Abstract
Background and Purpose:
The risk of arterial ischemic events after subdural hemorrhage (SDH) is poorly understood. This study aimed to evaluate the risk of acute ischemic stroke and myocardial infarction (MI) among patients with and without non-traumatic SDH.
Methods:
We performed a retrospective cohort study using claims data from 2008 through 2014 from a nationally representative sample of Medicare beneficiaries. The exposure was non-traumatic SDH. Our primary outcome was an arterial ischemic event, a composite of acute ischemic stroke and acute MI. Secondary outcomes were ischemic stroke alone and MI alone. We used validated ICD-9-CM diagnosis codes to identify our predictor and outcomes. Using Cox regression and corresponding survival probabilities, adjusted for demographics and vascular comorbidities, we computed the hazard ratio (HR) in 4-week intervals after SDH discharge. We performed secondary analyses stratified by strong indications for antithrombotic therapy (composite of atrial fibrillation, peripheral vascular disease, valvular heart disease, and venous thromboembolism).
Results:
Among 1.7 million Medicare beneficiaries, 2,939 were diagnosed with SDH. In the 4 weeks after SDH, patients’ risk of an arterial ischemic event was substantially increased (HR, 3.6; 95% CI, 1.9–5.5). There was no association between SDH diagnosis and arterial ischemic events beyond 4 weeks. In secondary analysis, during the 4 weeks after SDH, patients’ risk of ischemic stroke was increased (HR, 4.2; 95% CI, 2.1–7.3) but their risk of MI was not (HR, 0.8, 95% CI, 0.2–1.7). Patients with strong indications for antithrombotic therapy had increased risks for arterial ischemic events similar to patients in the primary analysis, but those without such indications did not demonstrate an increased risk for arterial ischemic events.
Conclusions:
Among Medicare beneficiaries, we found a heightened risk of arterial ischemic events driven by an increased risk of ischemic stroke, in the 4 weeks after non-traumatic SDH. This increased risk may be driven by interruption of antithrombotic therapy after SDH diagnosis.
Keywords: Subdural hematoma, Ischemic Stroke, Myocardial Infarction
Subdural hemorrhage (SDH) affects 125,000 individuals in the United States every year.1 SDH can be traumatic in etiology, with concurrent sequelae of traumatic brain injury such as cerebral contusions, axonal injury, and subarachnoid hemorrhage, or can occur spontaneously, without any obvious inciting trauma.1 Although both subtypes of SDH occur predominantly in elderly patients,2, 3 they often differ in clinical outcomes. We recently showed that patients with non-traumatic SDH have significantly higher cumulative rates of hospital re-admission, surgical hematoma evacuation, and in-hospital death at 90 days, compared to patients with traumatic SDH.4 These outcomes may be influenced by incident arterial thrombotic complications. For instance, emerging cohort studies suggest that traumatic brain injury is independently associated with an increased long-term risk of ischemic stroke.5, 6 Whether non-traumatic SDH also has a similar risk of ischemic cerebrovascular events is poorly understood. Given the aging population and increasing use of anticoagulant medications,7, 8 the incidence of SDH is expected to rise substantially in the coming decade, and delineation of this risk therefore has public health importance. We hence sought to evaluate the relationship between non-traumatic SDH and arterial ischemic events in a large, nationally representative sample of Medicare beneficiaries.
Methods
Study Design
We performed a retrospective cohort study using both inpatient and outpatient claims data on a 5% sample of Medicare beneficiaries. The U.S. Centers for Medicare and Medicaid Services (CMS) provides health insurance to a large majority of U.S. citizens 65 years of age or older. De-identified datasets based on claims data submitted by hospitals and providers are made available by CMS. The Medicare database allows for a comprehensive longitudinal analysis of each beneficiary’s care over time, since a unique and anonymous identifier code links multiple claims for a given patient. This study was approved by the Weill Cornell Medicine institutional review board. The claims data used in this analysis are restricted per the terms of Medicare’s data use agreement and therefore cannot be shared directly with other investigators. However, investigators can obtain access to these data by submitting a formal application to CMS.
Patient Population
We obtained data from inpatient and outpatient claims of Medicare beneficiaries between January 2008 and December 2014. In keeping with standard practice in analyzing Medicare data,9 we limited our cohort to beneficiaries with continuous coverage in traditional fee-for-service Medicare (both Parts A and B) for at least 1 year or until death, if applicable. Although Medicare eligibility generally begins at 65 years of age, we included only patients 66 years or older in order to allow time for beneficiaries to enter medical care and for their providers to document any pre-existing medical comorbidities.
