Highlights
► Some plant virus infections lead to the formation of large perinuclear globular structures. ► These structures contain viral replication complexes and an amalgamation of endoplasmic reticulum and Golgi bodies. ► Peripheral motile virus-induced vesicles involved in virus movement are linked to these perinuclear structures.
Abstract
Although there is a significant amount of literature that deals with the identification of plant viral proteins involved in membrane remodeling and vesicle production in infected cells, there are very few investigations that report on the impact that infection has on the overall architecture and dynamics of the early secretory endomembranes. Recent investigations have shown that for some viruses the endoplasmic reticulum, Golgi bodies and other organelles are heavily recruited into virus-induced perinuclear structures. These structures are not isolated organelles and are dynamically connected to the bulk of non-modified endomembranes. They also have a functional link with peripheral motile vesicles involved in virus intracellular movement. The full molecular events that consubstantiate with this endomembrane recruitment in virus-induced structures remain to be elucidated but viral genome replication and virion assembly are probably taking place within these structures.
Current Opinion in Virology 2012, 2:683–690
This review comes from a themed issue on Virus replication in animals and plants
Edited by Peter Nagy and Christopher Richardson
For a complete overview see the Issue and the Editorial
Available online 2nd November 2012
1879-6257/$ – see front matter, © 2012 Elsevier B.V. All rights reserved.
Introduction
Recent advances in cell fluorescent imaging and tomography coupled to electron microscopy are bringing growing interest in understanding the architecture and role of the cellular remodeling that is taking place upon infection by viruses (for extensive reviews on the subject, refer to [1, 2]). Because most investigations on cellular remodeling in plant cells have been conducted using positive-sense (+)RNA viruses, this review will mainly focus on this class of viruses. Replication by plant (+)RNA viruses, like their vertebrate homologs, leads to the formation in the infected cell of elaborate membranous, organelle-like, platforms that sustain viral RNA synthesis and cell-to-cell movement. These membrane modifications are believed to increase the local concentrations of viral and host proteins needed to produce new genomes, which probably enhance replication efficiency, and possibly to provide protection from host defense response [3]. They also act as vehicles for the egress of viral RNA for systemic infection throughout the plant (for a review on the subject, refer to [4]).
Different plant virus groups induce the formation of diverse structures from host endomembranes, both in terms of architecture and membrane/organelle origin. Endomembranes are defined here as a system of interconnected membranes that fills the cell interior and connects the cell boundary with the double membraned organelles – nucleus, plastids and mitochondria. Essentially, every single organelle found in a plant cell is targeted by one virus or another. For example, Tomato bushy stunt virus (TBSV) replicates in peroxisomes [5], Carnation Italian ringspot virus in mitochondria [6] and Turnip yellow mosaic virus in chloroplasts [7]. The significance of this organellar diversity is unknown, but specific membrane targeting appears not to be a strict requirement for efficient viral infection as replication complexes can be redirected to an alternate subcellular localization [8, 9]. There are also many examples indicating that membrane remodeling is the consequence of plant viruses replicating or moving on endoplasmic reticulum (ER)-derived membranes. This results in the formation of mini-organelles referred to as spherules, vesicles or multivesicular bodies [10, 11, 12, 13•, 14, 15•, 16, 17]. These vesicles may be single or double membrane structures that are often connected to the cytosol by a narrow neck, allowing exchange of components needed for replication [18]. The ER is a major component of the cell's secretory pathway, which is a series of steps a cell uses to move host components to their final functional location. The secretory pathway was thus shown to be used for intracellular and intercellular viral movement [19•, 20•, 21•, 22, 23••, 24, 25]. Virus use of the secretory pathway not only has a morphological impact on ER, it also leads to an inhibition of protein secretion [26] and may promote specific lipid synthesis [27]. Although there is a significant amount of literature that deals with the identification of the viral proteins involved in membrane remodeling and vesicle production, there are very few investigations that report on the impact that plant virus infection has on the overall architecture and dynamics of the early secretory endomembranes. This review will look at recent works that show that Potato virus X (PVX) and Turnip mosaic virus (TuMV) infections lead to endomembrane recruitment into large perinuclear globular structures that are functionally linked to smaller peripherally located motile vesicles that ultimately become associated with plasmodesmata. This connection would provide an assembly line for viral genome replication and virus egress into neighboring cells.
