Abstract
Objectives:
To evaluate the safety, pharmacokinetics, and preliminary efficacy of NT-814, a dual neurokinin 1,3 antagonist, in postmenopausal women with vasomotor symptoms (hot flashes).
Methods:
We completed a double-blind, randomized, placebo-controlled trial in three US clinical research units in 76 postmenopausal women with moderate/severe hot flashes. Participants were randomized to 14 days of once-daily NT-814 or placebo within each of four sequential dose cohorts; 50, 100, 150, and 300 mg. Participants completed diaries of hot flash frequency and severity and waking due to night sweats before (baseline) and during treatment.
Results:
All prespecified efficacy parameters (24-h hot flash frequency and severity, frequency of waking due to night sweats) decreased in all groups (including placebo). Mean reduction from baseline at week 2 in moderate/severe hot flash frequency was 37% in the placebo group and, respectively, 24% (P = 0.048 vs placebo), 59% (P = 0.155), 84% (P < 0.001) and 66% (P = 0.022) in the 50 mg, 100 mg, 150 mg, and 300 mg NT-814 groups; in waking due to night sweats reduction was 20% (P = 0.059), 55% (P = 0.135), 81% (P < 0.001), and 63% (P = 0.031) in the NT-814 groups and 32% in the placebo group. The improvement with NT-814 ≥150 mg was also evident in the first week of treatment. The most common treatment-related adverse events were mild somnolence and headache, more frequently in the 300 mg group. Safety monitoring identified no concerns.
Conclusions:
Once-daily NT-814 (≥150 mg/d) resulted in a rapid, marked improvement in hot flashes and waking due to night sweats. No safety concerns were identified. Doses up to 300 mg were well tolerated.
Keywords: Hot flash; Menopause; Neurokinin 1,3 antagonist; Night sweats
Up to 75% of women over 50 experience symptoms related to menopausal transition—particularly vasomotor symptoms (hot flashes and night sweats) and consequent sleep disturbances—that have a negative impact on daily activities and quality of life.1-3 Hot flashes are the most common and distressing symptom and the leading cause for seeking medical attention during this period.4,5 They are commonly associated with nighttime awakenings, causing insomnia and fatigue.6-8 Current treatment options are limited. Hormone therapy is effective in many women but associated with increased risk of hormone-dependent cancers and thrombosis and specifically contraindicated in women with history of hormone-sensitive cancers.9 Hormone therapy may also take several months to improve symptoms and may have limited effects on nighttime awakening.10,11 These limitations preclude its use in many women. Low-dose paroxetine is licensed for use in some countries but has modest efficacy and some tolerability concerns, as do other nonhormone therapies.12,13 A safe, effective alternative to hormonal treatments is urgently needed.
Evidence supports a functional role for neurokinin (NK) receptor systems in hot flash etiology through hypothalamic mechanisms.14-16 Kisspeptin, neurokinin B, and dynorphin (KNDy) neurons in the hypothalamus are hypertrophied in postmenopausal women and have elevated gene expression levels of kisspeptin, NKB (endogenous ligand for NK3 receptor), and substance P (SP) (endogenous ligand for NK1 receptor).17-22 The NK3 receptor and its NKB ligand in particular have been implicated in hot flash etiology.17,18,23,24 Proof-of-concept studies and more recently a fully powered phase 2b study with NK3 receptor antagonists have provided clinical evidence that NK3 receptor blockade rapidly reduces both the number and the severity of hot flashes.14,15,25,26 NK1 receptors and SP may also contribute to the control of vasomotor symptoms. SP immunoreactivity is frequently colocalized with kisspeptin and NKB in the infundibular region22,27 of the hypothalamus. Additionally, in the periphery, NK1 receptor desensitization attenuates cutaneous blood vessel dilatation28 and infusion of SP causes vasodilatation and flushing of the face and neck characteristic of postmenopausal hot flashes.29 We, therefore, hypothesized that NT-814, a selective antagonist of both NK1 and NK3 receptors, would reduce hot flashes and waking due to night sweats in postmenopausal women and conducted the RELENT-1 study to evaluate the pharmacokinetics and safety (primary endpoints) and efficacy (exploratory endpoints) of NT-814.
METHODS
RELENT-1 was a multicenter, double-blind, randomized, placebo-controlled, multiple-ascending-dose study, registered on Clinical Trials.gov (NCT02865538). The study was conducted in compliance with Good Clinical Practice and the principles of the Declaration of Helsinki and in accordance with a written protocol approved by the institutional review board for each participating center. The study was completed as planned. Postmenopausal women aged 40 to 65 years with an average of 7 to 20 moderate to severe hot flashes per day (as recorded in a 2-week patient-reported baseline diary), <50% change in average daily frequency between the first and second weeks of the baseline diary, and no active ongoing health condition that could have caused difficulty in interpreting hot flash symptoms were enrolled at three clinical research units in the United States. Menopause was defined as 12 months of spontaneous amenorrhea or 6 weeks after surgical bilateral oophorectomy, with or without hysterectomy. Participants were required to discontinue any medications that may have affected the number or severity of hot flashes or which may have interacted pharmacokinetically with NT-814 before the start of the baseline period and were not permitted to take these medications at any time during the study. All participants provided written informed consent.
