Abstract
The morbidity and mortality of HIV type‐1 (HIV‐1)‐related diseases were dramatically diminished by the grounds of the introduction of potent antiretroviral therapy, which induces persistent suppression of HIV‐1 replication and gradual recovery of CD4+ T‐cell counts. However, ∼10–40% of HIV‐1‐infected individuals fail to achieve normalization of CD4+ T‐cell counts despite persistent virological suppression. These patients are referred to as “inadequate immunological responders,” “immunodiscordant responders,” or “immunological non‐responders (INRs)” who show severe immunological dysfunction. Indeed, INRs are at an increased risk of clinical progression to AIDS and non‐AIDS events and present higher rates of mortality than HIV‐1‐infected individuals with adequate immune reconstitution. To date, the underlying mechanism of incomplete immune reconstitution in HIV‐1‐infected patients has not been fully elucidated. In light of this limitation, it is of substantial practical significance to deeply understand the mechanism of immune reconstitution and design effective individualized treatment strategies. Therefore, in this review, we aim to highlight the mechanism and risk factors of incomplete immune reconstitution and strategies to intervene.
Keywords: antiretroviral therapy, CD4+ T cells, HIV‐1 infection, immunological non‐responders, immune reconstitution
Highlights the mechanism and risk factors of incomplete immune reconstitution and strategies to intervene.

Abbreviations
- 3TC
Lamivudine
- ABC
abacavir
- ADCC
Ab‐dependent cell‐mediated cytotoxicity
- ART
antiretroviral therapy
- AZT
zidovudine
- BMI
body mass indexes
- CTLA‐4
cytotoxic T‐lymphocyte‐associated protein 4
- d4T
stavudine
- DC
dendritic cells
- DTG
dolutegravir
- EC
elite controller
- EFV
efavirenz
- FRCn
fibroblastic reticular cell network
- FTC
emtricitabine
- Glut1
glucose transporter 1
- HBV
hepatitis B virus
- HCV
hepatitis C virus
- HPC
hematopoietic progenitor cell
- HSC
hematopoietic stem cell
- ICRs
immune checkpoint receptors
- INR
immunological non‐responder
- IP‐10
IFN‐inducible protein‐10
- IR
immunological responder
- Lag‐3
lymphocyte activation gene‐3
- LT
lymphoid tissues
- MDSC
myeloid‐derived suppressor cell
- MSC
mesenchymal stem cell
- NLRP3
nucleotide‐binding oligomerization domain (NOD)‐like receptor (NLR) family, pyrin domain containing 3
- PD‐1
programmed cell death 1
- RAL
raltegravir
- RTE
recent thymic emigrant
- TDF
tenofovir
- TIGIT
T cell immunoglobulin and ITIM domain
- Tim‐3
T‐cell immunoglobulin and mucin domain‐containing protein‐3
- TREC
T‐cell receptor rearrangement excision circle
- VitD
Vitamin D
1. INTRODUCTION
The hallmark of HIV infection is the persistent destruction of CD4+ T cells, resulting in progressive immunodeficiency, opportunistic diseases, and death.1 It has been 32 years since the first antiretroviral drug, zidovudine (ZDV, formerly called AZT), was introduced to treat HIV infection. The increasing accessibility and use of antiretroviral therapy (ART) can suppress the HIV viral load to undetectable levels and to increase the CD4+ T‐cell counts; therefore, the acquired immunodeficiency syndrome (AIDS)‐related morbidity and mortality in HIV‐1‐infected individuals is sharply diminished.2, 3, 4 However, in some patients, optimal treatment and persistent suppression of viral replication fail to restore their CD4+ T‐cell counts. These patients are referred to as inadequate immunological responders, immunodiscordant responders, or immunological non‐responders (INRs), and an impaired immunological response is linked to an increased risk of disease progression and death for these patients.5, 6, 7 INRs present severe immune dysfunction, and the morbidity and mortality of AIDS and non‐AIDS events (such as metabolic syndrome, liver disease, nephropathy, cardiovascular disease, non‐AIDS‐related malignancies, and HIV‐1‐related neurocognitive disorder) were significantly elevated compared with those for HIV‐1‐infected patients who achieved complete immune reconstitution.8, 9, 10, 11, 12, 13 In this review, we focus on recent advances to identify the various mechanisms of poor immune reconstitution in HIV‐1‐infected patients and explore effective, newly specific therapeutic strategies to restore immunity and thus prevent AIDS‐related events.
2. DEFINITION OF INCOMPLETE IMMUNE RECONSTITUTION
Currently, there is no worldwide consensus on the definition of INRs (Table 1). Over the years, in different studies, INRs have been defined by either a failure to meet the prescribed CD4+ T‐cell count threshold (e.g., > 200 or > 250 or > 350 or > 400 or > 500/µl) or a prescribed percentage of CD4+ T‐cell increase over baseline (e.g., < 5% or < 20% or < 30%). In addition, some researchers defined INRs as those with an increase in CD4+ T‐cell counts from baseline, e.g., <50, <100, or <400/µl. However, the duration of ART in HIV‐1‐infected people varies substantially in different studies, ranging from 6 to 144 months (Table 1). The duration of ART can significantly affect the magnitude of immune reconstitution in HIV‐1‐infected patients, thus hindering the comparison of different findings. According to the heterogeneity among study populations and discrepancy in definitions, the prevalence of INRs varies from 10% to 40%.7, 14, 15 In contrast, an adequate immune response to ART is defined as CD4+ T‐cell counts >500 cells/µl, mainly because HIV‐1‐infected patients with this level of immune restoration have a morbidity and mortality rate approaching or comparable to those of HIV negative individuals.13, 16, 17, 18 In addition, the Department of Health and Human Services (DHHS) considered that patients with CD4+ T‐cell counts that had not increased to 350–500 cells/µl after 4–7 years of effective ART were defined as INRs.19 The various terms used to describe this poor immune reconstitution or immune reconstitution failure in the literature are outlined in Table 1. To date, it is commonly believed that the CD4+ T‐cell count is the most important predictor of immune recovery, treatment outcome, and disease progression in HIV‐1 infection, but recent reports clearly indicate the need for additional markers to supplement the CD4+ T‐cell count. Compared with the CD4+ T‐cell count and viral load, the CD4/CD8 ratio is potentially of higher predictive and evaluative value for the recovery of immunological function especially in patients who reached a CD4+ T‐cell count > 500/µl after initiation of ART.20, 21, 22
Table 1.
Definitions of immunological nonresponder and immunological responder from the literature
| Definition of “immunological nonresponder” | Definition of “immunological responder” | Reference |
|---|---|---|
| Total CD4+ T‐cell count < 500 cells/µl at 2–12 years after ART initiation, with an undetectable plasma VL.a | Total CD4+ T‐cell count > 500 cells/µl at 2–12 years after ART initiation, with an undetectable plasma VL. | 16, 179, 230, 231, 232, 233 |
| Increase in the CD4+ T‐cell count < 200 cells/µl from baseline at 7 years after ART initiation, with plasma HIV RNA < 200 copies/ml. | Increase in the CD4+ T‐cell count > 500 cells/µl from baseline at 7 years after ART initiation, with plasma HIV RNA < 200 copies/ml. | 54 |
| Increase in the CD4+ T‐cell count < 20% from baseline and/or CD4+ T‐cell counts < 200 cells/µl at 1–3 years after ART initiation, with plasma HIV RNA < 50 copies/ml. | Increase in the CD4+ T‐cell count > 20% from baseline and/or CD4+ T‐cell counts > 200 cells/µl at 1–3 years after ART initiation, with plasma HIV RNA < 50 copies/ml. | 35, 234, 235 |
| Increase in the CD4+ T‐cell count < 100 cells/µl from baseline at 1 year after ART initiation, with plasma HIV RNA < 50 copies/ml. | Increase in the CD4+ T‐cell count > 100 cells/µl from baseline at 1 year after ART initiation, with plasma HIV RNA < 50 copies/ml. | 236 |
| Increase in the CD4+ T‐cell count < 50 cells/µl from baseline at 3–9 months after ART initiation, with an undetectable plasma VL. | Increase in the CD4+ T‐cell count > 50 cells/µl from baseline at 3–9 months after ART initiation, with an undetectable plasma VL. | 9, 237 |
| Total CD4+ T‐cell count < 200 cells/µl at 2 years after ART initiation, with an undetectable plasma VL. | Total CD4+ T‐cell count > 500 cells/µL at 2 years after ART initiation, with an undetectable plasma VL. | 238, 239 |
| Increase in the CD4+ T‐cell count < 200 cells/µl from baseline at 1 year after ART initiation, with plasma HIV RNA < 50 copies/ml. | Increase in the CD4+ T‐cell count > 200 cells/µl from baseline at 1 year after ART initiation, with plasma HIV RNA < 50 copies/ml. | 240 |
| Total CD4+ T‐cell count < 350 cells/µl at 2 years after ART initiation, with plasma HIV RNA < 50 copies/ml. | Total CD4+ T‐cell count > 400 cells/µl at 2 years after ART initiation, with plasma HIV RNA < 50 copies/ml. | 48, 105, 106, 241 |
| Total CD4+ T‐cell count < 350 cells/µl and/or increase in the CD4+ T‐cell count < 30% from baseline at 1–10 years after ART initiation, with an undetectable plasma VL. | Total CD4+ T‐cell count > 350 cells/µl and/or increase in the CD4+ T‐cell count > 30% from baseline at 1–10 years after ART initiation, with an undetectable plasma VL. | 144, 177, 242, 243 |
| Total CD4+ T‐cell count < 500 cells/µl and CD4/CD8 ratio < 1 at 8 years after ART initiation, with plasma HIV RNA < 50 copies/ml. | Total CD4+ T‐cell count > 900 cells/µl and CD4/CD8 ratio < 1 at 8 years after ART initiation, with plasma HIV RNA < 50 copies/ml. | 37 |
| Increase in the CD4+ T‐cell count < 400 cells/µl from baseline at 5 years after ART initiation, with an undetectable plasma VL. | Increase in the CD4+ T‐cell count > 400 cells/µl from baseline at 5 years after ART initiation, with an undetectable plasma VL. | 117 |
| Total CD4+ T‐cell count < 350 cells/µl at 2 years after ART initiation, with an undetectable plasma VL | Total CD4+ T‐cell count > 500 cells/µl at 2 years after ART initiation, with an undetectable plasma VL. | 66, 116, 175, 244 |
| Total CD4+ T‐cell count < 400 cells/µl at 2 years after ART initiation, with plasma HIV RNA < 20 copies/ml. | Total CD4+ T‐cell count > 600 cells/µl at 2 years after ART initiation, with plasma HIV RNA < 20 copies/ml. | 110, 111 |
| Total CD4+ T‐cell count < 250 cells/µl at 2–3 years after ART initiation, with an undetectable plasma VL. | Total CD4+ T‐cell count > 250 cells/µl at 2–3 years after ART initiation, with an undetectable plasma VL. | 23, 170, 180, 245 |
| Increase in the CD4+ T‐cell count < 50 cells/µl from baseline at 1 year after ART initiation, with plasma HIV RNA < 40 copies/ml. | Increase in the CD4+ T‐cell count > 100 cells/µl from baseline at 1 year after ART initiation, with plasma HIV RNA < 40 copies/ml. | 47 |
| Total CD4+ T‐cell count < 270 cells/µl at 2 years after ART initiation, with an undetectable plasma VL. | Total CD4+ T‐cell count > 270 cells/µl at 2 years after ART initiation, with an undetectable plasma VL. | 176 |
Viral load.
3. POTENTIAL MECHANISMS OF INCOMPLETE IMMUNE RECONSTITUTION
The underlying mechanisms for this phenomenon are very complicated and may be multifactorial, including decreased hematopoiesis of bone marrow, insufficient thymic output, residual virus replication, aberrant immune activation, perturbations of cytokine secretion, and specific genetic or metabolic characteristics (Fig. 1).14, 23, 24, 25 However, none of these independent factors can fully explain the mechanism of incomplete immune reconstitution. At any time, the CD4+ T‐cell counts in HIV‐1‐infected individuals are associated with the production, destruction, and migration between secondary lymphoid organs and peripheral tissues.6, 26 INRs may have both reduced CD4 production and excessive destruction.
Figure 1.