Plant cell biology
Before looking at endomembrane recruitment during plant virus infections, it may be important to provide an overview of the general morphology of plant cells. For those interested in a more detailed account of plant cell morphology, they are invited to consult The Illuminated Plant Cell website (http://www.illuminatedcell.com/Home.html). First, it must be realized that for many plant cells there is limited free cytosolic space, which is restricted by the presence of large central vacuoles and by the sheer number of chloroplasts. Within this constricted space, the ER pervades the cell and has an extremely dynamic, multifunctional and pleomorphic nature [28, 29]. Morphologically, it is characterized by a nuclear envelope-connected ER and a peripheral (cortical) tubular and cisternal ER juxtaposing the plasma membrane. Transvacuolar ER strands provide a direct link between the perinuclear and the cortical ER and act as distribution routes for metabolites, organelles [30] and are also thought to be involved in anchoring the nucleus within the cell [31•]. The plant Golgi apparatus is present in the form of several motile bodies that are distributed throughout the cytoplasm and are associated with microfilaments [32]. Golgi bodies also move in close association with ER tubules and traffic rapidly within transvacuolar strands [33], whereas in animal cells the Golgi apparatus occupies a rather stationary perinuclear position [34]. Another important difference is the absence in plant cells of an intermediate compartment between ER and the Golgi apparatus, which is present in mammalian cells and known as the ER-Golgi intermediate compartment (ERGIC) [35]. Finally, plant cells have plasmodesmata that provide cytoplasmic continuity between neighboring cells that supports the cell-to-cell and long-distance trafficking of small molecules as well as of a wide spectrum of endogenous proteins and ribonucleoprotein complexes. These plasma membrane lined channels contain ER-derived desmotubules and actin filaments and are used for virus cell-to-cell spread (for a review on the topic see [4, 36]). These distinctive features may explain the relationship between ER-associated virus replication centers and virus egress through plasmodesmata [37].
Recruitment of endomembranes into virus-induced structures
Membrane-associated replication complexes contain viral RNA as well as viral and host replication factors but not much is known about their host endomembrane composition. This question has recently been addressed in the case of potato virus X (PVX, genus potexvirus). At late infection stages, PVX induces the formation of large inclusion bodies often localized next to the nucleus, which have historically been termed ‘X-bodies’, that contain viral RNA [38], PVX replication proteins, virions and ribosomes [12, 39]. Very recently, Tilsner et al. [40••] have analyzed the contribution of endomembranes to these X-bodies. First, they found that the triple gene block protein 1 (TGBp1) forms the core of the X-bodies, which have a layered structure with TGBp1 aggregates at the center, vRNA in the middle and virions at the cytoplasmic periphery (Figure 1 ). They found that the ER and Golgi (as well as actin filaments) are heavily recruited into these structures, apparently reorganized into densely stacked membranes (Figure 1). Since TGB proteins are not needed for PVX replication, the authors propose that this elaborate structure provides a restricted environment that would link replication with movement and possibly encapsidation.
Figure 1.
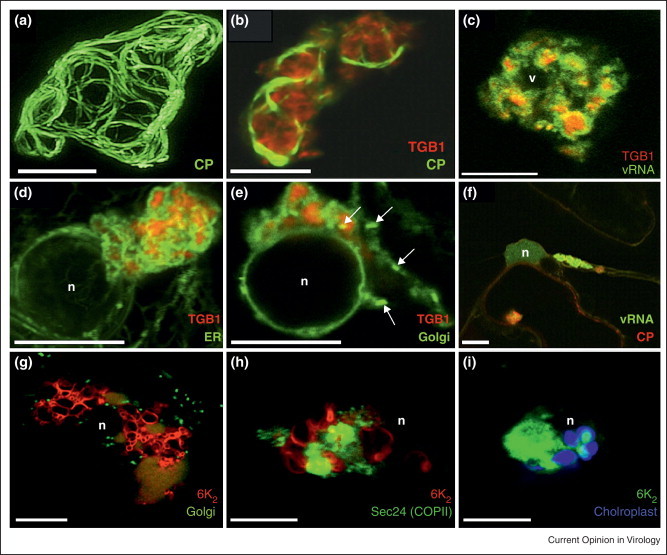
(a)–(f) Distribution of viral and host components in the PVX X-body. (a) GFP-CP expressed from a green overcoat virus construct labels virions that form cages around the X-body. (b) Aggregates of TGB1-mCherry localize within the cage formed by GFP-CP-decorated virions in green overcoat PVX-infected tissue. (c) TGB1-mCherry is located within PUM-BiFC-labeled vRNA whorls. (d) Recruitment of ER by perinuclear TGBp1-mCherry aggregates in uninfected tissue. The ER is wrapped tightly around the aggregates. (e) Recruitment of Golgi membranes and disassembly of Golgi stacks by TGB1-mCherry. Similar to the ER, Golgi membranes are wrapped tightly around the perinuclear aggregates. A few individual Golgi stacks are visible (arrows). (f) X-body containing vRNA and CP near the nucleus. (g)–(i) Organelle recruitment in TuMV globular structure. TuMV-infected cells expressing 6K2-mCherry (g, h) or 6K2-GFP (i), co-expressed with ST-GFP a Golgi marker (g), sec24-YFP (h) or with chloroplast autofluorescence (i). Bar = 10 μm. (n) indicates nucleus position.
(a)–(F) reproduced from [40••] with permission of the American Society of Plant Biologists. (g)–(i) reproduced from [41••] with permission of the American Society for Microbiology.
