Abstract
Photoresponsive materials that degrade, expand, change shape, or alter their microscopic topology in response to light have been studied for a wide range of applications. Such materials are typically in a metastable state during irradiation and return to their pre-irradiated state after removal of the light source. Strategies for the synthesis of materials that can reversibly photoswitch between two or more stable states, remaining in each state until a different stimulus is applied, could find applications as actuators, catalysts, and sensors. Here, we report a polymer gel comprising poly(ethylene glycol) star polymers linked by Cu24L24 cuboctahedral metal-organic cages/polyhedra (MOCs) decorated with coumarin ligands. Upon exposure to long-wavelength UV light in the presence of a photosensitizer and a hydrogen donor, this “polyMOC” material can be reversibly switched between three oxidation states of copper (Cu(II), Cu(I), and Cu(0)). The instability of the MOC junctions of these materials in the Cu(I) and Cu(0) states leads to network disassembly, providing Cu(I)/Cu(0) solutions, respectively, that are stable until exposure to a second stimulus, oxygen, induces re-oxidation to Cu(II) and rapid supramolecular gelation. This reversible supramolecular disassembly of the Cu-based polyMOC network is shown to occur in the presence of a fixed covalent second network generated in situ by copper-catalyzed azide-alkyne cycloaddition (CuAAC), providing interpenetrating supramolecular and covalent double networks with spatiotemporally enhanced mechanical properties. This work demonstrates that reversible disassembly of multicomponent MOCs can be used to switch bulk material properties, enabling functions that are difficult to access in traditional supramolecular networks.
Keywords: Gels, State-switching, Multi-material, PolyMOCs, stimuli responsive
Graphical Abstract

Supramolecular polymer metal-organic cage (polyMOC) gels with Cu24L24 cuboctahedral junctions featuring a high density of coumarin ligands were prepared. These robust, dynamic materials could be reversibly switched between Cu(II), Cu(I), and Cu(0) states, each producing distinct mechanical, optical, and catalytic properties. In particular, the Cu(I) state was leveraged to catalyze covalent network formation, providing novel polyMOC-covalent double networks
Introduction
Inspiration from biological tissues has led to the advent of many advanced synthetic material properties such as self-healing and enhanced toughness.[1–4] To achieve these and other properties on demand, researchers have relied on the incorporation of dynamic bonds that can be controlled through a specific external stimulus (e.g., light, force, heat, electricity).[4–19] These stimuli, however, typically produce a metastable material state; upon removal of the stimulus, the materials revert to their initial state. Systems that switch their properties in response to a given stimulus, and are subsequently stable in their new property state until exposure to a different stimulus, could find numerous applications as actuators, catalysts, scaffolds for additive manufacturing, biomaterials, sensors, etc.[20,21] Despite their significant potential, the scope of accessible properties with existing state-switchable materials is limited.
Seeking to develop a new material that is capable of achieving several complementary and useful stable states, we were inspired by the work of Park and coworkers who recently demonstrated a solution-phase reversible photoreduction of coumarin-functionalized Cu24L24 (L = benzenedicarboxylate, m-BDC) cuboctahedral metal-organic cages/polyhedra (MOC) between Cu(II) and Cu(I) states, showing that the Cu(I) state could be used for catalytic azide-alkyne cycloaddition (CuAAC) reactions.[22] In this system, the Cu(I) state was reported to be stable indefinitely until exposure to oxygen induced reversion to the Cu(II) state. Though the presence of the assembled MOC in the Cu(I) state was not studied in their work, we hypothesized that the geometrical requirements of Cu(I) complexes would lead to MOC disassembly. If true, and if such MOCs were used as the crosslinking junctions of polymer networks, then photoreduction of the resulting “polyMOCs” would lead to depolymerization and, thus, significant bulk material property changes. Notably, though there are previous reports of polyMOCs crosslinked by Cu24L24 MOCs in the dry state,[23,24] to our knowledge there are no reports of polyMOC gels crosslinked via Cu-based MOCs, nor are there studies that leverage the reversible assembly/disassembly of MOCs to drive bulk material property changes.
To achieve this goal, a 4-arm poly(ethylene glycol) (PEG) star polymer with m-BDC termini (PL) was synthesized from commercially available 10 kDa 4-arm PEG tetra-alcohol (Figure 1a). We have previously reported on the synthesis of polyMOCs derived from Pd12L24 and related MOCs crosslinked by 4-arm star polymers, showing that a large number of non-polymer ligands could be used in such systems to install functionality into the network junctions without sacrificing mechanical properties.[25,26] Here, we leverage this feature of polyMOC topology to install the same coumarin-based m-BDC ligands (CL) (Figure 1a) reported by Park and coworkers,[22] generating gels (referred to a c-Gel) composed of coumarin decorated Cu24L24 MOC junctions linked by PEG strands (Figure 1a). We demonstrate reversible switching of c-Gel between three functional, stable redox states (Figure 1b), transitioning between viscoelastic solid (gel) and fluid phases in a highly reversible manner. Moreover, we show that this material can be used to spatiotemporally generate double networks through CuAAC, providing a new strategy for gel additive manufacturing. This work demonstrates, to our knowledge, the first example of using reversible MOC assembly to drive both catalysis and reversible transitions between gel and liquid states, providing new mechanistic insights into the photoreduction of Cu24L24 MOCs, and a simple strategy for spatiotemporal covalent and double network fabrication.
