Abstract
Asthma is increasingly recognized as an underlying risk factor for severe respiratory disease in patients with coronavirus disease 2019 (COVID-19), particularly in the United States. Here, we report the postmortem lung findings from a 37-year-old man with asthma, who met the clinical criteria for severe acute respiratory distress syndrome and died of COVID-19 less than 2 weeks after presentation to the hospital. His lungs showed mucus plugging and other histologic changes attributable to asthma, as well as early diffuse alveolar damage and a fibrinous pneumonia. The presence of diffuse alveolar damage is similar to descriptions of autopsy lung findings from patients with severe acute respiratory syndrome coronavirus and Middle East respiratory syndrome coronavirus, and the absence of a neutrophil-rich acute bronchopneumonia differs from the histologic changes typical of influenza. The relative contribution of mucus plugging to his hypoxemia is unknown.
Key Words: asthma, coronavirus, diffuse alveolar damage
Abbreviations: COVID-19, coronavirus disease 2019; DAD, diffuse alveolar damage; HD, hospital day; MERS, Middle East respiratory syndrome; SARS, severe acute respiratory syndrome; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2
Novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of the respiratory illness coronavirus disease 2019 (COVID-19), emerged in Wuhan, China, in late 2019.1 Since then, it has rapidly spread to cause a worldwide health crisis. Although most patients experience mild symptoms, 5% of patients experience catastrophic respiratory failure and of those, approximately half die of the disease.2 , 3 In Chinese populations, diabetes, hypertension, and cardiovascular disease are the most commonly reported comorbidities and are associated with worse survival.4, 5, 6, 7 Early data from the Centers for Disease Control and Prevention showed similar findings, and also identified underlying lung disease as a risk factor in the United States. Nearly 10% of US patients with COVID-19 have chronic lung disease, including asthma, as a risk factor.8 In contrast, only 2% of patients in Chinese studies had chronic lung disease.4, 5, 6, 7 Here, we document the first case report, to our knowledge, of postmortem lung findings in a patient with asthma who died of COVID-19.
Case Report
A 37-year-old man presented with a 1-day history of fever, nonproductive cough, and myalgias, and was admitted for medical treatment of presumptive COVID-19. He later tested positive for SARS-CoV-2. His past medical history was most significant for asthma, treated with ipratropium bromide and albuterol inhaler, and type 2 diabetes, treated with sitagliptin. On admission, CT imaging of the chest showed multifocal ground-glass opacities. Treatment included hydroxychloroquine, empiric piperacillin/tazobactam and vancomycin, and corticosteroids. He experienced worsening hypoxemia, necessitating intubation and mechanical ventilation on hospital day (HD) 4, and met the Berlin criteria for severe ARDS.9 On HD 9, he underwent sudden decompensation in oxygenation followed by cardiogenic collapse, prompting transition to venous-arterial extracorporeal membrane oxygenation. Continuous renal replacement therapy was also initiated, but his severe lactic acidosis was unrelenting. Care was withdrawn on HD 9 and an autopsy was requested.