Grangeon et al. [41••] have reported that turnip mosaic virus (TuMV, genus potyvirus) infection also leads to the formation of perinuclear globular structures similar to X-bodies. The 6K2-VPg-Pro precursor protein of potyviruses has been shown to be a scaffold protein around which the viral replication complex assembles [15•, 42, 43, 44, 45, 46]. VPg binds several viral and host proteins, in particular translation factors (for a review, refer to [47]) while the 6K2 is responsible for membrane recruitment [47]. They examined the distribution of well characterized ER and Golgi organelle markers in TuMV-infected cells by confocal microscopy. In TuMV-infected cells, the cortical ER does not show any apparent modification but is speckled with 6K2-tagged vesicles (from now on designated as peripheral vesicles). On the contrary, the perinuclear ER is enlarged into a large irregular shaped globular-like structure that contained 6K2 and is linked to the cortical ER by transvacuolar strands. The ER is compacted within this structure and does not show a polygonal tubular pattern. Golgi bodies, COPII coatamers and chloroplasts are also recruited into this perinuclear globular structure (Figure 1). Disruption of the early secretory pathway by Brefeldin A (BFA) or by co-expression of a dominant-negative mutant of Arf1, which regulates membrane traffic between the Golgi and ER, does not affect the formation of the globular structure. Similarly, BFA does not affect replication of Melon necrotic spot virus whereas it has a negative impact on cell-to-cell movement [19•]. This situation is also observed during coronavirus infection where virus-induced remodeling of endoplasmic reticulum membranes and viral replication, albeit reduced, still take place in the presence of BFA [48].
However, despite their close association, ER and Golgi recruitment may not take place in tandem for all plant viruses. For instance, cowpea mosaic virus infection induces massive proliferation of ER and its recruitment into virus-induced vesicles, but not of Golgi membranes [14]. A similar situation is found for Grapevine fanleaf virus [11]. This noticeable dissimilarity suggests the existence of different mechanisms for host endomembrane recruitment during plant virus infection.
Dynamics of virus-induced structures
The plant ER and Golgi bodies are dynamic secretory organelles, constantly undergoing remodeling [49, 50]. Since the perinuclear globular structure observed in TuMV-infected cells contains an amalgam of condensed ER and Golgi membranes, investigations have been performed to observe if this compartment is nevertheless functionally linked to the bulk of non-modified endomembranes [41]. Fluorescence recovery after photobleaching (FRAP) experiments and the use of photoactivable GFP (PAGFP) [51] indicated that the TuMV-induced perinuclear structure is not an isolated subcellular compartment, Golgi and ER being connected to the bulk of the host cell endomembranes. It also appears that this compartment is a reservoir that can hold a large quantity of ER membranes. It has been reported that plant viral infections stimulate de novo membrane synthesis [14, 52, 53] and perhaps the bulk of newly synthesized lipids accumulate in these perinuclear structures. On the contrary, the perinuclear globular structure is not rapidly restocked in viral components following photobleaching, with no input of viral proteins from near-by perinuclear structures. Similarly, the internal architecture of Hepatitis C virus membranous webs appears relatively static, with limited exchange of viral proteins within and between neighboring replication complexes [54].
However, this apparent inactivity in restocking for viral components does not mean that the globular structure is a closed entity for viral proteins. PAGFP is used for fluorescent pulse labeling of fusion proteins at a specific position within a cell, which allows their subsequent cellular redistribution to be monitored [51]. When 6K2 was fused to PAGFP and expressed in infected cells and photoactivation performed within the globular structure, activated 6K2-PAGFP fluorescence was found to rapidly fill up the globular structure and motile 6K2-tagged vesicles were seen to originate and to move away from this same structure. These experiments then provide evidence for a functional link between the perinuclear globular structure and peripheral vesicles. Tilsner et al. [40••] also demonstrated that there is continuity between the X-bodies and peripheral vesicles associated with movement proteins and thus viral egress.
TuMV peripheral vesicles show rapid trafficking along transvacuolar strands as if they were traveling on a highway out and into the perinuclear globular structure [41••] (Figure 2 ). This trafficking is probably brought by rearrangements in the actin cytoskeleton [30]. Several groups have looked at the contribution of the cytoskeleton in the intracellular trafficking of virus-induced structures (for a review on the subject, refer to [22, 55]). For example, the group of Nelson analyzed the association of tobacco mosaic virus-induced bodies with microfilaments [16]. Time-lapse imaging shows that the peripheral bodies traffic along microfilaments with average velocities of 1 μm/s with top speed approaching 8 μm/s. The movement of these bodies has subsequently been shown to depend on myosin motors [15•, 20•, 21•]. Plasmodesmata are the ultimate destination of this trafficking.
Figure 2.
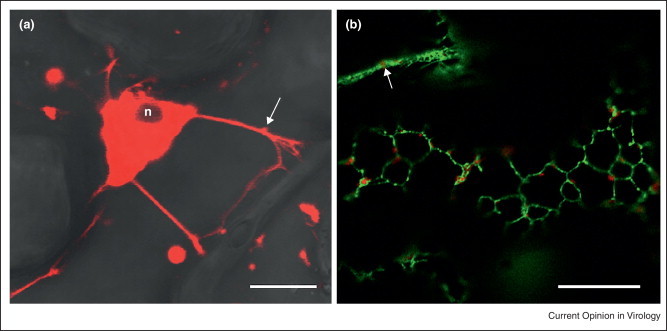
(a) TuMV-infected cells expressing 6K2-mCherry showing that transvacuolar strands provide a direct link between the perinuclear structure and the cell periphery highlighted by brightfield. (b) TuMV-infected cells expressing 6K2-mCherry showing association of peripheral vesicles with cortical ER (HDEL-GFP). n indicates nucleus position. Arrows indicate a peripheral vesicle moving within a transvacuolar strand. Bar = 10 μm.
