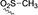Abstract
Rhodium‐catalyzed C−H insertions and cyclopropanations of donor/acceptor carbenes have been used for the synthesis of positional analogues of methylphenidate. The site selectivity is controlled by the catalyst and the amine protecting group. C−H functionalization of N‐Boc‐piperidine using Rh2(R‐TCPTAD)4, or N‐brosyl‐piperidine using Rh2(R‐TPPTTL)4 generated 2‐substitited analogues. In contrast, when N‐α‐oxoarylacetyl‐piperidines were used in combination with Rh2(S‐2‐Cl‐5‐BrTPCP)4, the C−H functionalization produced 4‐susbstiuted analogues. Finally, the 3‐substituted analogues were prepared indirectly by cyclopropanation of N‐Boc‐tetrahydropyridine followed by reductive regio‐ and stereoselective ring‐opening of the cyclopropanes.
Keywords: C−H functionalization, diastereoselectivity, piperidines, regioselectivity, rhodium
Attention, please: Regio, diastereo‐ and enantioselective C‐2, C‐3 and C‐4 functionalization on piperidine ring controlled by catalysts and protecting groups has been employed for the synthesis of positional analogues of methylphenidate (Ritalin).
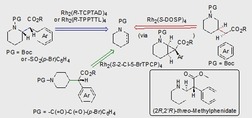
The piperidine ring with substituents at different positions is a prominent structural element in numerous pharmaceuticals,1 including Ritalin (methylphenidate), a therapeutic agent for attention deficit hyperactivity disorder.2 Traditional synthetic routes to these heterocycles typically involve ring construction or require functionalized piperidines,2, 3 with the latter being challenging owing to the lack of readily available enantiopure piperidine precursors. An alternative strategy would be the direct, site selective C−H functionalization, ideally at any position of the piperidine moiety at will. Many examples have been disclosed on the use of C−H functionalization as a key disconnection strategy for the synthesis of natural products and pharmaceutical targets.4 The majority of these applications rely on using either directing groups5 in the substrate or on the inherent reactivity6 of the substrate to control‐site selectivity. Considerable interest has also been shown in developing catalyst‐controlled6c, 7 or enzyme‐controlled8 C−H functionalization reactions. The C−H functionalization at the C2 position on piperidine derivatives has been achieved using several different approaches.9 However, selective functionalization at the remote positions of the piperidine moiety, that is, C3 and C4, is limited.10, 11
We have been exploring the rhodium‐catalyzed reactions of donor/acceptor carbenes for catalyst‐controlled C−H functionalization.6c, 7 Recently, we have designed catalysts that are capable of selective functionalization of inactivated primary, secondary, and tertiary C−H bonds,12 inactivated C−H bonds over electronically activated C−H bonds,13 and desymmetrization of alkylcyclohexanes.14 In this project, we describe the application of these catalysts to generate methylphenidate analogues with substituents at either C2, C3, or C4 of the piperidine rings starting from appropriate piperidine derivatives (Figure 1). The C−H functionalization at C2 is electronically preferred, because the build‐up of positive charge at carbon during the C−H functionalization would be stabilized by the nitrogen group.15, 16 The C−H bond at C3 would be deactivated through the inductive effect of nitrogen. The electronic deactivation would be less for C4, which should be sterically the most accessible position. Thus, a direct functionalization of the C−H bond at C4 should be feasible by sterically shielding at C2 position, whereas we envisioned that C−H activation at C3 might become possible by an indirect approach via regioselective ring‐opening of an appropriate cyclopropanated tetrahydropyridine.
Figure 1.
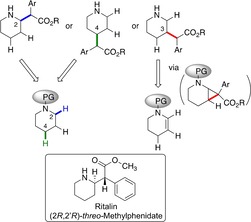
Synthetic strategies towards C−H functionalization of piperidines at C2, C3 and C4. C2−H: electronically activated but sterically hindered; C3−H: electronically deactivated through inductive effect of NPg, indirect approach through regio‐ and stereoselective cyclopropane ring opening; C4−H: accessible if the electronic preference for C2 can be overridden by steric shielding of catalyst and NPg.