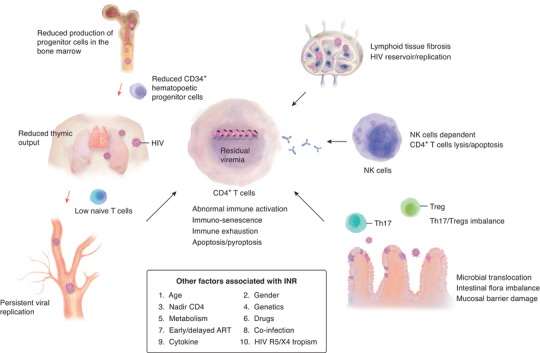
Factors associated with immunological non‐responders. Current understanding of the mechanism of incomplete immune reconstitution. INRs show severe immune dysfunction, including reduced production of progenitor cells in the bone marrow; thymic dysfunction; reduced CD34+ hematopoietic progenitor cells; abnormal immune activation; immune exhaustion; immunoregulatory cell imbalance, such as Treg and Th17 cells; increased immune‐senescence and cell apoptosis/pyroptosis, lymphoid tissue fibrosis, and microbial translocation; and persistent viral replication due to the HIV reservoir, and so on. Arrows in red highlight the maturation route of CD4+ T cells, while arrows in black indicate the factors associated with incomplete immune reconstitution. Th: helper T cell; Treg: regulatory T cell; NK: natural killer
3.1. CD4+ T‐cell production
3.1.1. Bone marrow and hematopoietic progenitor cells
T cells originate from bone marrow CD34+ hematopoietic progenitor cells (HPCs) and hematopoietic stem cells (HSCs), followed by their development and maturation in the thymus.27 Chelucci et al. found a proportion of CD34+ HPCs lineages can express the CD4 receptor together with the CXCR4 and/or CCR5 coreceptor and may thus be susceptible to HIV‐1.28, 29 It has been demonstrated that HIV can infect multiple subsets of bone marrow CD34+ HPCs in vivo and in humanized mice, establishing latent cellular reservoirs.30, 31, 32 Tsukamoto et al. utilized the in vitro OP9‐DL1/HIV‐1 model, cocultured cord‐derived CD34+ HPCs and CXCR4‐tropic HIV‐1 NL4‐3, and showed that CD34+CD7+CXCR4+ cells were rapidly depleted 1 week after HIV‐1 infection, accompanied by dramatically diminished numbers of CD34+CD7+CD4+ cells. These results suggest that the CXCR4‐tropic HIV‐1 strain may affect the differentiation rate or death rate of CD34+CD7+ lymphoid progenitor cells, resulting in impaired T‐cell production capacity.33 Li et al. found that CD34+CD38− early HPCs were preferentially depleted in HIV‐1‐infected individuals and humanized mice via plasmacytoid dendritic cell‐dependent mechanisms, accompanied with a significant reduction in proliferation capacity, while CD34+CD38+ intermediate HPCs were rarely affected.34 In addition, Isgro et al. found that the clonogenic capability in vitro and the level of more primitive CD34+ progenitor cells in INRs was reduced in parallel with reduced IL‐2 production and increased production of TNF‐α. Furthermore, Fas and Fas ligand expression was significantly up‐regulated, which could lead to the apoptotic depletion of CD34+ HPCs and decreased production of naïve CD4+ T cells.35 In addition, Sauce et al. found that the numbers of circulating CD34+ HPCs and mature lymphocyte numbers (i.e., CD8+ T cells, natural killer (NK) cells, or B cells) decreased dramatically with HIV disease progression and the number of circulating CD34+ HPCs was positively correlated with the number of CD4+ T cells, which may be due to the reduction in production of multiple lymphocyte lineages caused by bone marrow dysfunction.36 Menkova‐Garnier et al. reported that the capacity of CD34+ HPCs to differentiate into T cells is more significantly reduced in INRs than in immune responders (IRs) and healthy controls, which may be related to the significant up‐regulation of the ATP receptor P2 × 7 on CD34+ HPCs in INRs. Inhibition of the P2 × 7 pathway in vitro restores the potential of CD34+ HPC differentiation into T cells in INR patients, further confirming this view.37 The binding of ATP with its receptor, P2 × 7, induces the formation of inflammatory bodies, activates the caspase‐1 signaling pathway, and promotes the secretion of the proinflammatory cytokines IL‐1β and IL‐18, thus inducing host cell apoptosis and pyroptosis.38 Another study by Guo et al. found that circulating CD4+ T‐cell counts correlate with the proliferation ability of HPCs and HSCs. In addition, the colony‐forming ability of CD34+ HPCs and HSCs from INRs is much lower than that the colony‐forming ability of those from IRs.39 These studies suggest that incomplete immune reconstitution in HIV‐1‐infected individuals may be associated with impaired bone marrow hematopoietic function and decreased proliferative capacity.
3.1.2. Thymus and naïve cells
The thymus is crucial for the generation of naïve CD4 and CD8 cells with a broad T‐cell receptor repertoire. The most reliable method for evaluating thymic function is to perform a thymic biopsy. However, it is neither practical nor economical in HIV‐1‐infected individuals; therefore, thymic function is indirectly assessed by T‐cell receptor excision circles (TRECs), or recent thymus emigrants (RTEs) or naïve CD4+ T‐cell counts. After ART initiation, the thymic output improved significantly in HIV‐1‐infected adults and children, indicating that early ART initiation is essential for immune reconstitution in HIV‐1‐infected patients.40, 41 It has been reported that the thymic volume, as evaluated by computed tomographic scans, is a powerful independent predictor of the magnitude of CD4+ T‐cell recovery in HIV‐1‐infected individuals receiving ART.42, 43, 44 Simultaneously, other studies demonstrated that the thymic volume is associated with increased CD4+ TRECs content, CD4+ naïve cells and total CD4+ T‐cell counts in ART‐experienced HIV‐1‐infected individuals.45, 46 These studies suggest that in the case of lymphopenia, ongoing thymopoies may contribute to immune recovery in adult HIV‐1‐infected individuals. It has been shown that the frequency of CD4+ RTE cells, the numbers of sj‐TRECs and the sj/β‐TREC ratios are markedly lower in INRs than in IRs and healthy individuals, indicating that lower thymic output is the main cause of incomplete immune recovery in these patients.37, 47, 48, 49 Furthermore, Menkova‐Garnier et al. found that the frequency of CD4+ RTEs was positively correlated with the peripheral CD4+ T‐cell count.37 A study by Li et al. showed that INRs had a lower naïve CD4+ T‐cell increase and a lower percentage of CD4+ RTE than immunological responders and healthy controls, indicating that reduced thymic output may be a major mechanism of incomplete immune reconstitution.50 In accordance, it has been reported that thymic function, measured by the sj/β‐TREC ratio or CD4+ RTE%, can predict HIV‐1 disease progression in HIV‐1‐infected adults and adolescents with perinatally acquired HIV‐1.51, 52 These studies indicate that reduced thymic output may play an important role in the incomplete immune reconstitution of HIV‐1‐infected individuals. Conversely, Cobos Jiménez et al. found in HIV‐1‐infected ART‐experienced 45‐year‐old adults with detectable viremia (<50 copies/ml) for at least 1 year, the percentage of CD31+CD4+ cells and Sj‐TREC content in PBMCs are much higher than those in healthy controls. However, there was no correlation between Sj‐TREC content and CD4+ T‐cell recovery.53 In addition, a study by Delobel et al. found that the level of intrathymic proliferation, measured by the sj/β‐TREC ratio, as well as the frequency of CD31+ RTEs in INRs was comparable to that found in IRs despite their reduced numbers of naïve CD4+ T cells.54 These studies suggest that reduced thymic output does not help to explain the loss of CD4+ T‐cell counts. Our group recently found that INRs had a low number of CD3+CD4−CD8− T cells after long‐term ART and that the number of these cells positively correlated with the CD4+ T‐cell count. This study demonstrates that low double CD3+CD4−CD8− T‐cell counts may play a role in the incomplete restoration of CD4+ T cells.55 In conclusion, these studies indicate that a functional thymus is of vital importance for the maintenance of T‐cell homeostasis and achievement of optimal immune reconstitution.
3.1.3. Cytokines
Interleukin‐7 (IL‐7), which is mainly produced by stromal cells in primary and secondary lymphoid organs, such as bone marrow stromal cells, thymic epithelial cells, fibroblastic reticular cells, and lymphatic endothelial cells, and IL‐7 receptor (IL‐7R), consisting of a common γ‐chain (CD132) and an IL‐7R‐specific α‐chain (CD127), are crucial for CD4+ T‐cell homeostasis due to promotion of survival, proliferation, and de novo production of T cells.56, 57 It has been reported that in HIV‐1‐infected individuals, a decreased percentage of CD127+CD4+ T cells and an increased percentage of CD127+CD8+ central memory T cells are associated with incomplete immune reconstitution.58, 59 However, a study by Hartling et al. demonstrated that neither the plasma level of IL‐7 nor CD127 expression on CD4+ T cells was associated with an increase in the total CD4+ T‐cell count after initiation of ART. In contrast, they found positive associations between baseline CD127 density on CD4+ T cells and an increase in CD4+ RTE cell counts and naïve CD4+ T‐cell counts after 2 years of effective ART, indicating that CD127 expression on CD4+ T cells is a predictor of increased thymic output after 2 years of suppressive ART.60 Furthermore, a few studies have found that in INRs, plasma levels of IL‐7 were elevated and CD127 expression both on CD4+ and CD8+ T cells was decreased; however, there was no statistically significant correlation between the baseline plasma IL‐7 level and absolute CD4+ T‐cell count after successful ART.61 Furthermore, others have shown a positive association59, 62 or a negative association63, 64, 65 between baseline plasma IL‐7 levels and CD4 recovery in HIV‐1‐infected individuals after ART initiation. CD4+ T cells from INRs exhibited diminished CD127 expression and reduced IL‐7‐mediated proliferation responsiveness.66, 67 These studies demonstrated that IL‐7 responsiveness is impaired in INRs and may be related to downregulation of CD127. In addition, a study by Cote et al. showed that Th17 cells (a subset of CD4+ T cells) from aviremic HIV‐1‐infected individuals have increased CD127 expression but impaired IL‐7‐induced proliferation, indicating that this reduction in proliferation is not the result of the lack or dysfunction of the IL‐7 receptor.68 Accordingly, perturbations in the IL‐7/IL‐7R system may not be a reliable predictor of incomplete immune reconstitution. It has been reported that in HIV‐1‐infected individuals with incomplete immune reconstitution, elevated plasma levels of IL‐6, and increased CD4+ T cell turnover are already present before ART initiation.69, 70 A study by Shive et al. showed that pre‐exposure of PBMCs from healthy subjects to IL‐1β or IL‐6 can drive CD4+ T cell turnover, downregulate CD127 expression, and diminish CD4+ T‐cell responsiveness to IL‐7.71 While numerous studies have found that plasma IL‐6 levels69, 72, 73 and lymphoid tissue IL‐1 levels71 are elevated in HIV‐1‐infected individuals, these levels did not normalize even after effective ART. In addition, thymic atrophy and fibrosis in lymphoid tissues may hinder IL‐7 access, which seems to be related to the continuous expression of type I IFNs and decreased expression of IL‐7R induced by IL‐1 and IL‐6, which is linked to cell death and reduced thymopoiesis.74 Therefore, IL‐1β and IL‐6 induce thymic atrophy and fibrosis in lymphoid tissues, increasing CD4+ T‐cell turnover, and diminished T‐cell responsiveness to IL‐7 may partially explain the lack of CD4+ T‐cell recovery in aviremic HIV‐1‐infected individuals.
3.2. CD4+ T‐cell destruction
3.2.1. Coinhibitory receptors
Coinhibitory or immune checkpoint receptors (ICRs), including programmed cell death 1 (PD‐1), cytotoxic T lymphocyte‐associated protein 4 (CTLA‐4), T cell immunoglobulin and ITIM domain (TIGIT), T cell immunoglobulin and mucin domain‐containing molecule‐3 (Tim‐3), lymphocyte activation gene‐3 (Lag‐3), and 2B4 (CD244), play a vital role in regulating immune responses against HIV‐1 infection. In HIV‐1 infection up‐regulation of ICRs is associated with T‐cell exhaustion, which is characterized by decreased proliferation and production of cytokines.75, 76 Furthermore, ICRs also participate in the establishment and maintenance of viral reservoirs, which is the main obstacle of HIV‐1 eradication.77, 78, 79, 80 Numerous studies have shown that expression of ICRs (such as PD‐1, Tim‐3, CTLA‐4, and Lag‐3) on CD4 and CD8 T cells is substantially up‐regulated in untreated HIV‐1‐infected individuals and decreased after ART initiation, which is positively correlated with plasma viral load and negatively with CD4+ T‐cell count, and blockade of the corresponding pathways resulted in enhanced HIV‐specific CD4 and CD8 T‐cell proliferation and effector functions.81, 82, 83, 84, 85, 86, 87, 88 These studies indicate that ICR expression on T cells is associated with T‐cell exhaustion and disease progression.
Noyan et al. found that the frequencies of PD‐1, CTLA‐4, and TIGIT expression on CD4+ T cells in HIV‐1 elite controllers (ECs), who can spontaneously control HIV‐1 replication in the absence of ART and maintain a high CD4+ T‐cell count, were comparable with those in healthy individuals and significantly lower than those in untreated viremic subjects and ART‐treated aviremic subjects. In addition, all of these ICR coexpressed CD4+ T cells were positively correlated with pVL and negatively correlated with the CD4+ T‐cells count and CD4/CD8 ratio, suggesting that the state of CD4+ T‐cell exhaustion in ECs is equivalent to that in healthy subjects.88 It has been reported that INRs had significantly higher levels of PD‐1 than IRs on total CD4+ T cells,87, 89, 90, 91 and that PD‐1 expression on CD4+ T cells was significantly inversely associated with the CD4+ T‐cell count.87, 89 A study by Saidakova et al. showed that both CD4+ T‐cell cycling (expression of Ki‐67) and the exhaustion rate were significantly elevated in HIV‐1‐infected INRs on suppressive ART compared to those in IRs. In addition, the percentages of CD4+Ki‐67+ CM and EM T lymphocytes were inversely related to the CD4+ T‐cell counts, and the frequency of CD4+Ki−67+ CM T cells was significantly positively related to the proportion of CD4+PD‐1+ cells, suggesting that lymphopenia‐induced intensive homeostatic proliferation of CD4+ T cells is associated with CD4+ T‐cell exhaustion and poor CD4+ T‐cell recovery.91 In conclusion, these studies indicate that inhibitory receptor‐mediated T‐cell exhaustion may have an important role in incomplete immune reconstitution in HIV‐1‐infected individuals.