Measurements
Our exposure variable was non-traumatic SDH, identified by the ICD-9-CM code 432.1 in any diagnostic position. The ICD-9-CM code for SDH has been shown to have a sensitivity of 96% and a specificity of 89% as compared to medical record review.4 We excluded patients with diagnosis codes for trauma, and those with a diagnosis of acute myocardial infarction (MI) or acute cerebrovascular disease preceding the index hospitalization for non-traumatic SDH, to prevent misclassification of chronic events. We also excluded patients who had an arterial ischemic event concurrently with the SDH hospitalization, to prevent inclusion of patients who had SDH from treatment complications following stroke or MI. We collected data on demographic characteristics, such as age, sex, and race/ethnicity. Using previously used ICD-9-CM code algorithms, we also measured the following cardiovascular risk factors and relevant comorbidities: hypertension, diabetes, atrial fibrillation, congestive heart failure, valvular heart disease, peripheral vascular disease, chronic kidney disease, chronic obstructive pulmonary disease, and tobacco use. The Charlson comorbidity index, identified through ICD-9-CM codes, was used to quantify the burden of comorbidities.10, 11
Our primary outcome was a composite of acute ischemic stroke and MI, while secondary outcomes were ischemic stroke alone and MI alone. The diagnosis codes for acute ischemic stroke have a sensitivity of 86% and a specificity of 95%,12, 13 while those for MI have a sensitivity and specificity greater than 85%, with a positive predictive value of over 93%.14
Statistical Analysis
We compared patients’ baseline characteristics using the χ2 test and the Wilcoxon rank-sum test, as appropriate. To study the relationship between non-traumatic SDH and subsequent risk of arterial ischemic events, we fit separate Cox regression models for the groups with and without SDH. Patients entered observation after 1 year of continuous Medicare eligibility and were censored at the time of death, loss of Medicare insurance, or on December 31, 2014. The multivariable models adjusted for demographics, vascular risk factors such as hypertension, diabetes mellitus, dyslipidemia, chronic kidney disease, chronic obstructive pulmonary disease, valvular heart disease, atrial fibrillation, and Charlson comorbidities. The proportional hazard assumption was violated; therefore, we used the corresponding survival probabilities of these separate models to calculate hazard ratios (HR) in 4-week intervals after discharge from SDH hospitalization, and confidence intervals (CI) were computed using the nonparametric bootstrap method.
We performed pre-specified subgroup analyses stratified by strong indications for antithrombotic therapy, which included a composite of atrial fibrillation, peripheral vascular disease, valvular heart disease, and venous thromboembolism. All statistical analyses were performed using R software v 3.3.1 (R Foundation for Statistical Computing). The threshold for statistical significance was p<0.05.
Results
Study Population and Outcomes
Among 1,741,397 Medicare beneficiaries included in this study, 2,939 (0.1%) were diagnosed with a non-traumatic SDH. Patients with SDH were older and more often had hypertension, coronary artery disease, atrial fibrillation, and valvular heart disease than patients without SDH (Table 1). Arterial ischemic events were diagnosed in 114,942 patients (6.6%). Patients diagnosed with an arterial ischemic event were older and had more vascular risk factors than patients without an arterial ischemic event (Table 2). In multivariable Cox models, the risk of an arterial ischemic event was significantly increased in the first 4-weeks after SDH (HR, 3.6; 95% CI, 1.9–5.5) compared to patients without SDH. There was no association between SDH diagnosis and arterial ischemic event risk beyond 4 weeks. The slope of event-free survival was significantly lower in the first 5 months for patients with SDH than for those without (Figure 1). In secondary analysis, during the 4 weeks after SDH, patients’ risk of ischemic stroke was increased (HR, 4.2; 95% CI, 2.1–7.3) but not their risk of MI (HR, 0.8, 95% CI, 0.2–1.7) (Figure 2).
Table 1.
Characteristics of Patients Stratified by Presence of Non-Traumatic Subdural Hemorrhage
| Characteristica | SDH (N = 2,939) | No SDH (N = 1,738,458) | P value |
|---|---|---|---|
| Age, mean (SD), y | 79.4 (7.9) | 73.4 (7.7) | < 0.001 |
| Female | 1,178 (40.1) | 992,741 (57.1) | < 0.001 |
| Race | < 0.001 | ||
| White | 2,444 (83.2) | 1,497,556 (86.1) | |
| Black | 289 (9.8) | 136,059 (7.8) | |
| Other | 206 (7.0) | 104,843 (6.0) | |
| Hypertension | 2,667 (90.7) | 708,090 (40.7) | < 0.001 |
| Diabetes | 1,333 (45.4) | 279,719 (16.1) | < 0.001 |
| Congestive heart failure | 855 (29.1) | 94,824 (5.5) | < 0.001 |
| Peripheral vascular disease | 809 (27.5) | 92,237 (5.3) | < 0.001 |
| Chronic obstructive pulmonary disease | 912 (31.0) | 148,010 (8.5) | < 0.001 |
| Chronic kidney disease | 831 (28.3) | 70,855 (4.1) | < 0.001 |
| Atrial fibrillation | 1,035 (35.2) | 110,714 (6.4) | < 0.001 |
| Valvular disease | 811 (27.6) | 96,329 (5.5) | < 0.001 |
| Tobacco use | 696 (23.7) | 18,144 (1.0) | < 0.001 |
| Alcohol use | 383 (13.0) | 29,758 (1.7) | < 0.001 |
| Mild liver disease | 52 (1.8) | 654 (0.1) | < 0.001 |
| Moderate or severe liver disease | 43 (1.5) | 381 (0.1) | < 0.001 |
| Malignancy without metastasis | 560 (19.1) | 7,121 (0.4) | < 0.001 |
| Metastatic solid tumor | 178 (6.1) | 1,640 (0.1) | < 0.001 |
Abbreviations: SDH, subdural hemorrhage; SD, standard deviation
Data are presented as number (%) unless otherwise specified.