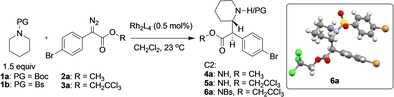
The first stage of this project was to optimize the C2 functionalization of piperidines. The basic transformation is one of the early classic C−H functionalization reactions of donor/acceptor carbenes, described independently by Davies15 and Winkler.16 In the original studies, the control of both the diastereoselectivity and enantioselectivity of the C−H functionalization was relatively moderate. Therefore, we decided to re‐examine this transformation using the specialized chiral dirhodium catalysts that have been recently developed. The key optimization studies are summarized in Table 1 and Scheme 1 (see the Supporting Information for more extensive details). The original Rh2(S‐DOSP)4‐catalyzed reaction of methyl aryldiazoacetate 2 a reacting with N‐Boc‐piperidine 1 a gives a 1:1 mixture of diastereomers.15 Several of the newer chiral dirhodium tetracarboxylate catalysts (Table 1<xtabr1) were tested under the same reaction conditions. Most of the catalysts furnished the C2‐functionalized product 4 a with 1:1 to 2:1 d.r. (entries 2–4) and low to moderate enantioselectivity (27–66 % ee), whereas the C4‐symmetric catalyst, Rh2(S‐2‐Cl‐5‐BrTPCP)4, enhanced the stereoselectivity to 5.3:1 d.r. and 83 % ee for the major diastereomer 4 a (entry 5). Another major advance in site‐selective C−H functionalization has been the use of aryldiazoacetates containing trichloroethyl esters instead of methyl esters as donor/acceptor carbene precursors.17 Hence, we evaluated the influence of the ester switch on the stereoselectivity of the C2 functionalization. The level of diastereoselectivity in the reaction of 1 a using trichloroethyl derivative 3 a, catalyzed by Rh2(S‐2‐Cl‐5‐BrTPCP)4, dropped considerably versus the methyl ester (entry 6). Fortunately, the Rh2(R‐TCPTAD)4‐catalyzed transformation to form 5 a lead to a considerable improvement in the stereoselectivity (11:1 d.r., 93 % ee) in 83 % yield (entry 7). The diastereoselectivity was greatly improved (27:1 d.r.) when Rh2(R‐TPPTTL)4 was used as catalyst, but with lower enantioselectivity (69 % ee, entry 8). Higher enantioselectivity (77 % ee) with Rh2(R‐TPPTTL)4 was obtained when an arylsulfonyl piperidine derivative 1 b was used (6 a, entry 9). Further optimization of the temperature showed improvement in yield with only a small decrease in stereoselectivity at higher temperature (39 °C: 87 % yield, 22:1 d.r., 76 % ee, entry 10), whereas the reaction at 0 °C caused declines in both yield and stereoselectivity (entry 11).
Table 1.
Optimization studies for C2 functionalization.[a]
|
| ||||||
|---|---|---|---|---|---|---|
|
Entry |
1 (PG) |
2 a/3 a (R) |
L |
Yield[b] [%] |
d.r.[c] |
ee [d] [%] |
|
1[e,f] |
1 a (Boc) |
2 a (CH3) |
S‐DOSP |
69 |
1.5:1 |
−69 |
|
2[f] |
1 a (Boc) |
2 a (CH3) |
R‐TCPTAD |
69 |
1.4:1 |
66 |
|
3[f] |
1 a (Boc) |
2 a (CH3) |
R‐p‐BrTPCP |
41 |
1.2:1 |
27 |
|
4[f] |
1 a (Boc) |
2 a (CH3) |
R‐TPPTTL |
69 |
1.5:1 |
54 |
|
5[f] |
1 a (Boc) |
2 a (CH3) |
S‐2‐Cl‐5‐BrTPCP |
83 |
5.3:1 |
83 |
|
6[f] |
1 a (Boc) |
3 a (CH2CCl3) |
S‐2‐Cl‐5‐BrTPCP |
73 |
3.6:1 |
65 |
|
7 [f] |
1 a (Boc) |
3 a (CH2CCl3) |
R ‐TCPTAD |
83 |
11:1 |
93 |
|
8[f] |
1 a (Boc) |
3 a (CH2CCl3) |
R‐TPPTTL |
80 |
27:1 |
69 |
|
9 |
1 b (Bs) |
3 a (CH2CCl3) |
R‐TPPTTL |
