Summary
Catatonia is a psychomotor disorder featuring stupor, posturing, and echophenomena. This Series paper examines the evidence for immune dysregulation in catatonia. Activation of the innate immune system is associated with mutism, withdrawal, and psychomotor retardation, which constitute the neurovegetative features of catatonia. Evidence is sparse and conflicting for acute-phase activation in catatonia, and whether this feature is secondary to immobility is unclear. Various viral, bacterial, and parasitic infections have been associated with catatonia, but it is primarily linked to CNS infections. The most common cause of autoimmune catatonia is N-methyl-D-aspartate receptor (NMDAR) encephalitis, which can account for the full spectrum of catatonic features. Autoimmunity appears to cause catatonia less by systemic inflammation than by the downstream effects of specific actions on extracellular antigens. The specific association with NMDAR encephalitis supports a hypothesis of glutamatergic hypofunction in catatonia.
This is the second in a Series of two papers on catatonia
Introduction
Catatonia is a psychomotor disorder characterised by diverse clinical signs, including mutism, negativism, ambitendency, stereotypy, posturing, waxy flexibility, and echophenomena.1 The structure and neural mechanisms of the disorder are reviewed elsewhere in this issue of The Lancet Psychiatry by Walther and colleagues.2 Understanding the pathophysiology of this severe disorder is crucial given its high rate of medical complications, including pressure ulcers, infections, and venous thromboembolism.3 Moreover, such understanding might aid the comprehension of other neuropsychiatric disorders.
Although catatonia has numerous possible symptom combinations,4 compelling reasons to study it as a single entity exist. Clinical and demographic factors can distinguish catatonia from other psychotic and affective disorders.5 Different forms of catatonia (retarded catatonia, malignant catatonia, and neuroleptic malignant syndrome) are highly comorbid.1 In terms of treatment, response rates to benzodiazepines and electroconvulsive therapy (ECT) are high, regardless of the cause of the catatonia.6 Moreover, catatonia is not a common disorder, so pragmatically, to study it in depth, considering it as a whole is useful.
Immune dysregulation is gaining interest as a pathophysiological mechanism underlying neuropsychiatric disorders as diverse as narcolepsy, some dementias, depression, and psychosis— with converging evidence from biochemical, neuroimaging, genetic, and postmortem studies.7, 8 Roles for both the innate immune system, which concerns the rapid, undirected response to pathogen-associated or injury-related signals, and the adaptive immune system, which functions over a longer timescale and involves the selection and maturation of antigen-specific T-cell and B-cell mediated responses, have been identified.
In this Series paper, we discuss the evidence for the involvement of the immune system in catatonia. This line of enquiry appears to be valuable, given the wide range of infective and inflammatory conditions that can cause catatonia (Table 1, Table 2 ). We address whether the immune system has a role in catatonia, using some direct and some more circumstantial evidence, and endeavour to establish specific models. We consider immunity in terms of innate and adaptive systems for the purposes of clarity, while acknowledging that strictly demarcating the two is not always possible.
Table 1.
Systematic review of infective causes of catatonia
| Cases (n) | Suspected organisms (cases, n) | |
|---|---|---|
| Bacterial meningitis or encephalitis | 5 | Borrelia burgdorferi (4), unspecified (1) |
| Viral meningitis or encephalitis | 26 | Adenovirus (1), cytomegalovirus (1), coronavirus (1), Epstein–Barr virus (1), human herpesvirus 6 (1), herpes simplex virus (8), Japanese encephalitis virus (1), measles virus (2), tick-borne encephalitis virus (1), varicella-zoster virus (1), unspecified (9) |
| Cerebral malaria | 2 | Plasmodium falciparum (1), unspecified (1) |
| CNS infection unspecified | 3 | Unspecified (3) |
| Respiratory tract infection | 10 | Influenza (1), Group A Streptococcus (2), Mycoplasma (1), Klebsiella (1), Epstein–Barr virus (1), unspecified (4) |
| HIV-related | 22 | HIV (20), HIV and John Cunningham virus (2) |
| Syphilis | 3 | Treponema pallidum (2) |
| Systemic bacterial infection | 31 | Coxiella burnetti (1), Salmonella typhi (29), unspecified (2) |
| Systemic viral infection | 4 | Cytomegalovirus (2), Epstein–Barr virus (1), flavivirus (1) |
| Prion-related disorders | 7 | Prion protein (7) |
| Other | 11 | Flavivirus vaccination (1), Tropheryma whipplei (1), Escherichia coli (1), Mycobacterium tuberculosis (1), Taenia solium (1), Chlamydia trachomatis (1), Trypanosoma cruzi (1), unspecified (4) |
| Total | 124 | .. |
Table 2.
Systematic review of autoimmune causes of catatonia
| Cases (n) | ||
|---|---|---|
| Autoimmune thyroid disorders | 13 | |
| Hyperthyroid state | 3 | |
| Hypothyroid state | 4 | |
| Euthyroid state with thyroid antibodies | 4 | |
| Thyroid state not stated | 2 | |
| Autoimmune encephalitis | 259 | |
| GABA-AR encephalitis | 2 | |
| NMDAR encephalitis | 249 | |
| Progressive encephalomyelitis with rigidity and myoclonus | 1 | |
| Voltage-gated potassium channel complex encephalitis | 4 | |
| Unspecified | 3 | |
| Demyelinating disorders | 13 | |
| Acute disseminated encephalomyelitis | 2 | |
| Multiple sclerosis | 10 | |
| Neuromyelitis optica | 1 | |
| Pernicious anaemia | 4 | |
| Systemic lupus erythematosus and related | 53 | |
| Antiphospholipid syndrome | 2 | |
| Systemic lupus erythematosus | 51 | |
| Other | 4 | |
| Addison's disease | 1 | |
| Crohn's disease | 1 | |
| MOG antibody-associated diseases | 1 | |
| PANDAS | 1 | |
| Total | 346 | |
GABA=γ-aminobutyric-acid. NMDAR=N-methyl-D-aspartate receptor. MOG=myelin oligodendrocyte protein. PANDAS=paediatric autoimmune neuropsychiatric disorders associated with streptococcal infections.
Innate immune system
Catatonia due to infection
A systematic review reported that 20% of catatonia has a general medical cause, of which CNS inflammation (comprising both infective and immune causes) accounts for 29%.9 Numerous infectious diseases have been reported to cause catatonia. Here, we present the results of a new systematic search of the literature (table 1; appendix). We identified 124 cases, the majority of which were published as case reports, with the remaining reported as case series. Laboratory evidence of infection (such as isolation of the organism in the serum, or viral DNA in the cerebrospinal fluid [CSF]) was reported in 85 of the cases (69%). A robust temporal association between the infection and catatonia was reported in 82 of the cases (66%). A previous psychiatric disorder was recorded in 16 cases (13%) and a previous medical disorder in 26 cases (21%), although the absence of a pre-existing condition was often not stated. Only 66 of the cases (53%) recorded the presence of at least two features from the Bush-Francis Catatonia Screening Instrument (BFCSI); the remainder had insufficient description or only one feature.10 In some cases, the catatonia resolved with antimicrobial therapy,11 but in others, it required treatment with benzodiazepines12 or ECT.13
How infection might result in catatonia is unclear from the literature. Possibilities include a direct neurotoxic effect, a psychological reaction to the infection, or mediation by an acute-phase response. Out of the 47 cases where a specific virus was implicated, 45 of these involved known neurotropic viruses, suggesting a direct neurotoxic effect. Some bacterial agents, such as Borrelia burgdorferi and Treponema pallidum are also known to infect the CNS.
