Abstract
Lens proteins become increasingly cross-linked through nondisulfide linkages during aging and cataract formation. One mechanism that has been implicated in this cross-linking is glycation through formation of advanced glycation end products (AGEs). Here, we found an age-associated increase in stiffness in human lenses that was directly correlated with levels of protein–cross-linking AGEs. α-Crystallin in the lens binds to other proteins and prevents their denaturation and aggregation through its chaperone-like activity. Using a FRET-based assay, we examined the stability of the αA-crystallin–γD-crystallin complex for up to 12 days and observed that this complex is stable in PBS and upon incubation with human lens–epithelial cell lysate or lens homogenate. Addition of 2 mm ATP to the lysate or homogenate did not decrease the stability of the complex. We also generated complexes of human αA-crystallin or αB-crystallin with alcohol dehydrogenase or citrate synthase by applying thermal stress. Upon glycation under physiological conditions, the chaperone–client complexes underwent greater extents of cross-linking than did uncomplexed protein mixtures. LC-MS/MS analyses revealed that the levels of cross-linking AGEs were significantly higher in the glycated chaperone–client complexes than in glycated but uncomplexed protein mixtures. Mouse lenses subjected to thermal stress followed by glycation lost resilience more extensively than lenses subjected to thermal stress or glycation alone, and this loss was accompanied by higher protein cross-linking and higher cross-linking AGE levels. These results uncover a protein cross-linking mechanism in the lens and suggest that AGE-mediated cross-linking of α-crystallin–client complexes could contribute to lens aging and presbyopia.
Keywords: protein cross-linking, small heat-shock protein (sHsp), chaperone, crystallin, cataract, advanced glycation end products (AGEs), lens protein, presbyopia, stiffness
Introduction
The eye lens is an avascular tissue. It has to remain transparent during aging to focus light onto the retina. It is a protein-rich tissue containing ∼35% protein by wet weight (1). Lens proteins, largely crystallins, have negligible turnover and therefore accumulate post-translational modifications throughout life. Some of the major modifications are deamidation, racemization, glycation, and oxidation (2–7).
Glycation in the lens is initiated by three major precursors: ascorbate, methylglyoxal (MGO),2 and glucose (6, 8). Although MGO and glucose react directly with proteins, ascorbate must undergo oxidation to produce glycating agents. Erythrulose and 3-deoxythreosone are considered major glycating agents from ascorbate oxidation in the lens (9). Through a series of intermediates, glycation produces stable advanced glycation end products (AGEs) in proteins. Lysine and arginine residues are the major sites of AGE formation in proteins (10, 11). As the lens ages, glutathione (GSH) levels are reduced (12), which could lead to increased ascorbate oxidation and consequently to the formation of glycation precursors. Additionally, the glyoxalase system that metabolizes MGO to d-lactate requires GSH as a cofactor (13). The loss of GSH could therefore decrease MGO metabolism and allow MGO to form AGEs in lens proteins.
Many AGEs have been detected in human lenses (14). Several of them, such as pentosidine, vesperlysine, K2P, and glucosepane, accumulate with advancing age and accumulate at a higher rate in senile cataractous lenses (15–19). Lens proteins become pigmented yellow-brown and cross-linked during aging, and these processes become accentuated in cataractous lenses (20, 21). Some AGEs are pigmented yellow-brown and form as amino acid cross-linking structures (22). In general, AGE levels are higher in water-insoluble proteins than in water-soluble proteins of human lenses (6, 18, 19). Together, these factors suggest that the milieu of the lens promotes the formation of AGEs, which contribute to lens aging and cataract formation.
α-Crystallin is a major component of lens proteins, accounting for ∼40% of total proteins. It consists of αA-crystallin (αAC) and αB-crystallin (αBC) subunits. They are typically present at a 3:1 ratio in the lens (23). They belong to the family of small heat-shock proteins. Similar to other members of this family, both αAC and αBC exhibit chaperone-like properties (24, 25). Through these properties, they prevent aggregation of denaturing proteins in an ATP-independent manner. This function of α-crystallin is suggested to be necessary to preserve lens transparency during aging. The noncovalent association between α-crystallin and other proteins, possibly through the chaperone-like activity of the former, has been demonstrated in human lenses (26). However, α-crystallin–client complexes in the lens, especially in lens fibers, are likely to be permanent. This is because metabolic activity in fiber cells, which make up a large part of the lens, is extremely low, and therefore, there is less likelihood for the existence of mechanisms that can dissociate α-crystallin–client complexes. We hypothesized that the proximity of two proteins in such complexes could render them susceptible to AGE-mediated nondisulfide intermolecular cross-linking and aggregation, especially when lens GSH levels are reduced during advanced aging. Such cross-linking and aggregation could explain the precipitous reduction of water-soluble α-crystallin in aged lenses. Moreover, cross-linking and aggregation of a major protein in the lens could decrease the accommodative ability of the lens, contributing to age-associated presbyopia. This study investigated AGE-mediated cross-linking of the α-crystallin–client complexes and the relationship between cross-linking AGEs and stiffness in aging human lenses.
Results
Levels of cross-linking AGEs correlate with stiffness in aging human lenses
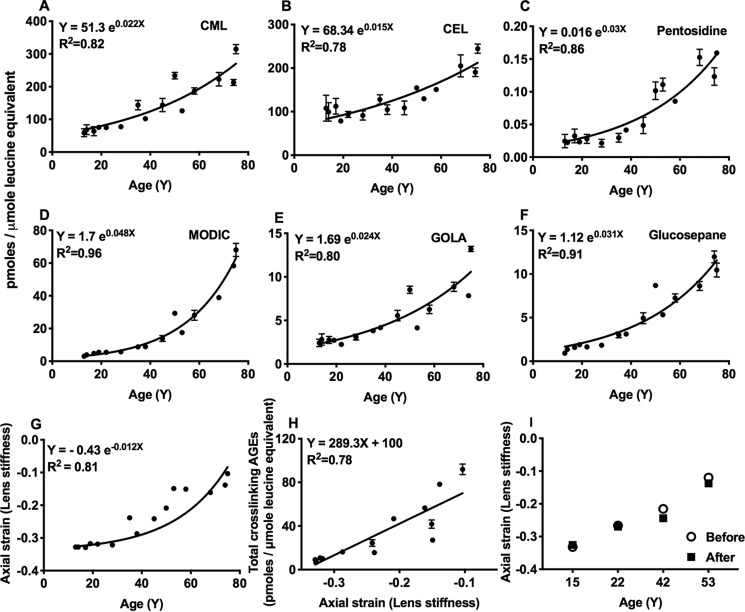
We quantified four cross-linking AGEs in proteins of aging human lenses (13–75 years). Three of them are formed as cross-links between lysine and arginine residues in proteins (methylglyoxal-derived imidazoline cross-link (MODIC), pentosidine, and glucosepane), and one is formed between two lysine residues (glyoxal lysine amide (GOLA)) along with N(6)-carboxymethyl-lysine (CML) and N(6)-carboxyethyl-lysine (CEL) (structures shown in Fig. 1). The CML and CEL levels were 68.3–314.7 and 78.5–244.6 pmol/μmol leucine equivalent, respectively (Fig. 2, A and B). Among the four cross-linking AGEs, MODIC was present at relatively high levels (3.9–68.1 pmol/μmol leucine equivalent) (Fig. 2D). Pentosidine, GOLA, and glucosepane levels were 0.022–0.159, 2.4–13.2, and 0.9–12 pmol/μmol leucine equivalent, respectively (Fig. 2, C, E, and F).
Figure 1.
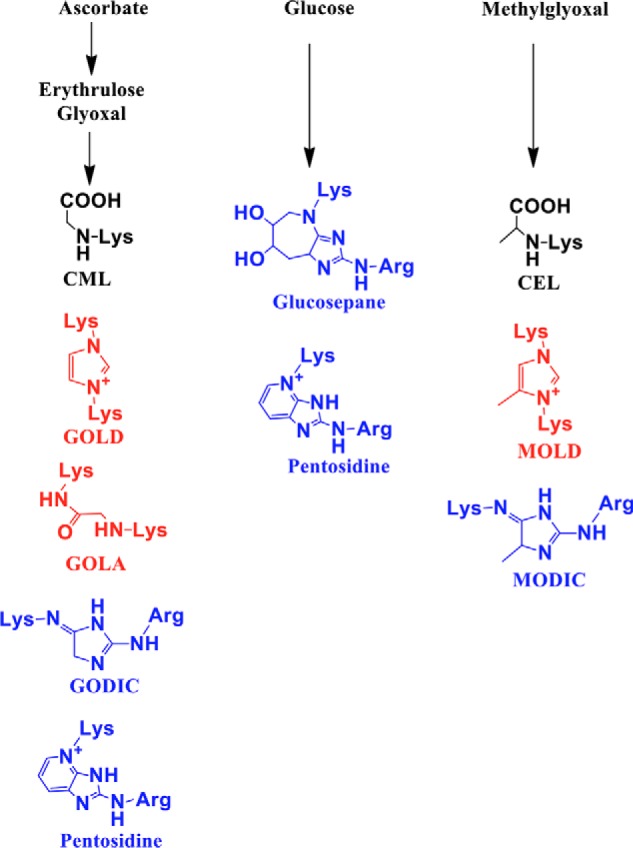
AGEs measured in this study by LC-MS/MS. Lys–Lys cross-linking AGEs are shown in red, and Lys–Arg cross-linking AGEs are shown in blue.
Figure 2.
Levels of cross-linking AGEs positively correlate with stiffness in aging human lenses. AGEs were quantified in human lens proteins by LC-MS/MS. CML and CEL are the dominant AGEs and were quantified in addition to cross-linking AGEs, pentosidine, glucosepane, MODIC, and GOLA (A–F). The data shown are means ± S.D. of three measurements from each sample. Lens stiffness increases during aging in humans (G), and the increase in stiffness positively correlates with levels of cross-linking AGEs (H). The stiffness measurements were not affected by freezing (2 days at −80 °C) followed by thawing (16 h at 4 °C) of lenses. Lens stiffness before and after freezing/thawing are shown in I. Axial compressive strain was used as a measure of lens stiffness.
Next, we determined the effect of age on human lens stiffness. Stiffness increased progressively until ∼40 years of age and then exponentially until ∼75 years of age (Fig. 2G). We found a strong correlation between the total cross-linking AGEs (the levels of MODIC, GOLA, glucosepane, and pentosidine together) and lens stiffness (Fig. 2H), suggesting a causal role for cross-linking AGEs in lens stiffness. In these measurements, we used thawed lenses that were stored frozen at −80 °C. To determine whether freezing of lenses affects stiffness measurements in our setting, we measured the axial strain of freshly-obtained human lenses (donor age: 15, 22, 42, and 53 years) and after their freezing at −80 °C for 2 days and thawing at 4 °C for 16 h. Although freezing and thawing slightly altered the axial strain (6.86 ± 6.24%, Fig. 2I), the age-associated increase in lens stiffness was still evident.