Table 2.
Characteristics of Patients Stratified by Presence of Ischemic Stroke or Myocardial Infarction
| Characteristica | Stroke/MI (N = 114,942) | No Stroke/MI (N = 1,626,455) | P value |
|---|---|---|---|
| Age, mean (SD), y | 77.3 (8.0) | 73.1 (7.6) | < 0.001 |
| Female | 63,139 (54.9) | 930,780 (57.2) | < 0.001 |
| Race | < 0.001 | ||
| White | 98,579 (85.8) | 1,401,421 (86.2) | |
| Black | 10,765 (9.4) | 125,583 (7.7) | |
| Other | 5,598 (4.9) | 99,451 (6.1) | |
| Hypertension | 67,491 (58.7) | 643,266 (39.6) | < 0.001 |
| Diabetes | 31,389 (27.3) | 249,663 (15.4) | < 0.001 |
| Congestive heart failure | 13,343 (11.6) | 82,336 (5.1) | < 0.001 |
| Peripheral vascular disease | 12,493 (10.9) | 80,553 (5.0) | < 0.001 |
| Chronic obstructive pulmonary disease | 15,757 (13.7) | 133,165 (8.2) | < 0.001 |
| Chronic kidney disease | 9,843 (8.6) | 61,843 (3.8) | < 0.001 |
| Atrial fibrillation | 13,827 (12.0) | 97,922 (6.0) | < 0.001 |
| Valvular disease | 11,070 (9.6) | 86,070 (5.3) | < 0.001 |
| Tobacco use | 1,873 (1.6) | 16,967 (1.0) | < 0.001 |
| Alcohol use | 3,154 (2.7) | 26,987 (1.7) | < 0.001 |
Abbreviations: MI, myocardial infarction; SD, standard deviation