3.2.2. Immune activation
Immune activation is manifested by mainly the acquisition of an activated phenotype by innate and adaptive immune cells and the secretion of soluble inflammatory mediators, such as IFN‐α, IL‐1β, IL‐6, IL‐8, TNF, TGF‐β, sCD14, sCD163, MIP‐1α, MIP‐1β, RANTES, and IP‐10. During HIV‐1 infection, persistent immune activation and inflammation are driven by multiple factors, including residual virus replication, inflammatory lipids, gut microbial translocation, and co‐infection.92, 93, 94 Although long‐term effective ART substantially reduces the level of immune activation and inflammation in HIV‐1‐infected individuals, it fails to normalize the activation and inflammation.95, 96, 97, 98, 99 It was reported that persistent T‐cell activation was associated with decreased CD4+ T‐cell gains in HIV‐1‐infected individuals during ART.53, 100, 101, 102, 103 Hunt et al. found that for every 5% increase in the percentage of activated CD4+ T cells, the CD4+ T‐cell counts decreased by 45 cells/µl in the first 3 months of ART. Similarly, for every 5% increase in the percentage of activated CD8+ T cells, the CD4+ T‐cell counts decreased by 35 cells/µl after 3 months of ART.100 In addition, a study by Cobos Jiménez et al. found that the plasma sCD14 and sCD163 levels and the percentages of activated (coexpressing CD38+HLA‐DR+) CD4+ and CD8+ T cells were higher in ART‐treated HIV‐1‐infected individuals than in healthy controls. The sCD14 and sCD163 levels were positively associated with the percentage of activated CD4+ T cells. Furthermore, the percentage of activated CD4+ T cells was significantly inversely associated with the CD4+ T‐cell count and CD4+ T‐cell recovery after starting ART. This study indicated that T‐cell activation driven by monocyte activation and bacterial translocation, as demonstrated by sCD14 and sCD163 levels, is associated with poor immune recovery in HIV‐1‐infected individuals.53 In support of this finding, Khoury et al. also found that activated CD4+ T cells were significantly inversely correlated with current and nadir CD4+ T‐cell counts.79 It was reported that INRs showed increased levels of immune activation, mainly in CD4+ T cells; increased levels of proliferation; and increased rates of spontaneous CD4+ T‐cell death by apoptosis.48, 104, 105, 106, 107, 108, 109 These studies indicate that activated‐mediated CD4+ T‐cell hyperproliferation and spontaneous cell death may impede immune recovery in HIV‐1‐infected individuals. Stiksrud et al. showed that INRs displayed higher activation of both monocytes and DCs than IRs and that this increase was correlated with enhanced CD4+ and CD8+ T‐cell activation. HIV‐1‐specific monocyte plasma IFN‐inducible protein‐10 (IP‐10, also known as CXCL‐10, a marker of monocyte activation) responses were shown to be negatively associated with future CD4 gain.110 This group also found that INRs had elevated plasma IP‐10 levels. Furthermore, the plasma IP‐10 levels and IDO‐1 enzyme activity, measured as the kynurenine/tryptophan ratio, were inversely associated with the CD4+ T‐cell count 2 years after inclusion.111 IDO is predominantly expressed in macrophages and DCs and is the rate‐limiting enzyme for the catabolism of tryptophan into kynurenines. Therefore, IDO activity may serve as a marker of inflammation and immune activation.112 Others have demonstrated that elevated IDO‐1 activity is associated with reduced CD4+ T‐cell recovery in HIV‐1‐infected individuals on ART.113, 114 A study by Luo et al. showed that the frequency of activated NK cells was significantly increased in IRs and healthy controls and that NK cell activation was inversely correlated with peripheral CD4+ T‐cell counts in HIV‐1‐infected individuals on ART. Furthermore, NK cells from INRs had the ability to induce uninfected CD4+ T‐cell death via cytotoxic effects. These results suggest that activated NK cells may play a role in unsatisfactory CD4+ T‐cell recovery in HIV‐1‐infected individuals on long‐term ART.115
In addition, Bandera et al. found that the nucleotide‐binding oligomerization domain (NOD)‐like receptor (NLR) family, pyrin domain containing 3 (NLRP3) inflammasome and caspase‐1 were significantly up‐regulated in INRs compared to those in IRs and that NLRP3‐mediated activation of caspase‐1 could induce CD4+ T‐cell loss via persistent immune activation and pyroptosis, resulting in unsatisfactory CD4+ T‐cells recovery.116 It was reported that INRs had a higher frequency of CXCR4‐tropic viruses than IRs and that CXCR4‐tropic virus can trigger persistent T‐cell activation and bystander apoptosis through the interaction of gp120‐CXCR4, thus resulting in the depletion of naïve T cells, and may play a role in the impaired immune reconstitution in INRs.54, 117
3.2.3. Microbial translocation and intestinal flora imbalance
In the early stage of HIV‐1 infection, massive amounts of CD4+ T cells in the gut are depleted, especially T helper (Th) 17 cells and Th22 cells, which play an important role in maintaining the integrity of the gut. HIV‐1 infection is also associated with gut epithelial barrier damage characterized by villous atrophy, enterocyte apoptosis, crypt hyperplasia, decreased expression of tight junction proteins, and increased gastrointestinal inflammation, which contributes to increased intestinal permeability.118, 119, 120 These abnormalities eventually result in alteration of the intestinal microbiota composition (dysbiosis) and release of bacterial products into the circulation (microbial translocation), leading to chronic immune activation and inflammation.74, 121, 122 A study by Jiang et al. found that bacterial ribosomal 16S RNA, a marker of microbial translocation from the gastrointestinal tract, was substantially elevated in HIV‐1‐infected subjects on effective ART compared with that in healthy controls and was positively associated with the levels of T‐cell activation and inversely associated with the levels of CD4+ T‐cell restoration.123 In addition, Mehraj et al. reported that plasma levels of the fungal antigen βDG, a component of fungal cell walls that serves as a potent pathogen‐associated molecular pattern in triggering antifungal immunity, were significantly elevated in HIV‐1‐infected individuals and did not normalize despite long‐term ART. βDG content correlated positively with activated CD4+ and CD8+ T cell levels, IDO‐1 enzyme activity, and plasma sCD14 and Lipopolysaccharide levels and inversely with CD4+ T‐cell counts.124 These studies suggest that microbial product translocation from the gut to the circulation was associated with immune activation and imply CD4+ T‐cell depletion during suppressive ART treatment. HIV‐1 infection is associated with a decrease in intestinal microbial diversity.118, 121 Nowak et al. found that the alpha‐diversity of the gut microbiota, measured as the number of observed bacterial species and Shannon index, was significantly lower in HIV‐1‐infected individuals, both at baseline and after ART initiation, than in healthy controls. The alpha diversity correlated with CD4+ T‐cell counts and inversely with markers of microbial translocation and monocyte activation. For every increase in the number of bacterial species, the CD4+ T‐cell count increased by 0.88 cells/µl. This study suggests a pivotal role of microbiota diversity in host immune homeostasis.125 Several studies have found that HIV‐1‐infected individuals have a significant increase in the relative abundance of the Gram‐negative bacteria Prevotella in conjunction with a decrease in Bacteroides abundance compared to those in healthy controls.126, 127, 128, 129, 130 A study by Kaur et al. also found that the abundance of Prevotella was significantly higher in perinatal HIV‐1‐infected children than in uninfected controls despite ART. The relative abundance of Prevotella positively correlated with the levels of IP‐10 and sCD14, a marker of monocyte activation and microbial translocation, and was inversely associated with the CD4+ T‐cell count.131 In addition, Dillon et al. reported that the relative abundance of Prevotella was strongly positively associated with the number of activated mucosal CD4+ and CD8+ T cells and the level of myeloid DC activation.127, 132 These studies suggest that enrichment of Prevotella may be detrimental to immune reconstitution by driving immune activation. Lee et al. found that INRs had a higher abundance of Fusobacterium than IRs and healthy controls. The relative abundance of Fusobacterium abundance was positively correlated with CD4+ T‐cell activation but negatively correlated with CD4+ T‐cell counts, suggesting that the enrichment of Fusobacterium may be associated with poor CD4+ T‐cell recovery.133 In addition, Lu et al. showed that INRs were enriched with Faecalibacterium prausnitzii, unclassified Subdoligranulum sp., and Coprococcus comes compared with those in IRs. Moreover, the relative abundances of unclassified Subdoligranulum sp. and C. comes were positively correlated with CD8+ T‐cell activation and inversely associated with CD4+ T‐cell counts.130 A study by Pérez‐Santiago et al. found that gut Lactobacillales was associated with an increased CD4 percentage, reduced microbial translocation, and decreased systemic immune activation during HIV infection, which may be related to the fact that Lactobacillus can regulate the anti‐inflammatory immune response and participate in maintenance of intestinal mucosal barrier integrity, thereby reducing the level of immune activation and the destruction of CD4+ T cells.134 These observations suggest that altered intestinal microbiota communities may be associated with systemic immune activation and microbial translocation, thus contributing to incomplete immune recovery in HIV‐1‐infected individuals. A study by Serpa et al. showed that long‐term use of proton pump inhibitors was associated with increased microbial translocation, innate immune activation, and poor immune reconstitution in HIV‐1‐infected individuals on suppressive ART.135
3.2.4. Coinfection
Numerous studies have found that hepatitis B virus (HBV),136, 137, 138, 139 hepatitis C virus (HCV),140, 141, 142 and CMV coinfections143, 144 were associated with poor CD4+ T‐cell immune recovery in HIV‐1‐infected individuals on ART. The precise mechanism by which HBV, HCV, and CMV coinfections may have deleterious effects on CD4+ T‐cell count recovery is unclear. The impaired immunological recovery in HBV‐, HCV‐, or CMV‐coinfected patients could be due to the destruction of CD4+ T cells by coinfection‐mediated CD4+ T‐cell activation, apoptosis, or exhaustion.145, 146, 147, 148, 149 Others studies did not show an association between HBV,142, 150, 151 HCV,152, 153 or CMV154 coinfection and immunological recovery. Demographic characteristics (such as age, sex, and ethnicity), baseline CD4+ T‐cell counts, follow‐up time, duration of ART, and coinfection status might have contributed to this discrepancy.
3.2.5. Secondary lymphatic organs
Lymphatic tissue structure and function is of vital importance in T‐cell homeostasis. HIV‐1 infection is associated with persistent chronic immune activation and inflammation, which results in progressive collagen deposition in the parafollicular T‐cell zone and lymphoid tissues fibrosis, which replaces the fibroblastic reticular cell network (FRCn), a structure that is vital to normal immune function, the FRCn also produce the T‐cell homeostatic cytokine IL‐7.155 Several studies demonstrated the evidence of dramatically paracortical T‐cell zone damage was associated with the deposition of collagen in lymphoid tissues (LT), and the magnitude of collagen deposition in LT was inversely correlated with both the size of the CD4+ T‐cell population in the LT and the increase of peripheral CD4+ T‐cell counts in HIV‐1‐infected individuals on effective ART.156, 157, 158 Consistently, the extent of loss of the FRCn and collagen in the LT predicts the degree of the restoration of both naïve T cells and peripheral total CD4+ T cells after 6 months of ART.159, 160 These studies suggest that collagen deposition and loss of the FRCn in the LT limit the magnitude of the CD4+ T‐cell recovery in HIV‐1‐infected individuals under long‐term ART.
3.3. Other factors associated with immune reconstitution
In addition to the factors mentioned above, older age,18, 161, 162, 163 male sex,164, 165, 166 lower nadir CD4 T‐cell counts,18, 167, 168 lower CD4/CD8 ratios, and a lower naïve/memory CD4+ T‐cell ratio167, 169, 170 have been associated with a blunted immunological response to ART treatment. Ethnic origin is also associated with immune recovery.171, 172, 173, 174 Furthermore, host genetic factors, metabolic characteristics, and specific ART regimens may play a role in incomplete immune recovery. Luo et al. reported that HIV‐1‐infected INRs had significantly increased surface‐bound IgG on CD4+ T cells compared to that in IRs and healthy controls. The percentage of auto‐IgG binding on CD4+ T‐cell surfaces was associated with increased CD4+ T‐cell apoptosis and inversely correlated with absolute CD4+ T‐cell counts. Furthermore, purified anti‐CD4 IgG from HIV‐1‐infected INRs bound to CD4+ T cells and induced cell apoptosis through NK‐mediated Ab‐dependent cell‐mediated cytotoxicity (ADCC) in vitro.175 This study suggests that autoreactive anti‐CD4 IgG may play a vital role in unsatisfactory CD4+ T‐cell reconstitution despite effective ART. In addition, Lisco et al. demonstrated that anti‐CD4 antibody‐mediated ADCC and aberrant inflammasome/caspase‐1 activation may be an important cause of extreme CD4+ T‐cell count decline in HIV‐1‐infected individuals with ART‐mediated viral suppression.176 A study by Tincati et al. showed that the level of HIV‐1 reservoirs, measured as the frequencies of cells harboring total and integrated HIV‐DNA as well as 2 long terminal repeat circles, in both peripheral CD4+ T cells and intestinal tissue was comparable between HIV‐1‐infected INRs and IRs. However, HIV reservoirs in both peripheral blood and the gut negatively correlate with CD4+ T‐cell reconstitution, suggesting that poor immune recovery on ART may be associated with increased HIV reservoirs.177 Agrati et al. reported that the frequency of myeloid‐derived suppressor cells (MDSCs) was significantly elevated during primary HIV‐1 infection and did not normalize after 48 weeks of successful ART. Furthermore, an inverse correlation was also observed between the frequency of MDSCs and the CD4+ T‐cell count at 48 weeks after ART initiation, indicating that the persistence of MDSCs may impede CD4+ T‐cell recovery.178 HIV‐1‐infected INRs had remarkably higher levels of α4β7, a marker of lymphocyte gut‐homing, on CD4+ T cells than healthy controls despite effective ART treatment, suggesting that an increase in the trafficking of CD4+ T cells to gut‐associated lymphatic tissue may contribute to unsatisfactory CD4+ T‐cell recovery.179, 180 A study by Sennepin et al. reported that the expression of NKp44L, the cellular ligand of an activating NK cell receptor, was remarkably up‐regulated on CD4+ T cells in HIV‐1‐infected INRs compared with that on CD4+ T cells in IRs and healthy controls. NKp44L expression was associated with a significant expansion and apoptosis of highly differentiated, multifunctional CD4+ T cells, indicating that a rapid CD4+ T‐cell turnover in HIV‐1‐infected individuals may prevent immune recovery.181
3.3.1. Host genetic factors
Previous studies have shown that host genetic factors can influence CD4+ T‐cell recovery during suppressive ART treatment. The CCL3L1‐CCR5 genotypes,182 polymorphisms in CD14 and TLR4,183 mitochondrial haplogroup H,184, 185 and IL18 G variant allele and genotype186 were associated with enhanced long‐term CD4+ T‐cell recovery in HIV‐1‐infected patients on suppressive ART. The IL‐7 receptor subunit alpha (IL7RA) rs6897932 CT/TT genotype was related to a faster and better CD4+ T‐cells recovery compared to that of the CC genotype; a potential mechanism is that signal transduction and proliferation in response to IL‐7 was is substantially higher in the "TT" genotype compared to that in the "CC" genotype in HIV‐infected individuals.187, 188, 189 A study by Greenblatt et al. showed that 41 genes harbored variations that were independently predictive of CD4 recovery. Many of these genes are associated with the cell cycle, apoptosis, lymphocyte migration, or CD4+ T‐cell homeostasis.163 Conversely, the CCR2 rs1799864‐AG genotypes,190 HLA‐A68 and HLA‐B15 alleles,191 TLR9 1635AA genotype,192 polymorphism rs1385129 in the glut1 gene SLC2A1,193 and polymorphisms in the IFN‐γ and IL19 genes194 are linked to poor CD4+ T‐cell recovery in HIV‐1‐infected individuals.