α-Crystallin–client complexes are stable under conditions of the lens
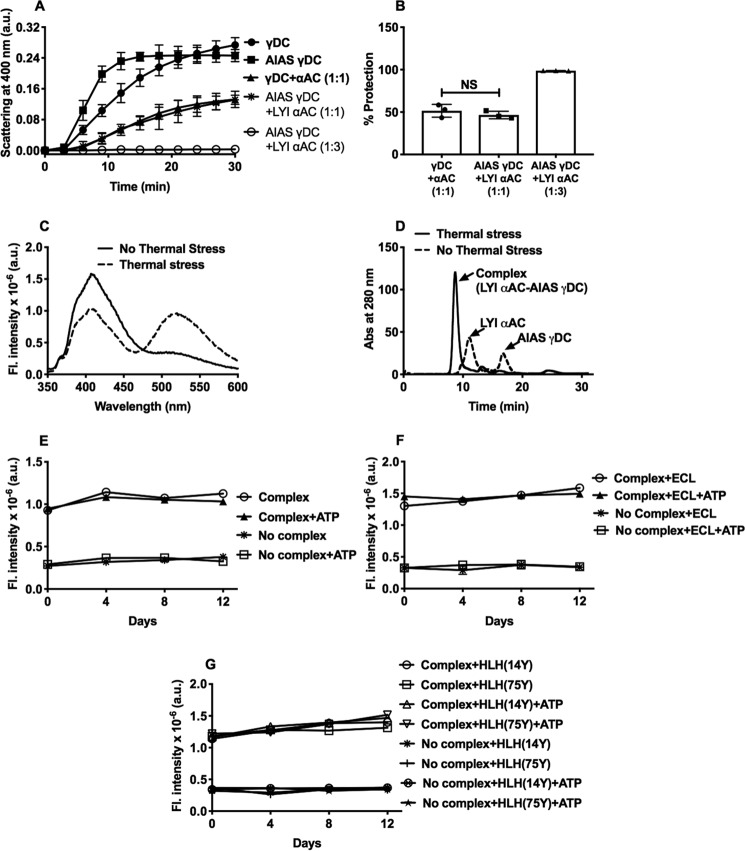
To determine the stability of α-crystallin–client complexes, a fluorescence resonance energy transfer (FRET)-based assay was developed. Human recombinant αAC was conjugated with Lucifer Yellow iodoacetamide (LYI), and human recombinant γD-crystallin (γDC) was conjugated with 4-acetamido-4′-((iodoacetyl)amino)-stilbene-2,2′-disulfonic acid (AIAS). We found 1 mol of LYI was bound to 1 mol of αAC, and 3 mol of AIAS was bound to 1 mol of γDC. The αAC–γDC complex was generated by incubating the two proteins at 70 °C for 0.5 h. Tagging AIAS to αAC or LYI to γDC did not appreciably alter the γDC aggregation or the ability of αAC to function as a chaperone (Fig. 3, A and B). FRET was observed in the αAC–γDC complex but not in the protein mixture that was not thermally stressed (Fig. 3C). The size-exclusion chromatographic (SEC) profile revealed that more than 95% LYI–αAC and AIAS–γDC were present in the complex (Fig. 3D). The αAC–γDC complex did not show a change in FRET intensity when incubated alone, with human lens epithelial cell lysate, or with human lens homogenate, even in the presence of 2 mm ATP, for up to 12 days tested (Fig. 3, E–G). Together, these observations suggest that the α-crystallin–client protein complexes are likely to be stable under the conditions of the human lens.
Figure 3.
α-Crystallin–client complexes are stable under conditions of the human lens. A FRET-based assay was used to investigate the stability of α-crystallin–client complexes. A, conjugation of AIAS to γDC did not appreciably change its thermal aggregation. AIAS–γDC or γDC at 0.1 mg/ml in 50 mm phosphate buffer, 1 mm EDTA, pH 7, was incubated at 70 °C for a period of 30 min. Conjugation of LYI to αAC did not alter chaperone activity toward thermally aggregating γDC. For this assay, αAC was used at 0.1 mg/ml, and/or LYI–αAC was used at 0.1 and 0.3 mg/ml in 50 mm phosphate buffer, 1 mm EDTA, pH 7, and incubated over a period of 30 min at 70 °C. The percent protection by αAC and LYI–αAC against aggregating γD and AIAS–γD is shown in B. C, FRET (excitation/emission wavelengths of 335/525 nm) was observed only when thermal stress (70 °C) was applied for 30 min to a protein mixture containing LYI–αAC and AIAS–γDC in 50 mm phosphate buffer, 1 mm EDTA, pH 7. Excitation and emission bandpasses used were 2.5 and 5 nm. D, FPLC elution profile for LYI–αAC (0.3 mg/ml) and AIAS–γDC (0.1 mg/ml) with and without thermal stress at 70 °C. Stability of the complex of LYI–αAC and AIAS–γDC (0.2 mg/ml) was verified by incubating the complex alone (E) or in the presence of lens epithelial cell lysate (ECL, 1 mg protein/ml) (F) and human lens homogenate (HLH, 6 mg protein/ml) (G) in the presence or absence of 2 mm ATP. Incubations were done at 37 °C in 50 mm phosphate buffer, pH 7.4. Samples were collected at regular intervals between 0 and 12 days, and FRET was measured as above. The bar graph represents the means ± S.D. of triplicate measurements. NS, not significant.
Chaperone–client complex formation increases with increasing temperature
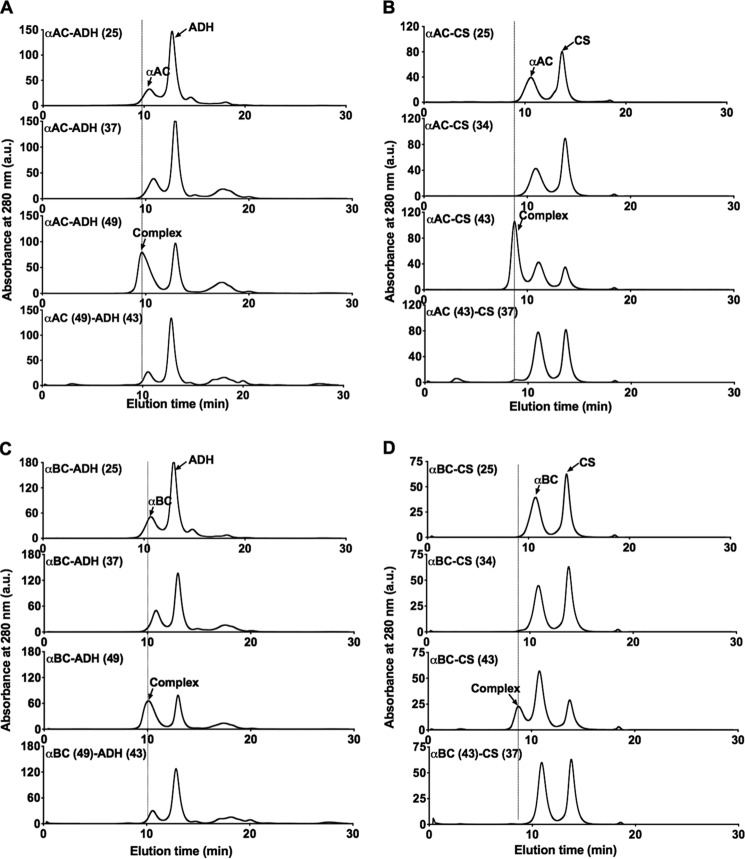
To optimize conditions for the formation of α-crystallin–client complexes, αAC or αBC and citrate synthase (CS) were mixed and incubated at 25, 34, or 43 °C for 1 h. αAC or αBC and alcohol dehydrogenase (ADH) were mixed and incubated at 25, 37, or 49 °C for 1 h. The samples were then passed through an FPLC–SEC column, and the elution profiles were monitored. Fig. 4, A–D, clearly shows the formation of complexes of αAC or αBC with ADH and αAC or αBC with CS. To determine whether prior thermal stress applied to proteins before they were mixed would lead to complex formation, αAC or αBC was incubated at 49 °C, and ADH was incubated at 43 °C for 1 h, cooled to room temperature for 1 h, and then mixed. Similarly, αAC or αBC was incubated at 43 °C, and CS was incubated at 37 °C for 1 h, cooled to room temperature for 1 h, and then mixed. Client proteins were thermally stressed at a slightly lower temperature than that used to promote complex formation to avoid their aggregation (occurs at 49 °C for ADH and 43 °C for CS). Formation of an α-crystallin–client complex was not observed in these protein mixtures, suggesting that the complex forms only when α-crystallin and the client protein are together and thermally stressed, but not when they were thermally stressed separately and then mixed.
Figure 4.
Complexes of α-crystallin and client proteins are formed upon thermal stress. A, size-exclusion chromatographic profile of the αAC–ADH; B, αAC–CS; C, αBC–ADH; D, αBC–CS protein mixtures that were thermally stressed for 1 h at temperatures shown in the figure. A vertical line is drawn to indicate the α-crystallin–client protein complex.
Inter-protein cross-linking by AGEs depends on the extent of formation of α-crystallin–client complex
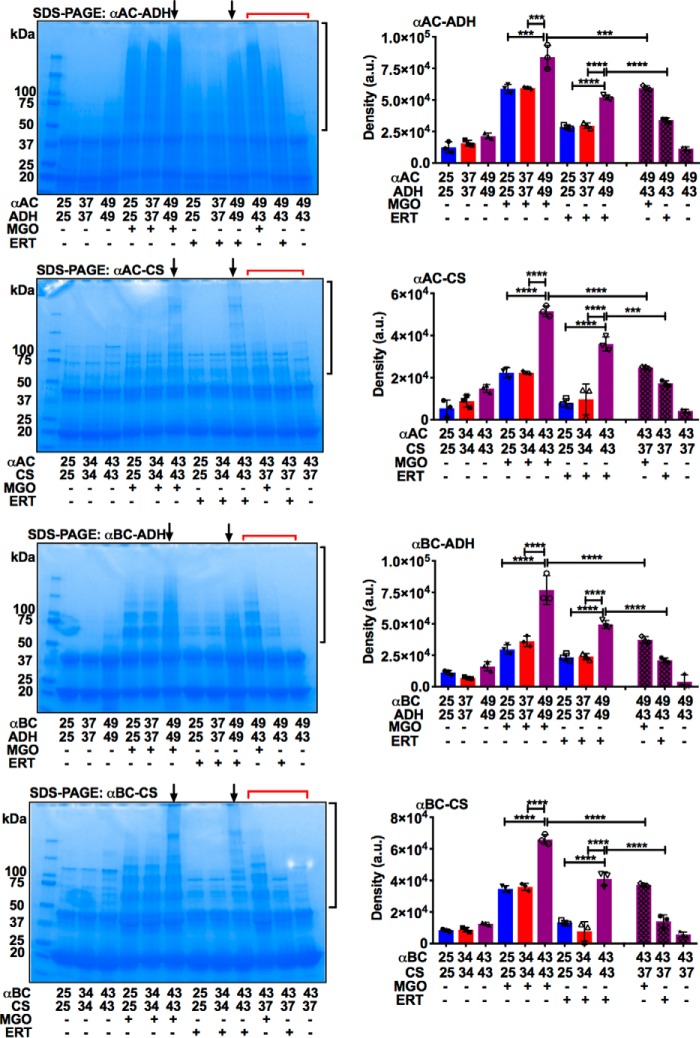
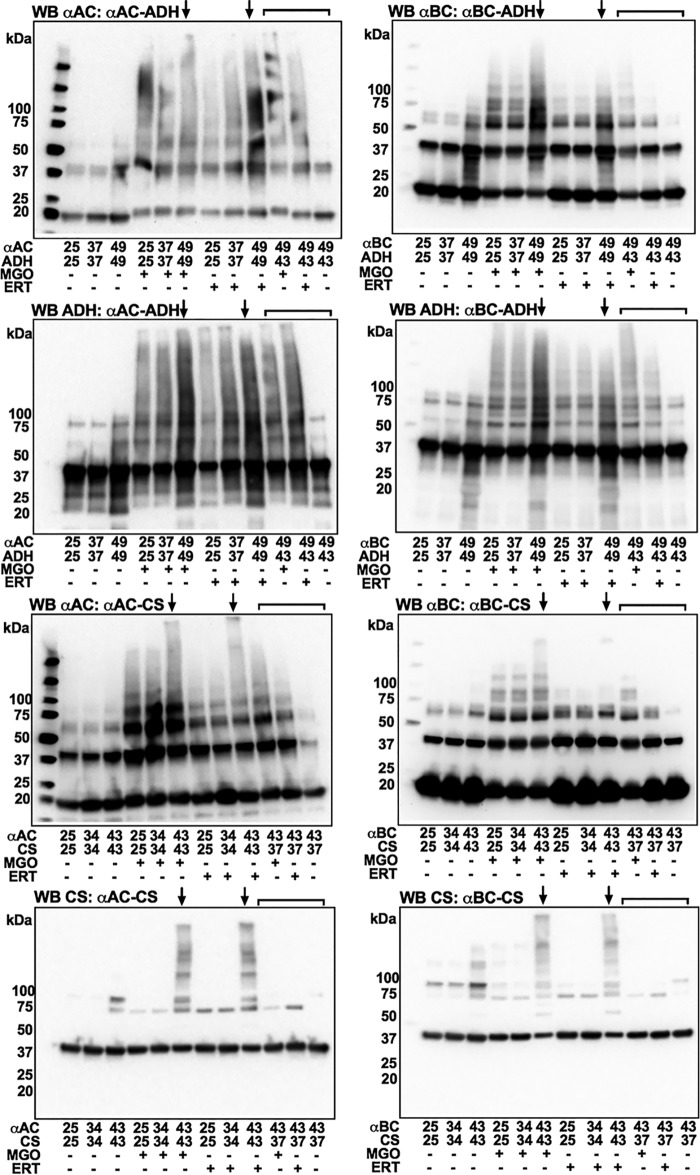
The formation of chaperone–client complexes of αAC or αBC with CS was determined by incubating the protein mixtures at 25, 34, and 43 °C for 1 h. Similarly, formation of complexes of αAC or αBC with ADH were determined at 25, 37, and 49 °C. The idea was that higher temperatures would promote complex formation and predispose them to greater levels of AGE-mediated inter-protein cross-linking. The samples were glycated by incubating with either 200 μm MGO or 250 μm erythrulose for 7 days under sterile conditions at pH 7.4 and 37 °C. The samples were then dialyzed against PBS and analyzed by SDS-PAGE using 4–20% gradient gels under reducing conditions (Fig. 5).