The immunological response might also be important, given that in some neurological disorders, such as meningoencephalitis, damage is caused primarily by an immune reaction.14 In several cases, an explicit immune response was suggested by the authors to explain the catatonia, such as in paediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS),15 or in N-methyl-D-aspartate receptor (NMDAR) encephalitis purportedly triggered by yellow fever vaccination,16 herpes simplex virus infection,17 or Epstein-Barr virus infection.18 In cases of pyrexia of unknown origin in which an infective cause was often assumed, a yet uncharacterised disorder might have been responsible.19, 20
Depression and inflammation
Although cases of overt catatonia in the context of infections are dramatic, the more common neuropsychiatric presentation of infection is a broader phenotype of illness behaviour that resembles depression. This presentation includes reductions in motor activity, oral intake, and social interaction,8 all of which are seen in catatonia. Psychomotor activity is also slowed in mild experimentally induced infection.21 This might be due to impaired spatial memory performance and aberrant activity in parts of the brain involved in interoception. Hence, the brain's response to inflammation, if severe, could result in a complex movement disorder such as catatonia.22, 23
In response to an acute stressor, immune cell trafficking occurs, with the movement of leukocytes to, and within, a target organ.24 However, in chronic stress, increased monocyte production and microglial activation result in neuroinflammation and are associated with depressive behaviour.8 Depression is often associated with raised levels of pro-inflammatory cytokines, granulocytes, and monocytes.8 Regarding subtypes of depression, atypical depression (characterised by mood reactivity, hyperphagia, hypersomnia, and leaden paralysis)25 is most associated with raised inflammatory markers.26 Conversely, psychomotor retardation is more commonly seen in melancholic depression, which is less associated with a peripheral pro-inflammatory state.26 Seasonal affective disorder has also been associated with a pro-inflammatory state, but there has been little research to date on the motor phenotype of this disorder.27
Neuroleptic malignant syndrome and inflammation
Neuroleptic malignant syndrome is a neurological emergency precipitated by antipsychotic use and characterised by muscular rigidity, autonomic dysfunction, and altered consciousness. Patients treated with antipsychotics who have pre-existing catatonia are at an increased risk of developing neuroleptic malignant syndrome compared with those who do not have catatonia (3·6% vs 0·07–1·8%).28 Given that no clinical features exist that can reliably distinguish neuroleptic malignant syndrome from malignant catatonia,29 some authors consider neuroleptic malignant syndrome to be a specific form of antipsychotic-induced malignant catatonia.30 Residual catatonia frequently remains after the resolution of the full syndrome of neuroleptic malignant syndrome.31
Some suggest that inflammation is important to the pathophysiology of neuroleptic malignant syndrome, with acute-phase responses such as leukocytosis, thrombocytosis, and low serum iron frequently reported.32, 33 Low serum iron has emerged as a promising biomarker.32, 33 It has been hypothesised that in neuroleptic malignant syndrome, pro-inflammatory cytokines might reduce the levels of the neuroprotective kynurenic acid, impairing the activity of dopaminergic neurons in the midbrain, causing exquisite sensitivity to a further antipsychotic-induced reduction in dopaminergic signalling.34 However, an inflammatory profile in the blood might be the consequence of rhabdomyolysis, rather than the primary pathology.
Serotonin syndrome, a rare adverse effect of antidepressant medication, has also been described as a form of drug-induced catatonia,1 but to the authors' knowledge no research has been published linking it to the immune system.35
Direct evidence for the acute-phase response
The acute-phase response is a core part of the innate immune system. The response is initiated by the activation of monocytes and macrophages by a stimulus, such as muscle breakdown, infection, physical injury, or psychological stress. In response to these stimuli, cells release pro-inflammatory cytokines such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF-α), which in turn act on receptors throughout the body to promote fever, anorexia, muscle catabolism, and activation of the hypothalamic-pituitary-adrenal axis. Importantly, these cytokines also alter protein synthesis in the liver, causing increased production of acute-phase proteins such as C-reactive protein (CRP), procalcitonin, ferritin, and fibrinogen.36, 37 Some features of malignant catatonia bear notable similarities to the acute-phase response, including fever, motor hypoactivity, and autonomic disturbance. Here, we include a summary of the evidence for the presence of systemic inflammation, as measured by acute-phase reactants and related proteins (table 3 ). Creatine kinase (CK) is not an acute-phase marker, but as it is a marker of muscle breakdown, the enzyme is sometimes raised as a downstream consequence of the acute-phase response. The evidence for CK elevation in catatonia is equivocal and could be argued to be the result of muscular rigidity and excessive immobilisation rather than indicating a primary muscular pathology. In one study, a raised CK predicted a good response to treatment with lorazepam.45
Table 3.
Systematic review of inflammatory markers in catatonia
| Study | Participants with catatonia | Controls | Results | |
|---|---|---|---|---|
| White blood cell count | Haouzir et al (2009)38 | 25 patients with acute catatonia | 50 patients without catatonia with similar diagnoses to patients with catatonia | No difference in white blood cell count |
| White blood cell count | Rao et al (2011)39 | 77 patients with catatonia | None | Responders to lorazepam had a statistically significantly lower monocyte count than non-responders; no difference in other cell counts |
| hsCRP | Akanji et al (2009)40 | 12 patients with schizophrenia with prominent catatonic features | 87 patients with schizophrenia without catatonia | hsCRP concentration statistically significantly higher in patients with catatonia |
| Iron | Haouzir et al (2009)38 | 25 patients with acute catatonia | 50 patients without catatonia with similar diagnoses to patients with catatonia | Iron concentration did not differ between patients with and without catatonia |
| Iron | Lee (1998)41 | 39 patients with catatonia in psychiatric intensive care units | None | 17 patients had iron concentration below reference range |
| Iron | Peralta et al (1999)42 | 40 patients with catatonia and psychosis | 40 patients with psychosis without catatonia | Iron concentration statistically significantly lower in patients without catatonia |
| Iron | Carroll and Goforth (1995)43 | 12 episodes of catatonia in 11 psychiatric inpatients | None | 3 patients had iron concentration below reference range |
| Iron | Lakshmana et al (2009)44 | 40 catatonic patients | Age-matched and sex-matched psychiatric patients | No difference in iron concentration between patients with and without catatonia |
| CK | Northoff et al (1996)45 | 32 hospital inpatients with catatonia | 32 dyskinetic psychiatric patients without catatonia, 32 non-dyskinetic psychiatric patients without catatonia, 32 healthy controls | CK concentration statistically significantly higher in individuals with catatonia than in healthy controls and non-dyskinetic patients without catatonia; no difference between patients with catatonia and dyskinetic patients without catatonia |
| CK | Haouzir et al (2009)38 | 25 patients with acute catatonia | 50 patients without catatonia with similar diagnoses to patients with catatonia | No difference in CK concentration |
| CK | Meltzer (1968)46 | Two patients with catatonia | 14 patients with non-catatonic psychoses | No difference in CK concentration |
| D-dimer | Haouzir et al (2009)38 | 25 patients with acute catatonia | 50 patients without catatonia with similar diagnoses to patients with catatonia | D-dimer concentration statistically significantly higher in patients with catatonia |
hsCRP=high-sensitivity C-reactive protein. CK=creatine kinase.
One study found the acute-phase marker, and fibrin degradation product, D-dimer to be raised in all 25 catatonic patients tested, with a mean value 3 times higher than in non-catatonic psychiatric patients.38 This finding is consistent with the increased risk of venous thromboembolism in catatonia, but has not yet been replicated.
High-sensitivity CRP concentration was measured in one study and found to be raised in catatonic patients, but the absolute concentration of CRP was not very elevated (1·23 mg/dL).40
Low serum iron was originally hypothesised to be present in catatonia given the similarities to neuroleptic malignant syndrome. Low serum iron is an established feature of the acute-phase response and arises because of the upregulated production of ferritin and hepcidin by the liver.47 Two uncontrolled studies have shown that between 25% and 44% of catatonic episodes were accompanied by serum iron concentrations below the reference range.41, 43 When catatonic patients have been compared with psychiatric controls however, the results have been ambiguous.38, 42, 44 The authors of one of the negative studies that used unmedicated patients speculated that iron might have been reduced in other reports because of the effect of antipsychotic medications.44 In several studies, low serum iron in catatonia has been associated with the subsequent development of neuroleptic malignant syndrome.41, 43, 48 This association might exist because iron is a cofactor for dopamine synthesis,49 so a combination of low iron impairing dopamine production and antipsychotic medications blocking dopamine receptors could result in the pathological hypodopaminergic signalling characteristic of neuroleptic malignant syndrome.
Glial dysfunction
Abnormalities of cerebral white matter, which is composed of glial cells, have been associated with schizophrenia, depression, and autism.50 2',3'-cyclic nucleotide 3'-phosphodiesterase (CNP) is a myelin protein that is specific to oligodendrocytes.50 In a mouse model, heterozygotes for a CNP loss-of-function genotype showed axonal degeneration and low-grade inflammation, along with a depressive and catatonic phenotype.51 Furthermore, this behaviour was alleviated by ablation of microglia, suggesting that microglia-mediated neuroinflammation was underlying the phenotype. When this polymorphism was examined in individuals with schizophrenia, a striking association existed with catatonic-depressive behaviour, a finding that was replicated in an independent cohort.52 Mutations of mouse genes encoding two other myelin proteins (myelin basic protein [MBP] and myelin proteolipid protein [PLP]) also result in a catatonic phenotype.52 How glial dysfunction—because of relevant polymorphisms or other factors—might contribute to the psychomotor features of catatonia clearly represents an important focus of future research.