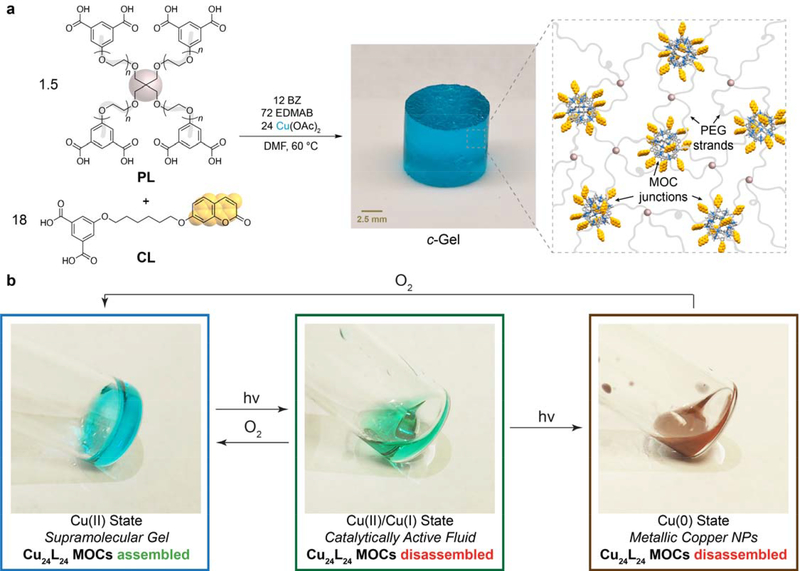
Figure 1. Design of Cu24L24-based polyMOCs featuring coumarin-functionalized junctions.
(a) m-BDC-functionalized PEG star polymer PL and coumarin-functionalized m-BDC (CL) are combined with Cu(OAc)2, dimethylformamide (DMF), benzophenone (BZ, a photosensitizer), and ethyl-4-(dimethylamino)benzoate (EDMAB, an H-Atom donor). Annealing provides coumarin-functionalized polyMOC c-Gel, which is composed of Cu24L24 junctions decorated with coumarins and polymer strands from CL and PL, respectively. (b) Photoreduction or air oxidation enables reversible interconversion of c-Gel between Cu(II), Cu(I), and Cu(0) functional states with corresponding to gel-sol transitions and differences in catalytic activity.
To explore the feasibility of using Cu24L24 MOC assembly to form supramolecular gels, we first explored the synthesis of Cu24L24-crosslinked polyMOCs lacking coumarin ligands as a proof-of-concept. When PL was combined with m-BDC in a 1:3 ratio with respect to m-BDC groups (each PL contributes 4 m-BDC groups) and mixed with a solution of Cu(II) acetate in dimethylformamide (DMF), gelation occurred immediately (Figure 2a, note: 5.3 wt. % polymer was used for these studies though polymer concentrations as low as 2 wt. % still formed robust gels). Annealing for 4 h at 60 °C provided a translucent blue gel (s-Gel) with a maximum absorption (λmax) at 724 nm (Figure 2b), which is diagnostic of the paddlewheel complexes in the desired m-BDC-based Cu24L24 MOCs.[27] Additionally, a robust polyMOC gel could be obtained using linear m-BDC terminated polymers, showing that the covalent crosslink in PL is not required for gelation (see supporting information for more detail).
Figure 2. Synthesis and characterization of s-Gel.
(a) Synthesis and schematic representation of s-Gel. DMF = N,N-dimethylformamide. (b) UV-vis spectrum of s-Gel (black line) showing the red-shifting of λmax to 724 nm compared to copper acetate (red line). This red shift is indicative of the formation of Cu-m-BDC paddlewheel complexes. (c) SAXS/WAXS curve depicting the spacing between Cu24L24 MOCs within s-Gel as well as the form factor of MOC junctions. (d) Frequency sweep rheology curve for s-Gel (5.3 wt. %) swollen to equilibrium in DMF. (e) Stress relaxation data for s-Gel. The red line corresponds to fitting of the data using the Kohlrausch stretched exponential model, which provide the characteristic relaxation time τ. Inset: Image of s-Gel cut into several pieces (left). Placing the mashed sample in a mold and applying pressure leads to complete healing (right).