76 |
>30:1 |
77 |
|
10[g] |
1 b (Bs) |
3 a (CH2CCl3) |
R‐TPPTTL |
87 |
22:1 |
76 |
|
11[h] |
1 b (Bs) |
3 a (CH2CCl3) |
R‐TPPTTL |
42 |
26:1 |
72 |
[a] Reaction conditions: a solution of 2 a–3 a (0.5 mmol) in 4 mL pentane/CH2Cl2 was added over 2 h to the solution of Rh2L4 (0.5 mol %) and 1 a,b (0.75 mmol) in 2 mL pentane/CH2Cl2. [b] Yield of isolated material. [c] Determined from crude 1H NMR. [d] Determined by chiral HPLC analysis of isolated product. [e] Reaction in pentane instead of CH2Cl2. [f] Analysis of yield, d.r. and ee were on free amine product after Boc‐deprotection via trifluoroacetic acid. [g] Reaction at refluxing CH2Cl2 (39 °C). [h] Reaction at 0 °C. Boc=tert‐butyloxycarbonyl, Bs=p‐bromo‐phenylsulfonyl. The absolute stereochemistry was deduced by comparison of products to those of the earlier study15a and confirmed by crystal structure of 6 a.
Scheme 1.
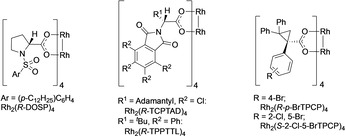
Catalyst structures.
The scope of the C2 functionalization of piperidine was examined using the two most promising conditions, N‐Boc‐piperidine functionalization catalyzed by Rh2(R‐TCPTAD)4 and N‐Bs‐piperidine functionalization catalyzed by Rh2(R‐TPPTTL)4 (Scheme 2). The Rh2(R‐TCPTAD)4‐catalyzed reactions gave moderate yield but variable stereoselectivity, reaching low levels with electron deficient aryldiazoacetates. In contrast, the Rh2(R‐TPPTTL)4‐catalyzed reactions were highly diastereoselective for all the substrates (29‐>30:1 d.r.) and maintained relatively constant levels of enantioselectivity (52–73 % ee).
Scheme 2.
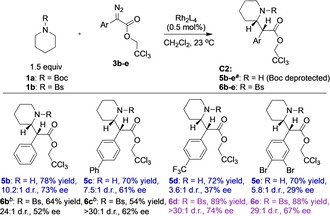
Substrate scope of C2 functionalization. The N‐Boc‐piperidine (1 a) functionalization was catalyzed by Rh2(R‐TCPTAD)4 to form 5 b–e and N‐Bs‐piperidine (1 b) functionalization was catalyzed by Rh2(R‐TPPTTL)4 to form 6 b–e. [a] Boc group was removed through trifluoroacetic acid treatment before analysis. [b] reaction conducted in refluxing CH2Cl2 (39 °C).
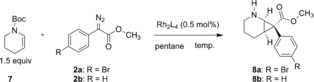
Having established the C2 functionalization of piperidine, we then explored how to introduce the arylacetate group at the C3 position. The direct C−H functionalization of piperidines was not considered to be a viable option, because the C3 position would be deactivated towards carbene C−H insertions caused by the inductively electron‐withdrawing effect of the nitrogen. Therefore, we explored an indirect approach through asymmetric cyclopropanation of a tetrahydropyridine followed by a reductive ring opening of the cyclopropane intermediate. A catalyst screen was conducted on the cyclopropanation of the N‐Boc‐tetrahydropyridine 7 to generate 8 and the key results are shown in Table 2 (see the Supporting Information for more extensive details). It is well established that Rh2(R‐DOSP)4 performs best when the methyl ester of aryldiazoacetates and hydrocarbon solvents are used.6c The classic catalyst, Rh2(R‐DOSP)4, is still unmatched for this type of cyclopropanation with methyl p‐bromophenyldiazoacetate 2 a, whereas other catalysts are considerably inferior (entry 1–4). A temperature screen revealed that 0 °C was the optimum condition (entries 5–7). Under these conditions, the cyclopropanation with methyl phenyldiazoacetate 2 b proceeded in 87 % yield, >30:1 d.r. and 95 % ee.