Implications of treatment
The mainstay of current treatment for catatonia is benzodiazepines and ECT, neither of which is classically understood as an immunomodulatory therapy. Benzodiazepines are positive allosteric modulators at the γ-aminobutyric-acid (GABA)-A receptor. Although research into the function of GABA in the immune system is at an early stage, evidence suggests that GABAergic signalling has a role in suppression of immune responses.53 Lymphocytes express GABA-A receptors, and activation of these receptors reduces production of pro-inflammatory cytokines.53 However, one study specifically on catatonia found higher monocyte counts predicted benzodiazepine non-response.39 Data distinguishing different benzodiazepines are sparse, but some benzodiazepines, such as diazepam and lorazepam (both recognised treatments for catatonia) but not clonazepam, also bind to translocator protein (TSPO), a mitochondrial protein associated with phagocyte activity, immune cell migration, and cytokine function.54, 55 In rats, diazepam reduces TSPO in the brain and decreases the number of CNS inflammatory cells, giving it a protective function against experimental autoimmune encephalomyelitis.55 Reports on other GABA-A receptor modulators are scarce, but epidemiological studies exist that indicate that zolpidem use is associated with higher rates of infections (including of pyelonephritis, which would be unlikely to be related to respiratory depression), suggesting the drug might also have an immunosuppressant role.56, 57
Regarding ECT, a single session appears to activate the immune system, increasing concentrations of the cytokines IL-1β, IL-6, IL-10, and TNF-α. However, a course of several sessions appears to down-regulate immune system activity, at least in animal studies.58
Minocycline is an antimicrobial drug that also has anti-inflammatory properties.59 The drug has been shown to prevent stress-induced microglial changes in rodents8 and has been proposed as an adjunctive treatment for schizophrenia.60 Some evidence suggests that minocycline might reduce negative symptoms in schizophrenia,61 some of which (such as poverty of speech, affective blunting, and avolition) overlap with catatonia. However, a 2018 double-blinded, randomised study which specifically aimed to examine the effect of this drug on negative symptoms did not find any benefit.62 No studies of which we are aware have investigated minocycline specifically for catatonia, but reports exist of two patients with schizophrenia and prominent catatonic features who responded well to minocycline in the absence of infection.63, 64
The evidence for innate immunity
We have argued that psychological stress and infection both cause a release of pro-inflammatory cytokines, which result in a state of motor hypoactivity. In a normal psychomotor response, this event might be adaptive, allowing conservation of energy for eliminating a pathogen or avoiding a stressor, and resolving when the stressor ends. However, in depression, a prolonged pro-inflammatory state might be maladaptive and cause further dysfunction. Immobilisation itself can also result in activation of the innate immune system.65
Studies specifically in catatonia have been sparse and conflicting. An argument could be made that catatonia is an exaggerated version of inflammatory depression, in which extreme psychomotor retardation culminates in stupor and mutism—neurovegetative features of catatonia hypothesised to be due to disordered top-down corticosubcortical signalling.66 However, this hypothesis would not explain the perseverative-compulsive behaviours exhibited in catatonia (posturing, stereotypy, mannerism, echophenomena, and perseveration), which have been proposed to arise due to disrupted corticocortical signalling. The infective causes of catatonia are largely pathogens that infect the CNS (table 1), which suggests that the causality is mediated by neurotoxic mechanisms, rather than by a systemic inflammatory response—although a maladaptive immune response to the pathogen might contribute.
Autoimmunity
Autoimmune neurological disorders resembling catatonia
A plethora of autoimmune neurological diseases exist, many of which, such as multiple sclerosis, neuromyotonia, and Sydenham's chorea, feature prominent movement disorders. We have chosen the examples of stiff person syndrome and narcolepsy to show some particular points of similarity to catatonia.
Stiff person syndrome is a rare neurological disorder characterised by gradually progressive increased muscle tone with the preservation of muscle power, sensation, and cognitive function. Most patients have autoantibodies against the enzyme glutamic acid decarboxylase (GAD2).67 GAD2 is an enzyme that converts glutamate to GABA, although the pathogenicity of GAD2 autoantibodies in stiff person syndrome is not fully established. Given that the disorder bears several similarities to catatonia, one author has suggested testing for GAD2 autoantibodies to distinguish between the two disorders.28 The syndromes share immobility, an emotionless facial expression, and marked anxiety. Moreover, hypertonic episodes in stiff person syndrome can have psychological triggers.67 As with catatonia, the mainstay of treatment for stiff person syndrome is benzodiazepines; however, immunotherapy in the form of intravenous immunoglobulin, corticosteroids and the anti-B-cell monoclonal antibody rituximab are increasingly used. A stiff person syndrome variant, progressive encephalomyelitis with rigidity and myoclonus, responds dramatically to immunosuppression.67, 68
Narcolepsy type 1 is a sleep disorder that arises due to depletion of the orexin-producing neurons in the hypothalamus. Evidence that this event is immune-mediated comes from linkage to HLA-DQB1*06:02 and outbreaks coinciding with epidemics of, and vaccination to, the H1N1 influenza virus, suggesting a possible role for molecular mimicry.69 A small study published in 2012 suggested that some patients with narcolepsy have autoantibodies to the NMDAR, without the seizures or autonomic disturbance characteristic of NMDAR autoimmune encephalitis.70 Narcolepsy type 1 also features cataplexy, a sudden loss of motor tone usually triggered by positive emotions. Cataplexy usually lasts for up to 2 min, but occasionally status cataplecticus lasting for hours to days can occur.71 This occurrence has been hypothesised to be due to either a prolonged emotional response to the original stimulus, or an emotional response to the cataplexy per se. Here, we show a comparison between cataplexy and catatonia (table 4 ).
Table 4.
Comparison of catatonia and cataplexy in the context of narcolepsy
| Catatonia | Cataplexy | |
|---|---|---|
| Trigger | Strong negative emotions | Strong positive emotions |
| Tone | Increased with posturing, but preservation of respiratory muscles | Atonic with preservation of respiratory muscles |
| Awareness | Retained | Retained |
| Main associated psychiatric disorders | Depression, psychosis | Depression, social anxiety |
| Pharmacological treatment | GABA-A receptor agonists | Antidepressants, sodium oxybate (a GABA-B receptor agonist) |
| Duration | Days to weeks | Up to 2 min (longer in status cataplecticus) |
GABA=γ-aminobutyric-acid.
Autoimmune disorders causing catatonia
We did a systematic literature search for autoimmune disorders causing catatonia (table 2; appendix). Most are presented in case reports and case series, with some larger case series for NMDAR encephalitis (table 5 ). 224 of the 346 cases (65%) recorded at least two features from the BFCSI.10 18 patients (5%) had previously had a psychiatric disorder and 33 (10%) had previously had a medical disorder, although an absence of a pre-existing condition was often not stated. In some cases, the autoimmune disorder appeared to be the proximal cause of the catatonia. In other cases, the autoimmune disorder was a more distal cause, as in one patient with autoimmune polyendocrine syndrome who developed autoimmune destruction of the adrenal gland (Addison's disease), resulting in hyponatraemia and subsequent extrapontine myelinosis, the latter precipitating catatonia.78
Table 5.
Prevalence of catatonia (as identified by authors) in case series of NMDAR encephalitis
| Participants (N) | Cases of catatonia (n [%]) | |
|---|---|---|
| Dalmau et al (2008)72 | 100 | 88 (88%) |
| Tsutsui et al (2012)70 | 3 | 2 (67%) |
| DeSena et al (2014)73* | 8 | 5 (63%) |
| Kruse et al (2015)74 | 12 | 9 (75%) |
| Duan et al (2016)75 | 28 | 19 (68%) |
| Granata et al (2018)76* | 18 | 8 (44%) |
| Herken and Prüss (2017)77† | 53 | 10 (19%) |
| Total | 222 | 141 (64%) |
NMDAR=N-methyl-D-aspartate receptor.
All paediatric cases.
Relied on retrospective analysis of charts, so probably underestimated prevalence of catatonia.
In addition, 22q11.2 deletion syndrome, which features thymic aplasia and a resultant absence of peripheral T cells,79 has also been linked to catatonia.80 Whether this association is due to immunodeficiency, the high rates of various autoimmune disorders present in the syndrome, or to another cause remains unclear.
The most noteworthy result from our systematic review is that 72% (249/346) of all cases of autoimmune catatonia reported were due to NMDAR encephalitis, despite the disorder only being described in 2007 (table 2).81 Before discussing this finding of autoimmunity directed against the CNS in depth, we will illustrate the complexity of autoimmune catatonia with three examples of peripheral autoimmunity.
Two cases of catatonia in pernicious anaemia have been reported, both of whom responded to vitamin B12 supplementation.82, 83 Dietary vitamin B12 deficiency might also cause catatonia.84, 85
In thyroid disease, catatonia has been reported in patients with thyroid autoantibodies with hyperthyroid,86, 87, 88 hypothyroid,90, 91 and euthyroid91, 92 states. However, catatonia has also occurred in hypothyroidism due to thyroidectomy;93 whether thyroid status or the presence of the autoantibodies is the causally relevant factor therefore remains unclear.
In systematic lupus erythematosus, 51 cases of catatonia have been reported, generally with high titres of antinuclear antibody and anti-double-stranded DNA; however, making further comparisons is difficult because testing panels have varied across studies (appendix).