Figure 5.
Thermal stress and glycation lead to nondisulfide cross-linking of α-crystallin and client proteins. αAC or αBC was mixed with ADH or CS and then incubated at temperatures ranging from 25 to 49 °C for 1 h. The protein mixture was then glycated using 200 μm MGO or 250 μm ERT for 7 days at 37 °C. The protein samples (15 μg) were analyzed by SDS-PAGE using 4–20% gradient gels under reducing conditions. Arrows point to lanes that have the highest level of cross-linked proteins. Densitometric analysis of the region (black brackets) was performed to quantify cross-linked proteins. The samples in lanes that have a red bracket had αAC or αBC or client proteins that were incubated separately at respective temperatures for 1 h as shown in the figure, cooled at room temperature for 1 h, mixed, and glycated. The bars represent means ± S.D. for three independent experiments. ***, p < 0.001; ****, p < 0.0001.
Densitometric analyses revealed that MGO promoted the formation of nondisulfide protein cross-linking in αAC–ADH samples (Fig. 5). The cross-linked protein content was higher by 28 and 29% in samples incubated at 49 °C than in samples incubated at 25 and 37 °C, respectively. The αAC–CS samples incubated at 43 °C showed 57 and 56% higher cross-linked protein content than those incubated at 25 and 34 °C, respectively. When glycated with erythrulose, the αAC–ADH samples incubated at 49 °C showed 45 and 42% higher cross-linked protein content than those incubated at 25 and 37 °C, and the αAC–CS samples incubated 43 °C showed 77 and 73% higher cross-linked protein content than those incubated at 25 and 34 °C.
With MGO as the glycating agent, αBC–ADH samples incubated at 49 °C showed 61 and 53% higher cross-linked protein content than samples incubated at 25 and 37 °C (Fig. 5). In αBC–CS samples incubated at 43 °C, the increase was 47 and 45% over samples incubated at 25 and 34 °C. With erythrulose as the glycating agent, αBC–ADH samples incubated at 49 °C showed 53 and 52% higher cross-linked proteins than samples incubated at 25 and 37 °C (Fig. 5). The increase was 67 and 81% in αBC–CS samples incubated at 43 °C compared with those incubated at 25 and 34 °C. Thus, inter-protein nondisulfide cross-linking in α-crystallin–client complexes by AGEs depended on the extent of the two proteins complexed. In contrast, samples in which α-crystallin and client proteins (ADH or CS) were incubated separately at temperatures as described in Fig. 5, cooled, and mixed and then glycated with either MGO or erythrulose as above, the cross-linked protein levels were relatively low (last 3 lanes in Fig. 5). These data reiterate the notion that only the complexes formed between α-crystallin and a client protein, but not individual proteins mixed together, become susceptible to AGE-mediated cross-linking.
To further confirm that the cross-linked proteins were formed as a result of inter-protein cross-linking, we performed Western blottings using specific antibodies for αAC, αBC, ADH, and CS. In all AGE-mediated cross-linked proteins, we observed α-crystallin and the client proteins (Fig. 6). The individual protein content in cross-linked proteins was highest in samples incubated at 49 °C and glycated (for ADH) and in samples incubated at 43 °C and glycated (for CS) (lanes marked with arrows in Fig. 6). This phenomenon was not observed in samples in which α-crystallin and client proteins were subjected to thermal stress separately (last three lanes, Fig. 6). Together, these data affirm that α-crystallin–client complexes are susceptible to inter-protein cross-linking by AGEs.
Figure 6.
α-Crystallin–client complexes undergo higher cross-linking when subjected to combined thermal stress and glycation than noncomplexed proteins. αAC or αBC and client proteins were mixed before subjecting them to thermal stress and glycation using ERT (250 μm) or MGO (200 μm) for 7 days at 37 °C. Subsequently, proteins (15 μg) were run on SDS-PAGE using 4–20% gradient gels under reducing conditions and subjected to Western blotting (WB) against αAC, αBC, CS, and ADH. Arrows above lanes indicate the highest levels of covalently cross-linked proteins. In lanes marked with brackets, proteins were first incubated at the indicated temperature for 1 h separately, cooled for 1 h at room temperature, mixed, and then glycated.
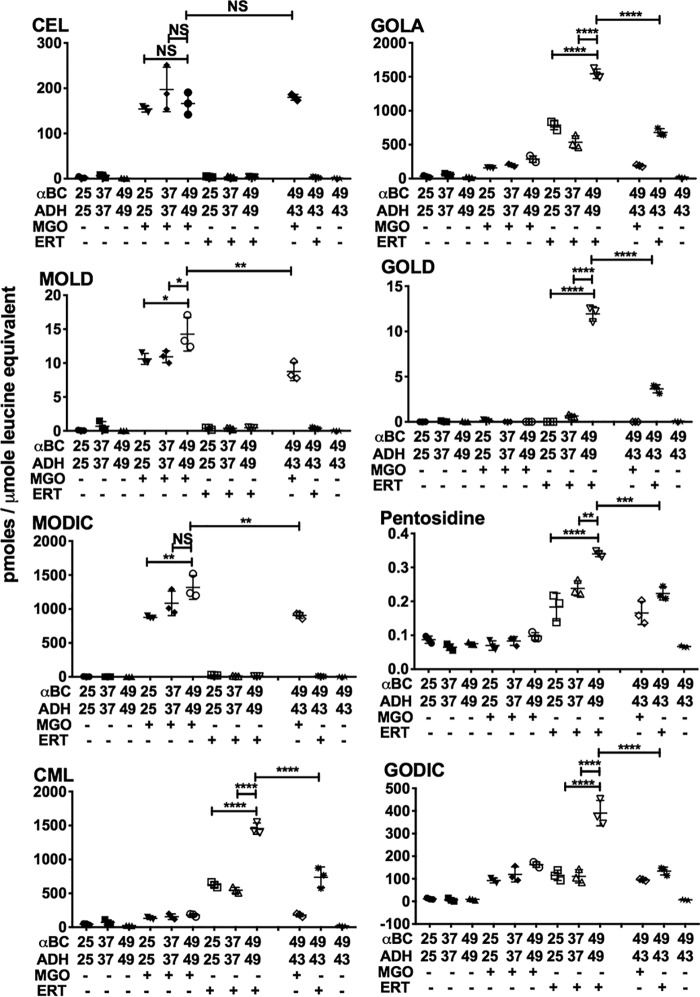
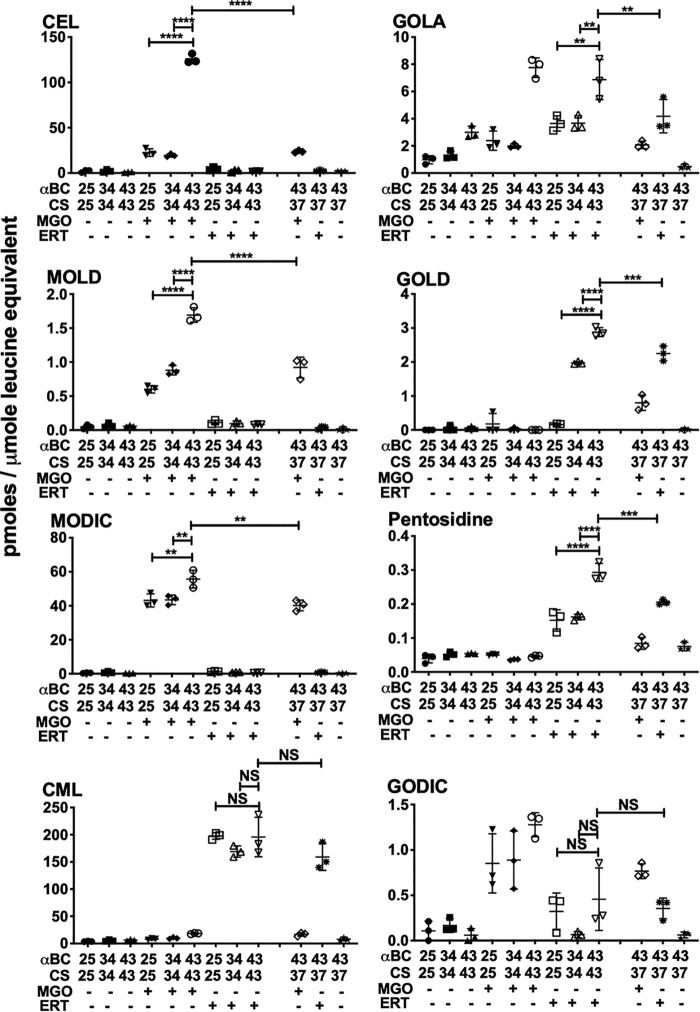
Cross-linking AGE levels are higher in cross-linked αBC–client protein complexes
In the MGO-glycated αBC–ADH samples, the levels of the two cross-linking AGEs, methylglyoxal lysine dimer (MOLD) (14.25 ± 2.48 pmol/μmol leucine equivalent) and MODIC (1317.27 ± 174.4) were higher in samples incubated at 49 °C than in samples incubated at 25 °C (MOLD: 10.6 ± 0.8, p < 0.05; MODIC: 877.7 ± 18.7, p < 0.01) and 37 °C (MOLD: 10.9 ± 0.9, p < 0.05; MODIC: 1083.7 ± 180, not significant) (Fig. 7). In αBC–CS protein complexes, glycation by MGO produced 1.69 ± 0.1 of MOLD and 55.7 ± 5.1 of MODIC in samples incubated at 43 °C, which were significantly higher than in samples incubated at 25 °C (MOLD: 0.6 ± 0.05, p < 0.001; MODIC: 43.1 ± 3.8, p < 0.01) and 34 °C (MOLD: 0.88 ± 0.07, p < 0.0001; MODIC: 43.5 ± 2.8, p < 0.1) (Fig. 8).
