Gough R., Hess M., Mawditt K., Britton P. Variation in the spike protein of the 793/B type of infectious bronchitis virus, in the field and during alternate passage in chickens and embryonated eggs. Avian Pathol. 2005;34:20–25. doi: 10.1080/03079450400025414. [DOI] [PubMed] [Google Scholar]
- Chen L., Zhang T., Han Z., Liang S., Xu Y., Xu Q., Chen Y., Zhao Y., Shao Y., Li H., Wang K., Kong X., Liu S. Molecular and antigenic characteristics of Massachusetts genotype infectious bronchitis coronavirus in China. Vet. Microbiol. 2015;181:241–251. doi: 10.1016/j.vetmic.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Jiang L., Zhao W., Liu L., Zhao Y., Shao Y., Li H., Han Z., Liu S. Identification and molecular characterization of a novel serotype infectious bronchitis virus (GI-28) in China. Vet. Microbiol. 2017;198:108–115. doi: 10.1016/j.vetmic.2016.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit J.J., Nieuwenhuisen-Van Wilgen J., Hoogkamer A., van de Sande H., Zuidam G.J., Fabri T.H. Induction of cystic oviducts and protection against early challenge with infectious bronchitis virus serotype D388 (genotype QX) by maternally derived antibodies and by early vaccination. Avian Pathol. 2011;40:463–471. doi: 10.1080/03079457.2011.599060. [DOI] [PubMed] [Google Scholar]
- de Wit J.J., Dijkman R., Guerrero P., Calvo J., Gonzalez A., Hidalgo H. Variability in biological behaviour, pathogenicity, protectotype and induction of virus neutralizing antibodies by different vaccination programmes to infectious bronchitis virus genotype Q1 strains from Chile. Avian Pathol. 2017;46:666–675. doi: 10.1080/03079457.2017.1346782. [DOI] [PubMed] [Google Scholar]
- Dwars R.M., Matthijs M.G., Daemen A.J., van Eck J.H., Vervelde L., Landman W.J. Progression of lesions in the respiratory tract of broilers after single infection with Escherichia coli compared to superinfection with E. coli after infection with infectious bronchitis virus. Vet. Immunol. Immunopathol. 2009;127:65–76. doi: 10.1016/j.vetimm.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Feng K., Wang F., Xue Y., Zhou Q., Chen F., Bi Y., Xie Q. Epidemiology and characterization of avian infectious bronchitis virus strains circulating in southern China during the period from 2013-2015. Sci. Rep. 2017;7:6576. doi: 10.1038/s41598-017-06987-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M., Wang Q., Zhao W., Chen Y., Zhang T., Han Z., Xu Q., Kong X., Liu S. Serotype, antigenicity, and pathogenicity of a naturally recombinant TW I genotype infectious bronchitis coronavirus in China. Vet. Microbiol. 2016;191:1–8. doi: 10.1016/j.vetmic.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades G., Iordanidis P., Koumbati M. Cases of swollen head syndrome in broiler chickens in Greece. Avian Dis. 2001;45:745–750. [PubMed] [Google Scholar]
- Haghighat-Jahromi, M.1., Asasi, K., Nili, H., Dadras, H., Shooshtari, A.H., 2008. Coinfection of avian influenza virus (H9N2 subtype) with infectious bronchitis live vaccine. Arch. Virol. 153, 651–655. [DOI] [PMC free article] [PubMed]
- Han Z., Sun C., Yan B., Zhang X., Wang Y., Li C., Zhang Q., Ma Y., Shao Y., Liu Q., Kong X., Liu S. A 15-year analysis of molecular epidemiology of avian infectious bronchitis coronavirus in China. Infect. Genet. Evol. 2011;11:190–200. doi: 10.1016/j.meegid.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Zhao W., Chen Y., Xu Q., Sun J., Zhang T., Zhao Y., Liang S., Gao M., Wang Q., Kong X., Liu S. Genetic, antigenic, and pathogenic characteristics of avian infectious bronchitis viruses genotypically related to 793/B in China. Vet. Microbiol. 2017;203:125–135. doi: 10.1016/j.vetmic.2017.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z., Jiang L., Zhao W., Chen Y., Xu L., Sun J., Zhao Y., Liu S. Isolation and Characteristics of the Arkansas-Type Infectious Bronchitis Virus in China. Avian Dis. 2018;62:18–27. doi: 10.1637/11719-072517-Reg.1. [DOI] [PubMed] [Google Scholar]