Data are presented as number (%) unless otherwise specified.
Figure 1:
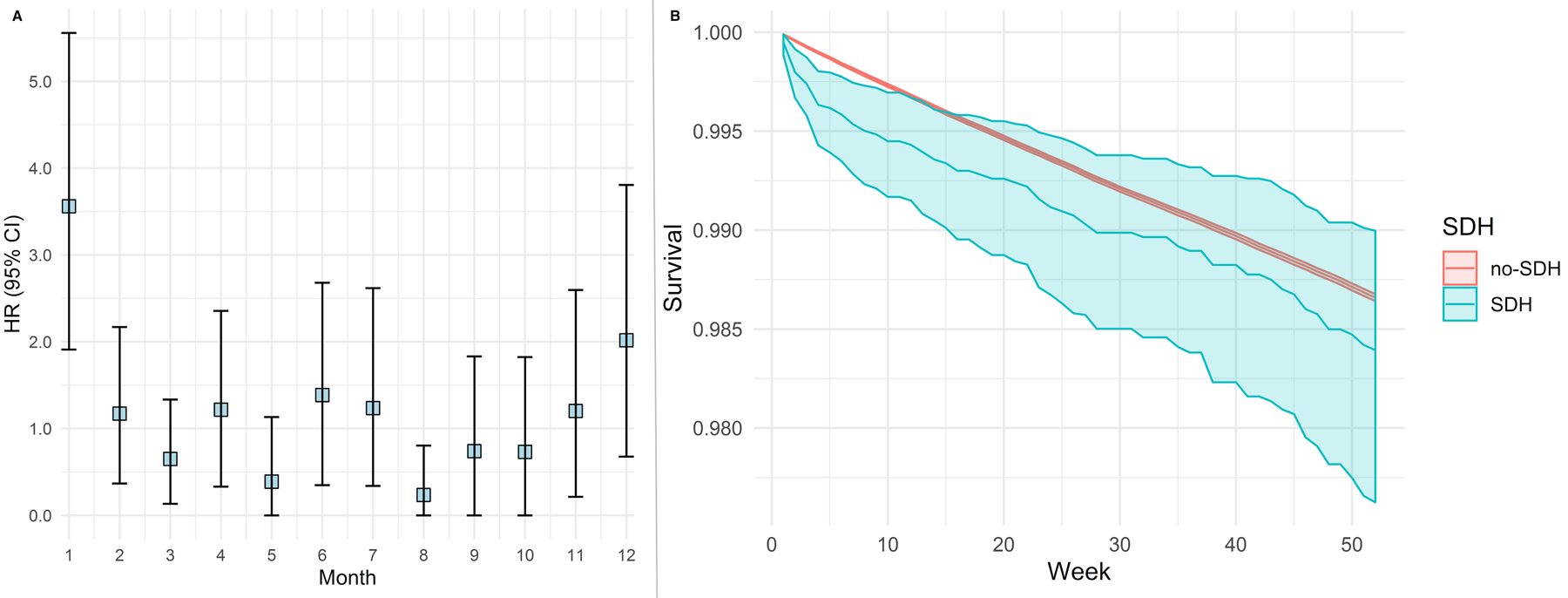
Risk of arterial thrombotic event after non-traumatic subdural hemorrhage. Cox regression model showing risk of an arterial thrombotic event after non-traumatic subdural hemorrhage (Panel A) and corresponding event-free probabilities (Panel B) among patients with and without subdural hemorrhage. Models adjusted for demographics, vascular risk factors, and Charlson comorbidity indices.
Abbreviations: CI, confidence interval; SDH, subdural hemorrhage.
Figure 2:
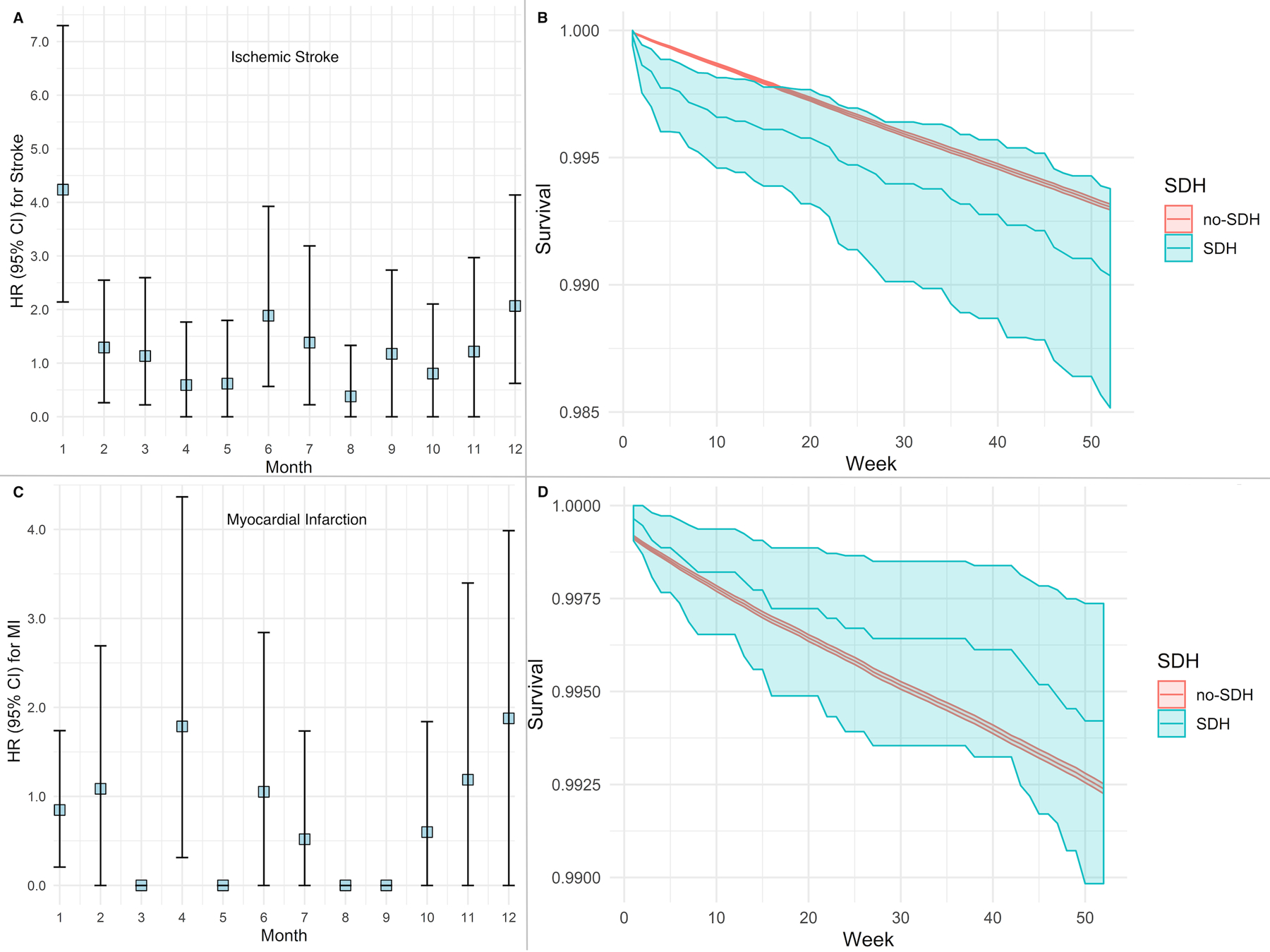
Risk of ischemic stroke and myocardial infarction after non-traumatic subdural hemorrhage. Cox regression model showing risk of an ischemic stroke (Panel A) and corresponding event-free probabilities (Panel B) among patients with and without non-traumatic subdural hemorrhage. Cox regression model showing risk of a myocardial infarction (Panel C) and corresponding event-free probabilities (Panel D) among patients with and without subdural hemorrhage. Models adjusted for demographics, vascular risk factors, and Charlson comorbidity indices.