3.3.2. Host metabolic factors
Numerous studies have shown that a higher baseline body mass index (BMI) was associated with enhanced immune reconstitution in HIV‐1‐infected individuals on suppressive ART.195, 196, 197, 198 In support of this finding, others have shown that an increasing BMI was an independent predictor of elevated CD4+ T‐cell counts in HIV‐1 uninfected individuals.199, 200, 201 These studies indicate that adipose tissue may affect peripheral CD4+ T‐cell recovery. Glucose metabolism plays a vital role in supporting the growth, proliferation, and effector functions of T cells. Glucose transporter‐1 (Glut1) is a kind of glucose transporter with high affinity for glucose and is the main glucose transporter in T cells. Glut1 can also serve as a marker of glycolysis activation. The percentage of circulating CD4+Glut1+ T cells was significantly elevated in HIV‐1‐infected individuals and did not normalize despite long‐term effective ART. In addition, the proportion of CD4+Glut1+ T cells correlates positively with the percentage of activated CD4+ T cells and inversely with the absolute CD4+ T‐cell count irrespective of HIV treatment status. This group also demonstrated that Glut1 was up‐regulated on exhausted and senescent CD4+ T cells. Thus, these observations indicate that hyperactivation of glycolysis in CD4+ T cells during HIV infection facilitates metabolic exhaustion that drives CD4+ T‐cell depletion.193, 202 Vitamin D (VitD) is a key regulator of host defense against infections by activating genes and pathways that enhance innate and adaptive immunity.203 Several studies have demonstrated that baseline VitD deficiency in HIV‐1‐infected individuals was associated with diminished CD4+ T‐cell recovery after ART initiation.204, 205, 206, 207 This effect may be partially explained by the fact that sufficient VitD levels can reduce inflammation and T cell activation, restrain HIV‐1 infection in T cells, and promote the proliferation of CD4+ T cells induced by APCs.207, 208 These observations suggest that the baseline metabolic characteristics may be associated with impaired immune reconstitution in HIV‐1‐infected individuals during ART.
3.3.3. Antiretroviral drugs and immune reconstitution
Numerous studies have demonstrated that the CD4+ T‐cell count recovery was superior under the raltegravir‐containing regimen compared with that under the efavirenz‐containing regimen when combined with tenofovir/emtricitabine in HIV‐1‐infected ART‐utilizing individuals after long‐term ART.209, 210, 211, 212 Others have shown that HIV‐1‐infected individuals receiving the dolutegravir‐abacavir‐lamivudine (DTG‐ABC‐3TC) regimen had a shorter median time to viral suppression, as well as greater increases in CD4+ T‐cell count, than those receiving the efavirenz–tenofovir disoproxil fumarate–emtricitabine (EFV‐TDF‐FTC) regimen.213, 214 Tanuma et al. reported that stavudine (d4T)‐based regimens (OR 0.51, vs AZT) and nevirapine (NVP)‐based regimens (OR 0.53, vs EFV) were associated with impaired immune recovery.165 Furthermore, a study by Zhang et al. showed that the risk of suboptimal immunologic recovery among patients with AZT‐containing regimens or d4T‐containing regimens was 2.1 and 2.4 times higher, respectively, compared with that among patients with TDF‐containing regimens.215 These studies suggest that a specific ART regimen may also affect the level of immune reconstitution in HIV‐1‐infected individuals.
4. INTERVENTION MEASURES FOR POOR IMMUNE RECONSTITUTION
Thus far, it is not clear which treatment can maximize the recovery of CD4+ T cells in INRs. Although researchers have made various attempts to improve the level of immune reconstitution in INRs, these specific interventions have not yet achieved convincing results except for standard ART regimes, mainly because the mechanism of immune reconstitution in INRs has not yet been clarified. As mentioned above, the occurrence of poor immune reconstitution in HIV‐1‐infected people may involve a variety of mechanisms, and the relative contributions of these mechanisms vary greatly among individuals. Therefore, precise individualized treatment should be selected according to the pathogenesis presented. In HIV‐1 infection, persistent immune activation contributes to rapid T‐cell turnover, immune exhaustion, and increased cell death. Several studies have evaluated the effect of other strategies, such as intensification with maraviroc or raltegravir, the immunomodulatory agents chloroquine and its analogue hydroxychloroquine, statins, aspirin, and prebiotics and probiotics in combination with a standard ART regimen, in limiting immune activation and immune reconstitution. As it is not possible to eradicate HIV, the current intervention strategies to limit residual immune activation are marginally successful.216
VitD deficiency is associated with an increased plasma HIV viral load, decreased peripheral blood CD4+ T cells, and rapid AIDS progression.203 Coelho et al. reported that plasma 25(OH)D levels were significantly positively correlated with CD4+ T‐cell counts after 24 weeks of VitD supplementation and that each 1.0 ng/ml increase in 25(OH)D during repletion therapy was associated with a 3.3 cell/µl increase in the CD4+ T‐cell count.217 Although others have found that VitD supplementation is associated with reduced immune activation and CD4+ T‐cell exhaustion levels, it has no effect on CD4+ T‐cell recovery in both untreated and treated HIV‐1‐infected individuals.218, 219, 220 Therefore, additional investigation is needed to determine whether VitD can promote immune reconstitution.
IL‐2 is a cytokine that regulates the proliferation and differentiation of lymphocytes and may help to reconstitute the immune system. A systematic review summarized the role of IL‐2 supplementation in HIV‐1‐infected individuals receiving ART treatment and found that IL‐2 in combination with ART increases the CD4+ T‐cell count in HIV‐1‐infected individuals compared with that of ART alone. However, combining IL‐2 therapy with ART does not confer any significant clinical benefit in terms of mortality and the occurrence of opportunistic infections and may increase grade 3 or 4 adverse effects. Therefore, IL‐2 is not recommended as a therapeutic adjunct in the treatment of HIV infection.221
IL‐7 plays a vital role in thymopoiesis as well as in the peripheral proliferation and survival of mature T cells. Multiple phase I/II clinical trials have evaluated the effect of recombinant human IL‐7 supplementation on immune reconstitution in HIV‐1‐infected individuals receiving ART treatment. The administration of r‐hIL‐7 resulted in a substantial and sustained increase in the numbers of circulating CD4+ and CD8+ T cells, as well as enhanced cell proliferation and thymic output.222, 223, 224, 225, 226, 227 IL‐7 therapy is also associated with apparent improvement in gut barrier integrity and decreased systemic inflammatory and immune activation. However, a study by Katlama et al. demonstrated that despite IL‐7 administration and dual ART intensification inducing a significant expansion of central memory CD4+ T‐cells, a mild HIV reactivation and an amplification of the HIV reservoir was also observed.228 Taken together, these studies revealed that patients may benefit from intermittent therapy with IL‐7 in combination with ART, but we should also pay attention to the side effects caused by HIV‐1 reactivation.
Mesenchymal stem cells (MSCs) can interact with cells of both the innate and adaptive immune systems and inhibit their activation and release of proinflammatory cytokines. A study by Zhang et al. found that umbilical cord‐MSCs transfusions are well tolerated and can substantially reduce the level of systemic immune activation, immune exhaustion, and the inflammatory response as well as efficiently increase circulating naïve and central memory CD4+ T‐cell counts in HIV‐1‐infected INRs, suggesting that such interventions may be helpful for immune reconstitution in INRs.229
5. CONCLUSIONS
In conclusion, numerous studies on the mechanism of INRs have focused on mainly isolated portions of the complex processes of CD4+ T‐cell production, differentiation, survival, and destruction. However, poor immune reconstitution in HIV‐1‐infected individuals is related to many factors. Thus far, there is no precise mechanism to explain INRs, which may be due to the combination of several factors leading to poor immune reconstitution. Moreover, it should be noted that different patients may have different dominant mechanisms of poor immune reconstitution. These two aspects can affect the best treatment choice for INRs. Therefore, precise individualized treatment should be selected according to the specific pathogenic mechanism presented. In summary, a further understanding and improvement of immune reconstitution in HIV‐1‐infected patients remains an important field of scientific research.
AUTHORSHIP
All authors made a substantial, direct, and intellectual contribution to the work. X.Y. and B.S. wrote the article, and T.Z. supervised the manuscript. All authors read and approved the final manuscript. X.Y. and B.S. contributed equally to the article.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (NSFC, 81772165 and 81974303 to B.S.), the National 13th Five‐Year Grand Program on Key Infectious Disease Control (2017ZX10202102‐005‐003 to B.S., 2017ZX10202101‐004‐001 to T.Z.), the NSFC‐NIH Biomedical collaborative research program (81761128001 to H.W.), the Beijing Municipal of Science and Technology Major Project (D161100000416003 to H.W.), and the Beijing Key Laboratory for HIV/AIDS Research (BZ0089). The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Yang X, Su B, Zhang X, Liu Y, Wu H, Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: Challenges of immunological non‐responders. J Leukoc Biol. 2020;107:597–612. 10.1002/JLB.4MR1019-189R
REFERENCES
- 1. Lucas S, Nelson AM. HIV and the spectrum of human disease. J Pathol. 2015;235:229‐241. [DOI] [PubMed] [Google Scholar]
- 2. Ghosn J, Taiwo B, Seedat S, et al. Hiv. Lancet. 2018;392:685‐697. [DOI] [PubMed] [Google Scholar]
- 3. Saag MS, Benson CA, Gandhi RT, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2018 recommendations of the International Antiviral Society‐USA panel. JAMA. 2018;320(4):379‐396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prabhu S, Harwell JI, Kumarasamy N. Advanced HIV: diagnosis, treatment, and prevention. Lancet HIV. 2019;6:PE540‐PE551. [DOI] [PubMed] [Google Scholar]
- 5. Battegay M, Nuesch R, Hirschel B, et al. Immunological recovery and antiretroviral therapy in HIV‐1 infection. Lancet Infect Dis. 2006;6:280‐287. [DOI] [PubMed] [Google Scholar]
- 6. Corbeau P, Reynes J. Immune reconstitution under antiretroviral therapy: the new challenge in HIV‐1 infection. Blood. 2011;117:5582‐5590. [DOI] [PubMed] [Google Scholar]
- 7. Gazzola L, Tincati C, Bellistri GM, et al. The absence of CD4+ T cell count recovery despite receipt of virologically suppressive highly active antiretroviral therapy: clinical risk, immunological gaps, and therapeutic options. Clin Infect Dis. 2009;48:328‐337. [DOI] [PubMed] [Google Scholar]
- 8. Young J, Psichogiou M, Ayayi S, et al Opportunistic Infections Project Team of the Collaboration of Observational HIVERiEiE . CD4 cell count and the risk of AIDS or death in HIV‐Infected adults on combination antiretroviral therapy with a suppressed viral load: a longitudinal cohort study from COHERE. PLoS Med. 2012;9:e1001194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baker JV, Peng G, Rapkin J, et al. Poor initial CD4+ recovery with antiretroviral therapy prolongs immune depletion and increases risk for AIDS and non‐AIDS diseases. J Acquir Immune Defic Syndr. 2008;48:541‐546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pacheco YM, Jarrin I, Rosado I, et al. Increased risk of non‐AIDS‐related events in HIV subjects with persistent low CD4 counts despite cART in the CoRIS cohort. Antiviral Res. 2015;117:69‐74. [DOI] [PubMed] [Google Scholar]
- 11. Engsig FN, Zangerle R, Katsarou O, et al. Long‐term mortality in HIV‐positive individuals virally suppressed for >3 years with incomplete CD4 recovery. Clin Infect Dis. 2014;58:1312‐1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Takuva S, Maskew M, Brennan AT, et al. Poor CD4 recovery and risk of subsequent progression to AIDS or death despite viral suppression in a South African cohort. J Int AIDS Soc. 2014;17:18651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. van Lelyveld SF, Gras L, Kesselring A, et al. Long‐term complications in patients with poor immunological recovery despite virological successful HAART in Dutch ATHENA cohort. AIDS. 2012;26:465‐474. [DOI] [PubMed] [Google Scholar]
- 14. Massanella M, Negredo E, Clotet B, et al. Immunodiscordant responses to HAART–mechanisms and consequences. Expert Rev Clin Immunol. 2013;9:1135‐1149. [DOI] [PubMed] [Google Scholar]
- 15. Nakanjako D, Kiragga AN, Musick BS, et al. Frequency and impact of suboptimal immune recovery on first‐line antiretroviral therapy within the international epidemiologic databases to evaluate AIDS in East Africa. AIDS. 2016;30:1913‐1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaufmann GR, Furrer H, Ledergerber B, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1‐infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41:361‐372. [DOI] [PubMed] [Google Scholar]
- 17. Lewden C, Chene G, Morlat P, et al. HIV‐infected adults with a CD4 cell count greater than 500 cells/mm3 on long‐term combination antiretroviral therapy reach same mortality rates as the general population. J Acquir Immune Defic Syndr. 2007;46:72‐77. [DOI] [PubMed] [Google Scholar]