Figure 7.
Cross-linking AGE levels are higher in glycated αBC–ADH complexes than in glycated noncomplexed proteins. LC-MS/MS quantification of AGEs in αBC–ADH protein mixtures that were incubated at the indicated temperature and glycated by either MGO or ERT. Means ± S.D. of triplicate measurements are shown.; *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Figure 8.
Cross-linking AGE levels are higher in glycated αBC–CS complexes than in glycated noncomplexed proteins. LC-MS/MS quantification of AGEs in αBC–CS protein mixtures that were incubated at the indicated temperature and glycated by either MGO or ERT. Means ± S.D. of triplicate measurements are shown. NS, not significant; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
In the erythrulose-glycated complexes, the levels of cross-linking AGEs, GOLA, GOLD (glyoxal lysine dimer), pentosidine, and GODIC (glyoxal-derived imidazoline cross-link) were significantly higher in αBC–ADH and αBC–CS protein complexes that were generated at 49 and 43 °C than in complexes generated at 25 and 37 or 34 °C (Figs. 7 and 8). The levels of GOLA and GOLD were 1543.4 ± 71.28 and 11.9 ± 0.8 pmol/μmol leucine equivalent in αBC–ADH complexes formed at 49 °C, compared with 786.9 ± 65.8 (p < 0.0001) and 0.003 ± 0.004 (p < 0.0001) for samples at 25 °C, and 536.4 ± 93.2 (p < 0.0001) and 0.65 ± 0.13, (p < 0.0001) for samples at 37 °C (Fig. 7). In αBC–CS samples, the GOLA and GOLD levels were 6.9 ± 1.5 and 2.9 ± 0.13 pmol/μmol leucine equivalent at 43 °C compared with samples at 25 °C (GOLA: 3.65 ± 0.55, p < 0.01; GOLD: 0.16 ± 0.04, p < 0.0001) and 34 °C (GOLA: 3.7 ± 0.55, p < 0.01; GOLD: 1.97 ± 0.04, p < 0.0001) (Fig. 8). The levels of pentosidine were 0.34 ± 0.008 pmol/μmol leucine equivalent in αBC–ADH samples at 49 °C, which were higher than those incubated at 25 °C (0.184 ± 0.04, p < 0.0001) and 37 °C (0.238 ± 0.022, p < 0.01) (Fig. 7). The levels were 0.293 ± 0.026 for the αBC–CS complex formed at 43 °C, and this was higher than samples incubated at 25 °C (0.152 ± 0.03, p < 0.0001) and 34 °C (0.162 ± 0.008, p < 0.0001) (Fig. 8). The GODIC levels were higher in αBC–ADH mixtures that were incubated at 49 °C (390.5 ± 56.1 pmol/μmol leucine equivalent) than in samples incubated at 25 °C (115.1 ± 22.5, p < 0.0001) and 37 °C (110.9 ± 29.4, p < 0.0001) (Fig. 7). No such change in GODIC levels was noticed in the αBC–CS protein mixtures incubated at 43 °C compared with those incubated at 25 and 34 °C (Fig. 8).
When αBC (49 or 43 °C), ADH (43 °C), or CS (37 °C) was subjected to thermal stress individually, mixed, and then glycated with either MGO or erythrulose, the levels of the cross-linking AGEs were similar to those observed for the protein mixtures (αBC + ADH or αBC + CS) incubated and glycated at 25 and 34 or 37 °C. Together, these results suggest that α-crystallin–client protein complexes, but not the noncomplexed proteins, are susceptible to AGE-mediated cross-linking.
Glycation-mediated protein cross-linking is higher in thermally-stressed and glycated mouse lenses
Mouse lenses were either exposed to high temperature (50 °C for 1.5 or 3 h) or to control conditions (37 °C) and then incubated with or without a mixture of glycating agents (25 mm glucose, 500 μm MGO, and 500 μm erythrulose) for 3 days at 37 °C in serum-free minimal essential medium (MEM) in a CO2 incubator. The lenses were then homogenized in PBS, and the water-soluble proteins were separated by centrifugation and subjected to SDS-PAGE on 4–20% gradient gels under reducing conditions to assess nondisulfide protein cross-linking. In lenses that were incubated at 50 °C for 1.5 and 3 h, the cross-linked protein levels were ∼30 and 28% higher compared with lenses incubated at 37 °C (Fig. 9, A and B). Compared with controls, lenses that were glycated alone showed ∼18% increase in cross-linked proteins. However, when lenses were first incubated at 50 °C and then glycated, the levels of cross-linked proteins were ∼41 and 44% higher that those in lenses that were glycated alone. The results clearly showed that the combination of thermal stress and glycation leads to more protein cross-linking than either thermal stress or glycation alone.
Figure 9.
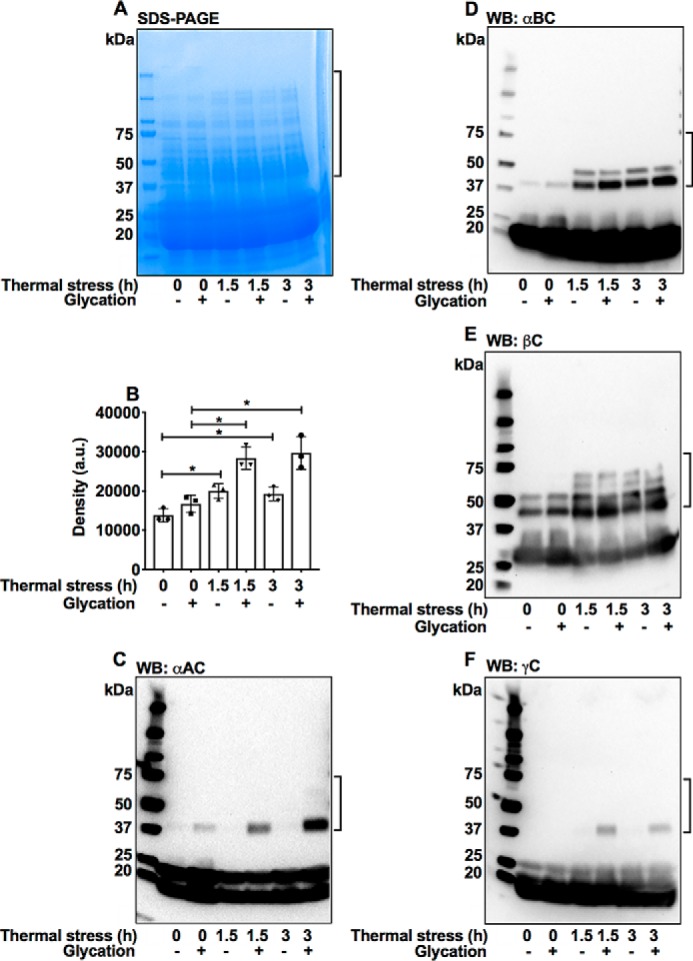
AGE-mediated protein cross-linking is higher in mouse lenses that were thermally stressed (50 °C) and glycated than in glycated or thermally stressed lenses. Lenses were glycated using a mixture of 25 mm glucose, 500 μm erythrulose, and 500 μm MGO in serum-free MEM for 3 days at 37 °C in a CO2 incubator. Water-soluble protein from mouse lenses (50 μg) was analyzed by SDS-PAGE using 4–20% gradient gels (A). The bar graph represents means ± S.D. of densitometric analyses of the intermolecular cross-linked proteins (in brackets) from triplicate measurements (B). Western blots (WB) for αAC (C), αBC (D), βC (E), and γC (F) reveal formation of high-molecular-weight covalently–cross-linked proteins, indicated by the brackets. *, p < 0.05.
Western blottings showed that αAC, αBC, β-crystallin (βC), and γ-crystallin (γC) become cross-linked by the combination of thermal stress and glycation in mouse lenses (Fig. 9, C–F). One observation to note is that in Western blottings the thermal stress alone induced nondisulfide intermolecular cross-linking of proteins. This could be due to glycation-mediated cross-linking by the pre-existing precursors in the lens, such as MGO and ascorbate oxidation products.
Thermally stressed and glycated lenses exhibit greater levels of cross-linking AGEs than glycated mouse lenses
Four cross-linking AGEs (MOLD, MODIC, GOLA, and pentosidine) were quantified in lens proteins. The levels of these AGEs were higher in lenses that were thermally stressed (1.5 h) and glycated than in lenses that were only glycated; the levels of MOLD, MODIC, GOLA, and pentosidine were 0.079 ± 0.016, 3.91 ± 0.5, 7.8 ± 2, and 0.33 ± 0.052 pmol/μmol leucine equivalent in thermally stressed and glycated lens proteins compared with 0.05 ± 0.03, 2.1 ± 0.8, 2.4 ± 1.1, and 0.175 ± 0.048 pmol/μmol leucine equivalent in just glycated lens proteins (Fig. 10). Furthermore, higher levels of cross-linking AGEs were observed in lenses subjected to 3 h of thermal stress and glycation (Fig. 10). Together, the results in Figs. 9 and 10 strongly suggest that the chaperone–client complexes that formed as a result of thermal stress underwent intermolecular protein cross-linking by AGEs.
Figure 10.
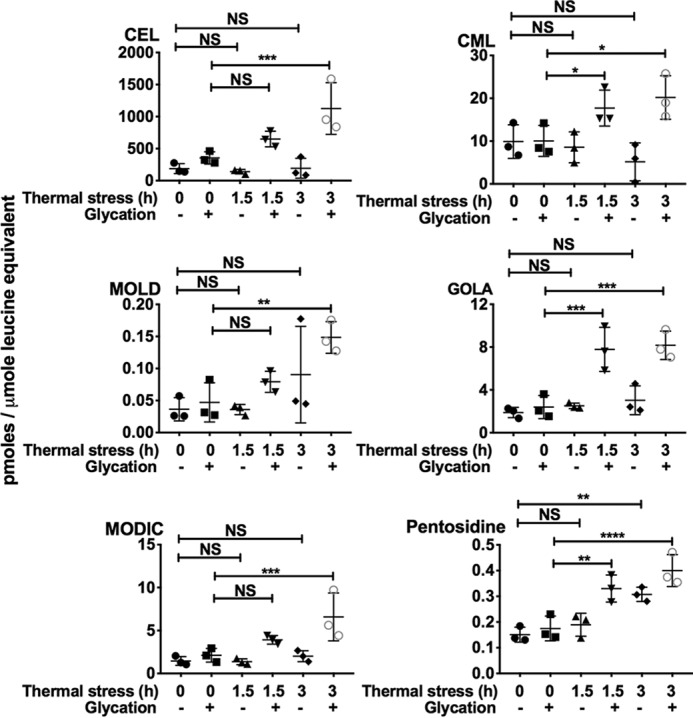
Cross-linking AGE levels are higher in mouse lenses that were thermally stressed (50 °C) and glycated. Lenses were glycated using a mixture of 25 mm glucose, 500 μm erythrulose, and 500 μm MGO in serum-free MEM for 3 days at 37 °C in a CO2 incubator. LC-MS/MS quantification of AGEs in WS protein from mouse lenses that were subjected to thermal stress and/or glycation. The bars represent means ± S.D. for three independent experiments. NS, not significant; *, p < 0.05; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Thermal stress and glycation increase mouse lens stiffness
The axial and equatorial strain in mouse lenses was unaltered upon glycation alone (Fig. 11, A and B). However, thermal stress at 50 °C (1.5 or 3 h) significantly reduced the axial strain (by 46 and 49%) and equatorial strain (by 61 and 58%). The combination of thermal stress and glycation further reduced the axial strain (by 57 and 58%) and equatorial strain (by 78 and 76%). These results suggest that AGE-mediated cross-linking of the α-crystallin–client complexes could increase lens stiffness and contribute to presbyopia.