Table 2.
Optimization studies for cyclopropanation.[a]
|
| ||||||
|---|---|---|---|---|---|---|
|
Entry |
2 |
L |
T [°C] |
Yield[b] [%] |
d.r.[c] |
ee [d] [%] |
|
1 |
2 a (Br) |
R‐TCPTAD |
23 |
75 |
>30:1 |
3 |
|
2 |
2 a (Br) |
R‐p‐BrTPCP |
23 |
73 |
>30:1 |
8 |
|
3 |
2 a (Br) |
S‐2‐Cl‐5‐BrTPCP |
23 |
77 |
>30:1 |
‐69 |
|
4 |
2 a (Br) |
R‐DOSP |
23 |
76 |
>30:1 |
‐89 |
|
5 |
2 b (H) |
S‐DOSP |
23 |
83 |
>30:1 |
‐92 |
|
6 |
2 b (H) |
S‐DOSP |
0 |
87 |
>30:1 |
95 |
|
7 |
2 b (H) |
S‐DOSP |
‐40 |
85 |
>30:1 |
95 |
[a] Reaction conditions: a solution of 2 a–e (0.5 mmol) in 12 mL of solvent was added over 2 h to the solution of Rh2L4 (0.5 mol %) and 7 (0.75 mmol) in 2 mL of solvent. [b] Yield of isolated material. [c] Determined from crude 1H NMR. [d] Determined by chiral HPLC analysis of isolated product. A negative sign indicates that the product is the opposite enantiomer to the one drawn in the Scheme. Boc=tert‐butyloxycarbonyl.
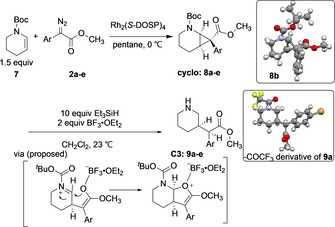
The next stage was to combine the asymmetric cyclopropanation with the reductive ring‐opening. This reaction was examined with five representative examples of aryldiazoacetates, and the results are summarized in Table 3. Rh2(S‐DOSP)4‐catalyzed cyclopropanation of aryldiazoacetates 2 a–e were examined and the cyclopropanes 8 a–e were produced in high yields (85–93 %) as single diastereomers (>30:1 d.r.) and moderate to high levels of enantiocontrol (81–95 % ee). The X‐ray structure of 8 b was consistent with cyclopropanation occurring at the Re face of the carbene, which is standard for Rh2(S‐DOSP)4‐catalyzed reactions. Reductive ring opening of the cyclopropanes 8 a–e using Et3SiH and BF3⋅Et2O18 resulting in concomitant removal of the N‐Boc protecting group and the generation of the desired C3‐substituted analogues 9 a–e in 67–92 % yield as single diastereomers (>30:1 dr) and retention of the asymmetric induction obtained in the cyclopropanation. The absolute stereochemistry was assigned basing on the crystal structure of trifluoroacetyl‐protected 9 a. The retention of the chirality at the benzylic carbon was proposed to arise from the formation of a bicyclic intermediate from the ring‐opened enolate, in which the bottom face cis to the bridging hydrogens is more accessible.
Table 3.
Substrate scope of C3‐functionalization.[a]
|
| ||||||
|---|---|---|---|---|---|---|
|
Entry |
Ar |
8 |
9 |
|||
|
|
|
Yield [%] |
ee [%] |
Yield [%] |
d.r. |
ee [%] |
|
1 |
|
93 (8 a) |
92 |
67 (9 a) |
>30:1 |
93 |
|
2 |
|
87 (8 b) |
95 |
70 (9 b) |
>30:1 |
92 |
|
3 |
|
86 (8 c) |
90 |
92 (9 c) |
>30:1 |
90 |
|
4 |
|
85 (8 d) |
86 |
77 (9 d) |
>30:1 |
87 |
|
5 |
|
90 (8 e) |
81 |
90 (9 e) |
>30:1 |
80 |
[a] Minimal amount of PhCF3 was added to dissolve the aryldiazoacetate.
