Community-onset bloodstream infections (CO-BSI) are major causes of severe febrile illness and death worldwide. In light of new data and the growing problem of antimicrobial resistance (AMR) among pathogens causing BSI, we undertook a systematic review of hospital-based studies of CO-BSI among patients hospitalized with fever.
KEYWORDS: antimicrobial resistance, bacteremia, bloodstream infections, community-onset infections
ABSTRACT
Community-onset bloodstream infections (CO-BSI) are major causes of severe febrile illness and death worldwide. In light of new data and the growing problem of antimicrobial resistance (AMR) among pathogens causing BSI, we undertook a systematic review of hospital-based studies of CO-BSI among patients hospitalized with fever. Without restriction to language or country, we searched PubMed, Web of Science, and Scopus for prospective hospital-based studies of culture-confirmed CO-BSI among febrile inpatients. We determined by study the prevalence of BSI among participants, the pathogens responsible for BSI, and the antimicrobial susceptibility patterns of pathogens causing BSI, according to place and time. Thirty-four (77.3%) of 44 eligible studies recruited 29,022 participants in Africa and Asia combined. Among participants in these two regions, the median prevalence of BSI was 12.5% (range, 2.0 to 48.4%); of 3,220 pathogens isolated, 1,119 (34.8%) were Salmonella enterica, 425 (13.2%) Streptococcus pneumoniae, and 282 (8.8%) Escherichia coli. Antimicrobial susceptibility testing was reported in 16 (36.4%) studies. When isolates collected prior to 2008 were compared to those collected in the period of 2008 through 2018, the proportions of typhoidal Salmonella and Staphylococcus aureus isolates resistant to several clinically relevant antimicrobials increased over time, while S. pneumoniae susceptibility was stable. CO-BSI remain a major cause of severe febrile illness among hospitalized patients in Africa and Asia, with S. enterica, S. pneumoniae, and E. coli predominating. There is a concerning increase in AMR among serious infections caused by community-onset pathogens. Ongoing surveillance is needed to inform empirical management and strategies to control AMR.
INTRODUCTION
Fever is a common reason for persons to seek health care at an emergency department (ED) or inpatient facility (1–4). While febrile illnesses seen at the community or primary care level are often self-limiting (5, 6), patients with severe illness commonly are among those presenting for hospital care (2, 7–11). With declines in malaria as a cause of febrile illness in low-resource areas (12, 13), attention to other causes of severe febrile illness, including bloodstream infections (BSI), has increased (14). Timely administration of appropriate empirical antimicrobial therapy can be life-saving, but designing the most appropriate empirical antimicrobial regimen requires a robust understanding of common causes of bacteremia and their patterns of antimicrobial resistance (AMR).
The patterns of organisms causing hospital-acquired and health care-associated BSI differ from those causing community-onset BSI (CO-BSI) (15). Hospital-acquired infections are defined as those with onset >48 h after hospital admission (16), and health care-associated infections are those associated with recent hospital admission or exposure to health care facilities (17, 18). AMR has been increasing among some pathogens in the community (19–21), risking mismatches between empirical antimicrobial regimens and etiological agents. For low-resource settings, standardized, high-quality laboratory services to identify AMR may be limited and local or national surveillance data may be unavailable (22, 23). In this context, sentinel site studies often provide the best evidence to inform management and to monitor trends in AMR (24).
Two systematic reviews described the epidemiology of CO-BSI in Africa and Asia, through 2009 and 2010, respectively (25, 26). Since then, new data have been published and concern has grown regarding AMR among community-onset pathogens, necessitating changes in international guidelines for empirical therapy of severe febrile illness (27, 28).
We performed a systematic review and meta-analysis to update and to expand previous reviews, to inform the empirical management of severe febrile illness, and control strategies for major pathogens. Our review was designed to inform the management of BSI in patients presenting with severe febrile illness, rather than BSI in patients meeting the definition of sepsis, which is a distinct clinical problem that been reviewed by others (29, 30).
(This work was presented in part at ASM Microbe 2019, San Francisco, CA, 20 to 24 June 2019.)
RESULTS
Our search of three online databases yielded 11,113 articles (Fig. 1). After the addition of 147 references from the two prior systematic reviews and the removal of 3,374 duplicates, a total of 7,886 titles and abstracts were screened for inclusion. We excluded 7,634 abstracts, leaving 252 full-text articles to screen. We excluded 190 articles based on study design, improper inclusion criteria, insufficient data to abstract, or inadequate reference standard diagnostics. Another 22 articles describing outpatient studies were excluded. Screening of the bibliographies of the included articles added 4 eligible articles, resulting in 44 articles included for analysis (31–74). Quality assessment is available in Table S1 in the supplemental material.
FIG 1.
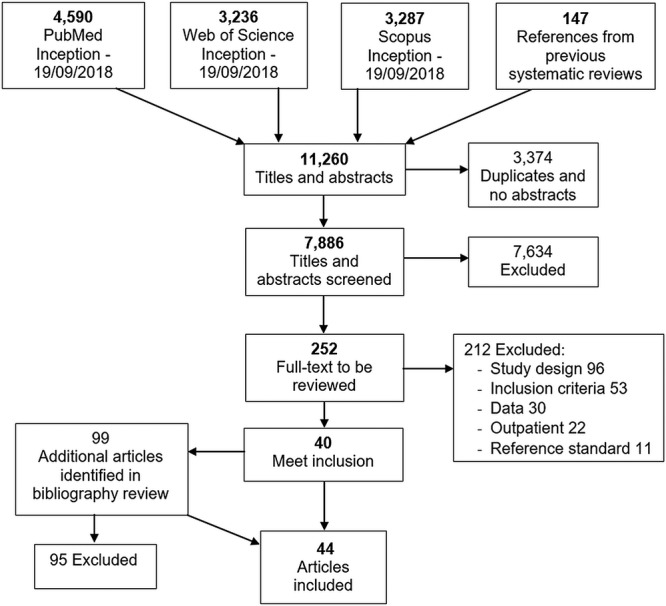
Flow diagram of search strategy and selection of articles reporting the prevalence of CO-BSI among febrile hospitalized patients in 1946 through 2018.
Study characteristics.
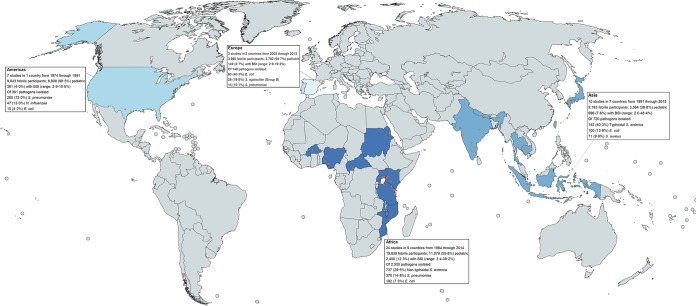
The 44 studies collected data from 1974 through 2015 in 19 countries, recruiting 42,060 participants, of whom 3,656 (8.7%) had BSI (Table 1). Eighteen studies were published from 2010 through 2018; among those, 1,418 (7.0%) of 20,399 participants had BSI, compared to 2,238 (10.3%) of 21,661 participants in studies published prior to 2010. According to United Nations geographical subregion, Eastern Africa had 19 studies performed in 5 countries, the most of any subregion (Fig. 2). All studies in Northern America collected data before 1991 and were performed in the United States (39, 47, 55, 57, 58, 70, 74). Two studies were performed in Southern Europe (42, 59) and 1 in Western Europe (53); no studies from the Southern Africa, Central and West Asia, Eastern and Northern Europe, Latin America and the Caribbean, or Oceania subregions were identified. Blood culture contamination prevalence was reported in 24 (54.5%) studies, and 3 (6.8%) studies reported blood culture volume adequacy (37, 40, 41).
TABLE 1.