One group reported 84 cases of paediatric catatonia of which they suspected 7 had an autoimmune origin, including two patients with evidence of inflammation who were responsive to immunosuppression but who could not be diagnosed with any known disorder.94
Autoimmune disorders directed at CNS targets causing catatonia
PANDAS and the broader concept of paediatric acute-onset neuropsychiatric syndrome (PANS) are characterised by an abrupt onset of obsessive behaviours or motor tics.27 This behaviour might be due to molecular mimicry, whereby antigens on the infective agent bear a similarity to and provoke a host immune response to self-CNS antigens.95 Antistreptococcal antibodies are often positive,96 although results of immunotherapy have been equivocal.97 One case has been reported of a boy who developed catatonic symptoms in addition to obsessionality following infection with group A Streptococcus; he responded well to lorazepam and plasmapheresis.98
Autoimmune encephalopathies, as examples of autoimmune disorders directed at CNS targets, merit special consideration. T-cell mediated disorders, such as acute demyelinating encephalomyelitis can occasionally present with catatonia.99 However, catatonia is more commonly a feature of autoimmune encephalitides associated with antineuronal antibodies. These antibodies can cause internalisation of the antigen, inhibiting its function.14
That catatonia has been reported in two patients with GABA-A receptor antibodies is unsurprising, given the centrality of benzodiazepines in treatment for catatonia.100, 101 Catatonia might be more common than these case reports would suggest, as careful psychiatric phenotyping has not been done among this population.102 In one of the patients reported with catatonia, GABA-A receptor antibodies were present in the serum on the original presentation, but not in the context of relapse, highlighting that testing serum only on a single occasion might increase the risk of missing a clinically significant syndrome.100
NMDAR encephalitis is increasingly considered as an organic cause of psychosis, although controversy exists as to whether this idea is only relevant in the context of the classical encephalitis or also in isolated psychiatric presentations.7, 103 The association NMDAR encephalitis with catatonia seems to be even stronger than the association with psychosis.72 Where catatonia is reported, it is often malignant catatonia and tends to co-occur with psychosis104 and mania.105 NMDAR encephalitis is strongly linked to neuroleptic malignant syndrome, with one study suggesting as many as 21 out of 36 (58%) patients with NMDAR antibody encephalitis who were administered antipsychotics developed suspected neuroleptic malignant syndrome.106 Of the total 222 cases of NMDAR encephalitis documented across seven studies where rates of catatonia were reported, 141 (64%) cases of catatonia were identified (table 5). The range of catatonic features reported is wide and includes echolalia, grimacing, posturing, and alternating hypermotor and hypomotor activity.72
A few studies have examined comparative rates of NMDAR autoantibody positivity among different diagnostic groups. Of 459 patients with psychiatric disorders, two had IgG antibodies against the NMDAR subunit NR1a in serum and CSF; both had catatonia and were ultimately reclassified as having NMDAR encephalitis.107 Among 49 inpatients with psychiatric disorders and serum antineuronal antibodies, nine of the 13 patients with NMDAR antibodies had catatonia, compared with only three of the remaining patients.74 Another study found higher NMDAR positivity among patients with catatonia than in a control group of healthy volunteers (although controls were younger than the patients and the investigators used an unusual continuous measure of NMDAR immunofluorescence).108 One study examined Bush-Francis Catatonia Rating Scale scores in patients with first-episode psychosis and found that catatonic features were actually less common in patients with antineuronal antibodies.109 A study published in 2018 by our group with individuals at ultra-high risk of psychosis suggests more severe catatonic features in individuals with NMDAR antibodies.110
NMDAR encephalitis has only been described in the last decade but has led to a re-evaluation of encephalitis lethargica,111 first recognised in 1917, due to notable similarities.112 Encephalitis lethargica is characterised by profound sleep impairment (insomnia, hypersomnia, or sleep inversion), extrapyramidal movement deficits, and neuropsychiatric symptoms.113 Although historically linked to the 1918 influenza pandemic, the evidence for a causal association is sparse.113 More recently, investigations have found a high prevalence of antibodies to the NMDAR and the dopamine D2 receptor in the serum of children with encephalitis lethargica, raising the intriguing prospect that many patients exhibiting catatonia previously diagnosed with the disorder, might have instead had antibody-mediated encephalitis.114
A model for autoimmunity in catatonia
When we considered the role of the innate immune system, we considered the possibility that inflammation itself was responsible for the stuporous aspects of catatonia. As far as adaptive immunity is concerned, the specificity of the antigen might be the most important determinant of the resulting neuropsychiatric phenotype, including catatonia. The downstream effect of immune activation is dependent on the antigen targeted. Autoimmune neurological disorders present differently depending on the target for autoantibodies or T cells; frequently these targets are neurotransmitter receptors with ensuing downstream effects on receptor dysfunction. In autoimmune encephalitis, the presentation depends on the specific antibodies present.115 In the specific case of NMDAR encephalitis, often little evidence exists of complement activation and neuronal degeneration.116 The fact that ketamine and phencyclidine—both NMDAR antagonists—cause catatonia117 suggests that NMDAR antagonism is responsible, the implication being that NMDAR antibody encephalitis is more usefully understood as a synaptopathy. Genetic hypofunction of the NMDAR due to GRIN1 mutation also appears to predispose to psychosis.118 Similarly, benzodiazepine withdrawal presents similarly to GABA-A receptor encephalitis.119, 120
Autoimmunity, therefore, appears to cause catatonia primarily by specific action against central or peripheral antigens; however, secondary inflammation could perpetuate a phenotype-relevant immune response.
A model for glutamatergic hypofunction in catatonia
The close association between NMDAR encephalitis and catatonia might provide valuable insight into the pathophysiology of catatonia. NMDAR encephalitis causes internalisation of the NMDAR, resulting in a reversible reduction in the number of receptors and impaired α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor-mediated long-term potentiation.121, 122 This idea is consistent with catatonia also resulting from use of the recreational non-competitive NMDAR antagonists, ketamine, and phencyclidine.117, 123
To integrate findings of effective treatment with GABA-A receptor agonists and NMDAR antagonists, Northoff66 has proposed a model of catatonia in which the normal inhibition of excitatory glutamatergic corticocortical association fibres by GABAergic neurons in the orbitofrontal region is impaired. In mice, the NMDAR antagonist dizocilpine (also known as MK-801) shows a bimodal effect on grooming and rearing behaviour: at low doses, this behaviour is suppressed, but as the dose increases, behaviour normalises, before being suppressed again at higher doses.124, 125 This effect might explain why catatonia is characterised not only by immobility, but also occasionally by catatonic excitement. An explanation for this finding might rely on the knowledge that the NMDAR is expressed by excitatory glutamatergic neurons and by inhibitory GABAergic neurons.126 Moreover, both reduced and excessive NMDAR activity can result in neuronal apoptosis,127 but at physiological levels can also promote neuronal survival.128
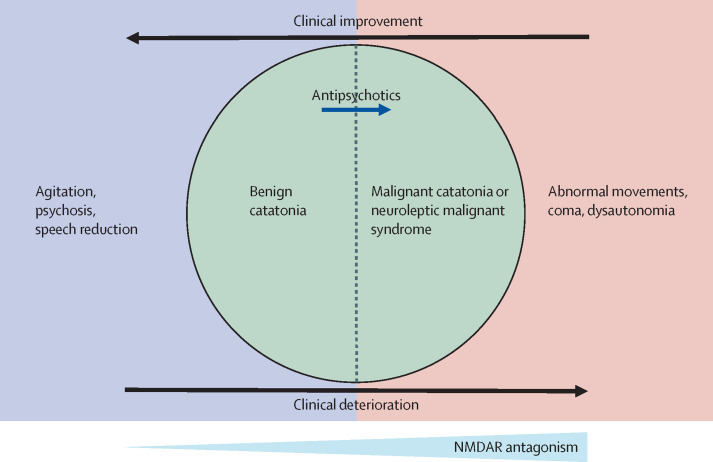
Dalmau and colleagues111 have proposed a model for antiNMDAR encephalitis, in which increasing NMDAR blockade results initially in behavioural and psychotic symptoms, and at higher antibody titres, neurological and autonomic dysfunction. One hypothesis would be that catatonia occupies the ground between these two states (figure ).10 Pharmacological or antibody-mediated NMDAR hypofunction could both cause catatonia and result in progression to malignant catatonia.
Figure.
A model for glutamatergic hypofunction in catatonia
NMDAR= N-methyl-D-aspartate receptor.
Conclusion
Catatonia is heterogeneous in presentation and cause. However, the generally favourable response to treatment with benzodiazepines or ECT suggests a common pathophysiology. Activation of the innate immune system can lead to the neurovegetative features of catatonia, but the evidence for the acute phase response in catatonia is preliminary and sometimes conflicting. Moreover, whether any peripheral inflammation in catatonia arises secondary to immobility and muscle breakdown is unknown. Examining the association of catatonia with the adaptive immune system reveals a strong and specific association with NMDAR encephalitis, which can cause the full range of catatonic features. This finding suggests that adaptive immunity can cause catatonia through action at specific extracellular antigens, rather than immune activation per se. Additionally, this illustrates the importance of glutamatergic function in catatonia. As more autoimmune disorders are characterised, more cases of catatonia might be explained in this way.