- Hewson K.A., Scott P.C., Devlin J.M., Ignjatovic J., Noormohammadi A.H. The presence of viral subpopulations in an infectious bronchitis virus vaccine with differing pathogenicity--a preliminary study. Vaccine. 2012;30:4190–4199. doi: 10.1016/j.vaccine.2012.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia W., Karaca K., Parrish C.R., Naqi S.A. A novel variant of avian infectious bronchitis virus resulting from recombination among three different strains. Arch. Virol. 1995;140:259–271. doi: 10.1007/BF01309861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Zhao W., Han Z., Chen Y., Zhao Y., Sun J., Li H., Shao Y., Liu L., Liu S. Genome characterization, antigenicity and pathogenicity of a novel infectious bronchitis virus type isolated from South China. Infect. Genet. Evol. 2017;54:437–446. doi: 10.1016/j.meegid.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant A., Koch G., van Roozelaar D.J., Kusters J.G., Poelwijk F.A., van der Zeijst B.A. Location of antigenic sites defined by neutralizing monoclonal antibodies on the S1 avian infectious bronchitis virus glycopolypeptide. J. Gen. Virol. 1992;73:591–596. doi: 10.1099/0022-1317-73-3-591. [DOI] [PubMed] [Google Scholar]
- Kusters J.G., Niesters H.G., Bleumink-Pluym N.M., Davelaar F.G., Horzinek M.C., Van der Zeijst B.A. Molecular epidemiology of infectious bronchitis virus in the Netherlands. J. Gen. Virol. 1987;68:343–352. doi: 10.1099/0022-1317-68-2-343. [DOI] [PubMed] [Google Scholar]
- Kwon H.M., Jackwood M.W., Gelb J., Jr. Differentiation of infectious bronchitis virus serotypes using polymerase chain reaction and restriction fragment length polymorphism analysis. Avian Dis. 1993;37:194–202. [PubMed] [Google Scholar]
- Lee C.W., Hilt D.A., Jackwood M.W. Typing of field isolates of infectious bronchitis virus based on the sequence of the hypervariable region in the S1 gene. J. Vet. Diagn. Investig. 2003;15:344–348. doi: 10.1177/104063870301500407. [DOI] [PubMed] [Google Scholar]
- Leghari R.A., Fan B., Wang H., Bai J., Zhang L., Abro S.H., Jiang P. Full-length genome sequencing analysis of avian infectious bronchitis virus isolate associated with nephropathogenic infection. Poult. Sci. 2016;95:2921–2929. doi: 10.3382/ps/pew259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Xue C., Chen F., Qin J., Xie Q., Bi Y., Cao Y. Isolation and genetic analysis revealed no predominant new strains of avian infectious bronchitis virus circulating in South China during 2004-2008. Vet. Microbiol. 2010;143:145–154. doi: 10.1016/j.vetmic.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim, T.H., Kim, M.S., Jang, J.H., Lee, D.H., Park, J.K., Youn, H.N., Lee, J.B., Park, S.Y., Choi, I.S., Song, C.S., 2012. Live attenuated nephropathogenic infectious bronchitis virus vaccine provides broad cross protection against new variant strains. Poult. Sci. 91, 89–94. [DOI] [PMC free article] [PubMed]
- Liu S., Kong X. A new genotype of nephropathogenic infectious bronchitis virus circulating in vaccinated and non-vaccinated flocks in China. Avian Pathol. 2004;33:321–327. doi: 10.1080/0307945042000220697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Han Z., Chen J., Liu X., Shao Y., Kong X., Tong G., Rong J. S1 gene sequence heterogeneity of a pathogenic infectious bronchitis virus strain and its embryo-passaged, attenuated derivatives. Avian Pathol. 2007;36:231–234. doi: 10.1080/03079450701338730. [DOI] [PubMed] [Google Scholar]
- Liu X., Ma H., Xu Q., Sun N., Han Z., Sun C., Guo H., Shao Y., Kong X., Liu S. Characterization of a recombinant coronavirus infectious bronchitis virus with distinct S1 subunits of spike and nucleocapsid genes and a 3' untranslated region. Vet. Microbiol. 2013;162:429–436. doi: 10.1016/j.vetmic.2012.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Xu Q., Han Z., Liu X., Li H., Guo H., Sun N., Shao Y., Kong X. Origin and characteristics of the recombinant novel avian infectious bronchitis coronavirus isolate ck/CH/LJL/111054. Infect. Genet. Evol. 2014;23:189–195. doi: 10.1016/j.meegid.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H., Shao Y., Sun C., Han Z., Liu X., Guo H., Liu X., Kong X., Liu S. Genetic diversity of avian infectious bronchitis coronavirus in recent years in China. Avian Dis. 2012;56:15–28. doi: 10.1637/9804-052011-Reg.1. [DOI] [PubMed] [Google Scholar]
- Mase M., Kawanishi N., Ootani Y., Murayama K., Karino A., Inoue T., Kawakami J. A novel genotype of avian infectious bronchitis virus isolated in Japan in 2009. J. Vet. Med. Sci. 2010;72:1265–1268. doi: 10.1292/jvms.10-0080. [DOI] [PubMed] [Google Scholar]
- Matthijs M.G., van Eck J.H., Landman W.J., Stegeman J.A. Ability of Massachusetts-type infectious bronchitis virus to increase colibacillosis susceptibility in commercial broilers: a comparison between vaccine and virulent field virus. Avian Pathol. 2003;32:473–481. doi: 10.1080/0307945031000154062. [DOI] [PubMed] [Google Scholar]