Characteristics of 44 included studies of global CO-BSI among febrile hospitalized patients, according to United Nations geographic region and subregion classification, collecting data 1974 through 2015
| Region and subregion | Locality and country | Data collection period | Inclusion age (median)a | Fever criterion | Recruitment setting | No. of febrile patients | No. of hospitalized patients with BSI |
|---|---|---|---|---|---|---|---|
| Africa | |||||||
| Eastern Africa | West Kenya, Kenya (66) | 1987–1990 | >8 yr (NR) | >38°C | 2 regional hospitals | 449 | 58 |
| Mumias, Kenya (43) | 1994 | >5 yr (NR) | ≥38°C | Private regional hospital | 229 | 51 | |
| Nairobi, Kenya (63) | 2001 | 3 mo to 12 yr (mean, 32 mo) | >37.5°C | University teaching hospital | 264 | 32 | |
| Multiple, Kenya (64) | 2013–2014 | 6 mo to 5 yr (3.1 yr) | ≥37.5°C | 1 teaching and referral hospital and 2 district hospitals | 148 | 5 | |
| Blantyre, Malawi (73) | 1996–1997 | Children (NR) | ≥38°C | Pediatric wards of 1,100-bed teaching hospital | 2,123 | NR (365 isolates) | |
| Blantyre, Malawi (44) | 1997–1998 | Adults (NR) | >37.5°C | Medical ward of large government teaching hospital | 2,789 | 449 | |
| Lilongwe, Malawi (35) | 1998 | ≥14 yr (29 yr) | ≥37.5°C | Medical service of 300-bed medical center | 238 | 67 | |
| Blantyre, Malawi (65) | 2000 | ≥14 yr (NR) | ≥37.4°C or shock or history of fever in past 4 days | Medical wards of large government hospital | 352 | 128 | |
| Maputo, Mozambique (67) | 2011–2012 | ≥18 yr (NR) | ≥38°C | Internal medicine ward of national referral hospital | 841 | 63 | |
| Dar es Salaam, Tanzania (32) | 1995 | ≥15 yr (38 yr) | ≥37.5°C | Adult medical unit of >1,000-bed hospital | 517 | 145 | |
| Dar es Salaam, Tanzania (36) | 2001–2002 | 0–7 yr (8.5 mo) | ≥38°C | >1,000-bed hospital | 1,787 | 127 | |
| Muheza, Tanzania (61) | 2006–2007 | 2 mo to 13 yr (1.6 yr) | Current fever or history of fever in past 48 h | District hospital | 3,639 | 341 | |
| Moshi, Tanzania (40) | 2007–2008 | ≥13 yr (38 yr) | ≥38°C | 2 regional hospitals | 403 | 68 | |
| Moshi, Tanzania (41) | 2007–2008 | ≥2 mo to <13 yr (2 yr) | History of fever in past 48 h or ≥37.5°C | Pediatric ward in large consultant hospital | 467 | 16 | |
| Muheza, Tanzania (62) | 2007 | ≥13 yr (36.5 yr) | Fever or history of fever | District hospital | 198 | 26 | |
| Pemba Island, Tanzania (71) | 2009–2010 | >2 mo (NR) | ≥37.5°C | 3 district hospitals | 2,209 | 79 | |
| Mwanza, Tanzania (38) | 2011–2012 | 2–60 mo (18 mo) | ≥37.5°C at time of admission | Pediatric ward of medical center | 317 | 21 | |
| Kampala, Uganda (69) | 1997 | 15–65 yr (30 yr) | >38°C | Medical wards of large public teaching hospital | 305 | 72 | |
| Jinja, Uganda (50) | 2012 | 6 to <60 mo (15.5 mo) | <37.5°C or history fever in past 24 h | ED of regional referral hospital | 250 | 45 | |
| Middle Africa | Bangui, Central African Republic (48) | 1999 | All ages (32 yr) | None given | Department of medicine of 44-bed reference community hospital | 131 | 46 |
| Northern Africa | Port Sudan, Sudan (46) | 1984 | ≥12 yr (mean, 29 yr) | ≥37.8°C | Regional hospital | 100 | 22 |
| Western Africa | Benin City, Nigeria (31) | 1988–1989 | 1 mo to 5 yr (NR) | ≥38°C | Pediatric ED at university hospital | 642 | 67 |
| Ibadan, Nigeria (34) | 1998 | 1–12 mo (4.6 mo for those with septicemia) | ≥38°C | Pediatric ED at university hospital | 102 | 39 | |
| Boulkiemde, Burkina Faso (45) | 2013–2014 | 2 mo to 15 yr (24.6 mo) | ≥37.5°C or history of fever in past 48 h | Pediatric ward of referral hospital and healthcare center | 1,339 | 118 | |
| Asia | |||||||
| East Asia | Tainan, Taiwan (51) | 2006–2007 | ≥18 yr (mean, 53.8 yr) | >38°C for <1 wk | ED of area medical center | 396 | 60 |
| Okinawa, Japan (72) | NR | ≥15 yr (mean, 57 yr) | ≥38°C | Large community hospital serving 400,000 | 526 | 40 | |
| Taipei, Taiwan (54) | NR | ≤15 yr (NR) | ≥39°C | Emergency services of hospital | 300 | 6 | |
| South-eastern Asia | Bangkok, Thailand (33) | 1997 | ≥15 yr (32 yr) | ≥38°C | Medical service of 500-bed hospital | 246 | 119 |
| Multiple, Thailand (52) | 1991–1993 | >2 yr (NR) | >38.3°C for 3–14 days | 10 community-based hospitals | 1,137 | 36 | |
| Jayapura, Northeastern Papua, Indonesia (68) | 1997–2000 | All ages (25 yr) | History of fever or ≥38°C at admission | Provincial hospital serving 286,000 | 226 | 34 | |
| Siem Reap, Cambodia (37) | 2009–2010 | <16 yr (2.0 yr) | ≥38°C within 48 h after admission | 50-bed children's hospital | 1,225 | 76 | |
| South Asia | Kathmandu, Nepal (49) | 2005–2006 | ≤12 yr (NR) | >38.3°C or afebrile with possible meningitis, pneumonia, or septicemia | Pediatric ward of large referral hospital | 2,039 | 142 |
| Multiple, India (60) | 2011–2012 | ≥5 yr (31 yr) | ≥38°C for 2–14 days | 8 secondary community (100–500-bed) hospitals | 1,564 | 124 | |
| Pune, India (56) | 2013–2015 | >6 mo (29 yr for adults; 2 yr in children) | ≥38°C for ≥24 h | Inpatient medicine and pediatric wards of large tertiary public teaching hospital | 1,524 | 59 | |
| Europe | |||||||
| Southern Europe | Bilbao and Barcelona, Spain (59) | 2003–2008 | <3 mo (NR) | ≥38°C | EDs of 2 tertiary teaching hospitals | 381 | 8 |
| Multiple, Spain (42) | 2011–2013 | <91 days (NR) | ≥38°C | 19 EDs | 3,401 | 100 | |
| Western Europe | Amsterdam, Netherlands (53) | 2008–2009 | Adults (66 yr) | >38.2°C | ED of general teaching hospital | 213 | NR (41 isolates) |
| Americas | |||||||
| Northern America | New Haven, Connecticut, USA (57) | 1974–1975 | <24 mo (NR) | ≥40°C | Pediatric ED of large area hospital | 330 | 24 |
| Texas, USA (39) | 1982–1984 | 6 mo to 2 yr (NR) | ≥39.4°C | EDs of 2 community hospitals | 201 | 21 | |
| Philadelphia, Pennsylvania, and Chicago, Illinois, USA (47) | 1982–1984 | 3–36 mo (mean, 16.7 mo) | ≥39°C | EDs of 2 children's hospitals | 955 | 42 | |
| Houston, Texas, USA (55) | 1983 | <24 mo (NR) | Acute febrile illness | ED of children's hospital | 570 | 44 | |
| New Haven, Connecticut, USA (58) | 1982–1983 | ≥16 yr (NR) | ≥37.9°C | Internal medicine department of ED at large hospital | 135 | 21 | |
| Chicago, Illinois, USA (74) | 1983–1984 | 3–24 mo (mean, 12.5 mo) | ≥40°C | EDs of 2 hospitals | 233 | 17 | |
| Multiple, USA (70) | 1987–1991 | 90 days to 36 mo (12.4 mo) | ≥39°C | EDs of 10 hospitals | 6,619 | 192 | |
| Totalb | 42,060 | 3,656b |
FIG 2.
World map of hospital-based study locations and summary findings on prevalent pathogens causing CO-BSI among febrile hospitalized patients in 1946 through 2018 (created using MapChart).
Prevalence of BSI in Africa and Asia.
Thirty-four studies recruited 29,022 participants in Africa and Asia combined, of whom 28,588 (98.5%) had an aerobic blood culture (Table 2). Among participants, 3,146 (10.8%) had BSI, and the median prevalence of BSI was 12.5% (range, 2.0 to 48.4%). There were 3,220 pathogenic organisms isolated among study participants with BSI. Of 3,220 pathogens, 1,996 (62.0%) were Gram-negative bacteria, 854 (26.5%) were Gram-positive bacteria, 94 (2.9%) were yeasts, and 276 (8.6%) were other pathogenic organisms.
TABLE 2.
Organisms isolated from blood cultures among febrile hospitalized patients in 34 studies in Africa and Asia in 1984 through 2018
| Organism group and species isolated | No. of isolates (% of total isolates) | No. of isolates from adults (% of isolates from adults) | No. of isolates from children (% of isolates from children) |
|---|---|---|---|
| Enterobacteriaceae | 1,676 (52.0) | 861 (48.2) | 815 (56.9) |
| Salmonella enterica | 1,119 (34.8) | 558 (31.2) | 561 (39.1) |
| Typhoidal Salmonellaa | 328 (10.2) | 195 (10.9) | 133 (9.3) |
| S. enterica serovar Typhi | 273 (8.5) | 146 (8.2) | 127 (8.9) |
| S. enterica serovar Paratyphi A | 11 (0.3) | 5 (0.3) | 6 (0.4) |
| Nontyphoidal Salmonella | 758 (23.5) | 333 (18.6) | 425 (29.7) |
| S. enterica serovar Typhimurium | 399 (12.4) | 221 (12.4) | 178 (12.4) |
| S. enterica serovar Enteritidis | 126 (3.9) | 73 (4.1) | 53 (3.7) |
| Other S. enterica serovarsb | 7 (0.2) | 4 (0.2) | 3 (0.2) |
| No serovar presented | 226 (7.0) | 35 (2.0) | 191 (13.5) |
| Unspecified Salmonella enterica | 33 (1.0) | 30 (1.7) | 3 (0.2) |
| Non-Salmonella enterica Enterobacteriaceae | 557 (17.3) | 303 (17.0) | 254 (17.7) |
| Escherichia coli | 282 (8.8) | 189 (10.6) | 93 (6.5) |
| Enterobacter spp. | 105 (3.3) | 20 (1.1) | 85 (5.9) |
| Klebsiella spp. | 91 (2.8) | 55 (3.1) | 36 (2.5) |
| Proteus spp. | 12 (0.4) | 9 (0.5) | 3 (0.2) |
| Proteus mirabilis | 6 (0.2) | 6 (0.3) | 0 (0.0) |
| Citrobacter spp. | 16 (0.5) | 3 (0.2) | 13 (0.9) |
| Shigella spp. | 8 (0.2) | 7 (0.4) | 1 (0.1) |
| Morganella morganii | 5 (0.2) | 5 (0.3) | 0 (0.0) |
| Other Enterobacteriaceaec | 38 (1.2) | 15 (0.8) | 23 (1.6) |