The small- and wide-angle X-ray scattering (SAXS/WAXS) curve (Figure 2c) for s-Gel displayed two peaks: one in the high q region (0.47 Å−1) that corresponds to the form factor of a ~2.7 nm spherical particle similar size to the cuboctahedral Cu24L24 MOC,[28] and a broad peak at low q (~0.07 Å−1) that we assign to the average distance between the MOC junctions (~9 nm; see supplemental text section I for discussion). These two peaks, which are unique to the MOC and the inter-MOC distance within the network, are strong evidence for the proposed polyMOC structure of c-Gel. In frequency sweep rheology studies s-Gel behaved as an elastic solid (G′ > G′′) at all measured frequencies (0.1 to 100 rad/s) with G′ = 6.6 kPa @ 10 rad/s (Figure 2d), which is consistent with the presence of high branch functionality junctions as expected for the proposed polyMOC structure (where there are ~6 strands per MOC on average). Simulations of s-Gel revealed that a significant fraction of the arms of each PL ligand are bridging across two different Cu24L24 MOCs to provide a high crosslinking density (Figure S3).The characteristic relaxation time (τ) of s-Gel was 132 ± 5 s at room temperature, which is ~100-fold less than previously reported polyMOCs with cuboctahedral Pd12L24 MOC junctions at 55 °C.[25] Cu24L24 MOCs are known to undergo rapid ligand exchange in solution,[27,29] which in the case of s-Gel provides a mechanism for rapid stress relaxation and healing ([dummy_]Figure 2f).
Next, samples of photoswitchable c-Gel were prepared by combining PL with CL[22] in a 1:3 ratio with respect to m-BDC groups and Cu(II) acetate (6.6 wt. % polymer in DMF) (Figure 1). H-atom donor ethyl-4-(dimethylamino)benzoate (EDMAB, 3 equiv relative to Cu) and photosensitizer benzophenone (BZ, 0.5 equiv relative to Cu) were added and the mixture was annealed for 4 h at 60 °C. The resulting c-Gel (Figure 1a), displayed the characteristic λmax = 724 nm of Cu(II) paddlewheel complexes (Figure 3a) while the SAXS/WAXS (Figure 3b) and frequency sweep rheology (Figure 3c) results were similar to those of s-Gel, suggesting that coumarin incorporation and the presence of EDMAB and BZ have little impact on the structure and topology of the polyMOC network. Strikingly, when c-Gel was exposed to 368 nm light (FL8BLB-368 8W bulb, 2 h) under N2 atmosphere, it converted into a transparent green liquid (Figure 1b, Figure 3d). This solid-to-liquid phase transition along with disappearance of the 724 nm absorbance peak (red line, Figure 3a) are consistent with photoinduced reduction of and MOC disassembly.
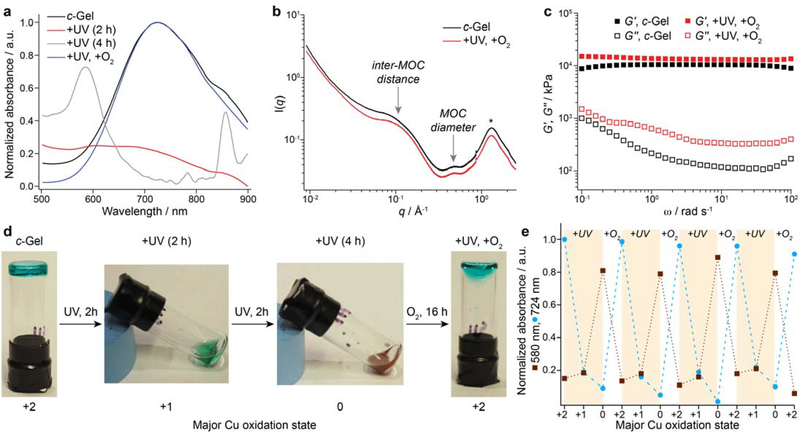
Figure 3. Multi-state switching of c-Gel in the presence of BZ and EDMAB.
(a) UV-vis spectra for c-Gel (6.6 wt. %) before UV exposure (black line), after switching to the Cu (I) (red line) and Cu (0) states (gray line), and after air oxidation (blue line). (b) SAXS/WAXS curves for c-Gel before (black) and after UV exposure and re-oxidation (red). The structure of the Cu(II) c-Gel is restored upon re-oxidation. (c) Frequency sweep rheology curves for c-Gel before (black) and after UV exposure and re-oxidation (red). The slight increases in the storage and loss moduli are attributed to solvent evaporation during the course of the switching and re-oxidation processes. (d) Images of c-Gel as it proceeds through a full switching cycle. After 2 h of UV exposure the gel converts into a green fluid, which further converts into a brown liquid after 2 additional hours of UV exposure. 16 h after exposure to air the blue Cu(II) c-Gel is re-formed. (e) Switching of c-Gel through 4 complete cycles (12 total state conversions) as monitored by UV-vis spectroscopy. Dashed lines are drawn between points to aid the viewer. Note: oxidation labels indicate the primary functional oxidation state of the material; they are not intended to imply that 100% of the Cu centers have a given oxidation state.