- 18. Kroeze S, Ondoa P, Kityo CM, et al. Suboptimal immune recovery during antiretroviral therapy with sustained HIV suppression in sub‐Saharan Africa. AIDS. 2018;32:1043‐1051. [DOI] [PubMed] [Google Scholar]
- 19. Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV‐1 infected adults and adolescents. Washington, DC: Department of Health and Human Services, 2008. [Google Scholar]
- 20. Lu W, Mehraj V, Vyboh K, et al. CD4:CD8 ratio as a frontier marker for clinical outcome, immune dysfunction and viral reservoir size in virologically suppressed HIV‐positive patients. J Int AIDS Soc. 2015;18:20052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Serrano‐Villar S, Sainz T, Lee SA, et al. HIV‐infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non‐AIDS morbidity and mortality. PLoS Pathog. 2014;10:e1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buggert M, Frederiksen J, Noyan K, et al. Multiparametric bioinformatics distinguish the CD4/CD8 ratio as a suitable laboratory predictor of combined T cell pathogenesis in HIV infection. J Immunol. 2014;192:2099‐2108. [DOI] [PubMed] [Google Scholar]
- 23. Rodriguez‐Gallego E, Gomez J, Pacheco YM, et al. A baseline metabolomic signature is associated with immunological CD4+ T‐cell recovery after 36 months of antiretroviral therapy in HIV‐infected patients. AIDS. 2018;32:565‐573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gunda DW, Kilonzo SB, Kamugisha E, et al. Prevalence and risk factors of poor immune recovery among adult HIV patients attending care and treatment centre in northwestern Tanzania following the use of highly active antiretroviral therapy: a retrospective study. BMC Res Notes. 2017;10:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Utay NS, Hunt PW. Role of immune activation in progression to AIDS. Curr Opin HIV AIDS. 2016;11:131‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gaardbo JC, Hartling HJ, Gerstoft J, et al. Incomplete immune recovery in HIV infection: mechanisms, relevance for clinical care, and possible solutions. Clin Dev Immunol. 2012;2012:670957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spits H, Blom B, Jaleco AC, et al. Early stages in the development of human T, natural killer and thymic dendritic cells. Immunol Rev. 1998;165:75‐86. [DOI] [PubMed] [Google Scholar]
- 28. Chelucci C, Casella I, Federico M, et al. Lineage‐specific expression of human immunodeficiency virus (HIV) receptor/coreceptors in differentiating hematopoietic precursors: correlation with susceptibility to T‐ and M‐tropic HIV and chemokine‐mediated HIV resistance. Blood. 1999;94:1590‐1600. [PubMed] [Google Scholar]
- 29. Chelucci C, Hassan HJ, Locardi C, et al. In vitro human immunodeficiency virus‐1 infection of purified hematopoietic progenitors in single‐cell culture. Blood. 1995;85:1181‐1187. [PubMed] [Google Scholar]
- 30. Nixon CC, Vatakis DN, Reichelderfer SN, et al. HIV‐1 infection of hematopoietic progenitor cells in vivo in humanized mice. Blood. 2013;122:2195‐2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carter CC, Onafuwa‐Nuga A, McNamara LA, et al. HIV‐1 infects multipotent progenitor cells causing cell death and establishing latent cellular reservoirs. Nat Med. 2010;16:446‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. McNamara LA, Collins KL. Hematopoietic stem/precursor cells as HIV reservoirs. Curr Opin HIV AIDS. 2011;6:43‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsukamoto T. HIV impacts CD34(+) progenitors involved in T‐cell differentiation during coculture with mouse stromal OP9‐DL1 cells. Front Immunol. 2019;10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li G, Zhao J, Cheng L, et al. HIV‐1 infection depletes human CD34+CD38‐ hematopoietic progenitor cells via pDC‐dependent mechanisms. PLoS Pathog. 2017;13:e1006505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Isgro A, Leti W, De Santis W, et al. Altered clonogenic capability and stromal cell function characterize bone marrow of HIV‐infected subjects with low CD4+ T cell counts despite viral suppression during HAART. Clin Infect Dis. 2008;46:1902‐1910. [DOI] [PubMed] [Google Scholar]
- 36. Sauce D, Larsen M, Fastenackels S, et al. HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood. 2011;117:5142‐5151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Menkova‐Garnier I, Hocini H, Foucat E, et al. P2X7 receptor inhibition improves CD34 T‐cell differentiation in HIV‐infected immunological nonresponders on c‐ART. PLoS Pathog. 2016;12:e1005571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mariathasan S, Weiss DS, Newton K, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228‐232. [DOI] [PubMed] [Google Scholar]
- 39. Guo X, He S, Lv X, et al. The role of HIV‐1 in affecting the proliferation ability of HPCs derived from BM. J Acquir Immune Defic Syndr. 2016;71:467‐473. [DOI] [PubMed] [Google Scholar]
- 40. Castro P, Torres B, Lopez A, et al. Effects of different antigenic stimuli on thymic function and interleukin‐7/CD127 system in patients with chronic HIV infection. J Acquir Immune Defic Syndr. 2014;66:466‐472. [DOI] [PubMed] [Google Scholar]
- 41. Sandgaard KS, Lewis J, Adams S, et al. Antiretroviral therapy increases thymic output in children with HIV. AIDS. 2014;28:209‐214. [DOI] [PubMed] [Google Scholar]
- 42. de la Rosa R, Leal M, Rubio A, et al. Baseline thymic volume is a predictor for CD4 T cell repopulation in adult HIV‐infected patients under highly active antiretroviral therapy. Antivir Ther. 2002;7:159‐163. [PubMed] [Google Scholar]
- 43. Ruiz‐Mateos E, Rubio A, Vallejo A, et al. Thymic volume is associated independently with the magnitude of short‐ and long‐term repopulation of CD4+ T cells in HIV‐infected adults after highly active antiretroviral therapy (HAART). Clin Exp Immunol. 2004;136:501‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rosado‐Sanchez I, Herrero‐Fernandez I, Genebat M, et al. Thymic function impacts the peripheral CD4/CD8 ratio of HIV‐infected subjects. Clin Infect Dis. 2017;64:152‐158. [DOI] [PubMed] [Google Scholar]
- 45. McCune JM, Loftus R, Schmidt DK, et al. High prevalence of thymic tissue in adults with human immunodeficiency virus‐1 infection. J Clin Invest. 1998;101:2301‐2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kolte L, Dreves AM, Ersboll AK, et al. Association between larger thymic size and higher thymic output in human immunodeficiency virus‐infected patients receiving highly active antiretroviral therapy. J Infect Dis. 2002;185:1578‐1585. [DOI] [PubMed] [Google Scholar]
- 47. Shete A, Dhayarkar S, Sangale S, et al. Incomplete functional T‐cell reconstitution in immunological non‐responders at one year after initiation of antiretroviral therapy possibly predisposes them to infectious diseases. Int J Infect Dis. 2019;81:114‐122. [DOI] [PubMed] [Google Scholar]
- 48. Massanella M, Negredo E, Perez‐Alvarez N, et al. CD4 T‐cell hyperactivation and susceptibility to cell death determine poor CD4 T‐cell recovery during suppressive HAART. AIDS. 2010;24:959‐968. [DOI] [PubMed] [Google Scholar]
- 49. Rb‐Silva R, Nobrega C, Azevedo C, et al. Thymic function as a predictor of immune recovery in chronically HIV‐infected patients initiating antiretroviral therapy. Front Immunol. 2019;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li T, Wu N, Dai Y, et al. Reduced thymic output is a major mechanism of immune reconstitution failure in HIV‐infected patients after long‐term antiretroviral therapy. Clin Infect Dis. 2011;53(9):944‐951. [DOI] [PubMed] [Google Scholar]
- 51. Zakhour R, Tran DQ, Degaffe G, et al. Recent thymus emigrant CD4+ T cells predict HIV disease progression in patients with perinatally acquired HIV. Clin Infect Dis. 2016;62(8):1029‐1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ferrando‐Martinez S, De Pablo‐Bernal RS, De Luna‐Romero M, et al. Thymic function failure is associated with human immunodeficiency virus disease progression. Clin Infect Dis. 2017;64(9):1191‐1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cobos Jimenez V, Wit FW, Joerink M, et al. T‐Cell activation independently associates with immune senescence in HIV‐infected recipients of long‐term antiretroviral treatment. J Infect Dis. 2016;214(2):216‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Delobel P, Nugeyre MT, Cazabat M, et al. Naive T‐cell depletion related to infection by X4 human immunodeficiency virus type 1 in poor immunological responders to highly active antiretroviral therapy. J Virol. 2006;80(20):10229‐10236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lu X, Su B, Xia H, et al. Low double‐negative CD3(+)CD4(‐)CD8(‐) T cells are associated with incomplete restoration of CD4(+) T cells and higher immune activation in HIV‐1 immunological non‐responders. Front Immunol. 2016;7:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lundstrom W, Fewkes NM, Mackall CL. IL‐7 in human health and disease. Semin Immunol. 2012;24(3):218‐224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Raeber ME, Zurbuchen Y, Impellizzieri D, et al. The role of cytokines in T‐cell memory in health and disease. Immunol Rev. 2018;283(1):176‐193. [DOI] [PubMed] [Google Scholar]
- 58. Bai F, Bellistri GM, Tincati C, et al. Reduced CD127 expression on peripheral CD4+ T cells impairs immunological recovery in course of suppressive highly active antiretroviral therapy. AIDS. 2010;24(16):2590‐2593. [DOI] [PubMed] [Google Scholar]
- 59. Benito JM, Lopez M, Lozano S, et al. Down‐regulation of interleukin‐7 receptor (CD127) in HIV infection is associated with T cell activation and is a main factor influencing restoration of CD4(+) cells after antiretroviral therapy. J Infect Dis. 2008;198(10):1466‐1473. [DOI] [PubMed] [Google Scholar]
- 60. Hartling HJ, Jespersen S, Gaardbo JC, et al. Reduced IL‐7R T cell expression and increased plasma sCD127 in late presenting HIV‐infected individuals. J Acquir Immune Defic Syndr. 2017;74(1):81‐90. [DOI] [PubMed] [Google Scholar]
- 61. Shive CL, Clagett B, McCausland MR, et al. Inflammation perturbs the IL‐7 axis, promoting senescence and exhaustion that broadly characterize immune failure in treated HIV infection. J Acquir Immune Defic Syndr. 2016;71(5):483‐492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Clark RD, Fox PC. Statistical variation in progressive scrambling. J Comput Aided Mol Des. 2004;18(7‐9):563‐576. [DOI] [PubMed] [Google Scholar]
- 63. Rajasuriar R, Booth D, Solomon A, et al. Biological determinants of immune reconstitution in HIV‐infected patients receiving antiretroviral therapy: the role of interleukin 7 and interleukin 7 receptor alpha and microbial translocation. J Infect Dis. 2010;202(8):1254‐1264. [DOI] [PubMed] [Google Scholar]
- 64. Rethi B, Fluur C, Atlas A, et al. Loss of IL‐7Ralpha is associated with CD4 T‐cell depletion, high interleukin‐7 levels and CD28 down‐regulation in HIV infected patients. AIDS. 2005;19(18):2077‐2086. [DOI] [PubMed] [Google Scholar]
- 65. Napolitano LA, Grant RM, Deeks SG, et al. Increased production of IL‐7 accompanies HIV‐1‐mediated T‐cell depletion: implications for T‐cell homeostasis. Nat Med. 2001;7(1):73‐79. [DOI] [PubMed] [Google Scholar]
- 66. Nguyen TP, Shukla S, Asaad R, et al. Responsiveness to IL‐7 but not to IFN‐alpha is diminished in CD4+ T cells from treated HIV infected patients who experience poor CD4+ T‐cell recovery. AIDS. 2016;30(13):2033‐2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bellistri GM, Casabianca A, Merlini E, et al. Increased bone marrow interleukin‐7 (IL‐7)/IL‐7R levels but reduced IL‐7 responsiveness in HIV‐positive patients lacking CD4+ gain on antiviral therapy. PLoS One. 2010;5(12):e15663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cote SC, Stilla A, Burke Schinkel SC, et al. IL‐7‐induced proliferation of peripheral Th17 cells is impaired in HAART‐controlled HIV infection. AIDS. 2019;33(6):985‐991. [DOI] [PubMed] [Google Scholar]
- 69. Lederman MM, Calabrese L, Funderburg NT, et al. Immunologic failure despite suppressive antiretroviral therapy is related to activation and turnover of memory CD4 cells. J Infect Dis. 2011;204(8):1217‐1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rosado‐Sanchez I, Jarrin I, Pozo‐Balado MM, et al. Higher levels of IL‐6, CD4 turnover and Treg frequency are already present before cART in HIV‐infected subjects with later low CD4 recovery. Antiviral Res. 2017;142:76‐82. [DOI] [PubMed] [Google Scholar]
- 71. Shive CL, Mudd JC, Funderburg NT, et al. Inflammatory cytokines drive CD4+ T‐cell cycling and impaired responsiveness to interleukin 7: implications for immune failure in HIV disease. J Infect Dis. 2014;210(4):619‐629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kalayjian RC, Machekano RN, Rizk N, et al. Pretreatment levels of soluble cellular receptors and interleukin‐6 are associated with HIV disease progression in subjects treated with highly active antiretroviral therapy. J Infect Dis. 2010;201(12):1796‐1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zicari S, Sessa L, Cotugno N, et al. Immune activation, inflammation, and Non‐AIDS co‐morbidities in HIV‐infected patients under long‐term ART. Viruses. 2019;11(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yasuma‐Mitobe K, Matsuoka M. The roles of coinhibitory receptors in pathogenesis of human retroviral infections. Front Immunol. 2018;9:2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sperk M, Domselaar RV, Neogi U. Immune checkpoints as the immune system regulators and potential biomarkers in HIV‐1 infection. Int J Mol Sci. 2018;19(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Banga R, Procopio FA, Noto A, et al. PD‐1(+) and follicular helper T cells are responsible for persistent HIV‐1 transcription in treated aviremic individuals. Nat Med. 2016;22(7):754‐761. [DOI] [PubMed] [Google Scholar]