Two approaches were examined to install the arylacetate functionality at the C4 position of the piperidine. The first attempt examined the allylic C−H functionalization of N‐Boc‐dihydropyridine 10 as the substrate (Scheme 3). Although the dihydropyridine might be expected to be susceptible to cyclopropanation rather than C−H functionalization, we had already established that 1,4‐cyclohexadiene strongly favors C−H functionalization.19 We expected the doubly allylic position in 10 to be similarly activated towards C−H functionalization, and this proved to be the case. The catalyst screen using the phenyldiazoacetate 2 b revealed that Rh2(R‐DOSP)4 is the optimum catalyst (see the Supporting Information for details). Due to the instability of the dihydropyridine 10 and the product 11, the reaction was somewhat challenging and neat conditions were used for the C−H insertion followed by immediate hydrogenation of 11. Under these conditions, the C4‐substituted product 12 was obtained in 54 % overall yields and 61 % ee.
Scheme 3.
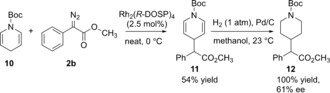
C4‐Analog from N‐Boc‐dihydropyridine.
A more innovative approach to C4‐substituted analogues would be the direct C−H functionalization on the saturated piperidine derivative. We have already proven that the rhodium‐stabilized donor/acceptor carbenes are sterically demanding and some of the new catalysts drive the site selectivity away from the electronically favored sites to the sterically most accessible sites. Therefore, by appropriate choice of catalyst and protecting group on nitrogen, we anticipated that it should be possible to alter the selectivity from C2 to C4 positions. The optimization study to achieve this goal is shown in Table 4. In the initial examination of the catalysts in reactions on N‐p‐bromophenylsulfonyl‐piperidine, most of the catalysts gave clean C2‐functionalization selectivity or no reaction (entries 1–3), while the Rh2(S‐2‐Cl‐5‐BrTPCP)4‐catalyzed reaction (entry 5) proceeded with 4.2:1 r.r. favoring the C4 insertion product 13 b in good yield (67 %) and enantiocontrol (90 % ee). As expected, the C2 position is less activated with electron‐withdrawing substituent on the arylsulfonyl group and gave slight improvement in the site selectivity (entry 6 vs., entry 5 and 4). A less bulky protecting group was expected to have a negative effect on the steric blocking of the C2 position; however, the smaller mesyl group caused an increased ratio for the C4 product (entry 7 vs. entry 4). With limited effect on the site selectivity with various sulfonyl groups, a more electron‐withdrawing protecting group, α‐oxoarylacetyl group as in 1 f, was utilized for better selectivity. With this adjustment, the site selectivity between C4 and C2 improved to >30:1 r.r. and 13 e was formed in 98 % ee, preferring the S configuration at the benzylic chiral center according to the crystal structure of 13 b. Switching the temperature and substrate ratio enhanced the yield (50 % at 23 °C and 1.5:1 1 f:3 a, entry 9 vs. 61 % at 39 °C and 1:1.5 1 f:3 a, entry 11) without influencing the site and enantioselectivity. The efficiency of Rh2(S‐2‐Cl‐5‐BrTPCP)4 in C‐4 functionalization of 1 f was explored using the optimized conditions (Scheme 4). When the substituents on the aryl ring in the diazoacetates were electron‐withdrawing (13 f, 13 h) high levels of enantiocontrol were retained (96–98 % ee) with moderate yields (50–57 %). When an electron‐rich aryl ring in the diazoacetate was used, both the yield and the enantioselectivity decreased (19 % yield, 75 % ee for 13 g).
Table 4.