Abbreviations: CI, confidence interval; SDH, subdural hemorrhage.
Note: Hazard ratio of 0 indicates very few events in the specified time interval.
Subgroup Analysis
A total of 2,188 SDH patients had strong indications for antithrombotic therapy (composite of atrial fibrillation, peripheral vascular disease, valvular heart disease, and venous thromboembolism) at the time of SDH diagnosis, and 751 SDH patients did not have any such indication. Patients with SDH who had strong indications for antithrombotic therapy had similarly increased risks for arterial ischemic events in the first 4-weeks as compared to patients in the primary analysis (HR, 3.2; 95% CI, 1.7–5.5), but this risk attenuated after the first month. However, patients without strong indications for antithrombotic medications did not demonstrate an increased risk for arterial ischemic events (Figure 3).
Figure 3:
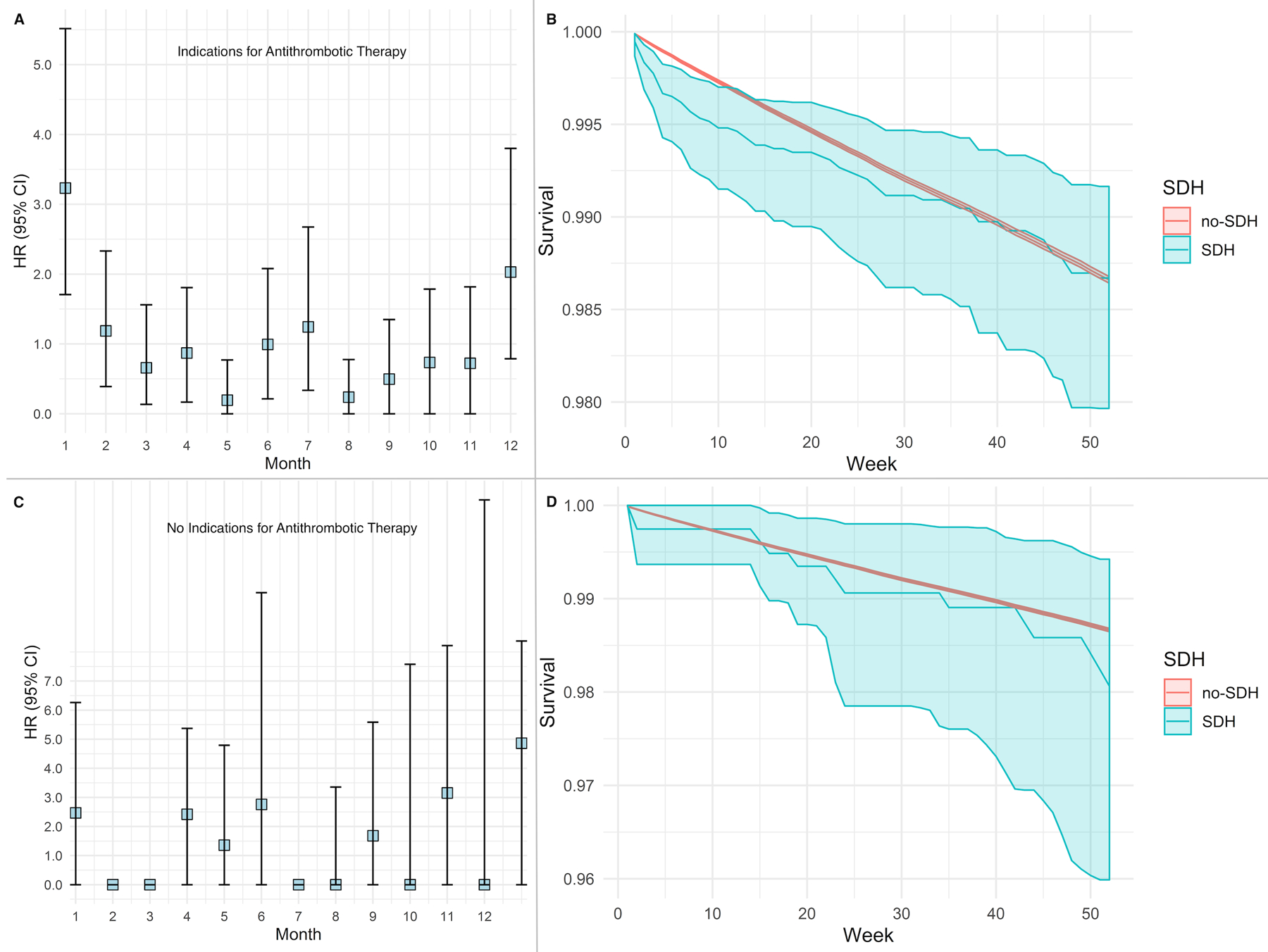
Risk of arterial thrombotic events after non-traumatic subdural hemorrhage, stratified by indications requiring antithrombotic therapy. Cox regression model showing the risk of an arterial ischemic event with corresponding survival probabilities after non-traumatic subdural hemorrhage (Panels A and B), compared to those without subdural hemorrhage (Panels C and D). Models adjusted for demographics, vascular risk factors, and Charlson comorbidity indices.
Abbreviations: CI, confidence interval; SDH, subdural hemorrhage.
Note: Hazard ratio of 0 indicates very few events in the specified time interval.
We subsequently performed post hoc analyses where we first evaluated the risk of ischemic stroke and MI in patients with atrial fibrillation or valvular heart disease, a population with an inherent higher risk of cardioembolic events. A total of 179,637 Medicare beneficiaries had atrial fibrillation or valvular heart disease, of whom 1,384 had SDH. The risk of an arterial ischemic event in the first month was over 4-fold higher among patients with SDH compared to those without (HR, 4.44; 95% CI, 2.30–6.59).
In the second post hoc analysis, we assessed the relationship between SDH and arterial ischemic events in patients with indications for anticoagulant and antiplatelet medications separately. SDH was associated with a 4-fold higher risk of an arterial ischemic event after SDH (HR 4.1, 95% CI, 2.3–6.4) in patients with indications for anticoagulation therapy, and a 3-fold elevated risk in patients with diagnoses that warrant antiplatelet therapy (HR, 3.3, 95% CI, 1.7–4.9).
Discussion
In a large cohort of Medicare beneficiaries, we observed a heightened short-term risk of an acute ischemic stroke in the first 4 weeks after SDH compared to patients without SDH. Furthermore, the increased risk of ischemic stroke after SDH was predominantly in patients with strong indications for antithrombotic medications, while patients without indications for antithrombotic therapy had no demonstrable increase in the risk of ischemic events.
Several prior studies including our own have evaluated the risk of arterial ischemic events after intracerebral hemorrhage,15–18 but there is a paucity of data on this risk after SDH. We have previously shown that patients with atrial fibrillation face an increased risk of ischemic stroke after intracranial hemorrhage including SDH, although the duration and magnitude of this risk was not evaluated.19 Additionally, it is unknown if this risk applies to all patients with SDH regardless of atrial fibrillation. In this context, our current study provides novel findings that indicate an acutely elevated risk of ischemic stroke in the short term after SDH. This risk was most perceivable among patients with strong indications for antithrombotic therapy, potentially implicating antithrombotic drug interruption after SDH as a mechanism. The current American Heart Association guidelines recommend withholding anticoagulant medications for at least 4 weeks after intracerebral hemorrhage among patients without mechanical valves.20 While no such guidelines exist for SDH, clinicians may extrapolate the same recommendations for SDH patients. This could explain the 4-week duration of heightened ischemic stroke risk observed in our study. Regardless, our results are important in the setting of recent studies in patients with intracerebral hemorrhage showing a reduction in the thromboembolic risk without an offsetting increase in the risk of recurrent brain hemorrhage after resumption of antithrombotic therapy.21, 22 Whether early reinstatement of antithrombotic therapy has a similar favorable risk-benefit profile in non-traumatic SDH remains poorly understood.
The lack of a relationship between SDH and risk of MI is probably multifactorial. In a retrospective cohort study of the Swedish Stroke Registry, rates of resumption of anticoagulant and antiplatelet medications after intracerebral hemorrhage were 8.5% and 36.6% at 6 months in patients with atrial fibrillation, with only modest increments at the end of 1 year.23 These data suggest clinician preference of antiplatelet agents over anticoagulant medications after intracranial hemorrhage presumably due to a more favorable hemorrhagic risk profile. It is possible that a similar trend in restarting antithrombotic medication may have occurred in our study. Further, antiplatelet therapy, a first-line recommendation for secondary prevention of MI, has been shown to be the inferior to anticoagulant therapy in alleviating the thromboembolic risk in atrial fibrillation.24 These factors likely explain the increased ischemic stroke risk after SDH, and lack of an association between SDH and MI risk observed in our study. Moreover, given the retrospective nature of claims data, subtle coronary ischemic events may have been missed resulting in an underestimation of the MI risk after SDH.
Our study has several important limitations. First, we lacked information on antithrombotic medication use and resumption. We tried to address this concern by performing a sensitivity analysis that excluded patients with strong indications for antithrombotic medications, a subset of patients in whom these medications were most likely to be reinstated. Second, our study was likely subject to surveillance bias since patients with SDH are more likely to undergo extensive neuroimaging than cardiac workup, which may have resulted in a higher incidental diagnosis of covert brain infarction than MI. Third, while errors in misclassification of events are possible, the ICD-9-CM codes for SDH, ischemic stroke, and MI used in this study have been previously validated to have high specificity and positive predictive value.25, 26 Fourth, given the nature of administrative claims data, we did not have information on the chronicity of SDH (acute vs. chronic), etiological classification of ischemic stroke, or the subtype of MI. Finally, inclusion of patients older than 66 years of age introduced selection bias in our study, which limits the generalizability of our results, although this age group accurately reflects the median age of SDH patients in the general population.
Conclusions
Among Medicare beneficiaries, we found a heightened risk of arterial ischemic events, particularly ischemic stroke, in the 4 weeks after SDH. This increased risk may be driven by interruption of antithrombotic therapy after SDH diagnosis. Further study of the link between SDH and subsequent ischemic stroke may yield new strategies for improving long-term recovery after SDH.
Funding/Support:
SBM is supported by the National Institutes of Health (NIH) (K23NS105948) and the Leon Levy Foundation. AM is supported by the NIH (grant KL2TR002385), the American Heart Association (18CDA34110419) and the Leon Levy Foundation. GJF is supported by the NIH (K76AG059992, R03NS112859), the American Heart Association (18IDDG34280056), the Yale Pepper Scholar Award (P30AG021342) and the Neurocritical Care Society Research Fellowship. AB is supported by the NIH (K23NS100816). BBN is supported by the NIH (K23NS091395) and the Florence Gould Endowment for Discovery in Stroke. KNS is supported by the NIH (U24NS107215, U24NS107136, RO1NR018335, and U01NS106513), and the American Heart Association (17CSA33550004). HK is supported by the NIH (R01NS097443 and R01HL144541).
Disclosures: BBN serves as a member of the data and safety monitoring board for the PCORI-funded Transeptal vs Retrograde Aortic Ventricular Entry to Reduce Systemic Emboli (TRAVERSE) trial, and has received personal fees for medicolegal consulting on stroke. KNS reports grants from Hyperfine, Biogen, and Astrocyte unrelated to this work. All other authors report no conflict of interest for this study. HK serves as the co-PI for the NIH-funded AtRial Cardiopathy and Antithrombotic Drugs In Prevention After Cryptogenic Stroke (ARCADIA) trial which receives in-kind study drug from the BMS-Pfizer Alliance and in-kind study assays from Roche Diagnostics, serves as a steering committee member of Medtronic’s Stroke AF trial (uncompensated), serves on an endpoint adjudication committee for a trial of empagliflozin for Boehringer-Ingelheim, and has served on an advisory board for Roivant Sciences related to Factor XI inhibition.
References
- 1.Frontera JA, Egorova N, Moskowitz AJ. National trend in prevalence, cost, and discharge disposition after subdural hematoma from 1998–2007. Crit Care Med. 2011;39:1619–1625 [DOI] [PubMed] [Google Scholar]
- 2.Bartek J Jr., Sjavik K, Kristiansson H, Stahl F, Fornebo I, Forander P, et al. Predictors of recurrence and complications after chronic subdural hematoma surgery: A population-based study. World Neurosurg. 2017;106:609–614 [DOI] [PubMed] [Google Scholar]
- 3.Dent DL, Croce MA, Menke PG, Young BH, Hinson MS, Kudsk KA, et al. Prognostic factors after acute subdural hematoma. J Trauma. 1995;39:36–42; discussion 42–33 [DOI] [PubMed] [Google Scholar]
- 4.Morris NA, Merkler AE, Parker WE, Claassen J, Connolly ES, Sheth KN, et al. Adverse outcomes after initial non-surgical management of subdural hematoma: A population-based study. Neurocrit Care. 2016;24:226–232 [DOI] [PubMed] [Google Scholar]
- 5.Kowalski RG, Haarbauer-Krupa JK, Bell JM, Corrigan JD, Hammond FM, Torbey MT, et al. Acute ischemic stroke after moderate to severe traumatic brain injury: Incidence and impact on outcome. Stroke. 2017;48:1802–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu SW, Huang LC, Chung WF, Chang HK, Wu JC, Chen LF, et al. Increased risk of stroke in patients of concussion: A nationwide cohort study. Int J Environ Res Public Health. 2017;14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hylek EM, Singer DE. Risk factors for intracranial hemorrhage in outpatients taking warfarin. Ann Intern Med. 1994;120:897–902 [DOI] [PubMed] [Google Scholar]
- 8.Wysowski DK, Nourjah P, Swartz L. Bleeding complications with warfarin use: A prevalent adverse effect resulting in regulatory action. Arch Intern Med. 2007;167:1414–1419 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Medicare and Medicaid Services. Medicare Limited Dataset Files. https://www.cms.gov/Research-Statistics-Data-and-Systems/Files-for-Order/LimitedDataSets/. Accessed on August 11, 2019.
- 10.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of chronic diseases. 1987;40:373–383 [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with icd-9-cm administrative databases. Journal of clinical epidemiology. 1992;45:613–619 [DOI] [PubMed] [Google Scholar]
- 12.McCormick N, Bhole V, Lacaille D, Avina-Zubieta JA. Validity of diagnostic codes for acute stroke in administrative databases: A systematic review. PLoS One. 2015;10:e0135834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tirschwell DL, Longstreth WT Jr. Validating administrative data in stroke research. Stroke. 2002;33:2465–2470 [DOI] [PubMed] [Google Scholar]
- 14.McCormick N, Lacaille D, Bhole V, Avina-Zubieta JA. Validity of myocardial infarction diagnoses in administrative databases: A systematic review. PLoS One. 2014;9:e92286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill MD, Silver FL, Austin PC, Tu JV. Rate of stroke recurrence in patients with primary intracerebral hemorrhage. Stroke. 2000;31:123–127 [DOI] [PubMed] [Google Scholar]
- 16.Casolla B, Moulin S, Kyheng M, Henon H, Labreuche J, Leys D, et al. Five-year risk of major ischemic and hemorrhagic events after intracerebral hemorrhage. Stroke. 2019;50:1100–1107 [DOI] [PubMed] [Google Scholar]
- 17.Hanger HC, Wilkinson TJ, Fayez-Iskander N, Sainsbury R. The risk of recurrent stroke after intracerebral haemorrhage. J Neurol Neurosurg Psychiatry. 2007;78:836–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weimar C, Benemann J, Terborg C, Walter U, Weber R, Diener HC, et al. Recurrent stroke after lobar and deep intracerebral hemorrhage: A hospital-based cohort study. Cerebrovasc Dis. 2011;32:283–288 [DOI] [PubMed] [Google Scholar]
- 19.Lerario MP, Gialdini G, Lapidus DM, Shaw MM, Navi BB, Merkler AE, et al. Risk of ischemic stroke after intracranial hemorrhage in patients with atrial fibrillation. PLoS One. 2015;10:e0145579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2015;46:2032–2060 [DOI] [PubMed] [Google Scholar]
- 21.Murthy SB, Gupta A, Merkler AE, Navi BB, Mandava P, Iadecola C, et al. Restarting anticoagulant therapy after intracranial hemorrhage: A systematic review and meta-analysis. Stroke. 2017;48:1594–1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Biffi A, Kuramatsu JB, Leasure A, Kamel H, Kourkoulis C, Schwab K, et al. Oral anticoagulation and functional outcome after intracerebral hemorrhage. Ann Neurol. 2017;82:755–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pennlert J, Asplund K, Carlberg B, Wiklund PG, Wisten A, Asberg S, et al. Antithrombotic treatment following intracerebral hemorrhage in patients with and without atrial fibrillation. Stroke. 2015;46:2094–2099 [DOI] [PubMed] [Google Scholar]
- 24.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, et al. Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817 [DOI] [PubMed] [Google Scholar]
- 25.Kokotailo RA, Hill MD. Coding of stroke and stroke risk factors using international classification of diseases, revisions 9 and 10. Stroke. 2005;36:1776–1781 [DOI] [PubMed] [Google Scholar]
- 26.Williams GR, Jiang JG, Matchar DB, Samsa GP. Incidence and occurrence of total (first-ever and recurrent) stroke. Stroke; a journal of cerebral circulation. 1999;30:2523–2528 [DOI] [PubMed] [Google Scholar]