- 78. Fromentin R, Bakeman W, Lawani MB, et al. CD4+ T cells expressing PD‐1, TIGIT and LAG‐3 contribute to HIV persistence during ART. PLoS Pathog. 2016;12(7):e1005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Khoury G, Fromentin R, Solomon A, et al. Human immunodeficiency virus persistence and T‐cell activation in blood, rectal, and lymph node tissue in human immunodeficiency virus‐infected individuals receiving suppressive antiretroviral therapy. J Infect Dis. 2017;215(6):911‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Evans VA, van der Sluis RM, Solomon A, et al. Programmed cell death‐1 contributes to the establishment and maintenance of HIV‐1 latency. AIDS. 2018;32(11):1491‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Day CL, Kaufmann DE, Kiepiela P, et al. PD‐1 expression on HIV‐specific T cells is associated with T‐cell exhaustion and disease progression. Nature. 2006;443(7109):350‐354. [DOI] [PubMed] [Google Scholar]
- 82. Hoffmann M, Pantazis N, Martin GE, et al. Exhaustion of activated CD8 T cells predicts disease progression in primary HIV‐1 infection. PLoS Pathog. 2016;12(7):e1005661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nunnari G, Fagone P, Condorelli F, et al. CD4+ T‐cell gene expression of healthy donors, HIV‐1 and elite controllers: immunological chaos. Cytokine. 2016;83:127‐135. [DOI] [PubMed] [Google Scholar]
- 84. Yamamoto T, Price DA, Casazza JP, et al. Surface expression patterns of negative regulatory molecules identify determinants of virus‐specific CD8+ T‐cell exhaustion in HIV infection. Blood. 2011;117(18):4805‐4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Tian X, Zhang A, Qiu C, et al. The upregulation of LAG‐3 on T cells defines a subpopulation with functional exhaustion and correlates with disease progression in HIV‐infected subjects. J Immunol. 2015;194(8):3873‐3882. [DOI] [PubMed] [Google Scholar]
- 86. Jones RB, Ndhlovu LC, Barbour JD, et al. Tim‐3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV‐1 infection. J Exp Med. 2008;205(12):2763‐2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cockerham LR, Jain V, Sinclair E, et al. Programmed death‐1 expression on CD4(+) and CD8(+) T cells in treated and untreated HIV disease. AIDS. 2014;28(12):1749‐1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Noyan K, Nguyen S, Betts MR, et al. Human immunodeficiency virus type‐1 elite controllers maintain low co‐expression of inhibitory receptors on CD4+ T cells. Front Immunol. 2018;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Grabmeier‐Pfistershammer K, Steinberger P, Rieger A, et al. Identification of PD‐1 as a unique marker for failing immune reconstitution in HIV‐1‐infected patients on treatment. J Acquir Immune Defic Syndr. 2011;56(2):118‐124. [DOI] [PubMed] [Google Scholar]
- 90. Hatano H, Jain V, Hunt PW, et al. Cell‐based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD‐1)‐expressing CD4+ T cells. J Infect Dis. 2013;208(1):50‐56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Saidakova EV, Shmagel KV, Korolevskaya LB, et al. Lymphopenia‐induced proliferation of CD4 T‐cells is associated with CD4 T‐lymphocyte exhaustion in treated HIV‐infected patients. Indian J Med Res. 2018;147(4):376‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Appay V, Kelleher AD. Immune activation and immune aging in HIV infection. Curr Opin HIV AIDS. 2016;11(2):242‐249. [DOI] [PubMed] [Google Scholar]
- 93. Bruzzesi E, Sereti I. Residual immune activation and latency. Curr Top Microbiol Immunol. 2018;417:157‐180. [DOI] [PubMed] [Google Scholar]
- 94. Hileman CO, Funderburg NT. Inflammation, immune activation, and antiretroviral therapy in HIV. Curr HIV/AIDS Rep. 2017;14(3):93‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sereti I, Krebs SJ, Phanuphak N, et al. Persistent, albeit reduced, chronic inflammation in persons starting antiretroviral therapy in acute HIV infection. Clin Infect Dis. 2017;64(2):124‐131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tanko RF, Soares AP, Masson L, et al. Residual T cell activation and skewed CD8+ T cell memory differentiation despite antiretroviral therapy‐induced HIV suppression. Clin Immunol. 2018;195:127‐138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Gandhi RT, McMahon DK, Bosch RJ, et al. Levels of HIV‐1 persistence on antiretroviral therapy are not associated with markers of inflammation or activation. PLoS Pathog. 2017;13(4):e1006285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Li JZ, Segal FP, Bosch RJ, et al. ART reduces T cell activation and immune exhaustion markers in HIV controllers. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kroeze S, Wit FW, Rossouw TM, et al. Plasma biomarkers of human immunodeficiency virus‐related systemic inflammation and immune activation in Sub‐Saharan Africa before and during suppressive antiretroviral therapy. J Infect Dis. 2019;220(6):1029‐1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Hunt PW, Martin JN, Sinclair E, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus‐infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187(10):1534‐1543. [DOI] [PubMed] [Google Scholar]
- 101. Vajpayee M, Kaushik S, Sreenivas V, et al. Role of immune activation in CD4+ T‐cell depletion in HIV‐1 infected Indian patients. Eur J Clin Microbiol Infect Dis. 2009;28(1):69‐73. [DOI] [PubMed] [Google Scholar]
- 102. Hunt PW, Cao HL, Muzoora C, et al. Impact of CD8+ T‐cell activation on CD4+ T‐cell recovery and mortality in HIV‐infected Ugandans initiating antiretroviral therapy. AIDS. 2011;25(17):2123‐2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Zhang X, Hunt PW, Hammer SM, et al. Immune activation while on potent antiretroviral therapy can predict subsequent CD4+ T‐cell increases through 15 years of treatment. HIV Clin Trials. 2013;14(2):61‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Marchetti G, Gori A, Casabianca A, et al. Comparative analysis of T‐cell turnover and homeostatic parameters in HIV‐infected patients with discordant immune‐virological responses to HAART. AIDS. 2006;20(13):1727‐1736. [DOI] [PubMed] [Google Scholar]
- 105. Negredo E, Massanella M, Puig J, et al. Nadir CD4 T cell count as predictor and high CD4 T cell intrinsic apoptosis as final mechanism of poor CD4 T cell recovery in virologically suppressed HIV‐infected patients: clinical implications. Clin Infect Dis. 2010;50(9):1300‐1308. [DOI] [PubMed] [Google Scholar]
- 106. Massanella M, Gomez‐Mora E, Carrillo J, et al. Increased ex vivo cell death of central memory CD4 T cells in treated HIV infected individuals with unsatisfactory immune recovery. J Transl Med. 2015;13:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Ramirez CM, Sinclair E, Epling L, et al. Immunologic profiles distinguish aviremic HIV‐infected adults. AIDS. 2016;30(10):1553‐1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Hansjee N, Kaufmann GR, Strub C, et al. Persistent apoptosis in HIV‐1‐infected individuals receiving potent antiretroviral therapy is associated with poor recovery of CD4 T lymphocytes. J Acquir Immune Defic Syndr. 2004;36(2):671‐677. [DOI] [PubMed] [Google Scholar]
- 109. Casetti R, Pinnetti C, Sacchi A, et al. HIV‐specific CD8 T cells producing CCL‐4 are associated with worse immune reconstitution during chronic infection. J Acquir Immune Defic Syndr. 2017;75(3):338‐344. [DOI] [PubMed] [Google Scholar]
- 110. Stiksrud B, Aass HCD, Lorvik KB, et al. Activated dendritic cells and monocytes in HIV immunological non‐responders; HIV‐induced IP‐10 correlates with low future CD4 recovery. AIDS. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Stiksrud B, Lorvik KB, Kvale D, et al. Plasma IP‐10 is increased in immunological nonresponders and associated with activated regulatory T cells and persisting low CD4 counts. J Acquir Immune Defic Syndr. 2016;73(2):138‐148. [DOI] [PubMed] [Google Scholar]
- 112. Favre D, Mold J, Hunt PW, et al. Tryptophan catabolism by indoleamine 2,3‐dioxygenase 1 alters the balance of TH17 to regulatory T cells in HIV disease. Sci Transl Med. 2010;2(32):32ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Byakwaga H, Boum Y, 2nd , Huang Y, et al. The kynurenine pathway of tryptophan catabolism, CD4+ T‐cell recovery, and mortality among HIV‐infected Ugandans initiating antiretroviral therapy. J Infect Dis. 2014;210(3):383‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gaardbo JC, Trosied M, Stiksrud B, et al. Increased tryptophan catabolism is associated with increased frequency of CD161+Tc17/MAIT cells and lower CD4+ T‐cell count in HIV‐1 infected patients on cART after 2 years of follow‐up. J Acquir Immune Defic Syndr. 2015;70(3):228‐235. [DOI] [PubMed] [Google Scholar]
- 115. Luo Z, Li Z, Martin L, et al. Increased natural killer cell activation in HIV‐infected immunologic non‐responders correlates with CD4+ T cell recovery after antiretroviral therapy and viral suppression. PLoS One. 2017;12(1):e0167640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Bandera A, Masetti M, Fabbiani M, et al. The NLRP3 inflammasome is upregulated in HIV‐infected antiretroviral therapy‐treated individuals with defective immune recovery. Front Immunol. 2018;9:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Bader J, Schoni‐Affolter F, Boni J, et al. Correlating HIV tropism with immunological response under combination antiretroviral therapy. HIV Med. 2016;17(8):615‐622. [DOI] [PubMed] [Google Scholar]
- 118. Dillon SM, Frank DN, Wilson CC. The gut microbiome and HIV‐1 pathogenesis: a two‐way street. AIDS. 2016;30(18):2737‐2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Mudd JC, Brenchley JM. Gut mucosal barrier dysfunction, microbial dysbiosis, and their role in HIV‐1 disease progression. J Infect Dis. 2016;214(Suppl 2):S58‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ramendra R, Isnard S, Mehraj V, et al. Circulating LPS and (1→3)‐beta‐D‐Glucan: a folie a deux contributing to HIV‐associated immune activation. Front Immunol. 2019;10:465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Gootenberg DB, Paer JM, Luevano JM, et al. HIV‐associated changes in the enteric microbial community: potential role in loss of homeostasis and development of systemic inflammation. Curr Opin Infect Dis. 2017;30(1):31‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sandler NG, Douek DC. Microbial translocation in HIV infection: causes, consequences and treatment opportunities. Nat Rev Microbiol. 2012;10(9):655‐666. [DOI] [PubMed] [Google Scholar]
- 123. Jiang W, Lederman MM, Hunt P, et al. Plasma levels of bacterial DNA correlate with immune activation and the magnitude of immune restoration in persons with antiretroviral‐treated HIV infection. J Infect Dis. 2009;199(8):1177‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Mehraj V, Ramendra R, Isnard S, et al. Circulating (1→3)‐beta‐D‐Glucan is associated with immune activation during HIV infection. Clin Infect Dis. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Nowak P, Troseid M, Avershina E, et al. Gut microbiota diversity predicts immune status in HIV‐1 infection. AIDS. 2015;29(18):2409‐2418. [DOI] [PubMed] [Google Scholar]
- 126. Lozupone CA, Li M, Campbell TB, et al. Alterations in the gut microbiota associated with HIV‐1 infection. Cell Host Microbe. 2013;14(3):329‐339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Dillon SM, Lee EJ, Kotter CV, et al. An altered intestinal mucosal microbiome in HIV‐1 infection is associated with mucosal and systemic immune activation and endotoxemia. Mucosal Immunol. 2014;7(4):983‐994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Mutlu EA, Keshavarzian A, Losurdo J, et al. A compositional look at the human gastrointestinal microbiome and immune activation parameters in HIV infected subjects. PLoS Pathog. 2014;10(2):e1003829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Vazquez‐Castellanos JF, Serrano‐Villar S, Latorre A, et al. Altered metabolism of gut microbiota contributes to chronic immune activation in HIV‐infected individuals. Mucosal Immunol. 2015;8(4):760‐772. [DOI] [PubMed] [Google Scholar]
- 130. Lu W, Feng Y, Jing F, et al. Association Between Gut Microbiota and CD4 Recovery in HIV‐1 Infected Patients. Front Microbiol. 2018;9:1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kaur US, Shet A, Rajnala N, et al. High abundance of genus Prevotella in the gut of perinatally HIV‐infected children is associated with IP‐10 levels despite therapy. Sci Rep. 2018;8(1):17679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Dillon SM, Lee EJ, Kotter CV, et al. Gut dendritic cell activation links an altered colonic microbiome to mucosal and systemic T‐cell activation in untreated HIV‐1 infection. Mucosal Immunol. 2016;9(1):24‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Lee SC, Chua LL, Yap SH, et al. Enrichment of gut‐derived Fusobacterium is associated with suboptimal immune recovery in HIV‐infected individuals. Sci Rep. 2018;8(1):14277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Perez‐Santiago J, Gianella S, Massanella M, et al. Gut Lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27(12):1921‐1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Serpa JA, Rueda AM, Somasunderam A, et al. Long‐term use of proton pump inhibitors is associated with increased microbial product translocation, innate immune activation, and reduced immunologic recovery in patients with chronic human immunodeficiency virus‐1 infection. Clin Infect Dis. 2017;65(10):1638‐1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hawkins C, Christian B, Ye J, et al. Prevalence of hepatitis B co‐infection and response to antiretroviral therapy among HIV‐infected patients in Tanzania. AIDS. 2013;27(6):919‐927. [DOI] [PubMed] [Google Scholar]