The reduced c-Gel solution was stable indefinitely under N2 atmosphere, while exposure to 368 nm light for an additional 2 h produced a brown liquid (Figure 3a) with λmax = 580 nm (grey line, Figure 3a), which is consistent with the formation of Cu(0) nanoparticles.[30] Exposing either the primarily Cu(I) or Cu(0) solutions to ambient air, however, led to their conversion back to Cu(II) c-Gel with λmax = 724 nm (blue line, Figure 3a; Figure 3d, supplementary video S1), an identical SAXS/WAXS profile (red line, Figure 3b), and a similar G′ value compared to c-Gel (red line, Figure 3c) (10.3 kPa vs 13.1 kPa @ 10 rad/s before and after photoreduction/re-oxidation, respectively. The increase in G′ is attributed to solvent evaporation during the oxidation process). This switching between Cu(II), Cu(I), Cu(0) and back to Cu(II) was repeated for 4 cycles without the addition of any new reagents, which corresponds to 12 separate state transformations for a single sample (Figure 3d). Interestingly, these results contrast with the report of Park and coworkers, who noted that under their conditions the Cu(0) state formed irreversibly. It should be noted, however, that our system is quite different (i.e., involving polymers) and different reagents were used for the photoreduction (EDMAB rather than MeOH/diethyl ether), which may explain the enhanced switchability in our system.
As noted above, while Park and coworkers confirmed the presence of Cu24m-BDC24 MOCs in the Cu(II) state, the identities of the species in the Cu(I) or Cu(0) states were not investigated. Given the geometric preferences of Cu(II) versus Cu(I) complexes, we hypothesized that photoreduction of Cu24m-BDC24 MOCs would necessitate MOC disassembly. Our observation of a gel to liquid transition upon photoreduction of c-Gel is consistent with this hypothesis (Figure 1b and 3d) as MOC disassembly lowers the system below the critical crosslinking density for gelation. Diffusion ordered spectroscopy (DOSY) and density functional theory (DFT) studies were conducted to provide further evidence of this proposed MOC disassembly mechanism. Solution DOSY studies of coumarin-decorated MOCs lacking PL in the Cu(II) state revealed the presence of ~3.9 ± 1.5 nm species as determined from the measured diffusion coefficient and the Stokes-Einstein equation. Photoreduction led to the disappearance of these slowly diffusing species and concomitant sharpening of the signals, which is indicative of both MOC disassembly and the transition from paramagnetic Cu(II) to diamagnetic Cu(I) (Figure 4a).[31–34] DFT calculations of a model bis-Cu(II) paddlewheel complex indicated that reduction of even one Cu center leads to cleavage of Cu-ligand bonds (Figure 4b) and further reduction leads to complete paddlewheel disassembly. Altogether, these results strongly support our hypothesis that photoreduction of these MOCs leads to their disassembly, explaining the observed gel-liquid transition upon photoreduction of c-Gel and providing a new mechanism to drive reversible self-assemble of supramolecular gels.
Figure 4. Mechanistic studies.
(a) Solution 1H DOSY measurements of a coumarin-decorated MOC before (blue) and after (green) irradiation. The faster diffusion times of peaks associated with the coumarin ligand (CL) indicate that the MOC disassembles in the Cu(I) state (b) DFT calculated stable structures of Cu2L4’ (L’ = C6H5COO–) for all possible oxidation states of Cu (blue: Cu; red: O; brown: C; pink: H). Free energies (ΔG) are reported in kcal/mol relative to Cu(II)/Cu(II). For the last two CuL2’ structures, energy is reported as ΔG = 2*G(CuL2’) – G(Cu2L4’). (c) Proposed mechanism for c-Gel switching. 3[BZ]* is deactivated by coumarin generating 3[coumarin]*. Subsequently, 3[coumarin]* reduces Cu(II) to Cu(I) (see Figure S6), which leads to MOC disassembly and a gel-sol transition. Further light exposure leads to reduction to Cu(0). Exposure to air at the Cu(I) or Cu(0) states regenerates Cu(II) leading to MOC reassembly (and gelation).
Park and coworkers suggested that coumarin acts to quench photoexcited BZ in their solution-based system, though direct experimental evidence to support this proposal was not provided. Here, we conducted a series of control experiments and DFT calculations to gain a deeper understanding of the related photoswitching process in our polyMOCs. First, a sample of s-Gel (Figure 2a, lacking coumarin ligand CL) was prepared in the presence of BZ and EDMAB and irradiated with 368 nm light. Regardless of the light intensity used, the material immediately converted to a brown fluid indicative of the Cu(0) state, confirming that coumarin functionalized MOC junctions are necessary to avoid overreduction and suggesting that coumarin plays a more complex role than simply acting as a quencher (Figure S4). When c-Gel (with coumarin) was irradiated for 24 h in the absence of BZ, no detectable changes were observed, which suggests that direct photoexcitation of coumarin (i.e., to the singlet state) does not induce reduction (Figure S5). Finally, when coumarin is added to s-Gel (i.e., not covalently attached to the polyMOC junctions) the material rapidly reduces directly to Cu(0), suggesting that a high local concentration of coumarin near the MOC junctions is critical.