Figure 11.
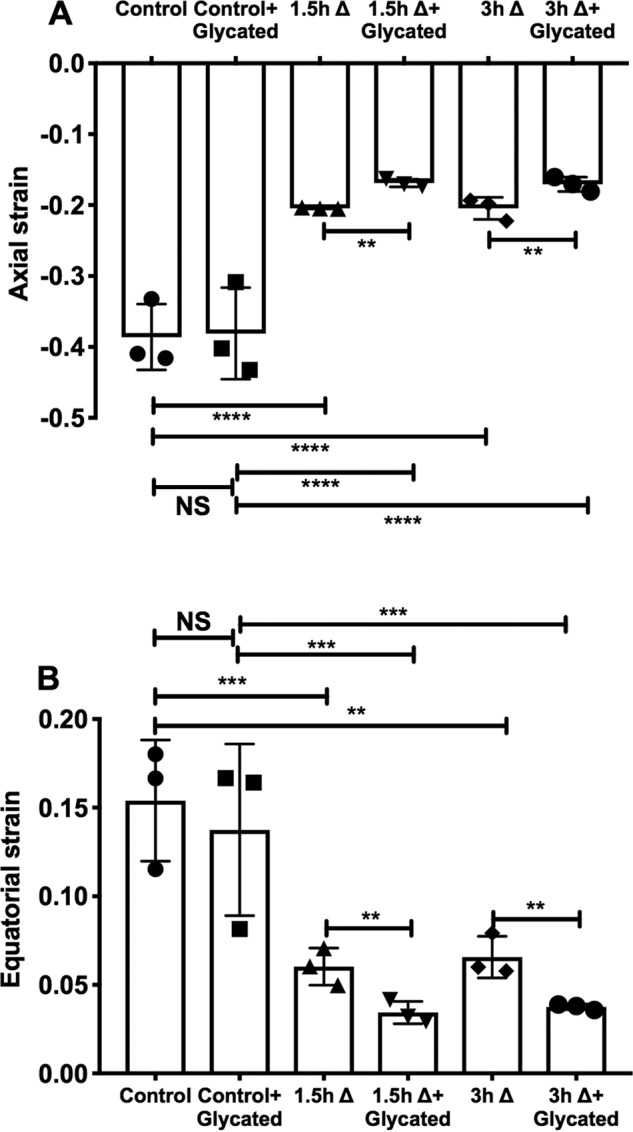
Stiffness increases in mouse lenses subjected to thermal stress and glycation. Mouse lenses (3–4 months, C57BL/6J mouse) in serum-free MEM were subjected to thermal stress (Δ) at 50 °C for 1.5 or 3 h. Subsequently, they were glycated using a mixture of 25 mm glucose, 500 μm erythrulose, and 500 μm MGO for 3 days at 37 °C in a CO2 incubator. Control experiments with lenses that were either thermally stressed or glycated alone were included. Axial (A) and equatorial (B) compressive strains are plotted against a fixed applied load. The bar graphs represent the means ± S.D. of triplicate measurements. NS, not significant; **, p < 0.01; ***, p < 0.001; ****, p < 0.0001.
Both cortical and nuclear proteins of human lens are AGE-modified upon glycation
We tested whether glycation of organ-cultured lenses leads to AGE formation in just cortical proteins or both cortical and nuclear proteins. We used human lenses in these experiments, as mouse lenses are difficult to separate into cortical and nuclear fractions. Freshly-obtained human lenses (donor age: 43, 44, and 48 years) were incubated for 3 h with serum-free MEM and then incubated with or without a glycation mixture (25 mm glucose, 500 μm MGO, and 500 μm erythrulose) for 3 days in a CO2 incubator. Lenses were then weighed, and the capsule was removed and stirred for 2 h in 10 ml of PBS at 4 °C in a 50-ml flask using a magnetic stirrer. The undissolved nucleus was weighed and homogenized in 3 ml of PBS using a hand homogenizer and centrifuged at 21,000 × g for 20 min at 4 °C. The soluble material from the lens after stirring (from lens cortex) was also centrifuged at 21,000 × g for 20 min at 4 °C. The supernatant protein from the nucleus and cortex (50 μg each) was subjected to Western blotting for CML and methylglyoxal-derived hydroimidazolone-1 (MGH-1). The dilutions were 1:5,000 for the mouse monoclonal anti-CML antibody (from Jes Thorn Clausen, Novo Nordisk, Germany) and 1:1,000 for the mouse monoclonal anti-MGH-1 antibody (generated in our laboratory). Secondary antibody used was HRP-conjugated anti-mouse IgG (diluted 1:5,000). Fig. S1 shows that lenses that were treated with the glycation mixture had 22.3 and 13.5% higher levels of MGH-1 and CML in the cortical proteins, and 22.7 and 24.5% higher in the nuclear proteins when compared with those in untreated lenses. These results suggest that both cortical and nuclear proteins can be AGE-modified by glycating agents and that the increased stiffness observed in mouse lenses upon glycation could be due to AGE modification of both cortical and nuclear proteins.
Discussion
The objectives of this study were as follows: 1) to investigate whether α-crystallin–client protein complexes are stable in human lenses; 2) to test whether α-crystallin–client protein complexes are more prone to intermolecular nondisulfide cross-linking by AGEs; and 3) to determine whether the accumulation of cross-linking AGEs is related to increase in lens stiffness.
αAC and αBC constitute ∼40% of total proteins in human lenses (27). They prevent aggregation of client proteins in the lens, thereby keeping the lens proteins soluble and maintaining lens transparency. α-Crystallin binds to aggregation-prone proteins, possibly through a “holdase” mechanism that does not require ATP hydrolysis and prevents further denaturation of the protein (28). Large heat-shock proteins bind to denaturing client proteins in an ATP-dependent manner and use ATP to dissociate chaperone–client complexes, resulting in the release of repaired client proteins (29, 30). Whether human lenses have such a mechanism to dissociate α-crystallin–client complexes is not known. Our FRET assay showed that under conditions of the human lens, the αAC–γDC complex was stable up to 12 days, the time period we tested. Addition of lens epithelial cell lysate, human lens homogenate or ATP did not cause a dissociation of that complex. This supports the idea that there is no mechanism to dissociate α-crystallin–client complexes in human lenses. This idea is further bolstered by the fact that α-crystallin cannot itself renature denatured client proteins. A recent study showed that BAG-3 mediates the association of client protein-bound small heat-shock proteins with a large heat-shock protein and such association helps in renaturation of the client protein (31). However, in that study, the release of the bound client protein was not demonstrated. Furthermore, this mechanism has not been demonstrated in the lens. Mogk et al. (32) demonstrated the role of ClpB/DnaK in the release of denatured client proteins bound to small heat-shock proteins, after which they can be renatured by the large heat-shock protein machinery. In the lens, large heat-shock proteins such as HSP70 are mostly present in the metabolically-active anterior epithelium, and possibly the outermost cortical fiber cells. The bulk of the lens comprises metabolically-inactive fiber cells. Therefore, HSP70-mediated dissociation of α-crystallin–client complexes in the inner regions of the lens is unlikely. Wang and Spector (33) demonstrated that ATP could alter conformation of α-crystallin–client complexes and trigger the release of denatured proteins, subsequently allowing the large heat-shock proteins to renature client proteins. In contrast, Biswas and Das (28) demonstrated that ATP improves chaperone activity of α-crystallin. Thus, it remains debatable whether ATP alone can dissociate α-crystallin–client protein complexes. Wang and Spector (34) showed that α-crystallin–bound enzyme can be activated and released in the presence of rabbit reticulocyte lysate and ATP, but such a mechanism is unlikely to occur in the lens. Moreover, there is no experimental evidence to date for the release of client proteins from the α-crystallin–client protein complex in the human lens. Thus, it is reasonable to assume that α-crystallin–client complexes formed especially in the inner fiber cells of human lenses are unlikely to dissociate and remain permanently tethered.
Human lenses accumulate several chemical modifications with aging. AGE modification is one of the dominant ones, and it can cross-link proteins and form HMW protein aggregates (35). AGE-mediated cross-linking is largely dependent on the proximity and concentrations of cross-linking components (amino groups) in a protein. We hypothesized that formation of α-crystallin–client complexes might increase the effective concentrations of critical components for glycation-mediated cross-linking of proteins. Our results clearly showed that α-crystallin–client complexes are susceptible to intermolecular cross-linking by glycation. We used thermal stress in this study to enhance formation of α-crystallin–client complexes. In human lenses, such complexes occur naturally during aging (26, 36).
Mass spectrometric analyses showed greater levels of cross-linking AGEs in glycated α-crystallin–client complexes than in glycated α-crystallin and client proteins that were not present in complex. Together, these data provide a strong basis for the notion that in the absence of any dissociation mechanism the proximity of α-crystallin and client proteins provides an ideal situation for AGE-mediated cross-linking. Furthermore, it is possible that the progressive accumulation of such cross-linked proteins leads to protein insolubilization. This could be the reason why AGE levels are higher in water-insoluble proteins than in water-soluble proteins of human lenses (6, 18, 19). Unlike disulfide bonds, inter-protein cross-linking by AGEs is permanent and could explain why addition of reducing agents cannot completely solubilize the highly–cross-linked water-insoluble proteins of aged and cataractous lenses (37).
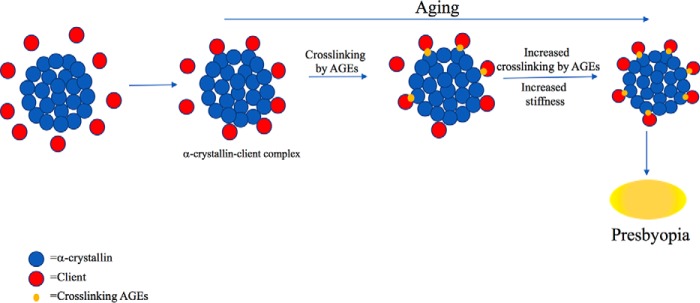
The age-associated increase in lens stiffness has been documented in many studies and has been implicated in the onset of presbyopia (38, 39). It has been established that the concentration of free α-crystallin, especially in the human lens nucleus, decreases with age as it becomes insoluble and is incorporated into HMW protein aggregates (26, 40). Whether loss of soluble chaperone protein is the primary trigger for presbyopia needs to be investigated. Many studies have implicated disulfide bond formation due to increased oxidative stress in the genesis of such protein aggregates (41, 42). Although there is compelling evidence for such a mechanism, our findings in this study offer an additional mechanism through AGE formation. A recent study showed that thermal stress promotes lens stiffness by enhancing aggregation of proteins (38). Whether promotion of AGE formation from in situ precursors is the reason for the increase in stiffness in that study has to be determined. Our human lens data in this study clearly establishes a positive correlation between cross-linking AGEs and age-associated lens stiffness. Furthermore, we demonstrated that mouse lenses subjected to combined thermal stress and glycation accumulate more nondisulfide cross-linked proteins than lenses subjected to either glycation or thermal stress alone. We also showed that the increase in cross-linked proteins by combined thermal stress and glycation is accompanied by an increase in cross-linking AGEs both in α-crystallin–client complexes and in mouse lenses. More importantly, we showed that mouse lenses subjected to thermal stress and glycation were less resilient than those subjected to thermal stress or glycation alone. Thus, our study provides a biochemical mechanism for the onset of presbyopia in humans (Fig. 12).