| Other Gram-negative organisms | 320 (9.9) | 122 (6.8) | 198 (13.8) |
| Haemophilus influenzae | 78 (2.4) | 4 (0.2) | 74 (5.2) |
| Haemophilus influenzae type b | 46 (1.4) | 4 (0.2) | 42 (2.9) |
| Acinetobacter spp. | 55 (1.7) | 36 (2.0) | 19 (1.3) |
| Acinetobacter baumannii | 8 (0.2) | 3 (0.2) | 5 (0.3) |
| Pseudomonas spp. | 54 (1.7) | 21 (1.2) | 33 (2.3) |
| Pseudomonas aeruginosa | 22 (0.7) | 4 (0.2) | 18 (1.3) |
| Neisseria spp. | 28 (0.9) | 24 (1.3) | 4 (0.3) |
| Neisseria meningitides | 21 (0.7) | 17 (1.0) | 14 (1.0) |
| Alcaligenes spp. | 11 (0.3) | 1 (0.1) | 10 (0.7) |
| Burkholderia pseudomallei | 7 (0.2) | 1 (0.1) | 6 (0.4) |
| Burkholderia cepacia | 6 (0.2) | 6 (0.4) | 0 (0.0) |
| Sphingomonas paucimobilis | 6 (0.2) | 4 (0.2) | 2 (0.1) |
| Additional Gram-negative organismsd | 20 (0.6) | 13 (0.7) | 7 (0.5) |
| Unspecified Gram-negative organisms | 55 (1.7) | 12 (0.7) | 43 (3.0) |
| Gram-positive organisms | 854 (26.5) | 450 (25.2) | 404 (28.2) |
| Streptococcus pneumoniae | 425 (13.2) | 248 (13.9) | 177 (12.4) |
| Staphylococcus aureus | 241 (7.5) | 113 (6.3) | 128 (8.9) |
| Enterococcus spp. | 56 (1.7) | 18 (1.0) | 38 (2.7) |
| Streptococcus agalactiae (group B) | 16 (0.5) | 1 (0.1) | 15 (1.0) |
| Streptococcus pyogenes (group A) | 29 (0.9) | 14 (0.8) | 15 (1.0) |
| Streptococcus group D | 13 (0.4) | 2 (0.1) | 11 (0.8) |
| Streptococcus group G | 2 (0.1) | 2 (0.1) | 0 (0.0) |
| Other Streptococcus spp.e | 42 (1.4) | 31 (1.7) | 11 (0.8) |
| Other Gram-positive organismsf | 11 (0.3) | 10 (0.6) | 1 (0.1) |
| Unspecified Gram-positive organisms | 19 (0.6) | 11 (0.6) | 8 (0.6) |
| Yeasts | 94 (2.9) | 78 (4.4) | 16 (1.1) |
| Cryptococcus neoformans | 61 (1.9) | 61 (3.4) | 0 (0.0) |
| Other Cryptococcus spp.k | 3 (0.2) | 3 (0.1) | 0 (0.0) |
| Candida spp. | 20 (0.6) | 5 (0.3) | 15 (1.0) |
| Histoplasma capsulatum | 5 (0.2) | 5 (0.3) | 0 (0.0) |
| Talaromyces marneffei | 4 (0.1) | 4 (0.2) | 0 (0.0) |
| Unspecified yeast | 1 (<0.1) | 0 (0.0) | 1 (0.1) |
| Mycobacteriag | 245 (7.6) | 245 (13.3) | 0 (0.0) |
| Mycobacterium tuberculosis complex | 206 (6.4) | 206 (11.1) | 0 (0.0) |
| Mycobacterium avium complex | 28 (0.9) | 28 (1.5) | 0 (0.0) |
| Mycobacterium simiae | 4 (0.1) | 4 (0.2) | 0 (0.0) |
| Other Mycobacterium spp.h | 3 (0.1) | 3 (0.2) | 0 (0.0) |
| Unspecified Mycobacterium | 4 (0.1) | 4 (0.2) | 0 (0.0) |
| Other unspecified or unidentified organisms | 31 (1.0) | 31 (1.7) | 0 (0.0) |
| Organisms isolated | 3,220i | 1,787 | 1,433 |
| BSIj | 3,146 (10.8) | 1,746 (12.1) | 1,400 (9.6) |
| Febrile inpatients | 29,022 | 14,380 | 14,642 |
Forty-four isolates were classified as serovar Typhi/Paratyphi by Morch et al. (60).
Including serovars Choleraesuis (n = 3), Newport (n = 1), Brancaster (n = 1), Freetown (n = 1), and Infantis (n = 1).
Including coliforms (n = 17), Klebsiella/Enterobacter unspecified (n = 15), Pantoea spp. (n = 3), Plesiomonas spp. (n = 2), and Providencia sp. (n = 1).
Including Serratia spp. (n = 5), Aeromonas spp. (n = 4), Campylobacter spp. (n = 2), Bacteroides spp. (n = 2), Moraxella catarrhalis (n = 1), Pasteurella sp. (n = 1), Xanthomonas maltophilia (n = 1), CDC group 3 (n = 1), Vibrio cholerae (n = 1), Stenotrophomonas maltophilia (n = 1), and Flavobacterium sp. (n = 1).
Including Streptococcus viridans (n = 3) when the study classified it as BSI, although it was likely a contaminant.
Including Aerococcus spp. (n = 5), Rhodococcus equi (n = 4), Nocardia sp. (n = 1), and Clostridium perfringens (n = 1).
Only 2,115 of 42,060 participants received mycobacterial blood cultures.
Including Mycobacterium scrofulaceum (n = 2) and Mycobacterium sherrisii (n = 1).
The number isolated is greater than the number of BSI due to polymicrobial infections.
Values in parentheses indicate proportions of febrile inpatients.
Including Cryptococcus laurentii (n = 2) and unspecified Cryptococcus spp. (n = 1).
Salmonella enterica accounted for 1,119 (34.8%) of 3,220 pathogens isolated, followed by 425 (13.2%) Streptococcus pneumoniae isolates and 282 (8.8%) Escherichia coli isolates. Of 1,119 S. enterica isolates, 328 (29.3%) were typhoidal Salmonella and 758 (67.7%) were nontyphoidal Salmonella (NTS). Of typhoidal Salmonella isolates, 273 (83.2%) were S. enterica serovar Typhi, 11 (3.4%) were S. enterica serovar Paratyphi A, and 44 (13.4%) were untyped typhoidal Salmonella. Of NTS isolates, 399 (52.6%) were S. enterica serovar Typhimurium and 126 (16.6%) were S. enterica serovar Enteritidis.
Of 29,022 febrile participants in Africa and Asia, 14,642 (50.5%) were from pediatric studies and 14,380 (49.5%) were from adult studies. BSI were identified in 1,400 (9.6%) children and 1,746 (12.1%) adult participants (χ2 = 49.7; P < 0.001). Of the 1,433 and 1,787 pathogens isolated in pediatric and adult studies, respectively, Haemophilus influenzae type b accounted for 42 (2.9%) from pediatric studies and 4 (0.2%) from adult studies (χ2 = 39.5; P < 0.001), S. pneumoniae for 177 (12.4%) from pediatric studies and 248 (13.9%) from adult studies (χ2 = 1.5; P = 0.223), and E. coli for 93 (6.5%) from pediatric studies and 189 (10.6%) from adult studies (χ2 = 16.1; P < 0.001). Mycobacteria were isolated exclusively in adult studies, and no mycobacteria were isolated in the 2 pediatric studies that used mycobacterial blood culture (36, 64).
Mycobacteria and malaria.
Of 9 studies with 4,036 participants reported using mycobacterial blood culture (32, 33, 35, 36, 40, 48, 64, 65, 69), 8 (88.9%) were performed in Africa and 1 (11.1%) in Asia (33). Among 4,036 participants for whom mycobacterial blood cultures were collected, 245 (6.1%) mycobacterial isolates were recovered. Of mycobacterial isolates, 206 (84.1%) belonged to Mycobacterium tuberculosis complex, 28 (11.4%) belonged to Mycobacterium avium complex, and 11 (4.5%) were other mycobacteria.
Fifteen studies (34.1%) used blood film microscopy to test 10,451 participants for malaria and identified parasitemia in 4,301 (41.2%) (31, 32, 35, 36, 38, 40, 41, 43, 45, 46, 60–65). Eight studies reported malaria and BSI coinfection (31, 32, 43, 45, 61–63, 65). Of 7,168 participants, 3,714 (51.8%) had malaria parasitemia. Of 3,714 participants with malaria parasitemia, 198 (5.3%) also had BSI; among the 3,454 participants with no malaria detected, 710 (20.6%) had BSI (odds ratio [OR], 0.22 [95% confidence interval [CI], 0.18 to 0.26]). Two studies reported parasitemia with specific BSI pathogens (62, 65). Twelve (27.3%) studies with 8,109 participants tested patients for HIV using either serological or nucleic acid amplification methods and described BSI coinfection (32, 33, 35, 36, 40, 41, 48, 61, 62, 65, 67, 69). Among the 2,513 HIV-infected participants, 676 (26.9%) had BSI; 566 (10.1%) of 5,596 HIV-uninfected participants had BSI (OR, 3.2 [95% CI, 2.8 to 3.7]). Associations of HIV with specific pathogens, such as M. tuberculosis and NTS, are provided in Table S2 in the supplemental material.
BSI prevalence by region.
When stratified by region, the median prevalence of BSI was 14.6% (range, 3.4 to 38.2%) in Africa, 7.3% (range, 2.0 to 48.4%) in Asia, 2.9% (range, 2.1 to 19.2%) in Europe, and 7.3% (range, 2.9 to 15.6%) in the Americas. Of the 2,500 pathogens isolated in Africa, 737 (29.5%) were NTS, followed by 370 (14.8%) S. pneumoniae and 182 (7.3%) E. coli. Of the 720 pathogens isolated in Asia, 142 (19.7%) were typhoidal Salmonella, 100 (13.9%) were E. coli, 71 (9.9%) were Staphylococcus aureus, and 55 (7.6%) were S. pneumoniae. Nine NTS pathogens (1.3%) were isolated in Asia, all from a single study (33).
Ten studies were performed in the Americas and Europe regions, of which 2 were adult studies (53, 58). In a multicenter study of 3,401 participants <3 months of age in Spain in 2011 through 2013, E. coli accounted for 46 (46.0%) of 100 pathogens isolated (42). S. pneumoniae represented 260 (72.0%) of the 361 pathogens isolated in 7 studies performed in the United States, followed by 47 H. influenzae pathogens (13.0%), of which 19 (40.0%) were specified as type b.
Antimicrobial susceptibility.
The results of antimicrobial susceptibility testing were reported in 16 studies (36.4%), all located in Eastern Africa and South and South-eastern Asia (Table 3) (32, 33, 35–38, 40, 41, 43, 44, 49, 61, 67, 68, 71, 73). Eight studies reported antimicrobial susceptibility results for typhoidal Salmonella (35, 37, 43, 44, 49, 68, 71, 73). The proportions of typhoidal Salmonella isolates susceptible to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole for the earlier period versus the contemporary period were 99 (100%) of 99 isolates versus 22 (48.9%) of 45 isolates, 125 (99.2%) of 126 isolates versus 23 (51.1%) of 45 isolates, and 52 (81.3%) of 64 isolates versus 23 (51.1%) of 45 isolates, respectively.