To collectively rationalize these observations, DFT calculations of a model junction were conducted (supplementary text section III, Figure S6). The results led us to propose an alternative mechanism to that proposed by Park and coworkers for the redox switching of coumarin-decorated Cu24L24 MOCs (Figure 4c). Following absorption and intersystem crossing, we propose that triplet excited BZ transfers energy to the coumarin groups within the MOC junctions of c-Gel generating triplet coumarin. The latter, based on our DFT calculations, is thermodynamically capable of reducing Cu(II) to Cu(I) but not Cu(I) to Cu(0); thus, we propose that the MOC is switched from Cu(II) to Cu(I) primarily by triplet coumarin rather than BZ. This photoreduction leads to MOC disassembly, which allows for direct approach of BZ to copper centers and further reduction of Cu(I) to Cu(0) (Figure 4c). Though many details of this mechanism deserve further study, e.g., the detailed elementary steps in each reduction process, we believe that our proposal is consistent with the experimental evidence, providing a framework for optimizing these materials in various applications.
To demonstrate the utility and versatility of c-Gel, we envisioned using the Cu(I) functional state to catalyze the formation of a covalent polymer network via CuAAC. Oxidation back to the Cu(II) state may provide an interpenetrating double network composed of both the supramolecular Cu24L24 polyMOC and a secondary covalent network. Related double networks featuring energy dissipating supramolecular networks and ductile covalent networks have received extensive attention due to their outstanding toughness.[35–38] Nevertheless, the use of a primary supramolecular network to catalyze the formation of a reinforcing secondary covalent network represents, to our knowledge, a fundamentally new way to generate such materials.
To confirm that c-Gel in its Cu(I) state could catalyze CuAAC, benzyl azide and tetrakis(prop-2-ynyloxymethyl)methane were added and the mixture was exposed to 368 nm light for 4 h. After oxidation the CuAAC triazole product was isolated from c-Gel in 85% yield (supplementary text section IV). Notably, incubation of these starting materials with c-Gel in the absence of light did not provide any detectable product, suggesting that Cu(I) is needed. Next, α,ω-azide-terminated PEG (Mn = 2.0 kDa) (A2) and tetrakis(prop-2-ynyloxymethyl)methane (B4) were added to a fresh sample of c-Gel (2:1 ratio of A2:B4, 13 wt. % of A2) as precursors to covalent A2 + B4 covalent networks (Figure 5a). The fast network dynamics allowed the mixture to be annealed into a uniform sheet via compression in a mold at room temperature over 6 h. The sheet was then cut into uniform strips, placed in nitrogen-purged vials (Figure 5a, i) and irradiated with 368 nm light for 4 h to achieve switching throughout the sample (Figure 5a, ii). In contrast to c-Gel alone, these samples retained their solid form, hinting at the creation of a new covalent network. UV-vis spectroscopy after switching showed disappearance of the characteristic 724 nm absorbance associated with the Cu(II) paddlewheel complex. Exposure of the materials to air restored their original blue color and λmax = 724 nm, providing putative double network “min-Gel” (Figure 5a, iii). SAXS/WAXS suggested that (Figure S7) the structure of min-Gel was very similar to that of the parent material c-Gel (Figure 5b). Thus, though a new covalent network is formed irreversibly in this process, c-Gel is able to reversibly switch between states (e.g., from Cu(I) back to Cu(II)) within the double network, making it available to catalyze subsequent reactions if desired.
Figure 5. Metal-organic cage interpenetrating network (min-Gel) fabrication enabled by photo-induced switching of c-Gel.