Endomembranes are not only recruited to virus replication complexes, they are actively remodeled. For example, TBSV recruits ESCRT (endosomal sorting complexes required for transport) factors [3] and Brome mosaic virus (BMV) host reticulon proteins [56, 57] to facilitate membrane curving during virus-induced structure formation.
Recruitment of endomembranes in Arabidopsis thaliana mutant lines
Interestingly, defects in the early secretory pathway can produce similar recruitment of endomembranes into perinuclear structures as those observed in PVX-infected and TuMV-infected cells. Faso et al. [58••] characterized an Arabidopsis thaliana mutant that partially accumulates Golgi membrane markers and a soluble secretory marker in perinuclear globular structures entwined with actin cables and composed of a mass of convoluted ER tubules that maintain a connection with the bulk ER. The mutation also leads to impaired traffic of proteins at the ER/Golgi interface. In the same vein, Nakano et al. [59••] isolated two A. thaliana mutants with defects in ER morphology and designated them endoplasmic reticulum morphology1 (ermo1) and ermo2. The cells of both mutants develop a number of ER-derived spherical bodies, approximately 1 μm in diameter, that also contain Golgi bodies. The above lines have a defect in one of the Sec24 isomers that causes a partial loss of function for the binding of cargo protein intended for secretion. Faso et al. [58••] hypothesized that if constitutive traffic is disrupted, inappropriate fusion of vesicles between the ER and the Golgi may occur, creating an aberrant compartment. It is then plausible that the formation of the perinuclear globular structure is the consequence of an interfering event between a viral protein and Sec24 or another host protein of that nature.
Such interfering events have been documented for vertebrate viruses. The viral proteins involved are membrane-associated and interact or interfere with cellular membrane trafficking proteins of the early secretory pathway [60•, 61, 62, 63]. However, there is as yet no report showing a specific interaction with a plant viral protein and a host secretory pathway component but there is one investigation indicating that this may be the case. The triple gene block protein 3 (TGBp3) of bamboo mosaic virus (genus potexvirus) induces by itself the production of peripheral vesicles that are associated with the cortical ER. Wu et al. [23••] showed that mutations in the C-terminal region of the protein no longer formed vesicles in the cortical ER but exhibited perinuclear ER localization and concluded that C-terminal region of TGBp3 probably contains a sorting signal specifying cortical ER localization, implying interaction with a secretory pathway component. The tobacco etch virus (genus potyvirus) 6K2 protein may also have an ER export signal [64]. It will be interesting to see if these viral proteins target a component of the early secretory pathway at the ER/Golgi interface that leads to inhibition of protein secretion and formation of the perinuclear globular structure.
We suggest the following model to describe the cellular remodeling taking place during TuMV infection that shares many features with PVX replication (Figure 3 ). Early in the infection process, the incoming viral RNA is translated and the viral gene products contribute to the formation of the perinuclear globular structure. Replication events (i.e., negative and positive-sense RNA transcription) take place within this globular structure and these events would still happen even if the ER-Golgi interface is disrupted during viral infection. After this step, viral egress is initiated by the budding of 6K2 vesicles at ERES in the globular structure, which then traffic along the ER/microfilaments towards the plasma membrane and plasmodesmata for ultimate delivery of the virus into neighboring cells.
Figure 3.
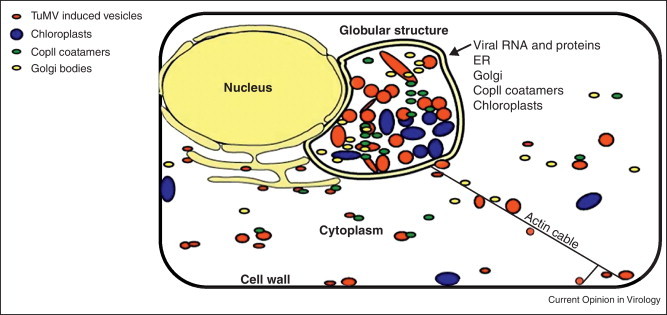
Model of perinuclear structure. Early in the infection process, the incoming viral RNA is translated and the viral gene products contribute to the formation of the perinuclear globular structure. Following replication events (i.e., negative and positive-sense RNA transcription) that take place within this globular structure, viral egress is initiated by vesicle budding. Vesicles then traffic along the ER/microfilaments towards the plasma membrane and plasmodesmata for ultimate delivery of the virus into neighboring cells.
Reproduced from [41••] with permission of the American Society for Microbiology.
Conclusion
The full molecular events that consubstantiate with this endomembrane recruitment in virus-induced structures remain however to be elucidated. Evidently viral genome replication and probably virion assembly are taking place within these structures. The fact that there is heavy recruitment of organelles into these structures would also reflect a need for sustained high synthetic activity that is required for virus production. Future investigations will thus aim at identifying host proteins that are involved in the formation of the perinuclear structure. Additionally, considering the large size of these structures, other events may take place concomitantly. For instance, the unfolded protein response has been proposed to be an element of PVX infection [65]. The active role of the host endomembranes in other plant (+)RNA virus replication should also be explored to broaden our understanding of general and unique aspects of these membranes in supporting viral processes.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We thank the anonymous reviewer for his/her constructive comments. Studies in J.-F. Laliberté laboratory are supported by grants from the Natural Sciences and Engineering Research Council of Canada and from Le Fonds québécois de recherche sur la nature et les technologies.