Figure 12.
Schematic representation of AGE-mediated cross-linking of α-crystallin–client protein complexes during aging leading to stiffening of the lens and presbyopia.
Given our findings in this study, an important question arises: is the chaperone activity of α-crystallin beneficial or detrimental to the lens, especially in aged lenses? It is likely that chaperone activity is essential to prevent protein aggregation and maintain transparency in aging lenses. This beneficial activity may have a negative consequence after a certain age, possibly after the 4th or 5th decade in humans, when lens defenses such as GSH and antioxidant enzymes become deficient or weak (12, 43). This could lead to reduced MGO metabolism by glyoxalase and enhanced oxidation of ascorbate to highly-reactive glycation precursors. In fact, these changes have been documented in aged human lenses (44, 45). The lack of dissociation mechanisms for the α-crystallin–client protein complexes could thus create a milieu in aged lenses favoring AGE-mediated cross-linking of α-crystallin–client protein complexes. Therefore, it is tempting to speculate that chaperone activity is beneficial in early life but could have a negative connotation later in life. The loss and gain of chaperone activity as a result of chemical modifications (46–50) could also dictate the kinetics of AGE-mediated cross-linking of α-crystallin–client protein complexes. The inter-protein association through disulfide bonds, which has been well-documented in human lenses, could also predispose them to AGE-mediated cross-linking, similar to α-crystallin–client complexes. This possibility needs to be investigated in future studies. It should be noted that we quantified only four protein cross-linking AGEs. We did not measure other known cross-linking AGEs in the lens such as vesperlysine (19) and K2P (16). Moreover, the human lens is expected to have many additional uncharacterized AGEs (51). Therefore, AGE-mediated cross-linking of α-crystallin–client complexes could be even more important than what is appreciated in this study. Finally, the proposed mechanism in this study might also explain the early onset of presbyopia in diabetics (52) because of higher levels of cross-linking AGEs than in nondiabetics (19).
Experimental procedures
Materials
CS (catalog no. C3260), ADH (catalog no. A7011), glucose (catalog no. G7528), MGO (catalog no. 67028), erythrulose (catalog no. 56845), Sephadex G-25 (catalog no. G25150), EDTA disodium salt dehydrate (catalog no. E5134), Pronase E (catalog no. P-5147), leucine aminopeptidase (catalog no. L5006), and carboxypeptidase Y (catalog no. C3888) were obtained from Sigma. LYI (catalog no. L1338) and AIAS (catalog no. A484) were obtained from Invitrogen. The antibody for αAC (catalog no. ADI-SPA-221-F) was obtained from Enzo Life Sciences (Farmingdale, NY). The antibody for αBC (catalog no. ABN185) was from Millipore-Sigma (Burlington, MA). Antibodies for βC (catalog no. SC22745) and γC (catalog no. SC22746) were from Santa Cruz Biotechnology (Dallas, TX). The CS antibody (catalog no. 14309S) and anti-rabbit IgG (catalog no. 7074S) were from Cell Signaling Technology (Danvers, MA), and the ADH antibody (catalog no. 200-4143S) was from Rockland Antibodies (Limerick, PA). All other chemicals were of analytical grade.
Measurement of lens stiffness
Human lenses (donor age: 13–75 years, n = 17) were obtained from Saving Sight, Kansas City, MO, and were stored at −80 °C until use. Stiffness was measured in thawed lenses using a setup described by Cheng et al. (53) with slight modifications. Briefly, the lens chamber was filled with 65–70 ml of PBS. A right-angled mirror was positioned for the side view of the lenses. Side-view images were captured using a dissecting microscope attached to a camera and computer. Lenses were pictured before placing a chamber slide on them. To increase the load on the lens, a premeasured volume of water was added using a pipette. Total load added was calculated by multiplying the volume of water added with the density of water. Lens stiffness was measured using a load of 2,030 mg. The axial diameter before and after loading a fixed volume of water was measured using ZEN 2.3 lite software (Carl Zeiss Microscopy, LLC, White Plains, NY). Axial strain was calculated as described previously (53).
LC-MS/MS measurement of AGEs
Samples were hydrolyzed with acid or enzymes, as described previously (14), with some minor modifications. For acid hydrolysis, samples were suspended in 6 n HCl and incubated at 110 °C for 16 h under argon in sealed glass ampules. The hydrolyzed samples were dried in a vacuum concentrator and suspended in 50 μl of water. For enzyme hydrolysis, protein solutions in PBS were incubated with 0.1 unit of Pronase E for 8 h followed by another addition of Pronase E for 16 h, 0.3 unit of leucine aminopeptidase for 8 h, and 0.3 unit of carboxypeptidase Y for 16 h. The enzyme-digested samples were passed through a 3-kDa molecular mass cutoff filter. The samples (acid- and enzyme-hydrolyzed) were analyzed by LC-MS/MS using the standard addition method, and the concentrations of AGEs were expressed relative to amino acid content (leucine equivalent, determined by the ninhydrin assay), as described previously (14). The LC-MS system consisted of a Waters Acquity UPLC system (Milford, MA) connected to a Sciex 4500 QTrap mass spectrometer (Redwood City, CA). Chromatographic separation was performed at 40 °C on a Waters Acquity HSS T3 1.8 μm 2.1 × 100-mm column using a gradient program at 0.6 ml/min consisting of solvent A = water and solvent B = 80% (v/v) acetonitrile, each containing 0.12% heptafluorobutyric acid. The gradient program was as follows: 2% B (0–2.2 min) to 8% B (3.3 min) to 34% B (7.6 min) to 100% B (7.8–9.5 min), after which the column was re-equilibrated with 2% B prior to the next analysis. For mass spectrometric detection, the scheduled multiple-reaction monitoring mode was used: utilizing collision-induced dissociation of the protonated molecules with compound-specific orifice potentials and fragment-specific collision energies (Table 1). Ion source parameters were as follows: temperature, 650 °C; ion spray voltage, 2.5 kV; curtain gas, 35 ml/min; nebulizer gas, 65 ml/min; heating gas, 70 ml/min. The MRM parameters for AGEs were measured in a positive-ion mode.
Table 1.
Mass spectrometric parameters for AGE quantification
CE, collision energy; CXP, collision cell exit potential; DP, declustering potential; amu, atomic mass unit.
| AGE | Retention time | Precursor ion |
Product ion 1a |
Product ion 2b |
Product ion 3b |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| m/z | DP | m/z | CE | CXP | m/z | CE | CXP | m/z | CE | CXP | ||
| min | amu | V | amu | eV | V | amu | eV | V | amu | eV | V | |
| CML | 3 | 205.1 | 40 | 130.2 | 17 | 11 | 84.1 | 25 | 13 | 56.1 | 50 | 10 |
| CEL | 4.8 | 219.1 | 54 | 84.1 | 33 | 11 | 130.1 | 18 | 12 | 56.1 | 59 | 8 |
| GOLA | 6.9 | 333.2 | 40 | 84.3 | 54 | 11 | 169.1 | 26 | 12 | 130.2 | 32 | 9 |
| Glucosepanec | 6.9/7.1 | 429.3 | 20 | 384.5 | 38 | 19 | 269.2 | 55 | 20 | 339.2 | 55 | 20 |
| GOLD | 7 | 327.2 | 60 | 84.1 | 55 | 13 | 282.3 | 29 | 11 | 198.1 | 25 | 19 |
| GODIC | 7.1 | 343.3 | 22 | 298.4 | 31 | 17 | 183.2 | 44 | 13 | 70.2 | 74 | 11 |
| MOLD | 7.3 | 341.3 | 45 | 296.3 | 33 | 18 | 84.2 | 52 | 14 | 212.3 | 29 | 21 |
| MODIC | 7.3 | 357.3 | 25 | 312.2 | 31 | 14 | 267.3 | 45 | 15 | 197.4 | 45 | 14 |
a MRM transition was used for quantitation (quantifier).
b MRM transition was used for confirmation (qualifier).
c Two diastereomeric compounds of glucosepane are present in the human lens.
Measurement of pentosidine
Proteins were acid-hydrolyzed and suspended in water as above. The acid-hydrolyzed material was analyzed for pentosidine using UPLC and a fluorescence detector. The column was a Waters Acquity HSS T3 1.8 μm 2.1 × 100 mm. A linear gradient program was used with solvent A: 100% water with 0.12% heptafluorobutyric acid, and solvent B: 60% acetonitrile with 0.12% heptafluorobutyric acid. The program was as follows: 0–5 min: 10% B; 5–7 min: 30% B; 7–7.5 min: 70% B; 7.5–10 min: 100% B; and 10–12.9 min: 10% B, at a flow rate of 0.5 ml/min. The column eluate was monitored in a fluorescence detector (excitation/emission wavelengths = 335/385 nm). Pentosidine content in samples was calculated based on the standard curve generated using a synthetic pentosidine and expressed as picomoles/μmol of leucine equivalent.
Labeling of recombinant αA- and γD-crystallin using fluorophores
Recombinant human αA- and γD-crystallin were purified as described previously (54, 55). The cysteine residues in αAC and γDC were tagged with LYI and AIAS, respectively, as described previously (56). Labeled LYI–αAC and AIAS–γDC were purified by passing through a Sephadex G-25 column equilibrated with 50 mm phosphate buffer, 100 mm NaCl, pH 7.4. The fluorescent peak containing the labeled protein was collected and dialyzed against 50 mm phosphate buffer, pH 7.4, for 16 h at 4 °C. The extent of labeling of the fluorophores was calculated as described previously (56).
Measurement of chaperone activity
γDC or AIAS–γDC (0.1 mg/ml) was incubated at 70 °C in the presence or absence of αAC (0.1 mg/ml) or LYI–αAC (0.1 and 0.3 mg/ml) in 50 mm phosphate buffer containing 1 mm EDTA, pH 7.4. Light scattering at 400 nm was monitored in the kinetic mode for 0.5 h in a UV spectrophotometer.
FRET assay
FRET was employed to determine the complex formation between LYI–αAC and AIAS–γDC. FRET was initiated by incubating LYI–αAC and AIAS–γDC as described in the chaperone activity assay above. The emission spectrum of the incubation mixture before and after thermal stress was measured in a spectrofluorometer (Spectramax-4, Horiba Jobin Mayer, Edison, NJ). An excitation wavelength of 335 nm was used to measure the emission spectra from 350 to 600 nm. The excitation and emission bandpasses were 2.5 and 5 nm, respectively.
Testing the stability of LYI–αAC and AIAS–γDC complex
Stability of the LYI–αAC-AIAS–γDC complex was tested (0.2 mg/ml) over a period of 12 days in the presence or absence of ATP (2 mm) or human lens homogenate (300 μl, 6 mg of protein/ml) from a young (17 years) or an aged donor (75 years) or human lens epithelial cell (FHL124) lysate (300 μl, 1 mg of protein/ml). FHL124 cells were from Dr. Michael Wormstone, University of East Anglia, UK, and cultured as described previously (57). Incubations were performed at 37 °C under sterile conditions in 50 mm phosphate buffer, pH 7.4. Samples were collected from the incubation mixtures at 0, 4, 8, and 12 days, and FRET was measured at excitation/emission wavelengths of 335/525 nm.
Generation and purification of α-crystallin–client protein complexes
Human αAC and αBC were cloned, bacterially expressed, and purified as described previously (58). Mixtures containing αAC or αBC and ADH were incubated at 25, 37, or 49 °C in 50 mm phosphate buffer, 100 mm NaCl, pH 7.4. Mixtures containing αAC or αBC and CS in 10 mm HEPES, pH 7.4, were incubated at 25, 34, or 43 °C. The ratios of αAC/ADH and αBC/ADH were 1:2 and 1:3 (w/w), respectively. These ratios were chosen for total inhibition of client protein aggregation by α-crystallin. The ratios of αAC/CS and αBC/CS were both 1:0.35 (w/w). The protein mixtures were dialyzed against 50 mm phosphate buffer, pH 7.4, for 16 h at 4 °C.
Purification of the α-crystallin–client protein complex was performed on a FPLC instrument (NGC Chromatography System, Bio-Rad) equipped with an analytical gel-filtration column (Enrich SEC-650, 10 × 300 mm, 24 ml). The column was equilibrated with 50 mm phosphate buffer, pH 7.4. Five hundred microliters of α-crystallin–client protein mixture was then loaded into the column and eluted using 50 mm phosphate buffer, pH 7.4, at a flow rate of 0.7 ml/min, and the eluent was monitored at 280 nm. LYI–αAC, AIAS–γDC, and the complex of LYI–αAC and AIAS–γDC (formed by incubating at 70 °C for 1 h) were similarly purified on FPLC.
Glycation of α-crystallin–client protein complexes
The α-crystallin–client protein complexes purified as described above were glycated with either 200 μm MGO or 250 μm erythrulose in 50 mm phosphate buffer, pH 7.4, at 37 °C for 7 days. Following incubation, the samples were dialyzed against PBS for 16 h at 4 °C.
Thermal stress and glycation in mouse lenses
All animal experiments were reviewed and approved by the University of Colorado, Aurora's Institutional Animal Care and Use Committee (IACUC), and performed under adherence to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Lenses from C57BL/6J mice (3–4 months old) were isolated from eyes and incubated at 50 °C for 1.5 or 3 h with serum and phenol-red-free MEM in a CO2 incubator. Control lenses were incubated at 37 °C. Lenses were then glycated with a mixture of 25 mm glucose, 500 μm MGO, and 500 μm erythrulose for 3 days at 37 °C in serum-free MEM in a CO2 incubator. Lenses incubated simultaneously without glycating mixture served as controls. Lens stiffness was measured as above. Lenses were then homogenized in PBS, and the water-soluble fraction was isolated by centrifugation at 20,000 × g for 30 min at 4 °C. Water-soluble protein was subjected to SDS-PAGE on 4–20% gradient gels under reducing conditions. To measure AGEs in proteins, the samples were processed and analyzed by LC-MS/MS as described above.
Western blotting detection of crystallin cross-linking
For Western blotting, water-soluble protein (50 μg) from mouse lenses was separated by SDS-PAGE on 4–20% gradient gels under reducing conditions and then electrophoretically transferred to a nitrocellulose membrane. The membrane was incubated overnight at 4 °C with one of the following antibodies: αAC antibody (diluted 1:5,000); αBC antibody (diluted 1:5,000); βC antibody (diluted 1:2,000), or γC antibody (diluted 1:2,000). The membrane was then incubated with HRP-conjugated anti-rabbit IgG (diluted 1:5,000) for 1 h at room temperature. For Western blotting of thermally stressed and glycated α-crystallin–client protein complexes (15 μg), the primary antibody (incubated overnight at 4 °C) dilutions for αAC and αBC are the same as those used above. The dilution for the CS antibody was 1:5,000 and that for the ADH antibody was 1:4,000.
Statistical analysis
All data are means ± S.D. and are representative of at least three independent experiments. Statistical significance of the data was analyzed using one-way ANOVA with Dunnett's multiple comparison tests using GraphPad Prism 7 software. A p value ≤0.05 was considered statistically significant.
Author contributions
S. K. N., R. B. N., J. R., and R. H. N. data curation; S. K. N. and R. H. N. formal analysis; S. K. N., R. B. N., J. R., and R. H. N. investigation; S. K. N. and M. A. G. methodology; S. K. N., R. B. N., J. R., M. A. G., and R. H. N. writing-original draft; S. K. N., R. B. N., J. R., M. A. G., and R. H. N. writing-review and editing; R. H. N. conceptualization; R. H. N. supervision; R. H. N. funding acquisition; R. H. N. project administration.
Supplementary Material
Acknowledgments
We thank Dr. Omid Masihzadeh for help with the setup of lens stiffness test equipment, Drs. Stefan Rakete and Christian Henning for help with the initial set up of LC-MS/MS analyses, and Dr. Mi-hyun Nam for critical reading of the manuscript. A Research to Prevent Blindness, NY Challenge Grant was awarded to the Department of Ophthalmology, University of Colorado.
This work was supported by National Institutes of Health Grants EY028836 and EY023286 (to R. H. N.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Fig. S1.
- MGO
- methylglyoxal
- AGE
- advanced glycation end product
- LYI
- Lucifer Yellow iodoacetamide
- AIAS
- 4-acetamido-4′-((iodoacetyl)amino)-stilbene-2,2′-disulfonic acid
- αAC
- αA-crystallin
- αBC
- αB-crystallin
- βC
- β-crystallin
- γC
- γ-crystallin
- γDC
- γD-crystallin
- HMW
- high molecular weight
- ADH
- alcohol dehydrogenase
- CS
- citrate synthase
- SEC
- size-exclusion chromatography
- CEL
- N(6)-carboxyethyl-lysine
- CML
- N(6)-carboxymethyl-lysine
- ERT
- erythrulose
- MOLD
- methylglyoxal lysine dimer
- MGH-1
- methylglyoxal-derived hydroimidazolone-1
- GOLD
- glyoxal lysine dimer
- GOLA
- glyoxal lysine amide
- MODIC
- methylglyoxal-derived imidazoline cross-link
- GODIC
- glyoxal-derived imidazoline cross-link
- MRM
- multiple-reaction monitoring
- FPLC
- fast-protein-liquid chromatography
- HRP
- horseradish peroxidase
- MEM
- minimal essential medium.
References
- 1. Heys K. R., Friedrich M. G., and Truscott R. J. (2008) Free and bound water in normal and cataractous human lenses. Invest. Ophthalmol. Vis. Sci. 49, 1991–1997 10.1167/iovs.07-1151 [DOI] [PubMed] [Google Scholar]
- 2. Gakamsky A., Duncan R. R., Howarth N. M., Dhillon B., Buttenschön K. K., Daly D. J., and Gakamsky D. (2017) Tryptophan and non-tryptophan fluorescence of the eye lens proteins provides diagnostics of cataract at the molecular level. Sci. Rep. 7, 40375 10.1038/srep40375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hains P. G., and Truscott R. J. (2010) Age-dependent deamidation of lifelong proteins in the human lens. Invest. Ophthalmol. Vis. Sci. 51, 3107–3114 10.1167/iovs.09-4308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hooi M. Y., and Truscott R. J. (2011) Racemisation and human cataract. d-Ser, d-Asp/Asn and d-Thr are higher in the lifelong proteins of cataract lenses than in age-matched normal lenses. Age 33, 131–141 10.1007/s11357-010-9171-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kodama T., and Takemoto L. (1988) Characterization of disulfide-linked crystallins associated with human cataractous lens membranes. Invest. Ophthalmol. Vis. Sci. 29, 145–149 [PubMed] [Google Scholar]
- 6. Nagaraj R. H., Linetsky M., and Stitt A. W. (2012) The pathogenic role of Maillard reaction in the aging eye. Amino Acids 42, 1205–1220 10.1007/s00726-010-0778-x [DOI] [PubMed] [Google Scholar]
- 7. Sharma K. K., and Santhoshkumar P. (2009) Lens aging: effects of crystallins. Biochim. Biophys. Acta 1790, 1095–1108 10.1016/j.bbagen.2009.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smuda M., and Glomb M. A. (2013) Maillard degradation pathways of vitamin C. Angew Chem. Int. Ed. Engl. 52, 4887–4891 10.1002/anie.201300399 [DOI] [PubMed] [Google Scholar]
- 9. Nemet I., and Monnier V. M. (2011) Vitamin C degradation products and pathways in the human lens. J. Biol. Chem. 286, 37128–37136 10.1074/jbc.M111.245100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rabbani N., Ashour A., and Thornalley P. J. (2016) Mass spectrometric determination of early and advanced glycation in biology. Glycoconj. J. 33, 553–568 10.1007/s10719-016-9709-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rabbani N., and Thornalley P. J. (2012) Glycation research in amino acids: a place to call home. Amino Acids 42, 1087–1096 10.1007/s00726-010-0782-1 [DOI] [PubMed] [Google Scholar]
- 12. Harding J. J. (1970) Free and protein-bound glutathione in normal and cataractous human lenses. Biochem. J. 117, 957–960 10.1042/bj1170957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vander Jagt D. L., Hassebrook R. K., Hunsaker L. A., Brown W. M., and Royer R. E. (2001) Metabolism of the 2-oxoaldehyde methylglyoxal by aldose reductase and by glyoxalase-I: roles for glutathione in both enzymes and implications for diabetic complications. Chem. Biol. Interact. 130, 549–562 10.1016/s0009-2797(00)00298-2 [DOI] [PubMed] [Google Scholar]
- 14. Smuda M., Henning C., Raghavan C. T., Johar K., Vasavada A. R., Nagaraj R. H., and Glomb M. A. (2015) Comprehensive analysis of maillard protein modifications in human lenses: effect of age and cataract. Biochemistry 54, 2500–2507 10.1021/bi5013194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Biemel K. M., Friedl D. A., and Lederer M. O. (2002) Identification and quantification of major maillard cross-links in human serum albumin and lens protein. Evidence for glucosepane as the dominant compound. J. Biol. Chem. 277, 24907–24915 10.1074/jbc.M202681200 [DOI] [PubMed] [Google Scholar]
- 16. Cheng R., Feng Q., Argirov O. K., and Ortwerth B. J. (2005) K2P–a novel cross-link from human lens protein. Ann. N.Y. Acad. Sci. 1043, 184–194 10.1196/annals.1333.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lyons T. J., Silvestri G., Dunn J. A., Dyer D. G., and Baynes J. W. (1991) Role of glycation in modification of lens crystallins in diabetic and nondiabetic senile cataracts. Diabetes 40, 1010–1015 10.2337/diab.40.8.1010 [DOI] [PubMed] [Google Scholar]