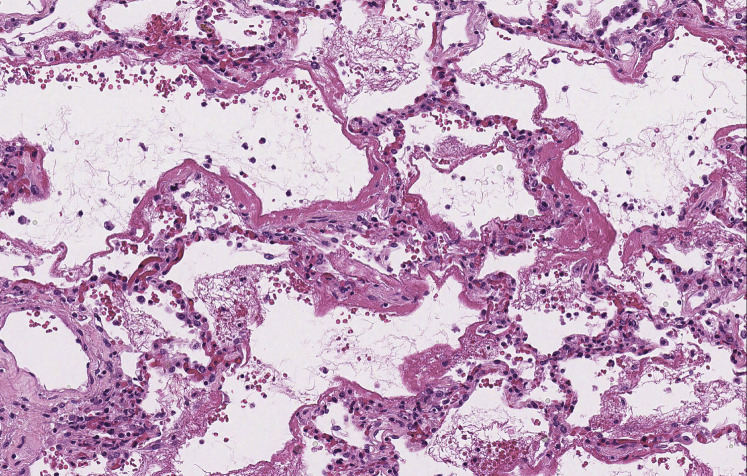
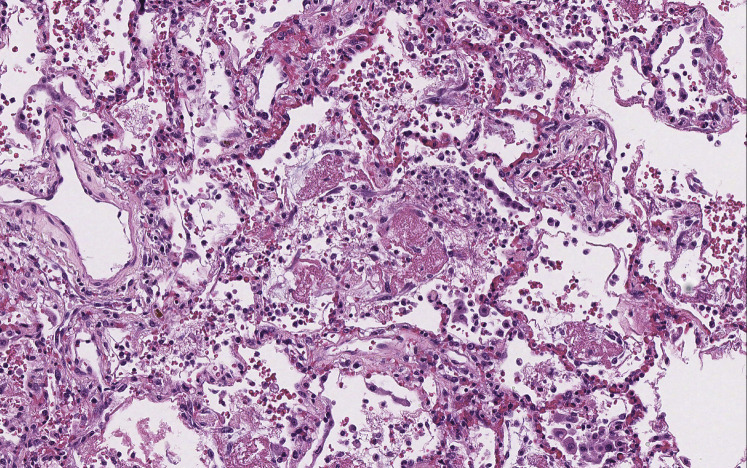
On gross examination, the lungs were heavy with mucus plugging of the conducting airways and consolidation of the lung parenchyma (Fig 1 ). Despite the presence of mucus plugs, there was no evidence of hyperinflation/air trapping, the anticipated finding in patients who die of status asthmaticus.10 Histologic sections of the proximal airways corroborated the presence of paucicellular mucus plugs, but without tissue eosinophilia. Goblet cell metaplasia, mucus gland hyperplasia, and thickening of subepithelial basement membranes in cartilaginous and noncartilaginous airways attested to the patient’s history of asthma. The distal alveolated lung tissue showed diffuse alveolar damage (DAD), characterized by patchy, mild interstitial thickening by edema, focal pneumocyte hyperplasia, and scattered hyaline membranes, the histologic hallmark of DAD (Fig 2 ). Rare fibrin thrombi were also identified within small vessels and a small muscular pulmonary artery, consistent with endothelial injury. This was accompanied by a mild patchy fibrinous airspace exudate in which mononuclear inflammatory cells predominated with scattered neutrophils (Fig 3 ). Although the inflammatory infiltrate was limited to distal airspaces without involvement of bronchi or bronchioles, this finding was morphologically similar to previous descriptions of COVID-19 pneumonia and may represent early bronchopneumonia.11, 12, 13
Figure 1.
Mucus plugs. Cut surface of the lung shows a thick intraluminal exudate within bronchi (gross image).
Figure 2.
Diffuse alveolar damage. Diffuse alveolar damage is characterized by epithelial and endothelial injury, resulting in the formation of hyaline membranes that outline distal alveolar airspaces (hematoxylin- and eosin-stained slide; original magnification, ×180).
Figure 3.
Fibrinous pneumonia. Distal alveolar spaces are focally filled with a fibrinous exudate accompanied by an inflammatory infiltrate, composed mainly of mononuclear inflammatory cells (hematoxylin- and eosin-stained slide; original magnification, ×176).
Discussion
DAD with a patchy fibrinous airspace exudate is the predominant finding in COVID-19-associated ARDS. The findings are histologically typical, including epithelial necrosis targeting the distal pulmonary acinus, endothelial injury with associated fibrin thrombi in small vessels, hyperplasia of reparative type 2 pneumocytes that lack viral inclusions, and hyaline membranes.14 Ours is, to our knowledge, among the earliest descriptions of pulmonary histologic changes in patients who die within weeks of symptom onset. Two previous reports also described DAD in patients with COVID-19 with premortem diagnoses of ARDS.12 , 13 DAD was also a consistent autopsy finding in patients from the earlier severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) coronavirus outbreaks.15, 16, 17 In some patients with SARS, as in the patient described here, a patchy, paucicellular fibrinous airspace pneumonia resembling acute fibrinous and organizing pneumonia was also reported.18
DAD is also a common finding in patients infected with influenza viruses, including influenza A/H1N1, and it is often accompanied by a combination of hemorrhage, acute bronchopneumonia in which neutrophils predominate, macroscopic thrombosis affecting peripheral pulmonary vessels, and hemophagocytosis.19 , 20 We identified none of these features in the patient described here, suggesting that the pathogenesis of COVID lung disease is different from these genetically distinct respiratory viruses.
Mucus plugs were a conspicuous feature in this patient and were likely attributable to his asthma history rather than a direct effect of viral infection. The extent to which this finding contributed to his respiratory failure is unclear, although it is conceivable that airflow limitation due to mucus plugging compounds the hypoxemia characteristic of DAD in patients with COVID-19 and underlying asthma.
Acknowledgments
Financial/nonfinancial disclosures: None declared.
Footnotes
Drs Konopka and Wilson are co-first authors.
References
- 1.Zhu N., Zhang D., Wang W. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 3.Wu C, Chen X, Cai Y, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. In press. 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed]
- 4.Chen T., Wu D., Chen H. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [published correction appears in BMJ. 2020;368:m1295] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. In press. 10.1164/rccm.202003-0543OC. [DOI] [PMC free article] [PubMed]
- 6.Guan W.J., Ni Z.Y., Hu Y. China Medical Treatment Expert Group for COVID-19. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC COVID-19 Response Team Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019—United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ARDS Definition Task Force; Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 10.Dunnill M.S. The pathology of asthma, with special reference to changes in the bronchial mucosa. J Clin Pathol. 1960;13:27–33. doi: 10.1136/jcp.13.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.Y. Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer. J Thorac Oncol. 2020;15(5):700–704. doi: 10.1016/j.jtho.2020.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Z., Shi L., Wang Y. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Repir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [published correction appears in Lancet Respir Med. 2020;8(4):e26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang H., Zhou P., Wei Y. Histopathologic changes and SARS-CoV-2 immunostaining in the lung of a patient with COVID-19. Ann Intern Med. 2020;172(9):629–632. doi: 10.7326/M20-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katzenstein A.L., Bloor C.M., Leibow A.A. Diffuse alveolar damage—the role of oxygen, shock, and related factors: a review. Am J Pathol. 1976;85(1):209–228. [PMC free article] [PubMed] [Google Scholar]
- 15.Franks T.J., Chong P.Y., Chui P. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol. 2003;34(8):743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng C.L., Al Hosani F., Keating M.K. Clinicopathologic, immunohistochemical, and ultrastructural findings of a fatal case of Middle East respiratory syndrome coronavirus infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186(3):652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alsaad K.O., Hajeer A.H., Al Balwi M. Histopathology of Middle East respiratory syndrome coronavirus (MERS-CoV) infection—clinicopathological and ultrastructural study. Histopathology. 2018;72(3):516–524. doi: 10.1111/his.13379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang D.M., Chamberlain D.W., Poutanen S.M., Low D.E., Asa S.L., Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18(1):1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harms P.W., Schmidt L.A., Smith L.B. Autopsy findings in eight patients with fatal H1N1 influenza. Am J Clin Pathol. 2010;134(1):27–35. doi: 10.1309/AJCP35KOZSAVNQZW. [DOI] [PubMed] [Google Scholar]
- 20.Rosen D.G., Lopez A.E., Anzalone M.L. Postmortem findings in eight cases of influenza A/H1N1. Mod Pathol. 2010;23(11):1449–1457. doi: 10.1038/modpathol.2010.148. [DOI] [PubMed] [Google Scholar]