Although we have considered the innate and adaptive immune systems separately, in reality, they are deeply interconnected. For instance, NMDAR encephalitis (a disorder of the adaptive immune system) entails a very high risk of neuroleptic malignant syndrome (a disorder with prominent activation of the innate immune system). Malignant catatonia remains an enigmatic entity that could possibly be accounted for by autoimmune disorders such as NMDAR encephalitis.
Finally, is it possible to conclude whether catatonia is due to activation of the immune system? In many cases, little compelling evidence exists for this. However, where infection or autoimmunity are directed at specific targets in the CNS or periphery, a high risk of catatonia is apparent. Further investigations based on this concept (panel ) might assist in elucidating pathophysiological mechanisms and improving the treatment of catatonia.
Panel. Questions for future research.
Epidemiology
-
•
Is catatonia overrepresented in individuals with autoimmune disorders?
-
•
What are the rates of catatonia in GABA-AR encephalitis when systematic detection methods are used?
Laboratory investigations
-
•
In studies of depression, is there evidence that raised inflammatory markers are associated with catatonic features?
-
•
Do relapsing and remitting inflammatory markers exist in periodic catatonia?
-
•
Are patients with catatonia more likely to have antibodies to N-methyl-D-aspartate (NMDA) receptor, γ-aminobutyric-acid (GABA)-A receptor, glutamic acid decarboxylase (GAD2) and other encephalitis-associated antibodies?
-
•
Will large genetic studies of catatonia show linkage to HLA or other immune-specific regions?
-
•
Do inflammatory markers predictors response to electroconvulsive therapy?
Neuroimaging
-
•
Does a reduced density of NMDA receptors exist in catatonia in single-photon-emission CT (SPECT) or PET studies?
-
•
Will the use of specific ligands reveal CNS immune activation (eg, mediated by microglia or astrocytes) in catatonia?
Therapy
-
•
Is immunomodulatory therapy (such as with corticosteroids, plasmapheresis, or rituximab) an appropriate treatment option in catatonia?
-
•
Is minocycline an effective treatment for catatonia?
-
•
Is immunosuppressant therapy effective for neuroleptic malignant syndrome?
Search strategy and selection criteria
We searched PubMed for articles published up to Sept 31, 2018, with the terms “catatoni*” in association with any of the following terms: “immune*”, “autoimmun*”, “inflame*”, “T-cell”, “B-cell”, “glia*”, “microglia*”, “acute phase”, “innate”, “adaptive”, “encephalitis”, “antibod*”, “infect*”, “interleukin”, “cytokine”, “monocyte”, “macrophage”, “leukocyte”, “lymphocyte”, “granulocyte”, “phagocyte”, “TNF”, “C-reactive protein”, “dendritic cell”, and “immunoglobulin”. This primary search along with the references of selected review articles revealed three areas that were suitable for systematic summaries of the literature: infective causes of catatonia, autoimmune causes of catatonia, and inflammatory markers in catatonia. To investigate these areas, we searched six databases (AMED, BNI, CNINAHL, Embase, MEDLINE, PsycINFO, and PubMed) for catatonia and MESH terms (or equivalent) in conjunction with relevant specific search terms (eg, “infect*”, “virus”, “bacteria”). After deduplication, articles were screened via their titles and abstracts before relevant full-text articles were reviewed by the first author and systematically included in tables in the manuscript. Only articles with full texts or sufficiently detailed abstracts written in English were included.
Acknowledgments
Acknowledgments
JPR and GB were supported by National Institute for Health Research (NIHR) academic clinical fellowships. TAP was supported by a clinical research training fellowship grant from the Wellcome Trust (number 105758/Z/14/Z). ASD received support from the NIHR Maudsley Biomedical Research Centre at South London and Maudsley National Health Service Foundation Trust and the Institute of Psychiatry, Psychology and Neuroscience, King's College London.
Contributors
The manuscript was planned by JPR, TAP, GB, and ASD. JR did the literature search and drafted the manuscript. TAP, GB, and ASD reviewed the manuscripts, made amendments, and added further references. All authors approved the final manuscript.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Fink M, Taylor M. Cambridge University Press; New York, NY: 2006. Catatonia: a clinician's guide to diagnosis and treatment. [Google Scholar]
- 2.Walther S, Stegmayer K, Wilson J, Heckers S. Structure and neural mechanisms of catatonia. Lancet Psychiatry. 2019 doi: 10.1016/S2215-0366(18)30474-7. published online June 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinebell K, Azzam PN, Gopalan P, Haskett R. Guidelines for preventing common medical complications of catatonia: case report and literature review. J Clin Psychiatry. 2014;75:644–651. doi: 10.4088/JCP.13r08870. [DOI] [PubMed] [Google Scholar]
- 4.Wilson JE, Niu K, Nicolson SE, Levine SZ, Heckers S. The diagnostic criteria and structure of catatonia. Schizophr Res. 2015;164:256–262. doi: 10.1016/j.schres.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 5.Peralta V, Cuesta MJ, Serrano JF, Mata I. The Kahlbaum syndrome: a study of its clinical validity, nosological status, and relationship with schizophrenia and mood disorder. Compr Psychiatry. 1997;38:61–67. doi: 10.1016/s0010-440x(97)90055-9. [DOI] [PubMed] [Google Scholar]
- 6.Barnes MP, Saunders M, Walls TJ, Saunders I, Kirk CA. The syndrome of Karl Ludwig Kahlbaum. J Neurol Neurosurg Psychiatry. 1986;49:991–996. doi: 10.1136/jnnp.49.9.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Al-Diwani AAJ, Pollak TA, Irani SR, Lennox BR. Psychosis: an autoimmune disease? Immunology. 2017;152:388–401. doi: 10.1111/imm.12795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci. 2016;17:497–511. doi: 10.1038/nrn.2016.69. [DOI] [PubMed] [Google Scholar]
- 9.Oldham MA. The probability that catatonia in the hospital has a medical cause and the relative proportions of its causes: a systematic review. Psychosomatics. 2018;59:333–340. doi: 10.1016/j.psym.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Bush G, Fink M, Petrides G, Dowling F, Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93:129–136. doi: 10.1111/j.1600-0447.1996.tb09814.x. [DOI] [PubMed] [Google Scholar]
- 11.Pfister HW, Preac-Mursic V, Wilske B. Catatonic syndrome in acute severe encephalitis due to Borrelia burgdorferi infection. Neurology. 1993;43:433–435. doi: 10.1212/wnl.43.2.433. [DOI] [PubMed] [Google Scholar]
- 12.Snyder S, Prenzlauer S, Maruyama N, Rose DN. Catatonia in a patient with AIDS-related dementia. J Clin Psychiatry. 1992;53:414. [PubMed] [Google Scholar]
- 13.Sall L, Salamon E, Allgulander C, Owe-Larsson B. Psychiatric symptoms and disorders in HIV infected mine workers in South Africa: a retrospective descriptive study of acute first admissions. African J Psychiatry (Johannesbg) 2009;12:206–212. doi: 10.4314/ajpsy.v12i3.48495. [DOI] [PubMed] [Google Scholar]
- 14.Waisman A, Liblau RS, Becher B. Innate and adaptive immune responses in the CNS. Lancet Neurol. 2015;14:945–955. doi: 10.1016/S1474-4422(15)00141-6. [DOI] [PubMed] [Google Scholar]
- 15.Elia J, Dell ML, Friedman DF. PANDAS with catatonia: a case report. Therapeutic response to lorazepam and plasmapheresis. J Am Acad Child Adolesc Psychiatry. 2005;44:1145–1150. doi: 10.1097/01.chi.0000179056.54419.5e. [DOI] [PubMed] [Google Scholar]
- 16.Hozakova L, Slonkova J, Blahutova S. Anti-NMDAR encephalitis as a serious adverse event probably related to yellow fever vaccination. Klin Mikrobiol Infekc Lek. 2018;24:17–19. [PubMed] [Google Scholar]
- 17.Schein F, Gagneux-Brunon A, Antoine JC. Anti-N-methyl-D-aspartate receptor encephalitis after herpes simplex virus-associated encephalitis: an emerging disease with diagnosis and therapeutic challenges. Infection. 2017;45:545–549. doi: 10.1007/s15010-016-0959-y. [DOI] [PubMed] [Google Scholar]
- 18.Derksen SJ, van der Hoeven JG, Goraj B, Molenaar JP. Severe anti NMDA encephalitis and EBV infection. Neth J Crit Care. 2013;17:19–21. [Google Scholar]