TABLE 3.
Earlier versus contemporary antimicrobial susceptibilities of prevalent BSI isolates in Africa and Asia in 1994 to 2018
| Organism, group,a and antimicrobial | Earlier period (pre-2008) |
Contemporary period (2008–2018) |
||||
|---|---|---|---|---|---|---|
| No. of studies | No. of isolates tested | Proportion of susceptible isolates (% [range]) | No. of studies | No. of isolates tested | Proportion of susceptible isolates (% [range]) | |
| Escherichia coli (36–38, 43, 44, 61, 67, 71) | ||||||
| Group A | ||||||
| Ampicillin | 2 | 67 | 6.0 (4.2–7.0) | 3 | 26 | 23.1 (14.3–40.0) |
| Gentamicin | 4 | 89 | 74.2 (0.0–93.0) | 3 | 26 | 76.9 (28.6–100) |
| Group B | ||||||
| Ceftriaxone | 0 | 0 | 3 | 26 | 92.3 (85.7–100) | |
| Ciprofloxacin | 1 | 24 | 91.7 (91.7) | 3 | 26 | 76.9 (57.1–80.0) |
| Imipenem-meropenem | 1 | 24 | 100 (100) | 2 | 21 | 100 (100) |
| Trimethoprim-sulfamethoxazole | 4 | 89 | 5.6 (0.0–12.5) | 3 | 26 | 34.6 (0–57.1) |
| Typhoidal Salmonella (35, 37, 43, 44, 49, 68, 71, 73) | ||||||
| Group A | ||||||
| Ampicillin | 4 | 99 | 100 (100) | 1 | 45 | 48.9 (48.9) |
| Group B | ||||||
| Chloramphenicol | 6 | 126 | 99.2 (95.8–100) | 1 | 45 | 51.1 (51.1) |
| Ciprofloxacin | 1 | 59 | 100 (100) | 2 | 66 | 95.5 (90.5–100) |
| Trimethoprim-sulfamethoxazole | 4 | 64 | 81.3 (50.0–100) | 1 | 45 | 51.1 (51.1) |
| Ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazoleb | 0 | 0 | 2 | 66 | 56.1 (42.2–85.7) | |
| Nontyphoidal Salmonella (33, 35, 43, 44, 61, 67, 73) | ||||||
| Group A | ||||||
| Ampicillin | 6 | 331 | 22.1 (0.0–100) | 1 | 10 | 10.0 (10.0) |
| Group B | ||||||
| Chloramphenicol | 5 | 482 | 77.8 (0.0–100) | 1 | 10 | 10.0 (10.0) |
| Ciprofloxacin | 0 | 0 | 1 | 10 | 90.0 (90.0) | |
| Trimethoprim-sulfamethoxazole | 6 | 486 | 31.3 (0.0–100) | 1 | 10 | 10.0 (10.0) |
| Ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazoleb | 0 | 0 | 0 | 0 | ||
| Staphylococcus aureus (33, 36–38, 43, 61, 67, 68, 71) | ||||||
| Group A | ||||||
| Erythromycin | 3 | 21 | 90.5 (71.4–100) | 1 | 5 | 100 (100) |
| Methicillin-oxacillin | 4 | 16 | 75.0 (0.0–100) | 4 | 29 | 69.0 (52.9–100) |
| Penicillin | 4 | 36 | 2.8 (0.0–6.7) | 2 | 22 | 4.5 (0.0–20.0) |
| Trimethoprim-sulfamethoxazole | 3 | 28 | 71.4 (69.2–100) | 3 | 23 | 60.9 (0.0–100) |
| Group B | ||||||
| Tetracycline | 1 | 1 | 100 (100) | 2 | 18 | 66.7 (0.0–70.6) |
| Vancomycin | 2 | 20 | 100 (100) | 2 | 22 | 100 (100) |
| Streptococcus pneumoniae (32, 37, 40, 41, 43, 44, 61, 67, 68, 71, 73) | ||||||
| Group A | ||||||
| Erythromycin | 3 | 192 | 99.0 (98.4–100) | 3 | 25 | 96.0 (85.7–100) |
| Penicillin | 5 | 254 | 86.6 (63.6–100) | 4 | 28 | 85.7 (66.7–100) |
| Trimethoprim-sulfamethoxazole | 4 | 233 | 16.3 (1.8–100) | 4 | 28 | 39.3 (16.7–66.7) |
| Chloramphenicol | 5 | 246 | 82.1 (74.4–100) | 4 | 28 | 100 (100) |
| Group B | ||||||
| Ceftriaxone | 0 | 0 | 2 | 11 | 100 (100) | |
| Tetracycline | 3 | 192 | 59.9 (50.4–70) | 1 | 3 | 66.7 (67.0) |
As defined by the Clinical and Laboratory Standards Institute (95).
Defines multiple drug resistance in Salmonella enterica.
There were 11 studies with antimicrobial susceptibility results for S. pneumoniae (32, 37, 40, 41, 43, 44, 61, 67, 68, 71, 73). The proportions of isolates susceptible to erythromycin, penicillin, and trimethoprim-sulfamethoxazole for the earlier period versus the contemporary period were 190 (99.0%) of 192 isolates versus 24 (96.0%) of 25 isolates, 220 (86.6%) of 254 isolates versus 24 (85.7%) of 28 isolates, and 38 (16.3%) of 233 isolates versus 11 (39.3%) of 28 isolates, respectively.
Eight studies reported antimicrobial susceptibility results for E. coli (36–38, 43, 44, 61, 67, 71). Among E. coli isolates with ampicillin susceptibility testing, 4 (6.0%) of 67 were susceptible in the earlier period versus 6 (23.1%) of 26 in the contemporary period. Among E. coli isolates with ciprofloxacin susceptibility testing, 22 (91.7%) of 24 were susceptible in the earlier period (36) versus 20 (76.9%) of 26 in the contemporary period (38, 67, 71). Eight studies tested S. aureus isolates for methicillin resistance (33, 36–38, 43, 67, 68, 71). The proportions of S. aureus isolates susceptible to methicillin were 12 (75.0%) of 16 in the earlier period versus 20 (69.0%) of 29 in the contemporary period. A meta-analysis of the proportions of organisms susceptible to all drugs reported regardless of clinical application in the earlier period versus the contemporary period showed 95.9% (95% CI, 89.7 to 99.5%) versus 77.1% (95% CI, 59.1 to 91.3%) for typhoidal Salmonella, 64.1% (95% CI, 49.7 to 77.3%) versus 51.4% (95% CI, 21.8 to 80.6%) for NTS, 76.0% (95% CI, 56.0 to 91.6%) versus 81.2% (95% CI, 68.1 to 91.6%) for S. pneumoniae, 44.5% (95% CI, 26.7 to 63.0%) versus 61.9% (95% CI, 46.8 to 76.0%) for E. coli, and 72.2% (95% CI, 60.8 to 85.7%) versus 57.4% (95% CI, 35.5 to 78.0%) for S. aureus.
DISCUSSION
We show that CO-BSIs continue to play a major role in febrile ED consultations and hospitalizations. S. enterica, S. pneumoniae, and E. coli were the leading pathogens in Africa and Asia, and BSI were more common in adult studies than in pediatric studies. Although our search was global, studies located in Africa and Asia predominated and are the focus of this review. We identified a number of regional differences, including greater proportions of CO-BSI in participants from studies in Africa, compared to studies in other locations. Several lines of evidence demonstrate increasing prevalence of AMR among community-onset pathogens causing bacteremia.
Salmonella enterica was the leading cause of CO-BSI in Africa and Asia, with nontyphoidal serovars playing a major role in African studies and typhoidal serovars being common in both African and Asian studies. We demonstrate that the proportions of typhoidal and nontyphoidal Salmonella isolates susceptible to the traditional first-line drugs ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole have all declined since 2008. While we did not demonstrate a change in the proportions of S. enterica isolates susceptible to fluoroquinolones between the two time periods, and susceptibility to extended-spectrum cephalosporins was rarely reported, outbreaks of S. enterica serovar Typhi and NTS disease resistant to many antimicrobial classes are of great concern (19, 20, 75, 76). Progress to improve access to microbiologically safe water and food and improved sanitation are needed to prevent Salmonella infections (77). Typhoid conjugate vaccines represent a new tool for typhoid fever control in areas in which the disease is endemic (78, 79).
Consistent with incidence data (80), we show that S. pneumoniae remains a leading cause of CO-BSI. S. pneumoniae was as common in pediatric studies as in adult studies in Africa and Asia. We identified no statistically significant changes in S. pneumoniae antimicrobial susceptibility between the time periods. Encouragingly, pneumococcal conjugate vaccine was introduced in 142 (73.2%) of 194 World Health Organization member states by 2018, and levels of pneumococcal conjugate vaccine coverage in these states have increased (81, 82).
E. coli was the third most frequently isolated cause of CO-BSI and was more common in adult studies than in pediatric studies. While we observed increases in the proportions of E. coli susceptible to penicillin and trimethoprim-sulfamethoxazole from the earlier period to the contemporary period, the prevalence of fluoroquinolone susceptibility appears to be declining. Of concern, resistance to extended-spectrum cephalosporins among a substantial minority of E. coli isolates causing CO-BSI was observed in studies from the contemporary period. Furthermore, vaccines and other measures to prevent community-onset extraintestinal pathogenic E. coli infections remain at an early stage of development (83).