(a) Synthesis of c-Gel (6.6 wt. %) in the presence of 2 kDa bis-azido-PEG A2 and tetrakis-alkyne B4. Images of the material immediately after annealing (i), after irradiation with UV light to induce the CuAAC reaction (ii), and re-oxidation (iii) to provide min-Gel. (b) Schematic depiction of the interpenetrating polyMOC and PEG supramolecular and covalent networks, respectively, within min-Gel. The covalent PEG network consists of linear strands (green) that can connect two junctions (green spheres) or be connected to the same junction. The latter “primary loop” defects, which are quantified using NDS, reduce the network connectivity giving rise to double-network-like properties. (c) Stress-strain plot from tensile testing showing the increased toughness of min-Gel compared to control samples. (d) Stress-strain curves showing hysteresis of min-Gel over 4 cycles. Strain rate = 1 mm/min; sample length = 5 mm. There was no resting period between cycles. (e) A fish-shaped sample of c-Gel with A2 and B4 (i) is covered with an aluminum foil photomask (ii). Irradiation of the sample with a 368 nm UV lamp for 8 h leads to the formation of Cu(I) in irradiated regions (iii). Washing the sample with aqueous ethylene diamine allows for isolation of the newly formed PEG-based covalent network (iv). (f) A rectangular sample of c-Gel (i) was first switched to the Cu(I) state to induce covalent network formation throughout the sample. Then, a photomask in the shape of a circuit was placed over the sample (ii), and the material was irradiated with a 365 nm UV LED to deposit Cu(0) in irradiated regions (iii). Washing the material with aqueous ethylene diamine allowed for isolation of the PEG covalent network with patterned Cu(0) regions (iv). Note: oxidation labels indicate the primary functional oxidation state of the material; they are not intended to imply that 100% of the Cu centers have a given oxidation state.
Uniaxial tensile testing (Figure 5c) and oscillatory rheology (Figure S8) studies of min-Gel and several control samples further supported the double network structure of min-Gel (see table S1 for values of toughness, Young’s moduli (E), extensibility, and ultimate strength). min-Gel displayed ~150-fold enhanced toughness (black line, Figure 5c) and increased moduli compared to both c-Gel with uncrosslinked A2 and B4 (blue line, Figure 5c) and c-Gel irradiated and re-oxidized in the absence of A2 and B4 (red line, Figure 5c). Following irradiation, the polyMOC network of min-Gel could be extracted with an aqueous ethylene diamine solution (10% v/v) (Figure S9) providing a colorless covalent PEG-based gel with much lower toughness (green line, Figure 5c). Thus, only the min-Gel displays the enhanced mechanical properties of double networks.
The characteristic relaxation time for min-Gel was 2460 ± 35 s (Figure S10), while hysteresis experiments demonstrated that this material dissipated stress as seen by the area between extension and relaxation (red line, Figure 5d). In repeated extensions, the material recovered a majority of its toughness despite a small amount of plastic deformation. The recovery of toughness on the timescale of the tensile testing experiment is attributed to the fast relaxation time the supramolecular polyMOC network component of min-Gel. Compressive testing corroborated these results, showing that min-Gel displayed significantly enhanced toughness compared to its component networks (Figure S11). In addition, a strain rate dependence was observed, as expected due to the fast relaxation time of the polyMOC network. Compression analysis of an irradiated sample before re-oxidation showed lower toughness than the oxidized min-Gel, which further demonstrates that an intact polyMOC network is necessary for the enhanced properties of this material.
In optimized interpenetrating networks, such as double networks and related systems, the secondary network typically comprises highly extensible, high molecular weight, lightly crosslinked polymers.[35,39] To characterize the covalent network within min-Gel, Fourier-transform infrared spectroscopy (FTIR) and network disassembly spectrometry (NDS) experiments were employed (Figure S12).[40–44] [45] Both methods showed the presence of unreacted azides within the covalent network, while NDS revealed that the average number of primary loops per B4 junction was ~0.6, which is near the limiting sol-gel threshold for tetra-functional end-linked networks.[41] These results suggest that though the PEG bis-azide used here has a relatively low molecular weight, the covalent network formed within min-Gel has a much larger effective molecular weight between crosslinks, making it more extensible and providing double network properties. When a sample of a min-Gel sample was prepared using a 20 kDa PEG bis-azide (13 wt. %), a more extensible material with a lower ultimate stress was obtained (Figure S13).
We reasoned that it should be possible to leverage the light-induced state-switching properties of c-Gel to spatiotemporally control double network fabrication. To demonstrate this concept, a fish-shaped slab (Figure 5e, i) of c-Gel containing A2 and B4 was covered by a fish skeleton-shaped photomask (Figure 5e, ii). After exposure to 368 nm light for 8 h, the Cu(I) functional state could be readily observed in the pattern set by the photomask (Figure 5e, iii). Re-oxidation provided a fish with a c-Gel (single network) body and a tough min-Gel (double network) skeleton. The covalent PEG gel of the latter could be isolated by dissolving away the polyMOC component with ethylene diamine (Figure 5e, iv), supplementary video S2). Given the liquid-like nature of the PEG network components this shape could only otherwise be generated using a mold or complex viscosity modification/processing techniques. Our approach provides a way to utilize the dynamic polyMOC as both a scaffold and a catalyst for covalent network formation.