- 19.Unni KE, Shivakumar V, Dutta TK, Chandrasekaran R. Fever of unknown cause presenting as Catatonia. J Assoc Physicians India. 1995;43:134–135. [PubMed] [Google Scholar]
- 20.Powers P, Douglass TS, Waziri R. Hyperpyrexia in catatonic states. Dis Nerv Syst. 1976;37:359–361. [PubMed] [Google Scholar]
- 21.Smith AP, Tyrrell DA, Al-Nakib W. Effects of experimentally induced respiratory virus infections and illness on psychomotor performance. Neuropsychobiology. 1987;18:144–148. doi: 10.1159/000118408. [DOI] [PubMed] [Google Scholar]
- 22.Harrison NA, Brydon L, Walker C. Neural origins of human sickness in interoceptive responses to inflammation. Biol Psychiatry. 2009;66:415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison NA, Doeller CF, Voon V, Burgess N, Critchley HD. Peripheral inflammation acutely impairs human spatial memory via actions on medial temporal lobe glucose metabolism. Biol Psychiatry. 2014;76:585–593. doi: 10.1016/j.biopsych.2014.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Workman JL, Nelson RJ. Potential animal models of seasonal affective disorder. Neurosci Biobehav Rev. 2011;35:669–679. doi: 10.1016/j.neubiorev.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 25.Singh T, Williams K. Atypical Depression. Psychiatry (Edgmont) 2006;3:33–39. [PMC free article] [PubMed] [Google Scholar]
- 26.Penninx BW, Milaneschi Y, Lamers F, Vogelzangs N. Understanding the somatic consequences of depression: biological mechanisms and the role of depression symptom profile. BMC Med. 2013;11:129. doi: 10.1186/1741-7015-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy TK, Patel PD, McGuire JF. Characterization of the pediatric acute-onset neuropsychiatric syndrome phenotype. J Child Adolesc Psychopharmacol. 2015;25:14–25. doi: 10.1089/cap.2014.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rasmussen SA, Mazurek MF, Rosebush PI. Catatonia: our current understanding of its diagnosis, treatment and pathophysiology. World J Psychiatry. 2016;6:391–398. doi: 10.5498/wjp.v6.i4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carroll BT, Taylor RE. The nondichotomy between lethal catatonia and neuroleptic malignant syndrome. J Clin Psychopharmacol. 1997;17:235–238. doi: 10.1097/00004714-199706000-00026. [DOI] [PubMed] [Google Scholar]
- 30.Fink M. Recognizing NMS as a type of catatonia. Neuropsychiatry Neuropsychol Behav Neurol. 1995;8:75–76. [Google Scholar]
- 31.Caroff SN, Mann SC, Keck PE, Jr, Francis A. Residual catatonic state following neuroleptic malignant syndrome. J Clin Psychopharmacol. 2000;20:257–259. doi: 10.1097/00004714-200004000-00021. [DOI] [PubMed] [Google Scholar]
- 32.Rosebush P, Stewart T. A prospective analysis of 24 episodes of neuroleptic malignant syndrome. Am J Psychiatry. 1989;146:717–725. doi: 10.1176/ajp.146.6.717. [DOI] [PubMed] [Google Scholar]
- 33.Anglin RE, Rosebush PI, Mazurek MF. Neuroleptic malignant syndrome: a neuroimmunologic hypothesis. CMAJ. 2010;182:E834–E838. doi: 10.1503/cmaj.091442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oruch R, Pryme IF, Engelsen BA, Lund A. Neuroleptic malignant syndrome: an easily overlooked neurologic emergency. Neuropsychiatr Dis Treat. 2017;13:161–175. doi: 10.2147/NDT.S118438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352:1112–1120. doi: 10.1056/NEJMra041867. [DOI] [PubMed] [Google Scholar]
- 36.Markanday A. Acute phase reactants in infections: evidence-based review and a guide for clinicians. Open Forum Infect Dis. 2015;2 doi: 10.1093/ofid/ofv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gruys E, Toussaint M, Niewold T, Koopmans S. Acute phase reaction and acute phase proteins. J Zhejiang Univ Sci B. 2005;6:1045–1056. doi: 10.1631/jzus.2005.B1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haouzir S, Lemoine X, Desbordes M. The role of coagulation marker fibrin D-dimer in early diagnosis of catatonia. Psychiatry Res. 2009;168:78–85. doi: 10.1016/j.psychres.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 39.Rao NP, Mutalik NR, Kasal V. Monocyte abnormality in catatonia: revisiting the immune theory of catatonia. J ECT. 2011;27:e53–e54. doi: 10.1097/YCT.0b013e318212ecaa. [DOI] [PubMed] [Google Scholar]
- 40.Akanji AO, Ohaeri JU, Al-Shammri S, Fatania HR. Association of blood levels of C-reactive protein with clinical phenotypes in Arab schizophrenic patients. Psychiatry Res. 2009;169:56–61. doi: 10.1016/j.psychres.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Lee JW. Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry. 1998;44:499–507. doi: 10.1016/s0006-3223(98)00109-7. [DOI] [PubMed] [Google Scholar]
- 42.Peralta V, Cuesta MJ, Mata I, Serrano JF, Perez-Nievas F, Natividad MC. Serum iron in catatonic and noncatatonic psychotic patients. Biol Psychiatry. 1999;45:788–790. doi: 10.1016/s0006-3223(98)00137-1. [DOI] [PubMed] [Google Scholar]
- 43.Carroll BT, Goforth HW. Serum iron in catatonia. Biol Psychiatry. 1995;38:776–777. doi: 10.1016/0006-3223(95)00361-4. [DOI] [PubMed] [Google Scholar]
- 44.Lakshmana R, Khanna S, Christopher R. Serum iron levels in catatonia. Indian J Psychiatry. 2009;51:153. [Google Scholar]
- 45.Northoff G, Wenke J, Pflug B. Increase of serum creatine phosphokinase in catatonia: an investigation in 32 acute catatonic patients. Psychol Med. 1996;26:547–553. doi: 10.1017/s0033291700035625. [DOI] [PubMed] [Google Scholar]
- 46.Meltzer H. Creatine kinase and aldolase in serum: abnormality common to acute psychoses. Science. 1968;159:1368–1370. doi: 10.1126/science.159.3821.1368. [DOI] [PubMed] [Google Scholar]
- 47.Northrop-Clewes CA. Interpreting indicators of iron status during an acute phase response—lessons from malaria and human immunodeficiency virus. Ann Clin Biochem. 2008;45:18–32. doi: 10.1258/acb.2007.007167. [DOI] [PubMed] [Google Scholar]
- 48.Raja M, Altavista MC, Cavallari S, Lubich L. Neuroleptic malignant syndrome and catatonia. Eur Arch Psychiatry Clin Neurosci. 1994;243:299–303. doi: 10.1007/BF02195723. [DOI] [PubMed] [Google Scholar]
- 49.Kim J, Wessling-Resnick M. Iron and mechanisms of emotional behavior. J Nutr Biochem. 2014;25:1101–1107. doi: 10.1016/j.jnutbio.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pease-Raissi SE, Chan JR. Micro(glial)-managing executive function: white matter inflammation drives catatonia. J Clin Invest. 2017;128:564–566. doi: 10.1172/JCI98761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hagemeyer N, Goebbels S, Papiol S. A myelin gene causative of a catatonia-depression syndrome upon aging. EMBO Mol Med. 2012;4:528–539. doi: 10.1002/emmm.201200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janova H, Arinrad S, Balmuth E. Microglia ablation alleviates myelin-associated catatonic signs in mice. J Clin Invest. 2017;128:734–745. doi: 10.1172/JCI97032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prud'homme GJ, Glinka Y, Wang Q. Immunological GABAergic interactions and therapeutic applications in autoimmune diseases. Autoimmun Rev. 2015;14:1048–1056. doi: 10.1016/j.autrev.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 54.Ramirez K, Niraula A, Sheridan JF. GABAergic modulation with classical benzodiazepines prevent stress-induced neuro-immune dysregulation and behavioral alterations. Brain Behav Immun. 2016;51:154–168. doi: 10.1016/j.bbi.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fernandez Hurst N, Zanetti SR, Baez NS, Bibolini MJ, Bouzat C, Roth GA. Diazepam treatment reduces inflammatory cells and mediators in the central nervous system of rats with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2017;313:145–151. doi: 10.1016/j.jneuroim.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 56.Huang CY, Chou FH, Huang YS. The association between zolpidem and infection in patients with sleep disturbance. J Psychiatr Res. 2014;54:116–120. doi: 10.1016/j.jpsychires.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 57.Hsu FG, Sheu MJ, Lin CL, Hsieh YW, Lai SW. Use of zolpidem and risk of acute pyelonephritis in women: a population-based case-control study in Taiwan. J Clin Pharmacol. 2017;57:376–381. doi: 10.1002/jcph.815. [DOI] [PubMed] [Google Scholar]