- Matthijs M.G., van Eck J.H., de Wit J.J., Bouma A., Stegeman J.A. Effect of IBV-H120 vaccination in broilers on colibacillosis susceptibility after infection with a virulent Massachusetts-type IBV strain. Avian Dis. 2005;49:540–545. doi: 10.1637/7380-051305R.1. [DOI] [PubMed] [Google Scholar]
- Matthijs M.G., Bouma A., Velkers F.C., van Eck J.H., Stegeman J.A. Transmissibility of infectious bronchitis virus H120 vaccine strain among broilers under experimental conditions. Avian Dis. 2008;52:461–466. doi: 10.1637/8204-010708-Reg.1. [DOI] [PubMed] [Google Scholar]
- Mo M.L., Li M., Huang B.C., Fan W.S., Wei P., Wei T.C., Cheng Q.Y., Wei Z.J., Lang Y.H. Molecular characterization of major structural protein genes of avian coronavirus infectious bronchitis virus isolates in southern China. Viruses. 2013;5:3007–3020. doi: 10.3390/v5123007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinteros J.A., Lee S.W., Markham P.F., Noormohammadi A.H., Hartley C.A., Legione A.R., Coppo M.J., Vaz P.K., Browning G.F. Full genome analysis of Australian infectious bronchitis viruses suggests frequent recombination events between vaccine strains and multiple phylogenetically distant avian coronaviruses of unknown origin. Vet. Microbiol. 2016;197:27–38. doi: 10.1016/j.vetmic.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro H., van Santen V.L., Li L., Lockaby S.B., van Santen E., Hoerr F.J. Epidemiological and experimental evidence for immunodeficiency affecting avian infectious bronchitis. Avian Pathol. 2006;35:455–464. doi: 10.1080/03079450601028811. [DOI] [PubMed] [Google Scholar]
- Valastro V., Holmes E.C., Britton P., Fusaro A., Jackwood M.W., Cattoli G., Monne I. S1 gene-based phylogeny of infectious bronchitis virus: an attempt to harmonize virus classification. Infect. Genet. Evol. 2016;39:349–364. doi: 10.1016/j.meegid.2016.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerchove D., Herdt P.D., Laevens H., Butaye P., Meulemans G., Pasmans F. Significance of interactions between Escherichia coli and respiratory pathogens in layer hen flocks suffering from colibacillosis-associated mortality. Avian Pathol. 2004;33:298–302. doi: 10.1080/030794504200020399. [DOI] [PubMed] [Google Scholar]
- Wang C.H., Tsai C.T. Genotypic grouping for the isolates of avian infectious bronchitis virus in Taiwan. Arch. Virol. 1996;141:1677–1688. doi: 10.1007/BF01718291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Junker D., Hock L., Ebiary E., Collisson E.W. Evolutionary implications of genetic variations in the S1 gene of infectious bronchitis virus. Virus Res. 1994;34:327–338. doi: 10.1016/0168-1702(94)90132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q., Han Z., Wang Q., Zhang T., Gao M., Zhao Y., Shao Y., Li H., Kong X., Liu S. Emergence of novel nephropathogenic infectious bronchitis viruses currently circulating in Chinese chicken flocks. Avian Pathol. 2016;45:54–65. doi: 10.1080/03079457.2015.1118435. [DOI] [PubMed] [Google Scholar]
- Zhang T., Han Z., Xu Q., Wang Q., Gao M., Wu W., Shao Y., Li H., Kong X., Liu S. Serotype shift of a 793/B genotype infectious bronchitis coronavirus by natural recombination. Infect. Genet. Evol. 2015;32:377–387. doi: 10.1016/j.meegid.2015.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F., Zou N., Wang F., Guo M., Liu P., Wen X., Cao S., Huang Y. Analysis of a QX-like avian infectious bronchitis virus genome identified recombination in the region containing the ORF 5a, ORF 5b, and nucleocapsid protein gene sequences. Virus Genes. 2013;46:454–464. doi: 10.1007/s11262-013-0884-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Gao M., Xu Q., Xu Y., Zhao Y., Chen Y., Zhang T., Wang Q., Han Z., Li H., Chen L., Liang S., Shao Y., Liu S. Origin and evolution of LX4 genotype infectious bronchitis coronavirus in China. Vet. Microbiol. 2017;198:9–16. doi: 10.1016/j.vetmic.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Tang M., Jiang Y., Chen X., Shen X., Li J., Dai Y., Zou J. Complete genome sequence of a novel infectious bronchitis virus strain circulating in China with a distinct S gene. Virus Genes. 2014;49:152–156. doi: 10.1007/s11262-014-1063-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Zhang M., Tian X., Shao H., Qian K., Ye J., Qin A. Identification of a novel recombinant virulent avian infectious bronchitis virus. Vet. Microbiol. 2017;199:120–127. doi: 10.1016/j.vetmic.2016.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary ma terial 1
Supplementary material 2