Our review had a number of limitations. First, although we expanded the search to encompass CO-BSI globally, there were still many regions and countries lacking eligible studies. Second, antimicrobial susceptibility data were available only from studies performed in Eastern Africa and South and South-eastern Asia, limiting our ability to make regional comparisons. Also, the total number of isolates undergoing susceptibility testing in studies included in our review was relatively small. Our AMR findings can be complemented by AMR data generated from national laboratory reporting surveillance networks (84) and other sources, such as large, single-center studies showing AMR trends for common organisms (85). Third, using prospective cohort studies as our data source meant that the substantial data from high-income countries with robust and routine local and national CO-BSI surveillance were not included. However, the primary purpose of this review was to provide data for settings that rely on sentinel site studies to understand the local and national epidemiology of CO-BSI. Fourth, our inclusion criteria of only febrile hospitalized patients did not capture the important group of individuals with nonfebrile CO-BSI, including those with sepsis (86, 87). Fifth, antimicrobial susceptibility standards and interpretive criteria change over time, which can contribute to changes in apparent antimicrobial susceptibility interpretations and results. Because we did not have access to raw antimicrobial susceptibility testing data, we were unable to reinterpret results with contemporary criteria. Sixth, some pathogens are likely underestimated due to incomplete identification. We identified incomplete use of mycobacterial blood cultures and malaria and HIV diagnostic testing for febrile inpatients. Pathogens such as Burkholderia pseudomallei may also be missed through limitations of media and identification methods in some locations (88, 89). Finally, while our review is focused on CO-BSI in the context of febrile illness sufficiently severe to present at an ED or admission to a hospital, we recognize that CO-BSI play an important role in the pathogenesis of the separate but overlapping clinical syndrome of sepsis. However, others have examined the infectious etiology of sepsis, and our study was designed to inform the management of febrile patients in the era of declining malaria incidence.
Our findings support the value of surveillance and high-quality research on CO-BSI to inform empirical treatment strategies, to help set priority pathogens to inform disease control measures, and to highlight the concerning growth of AMR among serious infections cause by community-onset pathogens. While the overall proportion of febrile participants with BSI declined in studies performed after 2008, compared to prior years, we confirm that CO-BSI remain a major cause of febrile presentation for emergency care or hospitalization and that AMR is a growing problem among CO-BSI. Our findings underscore the importance of both non-vaccine-based and vaccine-based control of community-onset pathogens such as S. enterica serovar Typhi and S. pneumoniae and highlight the prevention and control gap for E. coli acquired outside the health care system. The control of antimicrobial misuse in people, animals, and the environment is likely essential to slow the emergence of AMR in CO-BSI pathogens (90). Ongoing surveillance and further sentinel site studies remain invaluable for informing empirical management of severe febrile illnesses and bacteremia, as well as guiding strategies to control AMR.
MATERIALS AND METHODS
Search strategy and selection criteria.
We performed a systematic review by searching PubMed, Web of Science, and Scopus on 19 September 2018 to identify studies of CO-BSI. The search included key words of fever, bacteremia, septicemia, epidemiology, incidence, and prevalence, as well as spelling alternatives and related terms (see Text S1 in the supplemental material). No restrictions were placed on study setting (e.g., inpatient versus outpatient setting), language, country, or date. Selection criteria were set prior to the initial database searches.
Only prospective studies with consecutive series of febrile patients, with fever as the primary criterion for obtaining blood culture and aerobic or mycobacterial blood culture as the reference standard diagnostic test, were included. If a study enrolled a broader group of afebrile patients (e.g., suspicion of meningitis without fever), it was included only if the initial enrollment criterion was fever. However, we placed no restriction on how fever was defined. We defined CO-BSI as a pathogen-positive blood culture drawn from a febrile patient within 48 h after admission (16). Studies that did not present sufficient detail for calculation of the prevalence of isolates from blood cultures or that reported a single pathogen (e.g., only Streptococcus pneumoniae) as a cause of febrile illness, without describing other causes, were excluded.
Search results from each database were imported into Endnote X8 (Clarivate Analytics, Boston, MA). We also included all references from the bibliographies of the two previous systematic reviews on CO-BSI in Africa and Asia (25, 26). Endnote was used to remove duplicates, and a final deduplicated data set was uploaded to an online systematic review tool for abstract and full-text screening (91).
Two authors screened titles and abstracts for inclusion. Studies included by either author were moved forward to full-text review. All full-text articles were then independently screened in parallel by two authors. Discrepancies were resolved through discussion and, if necessary, by a separate author. Subsequent processes were also performed in this manner.
After the initial full-text review, studies were restricted to hospitalized patients, in keeping with the earlier reviews. Studies in an ED were deemed relevant and were included, on the basis that ED patients are part of a pathway to hospitalization. The full-text versions of included articles were rescreened in parallel, to exclude studies performed in an outpatient setting. Lastly, bibliographies of the final included articles were screened for additional relevant studies. We used the preferred reporting items for systematic reviews and meta-analyses (PRISMA) to record the search process (92). Descriptive study characteristics and quantitative data were abstracted in a shared Google (Mountain View, CA) spreadsheet document.
Data analysis.
Study quality was assessed by using criteria that aligned with the aims of this review. Our goal was to create an assessment tool that evaluated the quality of a study’s blood culture results and its recruitment procedures. We included two measures important to the growth of microorganisms in blood culture, namely, volume adequacy of culture bottles and the proportion of bottles reported as contaminated. Other questions assessed the possibility of selection bias, such as the study being performed in an ED setting where all patients may not be hospitalized. Quality assessment was performed by two authors in parallel, and discrepancies of the overall score (high, moderate, or low) were resolved through discussion.
Data on individual isolates were compiled and aggregated in Excel (Microsoft, Redmond, WA). If a study did not report the number of BSI, we made the assumption that the total number of pathogens isolated equaled the number participants with BSI and vice versa. No other data were imputed to account for missing values. For Salmonella enterica, when a serovar was provided, we grouped serovars Typhi, Paratyphi A, Paratyphi B, and Paratyphi C as typhoidal Salmonella and all others as NTS (93).
Isolates were stratified in two ways. First, studies were stratified by age group using the inclusion or median age. Studies with participants ≤15 years of age were defined as pediatric studies. Studies of populations of mixed ages or with median ages of >15 years were defined as adult studies. Comparisons between pediatric and adult studies were made using a two-sample test of proportions in R v3.5.1, with the prop.test function. Second, we stratified by United Nations region (94), describing the prevalence in studies performed in Africa and Asia and in studies performed outside those two regions using MapChart (https://mapchart.net/detworld.html). Additionally, we analyzed the association of HIV or malaria coinfection with BSI overall and also with specific causes of bacteremia. The significance of the associations was determined by the χ2 test or Fisher’s exact test.
As a secondary analysis, we abstracted data on antimicrobial susceptibility, when available. We defined an isolate as susceptible when a study reported its susceptibility to specific antimicrobial drugs as susceptible or intermediate and resistant when the study reported the isolate as resistant. We accepted the original study’s classification of isolate antimicrobial susceptibility and did not attempt to access and to reinterpret zone sizes or MIC values based on contemporary interpretive criteria.
Contemporary isolates were defined as those collected in 2008 through 2018. We compared the prevalence of susceptibility between contemporary isolates and earlier isolates (collected prior to 2008) for major drug-organism combinations. We used Clinical and Laboratory Standards Institute suggested antimicrobial agent groups A and B as a guide for reporting specific clinically relevant drugs according to organism group (95). To evaluate trends in overall susceptibility to all drugs that were tested, regardless of clinical importance or application, we also performed a random-effects meta-analysis of proportions of susceptibility over the two time periods, using MetaXL (EpiGear International Pty Ltd.). The study protocol was registered with PROSPERO (accession no. CRD42018109388). Because this was a study involving secondary analysis of published data, institutional review board approval was not required.
Supplementary Material
ACKNOWLEDGMENTS
J.A.C. and C.S.M. received support from Bill & Melinda Gates Foundation grant OPP1151153. J.A.C. also received support from the Bill & Melinda Gates Foundation (grants OPP1125993 and OPP1158210), the U.S. National Institutes of Health (grant R01AI121378), and the New Zealand Health Research Council through the e-ASIA Joint Research Program (grant 16/697). M.P.R. received support from the U.S. National Institutes of Health (grant K23AI116869).
J.A.C. conceived the study. C.S.M., M.P.R., and J.A.C. developed the research protocol. C.S.M. registered the review with PROSPERO and performed the initial literature database search. C.S.M. and A.P.D. screened titles and abstracts. C.S.M., A.P.D., and S.P. performed full-text reviews and data abstraction. M.P.R. assisted and mentored S.P. in reviewing and abstraction. C.S.M. and J.A.C. assessed the quality of the included studies. C.S.M. performed data analyses and prepared the first manuscript draft. J.A.C. provided major revisions and comments to the first draft. M.P.R. gave substantial feedback to subsequent drafts. All authors contributed to the final edits and revisions prior to submission.
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all of the data in the study and had final responsibility for the decision to submit the report for publication.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Petit PL, van Ginneken JK. 1995. Analysis of hospital records in four African countries, 1975–1990, with emphasis on infectious diseases. J Trop Med Hyg 98:217–227. [PubMed] [Google Scholar]
- 2.Feikin DR, Olack B, Bigogo GM, Audi A, Cosmas L, Aura B, Burke H, Njenga MK, Williamson J, Breiman RF. 2011. The burden of common infectious disease syndromes at the clinic and household level from population-based surveillance in rural and urban Kenya. PLoS One 6:e16085. doi: 10.1371/journal.pone.0016085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campbell JD, Sow SO, Levine MM, Kotloff KL. 2004. The causes of hospital admission and death among children in Bamako, Mali. J Trop Pediatr 50:158–163. doi: 10.1093/tropej/50.3.158. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. 2019. National Hospital Ambulatory Medical Care Survey: 2015 emergency department summary tables. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2015_ed_web_tables.pdf.