Women were enrolled into four sequential cohorts defined by the NT-814 daily dose (50, 100, 150, and 300 mg). The study protocol initially specified the dose for the first three cohorts and allowed an additional two cohorts with further escalation to as high as 400 mg. The study was completed after the fourth (300 mg) cohort because the prespecified NT-814 plasma concentration limit had been achieved. Within each cohort participants were randomized in a 3:1 ratio to a once-daily oral dose of NT-814 or matching placebo in the morning for 14 days. The randomization code (block size = 4) was computer generated by the Sponsor, communicated via an interactive Web-based randomization system, and implemented by an unblinded pharmacist at each site who packaged capsules in a blinded fashion and provided them to the blinded study staff dispensing medication to participants.
After being admitted the day before the first dose, participants remained as residents in the unit for the first 8 days of dosing (days 1 to 8) for safety, efficacy, and pharmacokinetic assessment and then continued to take 5 days of medication as outpatients (days 9-13) before being readmitted to the unit for the day 14 dose and safety, efficacy, and pharmacokinetic assessments for 24 hours postdose. Additional visits took place on days 16 and 17 for safety and pharmacokinetic assessments, and participants were contacted by telephone on day 21 for a final safety follow-up.
Participants recalled the number and severity (1 = mild, 2 = moderate, 3 = severe) of all hot flashes experienced and recorded these twice daily (morning and evening) in paper diaries for 2 weeks before treatment start (baseline), for the 14 days of treatment and on the morning of day 15. Participants also recorded the number of times that they were awakened through the night because of night sweats. Safety was monitored by recording of adverse events through 7 days after the last dose, physical examinations, vital signs, electrocardiograms (ECGs), and clinical laboratory tests.
The primary endpoints of this study were related to safety assessments and pharmacokinetic parameters (not reported here), but several exploratory efficacy endpoints were prespecified in the protocol and statistical analysis plan, including change from baseline in frequency of moderate and severe hot flashes, hot flash average severity, hot flash severity score (frequency × severity), and frequency of waking due to night sweats as recorded in the twice-daily diary.
A formal sample size estimate was not performed for efficacy, but a pilot study with another NK3 receptor antagonist undertaken by one of the authors (RAA) before the present study was able to distinguish clinically and statistically significant differences from placebo in hot flash frequency with eight participants per treatment group.14
All statistical tabulation and analyses were performed using SAS Version 9.3. Efficacy endpoints (frequency and severity of hot flashes, frequency of waking due to night sweats) were analyzed according to randomized treatment in participants who received at least one dose of study drug. Missing data were not imputed. Data from placebo participants from all cohorts were pooled for analysis. Statistical hypothesis tests and confidence intervals (CIs) were two-sided, using a 0.05 type I error rate. Each analysis was performed using a linear mixed effects model, with change from baseline as a dependent variable; treatment, week/day, and treatment by week/day interaction as fixed effects; participant as a random effect; and baseline (frequency/severity at week 1/day 1) and interaction with week/day as covariates. An unstructured covariance matrix was specified for the repeated measures at postdose time points for each participant. The least-squares mean and two-sided 95% CIs were calculated for NT-814 versus placebo for each treatment and each post-baseline week/day. Safety endpoints were analyzed descriptively in all participants who received at least 1 dose of study drug.
RESULTS
During this study conducted from August 1, 2016 to March 28, 2017, 316 women were screened, and 76 were randomized and treated (Fig. 1). The high screening exclusion rate resulted from the relatively stringent selection criteria for general health required by this first study of NT-814 in women and the onerous study requirements. All but two participants completed the study; 1 participant withdrew on day 8 for personal reasons unrelated to study medication, and one was lost to follow-up on day 15. All 76 participants were included in the efficacy and safety analyses. Participants had a mean age of 55 years; most were white and of Hispanic or Latino ethnicity (Table 1). Baseline hot flash symptoms were stable and similar in frequency and severity between all five groups (4 NT-814 dose levels and placebo) in the 2 weeks before treatment except that frequency of moderate and severe hot flashes, severity score, and frequency of waking due to night sweats were higher in the 50 mg NT-814 group than in the other groups.
FIG. 1.
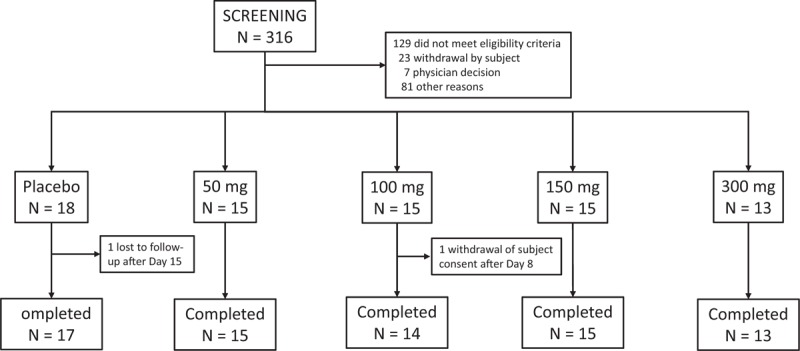
Disposition of participants.
TABLE 1.