References
- 1.den Boon J.A., Ahlquist P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication Factories. Annual Review of Microbiology. 2010;64:241–256. doi: 10.1146/annurev.micro.112408.134012. [DOI] [PubMed] [Google Scholar]
- 2.Laliberté J.-F., Sanfaçon H. Cellular remodeling during plant virus infection. Annual Review of Phytopathology. 2010;48:69–91. doi: 10.1146/annurev-phyto-073009-114239. [DOI] [PubMed] [Google Scholar]
- 3.Barajas D., Jiang Y., Nagy P.D. A unique role for the host ESCRT proteins in replication of Tomato bushy stunt virus. PLoS Pathogens. 2009;5:e1000705. doi: 10.1371/journal.ppat.1000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Niehl A., Heinlein M. Cellular pathways for viral transport through plasmodesmata. Protoplasma. 2011;248:75–99. doi: 10.1007/s00709-010-0246-1. [DOI] [PubMed] [Google Scholar]
- 5.McCartney A.W., Greenwood J.S., Fabian M.R., White K.A., Mullen R.T. Localization of the tomato bushy stunt virus replication protein p33 reveals a peroxisome-to-endoplasmic reticulum sorting pathway. The Plant Cell. 2005;17:3513–3531. doi: 10.1105/tpc.105.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang Y.T., McCartney A.W., Gidda S.K., Mullen R.T. Localization of the Carnation Italian ringspot virus replication protein p36 to the mitochondrial outer membrane is mediated by an internal targeting signal and the TOM complex. BMC Cell Biology. 2008;9:54. doi: 10.1186/1471-2121-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prod’homme D., Jakubiec A., Tournier V., Drugeon G., Jupin I. Targeting of the turnip yellow mosaic virus 66K replication protein to the chloroplast envelope is mediated by the 140K protein. Journal of Virology. 2003;77:9124–9135. doi: 10.1128/JVI.77.17.9124-9135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jonczyk M., Pathak K.B., Sharma M., Nagy P.D. Exploiting alternative subcellular location for replication: tombusvirus replication switches to the endoplasmic reticulum in the absence of peroxisomes. Virology. 2007;362:320–330. doi: 10.1016/j.virol.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 9.Miller D.J., Schwartz M.D., Dye B.T., Ahlquist P. Engineered retargeting of viral RNA replication complexes to an alternative intracellular membrane. Journal of Virology. 2003;77:12193–12202. doi: 10.1128/JVI.77.22.12193-12202.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz M., Chen J., Lee W.M., Janda M., Ahlquist P. Alternate, virus-induced membrane rearrangements support positive-strand RNA virus genome replication. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:11263–11268. doi: 10.1073/pnas.0404157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ritzenthaler C., Laporte C., Gaire F., Dunoyer P., Schmitt C., Duval S., Piequet A., Loudes A.M., Rohfritsch O., Stussi-Garaud C. Grapevine fanleaf virus replication occurs on endoplasmic reticulum-derived membranes. Journal of Virology. 2002;76:8808–8819. doi: 10.1128/JVI.76.17.8808-8819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju H.J., Samuels T.D., Wang Y.S., Blancaflor E., Payton M., Mitra R., Krishnamurthy K., Nelson R.S., Verchot-Lubicz J. The potato virus X TGBp2 movement protein associates with endoplasmic reticulum-derived vesicles during virus infection. Plant Physiology. 2005;138:1877–1895. doi: 10.1104/pp.105.066019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Bamunusinghe D., Seo J.-K., Rao A.L.N. Subcellular localization and rearrangement of endoplasmic reticulum by brome mosaic virus capsid protein. Journal of Virology. 2011;85:2953–2963. doi: 10.1128/JVI.02020-10. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors provides EM data of BMV vesicle production in infected cells.
- 14.Carette J.E., Stuiver M., Van Lent J., Wellink J., Van Kammen A.B. Cowpea mosaic virus infection induces a massive proliferation of endoplasmic reticulum but not Golgi membranes and is dependent on de novo membrane synthesis. Journal of Virology. 2000;74:6556–6563. doi: 10.1128/jvi.74.14.6556-6563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15•.Wei T.Y., Huang T.S., McNeil J., Laliberté J.F., Hong J., Nelson R.S., Wang A.M. Sequential recruitment of the endoplasmic reticulum and chloroplasts for plant potyvirus replication. Journal of Virology. 2010;84:799–809. doi: 10.1128/JVI.01824-09. [DOI] [PMC free article] [PubMed] [Google Scholar]; Authors provides evidence for the association of chloroplasts with replication vesicles.
- 16.Liu J.Z., Blancaflor E.B., Nelson R.S. The tobacco mosaic virus 126-kilodalton protein, a constituent of the virus replication complex, alone or within the complex aligns with and traffics along microfilaments. Plant Physiology. 2005;138:1853–1865. doi: 10.1104/pp.105.065722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kusumanegara K., Mine A., Hyodo K., Kaido M., Mise K., Okuno T. Identification of domains in p27 auxiliary replicase protein essential for its association with the endoplasmic reticulum membranes in Red clover necrotic mosaic virus. Virology. 2012;433:131–141. doi: 10.1016/j.virol.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Pogany J., Nagy P.D. Authentic replication and recombination of Tomato bushy stunt virus RNA in a cell-free extract from yeast. Journal of Virology. 2008;82:5967–5980. doi: 10.1128/JVI.02737-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19•.Genoves A., Navarro J.A., Pallas V. The intra- and intercellular movement of Melon necrotic spot virus (MNSV) depends on an active secretory pathway. Molecular Plant–Microbe Interactions. 2010;23:263–272. doi: 10.1094/MPMI-23-3-0263. [DOI] [PubMed] [Google Scholar]; The authors show that the Golgi apparatus is involved in virus movement.
- 20•.Amari K., Lerich A., Schmitt-Keichinger C., Dolja V.V., Ritzenthaler C. Tubule-guided cell-to-cell movement of a plant virus requires class XI myosin motors. PLoS Pathogens. 2011;7:e1002327. doi: 10.1371/journal.ppat.1002327. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show that intercellular movement through plasmodesmata depends on myosin motor.
- 21•.Harries P.A., Park J.W., Sasaki N., Ballard K.D., Maule A.J., Nelson R.S. Differing requirements for actin and myosin by plant viruses for sustained intercellular movement. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17594–17599. doi: 10.1073/pnas.0909239106. [DOI] [PMC free article] [PubMed] [Google Scholar]; First evidence for myosin motor involved in virus movement.
- 22.Harries P.A., Schoelz J.E., Nelson R.S. Intracellular transport of viruses and their components: utilizing the cytoskeleton and membrane highways. Molecular Plant–Microbe Interactions. 2010;23:1381–1393. doi: 10.1094/MPMI-05-10-0121. [DOI] [PubMed] [Google Scholar]
- 23••.Wu C.H., Lee S.C., Wang C.W. Viral protein targeting to the cortical endoplasmic reticulum is required for cell-cell spreading in plants. Journal of Cell Biology. 2011;193:521–535. doi: 10.1083/jcb.201006023. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors introduce the notion that vesicle-inducing viral proteins have a targeting signal for exit from perinuclear to cortical ER.
- 24.Schepetilnikov M.V., Solovyev A.G., Gorshkova E.N., Schiemann J., Prokhnevsky A.I., Dolja V.V., Morozov S.Y. Intracellular targeting of a hordeiviral membrane-spanning movement protein: sequence requirements and involvement of an unconventional mechanism. Journal of Virology. 2008;82:1284–1293. doi: 10.1128/JVI.01164-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haviv S., Moskovitz Y., Mawassi M. The ORF3-encoded proteins of vitiviruses GVA and GVB induce tubule-like and punctate structures during virus infection and localize to the plasmodesmata. Virus Research. 2012;163:291–301. doi: 10.1016/j.virusres.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Wei T.Y., Wang A.M. Biogenesis of cytoplasmic membranous vesicles for plant potyvirus replication occurs at endoplasmic reticulum exit sites in a COPI- and COPII-dependent manner. Journal of Virology. 2008;82:12252–12264. doi: 10.1128/JVI.01329-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Diaz A., Mao L., Ahlquist P., Wang X. Host acyl coenzyme A binding protein regulates replication complex assembly and activity of a positive-strand RNA virus. Journal of Virology. 2012;86:5110–5121. doi: 10.1128/JVI.06701-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sparkes I.A., Frigerio L., Tolley N., Hawes C. The plant endoplasmic reticulum: a cell-wide web. Biochemical Journal. 2009;423:145–155. doi: 10.1042/BJ20091113. [DOI] [PubMed] [Google Scholar]
- 29.Staehelin L.A. The plant ER: a dynamic organelle composed of a large number of discrete functional domains. Plant Journal. 1997;11:1151–1165. doi: 10.1046/j.1365-313x.1997.11061151.x. [DOI] [PubMed] [Google Scholar]
- 30.Hoffmann A., Nebenführ A. Dynamic rearrangements of transvacuolar strands in BY-2 cells imply a role of myosin in remodeling the plant actin cytoskeleton. Protoplasma. 2004;224:201–210. doi: 10.1007/s00709-004-0068-0. [DOI] [PubMed] [Google Scholar]
- 31•.Ruthardt N., Gulde N., Spiegel H., Fischer R., Emans N. Four-dimensional imaging of transvacuolar strand dynamics in tobacco BY-2 cells. Protoplasma. 2005;225:205–215. doi: 10.1007/s00709-005-0093-7. [DOI] [PubMed] [Google Scholar]; The authors show nice renderings of transvacuolar dynamics and involvement in nucleus positioning.