- 5.Keitel K, Kagoro F, Samaka J, Masimba J, Said Z, Temba H, Mlaganile T, Sangu W, Rambaud-Althaus C, Gervaix A, Genton B, D'Acremont V. 2017. A novel electronic algorithm using host biomarker point-of-care tests for the management of febrile illnesses in Tanzanian children (e-POCT): a randomized, controlled non-inferiority trial. PLoS Med 14:e1002411. doi: 10.1371/journal.pmed.1002411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Althaus T, Greer RC, Swe MMM, Cohen J, Tun NN, Heaton J, Nedsuwan S, Intralawan D, Sumpradit N, Dittrich S, Doran Z, Waithira N, Thu HM, Win H, Thaipadungpanit J, Srilohasin P, Mukaka M, Smit PW, Charoenboon EN, Haenssgen MJ, Wangrangsimakul T, Blacksell S, Limmathurotsakul D, Day N, Smithuis F, Lubell Y. 2019. Effect of point-of-care C-reactive protein testing on antibiotic prescription in febrile patients attending primary care in Thailand and Myanmar: an open-label, randomised, controlled trial. Lancet Glob Health 7:e119–e131. doi: 10.1016/S2214-109X(18)30444-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bigogo G, Audi A, Aura B, Aol G, Breiman RF, Feikin DR. 2010. Health-seeking patterns among participants of population-based morbidity surveillance in rural western Kenya: implications for calculating disease rates. Int J Infect Dis 14:e967–e973. doi: 10.1016/j.ijid.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Kenya National Bureau of Statistics. 2015. Kenya Demographic and Health Survey 2014, p 147–149. Kenya National Bureau of Statistics, Nairobi, Kenya: https://dhsprogram.com/pubs/pdf/fr308/fr308.pdf. [Google Scholar]
- 9.Amin AA, Marsh V, Noor AM, Ochola SA, Snow RW. 2003. The use of formal and informal curative services in the management of paediatric fevers in four districts in Kenya. Trop Med Int Health 8:1143–1152. doi: 10.1046/j.1360-2276.2003.01140.x. [DOI] [PubMed] [Google Scholar]
- 10.Peppa M, Edmunds WJ, Funk S. 2017. Disease severity determines health-seeking behaviour amongst individuals with influenza-like illness in an internet-based cohort. BMC Infect Dis 17:238. doi: 10.1186/s12879-017-2337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton DC, Flannery B, Onyango B, Larson C, Alaii J, Zhang X, Hamel MJ, Breiman RF, Feikin DR. 2011. Healthcare-seeking behaviour for common infectious disease-related illnesses in rural Kenya: a community-based house-to-house survey. J Health Popul Nutr 29:61–70. doi: 10.3329/jhpn.v29i1.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization. 2018. World malaria report 2018. World Health Organization, Geneva, Switzerland: https://apps.who.int/iris/bitstream/handle/10665/275867/9789241565653-eng.pdf. [Google Scholar]
- 13.Murray CJL, Ortblad KF, Duber HC, Naghavi M, Dicker D, Dandona L, Salomon JA, Heuton KR, Foreman K, Phillips DE, Fleming TD, Flaxman AD, Phillips BK, Johnson EK, Coggeshall MS, Abd-Allah F, Abera SF, Abraham JP, Abubakar I, Abu-Raddad LJ, Abu-Rmeileh NM, Achoki T, Adeyemo AO, Adou AK, Adsuar JC, Agardh EE, Akena D, Al Kahbouri MJ, Alasfoor D, Albittar MI, Alcalá-Cerra G, Alegretti MA, Alemu ZA, Alfonso-Cristancho R, Alhabib S, Ali R, Alla F, Allen PJ, Alsharif U, Alvarez E, Alvis-Guzman N, Amankwaa AA, Amare AT, Amini H, Ammar W, Anderson BO, Antonio CAT, Anwari P, Ärnlöv J, Arsenijevic VSA, Artaman A, Asghar RJ, Assadi R, Atkins LS, Badawi A, Balakrishnan K, Banerjee A, et al. 2014. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384:1005–1070. doi: 10.1016/S0140-6736(14)60844-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasad N, Murdoch DR, Reyburn H, Crump JA. 2015. Etiology of severe febrile illness in low- and middle-income countries: a systematic review. PLoS One 10:e0127962. doi: 10.1371/journal.pone.0127962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson KL, Müller-Pebody B, Johnson AP, Wade A, Sharland M, Gilbert R. 2013. Community-acquired, healthcare-associated and hospital-acquired bloodstream infection definitions in children: a systematic review demonstrating inconsistent criteria. J Hosp Infect 85:94–105. doi: 10.1016/j.jhin.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 16.Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. 1988. CDC definitions for nosocomial infections, 1988. Am J Infect Control 16:128–140. doi: 10.1016/0196-6553(88)90053-3. [DOI] [PubMed] [Google Scholar]
- 17.Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, Lamm W, Clark C, MacFarquhar J, Walton AL, Reller LB, Sexton DJ. 2002. Health care-associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med 137:791–797. doi: 10.7326/0003-4819-137-10-200211190-00007. [DOI] [PubMed] [Google Scholar]
- 18.Cardoso T, Almeida M, Friedman ND, Aragão I, Costa-Pereira A, Sarmento AE, Azevedo L. 2014. Classification of healthcare-associated infection: a systematic review 10 years after the first proposal. BMC Med 12:40. doi: 10.1186/1741-7015-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yousafzai MT, Qamar FN, Shakoor S, Saleem K, Lohana H, Karim S, Hotwani A, Qureshi S, Masood N, Rauf M, Khanzada JA, Kazi M, Hasan R. 2019. Ceftriaxone-resistant Salmonella Typhi outbreak in Hyderabad City of Sindh, Pakistan: high time for the introduction of typhoid conjugate vaccine. Clin Infect Dis 68(Suppl 1):S16–S21. doi: 10.1093/cid/ciy877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klemm EJ, Shakoor S, Page AJ, Qamar FN, Judge K, Saeed DK, Wong VK, Dallman TJ, Nair S, Baker S, Shaheen G, Qureshi S, Yousafzai MT, Saleem MK, Hasan Z, Dougan G, Hasan R. 2018. Emergence of an extensively drug-resistant Salmonella enterica serovar Typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third-generation cephalosporins. mBio 9:e00105-18. doi: 10.1128/mBio.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laupland KB, Ross T, Gregson DB. 2008. Staphylococcus aureus bloodstream infections: risk factors, outcomes, and the influence of methicillin resistance in Calgary, Canada, 2000–2006. J Infect Dis 198:336–343. doi: 10.1086/589717. [DOI] [PubMed] [Google Scholar]
- 22.Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. 2006. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis 42:377–382. doi: 10.1086/499363. [DOI] [PubMed] [Google Scholar]
- 23.Archibald LK, Reller LB. 2001. Clinical microbiology in developing countries. Emerg Infect Dis 7:302–305. doi: 10.3201/eid0702.010232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crump JA, Kirk MD. 2015. Estimating the burden of febrile illnesses. PLoS Negl Trop Dis 9:e0004040. doi: 10.1371/journal.pntd.0004040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddy EA, Shaw AV, Crump JA. 2010. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis 10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Deen J, von Seidlein L, Andersen F, Elle N, White NJ, Lubell Y. 2012. Community-acquired bacterial bloodstream infections in developing countries in south and southeast Asia: a systematic review. Lancet Infect Dis 12:480–487. doi: 10.1016/S1473-3099(12)70028-2. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. 2011. IMAI district clinician manual: hospital care for adolescents and adults: guidelines for the management of common illnesses with limited resources. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 28.World Health Organization. 2013. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses, 2nd ed World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 29.Fleischmann C, Scherag A, Adhikari NKJ, Hartog CS, Tsaganos T, Schlattmann P, Angus DC, Reinhart K. 2016. Assessment of global incidence and mortality of hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med 193:259–272. doi: 10.1164/rccm.201504-0781OC. [DOI] [PubMed] [Google Scholar]
- 30.Reinhart K, Daniels R, Kissoon N, Machado FR, Schachter RD, Finfer S. 2017. Recognizing sepsis as a global health priority: a WHO resolution. N Engl J Med 377:414–417. doi: 10.1056/NEJMp1707170. [DOI] [PubMed] [Google Scholar]
- 31.Akpede GO, Abiodun PO, Sykes RM. 1992. Relative contribution of bacteraemia and malaria to acute fever without localizing signs of infection in under-five children. J Trop Pediatr 38:295–298. doi: 10.1093/tropej/38.6.295. [DOI] [PubMed] [Google Scholar]
- 32.Archibald LK, den Dulk MO, Pallangyo KJ, Reller LB. 1998. Fatal Mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis 26:290–296. doi: 10.1086/516297. [DOI] [PubMed] [Google Scholar]
- 33.Archibald LK, McDonald LC, Rheanpumikankit S, Tansuphaswadikul S, Chaovanich A, Eampokalap B, Banerjee SN, Reller LB, Jarvis WR. 1999. Fever and human immunodeficiency virus infection as sentinels for emerging mycobacterial and fungal bloodstream infections in hospitalized patients ≥15 years old, Bangkok. J Infect Dis 180:87–92. doi: 10.1086/314836. [DOI] [PubMed] [Google Scholar]
- 34.Ayoola OO, Adeyemo AA, Osinusi K. 2003. Aetiological agents, clinical features and outcome of septicaemia in infants in Ibadan. West Afr J Med 22:30–34. [DOI] [PubMed] [Google Scholar]
- 35.Bell M, Archibald LK, Nwanyanwu O, Dobbie H, Tokars J, Kazembe PN, Reller LB, Jarvis WR. 2001. Seasonal variation in the etiology of bloodstream infections in a febrile inpatient population in a developing country. Int J Infect Dis 5:63–69. doi: 10.1016/S1201-9712(01)90027-X. [DOI] [PubMed] [Google Scholar]