Optimization studies for C4‐functionalization.[a]
|
| |||||||
|---|---|---|---|---|---|---|---|
|
Entry |
1 |
PG |
L |
T [%] |
r.r. (C4:C2)[b] |
Yield(C4)[c] [%] |
ee (C4)[d] [%] |
|
1 |
1 b |
|
R‐DOSP |
23 |
<1:30 |
– |
– |
|
2 |
1 b |
R‐TCPTAD |
23 |
<1:30 |
– |
– |
|
|
3 |
1 b |
R‐p‐BrTPCP |
23 |
–[e] |
– |
– |
|
|
4 |
1 b |
S‐2‐Cl‐5‐BrTPCP |
23 |
4.2:1 |
76 (13 a) |
90 |
|
|
5 |
1 c |
|
S‐2‐Cl‐5‐BrTPCP |
23 |
4.0:1 |
30[f] (13 b) |
96 |
|
6 |
1 d |
|
S‐2‐Cl‐5‐BrTPCP |
23 |
4.7:1 |
65 (13 c) |
96 |
|
7 |
1 e |
|
S‐2‐Cl‐5‐BrTPCP |
23 |
5.6:1 |
78 (13 d) |
97 |
|
8 |
1 f |
|
S‐2‐Cl‐5‐BrTPCP |
23 |
>30:1 |
50 (13 e) |
97 |
|
9 |
1 f |
S‐2‐Cl‐5‐BrTPCP |
39 |
>30:1 |
76 (13 e) |
97 |
|
|
10[g] |
1 f |
S‐2‐Cl‐5‐BrTPCP |
39 |
>30:1 |
76 (13 e) |
97 |
|
[a] Reaction conditions: a solution of 2 b (0.5 mmol) in 4 mL CH2Cl2 was added over 2 h to the solution of Rh2L4 (0.5 mol %) and 1 b–f (0.75 mmol) in 2 mL CH2Cl2. The reaction was allowed to stir for overnight. [b] Determined by crude 1H‐NMR. [c] Yield of isolated material. [d] Determined by chiral HPLC analysis. [e] No C−H functionalization products. [f] 40 % yield of primary C−H insertion on tosyl group. [g] 1.5 equiv of 3 a and 1.0 equiv of 1 f were used.
Scheme 4.
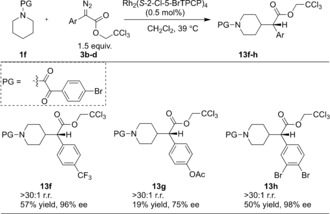
Substrate scope of C4 functionalization.
In summary, this study reveals that by appropriate considerations of the electronic and steric demands of the dirhodium catalysts, it is possible to functionalize piperidines at C2, C3 or C4. This leads to the synthesis of a small library of position analogues of methylphenidate.
Conflict of interest
HMLD is a named inventor on a patent entitled, Dirhodium Catalyst Compositions and Synthetic Processes Related Thereto (US 8,974,428, issued 3/10/2015). The other authors have no competing financial interests.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank Dr. John Bacsa for the X‐ray structure determination. Financial support (HMLD) was provided by NSF under the CCI Center for Selective C−H Functionalization (CHE‐1700982) and AbbVie. Financial support (OR) was provided by Elitenetzwerk Bayern (SYNCAT). Financial support for the development of the catalyst, Rh2(R‐TPPTTL)4 was provided by NIH (GM099142‐05). We thank Dr. Eric A. Voight and Mark A. Matulenko from AbbVie, and Shane W. Krska and Jaume Balsells‐Padros from Merck for helpful discussions. Funds to purchase the NMR and X‐ray spectrometers used in these studies were supported by NSF (CHE 1531620 and CHE 1626172).
W. Liu, T. Babl, A. Röther, O. Reiser, H. M. L. Davies, Chem. Eur. J. 2020, 26, 4236.
Contributor Information
Prof. Dr. Oliver Reiser, Email: oliver.reiser@chemie.uni-regensburg.de.
Prof. Dr. Huw M. L. Davies, Email: hmdavie@emory.edu.
References
- 1.