- 58.Guloksuz S, Rutten BP, Arts B, van Os J, Kenis G. The immune system and electroconvulsive therapy for depression. J ECT. 2014;30:132–137. doi: 10.1097/YCT.0000000000000127. [DOI] [PubMed] [Google Scholar]
- 59.Keller WR, Kum LM, Wehring HJ, Koola MM, Buchanan RW, Kelly DL. A review of anti-inflammatory agents for symptoms of schizophrenia. J Psychopharmacol. 2013;27:337–342. doi: 10.1177/0269881112467089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Solmi M, Veronese N, Thapa N. Systematic review and meta-analysis of the efficacy and safety of minocycline in schizophrenia. CNS Spectr. 2017;22:415–426. doi: 10.1017/S1092852916000638. [DOI] [PubMed] [Google Scholar]
- 61.Levkovitz Y, Mendlovich S, Riwkes S. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry. 2010;71:138–149. doi: 10.4088/JCP.08m04666yel. [DOI] [PubMed] [Google Scholar]
- 62.Deakin B, Suckling J, Barnes TRE. The benefit of minocycline on negative symptoms of schizophrenia in patients with recent-onset psychosis (BeneMin): a randomised, double-blind, placebo-controlled trial. Lancet Psychiatry. 2018;5:885–894. doi: 10.1016/S2215-0366(18)30345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J. Possible antipsychotic effects of minocycline in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:304–307. doi: 10.1016/j.pnpbp.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 64.Ahuja N, Carroll BT. Possible anti-catatonic effects of minocycline in patients with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:968–969. doi: 10.1016/j.pnpbp.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 65.Perez Nievas BG, Hammerschmidt T, Kummer MP, Terwel D, Leza JC, Heneka MT. Restraint stress increases neuroinflammation independently of amyloid beta levels in amyloid precursor protein/PS1 transgenic mice. J Neurochem. 2011;116:43–52. doi: 10.1111/j.1471-4159.2010.07083.x. [DOI] [PubMed] [Google Scholar]
- 66.Northoff G. What catatonia can tell us about “top-down modulation”: a neuropsychiatric hypothesis. Behav Brain Sci. 2002;25:555–577. doi: 10.1017/s0140525x02000109. [DOI] [PubMed] [Google Scholar]
- 67.Hadavi S, Noyce AJ, Leslie RD, Giovannoni G. Stiff person syndrome. Pract Neurol. 2011;11:272–282. doi: 10.1136/practneurol-2011-000071. [DOI] [PubMed] [Google Scholar]
- 68.Chang T, Alexopoulos H, McMenamin M. Neuronal surface and glutamic acid decarboxylase autoantibodies in Nonparaneoplastic stiff person syndrome. JAMA Neurol. 2013;70:1140–1149. doi: 10.1001/jamaneurol.2013.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scammell TE. Narcolepsy. N Engl J Med. 2015;373:2654–2662. doi: 10.1056/NEJMra1500587. [DOI] [PubMed] [Google Scholar]
- 70.Tsutsui K, Kanbayashi T, Tanaka K. Anti-NMDA-receptor antibody detected in encephalitis, schizophrenia, and narcolepsy with psychotic features. BMC Psychiatry. 2012;12:37. doi: 10.1186/1471-244X-12-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Antelmi E, Pizza F, Vandi S. The spectrum of REM sleep-related episodes in children with type 1 narcolepsy. Brain. 2017;140:1669–1679. doi: 10.1093/brain/awx096. [DOI] [PubMed] [Google Scholar]
- 72.Dalmau J, Gleichman AJ, Hughes EG. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1091–1098. doi: 10.1016/S1474-4422(08)70224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.DeSena AD, Greenberg BM, Graves D. Three phenotypes of anti-N-methyl-D-aspartate receptor antibody encephalitis in children: prevalence of symptoms and prognosis. Pediatric Neurology. 2014;51:542–549. doi: 10.1016/j.pediatrneurol.2014.04.030. [DOI] [PubMed] [Google Scholar]
- 74.Kruse JL, Lapid MI, Lennon VA. Psychiatric autoimmunity: N-methyl-D-aspartate receptor IgG and beyond. Psychosomatics. 2015;56:227–241. doi: 10.1016/j.psym.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 75.Duan BC, Weng WC, Lin KL. Variations of movement disorders in anti-N-methyl-D-aspartate receptor encephalitis: a nationwide study in Taiwan. Medicine (Baltimore) 2016;95 doi: 10.1097/MD.0000000000004365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Granata T, Matricardi S, Ragona F. Pediatric NMDAR encephalitis: a single center observation study with a closer look at movement disorders. Eur J Paediatr Neurol. 2018;22:301–307. doi: 10.1016/j.ejpn.2018.01.012. [DOI] [PubMed] [Google Scholar]
- 77.Herken J, Pruss H. Red flags: clinical signs for identifying autoimmune encephalitis in psychiatric patients. Front Psychiatry. 2017;8:25. doi: 10.3389/fpsyt.2017.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Koenig M, Duband S, Charmion S, Cathebras P, Camdessanche JP, Antoine JC. Extrapontine myelinolysis of favorable outcome in a patient with autoimmune polyglandular syndrome. Rev Med Interne. 2005;26:65–68. doi: 10.1016/j.revmed.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 79.Kobrynski LJ, Sullivan KE. Velocardiofacial syndrome, DiGeorge syndrome: the chromosome 22q11.2 deletion syndromes. Lancet. 2007;370:1443–1452. doi: 10.1016/S0140-6736(07)61601-8. [DOI] [PubMed] [Google Scholar]
- 80.Butcher NJ, Boot E, Lang AE. Neuropsychiatric expression and catatonia in 22q11.2 deletion syndrome: an overview and case series. Am J Med Genet A. 2018;176:2146–2159. doi: 10.1002/ajmg.a.38708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dalmau J, Tuzun E, Wu HY. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36. doi: 10.1002/ana.21050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bram D, Bubrovszky M, Durand JP, Lefevre G, Morell-Dubois S, Vaiva G. Pernicious anemia presenting as catatonia: correlating vitamin B12 levels and catatonic symptoms. Gen Hosp Psychiatry. 2015;37:273.e5–273.e7. doi: 10.1016/j.genhosppsych.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 83.Jauhar S, Blackett A, Srireddy P, McKenna PJ. Pernicious anaemia presenting as catatonia without signs of anaemia or macrocytosis. Br J Psychiatry. 2010;197:244–245. doi: 10.1192/bjp.bp.108.054072. [DOI] [PubMed] [Google Scholar]
- 84.Berry N, Sagar R, Tripathi BM. Catatonia and other psychiatric symptoms with vitamin B12 deficiency. Acta Psychiatr Scand. 2003;108:156–159. doi: 10.1034/j.1600-0447.2003.00089.x. [DOI] [PubMed] [Google Scholar]
- 85.Catalano G, Catalano MC, Rosenberg EI, Embi PJ, Embi CS. Catatonia. Another neuropsychiatric presentation of vitamin B12 deficiency? Psychosomatics. 1998;39:456–460. doi: 10.1016/S0033-3182(98)71307-6. [DOI] [PubMed] [Google Scholar]
- 86.Bharadwaj B, Sugaparaneetharan A, Rajkumar RP. Graves' disease presenting with catatonia: a probable case of encephalopathy associated with autoimmune thyroid disease. Acta Neuropsychiatr. 2012;24:374–379. doi: 10.1111/j.1601-5215.2012.00654.x. [DOI] [PubMed] [Google Scholar]
- 87.Urias-Uribe L, Valdez-Solis E, Gonzalez-Milan C, Ramirez-Renteria C, Ferreira-Hermosillo A. Psychosis crisis associated with thyrotoxicosis due to Graves' disease. Case Rep Psychiatry. 2017;2017 doi: 10.1155/2017/6803682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Saito T, Saito R, Suwa H, Yakushiji F, Takezawa K, Nakamura M. Differences in the treatment response to antithyroid drugs versus electroconvulsive therapy in a case of recurrent catatonia due to Graves' disease. Case Rep Psychiatry. 2012;2012 doi: 10.1155/2012/868490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shlykov MA, Rath S, Badger A, Winder GS. ‘Myxoedema madness’ with Capgras syndrome and catatonic features responsive to combination olanzapine and levothyroxine. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-215957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lalanne L, Meriot ME, Ruppert E, Zimmermann MA, Danion JM, Vidailhet P. Attempted infanticide and suicide inaugurating catatonia associated with Hashimoto's encephalopathy: a case report. BMC Psychiatry. 2016;16:13. doi: 10.1186/s12888-016-0719-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen YW, Hung PL, Wu CK, Tseng PT. Severe complication of catatonia in a young patient with Hashimoto's encephalopathy comorbid with Cornelia de Lange syndrome. Kaohsiung J Med Sci. 2015;31:60–61. doi: 10.1016/j.kjms.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 93.Iskandar M, Stepanova E, Francis A. Two cases of catatonia with thyroid dysfunction. Psychosomatics. 2014;55:703–707. doi: 10.1016/j.psym.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 94.Ferrafiat V, Raffin M, Deiva K. Catatonia and autoimmune conditions in children and adolescents: should we consider a therapeutic challenge? J Child Adolesc Psychopharmacol. 2017;27:167–176. doi: 10.1089/cap.2015.0086. [DOI] [PubMed] [Google Scholar]
- 95.Leon J, Hommer R, Grant P. Longitudinal outcomes of children with pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS) Eur Child Adolesc Psychiatry. 2018;27:637–643. doi: 10.1007/s00787-017-1077-9. [DOI] [PubMed] [Google Scholar]
- 96.Swedo SE, Leonard HL, Garvey M. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264–271. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- 97.Williams KA, Swedo SE, Farmer CA. Randomized, controlled trial of intravenous immunoglobulin for pediatric autoimmune neuropsychiatric disorders associated with Streptococcal infections. J Am Acad Child Adolesc Psychiatry. 2016;55:860. doi: 10.1016/j.jaac.2016.06.017. 67.e2. [DOI] [PubMed] [Google Scholar]
- 98.Elia J, Dell ML, Friedman DF. PANDAS with catatonia: a case report. Therapeutic response to lorazepam and plasmapheresis. J Am Acad Child Adolesc Psychiatry. 2005;44:1145–1150. doi: 10.1097/01.chi.0000179056.54419.5e. [DOI] [PubMed] [Google Scholar]
- 99.Bachmann S, Schroder J. Catatonic syndrome related to acute disseminated encephalomyelitis (ADEM) Schizophr Res. 2006;87:336–337. doi: 10.1016/j.schres.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 100.Pettingill P, Kramer HB, Coebergh JA. Antibodies to GABAA receptor alpha1 and gamma2 subunits: clinical and serologic characterization. Neurology. 2015;84:1233–1241. doi: 10.1212/WNL.0000000000001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nikolaus M, Knierim E, Meisel C. Severe GABAA receptor encephalitis without seizures: A paediatric case successfully treated with early immunomodulation. Eur J Paediatr Neurol. 2018;22:558–562. doi: 10.1016/j.ejpn.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 102.Petit-Pedrol M, Armangue T, Peng X. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol. 2014;13:276–286. doi: 10.1016/S1474-4422(13)70299-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dalmau J, Graus F. Antibody-mediated encephalitis. N Engl J Med. 2018;378:840–851. doi: 10.1056/NEJMra1708712. [DOI] [PubMed] [Google Scholar]
- 104.McCarthy A, Dineen J, McKenna P. Anti-NMDA receptor encephalitis with associated catatonia during pregnancy. J Neurol. 2012;259:2632–2635. doi: 10.1007/s00415-012-6561-z. [DOI] [PubMed] [Google Scholar]
- 105.Consoli A, Ronen K, An-Gourfinkel I. Malignant catatonia due to anti-NMDA-receptor encephalitis in a 17-year-old girl: case report. Child Adolesc Psychiatry Ment Health. 2011;5:15. doi: 10.1186/1753-2000-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lejuste F, Thomas L, Picard G. Neuroleptic intolerance in patients with anti-NMDAR encephalitis. Neurol Neuroimmunol Neuroinflamm. 2016;3:e280. doi: 10.1212/NXI.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steiner J, Walter M, Glanz W. Increased prevalence of diverse N-methyl-D-aspartate glutamate receptor antibodies in patients with an initial diagnosis of schizophrenia: specific relevance of IgG NR1a antibodies for distinction from N-methyl-D-aspartate glutamate receptor encephalitis. JAMA Psychiatry. 2013;70:271–278. doi: 10.1001/2013.jamapsychiatry.86. [DOI] [PubMed] [Google Scholar]
- 108.Lin CC, Hung YY, Tsai MC, Huang TL. Increased serum anti-N-methyl-D-aspartate receptor antibody immunofluorescence in psychiatric patients with past catatonia. PLoS One. 2017;12 doi: 10.1371/journal.pone.0187156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lennox BR, Palmer-Cooper EC, Pollak T. Prevalence and clinical characteristics of serum neuronal cell surface antibodies in first-episode psychosis: a case-control study. Lancet Psychiatry. 2017;4:42–48. doi: 10.1016/S2215-0366(16)30375-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pollak T, Iyegbe C, Kempton M. Neuronal autoantibodies shape symptomatology, cognitive function and brain structure in subjects at ultra-high risk for psychosis. Neurology Psychiatry Brain Res. 2018;29:19–20. (abstr). [Google Scholar]
- 111.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.von Economo C. Encephalitis lethargica. Wien Klin Wochenschr. 1917;30:581–585. [Google Scholar]
- 113.Reid AH, McCall S, Henry JM, Taubenberger JK. Experimenting on the past: the enigma of von Economo's encephalitis lethargica. J Neuropathol Exp Neurol. 2001;60:663–670. doi: 10.1093/jnen/60.7.663. [DOI] [PubMed] [Google Scholar]
- 114.Dale RC, Merheb V, Pillai S. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. 2012;135:3453–3468. doi: 10.1093/brain/aws256. [DOI] [PubMed] [Google Scholar]
- 115.Lancaster E. The diagnosis and treatment of autoimmune encephalitis. J Clin Neurol. 2016;12:1–13. doi: 10.3988/jcn.2016.12.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bauer J, Bien CG. Neuropathology of autoimmune encephalitides. Handb Clin Neurol. 2016;133:107–120. doi: 10.1016/B978-0-444-63432-0.00007-4. [DOI] [PubMed] [Google Scholar]
- 117.Corlett PR, Honey GD, Krystal JH, Fletcher PC. Glutamatergic model psychoses: prediction error, learning, and inference. Neuropsychopharmacology. 2011;36:294–315. doi: 10.1038/npp.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tani A, Kikuta R, Itoh K. Polymorphism analysis of the upstream region of the human N-methyl-D-aspartate receptor subunit NR1 gene (GRIN1): implications for schizophrenia. Schizophr Res. 2002;58:83–86. doi: 10.1016/s0920-9964(02)00161-5. [DOI] [PubMed] [Google Scholar]
- 119.Spatola M, Petit-Pedrol M, Simabukuro MM. Investigations in GABAA receptor antibody-associated encephalitis. Neurology. 2017;88:1012–1020. doi: 10.1212/WNL.0000000000003713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Khan A, Joyce P, Jones AV. Benzodiazepine withdrawal syndromes. N Z Med J. 1980;92:94–96. [PubMed] [Google Scholar]
- 121.Hughes EG, Peng X, Gleichman AJ. Cellular and synaptic mechanisms of anti-NMDA receptor encephalitis. J Neurosci. 2010;30:5866–5875. doi: 10.1523/JNEUROSCI.0167-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Jezequel J, Johansson EM, Dupuis JP. Dynamic disorganization of synaptic NMDA receptors triggered by autoantibodies from psychotic patients. Nat Comm. 2017;8:1791. doi: 10.1038/s41467-017-01700-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gouzoulis-Mayfrank E, Heekeren K, Neukirch A. Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double-blind, cross-over study in healthy volunteers. Pharmacopsychiatry. 2005;38:301–311. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- 124.Tang Y, Zou H, Strong JA. Paradoxical effects of very low dose MK-801. Eur J Pharmacol. 2006;537:77–84. doi: 10.1016/j.ejphar.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 125.Wu J, Zou H, Strong JA. Bimodal effects of MK-801 on locomotion and stereotypy in C57BL/6 mice. Psychopharmacology (Berl) 2005;177:256–263. doi: 10.1007/s00213-004-1944-1. [DOI] [PubMed] [Google Scholar]
- 126.Inta D, Sartorius A, Gass P. NMDA receptor blockade and catatonia: a complex relationship. Schizophr Res. 2015;168:581–582. doi: 10.1016/j.schres.2015.07.029. [DOI] [PubMed] [Google Scholar]
- 127.Chaves C, Marque CR, Trzesniak C. Glutamate-N-methyl-D-aspartate receptor modulation and minocycline for the treatment of patients with schizophrenia: an update. Braz J Med Biol Res. 2009;42:1002–1014. doi: 10.1590/S0100-879X2009001100002. [DOI] [PubMed] [Google Scholar]
- 128.Papadia S, Hardingham GE. The dichotomy of NMDA receptor signaling. Neuroscientist. 2007;13:572–579. doi: 10.1177/10738584070130060401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uncited Reference
- 89.Lee Y, House EM. Treatment of steroid-resistant hashimoto encephalopathy with misidentification delusions and catatonia. Psychosomatics. 2017;58:322–327. doi: 10.1016/j.psym.2016.10.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.