- 18. Nagaraj R. H., Sell D. R., Prabhakaram M., Ortwerth B. J., and Monnier V. M. (1991) High correlation between pentosidine protein cross-links and pigmentation implicates ascorbate oxidation in human lens senescence and cataractogenesis. Proc. Natl. Acad. Sci. U.S.A. 88, 10257–10261 10.1073/pnas.88.22.10257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tessier F., Obrenovich M., and Monnier V. M. (1999) Structure and mechanism of formation of human lens fluorophore LM-1. Relationship to vesperlysine A and the advanced Maillard reaction in aging, diabetes, and cataractogenesis. J. Biol. Chem. 274, 20796–20804 10.1074/jbc.274.30.20796 [DOI] [PubMed] [Google Scholar]
- 20. Bron A. J., Vrensen G. F., Koretz J., Maraini G., and Harding J. J. (2000) The ageing lens. Ophthalmologica 214, 86–104 10.1159/000027475 [DOI] [PubMed] [Google Scholar]
- 21. de Jong W. W., Mulders J. W., Voorter C. E., Berbers G. A., Hoekman W. A., and Bloemendal H. (1988) Post-translational modifications of eye lens crystallins: cross-linking, phosphorylation and deamidation. Adv. Exp. Med. Biol. 231, 95–108 10.1007/978-1-4684-9042-8_8 [DOI] [PubMed] [Google Scholar]
- 22. Cheng R., Lin B., Lee K. W., and Ortwerth B. J. (2001) Similarity of the yellow chromophores isolated from human cataracts with those from ascorbic acid-modified calf lens proteins: evidence for ascorbic acid glycation during cataract formation. Biochim. Biophys. Acta 1537, 14–26 10.1016/S0925-4439(01)00051-5 [DOI] [PubMed] [Google Scholar]
- 23. Srinivas P. N., Reddy P. Y., and Reddy G. B. (2008) Significance of α-crystallin heteropolymer with a 3:1 αA/αB ratio: chaperone-like activity, structure and hydrophobicity. Biochem. J. 414, 453–460 10.1042/BJ20080544 [DOI] [PubMed] [Google Scholar]
- 24. Horwitz J. (1992) α-Crystallin can function as a molecular chaperone. Proc. Natl. Acad. Sci. U.S.A. 89, 10449–10453 10.1073/pnas.89.21.10449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Horwitz J. (2003) α-Crystallin. Exp. Eye Res. 76, 145–153 10.1016/S0014-4835(02)00278-6 [DOI] [PubMed] [Google Scholar]
- 26. Srivastava K., Chaves J. M., Srivastava O. P., and Kirk M. (2008) Multi-crystallin complexes exist in the water-soluble high molecular weight protein fractions of aging normal and cataractous human lenses. Exp. Eye Res. 87, 356–366 10.1016/j.exer.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 27. Horwitz J., Bova M. P., Ding L. L., Haley D. A., and Stewart P. L. (1999) Lens α-crystallin: function and structure. Eye 13, 403–408 10.1038/eye.1999.114 [DOI] [PubMed] [Google Scholar]
- 28. Biswas A., and Das K. P. (2004) Role of ATP on the interaction of α-crystallin with its substrates and its implications for the molecular chaperone function. J. Biol. Chem. 279, 42648–42657 10.1074/jbc.M404444200 [DOI] [PubMed] [Google Scholar]
- 29. Beissinger M., and Buchner J. (1998) How chaperones fold proteins. Biol. Chem. 379, 245–259 [PubMed] [Google Scholar]
- 30. Bukau B., and Horwich A. L. (1998) The Hsp70 and Hsp60 chaperone machines. Cell 92, 351–366 10.1016/S0092-8674(00)80928-9 [DOI] [PubMed] [Google Scholar]
- 31. Rauch J. N., Tse E., Freilich R., Mok S. A., Makley L. N., Southworth D. R., and Gestwicki J. E. (2017) BAG3 is a modular, scaffolding protein that physically links heat-shock protein 70 (Hsp70) to the small heat-shock proteins. J. Mol. Biol. 429, 128–141 10.1016/j.jmb.2016.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mogk A., Deuerling E., Vorderwülbecke S., Vierling E., and Bukau B. (2003) Small heat-shock proteins, ClpB and the DnaK system form a functional triade in reversing protein aggregation. Mol. Microbiol. 50, 585–595 10.1046/j.1365-2958.2003.03710.x [DOI] [PubMed] [Google Scholar]
- 33. Wang K., and Spector A. (2001) ATP causes small heat-shock proteins to release denatured protein. Eur. J. Biochem. 268, 6335–6345 10.1046/j.0014-2956.2001.02580.x [DOI] [PubMed] [Google Scholar]
- 34. Wang K., and Spector A. (2000) α-Crystallin prevents irreversible protein denaturation and acts cooperatively with other heat-shock proteins to renature the stabilized partially denatured protein in an ATP-dependent manner. Eur. J. Biochem. 267, 4705–4712 10.1046/j.1432-1327.2000.01521.x [DOI] [PubMed] [Google Scholar]
- 35. Perry R. E., Swamy M. S., and Abraham E. C. (1987) Progressive changes in lens crystallin glycation and high-molecular-weight aggregate formation leading to cataract development in streptozotocin-diabetic rats. Exp. Eye Res. 44, 269–282 10.1016/S0014-4835(87)80011-8 [DOI] [PubMed] [Google Scholar]
- 36. Fu S. C., Su S. W., Wagner B. J., and Hart R. (1984) Characterization of lens proteins. IV. Analysis of soluble high molecular weight protein aggregates in human lenses. Exp. Eye Res. 38, 485–495 10.1016/0014-4835(84)90126-X [DOI] [PubMed] [Google Scholar]
- 37. Ortwerth B. J., and Olesen P. R. (1992) Studies on the solubilization of the water-insoluble fraction from human lens and cataract. Exp. Eye Res. 55, 777–783 10.1016/0014-4835(92)90004-C [DOI] [PubMed] [Google Scholar]
- 38. Heys K. R., Friedrich M. G., and Truscott R. J. (2007) Presbyopia and heat: changes associated with aging of the human lens suggest a functional role for the small heat-shock protein, α-crystallin, in maintaining lens flexibility. Aging Cell 6, 807–815 10.1111/j.1474-9726.2007.00342.x [DOI] [PubMed] [Google Scholar]
- 39. Heys K. R., Cram S. L., and Truscott R. J. (2004) Massive increase in the stiffness of the human lens nucleus with age: the basis for presbyopia? Mol. Vis. 10, 956–963 [PubMed] [Google Scholar]
- 40. Srivastava O. P., Kirk M. C., and Srivastava K. (2004) Characterization of covalent multimers of crystallins in aging human lenses. J. Biol. Chem. 279, 10901–10909 10.1074/jbc.M308884200 [DOI] [PubMed] [Google Scholar]
- 41. Serebryany E., Yu S., Trauger S. A., Budnik B., and Shakhnovich E. I. (2018) Dynamic disulfide exchange in a crystallin protein in the human eye lens promotes cataract-associated aggregation. J. Biol. Chem. 293, 17997–18009 10.1074/jbc.RA118.004551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yu N. T., DeNagel D. C., Pruett P. L., and Kuck J. F. Jr. (1985) Disulfide bond formation in the eye lens. Proc. Natl. Acad. Sci. U.S.A. 82, 7965–7968 10.1073/pnas.82.23.7965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xing K. Y., and Lou M. F. (2010) Effect of age on the thioltransferase (glutaredoxin) and thioredoxin systems in the human lens. Invest. Ophthalmol. Vis. Sci. 51, 6598–6604 10.1167/iovs.10-5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kisic B., Miric D., Zoric L., Ilic A., and Dragojevic I. (2012) Antioxidant capacity of lenses with age-related cataract. Oxid. Med. Cell. Longev. 2012, 467130 10.1155/2012/467130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mailankot M., Padmanabha S., Pasupuleti N., Major D., Howell S., and Nagaraj R. H. (2009) Glyoxalase I activity and immunoreactivity in the aging human lens. Biogerontology 10, 711–720 10.1007/s10522-009-9218-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gupta R., and Srivastava O. P. (2004) Deamidation affects structural and functional properties of human αA-crystallin and its oligomerization with αB-crystallin. J. Biol. Chem. 279, 44258–44269 10.1074/jbc.M405648200 [DOI] [PubMed] [Google Scholar]
- 47. Takata T., Nakamura-Hirota T., Inoue R., Morishima K., Sato N., Sugiyama M., and Fujii N. (2018) Asp-58 modulates lens αA-crystallin oligomer formation and chaperone function. FEBS J. 285, 2263–2277 10.1111/febs.14475 [DOI] [PubMed] [Google Scholar]
- 48. Rajan S., Horn C., and Abraham E. C. (2006) Effect of oxidation of αA- and αB-crystallins on their structure, oligomerization and chaperone function. Mol. Cell. Biochem. 288, 125–134 10.1007/s11010-006-9128-4 [DOI] [PubMed] [Google Scholar]
- 49. Nandi S. K., Nahomi R. B., Harris P. S., Michel C. R., Fritz K. S., and Nagaraj R. H. (2019) The absence of SIRT3 and SIRT5 promotes the acetylation of lens proteins and improves the chaperone activity of α-crystallin in mouse lenses. Exp. Eye Res. 182, 1–9 10.1016/j.exer.2019.02.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nandi S. K., Rakete S., Nahomi R. B., Michel C., Dunbar A., Fritz K. S., and Nagaraj R. H. (2019) Succinylation is a gain-of-function modification in human lens αB-crystallin. Biochemistry 58, 1260–1274 10.1021/acs.biochem.8b01053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cheng R., Feng Q., and Ortwerth B. J. (2006) LC-MS display of the total modified amino acids in cataract lens proteins and in lens proteins glycated by ascorbic acid in vitro. Biochim. Biophys. Acta 1762, 533–543 10.1016/j.bbadis.2006.01.009 [DOI] [PubMed] [Google Scholar]
- 52. Adnan, Efron N., Mathur A., Edwards K., Pritchard N., Suheimat M., and Atchison D. A. (2014) Amplitude of accommodation in type 1 diabetes. Invest. Ophthalmol. Vis. Sci. 55, 7014–7018 10.1167/iovs.14-15376 [DOI] [PubMed] [Google Scholar]
- 53. Cheng C., Gokhin D. S., Nowak R. B., and Fowler V. M. (2016) Sequential application of glass coverslips to assess the compressive stiffness of the mouse lens: strain and morphometric analyses. J. Vis. Exp. 2016, 10.3791/53986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. DiMauro M. A., Nandi S. K., Raghavan C. T., Kar R. K., Wang B., Bhunia A., Nagaraj R. H., and Biswas A. (2014) Acetylation of Gly1 and Lys2 promotes aggregation of human γD-crystallin. Biochemistry 53, 7269–7282 10.1021/bi501004y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nagaraj R. H., Panda A. K., Shanthakumar S., Santhoshkumar P., Pasupuleti N., Wang B., and Biswas A. (2012) Hydroimidazolone modification of the conserved Arg12 in small heat-shock proteins: studies on the structure and chaperone function using mutant mimics. PLoS ONE 7, e30257 10.1371/journal.pone.0030257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bova M. P., Ding L. L., Horwitz J., and Fung B. K. (1997) Subunit exchange of αA-crystallin. J. Biol. Chem. 272, 29511–29517 10.1074/jbc.272.47.29511 [DOI] [PubMed] [Google Scholar]
- 57. Nahomi R. B., Pantcheva M. B., and Nagaraj R. H. (2016) αB-crystallin is essential for the TGF-β2–mediated epithelial to mesenchymal transition of lens epithelial cells. Biochem. J. 473, 1455–1469 10.1042/BCJ20160128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nahomi R. B., Huang R., Nandi S. K., Wang B., Padmanabha S., Santhoshkumar P., Filipek S., Biswas A., and Nagaraj R. H. (2013) Acetylation of lysine 92 improves the chaperone and anti-apoptotic activities of human αB-crystallin. Biochemistry 52, 8126–8138 10.1021/bi400638s [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.