- 1a. Vitaku E., Smith D. T., Njardarson J. T., J. Med. Chem. 2014, 57, 10257–10274; [DOI] [PubMed] [Google Scholar]
- 1b. Taylor R. D., MacCoss M., Lawson A. D. G., J. Med. Chem. 2014, 57, 5845–5859. [DOI] [PubMed] [Google Scholar]
- 2. Prashad M., Adv. Synth. Catal. 2001, 343, 379–392. [Google Scholar]
- 3.
- 3a. Vo C.-V. T., Bode J. W., J. Org. Chem. 2014, 79, 2809–2815; [DOI] [PubMed] [Google Scholar]
- 3b. Eicher T., Hauptmann S., Speicher A., The Chemistry of Heterocycles: Structure, Reactions, Synthesis and Applications. Wiley-VCH, Weinheim, 2003; [Google Scholar]
- 3c. Wolfe J. P., Synthesis of Heterocycles via Metal-Catalyzed Reactions That Generate One or More Carbon-Heteroatom Bonds, Springer, Heidelberg, 2013. [Google Scholar]
- 4.Selected reviews of C−H functionalization applied to synthesis:
- 4a. Gutekunst W. R., Baran R. S., Chem. Soc. Rev. 2011, 40, 1976–1991; [DOI] [PubMed] [Google Scholar]
- 4b. McMurray L., O'Hara F., Gaunt M. J., Chem. Soc. Rev. 2011, 40, 1885–1898; [DOI] [PubMed] [Google Scholar]
- 4c. Yamaguchi J., Yamaguchi A. D., Itami K., Angew. Chem. Int. Ed. 2012, 51, 8960–9009; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 9092–9142; [Google Scholar]
- 4d. Wencel-Delord J., Glorius F., Nat. Chem. 2013, 5, 369–375; [DOI] [PubMed] [Google Scholar]
- 4e. Hartwig J. F., J. Am. Chem. Soc. 2016, 138, 2–24; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4f. Davies H. M. L., Morton D., J. Org. Chem. 2016, 81, 343–350; [DOI] [PubMed] [Google Scholar]
- 4g. Abrams D. J., Provencher P. A., Sorensen E. J., Chem. Soc. Rev. 2018, 47, 8925–8967. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. He J., Wasa M., Chan K. S. L., Shao Q., Yu J.-Q., Chem. Rev. 2017, 117, 8754–8786; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5b. Lyons T. W., Sanford M. S., Chem. Rev. 2010, 110, 1147–1169; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c. Chen Y.-Q., Wang Z., Wu Y., Wisniewski S. R., Qiao J. X., Ewing W. R., Eastgate M. D., Yu J.-Q., J. Am. Chem. Soc. 2018, 140, 17884–17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.
- 6a. Horn E. J., Rosen B. R., Chen Y., Tang J., Chen K., Eastgate M. D., Baran P. S., Nature 2016, 533, 77–81; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6b. Le C., Liang Y., Evans R. W., Li X., MacMillan D. W. C., Nature 2017, 547, 79–83; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6c. Davies H. M. L., Morton D., Chem. Soc. Rev. 2011, 40, 1857–1869. [DOI] [PubMed] [Google Scholar]
- 7.