- 137. Wandeler G, Gsponer T, Bihl F, et al. Hepatitis B virus infection is associated with impaired immunological recovery during antiretroviral therapy in the Swiss HIV cohort study. J Infect Dis. 2013;208(9):1454‐1458. [DOI] [PubMed] [Google Scholar]
- 138. van Griensven J, Phirum L, Choun K, et al. Hepatitis B and C co‐infection among HIV‐infected adults while on antiretroviral treatment: long‐term survival, CD4 cell count recovery and antiretroviral toxicity in Cambodia. PLoS One. 2014;9(2):e88552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Anderson M, Gaseitsiwe S, Moyo S, et al. Slow CD4(+) T‐cell recovery in human immunodeficiency virus/hepatitis B virus‐coinfected patients initiating truvada‐based combination antiretroviral therapy in Botswana. Open Forum Infect Dis. 2016;3(3):ofw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Potter M, Odueyungbo A, Yang H, et al. Impact of hepatitis C viral replication on CD4+ T‐lymphocyte progression in HIV‐HCV coinfection before and after antiretroviral therapy. AIDS. 2010;24(12):1857‐1865. [DOI] [PubMed] [Google Scholar]
- 141. Marcus JL, Leyden WA, Chao CR, et al. Differences in response to antiretroviral therapy by sex and hepatitis C infection status. AIDS Patient Care STDS. 2015;29(7):370‐378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chen M, Wong WW, Law MG, et al. Hepatitis B and C co‐infection in HIV patients from the TREAT Asia HIV observational database: analysis of risk factors and survival. PLoS One. 2016;11(3):e0150512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Appay V, Fastenackels S, Katlama C, et al. Old age and anti‐cytomegalovirus immunity are associated with altered T‐cell reconstitution in HIV‐1‐infected patients. AIDS. 2011;25(15):1813‐1822. [DOI] [PubMed] [Google Scholar]
- 144. Gomez‐Mora E, Massanella M, Garcia E, et al. Elevated humoral response to cytomegalovirus in HIV‐infected individuals with poor CD4+ T‐cell immune recovery. PLoS One. 2017;12(9):e0184433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Gonzalez VD, Falconer K, Blom KG, et al. High levels of chronic immune activation in the T‐cell compartments of patients coinfected with hepatitis C virus and human immunodeficiency virus type 1 and on highly active antiretroviral therapy are reverted by alpha interferon and ribavirin treatment. J Virol. 2009;83(21):11407‐11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Idoko J, Meloni S, Muazu M, et al. Impact of hepatitis B virus infection on human immunodeficiency virus response to antiretroviral therapy in Nigeria. Clin Infect Dis. 2009;49(8):1268‐1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Kovacs A, Al‐Harthi L, Christensen S, et al. CD8(+) T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis. 2008;197(10):1402‐1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Patel EU, Gianella S, Newell K, et al. Elevated cytomegalovirus IgG antibody levels are associated with HIV‐1 disease progression and immune activation. AIDS. 2017;31(6):807‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Christensen‐Quick A, Massanella M, Frick A, et al. Subclinical cytomegalovirus DNA is associated with CD4 T cell activation and impaired CD8 T cell CD107a expression in people living with HIV despite early antiretroviral therapy. J Virol. 2019;93(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Matthews GV, Manzini P, Hu Z, et al. Impact of lamivudine on HIV and hepatitis B virus‐related outcomes in HIV/hepatitis B virus individuals in a randomized clinical trial of antiretroviral therapy in southern Africa. AIDS. 2011;25(14):1727‐1735. [DOI] [PubMed] [Google Scholar]
- 151. Hamers RL, Zaaijer HL, Wallis CL, et al. HIV‐HBV coinfection in Southern Africa and the effect of lamivudine‐ versus tenofovir‐containing cART on HBV outcomes. J Acquir Immune Defic Syndr. 2013;64(2):174‐182. [DOI] [PubMed] [Google Scholar]
- 152. Anderson KB, Guest JL, Rimland D. Hepatitis C virus coinfection increases mortality in HIV‐infected patients in the highly active antiretroviral therapy era: data from the HIV Atlanta VA cohort study. Clin Infect Dis. 2004;39(10):1507‐1513. [DOI] [PubMed] [Google Scholar]
- 153. Peters L, Mocroft A, Soriano V, et al. Hepatitis C virus coinfection does not influence the CD4 cell recovery in HIV‐1‐infected patients with maximum virologic suppression. J Acquir Immune Defic Syndr. 2009;50(5):457‐463. [DOI] [PubMed] [Google Scholar]
- 154. Kapetanovic S, Aaron L, Montepiedra G, et al. Effect of cytomegalovirus co‐infection on normalization of selected T‐cell subsets in children with perinatally acquired HIV infection treated with combination antiretroviral therapy. PLoS One. 2015;10(3):e0120474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Estes JD. Pathobiology of HIV/SIV‐associated changes in secondary lymphoid tissues. Immunol Rev. 2013;254(1):65‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Schacker TW, Nguyen PL, Beilman GJ, et al. Collagen deposition in HIV‐1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110(8):1133‐1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Diaz A, Alos L, Leon A, et al. Factors associated with collagen deposition in lymphoid tissue in long‐term treated HIV‐infected patients. AIDS. 2010;24(13):2029‐2039. [DOI] [PubMed] [Google Scholar]
- 158. Diaz A, Garcia F, Mozos A, et al. Lymphoid tissue collagen deposition in HIV‐infected patients correlates with the imbalance between matrix metalloproteinases and their inhibitors. J Infect Dis. 2011;203(6):810‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Schacker TW, Reilly C, Beilman GJ, et al. Amount of lymphatic tissue fibrosis in HIV infection predicts magnitude of HAART‐associated change in peripheral CD4 cell count. AIDS. 2005;19(18):2169‐2171. [DOI] [PubMed] [Google Scholar]
- 160. Zeng M, Southern PJ, Reilly CS, et al. Lymphoid tissue damage in HIV‐1 infection depletes naive T cells and limits T cell reconstitution after antiretroviral therapy. PLoS Pathog. 2012;8(1):e1002437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Seng R, Goujard C, Krastinova E, et al. Influence of lifelong cumulative HIV viremia on long‐term recovery of CD4+ cell count and CD4+/CD8+ ratio among patients on combination antiretroviral therapy. AIDS. 2015;29(5):595‐607. [DOI] [PubMed] [Google Scholar]
- 162. Ahn MY, Jiamsakul A, Khusuwan S, et al. The influence of age‐associated comorbidities on responses to combination antiretroviral therapy in older people living with HIV. J Int AIDS Soc. 2019;22(2):e25228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Greenblatt R, Bacchetti P, Boylan R, et al. Genetic and clinical predictors of CD4 lymphocyte recovery during suppressive antiretroviral therapy: whole exome sequencing and antiretroviral therapy response phenotypes. PLoS One. 2019;14(8):e0219201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Lee SS, Wong NS, Wong BCK, et al. Combining CD4 recovery and CD4: cD8 ratio restoration as an indicator for evaluating the outcome of continued antiretroviral therapy: an observational cohort study. BMJ Open. 2017;7(9):e016886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tanuma J, Matsumoto S, Haneuse S, et al. Long‐term viral suppression and immune recovery during first‐line antiretroviral therapy: a study of an HIV‐infected adult cohort in Hanoi, Vietnam. J Int AIDS Soc. 2017;20(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Boatman JA, Baker JV, Emery S, et al. Risk factors for low CD4+ count recovery despite viral suppression among participants initiating antiretroviral treatment with CD4+ counts >500 cells/mm3: findings from the Strategic Timing of AntiRetroviral Therapy (START) trial. J Acquir Immune Defic Syndr. 2019;81(1):10‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Roul H, Mary‐Krause M, Ghosn J, et al. CD4+ cell count recovery after combined antiretroviral therapy in the modern combined antiretroviral therapy era. AIDS. 2018;32(17):2605‐2614. [DOI] [PubMed] [Google Scholar]
- 168. Davy‐Mendez T, Napravnik S, Zakharova O, et al. Effectiveness of integrase strand transfer inhibitors among treatment‐experienced patients in a clinical setting. AIDS. 2019;33(7):1187‐1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Gandhi RT, Spritzler J, Chan E, et al. Effect of baseline‐ and treatment‐related factors on immunologic recovery after initiation of antiretroviral therapy in HIV‐1‐positive subjects: results from ACTG 384. J Acquir Immune Defic Syndr. 2006;42(4):426‐434. [DOI] [PubMed] [Google Scholar]
- 170. Rosado‐Sanchez I, Herrero‐Fernandez I, Alvarez‐Rios AI, et al. A lower baseline CD4/CD8 T‐cell ratio is independently associated with immunodiscordant response to antiretroviral therapy in HIV‐infected subjects. Antimicrob Agents Chemother. 2017;61(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Geng EH, Neilands TB, Thiebaut R, et al. CD41 T cell recovery during suppression of HIV replication: an international comparison of the immunological efficacy of antiretroviral therapy in North America, Asia and Africa. Int J Epidemiol. 2015;44(1):251‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Luz PM, Belaunzaran‐Zamudio PF, Crabtree‐Ramirez B, et al. CD4 response up to 5 years after combination antiretroviral therapy in human immunodeficiency virus‐infected patients in Latin America and the Caribbean. Open Forum Infect Dis. 2015;2(2):ofv079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. de Monteynard LA, Matheron S, Gilquin J, et al. Influence of geographic origin, sex, and HIV transmission group on the outcome of first‐line combined antiretroviral therapy in France. AIDS. 2016;30(14):2235‐2246. [DOI] [PubMed] [Google Scholar]
- 174. Seng R, Ghislain M, Girard PM, et al. Sub‐Saharan African migrants have slower initial CD4+ cell recovery after combined antiretroviral treatment initiation than French natives. AIDS. 2017;31(9):1323‐1332. [DOI] [PubMed] [Google Scholar]
- 175. Luo Z, Li Z, Martin L, et al. Pathological role of anti‐CD4 antibodies in HIV‐infected immunologic nonresponders receiving virus‐suppressive antiretroviral therapy. J Infect Dis. 2017;216(1):82‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Lisco A, Wong CS, Lage SL, et al. Identification of rare HIV‐1‐infected patients with extreme CD4+ T cell decline despite ART‐mediated viral suppression. JCI Insight. 2019;4(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Tincati C, Merlini E, Braidotti P, et al. Impaired gut junctional complexes feature late‐treated individuals with suboptimal CD4+ T‐cell recovery upon virologically suppressive combination antiretroviral therapy. AIDS. 2016;30(7):991‐1003. [DOI] [PubMed] [Google Scholar]
- 178. Agrati C, Tumino N, Bordoni V, et al. Myeloid derived suppressor cells expansion persists after early ART and may affect CD4 T cell recovery. Front Immunol. 2019;10:1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Girard A, Vergnon‐Miszczycha D, Depince‐Berger AE, et al. Brief report: a high rate of beta7+ gut‐homing lymphocytes in HIV‐infected immunological nonresponders is associated with poor CD4 T‐cell recovery during suppressive HAART. J Acquir Immune Defic Syndr. 2016;72(3):259‐265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Rosado‐Sanchez I, Herrero‐Fernandez I, Genebat M, et al. HIV‐infected subjects with poor CD4 T‐cell recovery despite effective therapy express high levels of OX40 and alpha4beta7 on CD4 T‐cells prior therapy initiation. Front Immunol. 2018;9:1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Sennepin A, Baychelier F, Guihot A, et al. NKp44L expression on CD4+ T cells is associated with impaired immunological recovery in HIV‐infected patients under highly active antiretroviral therapy. AIDS. 2013;27(12):1857‐1866. [DOI] [PubMed] [Google Scholar]
- 182. Ahuja SK, Kulkarni H, Catano G, et al. CCL3L1‐CCR5 genotype influences durability of immune recovery during antiretroviral therapy of HIV‐1‐infected individuals. Nat Med. 2008;14(4):413‐420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Yong YK, Shankar EM, Solomon A, et al. Polymorphisms in the CD14 and TLR4 genes independently predict CD4+ T‐cell recovery in HIV‐infected individuals on antiretroviral therapy. AIDS. 2016;30(14):2159‐2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Guzman‐Fulgencio M, Berenguer J, Micheloud D, et al. European mitochondrial haplogroups are associated with CD4+ T cell recovery in HIV‐infected patients on combination antiretroviral therapy. J Antimicrob Chemother. 2013;68(10):2349‐2357. [DOI] [PubMed] [Google Scholar]
- 185. Medrano LM, Gutierrez‐Rivas M, Blanco J, et al. Mitochondrial haplogroup H is related to CD4+ T cell recovery in HIV infected patients starting combination antiretroviral therapy. J Transl Med. 2018;16(1):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Andrade‐Santos JL, Carvalho‐Silva WHV, Coelho AVC, et al. IL18 gene polymorphism and its influence on CD4+ T‐cell recovery in HIV‐positive patients receiving antiretroviral therapy. Infect Genet Evol. 2019;75:103997. [DOI] [PubMed] [Google Scholar]
- 187. Resino S, Navarrete‐Munoz MA, Blanco J, et al. IL7RA rs6897932 polymorphism is associated with better CD4(+) T‐cell recovery in HIV infected patients starting combination antiretroviral therapy. Biomolecules. 2019;9(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Hartling HJ, Thorner LW, Erikstrup C, et al. Polymorphism in interleukin‐7 receptor alpha gene is associated with faster CD4(+) T‐cell recovery after initiation of combination antiretroviral therapy. AIDS. 2014;28(12):1739‐1748. [DOI] [PubMed] [Google Scholar]
- 189. Hartling HJ, Ryder LP, Ullum H, et al. Gene variation in IL‐7 receptor (IL‐7R)alpha affects IL‐7R response in CD4+ T cells in HIV‐infected individuals. Sci Rep. 2017;7:42036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Restrepo C, Gutierrez‐Rivas M, Pacheco YM, et al. Genetic variation in CCR2 and CXCL12 genes impacts on CD4 restoration in patients initiating cART with advanced immunesupression. PLoS One. 2019;14(3):e0214421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. El‐Beeli M, Al‐Mahrooqi SH, Youssef RM, et al. HLA‐A68 and HLA‐B15 alleles correlate with poor immune response among AIDS patients on combined antiretroviral therapy. Hum Immunol. 2016;77(6):490‐497. [DOI] [PubMed] [Google Scholar]
- 192. Joshi A, Punke EB, Mehmetoglu‐Gurbuz T, et al. TLR9 polymorphism correlates with immune activation, CD4 decline and plasma IP10 levels in HIV patients. BMC Infect Dis. 2019;19(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Masson JJR, Cherry CL, Murphy NM, et al. Polymorphism rs1385129 within Glut1 gene SLC2A1 is linked to poor CD4+ T cell recovery in antiretroviral‐treated HIV+ individuals. Front Immunol. 2018;9:900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Garcia M, Jimenez‐Sousa MA, Blanco J, et al. CD4 recovery is associated with genetic variation in IFNgamma and IL19 genes. Antiviral Res. 2019;170:104577. [DOI] [PubMed] [Google Scholar]
- 195. Palermo B, Bosch RJ, Bennett K, et al. Body mass index and CD4+ T‐lymphocyte recovery in HIV‐infected men with viral suppression on antiretroviral therapy. HIV Clin Trials. 2011;12(4):222‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Koethe JR, Jenkins CA, Lau B, et al. Body mass index and early CD4 T‐cell recovery among adults initiating antiretroviral therapy in North America, 1998–2010. HIV Med. 2015;16(9):572‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Koethe JR, Jenkins CA, Lau B, et al. Higher time‐updated body mass index: association with improved CD4+ cell recovery on HIV treatment. J Acquir Immune Defic Syndr. 2016;73(2):197‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Li X, Ding H, Geng W, et al. Predictive effects of body mass index on immune reconstitution among HIV‐infected HAART users in China. BMC Infect Dis. 2019;19(1):373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zaldivar F, McMurray RG, Nemet D, et al. Body fat and circulating leukocytes in children. Int J Obes (Lond). 2006;30(6):906‐911. [DOI] [PubMed] [Google Scholar]
- 200. Nowicki MJ, Karim R, Mack WJ, et al. Correlates of CD4+ and CD8+ lymphocyte counts in high‐risk immunodeficiency virus (HIV)‐seronegative women enrolled in the women's interagency HIV study (WIHS). Hum Immunol. 2007;68(5):342‐349. [DOI] [PubMed] [Google Scholar]
- 201. Womack J, Tien PC, Feldman J, et al. Obesity and immune cell counts in women. Metabolism. 2007;56(7):998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Palmer CS, Ostrowski M, Gouillou M, et al. Increased glucose metabolic activity is associated with CD4+ T‐cell activation and depletion during chronic HIV infection. AIDS. 2014;28(3):297‐309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Jimenez‐Sousa MA, Martinez I, Medrano LM, et al. Vitamin D in human immunodeficiency virus infection: influence on immunity and disease. Front Immunol. 2018;9:458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Ross AC, Judd S, Kumari M, et al. Vitamin D is linked to carotid intima‐media thickness and immune reconstitution in HIV‐positive individuals. Antivir Ther. 2011;16(4):555‐563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Aziz M, Livak B, Burke‐Miller J, et al. Vitamin D insufficiency may impair CD4 recovery among women's interagency HIV study participants with advanced disease on HAART. AIDS. 2013;27(4):573‐578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Ezeamama AE, Guwatudde D, Wang M, et al. Vitamin‐D deficiency impairs CD4+T‐cell count recovery rate in HIV‐positive adults on highly active antiretroviral therapy: a longitudinal study. Clin Nutr. 2016;35(5):1110‐1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Shivakoti R, Ewald ER, Gupte N, et al. Effect of baseline micronutrient and inflammation status on CD4 recovery post‐cART initiation in the multinational PEARLS trial. Clin Nutr. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Aguilar‐Jimenez W, Villegas‐Ospina S, Gonzalez S, et al. Precursor forms of vitamin d reduce HIV‐1 infection in vitro. J Acquir Immune Defic Syndr. 2016;73(5):497‐506. [DOI] [PubMed] [Google Scholar]
- 209. Rockstroh JK, Lennox JL, Dejesus E, et al. Long‐term treatment with raltegravir or efavirenz combined with tenofovir/emtricitabine for treatment‐naive human immunodeficiency virus‐1‐infected patients: 156‐week results from STARTMRK. Clin Infect Dis. 2011;53(8):807‐816. [DOI] [PubMed] [Google Scholar]
- 210. Edwards JK, Cole SR, Hall HI, et al. Virologic suppression and CD4+ cell count recovery after initiation of raltegravir or efavirenz‐containing HIV treatment regimens. AIDS. 2018;32(2):261‐266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Lennox JL, DeJesus E, Lazzarin A, et al. Safety and efficacy of raltegravir‐based versus efavirenz‐based combination therapy in treatment‐naive patients with HIV‐1 infection: a multicentre, double‐blind randomised controlled trial. Lancet. 2009;374(9692):796‐806. [DOI] [PubMed] [Google Scholar]
- 212. Rockstroh JK, DeJesus E, Lennox JL, et al. Durable efficacy and safety of raltegravir versus efavirenz when combined with tenofovir/emtricitabine in treatment‐naive HIV‐1‐infected patients: final 5‐year results from STARTMRK. J Acquir Immune Defic Syndr. 2013;63(1):77‐85. [DOI] [PubMed] [Google Scholar]
- 213. Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir‐lamivudine for the treatment of HIV‐1 infection. N Engl J Med. 2013;369(19):1807‐1818. [DOI] [PubMed] [Google Scholar]
- 214. Blanco JR, Alejos B, Moreno S. Impact of dolutegravir and efavirenz on immune recovery markers: results from a randomized clinical trial. Clin Microbiol Infect. 2018;24(8):900‐907. [DOI] [PubMed] [Google Scholar]
- 215. Zhang F, Sun M, Sun J, et al. The risk factors for suboptimal CD4 recovery in HIV infected population: an observational and retrospective study in Shanghai, China. Biosci Trends. 2015;9(5):335‐341. [DOI] [PubMed] [Google Scholar]
- 216. Bandera A, Colella E, Rizzardini G, et al. Strategies to limit immune‐activation in HIV patients. Expert Rev Anti Infect Ther. 2017;15(1):43‐54. [DOI] [PubMed] [Google Scholar]
- 217. Coelho L, Cardoso SW, Luz PM, et al. Vitamin D3 supplementation in HIV infection: effectiveness and associations with antiretroviral therapy. Nutr J. 2015;14:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Fabre‐Mersseman V, Tubiana R, Papagno L, et al. Vitamin D supplementation is associated with reduced immune activation levels in HIV‐1‐infected patients on suppressive antiretroviral therapy. AIDS. 2014;28(18):2677‐2682. [DOI] [PubMed] [Google Scholar]
- 219. Eckard AR, O'Riordan MA, Rosebush JC, et al. Vitamin D supplementation decreases immune activation and exhaustion in HIV‐1‐infected youth. Antivir Ther. 2018;23(4):315‐324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Ashenafi S, Amogne W, Kassa E, et al. Daily nutritional supplementation with vitamin D(3) and phenylbutyrate to treatment‐naive HIV patients tested in a randomized placebo‐controlled trial. Nutrients. 2019;11(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Onwumeh J, Okwundu CI, Kredo T. Interleukin‐2 as an adjunct to antiretroviral therapy for HIV‐positive adults. Cochrane Database Syst Rev. 2017;5:CD009818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Sereti I, Dunham RM, Spritzler J, et al. IL‐7 administration drives T cell‐cycle entry and expansion in HIV‐1 infection. Blood. 2009;113(25):6304‐6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Levy Y, Lacabaratz C, Weiss L, et al. Enhanced T cell recovery in HIV‐1‐infected adults through IL‐7 treatment. J Clin Invest. 2009;119(4):997‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Levy Y, Sereti I, Tambussi G, et al. Effects of recombinant human interleukin 7 on T‐cell recovery and thymic output in HIV‐infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo‐controlled, multicenter study. Clin Infect Dis. 2012;55(2):291‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Thiebaut R, Drylewicz J, Prague M, et al. Quantifying and predicting the effect of exogenous interleukin‐7 on CD4+ T cells in HIV‐1 infection. PLoS Comput Biol. 2014;10(5):e1003630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Sereti I, Estes JD, Thompson WL, et al. Decreases in colonic and systemic inflammation in chronic HIV infection after IL‐7 administration. PLoS Pathog. 2014;10(1):e1003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Thiebaut R, Jarne A, Routy JP, et al. Repeated cycles of recombinant human interleukin 7 in HIV‐Infected patients with low CD4 T‐Cell Reconstitution on antiretroviral therapy: results of 2 phase II multicenter studies. Clin Infect Dis. 2016;62(9):1178‐1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Katlama C, Lambert‐Niclot S, Assoumou L, et al. Treatment intensification followed by interleukin‐7 reactivates HIV without reducing total HIV DNA: a randomized trial. AIDS. 2016;30(2):221‐230. [DOI] [PubMed] [Google Scholar]
- 229. Zhang Z, Fu J, Xu X, et al. Safety and immunological responses to human mesenchymal stem cell therapy in difficult‐to‐treat HIV‐1‐infected patients. AIDS. 2013;27(8):1283‐1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Saison J, Ferry T, Demaret J, et al. Association between discordant immunological response to highly active anti‐retroviral therapy, regulatory T cell percentage, immune cell activation and very low‐level viraemia in HIV‐infected patients. Clin Exp Immunol. 2014;176(3):401‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Jarrin I, Pantazis N, Dalmau J, et al. Does rapid HIV disease progression prior to combination antiretroviral therapy hinder optimal CD4+ T‐cell recovery once HIV‐1 suppression is achieved. AIDS. 2015;29(17):2323‐2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Kim KH, Yi J, Lee SH. The CD4 slope can be a predictor of immunologic recovery in advanced HIV patients: a case‐control study. Korean J Intern Med. 2015;30(5):705‐713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Norris PJ, Zhang J, Worlock A, et al. Systemic cytokine levels do not predict CD4(+) T‐cell recovery after suppressive combination antiretroviral therapy in chronic human immunodeficiency virus infection. Open Forum Infect Dis. 2016;3(1):ofw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Marziali M, De Santis W, Carello R, et al. T‐cell homeostasis alteration in HIV‐1 infected subjects with low CD4 T‐cell count despite undetectable virus load during HAART. AIDS. 2006;20(16):2033‐2041. [DOI] [PubMed] [Google Scholar]
- 235. Engsig FN, Gerstoft J, Kronborg G, et al. Long‐term mortality in HIV patients virally suppressed for more than three years with incomplete CD4 recovery: a cohort study. BMC Infect Dis. 2010;10:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Goicoechea M, Smith DM, Liu L, et al. Determinants of CD4+ T cell recovery during suppressive antiretroviral therapy: association of immune activation, T cell maturation markers, and cellular HIV‐1 DNA. J Infect Dis. 2006;194(1):29‐37. [DOI] [PubMed] [Google Scholar]
- 237. Tan R, Westfall AO, Willig JH, et al. Clinical outcome of HIV‐infected antiretroviral‐naive patients with discordant immunologic and virologic responses to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47(5):553‐558. [DOI] [PubMed] [Google Scholar]
- 238. Marchetti G, Gazzola L, Trabattoni D, et al. Skewed T‐cell maturation and function in HIV‐infected patients failing CD4+ recovery upon long‐term virologically suppressive HAART. AIDS. 2010;24(10):1455‐1460. [DOI] [PubMed] [Google Scholar]
- 239. Gaardbo JC, Hartling HJ, Ronit A, et al. Regulatory T cells in HIV‐infected immunological nonresponders are increased in blood but depleted in lymphoid tissue and predict immunological reconstitution. J Acquir Immune Defic Syndr. 2014;66(4):349‐357. [DOI] [PubMed] [Google Scholar]
- 240. Woelk CH, Beliakova‐Bethell N, Goicoechea M, et al. Gene expression before HAART initiation predicts HIV‐infected individuals at risk of poor CD4+ T‐cell recovery. AIDS. 2010;24(2):217‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Gomez‐Mora E, Garcia E, Urrea V, et al. Preserved immune functionality and high CMV‐specific T‐cell responses in HIV‐infected individuals with poor CD4(+) T‐cell immune recovery. Sci Rep. 2017;7(1):11711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Naftalin CM, Wong NS, Chan DP, et al. Three different patterns of CD4 recovery in a cohort of Chinese HIV patients following antiretroviral therapy ‐ a five‐year observational study. Int J STD AIDS. 2015;26(11):803‐809. [DOI] [PubMed] [Google Scholar]
- 243. Younes SA, Talla A, Pereira Ribeiro S, et al. Cycling CD4+ T cells in HIV‐infected immune nonresponders have mitochondrial dysfunction. J Clin Invest. 2018;128(11):5083‐5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Gomez‐Mora E, Robert‐Hebmann V, Garcia E, et al. Brief report: impaired CD4 T‐cell response to autophagy in treated HIV‐1‐infected individuals. J Acquir Immune Defic Syndr. 2017;74(2):201‐205. [DOI] [PubMed] [Google Scholar]
- 245. Rosado‐Sanchez I, Rodriguez‐Gallego E, Peraire J, et al. Glutaminolysis and lipoproteins are key factors in late immune recovery in successfully treated HIV‐infected patients. Clin Sci (Lond). 2019;133(8):997‐1010. [DOI] [PubMed] [Google Scholar]