Demographic and baseline characteristics
| NT-814 once-daily dose | |||||
| Characteristic | Placebo N = 18 | 50 mg N = 15 | 100 mg N = 15 | 150 mg N = 15 | 300 mg N = 13 |
| Age (years), mean ± SD | 55.2 ± 4.08 | 55.9 ± 5.57 | 55.3 ± 5.43 | 56.7 ± 4.37 | 53.8 ± 3.88 |
| Race, n (%) | |||||
| White | 16 (88.9) | 11 (73.3) | 11 (73.3) | 13 (86.7) | 11 (84.6) |
| Black or African-American | 2 (11.1) | 4 (26.7) | 4 (26.7) | 2 (13.3) | 2 (15.4) |
| Ethnicity, n (%) | |||||
| Hispanic/Latino | 13 (72.2) | 10 (66.7) | 8 (53.3) | 10 (66.7) | 9 (69.2) |
| Not Hispanic/Latino | 5 (27.8) | 5 (33.3) | 7 (46.7) | 5 (33.3) | 4 (30.8) |
| BMI (kg/m2), mean ± SD | 28.3 ± 3.71 | 27.6 ± 3.15 | 28.0 ± 4.51 | 28.6 ± 3.35 | 28.4 ± 4.28 |
BMI, body mass index; SD, standard deviation.
The safety profile observed for NT-814 in this study was favorable. No serious adverse events or adverse events leading to discontinuation from the study or study drug were reported. Most adverse events were mild; only one moderate adverse event was reported in each group. The most common adverse events were mild somnolence, contact dermatitis (from ECG electrode attachment), and headache (Table 2). Somnolence was always mild, usually intermittent, most commonly reported during the inpatient phase of the study, and most episodes resolved and did not recur during the second week of treatment. Overall adverse event incidence was similar in the placebo and 50, 100, and 150 mg NT-814 groups and slightly higher in 300 mg group. The proportion of adverse events related to study drug was also highest in the 300 mg group. The results of clinical laboratory tests, vital signs, and ECGs indicated no safety concerns for NT-814. ECG monitoring included extensive 24-hour Holter monitoring showed no difference between placebo and any dose of NT-814 in either the incidence or type of arrhythmias.
TABLE 2.
Most common adverse events (reported in ≥10% of participants in any treatment group)
| NT-814 once-daily dose | |||||
| Placebo N = 18 n (%) | 50 mg N = 15 n (%) | 100 mg N = 15 n (%) | 150 mg N = 15 n (%) | 300 mg N = 13 n (%) | |
| Any adverse event | 13 (72.2) | 7 (46.7) | 11 (73.3) | 10 (66.7) | 11 (84.6) |
| Somnolence | 3 (16.7) | 5 (33.3) | 2 (13.3) | 5 (33.3) | 9 (69.2) |
| Contact dermatitisa | 5 (27.8) | 2 (13.3) | 6 (40.0) | 5 (33.3) | 2 (15.4) |
| Headache | 3 (16.7) | 0 | 5 (33.3) | 1 (6.7) | 6 (46.2) |
| Diarrhea | 0 | 1 (6.7) | 0 | 1 (6.7) | 3 (23.1) |
| Pelvic pain | 2 (11.1) | 3 (20.0) | 0 | 0 | 0 |
| Viral upper respiratory tract infection | 1 (5.6) | 0 | 0 | 0 | 3 (23.1) |
| Dyspepsia | 2 (11.1) | 0 | 0 | 1 (6.7) | 0 |
| Alanine aminotransferase increased | 0 | 0 | 2 (13.3)b | 0 | 0 |
aFrom ECG electrodes.
bAlanine aminotransferase (ALT) increased to 1.3 × upper limit of normal (ULN) on day 8, decreasing to 1.1×ULN on day 14 in one participant; and to 2.6 × ULN on day 8, decreasing to 1.4×ULN on day 14 in the other participant. Four other participants (two receiving placebo and two receiving 300 mg NT-814) had similar changes in ALT that were not reported as adverse events.
After the start of treatment, improvements in all efficacy parameters; frequency (Fig. 2A), severity (Fig. 2B), severity score (Fig. 2C), and frequency of waking due to night sweats (Fig. 2D) were observed in all groups (including the placebo group). Reductions were greater in week 2 of treatment than in week 1. Mean reduction from baseline at week 2 in moderate/severe hot flash frequency was 37% in the placebo group and in the 50 mg, 100 mg, 150 mg, and 300 mg NT-814 was, respectively, 24% (P = 0.048 compared to placebo), 59% (P = 0.155), 84% (P < 0.001), and 66% (P = 0.022). Mean reduction in waking due to night sweats was 20% (P = 0.059), 55% (P = 0.135), 81% (P < 0.001), and 63% (P = 0.031) in the NT-814 groups and 32% in the placebo group. Mean improvements were greatest in the 150 mg and 300 mg NT-814 groups and smallest in the placebo and 50 mg NT-814 groups (Table 3). The 95% CIs for the mean differences showed a good separation between the two higher NT-814 doses and placebo. The changes in severity score mirrored those of the improvements in hot flash frequency, and the average severity was improved compared to placebo in participants treated with 150 mg NT-814 but not with the other doses (Table 2).
FIG. 2.