- 32.Akkerman M., Overdijk E.J.R., Schel J.H.N., Emons A.M.C., Ketelaar T. Golgi body motility in the plant cell cortex correlates with actin cytoskeleton organization. Plant and Cell Physiology. 2011;52:1844–1855. doi: 10.1093/pcp/pcr122. [DOI] [PubMed] [Google Scholar]
- 33.Yang Y.D., Elamawi R., Bubeck J., Pepperkok R., Ritzenthaler C., Robinson D.G. Dynamics of COPII vesicles and the Golgi apparatus in cultured Nicotiana tabacum BY-2 cells provides evidence for transient association of Golgi stacks with endoplasmic reticulum exit sites. The Plant Cell. 2005;17:1513–1531. doi: 10.1105/tpc.104.026757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neumann U., Brandizzi F., Hawes C. Protein transport in plant cells: in and out of the Golgi. Annals of Botany. 2003;92:167–180. doi: 10.1093/aob/mcg134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hanton S.L., Bortolotti L.E., Renna L., Stefano G., Brandizzi F. Crossing the divide – transport between the endoplasmic reticulum and Golgi apparatus in plants. Traffic. 2005;6:267–277. doi: 10.1111/j.1600-0854.2005.00278.x. [DOI] [PubMed] [Google Scholar]
- 36.Ueki S., Citovsky V. To gate, or not to gate: regulatory mechanisms for intercellular protein transport and virus movement in plants. Molecular Plant. 2011;4:782–793. doi: 10.1093/mp/ssr060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawakami S., Watanabe Y., Beachy R.N. Tobacco mosaic virus infection spreads cell to cell as intact replication complexes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6291–6296. doi: 10.1073/pnas.0401221101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tilsner J., Linnik O., Christensen N.M., Bell K., Roberts I.M., Lacomme C., Oparka K.J. Live-cell imaging of viral RNA genomes using a Pumilio-based reporter. Plant Journal. 2009;57:758–770. doi: 10.1111/j.1365-313X.2008.03720.x. [DOI] [PubMed] [Google Scholar]
- 39.Bamunusinghe D., Hemenway C.L., Nelson R.S., Sanderfoot A.A., Ye C.M., Silva M.A.T., Payton M., Verchot-Lubicz J. Analysis of potato virus X replicase and TGBp3 subcellular locations. Virology. 2009;393:272–285. doi: 10.1016/j.virol.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 40••.Tilsner J., Linnik O., Wright K.M., Bell K., Roberts A.G., Lacomme C., Santa Cruz S., Oparka K.J. The TGB1 movement protein of potato virus X reorganizes actin and endomembranes into the X-body, a viral replication factory. Plant Physiology. 2012;158:1359–1370. doi: 10.1104/pp.111.189605. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show endomembrane recruitment in PVX X-bodies.
- 41••.Grangeon R., Agbeci M., Chen J., Grondin G., Zheng H., Laliberté J.-F. Impact on the endoplasmic reticulum and Golgi apparatus during Turnip mosaic virus infection. Journal of Virology. 2012;86:9255–9265. doi: 10.1128/JVI.01146-12. [DOI] [PMC free article] [PubMed] [Google Scholar]; Similar to above contribution where endomembrane recruitment is shown for a potyvirus.
- 42.Beauchemin C., Boutet N., Laliberté J.-F. Visualization of the interaction between the precursors of VPg, the viral protein linked to the genome of turnip mosaic virus, and the translation eukaryotic initiation factor iso 4E in planta. Journal of Virology. 2007;81:775–782. doi: 10.1128/JVI.01277-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dufresne P.J., Thivierge K., Cotton S., Beauchemin C., Ide C., Ubalijoro E., Laliberté J.-F., Fortin M.G. Heat shock 70 protein interaction with Turnip mosaic virus RNA-dependent RNA polymerase within virus-induced membrane vesicles. Virology. 2008;374:217–227. doi: 10.1016/j.virol.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 44.Thivierge K., Cotton S., Dufresne P.J., Mathieu I., Beauchemin C., Ide C., Fortin M.G., Laliberté J.F. Eukaryotic elongation factor 1A interacts with Turnip mosaic virus RNA-dependent RNA polymerase and VPg-Pro in virus-induced vesicles. Virology. 2008;377:216–225. doi: 10.1016/j.virol.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Cotton S., Grangeon R., Thivierge K., Mathieu I., Ide C., Wei T., Wang A., Laliberté J.F. Turnip mosaic virus RNA replication complex vesicles are mobile, align with microfilaments, and are each derived from a single viral genome. Journal of Virology. 2009;83:10460–10471. doi: 10.1128/JVI.00819-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang T.S., Wei T., Laliberté J.F., Wang A. A host RNA helicase-like protein, AtRH8, interacts with the potyviral genome-linked protein, VPg, associates with the virus accumulation complex, and is essential for infection. Plant Physiology. 2010;152:255–266. doi: 10.1104/pp.109.147983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang J., Laliberté J.-F. The genome-linked protein VPg of plant viruses—a protein with many partners. Current Opinion in Virology. 2011;1:347–354. doi: 10.1016/j.coviro.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 48.Knoops K., Swett-Tapia C., van den Worm S.H.E., Velthuis A.J.W.T., Koster A.J., Mommaas A.M., Snijder E.J., Kikkert M. Integrity of the early secretory pathway promotes, but is not required for, severe acute respiratory syndrome coronavirus RNA synthesis and virus-induced remodeling of endoplasmic reticulum membranes. Journal of Virology. 2010;84:833–846. doi: 10.1128/JVI.01826-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boevink P., Oparka K., Cruz S.S., Martin B., Betteridge A., Hawes C. Stacks on tracks: the plant Golgi apparatus traffics on an actin/ER network. Plant Journal. 1998;15:441–447. doi: 10.1046/j.1365-313x.1998.00208.x. [DOI] [PubMed] [Google Scholar]
- 50.Nebenfuhr A., Gallagher L.A., Dunahay T.G., Frohlick J.A., Mazurkiewicz A.M., Meehl J.B., Staehelin L.A. Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiology. 1999;121:1127–1141. doi: 10.1104/pp.121.4.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Runions J., Brach T., Kuhner S., Hawes C. Photoactivation of GFP reveals protein dynamics within the endoplasmic reticulum membrane. Journal of Experimental Botany. 2006;57:43–50. doi: 10.1093/jxb/eri289. [DOI] [PubMed] [Google Scholar]
- 52.Lee W.M., Ahlquist P. Membrane synthesis, specific lipid requirements, and localized lipid composition changes associated with a positive-strand RNA virus RNA, replication protein. Journal of Virology. 2003;77:12819–12828. doi: 10.1128/JVI.77.23.12819-12828.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharma M., Sasvari Z., Nagy P.D. Inhibition of phospholipid biosynthesis decreases the activity of the tombusvirus replicase and alters the subcellular localization of replication proteins. Virology. 2011;415:141–152. doi: 10.1016/j.virol.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolk B., Buchele B., Moradpour D., Rice C.M. A dynamic view of Hepatitis C virus replication complexes. Journal of Virology. 2008;82:10519–10531. doi: 10.1128/JVI.00640-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schoelz J.E., Harries P.A., Nelson R.S. Intracellular transport of plant viruses: finding the door out of the cell. Molecular Plant. 2011;4:813–831. doi: 10.1093/mp/ssr070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diaz A., Ahlquist P. Role of host reticulon proteins in rearranging membranes for positive-strand RNA virus replication. Current Opinion in Microbiology. 2012;15:519–524. doi: 10.1016/j.mib.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Diaz A., Wang X., Ahlquist P. Membrane-shaping host reticulon proteins play crucial roles in viral RNA replication compartment formation and function. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:16291–16296. doi: 10.1073/pnas.1011105107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58••.Faso C., Chen Y.-N., Tamura K., Held M., Zemelis S., Marti L., Saravanan R., Hummel E., Kung L., Miller E. A missense mutation in the Arabidopsis COPII coat protein Sec24A induces the formation of clusters of the endoplasmic reticulum and golgi apparatus. The Plant Cell. 2009;21:3655–3671. doi: 10.1105/tpc.109.068262. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show that disruption of secretory pathway leads to the formation of aberrant structures similar to those seen with PVX and TuMV infection.
- 59••.Nakano R.T., Matsushima R., Ueda H., Tamura K., Shimada T., Li L., Hayashi Y., Kondo M., Nishimura M., Hara-Nishimura I. GNOM-LIKE1/ERMO1 and SEC24a/ERMO2 are required for maintenance of endoplasmic reticulum morphology in Arabidopsis thaliana. The Plant Cell. 2009;21:3672–3685. doi: 10.1105/tpc.109.068270. [DOI] [PMC free article] [PubMed] [Google Scholar]; Another example of the disruption of the secretory pathway may have on cellular remodeling.
- 60•.Sharp T.M., Guix S., Katayama K., Crawford S.E., Estes M.K. Inhibition of cellular protein secretion by Norwalk virus nonstructural protein p22 requires a mimic of an endoplasmic reticulum export signal. PLoS ONE. 2010;5:e13130. doi: 10.1371/journal.pone.0013130. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article shows many parallel with plant viruses of a vertebrate virus hijacking the secretory pathway.
- 61.Moffat K., Knox C., Howell G., Clark S.J., Yang H., Belsham G.J., Ryan M., Wileman T. Inhibition of the secretory pathway by foot-and-mouth disease virus 2BC protein is reproduced by coexpression of 2B with 2C, and the site of inhibition is determined by the subcellular location of 2C. Journal of Virology. 2007;81:1129–1139. doi: 10.1128/JVI.00393-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Belov G.A., Altan-Bonnet N., Kovtunovych G., Jackson C.L., Lippincott-Schwartz J., Ehrenfeld E. Hijacking components of the cellular secretory pathway for replication of poliovirus RNA. Journal of Virology. 2007;81:558–567. doi: 10.1128/JVI.01820-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Belov G.A., Feng Q., Nikovics K., Jackson C.L., Ehrenfeld E. A critical role of a cellular membrane traffic protein in poliovirus RNA replication. PLoS Pathogens. 2008;4:e1000216. doi: 10.1371/journal.ppat.1000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lerich A., Langhans M., Sturm S., Robinson D.G. Is the 6 kDa tobacco etch viral protein a bona fide ERES marker? Journal of Experimental Botany. 2011;62:5013–5023. doi: 10.1093/jxb/err200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye C., Dickman M.B., Whitham S.A., Payton M., Verchot J. The Unfolded Protein response is triggered by a plant viral movement protein. Plant Physiology. 2011;156:741–755. doi: 10.1104/pp.111.174110. [DOI] [PMC free article] [PubMed] [Google Scholar]