Further photoreduction using a 365 nm UV LED light source enabled the deposition of Cu(0) in irradiated regions (Figure S14 and S15). We reasoned that this phenomenon could provide a novel way to pattern metallic copper onto soft materials. To demonstrate this concept (supplementary text section V), a 2.1 cm square slab of c-Gel containing A2 and B4 (Figure 5f, i) was switched to the Cu(I) state to form a covalent network. A photomask was applied (Figure 5f, ii) and the sample was exposed to the UV-LED until a metallic pattern was apparent (~2 h, Figure 5f, iii). Removal of the polyMOC provided a covalent PEG network with a film of Cu(0) in a circuit-shaped pattern embedded on its surface (Figure 5f, iv). The resistance of the Cu(0) regions of this material was ~1000-fold lower than in non-irradiated regions, suggesting that with further engineering, sintering, and optimization the switchability of c-Gel could open new avenues for the fabrication of integrated soft-material circuits that could have applications in sensing or soft robotics [46,47].
This work describes, to our knowledge, the first examples of Cu-based polyMOC gels, significantly expanding the scope of this emerging class of hybrid materials. In addition, the use of photoredox-induced MOC disassembly is used for the first time to drive reversible sol-gel transitions and catalysis within polymer networks. In combination with the spatiotemporal control provided by light as an external stimulus, this material could find use in soft circuit fabrication, heterogeneous catalysis, and a range of other potential applications. Future studies aimed toward probing the photoswitching mechanism in detail, the impact of loops, the number of coumarins per cage, the cage density, etc. will further improve the properties of these systems and enable the rational design of next-generation state-switchable materials.
Materials and Methods
Details of all procedures can be found in the SI Appendix
Supplementary Material
Acknowledgments
N.J.O would like to thank J.R.L for artistic photomask contributions. N.J.O. acknowledges support from the National Institutes of Health (F32 fellowship GM123710). This work was funded by the Center for the Chemistry of Molecularly Optimized Networks, an NSF Center for Chemical Innovation (CHE-1832256).
Footnotes
Competing interests: Authors declare no competing interests
References
- [1].Montero de Espinosa L, Meesorn W, Moatsou D, Weder C, Chem. Rev 2017, 117, 12851–12892. [DOI] [PubMed] [Google Scholar]
- [2].Oliver K, Seddon A, Trask RS, J. Mater. Sci 2016, 51, 10663–10689. [Google Scholar]
- [3].Mendes PM, Chem. Soc. Rev 2008, 37, 2512. [DOI] [PubMed] [Google Scholar]
- [4].Wojtecki RJ, Meador MA, Rowan SJ, Nat. Mater 2011, 10, 14–27. [DOI] [PubMed] [Google Scholar]
- [5].Yan X, Wang F, Zheng B, Huang F, Chem. Soc. Rev 2012, 41, 6042. [DOI] [PubMed] [Google Scholar]
- [6].Datta S, Saha ML, Stang PJ, Acc. Chem. Res 2018, acs.accounts.8b00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li M, Chen LJ, Cai Y, Luo Q, Li W, Yang HB, Tian H, Zhu WH, Chem 2019, 5, 634–648. [Google Scholar]
- [8].Ji X, Yao Y, Li J, Yan X, Huang F, J. Am. Chem. Soc 2013, 135, 74–77. [DOI] [PubMed] [Google Scholar]
- [9].Ogden WA, Guan Z, J. Am. Chem. Soc 2018, 140, 6217–6220. [DOI] [PubMed] [Google Scholar]
- [10].V Accardo J, Kalow JA, Chem. Sci 2018, 9, 5987–5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Gu Y, Zhao J, Johnson JA, Angew. Chemie Int. Ed 2019, DOI 10.1002/anie.201902900. [Google Scholar]
- [12].Stuart MAC, Huck WTS, Genzer J, Müller M, Ober C, Stamm M, Sukhorukov GB, Szleifer I, V Tsukruk V, Urban M, et al. , Nat. Mater 2010, 9, 101–113. [DOI] [PubMed] [Google Scholar]
- [13].Li L, Scheiger JM, Levkin PA, Adv. Mater 2019, 1807333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Worrell BT, McBride MK, Lyon GB, Cox LM, Wang C, Mavila S, Lim C-H, Coley HM, Musgrave CB, Ding Y, et al. , Nat. Commun 2018, 9, 2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fiore GL, Rowan SJ, Weder C, Chem. Soc. Rev 2013, 42, 7278–7288. [DOI] [PubMed] [Google Scholar]
- [16].Lendlein A, Jiang H, Jünger O, Langer R, Nature 2005, 434, 879–882. [DOI] [PubMed] [Google Scholar]
- [17].Xie T, Nature 2010, 464, 267–270. [DOI] [PubMed] [Google Scholar]
- [18].Goor OJGM, Hendrikse SIS, Dankers PYW, Meijer EW, Chem. Soc. Rev 2017, 46, 6621–6637. [DOI] [PubMed] [Google Scholar]
- [19].Del Barrio J, Horton PN, Lairez D, Lloyd GO, Toprakcioglu C, Scherman OA, J. Am. Chem. Soc 2013, 135, 11760–11763. [DOI] [PubMed] [Google Scholar]
- [20].Freeman R, Han M, Álvarez Z, Lewis JA, Wester JR, Stephanopoulos N, McClendon MT, Lynsky C, Godbe JM, Sangji H, et al. , Science (80-. ). 2018, 362, 808–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Gu Y, Alt EA, Wang H, Li X, Willard AP, Johnson JA, Nature 2018, 560, 65–69. [DOI] [PubMed] [Google Scholar]
- [22].Bae J, Baek K, Yuan D, Kim W, Kim K, Zhou HC, Park J, Chem. Commun 2017, 53, 9250–9253. [DOI] [PubMed] [Google Scholar]
- [23].Lal G, Derakhshandeh M, Akhtar F, Spasyuk DM, Lin J-B, Trifkovic M, Shimizu GKH, J. Am. Chem. Soc 2019, 141, 1045–1053. [DOI] [PubMed] [Google Scholar]
- [24].Xie X-Y, Wu F, Liu X, Tao W-Q, Jiang Y, Liu X-Q, Sun L-B, Chem. Commun 2019, 55, 6177–6180. [DOI] [PubMed] [Google Scholar]
- [25].Wang Y, Gu Y, Keeler EG, Park JV, Griffin RG, Johnson JA, Angew. Chemie Int. Ed 2017, 56, 188–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhukhovitskiy AV, Zhong M, Keeler EG, Michaelis VK, Sun JEP, Hore MJA, Pochan DJ, Griffin RG, Willard AP, Johnson JA, Nat. Chem 2015, 8, 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li J-R, Zhou H-C, Nat. Chem 2010, 2, 893–898. [DOI] [PubMed] [Google Scholar]
- [28].Eddaoudi M, Kim J, Wachter JB, Chae HK, O’Keeffe M, Yaghi OM, J. Am. Chem. Soc 2001, 123, 4368–4369. [DOI] [PubMed] [Google Scholar]
- [29].Larsen RW, J. Am. Chem. Soc 2008, 130, 11246–11247. [DOI] [PubMed] [Google Scholar]
- [30].Henglein A, J. Phys. Chem. B 2000, 104, 1206–1211. [Google Scholar]
- [31].Kln P, Wirz J, Photochemistry of Organic Compounds, John Wiley & Sons, Ltd, Chichester, UK, 2009. [Google Scholar]
- [32].Romero NA, Nicewicz DA, Chem. Rev 2016, 116, 10075–10166. [DOI] [PubMed] [Google Scholar]
- [33].Chow YL, Buono-Core GE, Can. J. Chem 1982, 61, 795–800. [Google Scholar]
- [34].Buono-Core G, Iwai K, Chow YL, Koyanagi T, Kaji A, Hayami J-I, Can. J. Chem 1979, 57, 8–16. [Google Scholar]
- [35].Gong JP, Soft Matter 2010, 6, 2583–2590. [Google Scholar]
- [36].Sun JY, Zhao X, Illeperuma WRK, Chaudhuri O, Oh KH, Mooney DJ, Vlassak JJ, Suo Z, Nature 2012, 489, 133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gong JP, Katsuyama Y, Kurokawa T, Osada Y, Adv. Mater 2003, 15, 1155–1158. [Google Scholar]
- [38].Wang W, Narain R, Zeng H, Front. Chem 2018, 6, 497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brown HR, Macromolecules 2007, 40, 3815–3818. [Google Scholar]
- [40].Zhou H, Woo J, Cok AM, Wang M, Olsen BD, Johnson JA, Proc. Natl. Acad. Sci 2012, 109, 19119–19124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kawamoto K, Zhong M, Wang R, Olsen BD, Johnson JA, Macromolecules 2015, 48, 8980–8988. [Google Scholar]
- [42].Zhong M, Wang R, Kawamoto K, Olsen BD, Johnson JA, Science (80-. ). 2016, 353, 1264–1268. [DOI] [PubMed] [Google Scholar]
- [43].Gu Y, Kawamoto K, Zhong M, Chen M, Hore MJA, Jordan AM, Korley LTJ, Olsen BD, Johnson JA, Proc. Natl. Acad. Sci 2017, 114, 4875–4880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhou H, Schön E-M, Wang M, Glassman MJ, Liu J, Zhong M, Díaz Díaz D, Olsen BD, Johnson JA, J. Am. Chem. Soc 2014, 136, 9464–9470. [DOI] [PubMed] [Google Scholar]
- [45].Gu Y, Zhao J, Johnson JA, Trends Chem. 2019, DOI 10.1016/j.trechm.2019.02.017. [DOI] [Google Scholar]
- [46].Kamyshny A, Magdassi S, Chem. Soc. Rev 2018, 48, 1712. [DOI] [PubMed] [Google Scholar]
- [47].Choi S, Han SI, Kim D, Hyeon T, Kim D-H, Chem. Soc. Rev 2019, 48, 1566–1595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.