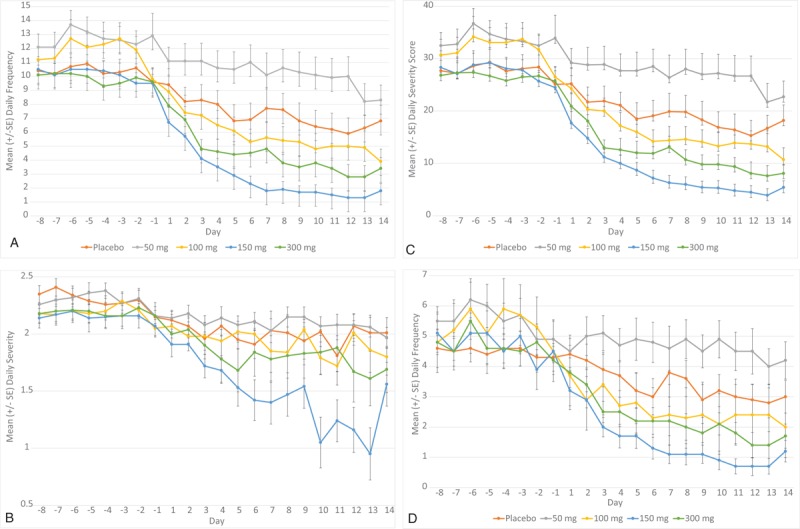
Daily frequency of: moderate and severe hot flashes (A), Severity of hot flashes (B), daily hot flash severity scorea (C), and waking at night due to night sweats (D), by day. Data shown are mean ± standard error (aseverity score = [number of mild hot flashes × 1] + [number of moderate hot flashes × 2] + [number of severe hot flashes × 3]).
TABLE 3.
Reduction in hot flash symptoms
| NT-814 once-daily dose | ||||||
| Time point | Statistica | Placebo N = 18 | 50 mg N = 15 | 100 mg N = 15 | 150 mg N = 15 | 300 mg N = 13 |
| Daily frequency of moderate and severe hot flashes | ||||||
| Week -1 (baseline) | Mean ± SD | 10.4 ± 3.87 | 12.7 ± 3.39 | 12.0 ± 3.61 | 10.2 ± 2.98 | 9.9 ± 2.11 |
| Median | 8.2 | 12.3 | 12.4 | 9.6 | 9.3 | |
| Week 1 | Mean ± SD | 7.9 ± 4.10 | 10.8 ± 4.50 | 6.7 ± 3.69 | 3.9 ± 1.85 | 5.4 ± 2.59 |
| Median | 7.0 | 10.7 | 5.6 | 3.3 | 6.1 | |
| Change from baseline vs placebo | NA | 2.2 (−0.3, 4.6) | −1.7 (−4.1, 0.7) | −4.0 (−6.4, −1.6) | −2.3 (−4.8, −0.1) | |
| P value | NA | 0.078 | 0.164 | 0.001 | 0.064 | |
| Week 2 | Mean ± SD | 6.6 ± 4.41 | 9.6 ± 4.70 | 4.9 ± 3.69 | 1.6 ± 1.68 | 3.4 ± 2.59 |
| Median | 6.8 | 7.9 | 4.2 | 1.0 | 2.4 | |
| Change from baseline vs placebo | NA | 2.6 (0.0, 5.2) | −1.9 (−4.4, 0.7) | −4.9 (−7.4, −2.4) | −3.1 (−5.7, −0.5) | |
| P value | NA | 0.048 | 0.155 | <0.001 | 0.022 | |
| Daily hot flash severity | ||||||
| Week -1 (baseline) | Mean ± SD | 2.3 ± 0.30 | 2.3 ± 0.32 | 2.2 ± 0.27 | 2.2 ± 0.38 | 2.2 ± 0.30 |
| Median | 2.3 | 2.3 | 2.2 | 2.1 | 2.3 | |
| Week 1 | Mean±SD | 2.0 ± 0.30 | 2.1 ± 0.32 | 2.0 ± 0.41 | 1.7 ± 0.38 | 1.9 ± 0.48 |
| Median | 2.1 | 2.2 | 2.1 | 1.6 | 1.8 | |
| Change from baseline vs placebo | NA | 0.1 (−0.2, 0.4) | 0.0 (−0.3, 0.3) | −0.3 (−0.6, −0.1) | −0.1 (−0.4, 0.2) | |
| P value | NA | 0.464 | 0.990 | 0.020 | 0.391 | |
| Week 2 | Mean ± SD | 2.0 ± 0.43 | 2.1 ± 0.32 | 1.9 ± 0.47 | 1.3 ± 0.53 | 1.8 ± 0.68 |
| Median | 2.1 | 2.0 | 1.9 | 1.3 | 1.9 | |
| Change from baseline vs placebo | NA | 0.1 (−0.2, 0.4) | 0.0 (−0.4, 0.3) | −0.6 (−1.0, −0.3) | −0.2 (−0.5, 0.2) | |
| P value | NA | 0.539 | 0.793 | <0.001 | 0.374 | |
| Daily hot flash severity score (frequency × severity)b | ||||||
| Week -1 (baseline) | Mean ± SD | 28.1 ± 11.63 | 33.7 ± 8.88 | 32.5 ± 11.20 | 27.9 ± 7.72 | 26.8 ± 5.42 |
| Median | 21.9 | 31.4 | 33.4 | 26.6 | 27.1 | |
| Week 1 | Mean±SD | 21.1 ± 10.52 | 28.2 ± 12.35 | 18.0 ± 8.31 | 10.8 ± 5.18 | 14.5 ± 6.41 |
| Median | 19.4 | 26.7 | 15.1 | 10.7 | 16.7 | |
| Change from baseline vs placebo | NA | 5.3 (0.9, 11.4) | −4.5 (−10.6, 1.6) | −10.2 (−16.2, −4.2) | −6.1 (−12.4, 0.1) | |
| P value | NA | 0.090 | 0.149 | 0.001 | 0.055 | |
| Week 2 | Mean±SD | 17.4 ± 11.60 | 25.7 ± 13.33 | 13.4 ± 9.37 | 5.1 ± 4.51 | 9.2 ± 6.31 |
| Median | 17.3 | 22.0 | 12.3 | 3.1 | 7.7 | |
| Change from baseline vs placebo | NA | 6.6 (−0.1, 13.3) | −5.1 (−11.7, 1.6) | −12.3 (−18.8, −5.8) | −7.8 (−14.6, −1.0) | |
| P value | NA | 0.052 | 0.133 | <0.001 | 0.025 | |
| Daily frequency of waking at night due to night sweats | ||||||
| Week -1 (baseline) | Mean ± SD | 4.5 ± 2.28 | 5.6 ± 1.79 | 5.4 ± 2.33 | 4.7 ± 2.11 | 4.8 ± 1.44 |
| Median | 4.0 | 5.4 | 5.7 | 4.4 | 4.6 | |
| Week 1 | Mean±SD | 3.7 ± 1.92 | 4.8 ± 2.25 | 2.9 ± 1.41 | 2.0 ± 1.00 | 2.7 ± 1.32 |
| Median | 3.6 | 4.4 | 2.6 | 1.9 | 2.6 | |
| Change from baseline vs placebo | NA | 0.8 (−0.3, 2.0) | −1.0 (−2.2, 0.1) | −1.8 (−2.9, −0.7) | −1.1 (−2.3, 0.1) | |
| P value | NA | 0.148 | 0.073 | 0.002 | 0.066 | |
| Week 2 | Mean±SD | 3.1 ± 2.16 | 4.5 ± 2.25 | 2.3 ± 1.74 | 0.9 ± 0.97 | 1.8 ± 1.24 |
| Median | 2.8 | 3.7 | 1.7 | 0.7 | 1.4 | |
| Change from baseline vs placebo | NA | 1.2 (−0.1, 2.4) | −0.9 (−2.2, 0.3) | −2.2 (−3.4, −1.0) | −1.4 (−2.6, −0.1) | |
| P value | NA | 0.059 | 0.135 | <0.001 | 0.031 | |
NA, not applicable; SD, standard deviation.
aChange from baseline is presented as change in least-squares mean and 95% confidence interval.
bSeverity score = (number of mild hot flashes × 1) + (number of moderate hot flashes × 2) + (number of severe hot flashes × 3).
Although mean reductions in symptoms in the 50 mg group were significantly smaller than those in the pooled placebo group, the median changes were in several instances larger on 50 mg than on placebo (Table 3). Overall, these data indicate that NT-814 50 mg is an ineffective dose.
The relationship between plasma NT-814 concentrations and effect was explored in two different pharmacokinetic-pharmacodynamic models, an inhibitory effect model and a sigmoid inhibitory effect model, each with a baseline effect parameter. Both models showed a maximum (plateau) effect on frequency of hot flashes and waking due to night sweats with plasma NT-814 concentrations that were achieved with doses ≥150 mg. An example exposure-response model (Emax model for hot flash frequency in week 2) is shown in Figure 3.
FIG. 3.
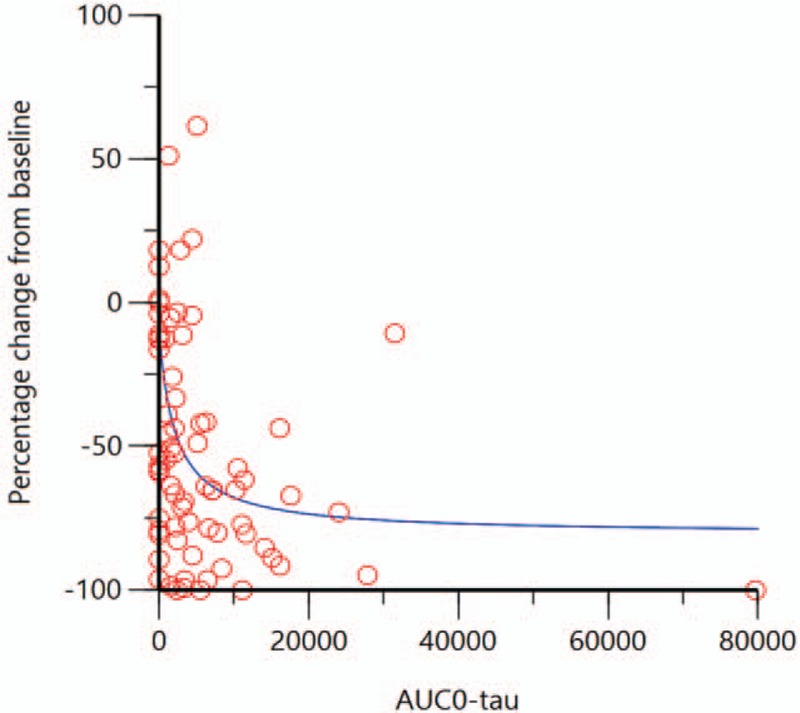
Exposure-response model of % change from baseline in frequency of moderate and severe hot flashes versus plasma concentration (area under the curve during the dose interval (AUC0-tau in ng∗h/mL) on day 14 of treatment. (the red circles show individual actual values; the blue line shows the predicted (modelled) response).
DISCUSSION
RELENT-1 was the first study in postmenopausal women with hot flash symptoms with NT-814, a novel, nonhormone dual NK1 and NK3 receptor antagonist that acts by directly modulating vasomotor neural pathways. Although the study was small (58 participants who received NT-814 and 18 who received placebo) and the treatment period was only 2 weeks, clear and rapid improvements in hot flash frequency and waking due to night sweats resulted from once-daily oral treatment with NT-814.
The observed improvements were sufficiently great to have been clinically meaningful for participants in the 150 mg and 300 mg groups. The response in the 150 mg and 300 mg groups, more than 40% improvement over placebo, is similar to that reported for the 2 NK3 antagonists evaluated in Phase 2 studies.14,15,25,26 It is also similar to the beneficial effects reported for hormone therapy over placebo.30,31 Improvements compared to placebo in severity were clearly apparent for the 150 mg dose but less so for the 300 mg dose. This finding most likely reflects the limitations of mean severity as an outcome without also taking into account the number of hot flashes. In contrast, severity score, which does take frequency into account, showed a clear separation from placebo for the effective doses. Doses up to 300 mg were well tolerated, and no safety concerns were identified.
A feature of the results is an apparent absence of a clear dose–response relationship on mean changes from baseline. However, review of the broader dataset suggests that there is a dose- and/or exposure-ordered response. The apparent advantage of placebo over the 50 mg dose is not present when median values are examined perhaps reflecting the small size of each treatment group in this early study of NT-814. At the higher end of the dose–response curve, the mean changes from baseline indicated that the 150 mg dose achieved a better effect than a 300 mg dose. This advantage is again not as apparent in the median values, and the exposure response modeling showed that once plasma concentrations of NT-814 equating to those achieved with a 150 mg dose are reached there is a plateau effect.
These findings further support modulation of NK receptors as a mechanism for treatment for hot flash and other menopause-related symptoms. The hypertrophy of hypothalamic KNDy neurons in postmenopausal women (with elevated gene expression levels of kisspeptin, NKB, and SP)17-22 are a consequence of estrogen withdrawal; in cynomolgus monkeys these features can be induced by oophorectomy and reversed by estrogen replacement.32-34 KNDy neurons branch extensively within the infundibular nucleus and are linked to the medial preoptic nucleus, which has been identified as the estrogen-sensitive thermoregulation control center.23,35 It is hypothesized that after menopause KNDy neurons are in a hyperactive state (consistent with the observed hypertrophy) that disrupts thermoregulation and triggers hot flashes.23 This hypothesis is supported by clinical studies with other NK3 receptor antagonists that showed NK3 receptor blockade rapidly reduces both the number and the severity of hot flashes.14,15,25,26 Additionally, a genome-wide association study demonstrated that NK3 receptor gene variation may influence hot flash risk.36 Hot flashes are also a symptom of carcinoid syndrome, in which SP levels are elevated.37 Although NK1 antagonism alone appears to be ineffective for hot flashes,38 Phase 2 studies have shown efficacy for NK1 receptor antagonists in mood39-41and primary insomnia,42 so a compound such as NT-814 that also has NK1 antagonist properties may also improve sleep and other comorbidities associated with menopausal symptoms.
Strengths of this study included its randomized, double-blind, placebo-controlled design, standardized procedures and assessments, and precise postmenopausal population definition. However, the relatively stringent selection criteria may have resulted in a less generalizable study population. Limitations of the study are the small group sizes and short duration, although both were sufficient to enable a preliminary assessment of the efficacy of NT-814 as a treatment for postmenopausal hot flashes to be made before progressing to larger and longer studies. Although diary recording of hot flash symptoms is the standard method for symptom assessment, it shares the limitations of other patient-reported outcome assessments. These assessments are subjective and, despite randomization and blinding, placebo responses of 50% or more are common.43,44 In RELENT-1, participants taking placebo had improvements of 37% in mean hot flash frequency and 15% in mean severity in the second week of treatment. On the basis of the results of this small, short duration study, further evaluation of NT-814 efficacy in women with bothersome menopausal hot flashes was considered appropriate, and a Phase 2b study evaluating a range of doses for 12 weeks of treatment in approximately 200 women is ongoing (ClinicalTrials.gov NCT03596762).
CONCLUSIONS
In this first study of NT-814 in postmenopausal women with hot flash symptoms, once-daily treatment resulted in a rapid, marked improvement in hot flashes and waking due to night sweats with statistically significant effects at the 150 mg and 300 mg doses. The effect at these doses was immediate, beginning in the first week of treatment, and also sustained, improving symptoms more in the second week of treatment than in the first. Lower doses (50 mg and 100 mg once daily) were not effective. No safety concerns were identified for NT-814. Doses up to 300 mg were well tolerated. Most adverse events were mild. The most common adverse events were headache and somnolence that was intermittent and limited in duration. The findings from the evaluation of this nonhormone dual NK1 and NK3 receptor antagonist support both modulation of NK receptors as a treatment for hot flashes and other menopause-related symptoms and the further assessment of NT-814 as a potential therapy for treating these symptoms.
Acknowledgments
The authors wish to thank Anne McDonough (McDonough Clinical Research Ltd) for medical writing assistance and Elise Dunzo (Parexel Quantitative Clinical Development) for undertaking the exposure-response modeling.
Footnotes
Funding/support: NeRRe Therapeutics, Ltd. sponsored the study and provided support for the medical writing assistance of Anne McDonough (McDonough Clinical Research Ltd).
Data Presentation: Presented at the 2018 Annual Meeting of The North American Menopause Society, October 3-6, 2018, San Diego, CA.
Clinical Trial Registration: ClinicalTrials.gov NCT02865538.
Financial disclosure/conflicts of interest: MT, MK, EB, and SP report holding stock in NeRRe Therapeutics, Ltd. and KaNDy Therapeutics, Ltd. (the current developer of NT-814). RAA reports receipt of personal fees from NeRRe, KaNDy, and Sojournix, Inc. for consultancy. HJ reports receipt of research grant support from NeRRe, KaNDy, Merck, QUE Oncology, Pfizer, and the US National Institutes of Health; receipt of personal fees from NeRRe, KaNDy, Eisai Co., Ltd., Sojournix, Inc., and Mitsubishi Tanabe Pharma for consultancy or service on advisory board; and a spouse who is an employee of Merck Research Laboratories, has equity in and received personal fees from Arsenal Biosciences, and has equity in Tango Therapeutics.
REFERENCES
- 1.Joffe H, Massler A, Sharkey KM. Evaluation and management of sleep disturbance during the menopause transition. Semin Reprod Med 2010; 28:404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santoro N, Epperson CN, Mathews SB. Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44:497–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams RE, Levine KB, Kalilani L, et al. Menopause-specific questionnaire assessment in US population-based study shows negative impact on health-related quality of life. Maturitas 2009; 62:153–159. [DOI] [PubMed] [Google Scholar]
- 4.Whiteley J, Wagner JS, Bushmakin A, et al. Impact of the severity of vasomotor symptoms on health status, resource use, and productivity. Menopause 2013; 20:518–524. [DOI] [PubMed] [Google Scholar]
- 5.Williams RE, Kalilani L, DiBenedetti DB, et al. Healthcare seeking and treatment for menopausal symptoms in the United States. Maturitas 2007; 58:348–358. [DOI] [PubMed] [Google Scholar]
- 6.Joffe H, Crawford S, Economou N, et al. A gonadotropin-releasing hormone agonist model demonstrates that nocturnal hot flashes interrupt objective sleep. Sleep 2013; 36:1977–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woods NF, Hohensee C, Carpenter JS, et al. Symptom clusters among MsFLASH clinical trial participants. Menopause 2016; 23:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multi-ethnic community sample of women. Sleep 2008; 31:979–990. [PMC free article] [PubMed] [Google Scholar]
- 9.Medicines and Healthcare Products Regulatory Agency. Hormone-replacement therapy: updated advice. Available at: https://www.gov.uk/drug-safety-update/hormone-replacement-therapy-updated-advice >Accessed February 15, 2019. [Google Scholar]
- 10.Pinkerton JV, Pan K, Abraham L, et al. Sleep parameters and health-related quality of life with bazedoxifene/conjugated estrogens: a randomized trial. Menopause 2014; 21:252–259. [DOI] [PubMed] [Google Scholar]
- 11.Santoro N, Allshouse A, Neal-Perry G, et al. Longitudinal changes in menopausal symptoms comparing women randomized to low-dose oral conjugated estrogens or transdermal estradiol plus micronized progesterone versus placebo: the Kronos Early Estrogen Prevention Study. Menopause 2017; 24:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Orleans RJ, Li L, Kim MJ, et al. FDA approval of paroxetine for menopausal hot flushes. N Engl J Med 2014; 370:1777–1779. [DOI] [PubMed] [Google Scholar]
- 13.Nelson HD, Vesco KK, Haney E, et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA 2006; 295:2057–2071. [DOI] [PubMed] [Google Scholar]
- 14.Skorupskaite K, George JT, Veldhuis JD, et al. Neurokinin B receptor antagonism decreases LH secretion and sensation of hot flushes in postmenopausal women. BJOG 2016; 132:79. [Google Scholar]
- 15.Prague JK, Roberts RE, Comninos AN, et al. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flashes: a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2017; 389:1809–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skorupskaite K, George JT, Veldhuis JD, et al. Neurokinin 3 receptor antagonism reveals roles for neurokinin B in the regulation of gonadotropin secretion and hot flashes in postmenopausal women. Neuroendocrinol 2018; 106:148–157. [DOI] [PubMed] [Google Scholar]
- 17.Padilla SL, Johnson CW, Barker FD, et al. A neural circuit underlying the generation of hot flushes. Cell Rep 2018; 24:271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krajewski-Hall SJ, Miranda Dos Santos F, McMullen NT, et al. Glutamatergic neurokinin 3 receptor neurons in the median preoptic nucleus modulate heat-defense pathways in female mice. Endocrinology 2019; 160:803–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rance NE, McMullen NT, Smialek JE, et al. Postmenopausal hypertrophy of neurons expressing the estrogen receptor gene in the human hypothalamus. J Clin Endocrinol Metab 1990; 71:79–85. [DOI] [PubMed] [Google Scholar]
- 20.Rance NE, Young WS. Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology 1991; 128:2239–2247. [DOI] [PubMed] [Google Scholar]
- 21.Rometo AM, Rance NE. Changes in prodynorphin gene expression and neuronal morphology in the hypothalamus of postmenopausal women. J Neuroendocrinol 2008; 20:1376–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Borsay B, Skrapits K, Herczeg L, et al. Hypophysiotropic gonadotropin-releasing hormone projections are exposed to dense plexuses of kisspeptin, neurokinin B and substance P immunoreactive fibers in the human: a study on tissues from postmenopausal women. Neuroendocrinology 2014; 100:141–152. [DOI] [PubMed] [Google Scholar]
- 23.Rance NE, Dacks PA, Mittelman-Smith MA, et al. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol 2013; 34:211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jayasena CN, Comninos AN, Stefanopoulou E, et al. Neurokinin B administration induces hot flushes in women. Sci Rep 2015; 5:8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.EU Clinical Trials Register. Clinical trial results: Pilot/phase IIa trial to investigate the effect of ESN364 in early postmenopausal women suffering from hot flashes. Available at: https://www.clinicaltrialsregister.eu/ctr-search/trial/2015-002578-20/results Retrieved February 15, 2019. [Google Scholar]
- 26.Fraser GL, Lederman S, Waldbaum A, et al. The neurokinin 3 receptor antagonist, fezolinetant, is effective in treatment of menopausal vasomotor symptoms: A randomized, placebo-controlled, double-blind, dose-ranging study. Presented at ENDO 2019 (March 23-26, 2019; New Orleans, LA). [Google Scholar]
- 27.Hrabovszky E, Borsay BÁ, Rácz K, et al. Substance P immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PLoS One 2013; 8:e7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilatation in humans. J Physiol 2006; 577:1043–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaffalitzky De Muckadell OB, Aggestrup S, Stentoft P. Flushing and plasma substance P concentration during infusion of synthetic substance P in normal man. Scand J Gastroenterol 1986; 21:498–502. [DOI] [PubMed] [Google Scholar]
- 30.Duavee [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2013. [Google Scholar]
- 31.Premarin [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc; 2011. [Google Scholar]
- 32.Abel TW, Voytko ML, Rance NE. The effects of hormone replacement therapy on hypothalamic neuropeptide gene expression in a primate model of menopause. J Clin Endocrinol Metab 1999; 84:2111–2118. [DOI] [PubMed] [Google Scholar]
- 33.Sandoval-Guzmán T, Stalcup ST, Krajewski SJ, et al. Effects of ovariectomy on the neuroendocrine axes regulating reproduction and energy balance in young cynomolgus macaques. J Neuroendocrinol 2004; 16:146–153. [DOI] [PubMed] [Google Scholar]
- 34.Rometo AM, Krajewski SJ, Voytko ML, et al. Hypertrophy and increased kisspeptin gene expression in the hypothalamic infundibular nucleus of postmenopausal women and ovariectomized monkeys. J Clin Endocrinol Metab 2007; 92:2744–2750. [DOI] [PubMed] [Google Scholar]
- 35.Boulant JA. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin Infect Dis 2000; 31: suppl 5: S157–S161. [DOI] [PubMed] [Google Scholar]
- 36.Crandall CJ, Manson JE, Hohensee C, et al. Association of genetic variation in the tachykinin receptor 3 locus with hot flashes and night sweats in the Women's Health Initiative Study. Menopause 2017; 24:252–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Skrabanek P, Cannon D, Kirrane J, et al. Substance P secretion by carcinoid tumours. Ir J Med Sci 1978; 147:47–49. [DOI] [PubMed] [Google Scholar]
- 38.Bardia A, Thompson S, Atherton P, et al. Pilot evaluation of aprepitant for the treatment of hot flashes. Support Cancer Ther 2006; 3:240–246. [DOI] [PubMed] [Google Scholar]
- 39.Kramer MS, Winokur A, Kelsey J, et al. Demonstration of the efficacy and safety of a novel substance P (NK1) receptor antagonist in major depression. Neuropsychopharmacology 2004; 29:385–392. [DOI] [PubMed] [Google Scholar]
- 40.Ratti E, Bellew K, Bettica P, et al. Results from 2 randomized, double-blind, placebo-controlled studies of the novel NK1 receptor antagonist casopitant in patients with major depressive disorder. J Clin Psychopharmacol 2011; 31:727–733. [DOI] [PubMed] [Google Scholar]
- 41.Ratti E, Bettica P, Alexander R, et al. Full central neurokinin-1 receptor blockade is required for efficacy in depression: evidence from orvepitant clinical studies. J Psychopharmacol 2013; 27:424–434. [DOI] [PubMed] [Google Scholar]
- 42.Ratti E, Carpenter DJ, Zamuner S, et al. Efficacy of vestipitant, a neurokinin-1 receptor antagonist, in primary insomnia. Sleep 2013; 36:1823–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bouchard P, Panay N, de Villiers TJ, et al. Randomized placebo- and active-controlled study of desvenlafaxine for menopausal vasomotor symptoms. Climacteric 2012; 15:12–20. [DOI] [PubMed] [Google Scholar]
- 44.Pinkerton JV, Utian WH, Constantine GD, et al. Relief of vasomotor symptoms with the tissue-selective estrogen complex containing bazedoxifene/conjugated estrogens: a randomized, controlled trial. Menopause 2009; 16:1116–1124. [DOI] [PubMed] [Google Scholar]