- 36.Blomberg B, Manji KP, Urassa WK, Tamim BS, Mwakagile DSM, Jureen R, Msangi V, Tellevik MG, Holberg-Petersen M, Harthug S, Maselle SY, Langeland N. 2007. Antimicrobial resistance predicts death in Tanzanian children with bloodstream infections: a prospective cohort study. BMC Infect Dis 7:43. doi: 10.1186/1471-2334-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chheng K, Carter MJ, Emary K, Chanpheaktra N, Moore CE, Stoesser N, Putchhat H, Sona S, Reaksmey S, Kitsutani P, Sar B, van Doorn HR, Uyen NH, Van Tan L, Paris DH, Paris D, Blacksell SD, Amornchai P, Wuthiekanun V, Parry CM, Day NPJ, Kumar V. 2013. A prospective study of the causes of febrile illness requiring hospitalization in children in Cambodia. PLoS One 8:e60634. doi: 10.1371/journal.pone.0060634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christopher A, Mshana SE, Kidenya BR, Hokororo A, Morona D. 2013. Bacteremia and resistant Gram-negative pathogens among under-fives in Tanzania. Ital J Pediatr 39:27. doi: 10.1186/1824-7288-39-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crocker PJ, Quick G, McCombs W. 1985. Occult bacteremia in the emergency department: diagnostic criteria for the young febrile child. Ann Emerg Med 14:1172–1177. doi: 10.1016/S0196-0644(85)81024-6. [DOI] [PubMed] [Google Scholar]
- 40.Crump JA, Ramadhani HO, Morrissey AB, Saganda W, Mwako MS, Yang L-Y, Chow S-C, Morpeth SC, Reyburn H, Njau BN, Shaw AV, Diefenthal HC, Shao JF, Bartlett JA, Maro VP. 2011. Invasive bacterial and fungal infections among hospitalized HIV-infected and HIV-uninfected adults and adolescents in northern Tanzania. Clin Infect Dis 52:341–348. doi: 10.1093/cid/ciq103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crump JA, Ramadhani HO, Morrissey AB, Msuya LJ, Yang L-Y, Chow S-C, Morpeth SC, Reyburn H, Njau BN, Shaw AV, Diefenthal HC, Bartlett JA, Shao JF, Schimana W, Cunningham CK, Kinabo GD. 2011. Invasive bacterial and fungal infections among hospitalized HIV‐infected and HIV‐uninfected children and infants in northern Tanzania. Trop Med Int Health 16:830–837. doi: 10.1111/j.1365-3156.2011.02774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de la Torre M, de Lucas N, Velasco R, Gómez B, Mintegi S. 2017. Aetiology and outcomes of potentially serious infections in febrile infants less than 3 months old. An Pediatr 87:42–49. (In Spanish.) doi: 10.1016/j.anpede.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 43.Dougle ML, Hendriks ER, Sanders EJ, Dorigo-Zetsma JW. 1997. Laboratory investigations in the diagnosis of septicaemia and malaria. East Afr Med J 74:353–356. [PubMed] [Google Scholar]
- 44.Gordon MA, Walsh AL, Chaponda M, Soko D, Mbvwinji M, Molyneux ME, Gordon SB. 2001. Bacteraemia and mortality among adult medical admissions in Malawi: predominance of non-typhi salmonellae and Streptococcus pneumoniae. J Infect 42:44–49. doi: 10.1053/jinf.2000.0779. [DOI] [PubMed] [Google Scholar]
- 45.Guiraud I, Post A, Diallo SN, Lompo P, Maltha J, Thriemer K, Tahita CM, Ley B, Derra K, Bottieau E, Kazienga A, Schurmans C, Ravinetto R, Rouamba E, Van Griensven J, Bertrand S, Tinto H, Jacobs J. 2017. Population-based incidence, seasonality and serotype distribution of invasive salmonellosis among children in Nanoro, rural Burkina Faso. PLoS One 12:e0178577. doi: 10.1371/journal.pone.0178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyams KC, Oldfield EC, Scott RM, Bourgeois AL, Gardiner H, Pazzaglia G, Moussa M, Saleh AS, Dawi OE, Daniell FD. 1986. Evaluation of febrile patients in Port Sudan, Sudan: isolation of dengue virus. Am J Trop Med Hyg 35:860–865. doi: 10.4269/ajtmh.1986.35.860. [DOI] [PubMed] [Google Scholar]
- 47.Jaffe DM, Tanz RR, Davis AT, Henretig F, Fleisher G. 1987. Antibiotic administration to treat possible occult bacteremia in febrile children. N Engl J Med 317:1175–1180. doi: 10.1056/NEJM198711053171902. [DOI] [PubMed] [Google Scholar]
- 48.Kassa-Kelembho E, Mbolidi C-D, Service Y-B, Morvan J, Minssart P. 2003. Bacteremia in adults admitted to the Department of Medicine of Bangui Community Hospital (Central African Republic). Acta Trop 89:67–72. doi: 10.1016/j.actatropica.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 49.Kelly DF, Thorson S, Maskey M, Mahat S, Shrestha U, Hamaluba M, Williams E, Dongol S, Werno AM, Portess H, Yadav BK, Adhikari N, Guiver M, Thomas K, Murdoch DR, Pollard AJ. 2011. The burden of vaccine-preventable invasive bacterial infections and pneumonia in children admitted to hospital in urban Nepal. Int J Infect Dis 15:e17–e23. doi: 10.1016/j.ijid.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 50.Kibuuka A, Byakika-Kibwika P, Achan J, Yeka A, Nalyazi JN, Mpimbaza A, Rosenthal PJ, Kamya MR. 2015. Bacteremia among febrile Ugandan children treated with antimalarials despite a negative malaria test. Am J Trop Med Hyg 93:276–280. doi: 10.4269/ajtmh.14-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee C-C, Wu C-J, Chi C-H, Lee N-Y, Chen P-L, Lee H-C, Chang C-M, Ko N-Y, Ko W-C. 2012. Prediction of community-onset bacteremia among febrile adults visiting an emergency department: rigor matters. Diagn Microbiol Infect Dis 73:168–173. doi: 10.1016/j.diagmicrobio.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Leelarasamee A, Chupaprawan C, Chenchittikul M, Udompanthurat S. 2004. Etiologies of acute undifferentiated febrile illness in Thailand. J Med Assoc Thai 87:464–472. [PubMed] [Google Scholar]
- 53.Limper M, Eeftinck Schattenkerk D, de Kruif MD, van Wissen M, Brandjes DPM, Duits AJ, van Gorp ECM. 2011. One-year epidemiology of fever at the emergency department. Neth J Med 69:124–128. [PubMed] [Google Scholar]
- 54.Lin TY. 1991. Bacteremia in febrile children. Changgeng Yi Xue Za Zhi 14:44–49. (In Chinese.) [PubMed] [Google Scholar]
- 55.Liu CH, Lehan C, Speer ME, Smith EO, Gutgesell ME, Fernbach DJ, Rudolph AJ. 1985. Early detection of bacteremia in an outpatient clinic. Pediatrics 75:827–831. [PubMed] [Google Scholar]
- 56.Mave V, Chandanwale A, Kagal A, Khadse S, Kadam D, Bharadwaj R, Dohe V, Robinson ML, Kinikar A, Joshi S, Raichur P, McIntire K, Kanade S, Sachs J, Valvi C, Balasubramanian U, Kulkarni V, Milstone AM, Marbaniang I, Zenilman J, Gupta A. 2017. High burden of antimicrobial resistance and mortality among adults and children with community-onset bacterial infections in India. J Infect Dis 215:1312–1320. doi: 10.1093/infdis/jix114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McCarthy PL, Jekel JF, Dolan TF. 1977. Temperature greater than or equal to 40 C in children less than 24 months of age: a prospective study. Pediatrics 59:663–668. [PubMed] [Google Scholar]
- 58.Mellors JW, Horwitz RI, Harvey MR, Horwitz SM. 1987. A simple index to identify occult bacterial infection in adults with acute unexplained fever. Arch Intern Med 147:666–671. doi: 10.1001/archinte.1987.00370040048009. [DOI] [PubMed] [Google Scholar]
- 59.Mintegi S, Garcia-Garcia JJ, Benito J, Carrasco-Colom J, Gomez B, Hernández-Bou S, Astobiza E, Luaces-Cubells C. 2009. Rapid influenza test in young febrile infants for the identification of low-risk patients. Pediatr Infect Dis J 28:1026–1028. doi: 10.1097/INF.0b013e3181ab603c. [DOI] [PubMed] [Google Scholar]
- 60.Morch K, Manoharan A, Chandy S, Chacko N, Alvarez-Uria G, Patil S, Henry A, Nesaraj J, Kuriakose C, Singh A, Kurian S, Gill Haanshuus C, Langeland N, Blomberg B, Vasanthan Antony G, Mathai D. 2017. Acute undifferentiated fever in India: a multicentre study of aetiology and diagnostic accuracy. BMC Infect Dis 17:665. doi: 10.1186/s12879-017-2764-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nadjm B, Amos B, Mtove G, Ostermann J, Chonya S, Wangai H, Kimera J, Msuya W, Mtei F, Dekker D, Malahiyo R, Olomi R, Crump JA, Whitty CJM, Reyburn H. 2010. WHO guidelines for antimicrobial treatment in children admitted to hospital in an area of intense Plasmodium falciparum transmission: prospective study. BMJ 340:c1350. doi: 10.1136/bmj.c1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nadjm B, Mtove G, Amos B, Walker NF, Diefendal H, Reyburn H, Whitty C. 2012. Severe febrile illness in adult hospital admissions in Tanzania: a prospective study in an area of high malaria transmission. Trans R Soc Trop Med Hyg 106:688–695. doi: 10.1016/j.trstmh.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 63.Okwara FN, Obimbo EM, Wafula EM, Murila FV. 2004. Bacteraemia, urinary tract infection and malaria in hospitalised febrile children in Nairobi: is there an association? East Afr Med J 81:47–51. doi: 10.4314/eamj.v81i1.8795. [DOI] [PubMed] [Google Scholar]
- 64.Pavlinac PB, Naulikha JM, John-Stewart GC, Onchiri FM, Okumu AO, Sitati RR, Cranmer LM, Lokken EM, Singa BO, Walson JL. 2015. Mycobacterium tuberculosis bacteremia among acutely febrile children in western Kenya. Am J Trop Med Hyg 93:1087–1091. doi: 10.4269/ajtmh.15-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peters RPH, Zijlstra EE, Schijffelen MJ, Walsh AL, Joaki G, Kumwenda JJ, Kublin JG, Molyneux ME, Lewis DK. 2004. A prospective study of bloodstream infections as cause of fever in Malawi: clinical predictors and implications for management. Trop Med Int Health 9:928–934. doi: 10.1111/j.1365-3156.2004.01288.x. [DOI] [PubMed] [Google Scholar]
- 66.Petit PL, Haarlem JV, Poelman M, Haverkamp MC, Wamola IA. 1995. Bacteraemia in patients presenting with fever. East Afr Med J 72:116–120. [PubMed] [Google Scholar]
- 67.Preziosi M, Zimba TF, Lee K, Tomas M, Kinlin S, Nhatave-Paiva C, Bene R, Paunde T, Lopes H, Kalkhoff S, Prathap V, Akrami K, Noormahomed EV, Schooley RT, Aronoff-Spencer E. 2015. A prospective observational study of bacteraemia in adults admitted to an urban Mozambican hospital. S Afr Med J 105:370–374. doi: 10.7196/samj.8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Punjabi NH, Taylor WRJ, Murphy GS, Purwaningsih S, Picarima H, Sisson J, Olson JG, Baso S, Wangsasaputra F, Lesmana M, Oyofo BA, Simanjuntak CH, Subekti D, Corwin AL, Richie TL. 2012. Etiology of acute, non-malaria, febrile illnesses in Jayapura, northeastern Papua, Indonesia. Am J Trop Med Hyg 86:46–51. doi: 10.4269/ajtmh.2012.10-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ssali FN, Kamya MR, Wabwire-Mangen F, Kasasa S, Joloba M, Williams D, Mugerwa RD, Ellner JJ, Johnson JL. 1998. A prospective study of community-acquired bloodstream infections among febrile adults admitted to Mulago Hospital in Kampala, Uganda. J Acquir Immune Defic Syndr Hum Retrovirol 19:484–489. doi: 10.1097/00042560-199812150-00007. [DOI] [PubMed] [Google Scholar]
- 70.Teach SJ, Fleisher GR. 1997. Duration of fever and its relationship to bacteremia in febrile outpatients three to 36 months old. Pediatr Emerg Care 13:317–319. doi: 10.1097/00006565-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 71.Thriemer K, Ley B, Ame S, von Seidlein L, Pak GD, Chang NY, Hashim R, Schmied WH, Busch C-L, Nixon S, Morrissey A, Puri MK, Ali M, Ochiai RL, Wierzba T, Jiddawi MS, Clemens JD, Ali SM, Deen JL. 2012. The burden of invasive bacterial infections in Pemba, Zanzibar. PLoS One 7:e30350. doi: 10.1371/journal.pone.0030350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tokuda Y, Miyasato H, Stein GH, Kishaba T. 2005. The degree of chills for risk of bacteremia in acute febrile illness. Am J Med 118:1417. doi: 10.1016/j.amjmed.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 73.Walsh AL, Phiri AJ, Graham SM, Molyneux EM, Molyneux ME. 2000. Bacteremia in febrile Malawian children: clinical and microbiologic features. Pediatr Infect Dis J 19:312–318. doi: 10.1097/00006454-200004000-00010. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto LT, Wigder HN, Fligner DJ, Rauen M, Dershewitz RA. 1987. Relationship of bacteremia to antipyretic therapy in febrile children. Pediatr Emerg Care 3:223–227. doi: 10.1097/00006565-198712000-00001. [DOI] [PubMed] [Google Scholar]
- 75.Wong VK, Baker S, Pickard DJ, Parkhill J, Page AJ, Feasey NA, Kingsley RA, Thomson NR, Keane JA, Weill F-X, Edwards DJ, Hawkey J, Harris SR, Mather AE, Cain AK, Hadfield J, Hart PJ, Thieu NTV, Klemm EJ, Glinos DA, Breiman RF, Watson CH, Kariuki S, Gordon MA, Heyderman RS, Okoro C, Jacobs J, Lunguya O, Edmunds WJ, Msefula C, Chabalgoity JA, Kama M, Jenkins K, Dutta S, Marks F, Campos J, Thompson C, Obaro S, MacLennan CA, Dolecek C, Keddy KH, Smith AM, Parry CM, Karkey A, Mulholland EK, Campbell JI, Dongol S, Basnyat B, Dufour M, Bandaranayake D, Naseri TT, Singh SP, Hatta M, Newton P, Onsare RS, Isaia L, Dance D, Davong V, Thwaites G, Wijedoru L, Crump JA, De Pinna E, Nair S, Nilles EJ, Thanh DP, Turner P, Soeng S, Valcanis M, Powling J, Dimovski K, Hogg G, Farrar J, Holt KE, Dougan G. 2015. Phylogeographical analysis of the dominant multidrug-resistant H58 clade of Salmonella Typhi identifies inter- and intracontinental transmission events. Nat Genet 47:632–639. doi: 10.1038/ng.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Muthumbi E, Morpeth SC, Ooko M, Mwanzu A, Mwarumba S, Mturi N, Etyang AO, Berkley JA, Williams TN, Kariuki S, Scott J. 2015. Invasive salmonellosis in Kilifi, Kenya. Clin Infect Dis 61:S290–S301. doi: 10.1093/cid/civ737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.UNICEF. 2015. Progress on sanitation and drinking water: 2015 update and MDG assessment. UNICEF, New York, NY. [Google Scholar]
- 78.World Health Organization. 3 January 2018. Typhoid vaccine prequalified. https://www.who.int/medicines/news/2018/WHOprequalifies-breakthrough-typhoid-vaccine/en.
- 79.World Health Organization. 2019. Typhoid vaccines: WHO position paper, March 2018: recommendations. Vaccine 37:214–216. doi: 10.1016/j.vaccine.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 80.Wahl B, O'Brien KL, Greenbaum A, Majumder A, Liu L, Chu Y, Lukšić I, Nair H, McAllister DA, Campbell H, Rudan I, Black R, Knoll MD. 2018. Burden of Streptococcus pneumoniae and Haemophilus influenzae type b disease in children in the era of conjugate vaccines: global, regional, and national estimates for 2000–15. Lancet Glob Health 6:e744–e757. doi: 10.1016/S2214-109X(18)30247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Centers for Disease Control and Prevention. Global pneumococcal disease and vaccine. https://www.cdc.gov/pneumococcal/global.html.
- 82.International Vaccine Access Center. 2018. VIEW-hub report: global vaccine introduction and implementation. https://view-hub.org/resourcesfile/VIEW-hubReports_Resources/2018_03/IVAC_VIEW-hub_Report%202018Mar.pdf.
- 83.Frenck RW, Ervin J, Chu L, Abbanat D, Spiessens B, Go O, Haazen W, van den Dobbelsteen G, Poolman J, Thoelen S, Ibarra de Palacios P. 2019. Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): a phase 2 randomised controlled trial. Lancet Infect Dis 19:631–640. doi: 10.1016/S1473-3099(18)30803-X. [DOI] [PubMed] [Google Scholar]
- 84.Dahiya S, Sharma P, Kumari B, Pandey S, Malik R, Manral N, Veeraraghavan B, Pragasam AK, Ray P, Gautam V, Sistla S, Parija SC, Walia K, Ohri V, Das BK, Sood S, Kapil A. 2017. Characterisation of antimicrobial resistance in Salmonellae during 2014–2015 from four centres across India: an ICMR antimicrobial resistance surveillance network report. Indian J Med Microbiol 35:61–68. doi: 10.4103/ijmm.IJMM_16_382. [DOI] [PubMed] [Google Scholar]
- 85.Zehra NM, Irfan F, Mirza IA, Imtiaz A, Nadeem S, Hameed F. 2017. Current trends of antimicrobial susceptibility of typhoidal Salmonellae isolated at tertiary care hospital. J Coll Physicians Surg Pak 27:690–692. [PubMed] [Google Scholar]
- 86.Berkley JA, Lowe BS, Mwangi I, Williams T, Bauni E, Mwarumba S, Ngetsa C, Slack MPE, Njenga S, Hart CA, Maitland K, English M, Marsh K, Scott J. 2005. Bacteremia among children admitted to a rural hospital in Kenya. N Engl J Med 352:39–47. doi: 10.1056/NEJMoa040275. [DOI] [PubMed] [Google Scholar]
- 87.Sigaúque B, Roca A, Mandomando I, Morais L, Quintó L, Sacarlal J, Macete E, Nhamposa T, Machevo S, Aide P, Bassat Q, Bardají A, Nhalungo D, Soriano-Gabarró M, Flannery B, Menendez C, Levine MM, Alonso PL. 2009. Community-acquired bacteremia among children admitted to a rural hospital in Mozambique. Pediatr Infect Dis J 28:108–113. doi: 10.1097/INF.0b013e318187a87d. [DOI] [PubMed] [Google Scholar]
- 88.Roesnita B, Tay ST, Puthucheary SD, Sam I-C. 2012. Diagnostic use of Burkholderia pseudomallei selective media in a low prevalence setting. Trans R Soc Trop Med Hyg 106:131–133. doi: 10.1016/j.trstmh.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 89.Hoffmaster AR, AuCoin D, Baccam P, Baggett HC, Baird R, Bhengsri S, Blaney DD, Brett PJ, Brooks TJG, Brown KA, Chantratita N, Cheng AC, Dance DAB, Decuypere S, Defenbaugh D, Gee JE, Houghton R, Jorakate P, Lertmemongkolchai G, Limmathurotsakul D, Merlin TL, Mukhopadhyay C, Norton R, Peacock SJ, Rolim DB, Simpson AJ, Steinmetz I, Stoddard RA, Stokes MM, Sue D, Tuanyok A, Whistler T, Wuthiekanun V, Walke HT. 2015. Melioidosis diagnostic workshop, 2013. Emerg Infect Dis 21:e141045. doi: 10.3201/eid2102.141045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.World Health Organization. 2015. Global action plan on antimicrobial resistance. World Health Organization, Geneva, Switzerland: http://www.wpro.who.int/entity/drug_resistance/resources/global_action_plan_eng.pdf. [Google Scholar]
- 91.Cheng SH, Augustin C, Bethel A, Gill D, Anzaroot S, Brun J, DeWilde B, Minnich RC, Garside R, Masuda YJ, Miller DC, Wilkie D, Wongbusarakum S, McKinnon MC. 2018. Using machine learning to advance synthesis and use of conservation and environmental evidence. Conserv Biol 32:762–764. doi: 10.1111/cobi.13117. [DOI] [PubMed] [Google Scholar]
- 92.Moher D, Liberati A, Tetzlaff J, Altman DG. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crump JA, Sjölund-Karlsson M, Gordon MA, Parry CM. 2015. Epidemiology, clinical presentation, laboratory diagnosis, antimicrobial resistance, and antimicrobial management of invasive Salmonella infections. Clin Microbiol Rev 28:901–937. doi: 10.1128/CMR.00002-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.United Nations Statistics Division. Standard country or area codes for statistical use (M49). https://unstats.un.org/unsd/methodology/m49.
- 95.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing, 29th ed CLSI document M100 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.