- 7a. Hartwig J. F., Acc. Chem. Res. 2017, 50, 549–555; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7b. Hartwig J. F., Larsen M. A., ACS Cent. Sci. 2016, 2, 281–292; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7c. Chiappini N. D., Mack J. B. C., Du Bois J., Angew. Chem. Int. Ed. 2018, 57, 4956–4959; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 5050–5053; [Google Scholar]
- 7d. White M. C., Zhao J., J. Am. Chem. Soc. 2018, 140, 13988–14009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7e. Qin C., Davies H. M. L., J. Am. Chem. Soc. 2014, 136, 9792–9796. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Lowell N. A., DeMars M. D., Slocum S. T., Yu F., Anand K., Chemler J. A., Korakavi N., Priessnitz J. K., Park S. R., Koch A. A., Schultz P. J., Sherman D. H., J. Am. Chem. Soc. 2017, 139, 7913–7920; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8b. Zhang R. K., Huang X., Arnold F. H., Curr. Opin. Chem. Biol. 2019, 49, 67–75; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8c. Narayan A. R. H., Jiménez-Osés G., Liu P., Negretti S., Zhao W., Gilbert M. M., Ramabhadran R. O., Yang Y. F., Furan L., Li Z., Podust L. M., Montgomery J., Houk K. N., Sherman D. H., Nat. Chem. 2015, 7, 653–660; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8d. Zhang R. K., Chen K., Huang X., Wohlschlager L., Renata H., Arnold F. H., Nature 2019, 565, 67–72; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8e. C. R. Zwick III , Renata H., J. Am. Chem. Soc. 2018, 140, 1165–1169. [DOI] [PubMed] [Google Scholar]
- 9.Selected examples for C2 functionalization:
- 9a. Chen W., Ma L., Paul A., Seidel D., Nat. Chem. 2018, 10, 165–169; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9b. Beak P., Kerrick S. T., Wu S., Chu J., J. Am. Chem. Soc. 1994, 116, 3231–3239; [Google Scholar]
- 9c. Seel S., Thaler T., Takatsu K., Zhang C., Zipse H., Straub B. F., Mayer P., Knochel P., J. Am. Chem. Soc. 2011, 133, 4774–4777. [DOI] [PubMed] [Google Scholar]
- 10.Selected examples for C3 functionalization: Millet A., Larini P., Clot E., Baudoin O., Chem. Sci. 2013, 4, 2241–2247. [Google Scholar]
- 11.C4 functionalization:
- 11a. Topczewski J. J., Cabrera P. J., Saper N. I., Sanford M. S., Nature 2016, 531, 220–224; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11b. Cabrera P. J., Lee M., Sanford M. S., J. Am. Chem. Soc. 2018, 140, 5599–5606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.
- 12a. Liao K., Yang Y.-F., Li Y., Sanders J., Houk K. N., Musaev D. G., Davies H. M. L., Nat. Chem. 2018, 10, 1048–1055; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12b. Liao K., Negretti S., Musaev D. G., Bacsa J., Davies H. M. L., Nature 2016, 533, 230–234; [DOI] [PubMed] [Google Scholar]
- 12c. Liao K., Pickel T. C., Boyarskikh V., Bacsa J., Musaev D. G., Davies H. M. L., Nature 2017, 551, 609–613. [DOI] [PubMed] [Google Scholar]
- 13. Liu W., Ren Z., Bosse A. T., Liao K., Goldstein E. L., Bacsa J., Musaev D. G., Stoltz B. M., Davies H. M. L., J. Am. Chem. Soc. 2018, 140, 12247–12255. [DOI] [PubMed] [Google Scholar]
- 14. Fu J., Ren Z., Bacsa J., Musaev D. G., Davies H. M. L., Nature 2018, 564, 395–399. [DOI] [PubMed] [Google Scholar]
- 15.
- 15a. Davies H. M. L., Hansen T., Hopper D. W., Panaro S. A., J. Am. Chem. Soc. 1999, 121, 6509–6510; [Google Scholar]
- 15b. Davies H. M. L., Venkataramani C., Hansen T., Hopper D. W., J. Am. Chem. Soc. 2003, 125, 6462–6468. [DOI] [PubMed] [Google Scholar]
- 16. Axten J. M., Krim L., Kung H. F., Winkler J. D., J. Org. Chem. 1998, 63, 9628–9629. [Google Scholar]
- 17. Guptill D. M., Davies H. M. L., J. Am. Chem. Soc. 2014, 136, 17718–17721. [DOI] [PubMed] [Google Scholar]
- 18. Pilsl L. K. A., Ertl T., Reiser O., Org. Lett. 2017, 19, 2754–2757. [DOI] [PubMed] [Google Scholar]
- 19.
- 19a. Davies H. M. L., Hansen T., Churchill M. R., J. Am. Chem. Soc. 2000, 122, 3063–3070; [Google Scholar]
- 19b. Hansen J., Autschbach J., Davies H. M. L., J. Org. Chem. 2009, 74, 6555–6563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary